- Department of Cardiology, Children's Hospital of Soochow University, Suzhou, China
Objectives: To evaluate the change of left ventricular (LV) systolic function after transcatheter patent ductus arteriosus (PDA) closure in children, and to identify whether echocardiography parameters could be the predictors of LV dysfunction post-PDA closure if present.
Methods: This study enrolled 191 pediatric PDA patients, and all of them underwent successful transcatheter PDA closure between January 2016 and December 2018. The patent ductus arteriosus diameter (PDAd), aortic root diameter (AOd), left atrial diameter (LAd), right ventricular outflow tract dimension (RVOT), LV end-diastolic dimension (LVEDD), and LV end-systolic dimension (LVESD) were all measured by echocardiography at pre-closure, post-closure (within 24 h after the procedure), and follow-up (3 months after the procedure). The ratio of PDAd to AOd (PDAd/AOd), the ratio of LAd to AOd (LAd/AOd), the left ventricular ejection fraction (LVEF), and the fractional shortening (FS) were calculated.
Results: The LAd, LVESD, LVEDD, FS, and LVEF decreased significantly in the 24 h after closure, compared to pre-closure levels. However, all echocardiography parameters recovered to pre-closure levels at 3 months after PDA closure in all patients. Moreover, the pre-closure LAd, LVEF, PDAd/AOd, and LAd/AOd were higher in the patients with post-closure LV systolic dysfunction than in those without post-closure LV systolic dysfunction. Furthermore, the pre-closure LVEF, PDAd/AOd, and LAd/AOd were correlated with the post-closure LVEF, and pre-closure LVEF ≤ 66.5%, PDAd/AOd ≥ 0.28, and LAd/AOd ≥ 1.54 predict the post-closure LV systolic dysfunction.
Conclusion: Transcatheter closure of PDA causes a significant deterioration in LV systolic function early after PDA closure, which recovered completely within 3 months of post-closure in children. Pre-closure LVEF, PDAd/AOd, and LAd/AOd can be the predictors of post-closure left ventricular systolic dysfunction.
Introduction
Patent ductus arteriosus (PDA) is a common form of congenital heart disease with a left-to-right shunt (1). It has a broad spectrum of clinical manifestations, and the natural history of PDA mainly depends upon its size. Hemodynamically significant PDA leads to a left ventricle (LV) volume overload and remodeling and ultimately leads to severe complications, such as congestive heart failure, Eisenmenger's syndrome, atrial arrhythmias, endarteritis, and ductus aneurysm (2–4).
Transcatheter closure of PDA has been in development for nearly 30 years. Now, transcatheter PDA closure has been proven to be safe and effective with short- and long-term results comparable to surgical closure, and it has become the leading approach to the closure of most instances of PDA (5). Recently, several reports demonstrated that left ventricular systolic properties altered in adult PDA patients (6, 7), and PDA closure also led to an immediate deterioration of LV systolic function in children (8, 9). However, pre-closure predictors of the LV systolic dysfunction after PDA closure have not yet been clearly demonstrated.
Echocardiography is the most common diagnostic method that provides information regarding PDA size and hemodynamics, and it is also the most frequently used method for the evaluation of cardiac chamber size and LV systolic performance (10). Moreover, these parameters obtained by echocardiography correlate well with those measured during cardiac catheterization and radionuclide angiography (11, 12), and the possibility of the use of echocardiographic parameters in risk assessment for adverse cardiac events has been proven in recent studies (13–15). Therefore, the present study aimed to investigate the changes of LV systolic function after PDA closure, and to identify whether echocardiography indicators, if present, could be the predictors of LV dysfunction after PDA closure.
Methods
Study Population
The study was approved by the institutional ethics committee at the Children's Hospital of Soochow University, and written informed consent was obtained from the patients' parents in all cases.
This study enrolled 191 children. All of them were diagnosed with isolated PDA and underwent successful transcatheter PDA closure between January 2016 and December 2018 in the cardiology department of the Children's Hospital of Soochow University. Patients of unsuitable PDA size for interventional closure were excluded.
