- 1Division of Neonatology, College of Medicine, University of Florida, Gainesville, FL, United States
- 2Analysis Group Inc., Boston, MA, United States
- 3Shire, A Takeda Company, Lexington, MA, United States
Background: Infants born extremely preterm are at high risk of developing bronchopulmonary dysplasia (BPD). This study aimed to assess the incremental health care burden of BPD and associated comorbidities among extremely preterm infants in the United States.
Methods: Health service claims in the Premier Perspective database were retrospectively analyzed for infants born at ≤28 weeks gestation who were admitted to neonatal intensive care during birth hospitalization and survived to a postmenstrual age of ≥36 weeks. Gestational age (GA) at birth and BPD status of infants was determined based on International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes recorded in the database.
Results: Of the 12,017 infants included, 4,904 (40.8%) had BPD. BPD increased with decreasing GA: 67.4% of infants born at <24 weeks GA had BPD vs. 28.7% of those born at 27–28 weeks. Infants with BPD had significantly longer hospital stays following birth than those without (mean [standard deviation (SD)] 102 [34] vs. 83 [24] days, respectively, P < 0.001), and incurred higher total charges (mean [SD] $799,499 [$535,528] vs. $588,949 [$377,137], respectively, P < 0.001). Mean total charges incurred during index hospitalization decreased as GA at birth increased, with GA having a bigger effect than presence or absence of BPD. During their first year, infants with BPD had a higher in-hospital late mortality rate than those without (1.9 vs. 0.6%), and were more likely to have two or more hospital encounters following birth hospitalization (58.0 vs. 48.2%). Among infants who had two or more encounters after discharge, those with BPD experienced a higher percentage of pulmonary symptoms than those without (46.3 vs. 38.9%). Comparison with infants who did not have BPD, retinopathy of prematurity, or intraventricular hemorrhage showed that BPD is the main complication contributing to increased length of stay, costs, in-hospital mortality, and additional health care encounters.
Conclusion: BPD is a key contributor to the large health care burden associated with extremely preterm birth. However, GA at birth has a bigger effect on health care costs for extremely preterm infants than the presence of BPD.
Introduction
Each year, >28,000 babies in the United States are born extremely preterm (<28 weeks gestation) (1). Survival of extremely preterm infants has steadily increased in recent years owing to advances in maternal and neonatal care (2, 3). In academic centers in the United States, the majority of infants born at a gestational age (GA) of ≥24 weeks will survive until discharge from their birth hospital (2). However, infants who survive extremely preterm birth are at increased risk for multiple morbidities that are associated with significant clinical burden during the newborn period, and require neonatal intensive care and long hospital stays (4, 5).
One of the most prevalent and clinically significant morbidities among infants born preterm is bronchopulmonary dysplasia (BPD), which can develop from the disruption of lung development (6–8). An increase in the incidence of BPD in extremely preterm infants in the United States has been reported, and this may be due in part to the increased survival of infants born at younger GAs (2). Infants with BPD require significantly longer hospital stays at birth and have a higher risk of delayed discharge than those without BPD (9). Because they may experience pulmonary issues for a long time following the neonatal period, infants with BPD are also more likely to require rehospitalization and make increased use of health care services during their first years of life (10). Over the long term, preterm infants who develop BPD are more commonly affected by chronic respiratory problems, cardiovascular impairment, and neurodevelopmental delay than those who do not develop BPD (8).
Other morbidities of extremely preterm birth, including intraventricular hemorrhage (IVH) and retinopathy of prematurity (ROP), may also have adverse outcomes. IVH is a notable cause of brain injury and is associated with developmental delay and neurosensory impairment; ROP is a significant cause of visual impairment, requiring specialized treatment in severe cases to mitigate permanent vision loss (11, 12). Because the incidence and severity of these morbidities increase with decreasing GA at birth, the same infants who develop BPD are also at high risk of developing ROP and IVH, adding to the already high burden of health care costs and resource utilization associated with extremely preterm infants (13, 14).
