- 1Department of Applied Computer Sciences, FH JOANNEUM - University of Applied Sciences, Graz, Austria
- 2Division of Pediatric Radiology, Department of Radiology, Medical University of Graz, Graz, Austria
- 3Department of Pediatric and Adolescent Surgery, Medical University of Graz, Graz, Austria
Introduction: In the field of pediatric trauma computer-aided detection (CADe) and computer-aided diagnosis (CADx) systems have emerged offering a promising avenue for improved patient care. Especially children with wrist fractures may benefit from machine learning (ML) solutions, since some of these lesions may be overlooked on conventional X-ray due to minimal compression without dislocation or mistaken for cartilaginous growth plates. In this article, we describe the development and optimization of AI algorithms for wrist fracture detection in children.
Methods: A team of IT-specialists, pediatric radiologists and pediatric surgeons used the freely available GRAZPEDWRI-DX dataset containing annotated pediatric trauma wrist radiographs of 6,091 patients, a total number of 10,643 studies (20,327 images). First, a basic object detection model, a You Only Look Once object detector of the seventh generation (YOLOv7) was trained and tested on these data. Then, team decisions were taken to adjust data preparation, image sizes used for training and testing, and configuration of the detection model. Furthermore, we investigated each of these models using an Explainable Artificial Intelligence (XAI) method called Gradient Class Activation Mapping (Grad-CAM). This method visualizes where a model directs its attention to before classifying and regressing a certain class through saliency maps.
Results: Mean average precision (mAP) improved when applying optimizations pre-processing the dataset images (maximum increases of 25.51% mAP@0.5 and 39.78% mAP@[0.5:0.95]), as well as the object detection model itself (maximum increases of 13.36% mAP@0.5 and 27.01% mAP@[0.5:0.95]). Generally, when analyzing the resulting models using XAI methods, higher scoring model variations in terms of mAP paid more attention to broader regions of the image, prioritizing detection accuracy over precision compared to the less accurate models.
Discussion: This paper supports the implementation of ML solutions for pediatric trauma care. Optimization of a large X-ray dataset and the YOLOv7 model improve the model’s ability to detect objects and provide valid diagnostic support to health care specialists. Such optimization protocols must be understood and advocated, before comparing ML performances against health care specialists.
1. Introduction
In pediatric medicine, artificial intelligence (AI) has found valuable applications to support healthcare professionals in tasks such as automating processes, retrieving information and providing decision support (1, 2). AI-based solutions may be extremely helpful, when a radiological question occurs frequently, so that considerably big data sets are available, but specific challenges still remain and require long-term experience (3). This constellation certainly applies to pediatric wrist fractures, since some of these lesions may be overlooked on conventional X-ray due to minimal compression without dislocation or mistaken for cartilaginous growth plates (4).
The plethora of terms used within the field of AI still causes difficulties or even confusion. Basically, the broad umbrella term is artificial intelligence (AI). Machine learning (ML) is one area of AI. Deep Learning (DL) is part of ML and makes use of artificial neural networks (ANN). A neural network is an AI variant that teaches computers to process data in a way that was originally inspired by the human brain and uses interconnected nodes or neurons, usually in a layered structure. Image classification regularly relies on convolutional neural networks (CNN), a special type of ANN (5). The main idea of CNNs, described well e.g. in Ch. 14 of Murphy (6), is to learn small filters, which (similar to those used in image processing) recognize certain features like horizontal or vertical edges. These features are combined, using higher-order filters on higher hierarchy levels (i.e. in later layers of the network), to identify first basic geometric structures (like rectangles, arcs and circles) and later on objects composed of these basic objects.
This holds true also for the “You Only Look Once” (YOLO) object detectors, first published in 2015/16, which has received several upgrades to newer versions (7, 8).
Regarding automated pediatric wrist fracture detection, computer-aided detection (CADe) and computer-aided diagnosis (CADx) systems based on big data sets contribute to the detection of rarities within the pool of available data (9). However, ensuring complete explainability and trustworthiness behind AI models remains a challenging endeavor as long as specific IT knowledge remains limited in healthcare professionals, both in general but also regarding the presented fracture detection problem.
Neural networks, especially deep neural networks, often operate in ways that are intricate and difficult for humans to comprehend (10). Consequently, Explainable Artificial Intelligence methods are employed to present machine learning outcomes in an understandable manner. These outcomes can take the form of visualizations, textual explanations, or examples (11, 12). A noteworthy method is Gradient-weighted class activation mapping (Grad-CAM), which adapts traditional Class Activation Mapping (CAM) in a model-agnostic manner, allowing for more than global average pooling. Additionally, Guided Grad-CAM acts as a hybrid technique, combining Grad-CAM and guided backpropagation through element-wise multiplication. This approach yields higher-resolution visualizations that are class-specific and discriminatory (11, 13).
