- Zhongjiang Maternal and Child Health-Care Hospital, Deyang, Sichuan, China,
Background: Anemia in premature infants, a high-risk category of patients, has been shown to impose significant economic and psychological burdens on families and society at large. Anemia is the most prevalent disease among pregnant women. The impact of anemia on the clinical high-risk status of premature infants remains elucidated; therefore, this study aims to investigate the risk factor of clinical high-risk infants in Chinese women based on anemia status.
Method: A retrospective analysis of the data from premature infants in four medical centres was conducted from January 2023–May 2025. The data, including demographic information, medical histories, gestational diseases, fetal development, and fetal position, were collected. The analysis to identify the factors contributing to the clinical high risk of premature infants was conducted between the anemia groups.
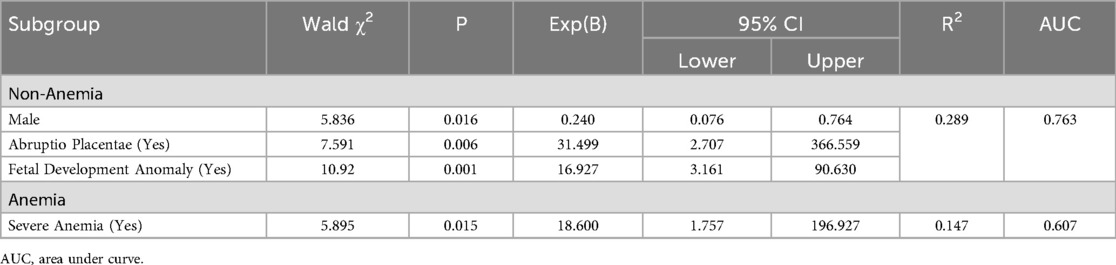
Results: A total of 191 subjects were involved, with an average age of 29.20 ± 5.00 years, but only 34 (17.8%) cases were classified as clinical high-risk. The mean weight of the infants was recorded as 2483 ± 458 g, and the mean gestational age was determined to be 34.96 ± 1.20 weeks, including 72(37.7%) females. A statistically significant variation was observed among the anemia groups concerning maternal age, hypertension, uterine abnormalities, scarred uterus, and placental abnormalities (p < 0.05). However, no statistically significant difference was found between high-risk and low-risk premature infants (p = 0.838). In the nonanemia group, a statistically significant difference was observed among the variables of gender, gestational hypertension, placental abnormalities, placental abruption, umbilical cord abnormalities, and fetal dysplasia (p < 0.05), with male was the protective factor [OR = 0.240, 95% CI = (0.076, 0.764)], while placental abruption [OR = 31.499, 95% CI = (2.707, 366.599)], and fetal dysplasia [OR = 16.927, 95% CI = (3.161, 90.630)] were risk factor. In the anemia group, mild anemia, severe anemia, and placenta previa were found to be statistically significant (p < 0.05), but only severe anemia was a high-risk factor [OR = 18.600, 95% CI = (1.757, 196.927)].
Conclusion: The findings of this study demonstrate that anemia exerts a significantly different influence on the clinical high-risk symptoms of premature infants. These differences can provide important reference points for managing pregnant women.
Introduction
Premature infants are defined as newborns born before 37 weeks of gestation. Current estimates indicate that the number of premature infants worldwide may reach 130 million by 2025, constituting approximately one-tenth of all live births (1). Moreover, the birth rate of premature infants in China is approximately 5% (2), and there has been no significant change in recent years (3). The incidence of premature infants varies geographically within China, which is higher in the regions with more developed economies (4) and is gradually increasing (2). These infants exhibit reduced intelligence (5) and low-risk behaviour (6), which adversely impact their academic performance during adolescence (7) and the establishment of social connections in adulthood (8). Premature infants are also accompanied by a high incidence of respiratory, gastrointestinal, endocrine, neurodevelopmental, and other systemic diseases (9). Furthermore, a negative correlation has been demonstrated between the incidence of premature birth and the level of economic development, with the highest rates in sub-Saharan Africa and South Asia (1). Moreover, premature birth was associated with increased medical expenditures and readmission rates (10), which was a significant contributing factor to the mortality rate of children under the age of five (11). Consequently, the prevalence of premature birth and premature infants has been demonstrated to impose a significant burden on families and society at large (12).
Anemia during pregnancy constitutes a significant global problem. Iron deficiency anemia (IDA) is considered to be the most common nutritional deficiency worldwide, affecting approximately 30% of the global population (13). Anemia during pregnancy has been associated with many adverse consequences for both mothers and infants, including an increased risk of premature birth, stillbirth, and neurocognitive impairment (14). The prevalence of anemia among pregnant women in China has been documented as high as 23.5% (15), which has been associated with adverse outcomes, including reduced birth weight (16), premature birth (17), and even postpartum hemorrhage due to tension loss (18), resulting in a considerable threat to the health of pregnant women in China. The prevalence of anemia among pregnant women in Northwest China has been documented as high as 65.1% (19), which may be attributed to the imbalanced economic development and cultural variations (20). The implementation of the national multiple birth policy has been associated with a consistent upward trend in the incidence of premature infants across various geographical regions (21). Consequently, the objective of this study is to investigate the correlation between anemia in pregnant women and the clinical high risk of premature infants through retrospective research, with the aim of providing novel insights for the protection of the health of regional pregnant women and newborns.