Insights into the health care burden of infants with BPD can help with management of medical care resources, counseling of parents, and development of effective treatments to improve patient outcomes and reduce care costs. The aim of this study was to assess the incremental health care burden of BPD and its interaction/association with comorbidities such as ROP and IVH among extremely preterm infants in urban centers in the United States, using data from the Premier Perspective health care services database. Presence or absence of BPD and comorbid conditions was determined based on diagnostic codes recorded in the database.
Materials and Methods
Data Source
This study was a retrospective analysis of the Premier Perspective database, which contains patient data in de-identified records from inpatient and outpatient visits to ~500 geographically diverse hospitals in the United States. Health care service utilization records in the database contain information on patients' demographic and clinical characteristics and details of billed services received during hospital visits, such as medications and therapeutic and diagnostic procedures (15).
Patient Selection
Data were retrieved on infants born between January 1, 2009 and June 30, 2015, who had an inpatient stay during their birth hospitalization of ≥1 day (index hospitalization). Of these infants, those selected for analysis were born at a GA of ≤28 weeks, admitted to the neonatal intensive care unit (NICU) during their index hospitalization, and discharged to home or died in the hospital after the postmenstrual age (PMA) of ≥36 weeks (Figure 1). To ensure that all birth hospitalization charges were captured, infants were only included if they had been discharged to home or died in the same hospital during index hospitalization, and did not transfer to another hospital. Infants who had zero costs during index hospitalization were excluded. GA at birth was determined from the highest classification code for GA [International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM)] recorded during index hospitalization (Supplementary Table 1). Although infants born at 28 completed weeks of gestation are outside the definition of extremely preterm, they were included in the analysis because they were captured under the ICD-9-CM code for 27–28 weeks GA. PMA at discharge or death was calculated as the GA at birth plus the number of weeks of index hospitalization until discharge or death.
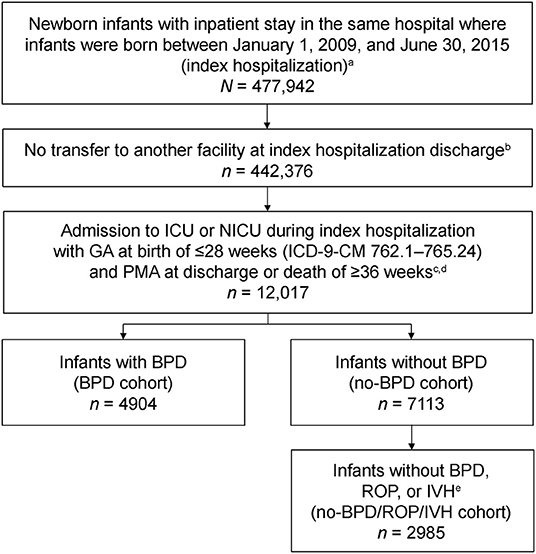
Figure 1. Selection of the analysis cohort from the Premier Perspective claims database. BPD, bronchopulmonary dysplasia; GA, gestational age; ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification; ICU, intensive care unit; IVH, intraventricular hemorrhage; NICU, neonatal intensive care unit; PMA, postmenstrual age; ROP, retinopathy of prematurity. aIndex hospitalization was defined as the newborn infant's first hospitalization. bOnly infants discharged to home or those who died during index hospitalization were included in the analysis. cOnly patients with non-zero costs during index hospitalization were included. dPMA at discharge was calculated as the GA at birth plus the length of stay during index hospitalization until discharge. ePresence of morbidities was identified from ICD-9-CM codes recorded during index hospitalization.
Analysis
Presence of BPD, ROP, IVH, and other select comorbidities were determined from ICD-9-CM codes recorded in the database during index hospitalization (Supplementary Table 2). Infants were categorized into two groups for analysis based on the presence or absence of BPD. As BPD and chronic lung disease (CLD) are coded using the same ICD-9-CM codes, the BPD cohort included both. For infants without BPD (no-BPD cohort), a subgroup was created to analyze those who also did not have ROP or IVH (no-BPD/ROP/IVH cohort; Figure 1). Statistical comparisons between cohorts were conducted for the study outcomes with the Wilcoxon rank-sum test.
