Abstract
Background:
Epidemiological studies suggest a link between eczema and attention deficit hyperactivity disorder (ADHD), but underlying mechanisms have not been examined.
Objective:
We aim to investigate the association between eczema and subsequent ADHD symptoms in the Growing Up in Singapore Towards healthy Outcomes cohort and explore the role of pro-inflammatory cytokines and gut microbiome.
Methods:
The modified International Study of Asthma and Allergies in Childhood questionnaire and Computerized Diagnostic Interview Schedule for Children Version IV were administered to assess reported eczema within the first 18 months and presence of ADHD symptoms at 54 months, respectively. Skin prick testing at 18 months, cytokines in maternal blood during pregnancy and cord blood and the mediating role of the gut microbiome at 24 months were assessed.
Results:
After adjusting for confounders, eczema with or without a positive skin prick test was associated with doubling the risk of ADHD symptoms. No differences in maternal and cord blood cytokines were observed in children with and without eczema, or children with and without ADHD. Gut microbiome dysbiosis was observed in children with eczema and children with ADHD. Children with eczema also had lower gut bacterial Shannon diversity. However, the relationship between eczema and ADHD was not mediated by gut microbiome.
Conclusion:
Early life eczema diagnosis is associated with a higher risk of subsequent ADHD symptoms in children. We found no evidence for underlying inflammatory mechanism or mediation by gut microbiome dysbiosis. Further research should evaluate other mechanisms underlying the link between eczema and ADHD.
Clinical Trial Registration:
[https://clinicaltrials.gov/ct2/show/NCT01174875], identifier [NCT01174875].
Introduction
The increasing incidence of eczema is paralleled by an increasing incidence of attention deficit hyperactivity disorder (ADHD) (1). Both eczema and ADHD are non-communicable diseases that carry a substantial economic burden and adversely affect quality of life (2, 3). Eczema is one of the earliest manifestations of allergic disease and affects approximately 20% of children; it generally manifests as dry and itchy skin on the face, elbow, and knee folds (4, 5). ADHD, characterized by inattention, hyperactivity, and impulsivity, is the most common mental health disorder in early childhood and occurs in 10% of children and adolescents (6).
Evidence from a number of studies suggests a link between eczema and ADHD. In a United States study including 354,416 children from 19 population-based surveys, eczema was associated with a 50% increased risk of developing ADHD compared to children without eczema (7). Similarly, in another United States study of 79,667 children aged 0–18 years, Yaghmaie and colleagues found that the odds of ADHD was two-fold higher in children with eczema than in those without eczema (5).
Despite the epidemiological associations between eczema and ADHD, studies have not evaluated the underlying mechanisms and common early-life factors that may mediate the association. Pro-inflammatory cytokines and the gut microbiome have been proposed to be involved in the development of both allergic and neurodevelopmental disorders (8). Eczema has been linked to increased production of Th2, Th17, and Th22 inflammatory cytokines (9). These pro-inflammatory cytokines can traverse the blood–brain barrier to activate the prefrontal cortex and increase susceptibility to cognitive disturbances (1). Inflammation may also be directly involved in the development of ADHD symptoms through microglial activation and tumor necrosis factor-α production, leading to altered central nervous system excitability (10). Also, alterations in gut microbiota composition have been reported in subjects with eczema (11) or ADHD (12).
Hence, the aim of our study is to investigate the association between eczema and subsequent ADHD symptoms in the Growing Up in Singapore Towards healthy Outcomes (GUSTO) cohort in a confirmatory analysis. We also explore associations of pro-inflammatory cytokines in maternal blood during pregnancy and cord blood with eczema and ADHD and the possible role of the gut microbiome in linking eczema and ADHD.
Materials and Methods
Study Design and Measurements
The GUSTO study is a prospective cohort study which recruited pregnant women attending their first-trimester antenatal dating ultrasound scan clinics at two major public maternity units in Singapore, KK Women’s and Children’s Hospital and National University Hospital from June 2009 to September 2010 (13). Pregnant women aged 18 years and above, from any one of the three major ethnic groups (Chinese, Malay, and Indian), Singapore citizen or permanent residents who had the intention of delivering in either hospital and staying in Singapore for at least the next 5 years, and who had agreed to donate their birth tissues were invited to participate. Women who had type 1 diabetes mellitus, or who were receiving chemotherapy or psychotropic drugs were excluded.
Trained interviewers gathered information on demographic characteristics, family history of allergy, socioeconomic data and lifestyle factors. Birth outcomes were measured and recorded after delivery. Ethical approval was obtained from the Domain Specific Review Board of Singapore National Healthcare Group (D/2009/021 approved on 26 February 2009) and the Centralised Institutional Review Board of SingHealth (2018/2767 approved on 2 March 2019). The conduct of this study was based on the guidelines in the Declaration of Helsinki. Written informed consent was obtained from the mothers after a detailed explanation of the study.
We used the modified International Study of Asthma and Allergies in Childhood (ISAAC) questionnaire at ages 3, 6, 9, 12, 15, and 18 months for evaluation of eczema, which was defined as maternally reported physician-diagnosed eczema based on the question: “Has your child ever been diagnosed with eczema?” at any timepoint, which included children with eczema ranging from mild to severe. The ISAAC questionnaire has been featured in several birth cohorts studying allergic outcomes (14, 15). We administered skin prick testing (SPT) at 18 months to assess hypersensitivity to the major relevant allergens in Singapore: cow’s milk, egg, peanut and house dust mites Dermatophagoides pteronyssinus, Dermatophagoides farina (Greer Laboratories, Lenoir, NC, United States), and Blomia tropicalis (developed in-house) (16). A positive SPT was defined as a positive SPT (wheal size ≥3 mm) to one or more of these allergens.
