- 1Division of Nephrology, Department of Pediatrics, Cohen Children’s Medical Center of New York, New Hyde Park, NY, United States
- 2Division of Critical Care, Department of Pediatrics, Cohen Children’s Medical Center of New York, New Hyde Park, NY, United States
- 3Department of Pediatrics, Zucker School of Medicine at Hofstra/Northwell, Uniondale, NY, United States
Introduction: To assess the prevalence of hyponatremia among pediatric patients with coronavirus disease 2019 (COVID-19) and Multisystem Inflammatory Syndrome in Children (MIS-C) and determine if pediatric hyponatremia was associated with an increased length of stay, higher rates of mechanical ventilation, and/or elevated inflammatory markers on admission as compared to eunatremic patients.
Methods: Electronic health records were retrospectively analyzed for 168 children less than 18 years old with COVID-19 or MIS-C who were admitted to pediatric units within the Northwell Health system. The primary exposure was hyponatremic status (serum sodium <135 mEq/L) and the primary outcomes were length of stay, mechanical ventilation usage and increased inflammatory markers.
Results: Of the 168 children in the study cohort, 95 (56%) were admitted for COVID-19 and 73 (43.5%) for MIS-C. Overall, 60 (35.7%) patients presented with hyponatremia on admission. Patients with hyponatremia had higher rates of intensive care unit admission when compared to eunatremic patients (32/60 [53.3%] vs. 39/108 [36.1%], p = 0.030). In regression models, hyponatremia was not significantly associated with increased length of stay or mechanical ventilation rates. After adjustment for relevant confounders, hyponatremia remained associated with an increased square root CRP (β = 1.79: 95% CI: 0.22–3.36) and lower albumin levels (β = −0.22: 95% CI: −0.42–−0.01).
Conclusion: Hyponatremia is common in pediatric COVID-19 and MIS-C. Hyponatremia was associated with a lower albumin and higher square root CRP levels. This may suggest an association of inflammation with lower serum sodium levels.
Introduction
As of May 2023, the United Stated reported 104 million cases of coronavirus disease 2019 (COVID-19); over four million of these cases occurred in children (1). Early on in the pandemic, the incidence of pediatric COVID-19 may have been significantly higher than reported due to the lack of widespread testing and decreased severity of disease in children as compared to adults (2–4). Only 1% of pediatric COVID-19 cases required hospitalization, however, 7.5% of these required pediatric intensive care unit admission (PICU) (5). Furthermore, the emergence of Multisystem Inflammatory Syndrome in Children (MIS-C), a serious disease associated with a recent COVID-19 infection involving inflammation of two or more organ systems, fever, severe illness, and epidemiologic evidence of SARS-CoV-2 infection, has posed increased risk to children (6–8). MIS-C is a dangerous and potentially fatal disease, with 68% of MIS-C cases requiring critical care support (9, 10). In addition to the morbidity above, in the pre-vaccine era, COVID-19 was reported to be associated with increased incidence of hyponatremia in adults (11).
Hyponatremia in critically ill adults and children has been shown to be an independent risk factor for mortality (12, 13). During the first wave of the pandemic, hyponatremia in COVID19 was found to be extremely prevelant and was associated with increased morbidity and mortality (12, 13). A prospective, observational, cohort study of adults with COVID 19 in Switzerland, found that COVID19 patients had higher incidence of hyponatremia compared to a control population of hospital patients who tested negative for SARS-CoV-2 (13). The exact etiology of hyponatremia in COVID-19 is unclear (14), however, inflammatory cytokines such as interlukein-1β and IL-6 induce secretion of arginine vasopressin which can cause the syndrome of inappropriate ADH secretion (SIADH) and thus hyponatremia. Disease severity was also correlated with sodium serum levels in adults with COVID-19 (14). Thus, hyponatremia may be a surrogate marker for disease severity or degree of inflammatory response (15). Moreover, hyponatremia was associated with a higher 30-day mortality rate, ICU admission, mechanical ventilation rates and length of stay (13).
While the associations of hyponatremia and COVID-19 is well established in adult populations, there are limited studies evaluating its prevalence and association with outcomes in pediatric patients (16, 17). In addition, while MIS-C is a new phenomenon, physicians have noted clinical similarities between MIS-C and Kawasaki disease (KD) (18). The severity of KD demonstrates strong association with hyponatremia and meta-analysis of MIS-C studies reported hyponatremia was present in 80.8% of MIS-C patients (19, 20). This meta-analysis aimed to determine the prevalence, outcomes and associated demographic and clinical variable in children with hyponatremia and COVID-19/MIS-C. We hypothesized that hyponatremia would be prevalent in pediatric patients with COVID-19 and MIS-C and would be associated with increased: length of stay (LOS), need for mechanical ventilation, and inflammatory markers at admission.
Methods
Patient population
A retrospective chart review of children less than 18 years old admitted to pediatric units within the Northwell Health system with acute COVID-19 or MIS-C was performed. Participant information was collected from hospitals within the New York metropolitan area; these included Cohen Children's Medical Center, an academic tertiary care children's hospital, as well as 3 tertiary hospitals: South Shore Hospital, Staten Island University Hospital, and Lenox Hill Hospital. Data from March 1, 2020, through February 28, 2021, was retrospectively collected using Sunrise Clinical Manager (Allscripts, Chicago, Illinois), an electronic health record system. The Institutional Review Board of Northwell Health approved this study.
