Abstract
Purpose:
The Fontan operation is commonly associated with alterations in heart rhythms, both tachycardic and bradycardic. Despite modifications to attempt to mitigate these complications, arrythmias still frequently occur. The purpose of this review is to examine the literature regarding the scope of the problem, therapeutic options, and current recommendations regarding screening and surveillance.
Recent findings:
Modifications to the original Fontan procedure, antiarrhythmic medications, and improvements in catheter ablation procedures have improved the management of patients with arrhythmias following Fontan palliation. There is growing interest in the role of junctional rhythm in the role of Fontan dysfunction. While chronotropic incompetence has often been blamed for poor exercise testing, there is evidence that decreased performance may be related to ventricular filling and Fontan hemodynamics.
Summary:
Tachyarrhythmias are an important cause of mortality and morbidity after the Fontan operation. Prompt and aggressive management of arrhythmias with the goal of maintaining sinus rhythm is vital. Management strategies such as anti-arrhythmic medications, ablation, anti-tachycardia pacing and Fontan conversion should be seen as complementary and used early to prevent hemodynamic deterioration. Bradyarrythmias likely also contribute to Fontan failure. Pacing is the primary management strategy with evidence supporting use of atrial pacing. However, ventricular pacing seems to often lead to deleterious effects. Current guidelines recommend surveillance with Holter monitor every 2–3 years in adolescents and every 1–2 years in adults. Future directions for research include further assessment of junctional rhythm and its management as well as further identifying patients in which pacing would be beneficial.
Introduction
The Fontan operation transformed the outlook for children born with a single ventricle. However, it is a fragile circulatory arrangement with a tendency to develop a multitude of problems (1). Specifically, arrhythmias are one of the commonest causes of morbidity and mortality in these patients (1). Despite surgical modifications like the intracardiac lateral tunnel (ILT) and the extracardiac conduit (ECC) Fontan, arrhythmias continue to occur in a significant number of patients (1). Arrhythmias may present in a wide variety of ways ranging from the subtle (vague or mild symptoms of fatigue) to the catastrophic (syncope or rarely, cardiac arrest). Some, with arrhythmias may even be detected only at routine follow up. Yet another important consideration in Fontan patients is their risk for acute thromboembolic events (1–3). Underlying structural problems including poor ventricular function, and valve regurgitation may predispose to arrhythmias. Arrhythmias also can lead to worsening of cardiac ventricular function. One report showed that ∼40% of patients developed ventricular dysfunction after the first onset of an arrhythmia (1, 4, 5). Supraventricular tachycardia (SVT) has also been shown to be associated with a six-fold increase in transplantation and death (1).
Physicians caring for these patients must be aware of these arrhythmias, including their unique presentations after the Fontan, the diagnostic approach one should take, and the treatment options available. Regular surveillance to detect pre-clinical arrhythmias and prompt recognition and management of clinical arrhythmias are keys to achieving this goal. In this review, we aim to review tachyarrhythmias and bradyarrhythmias that arise after Fontan palliation, including a summary of the literature and an up-to-date approach to these often-challenging clinical presentations.
Tachycardia in patients after fontan completion
In the original, so-called atrio-pulmonary connection (APC) Fontan, the presence of extensive surgical scars in the right atrium (RA) combined with pressure and volume overload of the RA (which lead to stretching of the scars) is thought to predispose to the development of arrhythmias (6–10). While surgical scars are still present after the ILT and the ECC, they are fewer. Also, the ILT leaves most of the RA in the lower pressure pulmonary venous side of the atrium (11, 12). The ECC leaves the entire RA on the low pressure pulmonary venous side (13). On the other hand ventricular tachyarrhythmia (VT) is predominantly seen in patients with ventricular surgical scars and in those with a dominant right ventricle (14, 15).
The development of any tachyarrhythmia is an independent predictor of poor clinical outcome and tachyarrhythmias are associated with Fontan failure, sudden cardiac death) SCD, and mortality (4, 5, 9, 16–20). In one study, the 15-year survival after development arrhythmias was 70% and freedom from Fontan failure was 44% (20). Atrial arrhythmias are associated with a six-fold increase in transplantation and death (21).
Given the fragile nature of the Fontan circulation, prompt recognition and management of tachyarrhythmias are key to improving patient quality and quantity of life. Some arrhythmias, especially intra atrial reentry tachycardia (IART) may appear innocuous and resemble either sinus or a “junctional” tachycardia on electrocardiogram (22, 23). An example of IART may be seen in Figure 1. The presence of extensive scarring in the atrium can lead to low amplitude and fractionated p waves which may be hard to see (22, 23). A high index of suspicion is important in making a timely diagnosis. In the next sections, we review the various types of tachyarrhythmias.
Figure 1
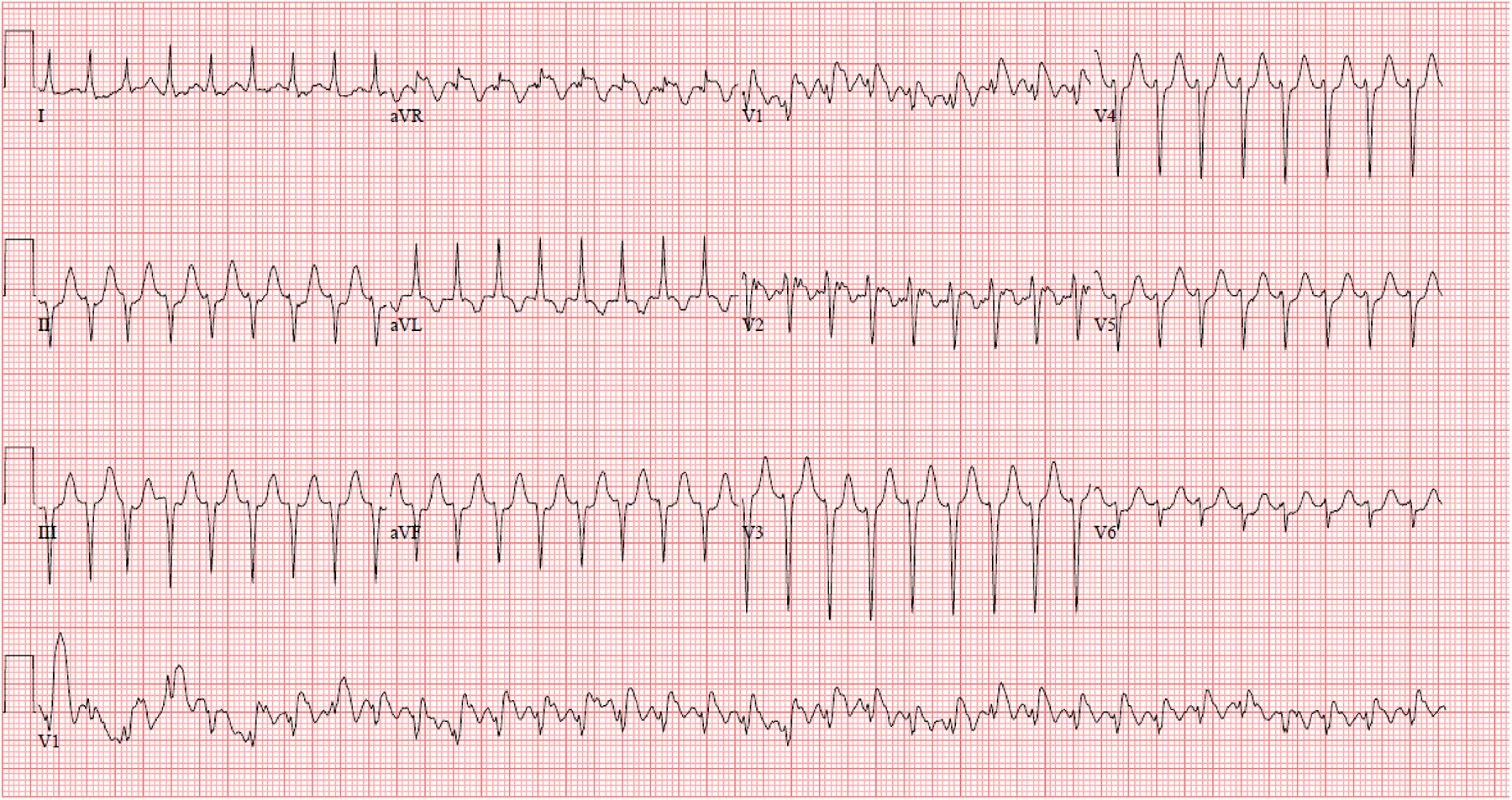
IART in a young adult with intra-cardiac lateral tunnel fontan.
Supraventricular tachycardia
The APC type of Fontan is associated with a high incidence of SVT. SVT occurred in the early post-operative period in 10%–30% of patients followed by a steady incidence during follow-up (24–28) Late arrhythmias occurred in up to 80% by >30 days post operative period (median time of 12 years after surgery) (27–29). Early post-operative SVT occurs in 25% patients after the ILT (30) and 14% after the ECC Fontan (29). As for late post-operative SVT, it has been noted in 32% of ILT and 6%–17% of ECC by 1–5 years post-operative (1, 24–28).
Much of what we know about the specific type of SVT's seen in these patients comes from studies describing outcomes after catheter ablation for SVT (21). The most common type of SVT in these patients is IART (∼93%) (21, 31, 32). Patients, however, can also have atrioventricular re-entrant tachycardia (AVRT) and atrioventricular nodal re-entrant tachycardia (AVNRT) which occur in ∼4% (21). As patients age, atrial fibrillation becomes the dominant arrhythmia, and has been noted in −2%–40% (4, 9, 33). However, other mechanisms such as AVRTs utilizing either an accessory pathway or twin atrioventricular nodes, AVNRT, ectopic atrial tachycardias, and junctional tachycardia are described (34).
Medical management of SVT
Prompt treatment is a must for episodes causing significant hemodynamic compromise. However, even in those who may tolerate the tachyarrhythmia acutely, conversion to sinus rhythm as soon as possible is of utmost importance (22). The importance of atrial kick in maintaining adequate cardiac output in the Fontan circulation cannot be overstated. Therefore, in patients with the Fontan physiology, the goal should be rhythm control rather than rate control (35).
For acute conversion, adenosine can be used for SVT involving the atrioventricular node after evaluating baseline ventricular function with an echocardiogram and electrocardiogram. However, such SVT are less common. Therefore, most patients with IART or atrial fibrillation need cardioversion (22). Most centers use direct current cardioversion. If the patient is stable with an adequate blood pressure, medications can be tried before cardioversion, although it is not considered first line therapy. Options for pharmacologic cardioversion include intravenous (IV) Ibutilide, IV sotalol, IV procainamide, and IV amiodarone (35–37). Due to the risk of development of intracardiac thrombi with untreated atrial arrhythmias >24–48 hour duration, pre-procedural transesophageal echocardiogram (to assess for thrombus), anticoagulation, and conscious sedation is necessary (38, 39).