The superordinate goal of this article, which extends and deepens the analysis presented in Till et al. (14), is to describe the development and optimization of AI algorithms for wrist fracture detection in children using a freely available dataset. It highlights the steps that lead to improved performance and the role that each of the medical and technical players must play to achieve this.
2. Materials and methods
This study assessed the influence of several variations and settings of the seventh generation YOLO (8) model (YOLOv7), a state-of-the-art object detector.
2.1. Dataset
The experiments were based on the openly available pediatric wrist trauma dataset “GRAZPEDWRI-DX” (15), published by the Division of Pediatric Radiology, Department of Radiology, Medical University of Graz in 2022. This dataset contains annotated pediatric trauma wrist radiographs of 6,091 patients, and a total number of 10,643 studies (20,327 images). All of the provided studies were included.
2.2. Model training
During training, the model learns by repeatedly making predictions, measuring the error between the prediction and the expected result, and adjusting internal parameters accordingly. Usually, some version of gradient descent is used for this, i.e. the process can be illustrated as “moving downhill” on an abstract landscape generated by the prediction error. The learning rate governs how fast this “movement” is, and often also some version of momentum is incorporated (16).
Care has to be taken not to overfit the model, i.e. not to have the model learn irrelevant details of the training data and losing its ability to generalize (17). This is usually achieved by performing a train-test-split, i.e. training the model only on some portion of the data, called the training set, and evaluating the prediction error also on the rest, called the test set or validation set. The ideal ratio of these splits vary depending on the data, with 80% typically being used for larger datasets (18). When the error decreases on the training set, but systematically increases on the validation set, this is a strong indication for the onset of overfitting (17).
When aiming for better model performance, this can be achieved, on the one hand, by enhancing the quality or amount of data fed into the network during training. In this study, we have varied image sizes and preprocessing procedures (19). On the other hand, directly altering the object detection model, like using different architectures (8) or adjusting hyperparameters during training (20), influence detection mean average precision as well. In this study, we agreed upon that the initial learning rate and momentum play a crucial role for optimizing clinical decision-making in pediatric wrist trauma care, which made them part of the analysis during experiments.
Models were trained on a Virtual Machine with 15GB RAM, 100GB hard drive storage, 4 CPU cores as well as a dedicated NVIDIA GPU. Training was performed using the code published with the original YOLOv7 paper (8) executing the scripts in the Command Line. Model training duration ranged from 12 to 24 h for all variations. However, it was agreed upon that differences in training time will not be taken into consideration for the upcoming analysis.
2.2.1. Hyperparameters
In machine learning, hyperparameters (like learning rate, influence of momentum or batch size) are different to standard model parameters, as they stay constant throughout the training process and are not contained in the final model. They greatly influence the speed of the training process and determine how the model is adjusted when new information becomes available. Thus, hyperparameter search often constitutes a major challenge in designing machine learning models. We decided to test 3 alternative learning rates and momentum settings in this manuscript (Figure 1).
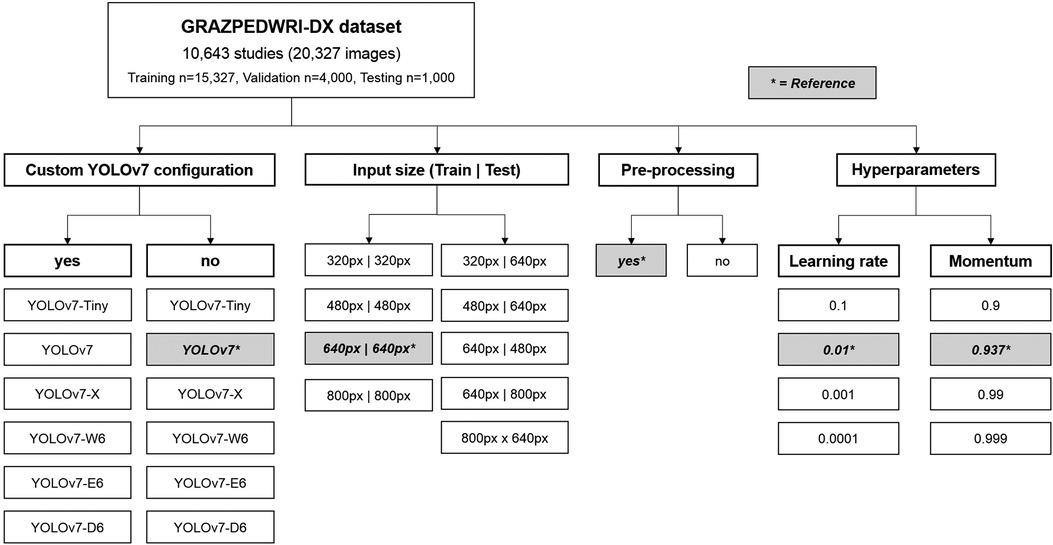
Figure 1. Flowchart depicting the variations of different YOLOv7 models, input sizes, pre-processing and hyperparameters assessed. The reference values are marked by an asterisk.
