- 1Chinese Evidence-Based Medicine Center, West China Hospital, Sichuan University, Chengdu, China
- 2Medical Device Regulatory Research and Evaluation Centre, West China Hospital, Sichuan University, Chengdu, China
- 3West China Medical Publishers, West China Hospital, Sichuan University, Chengdu, China
Objective: Childhood obesity is one of the most severe challenges of public health in the twenty-first century and may increase the risk of various physical and psychological diseases in adulthood. The prevalence and predictors of unreported results and premature termination in pediatric obesity research are not clear. We aimed to characterize childhood obesity trials registered on ClinicalTrials.gov and identify features associated with early termination and lack of results reporting.
Methods: Records were downloaded and screened for all childhood obesity trials from the inception of ClinicalTrials.gov to July 29, 2021. We performed descriptive analyses of characteristics, Cox regression for early termination, and logistic regression for lack of results reporting.
Results: We identified 1,312 trials registered at ClinicalTrials.gov. Among clinicalTrials.gov registered childhood obesity-related intervention trials, trial unreported results were 88.5 and 4.3% of trials were prematurely terminated. Additionally, the factors that reduced the risk of unreported outcomes were US-registered clinical studies and drug intervention trials. Factors associated with a reduced risk of early termination are National Institutes of Health (NIH) or other federal agency funding and large trials.
Conclusion: The problem of unreported results in clinical trials of childhood obesity is serious. Therefore, timely bulletin of the results and reasons for termination remain urgent aims for childhood obesity trials.
Introduction
Childhood obesity is one of the most severe challenges of public health in the twenty-first century, affecting ~41 million children under the age of 5 years (1, 2). The prevalence of overweight and obesity in children started to rise at the end of the 1980's and continues to rise in some countries (3). Childhood obesity increases the risk of having obesity in adulthood and health problems such as diabetes and cardiovascular diseases (4–10). Furthermore, growing prevalence of obesity can place a substantial cost burden to the health-care system. The overall economic cost of obesity worldwide was estimated at $2.0 trillion US dollars in 2012, with an estimated 5.1 billion a year spent on obesity-related diseases by the National Health Service (NHS) (11–13).
Clinical trials rely on the participation of volunteers, involving massive investment of human, material and financial resources (14). Meanwhile, trial researchers have an obligation to participants not only to minimize potential harm, but also to conduct trials scrupulously, and to publicly report their findings in time. Unpublished trial results could violate ethical obligations to participants and compromise existing medical evidence (15). Policies have been enacted to improve the transparency and accountability of trials in recent years. The International Committee of Medical Journal Editors has required registration of interventional trials in a public trials registry [such as ClinicalTrials.gov, a registry of clinical trials implemented in 2000 by the National Library of Medicine-National Institutes of Health (NIH)] as a condition for publication since July 1, 2005 (16). Furthermore, the Food and Drug Administration Amendments Act of 2007 (FDAAA) required that summary results of certain trials should be submitted within 1 year of final collection of data for the primary outcome, including whether the trials concluded according to the predetermined protocol or were discontinued (17). However, despite these increased ethical and legislative requirements, the discontinuation and unreported results of clinical trials remain common.
Although a limited number of studies have cross-sectional descriptions of pediatric trials (18–20), they have not further identified the factors of unreported results and premature termination in pediatric obesity research. In addition, analyses of trial discontinuation and result reporting have primarily focused on adult populations, and the prevalence of these outcomes for pediatric randomized controlled trials (RCTs) remains less well defined (21). We suppose that pediatric clinical trials are easier to stop early because of ethical and recruitment issues. Therefore, we aimed to investigate the factors of results reporting and premature termination of current childhood obesity trials registered in ClinicalTrials.gov and make corresponding recommendations.
Materials and Methods
Data Source
We used the terms “obesity,” “overweight,” “excess weight,” and “body mass index,” searched on ClinicalTrials.gov. To identify pediatric clinical studies, we used the “Age Group: birth-17” criteria. In the retrieval studies, the Age Group field included three types: (Child), (Child, Adult) and (Child, Adult, Older Adult) (18). Records were downloaded on July 29, 2021, for all childhood obesity trials submitted to ClinicalTrials.gov.
Study Selection
The childhood obesity clinical trial dataset was restricted to interventional studies registered up to July 29th. Interventional studies are defined by ClinicalTrials.gov as those in which an investigator assigns an intervention (including diagnostic, therapeutic or other types of interventions) based on a protocol. Noninterventional or observational trials were excluded because they are not currently required to be registered at ClinicalTrials.gov. Each trial was independently screened by two investigators, and disagreements were resolved by consensus or third-party adjudication.
Data Collection
The following data from each eligible trial were independently extracted by two investigators: general and design characteristics, country, interventions, study status, the results reporting, funding source and date. The duration of the trial was calculated from the beginning of the investigation to primary completion (22). Early discontinuation was defined as a study status including suspended, terminated, or withdrawn (23). In analyzing the reporting of results, we used the “primary completion date” which was interpreted as the date on which the primary outcome data for the last enrolled patient was collected (24). We put off the primary completion date by 1 year, as both NIH regulations and trial reporting policies required sponsors or researchers to submit results data within 1 year of the primary completion date (22, 25). Considering that some of these trials might still publish their results if their results could be reported within 1 year, we would only analyze results reporting for trials completed before July 29, 2020.