At 54 months, we administered the parent version of Computerized Diagnostic Interview Schedule for Children Version IV-Young Child (CDISC-YC), a highly structured diagnostic interview designed to be administered by lay interviewers to assess most of the commonly occurring mental health disorders in children (17). The mental health disorders in CDISC are based on the Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition Text Revised (DSM-IV) (18). CDISC is one of the most extensively tested structural interviews. Although cultural differences in psychiatric symptoms cannot be ruled out, the CDISC has been used in different countries, and its performance, sensitivity and criterion validity have been verified in both clinical and community samples (17, 19–23). If symptoms of ADHD were present and sufficient to justify a possible diagnosis of ADHD according to DSM-IV, we classified this as “presence of ADHD.” This means that the ADHD symptoms must have persisted for at least 6 months to a degree that is inconsistent with developmental level and present in two or more settings (for example, at home and in school or other activities). However, the severity of these symptoms and the impaired social or academic functioning were not taken into account, given the young age (18). If symptoms were present but insufficient to meet the diagnostic criteria of ADHD, this was classified as “no ADHD.”
Maternal and Cord Blood Cytokine Assays
Maternal blood samples were obtained between 26 and 28 weeks of pregnancy. Maternal and cord blood cytokines were assayed in plasma using customized Human ProcartaPlex Panels (Thermo Fisher Scientific, Massachusetts, United States), which uses Luminex xMAP technology in combination with DropArray bead plates (Curiox Biosystems, Singapore). Interferon gamma (IFNγ) and tumor necrosis factor-α (TNFα) were measured using single-molecule array assays on the SP-X platform (Quanterix Corp., United States). All values were corrected for plate-specific effects by centering at the median.
Gut Microbiome Analysis
Stool samples were collected at 24 months. DNA extraction, 16S rRNA gene V4 region sequencing and data processing were performed. We followed the methods for gut microbiome analysis in our previous publications, with minor modification (24, 25). Briefly, DNA was extracted from 250 mg of stool samples based on manufacturer’s instructions (PowerSoil DNA isolation kit, catalog number 12855–100; Mo Bio). 16S rRNA gene V4 region amplification, library preparation and sequencing were performed using 250 base-pair paired-end reads on an Illumina MiSeq instrument at Argonne National Laboratories (University of Chicago). Raw sequencing data were processed using QIIME 1.9.0 and USEARCH v9.2.64 (26, 27). Both ends of the forward and reverse reads were truncated at the base where Q-value was no more than 2. The forward and reverse reads were merged into a complete read with the default setting in QIIME 1.9.0. The reads with length between 252 base pairs and 254 base pairs were retained. OTU was delineated at a similarity threshold of 97% using USEARCH v 9.2.64, and respective relative abundance and Shannon diversity index were obtained subsequently. All raw microbiome data are available at NCBI1 (Accession Number PRJNA668050).
Statistical Analyses
Analyses were performed using the Statistical Package for the Social Sciences, Version 26 (IBM Cooperation, New York, NY, United States) and Stata version 16. Descriptive statistics for continuous variables are presented as mean (SD) when normality and homogeneity assumptions were satisfied, otherwise as median (IQR), and n (%) for categorical variables. Associations between maternally-reported diagnosis of eczema alone and in combination with a positive SPT, as well as presence of ADHD at 54 months as defined according to the CDISC structured interview, were assessed using Poisson regression, adjusting for demographic and other relevant covariates including mother history of allergy, maternal age, parity, child gender, ethnicity, childcare attendance in first year, exposure to tobacco smoke during pregnancy, gestational age, pre-pregnancy body mass index, child weight at birth, maternal education which have been shown to influence eczema and ADHD outcomes (28, 29). Differences in the levels of maternal and cord blood cytokines were assessed using linear regression with natural log transformation, adjusting for mother history of allergy, maternal age, parity, child gender, ethnicity, smoking status, pre-pregnancy body mass index (BMI) and maternal education. For gut microbiome analysis, the Mann–Whitney U test was performed to compare the bacterial Shannon diversity index. Linear regression was used to compare relative abundance of gut bacteria at 24 months between children with and without eczema within the first 18 months as well as abundance of gut bacteria at 24 months between children with and without ADHD at 54 months, with Bonferroni correction for multiple pairwise comparisons and adjustment for baseline values and three confounders (Fucosyltransferase 2, gender and duration of any breastfeeding that affects gut microbiome). Mediation analyses were performed using the Stata command Ldecomp to assess the role of the gut microbiome at 24 months in mediating the link between eczema in the first 18 months and ADHD at 54 months. We decomposed the total effect of eczema on ADHD into direct and indirect effects, adjusting for demographic and relevant covariates. Type 1 error rates for multiple outcomes of maternal and cord blood cytokines analysis and multiple mediators of mediation analysis were adjusted using the Benjamini-Hochberg procedure, with a false discovery rate at 0.20. Statistical significance was set at 2-sided p < 0.05.
Results
Study Population and Characteristics
A total of 288 mother–child pairs were included in the study after limiting the sample to children whose mothers completed the CDISC-YC at 54 months and the modified ISAAC questionnaire within the first 18 months. Of the 288 children, 158 (54.9%) were boys. Seventy-four (25.7%) children had at least once a reported diagnosis of eczema in the first 18 months. Seventy-two (25.0%) of the children had ADHD symptoms according to the CDISC at 54 months. The mothers’ median age at recruitment was 30.8 years (IQR 27.0–34.8). The majority of the mothers had 12 or fewer years of education [187 (65.6%)], were of Chinese ethnicity [142 (49.3%)], had no history of allergy [159 (56.8%)], and were not exposed to tobacco smoke during pregnancy [163 (60.4%)] (Table 1).
TABLE 1
| n (%) or median (IQR) | |
| Child sex | |
| Female | 130 (45.1%) |
| Male | 158 (54.9%) |
| Maternal age (years) | 30.8 (27.0 – 34.8) |
| Maternal education level | |
| ≤12 years | 187 (65.6%) |
| >12 years | 98 (34.4%) |
| Maternal ethnicity | |
| Chinese | 142 (49.3%) |
| Malay | 99 (34.4%) |
| Indian | 47 (16.3%) |
| Maternal history of allergy | 121 (43.2%) |
| Parity | |
| Parous | 159(55.2%) |
| Nulliparous | 129(44.8%) |
| Childcare attendance in first year | 18(6.5%) |
| Exposure to tobacco smoke during pregnancy | |
| Yes | 107(39.6%) |
| No | 163(60.4%) |
| Gestational age (years) | 38.9 (37.9–39.9) |
| Pre-pregnancy BMI (kg/m2) | 22.1 (19.7–25.5) |
| Child weight at birth (kg) | 3.1 (2.9–3.4) |
| Eczema by 18M | 74 (25.7%) |
| ADHD at 54 months | 72 (25.0%) |
| ADHD at 54 months + Eczema by 18M | 27 (9.4%) |
Characteristics of the study population (n = 288).