2.3. Explainable artificial intelligence
In many disciplines, including healthcare, but also for example finance, it is not sufficient for an AI model to just have a good predictive performance. For such models, it is also regarded as vitally important to be able to explain for which reasons a prediction has been made. Because of this, weaker, but transparent models (like linear scores or decision trees) are often preferred to more powerful but largely opaque models like neural networks.
For example, CNNs have achieved remarkable performance on many image classification tasks, but to explain why the network has come to a decision is no trivial task.
However, sacrificing predictive power in favor of transparency is considered as highly problematic as well, and considerable effort has been invested to even make complex models more transparent. The resulting field of Explainable Artificial Intelligence (XAI) encompasses both model-agnostic approaches, which can be used with any ML model, and approaches that are only applicable to specific ML methods.
In this article, we investigated our models using a XAI method called Gradient Class Activation Mapping (Grad-CAM) (13), ass exemplarily demonstrated in Figure 2, which is specifically designed for the analysis of image recognition processes. Through saliency maps, this method visualizes where a model directs its attention to in the process of predicting a certain class.
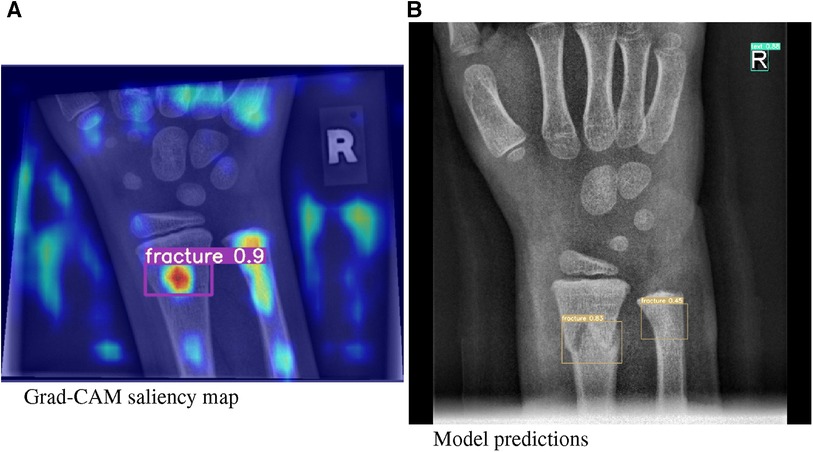
Figure 2. (A) Grad-CAM saliency maps for image 1430_0611557520_01_WRI-R1_M008.png. (B) Model predictions for image 1779_0477729910_01_WRI-R1_M004.png by means of a bounding box.
2.4. Model performance and metrics
It is crucial to analyse the performance of an AI model using adequate metrics and to provide the results in a comprehensible way. A number of different methods are available to accomplish these tasks. These have evolved over time and, depending on the application area, are now relatively standardised.
With regard to the recognition of objects, the most commonly used metrics are
• IoU (Intersection over Union). An IoU of 1 means a perfect match of the AI prediction and the ground truth. An IoU of 0 corresponds to no overlap of the labels.
• Precision: Number of true positives (TP) over sum of true positives (TP) and false positives (FP).
• Recall: Number of true positives (TP) over sum of true positives (TP) and false negatives (FN).
• Average precision (AP) is the area under a precision-recall curve for an object of interest.
• Mean average precision (mAP) is the AP over all tested classes. Typically, the mAP is calculated for an IoU threshold of 0.5 (50% mAP@[0.5]) and averaged between 0.5 and 0.95 with 5% steps (mAP@[0.5:0.95]) (21).
3. Results
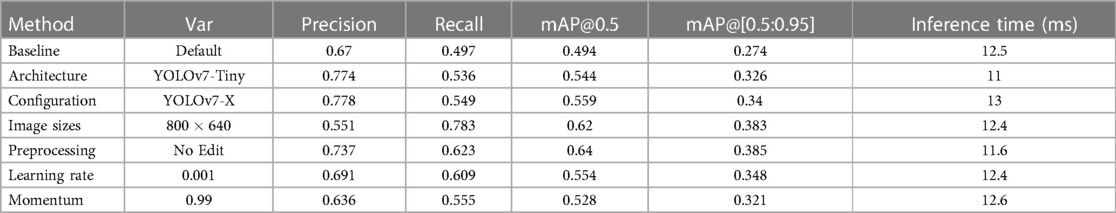
In comparison to the reference performance depicted in Figure 3, performance metrics benefited from employing more complex YOLOv7 architectures, which introduce more layers, parameters and gradients into the neural network. The increases in performance are displayed Table 1. However, training larger models did not automatically produce more precise or accurate models. mAP increases reached a maximum of 13.16% mAP@0.5 and 24.09% mAP@[0.5:0.95], respectively for the YOLOv7-X configuration, which is the 4th largest YOLOv7 variation analysed.