Outcomes
Length of stay (LOS), charges, and costs during index hospitalization were determined for each cohort, as were in-hospital mortality and rates of readmission during the first year after index hospitalization. Charges were calculated from coding of billable items for each patient, on the basis of health care providers' listed charges. Costs were computed from the actual cost to treat each patient, including supplies, labor, and depreciation of equipment; total costs were calculated as the sum of variable costs (direct) and fixed costs (overhead). Charges and costs were inflated to 2015 US dollars using the US Medical Service Consumer Price Index. Pulmonary symptoms reported during the first year of life were analyzed for infants who had at least two additional health care encounters of any type (outpatient visit, emergency department visit, or hospital readmission associated with a pulmonary diagnosis) following index hospitalization. Pulmonary-related medication use associated with outpatient visits during the first year was analyzed for infants who had at least two additional health care encounters following index hospitalization. Pulmonary symptoms and medications were identified from Current Procedural Terminology and ICD-9-CM codes, and by searching for the generic medication name in standard charge code descriptions (Supplementary Tables 2,3).
Results
Patient Characteristics
A total of 12,017 infants born at ≤28 weeks GA were included in the analysis. Of these, 4,904 (40.8%) had BPD and 7,113 (59.2%) did not have BPD (Table 1). Of the infants who did not have BPD, 2,985 (42.0%) also did not have ROP or IVH. BPD increased with decreasing GA: 353 of 524 (67.4%) infants born at <24 weeks GA had BPD vs. 1,747 of 6,091 (28.7%) infants born at 27–28 weeks GA.

Table 1. Demographics and clinical characteristics of extremely preterm infants with and without BPD at the time of index hospitalization (N = 12,017).
The incidence and severity of ROP and IVH were, respectively, higher in infants with BPD than in those without (Table 1). Among infants with BPD, 56.1% had ROP and 33.7% had IVH, whereas among those without BPD, 45.2% had ROP and 25.9% had IVH. For infants who were born at <24 weeks GA and survived to 36 weeks PMA, a similar pattern of worse outcomes was observed among infants with BPD vs. those without (data not shown in Table 1). Among 353 infants who were born at <24 weeks GA, survived to 36 weeks PMA, and had BPD, 75.4% had ROP, 58.4% had IVH, and 3.7% had late death in the hospital within a year of birth. The corresponding percentages among infants of <24 weeks GA who did not have BPD (n = 171) were ROP 71.3%, IVH 56.7%, and late mortality 2.9%. Where ROP stage was specified, the incidence of severe ROP (stages 3–5) among infants of all included GAs was 23.1% in the BPD cohort and 14.4% in the no-BPD cohort (P < 0.001). The incidence of severe IVH (grades 3–4) was 30.9% (482/1,560 cases with IVH grade specified) for infants with BPD, and 26.3% (450/1,710) for those without BPD (P = 0.004). Other complications that were more common in infants with BPD included necrotizing enterocolitis, bacterial sepsis, and patent ductus arteriosus (Table 1).
Resource Utilization
Preterm infants who had BPD remained in their hospital of birth significantly longer than those who did not have BPD (mean [standard deviation (SD)] LOS: 102 [34] vs. 83 [24] days, respectively; P < 0.001) and those who did not have BPD, ROP, or IVH (mean [SD] LOS: 102 [34] vs. 77 [20] days; P < 0.001; Table 2). The LOS for the no-BPD cohort was slightly longer than that for the no-BPD/ROP/IVH cohort (mean [SD] LOS: 83 [24] vs. 77 [20] days, respectively; P < 0.001). LOS decreased as GA at birth increased (Table 2).