Echocardiography
All echocardiographic examinations were carried out using the General Electric (GE) VIVID 7 ultrasound (Horten, Norway) with M4S and 5S probe and the GE EchoPAC workstation (BT 09, Horten, Norway). Some PDA children required sedation for echocardiography examination. For each PDA patient, echocardiography examinations were conducted at pre-closure, post-closure (within 24 h after PDA closure), and follow-up (3 months after PDA closure), respectively.
The patent ductus arteriosus diameter (PDAd) was measured in the high left parasternal short-axis view. The aortic root diameter (AOd), left atrial diameter (LAd), right ventricular outflow tract dimension (RVOT), LV end-diastolic dimension (LVEDD), and LV end-systolic dimension (LVESD) were obtained from the parasternal long-axis view. From these measurements, the following LV parameters were calculated: Fractional shortening , and LV ejection fraction (EF) = , where and (16). To normalized echocardiographic indicators, the ratio of LAd to AOd (LAd/AOd) as well as the ratio of PDAd to AOd (PDAd/AOd) was calculated. LV systolic dysfunction was defined as a post-PDA closure LVEF of <55% (8).
Cardiac Catheterization
Midazolam, ketamine, or propofol were used for sedation in all patients, and a dose of 100 unit/kg of heparin was given after vein and artery puncture. The lateral aortogram was performed at the distal aortic arch before PDA closure. Transcatheter closure of PDA was performed using an antegrade or retrograde technique. An additional aortogram was performed to confirm complete shutdown after the procedure.
Statistical Analysis
Data are expressed as mean ± standard deviation. Changes in echocardiographic parameters were analyzed with paired t-test. The correlation between two continuous variables was determined using linear regression analysis. Multiple stepwise linear regression analyses were used to identify pre-closure echocardiography indicators of post-closure LV systolic dysfunction. Firstly, several statistically significant risk factors were screened out with univariate analysis, and P < 0.05 was considered statistically significant. Afterwards, multivariate analysis was performed using variables that were significant on univariate analysis, and P < 0.1 was considered significant. Receiver operating characteristic (ROC) analysis was used to find optimal cut-offs for each parameter for when the post-closure LVEF was below 55%. All of the statistical analyses used the Statistical Package for the Social Sciences (SPSS), version 19.0 for Windows (SPSS, Chicago, IL, USA).
Result
Clinical Characteristics of Patients
The clinical features of these PDA patients are described in Table 1. There were 60 boys and 131 girls in this study, and the median age was 23 months (range 3–184 months) at the time of the procedure. The PDA pulmonic end size was 3.06 ± 1.25 mm. The closure was achieved in all 191 patients who underwent transcatheter, and Amplazter duct occluders were used in all cases. The heart rate, systolic blood pressure, and diastolic blood pressure levels of PDA patients were similar between pre- and post-closure (Supplemental Table 1).
The Comparison of Echocardiography Parameters Between Pre-closure and Post-closure
The LAd, LVESD, LVEDD, FS, and LVEF significantly decreased within 24 h after closure compared to pre-closure levels, while there was no difference in AOd and RVOT values between pre- and post-closure.
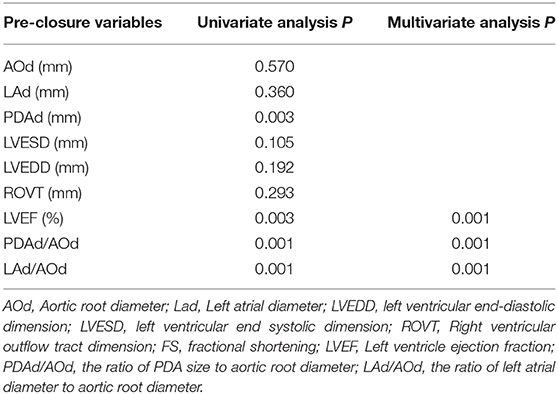
Of the 191 children, 27 of them showed LV systolic dysfunction within 24 h of PDA closure. The pre-closure LVEF, LAd, PDAd/AOd, and LAd/AOd were higher in the patients with post-closure LV systolic dysfunction than those without post-closure LV systolic dysfunction (Table 2).