ADHD, attention deficit hyperactivity disorder; BMI: body mass index. Column values may not always add up to total due to missing values.
Maternal and Cord Blood Cytokine Assays
No significant differences were observed in the maternal blood cytokines C-reactive protein (CRP), IFNγ and TNFα between children with and without eczema or between children with and without ADHD (Table 2). Nor did we find significant differences in these same cytokines measured in cord blood from children with and without eczema or those with and without ADHD (Table 3).
TABLE 2
| Unadjusted | Adjusted | |||||
| N | B (95% CI) | p-value | n | B (95% CI) | p-value | |
| Reference group: Subjects with no eczema | ||||||
| IFNγ | ||||||
| Eczema | 42 | 0.19(-0.37 to 0.75) | 0.507 | 37 | 0.14(−0.49 to 0.76) | 0.669 |
| No eczema | 132 | 107 | ||||
| TNFα | ||||||
| Eczema | 59 | −0.01(−0.34 to 0.31) | 0.929 | 49 | −0.11(−0.45 to 0.23) | 0.520 |
| No eczema | 178 | 147 | ||||
| CRP | ||||||
| Eczema | 73 | −0.25(−0.57 to 0.07) | 0.125 | 61 | 0.03(−0.29 to 0.34) | 0.870 |
| No eczema | 212 | 172 | ||||
| Reference group: Subjects with no ADHD | ||||||
| IFNγ | ||||||
| ADHD | 47 | 0.07(−0.47 to 0.61) | 0.806 | 40 | 0.02(−0.60 to 0.64) | 0.945 |
| No ADHD | 127 | 104 | ||||
| TNFα | ||||||
| ADHD | 58 | −0.23(−0.55 to 0.09) | 0.164 | 46 | −0.19(−0.54 to 0.16) | 0.290 |
| No ADHD | 179 | 150 | ||||
| CRP | ||||||
| ADHD | 71 | 0.05(−0.27 to 0.37) | 0.766 | 56 | 0.11(−0.21 to 0.44) | 0.489 |
| No ADHD | 214 | 177 | ||||
Differences in maternal blood cytokines.
ADHD, attention deficit hyperactivity disorder; CI, confidence interval; CRP, C-reactive protein; IFNγ, interferon gamma; TNFα, tumor necrosis factor α Benjamini–Hochberg correction with false discovery rate at 0.20 and n = 6 was applied.
TABLE 3
| Unadjusted | Adjusted | |||||
| N | B (95% CI) | p-value | n | B (95% CI) | p-value | |
| Reference group: Subjects with no eczema | ||||||
| IFNγ | ||||||
| Eczema | 30 | 0.07(−0.11 to 0.25) | 0.454 | 25 | 0.11(−0.08 to 0.30) | 0.257 |
| No eczema | 119 | 96 | ||||
| TNFα | ||||||
| Eczema | 47 | 0.07(−0.01 to 0.15) | 0.110 | 39 | 0.04(−0.04 to 0.13) | 0.317 |
| No eczema | 163 | 133 | ||||
| CRP | ||||||
| Eczema | 49 | −0.03(−0.31 −to 0.25) | 0.852 | 41 | 0.02(−0.27 to 0.30) | 0.903 |
| No eczema | 174 | 143 | ||||
| Reference group: Subjects with no ADHD | ||||||
| IFNγ | ||||||
| ADHD | 31 | −0.02(−0.21 to 0.16) | 0.786 | 21 | 0(−0.2 to 0.2) | 0.999 |
| No ADHD | 118 | 100 | ||||
| TNFα | ||||||
| ADHD | 52 | 0.01(−0.07 to 0.09) | 0.839 | 41 | 0.04(−0.05 to 0.13) | 0.367 |
| No ADHD | 158 | 131 | ||||
| CRP | ||||||
| ADHD | 52 | 0.11(−0.16 to 0.38) | 0.428 | 41 | −0.11(−0.40 to 0.18) | 0.446 |
| No ADHD | 171 | 143 | ||||
Differences in cord blood cytokines.
ADHD, attention deficit hyperactivity disorder; CI, confidence interval; CRP, C-reactive protein; IFNγ, interferon gamma; TNFα tumor necrosis factor α. Benjamini–Hochberg correction with false discovery rate at 0.20 and n = 6 was applied.
Associations Between Eczema and ADHD
A reported diagnosis of eczema within the first 18 months with and without a positive SPT was significantly associated with an increased risk of ADHD symptoms at 54 months [Adjusted relative risk (AdjRR) 2.5, 95%CI 1.1–6.0 and AdjRR 2.3, 95% CI 1.3–4.0, respectively] after adjusting for demographic and other covariates (Table 4).
TABLE 4
| Unadjusted | Adjusted | |||
| RR (95% CI) | p-value | RR (95% CI) | p-value | |
| Eczema by 18 months | 1.7 (1.1–2.8) | 0.024 | 2.3(1.3–4.0) | 0.005a |
| Eczema by 18 months + positive skin prick test | 1.9 (0.9–4.0) | 0.094 | 2.5(1.1–6.0) | 0.038b |
Poisson regression between eczema and ADHD at 54 months.
ADHD, attention deficit hyperactivity disorder; CI, confidence interval; RR: relative risk. Significant p value in bold. In the adjusted model,
a81.9% of subjects (236 out of 288) and
b65.6% of subjects (189 out of 288) were used.
Gut Microbiome Dysbiosis at 24 Months
A lower gut bacterial Shannon diversity at 24 months was observed in 72 children with reported eczema within the first 18 months compared to the 250 without reported eczema (adj p < 0.05). No differences were observed between the 65 children with ADHD symptoms at 54 months and the 196 without such symptoms (adj p < 0.05) (Supplementary Figure 1).