Acute COVID-19 was defined as a positive polymerase chain reaction testing for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (by Northwell Health Labs) within 24 h of admission. MIS-C was defined by the Center for Disease Control and Prevention case definition: fever, significant evidence of inflammation, evidence of greater than or equal to 2 organ systems with dysfunction, and a positive test for current or recent SARS-CoV-2 infection or serological confirmation of exposure to COVID-19 within 4 weeks of symptom onset (21). Patients with hypernatremia (serum sodium >145 mEq/L on admission), pregnant patients, kidney transplant recipients, patients with kidney failure (estimated glomerular filtration rate <15 ml/min per 1.73 m2 or dialysis dependent), or those transferred from outside of the health system were excluded.
Study variables
Demographic data including age, sex, self-reported race, and ethnicity, admission vital signs, laboratory values, in-hospital drug use, and echocardiogram data were extracted from medical records. Patient comorbidities including asthma, cancer, congestive heart failure, and immunosuppressed status were also collected.
Exposure and outcomes
The primary exposure was hyponatremia, defined as a serum or plasma sodium level less than 135 mEq/L within 24 h of admission. The Katz and Hillier formulas were used to correct serum sodium levels for hyperglycemia (22, 23). Serum sodium was measured using indirect potentiometry. Primary outcomes included length of stay in the hospital, length of mechanical ventilation time and elevated inflammatory markers.
Statistical analysis
Descriptive statistics were expressed as medians with 25th−75th percentiles reported for continuous variables and as counts with frequencies (%) for categorical variables. Mann–Whitney U-tests were used to compare continuous variables while Pearson's chi2 test or Fisher's exact test was used to compare categorical variables. Due to sample size limitations, adjusted models measuring evaluating hyponatremia. Logistic regression models assessed associations of the hyponatremia, the independent variable, with mortality, length of stay and need for mechanical ventilation. Variables included within the models were determined based on a priori variables of interest and potential confounders, including age, sex, race, PICU stay and primary diagnosis (COVID-19 or MIS-C), were added as clusters in a forward stepwise manner due to sample size limitation. Linear regression models were utilized to evaluate the association of hyponatremia with length of stay and levels of inflammatory markers. Standard assumptions of Gaussian residuals and quality of variance were tested. If the standard assumptions were not met, a transformation (i.e., natural logarithm, square route) was