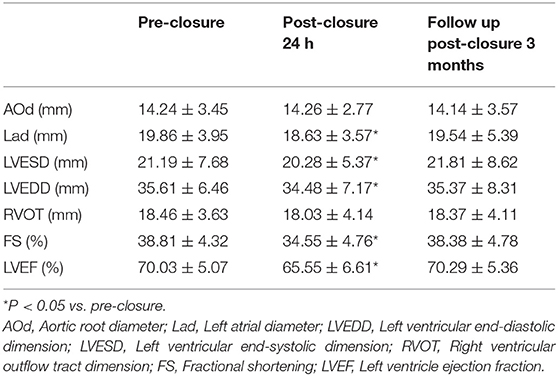
Table 2. Comparison of echocardiography parameters at pre-closure, post-PDA closure and follow up (n = 191).
At 3 months after PDA closure, the LAd, LVESD, and LVEDD were no different in comparison to the pre-closure baseline. Also, the LVEF and FS values recovered to pre-closure levels in all patients (Table 2).
The Correlation of Echocardiography Parameters With LVEF Post-closure
To identify the factors associated with post-closure LV systolic function, stepwise multiple linear regression analysis was conducted. Univariate linear regression analysis showed that pre-closure PDAd (r = −0.48, P < 0.01), PDAd/AOd (r = −0.50, P < 0.01), and LAd/AOd (r = −0.55, P < 0.01) negatively correlated with post-closure LVEF, and pre-closure LVEF (r = 0.66, P < 0.01) positively correlated with post-closure LVEF (Table 3).
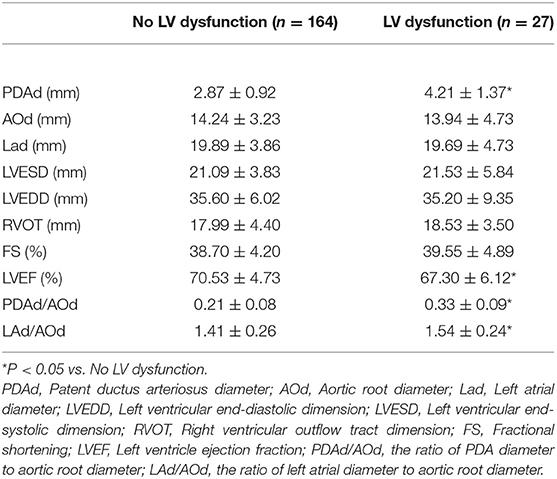
Table 3. Comparison of pre-closure echocardiography parameters in patients with or without post-closure LV systolic dysfunction.
Among these parameters, pre-closure LVEF (β = 0.443, P < 0.01), PDAd/AOd (β = −0.216, P < 0.01), and LAd/AOd (β = −0.211, P < 0.01) were statistically significant factors on multivariate stepwise linear regression analyses for the deterioration of post-closure LV (Table 4).
The Cutoff Value of Echocardiography Parameters Associated With Left Ventricular Dysfunction
By ROC analysis, the area under the curve (AUC) for pre-closure PDAd/AOd was 0.833, and pre-closure PDAd/AOd ≥ 0.28 showed a sensitivity of 81.5% and specificity of 98.5%. Moreover, pre-closure LAd/PDAd ≥ 1.54 showed a sensitivity of 81.5% and specificity of 68.5%. Its AUC was 0.692, whereas pre-closure LVEF ≤ 66.5% showed a sensitivity of 81.3% and specificity of 57.5% in predicting the immediate post-closure LV systolic dysfunction, and its AUC was 0.693 (Figure 1).
