- 1Centre for Evaluation of Vaccination, Vaccine and Infectious Disease Institute, University of Antwerp, Antwerp, Belgium
- 2Reference Centre for Pneumococci, University Hospitals Leuven, Leuven, Belgium
- 3Laboratory of Medical Microbiology, Vaccine and Infectious Disease Institute, University of Antwerp, Antwerp, Belgium
- 4Centre for Health Economics Research and Modelling Infectious Diseases, University of Antwerp, Antwerp, Belgium
Background: Streptococcus pneumoniae (Sp) is a major cause of acute otitis media (AOM). Pneumococcal conjugate vaccine (PCV) programs have altered pneumococcal serotype epidemiology in disease and carriage. In this study, we used samples collected during a cross-sectional study to examine if the clinical picture of acute otitis media (AOM) in young children exposed to the PCV program in Belgium was related to the carried pneumococcal strains, and if their carriage profile differed from healthy children attending daycare centers.
Material/Methods: In three collection periods from February 2016 to May 2018, nasopharyngeal swabs and background characteristics were collected from children aged 6–30 months either presenting at their physician with AOM (AOM-group) or healthy and attending day care (DCC-group). Clinical signs of AOM episodes and treatment schedule were registered by the physicians. Sp was detected, quantified, and characterized using both conventional culture analysis and real-time PCR analysis.
Results: Among 3,264 collected samples, overall pneumococcal carriage and density were found at similar rates in both AOM and DCC. As expected non-vaccine serotypes were most frequent: 23B (AOM: 12.3%; DCC: 17.4%), 11A (AOM: 7.5%; DCC: 7.4%) and 15B (AOM: 7.5%; DCC: 7.1%). Serotypes 3, 6C, 7B, 9N, 12F, 17F, and 29 were more often found in AOM than in DCC (p-value < 0.05), whereas 23A and 23B were less often present in AOM (p-value < 0.05). Antibiotic non-susceptibility of Sp strains was similar in both groups. No predictors of AOM severity were identified.
Conclusion: In the present study, overall carriage prevalence and density of S. pneumoniae were found similar in young children with AOM and in healthy children attending day-care centers in Belgium. Certain serotypes not currently included in the PCV vaccines were found to be carried more often in children with AOM than in DCC, a finding that might suggest a relationship between these serotypes and AOM.
Introduction
Acute otitis media (AOM) is one of the most common pediatric infections and has been described as one of the leading causes of antibiotic prescription in children in industrialized countries (1, 2). It is estimated that 80-90% of children have an episode of acute otitis media (AOM) before the age of three, with a peak incidence between 6 and 15 months (3, 4).
Streptococcus pneumoniae (Sp) is the most common OM pathogen and has been associated with first or early otitis media episodes, more severe AOM signs and symptoms, and potential middle-ear damage (5). It colonizes the nasopharynx within the first few weeks or months of life, and carriage is a highly dynamic process (6, 7). Sp is however commonly a quiescent colonizer of the upper respiratory tract (URT) that only becomes pathogenic when changes in the upper respiratory tract allow the organism to achieve a pathogenic inoculum that overcomes innate and adaptive host defense. Capacity to colonize and invade, differs and it is related to the capsular serotype as well as to the history of colonization with this serotype. So far, at least 100 distinct capsular serotypes have been described (8, 9). Inactivated pneumococcal conjugate vaccines (PCV) were developed to prevent invasive disease caused by the most pathogenic serotypes. Before PCV introduction, the most common pneumococcal serotypes causing AOM worldwide were 3, 6A, 6B, 9V, 14, 19A, 19F and 23F (10–13). Administration of PCV has also led to a reduction of AOM caused by Sp (3, 14).
Since PCV implementation in children, a tremendous decrease in vaccine serotype related carriage and invasive pneumococcal disease cases have been observed in countries with a high vaccine uptake (15). However, PCVs only target a limited number of frequently invasive serotypes. Furthermore, the protection against the vaccine serotypes opens new nasopharyngeal niches for colonization, which favors conditions for serotype replacement. Hence, PCV introduction induced significant changes worldwide in the epidemiology of Sp in disease, carriage and antimicrobial (non-)susceptibility. Belgium has a unique pneumococcal vaccine program history: serotype coverage moved from PCV7, implemented in 2007 to PCV13 in 2011 and from PCV13 to PCV10 in 2016. This switch happened because both PCV vaccines were considered equivalent by the National Immunization Technical Advisory Group (NITAG) at that time, based on invasive disease serotype epidemiology, and thus the outcome of the regular tender process (after expiry of the contract) was mainly guided by logistic and financial factors (16). At every change of vaccine brand, a cohort of children received mixed schedules. Although it was recommended to use the same vaccine for the primary doses in the first year of life, these vaccines were not automatically reserved per child and thus children received all kinds of mixed schedules with a total number of three PCV-doses. Monitoring nasopharyngeal carriage to complement invasive disease data started at the time of the switch to PCV10, and involved two populations of young children in whom pneumococcal carriage was known to be high, namely children attending daycare centers and children suffering from AOM (17).
No recent data were available on the serotype distribution and antibiotic susceptibility of pneumococcal isolates in Belgian children with AOM, and their relationship with clinical symptoms. The carriage study investigating them simultaneously with healthy infants attending daycare centers within a single study protocol created an opportunity to investigate whether there was a difference in serotype distribution, carriage density or antimicrobial non-susceptibility between children suffering from AOM and healthy children attending DCC. It also allowed to examine whether there is a link between the clinical severity of the AOM and the carried serotypes.
Materials and Methods
The data were collected during an ongoing study that monitors nasopharyngeal carriage in infants in Belgium since 2016, after the introduction of PCV10 in the PCV program. In the first 3 years, this study investigated nasopharyngeal carriage in two infant populations with high reported carriage of Sp, namely healthy infants attending DCC and infants suffering from AOM. The study protocol was described in detail by Wouters et al. (17, 18) and is summarized here. The study was in line with the Declaration of Helsinki, as revised in 2013. Approval to conduct the current study with ID 15/45/471 was obtained from the University of Antwerp and University Hospital of Antwerp ethics committee (Commissie voor Medische Ethiek van UZA/UA) on 30 November 2015.