The dominant gut bacterial candidates (>0.1% relative abundance) were further analyzed and those which showed significant differences between groups were plotted in term of relative abundance, as shown in Figure 1. Increases in relative abundance of Lachnoclostridium spp. and Tyzzerella spp. (Lachnospiraceae family); and Cronobacter spp. (Enterobacteriaceae family) and decreases in Faecalibacterium spp. (Ruminococcaceae family) and Fusicatenibacter spp. (Lachnospiraceae family) were observed in children with reported eczema compared to those without (adj p < 0.05) (Figure 1A). The relative abundance of Escherichia or Shigella spp. (Enterobacteriaceae family) and Haemophilus spp. (Pasteurellaceae family) was decreased, while that of Enterococcus devriesei (Enterococcaceae family), Streptococcus spp. (Streptococcaceae family), Eggerthella spp. (Coriobacteriaceae family), and Actinomyces spp. (Actinomycetaceae family) was increased in children with ADHD symptoms at 54 months compared to those without (adj p < 0.05) (Figure 1B).
FIGURE 1
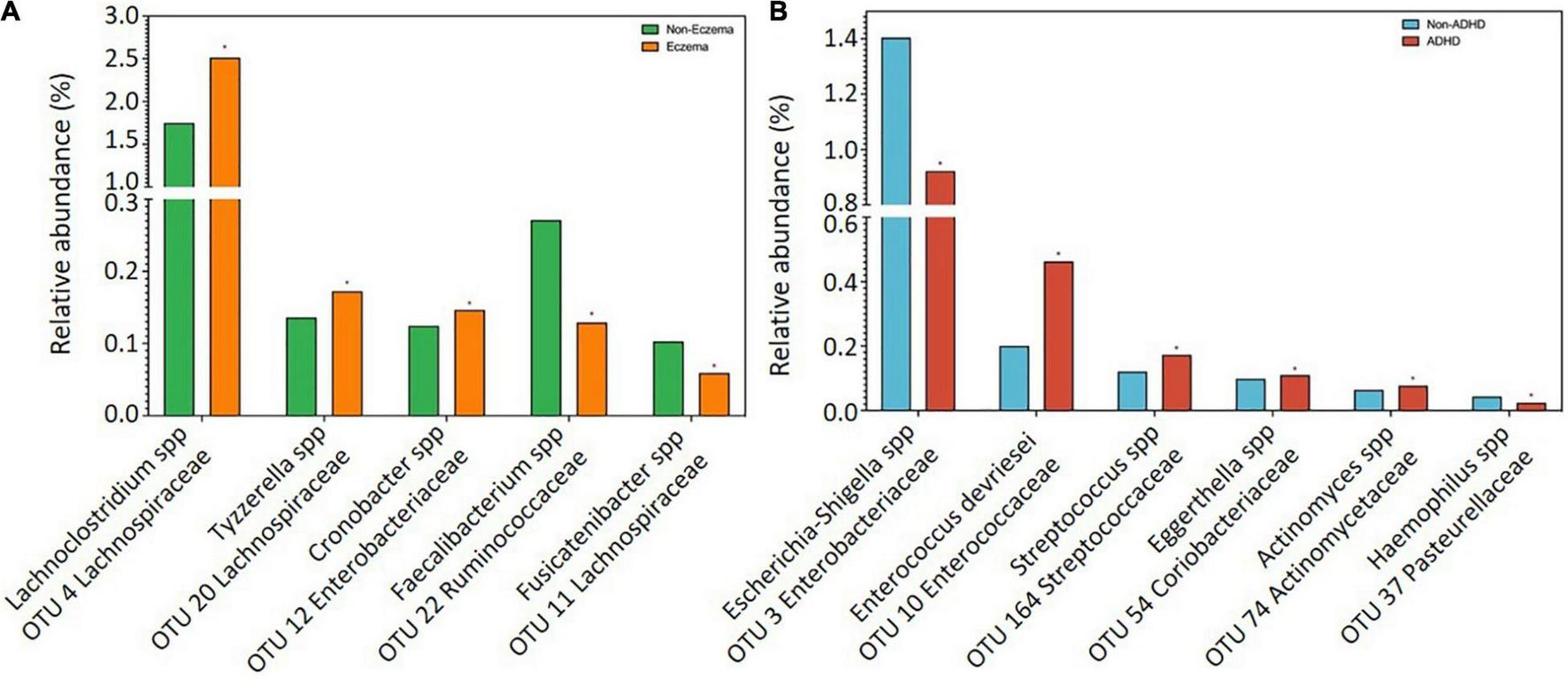
Comparisons of bacterial candidates in gut microbiome at 24 months between children with and without (A) eczema by 18 months (n = 72 vs 250), (B) ADHD symptoms at 54 months (n = 65 vs 196). Data are presented as median. Only significant bacteria (adjusted p < 0.05) with reference to controls were shown.
Mediating Role of Gut Microbiota in the Association Between Eczema and Subsequent ADHD
No mediation effect (overall indirect effect) was observed for any gut microbiota in the association between eczema reported within the first 18 months and ADHD symptoms at 54 months (Table 5).
TABLE 5
| Mediators at month 18 | Indirect effect | Adjusted p-value | % mediated |
| OTU3 | 0.02(−0.10 to 0.14) | 0.732 | 1.9% |
| OTU4 | 0.02(−0.09 to 0.13) | 0.705 | 1.9% |
| OTU5 | 0.01(−0.21 to 0.22) | 0.947 | 0.7% |
| OTU10 | 0.02(−0.15 to 0.19) | 0.834 | 1.6% |
| OTU11 | 0.04(−0.13 to 0.22) | 0.621 | 4.1% |
| OTU12 | −0.01(−0.14 to 0.11) | 0.823 | NA |
| OTU20 | −0.06(−0.26 to 0.13) | 0.534 | NA |
| OTU22 | 0.05(−0.08 to 0.18) | 0.466 | 4.6% |
| OTU37 | 0.02(−0.20 to 0.23) | 0.887 | 1.4% |
| OTU54 | 0.001(−0.107 to 0.109) | 0.989 | 0.1% |
| OTU68 | 0.05(−0.31 to 0.40) | 0.813 | 4.2% |
| OTU74 | −0.03(−0.18 to 0.11) | 0.666 | NA |
| OTU164 | 0.02(−0.37 to 0.41) | 0.904 | 2.1% |
| Overall | −0.01(−0.94 to 0.91) | 0.938 | NA |
Mediation effect of gut microbiome on ADHD at 54 months (n = 129).
