- 1Department of Neonatology Nursing, West China Second University Hospital, Sichuan University, Chengdu, Sichuan, China
- 2Key Laboratory of Birth Defects and Related Diseases of Women and Children (Sichuan University), Ministry of Education, Chengdu, Sichuan, China
- 3Institute of Sports Medicine and Health, Chengdu Sport University, Chengdu, Sichuan, China
Background: Bronchopulmonary dysplasia (BPD) represents the most widespread and severe form of chronic pulmonary disease in preterm infants. Studies have revealed an association between BPD and hematological parameters (HPs); however, the findings are inconsistent.
Objectives: This study utilized a systematic review and meta-analysis to summarize the association.
Methods: The Web of Science, Cochrane Library, Embase, and PubMed were retrieved up to May 4, 2024, with an update on April 10, 2025. Studies investigating the correlation between HPs and BPD in preterm infants were included. This study adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The quality of the studies was evaluated via the Newcastle-Ottawa Scale. Stata 18 and MetaDisc were utilized for statistical analysis.
Results: This meta-analysis encompassed 20 studies with a total of 4,752 participants and investigated the association between HPs and BPD in neonates. Statistically significant differences were found between BPD and non-BPD infants for eight parameters: neutrophil (NEU) count, hemoglobin (HGB), monocyte count, hematocrit (HCT), neutrophil-to-lymphocyte ratio (NLR), red blood cell (RBC) count, platelet (PLT) count, and systemic inflammatory response index (SIRI). Further correlation analysis and effect size evaluation revealed that, while some parameters differed between groups, the association between key parameters and BPD risk was not significant. Specifically, the odds ratios (ORs) for HCT (OR = 1.33, 95% confidence interval [CI]: −0.11 to 2.77), PLT (OR = 1.0, 95% CI: 0.98–1.03), and HGB (OR = 1.38, 95% CI: 0.42–4.49) did not reach statistical significance, suggesting these three parameters may not be independent influencing factors for BPD risk. Concurrently, diagnostic performance analysis demonstrated limited discriminatory ability for NLR, with a receiver operating characteristic-area under the curve (ROC-AUC) of 0.670 (standard error [SE] = 0.054), and for PLT, with an ROC-AUC of 0.675 (SE = 0.067).
Conclusions: The current study indicated significant differences in NEU, HGB, HCT, RBC, PLT, NLR, and SIRI between BPD and non-BPD patients, with elevated NEU, NLR, and SIRI and reduced HGB, HCT, RBC, and PLT. However, this study had its limitations. Further analysis requires more multicenter, large-sample prospective studies.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42024552716.
1 Introduction
Bronchopulmonary dysplasia (BPD) is the most common and serious chronic lung disorder affecting premature infants (1). The incidence of BPD ranges from 10% to 89% in extremely preterm infants (gestational age [GA] <28 weeks) worldwide, including 10%–73% in Europe, 30%–62% in Oceania, 18%–89% in North America, and 18%–82% in Asia (2). Furthermore, about 40% of infants with an ultra-low birth weight (less than 1,000 g) develop BPD (3). Although various therapies for BPD have significantly improved survival in neonates born before 28 weeks' GA, including surfactant therapy (4), postnatal steroid therapy (5), limiting the duration of invasive mechanical ventilation (6), and improved ventilation methods (e.g., noninvasive positive pressure ventilation [NIPPV] (7) or high-frequency oscillatory ventilation [HFOV]) (8), the incidence of BPD remains high. Studies have indicated that various factors, including the duration of mechanical ventilation, respiratory distress syndrome (RDS), early-onset sepsis, small for GA (SGA), patent ductus arteriosus (PDA), NLR, thrombocytopenia, and anemia, may contribute to the development of injury to the immature lungs, potentially leading to BPD. Nevertheless, the exact cause of BPD remains unclear, and effective prevention strategies are limited. Therefore, early identification and prevention of BPD are particularly important.
Current evidence suggests that preterm infants exhibit heightened pulmonary sensitivity to inflammatory stimuli due to immature alveolar development and incomplete pulmonary vascular bed structure. A persistent proinflammatory response, characterized by the infiltration of inflammatory mediators such as elastase and reactive oxygen species (ROS) released by neutrophils, can induce alveolar epithelial cell injury and pulmonary fibrosis. This ultimately culminates in BPD (9). Hematologic parameters (HPs), including neutrophil (NEU) count, neutrophil-to-lymphocyte ratio (NLR), and systemic inflammatory response index (SIRI), serve as ‘window biomarkers' that reflect systemic inflammatory status. This provides a pathophysiological basis for indirectly assessing pulmonary inflammatory imbalance via peripheral blood. Meanwhile, the current clinical practice for early BPD diagnosis relies heavily on imaging and respiratory support evaluation, lacking convenient, dynamically monitorable biomarkers. This unmet clinical need underscores the value of systematically investigating the association between HPs and BPD. Such research could facilitate the early identification of high-risk infants and inform targeted anti-inflammatory intervention strategies.
In recent years, several studies have examined the relationship between HPs and BPD. For instance, Chen et al. revealed a significant association between platelet (PLT) count and BPD in 115 preterm infants. The same study suggested that inhibiting PLT activation could relieve lung inflammation in extremely preterm infants (10). Duan et al.'s study on 149 preterm infants indicated that hemoglobin (HGB) levels ≤155 g/L during the initial three days of life were linked to an elevated risk of BPD (11). The researchers hypothesized that, during the transition from fetal to early neonatal life environment, HGB plays a significant role in adapting lung function to the new environment. Lower HGB levels may impair normal lung development and contribute to the development of BPD. Despite extensive studies on the association of HPs (such as NLR, PLT, HGB, and white blood cell [WBC] count) with BPD, the results are highly heterogeneous, and there is a lack of systematic reviews to comprehensively assess these parameters. Furthermore, the potential role of novel inflammatory composite indicators such as the monocyte-to-lymphocyte ratio (MLR) and the SIRI in BPD remains to be fully elucidated.
According to the present state of research, no study has systematically summarized and analyzed the use of HPs in BPD. Therefore, the current study adopted an evidence-based approach to evaluate the relationship between HPs and BPD via a meta-analysis that incorporated all relevant studies. The goal was to provide a reference basis for clinical treatment and prognosis.
2 Methods
2.1 Registration
This study's report was consistent with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (12). The study was also registered with the International Prospective Register of Systematic Reviews (PROSPERO) under the registration number CRD42024552716.
2.2 Data sources and search strategies
Four databases, including the Cochrane Library, Web of Science, PubMed, and Embase, were retrieved up to May 4, 2024, with no restrictions on language or publication date. The search was updated on April 10, 2025, to ensure timeliness. The search terms included “bronchopulmonary dysplasia”, “hematological parameters”, “platelet count”, “hemoglobin”, “white blood cell count”, and “hematocrit”. The specific strategy is described in Supplementary Table S1. To prevent omission, the reference lists of all relevant studies were also reviewed for additional publications.