The recruitment was restricted to non-summer seasons (November to end May) from 2016 to 2018. In period 1, sampling was performed between January and June 2016, i.e., during and shortly after the switch from PCV13 to PCV10 in the Belgian vaccination program, while in period 2 (2016–2017) and period 3 (2017–2018) samples were collected between November and May. Randomly selected DCCs and hospitals/ pediatric outpatient services were located over the three Belgian regions (the Flemish Region, the Walloon Region and the Brussels-Capital Region). Age limits for inclusion in both populations were 6 months and 30 months. In the first recruitment season, region-specific age criteria were added to include only infants who were offered PCV13 for both primary vaccine doses in the first year of life. AOM was defined explicitly as the acute onset of symptoms (within the preceding seven days). The clinical inclusion criteria were ear tugging, fever, crying, irritability, difficult sleeping, diminished activity and/or appetite together with otoscopic signs. Otoscopic signs are including tympanic opacity due to middle ear effusion combined with moderate or intense bulging, or slight bulging accompanied with either recent onset of otalgia/ear tugging or marked unilateral erythema of the membrane or new onset otorrhea (including through tympanic tube). Exclusion criteria for AOM infants as well as healthy infants in DCC were (1) use of antibiotics in the past seven days, (2) presence of a chronic and severe associated pathology and (3) previous inclusion in the study within the same collection period. AOM infants who were recruited in hospitals (outpatient services) had extra exclusion criteria: (1) referral by a GP or (2) having received three or more antibiotic treatments in the past 3 months.
Nasopharyngeal Samples (NP) and Questionnaire
Parents consenting to participate received a questionnaire regarding their infant's demographics and clinical characteristics, and pneumococcal vaccination status. A trained nurse or a general practitioner/pediatrician collected a single nasopharyngeal (NP) sample with a flocked nylon swab in 1 mL STGG (skim milk, tryptone, glucose and glycerol). Signs of common cold in DCC infants were defined as coughing and/or running nose at sampling.
The samples were stored frozen and assessed by culture at the National Reference Center for Pneumococci (University Hospitals in Leuven) and RT-PCR analysis at the Laboratory of Medical Microbiology (University of Antwerp). Cultured Sp strains were serotyped by performing Quellung-reaction and antimicrobial susceptibility to penicillin, tetracycline, erythromycin, levofloxacin and cotrimoxazole (the most frequently used antibiotics in respiratory disease over all ages) was determined by disc diffusion following CLSI 2016 guidelines. If non-susceptibility for levofloxacin or penicillin was identified, the minimal inhibitory concentration (MIC) was determined. A MIC of >2 mg/L and >0.06 mg/L was interpreted as levofloxacin and penicillin non-susceptible, respectively. Quantitative Taqman real-time PCR (qRT-PCR) targeting lytA, using StepOnePlusTM Real-Time PCR System (Applied Biosystems TM) was performed to screen the samples for presence of Sp. Bacterial densities were determined based on a standard curve that was set up using 10-fold serially diluted lytA PCR product of Sp strain ATCC 49619. Samples and standard curves were run in triplicate. Samples were considered positive for Sp if either culture or PCR (cut off cycle threshold value ≤ 35.0) was Sp-positive (18). LytA positive samples were pooled and screened for presence of PCV13-non-PCV10 serotypes (3, 6A, 19A) (2).
Statistical Analyses
All descriptives and statistical analyses were calculated in JMP Pro 14.3. To test for significant differences in proportions the Chi-Square Test (Chi2), or Fisher's Exact Test (FET) where appropriate, were used. To compare overall association between vaccination status and health status, bivariate logistic regression was performed using period and vaccination status as factors and AOM/DCC as outcome. To compare parameters that were not normally distributed the Mann-Whitney U Test (MWU) or Kruskal-Wallis Test (KW) were used as appropriate.
The sample size of the main study was calculated according with its primary aim to compare carriage prevalence of three pneumococcal vaccine serotypes over the first 3 years (2016–2018). For the current study, nasopharyngeal carriage of Sp in infants with AOM and in infants attending DCC, were compared using a post-hoc power analysis using R package power.
Results
Recruitment and Infant Characteristics
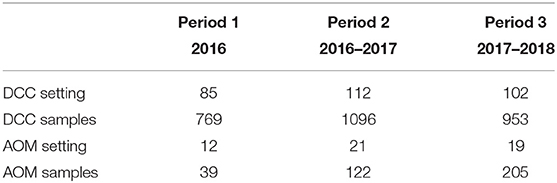
Over the 3-year study period, a total of 3,264 nasopharyngeal samples were collected of which 3,175 were included in the analysis (Table 1). Nasopharyngeal samples of 74 DCC children and 15 AOM children were excluded due to protocol violation. Moreover, four children with AOM and with incomplete questionnaires were excluded from the clinical analysis regarding AOM symptoms. A random selection of 194 DCC-samples collected in 2016–2017 was analyzed only by culture and not by PCR due to restriction at laboratory level.
A post-hoc power calculation was performed taking the total DCC (N = 2809) and AOM (N = 366) sample size into account. The sample size allows for the detection of a 15% difference in carriage prevalence of pneumococcal serotypes between healthy DCC infants and AOM infants with 77% power at a significance level of 5% (two-sided).
AOM children differed in several demographic and clinical characteristics from DCC children (Table 2). Overall, children with AOM tended to be younger than children recruited in DCCs. The convenience recruitment of AOM children resulted in a disproportionate regional distribution, which was not the case for DCC children. Overall, breastfeeding for more than 6 months was more frequent in DCC children and parental smoking was more frequent in AOM children. Children with AOM more often had a history of previous hospitalization than DCC children, and they more frequently had siblings. A significantly higher proportion of AOM children had a history of one or more previous AOM episodes over the 3-year study period compared to DCC children. Lastly, antibiotic use in the past 3 months was more frequent in DCC children than AOM children.