Statistical Analysis
Descriptive statistics were used to summarize trial data with frequencies and percentages. Furthermore, categorical variables were presented as frequencies and percentages. Univariable and multivariable Cox and logistic regression analyses were performed to investigate factors associated with early discontinuation and reporting results, respectively. In regression analysis, “Enrollment: 0-100”, “country: United States”, “Interventions: Drug”, “Funded: Non-industry”, “Allocation: Non-randomized”, “Masking: Yes”, “Primary Purpose: Treatment” was used as the reference group respectively. The hazard ratio (HR)/odds ratio (OR) and 95% confidence interval (CI) were reported. Then, subgroup analysis was conducted, and the trials were divided into two groups according to “both adults and children” and “children only,” and Cox and Logistic regression were performed respectively. For the statistical analysis, the significance level was set at 0.05, and the analysis was performed using STATA 16 software.
Results
Characteristics for All Studies
There were 384,836 clinical trials registered in ClinicalTrials.gov as of July 29, 2021.
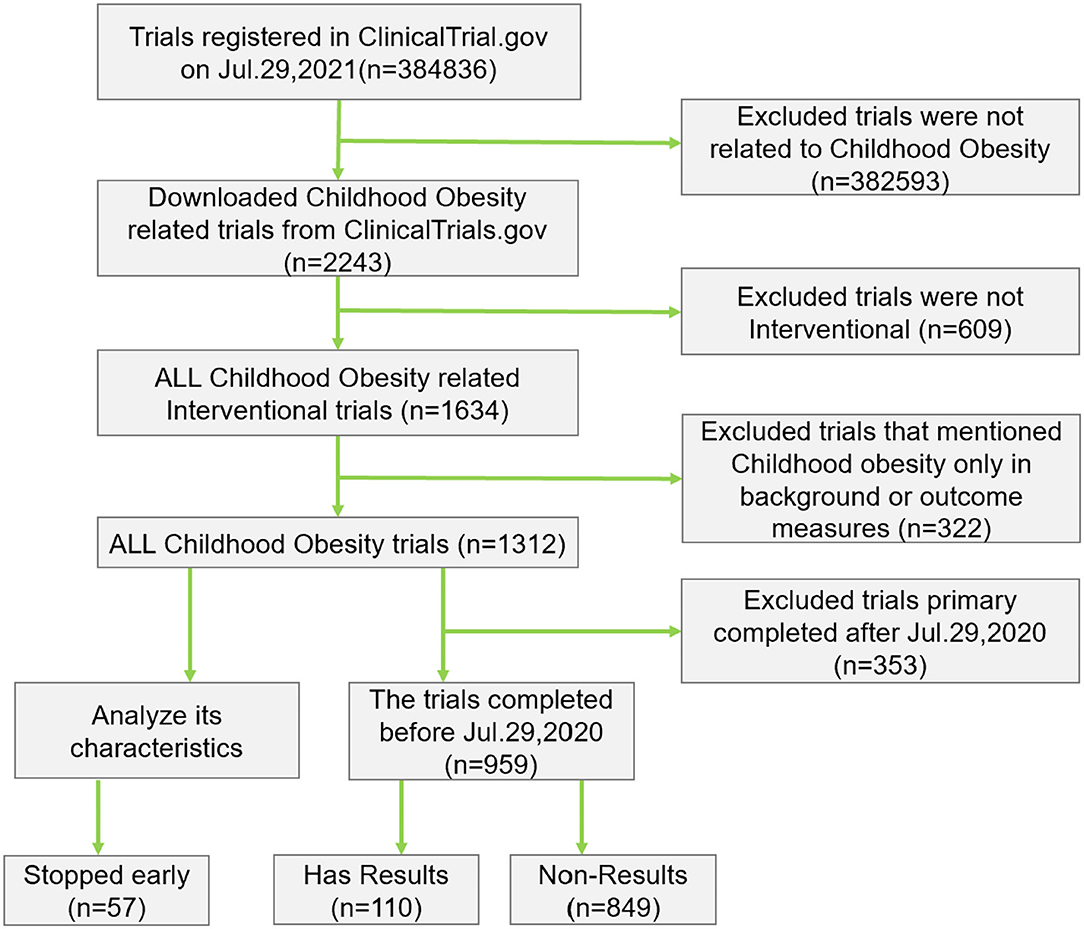
A total of 2,243 clinical trials were downloaded on childhood obesity among these trials. We excluded 609 trials because they were not interventional. Of the remaining 1,634 interventional trials within this period, we identified 1,312 trials after manual review (Figure 1).
Most trials included both males and females (n = 1163, 88.6%) (Table 1). 58.6% included only pediatric patients. The United States ranks first among 58 countries in the number of registered childhood obesity trials (51.7%). A total of 24.1% of the trials were completed within 1 year. Small studies involving <100 patients comprised 50.1% of childhood obesity trials. Only 16.9 and 6.2% of the childhood obesity trials were funded by the NIH and industries, respectively.
Of the 1,312 identified childhood obesity trials, 57 trials (nearly 4.3%) were terminated early for various reasons, including 5 suspended, 34 terminated, and 18 withdrawn trials. The two most common reasons reported in the terminated trials were enrollment problems (n = 16, 28.1%) and funding problems (n = 8, 14.0%), followed by (Head of the study left) (n = 6, 10.5%), COVID-19 (n = 4, 7.0%) and Drug/device availability (n = 2, 3.5%). Of the total number of terminated trials, 11 trials (19.3%) did not report the reason for their early termination.