Moreover, similar results could be achieved by decreasing the learning rate to 0.001 (12.15 mAP@0.5, 27.01 mAP@[0.5:0.95]), or increasing momentum to 0.99 (6.88 mAP@0.5, 17.15 mAP@[0.5:0.95]) for a model of equal size. mAP was improved when omitting image preprocessing procedures (17.65% mAP@0.5, 18.10% mAP@[0.5:0.95]) and increasing image sizes during training (25.51% mAP@0.5, 39.78% mAP@[0.5:0.95]) (compare Figure 4).
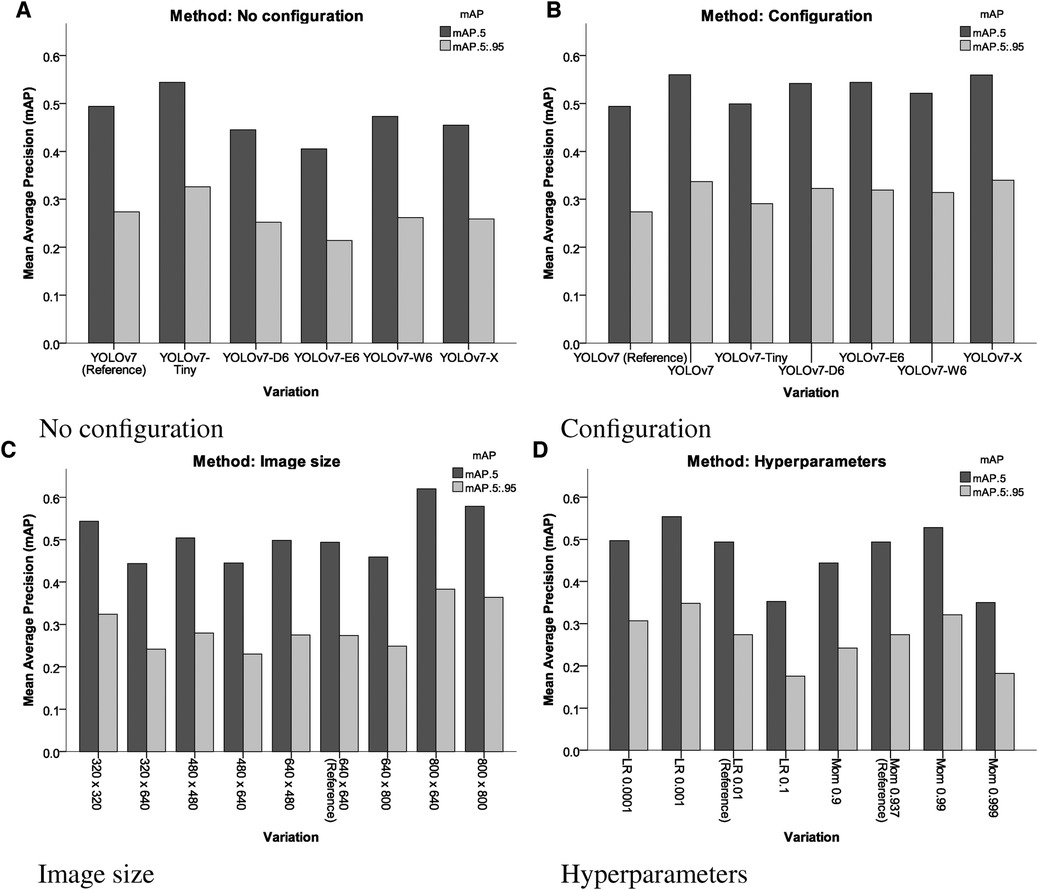
Figure 4. Results of the different variations of different YOLOv7 models, input sizes, pre-processing and hyperparameters assessed. LR, learning rate; Mom, momentum. (A) No configuration, (B) configuration, (C) image size and (D) hyperparameters.
Generally, when analysing the resulting models using XAI methods, higher scoring models in terms of mAP paid more attention to broader regions of the image, prioritizing detection accuracy over precision compared to the less accurate models.
The results in Table 1 indicate that the most important parameters are the avoidance of pre-processing procedures and a high image size (Figure 5). These two parameters more important than choosing different YOLOv7 architectures.
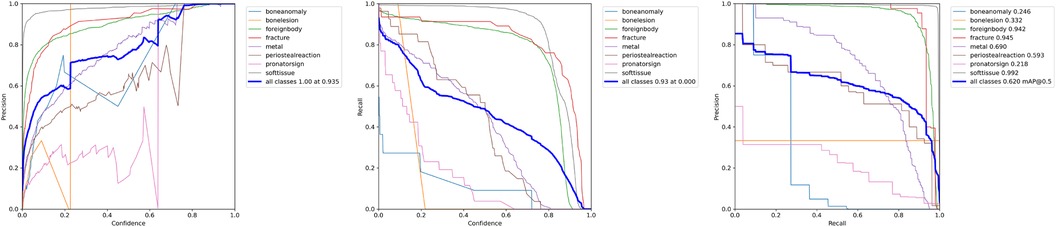
Figure 5. P-Curve, R-Curve and PR-Curve for the model trained with an increased image size of pixel.