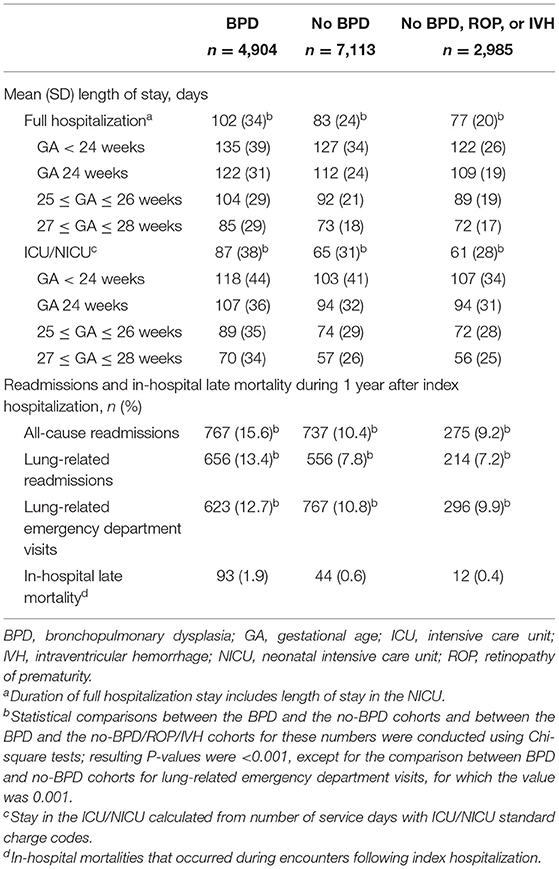
Table 2. Length of stay during index hospitalization and hospital readmission and mortality during 1 year after index hospitalization in extremely preterm infants with or without BPD (N = 12,017).
On average, preterm infants with BPD incurred higher total costs (mean [SD] $225,204 [$122,365]) than those without BPD (mean [SD] $171,097 [$93,498]; Figure 2). Infants without BPD, ROP, or IVH incurred a mean (SD) total cost of $153,306 ($83,324). Similar trends were seen in charges. Infants with BPD incurred higher total charges (mean [SD] $799,499 [$535,528]) than those without BPD (mean [SD] $588,949 [$377,137]; Figure 2). Infants without BPD, ROP, or IVH incurred the lowest mean (SD) total charge of $515,944 ($327,615), suggesting that BPD may be a bigger driver of costs than ROP or IVH.
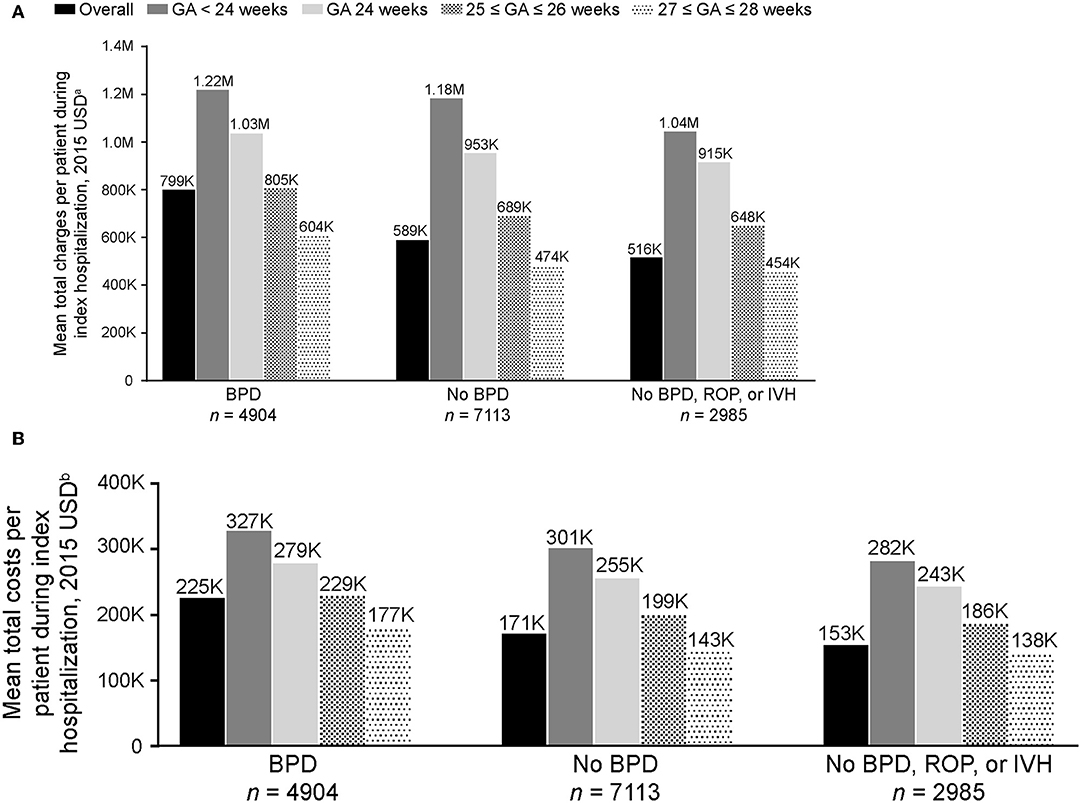
Figure 2. Mean (A) total charges and (B) costs per patient to treat extremely preterm infants with and without BPD during index hospitalization (N = 12,017). Charges and costs inflated to 2015 USD using the US Medical Service Consumer Price Index. BPD, bronchopulmonary dysplasia; GA, gestational age; IVH, intraventricular hemorrhage; ROP, retinopathy of prematurity; USD, United States dollars. aCharge of billable items for each patient. bActual cost to treat each patient, including supplies, labor, and depreciation of equipment; total costs were calculated as the sum of variable costs (direct) and fixed costs (overhead). The P-values for the comparisons of infants with overall BPD vs. no-BPD, and BPD vs. no-BPD/ROP/IVH were <0.001.
