Abstract
Objective:
Current knowledge on the global burden of infant sepsis is limited to population-level data. We aimed to summarize global case fatality rates (CFRs) of young infants with sepsis, stratified by gross national income (GNI) status and patient-level risk factors.
Methods:
We performed a systematic review and meta-analysis on CFRs among young infants < 90 days with sepsis. We searched PubMed, Cochrane Central, Embase, and Web of Science for studies published between January 2010 and September 2019. We obtained pooled CFRs estimates using the random effects model. We performed a univariate analysis at patient-level and a meta-regression to study the associations of gestational age, birth weight, onset of sepsis, GNI, age group and culture-proven sepsis with CFRs.
Results:
The search yielded 6314 publications, of which 240 studies (N = 437,796 patients) from 77 countries were included. Of 240 studies, 99 were conducted in high-income countries, 44 in upper-middle-income countries, 82 in lower-middle-income countries, 6 in low-income countries and 9 in multiple income-level countries. Overall pooled CFR was 18% (95% CI, 17–19%). The CFR was highest for low-income countries [25% (95% CI, 7–43%)], followed by lower-middle [25% (95% CI, 7–43%)], upper-middle [21% (95% CI, 18–24%)] and lowest for high-income countries [12% (95% CI, 11–13%)]. Factors associated with high CFRs included prematurity, low birth weight, age less than 28 days, early onset sepsis, hospital acquired infections and sepsis in middle- and low-income countries. Study setting in middle-income countries was an independent predictor of high CFRs. We found a widening disparity in CFRs between countries of different GNI over time.
Conclusion:
Young infant sepsis remains a major global health challenge. The widening disparity in young infant sepsis CFRs between GNI groups underscore the need to channel greater resources especially to the lower income regions.
Systematic Review Registration:
[www.crd.york.ac.uk/prospero], identifier [CRD42020164321].
Introduction
Infant sepsis is an important public health challenge with a significant burden of disease. Globally, an estimated 1.3–3.9 million young infants experience sepsis and 400,000–700,000 die from sepsis-related conditions annually (1). In the Sub-Saharan African region alone, young infant sepsis incurs an economic burden of US$10 – $469 billion annually (2).
Sepsis remains a significant cause for death and accounts for up to 15% of all young infant deaths (3). One of the targets in the United Nations Sustainable Developmental Goals 3 (SDG 3) is to reduce neonatal mortality to 12 per 1,000 livebirths and under-5 mortality to 25 per 1,000 livebirths by 2030 (4, 5). The World Health Organization (WHO) has reported that an estimated 84% of young infant deaths due to sepsis are preventable (1). Reducing young infant sepsis mortality can contribute to achieving the SDG3 targets by 2030. Pediatric sepsis survivors are at higher risk of poor neurodevelopmental sequelae including neurocognitive deficits and developmental delay (6–8). A better understanding of young infant sepsis can provide valuable insights to inform strategies that span prevention, diagnosis and intervention to mitigate long term mortality and morbidity.
The recent population-based systematic review and meta-analysis on young infant sepsis mortality performed by Fleischmann et al. reviewed data from 1979 to 2019 and reported a population rate of 2824 (95% CI, 1892–4194) neonatal sepsis cases per 100,000 livebirths worldwide with a mortality of 17.6% (95% CI, 10.3–28.6%) (9). Sepsis incidence was reported to be highest in preterm and very low birth weight (VLBW) infants. However, this seminal study only included 14 countries with available population-level data with majority originating from middle-income countries. In addition, there is still a gap in mortality data in low-income countries. This limits the accurate estimate of the global burden of young infant sepsis (10). A comprehensive systematic review and meta-analysis on young infant sepsis case fatality rates (CFRs) from different income level countries will allow for a timely update to previously published literature on the global burden of young infant sepsis, and provide a more complete understanding on the global burden of young infant sepsis.
We therefore aimed to summarize global CFRs for young infants (<90 days) with sepsis, published from January 2010 to September 2019. We also aimed to describe any differences in young infant sepsis mortality among countries of different gross national income (GNI) status over time.
Methods
We performed a systematic review and meta-analysis using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines (11). This study is registered with PROSPERO (CRD42020164321). The protocol is available online (12).
Eligibility Criteria
We included randomized controlled trials (RCTs), cohort studies and cross-sectional studies that were published between January 2010 and September 2019. This range was chosen to provide an update on a previously published systematic review on the global burden of neonatal sepsis (10). We excluded studies published before January 2010 in view of the substantial changes in neonatal sepsis diagnosis and management over time.
We identified studies that contained the following specified elements of population, exposure, outcome and study design. We defined the population as infants less than 90 days old, regardless of gestational age. The neonatal period was defined as the first 28 postnatal days in term and post-term newborns, and day of birth through the expected date of delivery plus 27 days for preterm newborns (13). We chose to study infants less than 90 days old to obtain a comprehensive picture of sepsis burden, as serious infections in the young infant population can present past 28 postnatal days (14). If a study included both pediatric and adult population, it was included only if data pertaining to the infant population (<90 days) could be extracted. We defined the exposure as sepsis, which could be of bacterial, viral or fungal origin (15). We included viral infections because young infants can suffer from long term deficits from invasive viral illnesses (16, 17). Due to the lack of a gold standard for diagnosis of young infant sepsis (18), we decided to include all studies with sepsis as defined by study authors. However, we documented if the study defined sepsis according to the International Paediatric Sepsis Consensus Conference (15). We defined our primary outcome as the CFR, which was computed based on the number of deaths divided by the number of infants with sepsis.
We excluded case-control studies, case reports, animal studies, laboratory studies and publications that were not in English. We excluded studies with a primary focus on necrotising enterocolitis, respiratory distress syndrome without a primary sepsis study population, leukemia or other malignancies. We also excluded studies with a sample size of less than 50 to avoid small study effects (19).
Information Sources and Search Strategy
We searched PubMed, Cochrane Central, Embase and Web of Science to identify eligible studies. The search was conducted on 17 September 2019 with a search strategy developed in consultation with research librarians experienced in systematic reviews and meta analyses. Strategic keywords used include “neonates,” “infants,” “sepsis,” “neonatal sepsis,” and “mortality.” The detailed search strategy can be found in Supplementary Table 1. We ensured that there were no completed or ongoing trials evaluating global burden of neonatal sepsis by searching PROSPERO, ClinicalTrials.gov, International Standard Randomized Controlled Trial Number (ISRCTN) registry, World Health Organization International Clinical Trials Registry Platform (ICTRP), and European Union Clinical Trials Register.
Study Selection Process
Covidence (Australia) was used for the review of articles. Three reviewers (MG, BY, SS) independently conducted the database search and screened the title and abstracts for relevance, and subsequently assessed the full-text of shortlisted articles for eligibility. Any conflict on study eligibility were resolved in discussion with the senior author (S-LC). Reason(s) for exclusion of each article was (were) recorded.
Data Collection Process and Data Items
Four reviewers (MG, BY, SS, and WL) independently carried out the data extraction using a standardized data collection form, and any conflict was resolved by discussion. Study variables included were study characteristics (e.g., study year, study design, geographical origin, sepsis definition, sample size), patient demographics (e.g., age, gender, gestational age, birth weight), patient characteristics (e.g., severity of sepsis, comorbidities, maternal risk factors, microbiological data, sources of infection, onset of sepsis, interventions, duration of hospital stay and blood markers), and outcome (deaths, timeframe to mortality). GNI was determined according to the World Bank Country Classification (20). We contacted the corresponding authors for any missing or unreported data via email. A second reminder email was sent 2 weeks later. When there was no reply 1 month from the first email we considered the team to be un-contactable.
Study Risk of Bias Assessment
We assessed the risk of bias using the Cochrane risk-of-bias tool for RCTs (21), and the Newcastle-Ottawa Scale for all observational studies (22). Two assessors (SS and WL) independently carried out the assessment, and any conflict was resolved by discussion or with input from a third independent reviewer (MG). An overall rating of high, moderate or low risk was given to each study.
Effect Measures and Synthesis Methods
Categorical variables were summarized as frequencies and percentages while continuous variables were summarized as means with standard deviations (SD). We generated pooled CFRs and the 95% confidence intervals (95% CI) using the DerSimonian and Laird method (23). We performed a univariate analysis at patient-level to assess the association between the CFRs and each variable (birth weight, age, gestational age, type of sepsis, source of infection either hospital or community acquired and culture-proven sepsis). We also performed a multivariable meta-regression at study level to assess the association between CFRs among infants less than 90 days and the following variables determined a priori – gestational age, birth weight, onset of sepsis, GNI, age group, and culture-proven sepsis. For each variable in the multivariable meta-regression analysis, only studies that exclusively looked at high risk groups [preterm infants, infants with low birth weight (LBW), early onset sepsis, countries with middle- and low-income, age <28 days and culture-proven sepsis] were selected. These studies were compared to reference groups of lower risk, and included studies that did not exclusively contain the high risk groups. For example, when studying the effect of birth weight, we compared studies that only included LBW and VLBW infants, as compared to studies that included infants of normal birth weight. For GNI status, we took high-income groups as the reference group. For study design, we took RCTs as the reference group.
All statistical analysis was done using Stata (v16.1, College Station, TX, United States). We used I2 statistics to quantify heterogeneity between studies. We performed two sensitivity analyses in which we included: (1) only studies with culture-proven sepsis; and (2) only studies with low risk of bias, evaluating CFRs and temporal trends limiting to these studies.
Results
Study Selection
Among 6314 articles screened, 240 studies (with a total of 437,796 patients) met the inclusion criteria and were included for analysis (Figure 1).
FIGURE 1
PRISMA flow diagram.
Study Characteristics
Characteristics of the included studies and study population are summarized in Tables 1, 2 and Supplementary Table 2. The studies originate from 77 countries and six continents (Figure 2).
TABLE 1
| High-income countries (n = 99) | Upper-middle-income countries (n = 44) | Lower-middle-income countries (n = 82) | Low-income countries (n = 6) | Multiple income level countries (n = 9) | All studies (n = 240) | |
| Study design | ||||||
| Cross sectional study, n (%) | 3 (1) | 5 (2) | 11 (5) | 1 (0.4) | 2 (0.8) | 22 (9) |
| Randomized controlled trial, n (%) | 2 (0.8) | 2 (0.8) | 9 (4) | 1 (0.4) | 1 (0.4) | 15 (6) |
| Prospective cohort study, n (%) | 31 (13) | 14 (6) | 34 (14) | 1 (0.4) | 5 (2) | 85 (35) |
| Retrospective cohort study, n (%) | 63 (26) | 23 (10) | 27 (11) | 3 (1) | 1 (0.4) | 117 (49) |
| Continents* | ||||||
| Africa, n (%) | 3 (1) | 8 (3) | 20 (8) | 3 (1) | 4 (2) | 38 (16) |
| Asia, n (%) | 33 (14) | 23 (10) | 70 (29) | 1 (0.4) | 6 (3) | 133 (55) |
| Australia, n (%) | 4 (2) | 0 | 0 | 0 | 3 (1) | 7 (3) |
| Europe, n (%) | 34 (14) | 13 (5) | 2 (0.8) | 0 | 4 (2) | 53 (22) |
| North America, n (%) | 28 (12) | 4 (2) | 0 | 2 (0.8) | 6 (3) | 40 (17) |
| South America, n (%) | 0 | 7 (3) | 0 | 0 | 6 (3) | 13 (5) |
| Studies that include culture-proven sepsis,n (%) | 91 (38) | 40 (17) | 74 (31) | 4 (2) | 6 (3) | 215 (90) |
Characteristics of studies.
*Some countries are transcontinental and hence total n for countries does not add up to 240.
TABLE 2
| High-income countries (n = 99) | Upper-middle-income countries (n = 44) | Lower-middle-income countries (n = 82) | Low-income countries (n = 6) | Multiple income level countries (n = 9) | All studies (n = 240) | |
| Age of patients | ||||||
| Age of patients, mean ± SD, days | 27.9 ± 20.4 | 16.2 ± 15.6 | 9.8 ± 10.7 | 1.7 | – | 13.2 ± 15.5 |
| Studies that exclusively studied neonates (<28 days), n (%) | 55 (23) | 31 (13) | 70 (29) | 5 (2) | 3 (1) | 164 (68) |
| Birth weight of patients | ||||||
| Birth weight of patients, mean (SD), g | 978 ± 356 | 1397 ± 522 | 2328 ± 666 | – | – | 1481 ± 517 |
| Studies that exclusively studied low birth weight infants (<2500 g), n (%) | 17 (7) | 4 (2) | 3 (1) | 0 | 2 (0.8) | 26 (11) |
| Gestational age of patients | ||||||
| Gestational age, mean (SD), weeks | 28.0 ± 3.0 | 29.8 ± 3.1 | 35.7 ± 2.9 | – | 37.0 ± 4.0 | 30.4 ± 3.0 |
| Studies that exclusively studied preterm infants (<37 weeks), n (%) | 13 (5) | 6 (3) | 5 (2) | 0 (0) | 0 (0) | 24 (10) |
| Types of sepsis | ||||||
| Studies that exclusively studied early onset sepsis (based on author’s definition), n (%) | 15 (6) | 3 (1) | 7 (3) | 0 | 1 (0.4) | 26 (11) |
| Predominant Causative Organisms | ||||||
| Gram-positive bacteria, n (%) | 62 (26) | 17 (7) | 15 (6) | 2 (0.8) | 6 (3) | 102 (43) |
| Gram-negative bacteria, n (%) | 20 (8) | 18 (8) | 48 (20) | 4 (2) | 1 (0.4) | 91 (38) |
| Viral, n (%) | 1 (0.4) | 0 | 0 | 0 | 0 | 1 (0.4) |
| Fungal, n (%) | 6 (3) | 2 (0.8) | 3 (1) | 0 | 1 (0.4) | 12 (5) |
Characteristics of study population.
FIGURE 2
Publication by countries.
The greatest number of studies were conducted in Asia with 133 studies (63042 patients), followed by Europe with 53 studies (23639 patients), North America (40 studies, 253786 patients), Africa (38 studies, 39554 patients), South America (13 studies, 160386 patients) and Australia (7 studies, 3270 patients).
Of the 240 studies, 99 (24–122) (41%) were conducted in high-income countries, 44 (123–166) (18%) in upper-middle-income countries, 82 (167–248) (34%) in lower-middle-income countries, and 6 (249–254) (3%) in low-income countries. Nine (255–263) (4%) studies were conducted in countries with multiple income levels.