2.3 Eligibility criteria
The eligibility criteria for the current study adhered strictly to the PECOS principles.
The following criteria were employed to include studies:
a. Population: The study population comprised neonates diagnosed with BPD using the following diagnostic criteria specified in the primary studies: the 2001 National Institute of Child Health and Human Development (NICHD) criteria, defining BPD as the need for oxygen or respiratory support at 28 days postnatal age (13); the 2018 NICHD criteria (14); and the 2019 Neonatal Research Network (NRN) definition, requiring oxygen or respiratory support at a postmenstrual age (PMA) of 36 weeks (15);
b. Exposure factors: all studied HPs, including WBC, NEU, MLR, HGB, lymphocytes, monocytes, hematocrit (HCT), red blood cell (RBC), PLT, NLR, platelet-to-lymphocyte ratio (PLR), mean platelet volume (MPV), red cell distribution width (RDW), platelet distribution width (PDW), C-reactive protein (CRP), SIRI;
c. Control: Non-BPD neonates;
d. Outcome indicators: Any of the following outcomes reported: i. Differences in HPs levels between the two groups; ii. Correlation data between HPs and outcomes; iii. Predictive data (e.g., specificity, sensitivity, or true-positive, false-positive, true-negative, false-negative), which can be utilized to calculate the diagnostic efficacy of HPs in relation to the outcomes;
e. Study type: Cohort or case-control study.
The following criteria were employed to exclude studies:
a. Conference abstracts, clinical trial registries, reviews, systematic reviews, meta-analyses, guidelines, animal experiments, and case reports;
b. BPD patients not involved;
c. Required HPs not covered;
d. Outcome indicators cannot be extracted or calculated;
e. Non-English articles.
2.4 Study selection
Two researchers (LHY and PJH) screened the articles based on eligibility criteria. After importing all relevant articles into EndNote X9 and removing duplicates, the rest were reviewed by titles and abstracts. The full texts of initially eligible articles were searched and reviewed to exclude ineligible studies. Any discrepancies were addressed via discussion, with unresolved issues adjudicated by a third researcher (DHH).
2.5 Extraction of data
Data were extracted via a predefined Microsoft Excel sheet. Two researchers (LHY and PJH) conducted the extraction independently and verified their respective results. Any discrepancies were settled via discussion, with unresolved issues adjudicated by a third researcher (DHH). The retrieved data included: (a) basic information: title, publication year, authors' names, population, country of study, source institution, and study type; (b) characteristics of included population: GA and weight of preterm infants at birth; number of BPD and non-BPD patients; weight, GA, and sex of BPD patients; and mother's gestational status; (c) type of HPs; (d) statistical methodology and outcome indicators.
2.6 Bias risk and rank certainty assessment
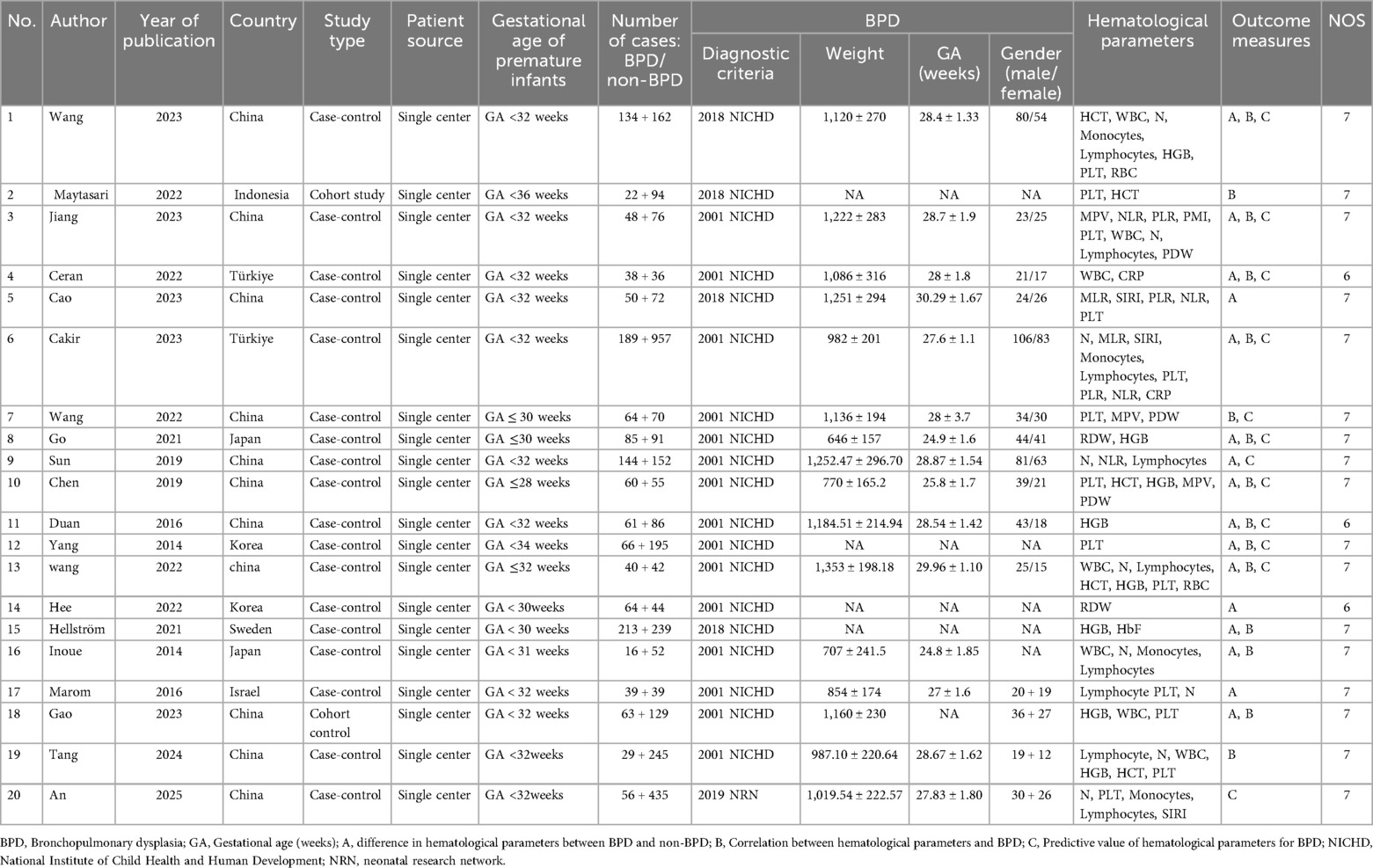
This meta-analysis included both cohort and case-control studies. Two researchers (LHY and PJH) evaluated the risk of bias (ROB) via the Newcastle-Ottawa Scale (NOS). The NOS comprises three domains: outcome, comparability, and selection (16). Any disagreements were resolved through discussion. Inter-rater agreement was evaluated using Cohen's kappa statistic, yielding a kappa coefficient exceeding 0.8. This indicates substantial consistency. All included studies had an NOS score of ≥6, indicating high quality (Table 1).