A total of 959 trials (73.1%) were completed before Jul. 29, 2020; nevertheless, only 110 trials (11.5%) submitted their results to the registry. Five trials (0.5%) submitted their results to the registry within 1 year from the primary completion date.
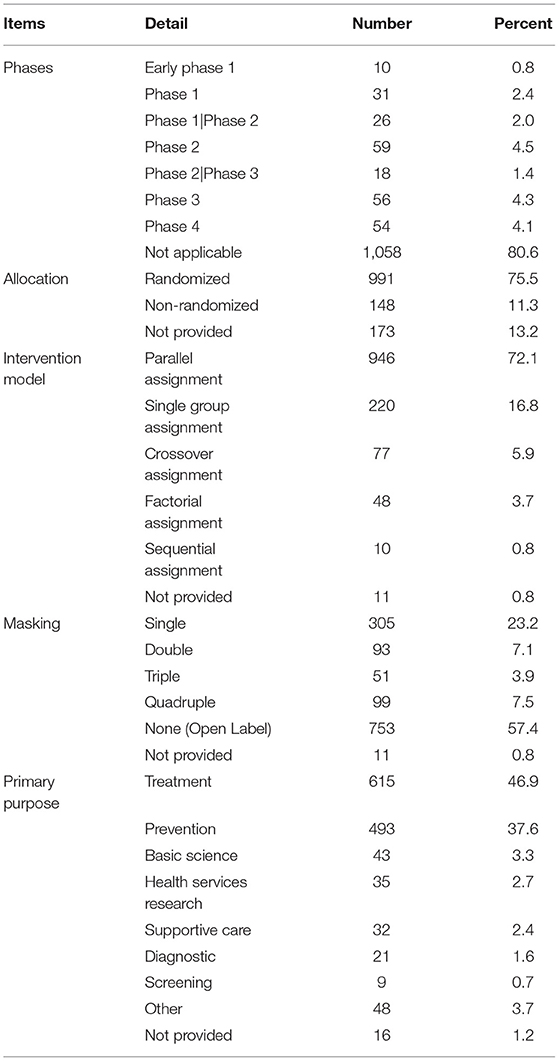
Design Characteristics
The design characteristics of the selected trials are shown in Table 2. Of the 1,312 trials, less than half provided information regarding their phase, as most of them were nondrug trials. Randomized design (75.5%) and parallel assignment (72.1%) were most commonly utilized. Some form of masking was used in 528 trials (41.8%), of which 22.9% were blinded to the participants. The primary purpose of the childhood obesity trials was treatment (46.9%), followed by prevention (37.6%).
Characteristics Associated With Early Trial Discontinuation
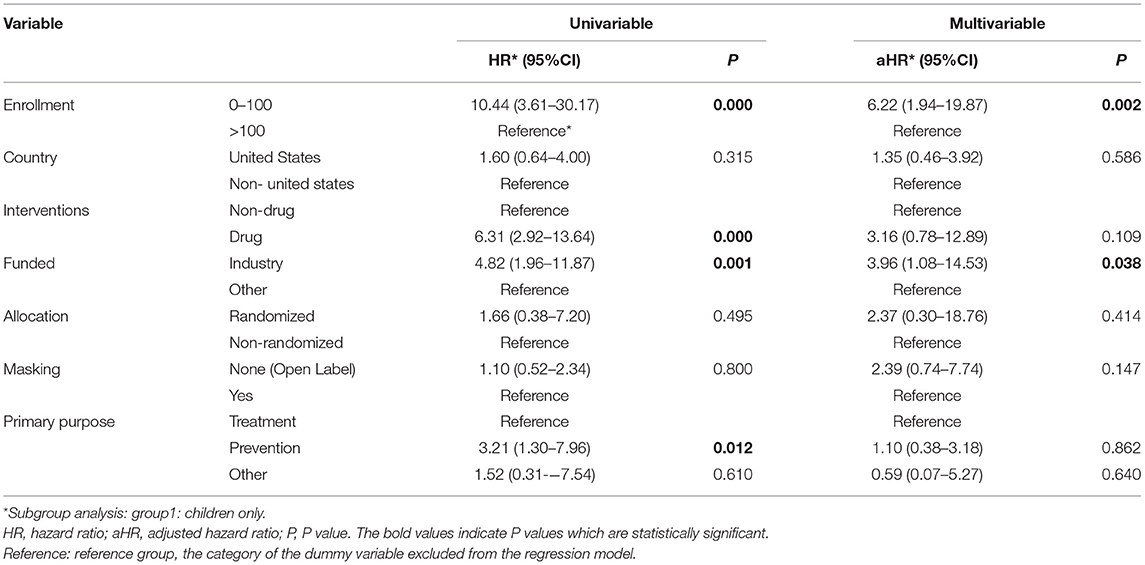
Univariable Cox regression analysis revealed a significant difference in the rate of early trial discontinuation between the different types of interventions, sample sizes, funding sources, and primary purposes (Table 3). Small trials (HR = 7.52, 95% CI: 3.53–16.04), drug trials (HR = 5.14, 95% CI: 2.96–8.91), industry trials (HR = 4.23, 95% CI: 2.12-8.44) and prevention trials (HR = 2.98, 95% CI: 1.43–6.21) were more frequently discontinued early than large trials, nondrug trials, NIH/other trials and treatment trials. In multivariable analysis, sample size and funding source remained significant determinants of trial discontinuation (Table 4). Trials primarily funded by industry were more likely to result in discontinuation compared with those funded by others (aHR = 3.09, 95% CI: 1.15–8.34, P = 0.026). Smaller trials were also more likely to be discontinued (aHR = 4.93, 95% CI: 2.02–12.01, P < 0.001). The subgroup analysis yielded consistent results (Table 5, Supplementary Table 1).