Methods and materials
Patients
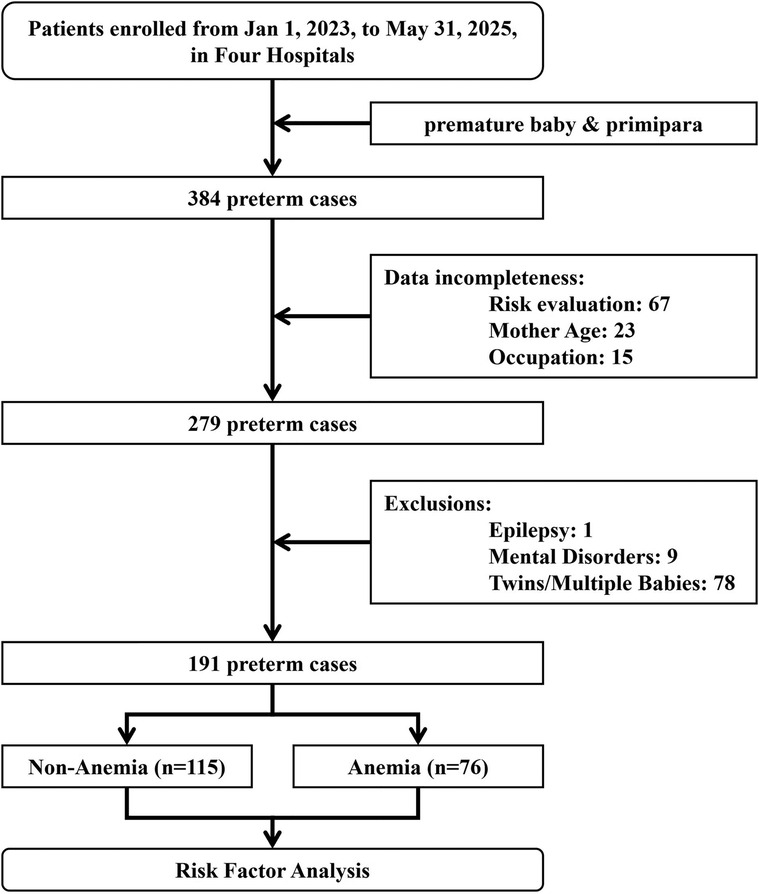
Preterm infants were defined as infants with gestational age <37 weeks, followed by Work Standards for Premature Infants’ Healthcare published by the National Health Commission of the People's Republic of China (https://www.nhc.gov.cn/fys/c100078/201703/bd1c0e0d58644bf3b234150cdf5cb4ca.shtml). Infants with a birthweight of less than 2,000 grams or a gestational age of less than 34 weeks were designated as the clinical high-risk group, while the remaining preterm infants were classified as the clinical low-risk group. A total of 384 premature infants and their mothers were collected from four medical institutions from January 2023 to May 2025. The study was reviewed and endorsed by the ethics committee of Zhongjiang Maternal and Child Health-Care Hospital (ID: 2024ky-001), and all subjects provided written consent in paper or electronic format. This report followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies (22), as shown in the Supplementary Appendix A1.
Data collection
Before the data collection procedure, Weiwei Ou and Zhu Xu were trained in the standardization of data collection (kappa = 0.823). A comprehensive data set was compiled, encompassing the demographic characteristics of subjects, including age, educational attainment, occupational status, and family history. Additional factors contributing to premature birth, such as diabetes, hypertension, hepatitis B, and pregnancy complications (e.g., eclampsia, diabetes, anemia, etc.), were also considered. The study further examined threatened abortion, placenta previa, placental abruption, abnormal fetal membranes, and infection as potential complicating factors. The infant's information is relevant for consideration: gender, birth weight, abnormal development (abnormal umbilical cord, abnormal fetal position, etc.), and other clinical indications. The subjects diagnosed with epilepsy, genetic diseases, twins/multiple births, or incomplete data were excluded. Following the screening process as shown in Figure 1, 191 cases were selected for data analysis. According to the World Health Organization (WHO), anemia is defined as a hemoglobin concentration below 110 g/L in any trimester of pregnancy (23), with mild anemia (100–109 g/L), moderate anemia (70–99 g/L) and severe anemia (<70 g/L).
Statistical analysis
SPSS 26 (IBM Corporation, New Orchard Road, Armonk, NY 10,504, USA) was used for statistics. Intra-group differences were compared by t-test, 2-sample Kolmogorov–Smirnov test, χ2 or Fisher's exact test. Binary logistic regression was used for independent risk factor analysis of high-risk neonates. To address the issue of multiple comparisons, the False Discovery Rate (FDR) was implemented to adjust for multiple comparisons. Continuous variables were shown as mean ± standard deviation (). A p-value of less than 0.05 was considered statistically significant.
Results
Patients
The mean age of the 191 mothers was 29.2 ± 5.0 years old, 76 (39.8%) of whom had anemia, 36 (18.8%) received a higher education, and 11 (5.8%) had a family history. The sample included 34(17.8%) clinical high-risk premature infants, with a weight of 2,483 ± 458 g, aged 35.0 ± 1.3 weeks, and 72 (37.7%) female infants.