Among all studies, 215/240 studies (90%) included culture-proven sepsis in their study population while 175/240 (73%) used a combination of clinical and laboratory criteria. 164/240 (68%) studies exclusively studied neonates (<28 days), 24/116 (21%) studies exclusively studied preterm infants, 26/125 (21%) exclusively studied LBW infants and 26/160 (16%) exclusively studied early onset sepsis. Out of 240 studies, 55 (23%) studies reported CFRs stratified by preterm versus term, 28 (12%) studies reported CFRs in community versus hospital acquired sepsis, 107 (45%) studies reported CFRs defined by early or late onset sepsis, and 56 (23%) studies reported birth weight related specific CFRs.
Patient Characteristics
Overall mean age of the study population was 13.2 ± 15.5 days. Mean birth weight and gestational age of the study population was 1480 ± 516 grams and 30.4 ± 3.0 weeks, respectively.
One hundred and two studies (43%) reported gram positive organisms as the predominant organisms causing sepsis. Among the studies conducted in high-income countries, 62 of 99 studies (63%) reported gram positive organisms as the predominant causative organisms, while gram negative organisms were the predominant causative organisms in studies conducted in middle- or low-income countries – 18/44 (41%) in upper-middle-income countries, 48/82 (59%) in lower-middle-income countries, 4/6 (67%) in low-income countries.
Amongst the 200 studies that reported pathogens causing infant sepsis, the most common organisms reported were Coagulase-negative staphylococci (28%), followed by Klebsiella pneumoniae (24), Staphylococcus aureus (10%), Group B Streptococcus (8%), Escherichia coli (7%), and Candida albicans (6%). Among 24 studies that exclusively studied preterm infants, 21 studies reported the most common organism, in which the most common organism was coagulase-negative staphylococci, reported by 10/21 (48%) studies (Figure 3). Among 26 studies that exclusively studied LBW infants, 24 studies reported the most common organism, in which the most common organism was coagulase-negative staphylococci, reported by 13/24 (54%) studies.
FIGURE 3
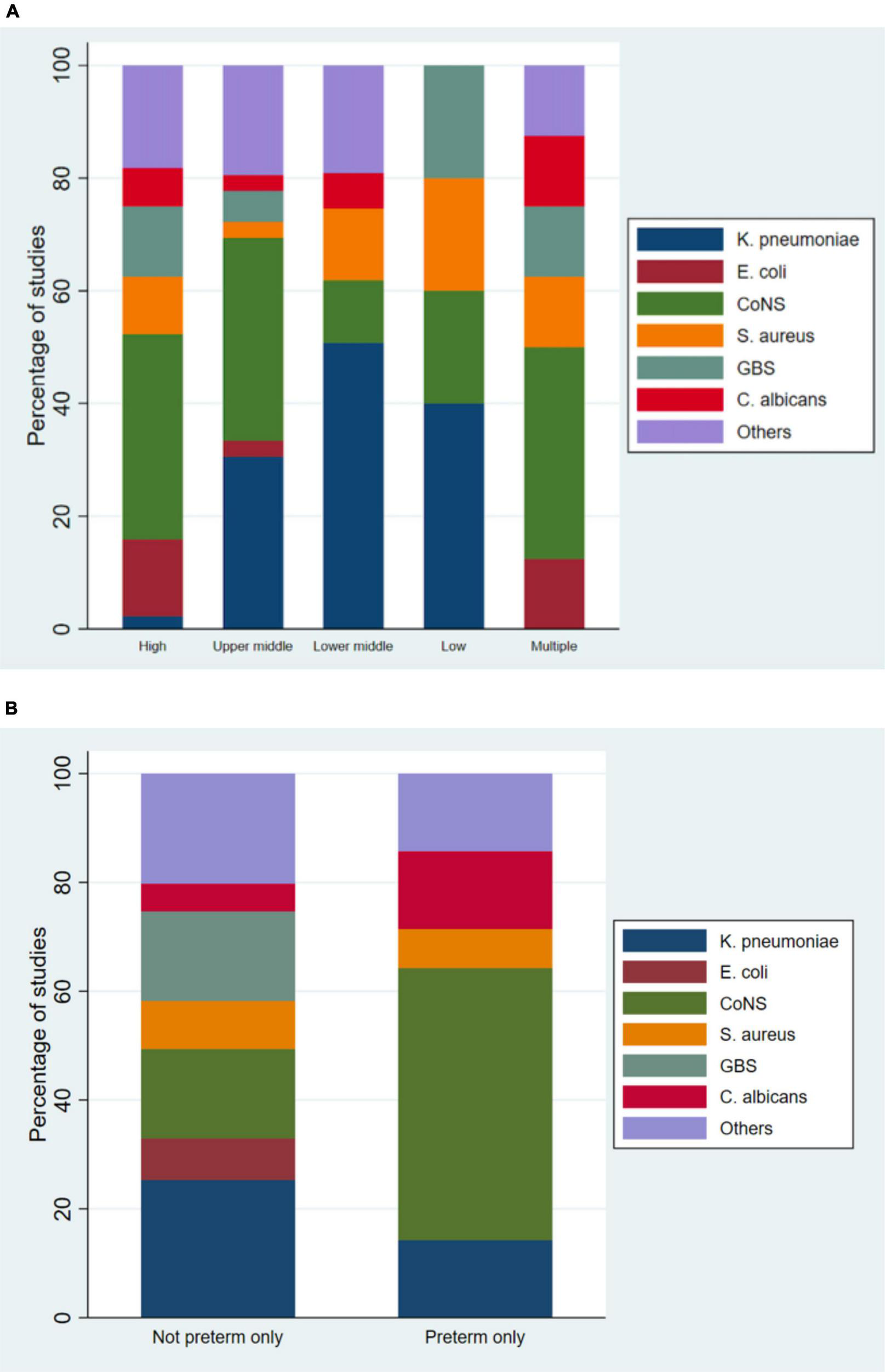
Causative organisms categorized by gross national income (A) and gestational age (B).
Outcomes
Overall, the pooled CFR was 18% (95% CI, 17–19%). The CFR was lowest for high-income countries [12% (95% CI, 11–13%)], as compared to upper-middle-income countries [21% (95% CI, 18–24%)] and lower-middle-income countries [24% (95% CI, 21–26%)]. Low-income countries had the highest CFR [25% (95% CI, 7–43%)]. Pooled CFR for continents was highest for Africa 24% (95% CI, 21–27%) and lowest in Australia 14% (95% CI, 10–18%) (Table 3). For study designs, cross sectional studies had a higher CFR of 22% (95% CI, 16–28%), while RCTs had a CFR of 13% (95% CI, 10–17%). Studies with a low risk of bias had a pooled CFR of 17% (95% CI, 16–18%).
TABLE 3
| Pooled CFRs (95% Confidence Interval) | |
| Gross National Income | |
| Low-income countries (6)† | 25% (7–43%) |
| Lower-middle-income countries (82) | 24% (21–26%) |
| Upper-middle-income countries (44) | 21% (18–24%) |
| High-income countries (99) | 12% (11–13%) |
| Study designs | |
| Cross-sectional studies (22) | 22% (16–28%) |
| Prospective studies (85) | 20% (18–22%) |
| Retrospective studies (117) | 17% (16–18%) |
| Randomized controlled trials (15) | 13% (10–17%) |
| Study continent | |
| Africa (38) | 24% (21–27%) |
| South America (13) | 20% (17–22%) |
| Asia (133) | 19% (18–21%) |
| North America (40) | 18% (15–20%) |
| Europe (53) | 16% (14–17%) |
| Australia (7) | 14% (10–18%) |
| Studies with low risk of bias (184) | 17% (16–18%) |
| Overall pooled CFR | 18% (17–19%) |
Pooled Case Fatality Rates based on study characteristics.
†(N) refers to the number of studies that studied the variable and reported mortality.
Among studies that reported CFRs by birth weight, the highest CFR was for VLBW infants [24% (95% CI, 21–26%] in comparison to LBW infants [23% (95% CI, 21–26%) and normal birth weight infants [15% (95% CI, 10–21%)]. Studies exclusively on neonates had a higher CFR of 18% (95% CI, 17–19%) compared to studies that included older infants [15% (95% CI, 14–17%)]. Studies on preterm infants had a CFR of 23% (95% CI, 19–26%), more than double compared to term infants with a CFR of 10% (95% CI, 8–13%) (Figure 4). Infants with early onset sepsis (<72 h of life) had a CFR of 20% (95% CI, 17–24%), while infants with early and late onset sepsis had a CFR of 16% (95% CI, 14–18%). Infants with hospital-acquired infections had a CFR of 23% (95% CI, 17–29%), higher than those with community-acquired infections [14% (95% CI, 9–19%)]. Infants with culture- proven sepsis had a CFR of 20% (95% CI, 19–22%) (Table 4).
FIGURE 4
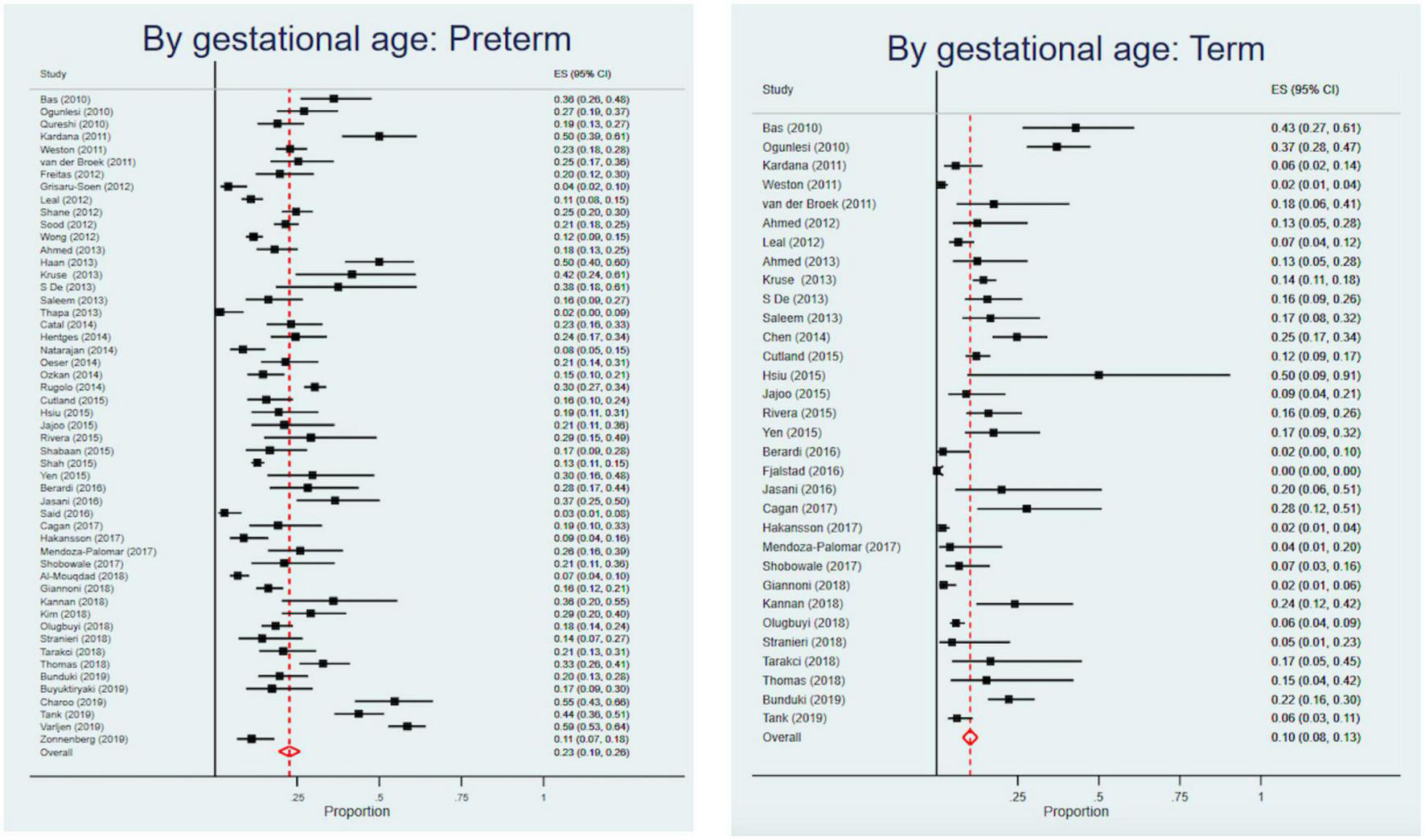
Forest plots of preterm and term Case Fatality Rates.
TABLE 4
| Pooled CFRs (95% Confidence Interval) | |
| Birth weight | |
| <1500 g (45)‡ | 24% (21–26%) |
| <2500 g (51) | 23% (21–26%) |
| ≥2500 g (16) | 15% (10–21%) |
| Age | |
| Studies that exclusively studied neonates < 28 days (164) | 18% (17–19%) |
| Gestational age | |
| Preterm (52) | 23% (19–26%) |
| Term (32) | 10% (8–13%) |
| Type of sepsis | |
| Early onset (<72 h) (44) | 20% (17–24%) |
| All (both) (83) | 16% (14–18%) |
| Source of infection | |
| Hospital acquired (22) | 23% (17–29%) |
| Community acquired (14) | 14% (9–19%) |
| Culture-proven sepsis (178) | 20% (19–22%) |
| Overall pooled case fatality rate (240) | 18% (17–19%) |
Pooled Case Fatality Rates for study population characteristics.
‡(N) refers to the number of studies that studied the variable and reported mortality.
There was an overall increasing trend in young infant sepsis CFRs over time (Figure 5 and Supplementary Figure 1). When annual pooled CFRs were stratified according to GNI status, there was a widening of the disparity between GNI and young infant CFRs, with time. This is consistent with the sensitivity analysis including only studies with low risk of bias (Figure 5).