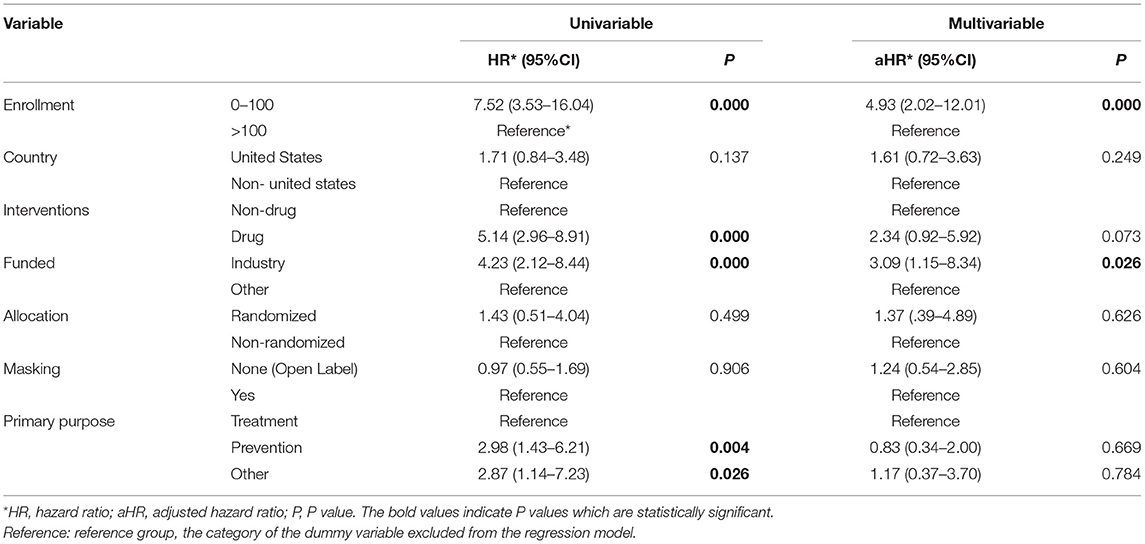
Table 3. Cox regression analysis of childhood obesity trials characteristics associated with early trial discontinuation.
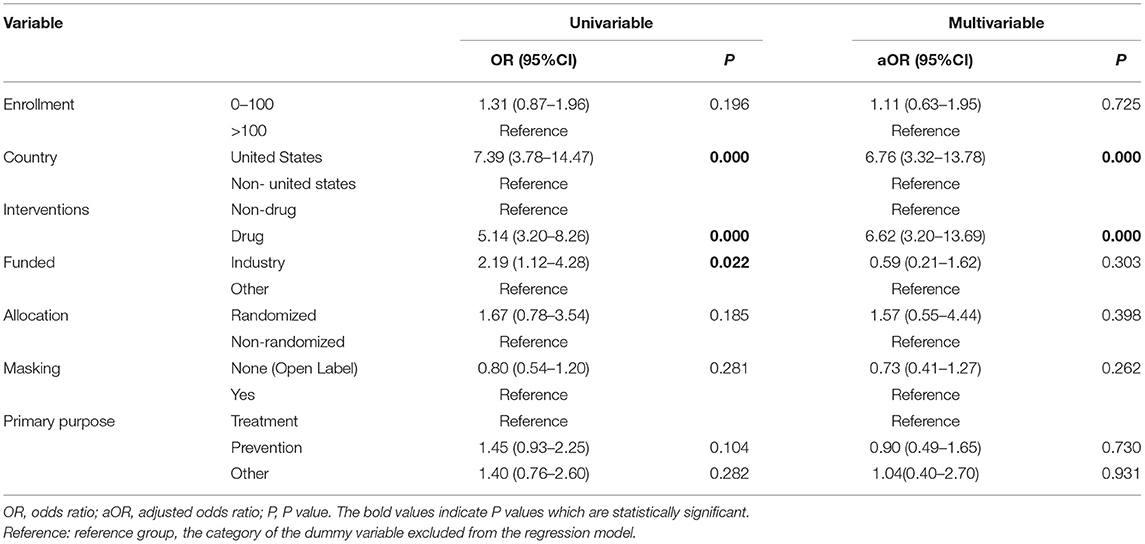
Table 4. Logistic regression analysis of childhood obesity trials characteristics associated with study results for completed trials.