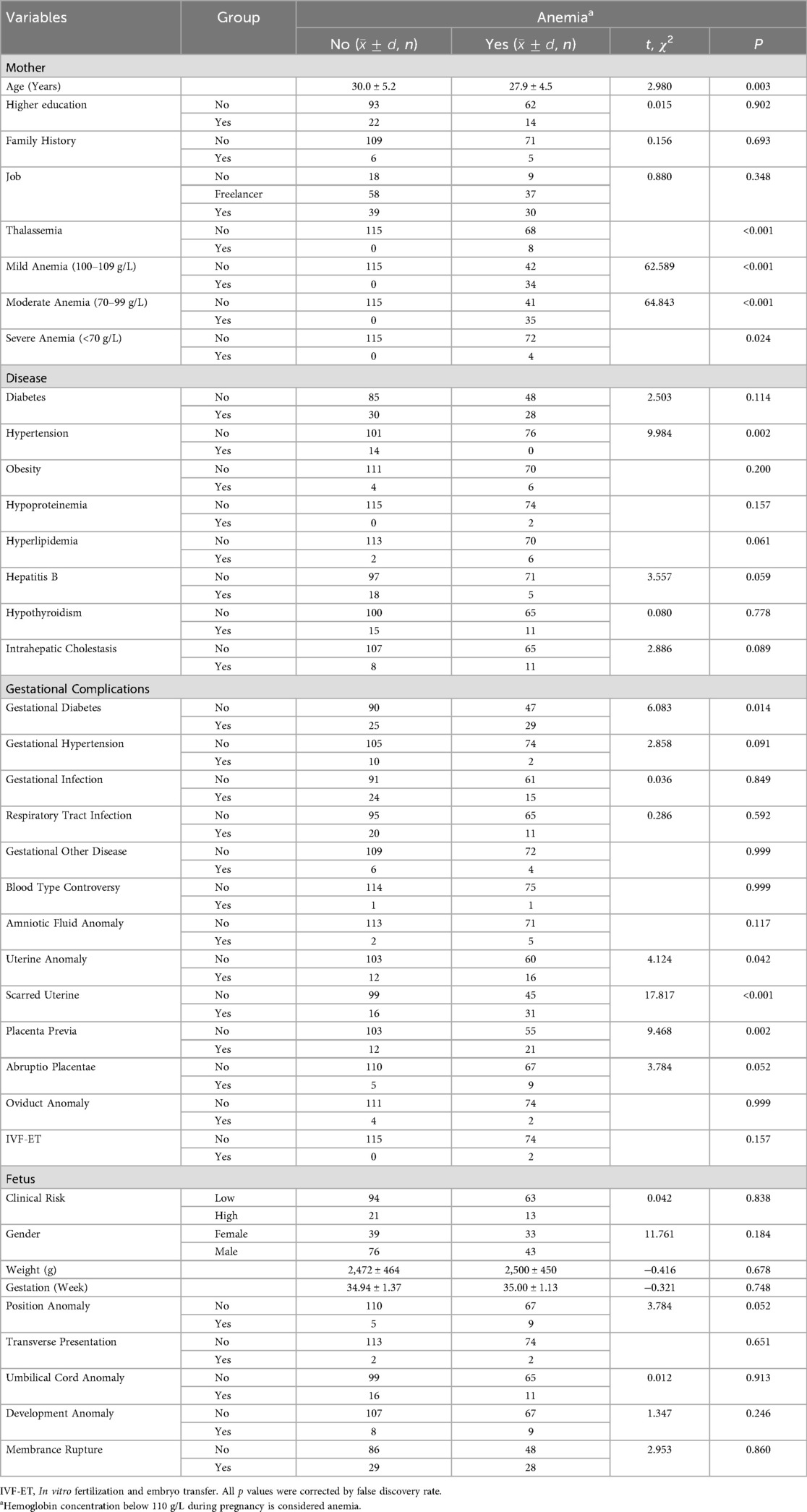
A subsequent analysis revealed that there were statistically significant differences between the age of the mother, the presence of hypertension, gestational diabetes, uterine abnormalities, scarred uterus, and placental abnormalities between the anemia group and the nonanemia group (p < 0.05), as demonstrated in Table 1, but the age of mother (p = 0.016), scarred uterus (p < 0.001), hypertension (p = 0.013) and placenta previa (p = 0.013) passed the FDR correction. Additionally, the analysis reveals that there is no statistically significant difference between premature infants in the clinical high-risk and low-risk groups [OR = 0.924, 95% CI = (0.431, 1.978), p = 0.838].
Risk factor analysis
Nonanemia group: Table 2 showed that statistical significances were found in gender, gestational hypertension, placental abnormalities, placental abruption, umbilical cord abnormalities, and fetal development abnormalities (all p < 0.05), but only development abnormalities (p = 0.037) passed FDR correction. Binary logistic regression analysis demonstrated that men exhibited a protective effect [OR = 0.240, 95% CI = (0.076, 0.764)], while placental abruption [OR = 31.499, 95% CI = (2.707, 366.599)] and fetal dysplasia [OR = 16.927, 95% CI = (3.161, 90.630)] were identified as significant contributors to the risk of severe premature births, as illustrated in Table 3.