performed. Variables included in the models were determined based on a priori variables of interest and potential confounders and added as clusters in a forward stepwise manner due to sample size limitations. Missing values were rare in the dataset and were not imputed. Results were considered statistically significant at the p < 0.05 level of significance. All analyses were performed using Stata 15.3 and JASP 0.16.
Results
Baseline results
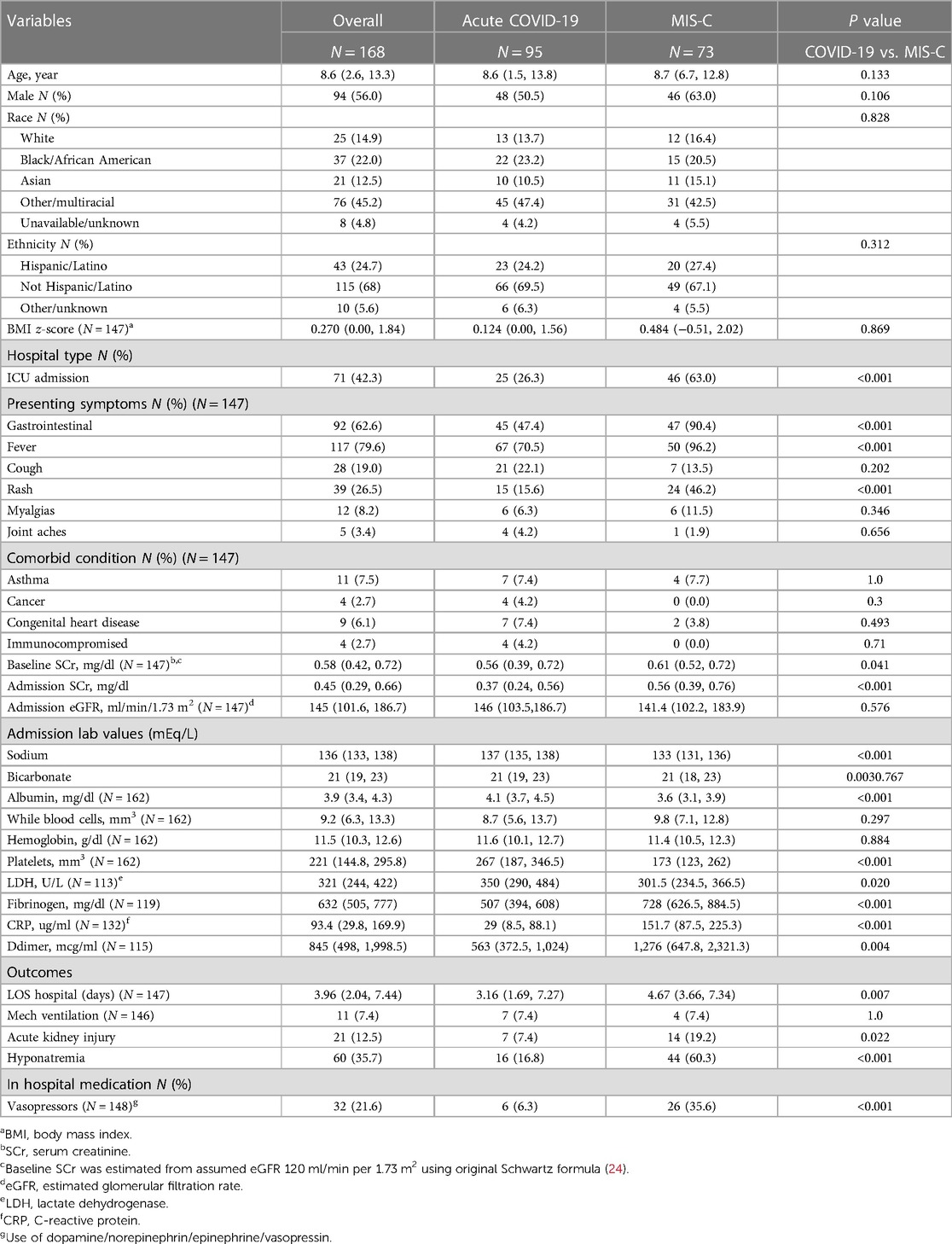
Of the 168 children in the study cohort, 95 (56%) were admitted for acute COVID-19 and 73 (43.5%) were admitted for MIS-C. Both patient populations had similar ages, gender, BMI z-scores, racial and ethnic demographics. Overall, 60 (35.7%) patients presented with hyponatremia, 95 (56%) with COVID and 73 (35.7%) with MIS-C (Table 1). Two patients re-classified from hyponatremia to eunatremia after correcting for hyperglycemia, using both the Katz and Hillier methods. No eunatremic patients were reclassified as hyponatremic. A larger proportion of MIS-C patients required intensive care; MIS-C patients also presented more often with fever, rash, and gastrointestinal symptoms as compared to patients with acute COVID-19 (p-value < 0.001). Admission sodium, potassium, calcium, and albumin were all significantly lower in the MIS-C population. Inflammatory markers such as fibrinogen, c-reactive protein (CRP), D-dimer, and treatment with vasoactive infusions were higher in the MIS-C cohort. MIS-C patients had a greater length of stay and presented with a higher prevalence of hyponatremia at admission, p = 0.007 and p < 0.001 respectively (Table 1). Two patients in the cohort died, both with acute Covid-19.
Hyponatremia and acute COVID-19
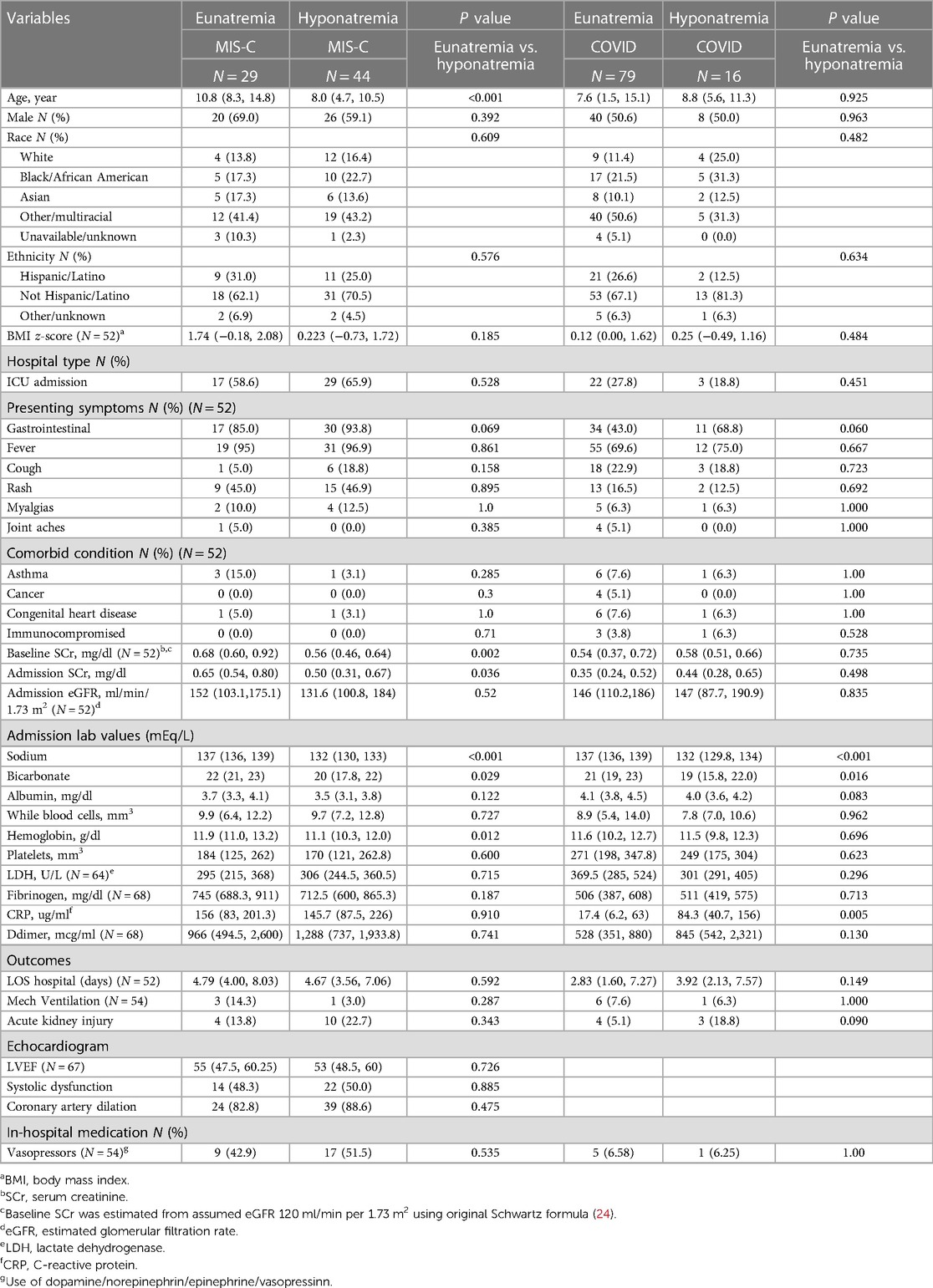
The median age of children with acute-COVID-19 was 8.6 years (IQR 1.5–13.8) and over half were male. Sixteen (16.8%) patients presented with hyponatremia [132 mEq/L (IQR 129.8–134.0)] (Table 2). There were no significant differences in age, sex, race, ethnicity, or BMI z-score between children with and without hyponatremia. Moreover, there were no significant differences between presenting symptoms, comorbid conditions, hospital vasoactive infusion use, length of stay, or mechanical ventilation between the hyponatremic and eunatremic populations. Admission bicarbonate levels in hyponatremic patients were significantly lower than median admission bicarbonate levels in eunatremic patients (19 mEq/L (15.8–22.0) vs. 21 mEq/L (19.0–23.0), p = 0.016). Admission CRP levels were elevated in the hyponatremic population [84.3 ug/ml (IQR 40.7–155.8)] when compared to eunatremic patients [17.4 ug/ml (IQR 6.2–63)], p = 0.005 (Table 2). One of the two patients who died was hyponatremic on admission (serum sodium 129 mEq/L).