Chronic therapy options include anti-arrhythmic drugs, catheter ablation, anti-tachycardia pacing and the surgical MAZE procedure (35). A summary of medication options for long term management of IART including dosage, side effects, and monitoring considerations can be found in Table 1. The goal of chronic medical management is maintenance of sinus rhythm. The choice of medication primarily depends upon whether or not there is normal ventricular function. If so, class 1c agents (propafenone or flecainide) or sotalol (class III agent) are typically chosen as first line medication. If not, amiodarone or dofetilide may be considered. Amiodarone may also be employed as a second line agent in patients with normal ventricular function (35).
Table 1
| Medication | Indication | Dose | Side effects contraindications | Monitoring issues |
|---|---|---|---|---|
| Flecainide (class IC) | First line in patients with normal ventricular function | Dose adjusted based on serum levels: Initial: 50 mg bid Increase by 50 mg bid at 4-day intervals; maximum dose: 300 mg/day |
Black box warning with increased risk of ventricular arrhythmias as well as mortality in patients with ventricular dysfunction or coronary artery disease Proarrhythmic (VT, bradycardia, AV block) QRS prolongation Has potential to convert atrial arrhythmias into 1:1 conduction due to negative chronotropic effects—should initiate concurrent AV node blocking medication |
Dosing based on serum levels which need to be intermittently monitored: therapeutic trough concentration is between 0.2 and 1 mcg/ml Follow daily ECG for QRS prolongation while inpatient until therapeutic |
| Propafenone (class IC) | First line in patients with normal ventricular function | Immediate release: Initial: 150 mg every 8 h; may increase after 3- to 4-day intervals; may increase to 300 mg every 8 h. Extended release: Initial: 225 mg every 12 h; may increase after 5-day intervals; may increase to 425 mg every 12 h |
Black box warning with increased risk of ventricular arrhythmias as well as mortality in patients with ventricular dysfunction or coronary artery disease Proarrhythmic (VT, bradycardia, AV block) QRS prolongation Has potential to convert atrial arrhythmias into 1:1 conduction due to negative chronotropic effects—should initiate concurrent AV node blocking medication Central nervous system side effects such as dizziness, nausea, unusual taste, and blurred vision |
Check ECG for QRS prolongation daily while inpatient until therapeutic |
| Sotalol (class III) | First line in patients with normal ventricular function | Initial dose: 80 mg Dosing frequency based on calculated creatinine clearance: > 60 ml/min: bid dosing 40–60 ml/min: daily dosing < 40 ml/min: contraindicated Adjustment: may increase every 2–3 days up to 320 mg, given in 2 or 3 divided doses |
Can prolong the QT interval, so check QT interval before administering (contraindicated if > 450 ms) Proarrhythmic (torsade); exercise caution when combining with other QT-prolonging medications (antimicrobial, antiemetic, etc) |
Check ECG for QRS prolongation daily while inpatient (adjust dose if QTc > 500 ms) Monitor ECG intermittently once stable |
| Amiodarone (class III) | First line in patients with ventricular dysfunction Second line if function is normal |
200 mg daily | Pulmonary and liver toxicity, corneal microdeposits, photosensitivity, thyroid dysfunction [hypo- or hyperthyroidism, especially in women post Fontan and those with BMI < 21 kg/m2 (35)], and adverse cardiac effects (e.g., bradycardia, torsades de pointes) | Cardiac: baseline testing of ICD threshold if one present Pulmonary: chest radiograph at baseline and yearly for asymptomatic patients; PFT if symptoms develop Thyroid: baseline TSH and FT4 at baseline; serial testing 3–4 months post initiation, then yearly Liver: baseline AST/ALT, repeat 6 months post initiation, then yearly Ophthalmologic: yearly eye exam |
| Dofetilide (class III) | Second line therapy as alternative to amiodarone in patients with ventricular dysfunction | Dofetilide dosing is based on creatinine clearance and calculated QTc: Initial dose with calculated creatinine clearance: > 60 ml/min: 500 mcg bid 40 –60 ml/min: 250 mcg bid 20 –40 ml/min: 125 mcg bid < 20 ml/min: contraindicated Subsequent doses adjusted if QTc increases > 15% or QTc > 500 ms Starting dose: adjusted dose: 500 mcg bid 250 mcg bid 250 mcg bid 125 mcg bid 123 mcg daily 125 mcg daily |
Contraindicated in pregnancy (class C), patients with LQTS, patients on dialysis or with renal disease, or with vomiting or electrolyte derangements Adverse Effects: Proarrhythmic—Torsades de pointes in 1–3% of patients, may induce of worsen ventricular dysrhythmias, possibly inducing PMVT Side Effects: Rash, diarrhea, dizziness, sweating, vomiting, loss of appetite |
Follow QTc to monitor for signs of prolongation. FDA recommends admission for initiation with continuous ECG and calculating QTc 2–3 h after doses 2–5 after starting and monitor for minimum of 3 days |
Chronic rhythm control in adults with fontan circulation and IART.
IART, intra atrial reentry tachycardia; AV, atrioventricular; ECG, electrocardiogram; VT, ventricular tachycardia; PMVT, polymorphic ventricular tachycardia; QTc, adjusted QT interval; LQTS, long QT syndrome; PFT, pulmonary function testing.
Catheter ablation
Catheter ablation of SVT in Fontan patients is a highly specialized procedure performed by electrophysiology physicians with training and experience in congenital heart disease (18). These procedures entail prolonged case time, are complex, and require highly specialized equipment including three-dimensional mapping (3D) technology, intracardiac echocardiogram and the use of specialized catheters. With large atrial size, multiple scars and pathways, the APC Fontan patients pose significant challenges during ablation as there may be multiple circuits with the rhythm morphing from one to the other (36, 40). In APC Fontan patients, studies have described an acute success rate varying from 78%–94%, with partial success achieved in 6%–13%. The recurrence rate is high (20%–50%), and multiple ablations are needed in ∼5% of patients (21, 40–42). Complications of ablation include complete atrioventricular block requiring pacemaker placement, thrombus and embolism, rarely fatality (36, 40–42). Despite these risks at 24 months following ablation, there was an improvement in overall arrhythmia score. This is likely postulated due to either ablation of all tachycardia foci or modification of the dominant arrhythmia substrate (21, 42). The acute success rate has been described to be higher in ILT patients (18), however, they also have a high recurrence rate (18). ECC patients require puncture of the Fontan baffle to access the atrial myocardium, without significant consequent risks (42). In the ECC group, the most common site of arrhythmias is the cavo-tricuspid isthmus, with consequently higher success rates for ablation (43). Reports of ablation of AVNRT and AVRT using an accessory pathway or twin AV nodes are confined to small case series (42). Any ablation in a Fontan patient must surmount a few anatomical challenges and therefore must be performed at specialized centers with experienced personnel.
Summary of SVT management
It is our practice that an in-depth discussion is pursued with the family regarding ablation vs. medical management, emphasizing that these therapies are complementary and not contradictory; most patients will need both. After a detailed conversion with shared decision making, we would proceed with either a class 1C or class III medication for rhythm control. It is our practice to typically admit patients for initiation of medication over the course of 2–3 days to ensure stability. If they tolerate this, we continue therapy as an outpatient with frequent ambulatory monitoring to assess adequacy of treatment. In select patients, catheter ablation may be considered as a first line therapy. When ablation is successful, patients may be monitored without need for initiating anti-arrhythmic therapy. If unsuccessful or only partially successful, we would initiated medications and monitor as above.
Ventricular tachycardia
Ventricular tachycardias are less frequent in the Fontan population (3%–10%) and non-sustained ventricular arrhythmias are typically discovered during surveillance (6, 13). However, the long-term implications of this arrhythmia, especially in relation to risk for sudden death, is unclear. This is an important consideration given that reports show that the mode of death is sudden (and presumably arrhythmic) in −3%–12% of Fontan patients (2, 24, 25, 33).
VT can be difficult to manage in Fontan patients. While beta blockers may provide some protective effect, they cannot be relied upon to be the mainstay of treatment in patients who have VT associated with major symptoms such as syncope or near-syncope (13, 16, 44). ICD placement may need to be considered. Given the anatomical constraints imposed by the Fontan circulation, ICD's are hard to place (41, 42, 45–47). The subcutaneous ICD may be an excellent option in many patients (42). Major anti-arrhythmic drugs such as sotalol and amiodarone should be considered in patients with VT.
Anti-arrhythmic drugs
Vaughan-Williams class Ic/III (Sodium and Potassium channel blockade) rhythm-control agents are initiated in the hospital with electrocardiogram monitoring. Class I agents include flecainide and propafenone and class III agents commonly used are Amiodarone, Sotalol and Dofetilide (36). Sotalol and Dofetilide can cause prolongation of the QT interval and torsades as a lethal proarrhythmia (35, 48, 49). Hence, careful monitoring of the QT at the time of initiation of these two drugs is critical to long term use (49). Amiodarone, even though is most effective drug in the long-term, is disadvantageous for multiple reasons, especially its association with multiple side effects when used long term (35, 50). Therefore, it is important to think of an “exit-strategy” (such as ablation, Fontan conversion surgery, and transplantation) out of chronic amiodarone use. If possible long-term use should be avoided.
Pharmacologic therapy is associated with a >90% arrhythmia recurrence rate within 3 years (36). Complete control of arrhythmia was seen in 63% whereas 35% of partial benefit was seen with medical therapy alone (50). Discontinuation due to toxicity is common accounting for 42% of AAD (50). In one cohort on Amiodarone, 30% developed thyrotoxicosis and 14% hypothyroidism (35, 50).
Class 1 c agents must be used with caution for two main reasons. Firstly, they can cause QRS duration prolongation and ventricular arrhythmias (35). Secondly, they can slow down the rate of atrial arrhythmias, which can be conducted at a higher ratio to the ventricle. For instance, a stable patient with a IART rate of 300, and 2:1 conduction giving a ventricular rate of 150 may become unstable if their IART rate is reduced (for example to 200) with consequent 1:1 atrioventricular (AV) nodal conduction. Hence class1c agents should always be combined with high doses of AV node blocking agents when used in this patient population (35).
Fontan conversion
The Fontan conversion operation is an option for patients with an APC faced with intractable arrhythmias. This includes resection of the enlarged RA, atrial septectomy, right-sided or bi-atrial maze cryoablation, extracardiac conduit placement (inferior vena cava to pulmonary artery), bidirectional Glenn, and, if needed, placement of an epicardial pacemaker (8). This surgery has a procedural mortality risk of 0.9%–3% in the perioperative period and a further late mortality 3–5.4% (36, 51). However, patients have been shown to have a freedom from atrial tachycardia of 77% at 10 years and freedom from cardiac death/transplant of 90% at 5 years, 84% at 10 years, and 66% at 15 years (52). High-risk characteristics for the operation at the conversion include right dominant ventricle, cardiopulmonary bypass (CPB) >240 min, ascites, protein losing enteropathy (PLE) and those having bi-atrial MAZE operation (52).