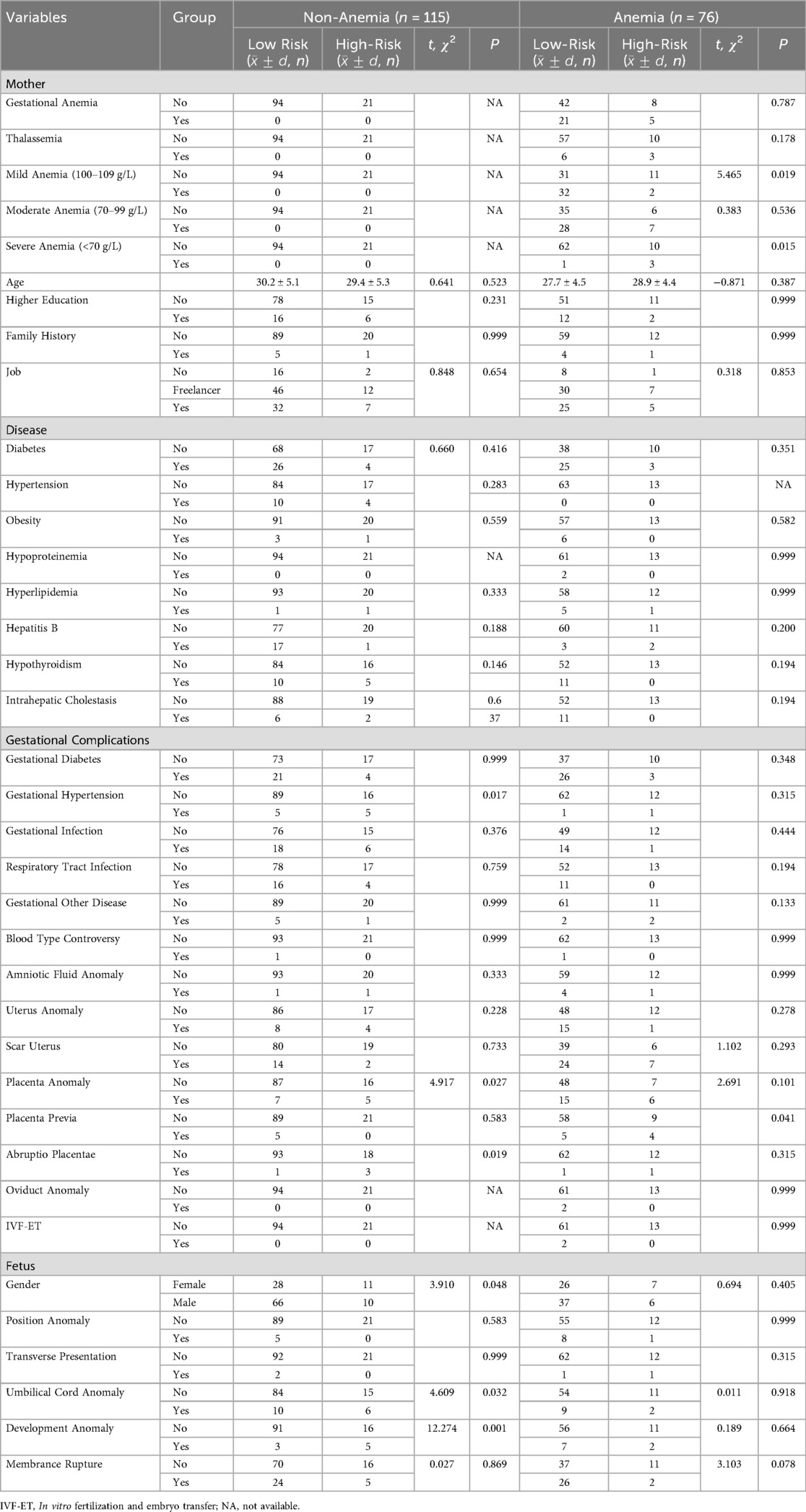
Table 2. Analysis of related factors for clinical high-risk levels in neonates based on anemia groups.
Anemia group: As illustrated in Table 2, there were statistically significant differences found in mild anemia, severe anemia, and placenta previa (all p < 0.05), but none passed FDR correction. Only severe anemia [OR = 18.600, 95% CI = (1.757, 196.927)] was the risk factor for clinical high-risk in preterm neonates, as illustrated in Table 3.
Discussion
Despite the absence of a statistically significant discrepancy in the prevalence of anemia between premature infants classified as high-risk and low-risk infants, a discernible divergence in the contributing factors emerged when comparing the anemia group to the nonanemia group. The former exhibited a preponderance of female infants, placental abruption, and fetal dysplasia. In contrast, the latter demonstrated a notable absence of severe anemia. These observations underscore the potential for targeted clinical management interventions and health guidance for expectant mothers, thereby enhancing maternal and fetal well-being.
Female and clinical high-risk
Fetal gender differences may also be a factor leading to the risk of premature birth (24). Pregnant women carrying female fetuses may be susceptible to premature delivery due to other complications, such as hypothyroidism (25). Furthermore, female fetuses demonstrate a heightened susceptibility to the impact of ambient temperature, particularly in instances of extreme temperature, which can result in preterm birth (26). For example, female fetuses in Germany are more susceptible to heat stress during pregnancy, and extremely high temperatures and long-term high temperature exposure increase the risk of premature birth, which may be due to the change of vascular resistance in the uterine artery caused by heat exposure (27). However, research indicates that pregnant women in Western and Northern China exhibit heightened sensitivity to the cold wave; in particular, pregnant women carrying female fetuses may experience an elevated risk of premature birth when exposed to the cold wave (28). Consequently, the clinical management of fetuses may be contingent on gender, with mothers carrying female fetuses being advised to focus on the external factors, particularly temperature.
Placental abruption and clinical high-risk
Placental abruption is defined as the premature separation of the placenta and uterine attachments prior to fetal delivery (29), which may increase the risk of premature delivery, severe maternal complications, and adverse neonatal outcomes (30). Placental abruption has been associated with several potential etiologies, including thrombosis, inflammation, infection, and uterine placental vascular lesions (31), leading to a range of chronic pathological changes gradually, such as placental perfusion insufficiency, placental infarction, and spiral artery remodelling defects (32). It is important to note that these defects may, in turn, contribute to the development of placental abruption. Consequently, premature births accompanied by lesions may manifest symptoms such as vaginal bleeding (33) and discomfort. We recommend that a meticulous blood and ultrasound examination be conducted for confirmation in the early clinical suspicion (34). Furthermore, the substantial mechanical force and shear force on the abdomen may result in placental abruption (35). This condition can be further influenced by maternal factors, including age >35, race, low BMI, smoking during pregnancy, and previous placental abruption history (36). Additional contributing factors include placenta previa, pregnancy-induced hypertension, and other elements. Consequently, in clinical practice, it is imperative to promptly assess pregnant women experiencing abdominal pain, vaginal bleeding, and abnormal fetal heart rate patterns, intending to enhance the management of maternal high-risk factors, and aiming at mitigating the risk of premature birth attributable to placental abruption.