Hyponatremia and MIS-C
The median age of children with MIS-C was 8.7 years (IQR 6.7–12.8) with slightly more than half being male. Forty-four (60.3%) patients with MIS-C presented with hyponatremia. MIS-C patients with hyponatremia had a median age of 8.0 (IQR 4.7, 10.5); this was significantly younger than the eunatremic population with a median age of 10.8 (IQR 8.3, 14.8), p < 0.001 (Table 2). There were no significant differences in sex, race, ethnicity, and BMI z-score between children with and without hyponatremia. There were no significant differences in presenting symptoms, comorbid conditions and in hospital vasoactive medication usage between the hyponatremic and eunatremic populations. Echocardiographic data was available and analyzed for all MIS-C patients (n = 73) (Table 2). There were no significant differences between hyponatremic and eunatremic populations for left ventricle ejection fraction (LVEF), systolic dysfunction status, or coronary artery dilation.
Hyponatremia in the overall
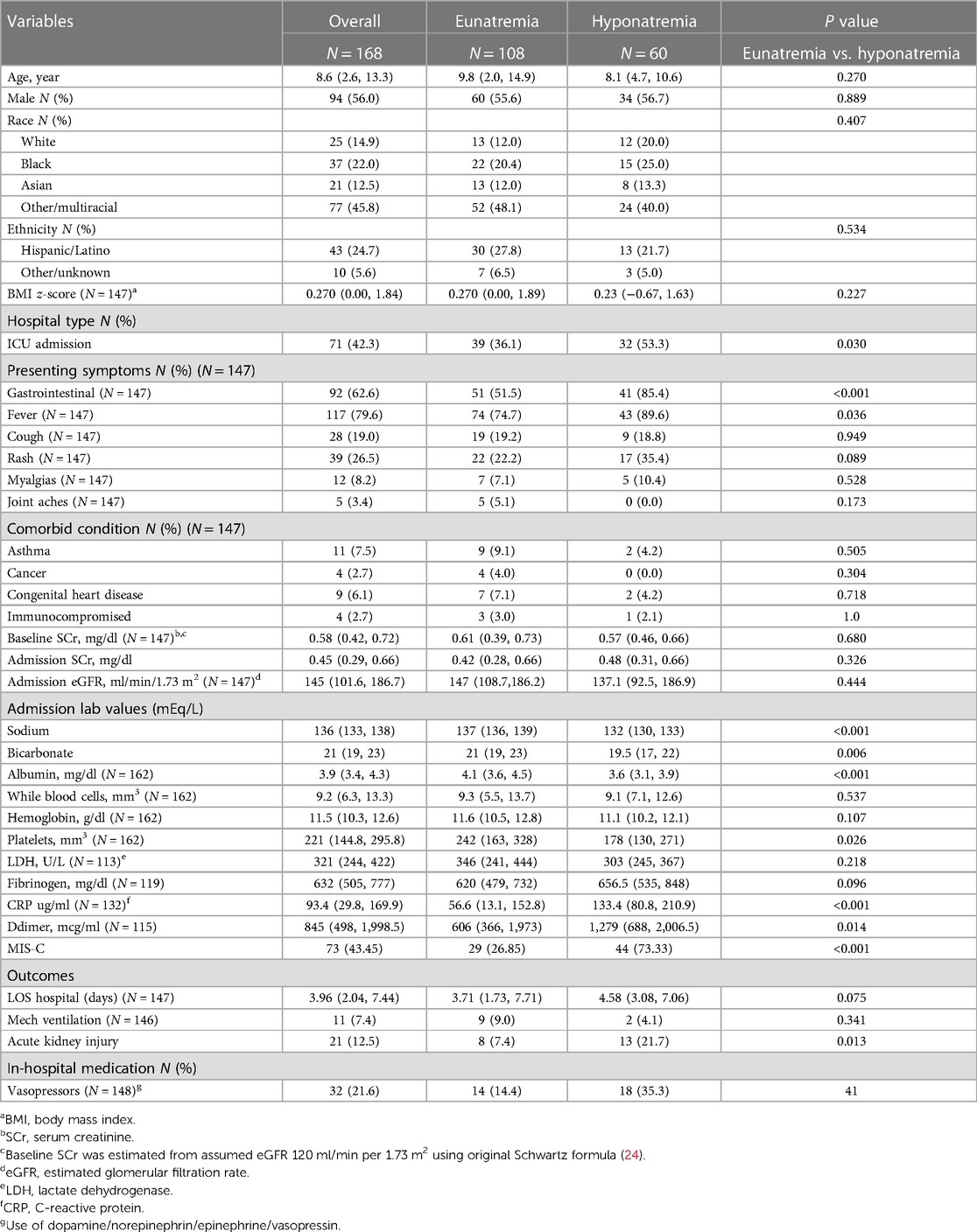
Hyponatremic patients had lower admission calcium, bicarbonate, albumin, and platelet levels as compared to eunatremic patients. However, hyponatremic patients presented with significantly higher rates of gastrointestinal symptoms (p < 0.001), fever (p = 0.036), and D-dimer levels (p = 0.014) (Table 3). Hyponatremic patients had an elevated median CRP level as compared to eunatremic patients (133.4 ug/ml (IQR 80.8, 210.9), while eunatremic patients had a median CRP level of 56.6 ug/ml (IQR 13.1, 152.8), p < 0.001 (Table 3). There were no significant differences in total length of stay or mechanical ventilation usage between the hyponatremic and eunatremic populations. Of the total cohort, 71 (42.2%) were admitted to the pediatric intensive care unit (PICU). The median age of the PICU population was (10.8 (IQR 6.4, 14.7) vs. 7.4 (IQR 1.9, 11), p = 0.002) (Supplementary Table S1). PICU patients had higher prevalence of hyponatremia, elevated admission CRP levels, and elevated D-dimer; they also had lower albumin, potassium, calcium, sodium, and platelet count (Supplementary Table S1). The elevated CRP levels and diminished albumin level of hyponatremic vs. eunatremic patients were maintained in analysis of only patients admitted to the intensive care unit (Supplementary Table S2). In addition, within the PICU population, those with hyponatremia were more likely to have gastrointestinal symptoms, in hospital vasoactive medication usage and elevated bicarbonate levels (Supplementary Table S2).
Hyponatremia and outcomes
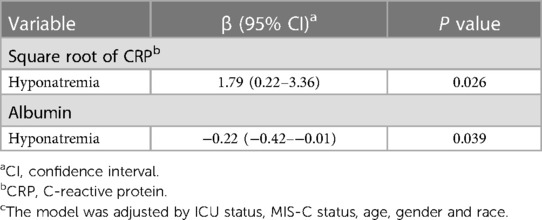
There was no association of hyponatremia with LOS or mechanical ventilation in bivariate associations or in regression models. However, in linear regression models, adjusted for age, sex, race, ICU stay and primary diagnosis (COVID-19 or MIS-C), the square root CRP was significantly increased (β = 1.79: 95% CI: 0.22–3.36) and the albumin level was 0.22 mg/dl lower (β = −0.22: 95% CI: −0.42–−0.01), as compared to those with eunatremia (Table 4, Supplementary Table S3).
Discussion
