- 1Department of Neonatology, Bharati Vidyapeeth Deemed to be University Medical College, Pune, Maharashtra, India
- 2Department of Pediatrics, KGMU, Lucknow, Uttar Pradesh, India
- 3Department of Neonatology and Pediatrics, Cloudnine Hospital, Bengaluru, India
- 4Department of Neonatology, Motherhood Women and Children’s Hospital, Bengaluru, India
Background: Blood transfusion, a vital procedure in neonatal intensive care units (NICUs), also poses risks such as necrotizing enterocolitis, intraventricular hemorrhage, and death. We conducted a nationwide survey to evaluate current neonatal transfusion practices among clinicians in India.
Methods: A cross-sectional survey with a structured 23-item questionnaire was conducted online using Google Forms during February 2024. The questionnaire covered key elements of transfusion practices, including threshold, dosing, and duration of blood product administration. Five hundred forty clinicians working in NICUs across India were invited to participate in the survey.
Results: Responses were received from 368 clinicians, most of whom practice in Level 3 Neonatal Intensive Care Units. Approximately 67% reported adherence to the guidelines established by the National Neonatal Forum of India 2020. Packed red blood cells were predominantly transfused at a volume of 15 mL/kg (79%) over a duration of four hours (69%), with a hemoglobin threshold of 7.5 g/dL (48%) employed after two weeks of life in stable preterm neonates younger than 32 weeks. The majority of practitioners (59%) did not utilize diuretics, and half (50%) withheld feeds during red blood cell transfusions. Platelet transfusions were most frequently administered at 10 mL/kg (51%) over a period of 0.5 h (60%), with a threshold platelet count of 50,000/µL in cases of bleeding (60%) or 25,000/µL in the absence of bleeding (58%). Fresh frozen plasma was used in neonates presenting with coagulopathy and bleeding (73%) and also in cases without bleeding (25%), most commonly at 10 mL/kg (47%) administered over 1 h (43%).
Conclusion: Transfusion practices varied across Indian NICUs despite adherence to NNF guidelines and generally adopting a restrictive approach. The standardization of protocols and enhancement of compliance could potentially improve clinical outcomes and diminish complications associated with transfusion.
Introduction
Blood transfusion is a common intervention used in the neonatal intensive care unit (NICU) for premature and sick newborns. During the transition from a fetus to a neonate, several physiologic changes occur, including changes in blood volume and other hematologic parameters (1). In extremely low birth weight infants, maintaining a higher hemoglobin level results in more infants receiving transfusions, although there is little evidence that this is beneficial (2). Anemia may result in impaired oxygen supply to the brain and prematurity-related brain injury, especially in the setting of apnea and intermittent hypoxemia or circulatory insufficiency during a period of rapid brain growth and development. However, red blood cell (RBC) transfusions can have complications, especially in preterm infants. They have been associated with intraventricular hemorrhage, necrotizing enterocolitis (NEC), bronchopulmonary dysplasia, retinopathy of prematurity, and increased mortality (3).Transfusions in preterm neonates may be harmful due to pro-inflammatory and immunosuppressive effects or, as recently proposed, from repeated adult red blood cell (RBC) transfusions that replace fetal hemoglobin (HbF) with adult hemoglobin, increasing oxidative stress. This oxidative stress promotes band 3 oxidation and neoantigen formation, facilitating phagocytic recognition (4). Supporting this, Pellegrino et al. showed that adult donor RBCs deliver more oxygen to preterm neonatal brains than cord blood (5).
Many clinicians use a specific hematocrit or hemoglobin level to assess the need for RBC transfusion, which varies based on the degree of prematurity, the postnatal age, and the clinical state of the infant, including oxygen requirement and need for ventilatory support (6). Two large randomized clinical trials (ETTNO and TOP) have been published since 2019 regarding transfusion threshold and neurodevelopmental outcome in neonates (7). In extremely low birth weight infants, a higher hemoglobin threshold for red blood cell transfusion did not improve survival without neurodevelopmental impairment at 22–26 months of age, corrected for prematurity (TOP trial) (8). Among infants with birthweight <1,000 g, a liberal blood transfusions strategy compared to a restrictive approach did not reduce the risk of death or disability at 24 months of corrected age (9).