Fetal dysplasia and clinical high-risk
In the present study, fetal dysplasia was combined with a variety of other anomalies, including fetal distress, fetal growth restriction, fetal tricuspid regurgitation, and fetal permanent right umbilical vein. Fetal distress is a symptom of fetal intrauterine hypoxia, which may be manifested by umbilical artery and cerebral artery abnormalities (37). For instance, umbilical artery thrombosis has been associated with premature birth (38) and a significant escalation in the risk of refeeding syndrome in premature infants (39). Maternal exposure to air pollution (40) and fetal umbilical cord abnormalities (41) have been demonstrated to increase the risk of fetal distress significantly. Fortunately, a variety of diagnostic procedures, including fetal heart rate monitoring, ultrasound arterial examination, and meconium-stained amniotic fluid assessment, can be utilized to evaluate the risk of fetal distress in a clinical setting. Therefore, a comprehensive review of the patient's medical history is imperative to ascertain the risk of fetal development distress. This requires a meticulous monitoring regimen during pregnancy to mitigate the potential complications.
Fetal growth restriction (FGR) may be attributed to chronic fetal hypoxia resulting from inadequate placental perfusion (42). Abnormal fetal metabolism or malnutrition may also lead to FGR (43). Negative emotions of mother may have the potential to disrupt normal metabolic pathways (44), which can have adverse effects on the cardiovascular health of fetus (45) and resulting in FGR and preterm. Fortunately, the abnormal characteristics of premature infants’ growth restriction performance can be identified by Doppler ultrasound (46), and the risk of fetal growth restriction may be reduced by supplementing 50–150 mg of aspirin daily before 16 weeks of gestation (47). Consequently, within the framework of clinical management, it is recommended that fetal growth restriction be regarded as a pivotal monitoring index and management objective during routine examinations.
Although the incidence of other fetal developmental abnormalities, such as tricuspid regurgitation and fetal persistent right umbilical vein, is low, they nevertheless require consideration in the clinical management of pregnant women. Fetal tricuspid regurgitation has been associated with chromosome defects, including Down syndrome (48), which has been linked to an increased risk of coronary heart disease (49). However, ultrasound examination findings indicate that patients with mild fetal tricuspid regurgitation or without structural abnormalities in the early stage of pregnancy are less likely to suffer from severe congenital heart disease after delivery (50). Therefore, accurate identification of tricuspid regurgitation in clinical management is imperative to mitigate the potential anxiety experienced by pregnant women. The prevalence of fetal persistent right umbilical vein (PRUV) is approximately 0.17%, a condition that can be identified through prenatal echocardiography. Although the prognosis for isolated PRUV is favorable, patients with severe PRUV symptoms and structural abnormalities may develop heart-related diseases, thereby increasing the likelihood of premature birth (51). Consequently, it is imperative to allocate clinical attention to these diseases. However, the question of whether these diseases result in the manifestation of high-risk clinical symptoms associated with premature birth requires further investigation.
Severe anemia
Our research indicates that severe anemia is a crucial factor in the development of adverse clinical manifestations in preterm infants with anemia. Global data show that the prevalence of anemia among pregnant women is 36.8% (458,067/1,244,747) (52), which is consistent with our study's findings (39.8% = 76/191) (P = 0.391). The majority of the patients are mildly anemic. However, severe anemia during pregnancy can lead to a decrease in blood volume and oxygen-carrying capacity, potentially resulting in postpartum hemorrhage, premature rupture of the membranes, preterm delivery, low birth weight, cesarean section, and neonatal respiratory distress syndrome (53). Research indicates that Chinese anemia in pregnant women may be associated with nutritional deficiencies, regional differences, dietary habits, economic status (54), and genetics (55). The severity of anemia is directly related to the risk of preterm delivery (56). Although meta-analyses have not demonstrated a substantial clinical benefit from antenatal nutritional supplementation (57), they have been shown to improve the outcomes of other pregnancy complications (58). Therefore, it is essential for healthcare providers to thoroughly investigate the underlying causes of anemia in pregnant women and to implement appropriate nutritional interventions to improve the outcomes for both the mother and the fetus.
Limitation
Despite conducting a multicenter retrospective study, the limited sample size precluded the execution of a comprehensive hierarchical analysis of gender or anemia types, including thalassemia and iron deficiency anemia. This discrepancy can be attributed, at least in part, to the study's primary objective, which was to ascertain whether premature infants are clinically high-risk. The number of infants exhibiting high-risk clinical symptoms is minimal, with only 34 cases identified. This may potentially lead to an inadequate statistical efficacy in the subgroup analysis of anemia (i.e., 4 severe anemia cases, with only 3 in high-risk group). Furthermore, cases of lower weight (i.e., <2,000 g) or gestational weeks (i.e., <34) may have implications for the sensitivity analysis focused on the anemia group. Secondly, the decline in fertility in China in recent years has also led to a decrease in the number of premature infants (59). Thirdly, we excluded twins from our sample because the identical maternal environment in twins may result in inconsistent premature infant statuses. Furthermore, the weight of each twin will be significantly lower than that of a non-twin (2,211 ± 423 g vs. 2,483 ± 457 g, p < 0.001). The exclusion of these variables from the study design may have resulted in significant alterations to the study's findings. A fourth limitation of our study is that it did not include data on minority nationality, living habits, dietary habits, family economic level, anemia gene screening results, or father's disease history (54, 55). These factors may have impacted the results. Consequently, this study constitutes a preliminary exploration of the relationship between anemia and clinical risk factors in premature infants, thereby establishing a foundation for more in-depth research.
