- 1Department of Allergy and Clinical Immunology, Children's Hospital of Fudan University, Shanghai, China
- 2Department of Pediatrics, Shanghai Songjiang District Central Hospital, Shanghai, China
Dermatological disorders are the most common extrapulmonary complications of Mycoplasma pneumoniae, of which Mycoplasma-induced rash and mucositis (MIRM) has recently been proposed to be a separate diagnostic entity. MIRM could easily be misdiagnosed as atypical Stevens-Johnson syndrome by clinicians due to the unawareness of this rare disease. We retrospectively reviewed the inpatient database from Jan. 2016 to Dec. 2019 of the Children's Hospital of Fudan University. In total, five patients (mean age 5.5 years, three male) matched the diagnostic criteria of MIRM. All patients had scattered lesions and more than two sites of mucosal involvement. The serum IgA level of three patients was higher than normal. Two patients had a significant decrease in peripheral blood CD3+ T and CD4+ T cells that improved with recovery. The percentage of TCRαβ+ CD4–CD8–T cells of Patient five was higher than normal. All patients received treatments with antibiotics and corticosteroids, 3 patients received intravenous immunoglobulin. Among five patients, three patients complained of dyspigmentation, and two patients had an uneventful recovery. MIRM is a separate entity with predominant mucosal involvement and excellent prognosis that more often affects younger patients. Excessive inflammatory reactions may lead to immune disorders, including lymphopenia and a redistribution of CD4+ T cells. We recommend that pneumonia accompanied by mucocutaneous eruptions, especially in young patients, should raise clinical suspicion of MIRM.
Introduction
Mycoplasma pneumoniae is a common cause of respiratory tract infections (1). Although the majority of infections are mild, 25% of patients experience a wide variety of extrapulmonary complications (2). Dermatological disorders, including erythematous, maculopapular and vesicular rashes, are the most common extrapulmonary complications, occurring in up to 25% of patients (1).
Stevens-Johnson syndrome (SJS) and erythema multiforme (EM) have frequently been reported in association with M. pneumoniae infection (3, 4). Both of them are thought to be a spectrum of epidermolytic dermopathies (5). Atypical SJS has been described as prominent mucositis and scarce or absent cutaneous involvement (6–10). In 2015, Canavan et al. (11) prompted an additional Mycoplasma-induced mucocutaneous eruption entity, presenting as predominant mucositis, with sparse or nonexistent cutaneous involvement, and named it Mycoplasma-induced rash and mucositis (MIRM).
The classic MIRM diagnostic criteria are as follows (11): 1. skin detachment <10% of the body surface area (BSA); 2. involvement of at least two mucosal sites; 3. few vesiculobullous lesions or scattered atypical targets; and 4. evidence of M. pneumoniae infection. Regarding the last criterion, the evidence of M. pneumoniae infection must be supported by the clinical findings of atypical pneumonia, such as fever, cough and positive auscultatory findings, and by laboratory findings, including elevated M. pneumoniae IgM antibodies, positive cultures or polymerase chain reaction for M. pneumoniae from the oropharynx or bullae, and/or serial cold agglutinins. However, the pathogenesis of MIRM and the reasons of different dermatological manifestations in MP infection are unknown.
MIRM may be formerly have been misdiagnosed as atypical SJS. To date, no MIRM cases have been reported in China. Here, we reported five Chinese cases that matched the MIRM diagnostic criteria and summarized the clinical and immunological characteristics to enhance the awareness of this relatively rare disease.
Cases Presentation
We retrospectively reviewed the inpatient electronic database from Jan. 2016 to Dec. 2019 in the Children's Hospital of Fudan University. As the entity of “Mycoplasma induced rash and mucositis” was not included in the code of the International Classification of Diseases (ICD 10), we searched the database using the terms “Stevens-Johnson syndrome” or “Erythema multiforme” or “Erythema multiforme exudativum” or “Toxic epidermal necrolysis” and overviewed the clinical information of all the above cases. In total, 194 cases were identified and retrospectively analyzed, among which five patients satisfied the MIRM diagnostic criteria proposed by Canavan et al. (11). Of these five patients, three patients were previously diagnosed with “Erythema multiforme,” and another two patients were diagnosed with “Erythema multiforme exudativum.”
Clinical Presentation
All five patients were previously healthy. None of them had a history of allergic drug exposure or infection prior to the lesion. The mean age was 5.5 years (range: 2 years, 5 months to 7 years), and three patients (60%) were male.
All of the MIRM groups had universal prodromal symptoms preceding the eruption by an average of 2 days (range: 1–4 days), including fever, cough or sore throat. Fever was the main complaint, with a maximum axillary temperature of 39–40°C. Two patients had no obvious respiratory symptoms, while the other three patients had cough during the disease process, and the chest radiograph of two patients suggested bronchial pneumonia (Table 1).