This study adds to the current literature about the epidemiology of pediatric hyponatremia in COVID19 and MIS-C and is, to our knowledge, the first to look at the associations of hyponatremia in pediatric acute COVID-19 and MIS-C. Hyponatremia was present on admission in over a third of the cohort (35.7%), 44 (60.3%) of the patients with MIS-C and 16 (16.8%) of children with acute COVID-19. Contrary to our hypothesis, hyponatremia on admission was not associated greater length of stay or need for mechanical ventilation (25). There was a significant association between hyponatremia and higher CRP and lower serum albumin levels which may suggest increased inflammation in children with hyponatremia and COVID19/MISC.
The incidence of hyponatremia in over a third (37.5%) of our cohort was not dissimilar to reported incidences of hyponatremia in adults with COVID19. Hirsch et al. evaluated a cohort of 9,943 adults with COVID-19 from Northwell Health and reported a prevalence of admission hyponatremia in over a third of hospitalizations (16). The incidence of hyponatremia was higher than that expected in a general hospital cohort. Another European study estimated the incidence of COVID19 related hyponatremia in adults as 16.8% higher as compared to control patients without COVID19 (13). While reports from the US and Europe differed, they almost all report a higher incidence of hyponatremia in COVID19 patients. Looking at the children in this study's cohort with COVID19, the incidence of hyponatremia is just 16.8% and approximates the reported prevalence of hyponatremia in hospitalized children (26). The lower incidence of hyponatremia in pediatric COVID-19 patients may be in part due to the fact that children have less severe disease manifestations as compared to adults (14).
The etiology of hyponatremia in pediatric COVID-19 may be caused by decreased effective circulating blood volume, extracellular fluid volume depletion or SIADH (27, 28). Children with hyponatremia overall had more GI issues. Hypovolemic hyponatremia is often attributed to gastrointestinal sodium loss through vomiting and diarrhea, although SAR-CoV-2 patients with acute gastroenteritis were found to have a lower risk of hyponatremia when compared to other pathogens (29, 30). In addition, patients with pain, nausea or pneumonia related to COVID-19 can develop SIADH through the non-osmotic release of arginine vasopressin induced by increased levels of cytokines and inflammation (31, 32). Children with hyponatremia had higher CRP and hypoalbuminemia, both markers for inflammation, as compared to eunatremic patients which may suggest potentiation of inflammation and in some, SIADH.
A meta-analysis of MIS-C studies reported hyponatremia rates as high as 80.8% (20). Similarly, this study found hyponatremia rates in children with MISC (60.3%) analogous to adults with severe COVID-19. This may be due to the similarity between the pathophysiology of MISC and the inflammatory response of adults with severe COVID-19 (16). Given the severity of presentation in MIS-C it is not surprising that the majority (n = 46, 63%) of our MIS-C population were admitted to the intensive care unit (9). Throughout the pandemic this remained true as the clinical severity of MIS-C was similar across Alpha, Delta and Omricon SARS-CoV-2 strains (33).