2.7 Statistical analysis
Primary outcome indicators included WBC, NEU, MLR, HGB, lymphocytes, monocytes, HCT, RBC, PLT, NLR, PLR, MPV, RDW, PDW, CRP, and SIRI in BPD and non-BPD patients. Secondary outcome indicators were the correlation analysis of PLT, HGB, and HCT with BPD and the predictive value of PLT and NLR in BPD.
Statistical heterogeneity was quantified via Cochran's Q test and Higgins' I2. P < 0.1 or I2 > 50% indicated significant heterogeneity, in which case a random-effects model (REM) was employed. Otherwise, a fixed-effects model was utilized. In case of high heterogeneity, subgroup and meta-regression analyses were performed to identify the source. For analyses of differences in HPs levels between the two groups and the associations between HPs and BPD, a meta-analysis was conducted via Stata 18.0. Weighted mean differences (WMD) and 95% confidence intervals (CIs) were computed to assess possible differences in WBC, NEU, MLR, HGB, lymphocytes, monocytes, HCT, RBC, PLT, NLR, PLR, MPV, RDW, PDW, CRP, SIRI, between the BPD and non-BPD groups. Furthermore, 95% CIs and odds ratios (ORs) were calculated to evaluate the correlation between HPs and BPD. P < 0.05 indicated statistical significance for the pooled statistics of included studies. A diagnostic meta-analysis was conducted via Meta-Disc 1.4 and Stata software (version 18.0, Stata Corporation, TX, USA). The MIDAS module of the bivariate mixed-effects model was adopted for the analysis. Pooled sensitivity, specificity, diagnostic score (DS), diagnostic odds ratio (DOR), negative likelihood ratio (LR-), and positive likelihood ratio (LR+) were calculated via forest plots. Higher DOR and DS values were linked to better diagnostic performance. The area under the curve (AUC) was obtained by plotting the summarized receiver operating characteristic (SROC) curve. The diagnostic performance was low, medium, or high when the AUC was 0.5–0.7, 0.7–0.9, or 0.9–1.0, respectively. Deeks' funnel plot was drawn to assess potential publication bias in the diagnostic outcomes across studies. A p-value greater than 0.05 suggested a lack of substantial publication bias. To investigate potential publication bias in the correlation analysis, we generated funnel plots and performed an Egger test. When significant publication bias was detected and the number of studies exceeded ten, the trim-and-fill method was implemented to assess the impact of the bias on the results.
3 Results
3.1 Article search and study selection
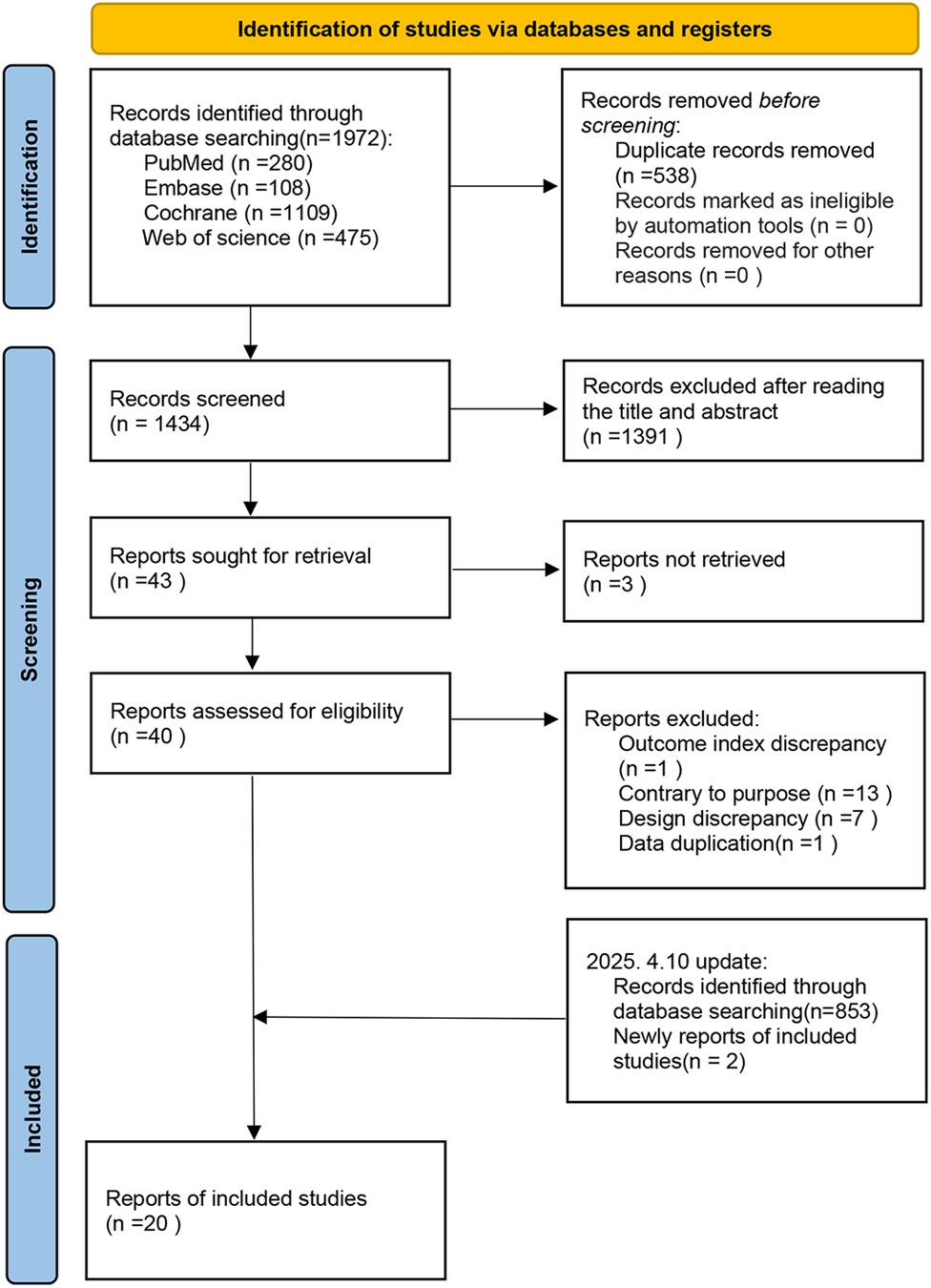
Initially, 1,972 articles were identified. After removing 538 duplicates, 1,434 articles were screened by title and abstract, excluding 1,391 records. The full texts of the remaining 43 articles were reviewed, excluding 3 for unavailable full texts, 21 for ineligibility, and 1 for duplication. Ultimately, 18 eligible articles were selected. In addition, an updated search on April 10, 2025, yielded 853 new records, and 2 additional eligible studies were found. Finally, 20 articles were included in this meta-analysis, as illustrated in Figure 1.