ADHD, attention deficit hyperactivity disorder; NA, not applicable; OTU, operational taxonomic unit. Benjamini–Hochberg correction with false discovery rate at 0.20 and n = 13 was applied. NA:% mediated is not computed due to inconsistent mediation.
Discussion
In the prospective GUSTO cohort, we observed that maternally reported diagnosis of eczema within the first 18 months of life with or without a positive SPT was associated with a doubling of the risk of ADHD symptoms. These findings are consistent with previous reports of this association (30, 31). While there are studies investigating the effects of cytokine levels and gut microbiome on eczema and ADHD separately (32, 33), to our knowledge, this is the first study to examine the roles of cytokines and gut microbiome in mediating the association between eczema and ADHD.
A cross-sectional study by Schmitt et al. found that children with eczema were approximately 1.5 times more likely to have ADHD (30). Another prospective birth cohort study in Germany reported that eczema by 4 years was associated with ADHD diagnosis by 8 years (31). Conversely, other studies did not report significant associations between eczema and ADHD (34, 35). These include the BAMSE birth cohort, which reported that preschool eczema was not associated with use of ADHD medication at school age (34). One possible reason for the difference is that some children with ADHD symptoms did not receive medication. Suwan et al. reported no significant association between eczema and ADHD in a cross-sectional case-control study of children aged 5–15 years (35). However, the design of the latter study was unable to assess an association with eczema at younger ages.
Eczema and ADHD are linked to the release of pro-inflammatory cytokines (8). In our study, however, we observed no differences in maternal or cord blood cytokines between children with and without eczema, nor between children with and without ADHD symptoms. Our findings are contrary to results reported by other studies (36, 37). A possible reason for the different results may be that we assayed cord blood without stimulation and cord blood levels of IFNγ without stimulation are generally low (38). Supportive evidence is provided by an Indonesian birth cohort study that also found no association between unstimulated cord blood IFNγ levels and eczema development by six months (39). Similarly, the Hokkaido Study on Environment and Children’s Health reported no associations between unstimulated cord blood TNFα levels and hyperactivity or inattention issues in children (40). Another reason for the absence of differences in maternal or cord cytokines between children with and without eczema, nor between children with and without ADHD symptoms that we observed may be due to limited immune profiling. While the Newborn Epigenetics Study and LINA cohort reported associations between maternal IL-12p70, IL-17A, IL-1β, and IL-13 levels and child neurodevelopment (36, 37), we did not measure these cytokines. We also assayed cytokines from maternal blood during pregnancy and cord blood which is not indicative of subsequent inflammation that may be brought about by gut microbiome dysbiosis that we have observed in children with eczema as well as in children with ADHD.
Gut microbiome dysbiosis may be linked to allergic and neurodevelopmental diseases. We observed that relative abundance of Lachnoclostridium spp. was substantially higher in the gut microbiome of subjects with eczema in comparison to subjects without eczema. The pro-inflammatory role of Lachnoclostridium had been shown in murine models in which gut Lachnoclostridium abundance was higher in wild-type eczema mouse models as compared to anti-inflammatory interleukin-37b knock-in mice (41). Gut Lachnoclostridium is also found in higher abundance in patients with inflammatory Crohn’s disease (42). We propose that higher relative abundances of Lachnoclostridium may be linked to inflammation and result in eczema. We also observed lower relative abundance of Faecalibacterium spp. and Fusicatenibacter spp. in the gut microbiome of children with eczema. Lower abundance of Fusicatenibacter in the gut is linked to higher levels of inflammatory fecal calprotectin (43). High fecal calprotectin levels in offspring at 2 months of age has been associated with eczema development by age 6 years (44). Faecalibacterium and Fusicatenibacter are also short-chain fatty acid (SCFA) producers which serve as an important energy source and exhibit anti-inflammatory properties in the host (45).
In children with ADHD symptoms compared to children without ADHD symptoms, we observed a substantial reduction in relative abundance of gut Escherichia-Shigella spp. and increase in gut Enterococcus devriesei. Supportive evidence is provided by murine models where mice colonized with gut microbiota from ADHD patients had lower concentrations of gut Escherichia or Shigella spp. as compared to controls (46). Escherichia is also a SCFA producer that can modulate neurotransmitters and neurotrophic factors, such as brain-derived neurotrophic factor, which is important for neurogenesis and in turn may influence the development of ADHD (45). A study of Chinese schoolchildren reported higher Enterococcus abundance in children with ADHD (47).
We found no indications that the gut microbiome mediates the association we observed between eczema and later ADHD symptoms. Further research would help to understand the role of the gut microbiome in these diseases.
Strengths of our study include the frequent, prospective follow-up of subjects using validated questionnaires and a structured clinical interview to assess presence of ADHD symptoms. Limitations of our study include maternally reported diagnosis of eczema, although we objectively assessed atopy using SPT in a subsample. Also, we have explored the relation with a “presence of ADHD” based on the number of ADHD symptoms present, but without taking into account the degree of impairment in functioning. Also, the symptoms of ADHD were assessed using parental reports without clinical observation of the children. The cytokines were only evaluated in maternal blood during pregnancy and in cord blood and not in later timepoints. Finally, we did not study other factors and possible mechanisms, such as maternal mental health state, sleep disturbances, epigenetics, gene expression and mitochondrial dysfunction, that may underlie the pathogenesis of allergic and neurodevelopmental disorders (8). For instance, a study of Italian children with autism spectrum disorder by Lamanna et al. reported that presence of allergies and family history of psychosis accounted for 25% of variance in ADHD severity, hence highlighting the importance of examining comorbidities in understanding the mechanisms linking eczema and ADHD development (48).
In conclusion, we observed that early life eczema is associated with a higher risk of later presence of ADHD symptoms in children. We found no indications for an association with maternal blood or cord cytokines. Gut microbiome dysbiosis was a common feature of both eczema and ADHD, but association between eczema and ADHD was not mediated by the gut microbiome. More studies are clearly warranted to elucidate the link between eczema and ADHD, as well as the biological mechanisms underlying the link.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Data availability statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions. All raw microbiome data are available at NCBI (https://www.ncbi.nlm.nih.gov) (accession number PRJNA668050).