4. Discussion
Wrist fractures are the most common fractures diagnosed in children and adolescents (22). While some fractures of the distal forearm do not even require conventional X-ray to confirm the diagnosis because of an obvious clinical malalignment, minor fractures in young children may easily be overlooked, because they cause only minimal radiological signs of bony compressions. Furthermore, cartilaginous growth zones may mimic or deceive actual fractures (23). Thus, specific medical training as a pediatric radiologist or pediatric surgeon is required to be familiar with age-specific details of the growing skeleton. Nevertheless, all forms of pediatric wrist fractures require adequate diagnosis and therapy to minimise potential subsequent growth disturbances (24). Paediatric trauma radiographs are often interpreted by emergency physicians and adult radiologists, sometimes without specialization in pediatric X-rays or back-up by experienced pediatric radiologists. Even in developed countries, shortages of radiologists were reported, posing a risk to pediatric patient care. In some parts of the world, access to pediatric radiologists is considerably restricted, if not unavailable (25).
Thus, particularly in the field of pediatric radiology, computer-aided detection (CADe) and computer-aided diagnosis (CADx) systems could contribute to the interpretation of patient data and scans (9). The roots of CAD systems trace back to the late 1950s when biomedical researchers first explored the potential of expert systems in medicine. These early endeavors involved computer programs that took patient data as input and generated diagnostic outputs. As technology progressed, these initial approaches evolved and were refined, incorporating AI and specialized algorithms to enhance predictive capabilities (26). In medical imaging there are several approaches to help physicians making a correct diagnosis. In addition to simple classification, i.e. predicting, whether a feature is present or not in the respective image, methods for localizing pathologies have also proven successful. A common approach involves identifying and delineating objects through bounding boxes (boxes around a predicted object), or image segmentation (masks or polygonal shapes around a predicted object) (27).
Medicine, and especially imaging are in the midst of a transformation towards AI, and AI algorithms are playing an increasingly important role in diagnostics (28). Some solutions are visible to the user in terms of computer assistance or interaction, while others work in the background to make radiological examinations even better (29). The clinical relevance of the available AI solutions is growing steadily. In a few years’ time, the use of optimised assistance solutions - such as the one presented in this manuscript - could become indispensable. It is not unlikely that CAD systems for recognising fractures or pathologies might also be available as open source solutions, community projects or educational resources in the future (30), alongside commercial products. Language models and generative AI have recently shown us this possibility (31). Seamless integration of useful AI tools will play a decisive role in their acceptance.
Given the complexity of the challenges in this domain, deep learning algorithms have risen to prominence, surpassing traditional neural networks in various aspects (32). In some cases, deep learning algorithms demonstrate comparable or even superior performance to human medical professionals (33). However, the increasing complexity of these models, the reliance on high-quality data, and concerns related to explainability and ethics have posed hurdles to their full-scale practical implementation. Any technology in the medical field must embody simplicity, safety, and trustworthiness to be of true benefit to healthcare practitioners (10).
In this study, we delved into the examination of a YOLOv7 object detector with a focus on detecting pediatric wrist fractures. The dataset and baseline parameters used here closely followed a study by Nagy et al. (15), ensuring a realistic scope and resource context. Accordingly, this paper undertook an ablation study, established a baseline model, and subsequently presents the research findings (10).
Overall, using configuration files improved model performance, except for YOLOv7-Tiny. Different architectures scaled similarly to or above YOLOv7 performed similarly well. Yet, configuration adjustments did not consistently enhance all models. Further investigation is needed to understand their relationship. Adjusting learning rate and momentum had significant impacts on performance. Lower learning rates led to better precision, compensating for imbalanced classes. An initial learning rate of 0.001 worked best. A momentum of 0.99 yielded optimal results overall.
Experimenting with image sizes during testing and training resulted in more variable outcomes. Training on larger images and testing on smaller ones yielded better results for all classes. Testing on larger images reduced performance, similar to omitting configurations. Unedited data improved average precision and reduced inference time, especially for fractures. Adjusting image size for training to produced the best results overall, benefiting average precision. Smaller image sizes helped object detection in general, but were less effective for fractures.
When analysed with Grad-CAM, models that outperformed the reference model in terms of mean average precision tended to generate noisier saliency maps, directing attention to the predicted object areas. Worse models produced more focused maps around actual fractures (compare Figure 6). Higher scoring models performed better due to their ability to minimize the amount of false negatives during detection, rather than maximizing IoU scores during regression, thus valuing detection accuracy over detection precision (Figure 7).