3.2 Characteristics of included studies
Twenty studies (10, 11, 17–34) were included in this meta-analysis. The analysis involved 4,752 participants, of whom 1,554 were BPD patients and 3,198 were non-BPD patients. Seven countries were involved, i.e., China, Indonesia, Turkey, Japan, South Korea, Sweden, and Israel. All the studies included were single-center studies, encompassing 18 case-control and 2 cohort studies. The mean weight of BPD patients was 646–1,353 g, the mean GA was 24.9–30.29 weeks, and there were more males than females. For differences in HPs with respect to BPD, seven studies examined WBC (18, 20, 25, 26, 29, 30, 34), 9 examined NEU (18, 20, 21, 24–26, 32–34), 2 examined MLR (31, 32), 9 examined lymphocytes (18, 20, 21, 24–26, 32–34), 4 examined monocytes (18, 26, 32, 33), 8 examined HGB (10, 11, 18, 20, 27–29, 34), 4 examined HCT (10, 18, 20), 2 examined RBC (18, 20), 8 examined PLT (18, 20, 24, 25, 29, 32–34), 3 examined PLR (19, 25, 32), 4 examined NLR (21, 25, 31, 32), 3 examined MPV (10, 19, 25), 3 examined PDW (10, 19, 30), 2 examined CRP (30, 32), and 3 examined SIRI (31–33). The NOS assessment demonstrated good overall quality, as detailed in Table 1.
3.3 Meta-analysis
3.3.1 Differences in HPs between the BPD and non-BPD groups
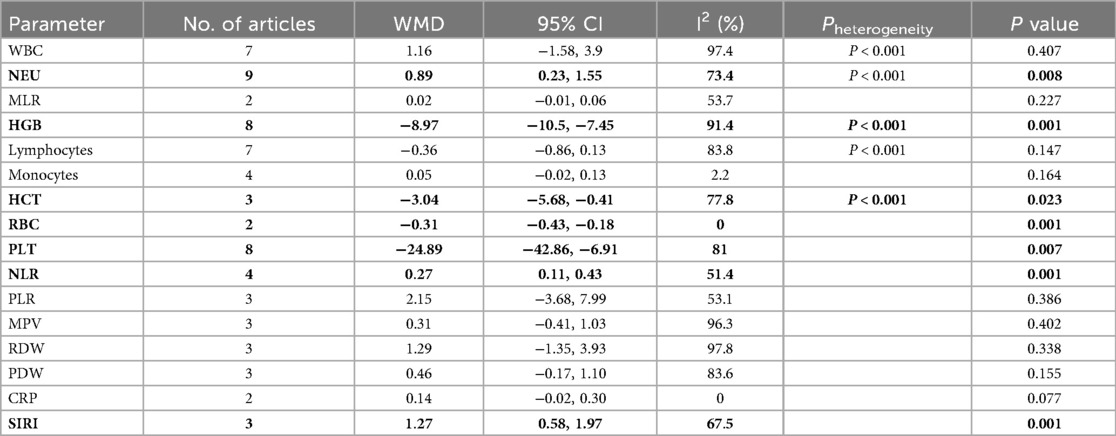
Differences in WBC, NEU, MLR, HGB, lymphocytes, monocytes, HCT, RBC, PLT, NLR, PLR, MPV, RDW, PDW, CRP, and SIRI in BPD and non-BPD patients were analyzed in the BPD and non-BPD groups. The results indicated statistical significance for NEU (WMD: 0.89, 95% CI: 0.23, 1.55, P = 0.008; Supplementary Figure 1A), HGB (WMD: −8.97, 95% CI: −10.5, −7.45, P = 0.001; Supplementary Figure 1B), HCT (WMD: −3.04, 95% CI: −5.68, −0.41, P = 0.023; Supplementary Figure 1C), RBC (WMD: −0.31, 95% CI: −0.43, −0.18, P = 0.001; Supplementary Figure 1D), PLT (WMD: −24.89, 95% CI: −42.86, −6.91, P = 0.007; Supplementary Figure 2A), NLR (WMD: 0.27, 95% CI: 0.11, 0.43, P = 0.001; Supplementary Figure 2B), and SIRI (WMD: 1.27, 95% CI: 0.58, 1.97, P = 0.001; Supplementary Figure 2C) with P < 0.05. Compared to the non-BPD group, the BPD group had elevated NEU, NLR, and SIRI, as well as reduced HGB, HCT, RBC, and PLT (Table 2).
3.3.2 Correlation analysis between HPs and BPD
Five studies examined the relationship between PLT and BPD, three studies focused on the relationship between HGB and BPD, and another three studies explored the relationship between HCT and BPD. All three sets of analyses employed an REM owing to significant statistical heterogeneity (PLT: I2 = 84.3%, P < 0.0001; HGB: I2 = 92.2%, P < 0.0001; HCT: I2 = 95.5%, P < 0.0001). The results showed that none of these indicators were significantly linked to BPD. For PLT, the OR was 1.0 with a 95% CI of (0.98, 1.03) (P > 0.05). For HGB, the OR was 1.38 with a 95% CI of (0.42, 4.49) (P > 0.05). For HCT, the OR was 1.33 with a 95% CI of (0.11, 2.77) (P > 0.05). Details are presented in Supplementary Figure 3A–C, respectively.
3.3.3 Heterogeneity exploration
To clarify the sources of heterogeneity, a stratified analysis was conducted based on two key clinical variables potentially affecting result consistency: BPD diagnostic criteria and GA, Supplementary Figures 4, 5. Stratification by diagnostic criteria significantly reduced heterogeneity solely for the NEU parameter. The overall analysis indicated high heterogeneity for NEU (I2 = 73.4%, P < 0.01). After stratifying by criteria, heterogeneity markedly decreased in the subgroup employing the “2018 NICHD criteria” (I2 = 17%, P = 0.272) and also declined in the “2001 NICHD criteria” subgroup (I2 = 40.7%, P = 0.134). Conversely, other HPs maintained high heterogeneity post-stratification: HCT (I2 = 90.1%, P < 0.01), HGB (I2 = 91.4%, P < 0.01), lymphocytes (I2 = 83.8%, P < 0.01), PLT (I2 = 81.0%, P < 0.01), and WBC (I2 = 97.4%, P < 0.01). These parameters exhibited persistently high heterogeneity across subgroups defined by the “2018 NICHD,” “2001 NICHD,” and “2019 NRN” criteria. Stratification by GA failed to substantially improve heterogeneity for any parameter, suggesting that GA differences may not be a primary driver of the observed heterogeneity.
Subgroup analysis further revealed that the diagnostic criteria directly influenced the statistical significance of the pooled results. For instance, the WMD for NEU was 2.12 (95% CI: 1.37, 2.88) under the 2018 NICHD criteria and 0.47 (95% CI: −0.07, 1.00) under the 2001 criteria. This discrepancy underscores how divergent conclusions for the same parameter can arise from different standards, potentially leading to conflicting interpretations and hindering evidence synthesis. Consequently, these findings highlight the urgent need for standardized diagnostic criteria in BPD research.