FIGURE 5
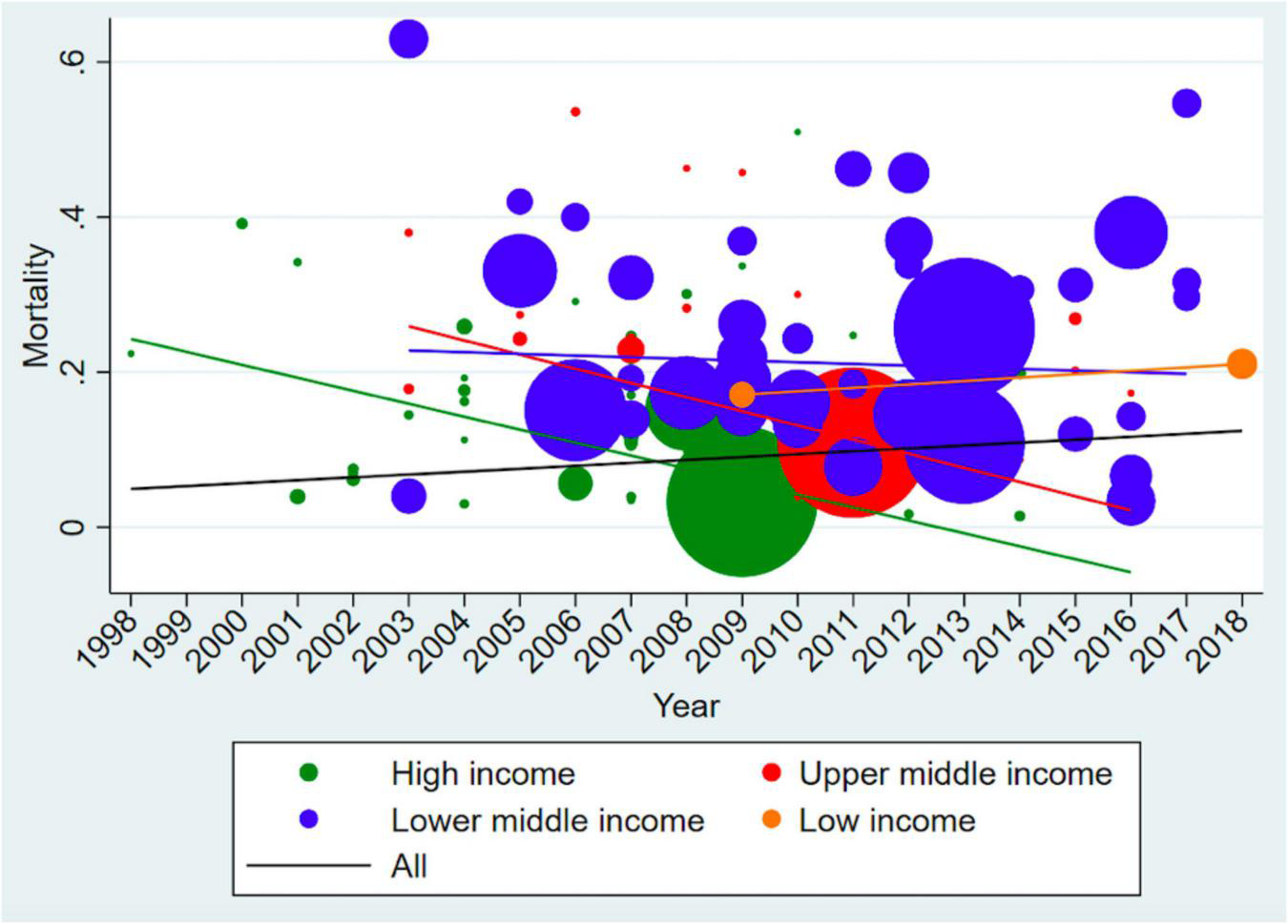
Time trend analysis of Case Fatality Rates (including only low risk of bias studies). The size of the bubble is proportionate to the number of infants in the study, while the line represents the trends of case fatality rates over time. There is an increasing trend for low-income countries and decreasing trend for middle- and high-income countries overtime. The overall trend for young infant sepsis case fatality rates is increasing.
Meta Regression
Nine (4%) studies that were conducted in countries with multiple income levels were excluded from the analysis. Independent predictors of higher CFRs include upper-middle- and lower-middle-income countries [regression coefficient 0.12 (95% CI, 0.04–0.21); p = 0.003 and 0.14 (95% CI, 0.06–0.22); p = 0.001 respectively] (Table 5).
TABLE 5
| Characteristics | Univariate analysis | Multivariable analysis | ||
| Coefficient (95% CI) | P-value | Coefficient (95% CI) | P-value | |
| Gestational age§ | ||||
| Term | 1 [Reference] | NA | 1 [Reference] | NA |
| Preterm | 0.04 (–0.01, 0.09) | 0.11 | 0.11 (–0.02, 0.23) | 0.09 |
| Birth weight** | ||||
| Normal birth weight | 1 [Reference] | NA | 1 [Reference] | NA |
| Low birth weight | 0.08 (0.03, 0.12) | 0.001 | –0.02 (–0.16, 0.13) | 0.83 |
| Type of sepsis†† | ||||
| Late onset sepsis | 1 [Reference] | NA | 1 [Reference] | NA |
| Early onset sepsis | 0.0005 (–0.07, 0.07) | 0.99 | 0.05 (–0.05, 0.15) | 0.35 |
| Both early and late onset sepsis | 0.05 (–0.006, 0.10) | 0.08 | 0.04 (–0.04, 0.12) | 0.33 |
| Gross national income | ||||
| High-income | 1 [Reference] | NA | 1 [Reference] | NA |
| Upper-middle-income | 0.05 (0.03, 0.07) | <0.001 | 0.12 (0.04, 0.21) | 0.003 |
| Lower-middle-income | 0.15 (0.11, 0.19) | <0.001 | 0.14 (0.06, 0.22) | 0.001 |
| Low-income | 0.20 (0.08, 0.31) | 0.001 | 0.04 (–0.13, 0.21) | 0.65 |
| Age < 28 days | –0.06 (–0.08, –0.05) | <0.001 | –0.06 (–0.15, 0.03) | 0.17 |
| Culture-proven sepsis | 0.07 (0.04, 0.09) | <0.001 | 0.08 (–0.04, 0.20) | 0.20 |
Meta regression predicting Case Fatality Rates among infants less than 90 days.
§Term is defined as 37 weeks or more, preterm is defined as less than 37 weeks.
**Normal birthweight is defined as ≥ 2500 g, low birthweight is defined as < 2500 g.
††Early onset sepsis is defined as sepsis onset < 72 h.
Heterogeneity among studies were high with I2 ranging 91–99%. This was persistent even with our sensitivity analyses that included only studies with culture-proven sepsis, and only studies with low risk of bias.
Reporting Biases
Five (33%) of the 15 RCTs and 179 (80%) of the 225 observational studies were assigned low risk of bias respectively (Supplementary Tables 3, 4). RCTs that were assigned moderate or high risk of bias were mainly due to the lack of blinding of participants or outcome assessors. Observational studies that were assigned moderate or high risk of bias were mainly due to either undefined or poorly defined clinical sepsis criteria, lack of a sufficient duration of follow up for assessing mortality or lack of adequate follow up rate.
Discussion
In this systematic review and meta-analysis, we provide an update to previously published literature on the global burden of young infant sepsis. To the best of our knowledge, this is the largest systematic review and meta-analysis of young infant sepsis CFRs, comprising 240 studies from 77 countries over six continents, which are not limited to population-based studies. Of the 240 studies included, 132 (55%) studies from 36 countries were conducted in middle and low-income countries, compared to 22 studies from 11 countries that were included in the prior systematic review by Fleischmann et al. (9). Our data bridge the knowledge gap on young infant sepsis particularly from low- and middle-income countries. This is also the first study that reflects global young infant sepsis CFRs over time stratified by GNI – with a decreasing trend in high-income countries but a worrying increasing trend in low-income countries, providing new insights into global young infant sepsis CFR trajectories.
We found a widening disparity in young infant sepsis outcomes between countries of different GNI. Our study revealed that Africa with a majority of low-income countries bore the highest burden of young infant sepsis with a CFR of 24% compared to Europe and Australia with a CFR burden of 16 and 14% respectively. The WHO Global Report on the Epidemiology and Burden of Sepsis 2020 showed similar results where low- and middle-income countries shoulder a higher incidence and mortality burden of young infant sepsis, particularly in Sub-Saharan Africa and South East Asia (1). Fleischmann-Struzek et al. also reported that young infant sepsis mortality rates were two times higher in middle-income countries than high-income countries (10). Young infants from lower middle- and low-income countries often present with comorbidities such as malnutrition and dehydration that can increase the risk of severe sepsis and death (264). Furthermore, unrecognized or untreated perinatal infections increase the risk of young infant sepsis (264). Limited access to high-quality healthcare results in delays to diagnosis and treatment of young infant sepsis (265–267). This includes the lack of adequately equipped and staffed healthcare institutions (19), and insufficient subsidized healthcare services for the poor (268).
There is currently limited epidemiological data on infant sepsis, especially in low-middle- and low-income countries (9, 10). The WHO has repeatedly called for a global effort to generate sepsis epidemiological data, especially in low-income countries (1). This requires early and accurate diagnosis of sepsis, as well as an improved coordination between like-minded groups doing research in these countries. Systems-based changes to strengthen each country’s health system should target the achievement of universal health coverage (269). This will ensure the availability of essential health-care services that are safe, effective and affordable to all in low-income countries (270). With a global commitment to robust infant sepsis surveillance, universal health coverage and greater resources channeled to low-income countries, we can work toward reducing young infant CFRs globally, especially in low-income countries.
Our overall CFR of 18% for young infants with sepsis was consistent with prior reports (11–29%) (9, 10). Of potential concern is that our time trend analysis of young infant sepsis CFRs showed an overall increasing trend over time from 1998 to 2018. In contrast, the Global Burden of Disease study 2019 showed a decreasing trend of –11.5% (95% CI, –23.6% to –4.3%) change in deaths due to neonatal sepsis from 2009 to 2019 (271). The limited number of studies from middle- and low-income countries represented in the earlier time period in our meta-analysis could have resulted in falsely low global young infant CFRs. Notably, all studies with a reference year prior to 2002 (Figure 5) were conducted in high-income countries. In addition, a global shift in focus to better understand young infant sepsis epidemiology in recent years could have resulted in an increased reporting of young infant sepsis mortality in lower-middle- and low-income countries (1, 272), contributing to the increasing trend of young infant CFRs in the lower-middle- and low-income countries, and also the overall trend.
Our study highlighted several factors associated with higher CFRs in young infant sepsis, namely prematurity, LBW, age less than 28 days, early onset sepsis and hospital acquired infections and sepsis in middle- and low-income countries. Previous literature similarly demonstrated prematurity and LBW to be major risk factors of young infant sepsis (273). In addition, neonates aged less than 28 days reported higher CFRs. This could be attributed to a relatively immature immune system resulting in neonates being more susceptible to infections (274). Studies have also shown that early onset sepsis disproportionately affects preterm infants who are already at a higher risk for mortality, and can result in fulminant, multisystemic infections (275). Infants who develop hospital acquired infections often have lower birth weights, lower gestational age and greater comorbidities compared to community acquired infections (37, 276). This translates to lower functional reserves and decreased immunity defenses, resulting in infants who develop hospital acquired infections having more severe infections and poorer outcomes.
Our study focused on the association between geographical gross income status and CFRs. Nonetheless, we recognize the impact of social determinants of health on young infant sepsis CFRs. A previous study reported that patients of lower socioeconomic status and those who paid out-of-pocket (as compared to privately insured patients) experienced a greater risk of young infant sepsis mortality (39). In another study, it was reported that young infants born to mothers with lower education experienced higher young infant sepsis death rate (101). Young infants of non-White ethnicity have been reported to have higher odds ratio of young infant sepsis mortality as compared to infants of White ethnicity (109). Currently, few studies detail the impact of specific social determinants of health on young infant sepsis incidence and CFRs. Further research in this area may be helpful in informing strategies regarding resource distribution and utilization.
Our attempt to provide a global scope on young infant sepsis with an inclusion criteria that accepted a wide range of study populations and sepsis definition may have led to considerable heterogeneity (1). This is likely to be contributed by the differing underlying parameters used in each study – such as differences in study design, study period, geographical region as well as the predominant organisms. Previous studies have shown that meta-analyses addressing broad review questions may result in highly heterogenous studies (277). We generated pooled CFRs using the random effects model which accounts for total study variation including between-study variations. Nevertheless, we recognize wide variation between study populations, social determinants of health, resource availability and outcome measures used, so much so that the high heterogeneity limited precise answers on sepsis mortality among young infants (277).
Limitations
Firstly, due to the lack of a gold standard for diagnosis of young infant sepsis, we accepted a wide range of sepsis definitions but categorized them into clinical, laboratory or culture-proven (18). Future studies utilizing a standardized sepsis definition that can be widely applied to all regions will facilitate comparative studies. Secondly, there was considerable heterogeneity in our meta-analysis, ranging from 91 to 99%. A sensitivity analysis including only studies with low risk of bias revealed similar results. We attributed this to variability in study populations, availability and delivery of health services, and outcome measures used. Thirdly, we excluded non-English studies which may have resulted in us missing useful data. We also excluded any studies with sample size less than 50 to reduce small study effects (19), which may have resulted in relevant studies being excluded. Finally, a lack of studies from the low- and middle-income countries in the earlier time period resulted in limited interpretation of the overall trend of young infant sepsis.
Conclusion
Our review showed an overall global burden of young infant sepsis CFR of 18%, with an increasing disparity between low- and middle-income countries compared to high-income countries over time. Factors associated with higher CFRs included prematurity, LBW, age less than 28 days, early onset sepsis, hospital acquired infections and sepsis in middle- and low-income countries, of which sepsis in middle-income countries was an independent predictor of higher CFRs. The findings from our study serve as a global call to action to achieve further reductions in young infant sepsis mortality. Future initiatives should focus on improving the delivery of care to infants at higher risk of sepsis, especially in the low- and middle-income countries.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
MG, WL, BY, and S-LC coordinated the study. MG, BY, JP, BT, and S-LC developed the search strategy and registered the protocol. MG, WL, BY, SS, and BT reviewed the studies and extracted data from the studies. WL and SS conducted risk of bias assessments. RG conducted the statistical analyses. MG, WL, RG, CH, JL, and S-LC performed the data interpretation. MG and WL wrote the original draft of the manuscript with revision from BY and SS. BY, SS, RG, JP, BT, CH, JL, and S-LC helped to revise the manuscript. All authors read and approved the final manuscript.
Acknowledgments
We would like to thank Ms. Toh Kim Kee, senior librarian from National University of Singapore, for tirelessly helping us with the search strategy.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.890767/full#supplementary-material
References
1.