In India, large variation in platelet transfusion practice has been demonstrated, regarding the thresholds used, the volume administered, and the transfusion rate (10). In the PLANET 2 trial, preterm infants with low platelet count who received platelet transfusions at a higher threshold were found to have an increased risk of death or major bleeding compared to those who were transfused at a lower threshold (11).
Over the past 20 years, several recommendations for blood transfusion in newborns have been published (11–13). Despite the availability of neonatal transfusion guidelines, there is a lack of Indian data on transfusion practices like threshold, dosing, and duration of different blood products used in NICUs across India. Therefore, this study aimed to evaluate current neonatal transfusion policies and practices in Indian neonatal critical care units.
Material and methods
The study started in February 2024 after getting approval from the institutional ethical committee. An online survey with 23 multiple-choice questions about blood transfusion practices and policies was created using Google Forms and tested for content validity by five practicing neonatologists. After finalizing, clinicians across India (n = 540), listed in the directories of the National Neonatology Forum (NNF) of India and the Neonatology Chapter of the Indian Academy of Pediatrics, were contacted by phone, text, and email to participate. Ten of the twenty-three questions were examined to see if public and private hospitals followed the NNF recommendations for transfusion practices. Adherence to the NNF guidelines was evaluated using a 10-item questionnaire. Each item was scored as 1 if the NICU followed the respective guideline and 0 if it did not. The total adherence score was calculated by summing the responses across all items, with higher scores indicating better compliance. Participants implicitly consented to the survey. All information received was kept confidential. After a month of weekly reminders, the survey was closed. No incentives were offered. The responses are summarized in Tables 1–4. The questionnaire is provided in the Supplementary File.
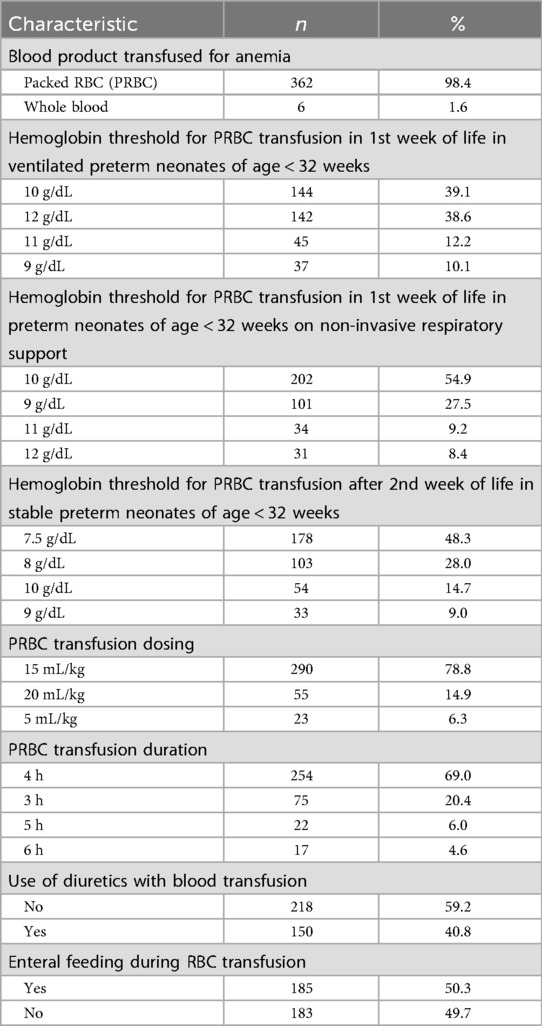
Table 2. Red blood cell (RBC) transfusion practices of survey participants in their neonatal intensive care units (n = 368).
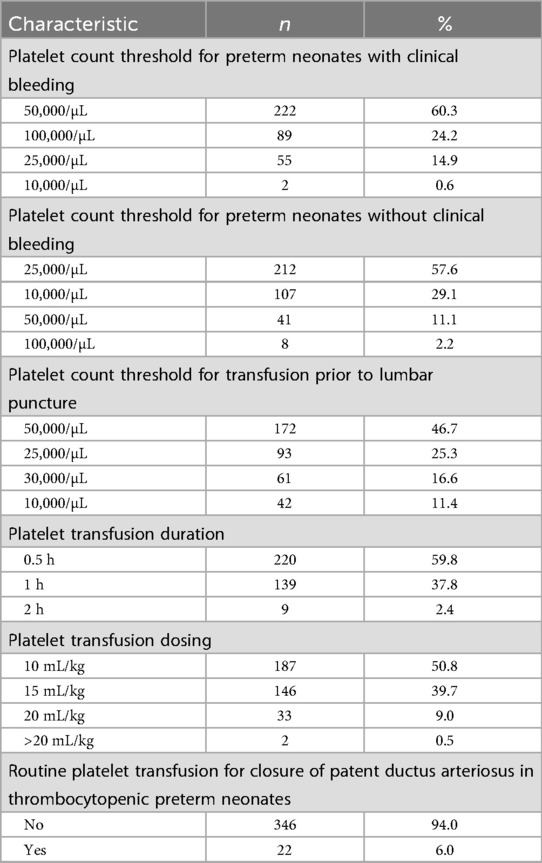
Table 3. Platelet transfusion practices of survey participants in their neonatal intensive care units (n = 368).