The 2014 HRS/PACE guidelines recommended a modified right atrial MAZE procedure in all Fontan conversion surgeries, including those without prior atrial arrhythmias (35). On the other hand, a left atrial/biatrial Cox MAZE III procedure is chiefly used in those with a known left atrial arrhythmia or atrial fibrillation (AF) (35, 51). Patients who are felt to be too high risk for a Fontan conversion operation, especially those with severe ventricular dysfunction or PLE, should be referred for transplantation.
Sudden death
Arrhythmias as a cause of SCD has been noted to have an incidence of 3%–12% and occur during late follow-up (6, 13, 16, 53). Risk factors for sudden death include atrioventricular valve replacement at time of Fontan operation and a Fontan pressure >20 mmHg (6). Conversely, the presence of pre-operative sinus rhythm has been shown to be protective (17). Risk stratification to predict SCD is still in its infancy and further studies are needed to identify which patients would benefit from implantable cardioverter defibrillator (ICD) placement.
Antitachycardia pacing & ICD
Antitachycardia pacing can be used to overdrive pace the patient out of SVT, specifically IART (3). The enthusiasm for antitachycardia pacing has waned after the introduction of catheter ablation. However, it can still have a role in select patients with recalcitrant arrhythmias (3). Patients with resuscitated cardiac arrest or hemodynamically unstable ventricular arrhythmia are usually treated with an ICD. Despite SCD being the cause of death in −20%–25% of adult patients with a Fontan, the lack of adequate risk prediction methods makes it difficult to place a prophylactic ICD in these patients.
Conclusions
Tachyarrhythmias are an important cause of mortality and morbidity after the Fontan operation. While the change from APC to ILT/ECC has reduced the incidence and hemodynamic deterioration associated with atrial arrhythmias, they have not eliminated the problem. An important key to early detection is a high index of suspicion. Prompt and aggressive management of arrhythmias with the goal of maintaining sinus rhythm is vital. Management strategies such as anti-arrhythmic medications, ablation, anti-tachycardia pacing and Fontan conversion should be seen as complementary and used early to prevent hemodynamic deterioration.
Bradycardia in patients after the fontan procedure
Bradyarrhythmias present a challenge in patients after completion of the Fontan procedure with clinical complications that can be highly consequential. While the association between tachyarrhythmias and poor outcomes is well-established in the Fontan population, bradyarrhythmias may also have serious clinical consequences, as even small alterations in the hemodynamics of these patients can lead to adverse outcomes including Fontan failure, Fontan associated liver disease, and even mortality (54, 55). While tachyarrhythmias are often easily recognized (due to the severity of their symptoms), bradyarrhythmias can be insidious, with prolonged periods of being asymptomatic prior to discovery (33, 56). A study by Carins et al. from the Australia-New Zealand Fontan registry demonstrated a high incidence of Fontan failure after onset of bradyarrhythmias including sinus node dysfunction (SND) and AV node disease causing heart block (33). In this section, we will review the existing literature on bradyarrhythmias in patients with Fontan physiology.
Sinus node dysfunction
SND is the most commonly encountered bradyarrhythmia in patients after Fontan completion with an estimated incidence between 9%–60% (56–62). However, this underestimates the issue, as patients with sinus node dysfunction can be asymptomatic (33). SND can be defined as an average resting heart rate greater than two standard deviations below the mean, predominant junctional rhythm, and/or sinus pauses of 3 or more seconds (63). SND typically begins subtly with progressively decreasing periods of sinus rhythm and greater periods spent in bradycardia and junctional rhythm. While sinus bradycardia and pauses may cause symptoms such as fatigue, exertional shortness of breath or dizziness/syncope, many patients “adapt” to their chronic disability and may not endorse any symptoms (64).
The APC Fontan is known to have a high incidence of SND, up to 50% at 10–15 years (12). The high frequency is attributed to the extensive atrial tissue manipulation required during surgery, which likely leads to trauma to the sinus node or compromise of the arterial supply (58, 63). Others have proposed theories to explain why the incidence of sinus node dysfunction increases with including abnormal right-sided hemodynamics, atrioventricular valve regurgitation leading to atrial distention, and multiple suture lines near or around the sinus node (63). Alternative surgical techniques including the ILT and ECC have sought, in part, to mitigate against these risk factors (63, 65–67).
With the ILT, the placement of an intra-atrial baffle leads to less of the atrial wall exposed to higher pressure and thus arrhythmia-generating distension (68). The downside is that there is suturing close to the sinus node during the baffling process. In contrast, the ECC eliminates the need for dissection and suture lines near the SA node as well as protecting against atrial distention (68). However, the need for harvesting an atrial cuff to allow for inferior anastomosis may lead to a higher-than-expected incidence of arrhythmias (18, 69). Despite these alterations in surgical technique, SND occurs in both the immediate post-operative period as well as late term follow-up.
Early SND
The incidence of SND in the immediate post-operative (Fontan) period is variably estimated between 2% and 25% (56, 59, 63, 68, 70, 71). While a majority of patients do not require pacing prior to discharge, there is evidence that the presence of SND within the first several days post-operatively may predict the development of both late onset sinus node dysfunction and tachyarrhythmias (62, 65, 70, 72). Studies have compared the rates of early SND after each approach to Fontan completion, with conflicting results. Three groups found a higher incidence in the ILT compared to ECC patients (60, 73, 74), with each of these being retrospective single center studies. Conversely, several studies have demonstrated no significant difference between the techniques or even a higher incidence in the ECC patients (70, 71, 75–78). These were also retrospective studies, but include several multicenter and multi-national cohorts. A metanalysis from Li et al. in 2017 included each of the afore-mentioned studies and examined the existing data from these studies (18). The conclusion was that while odds of SND was lower in the ECC group, this did not reach statistical significance (p = 0.06) (18).
Late SND
Late onset SND has a reported incidence between 15% and 44% (56, 57, 79). There is evidence that the presence of early SND predicts the development of late SND (63), and several studies have demonstrated that SND becomes more prevalent over time (63, 70, 80). Any of the estimate of the incidence of SND likely underestimates the true frequency, given to the presence of asymptomatic patients.
Late SND associated with an APC Fontan has been reported in 10%–12% of patients (80). As with early onset SND, multiple studies have compared the incidence of late SND associated with the ILT vs. ECC procedure. Dilawar et al. reported a higher frequency of SND after ECC over a seven-year period in 4/19 ILT patients (21%) vs. 10/17 ECC patients (59%; p = 0.04) (58). However, this was a single-center retrospective study with longer mean follow-up in the ECC compared to the ILT group. In contrast, Nürnberg et al. found that late SND was higher in patients after ILT, with 50% of patients following ILT being in a non-sinus rhythm at follow up compared to 14% for ECC patients (60). However, in this study, the median follow up post-ECC was 4.4 years compared to 7.9 years for ILT (60). Overall, a majority of studies including multi-center studies and the meta-analysis from Li et al. have demonstrated no statistical difference in late SND between the two surgical approaches (18, 23, 68, 71, 75, 77, 78).
Sinus node dysfunction or secondary limitation—insights and the role of exercise testing
Multiple studies have shown that Fontan patients have an abnormal heart rate response to exercise. However, it is unclear if this is a primary rhythm problem or a secondary adaptive response. Powell et al. showed that in teenage Fontan patients, chronotropic limitation to exercise, as defined by chronotropic index [CI = actual peak heart rate (HR)—resting HR/expected peak HR for age—resting HR], was prevalent, with the mean CI being 0.72 as compared to a typical definition of chronotropic incompetence, which is a CI <0.8 (81). The CI, however, is an incomplete assessment of chronotropic function, as it only uses the peak HR (and not slope of HR change) and does not consider secondary reasons for peak HR truncation.
An alternative proposal is that in select Fontan patients, limitation in HR, especially during exercise, is a secondary and even adaptive phenomenon. Support for this comes from Claessen et al, who compared ten patients after Fontan palliation (mean age 20 years, 60% male) to healthy controls utilizing MRI and simultaneous invasive arterial pressure recording during exercise (82). Exercise associated cardiac index, stroke volume, and HR relative to peak oxygen consumption (VO2) were determined and compared amongst the groups. As expected, heart rate reserve (peak HR—resting HR) was lower in Fontan patients compared to controls (71+/−21 vs. 92+/−15 bpm; p = 0.01) (82). However, increase in HR relative to workload and peak VO2 were actually higher in the Fontan patients. Furthermore, the Fontan patients had reduced augmentation of stroke volume for any given change in VO2 (82). Finally, the Fontan patients had a pronounced plateau in cardiac output at a lower HR than controls (82). The authors concluded that HR response to exercise was actually appropriate relative to exercise intensity (implying lack of chronotropic incompetence). However, premature reduction in ventricular filling and thus stroke volume led to leveling off of cardiac output during exercise, thus making peak HR secondarily truncated (82). Hedlund et al. reported similar outcomes from their prospective cohort study. When comparing 27 teenage Fontan patients to healthy controls, the Fontan cohort's mean HR for any given workload was actually higher than the controls (83). Furthermore, oxygen pulse, a surrogate marker for stroke volume, was reduced at maximal effort in the Fontan patients comparatively (83). The authors postulated that this was related to reduced ability to augment stroke volume with more intensive exercise and thus the reduced HR was appropriate for the degree of stroke volume (83). Hebert et al. similarly concluded that low stroke volume index was the most important limiting factor for exercise capacity in Fontan patients (84). But how might this heart rate modulation actually occur? A proposal is that this occurs via the Bainbridge and “Reverse” Bainbridge reflexes. These reflexes are described within the anesthesia literature (85), given the HR changes that are routinely observed with changes in peri-procedural fluid status. Via stretch fibers near the vena cava and within the right atrium, excessive cardiac filling and overdistension is prevented via reflex tachycardia (in turn via inhibition of vagal outflow and enhancement of sympathetic stimulus to the sinus node). The “Reverse” Bainbridge is the opposite—reduced venous return leads to deactivation of sinus node stimulation and in the midst of exercise, a negative stimulus for further HR elevation (85). In patients with worse Fontan hemodynamics (elevated central venous pressure and ventricular filling pressure), the already preload-deficient systemic ventricle (given the lack of a subpulmonary ventricle to augment pulmonary blood flow and thus pulmonary venous return to the heart) would be even more lacking in preload, thus leading to a lack of further stimulus to increase HR further during exercise (85).
Exercise testing is an excellent modality to utilize to help decipher whether HR limitation during exercise is primary or secondary to poor Fontan hemodynamics. In patients with primary sinus node dysfunction, heart rate during exercise would be expected to lag in general, and in comparison with oxygen consumption (VO2). Heart rate might even plateau prior to peak VO2 doing so (Figure 2). On the other hand, limitation in HR secondary to poor Fontan hemodynamics would be expected to show appropriate HR acceleration for any given increase in peak VO2, but abrupt truncation of HR at peak exercise rather than any plateau (Figure 3). We believe complete evaluation of Fontan status, including exercise testing, has a role in deducing the etiology of HR limitation in a given patient and should be considered prior to pacemaker placement.