Mean total charges and costs incurred during index hospitalization decreased as GA at birth increased (Figure 2). The in-hospital rate of deaths during the first year after index hospitalization was higher for preterm infants with BPD (93/4,904 [1.9%]), compared with those without BPD (44/7113 [0.6%]) and those without BPD, ROP, or IVH (12/2985 [0.4%]; Table 2). Rates of all-cause and pulmonary-related readmissions to the hospital were significantly higher in infants with BPD compared with those without. Emergency room visits for pulmonary-related issues were also higher in infants with BPD (Table 2).
Pulmonary Symptoms and Pulmonary-Related Medication Use
Of the 12,017 preterm infants included in the analysis, 6,329 returned at least twice for an outpatient visit, emergency department visit, or readmission during the first year following their index hospitalization. It was more common for infants with BPD to have at least two of these additional health care encounters (2,845/4,904 [58.0%]) than for those without BPD (3,484/7,113 [49.0%]; BPD vs. no-BPD P < 0.001) and those without BPD, ROP, or IVH (1,439/2,985 [48.2%]; BPD vs. no-BPD/ROP/IVH P < 0.001). Among infants who had at least two additional encounters, those with BPD experienced a higher percentage of pulmonary symptoms (1,317/2,845 [46.3%]) than those without BPD (1,356/3,484 [38.9%]; BPD vs. no-BPD P < 0.001) and those without BPD, ROP, or IVH (529/1,439 [36.8%]; BPD vs. no-BPD/ROP/IVH P < 0.001). Although the five most common pulmonary symptoms/disorders reported were the same in all three cohorts (apnea, dyspnea, bronchiolitis, cough, and upper respiratory tract infections), pneumonia, asthma, reactive airway disease, and hypoxemia were notably more common in infants with BPD (Table 3). Among infants with at least two encounters, pulmonary medication use associated with outpatient visits during the first year after index hospitalization was reported in 474 (16.7%) infants with BPD, 536 (15.4%) infants without BPD, and 244 (17.0%) infants without BPD, ROP, or IVH (Figure 3). β2 agonists were the most common pulmonary medications used.
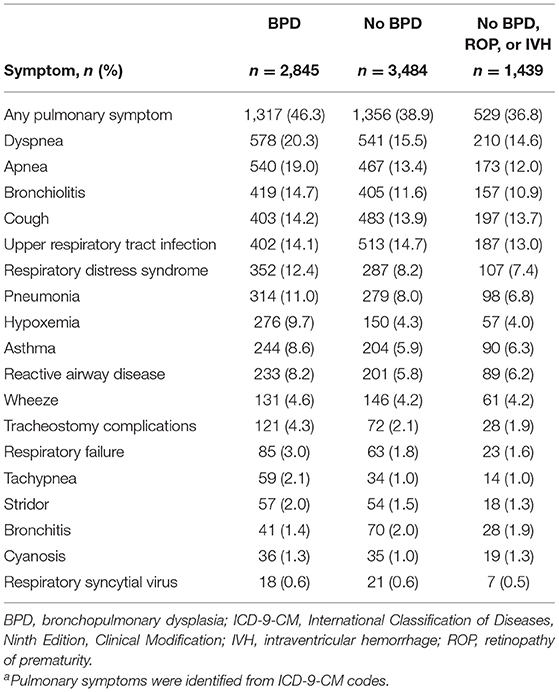
Table 3. Incidence of select pulmonary symptoms during 1 year after index hospitalization in preterm infants who had at least two additional health care encounters following index hospitalization (N = 6,329)a.