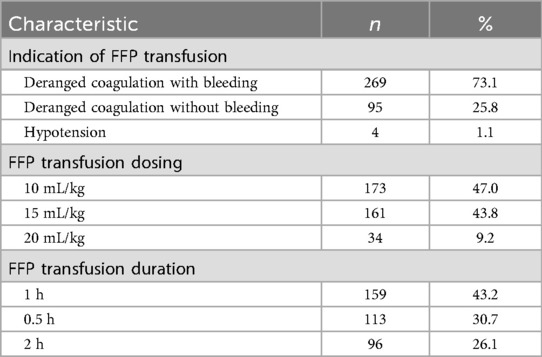
Table 4. Fresh frozen plasma (FFP) transfusion practices of survey participants in their neonatal intensive care units (n = 368).
Results
Characteristics of survey respondents
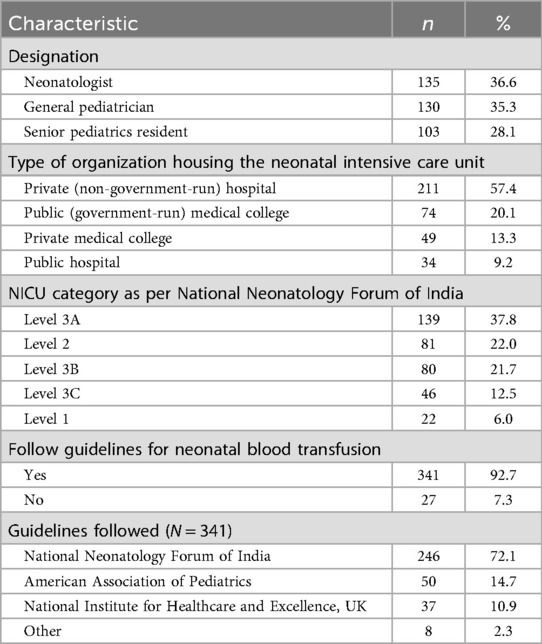
Of the 540 clinicians invited to participate, 368 (68.1%) responded to the online survey. They came from 260 institutions and hospitals across 65 cities in 25 states of India. Table 1 shows the characteristics of the survey respondents. Most respondents were neonatologists (37%) or general pediatricians (35%). The importance of private healthcare in newborn services was reflected in institutional statistics showing that private (non-government) hospitals housed the majority of NICUs (57%), where the respondents worked, followed by government (public) medical colleges (20%) and private medical colleges (13%).The mean adherence score to the NNF guidelines was 6.27 ± 1.44 among clinicians working in private hospital (n = 263) and 6.31 ± 1.51 among those in government hospital(n = 105), with no statistically significant difference between the two groups (p = 0.81) (Figure 1). Seventy-two percent of the NICUs were classified as NNF India category Level 3A or higher, indicating a strong focus on intensive and specialized neonatal care according to NNF accreditation standards. The survey respondents used various transfusion protocols, with 90.6% following guidelines from either NNF India (67%), the American Academy of Pediatrics (13.6%), or the National Institute for Healthcare and Excellence (NICE) of the UK (10%).