Figure 2
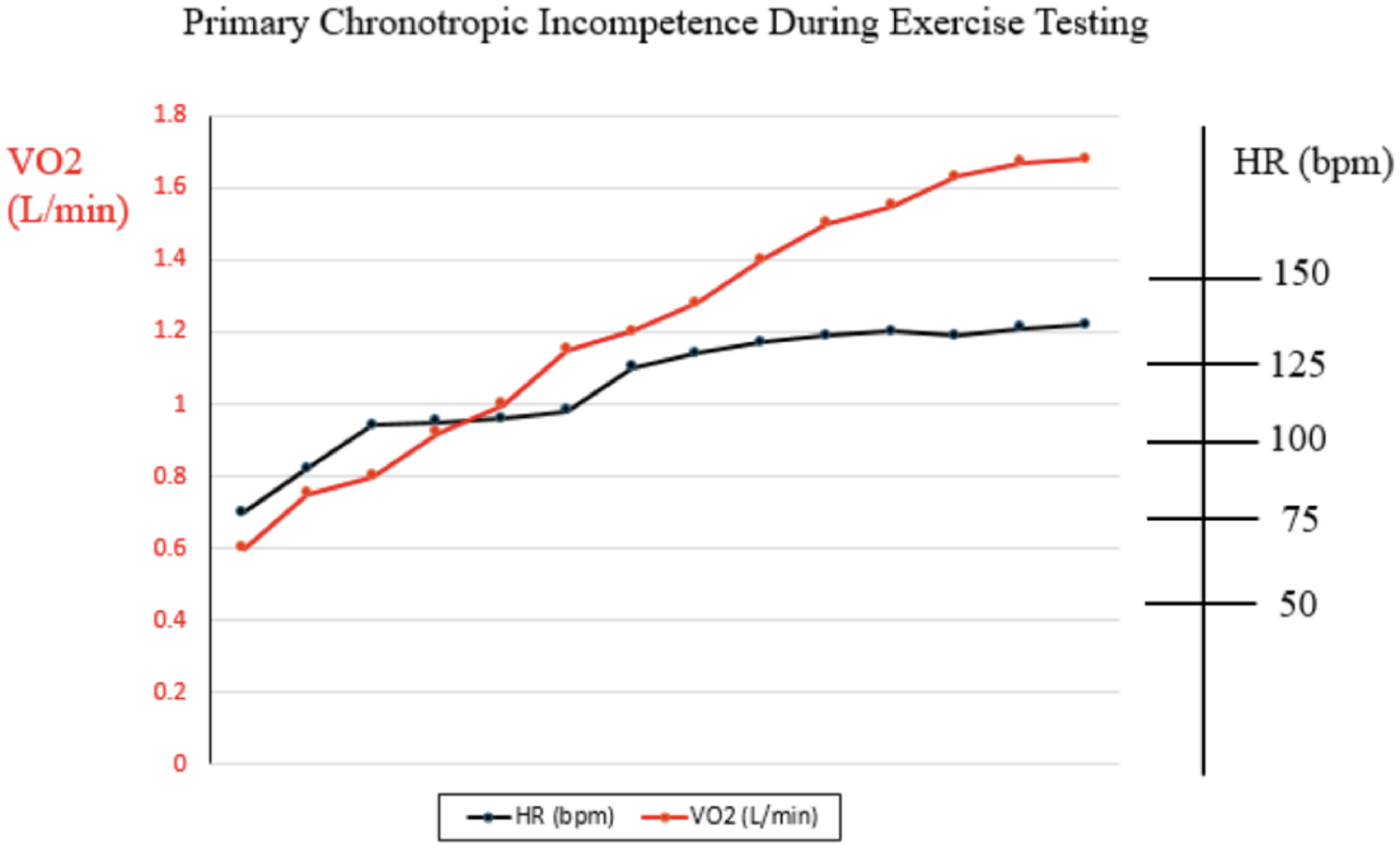
Example of relationship between heart rate and oxygen consumption during cardiopulmonary exercise test in patient with primary chronotropic incompetence.
Figure 3
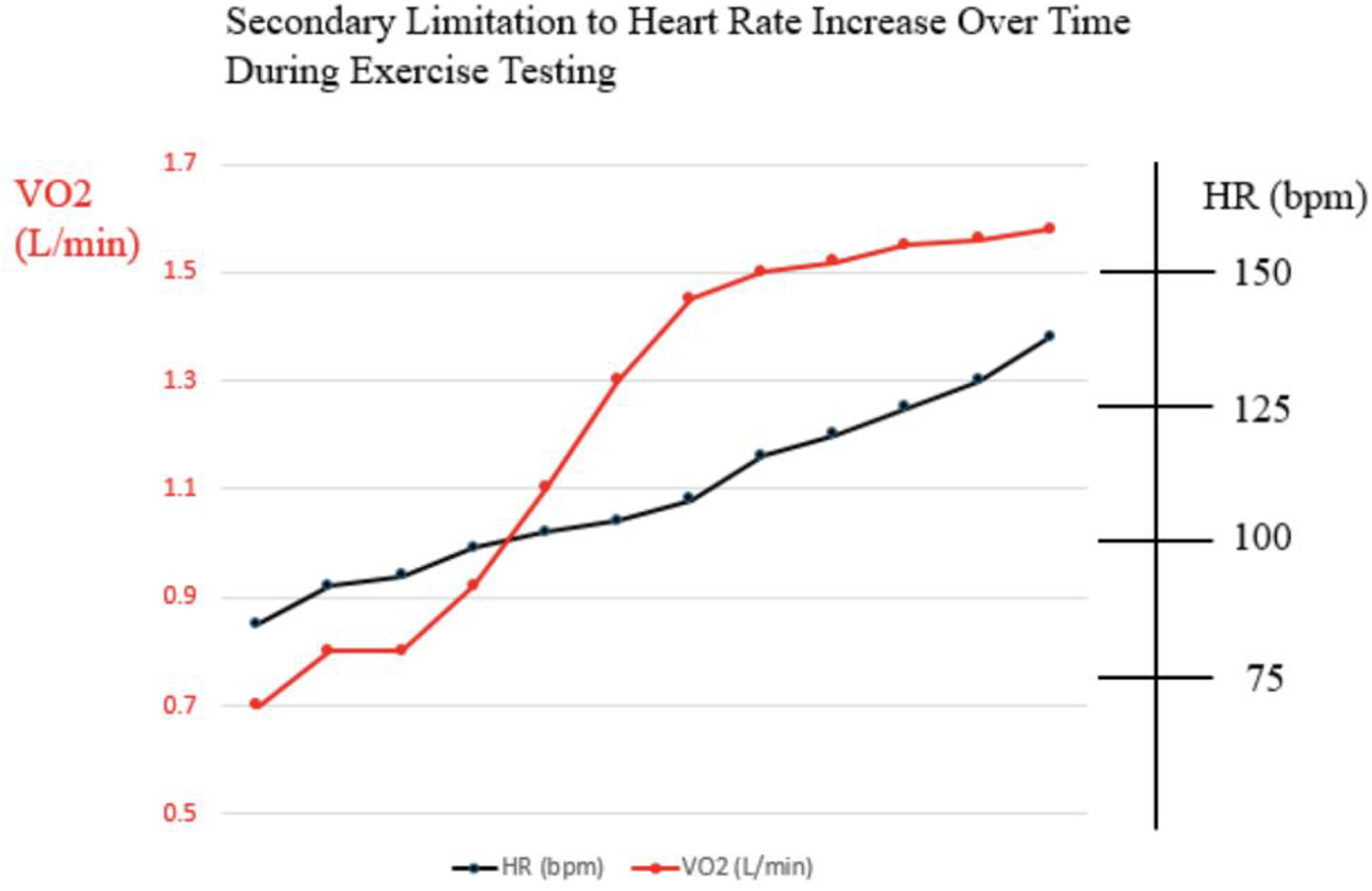
Example of relationship between heart rate and oxygen consumption during cardiopulmonary exercise test in patient with secondary limitation to heart rate such as poor fontan hemodynamics.
Junctional rhythm
SND manifests as sinus bradycardia, pauses, and junctional rhythm (JR). JR can lead to asynchrony and loss of atrial kick, with further decrease in cardiac output (86). This is particularly important in the Fontan circulation given the tenuous hemodynamics present. JR is also often characterized by an insidious onset. Even more problematically, patients with JR may have a relatively normal heart rate which can further delay the recognition of this arrhythmia (33). An example of an ECG with JR in a Fontan patient is presented in Figure 4. The frequency of JR after Fontan also tends to increase with time, with estimated incidence between 15% and 46% (56, 62, 65, 77, 80, 87). Furthermore, Januszewska et al. compared the rates of JR between the ILT and ECC. In this retrospective cohort study, ILT demonstrated significantly higher rates of JR early postoperatively (p < 0.001), during hospitalization (p = 0.004), and at discharge (p < 0.001) (87).
Figure 4
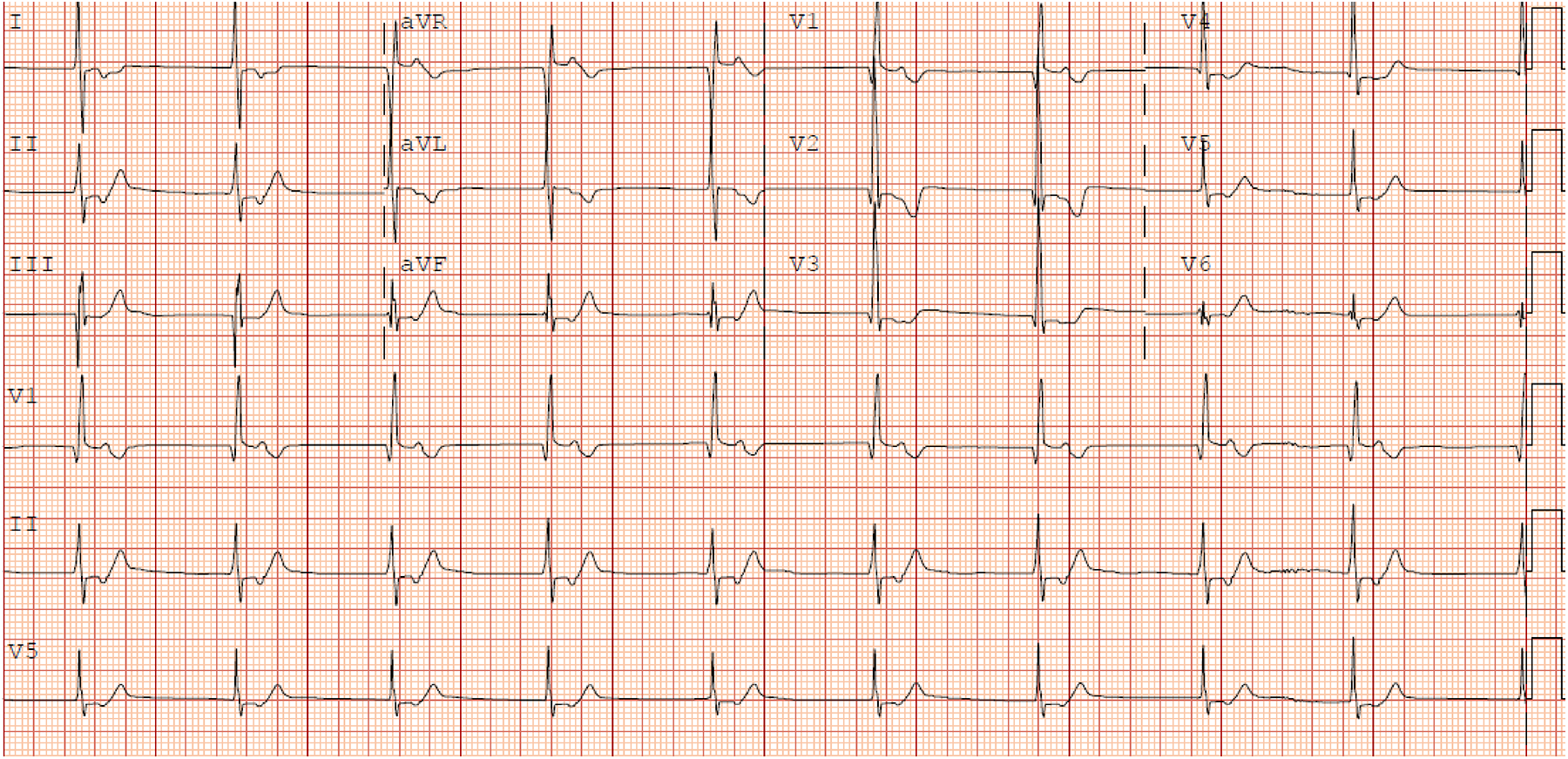
12 lead of junctional rhythm in a fontan patient.