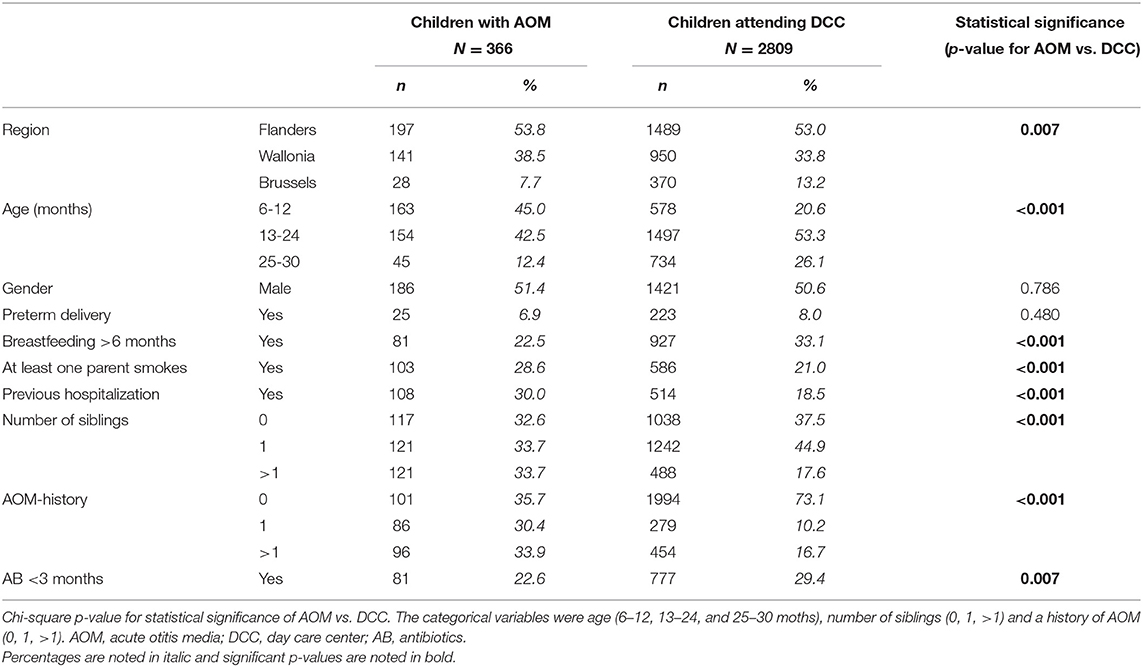
Table 2. Overall demographic and clinical characteristics of children with AOM and children attending DCC.
The PCV10-vaccinated proportion increased over the study period from 2.6 to 83.9% and from 0.0 to 75.8%, in AOM children and DCC children respectively. The proportion of PCV13-vaccinated children decreased from 74.4 to 1.0% in AOM and 75.7 to 2.7% in DCCs (Figure 1). In each period the overall difference in vaccination status (as defined in Figure 1) between AOM and DCC was significant.
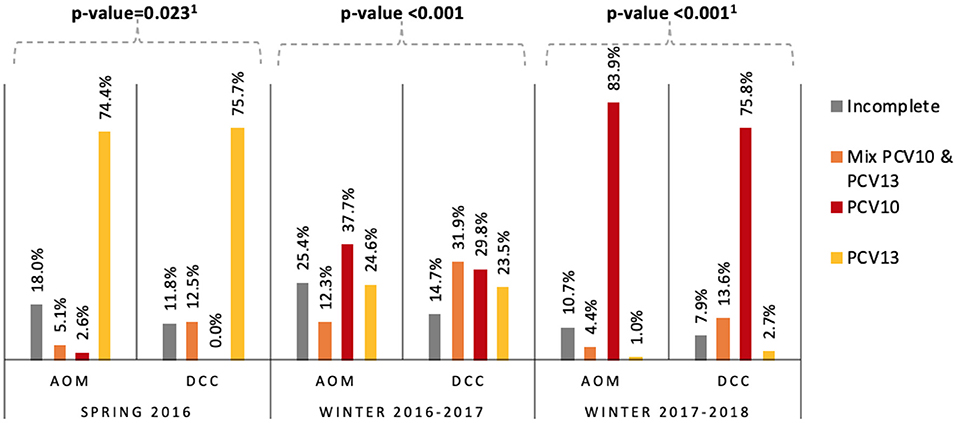
Figure 1. Vaccination status of children with AOM and children attending DCCs per season in Belgium in 2016–2018. Per season, the Chi-square or Fisher's Exact Test p-values for statistical significance of AOM vs. DCC are noted above. 1Fishers Exact Test. AOM = acute otitis media, DCC = day care center, PCV10 = age-appropriately vaccinated with 10-valent pneumococcal conjugate vaccine, PCV13 = age-appropriately vaccinated with 13-valent pneumococcal conjugate vaccine; Mix PCV10&PCV13 = age-appropriately vaccinated with a schedule combining both vaccines; incomplete = not vaccinated age-appropriately.
Carriage Prevalence Was Similar in AOM Children and in DCC Children
Pneumococcal carriage prevalence was similar in AOM children and in DCC children (AOM: 79.2%; DCC: 77.5%; p-value = 0.454), and also among different age categories, for both AOM (6–12 m: 76.7%, 13–24 m: 82.5%, 25–30 m: 77.8%; p-value = 0.431) and DCC (6–12 m: 75.8%, 13–24 m: 79.1%, 25–30 m: 75.6%; p-value = 0.098).
Serotype Distribution Slightly Differed Between AOM Children and DCC Children
A total of 2241 Sp strains were cultured and 51 different serotypes were identified. In AOM children, 34 serotypes in 268 strains were identified and in DCC children 49 serotypes in 1973 strains. PCV13 serotypes increased over time in AOM (Y1: 0.0%, Y2: 8.4%, Y3: 8.3%) and DCC (Y1: 1.8%, Y2: 2.6%, Y3: 9.8%), mainly because of an increased prevalence of serotype 19A (AOM: Y1: 0.0%, Y2: 3.6%, Y3: 6.4%; DCC: Y1: 0.6%, Y2: 1.9%, Y3: 8.8%). Serotype 23B, a non-vaccine type, was most frequent in AOM (12.3%) and DCC (17.4%), followed by 11A (7.5%), 15B (7.5%) and 6C (6.3%) in AOM and 23A (8.0%), 11A (7.4%) and 15B (7.1%) in DCC. Serotypes 8 and 19B were found in AOM children (both 0.4%), but not in DCC children. For 9 serotypes, frequency differed significantly between AOM and DCC (Table 3), although the difference was not significant in every separate period. Frequency of vaccine serotypes over time is summarized in Table 8: Appendix 3 in Supplementary Material.
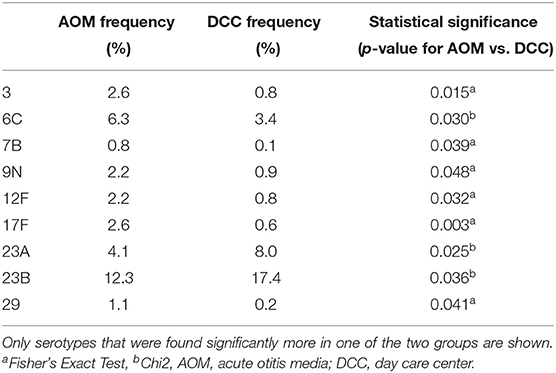
Table 3. Difference in serotype distribution of S. pneumoniae between children with AOM and children attending DCC in Belgium in 2016–2018.
Carriage Density of Sp Positive Samples Was Similar in AOM Children and DCC Children
The overall median pneumococcal DNA density in AOM-infants was 5.0 × 105 copies/μl (3.3 × 105-7.6 × 105 copies/μl) and in DCC-infants 4.2 × 105 copies/μl (3.8 × 105-5.7 × 105 copies/μl) (MWU for median; p-value = 0.154). In an age-specific analysis, median density was higher in carriers with AOM in the 6–12 months and 25–30 months age category compared to carriers in DCC in the same age categories (Table 4).