The higher rates of hyponatremia in MIS-C patients can be due to the aforementioned suggested mechanisms, of dehydration through GI loss and SIADH from the high levels of inflammation as discussed above. Inflammation as discussed above may also play a key role to be most similar to MIS-C. MIS-C shares several clinical features with both KD and Toxic Shock Syndrome, two phenomena associated with higher rates of hyponatremia (21). In a study on Kawasaki disease, elevated CRP was associated with elevated IL-6, elevated IL-1β, and subsequently rates of hyponatremia (34).
The association between COVID-19 and hyponatremia in adult populations demonstrated increased length of hospital stay, need for mechanical ventilation, and mortality in hyponatremic patients (16). Our study did not find any relationship between hyponatremia and the aforementioned outcomes in the COVID-19 or MIS-C population. Given the low mortality rates risk of mortality was not evaluated but it should be noted that we had a small sample size and thus are limited to test and measure strong associations.
The analysis suggests that hyponatremia was associated with elevated CRP levels and lower albumin levels, markers of inflammation. CRP is a non-specific protein induced by IL-6 in the liver and is a sensitive biomarker for inflammation and tissue damage (35). The pathogenesis of COVID-19 involves a potent inflammatory response, and CRP is an early predictor of severe COVID-19 and extremely elevated CRP levels may precede or reflect cytokine storm (36–38).
Similarly, in adults, hypoalbuminemia is associated with severe COVID-19 and researchers have found an inverse relationship between the levels of albumin and the risk of death in COVID-19 patients (39). While the mechanism behind hypoalbuminemia in COVID-19 is likely multifactorial and low albumin levels were seen predominately in adults with severe COVID-19 suggesting a phenomenon beyond hepatocellular dysfunction (39). Inflammation increases capillary permeability, leading to serum albumin release into interstitial space (40). Hypoalbuminemia often leads to lowered plasma oncotic pressure which leads to antidiuretic hormone release which can contribute to hyponatremia as well.
There are several limitations to this study. Due to the retrospective nature of the study, causality cannot be determined. Our sample size was small and thus conclusions should be made with caution. Moreover, serum sodium was measured using indirect potentiometry which is frequently utilized yet less accurate then direct potentiometry in pediatric populations (41–43). However, the study corrected for hyperglycemia the most common cause of pseudohyponatremia. Indirect potentiometry measurements are falsely depressed as total protein increases, as seen in our study with albumin, which could have resulted in an overdiagnosis of hyponatremia (44). Furthermore, the study lacked the data and serotyping of viral strains (alpha vs. delta variant) and thus models were not unable to adjust for strains. A strength of our study is that it represents patients from the greater New York City area at the epicenter of the COVID-19 outbreak, thus including a diverse racial, ethnic, and socioeconomic population. As noted above it took into account common confounders such as hyperglycemia and adjusted for these findings using validated equations.
In conclusion, hyponatremia occurred in over a third of children with acute COVID-19 and MIS-C. There was no association between hyponatremia and length of hospital stay or need for mechanical ventilation. Yet hyponatremia was associated with a higher serum CRP and lower serum albumin, which may reflect increased state of inflammation. Serum sodium on admission may be a useful and easily accessible marker to distinguish which children with COVID19 and MISC develop a higher immune response. Further prospective studies in larger cohorts are needed is needed to evaluate the prognostic use of admission hyponatremia, validated with serum cytokine levels in children with acute COVID-19 and MIS-C.
Data availability statement
The datasets presented in this article are not readily available because of PHI and is sensitive to the patients involved. Requests to access the datasets should be directed toYWJhc2FsZWx5QG5vcnRod2VsbC5lZHU=.
Ethics statement
The studies involving human participants were reviewed and approved by Northwell Health IRB. Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
Study conception and design: ND, CS, AB; data collection: ND Author; analysis and interpretation of results: draft manuscript preparation: ND, MP, LS, CS, LG. All authors contributed to the article and approved the submitted version.
Conflict of interest
ND is a consultant for Triangle Insights Group (TIG), a life science consulting company.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2023.1209587/full#supplementary-material
References
1. Engineering CfSSa. COVID-19 Dashboard. Available at: https://gisanddata.maps.arcgis.com/apps/dashboards/bda7594740fd40299423467b48e9ecf6 (Accessed August 8, 2021).
2. Marlais M, Wlodkowski T, Vivarelli M, Pape L, Tonshoff B, Schaefer F, et al. The severity of COVID-19 in children on immunosuppressive medication. Lancet Child Adolesc Health. (2020) 4(7):e17–8. doi: 10.1016/S2352-4642(20)30145-0
3. Bixler D, Miller AD, Mattison CP, Taylor B, Komatsu K, Pompa XP, et al. SARS-CoV-2-Associated deaths among persons aged <21 years—United States, February 12–July 31, 2020. MMWR Morb Mortal Wkly Rep. (2020) 69(37):1324–9. doi: 10.15585/mmwr.mm6937e4
4. Bi Q, Wu Y, Mei S, Ye C, Zou X, Zhang Z, et al. Epidemiology and transmission of COVID-19 in 391 cases and 1,286 of their close contacts in Shenzhen, China: a retrospective cohort study. Lancet Infect Dis. (2020) 20(8):911–9. doi: 10.1016/s1473-3099(20)30287-5
5. Zhou B, Yuan Y, Wang S, Zhang Z, Yang M, Deng X, et al. Risk profiles of severe illness in children with COVID-19: a meta-analysis of individual patients. Pediatr Res. (2021) 90:347–52. doi: 10.1038/s41390-021-01429-2
6. Simpson JM, Newburger JW. Multisystem inflammatory syndrome in children in association with COVID-19. Circulation. (2020) 142(5):437–40. doi: 10.1161/CIRCULATIONAHA.120.048726
7. Dong Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z, et al. Epidemiology of COVID-19 among children in China. Pediatrics. (2020) 145(6). doi: 10.1542/peds.2020-0702. [Epub ahead of print]32179660