WHO.Global Report on the Epidemiology and Burden of Sepsis: Current Evidence, Identifying Gaps and Future Directions.Geneva: WHO (2020).
2.
RanjevaSLWarfBCSchiffSJ. Economic burden of neonatal sepsis in sub-Saharan Africa.BMJ Glob Health. (2018) 3:e000347. 10.1136/bmjgh-2017-000347
3.
UN-IGME, UNICEF.Levels & Trends in Child Mortality: Report 2019, Estimates Developed by the United Nations Inter-Agency Group for Child Mortality Estimation.New York, NY: United Nations Children’s Fund (2019).
4.
WHO.Sustainable Development Goals (SDGs). (2020). Available online at:https://www.who.int/health-topics/sustainable-development-goals#tab=tab_2 (accessed October 18, 2020).
5.
HugLAlexanderMYouDAlkemaL.National, regional, and global levels and trends in neonatal mortality between 1990 and 2017, with scenario-based projections to 2030: a systematic analysis.Lancet Glob Health. (2019) 7:e710–20. 10.1016/S2214-109X(19)30163-9
6.
SavioliKRouseCSusiAGormanGHisle-GormanE.Suspected or known neonatal sepsis and neurodevelopmental delay by 5 years.J Perinatol. (2018) 38:1573–80. 10.1038/s41372-018-0217-5
7.
CaiSThompsonDKAndersonPJYangJY-M.Short-and long-term neurodevelopmental outcomes of very preterm infants with neonatal sepsis: a systematic review and meta-analysis.Children. (2019) 6:131.
8.
AlshaikhBYusufKSauveR.Neurodevelopmental outcomes of very low birth weight infants with neonatal sepsis: systematic review and meta-analysis.J Perinatol. (2013) 33:558–64. 10.1038/jp.2012.167
9.
FleischmannCReichertFCassiniAHornerRHarderTMarkwartRet alGlobal incidence and mortality of neonatal sepsis: a systematic review and meta-analysis.Arch Dis Child. (2021) 106:745–52. 10.1136/archdischild-2020-320217
10.
Fleischmann-StruzekCGoldfarbDMSchlattmannPSchlapbachLJReinhartKKissoonN.The global burden of paediatric and neonatal sepsis: a systematic review.Lancet Respir Med. (2018) 6:223–30. 10.1016/S2213-2600(18)30063-8
11.
PageMJMcKenzieJEBossuytPMBoutronIHoffmannTCMulrowCDet alThe PRISMA 2020 statement: an updated guideline for reporting systematic reviews.BMJ. (2021) 372:n71.
12.
PekJHGanMYYapBJSeethorSTTGreenbergRGHornikCPVet alContemporary trends in global mortality of sepsis among young infants less than 90 days old: protocol for a systematic review and meta-analysis.BMJ Open. (2020) 10:e038815. 10.1136/bmjopen-2020-038815
13.
U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER).General Clinical Pharmacology Considerations for Neonatal Studies for Drugs and Biological Products Guidance for Industry. (2019). Available online at:https://www.fda.gov/media/129532/download (accessed May 2021).
14.
BizzarroMJShabanovaVBaltimoreRSDembryL-MEhrenkranzRAGallagherPG.Neonatal sepsis 2004-2013: the rise and fall of coagulase-negative staphylococci.J Pediatr. (2015) 166:1193–9. 10.1016/j.jpeds.2015.02.009
15.
GoldsteinBGiroirBRandolphA.International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics.Pediatr Crit Care Med. (2005) 6:2–8.
16.
ShaneALSánchezPJStollBJ.Neonatal sepsis.Lancet. (2017) 390:1770–80.
17.
SatarMOzlüF.Neonatal sepsis: a continuing disease burden.Turk J Pediatr. (2012) 54:449–57.
18.
CoopersmithCMDe BackerDDeutschmanCSFerrerRLatIMachadoFRet alSurviving sepsis campaign: research priorities for sepsis and septic shock.Intensive Care Med. (2018) 44:1400–26.
19.
TanBWongJJSultanaRKohJJitMMokYHet alGlobal case-fatality rates in pediatric severe sepsis and septic shock: a systematic review and meta-analysis.JAMA Pediatr. (2019) 173:352–62. 10.1001/jamapediatrics.2018.4839
20.
The World Bank.World Bank Country and Lending Groups. (2020). Available online at:https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups (accessed October 18, 2020).
21.
SterneJASavovićJPageMJElbersRGBlencoweNSBoutronIet alRoB 2: a revised tool for assessing risk of bias in randomised trials.BMJ. (2019) 366:l4898. 10.1136/bmj.l4898
22.
GA WellsBSO’ConnellDPetersonJWelchVLososMTugwellP.The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. (2021) Available online at:http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed September 12, 2021).
23.
SterneJBradburnMEggerM.Meta-Analysis in Stata: Systematic Reviews in Health Care.London: BMJ Publishing Group (2008).
24.
ZonnenbergIAvan Dijk-LokkartEMvan den DungenFAMVermeulenRJvan WeissenbruchMM.Neurodevelopmental outcome at 2 years of age in preterm infants with late-onset sepsis.Eur J Pediatr. (2019) 178:673–80. 10.1007/s00431-019-03339-2
25.
SinghTBarnesEHIsaacsDAustralian Study Group for Neonatal Infections.Early-onset neonatal infections in Australia and New Zealand, 2002-2012.Arch Dis Child Fetal Neonatal Ed. (2019) 104:F248–52. 10.1136/archdischild-2017-314671
26.
PruittCMNeumanMIShahSSShabanovaVWollCWangMEet alFactors associated with adverse outcomes among febrile young infants with invasive bacterial infections.J Pediatr. (2019) 204:177–82.e1. 10.1016/j.jpeds.2018.08.066
27.
HamdyRFDonaDJacobsMBGerberJS.Risk Factors for complications in children with staphylococcus aureus bacteremia.J Pediatr. (2019) 208:214–20.e2. 10.1016/j.jpeds.2018.12.002
28.
GlikmanDCurielNGlatman-FreedmanAMeggedOYoungsterIMaromRet alNationwide epidemiology of early-onset sepsis in Israel 2010-2015, time to re-evaluate empiric treatment.Acta Paediatr. (2019) 108:2192–8. 10.1111/apa.14889
29.
BerardiASpadaCReggianiMLBCretiRBaroniLCaprettiMGet alGroup B Streptococcus early-onset disease and observation of well-appearing newborns.PLoS One. (2019) 14:e0212784. 10.1371/journal.pone.0212784
30.
Al-MataryAHeenaHAlSarheedASOudaWAlShahraniDAWaniTAet alCharacteristics of neonatal sepsis at a tertiary care hospital in Saudi Arabia.J Infect Public Health. (2019) 12:666–72. 10.1016/j.jiph.2019.03.007
31.
Al LuhidanLMadaniAAlbanyanEAAl SaifSNasefMAlJohaniSet alNeonatal group B streptococcal infection in a tertiary care hospital in Saudi Arabia: a 13-year experience.Pediatr Infect Dis J. (2019) 38:731–4. 10.1097/INF.0000000000002269
32.
AbdellatifMAl-KhaboriMRahmanAUKhanAAAl-FarsiAAliK.Outcome of late-onset neonatal sepsis at a tertiary hospital in Oman.Oman Med J. (2019) 34:302–7. 10.5001/omj.2019.60
33.
TingJYRobertsASynnesACanningRBodaniJMonterossaLet alInvasive fungal infections in neonates in Canada: epidemiology and outcomes.Pediatr Infect Dis J. (2018) 37:1154–9. 10.1097/INF.0000000000001968
34.
KimJKChangYSSungSAhnSYParkWS.Trends in the incidence and associated factors of late-onset sepsis associated with improved survival in extremely preterm infants born at 23-26 weeks’ gestation: a retrospective study.BMC Pediatr. (2018) 18:172. 10.1186/s12887-018-1130-y
35.
HsuJFLaiMYLeeCWChuSMWuIHHuangHRet alComparison of the incidence, clinical features and outcomes of invasive candidiasis in children and neonates.BMC Infect Dis. (2018) 18:194. 10.1186/s12879-018-3100-2
36.
GlikmanDDaganRBarkaiGAverbuchDGuriAGivon-LaviNet alDynamics of severe and non-severe invasive pneumococcal disease in young children in israel following PCV7/PCV13 introduction.Pediatr Infect Dis J. (2018) 37:1048–53. 10.1097/INF.0000000000002100
37.
GiannoniEAgyemanPKAStockerMPosfay-BarbeKMHeiningerUSpycherBDet alNeonatal sepsis of early onset, and hospital-acquired and community-acquired late onset: a prospective population-based cohort study.J Pediatr. (2018) 201:106–14.e4. 10.1016/j.jpeds.2018.05.048
38.
CanteyJBAndersonKRKalagiriRRMallettLH.Morbidity and mortality of coagulase-negative staphylococcal sepsis in very-low-birth-weight infants.World J Pediatr. (2018) 14:269–73. 10.1007/s12519-018-0145-7
39.
BohanonFJNunez LopezOAdhikariDMehtaHBRojas-KhalilYBowen-JallowKAet alRace, income and insurance status affect neonatal sepsis mortality and healthcare resource utilization.Pediatr Infect Dis J. (2018) 37:e178–84. 10.1097/INF.0000000000001846
40.
BenedictKRoyMKabbaniSAndersonEJFarleyMMHarbSet alNeonatal and pediatric candidemia: results from population-based active laboratory surveillance in four US locations, 2009-2015.J Pediatr Infect Dis Soc. (2018) 7:e78–85. 10.1093/jpids/piy009
41.
Al-MouqdadMMAljobairFAlaklobiFATahaMYAbdelrahimAAsfourSS.The consequences of prolonged duration of antibiotics in premature infants with suspected sepsis in a large tertiary referral hospital: a retrospective cohort study.Int J Pediatr Adolesc Med. (2018) 5:110–5. 10.1016/j.ijpam.2018.08.003
42.
WuIHTsaiMHLaiMYHsuLFChiangMCLienRet alIncidence, clinical features, and implications on outcomes of neonatal late-onset sepsis with concurrent infectious focus.BMC Infect Dis. (2017) 17:465. 10.1186/s12879-017-2574-7
43.
ReeIMCFustolo-GunninkSFBekkerVFijnvandraatKJSteggerdaSJLoprioreE.Thrombocytopenia in neonatal sepsis: incidence, severity and risk factors.PLoS One. (2017) 12:e0185581. 10.1371/journal.pone.0185581
44.
PieningBCGeffersCGastmeierPSchwabF.Pathogen-specific mortality in very low birth weight infants with primary bloodstream infection.PLoS One. (2017) 12:e0180134. 10.1371/journal.pone.0180134
45.
Mendoza-PalomarNBalasch-CarullaMGonzalez-Di LauroSCespedesMCAndreuAFrickMAet alEscherichia coli early-onset sepsis: trends over two decades.Eur J Pediatr. (2017) 176:1227–34. 10.1007/s00431-017-2975-z
46.
MaticsTJSanchez-PintoLN.Adaptation and validation of a pediatric sequential organ failure assessment score and evaluation of the sepsis-3 definitions in critically Ill children.JAMA Pediatr. (2017) 171:e172352. 10.1001/jamapediatrics.2017.2352
47.
KuzniewiczMWPuopoloKMFischerAWalshEMLiSNewmanTBet alRisk-based approach to the management of neonatal early-onset sepsis.JAMA Pediatr. (2017) 171:365–71.
48.
HakanssonSLiljaMJacobssonBKallenK.Reduced incidence of neonatal early-onset group B streptococcal infection after promulgation of guidelines for risk-based intrapartum antibiotic prophylaxis in Sweden: analysis of a national population-based cohort.Acta Obstet Gynecol Scand. (2017) 96:1475–83. 10.1111/aogs.13211
49.
GowdaHNortonRWhiteAKandasamyY.Late-onset neonatal sepsis-a 10-year review from north Queensland, Australia.Pediatr Infect Dis J. (2017) 36:883–8. 10.1097/INF.0000000000001568
50.
DragesetMFjalstadJWMortensenSKlingenbergC.Management of early-onset neonatal sepsis differs in the north and south of Scandinavia.Acta Paediatr. (2017) 106:375–81. 10.1111/apa.13698
51.
ChenILChiuNCChiHHsuCHChangJHHuangDTet alChanging of bloodstream infections in a medical center neonatal intensive care unit.J Microbiol Immunol Infect. (2017) 50:514–20. 10.1016/j.jmii.2015.08.023
52.
CarrDBarnesEHGordonAIsaacsD.Effect of antibiotic use on antimicrobial antibiotic resistance and late-onset neonatal infections over 25 years in an Australian tertiary neonatal unit.Arch Dis Child Fetal Neonatal Ed. (2017) 102:F244–50. 10.1136/archdischild-2016-310905
53.
BlanchardACFortinELaferriereCGoyerIMoussaAAutmizguineJet alComparative effectiveness of linezolid versus vancomycin as definitive antibiotic therapy for heterogeneously resistant vancomycin-intermediate coagulase-negative staphylococcal central-line-associated bloodstream infections in a neonatal intensive care unit.J Antimicrob Chemother. (2017) 72:1812–7. 10.1093/jac/dkx059
54.
AgyemanPKASchlapbachLJGiannoniEStockerMPosfay-BarbeKMHeiningerUet alEpidemiology of blood culture-proven bacterial sepsis in children in Switzerland: a population-based cohort study.Lancet Child Adolesc Health. (2017) 1:124–33. 10.1016/S2352-4642(17)30010-X
55.
VerstraeteEHMahieuLDe CoenKVogelaersDBlotS.Impact of healthcare-associated sepsis on mortality in critically ill infants.Eur J Pediatr. (2016) 175:943–52. 10.1007/s00431-016-2726-6
56.
TsaiMHLeeCWChuSMLeeITLienRHuangHRet alInfectious complications and morbidities after neonatal bloodstream infections: an observational cohort study.Medicine (Baltimore). (2016) 95:e3078.
57.
SchragSJFarleyMMPetitSReingoldAWestonEJPondoTet alEpidemiology of invasive early-onset neonatal sepsis, 2005 to 2014.Pediatrics. (2016) 138:e20162013. 10.1542/peds.2016-2013
58.
PugniLRonchiABizzarriBConsonniDPietrasantaCGhirardiBet alExchange transfusion in the treatment of neonatal septic shock: a ten-year experience in a neonatal intensive care unit.Int J Mol Sci. (2016) 17:695. 10.3390/ijms17050695
59.