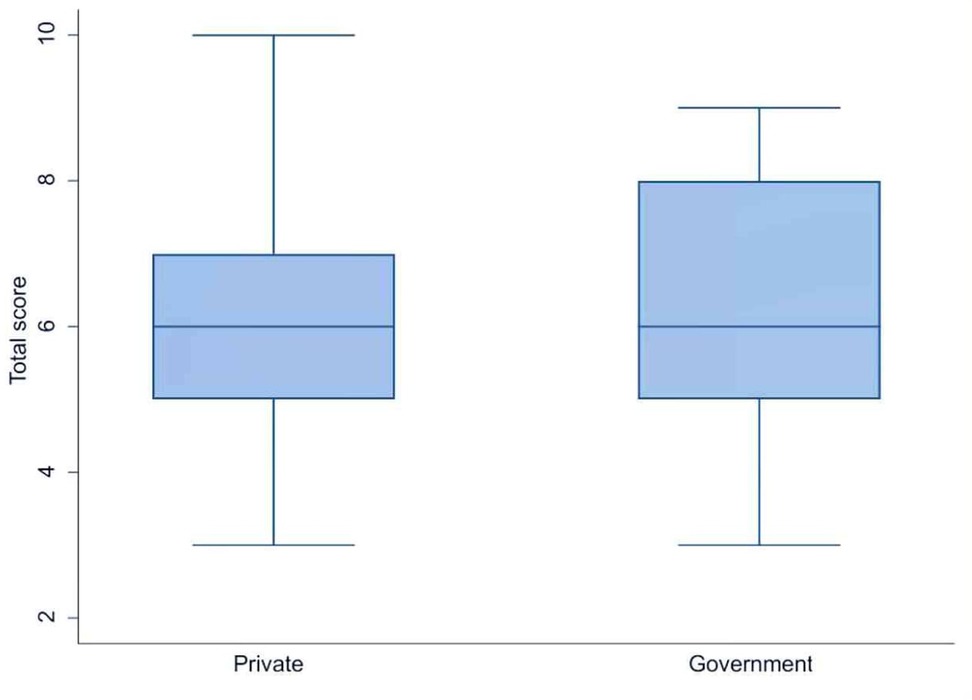
Figure 1. Comparision of adherence to transfusion practices in private and public hospitals in India.
RBC transfusion practices
Survey responses on RBC transfusion practice are summarized in Table 2. Packed Red blood cell (PRBC) was the blood product of choice of 98% of respondents for the management of anemia. As suggested in NNF India guidelines, PRBC transfusion at a hemoglobin threshold of 7.5 g/dL after the second week of life in stable preterm infants of age < 32 weeks was performed by 48% of respondents. For preterm infants of age < 32 weeks in first week of life, hemoglobin threshold that was used was 10 g/dL (39%) or 12 g/dL (39%) for those on ventilator, while it was 10 g/dL (54.9%) for those on non-invasive support. There is limited evidence to determine the ideal PRBC transfusion dosing for newborns, particularly concerning long-term outcomes. In our survey, PRBCs were transfused most frequently (79%) at 15 mL/kg, as recommended in the NNF 2020 guidelines, with 15% and 6% of respondents respectively choosing dosings of 20 mL/kg or 5 mL/kg. PRBC was typically transfused over four hours in most cases (69%). Diuretics were used by 41% of respondents during transfusion, whereas 50% of respondents delayed enteral feeding during RBC transfusion, in line with NNF India guidelines.
Platelet transfusion practices
About 60% of respondents transfused platelets at a threshold platelet count of 50,000/µL in preterm newborns with clinical bleeding (Table 3). In the absence of such bleeding, a threshold of 25,000/µL was used by a majority (58%), as recommended in the guidelines of NNF India as well as British Society of Haematology (BSH) (12). Additionally, as per BSH guidelines, a threshold of 50,000/µL was used by 47% of respondents for platelet transfusions prior to lumbar puncture. Most (94%) respondents did not routinely transfuse platelets in thrombocytopenic preterm newborns for closure of patent ductus arteriosus (PDA), in accordance with NNF India guidelines. Platelet transfusions were administered at a dosing of 10 mL/kg (51%), 15 mL/kg (40%), or 20 mL/kg (9%). Sixty percent of respondents administered transfusions over 0.5 h and 40% over one hour.
Fresh frozen plasma (FFP) transfusion practices
For FFP transfusion, 73% of respondents followed NNF India guidelines by transfusing in neonates with deranged coagulation and active bleeding, while 26% transfused in cases of deranged coagulation without bleeding (Table 4). FFP was transfused at a dose of 10 mL/kg (47%), 15 mL/kg (44%), or 20 mL/kg (9%), over a period of 1 h (43%), 0.5 h (31%), or 2 h (26%).