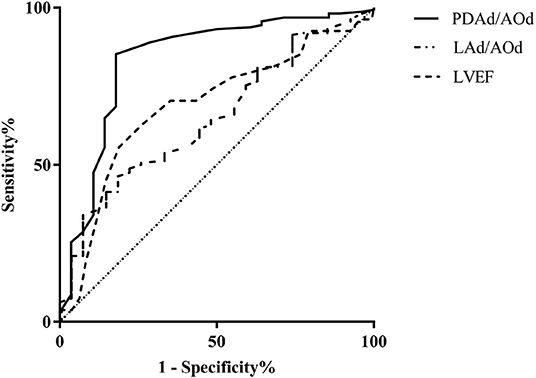
Figure 1. Receiver operating characteristic curve of pre-closure LVEF, PDAd/AOd, and LAd/AOd echocardiography parameters for predicting LV systolic dysfunction.
Discussion
PDA is a relatively common congenital heart defect. The incidence of PDA is ~1 per 2,000 live births in full-term newborns, and accounts for 5–10% of all congenital heart diseases (17). Now, the prevalence rate of PDA is 0.78 per 1,000 in China according to a recent report (18). Since the first transcatheter PDA closure was conducted by Porstmann et al. in 1967 (19), there have been many significant developments in PDA closure. Now, transcatheter PDA occlusion has become the priority choice to treat most PDA in both children and adults (5). The current study demonstrated an early deterioration of LV function following successful transcatheter ductal closure, which recovered completely within 3 months post-closure. Moreover, pre-closure PDAd/AOd, LAd/AOd, and LVEF were the predictors of post-closure LV dysfunction.
As is known, PDA frequently results in left ventricular volume overload, which is required to increases left ventricular output by Frank-Starling response, and it can therefore overcome the left-to-right shunt and maintain systemic circulation (20, 21). Because the left ventricular remodeling is caused by a significant left-to-right shunt through PDA, it is conceivable that left ventricular reverse remodeling occurs after ductal closure. In the current study, the LAd, LVESD, LVEDD, FS, and LVEF significantly decreased in the 24 h after PDA closure. Consistently, Gupta et al. also found there was a significant reduction in LVEDD and LVESD in immediate post-closure as compared to pre-closure baseline (22); these results confirmed that early PDA closure in childhood may benefit the remodeling of LV.
In 2005, Eerola et al. demonstrated that changes in LV systolic function were caused by PDA closure in children (9). Similarly, Galal et al. found that the closure of relatively large PDA led to a significant immediate deterioration of LV systolic performance in children (23). Consistent with these previous reports, the current study demonstrated that LVEF reduced immediately 24 h after PDA closure, which indicated impaired LV systolic function. The possible explanation for this is that when a hemodynamically significant PDA is closed it abolishes the left-to-right shunt, thereby reducing the preload of the LV. However, it also increases the afterload by eliminating the low-resistance pulmonary circulation from LV outflow circulation. Due to a phenomenon called “afterload mismatch,” this simultaneous reduction in the LV preload and increase in the afterload may lead to LV systolic dysfunction (24).
Furthermore, all of the LV dysfunction recovered to baseline in the follow-up periods in the current study, which is consistent with previous children studies (9, 23). However, about 11% of transcatheter PDA adult patients showed the persistent long-term deterioration of LV systolic function, according to Jeong's study (6). This discrepancy is probably due to the longer duration of volume overload and consequently more extensive and irreversible changes in LV in adults compared to children.
In a study conducted by Agha et al., PDAd was proved to be the predictor of post-closure LV systolic function (25). However, the current study found that PDAd/AOd is a predictor of post-closure LV systolic dysfunction, rather than the absolute diameter of PDA. This inconsistency may be due to age, which can affect the PDA size; “PDA size” is also a relative term with no standardization attached to it, which may limit its practical value somewhat. “PDAd/AOd,” therefore, is a better indicator in predicting post-closure LV systolic function, and these results suggest that the larger the PDAd/AOd value the more likely post-closure LV dysfunction would be. Interestingly, the LAd/AOd ratio also predicted the post-closure LV dysfunction in our study. As is known, a significant hemodynamic shunt via a PDA leads to the enlargement of the left heart and a decreased LV ejection fraction (26). LAd/AOd may thus reflect the severity of ductal shunting in these PDA patients; Iyer et al. also reported that a LAd/AOd of >1.4:1 was associated with a hemodynamically significant ductal flow in a preterm infant (27). Our results suggest that PDA closure should be conducted before LAd/AOd reaches more than 1.54 to achieve a normal LVEF after PDA closure.