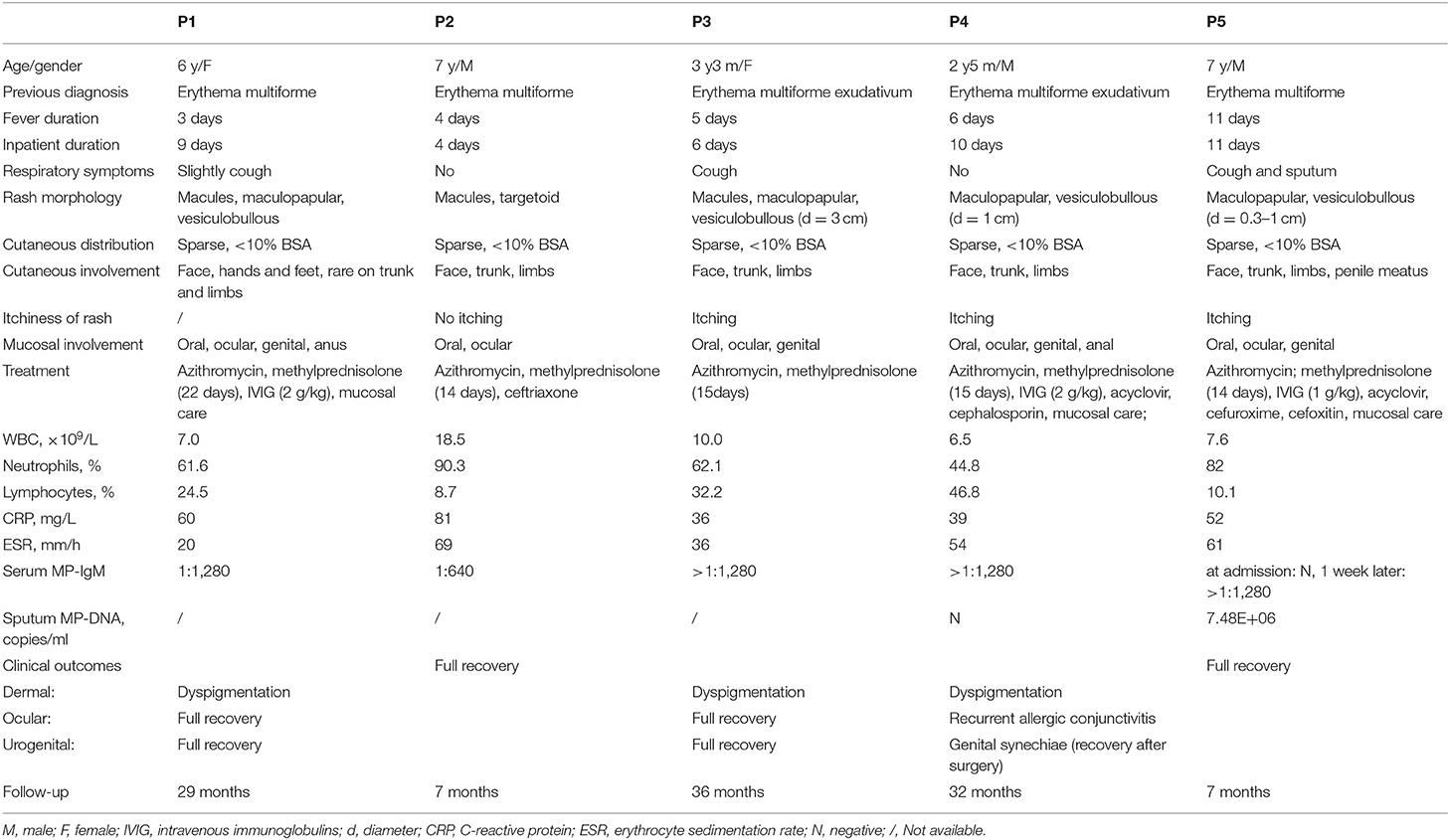
Table 1. Demographic characteristics and clinical presentations of Mycoplasma-induced rash and mucositis.
Cutaneous Morphology
All five patients presented with polymorphic lesions (Figure 1). The extent of cutaneous involvement was <10% of the body surface area in all patients. Vesiculobullous and maculopapular rash were the most common morphology (80%), followed by macules (60%) and targetoid lesions (20%). The rash was scattered throughout the body, with some fused into slices, and the vesiculobullous size differed from 0.3 to 3 cm in diameter. They had cutaneous involvement in the same stage of evolution on the face, trunk and limbs; one patient's penile meatus was also involved. The rash of three patients was obvious with itchiness (Table 1).
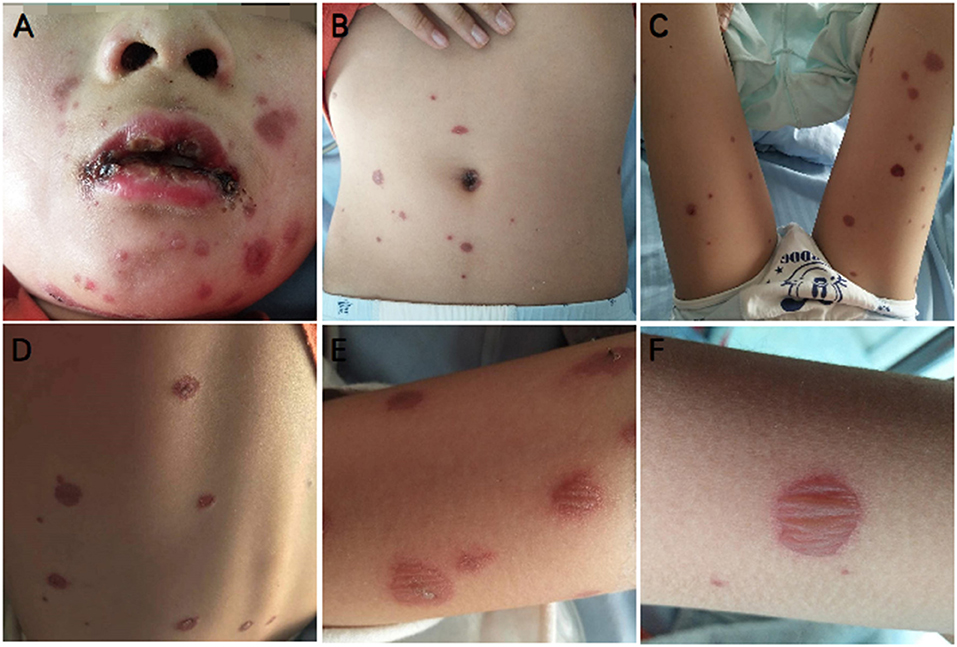
Figure 1. Typical mucocutaneous findings in P5 with Mycoplasma-induced rash and mucositis (MIRM). Severe oral mucositis partially covered by hemorrhagic crusts, some atypical targets with central bulla on the face (A), sparse vesiculobullous eruption, and scattered rash on the trunk and limbs (A–F).