Evidence for the deleterious effects of JR in the Fontan population comes from several studies. Ferrari et al. reported cardiac magnetic resonance-derived flow patterns showing retrograde flow in the entire Fontan system in a patient with JR (88). This flow reversal increases the pulmonary artery pressure, which in turn increases the hepatic and splanchnic venous pressures, which is particularly deleterious in the Fontan circulation (89). These studies suggest JR chronically decreases cardiac output and further elevates central venous pressure, known risk factors for developing Fontan failure (54). However, it is worth noting that each of these studies are either case reports or small series and the long-term impact of JR remains to be studied.
The optimal management of JR is unclear. In a recent survey of congenital cardiologists, 80% would not implant an atrial pacemaker in an asymptomatic patient with JR (90). On the other hand, more than 83% indicated that they would pursue pacemaker placement if the asymptomatic patient was already undergoing a sternotomy for another reason (90). This suggests that congenital cardiologists feel the risk of pacemaker placement is not worth the uncertain benefits to a patient with JR, although they also understand intuitively the possible benefit of treatment (90). There was general agreement that Fontan patients with symptomatic JR including evidence of Fontan failure should receive pacing, as 90% indicated they would support placement of a device (90).
Is pacing the solution for the bradycardic fontan?
The direct solution to managing significant bradycardia is pacing. The incidence of permanent pacemaker placement in the Fontan populations is 8%–13% (91–93). Additional reasons for placement following Fontan completion include anti-tachycardia management, ventricular tachycardia, and cardiac resynchronization (92).
There is evidence that permanent pacing is associated with clinical improvement and is recommended in symptomatic patients and those with exercise intolerance, or if there are hemodynamic derangements worsened by bradycardia (1, 35, 91). Cohen et al. reported two patients in whom atrial pacing (AP) resolved symptoms of PLE (94). In these two patients, symptoms remained despite aggressive diuresis and creation of a fenestration in the Fontan circuit (94). In contrast, there was complete resolution of PLE within 6 weeks of AP (1). Heinemann et al. also reported improvement in PLE symptoms after placement of dual-chamber pacemakers in their case series (95). A separate report also described resolution of plastic bronchitis with AP (96). While this total experience is small, AP seems to offer Fontan patients clinical improvement when appropriately utilized.
Pacing has also been shown, via restoration of AV synchrony, to eliminate the reversal of fenestration flow, lower left atrial pressure, and improve cardiac output and clinical status in Fontan patients previously in junctional rhythm or undergoing ventricular pacing (89). In a recent study, Alnoor et al. reported 7 patients with Fontan physiology and JR undergoing cardiac catheterization (97). Hemodynamic measurements were performed in JR and during AP∼10 beats faster than the JR rate. JR was associated with lower cardiac output and elevated central venous pressure, which improved with AP (97). AP increased CO (by∼23%), from 2.7 ± 0.8 (in JR) to 3.5 ± 1 L/min/m2 (97). AP also decreased left atrial pressure (from 8.8 ± 2.6 to 5.5 ± 2.9 mmHg) and increased the pulmonary blood flow (97).
Not all studies, however, have shown a benefit to pacing. Ventricular pacing, in particular, seems to be problematic. A large Pediatric Heart Network study showed that ventricularly paced Fontan patients had worse clinical outcomes and decreased ventricular function compared to those who were not paced (93). Multiple studies have concluded that asynchronous ventricular pacing is particularly deleterious (91, 92, 98–100). Barber et al. found that systemic blood flow was significantly lower with VOO vs. AOO or DOO pacing and theorized that pacing from the ventricle leads to similar physiology to junctional rhythm, where the loss of synchrony leads to a loss of atrial kick (and thus decreased preload to the single ventricle and further reduction in stroke volume) (91). Poh et al. examined the Australian and New Zealand Fontan registry and demonstrated that amongst all paced patients, those with QRS interval >130 ms on electrocardiogram were at greater risk of death and transplantation (92). Furthermore, they found that patients who had ventricular pacing >50% of the time were at higher risk of death, transplantation, and moderate to severe systemic ventricular dysfunction at latest follow-up (92). Poh also showed that atrial pacing was not associated with any of these deleterious effects (92).
Although there is evidence of benefit to AP in Fontan patients, pacemaker placement itself can be technically complex in this population. In ECC Fontan patients, where the conduit completely bypasses atrial tissue, lead placement requires a thoracotomy or other innovative approaches to reach the atria (101). Therefore, endocardial pacing (with transvenous leads to the atrium) often can only be accomplished in patients whom atrial tissue is present inside the Fontan circuit—the APC or ILT Fontan. Endocardial leads also promote thrombus formation around the lead (which sits inside the sluggish and passive venous blood flow of the Fontan circulation) with embolization to the lungs or the systemic circulation being a possibility (102, 103). Given the more significant risk for morbidity and, potentially, mortality in this population, the decision to pace must be done with much thought and consideration.
Heart block
In single ventricle patients, who tend to be even more heart rate dependent (due to lack of a subpulmonary ventricle to aid preload to the systemic ventricle and thus stroke volume), it follows that heart block would be particularly poorly tolerated. NPC-QIC single-ventricle database studies have supported this, with reports of 39% mortality by 12 months of age in those with surgical heart block—a more likely event compared to those single ventricle patients without heart block (OR 4.9, 95% GI 1.4–17.5, p = 0.01) (104). The resulting loss of atrioventricular synchrony is additionally problematic in Fontan patients, with studies showing association with increased hepatic pressures and clinical worsening (89). Chronic ventricular pacing has also been shown to be detrimental to single ventricle function over time. Bulic et al. evaluated 22 paced vs. 53 control (non-paced single ventricle) patients longitudinally in terms of clinical and echocardiographic parameter changes (100). The authors found that the paced patients were more likely to develop moderate to severe systolic dysfunction (68 vs. 15%, p < 0.01) and atrioventricular valve regurgitation (65 vs. 21%, p < 0.01) as well as require heart failure medications (65 vs. 21%, p < 0.01) or experience death or heart transplantation (odds ratio 4.9, 9% CI 1.05–22.7, p = 0.04) (100).
Histologic and pathologic causes for worsening ventricular function and outcomes in ventricularly paced patients have been described (105) and single ventricles with unique pressure and volume-loading challenges would seem at particular risk. To navigate this “rock and a hard place,” cardiac resynchronization therapy (CRT) has been proposed. Effectiveness, however, at least compared to in other patient populations, has not been extensively described. As an example, Dubin et al. reported that amongst 7 single-ventricle patients who underwent CRT/multisite resynchronization in their study, no significant improvement in systolic function was seen (106). Difficulties in understanding dyssynchrony in this patient population, as well as concrete issues such as exact location of lead placement, were postulated to be reasons for this lack of response (106). A larger review compared n = 19 CRT paced Fontan patients vs. n = 43 who were single-site ventricular paced (19). While there was no statistical difference in long-term outcomes, including ventricular systolic function and mortality, the authors acknowledged their study was likely underpowered to demonstrate the trend toward better outcomes in the CRT Fontans (19). Published guidelines including the 2021 PACES Expert Consensus Statement help with decision making in these complex patients (107). Pacing is a class I indication in patients with complex congenital heart disease with heart block resulting in hemodynamic compromise or a mean ventricular rate less than 60–70 bpm (107). This is a lower threshold than in patients without congenital heart disease, where a recommended mean ventricular rate <50 bpm is advised (107). The expert panel notes the additional functional and structural lesions, as well as more easily compromised hemodynamics, as reasons behind this difference.
Monitoring and surveillance
There is little evidence to guide the frequency of monitoring for arrhythmias in Fontan patients. The 2019 American Heart Association guidelines recommend Holter monitoring every 2–3 years in children and every 1–2 years in adolescents and adults (1). However, the guidelines were not rooted in strong evidence and instead on the consensus of the authors (1). In a survey of centers with existing Fontan care programs, 27% obtain Holter monitoring annually while 36% of centers perform them every other year (108).
Saley et al. conducted a retrospective review of their institutional Fontan ambulatory rhythm surveillance program to study its utility (109). The protocol included routine use of ambulatory rhythm monitors at ages 6, 10, 13, 16, and 19 years of age (109). Eighty-three patients were included in the study with a total of 134 unique studies. Routine surveillance was the indication for 56% of the studies, with the remainder ordered for an abnormal electrocardiogram (28%), prior arrhythmias (36%), or reported symptoms (36%) (109). Indicated studies were more likely to find arrhythmia than surveillance studies (52% vs. 26%, p < 0.01) (109). Occult arrhythmias (not anticipated, found on surveillance studies) were responsible for 39% of positive ambulatory tests. The most common occult arrhythmia was SVT (10/23, 43%) with ventricular ectopy (9/23, 39%), accelerated junctional rhythm (3/23, 13%), and SND (1/23, 4%) also found (109). The most common arrhythmia on clinically indicated studies was accelerated junctional rhythm (15/45, 33%), with SVT (13/45, 29%), complex ventricular ectopy (11/45, 24%), Wenckebach rhythm (4/45, 9%), and SND (2/45, 4%) also being reported (109). Arrhythmia surveillance was increased in response to three (15%) studies with occult arrhythmias (1 SVT, 2 complex ventricular ectopy), and detection of any arrhythmia (occult or symptomatic) led to a change in clinical management in 31% of positive tests (14 patients with increased surveillance with the remaining two resulting in medications changes) (109).