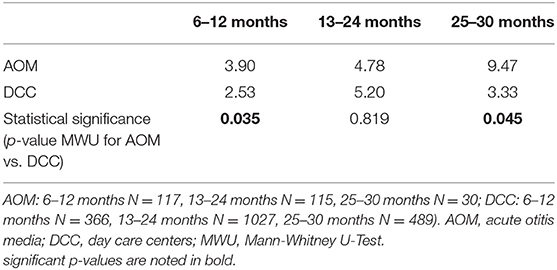
Table 4. Median S. pneumoniae carriage density, expressed in × 105 copies/μl, per age group for AOM and DCC in 2016–2018.
Antimicrobial Non-susceptibility Was Similar in AOM Children and DCC Children
In the AOM population, 19A strains were more frequently non-susceptible to penicillin compared to the DCC population (AOM: 8.8%; DCC: 1.2%; p-value = 0.002). The susceptibility profile of other serotypes was similar in both populations.
Over time, non-susceptibility of Sp strains to more than one antibiotic increased borderline non-significantly in the AOM population (Y1: 18.5%, Y2: 25.3%, Y3: 35.7%; p-value = 0.092) and increased significantly in the DCC population (Y1: 18.4%, Y2: 26.3%, Y3: 30.5%; p-value < 0.001).
Clinical Signs in Children With AOM Were Not Associated With Vaccine Type Carriage
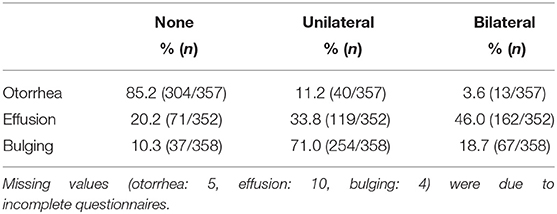
Clinical signs were analyzed to look for differences between AOM infants carrying vaccine serotypes and non-vaccine serotypes. Otoscopic signs are summarized in Table 5. The average temperature was 39.1°C (36.0–41.6°C). A small number of patients had no fever (8.7%, 30/347), while 47.8% (166/347) had a temperature of >39°C.
Median recovery time was 4 days; 47.5% (124/261) of children recovered within 3 days, and 8.8% (23/261) of children needed more than 8 days. A second visit for AOM within 1 month was reported for 18% (65/362) of the children. Reasons for a second visit were: deterioration established by a physician (9.2%, 6/65), deterioration according to the parents during a follow-up phone call (10.8%, 7/65), additional infection established by a physician (24.6%, 16/65), relapse according to the parents during a follow-up phone call (35.4%, 23/65) or unknown reasons (20.0%, 13/65).
No differences in clinical signs were observed between children carrying vaccine serotypes and non-vaccine serotypes, except for antibiotic use. Antibiotic prescription occurred in 77.3% of all cases (279/361, 1 missing value). Children carrying non-vaccine serotypes (82.3%, 191/232) more often received antibiotics than children carrying vaccine serotypes (64.3%, 18/28) (p-value = 0.023).
Discussion
In this 3 year nasopharyngeal carriage study, differences were identified in Sp serotype distribution, but not in carriage density or antimicrobial non-susceptibility between children with AOM and healthy children attending DCC.
Overall Sp carriage prevalence was similar and high in the two populations (AOM: 79.2%; DCC: 77.5%; p-value = 0.454). Carriage prevalence of Sp vary between studies from 11.8 to 71.2% in PCV vaccinated AOM children and from 8.3 to 89.5% in healthy PCV vaccinated children (18–21). The carriage prevalence in the current study is at the higher end of the reported range as in this study two child populations that are known to have a higher pneumococcal carriage prevalence were selected (22–25).
Serotypes 3, 6C, 9N, 12F, 17F, 7B, and 29 were found more frequently in AOM children than in DCC, although they were all together uncommon (AOM: 17.9%; DCC: 6.7%). Serotype 3 was thus the only vaccine serotype that was identified significantly more frequently in children with AOM than in children attending DCC. This is in line with previous findings that associated serotype 3 to a high invasive capacity and more-severe disease (26–30). Serotypes 9N and 12F have not previously been associated with AOM, though they have been associated with invasive pneumococcal disease (IPD) (27, 29, 31–33). Serotype 17F has previously been associated with serious clinical outcomes and higher case-fatality, but not with AOM (34, 35).
However, as the prevalence of PCV13 vaccine serotypes increased over the 3 year study period, serotype 19A was the most common PCV13 vaccine serotype in the third period of our study for both populations. Serotype 19A was also the most commonly identified serotype in IPD (27%) and middle ear fluid (MEF) (26%) in 2018 in Belgian children (36). In other studies looking at clinical manifestation of AOM episodes by serotype, serotype 19A was (one of) the most frequent serotype(s) identified in MEF (36–39), and 19A was also found among the most frequently carried serotypes in children with AOM in other nasopharyngeal carriage studies (38, 40). After PCV10 introduction in Bulgaria, the prevalence of 19A in the MEF of children diagnosed with AOM was 5.6% (41). This is similar as the nasopharyngeal carriage prevalence of serotype 19A (6.4%) that we found in children with AOM 2–3 years after PCV10 introduction in Belgium. Serotype 19A has been reported as a serotype that is frequently non-susceptible to antibiotics, especially after PCV7 or PCV10 introduction (10, 42, 43). After PCV7 introduction in France, penicillin non-susceptibility for serotype 19A in children with AOM ranged from 77.6 to 92.0% (40). However, in the present study non-susceptibility to penicillin of serotype 19A strains was a lot lower (AOM: 8.8%; DCC: 1.2%). Since children who were treated with oral antibiotics in the seven days prior to sampling were excluded, some non-susceptible strains could have been missed. In the current study, non-susceptibility to penicillin was investigated and overall no difference was found in non-susceptibility to penicillin in the AOM group compared to the DCC group. The current guideline for antibiotic use for AOM can be maintained as non-susceptibility to penicillin is still limited, but if this should increase as a result of changes in the serotype prevalence, then this needs to be reconsidered.
The PCV7 serotype 19F also continues to circulate and was found in children with AOM in all periods, as was the case in other PCV7-, PCV10- and PCV13-administering countries (10, 30, 37, 44–49). Among non-PCV13 serotypes, serotype 23B was most prevalent in both groups of children, and has also been reported as the most common colonizer in other studies (10, 30, 45). Other studies showed that serotypes 23A and 23B are carried for a longer period and therefore are detected more frequently (28). In the current study, 23A and 23B were found more frequently in healthy children attending DCC than in children with AOM.