Mucositis Morphology
Severe mucositis was present in all five patients, with ≥2 sites of mucosal involvement. The most common mucosal involvement areas were oral and ocular sites (100%), followed by genital (80%) and anal (40%) sites. The clinical observation revealed painful erosions on the lips and oral mucosa that were partially covered by hemorrhagic crusts (Figure 1) as well as bilateral conjunctival hyperemia and genital and perianal mucosal erosion with painful urination or defecation (Table 1).
Laboratory Findings
Routine blood examinations showed normal or elevated levels of white blood cells (6.5–18.5 × 109/L) with neutrophilia (44.8–90.3%), elevated C-reactive protein levels (29–81 mg/L), and elevated erythrocyte sedimentation rates (20–66 mm/L). Acute herpes simplex virus (HSV) and Epstein-Barr virus infection was excluded in all patients. P5 exhibited a seroconversion of specific M. pneumoniae IgM titer (from negative results on admission to a specific M. pneumoniae IgM titer ≥1:1,280 at the 2nd examination). The result of sputum pathogen examination showed high M. pneumoniae DNA copies of 7.48E+06 copies/ml (normal value <2,500 copies/ml). The remaining four cases had the serological examination at presentation, with a specific M.pneumoniae IgM titer ≥1:1,280 in three cases and 1:640 in one case. All patients were excluded other pathogens infection and then diagnosed with M. pneumoniae infection (Table 1).
Immune Function Evaluation
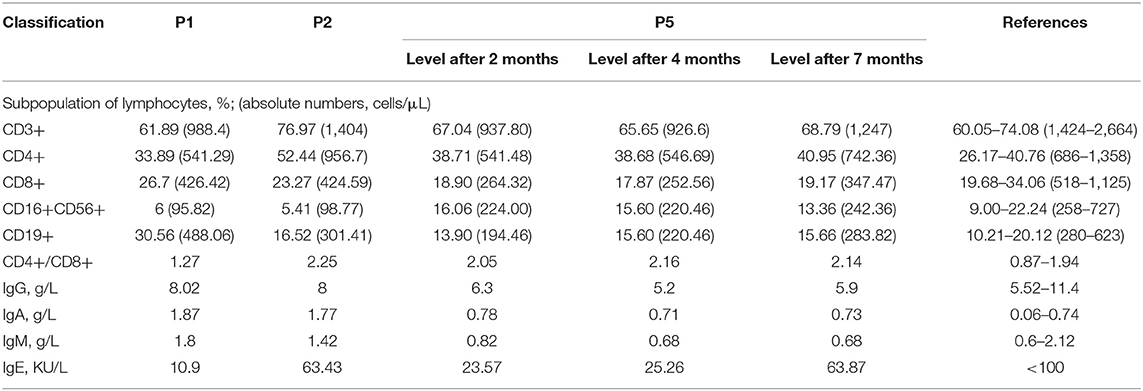
Immune function evaluation was conducted in three patients. The serum IgA level of the three patients was higher than normal, and gradually returned to the normal range after 7 months, while IgM, IgG and IgE were in the normal range. Two patients had a significant decrease in peripheral blood CD3+ and CD4+ T lymphocytes (Table 2). We performed multiple follow-ups of P5 and found that CD3+ T cells significantly increased in 7 months after recovery, and the number of CD4+ T cells, CD8+ T cells and B cells gradually increased (Table 2).
To further explore the immunological features, we performed immunophenotyping of peripheral blood lymphocyte subsets of P5 after 2 and 4 months (Figure 2). The distribution of the percentage of TCRαβ+ CD4–CD8–double-negative (DN) T cells in both experiments was higher than the normal range and gradually decreased with time (Table 3). The distribution of the percentage of transitional B lymphocyte subsets was higher than the normal range, while the remaining B lymphocyte subsets were in the normal range in both experiments (Figure 2, for flow cytometry gating strategy, see Figure S1 in Supplementary File).