LauterbachRWilkBBochenskaAHurkalaJRadziszewskaR.Nonactivated protein C in the treatment of neonatal sepsis: a retrospective analysis of outcome.Pediatr Infect Dis J. (2016) 35:967–71. 10.1097/INF.0000000000001247
60.
IvadyBKeneseiEToth-HeynPKerteszGTarkanyiKKassaCet alFactors influencing antimicrobial resistance and outcome of Gram-negative bloodstream infections in children.Infection. (2016) 44:309–21. 10.1007/s15010-015-0857-8
61.
FjalstadJWStensvoldHJBergsengHSimonsenGSSalvesenBRonnestadAEet alEarly-onset sepsis and antibiotic exposure in term infants: a nationwide population-based study in Norway.Pediatr Infect Dis J. (2016) 35:1–6. 10.1097/INF.0000000000000906
62.
DeshpandePJainAShahPS.Outcomes associated with early removal versus retention of peripherally inserted central catheters after diagnosis of catheter-associated infections in neonates.J Matern Fetal Neonatal Med. (2016) 29:4082–7. 10.3109/14767058.2016.1157578
63.
BulkowsteinSBen-ShimolSGivon-LaviNMelamedRShanyEGreenbergD.Comparison of early onset sepsis and community-acquired late onset sepsis in infants less than 3 months of age.BMC Pediatr. (2016) 16:82. 10.1186/s12887-016-0618-6
64.
BerardiABaroniLBacchi ReggianiMLAmbrettiSBiasucciGBolognesiSet alThe burden of early-onset sepsis in Emilia-Romagna (Italy): a 4-year, population-based study.J Matern Fetal Neonatal Med. (2016) 29:3126–31. 10.3109/14767058.2015.1114093
65.
Ben SaidMHaysSBonfilsMJourdesERasigadeJPLaurentFet alLate-onset sepsis due to Staphylococcus capitis ‘neonatalis’ in low-birthweight infants: a new entity?J Hosp Infect. (2016) 94:95–8. 10.1016/j.jhin.2016.06.008
66.
YenMHHuangYCChenMCLiuCCChiuNCLienRet alEffect of intravenous immunoglobulin for neonates with severe enteroviral infections with emphasis on the timing of administration.J Clin Virol. (2015) 64:92–6. 10.1016/j.jcv.2015.01.013
67.
WeiHMHsuYLLinHCHsiehTHYenTYLinHCet alMultidrug-resistant Acinetobacter baumannii infection among neonates in a neonatal intensive care unit at a medical center in central Taiwan.J Microbiol Immunol Infect. (2015) 48:531–9. 10.1016/j.jmii.2014.08.025
68.
TsaiMHChuSMHsuJFLienRHuangHRChiangMCet alBreakthrough bacteremia in the neonatal intensive care unit: incidence, risk factors, and attributable mortality.Am J Infect Control. (2015) 43:20–5. 10.1016/j.ajic.2014.09.022
69.
ShahJJefferiesALYoonEWLeeSKShahPSCanadian NeonatalN.Risk factors and outcomes of late-onset bacterial sepsis in preterm neonates born at < 32 weeks’.Gestation. Am J Perinatol. (2015) 32:675–82. 10.1055/s-0034-1393936
70.
SchwabFZibellRPieningBGeffersCGastmeierP.Mortality due to bloodstream infections and necrotizing enterocolitis in very low birth weight infants.Pediatr Infect Dis J. (2015) 34:235–40. 10.1097/INF.0000000000000532
71.
SchlapbachLJStraneyLAlexanderJMacLarenGFestaMSchiblerAet alMortality related to invasive infections, sepsis, and septic shock in critically ill children in Australia and New Zealand, 2002–13: a multicentre retrospective cohort study.Lancet Infect Dis. (2015) 15:46–54. 10.1016/S1473-3099(14)71003-5
72.
MitsiakosGPanaZ-DChatziioannidisIPiltsouliDLazaridouEKoulouridaVet alPlatelet mass predicts intracranial hemorrhage in neonates with gram-negative sepsis.J Pediatr Hematol Oncol. (2015) 37:519–23. 10.1097/MPH.0000000000000367
73.
LeeSMChangMKimKS.Blood culture proven early onset sepsis and late onset sepsis in very-low-birth-weight infants in Korea.J Korean Med Sci. (2015) 30(Suppl. 1):S67–74. 10.3346/jkms.2015.30.S1.S67
74.
LaiMYTsaiMHLeeCWChiangMCLienRFuRHet alCharacteristics of neonates with culture-proven bloodstream infection who have low levels of C-reactive protein (<==10 mg/L).BMC Infect Dis. (2015) 15:320. 10.1186/s12879-015-1069-7
75.
HsuJFChuSMLeeCWYangPHLienRChiangMCet alIncidence, clinical characteristics and attributable mortality of persistent bloodstream infection in the neonatal intensive care unit.PLoS One. (2015) 10:e0124567. 10.1371/journal.pone.0124567
76.
Cobos-CarrascosaESoler-PalacínPNieves LarrosaMBartoloméRMartín-NaldaAAntoinette FrickMet alStaphylococcus aureus bacteremia in children: changes during eighteen years.Pediatr Infect Dis J. (2015) 34:1329–34. 10.1097/INF.0000000000000907
77.
BerginSPThadenJTEricsonJECrossHMessinaJClarkRHet alLeadership, neonatal Escherichia coli bloodstream infections: clinical outcomes and impact of initial antibiotic therapy.Pediatr Infect Dis J. (2015) 34:933–6. 10.1097/INF.0000000000000769
78.
ChmielarczykAPobiegaMWójkowska-MachJRomaniszynDHeczkoPBBulandaM.Bloodstream infections due to Enterobacteriaceae among neonates in poland – molecular analysis of the isolates (Chmielarczyk) (2015).pdf.Polish J Microbiol. (2015) 64:217–25.
79.
VerstraeteEBoelensJDe CoenKClaeysGVogelaersDVanhaesebrouckPet alHealthcare-associated bloodstream infections in a neonatal intensive care unit over a 20-year period (1992-2011): trends in incidence, pathogens, and mortality.Infect Control Hosp Epidemiol. (2014) 35:511–8. 10.1086/675836
80.
TsaiMHChuSMHsuJFLienRHuangHRChiangMCet alRisk factors and outcomes for multidrug-resistant gram-negative bacteremia in the NICU.Pediatrics. (2014) 133:e322–9. 10.1542/peds.2013-1248
81.
TsaiM-HHsuJ-FChuS-MLienRHuangH-RChiangM-Cet alIncidence, clinical characteristics and risk factors for adverse outcome in neonates with late-onset sepsis.Pediatr Infect Dis J. (2014) 33:e7–13. 10.1097/INF.0b013e3182a72ee0
82.
OeserCVergnanoSNaidooRAnthonyMChangJChowPet alNeonatal invasive fungal infection in England 2004–2010.Clin Microbiol Infect. (2014) 20:936–41. 10.1111/1469-0691.12578
83.
NatarajanGMondayLScheerTLulic-BoticaM.Timely empiric antimicrobials are associated with faster microbiologic clearance in preterm neonates with late-onset bloodstream infections.Acta Paediatr. (2014) 103:e418–23. 10.1111/apa.12734
84.
MittPMetsvahtTAdamsonVTellingKNaaberPLutsarIet alFive-year prospective surveillance of nosocomial bloodstream infections in an Estonian paediatric intensive care unit.J Hosp Infect. (2014) 86:95–9. 10.1016/j.jhin.2013.11.002
85.
LutsarIChazallonCCarducciFITrafojerUAbdelkaderBde CabreVMet alCurrent management of late onset neonatal bacterial sepsis in five European countries.Eur J Pediatr. (2014) 173:997–1004. 10.1007/s00431-014-2279-5
86.
LevitOBhandariVLiFYShabanovaVGallagherPGBizzarroMJ.Clinical and laboratory factors that predict death in very low birth weight infants presenting with late-onset sepsis.Pediatr Infect Dis J. (2014) 33:143–6. 10.1097/INF.0000000000000024
87.
DolapoODhanireddyRTalatiAJ.Trends of Staphylococcus aureus bloodstream infections in a neonatal intensive care unit from 2000-2009.BMC Pediatr. (2014) 14:121. 10.1186/1471-2431-14-121
88.
ChuSMHsuJFLeeCWLienRHuangHRChiangMCet alNeurological complications after neonatal bacteremia: the clinical characteristics, risk factors, and outcomes.PLoS One. (2014) 9:e105294. 10.1371/journal.pone.0105294
89.
ChenJJinLYangT.Clinical study of RDW and prognosis in sepsis new borns.Biomed Res. (2014) 25:576–79.
90.
BizzarroMJEhrenkranzRAGallagherPG.Concurrent bloodstream infections in infants with necrotizing enterocolitis.J Pediatr. (2014) 164:61–6. 10.1016/j.jpeds.2013.09.020
91.
BambergerEEBen-ShimolSRayaBAKatzAGivon-LaviNDaganRet alPediatric invasive Haemophilus influenzae infections in Israel in the era of Haemophilus influenzae type b vaccine: a nationwide prospective study.Pediatr Infect Dis J. (2014) 33:477–81. 10.1097/INF.0000000000000193
92.
BalamuthFWeissSLNeumanMIScottHBradyPWPaulRet alPediatric severe sepsis in U.S. children’s hospitals.Pediatr Crit Care Med. (2014) 15:798–805. 10.1097/PCC.0000000000000225
93.
WynnJLHansenNIDasACottenCMGoldbergRNSanchezPJet alEarly sepsis does not increase the risk of late sepsis in very low birth weight neonates.J Pediatr. (2013) 162:942–8.e1–3. 10.1016/j.jpeds.2012.11.027
94.
PrietoCLColomerBFSastreJB.Prognostic factors of mortality in very low-birth-weight infants with neonatal sepsis of nosocomial origin.Am J Perinatol. (2013) 30:353–8. 10.1055/s-0032-1324701
95.
LuthanderJBennetRGiskeCGNilssonAErikssonM.Age and risk factors influence the microbial aetiology of bloodstream infection in children.Acta Paediatr. (2013) 102:182–6. 10.1111/apa.12077
96.
HammoudMSAl-TaiarAFouadMRainaAKhanZ.Persistent candidemia in neonatal care units: risk factors and clinical significance.Int J Infect Dis. (2013) 17:e624–8. 10.1016/j.ijid.2012.11.020
97.
FairchildKDSchelonkaRLKaufmanDACarloWAKattwinkelJPorcelliPJet alSepticemia mortality reduction in neonates in a heart rate characteristics monitoring trial.Pediatr Res. (2013) 74:570–5. 10.1038/pr.2013.136
98.
ErgazZBenensonSCohenMJBraunsteinRBar-OzB.No change in antibiotic susceptibility patterns in the neonatal ICU over two decades.Pediatr Crit Care Med. (2013) 14:164–70. 10.1097/PCC.0b013e31824fbc19
99.
de HaanTRBeckersLde JongeRCSpanjaardLvan ToledoLPajkrtDet alNeonatal gram negative and Candida sepsis survival and neurodevelopmental outcome at the corrected age of 24 months.PLoS One. (2013) 8:e59214. 10.1371/journal.pone.0059214
100.
AhmedALutfiSAl-HailMAl-SaadiM.Antibiotic susceptibility patterns of microbial isolates from blood culture in the neonatal intensive care unit of Hamad Medical Corporation (HMC), Doha, Qatar.Asian J Pharm Clin Res. (2013) 6(Suppl. 2):191–5.
101.
dams-ChapmanIABannCMDasAGoldbergRNStollBJWalshMCet alNeurodevelopmental outcome of extremely low birth weight infants with Candida infection.J Pediatr. (2013) 163:961–7.e3.
102.
WongJDowKShahPSAndrewsWLeeS.Percutaneously placed central venous catheter-related sepsis in Canadian neonatal intensive care units.Am J Perinatol. (2012) 29:629–34. 10.1055/s-0032-1311978
103.
VillaJAlbaCBarradoLSanzFDel CastilloEGViedmaEet alLong-term evolution of multiple outbreaks of Serratia marcescens bacteremia in a neonatal intensive care unit.Pediatr Infect Dis J. (2012) 31:1298–300. 10.1097/INF.0b013e318267f441
104.
TsaiMHHsuJFLienRHuangHRChiangCCChuSMet alCatheter management in neonates with bloodstream infection and a percutaneously inserted central venous catheter in situ: removal or not?Am J Infect Control. (2012) 40:59–64. 10.1016/j.ajic.2011.04.051
105.
SoodBGShankaranSSchelonkaRLSahaSBenjaminDKJrSanchezPJet alCytokine profiles of preterm neonates with fungal and bacterial sepsis.Pediatr Res. (2012) 72:212–20. 10.1038/pr.2012.56
106.
ShaneALHansenNIStollBJBellEFSánchezPJShankaranSet alMethicillin-resistant and susceptible Staphylococcus aureus bacteremia and meningitis in preterm infants.Pediatrics. (2012) 129:e914–22. 10.1542/peds.2011-0966
107.
MoriokaIMorikawaSMiwaAMinamiHYoshiiKKugoMet alCulture-proven neonatal sepsis in Japanese neonatal care units in 2006-2008.Neonatology. (2012) 102:75–80. 10.1159/000337833
108.
LivorsiDJMacneilJRCohnACBaretaJZanskySPetitSet alInvasive Haemophilus influenzae in the United States, 1999-2008: epidemiology and outcomes.J Infect. (2012) 65:496–504.
109.
HornikCPFortPClarkRHWattKBenjaminDKSmithPBet alEarly and late onset sepsis in very-low-birth-weight infants from a large group of neonatal intensive care units.Early Hum Dev. (2012) 88:S69–74. 10.1016/S0378-3782(12)70019-1
110.
HammoudMSAl-TaiarAThalibLAl-SweihNPathanSIsaacsD.Incidence, aetiology and resistance of late-onset neonatal sepsis: a five-year prospective study.J Paediatr Child Health. (2012) 48:604–9. 10.1111/j.1440-1754.2012.02432.x
111.
Grisaru-SoenGFriedmanTDollbergSMishaliHCarmeliY.Late-onset bloodstream infections in preterm infants: a 2-year survey.Pediatr Int. (2012) 54:748–53. 10.1111/j.1442-200X.2012.03679.x
112.