3.4 HPs for BPD diagnosis
3.4.1 NLR
Three studies examined the performance of NLR for BPD diagnosis. The results indicated that the pooled sensitivity, specificity, LR+, and LR- were 0.66 (0.60, 0.72), 0.6 (0.54, 0.65), 1.88 (1.1, 3.2), and 0.6 (0.43, 0.84), respectively (Supplementary Figures 6A–E). The ROC-AUC was 0.67 (standard error [SE] = 0.0536), as shown in Supplementary Figure 6F and Table 3.
3.4.2 PLT
Four studies examined the performance of PLT for BPD diagnosis. The results indicated that the pooled sensitivity, specificity, LR+, and LR− were 0.51 (0.45, 0.57), 0.7 (0.65, 0.75), 1.82 (1.29, 2.57), and 0.65 (0.5, 0.85), respectively (Supplementary Figures 7A–E). The ROC-AUC was 0.6745 (SE = 0.0670), as illustrated in Supplementary Figure 7F and Table 3.
3.5 Sensitivity analysis
Sensitivity analyses of WBC, NEU, HGB, lymphocytes, monocytes, HCT, PLT, NLR, PLR, MPV, RDW, PDW, and SIRI produced stable and reliable results, as displayed in Supplementary Figures 8, 9.
3.6 Publication bias
A publication bias analysis was performed using funnel plots (Supplementary Figures 10–12) for WBC, NEU, MLR, HGB, lymphocytes, monocytes, HCT, RBC, PLT, NLR, PLR, MPV, RDW, PDW, CRP, and SIRI. The results indicated possible publication bias.
4 Discussion
Bronchopulmonary dysplasia (BPD) is a frequent respiratory complication in preterm infants. It is closely linked to higher mortality, long-term lung function impairment, and lifelong effects on children (35–38). Studies have shown that systemic inflammation indicators contribute significantly to many respiratory diseases. The levels of hyperoxia exposure and inflammatory response in infants with BPD vary based on the degree of hypoxia, and the number and function of NEU, monocytes, and lymphocytes may change (39, 40). Therefore, identifying high-risk infants early and intervening promptly are crucial to reduce neonatal mortality and minimizing the incidence of BPD. Hematological tests are routinely performed on preterm infants after birth to assess their health status. Studies have demonstrated that different blood cells significantly contribute to lung inflammation and subsequent injury in preterm infants (21, 41). However, the relationship between HPs and BPD remains unclear. Further studies are urgently needed to elucidate the potential associations and clinical implications. The current study investigated the association of 16 HPs with moderate-to-severe BPD using a meta-analysis.
According to the study results, the BPD group exhibited markedly higher levels of NEU, NLR, and SIRI compared to the non-BPD group. The p-value for CRP was 0.077, approaching statistical significance and suggesting a potential upward trend in the BPD group that requires further validation. These findings support the inflammation-driven hypothesis of BPD pathogenesis and align with earlier studies indicating that BPD's pathological process is rooted in an imbalance between pro-inflammatory and anti-inflammatory factors that triggers chronic lung tissue damage (42).
To elaborate, the inflammatory response contributes to BPD development through two key pathways. First, it directly damages lung tissue. Second, it exacerbates pulmonary pathological changes by releasing cytokines and growth factors (43, 44). This effect is particularly pronounced in preterm infants whose immature lungs are highly sensitive to inflammatory factors, making them more susceptible to inflammatory injury and further promoting BPD onset (45, 46). As the main effector cells of innate immunity, NEU can directly aggravate lung tissue damage by releasing ROS and proteases (47). This explains why elevated NEU in the BPD group further reinforces the inflammation-driven mechanism of BPD (9, 47).
Notably, composite inflammatory indicators, such as NLR and SIRI, are more sensitive to systemic inflammatory imbalances than single markers (48). While the BPD group showed statistically significant differences in NLR, the limited predictive value, evidenced by a pooled effect size of WMD, may stem from inconsistent detection time points and GA across studies. From a clinical perspective, this highlights two key implications. First, the study's results reinforce the clinical relevance of targeting inflammatory pathways for BPD prevention or intervention. Second, future research must standardize study designs (e.g., unified detection time points and stratified analyses by GA) to clarify the clinical threshold of NLR and validate its utility in predicting BPD. This will address current limitations and enhance the translational value of NLR in clinical practice.
The current study indicated that the BPD group had significantly lower HGB, HCT, RBC, and PLT than the non-BPD group. While levels of PLT, HGB, and HCT were significantly lower in the BPD group, their pooled ORs did not show a significant correlational association with the risk of developing BPD. These findings might reflect deficiencies in oxygenation function in children with BPD. Chronic hypoxia stimulates an increase in erythropoietin (EPO) production (49). EPO is a key factor in regulating erythropoiesis. Its compensatory elevation may suggest an adaptive response to hypoxia (48). However, persistent hypoxia and the release of inflammatory factors can inhibit or accelerate the destruction of erythropoiesis. This exacerbates anemia and hypoxia, creating a vicious cycle. Furthermore, lower HGB levels compromise the blood's oxygen-carrying capacity (50, 51), leading to an inadequate supply of oxygen to tissues. This, in turn, increases heart rate and cardiac output, which may induce organ dysfunction (52). Preterm infants are prone to anemia due to frequent blood collection, inadequate nutrition, and other factors. Anemia can decrease the tissue oxygen supply, which increases lactate levels through anaerobic metabolism. This exacerbates pulmonary vascular remodeling and fibrosis, contributing to the progression of BPD (53, 54). Although we observed elevated NEU, NLR, and SIRI, as well as reduced HGB, HCT, RBC, and PLT, our correlation analysis indicated no significant correlation between HGB and BPD. Thus, anemia may be a concomitant phenomenon of BPD. Future studies must incorporate stratified analyses of oxygen therapy strategies, transfusion history, and other clinical factors to elucidate the complex relationship between anemia and BPD. For instance, investigating the correlation of anemia with the severity of BPD or examining how different oxygen therapy strategies affect anemia and BPD progression could provide new insights for clinical intervention.
This meta-analysis found no significant correlation of PLT with the risk of BPD. Although PLT contributes significantly to inflammation and vascular remodeling by releasing growth factors and chemokines, this study revealed no significant association between PLT and BPD. This finding might be related to the following factors: (i) PLT dynamics are influenced by various confounders (e.g., infection in preterm infants, history of transfusion, or maternal pregnancy complications), which may obscure its specific association with BPD; (ii) PLT function (rather than number) may be more directly involved in BPD pathology (e.g., PLT activation-mediated pulmonary microvascular injury); however, existing studies are limited to PLT count and lack functional data; (iii) The time window for PLT detection may affect the results. PLT was detected in the early postnatal period (e.g., within 72 h) in some studies. However, the chronic inflammatory process of BPD may not significantly affect PLT kinetics until later. Further diagnostic performance analyses indicated that PLT had limited predictive power for BPD. Its pooled sensitivity, specificity, and ROC-AUC were 0.51 (95% CI: 0.45–0.57), 0.70 (95% CI: 0.65–0.75), and 0.67 (95% CI: 0.0670), respectively, suggesting low clinical value as a biomarker alone. Nevertheless, the specificity (0.70) and LR+ (1.82) of PLT indicated that it could help exclude some non-BPD cases (e.g., when combined with other indicators). Its low sensitivity (0.51) and high LR− (0.65) suggested that relying on PLT alone resulted in a higher risk of false negatives. An ROC-AUC value of 0.67 confirmed that its predictive efficacy was insufficient, with a significant gap compared to the composite inflammation indicators (NLR, SIRI, etc.) reported in previous studies (AUC > 0.70 in general) (52). These results suggest that PLT has a limited role in predicting BPD as a single parameter, but future studies may explore combining PLT with inflammation or hypoxia indicators to improve the discriminative ability of comprehensive models.