Ethics statement
Ethical approval was obtained from the Domain Specific Review Board of Singapore National Healthcare Group (D/2009/021 approved on 26 February 2009) and the Centralised Institutional Review Board of SingHealth (2018/2767 approved on 2 March 2019). The conduct of this study was based on the guidelines in the Declaration of Helsinki. Written informed consent was obtained from the mothers after a detailed explanation of the study. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
EL conceptualized and designed the study, designed the methodology, performed data analysis, drafted the initial manuscript as well as reviewed and revised the manuscript. DO, MO, HL, and MT drafted the initial manuscript as well as reviewed and revised the manuscript. LT performed the data analysis, drafted the initial manuscript as well as reviewed and revised the manuscript. QY designed the methodology, performed data analysis, drafted the initial manuscript as well as reviewed and revised the manuscript. YHC designed the methodology, performed data analysis as well as reviewed and revised the manuscript. ET, AG, HV, OT, JE, YSC, PG, FY, KMT, KHT, NK, BL, LS, and MM conceptualized and designed the study as well as reviewed and revised the manuscript. JX performed the data analysis as well as reviewed and revised the manuscript. MK designed the methodology, drafted the initial manuscript as well as reviewed and revised the manuscript. BB conceptualized and designed the study, supervised the project as well as reviewed and revised the manuscript. All authors agree to be accountable for the work.
Funding
This work was supported by the Singapore National Research Foundation under its Translational and Clinical Research (TCR) Flagship Programme and administered by the Singapore Ministry of Health’s National Medical Research Council (NMRC), Singapore-NMRC/TCR/004-NUS/2008; NMRC/TCR/012-NUHS/2014. ET was supported by the National Medical Research Council (NMRC) Transition Award grant [MOH-TA18nov-003] from NMRC, Singapore. All authors declare that the study sponsor had no role in the design or conduct of the study; in the collection, management, analysis, or interpretation of data; or in the preparation, review or approval of the article. We thank the GUSTO Study Group for their additional contributions.
Acknowledgments
We thank the GUSTO study group and all clinical and home-visit staff involved. The voluntary participation of all subjects is greatly appreciated. The GUSTO study group includes Allan Sheppard, Amutha Chinnadurai, Anne Eng Neo Goh, Anne Rifkin-Graboi, Anqi Qiu, Arijit Biswas, BL, Birit Froukje Philipp Broekman, Boon Long Quah, Chai Kiat Chng, Cheryl Shufen Ngo, Choon Looi Bong, Christiani Jeyakumar Henry, Daniel Yam Thiam Goh, Doris Ngiuk Lan Loh, FY, George Seow Heong Yeo, Helen Yu Chen, Hugo P. S. van Bever, Iliana Magiati, Inez Bik Yun Wong, Ivy Yee-Man Lau, Jeevesh Kapur, Jenny L. Richmond, Jerry Kok Yen Chan, Joanna Dawn Holbrook, Joshua J. Gooley, Keith M. Godfrey, Kenneth Yung Chiang Kwek, KHT, Krishnamoorthy Naiduvaje, Leher Singh, Lin Lin Su, Lourdes Mary Daniel, LS, Marielle V. Fortier, Mark Hanson, Mary Foong-Fong Chong, Mary Rauff, Mei Chien Chua, MM, Mya Thway Tint, Neerja Karnani, Ngee Lek, OT, P. C. Wong, Peter David Gluckman, Pratibha Keshav Agarwal, Rob Martinus van Dam, Salome A. Rebello, Seang Mei Saw, Shang Chee Chong, Shirong Cai, Shu-E Soh, Sok Bee Lim, Stephen Chin-Ying Hsu, Victor Samuel Rajadurai, Walter Stunkel, Wee Meng Han, Wei Wei Pang, YSC, Yin Bun Cheung, YHC, and Yung Seng Lee.
Conflict of interest
YSC and NK are part of an academic consortium that has received research funding from Abbot Nutrition, Nestle and Danone. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.837741/full#supplementary-material
Abbreviations
- AdjRR
adjusted RR
- ADHD
attention deficit hyperactivity disorder
- BMI
body mass index
- CDISC-YC
Computerized Diagnostic Interview Schedule for Children Version IV Young Child
- CRP
C-reactive protein
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition Text Revised
- GUSTO
Growing Up in Singapore Towards healthy Outcomes
- IFN γ
interferon gamma
- SCFA
short-chain fatty acid
- SPT
skin prick testing
- TNF α
tumor necrosis factor α.
Footnotes
References
1.
SchmittJBuske-KirschbaumARoessnerV. Is atopic disease a risk factor for attention-deficit/hyperactivity disorder? A systematic review.Allergy. (2010) 65:1506–24. 10.1111/j.1398-9995.2010.02449.x
2.
GuptaRSheikhAStrachanDPAndersonHR. Burden of allergic disease in the UK: secondary analyses of national databases.Clin Exp Allergy. (2004) 34:520–6. 10.1111/j.1365-2222.2004.1935.x
3.
MatzaLSParamoreCPrasadM. A review of the economic burden of ADHD.Cost Eff Resour Alloc. (2005) 3:5. 10.1186/1478-7547-3-5
4.
CamffermanDKennedyJDGoldMMartinAJWinwoodPLushingtonK. Eczema, sleep, and behavior in children.J Clin Sleep Med. (2010) 6:581–8. 10.5664/jcsm.27992
5.
YaghmaiePKoudelkaCWSimpsonEL. Mental health comorbidity in patients with atopic dermatitis.J Allergy Clin Immunol. (2013) 131:428–33. 10.1016/j.jaci.2012.10.041
6.
XuGStrathearnLLiuBYangBBaoW. Twenty-year trends in diagnosed attention-deficit/hyperactivity disorder among US children and adolescents, 1997-2016.JAMA Netw Open. (2018) 1:e181471. 10.1001/jamanetworkopen.2018.1471
7.
StromMAFishbeinABPallerASSilverbergJI. Association between atopic dermatitis and attention deficit hyperactivity disorder in U.S. children and adults.Br J Dermatol. (2016) 175:920–9. 10.1111/bjd.14697
8.
ChuaRXYTayMJYOoiDSQSiahKTHThamEHShekLPCet alUnderstanding the link between allergy and neurodevelopmental disorders: a current review of factors and mechanisms.Front Neurol. (2020) 11:603571. 10.3389/fneur.2020.603571
9.