AhmedALutfiSPvRAHailMRahmanSKassimW.Incidence of bacterial isolates from blood culture in the neonatal intensive care unit of tertiary care hospital.Int J Drug Dev Res. (2012) 4:359–67.
113.
WestonEJPondoTLewisMMMartell-ClearyPMorinCJewellBet alThe burden of invasive early-onset neonatal sepsis in the United States, 2005-2008.Pediatr Infect Dis J. (2011) 30:937–41. 10.1097/INF.0b013e318223bad2
114.
VergnanoSMensonESmithZKenneaNEmbletonNClarkePet alCharacteristics of invasive Staphylococcus aureus in United Kingdom neonatal units.Pediatr Infect Dis J. (2011) 30:850–4.
115.
van den BroekPVerkadeHJHulzebosCV.Hyperbilirubinemia in infants with gram-negative sepsis does not affect mortality.Early Hum Dev. (2011) 87:515–9.
116.
StollBJHansenNISánchezPJFaixRGPoindexterBBMeursK.P. Vanet alEarly onset neonatal sepsis: the burden of group B Streptococcal and E. coli disease continues.Pediatrics. (2011) 127:817–26.
117.
SchlapbachLJAebischerMAdamsMNatalucciGBonhoefferJLatzinPet alImpact of sepsis on neurodevelopmental outcome in a swiss national cohort of extremely premature infants.Pediatrics. (2011) 128:e348–57. 10.1542/peds.2010-3338
118.
Al-TaiarAHammoudMSThalibLIsaacsD.Pattern and etiology of culture-proven early-onset neonatal sepsis: a five-year prospective study.Int J Infect Dis. (2011) 15:e631–4. 10.1016/j.ijid.2011.05.004
119.
MetsvahtTIlmojaMLParmUMaipuuLMerilaMLutsarI.Comparison of ampicillin plus gentamicin vs. penicillin plus gentamicin in empiric treatment of neonates at risk of early onset sepsis.Acta Paediatr. (2010) 99:665–72. 10.1111/j.1651-2227.2010.01687.x
120.
KlingerGLevyISirotaLBoykoVLerner-GevaLReichmanBet alOutcome of early-onset sepsis in a national cohort of very low birth weight infants.Pediatrics. (2010) 125:e736–40. 10.1542/peds.2009-2017
121.
GuilbertJLevyCCohenRBacterial Meningitis groupDelacourtCRenolleauSet alLate and ultra late onset Streptococcus B meningitis: clinical and bacteriological data over 6 years in France.Acta Paediatr. (2010) 99:47–51. 10.1111/j.1651-2227.2009.01510.x
122.
AuritiCPrencipeGIngleseRAzzariCRonchettiMPTozziAet alRole of mannose-binding lectin in nosocomial sepsis in critically ill neonates.Hum Immunol. (2010) 71:1084–8. 10.1016/j.humimm.2010.08.012
123.
VarljenTRakicOSekulovicGJekicBMaksimovicNJanevskiMRet alAssociation between tumor necrosis factor-alpha promoter -308 G/A polymorphism and early onset sepsis in preterm infants.Tohoku J Exp Med. (2019) 247:259–64. 10.1620/tjem.247.259
124.
MacooieAAFakhraieSHAfjehSAKazemianMKolahiAA.Comparing the values of serum high density lipoprotein (Hdl) level in neonatal sepsis.J Evol Med Dent Sci. (2019) 8:1772–6. 10.1097/INF.0000000000002682
125.
LealYAAlvarez-NemegyeiJLavadores-MayAIGiron-CarrilloJLCedillo-RiveraRVelazquezJR.Cytokine profile as diagnostic and prognostic factor in neonatal sepsis.J Matern Fetal Neonatal Med. (2019) 32:2830–6. 10.1080/14767058.2018.1449828
126.
FreitasFTMAraujoAMeloMISRomeroGAS.Late-onset sepsis and mortality among neonates in a Brazilian intensive care unit: a cohort study and survival analysis.Epidemiol Infect. (2019) 147:e208. 10.1017/S095026881900092X
127.
CelikIHArifogluIArslanZAksuGBasAYDemirelN.The value of delta neutrophil index in neonatal sepsis diagnosis, follow-up and mortality prediction.Early Hum Dev. (2019) 131:6–9. 10.1016/j.earlhumdev.2019.02.003
128.
YusefDShalakhtiTAwadSAlgharaibehHKhasawnehW.Clinical characteristics and epidemiology of sepsis in the neonatal intensive care unit in the era of multi-drug resistant organisms: a retrospective review.Pediatr Neonatol. (2018) 59:35–41. 10.1016/j.pedneo.2017.06.001
129.
ThomasRWadulaJSeetharamSVelaphiS.Prevalence, antimicrobial susceptibility profiles and case fatality rates of Acinetobacter baumannii sepsis in a neonatal unit.J Infect Dev Ctries. (2018) 12:211–9. 10.3855/jidc.9543
130.
TarakcıN.Neonatal septicemia in tertiary hospitals in Konya, Turkey.J Clin Anal Med. (2018) 9:187–91.
131.
StranieriIKanunfreKARodriguesJCYamamotoLNadafMIVPalmeiraPet alAssessment and comparison of bacterial load levels determined by quantitative amplifications in blood culture-positive and negative neonatal sepsis.Rev Inst Med Trop Sao Paulo. (2018) 60:e61. 10.1590/S1678-9946201860061
132.
Quintano NeiraRAHamacherSJapiassuAM.Epidemiology of sepsis in Brazil: incidence, lethality, costs, and other indicators for Brazilian unified health system hospitalizations from 2006 to 2015.PLoS One. (2018) 13:e0195873. 10.1371/journal.pone.0195873
133.
OguzSOncelMHalilHCakirUSerkantUOkurNet alCan endocan predict late-onset neonatal sepsis?J Pediatr Infect Dis. (2018) 14:096–102. 10.3855/jidc.11660
134.
EscalanteMJCeriani-CernadasJMD’ApremontIBancalariAWebbVGenesLet alLate onset sepsis in very low birth weight infants in the South American NEOCOSUR network.Pediatr Infect Dis J. (2018) 37:1022–7. 10.1097/INF.0000000000001958
135.
SofticITahirovicHDi CiommoVAuritiC.Bacterial sepsis in neonates: single centre study in a Neonatal intensive care unit in Bosnia and Herzegovina.Acta Med Acad. (2017) 46:7–15. 10.5644/ama2006-124.181
136.
SiavashiVAsadianSTaheri-AslMKeshavarzSZamani-AhmadmahmudiMNassiriSM.Endothelial progenitor cell mobilization in preterm infants with sepsis is associated with improved survival.J Cell Biochem. (2017) 118:3299–307. 10.1002/jcb.25981
137.
OlugbuyiOSwabyKRobertsPChristieCKissoonN.Burden of paediatric sepsis in a tertiary centre from a developing country.West Indian Med J. (2018) 67:137–42.
138.
FuJDingYWeiBWangLXuSQinPet alEpidemiology of Candida albicans and non-C.albicans of neonatal candidemia at a tertiary care hospital in western China.BMC Infect Dis. (2017) 17:329. 10.1186/s12879-017-2423-8
139.
CaganEKiray BasEAskerHS.Use of colistin in a neonatal intensive care unit: a cohort study of 65 patients.Med Sci Monit. (2017) 23:548–54. 10.12659/msm.898213
140.
ZhouBLiuXWuJBJinBZhangYY.Clinical and microbiological profile of babies born with risk of neonatal sepsis.Exp Ther Med. (2016) 12:3621–5. 10.3892/etm.2016.3836
141.
TurhanEEGursoyTOvaliF.Factors which affect mortality in neonatal sepsis.Turk Pediatri Ars. (2015) 50:170–5. 10.5152/TurkPediatriArs.2015.2627
142.
MosayebiZMovahedianAHEbrahimB.Epidemiologic features of early onset sepsis in neonatal ward of Shabih Khani hospital in Kashan.Iran J Neonatol. (2015) 5:19–23.
143.
DramowskiAMadideABekkerA.Neonatal nosocomial bloodstream infections at a referral hospital in a middle-income country: burden, pathogens, antimicrobial resistance and mortality.Paediatr Int Child Health. (2015) 35:265–72. 10.1179/2046905515Y.0000000029
144.
DramowskiACottonMFRabieHWhitelawA.Trends in paediatric bloodstream infections at a South African referral hospital.BMC Pediatr. (2015) 15:33. 10.1186/s12887-015-0354-3
145.
CutlandCLSchragSJThigpenMCVelaphiSCWadulaJAdrianPVet alIncreased risk for group B Streptococcus sepsis in young infants exposed to HIV, Soweto, South Africa, 2004-2008(1).Emerg Infect Dis. (2015) 21:638–45.
146.
Arizaga-BallesterosVAlcorta-GarciaMRLazaro-MartinezLCAmezquita-GomezJMAlanis-CajeroJMVillelaLet alCan sTREM-1 predict septic shock & death in late-onset neonatal sepsis? A pilot study.Int J Infect Dis. (2015) 30:27–32.
147.
ThatrimontrichaiAChanvitanPJanjindamaiWDissaneevateSJefferiesAShahV.Trends in neonatal sepsis in a neonatal intensive care unit in Thailand before and after construction of a new facility.Asian Biomed. (2014) 8:771–8.
148.
OzkanHCetinkayaMKoksalNCelebiSHacimustafaogluM.Culture-proven neonatal sepsis in preterm infants in a neonatal intensive care unit over a 7 year period: coagulase-negative Staphylococcus as the predominant pathogen.Pediatr Int. (2014) 56:60–6. 10.1111/ped.12218
149.
MorkelGBekkerAMaraisBJKirstenGvan WykJDramowskiA.Bloodstream infections and antimicrobial resistance patterns in a South African neonatal intensive care unit.Paediatr Int Child Health. (2014) 34:108–14. 10.1179/2046905513Y.0000000082
150.
HentgesCRSilveiraRCProcianoyRSCarvalhoCGFilipouskiGRFuentefriaRNet alAssociation of late-onset neonatal sepsis with late neurodevelopment in the first two years of life of preterm infants with very low birth weight.J Pediatr (Rio J). (2014) 90:50–7. 10.1016/j.jped.2013.10.002
151.
de Souza RugoloLMBentlinMRMussi-PinhataMde AlmeidaMFLopesJMMarbaSTet alLate-onset sepsis in very low birth weight infants: a Brazilian neonatal research network study.J Trop Pediatr. (2014) 60:415–21. 10.1093/tropej/fmu038
152.
AkdagADilmenUHaqueKDilliDErdeveOGoekmenT.Role of pentoxifylline and/or IgM-enriched intravenous immunoglobulin in the management of neonatal sepsis.Am J Perinatol. (2014) 31:905–12. 10.1055/s-0033-1363771
153.
TurnerCTurnerPHoogenboomGAye Mya TheinNMcGreadyRPhakaudomKet alA three year descriptive study of early onset neonatal sepsis in a refugee population on the Thailand Myanmar border.BMC Infect Dis. (2013) 13:601. 10.1186/1471-2334-13-601
154.
BallotDEBosmanNNanaTRamdinTCooperPA.Background changing patterns of neonatal fungal sepsis in a developing country.J Trop Pediatr. (2013) 59:460–4. 10.1093/tropej/fmt053
155.
Al-TalibHAl-KhateebAAl-KhalidiR.Neonatal septicemia in neonatal intensive care units: epidemiological and microbiological analysis of causative organisms and antimicrobial susceptibility.Int Med J. (2013) 20:36–40.
156.
SchragSJCutlandCLZellERKuwandaLBuchmannEJVelaphiSCet alRisk factors for neonatal sepsis and perinatal death among infants enrolled in the prevention of perinatal sepsis trial, Soweto, South Africa.Pediatr Infect Dis J. (2012) 31:821–6. 10.1097/INF.0b013e31825c4b5a
157.
LealYAAlvarez-NemegyeiJVelazquezJRRosado-QuiabUDiego-RodriguezNPaz-BaezaEet alRisk factors and prognosis for neonatal sepsis in southeastern Mexico: analysis of a four-year historic cohort follow-up.BMC Pregnancy Childbirth. (2012) 12:48. 10.1186/1471-2393-12-48
158.
FreitasBAPelosoMManellaLDFranceschini SdoCLongoGZGomesAPet alLate-onset sepsis in preterm children in a neonatal intensive care unit: a three-year analysis.Rev Bras Ter Intensiva. (2012) 24:79–85.
159.
BallotDENanaTSriruttanCCooperPA.Bacterial bloodstream infections in neonates in a developing country.ISRN Pediatr. (2012) 2012:508512. 10.5402/2012/508512
160.
AfsharpaimanSTorkamanMSaburiAFarzaampurAAmirsalariSKavehmaneshZ.Trends in incidence of neonatal sepsis and antibiotic susceptibility of causative agents in two neonatal intensive care units in tehran, I.R iran.J Clin Neonatol. (2012) 1:124–30. 10.4103/2249-4847.101692
161.
MutluYAMSayginBYilmazGBayramoðluGKöksalI.Neonatal sepsis caused by gram-negative bacteria in a neonatal intensive care unit- a six years analysis.Hong Kong J Paediatr. (2011) 16:253–7.
162.
KardanaIM.Incidence and factors associated with mortality of neonatal sepsis.Paediatr Indones. (2011) 51:144–8.
163.
AletayebSMHKhosraviADDehdashtianMKompaniFArameshMR.Identification of bacterial agents and antimicrobial susceptibility of neonatal sepsis: a 54-month study in a tertiary hospital.Afr J Microbiol Res. (2011) 5:528–31.
164.
YilmazNOAgusNHelvaciMKoseSOzerESahbudakZ.Change in pathogens causing late-onset sepsis in neonatal intensive care unit in Izmir, Turkey.Iran J Pediatr. (2010) 20:451.
165.
Turkish Neonatal Society, Nosocomial Infections Study Group.Nosocomial infections in neonatal units in Turkey: epidemiology, problems, unit policies and opinions of healthcare workers.Turk J Pediatr. (2010) 52:50–7.
166.
BasAYDemirelNZencirogluAGölNTanirG.Nosocomial blood stream infections in a neonatal intensive care unit in Ankara, Turkey.Turk J Pediatr. (2010) 52:464.
167.
FahmeySSHodeibMRefaatKMohammedW.Evaluation of myocardial function in neonatal sepsis using tissue Doppler imaging.J Matern Fetal Neonatal Med. (2019) 33:3752–6. 10.1080/14767058.2019.1583739
168.