The current study has the following strengths. First, it is the first meta-analysis to systematically assess the correlation between 16 HPs and moderate-to-severe BPD. This approach overcame the limitations of previous single-indicator or small-sample studies, potentially improving the level of evidence by integrating multicenter data. Second, this meta-analysis innovatively analyzed the composite inflammation indicators (NLR and SIRI) in combination with classical inflammation indicators (NEU). This approach potentially verified the centrality of inflammation in the pathological mechanism of BPD and provided a new direction for future biomarker studies. Third, the meta-analysis identified a significant correlation between BPD and lower HGB levels, which is consistent with the pathophysiological model of erythropoietic suppression under chronic hypoxia. Although anemia could theoretically exacerbate impaired lung development by limiting oxygen delivery, the relationship is more likely the result of shared underlying pathological processes, such as inflammation and oxidative stress. Current evidence is insufficient to establish HGB reduction as an independent factor directly impairing alveologenesis or pulmonary vascular maturation. Instead, its clinical significance may lie in signifying synergistic systemic hypoxia aggravation or serving as a surrogate marker of disease severity. Future mechanistic studies are warranted to elucidate the role of anemia in lung development.
Several key limitations warrant consideration when interpreting the associations between HPs and BPD. First, significant heterogeneity was observed in nearly all inter-study comparisons. Despite employing an REM and conducting exploratory analyses for BPD diagnostic criteria and GA, the sources of heterogeneity were not fully resolved. Potential contributors include differences in baseline birth weight and maternal pregnancy conditions, inconsistent timing of HPs assessments, and insufficient exclusion of confounding effects from neonatal infections. Incomplete reporting in the primary studies precluded further stratification to clarify these factors and may have undermined the reliability of the pooled estimates. Second, the limited number of available studies globally resulted in a small overall sample size susceptible to small-study effects and a general lack of critical clinical data, such as threshold values for HPs. This gap impedes the translation of findings into actionable clinical decision-making tools. Furthermore, the small number of included studies and inconsistent timing of blood sampling across studies precluded a meta-regression analysis comparing early vs. late time points, considering both analytical constraints and clinical applicability. Third, restricting the analysis to English-language publications may have introduced selection bias by omitting relevant non-English studies and potentially overemphasizing positive results. Finally, the confounding effect of neonatal transfusion therapy requires particular attention. Transfusion of blood products alters levels of parameters such as HGB and PLT. Inconsistent recording of transfusion timing, product type, and the interval between transfusion and blood sampling across the primary studies, coupled with a lack of statistical adjustment for this confounder, may obscure the true relationship between parameter abnormalities and transfusion-induced fluctuations on BPD risk.
5 Conclusion
This meta-analysis suggests an association between BPD and several HPs, including NEU, PLT, HGB, HCT, RBC, NLR, and SIRI. However, the effectiveness of NLR and PLT as predictive biomarkers for BPD appears limited. These findings imply that early monitoring and targeted management of inflammation-associated parameters, such as NEU and SIRI, might offer potential avenues for improving BPD outcomes in preterm infants. Nevertheless, given the outlined limitations, the pooled results primarily indicate associations rather than confirm causality and should not be directly adopted as a basis for clinical intervention. Interpretation requires caution to avoid overgeneralization.
To further elucidate the relationship between HPs and BPD and facilitate clinical translation, three key directions for future research are proposed: (i) Conduct large-sample, multicenter, high-quality case-control studies employing uniform diagnostic criteria, such as the 2018 NICHD definition, to minimize heterogeneity from criterion differences; (ii) Mandate detailed documentation of transfusion-related specifics in study designs (e.g., transfusion timing, product type, and transfusion-to-blood collection interval) and incorporate serial measurements of HPs to enable dose-response analyses for establishing critical thresholds and validating the preventive efficacy of parameter modulation through clinical intervention trials; (iii) Employ multi-language search strategies in subsequent meta-analyses to include non-English and gray literature (e.g., conference abstracts), complemented by subgroup (stratified by GA and transfusion status) and sensitivity analyses to verify the robustness of the associations. This will ultimately provide more reliable evidence for BPD prediction and intervention in neonates.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
HL: Writing – original draft, Software, Data curation, Methodology, Conceptualization. JF: Data curation, Supervision, Writing – review & editing. HD: Validation, Writing – review & editing, Software. JP: Writing – review & editing, Data curation, Visualization.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2025.1657314/full#supplementary-material
Abbreviations
BPD, bronchopulmonary dysplasia; HPs, hematological parameters; GA, gestational age; NEU, neutrophil; WBC, white blood cell; PLT, platelet; HGB, hemoglobin; RBC, red blood cel; NLR, neutrophil-to-lymphocyte ratio; HCT, hematocrit; MCV, mean corpuscular volume; RDW, red cell distribution width; MPV, mean platelet volume; PDW, platelet distribution width; MLR, monocyte to lymphocyte ratio; CRP, C-reactive protein; PLR, platelet-to-lymphocyte ratio; SIRI, systemic inflammation response index.