Characteristics Associated With Reporting Results in the Registry
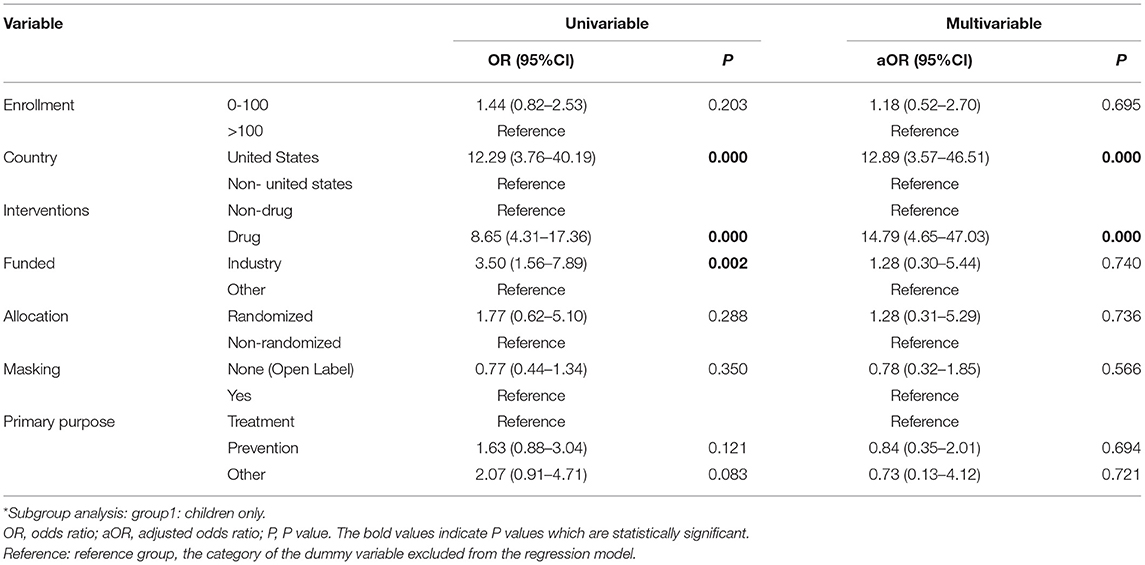
Univariable logistic regression showed significant differences in the rate of reporting results in the registry, according to different types of countries, interventions, and funding sources. United States trials (OR = 7.14, 95% CI: 3.04–16.7), drug trials (OR = 5.91, 95% CI: 2.61–13.36), and industry trials (OR = 7.70, 95% CI: 1.67–35.42) were more frequently reported than non-United States trials, nondrug trials, and NIH/other trials. Further multivariable logistic regression showed that United States trials were more likely to report results in ClinicalTrials.gov than all other trials (aOR = 6.76, 95% CI: 3.32–13.78). Drug trials were more likely to report results than Nondrug trials (aOR = 6.62, 95% CI: 3.20–13.69). The subgroup analysis yielded consistent results (Table 6, Supplementary Table 2).
Discussion
Our study showed that among clinicalTrials.gov registered childhood obesity-related intervention trials, trial unreported results were 88.5%, and 4.3% of trials were prematurely terminated. The factors that reduced the risk of unreported outcomes were US-registered clinical studies and drug intervention trials. Factors associated with reduced risk of early termination are NIH or other federal agency funding and large trials. The study also suggests that behavioral interventions play an important role in pediatric obesity clinical trials. Fewer than half of the trials provided information about their trial phase, and fewer than half used blinding.
Like obesity adults, obesity children have the right to receive the highest attainable standard of health care, but there is a lack of evidence regarding the safety and efficacy of treatments for overweight and obesity in children (9). For drug interventions, scientific data from only a limited number of short-term clinical trials are available, therefore, clinical trials with children are of the utmost importance (8–10). Ethical issues and policy requirements make it more difficult to recruit children, leading to the possible early termination of many clinical trials. Previous studies have found that pediatric clinical trials are terminated frequently (19, 20, 26). Thousands of children have participated in these trials, which means considerable inefficiency and a waste of financial resources (18). However, the early termination rate of pediatric obesity trials (4.3%) was lower than that of neonatal clinical trials, pediatric cardiovascular trials, pediatric psychological trials, etc. (21, 27, 28). This was an unexpected result, which may be due to a large number of obese children and the fact that most of the trial interventions are behavioral interventions that are easily accepted by patients, so clinical trials can better recruit patients. Furthermore, our analysis showed that industry-funded trials were more likely to stop early than government-funded or others, which may be because industry-funded clinical trials are more subject to financial and commercial influence than government-funded trials (21). Stefan et al. conducted a retrospective cohort study of pediatric randomized controlled trials in Switzerland, Germany, and Canada and found that the larger sample sizes (Enrollment>100) were less likely to stop early (29), which is consistent with our results. Perhaps because these trials were better organized from the start, the trial design systems built around experienced researchers may be better able to cope with recruitment challenges. In univariate analyses, drug trials were more likely to stop early, which may be due to the high ethical and regulatory requirements of drug trials for children, which may affect enrollment. Prevention trials are also risk factors for early termination, which is consistent with Turner et al. (30). The possible explanation is that prevention trials. One possible explanation is that prevention trials have a comparatively higher time cost to achieve clinical benefits than treatment trials (31). In addition, our analysis found that 11 of 57 (19.3%) intervention trials on childhood obesity did not report the reason for their early termination on ClinicalTrials.gov. The results reporting is especially important if the study is discontinued because of the detection of harm caused by the intervention. Trials involving children should always post reasons for early discontinuation on ClinicalTrials.gov (26). Studies have shown that failure to recruit sufficient numbers of participants and funding problems are the most common reasons for premature termination of clinical trials (32). Our study confirms these findings in childhood obesity trials.