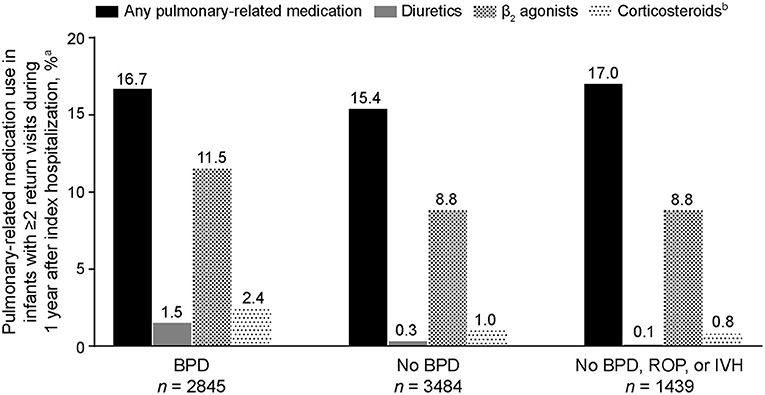
Figure 3. Pulmonary-related medication use during the first year of life in extremely preterm infants who had at least two additional health care encounters following index hospitalization (n = 6,329). BPD, bronchopulmonary dysplasia; IVH, intraventricular hemorrhage; ROP, retinopathy of prematurity. aInfants who had at least two encounters (outpatient visit, emergency room visit, or hospital readmission) after their index hospitalization; only pulmonary-related medications reported during outpatient visits are included. bInhaled formulations.
Discussion
Advances in neonatal care have led to increased survival of preterm infants born at earlier GAs. However, infants born before 28 weeks GA remain at high risk of developing BPD owing to lung development being interrupted as a consequence of extremely preterm birth (6, 7). The incidence of BPD in our cohort of infants born at or before 28 weeks GA was 40.8%, which is similar to the incidences of 44.7 and 40.8% reported in other multicenter studies in the United States, where BPD was defined as oxygen use at 36 weeks PMA (2, 16). The incidence of BPD decreased dramatically with increasing birth GA: a result that is reported consistently in studies of extremely preterm infants (2, 13).
In the present study, infants with BPD had significantly longer stays in their hospital of birth compared with those without BPD and those without BPD, ROP, or IVH. Observationally, however, LOS was more closely linked (inversely) to GA at birth than to presence of BPD. Infants born at earlier GAs had longer LOS than those born at later GAs, regardless of whether or not they had BPD. Prematurity itself is a strong risk factor for long hospital stays, although a study of infants with very low birth weights reported that infants with BPD required hospital stays that were ~4 weeks longer than those for infants without BPD (9, 17).
Analysis of the total costs and charges to treat extremely preterm infants during index hospitalization showed results in line with those for the length of hospital stay. Total costs and charges were influenced more by GA at birth than by the presence or absence of BPD, with infants born before 24 weeks GA incurring substantially higher costs and charges than those born at 27–28 weeks GA. For each GA category, costs and charges for infants with BPD were higher than for those without, and were lowest for those without BPD, ROP, or IVH. Beyond index hospitalization, infants in the BPD cohort had, on average, more hospital readmissions for lung-related and other issues during their first year of life than those in the no-BPD cohort. Other researchers have reported a rehospitalization rate that is more than two times higher in preterm infants with BPD than in those without BPD (10).
Extremely preterm infants with BPD were more likely to have at least two additional hospital encounters after their index hospitalization during their first year of life compared with those without BPD, and the encounters were often related to a pulmonary issue. Among infants who returned at least twice to the hospital during their first year, pneumonia, asthma, reactive airway disease, and hypoxemia were reported more frequently in infants with BPD than in those without, whereas rates of cough and upper respiratory tract infections were similar in both cohorts. Pulmonary-related medications during the first year were used in similar percentages of patients with BPD (16.7%), without BPD (15.4%), and without BPD, ROP, or IVH (17.0%), which could indicate that medication use may be related to conditions other than BPD and might provide persistent beneficial effects. Moreover, use of these medications has not been associated with any meaningful impact on the incidence or severity of BPD (18).