No significant difference in carriage density of Sp between children with AOM and children attending DCC was found. A higher density was expected in children with AOM, since a higher pneumococcal load has been found associated with acute respiratory disease (50–52). The study may be underpowered, as in period three, when the highest number of children with AOM were recruited, the difference in density was borderline non-significant with a higher value in AOM (AOM: 5.3 × 105; DCC: 3.3 × 105; p-value = 0.086). A possible explanation is that Sp transmission is high in Belgian DCCs, as high density of Sp in the nasopharynx is suggested to be a predictor of both transmission and disease probability (53, 54). Dunne et al. found that having upper respiratory tract infection symptoms was associated with an increased pneumococcal density (55–57). In the current study, 36.4% of children in DCCs presented common cold symptoms, which associates with the high pneumococcal density in DCC-children. For children aged between 25 and 30-months, a three times higher carriage density was observed in the AOM-population compared to the DCC-population. Since risk of AOM development decreases with age (58), this finding suggests a role of carriage density in disease development.
In the current study, we compared children carrying vaccine serotypes and non-vaccine serotypes, and did not find a difference for any of the mentioned symptoms, neither in a separate analysis nor using a summarizing score that combined the different elements of the clinical evaluation by the treating physician (Table 9: Appendix 4 in Supplementary Material). This can be due to the low number of vaccine type carriers in this study that do not allow to detect a clear difference in AOM children carrying vaccine serotypes and non-vaccine serotypes.
A major strength of this study is the combined use of culture and molecular methods to explore pneumococcal carriage in nasopharyngeal microbiota. In addition, this study started after a switch from PCV13 to PCV10, which has rarely occurred worldwide and with different preceding PCV program history. Another strength is the that the two populations, children with AOM and healthy carriers, were recruited simultaneously within the same study protocol and using the same methods. However, some limitations should be considered when interpreting the presented results. First, in children with AOM we cannot be sure that the carried Sp serotype was the cause of the AOM episode, especially since 91.4 and 95.5% of Sp positive samples also carried Haemophilus influenzae and Moraxella catarrhalis respectively. MEF samples allow for more accurate identification of the causative agent, but are not standard practice in first-line AOM approach in Belgium. Secondly, findings refer to the specific child populations that were studied and cannot be generalized to non-responsive or chronic otitis media, or to children of all ages. In addition, 148 DCC children representing 303 isolates participated more than once, but in different study periods. A sensitivity analysis that only used their first sample confirmed all findings for child characteristics, carriage prevalence and carriage density. Finally, any trends in time should be interpreted cautiously. Study population characteristics were not constant over time, especially in the AOM population in whom recruitment was more successful toward the end of the study. Moreover, calendar time was different in the first collection period than in the later ones, which might have exaggerated season-dependent changes in serotype distribution. However, the marked 19A increase was the only time trend we identified. A trend we suppose not to be due to differences in season as no season-dependency of 19A carriage has been reported so far. After the PCV13-to-PCV10 switch, the carriage proportion of PCV13-non-PCV10 vaccine serotype 19A showed an increasing trend in healthy children attending day care centers (DCC) (2). This increasing trend of serotype 19A was also observed in Belgian children with invasive pneumococcal disease (59). Consequently, PCV13 has been implemented again in 2019.
We can conclude that the change in the Belgian PCV program has induced similar changes in both AOM and DCC infants, with an increase of PCV13 vaccine serotype 19A from 0.0% in year 1 to 6.4% in year 3 for the AOM population and from 0.6% in year 1 to 8.8% in year 3 for the DCC population. This study shows that young children with AOM did not carry S. pneumoniae more frequently or at higher load than healthy children attending DCC. Nevertheless, the serotypes that were found more common in AOM children were mainly non-vaccine serotypes. Although an interesting finding, it should be taken with caution as only nasopharyngeal swabs were collected in the present study, and it is known that results from nasopharyngeal swabs and MEF swabs in AOM patients are not fully consistent. Lastly, no differences were observed in the clinical outcome of AOM in children carrying vaccine types compared to children carrying non-vaccine types.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
Approval to conduct the current study with ID 15/45/471 was obtained from the University of Antwerp and University Hospital of Antwerp ethics committee (Commissie voor Medische Ethiek van UZA/UA). Parental consent was obtained.
NPcarriage Study Group
David Tuerlinckx, CHU Dinant-Godinne, Université Catholique de Louvain, Yvoir, Belgium; Adam Finn, University of Bristol, School of Cellular and Molecular Medicine, Bristol, UK; Koen Van Herck, Department of Public Health and Primary Care, Ghent University, Gent, Belgium, Center for the Evaluation of Vaccination, Vaccine and Infectious Disease Institute, University of Antwerp, Wilrijk, Belgium; Robert Cohen, Université Paris Est, IMRB-GRC GEMINI, 94000 Créteil, France; Christine Lammens, Laboratory of Medical Microbiology, Vaccine and Infectious Disease Institute, University of Antwerp, Wilrijk, Belgium; Marc Verghote (Private pediatrician); Marc De Meulemeester (Private pediatrician); Barbara De Wilde (Private pediatrician); Kate Sauer, Pediatric Department, AZ Sint-Jan, Brugge, Belgium; Luc Pattyn, Pediatric Department, AZ Turnhout, Belgium; Bart Rutteman, Pediatric Department, AZ Sint-Blasius, Dendermonde, Belgium; Caroline Genin, Pediatric Department, C.H.C Espérance, Saint-Nicolas, Belgium; Diane Stroobant, Pediatric Department, Grand Hôpital de Charleroi, Charleroi, Belgium; Ilse Ryckaert, Pediatric Department AZ-Nikolaas, Sint-Niklaas, Belgium; Tessa Goetghebuer, Pediatric Department, CHU Saint-Pierre, Brussels, Belgium; Zornitsa Vassileva, Pediatric Department, AZ Lokeren, Lokeren, Belgium.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
IW acknowledges Research grant from Research Foundation Flanders (1150017N); investigator-initiated research grant from Pfizer.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank the members of the expert advisory board for their contribution to the interpretation of the results; Research Link–ECSOR operating as the CRO; the cooperating nurses, physicians, the participating baby clinics for assistance in the recruitment and sampling; Kind en Gezin and Office de la Naissance et de l'Enfance (ONE) for their support; the children and their parents for their participation.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2021.664083/full#supplementary-material
References
1. Monasta L, Ronfani L, Marchetti F, Montico M, Vecchi Brumatti L, Bavcar A, et al. Burden of disease caused by otitis media: systematic review and global estimates. PLoS ONE. (2012) 7:e36226. doi: 10.1371/journal.pone.0036226
2. Wouters I, Desmet S, Van Heirstraeten L, Herzog SA, Beutels P, Verhaegen J, et al. How nasopharyngeal pneumococcal carriage evolved during and after a PCV13-to-PCV10 vaccination programme switch in Belgium, 2016 to 2018. Euro Surveill. (2020) 25:1900303. doi: 10.2807/1560-7917.ES.2020.25.5.1900303