The ethics and regulations of research involving children are widely discussed in the current literature (33–37); however, the reporting of results is ignored. Underreporting remains an important issue in the field of childhood obesity. Therefore, this issue should raise concerns about the overall quality of pediatric clinical trials. Despite the policy of the International Council of Medical Journal Editors (ICMJE), which recommends that all investigators report results (38), we found that the quality of results reported in the childhood obesity registry is still poor. These conclusions are consistent with Yang et al. (18). In clinical studies of mental health and neurology (30, 39). the reporting rates of clinical trial results were 42.3% and 32.2% respectively, both higher than our study results (11.5%). Food and Drugs Administration Amendments Act (FDAAA) and the Final Rule do not mandate that all studies report their results to the registry, which may account for the different reporting rates of study results (15). Similarly, USA-trials and drug trials were more likely to report results on ClinicalTrials.gov, which is related to the policy requiring reporting of the Food and Drugs Administration Amendments Act (FDAAA) and the Final Rule (15). In univariate analyses, industry-sponsored trials are more likely to report results, possibly because industry-sponsored trials more often have favorable results and conclusions (40).
Recommendations for Future Studies
First, study protocols and designs should be sternly controlled by supervisors, with particular emphasis on feasibility analysis of the conditions and expected numbers of subjects to be enrolled in trials, to ensure that studies with both reliable sample estimation and feasibility are available to allow patients to be enrolled. Second, childhood obesity trials should be routinely monitored by policymakers for registration and results on ClinicalTrials.gov, while ensuring trials are discontinued only for legitimate reasons. Third, due to the particularity, stringent ethical requirements, and lack of commercial benefits of clinical trials in children, most results of adult trials cannot be directly extended to children (41). Therefore, researchers need to publish trial results in a timely manner, especially for pediatric diseases. Fourth, the issue of inadequate enrollment emphasizes the importance of establishing global networks to coordinate recruitment efforts. Therefore, when designing a study, if the patient population is geographically dispersed and limited, researchers should positively collaborate with both clinicians and patient groups in each region and communicate with potential participants to maximize the number of enrollments needed to complete the trial.
Limitations
Our study has several limitations. First, this study only analyzed trials registered at ClincialTrials.gov, and our analysis did not include trials in other areas of pediatrics. Moreover, it should also be noted that information in the registry is provided by investigators and sponsors, and we were not able to check the degree of accuracy of the trial data. However, this issue is mitigated in part by automated data validity checks and manual review by ClinicalTrials.gov staff to ensure data reliability before public posting (42).
Conclusions
In summary, the problem of unreported results in clinical trials of childhood obesity is serious, and the factors that reduced the risk of unreported outcomes were US-registered clinical studies and drug intervention trials. Furthermore, the factors associated with a reduced risk of early termination are NIH or other federal agency funding and large trials. Our findings may contribute to a better understanding of the etiologies of premature termination and unreported results, thus setting the stage for targeted approaches to improve clinical research.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
LD and JH conceptualized and designed the study and reviewed and revised the manuscript. XW and YL collected and screened the data, carried out the initial analyses, drafted the initial manuscript, and reviewed and revised the manuscript. LY coordinated and supervised data collection and critically reviewed the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Funding
This study was supported by the Key Program of Sichuan Provincial Science and Technology Department, China (No. 2019YFS0194) and the National Natural Science Foundation of China (No. 81873197, No. 72074161, and No.81403276).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We gratefully thank for the help of data collection from Li Tang, MD student from state Key Laboratory of Oral Diseases and National Clinical Research Center for Oral Diseases West China Hospital of Stomatology, Sichuan University and data processing from Ran Gu, a Ph.D. student from the University of Electronic Science and Technology of China.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.860610/full#supplementary-material
Abbreviations
NIH, national institutes of health; HR, hazard ratio; OR, odds ratio; CI, confidence interval; FDA, food and drug administration; NCT, national clinical trial; FDAAA, food and drug administration amendments act; RCT, randomized controlled trial; ICMJE, international council of medical journal editors.