Another study from Czosek et al. examined the utility of Holter monitoring in several different patient populations including those with Fontan physiology (110). Of the n = 51 Fontan patients undergoing a total of 148 Holter studies, 79% of the studies were obtained in asymptomatic patients (110). Of this Fontan cohort, the researchers found that only 9 of the 148 (6%) Holters lead to a change in clinical management (110). Of these, 5 of the patients underwent electrophysiology studies, four received pacemakers, and one had an implantable cardioverter-defibrillator device placed (110). The study examined the predictive values of Holter monitoring for detection of a significant arrhythmia event, including SVT, VT, SCD, and a history of appropriate ICD device therapy. The positive predictive value was poor (8%), however the negative predictive value was high (94%) (110). The study did not comment on a suggested frequency of monitoring. As such, following reported guidelines remains reasonable for now, with the hope that further studies will better inform these decisions over time.
Conclusions
While the full scope of both the prevalence as well as the clinical implications of bradyarrhythmias in the Fontan population are still poorly defined, there is undoubtedly evidence that it is a common complication of the procedure and is likely underappreciated. Despite modifications to the Fontan procedure, arrythmias persist. It appears that the incidence of sinus node dysfunction increases as a function of time with many progressing to junctional rhythm. While pacing has been considered as a therapy for this patient population, the evidence to support this practice is inconclusive. Current guidelines recommend surveillance with Holter monitor every 2–3 years in adolescents and every 1–2 years in adults. Future directions for research include further assessment of junctional rhythm and its management as well as further identifying patients in which pacing would be beneficial.
Statements
Author contributions
KW: Conceptualization, Writing – original draft, Writing – review & editing. CH: Conceptualization, Supervision, Writing – original draft, Writing – review & editing. RD: Conceptualization, Writing – original draft, Writing – review & editing. SB: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
SB is a consultant to Milestone pharmaceuticals and Tenaya pharmaceuticals.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Rychik J Atz AM Celermajer DS Deal BJ Gatzoulis MA Gewillig MH et al Evaluation and management of the child and adult with fontan circulation: a scientific statement from the American Heart Association. Circulation. (2019) 140(6):e234–84. 10.1161/CIR.0000000000000696
2.
Deal BJ Mavroudis C Backer CL . Arrhythmia management in the fontan patient. Pediatr Cardiol. (2007) 28:448–56. 10.1007/s00246-007-9005-2
3.
Kramer CC Maldonado JR Olson MD Gingerich JC Ochoa LA Law IH . Atrial antitachycardia pacing in complex congenital heart disease: a case series. J Innov Card Rhythm Manag. (2018) 9(3):3079. 10.19102/icrm.2018.090304
4.
Atallah J Collins KK Jonas RA Mayer Jr JE Triedman JK . Follow-up of a modified fontan randomized trial for intraatrial reentrant tachycardia prophylaxis. Congenit Heart Dis. (2012) 7(3):219–25. 10.1111/j.1747-0803.2012.00636.x
5.
Quinton E Nightingale P Hudsmith L Thorne S Marshall H Clift P et al Prevalence of atrial tachyarrhythmia in adults after fontan operation. Heart. (2015) 101(20):1672–7. 10.1136/heartjnl-2015-307514
6.
Pundi KN Pundi KN Johnson JN Dearani JA Li Z Driscoll DJ et al Sudden cardiac death and late arrhythmias after the fontan operation. Congenit Heart Dis. (2017) 12(1):17–23. 10.1111/chd.12401
7.
Dennis M Zannino D du Plessis K Bullock A Disney PJ Radford DJ et al Clinical outcomes in adolescents and adults after the fontan procedure. J Am Coll Cardiol. (2018) 71(9):1009–17. 10.1016/j.jacc.2017.12.054
8.
Backer CL Mavroudis C . 149 fontan conversions. Methodist Debakey Cardiovasc J. (2021) 15(2):105. 10.14797/mdcj-15-2-105
9.
Poh CL Zannino D Weintraub RG Winlaw DS Grigg LE Cordina R et al Three decades later: the fate of the population of patients who underwent the atriopulmonary fontan procedure. Int J Cardiol. (2017) 231:99–104. 10.1016/j.ijcard.2017.01.057
10.
Rijnberg FM Blom NA Sojak V Bruggemans EF Kuipers IM Rammeloo LA et al A 45-year experience with the fontan procedure: tachyarrhythmia, an important sign for adverse outcome. Interact Cardiovasc Thorac Surg. (2019) 29(3):461–8. 10.1093/icvts/ivz111
11.
Kilner P de Leval M . A model to simulate the effects of right heart pulsatile flow after modified fontain procedure. Br Heart J. (1993) 69(1):93. 10.1136/hrt.69.1.93-a
12.
Gewillig M Wyse RK de Leval MR Deanfield JE . Early and late arrhythmias after the fontan operation: predisposing factors and clinical consequences. Heart. (1992) 67(1):72–9. 10.1136/hrt.67.1.72
13.
Giacone HM Chubb H Dubin AM Motonaga KS Ceresnak SR Goodyer WR et al Outcomes after development of ventricular arrhythmias in single ventricular heart disease patients with fontan palliation. Circ Arrhythm Electrophysiol. (2023) 16(6):e011143. 10.1161/CIRCEP.122.011143
14.
Marcelletti CF Iorio FS Abella RF . Late results of extracardiac fontan repair. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. (1999) 2:131–41. 10.1016/S1092-9126(99)70014-1
15.
Rathod RH Prakash A Powell AJ Geva T . Myocardial fibrosis identified by cardiac magnetic resonance late gadolinium enhancement is associated with adverse ventricular mechanics and ventricular tachycardia late after fontan operation. J Am Coll Cardiol. (2010) 55(16):1721–8. 10.1016/j.jacc.2009.12.036
16.
Diller GP Giardini A Dimopoulos K Gargiulo G Müller J Derrick G et al Predictors of morbidity and mortality in contemporary fontan patients: results from a multicenter study including cardiopulmonary exercise testing in 321 patients. Eur Heart J. (2010) 31(24):3073–83. 10.1093/eurheartj/ehq356
17.
Stout KK Daniels CJ Aboulhosn JA Bozkurt B Broberg CS Colman JM et al 2018 AHA/ACC guideline for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. (2019) 73(12):e81–e192. 10.1016/j.jacc.2018.08.1029
18.
Li D Fan Q Hirata Y Ono M An Q . Arrhythmias after fontan operation with intra-atrial lateral tunnel versus extra-cardiac conduit: a systematic review and meta-analysis. Pediatr Cardiol. (2017) 38:873–80. 10.1007/s00246-017-1595-8
19.
O’Leary ET Gauvreau K Alexander ME Banka P Bezzerides VJ Fynn-Thompson F et al Dual-site ventricular pacing in patients with fontan physiology and heart block: does it mitigate the detrimental effects of single-site ventricular pacing? JACC Clin Electrophysiol. (2018) 4(10):1289–97. 10.1016/j.jacep.2018.07.004
20.
Li D Li M Zhou X An Q . Comparison of the fenestrated and non-fenestrated fontan procedures: a meta-analysis. Medicine (Baltimore). (2019) 98(29):e16554. 10.1097/MD.0000000000016554
21.
Moore BM Anderson R Nisbet AM Kalla M du Plessis K d’Udekem Y et al Ablation of atrial arrhythmias after the atriopulmonary fontan procedure: mechanisms of arrhythmia and outcomes. JACC. (2018) 4(10):1338–46. 10.1016/j.jacep.2018.08.012
22.
Laubham M Blais B Kamp AN . Atrial arrhythmias in adults with fontan palliation. Cardiol Ther. (2023) 12(3):473–87. 10.1007/s40119-023-00326-5
23.
Stephenson EA Lu M Berul CI Etheridge SP Idriss SF Margossian R et al Arrhythmias in a contemporary fontan cohort: prevalence and clinical associations in a multicenter cross-sectional study. J Am Coll Cardiol. (2010) 56(11):890–6. 10.1016/j.jacc.2010.03.079
24.
Giannakoulas G Dimopoulos K Yuksel S Inuzuka R Pijuan-Domenech A Hussain W et al Atrial tachyarrhythmias late after fontan operation are related to increase in mortality and hospitalization. Int J Cardiol. (2012) 157(2):221–6. 10.1016/j.ijcard.2010.12.049
25.
d’Udekem Y Iyengar AJ Galati JC Forsdick V Weintraub RG Wheaton GR et al Redefining expectations of long-term survival after the fontan procedure: twenty-five years of follow-up from the entire population of Australia and New Zealand. Circulation. (2014) 130(11_suppl_1):S32–8. 10.1161/CIRCULATIONAHA.113.007764
26.
Lin Z Ge H Xue J Wu G Du J Hu X et al Comparison of extracardiac conduit and lateral tunnel for functional single-ventricle patients: a meta-analysis. Congenit Heart Dis. (2017) 12(6):711–20. 10.1111/chd.12503
27.
Bossers SS Duppen N Kapusta L Maan AC Duim AR Bogers AJ et al Comprehensive rhythm evaluation in a large contemporary fontan population. Eur J Cardiothorac Surg. (2015) 48(6):833–41. 10.1093/ejcts/ezu548
28.
Atz AM Zak V Mahony L Uzark K D’agincourt N Goldberg DJ et al Longitudinal outcomes of patients with single ventricle after the fontan procedure. J Am Coll Cardiol. (2017) 69(22):2735–44. 10.1016/j.jacc.2017.03.582
29.
Di Mambro C Yammine ML Tamborrino PP Giordano U Righi D Unolt M et al Long-term incidence of arrhythmias in extracardiac conduit fontan and comparison between systemic left and right ventricle. Europace. (2024) 26(5):euae097. 10.1093/europace/euae097
30.
Durongpisitkul K Porter CBJ Cetta F Offord KP Slezak JM Puga FJ et al Predictors of early-and late-onset supraventricular tachyarrhythmias after fontan operation. Circulation. (1998) 98(11):1099–107. 10.1161/01.CIR.98.11.1099
31.
Ohuchi H . Adult patients with fontan circulation: what we know and how to manage adults with fontan circulation?J Cardiol. (2016) 68(3):181–9. 10.1016/j.jjcc.2016.04.001
32.
Kürer CC Tanner CS Vetter VL . Electrophysiologic findings after fontan repair of functional single ventricle. J Am Coll Cardiol. (1991) 17(1):174–81. 10.1016/0735-1097(91)90723-M
33.
Carins TA Shi WY Iyengar AJ Nisbet A Forsdick V Zannino D et al Long-term outcomes after first-onset arrhythmia in fontan physiology. J Thorac Cardiovasc Surg. (2016) 152(5):1355–63. 10.1016/j.jtcvs.2016.07.073
34.
Takeuchi D Toyohara K Kudo Y Nishimura T Shoda M . Impact of preoperative electrophysiological intervention on occurrence of peri/postoperative supraventricular tachycardia following fontan surgery. Heart Rhythm. (2021) 18(1):34–40. 10.1016/j.hrthm.2020.08.003
35.