The current study found that the incidence of LV systolic dysfunction was 14.1% in this cohort (n = 27/191). This is comparable with the studies conducted by Jeong et al. (6) (11.1%), Kim et al. (28) (18.6%) on the Korean population, and Eerola et al. (9) (15.2%) on the Finland cohort. However, it is much lower than a study conducted by Kiran et al., which reported the incidence of LV dysfunction is 22.8% in Indian PDA patients (29). These studies suggest the influencing factors of LV systolic dysfunction may also involve ethnicity and social factors in addition to the heart hemodynamics parameters.
The present study had some limitations. Firstly, this study was retrospective and in a single center despite having a relatively large sample size. Secondly, the study only involved the assessment of the systolic function of the LV by two-dimension echocardiography method.
In conclusion, transcatheter closure of PDA is associated with reversible LV systolic dysfunction in children patients. Pre-closure PDAd/AOd ≥ 0.28, LAd/AOd ≥ 1.54, and LVEF ≤ 66.5%, measured by echocardiography, were the cutoff values used to predict post-PDA closure LV systolic dysfunction. The results of the present study can provide a useful and convenient strategy to predict who (of the patients undergoing PDA device closure) is likely to have LV dysfunction after PDA closure in clinical practice.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the ethics committee of the Children's Hospital of Soochow University. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author's Note
The authors declare that neither this manuscript nor any similar paper, in whole or in part, have been or will be submitted to or published in any other scientific journal.
Author Contributions
MH, WQ, and LS conceived and designed the study and analyzed data and wrote the manuscript. MH, WQ, BW, WZ, JZ, YD, QX, JH, JS, LC, HL, and LS performed this study. BW, WZ, JZ, YD, QX, JH, JS, LC, and HL reviewed and edited the manuscript. All authors read and approved the manuscript.
Funding
This work was supported by a grant from the National Natural Science Foundation of China (ID: 81300692) and the Natural Sciences Foundation of the Jiangsu Higher Education Institutions of China (ID: 17KJB320014).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Dr. Cui Hou and Dr. Hui Wang (Department of echocardiography, Children's Hospital of Soochow University) for their technical assistance.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2019.00409/full#supplementary-material
References
1. Baruteau AE, Hascoet S, Baruteau J, Boudjemline Y, Lambert V, Angel CY, et al. Transcatheter closure of patent ductus arteriosus: past, present and future. Arch Cardiovasc Dis. (2014) 107:122–32. doi: 10.1016/j.acvd.2014.01.008
2. Bessinger FJ, Blieden LC, Edwards JE. Hypertensive pulmonary vascular disease associated with patent ductus arteriosus. Primary or secondary? Circulation. (1975) 52:157–61. doi: 10.1161/01.CIR.52.1.157
3. Espino-Vela J, Cardenas N, Cruz R. Patent ductus arteriosus. With special reference to patients with pulmonary hypertension. Circulation. (1968) 38:45–60. doi: 10.1161/01.CIR.38.1S5.V-45
4. Schneider DJ. The patent ductus arteriosus in term infants, children, and adults. Semin Perinatol. (2012) 36:146–53. doi: 10.1053/j.semperi.2011.09.025
5. Baumgartner H, Bonhoeffer P, De Groot NM, de Haan F, Deanfield JE, Galie N, et al. ESC Guidelines for the management of grown-up congenital heart disease (new version 2010). Eur Heart J. (2010) 31:2915–57. doi: 10.1093/eurheartj/ehq249