8. Network CHA. Multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease 2019 (COVID-19).
9. Radia T, Williams N, Agrawal P, Harman K, Weale J, Cook J, et al. Multi-system inflammatory syndrome in children & adolescents (MIS-C): a systematic review of clinical features and presentation. Paediatr Respir Rev. (2021) 38:51–7. doi: 10.1016/j.prrv.2020.08.001
10. Godfred-Cato S, Bryant B, Leung J, Oster ME, Conklin L, Abrams J, et al. COVID-19-Associated multisystem inflammatory syndrome in children—United States, March–July 2020. MMWR Morb Mortal Wkly Rep. (2020) 69(32):1074–80. doi: 10.15585/mmwr.mm6932e2
11. Berni A, Malandrino D, Parenti G, Maggi M, Poggesi L, Hyponatremia PA. IL-6, and SARS-CoV-2 (COVID-19) infection: may all fit together? J Endocrinol Invest. (2020) 43(8):1137–9. doi: 10.1007/s40618-020-01301-w
12. Hu J, Wang Y, Geng Z, Chen R, Zhang P, Lin J, et al. Dysnatremia is an independent indicator of mortality in hospitalized patients. Med Sci Monit. (2017) 23:2408–25. doi: 10.12659/msm.902032
13. Atila C, Sailer CO, Bassetti S, Tschudin-Sutter S, Bingisser R, Siegemund M, et al. Prevalence and outcome of dysnatremia in patients with COVID-19 compared to controls. Eur J Endocrinol. (2021) 184(3):409–18. doi: 10.1530/eje-20-1374
14. Post A, Dullaart RPF, Bakker SJL. Is low sodium intake a risk factor for severe and fatal COVID-19 infection? Eur J Intern Med. (2020) 75:109. doi: 10.1016/j.ejim.2020.04.003
15. Tzoulis P, Waung JA, Bagkeris E, Hussein Z, Biddanda A, Cousins J, et al. Dysnatremia is a predictor for morbidity and mortality in hospitalized patients with COVID-19. J Clin Endocrinol Metab. (2021) 106(6):1637–48. doi: 10.1210/clinem/dgab107
16. Hirsch JS, Uppal NN, Sharma P, Khanin Y, Shah HH, Maliekal DA, et al. Prevalence and outcomes of hyponatremia and hypernatremia in patients hospitalized with COVID-19. Nephrol Dial Transplant. (2021) 36(6):1135–8. doi: 10.1093/ndt/gfab067
17. Tzoulis P. Prevalence, prognostic value, pathophysiology, and management of hyponatraemia in children and adolescents with COVID-19. Acta Biomed. (2021) 92(5):e2021474. doi: 10.23750/abm.v92i5.12330
18. Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Mary BF, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. (2020) 383(4):334–46. doi: 10.1056/NEJMoa2021680
19. Tsuji S. Significance of hyponatremia in Kawasaki disease. Pediatr Int. (2020) 62(3):307. doi: 10.1111/ped.14169
20. Jiang L, Tang K, Levin M, Irfan O, Morris SK, Wilson K, et al. COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect Dis. (2020) 20(11):e276–88. doi: 10.1016/s1473-3099(20)30651-4
21. Basalely A, Gurusingh S, Schneider J, Shah SS, Siegel LB, Pollack G, et al. Acute kidney injury in pediatric patients hospitalized with acute COVID-19 and multisystem inflammatory syndrome in children associated with COVID-19. Kidney Int. (2021) 100(1):138–45. doi: 10.1016/j.kint.2021.02.026
22. Katz MA. Hyperglycemia-induced hyponatremia–calculation of expected serum sodium depression. N Engl J Med. (1973) 289(16):843–4. doi: 10.1056/nejm197310182891607
23. Hillier TA, Abbott RD, Barrett EJ. Hyponatremia: evaluating the correction factor for hyperglycemia. Am J Med. (1999) 106(4):399–403. doi: 10.1016/s0002-9343(99)00055-8
24. Zappitelli M, Parikh CR, Akcan-Arikan A, Washburn KK, Moffett BS, Goldstein SL. Ascertainment and epidemiology of acute kidney injury varies with definition interpretation. Clin J Am Soc Nephrol. (2008) 3(4):948–54. doi: 10.2215/cjn.05431207
25. De Carvalho H, Letellier T, Karakachoff M, Desvaux G, Caillon H, Papuchon E, et al. Hyponatremia is associated with poor outcome in COVID-19. J Nephrol. (2021) 34(4):991–8. doi: 10.1007/s40620-021-01036-8
26. Park SW, Shin SM, Jeong M, Cho DH, Lee Keum Hwa KH, Eisenhut M, et al. Hyponatremia in children with respiratory infections: a cross-sectional analysis of a cohort of 3,938 patients. Sci Rep. (2018) 8(1):16494. doi: 10.1038/s41598-018-34703-1
27. Lavagno C, Milani GP, Uestuener P, Simonetti GD, Casaulta C, Bianchetti MG, et al. Hyponatremia in children with acute respiratory infections: a reappraisal. Pediatr Pulmonol. (2017) 52(7):962–7. doi: 10.1002/ppul.23671
28. Milani GP, Rocchi A, Teatini T, Bianchetti MG, Amelio G, Mirra N, et al. Hyponatremia in infants with new onset moderate-severe bronchiolitis: a cross-sectional study. Respir Med. (2017) 133:48–50. doi: 10.1016/j.rmed.2017.10.028