Conclusion
Anemia, as a prevalent clinical ailment among pregnant women, poses a significant threat to the health of both the mother and the fetus, particularly in cases of premature infants. The findings of this study demonstrate that anemia exerts a significantly different influence on the clinical high-risk symptoms of premature infants. These differences can provide important reference points for the individual clinical management of pregnant women.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethical Committee of Zhongjiang Maternal and Child Health-Care Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
WO: Funding acquisition, Writing – original draft, Formal analysis, Validation, Supervision, Methodology, Writing – review & editing, Conceptualization. ZX: Data curation, Writing – review & editing. DL: Validation, Project administration, Writing – review & editing, Conceptualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This project was supported by Deyang Science and Technology Bureau [granted number: 2024SZY109].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2025.1690771/full#supplementary-material
References
1. Ohuma EO, Moller AB, Bradley E, Chakwera S, Hussain-Alkhateeb L, Lewin A, et al. National, regional, and global estimates of preterm birth in 2020, with trends from 2010: a systematic analysis. Lancet. (2023) 402(10409):1261–71. doi: 10.1016/S0140-6736(23)00878-4
2. Deng K, Liang J, Mu Y, Liu Z, Wang Y, Li M, et al. Preterm births in China between 2012 and 2018: an observational study of more than 9 million women. Lancet Glob Health. (2021) 9(9):e1226–41. doi: 10.1016/S2214-109X(21)00298-9
3. Song Q, Chen J, Zhou Y, Li Z, Li H, Liu J. Preterm delivery rate in China: a systematic review and meta-analysis. BMC Pregnancy Childbirth. (2022) 22(1):383. doi: 10.1186/s12884-022-04713-z
4. Miao H, He H, Nie C, Ren J, Luo X. Spatiotemporal characteristics and risk factors for all and severity-specific preterm births in Southern China, 2014–2021: large population-based study. JMIR Public Health Surveill. (2024) 10:e48815. doi: 10.2196/48815
5. Eves R, Mendonca M, Baumann N, Ni Y, Darlow BA, Horwood J, et al. Association of very preterm birth or very low birth weight with intelligence in adulthood: an individual participant data meta-analysis. JAMA Pediatr. (2021) 175(8):e211058. doi: 10.1001/jamapediatrics.2021.1058
6. Alenius S, Kajantie E, Sund R, Nurhonen M, Haaramo P, Näsänen-Gilmore P, et al. Risk-taking behavior of adolescents and young adults born preterm. J Pediatr. (2023) 253:135–43.e6. doi: 10.1016/j.jpeds.2022.09.032
7. Alenius S, Kajantie E, Sund R, Nurhonen M, Haaramo P, Nasanen-Gilmore P, et al. Author correction: school grades and educational attainments of adolescents and young adults born preterm. Sci Rep. (2023) 13(1):231. doi: 10.1038/s41598-023-30739-0
8. Mendonça M, Bilgin A, Wolke D. Association of preterm birth and low birth weight with romantic partnership, sexual intercourse, and parenthood in adulthood: a systematic review and meta-analysis. JAMA Netw Open. (2019) 2(7):e196961. doi: 10.1001/jamanetworkopen.2019.6961
9. Gette F, Ali A, Ho S, Richter MSP, Chan LL, Yang ES, et al. Long-term health outcomes of preterm birth: a narrative review. Front Pediatr. (2025) 13:1565897. doi: 10.3389/fped.2025.1565897
10. Lee JY, Park J, Lee M, Han M, Lim SM, Baek JY, et al. Rising public costs of preterm infant hospitalization in South Korea from a nationwide observational study. Sci Rep. (2025) 15(1):14357. doi: 10.1038/s41598-025-98868-2
11. Perin J, Mulick A, Yeung D, Villavicencio F, Lopez G, Strong KL, et al. Global, regional, and national causes of under-5 mortality in 2000–19: an updated systematic analysis with implications for the sustainable development goals. Lancet Child Adolesc Health. (2022) 6(2):106–15. doi: 10.1016/S2352-4642(21)00311-4
12. Bradley E, Blencowe H, Moller AB, Okwaraji YB, Sadler F, Gruending A, et al. Born too soon: global epidemiology of preterm birth and drivers for change. Reprod Health. (2025) 22(Suppl 2):105. doi: 10.1186/s12978-025-02033-x
13. Kirthan JPA, Somannavar MS. Pathophysiology and management of iron deficiency anaemia in pregnancy: a review. Ann Hematol. (2024) 103(8):2637–46. doi: 10.1007/s00277-023-05481-2
14. Obianeli C, Afifi K, Stanworth S, Churchill D. Iron deficiency anaemia in pregnancy: a narrative review from a clinical perspective. Diagnostics. (2024) 14(20):2306. doi: 10.3390/diagnostics14202306
15. Lin L, Wei Y, Zhu W, Wang C, Su R, Feng H, et al. Prevalence, risk factors and associated adverse pregnancy outcomes of anaemia in Chinese pregnant women: a multicentre retrospective study. BMC Pregnancy Childbirth. (2018) 18(1):111. doi: 10.1186/s12884-018-1739-8
16. Xiong J, Zhou W, Huang S, Xu K, Xu Y, He X. Maternal anaemia and birth weight: a cross-sectional study from Jiangxi province, China. Fam Pract. (2023) 40(5-6):722–7. doi: 10.1093/fampra/cmac148
17. Liu X, An H, Li N, Li Z, Zhang Y, Zhang L, et al. Preconception hemoglobin concentration and risk of low birth weight and small-for-gestational-age: a large prospective cohort study in China. Nutrients. (2022) 14(2):271. doi: 10.3390/nu14020271
18. Lao TT, Wong LL, Hui SYA, Sahota DS. Iron deficiency anaemia and atonic postpartum haemorrhage following labour. Reprod Sci. (2022) 29(4):1102–10. doi: 10.1007/s43032-021-00534-1
19. Liu D, Li S, Zhang B, Kang Y, Cheng Y, Zeng L, et al. Maternal hemoglobin concentrations and birth weight, low birth weight (Lbw), and small for gestational age (Sga): findings from a prospective study in Northwest China. Nutrients. (2022) 14(4), 858. doi: 10.3390/nu14040858
20. Chung GK, Sharma B, Vargas DC, Lee W, Sun KS, Hung H, et al. Prevalence and determinants of anaemia in south Asian diaspora women residing in Hong Kong: an exploratory cross-sectional study. J Migr Health. (2025) 11:100312. doi: 10.1016/j.jmh.2025.100312
21. Geng Y, Zhuo L, Zhang R, Zhao H, Hou X, Chen H, et al. The impact of China’s universal two-child policy on total, preterm, and multiple births: a nationwide interrupted time-series analysis. BMC Public Health. (2024) 24(1):236. doi: 10.1186/s12889-023-17620-5
22. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The strengthening the reporting of observational studies in epidemiology (strobe) statement: guidelines for reporting observational studies. Int J Surg. (2014) 12(12):1495–9. doi: 10.1016/j.ijsu.2014.07.013
23. WHO. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity Geneva: World Health Organization (2011). Available online at: https://www.who.int/publications/i/item/WHO-NMH-NHD-MNM-11.1 (Updated May 31, 2011; Accessed August 13, 2025).