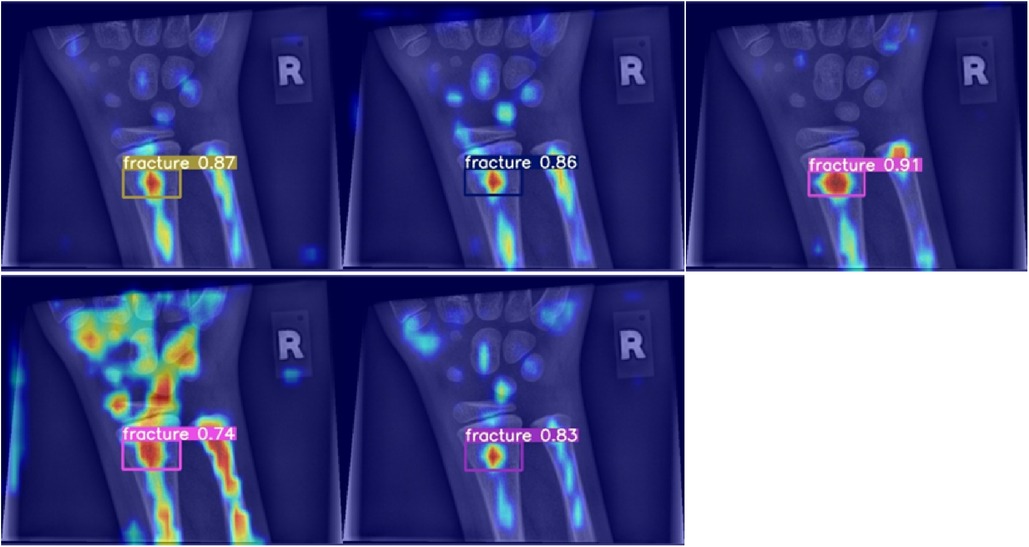
Figure 6. Grad-CAM for models performing worse than the baseline in terms of mean average precision.
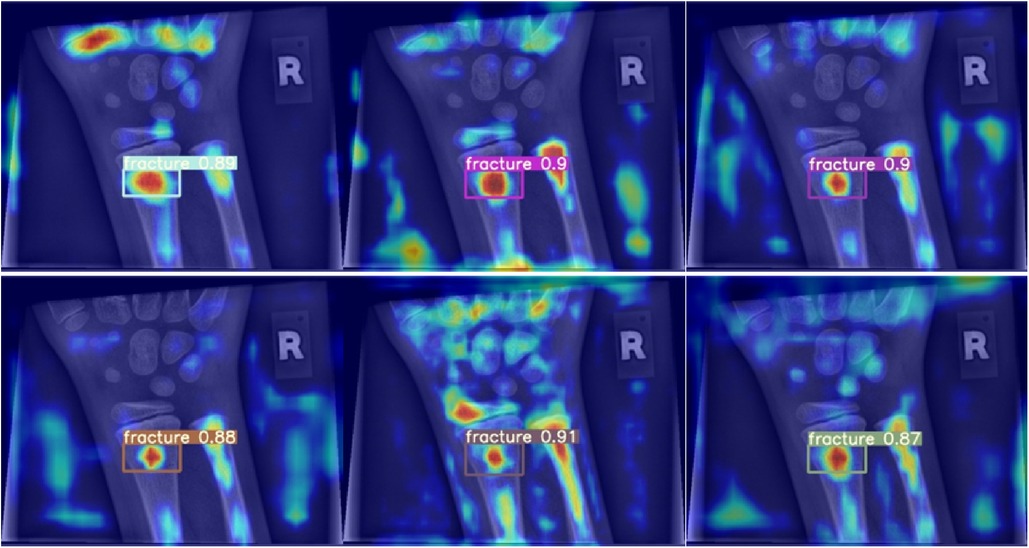
Figure 7. Grad-CAM for models performing better than the baseline in terms of mean average precision.
Some limitations of the manuscript need to be mentioned: We analysed the seventh iteration of the YOLO algorithm for object detection, while, in the meantime, a newer version has been released. It has to be understood that there might be small improvements in detection performance. Moreover, we did not compare the model results with medical experts. The reason is that we wanted to highlight the various technical possibilities to improve fracture detection performance during model development. We did not include statistical analyses in this manuscript, primarily because a comparison of model performances on image or bounding box levels with a test set of always result in p values below 0.05, even in minor group differences. On the other hand, a statistical comparison of the mAP values are not possible, because of the single metric value for each group. Thus, the presented differences between the individual architectures and settings need to be judged for their clinical and technical relevance. We were not able to test higher image sizes above pixels due to computational restraints. The results showed a dependency of image size with model performance, and higher image sizes might lead to further improvements in model performances.