References
1. Kair LR, Leonard DT, Anderson JM. Bronchopulmonary dysplasia. Pediatr Rev. (2012) 33(6):255–63. doi: 10.1542/pir.33-6-255
2. Siffel C, Kistler KD, Lewis JFM, Sarda SP. Global incidence of bronchopulmonary dysplasia among extremely preterm infants: a systematic literature review. J Matern Fetal Neonatal Med. (2021) 34(11):1721–31. doi: 10.1080/14767058.2019.1646240
3. Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Neonatal outcomes of extremely preterm infants from the NICHD neonatal research network. Pediatrics. (2010) 126(3):443–56. doi: 10.1542/peds.2009-2959
4. Bahadue FL, Soll R. Early versus delayed selective surfactant treatment for neonatal respiratory distress syndrome. Cochrane Database Syst Rev. (2012) 11(11):Cd001456. doi: 10.1002/14651858.CD001456
5. Halliday HL, Ehrenkranz RA, Doyle LW. Early (<8 days) postnatal corticosteroids for preventing chronic lung disease in preterm infants. Cochrane Database Syst Rev. (2010) 5(1):CD001146. doi: 10.1002/14651858.CD001146.pub3
6. Vliegenthart RJS, Onland W, van Wassenaer-Leemhuis AG, De Jaegere APM, Aarnoudse-Moens CSH, van Kaam AH. Restricted ventilation associated with reduced neurodevelopmental impairment in preterm infants. Neonatology. (2017) 112(2):172–9. doi: 10.1159/000471841
7. Morley CJ, Davis PG, Doyle LW, Brion LP, Hascoet J-M, Carlin JB. Nasal CPAP or intubation at birth for very preterm infants. New Engl J Med. (2008) 358(7):700–8. doi: 10.1056/NEJMoa072788
8. Sun H, Cheng R, Kang W, Xiong H, Zhou C, Zhang Y, et al. High-frequency oscillatory ventilation versus synchronized intermittent mandatory ventilation plus pressure support in preterm infants with severe respiratory distress syndrome. Respir Care. (2014) 59(2):159–69. doi: 10.4187/respcare.02382
9. Holzfurtner L, Shahzad T, Dong Y, Rekers L, Selting A, Staude B, et al. When inflammation meets lung development—an update on the pathogenesis of bronchopulmonary dysplasia. Mol Cell Pediatr. (2022) 9(1):7. doi: 10.1186/s40348-022-00137-z
10. Chen X, Li H, Qiu X, Yang C, Walther FJ. Neonatal hematological parameters and the risk of moderate-severe bronchopulmonary dysplasia in extremely premature infants. BMC Pediatr. (2019) 19(1):138. doi: 10.1186/s12887-019-1515-6
11. Duan J, Kong X, Hua S, Zhang S, Feng Z, Zhang X. Association between hemoglobin levels in the first 3 days of life and bronchopulmonary dysplasia in preterm infants. Am J Perinatol. (2016) 33(10):998–1002. doi: 10.1055/s-0036-1583189
12. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. (2021) 10(1):89. doi: 10.1186/s13643-021-01626-4
13. Schmidt AR, Ramamoorthy C. Bronchopulmonary dysplasia. Paediatr Anaesth. (2022) 32(2):174–80. doi: 10.1111/pan.14365
14. Higgins RD, Jobe AH, Koso-Thomas M, Bancalari E, Viscardi RM, Hartert TV, et al. Bronchopulmonary dysplasia: executive summary of a workshop. J Pediatr. (2018) 197:300–8. doi: 10.1016/j.jpeds.2018.01.043
15. Jensen EA, Dysart K, Gantz MG, McDonald S, Bamat NA, Keszler M, et al. The diagnosis of bronchopulmonary dysplasia in very preterm infants. An evidence-based approach. Am J Respir Crit Care Med. (2019) 200(6):751–9. doi: 10.1164/rccm.201812-2348OC
16. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z
17. Yang JY, Cha J, Shim S-Y, Park EA. The relationship between eosinophilia and bronchopulmonary dysplasia in premature infants at less than 34 weeks’ gestation. Korean J Pediatr. (2014) 57(4):171–7. doi: 10.3345/kjp.2014.57.4.171
18. Wang X, Wang S, Chen M, Lv Y, Chen X, Yang C. The value of hematocrit for predicting bronchopulmonary dysplasia in very low birth weight preterm infants. Medicine (Baltimore). (2023) 102(39):e35056. doi: 10.1097/MD.0000000000035056
19. Wang X, Ma Y, Wang S, Dong W, Lei X. Platelet is the early predictor of bronchopulmonary dysplasia in very premature infants: an observational cohort study. BMC Pulm Med. (2022) 22(1):109. doi: 10.1186/s12890-022-01895-2
20. Wang H, Yan D, Wu Z, Geng H, Zhu X, Zhu X. Predictive values of clinical data, molecular biomarkers, and echocardiographic measurements in preterm infants with bronchopulmonary dysplasia. Front Pediatr. (2023) 10:1070858. doi: 10.3389/fped.2022.1070858
21. Sun Y, Chen C, Zhang X, Weng X, Sheng A, Zhu Y, et al. High neutrophil-to-lymphocyte ratio is an early predictor of bronchopulmonary dysplasia. Front Pediatr. (2019) 7:464. doi: 10.3389/fped.2019.00464
22. Oh SH, Do HJ, Park JS, Cho JY, Park CH. Can red cell distribution width in very low birth weight infants predict bronchopulmonary dysplasia? Medicine (United States). (2022) 101(3):e28640. doi: 10.1097/MD.0000000000028640
23. Maytasari GM, Haksari EL, Prawirohartono EP. Predictors of bronchopulmonary dysplasia in infants with birth weight less than 1500 g. Glob Pediatr Health. (2023) 10:2333794X231152199. doi: 10.1177/2333794X231152199
24. Marom R, Mimouni FB, Lubetzky R, Deutsch V, Mandel D. Absolute nucleated red blood cells counts do not predict the development of bronchopulmonary dysplasia. J Matern Fetal Neonatal Med. (2016) 29(10):1603–6. doi: 10.3109/14767058.2015.1056145
25. Jiang J, Mao Y, Wu J, Zhou Q. Relationship between hematological parameters and bronchopulmonary dysplasia in premature infants. J Int Med Res. (2023) 51(7):3000605231187802. doi: 10.1177/03000605231187802
26. Inoue H, Ohga S, Kusuda T, Kitajima J, Kinjo T, Ochiai M, et al. Serum neutrophil gelatinase-associated lipocalin as a predictor of the development of bronchopulmonary dysplasia in preterm infants. Early Hum Dev. (2013) 89(6):425–9. doi: 10.1016/j.earlhumdev.2012.12.011
27. Hellström W, Martinsson T, Hellstrom A, Morsing E, Ley D. Fetal haemoglobin and bronchopulmonary dysplasia in neonates: an observational study. Arch Dis Childhood Fetal Neonatal Ed. (2021) 106(1):F88–92. doi: 10.1136/archdischild-2020-319181
28. Go H, Ohto H, Nollet KE, Sato K, Ichikawa H, Kume Y, et al. Red cell distribution width as a predictor for bronchopulmonary dysplasia in premature infants. Sci Rep. (2021) 11(1):7221. doi: 10.1038/s41598-021-86752-8
29. Gao Y, Liu D, Guo Y, Cao M. Risk prediction of bronchopulmonary dysplasia in preterm infants by the nomogram model. Front Pediatr. (2023) 11:1117142. doi: 10.3389/fped.2023.1117142