EllahonyDMEl-MekkawyMSFaragMM.A study of red cell distribution width in neonatal sepsis.Pediatr Emerg Care. (2017) 36:378–83.
169.
TankPJOmarAMusokeR.Audit of antibiotic prescribing practices for neonatal sepsis and measurement of outcome in new born unit at Kenyatta national hospital.Int J Pediatr. (2019) 2019:7930238. 10.1155/2019/7930238
170.
MartinSLDesaiSNanavatiRColahRBGhoshKMukherjeeMB.Red cell distribution width and its association with mortality in neonatal sepsis.J Matern Fetal Neonatal Med. (2019) 32:1925–30. 10.1080/14767058.2017.1421932
171.
BhatJCharooBAshrafYQaziI.Systemic Candida infection in preterm babies: experience from a tertiary care hospital of North India.J Clin Neonatol. (2019) 8:151–4.
172.
SinghLDasSBhatVBPlakkalN.Early neurodevelopmental outcome of very low birthweight neonates with culture-positive blood stream infection: a prospective cohort study.Cureus. (2018) 10:e3492. 10.7759/cureus.3492
173.
KumarSShankarBAryaSDebMChellaniH.Healthcare associated infections in neonatal intensive care unit and its correlation with environmental surveillance.J Infect Public Health. (2018) 11:275–9. 10.1016/j.jiph.2017.08.005
174.
KhattabAAEl-MekkawyMSHelwaMAOmarES.Utility of serum resistin in the diagnosis of neonatal sepsis and prediction of disease severity in term and late preterm infants.J Perinat Med. (2018) 46:919–25. 10.1515/jpm-2018-0018
175.
KannanRRaoSSMithraPDhanashreeBBaligaSBhatKG.Diagnostic and prognostic validity of proadrenomedullin among neonates with sepsis in tertiary care hospitals of Southern India.Int J Pediatr. (2018) 2018:7908148. 10.1155/2018/7908148
176.
JajooMManchandaVChaurasiaSSankarMJGautamHAgarwalRet alAlarming rates of antimicrobial resistance and fungal sepsis in outborn neonates in North India.PLoS One. (2018) 13:e0180705. 10.1371/journal.pone.0180705
177.
FahmeySSMostafaHElhafeezNAHussainH.Diagnostic and prognostic value of proadrenomedullin in neonatal sepsis.Korean J Pediatr. (2018) 61:156–9. 10.3345/kjp.2018.61.5.156
178.
El ShimyMSEl-RaggalNMEl-FarrashRAShaabanHAMohamedHEBarakatNMet alCerebral blood flow and serum neuron-specific enolase in early-onset neonatal sepsis.Pediatr Res. (2018) 84:261–6. 10.1038/s41390-018-0062-4
179.
BanupriyaNBhatBVBenetBDCatherineCSridharMGParijaSC.Short term oral zinc supplementation among babies with neonatal sepsis for reducing mortality and improving outcome – a double-blind randomized controlled trial.Indian J Pediatr. (2018) 85:5–9. 10.1007/s12098-017-2444-8
180.
BandyopadhyayTKumarASailiARandhawaVS.Distribution, antimicrobial resistance and predictors of mortality in neonatal sepsis.J Neonatal Perinatal Med. (2018) 11:145–53. 10.3233/NPM-1765
181.
AhmadMSAhmadDMedhatNZaidiSAHFarooqHTabraizSA.Electrolyte abnormalities in neonates with probable and culture-proven sepsis and its association with neonatal mortality.J Coll Physicians Surg Pak. (2018) 28:206–9. 10.29271/jcpsp.2018.03.206
182.
ShobowaleEOSolarinAUElikwuCJOnyedibeKIAkinolaIJFaniranAA.Neonatal sepsis in a Nigerian private tertiary hospital: bacterial isolates, risk factors, and antibiotic susceptibility patterns.Ann Afr Med. (2017) 16:52–8.
183.
ShabaanAENourIElsayed EldeglaHNasefNShoumanBAbdel-HadyH.Conventional versus prolonged infusion of meropenem in neonates with gram-negative late-onset sepsis: a randomized controlled trial.Pediatr Infect Dis J. (2017) 36:358–63.
184.
SealeACObieroCWJonesKDBarsosioHCThitiriJNgariMet alShould first-line empiric treatment strategies for neonates cover coagulase-negative staphylococcal infections in Kenya?Pediatr Infect Dis J. (2017) 36:1073–8. 10.1097/INF.0000000000001699
185.
ChohanMNDassDTalrejaSAhmedN.Bacterial resistance in neonates with sepsis, not responding to first line antibiotics.Med Forum Mon. (2017) 28:30–3.
186.
AhmadMSMemoodRWahidAMehboobNAliNNasirW.Prevalence of neutropenia in cases of neonatal sepsis.Pak Pediatr J. (2017) 41:116–8.
187.
ChellaniHKaurCKumarSAryaS.Acute kidney injury in neonatal sepsis: risk factors, clinical profile, and outcome.J Pediatr Infect Dis. (2017) 13:32–6. 10.4103/1319-2442.338284
188.
BanupriyaNVishnu BhatBBenetBDSridharMGParijaSC.Efficacy of zinc supplementation on serum calprotectin, inflammatory cytokines and outcome in neonatal sepsis – a randomized controlled trial.J Matern Fetal Neonatal Med. (2017) 30:1627–31. 10.1080/14767058.2016.1220524
189.
ArowosegbeAOOjoDADedekeIOShittuOBAkingbadeOA.Neonatal sepsis in a Nigerian tertiary hospital: clinical features, clinical outcome, aetiology and antibiotic susceptibility pattern.Southern Afr J Infect Dis. (2017) 32:127–31.
190.
PradhanRJainPPariaASahaASahooJSenAet alRatio of neutrophilic CD64 and monocytic HLA-DR: a novel parameter in diagnosis and prognostication of neonatal sepsis.Cytometry B Clin Cytom. (2016) 90:295–302. 10.1002/cyto.b.21244
191.
Kumar DebbarmaSMajumdarTKumar ChakrabartiSIslamN.Proportion of neonatal sepsis among study subjects with or without jaundice in a tertiary care centre of Agartala, Tripura.J Evol Med Dent Sci. (2016) 5:7141–5.
192.
KabweMTemboJChilukutuLChilufyaMNgulubeFLukwesaCet alEtiology, antibiotic resistance and risk factors for neonatal sepsis in a large referral center in Zambia.Pediatr Infect Dis J. (2016) 35:e191–8. 10.1097/INF.0000000000001154
193.
JasaniBKannanSNanavatiRGogtayNJThatteU.An audit of colistin use in neonatal sepsis from a tertiary care centre of a resource-limited country.Indian J Med Res. (2016) 144:433–9. 10.4103/0971-5916.198682
194.
Investigators of the Delhi Neonatal Infection Study (DeNIS) collaboration.Characterisation and antimicrobial resistance of sepsis pathogens in neonates born in tertiary care centres in Delhi, India: a cohort study.Lancet Glob Health. (2016) 4:e752–60. 10.1016/S2214-109X(16)30148-6
195.
ChandrasekaranASubburajuNMustafaMPutlibaiS.Profile of neonatal sepsis due to Burkholderia capacia complex.Indian Pediatr. (2016) 53:1109–10.
196.
BhatBVPrasadPRavi KumarVBHarishBNKrishnakumariKRekhaAet alSyndrome evaluation system (SES) versus blood culture (BACTEC) in the diagnosis and management of neonatal sepsis–a randomized controlled trial.Indian J Pediatr. (2016) 83:370–9. 10.1007/s12098-015-1956-3
197.
AhmadMSAliNMehboobNMehmoodRAhmadMWahidA.Temperature on admission among cases of neonatal sepsis and its association with mortality.J Pak Med Assoc. (2016) 66:1303–6.
198.
WadileRGBhateVM.Study of clinical spectrum and risk factors of neonatal candidemia.Indian J Pathol Microbiol. (2015) 58:472–4. 10.4103/0377-4929.168888
199.
TranHTDoyleLWLeeKJDangNMGrahamSM.A high burden of late-onset sepsis among newborns admitted to the largest neonatal unit in central Vietnam.J Perinatol. (2015) 35:846–51. 10.1038/jp.2015.78
200.
El-DinSRabieEMEl-SokkaryMMBassiounyMRHassanR.Epidemiology of neonatal sepsis and implicated pathogens: a study from Egypt.Biomed Res Int. (2015) 2015:509484. 10.1155/2015/509484
201.
ShabaanAENasefNShoumanBNourIMesbahAAbdel-HadyH.Pentoxifylline therapy for late-onset sepsis in preterm infants: a randomized controlled trial.Pediatr Infect Dis J. (2015) 34:e143–8. 10.1097/INF.0000000000000698
202.
MandaviDJainN.Clinico – bacteriological profile of late onset septicemia in M. Y. H. nursery.J Evol Med Dent Sci. (2015) 4:16520–4.
203.
JajooMKapoorKGargLKManchandaVMittalSK.To study the incidence and risk factors of early onset neonatal sepsis in an out born neonatal intensive care unit of India.J Clin Neonatol. (2015) 4:91–5.
204.
AundhakarCDPatilADagarJKarandeGS.Bacteriological profile of neonatal sepsis: a hospital based study of Nicu, Krishna institute of medical sciences, Karad, Maharashtra.J Evol Med Dent Sci. (2015) 04:3222–8.
205.
SheikhASajjadAHanifS.Neonatal sepsis: an evaluation of bacteriological spectrum, antibiotic susceptibilities and prognostic predictors at civil hospital, Karachi.Pak Paediatr J. (2014) 38:143–55.
206.
Saima UmarMFAJaved IqbalSMSultanMA.Diagnostic accuracy of score for neonatal acute physiology II (SNAP II) in prediction of mortality in neonates with sepsis.Pak Pediatr J. (2014) 38:139–42.
207.
RamasamySBiswalNBethouAMathaiB.Comparison of two empiric antibiotic regimen in late onset neonatal sepsis–a randomized controlled trial.J Trop Pediatr. (2014) 60:83–6. 10.1093/tropej/fmt080
208.
PatelDNimbalkarASethiAKungwaniANimbalkarS.Blood culture isolates in neonatal sepsis and their sensitivity in Anand district of India.Indian J Pediatr. (2014) 81:785–90. 10.1007/s12098-013-1314-2
209.
KumarARandhawaVSNirupamNRaiYSailiA.Risk factors for carbapenem-resistant Acinetobacter baumanii blood stream infections in a neonatal intensive care unit, Delhi, India.J Infect Dev Ctries. (2014) 8:1049–54. 10.3855/jidc.4248
210.
GoheerLKhattakS.Early onset neonatal sepsis (risk factors & clinic-bacteriological profile).Pak Pediatr J. (2014) 38:205–10.
211.
GillCJMacLeodWBPhiri-MazalaGGuerinaNGMirochnickMKnappABet alCan traditional birth attendants be trained to accurately identify septic infants, initiate antibiotics, and refer in a rural African setting?Glob Health Sci Pract. (2014) 2:318–27. 10.9745/GHSP-D-14-00045
212.
DattaSRoySChatterjeeSSahaASenBPalTet alA five-year experience of carbapenem resistance in Enterobacteriaceae causing neonatal septicaemia: predominance of NDM-1.PLoS One. (2014) 9:e112101. 10.1371/journal.pone.0112101
213.
CatalFTaymanCTonbulAAkcaHKaraSTatliMMet alMean platelet volume (MPV) may simply predict the severity of sepsis in preterm infants.Clin Lab. (2014) 60:1193–200. 10.7754/clin.lab.2013.130501
214.
AhmadMSWaheedA.Platelet counts, MPV and PDW in culture proven and probable neonatal sepsis and association of platelet counts with mortality rate.J Coll Physicians Surg Pak. (2014) 24:340–4.
215.
AdlyAAIsmailEAAndrawesNGEl-SaadanyMA.Circulating soluble triggering receptor expressed on myeloid cells-1 (sTREM-1) as diagnostic and prognostic marker in neonatal sepsis.Cytokine. (2014) 65:184–91. 10.1016/j.cyto.2013.11.004
216.
ThapaBThapaAAryalDRThapaKPunAKhanalSet alNeonatal sepsis as a major cause of morbidity in a tertiary center in Kathmandu.J Nepal Med Assoc. (2013) 52:549–56.
217.
StoesserNMooreCEPocockJMAnKPEmaryKCarterMet alPediatric bloodstream infections in Cambodia, 2007 to 2011.Pediatr Infect Dis J. (2013) 32:e272–6. 10.1097/INF.0b013e31828ba7c6
218.
ShresthaSSinghSDShresthaNShresthaRMadhupS.Comparision of clinical and laboratory parameters in culture proven and unproven early onset sepsis in NICU.Kathmandu Univ Med J. (2013) 11:310–4. 10.3126/kumj.v11i4.12528
219.
SaleemAFQamarFNShahzadHQadirMZaidiAK.Trends in antibiotic susceptibility and incidence of late-onset Klebsiella pneumoniae neonatal sepsis over a six-year period in a neonatal intensive care unit in Karachi, Pakistan.Int J Infect Dis. (2013) 17:e961–5. 10.1016/j.ijid.2013.04.007
220.
RajendraprasadMBasavarajKAntonyBPatelB.Bacterial spectrum of neonatal septicemia with their antibiogram with reference to various predisposing factors in a tertiary care hospital in Southern India.Ann Trop Med Public Health. (2013) 6:96–9.
221.
MehtaKBhattaNMajhiSShrivastavaMSinghRR.Oral zinc supplementation for reducing mortality in probable neonatal sepsis: a double blind randomized placebo controlled trial.Indian Pediatr. (2013) 50:390–3. 10.1007/s13312-013-0120-2
222.
MeharVYadavDSomaniPBhatambareGMulyeSSinghK.Neonatal sepsis in a tertiary care center in central India: microbiological profile, antimicrobial sensitivity pattern and outcome.J Neonatal Perinatal Med. (2013) 6:165–72. 10.3233/NPM-1367312
223.
KruseAYThieu Chuong doHPhuongCNDucTGraff StensballeLPragJet alNeonatal bloodstream infections in a pediatric hospital in Vietnam: a cohort study.J Trop Pediatr. (2013) 59:483–8. 10.1093/tropej/fmt056
224.