Suárez-FariñasMDhingraNGittlerJShemerACardinaleIde Guzman StrongCet alIntrinsic atopic dermatitis shows similar TH2 and higher TH17 immune activation compared with extrinsic atopic dermatitis.J Allergy Clin Immunol. (2013) 132:361–70. 10.1016/j.jaci.2013.04.046
10.
RiaziKGalicMAKuzmiskiJBHoWSharkeyKAPittmanQJ. Microglial activation and TNFalpha production mediate altered CNS excitability following peripheral inflammation.Proc Natl Acad Sci USA. (2008) 105:17151–6. 10.1073/pnas.0806682105
11.
ZhengHLiangHWangYMiaoMShiTYangFet alAltered gut microbiota composition associated with eczema in infants.PLoS One. (2016) 11:e0166026. 10.1371/journal.pone.0166026
12.
Bundgaard-NielsenCKnudsenJLeutscherPDCLauritsenMBNyegaardMHagstrømSet alGut microbiota profiles of autism spectrum disorder and attention deficit/hyperactivity disorder: a systematic literature review.Gut Microbes. (2020) 11:1172–87. 10.1080/19490976.2020.1748258
13.
SohS-ETintMTGluckmanPDGodfreyKMRifkin-GraboiAChanYHet alCohort profile: growing up in singapore towards healthy outcomes (GUSTO) birth cohort study.Int J Epidemiol. (2014) 43:1401–9. 10.1093/ije/dyt125
14.
AsherMIKeilUAndersonHRBeasleyRCraneJMartinezFet alInternational study of asthma and allergies in childhood (ISAAC): rationale and methods.Eur Respir J. (1995) 8:483. 10.1183/09031936.95.08030483
15.
KeilTKuligMSimpsonACustovicAWickmanMKullIet alEuropean birth cohort studies on asthma and atopic diseases: II. Comparison of outcomes and exposures – a GA2LEN initiative.Allergy. (2006) 61:1104–11. 10.1111/j.1398-9995.2006.01167.x
16.
YiFChewFJimenezSChuaKLeeB. Culture of Biomia tropicalis and IgE immunoblot characterization of its allergenicity.Asian Pac J Allergy Immunol. (1999) 17:189.
17.
FisherPWShafferDPiacentiniJCLapkinJKafantarisVLeonardHet alSensitivity of the diagnostic interview schedule for children, 2nd edition (DISC-2.1) for specific diagnoses of children and adolescents.J Am Acad Child Adolesc Psychiatry. (1993) 32:666–73. 10.1097/00004583-199305000-00026
18.
SegalDL. Diagnostic and statistical manual of mental disorders (DSM-IV-TR). In: WeinerIBCraigheadWE. The Corsini Encyclopedia of Psychology.Washington, DC: American Psychiatric Association (2010). p. 1–3. 10.1002/9780470479216.corpsy0271
19.
JensenPRoperMFisherPPiacentiniJCaninoGRichtersJet alTest-retest reliability of the diagnostic interview schedule for children (DISC 2.1). Parent, child combined algorithms.Arch Gen Psychiatry. (1995) 52:61–71. 10.1001/archpsyc.1995.03950130061007
20.
Schwab-StoneMEShafferDDulcanMKJensenPSFisherPBirdHRet alCriterion validity of the NIMH diagnostic interview schedule for children version 2.3 (DISC-2.3).J Am Acad Child Adolesc Psychiatry. (1996) 35:878–88. 10.1097/00004583-199607000-00013
21.
ShafferDFisherPLucasCPDulcanMKSchwab-StoneMENIMH. Diagnostic interview schedule for children version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses.J Am Acad Child Adolesc Psychiatry. (2000) 39:28–38. 10.1097/00004583-200001000-00014
22.
ShafferDSchwab-StoneMFisherPCohenPPiacentiniJDaviesMet alThe diagnostic interview schedule for children-revised version (DISC-R): I. preparation, field testing, interrater reliability, and acceptability.J Am Acad Child Adolesc Psychiatry. (1993) 32:643–50. 10.1097/00004583-199305000-00023
23.
Rolon-ArroyoBArnoldDHHarveyEAMarshallN. Assessing attention and disruptive behavior symptoms in preschool-age children: the utility of the diagnostic interview schedule for children.J Child Fam Stud. (2016) 25:65–76. 10.1007/s10826-015-0203-x
24.
XuJLawleyBWongGOtalAChenLYingTJet alEthnic diversity in infant gut microbiota is apparent before the introduction of complementary diets.Gut Microbes. (2020) 11:1362–73. 10.1080/19490976.2020.1756150
25.
ChenL-WXuJSohSEArisIMTintMTGluckmanPDet alImplication of gut microbiota in the association between infant antibiotic exposure and childhood obesity and adiposity accumulation.Int J Obes. (2020) 44:1508–20. 10.1038/s41366-020-0572-0
26.
CaporasoJGKuczynskiJStombaughJBittingerKBushmanFDCostelloEKet alQIIME allows analysis of high-throughput community sequencing data.Nat Methods. (2010) 7:335–6. 10.1038/nmeth.f.303
27.
EdgarRC. Search and clustering orders of magnitude faster than BLAST.Bioinformatics. (2010) 26:2460–1. 10.1093/bioinformatics/btq461
28.
NgYTChewFT. A systematic review and meta-analysis of risk factors associated with atopic dermatitis in Asia.World Allergy Organ J. (2020) 13:100477. 10.1016/j.waojou.2020.100477
29.
ThaparACooperMJefferiesRStergiakouliE. What causes attention deficit hyperactivity disorder?Arch Dis Child. (2012) 97:260. 10.1136/archdischild-2011-300482
30.
SchmittJRomanosMSchmittNMMeurerMKirchW. Atopic eczema and attention-deficit/hyperactivity disorder in a population-based sample of children and adolescents.JAMA. (2009) 301:724–6. 10.1001/jama.2009.136
31.
GenuneitJBraigSBrandtSWabitschMFlorathIBrennerHet alInfant atopic eczema and subsequent attention-deficit/hyperactivity disorder–a prospective birth cohort study.Pediatr Allergy Immunol. (2014) 25:51–6. 10.1111/pai.12152
32.