24. Lee G, Andrade GM, Kim YJ, Anumba DOC. The sex difference in the pathophysiology of preterm birth. Cells. (2025) 14(14), 1084. doi: 10.3390/cells14141084
25. Yang X, Yu Y, Zhang C, Zhang Y, Chen Z, Dubois L, et al. The association between isolated maternal hypothyroxinemia in early pregnancy and preterm birth. Thyroid. (2020) 30(12):1724–31. doi: 10.1089/thy.2019.0818
26. Zheng X, Zhang W, Lu C, Norback D, Deng Q. An epidemiological assessment of the effect of ambient temperature on the incidence of preterm births: identifying windows of susceptibility during pregnancy. J Therm Biol. (2018) 74:201–7. doi: 10.1016/j.jtherbio.2018.04.001
27. Yuzen D, Graf I, Tallarek AC, Hollwitz B, Wiessner C, Schleussner E, et al. Increased late preterm birth risk and altered uterine blood flow upon exposure to heat stress. EBioMedicine. (2023) 93:104651. doi: 10.1016/j.ebiom.2023.104651
28. Yu G, Yang L, Liu M, Wang C, Shen X, Fan L, et al. Extreme temperature exposure and risks of preterm birth subtypes based on a nationwide survey in China. Environ Health Perspect. (2023) 131(8):87009. doi: 10.1289/EHP10831
29. Brandt JS, Ananth CV. Placental abruption at near-term and term gestations: pathophysiology, epidemiology, diagnosis, and management. Am J Obstet Gynecol. (2023) 228(5S):S1313–S29. doi: 10.1016/j.ajog.2022.06.059
30. Zhang L, Yang H, Sun Y, Liu S. Preterm placental abruption and its association with adverse maternal and neonatal outcomes: a retrospective study. BMC Pregnancy Childbirth. (2025) 25(1):620. doi: 10.1186/s12884-025-07718-6
31. Ananth CV, Oyelese Y, Prasad V, Getahun D, Smulian JC. Evidence of placental abruption as a chronic process: associations with vaginal bleeding early in pregnancy and placental lesions. Eur J Obstet Gynecol Reprod Biol. (2006) 128(1-2):15–21. doi: 10.1016/j.ejogrb.2006.01.016
32. Krikun G, Huang ST, Schatz F, Salafia C, Stocco C, Lockwood CJ. Thrombin activation of endometrial endothelial cells: a possible role in intrauterine growth restriction. Thromb Haemost. (2007) 97(2):245–53. doi: 10.1160/TH06-07-0387
33. Gonen N, Mor L, Rabinovich D, Kleiner I, Schreiber L, Barda G, et al. Obstetric and neonatal outcomes in clinically diagnosed placental abruption with and without placental histopathologic confirmation-a retrospective study. Int J Gynaecol Obstet. (2025) 170(2):760–7. doi: 10.1002/ijgo.70079
34. Sener S, Uysal G, Adiguzel C, Okcu NT. Analysis of maternal fetal outcomes and complete blood count parameters according to the stages of placental abruption: a retrospective study. Eur J Med Res. (2025) 30(1):489. doi: 10.1186/s40001-025-02778-y
35. Cheng HT, Wang YC, Lo HC, Su LT, Lin CH, Sung FC, et al. Trauma during pregnancy: a population-based analysis of maternal outcome. World J Surg. (2012) 36(12):2767–75. doi: 10.1007/s00268-012-1750-6
36. Chen D, Gao X, Yang T, Xin X, Wang G, Wang H, et al. Independent risk factors for placental abruption: a systematic review and meta-analysis. BMC Pregnancy Childbirth. (2025) 25(1):351. doi: 10.1186/s12884-025-07482-7
37. Chen J, Liu FX, Tao RX. Relationship between ultrasound parameters of the umbilical and middle cerebral arteries and intrauterine fetal distress. World J Clin Cases. (2024) 12(16):2745–50. doi: 10.12998/wjcc.v12.i16.2745
38. Li J, Ijaz I, Zhao L. Umbilical artery thrombosis causing fetal distress: a case report. Cureus. (2024) 16(7):e64624. doi: 10.7759/cureus.64624
39. Di Chiara M, Spiriti C, Gloria F, Laccetta G, Dito L, Gharbiya M, et al. Fetal distress as a determinant for refeeding syndrome in preterm neonates. Nutrients. (2025) 17(9), 1417. doi: 10.3390/nu17091417
40. Xiao H, Yao C, Qi Z, Liu J, Liu X, Zhou Y, et al. Association between maternal short-term exposure to ambient air pollution and the risk of fetal distress: a matched case-control study. Sci Total Environ. (2023) 860:160438. doi: 10.1016/j.scitotenv.2022.160438
41. Meskele S, Mulu A, GebreMickael A, Ena L. Placental and umbilical cord indices and their association with fetal distress in Hadiya zone public hospitals, Southern Ethiopia: a cross-sectional study. Int J Gen Med. (2021) 14:10045–53. doi: 10.2147/IJGM.S346544
42. Zur RL, Kingdom JC, Parks WT, Hobson SR. The placental basis of fetal growth restriction. Obstet Gynecol Clin North Am. (2020) 47(1):81–98. doi: 10.1016/j.ogc.2019.10.008
43. Conde-Agudelo A, Villar J, Risso M, Papageorghiou AT, Roberts LD, Kennedy SH. Metabolomic signatures associated with fetal growth restriction and small for gestational age: a systematic review. Nat Commun. (2024) 15(1):9752. doi: 10.1038/s41467-024-53597-4