KaulVHarishRGanjooSMahajanBRainaSKKoulD.Importance of obtaining lumbar puncture in neonates with late onset septicemia a hospital based observational study from north-west India.J Clin Neonatol. (2013) 2:83–7. 10.4103/2249-4847.116407
225.
DeASRathiMRMathurMM.Mortality audit of neonatal sepsis secondary to acinetobacter.J Glob Infect Dis. (2013) 5:3–7. 10.4103/0974-777X.107165
226.
ZakariyaBPBhatBVHarishBNArun BabuTJosephNM.Risk factors and predictors of mortality in culture proven neonatal sepsis.Indian J Pediatr. (2012) 79:358–61. 10.1007/s12098-011-0584-9
227.
ZaidiAKTikmaniSSWarraichHJDarmstadtGLBhuttaZASultanaSet alCommunity-based treatment of serious bacterial infections in newborns and young infants: a randomized controlled trial assessing three antibiotic regimens.Pediatr Infect Dis J. (2012) 31:667–72. 10.1097/INF.0b013e318256f86c
228.
ViswanathanRSinghAKGhoshCDasguptaSMukherjeeSBasuS.Profile of neonatal septicaemia at a district-level sick newborn care unit.J Health Popul Nutr. (2012) 30:41. 10.3329/jhpn.v30i1.11274
229.
ViswanathanRSinghAKBasuSChatterjeeSSardarSIsaacsD.Multi-drug resistant gram negative bacilli causing early neonatal sepsis in India.Arch Dis Child Fetal Neonatal Ed. (2012) 97:F182–7. 10.1136/archdischild-2011-300097
230.
ShawCKShawPMallaTMallaKK.The clinical spectrum and outcome of neonatal sepsis in a neonatal intensive care unit at a tertiary care hospital in western Nepal: January 2000 to December 2005-a retrospective study.Eastern J Med. (2012) 17:119.
231.
RaoYKMidhaTGargAGargJDwivediGSinghNet alNeonatal septicemia in north india due to extended spectrum beta lactamase (ESBL) producing gram negative bacteria.Int J Pharma Bio Sci. (2012) 3:B282–90.
232.
MhadaTVFredrickFMateeMIMassaweA.Neonatal sepsis at Muhimbili National Hospital, Dar es Salaam, Tanzania; aetiology, antimicrobial sensitivity pattern and clinical outcome.BMC Public Health. (2012) 12:904. 10.1186/1471-2458-12-904
233.
BasuSDewanganSShuklaRCAnupurvaSKumarA.Cerebral blood flow velocity in early-onset neonatal sepsis and its clinical significance.Eur J Pediatr. (2012) 171:901–9. 10.1007/s00431-011-1643-y
234.
AhmadSKhalidR.Blood glucose levels in neonatal sepsis and probable sepsis and its association with mortality.J coll physicians Surg Pak. (2012) 22:15–8.
235.
ChiabiADjoupombMMahENguefackSMbuagbawLZafackJet alThe clinical and bacteriogical spectrum of neonatal sepsis in a tertiary hospital in Yaounde, Cameroon.Iran J Pediatr. (2011) 21:441.
236.
BhatYRBabyLP.Early onset of neonatal sepsis: analysis of the risk factors and the bacterial isolates by using the BacT alert system.J Clin Diagn Res. (2011) 5:1385–8.
237.
TalbertAWMwanikiMMwarumbaSNewtonCRBerkleyJA.Invasive bacterial infections in neonates and young infants born outside hospital admitted to a rural hospital in Kenya.Pediatr Infect Dis J. (2010) 29:945–9. 10.1097/INF.0b013e3181dfca8c
238.
SheikhAMJavedTAfzalMFSheikhGA.Course and complications of early onset nonatal sepsis: a descriptive study.Ann King Ed Med Univ. (2010) 16:307.
239.
QureshiDJaffarAJMajeedR.Risk factors for neonatal sepsis.Med Forum Monthly. (2010) 21:7–11.
240.
OgunlesiTAOgunfoworaOB.Predictors of mortality in neonatal septicemia in an underresourced setting.J Natl Med Assoc. (2010) 102:915–22. 10.1016/s0027-9684(15)30710-0
241.
MathurNBMarmuKKumarS.Systemic inflammatory response syndrome in home delivered neonates: a prospective observational study.Indian J Pediatr. (2010) 77:1109–13. 10.1007/s12098-010-0123-0
242.
KayangeNKamugishaEMwizamholyaDLJeremiahSMshanaSE.Predictors of positive blood culture and deaths among neonates with suspected neonatal sepsis in a tertiary hospital, Mwanza-Tanzania.BMC Pediatr. (2010) 10:39. 10.1186/1471-2431-10-39
243.
ShahSGadreKTagareADhanawadeS.Pattern and antimicrobial susceptibility of neonatal sepsis at a tertiary care center in Western India.J Pediatr Infect Dis. (2015) 10:76–81.
244.
GargBUmateSKabraN.Incidence of meningitis in neonates with late-onset sepsis at a tertiary care center in Western India: an observational study.J Clin Neonatol. (2014) 8:67–70.
245.
BabaAAhmadAMushtaqSBashirCIqbalQ.Thrombocytopenia and other hematological parameters in culture positive neonatal sepsis and their impact.J Pediatr Infect Dis. (2013) 8:25–9.
246.
Ben AbdeljelilJSaghrouniFNouriSGeithSKhammariIFathallahAet alNeonatal invasive candidiasis in Tunisian hospital: incidence, risk factors, distribution of species and antifungal susceptibility.Mycoses. (2012) 55:493–500. 10.1111/j.1439-0507.2012.02189.x
247.
RajniGSugandhaAGauravJKumarC.Sepsis with extended-spectrum β-lactamase producing gram-negative bacteria in neonates – risk factors, clinical profile and outcome.J Pediatr Infect Dis. (2011) 06:195–200.
248.
PanditADeodharJVaidyaUTagareAKadamS.Multidrug resistant Klebsiella pneumoniae in NICU – what next? Trend of antibiotic resistance.J Pediatr Infect Dis. (2010) 05:119–24.
249.
BundukiGKAdu-SarkodieY.Clinical outcome and isolated pathogens among neonates with sepsis in democratic republic of the Congo: a cross-sectional study.BMC Res Notes. (2019) 12:303. 10.1186/s13104-019-4346-5
250.
LengletAFaniyanOHopmanJ.A nosocomial outbreak of clinical sepsis in a neonatal care unit (NCU) in Port-Au-Prince Haiti, July 2014 - September 2015.PLoS Curr. (2018) 10. 10.1371/currents.outbreaks.58723332ec0de952adefd9a9b6905932
251.
TewabeTMohammedSTilahunYMelakuBFentaMDagnawTet alClinical outcome and risk factors of neonatal sepsis among neonates in Felege Hiwot referral Hospital, Bahir Dar, Amhara Regional State, North West Ethiopia 2016: a retrospective chart review.BMC Res Notes. (2017) 10:265. 10.1186/s13104-017-2573-1
252.
MolyneuxEMDubeQBandaFMChiumeMSinginiIMallewaMet alThe treatment of possible severe infection in infants: an open randomized safety trial of parenteral benzylpenicillin and gentamicin versus ceftriaxone in infants <60 days of age in Malawi.Pediatr Infect Dis J. (2017) 36:e328–33. 10.1097/INF.0000000000001576
253.
BoulosARandKJohnsonJAGautierJKosterM.Neonatal Sepsis in Haiti.J Trop Pediatr. (2017) 63:70–3. 10.1093/tropej/fmw077
254.
Al-ShamahyHASabrahAAAl-RobasiABNaserSM. Types of bacteria associated with neonatal sepsis in Al-Thawra university hospital, Sana’a, Yemen, and their antimicrobial profile.Sultan Qaboos Univ Med J. (2012) 12:48–54. 10.12816/0003087
255.
HibberdPLHansenNIWangMEGoudarSSPashaOEsamaiFet alTrends in the incidence of possible severe bacterial infection and case fatality rates in rural communities in Sub-Saharan Africa, South Asia and Latin America, 2010-2013: a multicenter prospective cohort study.Reprod Health. (2016) 13:65. 10.1186/s12978-016-0177-1
256.
FitzgeraldJCBasuRKAkcan-ArikanAIzquierdoLMPineres OlaveBEHassingerABet alAcute kidney injury in pediatric severe sepsis: an independent risk factor for death and new disability.Crit Care Med. (2016) 44:2241–50. 10.1097/CCM.0000000000002007
257.
WeissSLFitzgeraldJCPappachanJWheelerDJaramillo-BustamanteJCSallooAet alGlobal epidemiology of pediatric severe sepsis: the sepsis prevalence, outcomes, and therapies study.Am J Respir Crit Care Med. (2015) 191:1147–57.
258.
RiveraLSaez-LlorensXFeris-IglesiasJIpMSahaSAdrianPVet alIncidence and serotype distribution of invasive group B streptococcal disease in young infants: a multi-country observational study.BMC Pediatr. (2015) 15:143. 10.1186/s12887-015-0460-2
259.
HamerDHDarmstadtGLCarlinJBZaidiAKYeboah-AntwiKSahaSKet alEtiology of bacteremia in young infants in six countries.Pediatr Infect Dis J. (2015) 34:e1–8. 10.1097/INF.0000000000000549
260.
SantolayaMEAlvaradoTQueiroz-TellesFColomboALZuritaJTiraboschiINet alActive surveillance of candidemia in children from Latin America: a key requirement for improving disease outcome.Pediatr Infect Dis J. (2018) 33:e40–4. 10.1097/INF.0000000000000039
261.
MularoniAMadridMAzpeitiaAValls i SolerA.The role of coagulase-negative staphylococci in early onset sepsis in a large European cohort of very low birth weight infants.Pediatr Infect Dis J. (2014) 33:e121–5. 10.1097/INF.0000000000000175
262.
Al-TaiarAHammoudMSCuiqingLLeeJKLuiKMNakwanNet alNeonatal infections in China, Malaysia, Hong Kong and Thailand.Arch Dis Child Fetal Neonatal Ed. (2013) 98:F249–55. 10.1136/archdischild-2012-301767
263.
Inis Collaborative GroupBrocklehurstPFarrellBKingAJuszczakEDarlowBet alTreatment of neonatal sepsis with intravenous immune globulin.N Engl J Med. (2011) 365:1201–11.
264.
PopescuCRCavanaghMMMTemboBChiumeMLufesiNGoldfarbDMet alNeonatal sepsis in low-income countries: epidemiology, diagnosis and prevention.Expert Rev Anti Infect Ther. (2020) 18:443–52. 10.1080/14787210.2020.1732818
265.
WatersDJawadIAhmadALukšićINairHZgagaLet alAetiology of community-acquired neonatal sepsis in low and middle income countries.J Glob Health. (2011) 1:154–70.
266.
EdmondKZaidiA.New approaches to preventing, diagnosing, and treating neonatal sepsis.PLoS Med. (2010) 7:e1000213. 10.1371/journal.pmed.1000213
267.
Zea-VeraAOchoaTJ.Challenges in the diagnosis and management of neonatal sepsis.J Trop Pediatr. (2015) 61:1–13. 10.1093/tropej/fmu079
268.
OrachCG.Health equity: challenges in low income countries.Afr Health Sci. (2009) 9(Suppl. 2):S49–51.
269.
United Nations.Universal Health Coverage. (2019). Available online at:https://www.un.org/pga/73/event/universal-health-coverage/#:~:text=As%20part%20of%20the%202030,and%20affordable%20essential%20medicines%20and (accessed November 2, 2021).
270.
PearsonMColomboFMurakamiYJamesC.Universal health coverage and health outcomes in Paris. In: Final Report for the G7 Health Ministerial Meeting.Organisation for Economic Co-operation and Development (2016).
271.
GBD.GBD Cause and Risk Summaries: Neonatal Sepsis and Other Neonatal Infections – Level 4 Cause. (2019). Available online at:https://www.thelancet.com/gbd/summaries (accessed April 12, 2021)
272.
WHO.World Health Assembly Secretariat Report A70/13: Improving the Prevention, Diagnosis and Clinical Management of Sepsis.Geneva: WHO (2017).
273.
LiangLDKotadiaNEnglishLKissoonNAnserminoJMKabakyengaJet alPredictors of mortality in neonates and infants hospitalized with sepsis or serious infections in developing countries: a systematic review.Front Pediatr. (2018) 6:277. 10.3389/fped.2018.00277
274.
Vishnu BhatBManoj Kumar KingsleyS.Chapter 2 – innate immunity at birth: implications for inflammation and infection in Newborns. In: ChatterjeeSJungraithmayrWBagchiDeditors. Immunity and Inflammation in Health and Disease.London: Academic Press (2018). p. 15–35.
275.
StollBJPuopoloKMHansenNISánchezPJBellEFCarloWAet alEarly-onset neonatal sepsis 2015 to 2017, the rise of Escherichia coli, and the need for novel prevention strategies.JAMA Pediatr. (2020) 174:e200593.
276.
VergnanoSSharlandMKazembePMwansamboCHeathPT.Neonatal sepsis: an international perspective.Arch Dis Childhood Fetal Neonatal Ed. (2005) 90:F220–4.
277.
ImreyPB.Limitations of meta-analyses of studies with high heterogeneity.JAMA Netw Open. (2020) 3:e1919325. 10.1001/jamanetworkopen.2019.19325
Summary
Keywords
pediatrics, infant, mortality, infections, sepsis, global health
Citation
Gan MY, Lee WL, Yap BJ, Seethor STT, Greenberg RG, Pek JH, Tan B, Hornik CPV, Lee JH and Chong S-L (2022) Contemporary Trends in Global Mortality of Sepsis Among Young Infants Less Than 90 Days: A Systematic Review and Meta-Analysis. Front. Pediatr. 10:890767. doi: 10.3389/fped.2022.890767
Received
06 March 2022
Accepted
26 April 2022
Published
03 June 2022
Volume
10 - 2022
Edited by
Luregn J. Schlapbach, University Children’s Hospital Zurich, Switzerland
Reviewed by
James Lawrence Wynn, University of Florida, United States; Martin Stocker, Luzerner Kantonsspital, Switzerland
Updates
Copyright
© 2022 Gan, Lee, Yap, Seethor, Greenberg, Pek, Tan, Hornik, Lee and Chong.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shu-Ling Chong, Chong.Shu-Ling@kkh.com.sg
This article was submitted to Children and Health, a section of the journal Frontiers in Pediatrics
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.