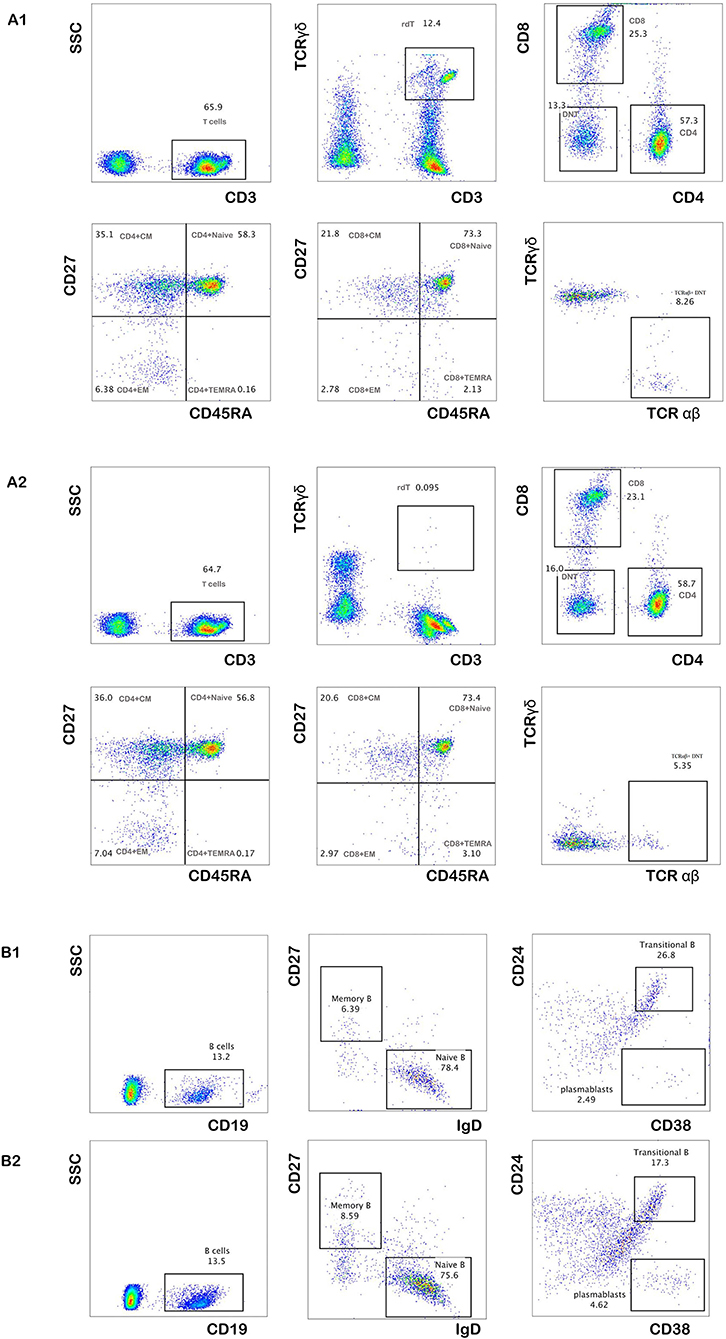
Figure 2. The percentage of peripheral blood lymphocyte subsets of P5. The distribution of the percentage of total T cells and their subsets after 2 and 4 months of follow-up (A1,A2). The distribution of the percentage of total B cells and their subsets after 2 and 4 months of follow-up (B1,B2).
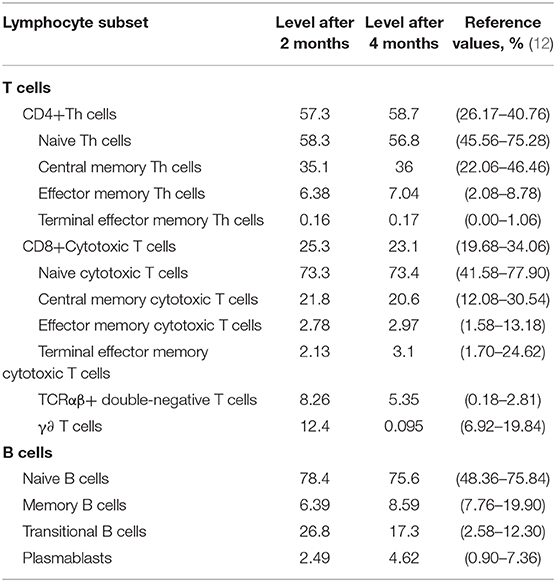
Table 3. Distribution of the percentage of total T and B cells and their subsets in the peripheral blood of P5 (%).
Treatment
All patients received treatments with azithromycin (10 mg/kg IV once daily). Two patients underwent intravenous acyclovir treatment for 5–7 days. Three patients received cephalosporin treatment for 3–10 days. Three patients (60%) with more severe clinical manifestations received IVIG therapy at a dosage of 1–2 g/kg on the second to fifth day of the disease course. These three patients also received local mucosal care. All patients (100%) were treated with corticosteroids. The initial dose of corticosteroids varied between 1.5 and 4 mg/kg depending on the severity of the disease. As clinical symptoms improved, the dose of corticosteroids was gradually reduced, and the total course of corticosteroid treatment was 13–22 days.
After IVIG combined with glucocorticoid therapy, the body temperature of two patients returned to normal within 1–2 days, while the body temperature of P5 returned to normal on the 5th day. Two patients received corticosteroid treatment alone, and the temperature gradually decreased to normal after 2–3 days. All patients had rapid oral, ocular and genital mucositis improvements, and the skin lesions improved.
Follow-Up and Outcomes
The average duration of hospital stay was 8 days (range 4–11 days). Two patients had a full recovery. The longest follow-up we conducted was 3 years. Dyspigmentation (60%) was the most common skin sequelae. Two patients had double meibomian gland opening embolization and conjunctivitis after 2 weeks. The ocular symptoms of P1 gradually disappeared during follow-up, while P4 had recurrent allergic conjunctivitis. No patients had corneal involvement, and visual outcomes were excellent. One patient (20%) had genital synechiae and underwent circumcision 4 months later.
Discussion
Mycoplasma pneumoniae is the most common infectious agent associated with acute epidermolytic dermopathies (13). The M. pneumoniae spectrum of dermatological manifestations varies and includes Raynaud's disease, erythema nodosum, Kawasaki disease, EM and SJS/toxic epidermal necrolysis (TEN) (2, 5). Mycoplasma-induced rash and mucositis is rare. Previously, this clinical condition was classified within the epidermolytic dermopathy spectrum as “atypical SJS,” “incomplete SJS,” or “Fuchs syndrome” (5, 10, 11, 14, 15). Recently, a systematic review concluded that this condition was a distinct entity called Mycoplasma pneumoniae-induced rash and mucositis (MIRM) (11, 14–17). However, no patient with MIRM has been reported in China until now, indicating that many clinicians had no knowledge of this disease and that such patients could be misdiagnosed.