KimJKimBELeungDYM. Pathophysiology of atopic dermatitis: clinical implications.Allergy Asthma Proc. (2019) 40:84–92. 10.2500/aap.2019.40.4202
33.
ChangJP-CSuK-PMondelliVParianteCM. Cortisol and inflammatory biomarker levels in youths with attention deficit hyperactivity disorder (ADHD): evidence from a systematic review with meta-analysis.Transl Psychiatry. (2021) 11:430. 10.1038/s41398-021-01550-0
34.
JohanssonEKBallardiniNKullIBergströmAWahlgrenC-F. Association between preschool eczema and medication for attention-deficit/hyperactivity disorder in school age.Pediatr Allergy Immunol. (2017) 28:44–50. 10.1111/pai.12657
35.
SuwanPAkaramethathipDNoipayakP. Association between allergic sensitization and attention deficit hyperactivity disorder (ADHD).Asian Pac J Allergy Immunol. (2011) 29:57–65.
36.
DozmorovMGBilboSDKollinsSHZuckerNDoEKSchechterJCet alAssociations between maternal cytokine levels during gestation and measures of child cognitive abilities and executive functioning.Brain Behav Immun. (2018) 70:390–7. 10.1016/j.bbi.2018.03.029
37.
ThürmannLHerberthGRolle-KampczykURöderSBorteMvon BergenMet alElevated gestational IL-13 during fetal development is associated with hyperactivity and inattention in eight-year-old children.Front Immunol. (2019) 10:1658. 10.3389/fimmu.2019.01658
38.
SchaubBCampoMHeHPerkinsDGillmanMWGoldDRet alNeonatal immune responses to TLR2 stimulation: influence of maternal atopy on Foxp3 and IL-10 expression.Respir Res. (2006) 7:40. 10.1186/1465-9921-7-40
39.
MunasirZSastroasmoroSDjauziSWaspadjiSRamelanWAminullahAet alThe role of allergic risk and other factors that affect the occurrence of atopic dermatitis in the first 6 months of life.Asia Pac Allergy. (2011) 1:73–9. 10.5415/apallergy.2011.1.2.73
40.
MinatoyaMItohSArakiATamuraNYamazakiKMiyashitaCet alAssociation between fetal adipokines and child behavioral problems at preschool age: the Hokkaido study on environment and children’s health.Int J Environ Res Public Health. (2018) 15:120. 10.3390/ijerph15010120
41.
HouTSunXZhuJHonKLJiangPChuIMTet alIL-37 ameliorating allergic inflammation in atopic dermatitis through regulating microbiota and AMPK-mTOR signaling pathway-modulated autophagy mechanism.Front Immunol. (2020) 11:752. 10.3389/fimmu.2020.00752
42.
QiuZYangHRongLDingWChenJZhongL. Targeted metagenome based analyses show gut microbial diversity of inflammatory bowel disease patients.Indian J Microbiol. (2017) 57:307–15. 10.1007/s12088-017-0652-6
43.
WeisSSchwiertzAUngerMMBeckerAFaßbenderKRateringSet alEffect of Parkinson’s disease and related medications on the composition of the fecal bacterial microbiota.NPJ Parkinsons Dis. (2019) 5:28. 10.1038/s41531-019-0100-x
44.
OrivuoriLMustonenKde GoffauMCHakalaSPaaeslaMRoduitCet alHigh level of fecal calprotectin at age 2 months as a marker of intestinal inflammation predicts atopic dermatitis and asthma by age 6.Clin Exp Allergy. (2015) 45:928–39. 10.1111/cea.12522
45.
SilvaYPBernardiAFrozzaRL. The role of short-chain fatty acids from gut microbiota in gut-brain communication.Front Endocrinol. (2020) 11:25. 10.3389/fendo.2020.00025
46.
TengelerACDamSAWiesmannMNaaijenJvon BodegomMBelzerCet alGut microbiota from persons with attention-deficit/hyperactivity disorder affects the brain in mice.Microbiome. (2020) 8:44. 10.1186/s40168-020-00816-x
47.
WanLGeW-RZhangSSunY-LWangBYangG. Case-control study of the effects of gut microbiota composition on neurotransmitter metabolic pathways in children with attention deficit hyperactivity disorder.Front Neurosci. (2020) 14:127. 10.3389/fnins.2020.00127
48.
LamannaALCraigFMateraESimoneMButtiglioneMMargariL. Risk factors for the existence of attention deficit hyperactivity disorder symptoms in children with autism spectrum disorders.Neuropsychiatr Dis Treat. (2017) 13:1559–67. 10.2147/NDT.S132214
Summary
Keywords
atopy, attention deficit hyperactivity disorder, cytokines, eczema, gut microbiome dysbiosis
Citation
Loo EXL, Ooi DSQ, Ong M, Ta LDH, Lau HX, Tay MJY, Yap QV, Chan YH, Tham EH, Goh AEN, Van Bever H, Teoh OH, Eriksson JG, Chong YS, Gluckman P, Yap FKP, Karnani N, Xu J, Tan KML, Tan KH, Lee BW, Kramer M, Shek LP-c, Meaney MJ and Broekman BFP (2022) Associations Between Eczema and Attention Deficit Hyperactivity Disorder Symptoms in Children. Front. Pediatr. 10:837741. doi: 10.3389/fped.2022.837741
Received
17 December 2021
Accepted
15 February 2022
Published
30 March 2022
Volume
10 - 2022
Edited by
Raz Gross, Sheba Medical Center, Israel
Reviewed by
Emilia Matera, University of Bari Aldo Moro, Italy; Tudor Lucian Pop, Iuliu Haţieganu University of Medicine and Pharmacy, Romania
Updates
Copyright
© 2022 Loo, Ooi, Ong, Ta, Lau, Tay, Yap, Chan, Tham, Goh, Van Bever, Teoh, Eriksson, Chong, Gluckman, Yap, Karnani, Xu, Tan, Tan, Lee, Kramer, Shek, Meaney and Broekman.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Birit F. P. Broekman, b.broekman@amsterdamumc.nl
This article was submitted to Children and Health, a section of the journal Frontiers in Pediatrics
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.