References
1. Adab P, Pallan MJ, Lancashire ER, Hemming K, Frew E, Barrett T, et al. Effectiveness of a childhood obesity prevention programme delivered through schools, targeting 6 and 7 year olds: cluster randomised controlled trial (WAVES study). BMJ. (2018) 360:k211. doi: 10.1136/bmj.k211
2. Caprio S, Santoro N, Weiss R. Childhood obesity and the associated rise in cardiometabolic complications. Nat Metabol. (2020) 2:223–32. doi: 10.1038/s42255-020-0183-z
3. Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. (2014) 384:766–81. doi: 10.1016/S0140-6736(14)60460-8
4. Morales Camacho WJ, Molina Díaz JM, Plata Ortiz S, Plata Ortiz JE, Morales Camacho MA, Calderón BP. Childhood obesity: Aetiology, comorbidities, and treatment. Diabetes Metab Res Rev. (2019) 35:e3203. doi: 10.1002/dmrr.3203
5. Donoso Fuentes A, Córdova LP, Hevia JP, Arriagada SD. The obese child in the Intensive Care Unit. Update. Arch Argent Pediatr. (2016) 114:258–166. doi: 10.5546/aap.2016.eng.258
6. Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. (2006) 295:1549–55. doi: 10.1001/jama.295.13.1549
7. Cao Z, Hua J, Zhang D, Thapa JR, Wang S. A cohort study assessing the sustainable long-term effectiveness of a childhood-obesity intervention in China. Int J Epidemiol. (2019) 48:108–15. doi: 10.1093/ije/dyy145
8. Weihrauch-Blüher S, Wiegand S. Risk factors and implications of childhood obesity. Curr Obes Rep. (2018) 7:254–59. doi: 10.1007/s13679-018-0320-0
9. Oude Luttikhuis H, Baur L, Jansen H, Shrewsbury VA, O'Malley C, Stolk RP, et al. WITHDRAWN: Interventions for treating obesity in children. Cochrane Database Syst Rev. (2019) 3:Cd001872. doi: 10.1002/14651858.CD001872.pub3
10. Bjerregaard LG, Jensen BW, Ängquist L, Osler M, Sørensen TIA, Baker JL. Change in Overweight from Childhood to Early Adulthood and Risk of Type 2 Diabetes. N Engl J Med. (2018) 378:1302–12. doi: 10.1056/NEJMoa1713231
11. Brown T, Moore TH, Hooper L, Gao Y, Zayegh A, Ijaz S, et al. Interventions for preventing obesity in children. Cochrane Database Syst Rev. (2019) 7:Cd001871. doi: 10.1002/14651858.CD001871.pub4
12. Withrow D, Alter DA. The economic burden of obesity worldwide: a systematic review of the direct costs of obesity. Obes Rev. (2011) 12:131–41. doi: 10.1111/j.1467-789X.2009.00712.x
13. Segal AB, Huerta MC, Aurino E, Sassi F. The impact of childhood obesity on human capital in high-income countries: a systematic review. Obes Rev. (2021) 22:e13104. doi: 10.1111/obr.13104
14. Pasquali SK, Lam WK, Chiswell K, Kemper AR, Li JS. Status of the pediatric clinical trials enterprise: an analysis of the US ClinicalTrials.gov registry. Pediatrics. (2012) 130:e1269–77. doi: 10.1542/peds.2011-3565
15. Tse T, Fain KM, Zarin DA. How to avoid common problems when using ClinicalTrials. gov in research: 10 issues to consider. BMJ. (2018) 361:k1452. doi: 10.1136/bmj.k1452
16. Food and Drug Administration Amendments Act of 2007. Pub. L. 110–85, Title VIII—Clinical Trial Databases: 121 STAT. 904. Available online at: http://www.gpo.gov/fdsys/pkg/PLAW-110publ85/pdf/PLAW-110publ85.pdf#page=82 (accessed July. 1, 2021).
17. Zarin DA, Fain KM, Dobbins HD, Tse T, Williams RJ. 10-Year Update on Study Results Submitted to ClinicalTrials. gov. N Engl J Med. (2019) 381:1966–74. doi: 10.1056/NEJMsr1907644
18. Zhong Y, Zhang X, Zhou L, Li L, Zhang T. Updated analysis of pediatric clinical studies registered in ClinicalTrials.gov, 2008-2019. BMC Pediatr. (2021) 21:212. doi: 10.1186/s12887-021-02658-4
19. Fernandes RM, van der Lee JH, Offringa M. A systematic review of the reporting of Data Monitoring Committees' roles, interim analysis and early termination in pediatric clinical trials. BMC Pediatr. (2009) 9:77. doi: 10.1186/1471-2431-9-77
20. Fernandes RM, van der Lee JH, Offringa M. Data monitoring committees, interim analysis and early termination in paediatric trials. Acta Paediatr. (2011) 100:1386–92. doi: 10.1111/j.1651-2227.2011.02282.x
21. Wortzel JR, Turner BE, Weeks BT, Fragassi C, Ramos V, Truong T, et al. Trends in US pediatric mental health clinical trials: An analysis of ClinicalTrials.gov from 2007-2018. PloS ONE. (2021) 16:e0248898. doi: 10.1371/journal.pone.0248898
22. DeVito NJ, Bacon S, Goldacre B. Compliance with legal requirement to report clinical trial results on ClinicalTrials. gov: a cohort study. Lancet. (2020) 395:361–69. doi: 10.1016/S0140-6736(19)33220-9
23. Turner B, Rajeshuni N, Tran EM, Ludwig CA, Tauqeer Z, Weeks B, et al. Characteristics of Ophthalmology Trials Registered in ClinicalTrials.gov, 2007-2018. Am J Ophthalmol. (2020) 211:132–41. doi: 10.1016/j.ajo.2019.11.004
24. Maruani A, Boutron I, Baron G, Ravaud P. Impact of sending email reminders of the legal requirement for posting results on ClinicalTrials. gov: cohort embedded pragmatic randomized controlled trial. BMJ. (2014) 349:g5579. doi: 10.1136/bmj.g5579