Discussion
Transfusion of blood products is a common intervention used in the NICU to treat premature and ill newborns. RBC transfusions are often recommended in the early weeks of infancy to treat anemia, which improves oxygen delivery and cardio-respiratory function status. Conversely, the adverse effects of RBC transfusion are increasingly recognized (13). Despite the growth in the number of Neonatal Intensive Care Units (NICUs), the specific operational practices of these facilities remain inadequate. In the absence of a system of mandatory registration and assessment by authorities, one has to rely on periodic surveys for an overview of the current situation regarding transfusion procedures and policies in Indian NICUs (14–16). Our study provides an insight into different neonatal blood transfusion practices among clinicians in India.
A small percentage of respondents reported continued use of whole blood for neonatal anemia even though it has largely been replaced by packed red blood cells (PRBCs). This may indicate difficulty in blood component separation or logistical issues in preparing PRBCs, particularly in smaller centers. In our survey, 40% of clinicians reported using diuretics during transfusions (Table 2), despite limited evidence of benefit. Randomized trials, including Balegar et al., show minimal clinical advantage, suggesting this practice persists due to concern about fluid overload or institutional policies rather than evidence-based guidance (17).
In our cohort, 50% of clinicians withheld feeds during PRBC transfusions, reflecting moderate guideline adherence, while evidence indicates uncertain NEC protection and possible harms such as disrupted nutrition and additional interventions (13, 14, 18, 19).
Beyond variations in diuretic use and enteral feeding practices, survey responses also revealed differences in transfusion thresholds, reflecting ongoing uncertainty and heterogeneity in clinical decision-making despite established guidelines. In stable preterm neonates <32 weeks, 48% of clinicians reported using a hemoglobin threshold of 7.5 g/dL for transfusion after the second week of life. Evidence indicates that restrictive thresholds (7–8 g/dL) do not increase mortality or adverse outcomes compared to liberal thresholds (9–10 g/dL), and higher thresholds increase transfusion frequency without improving long-term neurodevelopmental outcomes (9, 20). These findings highlight the need to individualize transfusion decisions based on clinical condition, oxygen requirements, and hemodynamic stability.
For preterm infants, maximum number of transfusions occurred during first and second weeks of life (21). For PRBC transfusion in the first week of life in ventilated preterm neonates of age < 32 weeks, the majority of practitioners in our survey used a hemoglobin threshold of 10 g/dL, in contrast to 12 g/dL suggested in NNF India guidelines (19). However, a majority used a threshold of 10 g/dL in case of neonates on non-invasive respiratory support, in accordance with the NNF guidelines. Similar results were found by Bell et al. in their investigation (22).
In VLBW infants, most RBC transfusions are top-ups to replace phlebotomy losses, and volumes >20 mL/kg in non-bleeding neonates may increase the risk of fluid overload (21). Conservative transfusion volumes of 15 mL/kg are generally appropriate, as studies show that larger volumes (15–20 mL/kg) can reduce the need for multiple transfusions and donor exposure without increasing adverse events or affecting pulmonary function (23–25). These findings support individualized, conservative transfusion strategies that balance efficacy with safety in this vulnerable population.
Alongside variations in RBC transfusion practices, our survey also revealed conservative platelet transfusion thresholds. with 58% of clinicians using 25,000/µL in non-bleeding neonates. This is lower than thresholds reported in previous studies (30,000–50,000/µL) (26–28), indicating closer adherence to NNF guidelines and a more conservative, evidence-informed approach. The BSH-recommended platelet threshold of 25,000/µL for prophylactic transfusions in non-bleeding preterm neonates is supported by the PLANET-2 trial and recent RCTs, which reported higher mortality and neurodevelopmental impairment at 50,000/µL (11, 29) In our survey, 89% of respondents used platelet thresholds of 25,000–50,000/µL, consistent with previous reports (30), although a majority (47%) used 50,000/µL prior to lumbar puncture, reflecting cautious practice in procedures with potential bleeding risk (31). Only 6% reported transfusing platelets for PDA closure, in line with evidence showing that higher platelet counts do not facilitate early PDA closure (32). Clinicians remain aware of transfusion-related risks, including acute lung injury and allergic reactions (33).