3. Blanchard-Rohner G. Impact de la vaccination sur les otites moyennes aiguës. Rev Méd Suisse. (2016) 12:350–53.
4. Grabenstein JD, Musher DM. Pneumococcal polysaccharide vaccines. In: Edwards KM, Offit PA, Plotkin SA, Orenstein WA, editors. Plotkin's Vaccines. Elsevier (2018). p. 816–40.e13. doi: 10.1016/B978-0-323-35761-6.00046-8
5. Dagan R, Pelton S, Bakaletz L, Cohen R. Prevention of early episodes of otitis media by pneumococcal vaccines might reduce progression to complex disease. Lancet Infect Dis. (2016) 16:480–92. doi: 10.1016/S1473-3099(15)00549-6
6. Bogaert D, De Groot R, Hermans PW. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis. (2004) 4:144–54. doi: 10.1016/S1473-3099(04)00938-7
7. Simell B, Auranen K, Kayhty H, Goldblatt D, Dagan R, O'Brien KL, et al. The fundamental link between pneumococcal carriage and disease. Expert Rev Vaccines. (2012) 11:841–55. doi: 10.1586/erv.12.53
8. Ganaie F, Saad JS, McGee L, van Tonder AJ, Bentley SD, Lo SW, et al. A new pneumococcal capsule type, 10D, is the 100th Serotype and has a large cps fragment from an oral Streptococcus. mBio. (2020) 11:e00937–20. doi: 10.1128/mBio.00937-20
9. Pichichero ME, Khan MN, Xu Q. Next generation protein based Streptococcus pneumoniae vaccines. Hum Vaccin Immunother. (2016) 12:194–205. doi: 10.1080/21645515.2015.1052198
10. Kempf M, Varon E, Lepoutre A, Gravet A, Baraduc R, Brun M, et al. Decline in antibiotic resistance and changes in the serotype distribution of Streptococcus pneumoniae isolates from children with acute otitis media; a 2001-2011 survey by the French pneumococcal network. Clin Microbiol Infect. (2015) 21:35–42. doi: 10.1016/j.cmi.2014.08.009
11. Malfroot A, Verhaegen J, Dubru JM, Van Kerschaver E, Leyman S. A cross-sectional survey of the prevalence of Streptococcus pneumoniae nasopharyngeal carriage in Belgian infants attending day care centres. Clin Microbiol Infect. (2004) 10:797–803. doi: 10.1111/j.1198-743X.2004.00926.x
12. Rodgers GL, Arguedas A, Cohen R, Dagan R. Global serotype distribution among Streptococcus pneumoniae isolates causing otitis media in children: potential implications for pneumococcal conjugate vaccines. Vaccine. (2009) 27:3802–10. doi: 10.1016/j.vaccine.2009.04.021
13. Hausdorff WP, Yothers G, Dagan R, Kilpi T, Pelton SI, Cohen R, et al. Multinational study of pneumococcal serotypes causing acute otitis media in children. Pediatric Infect Dis J. (2002) 21:1008–16. doi: 10.1097/00006454-200211000-00007
14. Eskola J, Kilpi T, Palmu A, Jokinen J, Haapakoski J, Herva E, et al. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N Engl J Med. (2001) 344:403–9. doi: 10.1056/NEJM200102083440602
15. Fitzwater SP, Chandran A, Santosham M, Johnson HL. The worldwide impact of the seven-valent pneumococcal conjugate vaccine. Pediatr Infect Dis J. (2012) 31:501–8. doi: 10.1097/INF.0b013e31824de9f6
16. Beutels P, Hanquet G., Bilcke J., Thiry N., Sabbe M., Verhaegen J, et al. Cost-Effectiveness of 10- and 13-Valent Pneumococcal Conjugate Vaccines In Childhood. Brussels: Belgian HealthCare Knowledge Centre (KCE) (2011). p. 101.
17. Wouters I, Desmet S, Van Heirstraeten L, Blaizot S, Verhaegen J, Van Damme P, et al. Follow-up of serotype distribution and antimicrobial susceptibility of Streptococcus pneumoniae in child carriage after a PCV13-to-PCV10 vaccine switch in Belgium. Vaccine. (2019) 37:1080–6. doi: 10.1016/j.vaccine.2018.12.068
18. Wouters I, Van Heirstraeten L, Desmet S, Blaizot S, Verhaegen J, Goossens H, et al. Nasopharyngeal S. pneumoniae carriage and density in Belgian infants after 9 years of pneumococcal conjugate vaccine programme. Vaccine. (2018) 36:15–22. doi: 10.1016/j.vaccine.2017.11.052
19. Dagan R, Melamed R, Muallem M, Piglansky L, Yagupsky P. Nasopharyngeal colonization in southern Israel with antibiotic-resistant pneumococci during the first 2 years of life: relation to serotypes likely to be included in pneumococcal conjugate vaccines. J Infect Dis. (1996) 174:1352–5. doi: 10.1093/infdis/174.6.1352
20. Dunais B, Bruno-Bazureault P, Carsenti-Dellamonica H, Touboul P, Pradier C. A decade-long surveillance of nasopharyngeal colonisation with Streptococcus pneumoniae among children attending day-care centres in south-eastern France: 1999-2008. Eur J Clin Microbiol Infect Dis. (2011) 30:837–43. doi: 10.1007/s10096-011-1154-9
21. Labout JA, Duijts L, Arends LR, Jaddoe VW, Hofman A, de Groot R, et al. Factors associated with pneumococcal carriage in healthy Dutch infants: the generation R study. J Pediatr. (2008) 153:771–6. doi: 10.1016/j.jpeds.2008.05.061
22. Cohen R, Varon E, Doit C, Schlemmer C, Romain O, Thollot F, et al. A 13-year survey of pneumococcal nasopharyngeal carriage in children with acute otitis media following PCV7 and PCV13 implementation. Vaccine. (2015) 33:5118–26. doi: 10.1016/j.vaccine.2015.08.010
23. Principi N, Marchisio P, Schito GC, Mannelli S. Risk factors for carriage of respiratory pathogens in the nasopharynx of healthy children. Ascanius project collaborative group. Pediatr Infect Dis J. (1999) 18:517–23. doi: 10.1097/00006454-199906000-00008
24. Dagan R, O'Brien KL. Modeling the association between pneumococcal carriage and child-care center attendance. Clin Infect Dis. (2005) 40:1223–6. doi: 10.1086/428585