6. Jeong YH, Yun TJ, Song JM, Park JJ, Seo DM, Koh JK, et al. Left ventricular remodeling and change of systolic function after closure of patent ductus arteriosus in adults: device and surgical closure. Am Heart J. (2007) 154:436–40. doi: 10.1016/j.ahj.2007.04.045
7. Zhang CJ, Huang YG, Huang XS, Huang T, Huang WH, Xia CL, et al. Transcatheter closure of large patent ductus arteriosus with severe pulmonary arterial hypertension in adults: immediate and two-year follow-up results. Chin Med J. (2012) 125:3844–50. doi: 10.1136/hrt.2010.208967.495
8. Galal MO, Amin M, Hussein A, Kouatli A, Al-Ata J, Jamjoom A. Left ventricular dysfunction after closure of large patent ductus arteriosus. Asian Cardiovasc Thorac Ann. (2005) 13: 24–9. doi: 10.1177/021849230501300106
9. Eerola A, Jokinen E, Boldt T, Pihkala J. The influence of percutaneous closure of patent ductus arteriosus on left ventricular size and function: a prospective study using two- and three-dimensional echocardiography and measurements of serum natriuretic peptides. J Am Coll Cardiol. (2006) 47:1060–6. doi: 10.1016/j.jacc.2005.09.067
10. Kindler A, Seipolt B, Heilmann A, Range U, Rudiger M, Hofmann SR. Development of a diagnostic clinical score for hemodynamically significant patent ductus arteriosus. Front Pediatr. (2017) 5:280. doi: 10.3389/fped.2017.00280
11. Martin RW, Graham MM, Kao R, Bashein G. Measurement of left ventricular ejection fraction and volumes with three-dimensional reconstructed transesophageal ultrasound scans: comparison to radionuclide and thermal dilution measurements. J Cardiothorac Anesth. (1989) 3:260–8. doi: 10.1016/0888-6296(89)90105-1
12. Gottsauner-Wolf M, Schedlmayer-Duit J, Porenta G, Gwechenberger M, Huber K, Glogar D, et al. Assessment of left ventricular function: comparison between radionuclide angiography and semiquantitative two-dimensional echocardiographic analysis. Eur J Nucl Med. (1996) 23:1613–8. doi: 10.1007/BF01249624
13. van Everdingen WM, Walmsley J, Cramer MJ, van Hagen I, De Boeck B, Meine M, et al. Echocardiographic prediction of cardiac resynchronization therapy response requires analysis of both mechanical dyssynchrony and right ventricular function: a combined analysis of patient data and computer simulations. J Am Soc Echocardiogr. (2017) 30:1012–1020.e2. doi: 10.1016/j.echo.2017.06.004
14. Untersteller K, Girerd N, Duarte K, Rogacev KS, Seiler-Mussler S, Fliser D, et al. NT-proBNP and echocardiographic parameters for prediction of cardiovascular outcomes in patients with CKD stages G2-G4. Clin J Am Soc Nephrol. (2016) 11:1978–88. doi: 10.2215/CJN.01660216
15. Yang LT, Tsai WC, Su HM. Echocardiographic parameters versus CHA2DS2-VASc score in prediction of overall cardiac events, heart failure, and stroke in non-valvular atrial fibrillation. Cardiol J. (2018) 25:60–71. doi: 10.5603/CJ.a2017.0086
16. Akiba T, Yoshikawa M, Otaki S, Kobayashi Y, Nakasato M, Suzuki H, et al. Echocardiographic measurements of left ventricle in normal infants and children. Tohoku J Exp Med. (1986) 149: 31–37. doi: 10.1620/tjem.149.31
17. Mitchell SC, Korones SB, Berendes HW. Congenital heart disease in 56,109 births. Incidence and natural history. Circulation. (1971) 43:323–32. doi: 10.1161/01.CIR.43.3.323
18. Zhao QM, Liu F, Wu L, Ma XJ, Niu C, Huang GY. Prevalence of congenital heart disease at live birth in China. J Pediatr. (2019) 204:53–8. doi: 10.1016/j.jpeds.2018.08.040
19. Porstmann W, Wierny L, Warnke H. Closure of persistent ductus arteriosus without thoracotomy. Ger Med Mon. (1967) 12:259–61.