5. Conclusions
Adjusting the dataset and image size had the most significant impact on average precision during testing. Sharpening and contrast enhancement hindered feature learning, and larger image sizes improved results despite testing on smaller images. Larger architectures did not always guarantee better performance. Hyperparameter adjustments also influenced results, with customized hyperparameters improving average precision by up to 27.01%. Grad-CAM analysis highlighted model strengths and weaknesses. This study contributes to understanding machine learning’s potential in pediatric healthcare, emphasizing the importance of data and configuration considerations. Such optimization protocols must be understood and advocated, before comparing ML performances against health care specialists.
Data availability statement
A publicly available dataset was used in this study, accessible under: https://doi.org/10.1038/s41597-022-01328-z
Ethics statement
The studies involving humans were approved by Ethics committee of the Medical University of Graz, Austria. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
TT: Data curation, Investigation, Methodology, Visualization, Writing – original draft, Conceptualization, Software; ST: Formal analysis, Methodology, Supervision, Writing – review & editing, Project administration, Resources, Data curation, Investigation, Visualization, Writing – original draft; GS: Formal analysis, Writing – review & editing, Methodology, Supervision, Validation; KL: Investigation, Software, Formal analysis, Project administration, Resources, Supervision, Validation, Writing – review & editing; HT: Formal analysis, Project administration, Resources, Writing – review & editing, Conceptualization, Funding acquisition.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Tobler P, Cyriac J, Kovacs BK, Hofmann V, Sexauer R, Paciolla F, et al. AI-based detection and classification of distal radius fractures using low-effort data labeling: evaluation of applicability and effect of training set size. Eur Radiol. (2021) 31(9):6816–24. PMID: 33742228; PMCID: PMC8379111. doi: 10.1007/s00330-021-07811-2
2. Raisuddin AM, Vaattovaara E, Nevalainen M, Nikki M, Jarvenpaa E, Makkonen K, et al. Critical evaluation of deep neural networks for wrist fracture detection. Sci Rep. (2021) 11:6006. doi: 10.1038/s41598-021-85570-2
3. Janisch M, Apfaltrer G, Hrzic F, Castellani C, Mittl B, Singer G, et al. Pediatric radius torus fractures in x-rays-how computer vision could render lateral projections obsolete. Front Pediatr. (2022) 10:1005099. doi: 10.3389/fped.2022.1005099
4. Mounts J, Clingenpeel J, McGuire E, Byers E, Kireeva Y. Most frequently missed fractures in the emergency department. Clin Pediatr (Phila). (2011) 50:183–6. doi: 10.1177/0009922810384725
5. Mahadevkar SV, Khemani B, Patil S, Kotecha K, Vora DR, Abraham A, et al. A review on machine learning styles in computer vision—techniques and future directions. IEEE Access. (2022) 10:107293–329. doi: 10.1109/ACCESS.2022.3209825
7. Redmon J, Divvala S, Girshick R, Farhadi A. You only look once: unified, real-time object detection. In: 2016 IEEE Conference on Computer Vision and Pattern Recognition (CVPR). Las Vegas, NV (2016). p. 779–88. doi: 10.1109/CVPR.2016.91
8. Wang CY, Bochkovskiy A, Liao HYM. YOLOv7: trainable bag-of-freebies sets new state-of-the-art for real-time object detectors. arXiv [preprint]. (2022). doi: 10.48550/arXiv.2207.02696
9. Silva W, Gonçalves T, Härmä K, Schröder E, Obmann V, Barroso M, et al. Computer-aided diagnosis through medical image retrieval in radiology (2022). Available from: https://doi.org/10.21203/rs.3.rs-1661223/v1
10. Gui C, Chan V. Machine learning in medicine. Univ West Ont Med J. (2017) 86:76–8. doi: 10.5206/uwomj.v86i2.2060
11. van der Velden BH, Kuijf HJ, Gilhuijs KG, Viergever MA. Explainable artificial intelligence (XAI) in deep learning-based medical image analysis. Med Image Anal. (2022) 79:102470. doi: 10.1016/j.media.2022.102470
12. Singh A, Sengupta S, Lakshminarayanan V. Explainable deep learning models in medical image analysis. J Imaging. (2020) 6:52. doi: 10.3390/jimaging6060052
13. Selvaraju RR, Cogswell M, Das A, Vedantam R, Parikh D, Batra D. Grad-CAM: visual explanations from deep networks via gradient-based localization. In: 2017 IEEE International Conference on Computer Vision (ICCV). New York, NY: IEEE (2017). p. 618–26. doi: 10.1109/ICCV.2017.74
14. Till T, Tschauner S, Singer G, Till H, Lichtenegger K. Machine learning in human healthcare: optimizing computer-aided detection, diagnosis systems using an x-ray data set of pediatric wrist fractures. In: Granigg W, editor. Scientific computing. FH JOANNEUM Gesellschaft mbH (2023). p. 60–7.