MIRM is more often characterized by prominent mucositis with a single lesion or a few scattered skin lesions (10, 13, 14, 17, 18), commonly in children and young adolescents (11). The mean age of onset in our cohort was 5.5 years. However, cases of MIRM in young adults have also been reported (16, 19). MIRM is relatively predominant in males (66%) (11), which was similar to our cohort. As reported, all patients had prodromal symptoms by approximately 1 week (11). In our cohort, the eruption appeared approximately on the second day of the disease, earlier than reported in the literature.
Mucosal involvement is critical to the diagnosis, with a mean of 2.5 mucosal sites affected, such as oral mucosal (94%), ocular (82%) and urogenital (63%) sites (11). Other mucosal sites involved include the nares and anus. Mucosal lesions are typically described as ulcerative or hemorrhagic and may be painful (20). Additionally, a cutaneous rash is present in 47% of cases, which are classified as classic MIRM rash. The cutaneous rash of MIRM is sparse in overall distribution and, specifically, is located more in the acral regions (46%) than in the trunk (23%). The cutaneous rash morphology is commonly described as vesiculobullous (77%) and typical target lesions (48%). Less commonly, rashes are described as papules (14%), macules (12%), or morbilliform rashes (9%) (11). The mucocutaneous manifestations of our patients were similar to those of previously reported cases. Interestingly, we found that only the vesiculobullous morphology had obvious itchiness; therefore, some patients were misdiagnosed with varicella in the early stage. Itchiness had not been mentioned in previous literature reports. Therefore, MIRM patients with itchy vesiculobullous lesions should be differentiated from varicella.
Most important is the scarce cutaneous involvement in MIRM. The amount of skin detached is usually <10% BSA, which is critical for clinical distinction. The amount of skin detachment differentiates the extent of SJS/TEN. SJS has <10% skin detachment. Skin detachment of 10–30% indicates SJS/TEN overlap. More than 30% skin detachment indicates TEN (21–23). There are additional features that may help to distinguish MIRM from EM or SJS/TEN. First, the young age characteristic of most patients with MIRM. Second, the etiology of atypical pneumonia in MIRM could be opposed to the HSV associated with EM and the medication etiology of SJS/TEN. It is also notable that there is a better prognosis of MIRM even with support treatment alone (11, 21–25). All these clinical features may help physicians distinguish the clinical spectrum of EM, SJS/TEN and MIRM, where skin lesions are different and milder, explaining its previous and obsolete name of incomplete SJS (11, 26).
Children with M. pneumoniae infection may have extrapulmonary manifestations alone. In our study, two patients had no obvious respiratory symptoms. Therefore, serology can help indicating a recent infection with M. pneumonia (1). All patients had an M. pneumoniae-specific IgM titer higher than normal. We should pay attention to identifying M. pneumoniae infection, even if the patient has no respiratory symptoms.
M. pneumoniae is a mucosal pathogen; interestingly, several studies demonstrated that IgA antibodies are produced early, peak quickly, and decline earlier than IgM or IgG antibodies (1, 18, 27, 28).
Research on specific T cell-mediated immunity is also involved in the host reaction to M. pneumoniae infection, and excessive inflammatory reactions may lead to immune disorders (29–31). By now, it is clear that once M. pneumoniae reaches the lower respiratory tract, it can trigger and amplify the production of chemokines promoting lymphocyte and neutrophil tracking and inflammation in the lung. High percentages of neutrophils and lymphocytes are present in alveolar fluid. CD4+ T lymphocytes, B lymphocytes, and plasma cellsinfiltrate the lung (1, 32, 33). Wang et al. (34) reported a case of Stevens-Johnson syndrome associated with M. pneumoniae infection, in which lymphopenia, with a significant decrease in CD4+ T cells in the blood and predominant CD4+ T cells in the skin vesicular fluid, was found, and improvement in lymphopenia was associated with disease recovery. In our cohort, we found that two patients with more severe mucocutaneous manifestations had a significant decrease in peripheral blood CD3+ and CD4+ T lymphocytes. Of note, we performed multiple follow-ups of P5 and found that the lymphocyte subsets gradually increased after 7 months, which is similar to the report of Wang et al. However, we did not perform a mucocutaneous biopsy, and the number of lymphocytes in the mucosal lesions is unclear. Therefore, we speculate that patients with MIRM may also have lymphopenia and a redistribution of CD4+ T cells. The mechanism of CD4+ T cell redistribution is not clear.
DNTs are mature post-thymic T cells that express the CD3+TCRαβ+ receptor but lack CD4+CD8+ (35); in healthy humans, approximately 1–5% of all peripheral T cells are of the TCR αβ+DNT phenotype (36, 37). Several studies have demonstrated that various T cell subsets possess immunoregulatory properties (38, 39), and the TCR αβ+ DNT cells was found have the ability to inhibit immune responses (40). Voelkl et al. revealed that human DNT cells exert strong immunosuppressive effects on both CD4+ and CD8+ T cells (40). We performed multiple follow-ups of P5 and found that the distribution of the percentage of TCR αβ+ DNT cells in P5 was higher than normal and gradually decreased with time, and the number of CD4+ T cells, CD8+ T cells gradually increased with disease recovery. Regarding the cause of CD4+ T cell reduction, we speculate that this may be caused by the inhibition of TCR αβ+ DNT cells, and CD4+ T cell redistribution may also play an important role. The distribution of the percentage of transitional B lymphocyte subsets was higher than the normal range, while the remaining B lymphocyte subsets were normal. There were currently no reports on the immunological characteristics of MIRM disease. A limitation is the small number of cases because MIRM remains rare, further investigation is required to reveal the immunological mechanism.