We acknowledge a number of limitations in our study. The Premier Perspective database only captures data from outpatient clinics and emergency department services associated with hospitals in the database network. Rates of outpatient visits, emergency department visits, hospital readmissions, and medication use are thus likely to be underestimated in our analysis owing to patients being lost to facilities outside of the database network for follow-up. Pulmonary medication use may be further underestimated because estimations were based on reported procedural codes and charge descriptions. In addition, the presence or absence of BPD was determined from ICD-9-CM codes reported in patient files. No information was obtained on the definition used to diagnose BPD or on the severity of the disease, and results may be influenced by clinician judgement. It should also be noted that BPD and CLD are coded using the same ICD-9-CM codes. Nonetheless, the BPD rates found are consistent with those based on clinical criteria, suggesting that coding issues for BPD were not significant in this cohort.
In conclusion, extremely preterm infants with BPD had longer hospital stays and higher costs during their initial hospitalization compared with those without BPD, and had higher rates of in-hospital mortality, hospital readmission, and pulmonary morbidities during their first year of life. Strategies to improve clinical outcomes for infants with BPD are important to reduce the large health care burden associated with extremely preterm birth. Our results also indicate that increasing the GA at birth, even by 1 week, has a larger impact on health care burden than presence of BPD.
Data Availability Statement
The data that support the findings of this study are available from Premier Perspective but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. However, data are available from the authors upon reasonable request and with permission of SS.
Author Contributions
MM, RA, AM, and SS contributed to the study design and interpretation of the results. RA, WG, and JZ contributed to data analysis and interpretation of the results. All authors provided sustained intellectual contributions to the writing of this article, revised it critically for intellectual content, and read and approved the final manuscript.
Conflict of Interest
MM has no disclosures in relation to this study. RA, WG, and JZ are employees of Analysis Group Inc., who were paid consultants to Shire, a Takeda company, in relation to this study. AM and SS are employees of Shire, a member of the Takeda group of companies, and own stock/stock options in Takeda.
Acknowledgments
Under direction of the authors, Julia Cope and Ira Probodh of Excel Medical Affairs provided writing assistance for this publication. Editorial assistance in formatting, proofreading, and copy editing was also provided by Excel Medical Affairs. Shire, a member of the Takeda group of companies, provided funding to Excel Medical Affairs for support in writing and editing this manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2019.00510/full#supplementary-material
Abbreviations
BPD, bronchopulmonary dysplasia; CLD, chronic lung disease; GA, gestational age; ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification; ICU, intensive care unit; IVH, intraventricular hemorrhage; LOS, length of stay; NICU, neonatal intensive care unit; PMA, postmenstrual age; ROP, retinopathy of prematurity; SD, standard deviation.
References
1. Martin JA, Hamilton BE, Osteman MJK, Curtin SC, Mathews TJ. Births: final data for 2013. Natl Vital Stat Rep. (2015) 64:1–65. Available online at: https://www.cdc.gov/nchs/data/nvsr/nvsr64/nvsr64_01.pdf
2. Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA. (2015) 314:1039–51. doi: 10.1001/jama.2015.10244
3. Glass HC, Costarino AT, Stayer SA, Brett CM, Cladis F, Davis PJ. Outcomes for extremely premature infants. Anesth Analg. (2015) 120:1337–51. doi: 10.1213/ANE.0000000000000705
4. Johnson TJ, Patel AL, Jegier BJ, Engstrom JL, Meier PP. Cost of morbidities in very low birth weight infants. J Pediatr. (2013) 162:243–9. doi: 10.1016/j.jpeds.2012.07.013
5. Institute of Medicine (US) Committee on Understanding Premature Birth and Assuring Healthy Outcomes. Preterm Birth: Causes, Consequences, and Prevention. In: Behrman RE, Butler AS, editors. Washington, DC: National Academies Press (US) (2007).