30. Ceran B, Kadıoğlu Şimşek G, Beşer E, Tayman C, Canpolat FE, Kutman HGK. The association between βeta 2-microglobulin and bronchopulmonary dysplasia. Turkish J Biochem. (2023) 48:128–34. doi: 10.1515/tjb-2022-0133
31. Cao L, Liu X, Sun T, Zhang Y, Bao T, Cheng H, Tian Z. Predictive and diagnostic values of systemic inflammatory indices in bronchopulmonary dysplasia. Children (Basel). (2023) 11(1):24. doi: 10.3390/children11010024
32. Cakir U, Tayman C, Tugcu AU, Yildiz D. Role of systemic inflammatory indices in the prediction of moderate to severe bronchopulmonary dysplasia in preterm infants. Arch Bronconeumol. (2023) 59(4):216–22. doi: 10.1016/j.arbres.2023.01.003
33. An N, Li J, Li M. Role of systemic inflammation response index and prognostic nutritional index in the prediction of moderate-to-severe bronchopulmonary dysplasia in very preterm infants. Transl Pediatr. (2025) 14(1):52–60. doi: 10.21037/tp-24-381
34. Zhang X, Chen C, Chen C, Chen S, Lu N, Sun Y. Platelet-Related indicators: potential role in early prediction of bronchopulmonary dysplasia. Clin Exp Obstet Gynecol. (2024) 51(10):216. doi: 10.31083/j.ceog5110216
35. Lignelli E, Palumbo F, Myti D, Morty RE. Recent advances in our understanding of the mechanisms of lung alveolarization and bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. (2019) 317(6):L832–87. doi: 10.1152/ajplung.00369.2019
36. Kalikkot Thekkeveedu R, Guaman MC, Shivanna B. Bronchopulmonary dysplasia: a review of pathogenesis and pathophysiology. Respir Med. (2017) 132:170–7. doi: 10.1016/j.rmed.2017.10.014
37. Yao L, Shi Y, Zhao X, Hou A, Xing Y, Fu J, et al. Vitamin D attenuates hyperoxia-induced lung injury through downregulation of toll-like receptor 4. Int J Mol Med. (2017) 39(6):1403–8. doi: 10.3892/ijmm.2017.2961
38. Hurst JR, Beckmann J, Ni Y, Bolton CE, McEniery CM, Cockcroft JR, et al. Respiratory and cardiovascular outcomes in survivors of extremely preterm birth at 19 years. Am J Respir Crit Care Med. (2020) 202(3):422–32. doi: 10.1164/rccm.202001-0016OC
39. Cannavò L, Perrone S, Viola V, Marseglia L, Di Rosa G, Gitto E. Oxidative stress and respiratory diseases in preterm newborns. Int J Mol Sci. (2021) 22(22):12504. doi: 10.3390/ijms222212504
40. Ramani M, Bradley WE, Dell’Italia LJ, Ambalavanan N. Early exposure to hyperoxia or hypoxia adversely impacts cardiopulmonary development. Am J Respir Cell Mol Biol. (2015) 52(5):594–602. doi: 10.1165/rcmb.2013-0491OC
41. Klinger G, Levy I, Sirota L, Boyko V, Lerner-Geva L, Reichman B. Outcome of early-onset sepsis in a national cohort of very low birth weight infants. Pediatrics. (2010) 125(4):e736–40. doi: 10.1542/peds.2009-2017
42. Read B, Ethier G, Mehrem AA, Dunn M, Pelletier-Veilleux S, Davidson J, et al. Prevention of bronchopulmonary dysplasia: a cross-sectional survey of clinical practices in Canada. J Perinatol. (2022) 42(9):1255–7. doi: 10.1038/s41372-022-01395-5
43. Li H, Rosas L, Bian Z, Choi K, Cai C, Zhou X, et al. MG53 Attenuates nitrogen mustard-induced acute lung injury. J Cell Mol Med. (2022) 26(7):1886–95. doi: 10.1111/jcmm.16917
44. Savani RC. Modulators of inflammation in bronchopulmonary dysplasia. Semin Perinatol. (2018) 42(7):459–70. doi: 10.1053/j.semperi.2018.09.009
45. Zhang Z, Jiang J, Li Z, Wan W. The change of cytokines and gut microbiome in preterm infants for bronchopulmonary dysplasia. Front Microbiol. (2022) 13:804887. doi: 10.3389/fmicb.2022.804887
46. Liu YB, Yan CB, Zhang YY, Weng BW, Cai C. Risk factors for bronchopulmonary dysplasia in preterm infants and establishment of a prediction model. Zhongguo Dang Dai Er Ke Za Zhi. (2024) 26(11):1148–54. doi: 10.7499/j.issn.1008-8830.2404065
47. Huang Z, Wang H, Long J, Chun C. Neutrophil membrane-coated therapeutic liposomes for targeted treatment in acute lung injury. Int J Pharm. (2022) 624:121971. doi: 10.1016/j.ijpharm.2022.121971
48. Ninla-Aesong P, Kietdumrongwong P, Neupane SP, Puangsri P, Jongkrijak H, Chotipong P, et al. Relative value of novel systemic immune-inflammatory indices and classical hematological parameters in predicting depression, suicide attempts and treatment response. Sci Rep. (2024) 14(1):19018. doi: 10.1038/s41598-024-70097-z
49. Subahi EA, Ata F, Choudry H, Iqbal P, AlHiyari MA, Soliman AT, et al. Extramedullary haematopoiesis in patients with transfusion dependent β-thalassaemia (TDT): a systematic review. Ann Med. (2022) 54(1):764–74. doi: 10.1080/07853890.2022.2048065
50. Obeagu EI, Obeagu GU. Anemia and cerebrovascular disease: pathophysiological insights and clinical implications. Ann Med Surg (Lond). (2025) 87(6):3254–67. doi: 10.1097/MS9.0000000000002907
51. Xiu WJ, Zheng Y-Y, Wu T-T, Hou X-G, Yang Y, Ma Y-T, et al. Hemoglobin-to-Red-Cell distribution width ratio is a novel predictor of long-term patient outcomes after percutaneous coronary intervention: a retrospective cohort study. Front Cardiovasc Med. (2022) 9:726025. doi: 10.3389/fcvm.2022.726025
52. Hosmer DW Jr, Sturdivant RX. Applied Logistic Regression. Wiley Series in Probability and Statistics. Hoboken, NJ: John Wiley & Sons, Inc. (2013).
53. Singh R, Visintainer PF, Frantz ID, Shah BL, Meyer KM, Favila SA, et al. Association of necrotizing enterocolitis with anemia and packed red blood cell transfusions in preterm infants. J Perinatol. (2011) 31(3):176–82. doi: 10.1038/jp.2010.145
Keywords: bronchopulmonary dysplasia, lung dysplasia, platelets, meta-analysis, preterm infants
Citation: Li H, Fan J, Du H and Pan J (2025) Hematological parameters of bronchopulmonary dysplasia in preterm infants: a meta-analysis. Front. Pediatr. 13:1657314. doi: 10.3389/fped.2025.1657314
Received: 1 July 2025; Accepted: 30 October 2025;
Published: 17 November 2025.
Edited by:
Luca Bonadies, University of Padua, ItalyReviewed by:
San-Nan Yang, E-Da Hospital, TaiwanKristen Leeman, Boston Children’s Hospital and Harvard Medical School, United States
Copyright: © 2025 Li, Fan, Du and Pan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jihong Pan, NzkyMjcxMjI1QHFxLmNvbQ==
 Hongyu Li1,2
Hongyu Li1,2 Jia Fan
Jia Fan Jihong Pan
Jihong Pan