25. Gessner BD, Ussery XT, Parkinson AJ, Breiman RF. Risk factors for invasive disease caused by Streptococcus pneumoniae among Alaska native children younger than two years of age. Pediatr Infect Dis J. (1995) 14:123–8. doi: 10.1097/00006454-199502000-00008
26. Sjöström K, Spindler C, Ortqvist A, Kalin M, Sandgren A, Kühlmann-Berenzon S, et al. Clonal and capsular types decide whether pneumococci will act as a primary or opportunistic pathogen. Clin Infect Dis. (2006) 42:451–9. doi: 10.1086/499242
27. Hanage WP, Kaijalainen TH, Syrjänen RK, Auranen K, Leinonen M, Mäkelä PH, et al. Invasiveness of serotypes and clones of Streptococcus pneumoniae among children in Finland. Infect Immun. (2005) 73:431–5. doi: 10.1128/IAI.73.1.431-435.2005
28. Yildirim I, Hanage WP, Lipsitch M, Shea KM, Stevenson A, Finkelstein J, et al. Serotype specific invasive capacity and persistent reduction in invasive pneumococcal disease. Vaccine. (2010) 29:283–8. doi: 10.1016/j.vaccine.2010.10.032
29. Martens P, Worm SW, Lundgren B, Konradsen HB, Benfield T. Serotype-specific mortality from invasive Streptococcus pneumoniae disease revisited. BMC Infect Dis. (2004) 4:21. doi: 10.1186/1471-2334-4-21
30. Allemann A, Frey PM, Brugger SD, Hilty M. Pneumococcal carriage and serotype variation before and after introduction of pneumococcal conjugate vaccines in patients with acute otitis media in Switzerland. Vaccine. (2017) 35:1946–53. doi: 10.1016/j.vaccine.2017.02.010
31. Brueggemann AB, Griffiths DT, Meats E, Peto T, Crook DW, Spratt BG. Clonal relationships between invasive and carriage Streptococcus pneumoniae and serotype- and clone-specific differences in invasive disease potential. J Infect Dis. (2003) 187:1424–32. doi: 10.1086/374624
32. Ladhani SN, Collins S, Djennad A, Sheppard CL, Borrow R, Fry NK, et al. Rapid increase in non-vaccine serotypes causing invasive pneumococcal disease in England and Wales, 2000-17: a prospective national observational cohort study. Lancet Infect Dis. (2018) 18:441–51. doi: 10.1016/S1473-3099(18)30052-5
33. Jansen AG, Rodenburg GD, van der Ende A, van Alphen L, Veenhoven RH, Spanjaard L, et al. Invasive pneumococcal disease among adults: associations among serotypes, disease characteristics, outcome. Clin Infect Dis. (2009) 49:e23–9. doi: 10.1086/600045
34. Ciprero KL, Marchese RD, Richard P, Baudin M, Sterling TM, Manoff SB, et al. Vaccination of adults with 23-valent pneumococcal polysaccharide vaccine induces robust antibody responses against pneumococcal serotypes associated with serious clinical outcomes. Hum Vaccin Immunother. (2016) 12:2135–141. doi: 10.1080/21645515.2016.1156270
35. Harboe ZB, Thomsen RW, Riis A, Valentiner-Branth P, Christensen JJ, Lambertsen L, et al. Pneumococcal serotypes and mortality following invasive pneumococcal disease: a population-based cohort study. PLoS Med. (2009) 6:e1000081. doi: 10.1371/journal.pmed.1000081
36. Braeye T, Wyndham-Thomas C, Lagrou K, Grammens T, Desmet S. Epidemiologische Surveillance Van Invasieve Pneumokokkeninfecties (IPD)−2018. Brussel: Sciensano (2018). p. 10.
37. Chi H, Chiu NC, Huang FY, Hsu CH, Lee KS, Huang LM, et al. Acute otitis media caused by Streptococcus pneumoniae serotype 19A ST320 clone: epidemiological and clinical characteristics. J Microbiol Immunol Infect. (2018) 51:337–43. doi: 10.1016/j.jmii.2016.08.002
38. Chen YJ, Hsieh YC, Huang YC, Chiu CH. Clinical manifestations and microbiology of acute otitis media with spontaneous otorrhea in children. J Microbiol Immunol Infect. (2013) 46:382–8. doi: 10.1016/j.jmii.2013.04.001
39. Palmu AA, Jokinen JT, Kaijalainen T, Leinonen M, Karma P, Kilpi TM. Association of clinical signs and symptoms with pneumococcal acute otitis media by serotype–implications for vaccine effect. Clin Infect Dis. (2005) 40:52–7. doi: 10.1086/426446
40. Cohen R, Levy C, Bonnet E, Grondin S, Desvignes V, Lecuyer A, et al. Dynamic of pneumococcal nasopharyngeal carriage in children with acute otitis media following PCV7 introduction in France. Vaccine. (2010) 28:6114–21. doi: 10.1016/j.vaccine.2009.05.037
41. Setchanova L, Murdjeva M, Stancheva I, Alexandrova A, Sredkova M, Stoeva T, et al. Serotype changes and antimicrobial nonsusceptibility rates of invasive and non-invasive Streptococcus pneumoniae isolates after implementation of 10-valent pneumococcal nontypeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) in Bulgaria. Braz J Infect Dis. (2017) 21:433–40. doi: 10.1016/j.bjid.2017.03.011
42. Manenzhe RI, Moodley C, Abdulgader SM, Robberts FJL, Zar HJ, Nicol MP, et al. Nasopharyngeal carriage of antimicrobial-resistant pneumococci in an intensively sampled South African Birth cohort. Front Microbiol. (2019) 10:610. doi: 10.3389/fmicb.2019.00610
43. Pumarola F, Marès J, Losada I, Minguella I, Moraga F, Tarragó D, et al. Microbiology of bacteria causing recurrent acute otitis media (AOM) and AOM treatment failure in young children in Spain: shifting pathogens in the post-pneumococcal conjugate vaccination era. Int J Pediatr Otorhinolaryngol. (2013) 77:1231–6. doi: 10.1016/j.ijporl.2013.04.002
44. Neves FP, Pinto TC, Corrêa MA, dos Anjos Barreto R, de Souza Gouveia Moreira L, Rodrigues HG, et al. Nasopharyngeal carriage, serotype distribution and antimicrobial resistance of Streptococcus pneumoniae among children from Brazil before the introduction of the 10-valent conjugate vaccine. BMC Infect Dis. (2013) 13:318. doi: 10.1186/1471-2334-13-318