The pathological mechanism of MIRM is currently unclear. MIRM is currently believed to be caused by polyclonal B-cell proliferation, and antibody production may theoretically result in skin damage stemming from immune complex and complement deposition (14, 41, 42). And there was a protein-homeostasis-system hypothesis proposed, it is possible that inflammation-inducing substances in M. pneumoniae infection are produced when pathogens are replicated within host cells, including toxins and pathogen-associated molecular patterns (PAMPs), and/or those originated from injured infected-host cells including damage-associated molecular patterns (DAMPs), pathogenic proteins, and pathogenic peptides. When these substances spread systemically and locally and bind to target organ cells, such as pneumonia and other extrapulmonary manifestations were induced, due to the activation of corresponding immune cells and immune proteins, and the substances produced from injured host cells induce further inflammation (43). Importantly, the etiological substances are different from those of EM or SJS/TEN, which are mediated by delayed hypersensitivity reactions and Fas ligand-mediated toxicity (14), leading to the differentiation of MIRM from other cutaneous diseases. Molecular mimicry between Mycoplasma P1-adhesion molecules and a keratinocyte antigen has also been speculated (4, 41). The fact that M. pneumoniae was isolated from the skin blister fluid on at least two independent occasions could not be ignored, which suggests the possibility of a direct type mechanism (3, 44, 45), and the skin inflammatory bullous might induced by cytokines (2). But in our cohort, the PCR for M. pneumoniae from the skin blister fluid of P5 was negative. The pathogenic mechanism and how M. pneumoniae transfer from the respiratory tract to the skin need more study.
Currently, there are no guidelines for MIRM treatment. However, most patients are treated with antibiotics, systemic corticosteroids, intravenous immunoglobulin (IVIG), supportive care or a combination of the above (11). Although antibiotic treatment directed at M. pneumoniae eliminates the causative agent and limits the duration and severity of the pulmonary disease, the paucity of data does not indicate whether the incidence or severity of the mucocutaneous eruption is reduced (16). Additionally, the role of immunomodulatory therapies is not clear, and there is clinical evidence that IVIG may be helpful for MIRM patients with severe mucositis (11, 14, 17, 20, 26). In our report, three patients with severe mucositis had been treated with IVIG, and only one patient had genital synechiae and underwent circumcision. Future studies would be necessary to investigate the clinical efficacy of immunosuppressants of the aberrant host immune response in cases of severe extrapulmonary manifestations.
Conclusion
MIRM may a separate clinical entity due to the young age of the patients, the predominant mucosal involvement and the excellent prognosis. Pneumonia accompanied by mucocutaneous eruptions, especially in young patients, should raise clinical suspicion of MIRM. Immunomodulatory therapies may be helpful for MIRM patients with severe mucositis
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Review Committee of Children's Hospital of Fudan University. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin. Written informed consent was obtained from the minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author Contributions
LL and JH contributed to conceptualize and design the study, conduct the investigation and formal analysis, draft the initial manuscript, and review and revise the manuscript. XW contributed to conceptualize and design the study, supervise the methodology, and critically review and revise the manuscript. WW contributed to the collection the clinical data, conduct investigation, carry out the initial analyses, and review and revise the manuscript. YW and JS contributed to design the study and methodology, supervise clinical data collection, and critically review and revise the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Science and Technology Research project of Songjiang District, Shanghai in 2019 (19SJKJGG137).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to acknowledge the patients and their families for their cooperation.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2020.00402/full#supplementary-material
Figure S1. Flow cytometry gating strategy for T-cell and B-cell subsets.
References
1. Waites KB, Talkington DF. Mycoplasma pneumoniae and its role as a human pathogen. Clin Microbiol Rev. (2004) 17:697–728. doi: 10.1128/CMR.17.4.697-728.2004
2. Narita M. Classification of extrapulmonary manifestations due to Mycoplasma pneumoniae infection on the basis of possible pathogenesis. Front Microbiol. (2016) 7:23. doi: 10.3389/fmicb.2016.00023
3. Meseguer MA, de Rafael L, Vidal ML. Stevens-Johnson syndrome with isolation of Mycoplasma pneumoniae from skin lesions. Eur J Clin Microbiol. (1986) 5:167–8. doi: 10.1007/BF02013977
4. Schalock PC, Dinulos JG, Pace N, Schwarzenberger K, Wenger JK. Erythema multiforme due to Mycoplasma pneumoniae infection in two children. Pediatr Dermatol. (2006) 23:546–55. doi: 10.1111/j.1525-1470.2006.00307.x