Khairy P Van Hare GF Balaji S Berul CI Cecchin F Cohen MI et al PACES/HRS expert consensus statement on the recognition and management of arrhythmias in adult congenital heart disease: developed in partnership between the pediatric and congenital electrophysiology society (PACES) and the heart rhythm society (HRS). endorsed by the governing bodies of PACES, HRS, the American College of Cardiology (ACC), the American Heart Association (AHA), the European heart rhythm association (EHRA), the Canadian heart rhythm society (CHRS), and the international society for adult. Heart Rhythm. (2014) 11(10):e102–65. 10.1016/j.hrthm.2014.05.009
36.
Egbe AC Connolly HM Khan AR Niaz T Said SS Dearani JA et al Outcomes in adult fontan patients with atrial tachyarrhythmias. Am Heart J. (2017) 186:12–20. 10.1016/j.ahj.2016.12.015
37.
Koyak Z Kroon B de Groot JR Wagenaar LJ van Dijk AP Mulder BA et al Efficacy of antiarrhythmic drugs in adults with congenital heart disease and supraventricular tachycardias. Am J Cardiol. (2013) 112(9):1461–7. 10.1016/j.amjcard.2013.07.029
38.
Egbe AC Asirvatham SJ Connolly HM Kapa S Desimone CV Vaidya VR et al Outcomes of direct current cardioversion in adults with congenital heart disease. Am J Cardiol. (2017) 119(9):1468–72. 10.1016/j.amjcard.2017.01.018
39.
Egbe AC Connolly HM Niaz T McLeod CJ . Outcome of direct current cardioversion for atrial arrhythmia in adult fontan patients. Int J Cardiol. (2016) 208:115–9. 10.1016/j.ijcard.2016.01.209
40.
Hebe J Hansen P Ouyang F Volkmer M Kuck KH . Radiofrequency catheter ablation of tachycardia in patients with congenital heart disease. Pediatr Cardiol. (2000) 21:557–75. 10.1007/s002460010134
41.
Weipert J Noebauer C Schreiber C Kostolny M Zrenner B Wacker A et al Occurrence and management of atrial arrhythmia after long-term fontan circulation. J Thorac Cardiovasc Surg. (2004) 127(2):457–64. 10.1016/j.jtcvs.2003.08.054
42.
Correa R Sherwin ED Kovach J Mah DY Alexander ME Cecchin F et al Mechanism and ablation of arrhythmia following total cavopulmonary connection. Circ Arrhythm Electrophysiol. (2015) 8(2):318–25. 10.1161/CIRCEP.114.001758
43.
Radbill AE Triedman JK Berul CI Fynn-Thompson F Atallah J Alexander ME et al System survival of nontransvenous implantable cardioverter-defibrillators compared to transvenous implantable cardioverter-defibrillators in pediatric and congenital heart disease patients. Heart Rhythm. (2010) 7(2):193–8. 10.1016/j.hrthm.2009.10.014
44.
Nery PB Green MS Khairy P Alhebaishi Y Hendry P Birnie DH . Implantable cardioverter-defibrillator insertion in congenital heart disease without transvenous access to the heart. Can J Cardiol. (2013) 29(2):254–e1. 10.1016/j.cjca.2012.04.020
45.
Chubb H Rosenthal E . Implantable cardioverter-defibrillators in congenital heart disease. Herzschrittmacherther Elektrophysiol. (2016) 27(2):95–103. 10.1007/s00399-016-0437-3
46.
Cannon BC Friedman RA Fenrich AL Fraser CD McKENZIE ED Kertesz NJ . Innovative techniques for placement of implantable cardioverter-defibrillator leads in patients with limited venous access to the heart. Pacing Clin Electrophysiol. (2006) 29(2):181–7. 10.1111/j.1540-8159.2006.00314.x
47.
Moore JP Mondésert B Lloyd MS Cook SC Zaidi AN Pass RH et al Clinical experience with the subcutaneous implantable cardioverter–defibrillator in adults with congenital heart disease. Circ Arrhythm Electrophysiol. (2016) 9(9):e004338. 10.1161/CIRCEP.116.004338
48.
Miyazaki A Ohuchi H Kurosaki KI Kamakura S Yagihara T Yamada O . Efficacy and safety of sotalol for refractory tachyarrhythmias in congenital heart disease. Circ J. (2008) 72(12):1998–2003. 10.1253/circj.CJ-08-0194
49.
Ibrahim MA Kerndt CC Tivakaran VS . Dofetilide. (2017).
50.
Moore BM Cordina RL McGuire MA Celermajer DS . Adverse effects of amiodarone therapy in adults with congenital heart disease. Congenit Heart Dis. (2018) 13(6):944–51. 10.1111/chd.12657
51.
Mavroudis C Deal BJ Backer CL Stewart RD Franklin WH Tsao S et al 111 fontan conversions with arrhythmia surgery: surgical lessons and outcomes. Ann Thorac Surg. (2007) 84(5):1457–66. 10.1016/j.athoracsur.2007.06.079
52.
Deal BJ Costello JM Webster G Tsao S Backer CL Mavroudis C . Intermediate-term outcome of 140 consecutive fontan conversions with arrhythmia operations. Ann Thorac Surg. (2016) 101(2):717–24. 10.1016/j.athoracsur.2015.09.017
53.
Khairy P Fernandes SM Mayer JE Jr Triedman JK Walsh EP Lock JE et al Long-term survival, modes of death, and predictors of mortality in patients with fontan surgery. Circulation. (2008) 117(1):85–92. 10.1161/CIRCULATIONAHA.107.738559
54.
Van De Bruaene A Claessen G Salaets T Gewillig M . Late fontan circulatory failure. What drives systemic venous congestion and low cardiac output in adult fontan patients?. Frontiers in Cardiovascular Medicine. (2022) 9:825472. 10.3389/fcvm.2022.825472
55.
Gordon-Walker TT Bove K Veldtman G . Fontan-associated liver disease: a review. J Cardiol. (2019) 74(3):223–32. 10.1016/j.jjcc.2019.02.016
56.
Bae EJ Lee JY Noh CI Kim WH Kim YJ . Sinus node dysfunction after fontan modifications—influence of surgical method. Int J Cardiol. (2003) 88(2-3):285–91. 10.1016/S0167-5273(02)00530-2
57.
Blaufox AD Sleeper LA Bradley DJ Breitbart RE Hordof A Kanter RJ et al Functional status, heart rate, and rhythm abnormalities in 521 fontan patients 6 to 18 years of age. J Thorac Cardiovasc Surg. (2008) 136(1):100–7. 10.1016/j.jtcvs.2007.12.024
58.
Dilawar M Bradley SM Saul JP Stroud MR Balaji S . Sinus node dysfunction after intraatrial lateral tunnel and extracardiac conduit fontan procedures: a study of 24-hour holter recordings. Pediatr Cardiol. (2003) 24(3):284–9. 10.1007/s00246-002-0238-9
59.
Kumar SP Rubinstein CS Simsic JM Taylor AB Saul JP Bradley SM . Lateral tunnel versus extracardiac conduit fontan procedure: a concurrent comparison. Ann Thorac Surg. (2003) 76(5):1389–97. 10.1016/S0003-4975(03)01010-5
60.
Nürnberg JH Ovroutski S Alexi-Meskishvili V Ewert P Hetzer R Lange PE . New onset arrhythmias after the extracardiac conduit fontan operation compared with the intraatrial lateral tunnel procedure: early and midterm results. Ann Thorac Surg. (2004) 78(6):1979–88. 10.1016/j.athoracsur.2004.02.107
61.
Okólska M Karkowski G Kuniewicz M Bednarek J Pająk J Róg B et al Prevalence of arrhythmia in adults after fontan operation. J Clin Med. (2022) 11(7):1968. 10.3390/jcm11071968
62.
Fishberger SB Wernovsky G Gentles TL Gauvreau K Burnetta J Mayer Jr JE et al Factors that influence the development of atrial flutter after the fontan operation. J Thorac Cardiovasc Surg. (1997) 113(1):80–6. 10.1016/S0022-5223(97)70402-1
63.
Cohen MI Wernovsky G Vetter VL Wieand TS Gaynor JW Jacobs ML et al Sinus node function after a systematically staged fontan procedure. Circulation. (1998) 98(19 Suppl):II352–8.
64.
Dulfer K Bossers SS Utens EM Duppen N Kuipers IM Kapusta L et al Does functional health status predict health-related quality of life in children after fontan operation? Cardiol Young. (2016) 26(3):459–68. 10.1017/S1047951115000426
65.
Amodeo A Galletti L Marianeschi S Picardo S Giannico S Di Renzi P et al Extracardiac fontan operation for complex cardiac anomalies: seven years’ experience. J Thorac Cardiovasc Surg. (1997) 114(6):1020–31. 10.1016/S0022-5223(97)70016-3
66.
Petrossian ED Reddy VM McElhinney DB Akkersdijk GP Moore P Parry AJ et al Early results of the extracardiac conduit fontan operation. J Thorac Cardiovasc Surg. (1999) 117(4):688–96. 10.1016/S0022-5223(99)70288-6
67.
Giannico S Hammad F Amodeo A Michielon G Drago F Turchetta A et al Clinical outcome of 193 extracardiac fontan patients: the first 15 years. J Am Coll Cardiol. (2006) 47(10):2065–73. 10.1016/j.jacc.2005.12.065
68.
Lee JR Kwak J Kim KC Min SK Kim WH Kim YJ et al Comparison of lateral tunnel and extracardiac conduit fontan procedure. Interact Cardiovasc Thorac Surg. (2007) 6(3):328–30. 10.1510/icvts.2006.146928
69.
Rajanbabu BB Gangopadhyay D . Sinus node dysfunction after extracardiac conduit and lateral tunnel fontan operation: the importance of the type of prior superior cavopulmonary anastomosis. World J Pediatr Congenit Heart Surg. (2016) 7(2):210–5. 10.1177/2150135115616370
70.
Cohen MI Bridges ND Gaynor JW Hoffman TM Wernovsky G Vetter VL et al Modifications to the cavopulmonary anastomosis do not eliminate early sinus node dysfunction. J Thorac Cardiovasc Surg. (2000) 120(5):891–901. 10.1067/mtc.2000.109708
71.
Balaji S Daga A Bradley DJ Etheridge SP Law IH Batra AS et al An international multicenter study comparing arrhythmia prevalence between the intracardiac lateral tunnel and the extracardiac conduit type of fontan operations. J Thorac Cardiovasc Surg. (2014) 148(2):576–81. 10.1016/j.jtcvs.2013.08.070
72.
Brown JW Ruzmetov M Deschner BW Rodefeld MD Turrentine MW . Lateral tunnel fontan in the current era: is it still a good option?Ann Thorac Surg. (2010) 89(2):556–63. 10.1016/j.athoracsur.2009.10.050
73.
Sarkis V Sreeram N Trieschmann U Mime LB Bennink G . Comparison of arrhythmia incidence after the extracardiac conduit versus the intracardiac lateral tunnel fontan completion. Int J Cardiol. (2011) 146(2):258–9. 10.1016/j.ijcard.2010.10.070
74.