45. Pichichero M, Kaur R, Scott DA, Gruber WC, Trammel J, Almudevar A, et al. Effectiveness of 13-valent pneumococcal conjugate vaccination for protection against acute otitis media caused by Streptococcus pneumoniae in healthy young children: a prospective observational study. Lancet Child Adolesc Health. (2018) 2:561–568. doi: 10.1016/S2352-4642(18)30168-8
46. Soysal A, Karabag-Yilmaz E, Kepenekli E, Karaaslan A, Cagan E, Atici S, et al. The impact of a pneumococcal conjugate vaccination program on the nasopharyngeal carriage, serotype distribution and antimicrobial resistance of Streptococcus pneumoniae among healthy children in Turkey. Vaccine. (2016) 34:3894–900. doi: 10.1016/j.vaccine.2016.05.043
47. Nguyen HAT, Fujii H, Vu HTT, Parry CM, Dang AD, Ariyoshi K, et al. An alarmingly high nasal carriage rate of Streptococcus pneumoniae serotype 19F non-susceptible to multiple beta-lactam antimicrobials among Vietnamese children. BMC Infect Dis. (2019) 19:241. doi: 10.1186/s12879-019-3861-2
48. Hays C, Vermee Q, Agathine A, Dupuis A, Varon E, Poyart C, et al. Demonstration of the herd effect in adults after the implementation of pneumococcal vaccination with PCV13 in children. Eur J Clin Microbiol Infect Dis. (2017) 36:831–8. doi: 10.1007/s10096-016-2868-5
49. Melin M, Jarva H, Siira L, Meri S, Käyhty H, Väkeväinen M. Streptococcus pneumoniae capsular serotype 19F is more resistant to C3 deposition and less sensitive to opsonophagocytosis than serotype 6B. Infect Immun. (2009) 77:676–84. doi: 10.1128/IAI.01186-08
50. Baggett HC, Watson NL, Deloria Knoll M, Brooks WA, Feikin DR, Hammitt LL, et al. Density of upper respiratory colonization with Streptococcus pneumoniae and its role in the diagnosis of pneumococcal pneumonia among children aged <5 years in the PERCH study. Clin Infect Dis. (2017) 64:S317–27. doi: 10.1093/cid/cix100
51. Howard LM, Zhu Y, Griffin MR, Edwards KM, Williams JV, Gil AI, et al. Nasopharyngeal pneumococcal density during asymptomatic respiratory virus infection and risk for subsequent acute respiratory illness. Emerg Infect Dis. (2019) 25:2040–7. doi: 10.3201/eid2511.190157
52. Brotons P, Bassat Q, Lanaspa M, Henares D, Perez-Arguello A, Madrid L, et al. Nasopharyngeal bacterial load as a marker for rapid and easy diagnosis of invasive pneumococcal disease in children from Mozambique. PLoS ONE. (2017) 12:e0184762. doi: 10.1371/journal.pone.0184762
53. Thors V, Morales-Aza B, Pidwill G, Vipond I, Muir P, Finn A. Population density profiles of nasopharyngeal carriage of 5 bacterial species in pre-school children measured using quantitative PCR offer potential insights into the dynamics of transmission. Hum Vaccin Immunother. (2016) 12:375–82. doi: 10.1080/21645515.2015.1090069
54. Fadlyana E, Dunne EM, Rusmil K, Tarigan R, Sudigdoadi S, Murad C, et al. Risk factors associated with nasopharyngeal carriage and density of Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, and Staphylococcus aureus in young children living in Indonesia. Pneumonia. (2018) 10:14. doi: 10.1186/s41479-018-0058-1
55. Murad C, Dunne EM, Sudigdoadi S, Fadlyana E, Tarigan R, Pell CL, et al. Pneumococcal carriage, density, and co-colonization dynamics: a longitudinal study in Indonesian infants. Int J Infect Dis. (2019) 86:73–81. doi: 10.1016/j.ijid.2019.06.024
56. Dunne EM, Choummanivong M, Neal EFG, Stanhope K, Nguyen CD, Xeuatvongsa A, et al. Factors associated with pneumococcal carriage and density in infants and young children in Laos PDR. PLoS ONE. (2019) 14:e0224392. doi: 10.1371/journal.pone.0224392
57. Neal EFG, Nguyen CD, Ratu FT, Dunne EM, Kama M, Ortika BD, et al. Factors associated with pneumococcal carriage and density in children and adults in Fiji, using four cross-sectional surveys. PLoS ONE. (2020) 15:e0231041. doi: 10.1371/journal.pone.0231041
58. Kaur R, Morris M, Pichichero ME. Epidemiology of acute otitis media in the postpneumococcal conjugate vaccine era. Pediatrics. (2017) 140:e20170181. doi: 10.1542/peds.2017-0181
59. Desmet S, Wouters I, Heirstraeten LV, Beutels P, Van Damme P, Malhotra-Kumar S, et al. In-depth analysis of pneumococcal serotypes in Belgian children (2015-2018): diversity, invasive disease potential, and antimicrobial susceptibility in carriage and disease. Vaccine. (2021) 39:372–9. doi: 10.1016/j.vaccine.2020.11.044
Keywords: pneumococcal acute otitis media, serotypes, children, pneumococcal conjugate vaccine, AOM, PCV, Streptococcus pneumoniae
Citation: Ekinci E, Desmet S, Van Heirstraeten L, Mertens C, Wouters I, Beutels P, Verhaegen J, Malhotra-Kumar S, Theeten H and NPcarriage Group (2021) Streptococcus pneumoniae Serotypes Carried by Young Children and Their Association With Acute Otitis Media During the Period 2016–2019. Front. Pediatr. 9:664083. doi: 10.3389/fped.2021.664083
Received: 12 February 2021; Accepted: 07 June 2021;
Published: 05 July 2021.
Edited by:
Chien-Shun Chiou, Taiwan Centers for Disease Control (CDC), TaiwanReviewed by:
Cheng-Hsun Chiu, Chang Gung Children's Hospital, TaiwanTal Marom, Assuta Ashdod University Hospital, Israel
Copyright © 2021 Ekinci, Desmet, Van Heirstraeten, Mertens, Wouters, Beutels, Verhaegen, Malhotra-Kumar, Theeten and NPcarriage Group. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Esra Ekinci, ZXNyYS5la2luY2lAdWFudHdlcnBlbi5iZQ==
 Esra Ekinci
Esra Ekinci Stefanie Desmet
Stefanie Desmet Liesbet Van Heirstraeten3
Liesbet Van Heirstraeten3 Surbhi Malhotra-Kumar
Surbhi Malhotra-Kumar