Following platelet transfusion practices, our survey assessed FFP use, which is particularly relevant in extremely low birth-weight neonates with coagulopathy or active bleeding (34). We observed that 73% of clinicians administered FFP for deranged coagulation with active bleeding, while 26% transfused in the absence of bleeding (Table 4), highlighting variability in practice and potential overuse. The lack of defined optimal dosing guidelines further underscores the need for evidence-based protocols to guide FFP transfusion and minimize unnecessary exposure in this vulnerable population.
To our knowledge this is the first cross-sectional study among practicing clinicians and neonatologists depicting transfusion practices and policies from various NICUs across India. The responses from clinicians nationwide provide broad geographic coverage. Although this is a comprehensive questionnaire-based study, most questions were answered by respondents, with an option for free-text input to share opinions on different practices beyond the provided alternatives. There are several limitations to our study. Firstly, its findings may not be a true reflection of practices across the country because of inadequate sampling. Secondly, some responses regarding transfusion policies were based on personal opinions rather than standardized national guidelines. Seventy-one percent of participants were affiliated with private hospitals or medical colleges. This bias can skew our findings as the practices in private institutions may differ from those in public ones, particularly in terms of resource availability, patient population, and staff training. The choices for transfusion thresholds, dosings, and rates in the survey questionnaire were not objectively validated, which may restrict the accuracy and practicality of the survey's findings. Furthermore, variables such as hospital infrastructure, staff experience, and financial status of patients that may have an impact on transfusion procedures were not taken into consideration in this study. The cross-sectional nature of the study limits the ability to establish temporal relationships or infer causality between the observed variables.
In conclusion, our study emphasizes the differences in neonatal blood transfusion practices across India, even though most practitioners report following NNF India guidelines. Its findings indicate that healthcare providers should regularly review and update their transfusion protocols based on the latest evidence and guidelines, while also ensuring compliance and conducting regular audits of their transfusion practices. The study further underscores the need for greater standardization of neonatal transfusion protocols to reduce transfusion-related complications and enhance neonatal outcomes.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Institutional Ethics Committee,Bharati Vidyapeeth(Deemed to be university) Medical college,Pune Satara Road,Pune-411043;India (Letter No. BVDUMC/IEC/4). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
KK: Writing – original draft, Formal analysis, Writing – review & editing, Visualization. SP: Supervision, Project administration, Writing – review & editing, Conceptualization. NM: Conceptualization, Writing – review & editing, Methodology, Formal analysis. VD: Writing – review & editing. ST: Data curation, Writing – review & editing. NN: Writing – review & editing, Data curation. SS: Data curation, Writing – review & editing. PS: Supervision, Conceptualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We thank the survey respondents.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2025.1692244/full#supplementary-material
References
1. Amrutiya RJ, Mungala BM, Patel VT, Ganjiwale JD, Nimbalkar SM. Blood component transfusion in tertiary care neonatal intensive care unit and neonatal intermediate care unit: an audit. Cureus. (2020) 12:e9952. doi: 10.7759/cureus.9952
2. Kirpalani H, Whyte RK, Andersen C, Asztalos EV, Heddle N, Blajchman MA, et al. The premature infants in need of transfusion (pint) study: a randomized, controlled trial of a restrictive (low) versus liberal (high) transfusion threshold for extremely low birth weight infants. J Pediatr. (2006) 149(3):301–7. doi: 10.1016/j.jpeds.2006.05.011
3. Lee EY, Kim SS, Park GY, Lee SH. Effect of red blood cell transfusion on short-term outcomes in very low birth weight infants. Clin Exp Pediatr. (2020) 63(2):56–62. doi: 10.3345/kjp.2019.00990
4. Pellegrino C, Stone EF, Valentini CG, Teofili L. Fetal red blood cells: a comprehensive review of biological properties and implications for neonatal transfusion. Cells. (2024) 13(22):1843. doi: 10.3390/cells13221843
5. Pellegrino C, Papacci P, Beccia F, Serrao F, Cantone GV, Cannetti G, et al. Differences in cerebral tissue oxygenation in preterm neonates receiving adult or cord blood red blood cell transfusions. JAMA Network Open. (2023) 6(11):e2341643. doi: 10.1001/jamanetworkopen.2023.41643
6. Arlettaz Mieth R, Gosztonyi L, Hegemann I, Bassler D, Ruegger C. Neonatal red blood cell transfusion practices in Switzerland: national survey and review of international recommendations. Swiss Med Wkly. (2020) 150:w20178. doi: 10.4414/smw.2020.20178
7. Investigators E. The ‘effects of transfusion thresholds on neurocognitive outcome of extremely low birth-weight infants (ettno)’ study: background, aims, and study protocol. Neonatology. (2012) 101(4):301–5. doi: 10.1159/000335030
8. Kirpalani H, Bell EF, Hintz SR, Tan S, Schmidt B, Chaudhary AS, et al. Higher or lower hemoglobin transfusion thresholds for preterm infants. N Engl J Med. (2020) 383(27):2639–51. doi: 10.1056/nejmoa2020248
9. Franz AR, Engel C, Bassler D, Rüdiger M, Thome UH, Maier RF, et al. Effects of liberal versus restrictive transfusion strategies on intermittent hypoxaemia in extremely low birthweight infants: secondary analyses of the ettno randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. (2025) 116:523. doi: 10.1136/archdischild-2024-327643
10. Cortesi V, Lopriore E, Fustolo-Gunnink S. Platelet transfusion and bleeding risk. In: Seminars in Fetal and Neonatal Medicine. WB Saunders (2025). p. 101608.