25. Riveros C, Dechartres A, Perrodeau E, Haneef R, Boutron I, Ravaud P. Timing and completeness of trial results posted at ClinicalTrials.gov and published in journals. PLoS Med. (2013) 10:e1001566; discussion e66. doi: 10.1371/journal.pmed.1001566
26. Shamliyan T, Kane RL. Clinical research involving children: registration, completeness, and publication. Pediatrics. (2012) 129:e1291–300. doi: 10.1542/peds.2010-2847
27. Perrem LM, Gosling S, Ravikumar I, Khashan AS, Miletin J, Ryan CA, et al. Reporting on data monitoring committees in neonatal randomised controlled trials is inconsistent. Acta Paediatr. (2017) 106:30–3. doi: 10.1111/apa.13593
28. Hill KD, Chiswell K, Califf RM, Pearson G, Li JS. Characteristics of pediatric cardiovascular clinical trials registered on ClinicalTrials.gov. Am Heart J. (2014) 167:921–9.e2. doi: 10.1016/j.ahj.2014.02.002
29. Schandelmaier S, Tomonaga Y, Bassler D, Meerpohl JJ, von Elm E, You JJ, et al. Premature discontinuation of pediatric randomized controlled trials: a retrospective cohort study. J Pediatr. (2017) 184:209–14.e1. doi: 10.1016/j.jpeds.2017.01.071
30. Turner BE, Magnani CJ, Frolov A, Weeks BT, Steinberg JR, Huda N, et al. Neurology trial registrations on ClinicalTrials.gov between 2007 and 2018: A cross-sectional analysis of characteristics, early discontinuation, and results reporting. J Neurol Sci. (2021) 428:117579. doi: 10.1016/j.jns.2021.117579
31. Cooper CL, Hind D, Duncan R, Walters S, Lartey A, Lee E, et al. A rapid review indicated higher recruitment rates in treatment trials than in prevention trials. J Clin Epidemiol. (2015) 68:347–54. doi: 10.1016/j.jclinepi.2014.10.007
32. Bernardez-Pereira S, Lopes RD, Carrion MJ, Santucci EV, Soares RM, de Oliveira Abreu M, et al. Prevalence, characteristics, and predictors of early termination of cardiovascular clinical trials due to low recruitment: insights from the ClinicalTrials.gov registry. Am Heart J. (2014) 168:213–9.e1. doi: 10.1016/j.ahj.2014.04.013
33. Botkin J. Protecting the privacy of family members in survey and pedigree research. JAMA. (2001) 285:207–11. doi: 10.1001/jama.285.2.207
34. Millum J, Emanuel EJ. Ethics. The ethics of international research with abandoned children. Science. (2007) 318:1874–5. doi: 10.1126/science.1153822
35. Fisher CB, Kornetsky SZ, Prentice ED. Determining risk in pediatric research with no prospect of direct benefit: time for a national consensus on the interpretation of federal regulations. Am J Bioeth. (2007) 7:5–10. doi: 10.1080/15265160601171572
36. Rose CD. Ethical Conduct of Research in Children: Pediatricians and Their IRB (Part 1 of 2). Pediatrics. (2017) 139:e20163648. doi: 10.1542/peds.2016-3648
37. Park SS, Grayson MH. Clinical research: protection of the “vulnerable”? J Allergy Clin Immunol. (2008) 121:1103–7. doi: 10.1016/j.jaci.2008.01.014
38. Zarin DA, Tse T, Williams RJ, Califf RM, Ide NC. The ClinicalTrials. gov results database–update and key issues. N Engl J Med. (2011) 364:852–60. doi: 10.1056/NEJMsa1012065
39. Wortzel JR, Turner BE, Weeks BT, Fragassi C, Ramos V, Truong T, et al. Trends in mental health clinical research: Characterizing the ClinicalTrials.gov registry from 2007-2018. PloS ONE. (2020) 15:e0233996. doi: 10.1371/journal.pone.0233996
40. Lundh A, Lexchin J, Mintzes B, Schroll JB, Bero L. Industry sponsorship and research outcome: systematic review with meta-analysis. Intensive Care Med. (2018) 44:1603–12. doi: 10.1007/s00134-018-5293-7
41. Janiaud P, Lajoinie A, Cour-Andlauer F, Cornu C, Cochat P, Cucherat M, et al. Different treatment benefits were estimated by clinical trials performed in adults compared with those performed in children. J Clin Epidemiol. (2015) 68:1221–31. doi: 10.1016/j.jclinepi.2015.06.021
42. Medicine, UNLo,. ClinicalTrials.gov and related projects: improving access to information about clinical studies. press release LHNCBC. Available online at: https://lhncbc.nlm.nih.gov/LHC-publications/pubs/ClinicalTrialsgovandRelatedProjectsImprovingAccesstoInformationaboutClinicalTrials.html (accessed July 1, 2021).
Keywords: childhood obesity, early termination, results reporting, underreporting, ClinicalTrials.gov
Citation: Wang X, Long Y, Yang L, Huang J and Du L (2022) Results Reporting and Early Termination of Childhood Obesity Trials Registered on ClinicalTrials.gov. Front. Pediatr. 10:860610. doi: 10.3389/fped.2022.860610
Received: 24 January 2022; Accepted: 28 February 2022;
Published: 24 March 2022.
Edited by:
Pietro Vajro, University of Salerno, ItalyReviewed by:
Maria Chiara Rocco, Università degli Studi di Salerno, ItalyAnna Pia Delli Bovi, University of Siena, Italy
Copyright © 2022 Wang, Long, Yang, Huang and Du. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liang Du, ZHVsaWFuZzA2MDZAdmlwLnNpbmEuY29t; Jin Huang, bWljaGFlbF9odWFuZ2ppbkAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Xinyi Wang
Xinyi Wang Youlin Long
Youlin Long Liu Yang
Liu Yang Jin Huang
Jin Huang Liang Du
Liang Du