6. Baker CD, Alvira CM. Disrupted lung development and bronchopulmonary dysplasia: opportunities for lung repair and regeneration. Curr Opin Pediatr. (2014) 26:306–14. doi: 10.1097/MOP.0000000000000095
7. Islam JY, Keller RL, Aschner JL, Hartert TV, Moore PE. Understanding the short- and long-term respiratory outcomes of prematurity and bronchopulmonary dysplasia. Am J Respir Crit Care Med. (2015) 192:134–56. doi: 10.1164/rccm.201412-2142PP
8. Jensen EA, Schmidt B. Epidemiology of bronchopulmonary dysplasia. Birth Defects Res A Clin Mol Teratol. (2014) 100:145–57. doi: 10.1002/bdra.23235
9. Klinger G, Sirota L, Lusky A, Reichman B. Bronchopulmonary dysplasia in very low birth weight infants is associated with prolonged hospital stay. J Perinatol. (2006) 26:640–4. doi: 10.1038/sj.jp.7211580
10. Smith VC, Zupancic JAF, McCormick MC, Croen LA, Greene J, Escobar GJ, et al. Rehospitalization in the first year of life among infants with bronchopulmonary dysplasia. J Pediatr. (2004) 144:799–803. doi: 10.1016/j.jpeds.2004.03.026
11. Blencowe H, Lawn JE, Vazquez T, Fielder A, Gilbert C. Preterm-associated visual impairment and estimates of retinopathy of prematurity at regional and global levels for 2010. Pediatr Res. (2013) 74:35–49. doi: 10.1038/pr.2013.205
12. Bolisetty S, Dhawan A, Abdel-Latif M, Bajuk B, Stack J, Lui K. Intraventricular hemorrhage and neurodevelopmental outcomes in extreme preterm infants. Pediatrics. (2014) 133:55–62. doi: 10.1542/peds.2013-0372
13. Anderson JG, Baer RJ, Partridge JC, Kuppermann M, Franck LS, Rand L, et al. Survival and major morbidity of extremely preterm infants: a population-based study. Pediatrics. (2016) 138:e20154434. doi: 10.1542/peds.2015-4434
14. Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. (2010) 126:443–56. doi: 10.1542/peds.2009-2959
15. Makadia R, Ryan PB. Transforming the Premier Perspective® hospital database into the Observational Medical Outcomes Partnership (OMOP) common data model. EGEMS (Wash DC). (2014) 2:1110. doi: 10.13063/2327-9214.1110
16. Poindexter BB, Feng R, Schmidt B, Aschner JL, Ballard RA, Hamvas A, et al. Comparisons and limitations of current definitions of bronchopulmonary dysplasia for the Prematurity and Respiratory Outcomes Program. Ann Am Thorac Soc. (2015) 12:1822–30. doi: 10.1513/AnnalsATS.201504-218OC
17. Seaton SE, Barker L, Jenkins D, Draper ES, Abrams KR, Manktelow BN. What factors predict length of stay in a neonatal unit: a systematic review. BMJ Open. (2016) 6:e010466. doi: 10.1136/bmjopen-2015-010466
Keywords: bronchopulmonary dysplasia, comorbidities of prematurity, cost analysis, health care resource utilization, neonatology
Citation: Mowitz ME, Ayyagari R, Gao W, Zhao J, Mangili A and Sarda SP (2019) Health Care Burden of Bronchopulmonary Dysplasia Among Extremely Preterm Infants. Front. Pediatr. 7:510. doi: 10.3389/fped.2019.00510
Received: 11 July 2019; Accepted: 25 November 2019;
Published: 12 December 2019.
Edited by:
Arjan Te Pas, Leiden University, NetherlandsReviewed by:
Jonathan Michael Klein, The University of Iowa, United StatesAstri Maria Lang, Oslo University Hospital, Norway
Copyright © 2019 Mowitz, Ayyagari, Gao, Zhao, Mangili and Sarda. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sujata P. Sarda, c3VqYXRhLnNhcmRhQHRha2VkYS5jb20=
 Meredith E. Mowitz
Meredith E. Mowitz Rajeev Ayyagari2
Rajeev Ayyagari2 Wei Gao
Wei Gao Jing Zhao
Jing Zhao Sujata P. Sarda
Sujata P. Sarda