11. Moore CM, D’Amore A, Fustolo-Gunnink S, Hudson C, Newton A, Santamaria BL, et al. Two-year outcomes following a randomised platelet transfusion trial in preterm infants. Arch Dis Child Fetal Neonatal Ed. (2023) 108(5):452–7. doi: 10.1136/archdischild-2022-324915
12. Dogra K, Kaur G, Basu S, Chawla D. Red cell transfusion practices in neonatal intensive care unit: an experience from tertiary care centre. Indian J Hematol Blood Transfus. (2018) 34(4):671–6. doi: 10.1007/s12288-018-0959-4
13. Lee JW. Transfusion guidelines in pediatric patients. Anesth Pain Med. (2015) 10(3):141–8. doi: 10.17085/apm.2015.10.3.141
14. Garg PM, Ravisankar S, Bian H, Macgilvray S, Shekhawat PS. Relationship between packed red blood cell transfusion and severe form of necrotizing enterocolitis: a case control study. Indian Pediatr. (2015) 52(12):1041–5. doi: 10.1007/s13312-015-0770-3
15. Pandita A, Kumar A, Gupta G, Taligasalam V, Tewari VV. Use of blood components in newborns. J Neonatol. (2020) 34(4):199–217. doi: 10.1177/0973217921989856
16. Sundaram V, Chirla D, Panigrahy N, Kumar P. Current status of nicus in India: a nationwide survey and the way forward. Indian J Pediatr. (2014) 81(11):1198–204. doi: 10.1007/s12098-014-1489-1
17. Kluckow M. Furosemide for packed red cell transfusion in preterm infants: a randomized controlled trial. J Pediatr. (2011) 159(6):913–8. doi: 10.1016/j.jpeds.2011.05.022
18. Jasani B, Rao S, Patole S. Withholding feeds and transfusion-associated necrotizing enterocolitis in preterm infants: a systematic review. Adv Nutr. (2017) 8(5):764–9. doi: 10.3945/an.117.015818
19. Wade C, Farr J, Gomezcoello V, Martin G, Nasr T, Doty M. Feeding during blood transfusions and the association with necrotizing enterocolitis. Am J Perinatol. (2016) 33(09):882–6. doi: 10.1055/s-0036-1579651
20. Carson JL, Stanworth SJ, Dennis JA, Trivella M, Roubinian N, Fergusson DA, et al. Transfusion thresholds for guiding red blood cell transfusion. Cochrane Database Syst Rev. (2021) 2022(1):913–8. doi: 10.1002/14651858.cd002042.pub5
21. New HV, Berryman J, Bolton-Maggs PH, Cantwell C, Chalmers EA, Davies T, et al. British Committee for standards in haematology. Guidelines on transfusion for fetuses, neonates and older children. Br J Haematol. (2016) 175(5):784–828. doi: 10.1111/bjh.14233
22. Bell EF. Red cell transfusion thresholds for preterm infants: finally some answers. Arch Dis Child Fetal Neonatal Ed. (2022) 107(2):126–30. doi: 10.1136/archdischild-2020-320495
23. Wong H, Connelly R, Day A, Flavin MP. A comparison of high and standard blood transfusion volumes in premature infants. Acta Paediatr. (2005) 94(5):624–5. doi: 10.1111/j.1651-2227.2005.tb01949.x
24. Paul DA, Leef KH, Locke RG, Stefano JL. Transfusion volume in infants with very low birth weight: a randomized trial of 10 versus 20 mL/kg. J Pediatr Hematol Oncol. (2002) 24(1):43–6. doi: 10.1097/00043426-200201000-00012
25. Mallett LH, Govande VP, Shetty A, Beeram MR. Safety and efficacy of packed red blood cell transfusions at different doses in very low birth weight infants. Bayl Univ Med Cent Proc. (2016) 29(2):128–30. doi: 10.1080/08998280.2016.11929387