15. [Dataset] Nagy E, Janisch M, Hržić F, Sorantin E, Tschauner S. A pediatric wrist trauma x-ray dataset (GRAZPEDWRI-DX) for machine learning. Sci Data (2022) 9:222. doi: 10.1038/s41597-022-01328-z
16. Li Z, Liu F, Yang W, Peng S, Zhou J. A survey of convolutional neural networks: analysis, applications,, prospects. IEEE Trans Neural Netw Learn Syst. (2022) 33:6999–7019. doi: 10.1109/TNNLS.2021.3084827
17. Ying X. An overview of overfitting, its solutions. J Phys Conf Ser. (2019) 1168:022022. doi: 10.1088/1742-6596/1168/2/022022
18. Rácz A, Bajusz D, Héberger K. Effect of dataset size and train/test split ratios in QSAR/QSPR multiclass classification. Molecules. (2021) 26(4):1111. PMID: 33669834; PMCID: PMC7922354. doi: 10.3390/molecules26041111
19. Ojo MO, Zahid A. Improving deep learning classifiers performance via preprocessing and class imbalance approaches in a plant disease detection pipeline. Agronomy. (2023) 13(3):887. doi: 10.3390/agronomy13030887
20. Isa IS, Rosli MSA, Yusof UK, Maruzuki MIF, Sulaiman SN. Optimizing the hyperparameter tuning of YOLOv5 for underwater detection. IEEE Access. (2022) 10:52818–31. doi: 10.1109/ACCESS.2022.3174583
21. Henderson P, Ferrari V. End-to-end training of object class detectors for mean average precision. In: Lai S-H, Lepetit V, Nishino K, Sato Y, editors. Computer Vision – ACCV 2016. Cham: Springer International Publishing (2017). p. 198–213.
22. Schalamon J, Dampf S, Singer G, Ainoedhofer H, Petnehazy T, Hoellwarth ME, et al. Evaluation of fractures in children and adolescents in a Level I Trauma Center in Austria. J Trauma. (2011) 71:19–25. doi: 10.1097/TA.0b013e3181f8a903
23. George MP, Bixby S. Frequently missed fractures in pediatric trauma: a pictorial review of plain film radiography. Radiol Clin North Am. (2019) 57:843–55. doi: 10.1016/j.rcl.2019.02.009
24. Liao JCY, Chong AKS. Pediatric hand and wrist fractures. Clin Plast Surg. (2019) 46:425–36. doi: 10.1016/j.cps.2019.02.012
25. Farmakis SG, Chertoff JD, Barth RA. Pediatric radiologist workforce shortage: action steps to resolve. J Am Coll Radiol. (2021) 18:1675–7. doi: 10.1016/j.jacr.2021.07.026
26. Yanase J, Triantaphyllou E. A systematic survey of computer-aided diagnosis in medicine: past and present developments. Expert Syst Appl. (2019) 138:112821. doi: 10.1016/j.eswa.2019.112821
27. Wang S, Summers RM. Machine learning and radiology. Med Image Anal. (2012) 16:933–51. doi: 10.1016/j.media.2012.02.005
28. Dundamadappa SK. AI tools in Emergency Radiology reading room: a new era of Radiology. Emerg Radiol. (2023) 30:647–57. doi: 10.1007/s10140-023-02154-5
29. Langlotz CP. The future of AI and informatics in radiology: 10 predictions. Radiology. (2023) 309:e231114. doi: 10.1148/radiol.231114
30. Borgbjerg J, Thompson JD, Salte IM, Frøkjæ JB. Towards AI-augmented radiology education: a web-based application for perception training in chest X-ray nodule detection. Br J Radiol. (2023) 96:20230299. doi: 10.1259/bjr.20230299
31. Gordon EB, Towbin AJ, Wingrove P, Shafique U, Haas B, Kitts AB, et al. Enhancing patient communication with Chat-GPT in radiology: evaluating the efficacy and readability of answers to common imaging-related questions. J Am Coll Radiol. (2023). doi: 10.1016/j.jacr.2023.09.011
Keywords: artificial intelligence, machine learning, children, trauma, wrist, fracture, training, YOLO
Citation: Till T, Tschauner S, Singer G, Lichtenegger K and Till H (2023) Development and optimization of AI algorithms for wrist fracture detection in children using a freely available dataset. Front. Pediatr. 11:1291804. doi: 10.3389/fped.2023.1291804
Received: 10 September 2023; Accepted: 5 December 2023;
Published: 21 December 2023.
Edited by:
Mario Lima, University of Bologna, ItalyReviewed by:
Mohsen Norouzinia, Shahid Beheshti University of Medical Sciences, IranSteffen Berger, University of Bern, Switzerland
Udo Rolle, University Hospital Frankfurt, Germany
© 2023 Till, Tschauner, Singer, Lichtenegger and Till. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sebastian Tschauner c2ViYXN0aWFuLnRzY2hhdW5lckBtZWR1bmlncmF6LmF0
 Tristan Till1,2
Tristan Till1,2 Sebastian Tschauner
Sebastian Tschauner Georg Singer
Georg Singer Holger Till
Holger Till