5. Meyer SP, Goetschel P, Lautenschlager S. Mycoplasma pneumoniae and mucositis–part of the Stevens-Johnson syndrome spectrum. J Dtsch Dermatol Ges. (2012) 10:740–6. doi: 10.1111/j.1610-0387.2012.07951.x
6. Zao I, Ribeiro F, Rocha V, Neto P, Matias C, Jesus G. Mycoplasma pneumoniae-associated mucositis: a recently described entity. Eur J Case Rep Intern Med. (2018) 5:000977. doi: 10.12890/2018_000977
7. Li K, Haber RM. Stevens-Johnson syndrome without skin lesions (Fuchs syndrome): a literature review of adult cases with Mycoplasma cause. Arch Dermatol. (2012) 148:963–4. doi: 10.1001/archdermatol.2012.681
8. Latsch K, Girschick HJ, Abele-Horn M. Stevens-Johnson syndrome without skin lesions. J Med Microbiol. (2007) 56:1696–9. doi: 10.1099/jmm.0.47318-0
9. Hillebrand-Haverkort ME, Budding AE, Bij DVL, van Agtmael MA. Mycoplasma pneumoniae infection with incomplete Stevens-Johnson syndrome. Lancet Infect Dis. (2008) 8:586–7. doi: 10.1016/S1473-3099(08)70209-3
10. Ravin KA, Rappaport LD, Zuckerbraun NS, Wadowsky RM, Wald ER, Michaels MM. Mycoplasma pneumoniae and atypical Stevens-Johnson syndrome: a case series. Pediatrics. (2007) 119:e1002–5. doi: 10.1542/peds.2007-1407
11. Canavan TN, Mathes EF, Frieden I, Shinkai K. Mycoplasma pneumoniae-induced rash and mucositis as a syndrome distinct from Stevens-Johnson syndrome and erythema multiforme: a systematic review. J Am Acad Dermatol. (2015) 72:239–45. doi: 10.1016/j.jaad.2014.06.026
12. Ding Y, Zhou L, Xia Y, Wang W, Wang Y, Li L, et al. Reference values for peripheral blood lymphocyte subsets of healthy children in China. J Allergy Clin Immunol. (2018) 142:970–3.e8. doi: 10.1016/j.jaci.2018.04.022
13. Olson D, Watkins LK, Demirjian A, Lin X, Robinson CC, Pretty K, et al. Outbreak of Mycoplasma pneumoniae-Associated Stevens-Johnson Syndrome. Pediatrics. (2015) 136:e386–94. doi: 10.1542/peds.2015-0278
14. Martinez-Perez M, Imbernon-Moya A, Lobato-Berezo A, Churruca-Grijelmo M. Mycoplasma pneumoniae-induced mucocutaneous rash: a new syndrome distinct from erythema multiforme? Report of a new case and review of the literature. Actas Dermosifiliogr. (2016) 107:e47–51. doi: 10.1016/j.adengl.2016.06.005
15. Kheiri B, Alhesan NA, Madala S, Assasa O, Shen M, Dawood T. Mycoplasma pneumoniae-associated Fuchs syndrome. Clin Case Rep. (2018) 6:434–35. doi: 10.1002/ccr3.1350
16. Li T, Lee N. Mycoplasma pneumoniae-associated mucositis. N Engl J Med. (2018) 379:1262. doi: 10.1056/NEJMicm1614484
17. Demitsu T, Kawase M, Nagashima K, Takazawa M, Yamada T, Kakurai M, et al. Mycoplasma pneumoniae-associated mucositis with severe blistering stomatitis and pneumonia successfully treated with azithromycin and infusion therapy. J Dermatol. (2019) 46:e38–9. doi: 10.1111/1346-8138.14510
18. Vujic I, Shroff A, Grzelka M, Posch C, Monshi B, Sanlorenzo M, et al. Mycoplasma pneumoniae-associated mucositis–case report and systematic review of literature. J Eur Acad Dermatol Venereol. (2015) 29:595–8. doi: 10.1111/jdv.12392
19. Figueira-Coelho J, Lourenco S, Pires AC, Mendonca P, Malhado JA. Mycoplasma pneumoniae-associated mucositis with minimal skin manifestations. Am J Clin Dermatol. (2008) 9:399–403. doi: 10.2165/0128071-200809060-00008
20. Norton SA. Diagnosing Mycoplasma pneumoniae-induced rash and mucositis (MIRM) in the emergency room. J Am Acad Dermatol. (2015) 73:e67. doi: 10.1016/j.jaad.2015.03.060
21. Downey A, Jackson C, Harun N, Cooper A. Toxic epidermal necrolysis: review of pathogenesis and management. J Am Acad Dermatol. (2012) 66:995–1003. doi: 10.1016/j.jaad.2011.09.029
22. Miliszewski MA, Kirchhof MG, Sikora S, Papp A, Dutz JP. Stevens-Johnson syndrome and toxic epidermal necrolysis: an analysis of triggers and implications for improving prevention. Am J Med. (2016) 129:1221–5. doi: 10.1016/j.amjmed.2016.03.022
23. French LE, Trent JT, Kerdel FA. Use of intravenous immunoglobulin in toxic epidermal necrolysis and Stevens-Johnson syndrome: our current understanding. Int Immunopharmacol. (2006) 6:543–9. doi: 10.1016/j.intimp.2005.11.012
24. Chatproedprai S, Wutticharoenwong V, Tempark T, Wananukul S. Clinical features and treatment outcomes among children with Stevens-Johnson syndrome and toxic epidermal necrolysis: a 20-year study in a Tertiary Referral Hospital. Dermatol Res Pract. (2018) 2018:3061084. doi: 10.1155/2018/3061084
25. Zhang AJ, Nygaard RM, Endorf FW, Hylwa SA. Stevens-Johnson syndrome and toxic epidermal necrolysis: retrospective review of 10-year experience. Int J Dermatol. (2019) 58:1069–77. doi: 10.1111/ijd.14409
26. Poddighe D, Bruni P. Mycoplasma pneumoniae-induced rash and mucositis (MIRM): an unusual mild skin rash associated with severe mucosal involvement. BMJ Case Rep. (2017) 2017:bcr2017220749. doi: 10.1136/bcr-2017-220749
27. Granstrom M, Holme T, Sjogren AM, Ortqvist A, Kalin M. The role of IgA determination by ELISA in the early serodiagnosis of Mycoplasma pneumoniae infection, in relation to IgG and mu-capture IgM methods. J Med Microbiol. (1994) 40:288–92. doi: 10.1099/00222615-40-4-288
28. Watkins-Riedel T, Stanek G, Daxboeck F. Comparison of SeroMP IgA with four other commercial assays for serodiagnosis of Mycoplasma pneumoniae pneumonia. Diagn Microbiol Infect Dis. (2001) 40:21–5. doi: 10.1016/S0732-8893(01)00250-4
29. Lisin VV, Nazarov PG, Katorgina LG, Kim TN. [Characteristics of the immunologic response to Mycoplasma pneumoniae as a polyclonal lymphocyte activator]. Zh Mikrobiol Epidemiol Immunobiol. 1988:108–11.