26. Ribeiro HS, Assunção A, Vieira RJ, Soares P, Guimarães H, Flor-de-Lima F. Platelet transfusions in preterm infants: current concepts and controversies—a systematic review and meta-analysis. Eur J Pediatr. (2023) 182(8):3433–43. doi: 10.1007/s00431-023-05031-y
27. Christensen RD, Henry E, Wiedmeier SE, Stoddard RA, Sola-Visner MC, Lambert DK, et al. Thrombocytopenia among extremely low birth weight neonates: data from a multihospital healthcare system. J Perinatol. (2006) 26(6):348–53. doi: 10.1038/sj.jp.7211509
28. Josephson CD, Su LL, Christensen RD, Hillyer CD, Castillejo M-I, Emory MR, et al. Platelet transfusion practices among neonatologists in the United States and Canada: results of a survey. Pediatrics. (2009) 123(1):278–85. doi: 10.1542/peds.2007-2850
29. Patel RM. Effect of a Higher vs. Lower Platelet Transfusion Threshold on Death or Major Bleeding in Preterm Infants. 50 Studies Every Neonatologist Should Know. New York: Oxford University Press (2024). p. 207–12. doi: 10.1093/med/9780197646953.003.0033
30. Murray NA, Howarth LJ, McCloy MP, Letsky EA, Roberts IAG. Platelet transfusion in the management of severe thrombocytopenia in neonatal intensive care unit patients. Transfus Med. (2002) 12(1):35–41. doi: 10.1046/j.1365-3148.2002.00343.x
31. Kozak M, Hallan DR, Stoltzfus M, Rizk E. Lumbar puncture in thrombocytopenia: the floor is not firm. Cureus. (2023) 15:e42019. doi: 10.7759/cureus.42019
32. Kumar J, Dutta S, Sundaram V, Saini SS, Sharma RR, Varma N. Platelet transfusion for Pda closure in preterm infants: a randomized controlled trial. Pediatrics. (2019) 143(5):e20182565. doi: 10.1542/peds.2018-2565
33. Dannaway DC, Noori S. A randomized trial of platelet transfusions over 30 vs. 120 minutes: is there an effect on post-transfusion platelet counts? J Perinatol. (2013) 33(9):703–6. doi: 10.1038/jp.2013.46
Keywords: blood transfusion, India, neonate, NICU, survey
Citation: Kolkur KS, Patnaik SK, Malshe N, Deshmukh V, Tripathi S, Nagar N, Sreekantha S and Suryawanshi P (2025) Neonatal blood transfusion practices in India: a nationwide survey of clinicians. Front. Pediatr. 13:1692244. doi: 10.3389/fped.2025.1692244
Received: 25 August 2025; Accepted: 5 November 2025;
Published: 18 November 2025.
Edited by:
Mehmet Satar, Çukurova University, TürkiyeReviewed by:
Claudio Pellegrino, Agostino Gemelli University Polyclinic (IRCCS), ItalyAysegul Zenciroglu, Dr Sami Ulus Child Health and Diseases Training and Research Hospital, Türkiye
Copyright: © 2025 Kolkur, Patnaik, Malshe, Deshmukh, Tripathi, Nagar, Sreekantha and Suryawanshi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pradeep Suryawanshi, UHJhZGVlcC5zdXJ5YXdhbnNoaUBiaGFyYXRpdmlkeWFwZWV0aC5lZHU=
 Karthik S. Kolkur
Karthik S. Kolkur Suprabha K. Patnaik
Suprabha K. Patnaik Nandini Malshe
Nandini Malshe Vikrant Deshmukh
Vikrant Deshmukh Shalini Tripathi
Shalini Tripathi Nandini Nagar3
Nandini Nagar3 Pradeep Suryawanshi
Pradeep Suryawanshi