Azakie A McCrindle BW Van Arsdell G Benson LN Coles J Hamilton R et al Extracardiac conduit versus lateral tunnel cavopulmonary connections at a single institution: impact on outcomes. J Thorac Cardiovasc Surg. (2001) 122(6):1219–28. 10.1067/mtc.2001.116947
75.
Fiore AC Turrentine M Rodefeld M Vijay P Schwartz TL Virgo KS et al Fontan operation: a comparison of lateral tunnel with extracardiac conduit. Ann Thorac Surg. (2007) 83(2):622–30. 10.1016/j.athoracsur.2006.09.070
76.
Morales DL Dibardino DJ Braud BE Fenrich AL Heinle JS Vaughn WK et al Salvaging the failing fontan: lateral tunnel versus extracardiac conduit. Ann Thorac Surg. (2005) 80(4):1445–52. 10.1016/j.athoracsur.2005.03.112
77.
Hakacova N Lakomy M Kovacikova L . Arrhythmias after fontan operation: comparison of lateral tunnel and extracardiac conduit. J Electrocardiol. (2008) 41(2):173–7. 10.1016/j.jelectrocard.2007.10.007
78.
Hirsch JC Goldberg C Bove EL Salehian S Lee T Ohye RG et al Fontan operation in the current era: a 15-year single institution experience. Ann Surg. (2008) 248(3):402–10. 10.1097/SLA.0b013e3181858286
79.
Shirai LK Rosenthal DN Reitz BA Robbins RC Dubin AM . Arrhythmias and thromboembolic complications after the extracardiac fontan operation. J Thorac Cardiovasc Surg. (1998) 115(3):499–505. 10.1016/S0022-5223(98)70311-3
80.
Kavey REW Gaum WE Byrum CJ Smith FC Kveselis DA . Loss of sinus rhythm after total cavopulmonary connection. Circulation. (1995) 92(9):304–8. 10.1161/01.CIR.92.9.304
81.
Powell AW Veldtman G . Heart rate responses during exercise by dominant ventricle in pediatric and young adult patients with a fontan circulation. Can J Cardiol. (2020) 36(9):1508–15. 10.1016/j.cjca.2019.10.042
82.
Claessen G La Gerche A Van De Bruaene A Claeys M Willems R Dymarkowski S et al Heart rate reserve in fontan patients: chronotropic incompetence or hemodynamic limitation? J Am Heart Assoc. (2019) 8(9):e012008. 10.1161/JAHA.119.012008
83.
Hedlund ER Söderström L Lundell B . Appropriate heart rate during exercise in fontan patients. Cardiol Young. (2020) 30(5):674–80. 10.1017/S1047951120000761
84.
Hebert A Jensen AS Mikkelsen UR Idorn L Sørensen KE Thilen U et al Hemodynamic causes of exercise intolerance in fontan patients. Int J Cardiol. (2014) 175(3):478–83. 10.1016/j.ijcard.2014.06.015
85.
Crystal GJ Salem MR . The bainbridge and the “reverse” bainbridge reflexes: history, physiology, and clinical relevance. Anesth Analg. (2012) 114(3):520–32. 10.1213/ANE.0b013e3182312e21
86.
De Azevedo IM Watanabe Y Dreifus LS . Atrioventricular junctional rhythm: classification and clinical significance. Chest. (1973) 64:732–40. 10.1378/chest.64.6.732
87.
Januszewska K Schuh A Lehner A Dalla-Pozza R Malec E . Lateral atrial tunnel fontan operation predisposes to the junctional rhythm. Pediatr Cardiol. (2017) 38:712–8. 10.1007/s00246-017-1571-3
88.
Ferrari I Shehu N Stern H Meierhofer C . Junctional rhythm produces retrograde flow in the whole fontan system: cardiac magnetic resonance–derived flow documentation—a case report. Eur Heart J Case Rep. (2023) 7(3):ytad126. 10.1093/ehjcr/ytad126
89.
Hasselman T Schneider D Madan N Jacobs M . Reversal of fenestration flow during ventricular systole in fontan patients in junctional or ventricular paced rhythm. Pediatr Cardiol. (2005) 26:638–41. 10.1007/s00246-005-0879-6
90.
Balaji S Evers PD Batra AS Moore J . Management of junctional rhythm in patients after the fontan operation: a multicenter congenital cardiology survey. Pediatr Cardiol. (2024) 45(1):63–7. 10.1007/s00246-023-03296-6
91.
Barber BJ Batra AS Burch GH Shen I Ungerleider RM Brown JW et al Acute hemodynamic effects of pacing in patients with fontan physiology: a prospective study. J Am Coll Cardiol. (2005) 46(10):1937–42. 10.1016/j.jacc.2005.07.045
92.
Poh CL Celermajer DS Grigg LE Kalman JM McGuire MA Gentles TL et al Pacemakers are associated with a higher risk of late death and transplantation in the fontan population. Int J Cardiol. (2019) 282:33–7. 10.1016/j.ijcard.2019.01.088
93.
Williams RV Travison T Kaltman JR Cecchin F Colan SD Idriss SF et al Comparison of fontan survivors with and without pacemakers: a report from the pediatric heart network fontan cross-sectional study. Congenit Heart Dis. (2013) 8(1):32–9. 10.1111/j.1747-0803.2012.00692.x
94.
Cohen MI Rhodes LA Wernovsky G Gaynor JW Spray TL Rychik J . Atrial pacing: an alternative treatment for protein-losing enteropathy after the fontan operation. J Thorac Cardiovasc Surg. (2001) 121(3):582–3. 10.1067/mtc.2001.110681
95.
Heinemann MK Gass M Breuer J Ziemer G . DDD pacemaker implantation after fontan-type operations. Pacing Clin Electrophysiol. (2003) 26(1p2):492–5. 10.1046/j.1460-9592.2003.00079.x
96.
Barber BJ Burch GH Tripple D Balaji S . Resolution of plastic bronchitis with atrial pacing in a patient with fontan physiology. Pediatr Cardiol. (2004) 25:73–6. 10.1007/s00246-003-0529-9
97.
Alnoor M Burch G Armsby L Batra A Balaji S . Hemodynamic impact of atrial pacing in patients with fontan physiology and junctional rhythm: a cardiac catheterization study. Pediatr Cardiol. (2022) 43:508–14. 10.1007/s00246-021-02747-2
98.
Kodama Y Kuraoka A Ishikawa Y Nakamura M Ushinohama H Sagawa K et al Outcome of patients with functional single ventricular heart after pacemaker implantation: what makes it poor, and what can we do? Heart Rhythm. (2019) 16(12):1870–4. 10.1016/j.hrthm.2019.06.019
99.
Chubb H Bulic A Mah D Moore JP Janousek J Fumanelli J et al Impact and modifiers of ventricular pacing in patients with single ventricle circulation. J Am Coll Cardiol. (2022) 80(9):902–14. 10.1016/j.jacc.2022.05.053
100.
Bulic A Zimmerman FJ Ceresnak SR Shetty I Motonaga KS Freter A et al Ventricular pacing in single ventricles—a bad combination. Heart Rhythm. (2017) 14(6):853–7. 10.1016/j.hrthm.2017.03.035
101.
Moore JP Shannon KM . Transpulmonary atrial pacing: an approach to transvenous pacemaker implantation after extracardiac conduit fontan surgery. J Cardiovasc Electrophysiol. (2014) 25(9):1028–31. 10.1111/jce.12447
102.
Khairy P Landzberg MJ Gatzoulis MA Mercier LA Fernandes SM Côté JM et al Transvenous pacing leads and systemic thromboemboli in patients with intracardiac shunts: a multicenter study. Circulation. (2006) 113(20):2391–7. 10.1161/CIRCULATIONAHA.106.622076
103.
El Assaad I Pastor T O’Leary E Gauvreau K Rathod RH Gurvitz M et al Atrial pacing in fontan patients: the effect of transvenous lead on clot burden. Heart Rhythm. (2021) 18(11):1860–7. 10.1016/j.hrthm.2021.06.1191
104.
Czosek RJ Anderson JB Baskar S Khoury PR Jayaram N Spar DS . Predictors and outcomes of heart block during surgical stage I palliation of patients with a single ventricle: a report from the NPC-QIC. Heart Rhythm. (2021) 18(11):1876–83. 10.1016/j.hrthm.2021.05.019
105.
Karpawich PP Rabah R Haas JE . Altered cardiac histology following apical right ventricular pacing in patients with congenital atrioventricular block. Pacing Clin Electrophysiol. (1999) 22(9):1372–7. 10.1111/j.1540-8159.1999.tb00631.x
106.
Dubin AM Janousek J Rhee E Strieper MJ Cecchin F Law IH et al Resynchronization therapy in pediatric and congenital heart disease patients: an international multicenter study. J Am Coll Cardiol. (2005) 46(12):2277–83. 10.1016/j.jacc.2005.05.096
107.
Silka MJ Shah MJ Silva JNA Balaji S Beach CM Benjamin MN et al 2021 PACES expert consensus statement on the indications and management of cardiovascular implantable electronic devices in pediatric patients: executive summary. Ann Pediatr Cardiol. (2022) 15(3):323–46. 10.4103/0974-2069.361245
108.
Di Maria MV Brown DW Cetta F Ginde S Goldberg D Menon SC et al Surveillance testing and preventive care after fontan operation: a multi-institutional survey. Pediatr Cardiol. (2019) 40:110–5. 10.1007/s00246-018-1966-9
109.
Saley TP Patel ND Bar-Cohen Y Silka MJ Hill AC . Utility of surveillance ambulatory rhythm monitoring in the pediatric fontan population. Pediatr Cardiol. (2021) 42(6):1442–8. 10.1007/s00246-021-02630-0
110.
Czosek RJ Anderson J Khoury PR Knilans TK Spar DS Marino BS . Utility of ambulatory monitoring in patients with congenital heart disease. Am J Cardiol. (2013) 111(5):723–30. 10.1016/j.amjcard.2012.11.021
Summary
Keywords
fontan, junctional rhythm, tachyarrhythmia, antiarrhythmic drugs, arrhythmia, congenital heart disease, surveillance
Citation
Wall K, Hebson C, D’Souza R and Balaji S (2025) Review of rhythm disturbances in patient after fontan completion: epidemiology, management, and surveillance. Front. Pediatr. 13:1506690. doi: 10.3389/fped.2025.1506690
Received
05 October 2024
Accepted
20 January 2025
Published
12 February 2025
Volume
13 - 2025
Edited by
Nathalie Dedieu, Paediatric Cardiology Consultant at Great Ormond Street Hospital, United Kingdom
Reviewed by
Roman A. Gebauer, Leipzig University, Germany
Floris E.A. Udink ten Cate, Radboud University Medical Center, Netherlands
Updates
Copyright
© 2025 Wall, Hebson, D'Souza and Balaji.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Seshadri Balaji balajis@ohsu.edu
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.