44. Yao M, Yang Z, Rong X, Hu X, Yao N, Zhu M, et al. The exploration of fetal growth restriction based on metabolomics: a systematic review. Metabolites. (2022) 12(9), 860. doi: 10.3390/metabo12090860
45. Course CW, Kotecha SJ, Cousins M, Hart K, Lowe J, Watkins WJ, et al. Association of gestation and fetal growth restriction on cardiovascular health in preterm-born children. J Pediatr. (2023) 255:42–9.e4. doi: 10.1016/j.jpeds.2022.09.057
46. Kim F, Bateman DA, Goldshtrom N, Sheen JJ, Garey D. Intracranial ultrasound abnormalities and mortality in preterm infants with and without fetal growth restriction stratified by fetal doppler study results. J Perinatol. (2023) 43(5):560–7. doi: 10.1038/s41372-023-01621-8
47. Roberge S, Nicolaides K, Demers S, Hyett J, Chaillet N, Bujold E. The role of aspirin dose on the prevention of preeclampsia and fetal growth restriction: systematic review and meta-analysis. Am J Obstet Gynecol. (2017) 216(2):110–20.e6. doi: 10.1016/j.ajog.2016.09.076
48. Benchamanon R, Suwanrath C, Pranpanus S. Fetal tricuspid regurgitation in second trimester of pregnancies at risk for fetal chromosomal defects. J Clin Ultrasound. (2020) 48(2):97–101. doi: 10.1002/jcu.22753
49. Scala C, Morlando M, Familiari A, Leone Roberti Maggiore U, Ferrero S, D'Antonio F, et al. Fetal tricuspid regurgitation in the first trimester as a screening marker for congenital heart defects: systematic review and meta-analysis. Fetal Diagn Ther. (2017) 42(1):1–8. doi: 10.1159/000455947
50. Kavgaci A, Örün UA, Kaya Ö, Ari ME. Isolated mild fetal tricuspid regurgitation in low-risk pregnancies: an incidental doppler finding or a marker of postnatal cardiac risk? Children (Basel). (2025) 12(7):879. doi: 10.3390/children12070879
51. Li J, Yuan Q, Ding H, Yang Z, Wang B, Wang B. Ultrasonic detection of fetal persistent right umbilical vein and incidence and significance of concomitant anomalies. BMC Pregnancy Childbirth. (2020) 20(1):610. doi: 10.1186/s12884-020-03310-2
52. Karami M, Chaleshgar M, Salari N, Akbari H, Mohammadi M. Global prevalence of anemia in pregnant women: a comprehensive systematic review and meta-analysis. Matern Child Health J. (2022) 26(7):1473–87. doi: 10.1007/s10995-022-03450-1
53. Wang R, Xu S, Hao X, Jin X, Pan D, Xia H, et al. Anemia during pregnancy and adverse pregnancy outcomes: a systematic review and meta-analysis of cohort studies. Front Glob Womens Health. (2025) 6:1502585. doi: 10.3389/fgwh.2025.1502585
54. Zhou Y, Lyu Y, Ye W, Shi H, Peng Y, Wen Z, et al. The prevalence of anemia among pregnant women in China: a systematic review and meta-analysis. Nutrients. (2024) 16(12):1854. doi: 10.3390/nu16121854
55. Gan W, Guan Y, Wu Q, An P, Zhu J, Lu L, et al. Association of Tmprss6 polymorphisms with ferritin, hemoglobin, and type 2 diabetes risk in a Chinese han population. Am J Clin Nutr. (2012) 95(3):626–32. doi: 10.3945/ajcn.111.025684
56. Shi H, Chen L, Wang Y, Sun M, Guo Y, Ma S, et al. Severity of anemia during pregnancy and adverse maternal and fetal outcomes. JAMA Netw Open. (2022) 5(2):e2147046. doi: 10.1001/jamanetworkopen.2021.47046
57. Keats EC, Haider BA, Tam E, Bhutta ZA. Multiple-micronutrient supplementation for women during pregnancy. Cochrane Database Syst Rev. (2019) 3(3):CD004905. doi: 10.1002/14651858.CD004905.pub6
58. Lassi ZS, Padhani ZA, Rabbani A, Rind F, Salam RA, Bhutta ZA. Effects of nutritional interventions during pregnancy on birth, child health and development outcomes: a systematic review of evidence from low- and middle-income countries. Campbell Syst Rev. (2021) 17(2):e1150. doi: 10.1002/cl2.1150
Keywords: preterm infant, anemia, clinical high-risk, fetal dysplasia, placental abruption, severe anemia
Citation: Ou W, Xu Z and Liu D (2025) Analysis of clinical risk factors of premature infants in Chinese women based on anemia status. Front. Pediatr. 13:1690771. doi: 10.3389/fped.2025.1690771
Received: 22 August 2025; Accepted: 31 October 2025;
Published: 13 November 2025.
Edited by:
Licia Lugli, University Hospital of Modena, ItalyReviewed by:
Jackline De Paula Ayres-Silva, Oswaldo Cruz Foundation (Fiocruz), BrazilShubha Davalagi, JJM Medical College, India
Copyright: © 2025 Ou, Xu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weiwei Ou, MTUxOTYzMjM4NTNAMTYzLmNvbQ==
 Weiwei Ou
Weiwei Ou Zhu Xu
Zhu Xu