29. Frontera J, Valdes E, Huang J, Lewis A, Lord AS, Zhou T, et al. Prevalence and impact of hyponatremia in patients with coronavirus disease 2019 in New York City. Crit Care Med. (2020) 48(12):e1211–7. doi: 10.1097/ccm.0000000000004605
30. Milani G, Buonsenso D, Marchisio P, Agostoni C, Corso MC, Guarino A, et al. Gastroenteritis is less severe but is more often associated with systemic inflammation in SARS-CoV-2-positive than in SARS-CoV-2-negative children. Pediatr Infect Dis J. (2023). doi: 10.1097/inf.0000000000004001. [Epub ahead of print]
31. Dhawan A, Narang A, Singhi S. Hyponatraemia and the inappropriate ADH syndrome in pneumonia. Ann Trop Paediatr. (1992) 12(4):455–62. doi: 10.1080/02724936.1992.11747614
32. Ellison DH, Berl T. The syndrome of inappropriate antidiuresis. N Engl J Med. (2007) 356(20):2064–72. doi: 10.1056/NEJMcp066837
33. Laird-Gion J, Dionne A, Gauvreau K, Baker A, Day-Lewis M, de Ferranti S, et al. MIS-C across three SARS-CoV-2 variants: changes in COVID-19 testing and clinical characteristics in a cohort of U.S. children. Eur J Pediatr. (2023) 182:1–8. doi: 10.1007/s00431-023-04968-4
34. Lim GW, Lee M, Kim HS, Hong YM, Sohn S. Hyponatremia and syndrome of inappropriate antidiuretic hormone secretion in Kawasaki disease. Korean Circ J. (2010) 40(10):507–13. doi: 10.4070/kcj.2010.40.10.507
35. Liu F, Li L, Xu M, Wu J, Luo D, Zhu Y, et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol. (2020) 127:104370. doi: 10.1016/j.jcv.2020.104370
36. Tan C, Huang Y, Shi F, Tan K, Ma Q, Chen Y, et al. C-reactive protein correlates with computed tomographic findings and predicts severe COVID-19 early. J Med Virol. (2020) 92(7):856–62. doi: 10.1002/jmv.25871
37. Ji P, Zhu J, Zhong Z, Li H, Pang J, Li B, et al. Association of elevated inflammatory markers and severe COVID-19: a meta-analysis. Medicine (Baltimore). (2020) 99(47):e23315. doi: 10.1097/md.0000000000023315
38. Han H, Ma Q, Li C, Liu R, Zhao L, Wang W, et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg Microbes Infect. (2020) 9(1):1123–30. doi: 10.1080/22221751.2020.1770129
39. Huang J, Cheng A, Kumar R, Fang Y, Chen G, Zhu Y, et al. Hypoalbuminemia predicts the outcome of COVID-19 independent of age and co-morbidity. J Med Virol. (2020) 92(10):2152–8. doi: 10.1002/jmv.26003
40. Fleck A, Hawker F, Wallace PI, Raines G, Trotter J, Ledingham IM, et al. Increased vascular permeability: a major cause of hypoalbuminaemia in disease and injury. Lancet. (1985) 1(8432):781–4. doi: 10.1016/s0140-6736(85)91447-3
41. Corsello A, Malandrini S, Bianchetti MG, Agostoni C, Cantoni B, Meani F, et al. Sodium assessment in neonates, infants, and children: a systematic review. Eur J Pediatr. (2022) 181(9):3413–9. doi: 10.1007/s00431-022-04543-3
42. Malandrini S, Lava SAG, Bianchetti MG, Meani F, Fare PB, Camozzi P, et al. Which laboratory technique is used for the blood sodium analysis in clinical research? A systematic review. Clin Chem Lab Med. (2021) 59(9):1501–6. doi: 10.1515/cclm-2021-0293
43. Milani GP, Edefonti V, Santis RD, Agostoni C, Spolidoro C, Pelucchi C, et al. Disagreement between direct and indirect potentiometric Na + determination in infancy and childhood. Clin Chem Lab Med. (2020) 58(4):e117–9. doi: 10.1515/cclm-2019-0931
Keywords: COVID-19, hyponatremia, MIS-C multisystem inflammatory syndrome in children, pediatric, prevalence, outcomes
Citation: Dalal N, Pfaff M, Silver L, Glater-Welt L, Sethna C, Singer P, Castellanos-Reyes L and Basalely A (2023) The prevalence and outcomes of hyponatremia in children with COVID-19 and multisystem inflammatory syndrome in children (MIS-C). Front. Pediatr. 11:1209587. doi: 10.3389/fped.2023.1209587
Received: 20 April 2023; Accepted: 24 July 2023;
Published: 7 September 2023.
Edited by:
Orkun Tolunay, Adana Faculty of Medicine/University of Health Sciences, TürkiyeReviewed by:
Sevgin Taner, Ministry of Health, TürkiyeGregorio Paolo Milani, University of Milan, Italy
© 2023 Dalal, Pfaff, Silver, Glater-Welt, Sethna, Singer, Castellanos-Reyes and Basalely. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abby Basalely YWJhc2FsZWx5QG5vcnRod2VsbC5lZHU=
 Neal Dalal1
Neal Dalal1 Lily Glater-Welt
Lily Glater-Welt Christine Sethna
Christine Sethna Abby Basalely
Abby Basalely