30. Mogensen HH, Lind K. In vitro stimulation of blood lymphocytes from Mycoplasma pneumoniae infected patients with pneumonia and with disorders of the central nervous system. Acta Pathol Microbiol Scand C. (1980) 88:61–5. doi: 10.1111/j.1699-0463.1980.tb00073.x
31. Zhang Y, Zhou Y, Li S, Yang D, Wu X, Chen Z. The clinical characteristics and predictors of refractory Mycoplasma pneumoniae pneumonia in children. PLoS ONE. (2016) 11:e0156465. doi: 10.1371/journal.pone.0156465
33. Opitz O, Pietsch K, Ehlers S, Jacobs E. Cytokine gene expression in immune mice reinfected with Mycoplasma pneumoniae: the role of T cell subsets in aggravating the inflammatory response. Immunobiology. (1996) 196:575–87. doi: 10.1016/S0171-2985(97)80073-3
34. Wang L, Hong KC, Lin FC, Yang KD. Mycoplasma pneumoniae-associated Stevens-Johnson syndrome exhibits lymphopenia and redistribution of CD4+ T cells. J Formos Med Assoc. (2003) 102:55–8.
35. Fleisher TA, Oliveira JB. Monogenic defects in lymphocyte apoptosis. Curr Opin Allergy Clin Immunol. (2012) 12:609–15. doi: 10.1097/ACI.0b013e3283588da0
36. Zhang ZX, Yang L, Young KJ, DuTemple B, Zhang L. Identification of a previously unknown antigen-specific regulatory T cell and its mechanism of suppression. Nat Med. (2000) 6:782–9. doi: 10.1038/77513
37. Fischer K, Voelkl S, Heymann J, Przybylski GK, Mondal K, Laumer M, et al. Isolation and characterization of human antigen-specific TCR alpha beta+ CD4(-)CD8- double-negative regulatory T cells. Blood. (2005) 105:2828–35. doi: 10.1182/blood-2004-07-2583
38. Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. (2001) 27:20–1. doi: 10.1038/83713
39. Kriegel MA, Lohmann T, Gabler C, Blank N, Kalden JR, Lorenz HM. Defective suppressor function of human CD4+ CD25+ regulatory T cells in autoimmune polyglandular syndrome type II. J Exp Med. (2004) 199:1285–91. doi: 10.1084/jem.20032158
40. Voelkl S, Gary R, Mackensen A. Characterization of the immunoregulatory function of human TCR-αβ+ CD4- CD8- double-negative T cells. Eur J Immunol. (2011) 41:739–48. doi: 10.1002/eji.201040982
41. Fernald GW. Immunological interactions between host cells and mycoplasmas: an introduction. Rev Infect Dis. (1982) 4 (Suppl.):S201–4. doi: 10.1093/clinids/4.Supplement_1.S201
42. Simecka JW, Ross SE, Cassell GH, Davis JK. Interactions of mycoplasmas with B cells: antibody production and nonspecific effects. Clin Infect Dis. (1993) 17 (Suppl. 1):S176–82. doi: 10.1093/clinids/17.Supplement_1.S176
43. Lee KY. A common immunopathogenesis mechanism for infectious diseases: the protein-homeostasis-system hypothesis. Infect Chemother. (2015) 47:12–26. doi: 10.3947/ic.2015.47.1.12
44. Stutman HR. Stevens-Johnson syndrome and Mycoplasma pneumoniae: evidence for cutaneous infection. J Pediatr. (1987) 111:845–7. doi: 10.1016/S0022-3476(87)80200-7
Keywords: Mycoplasma pneumoniae, rash, mucositis, lymphopenia, atypical Stevens-Johnson syndrome
Citation: Liu L, Wang Y, Sun J, Wang W, Hou J and Wang X (2020) Case Report: Clinical and Immunological Features of a Chinese Cohort With Mycoplasma-Induced Rash and Mucositis. Front. Pediatr. 8:402. doi: 10.3389/fped.2020.00402
Received: 05 March 2020; Accepted: 11 June 2020;
Published: 22 July 2020.
Edited by:
Kyung-Yil Lee, The Catholic University of Korea, South KoreaReviewed by:
Beatriz Elena Marciano, National Institutes of Health (NIH), United StatesEugênia Terra Granado, Laboratório de Investigação em Genética e Hematologia Translacional, Instituto Gonçalo Moniz (IGM), Brazil
Copyright © 2020 Liu, Wang, Sun, Wang, Hou and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jia Hou, ZG9jdG9yaG91amlhQGhvdG1haWwuY29t
 Lipin Liu
Lipin Liu Ying Wang
Ying Wang Jinqiao Sun
Jinqiao Sun Wenjie Wang
Wenjie Wang Jia Hou
Jia Hou Xiaochuan Wang
Xiaochuan Wang