20. Kluckow M, Lemmers P. Hemodynamic assessment of the patent ductus arteriosus: beyond ultrasound. Semin Fetal Neonatal Med. (2018) 23:239–44. doi: 10.1016/j.siny.2018.04.002
21. Anilkumar M. Patent ductus arteriosus. Cardiol Clin. (2013) 31:417–30. doi: 10.1016/j.ccl.2013.05.006
22. Gupta SK, Krishnamoorthy K, Tharakan JA, Sivasankaran S, Sanjay G, Bijulal S, et al. Percutaneous closure of patent ductus arteriosus in children: Immediate and short-term changes in left ventricular systolic and diastolic function. Ann Pediatr Cardiol. (2011) 4:139–44. doi: 10.4103/0974-2069.84652
23. Galal MO, Arfi MA, Nicole S, Payot M, Hussain A, Qureshi S. Left ventricular systolic dysfunction after transcatheter closure of a large patent ductus arteriosus. J Coll Physicians Surg Pak. (2005) 15:723–5. doi: 10.11.2005/JCPSP.723725
24. Ross JJ. Afterload mismatch and preload reserve: a conceptual framework for the analysis of ventricular function. Prog Cardiovasc Dis. (1976) 18:255–64. doi: 10.1016/0033-0620(76)90021-9
25. Agha HM, Hamza HS, Kotby A, Ganzoury M, Soliman N. Predictors of transient left ventricular dysfunction following transcatheter patent ductus arteriosus closure in pediatric age. J Saudi Heart Assoc. (2017) 29:244–51. doi: 10.1016/j.jsha.2017.02.002
26. Jarmakani MM, Graham TJ, Canent RJ, Spach MS, Capp MP. Effect of site of shunt on left heart-volume characteristics in children with ventricular septal defect and patent ductus arteriosus. Circulation. (1969) 40:411–8. doi: 10.1161/01.CIR.40.3.411
27. Iyer P, Evans N. Re-evaluation of the left atrial to aortic root ratio as a marker of patent ductus arteriosus. Arch Dis Child Fetal Neonatal Ed. (1994) 70:F112–7. doi: 10.1136/fn.70.2.F112
28. Kim YH, Choi HJ, Cho Y, Lee SB, Hyun MC. Transient left ventricular dysfunction after percutaneous patent ductus arteriosus closure in children. Korean Circ J. (2008) 38:596–600. doi: 10.4070/kcj.2008.38.11.596
Keywords: patent ductus arteriosus, transcatheter closure, left ventricular dysfunction, echocardiography, children
Citation: Hou M, Qian W, Wang B, Zhou W, Zhang J, Ding Y, Xu Q, Huang J, Shen J, Cao L, Lv H and Sun L (2019) Echocardiographic Prediction of Left Ventricular Dysfunction After Transcatheter Patent Ductus Arteriosus Closure in Children. Front. Pediatr. 7:409. doi: 10.3389/fped.2019.00409
Received: 26 June 2019; Accepted: 24 September 2019;
Published: 15 October 2019.
Edited by:
Cecile Tissot, Clinique des Grangettes, SwitzerlandReviewed by:
Hopewell Nkosipendule Ntsinjana, University of the Witwatersrand, South AfricaFederico Gutierrez-Larraiya, University Hospital La Paz, Spain
Copyright © 2019 Hou, Qian, Wang, Zhou, Zhang, Ding, Xu, Huang, Shen, Cao, Lv and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ling Sun, c3Vubnk3MG1haWxAMTYzLmNvbQ==
 Miao Hou
Miao Hou Weiguo Qian
Weiguo Qian Bo Wang
Bo Wang Wanping Zhou
Wanping Zhou Jianmin Zhang
Jianmin Zhang Jie Huang
Jie Huang Jie Shen
Jie Shen Lei Cao
Lei Cao Ling Sun
Ling Sun