- Department of Pediatric Infectious Diseases, West China Second University Hospital, Key Laboratory of Birth Defects and Related Diseases of Women and Children (Sichuan University), Ministry of Education, Chengdu, China
Salmonella enterica-induced arthritis frequently manifests in children with malignancies, sickle cell disease, immunodeficiency, or undergoing immunosuppressive therapy; however, its incidence in healthy children is rare. Here, we present a case of septic arthritis resulting from S. enterica infection in a previously healthy child, diagnosed utilizing metagenomic next-generation sequencing (mNGS). This case underscores the utility of mNGS in the clinical identification of S. enterica arthritis. Particularly in scenarios where the causative pathogen remains unidentified, mNGS emerges as a pivotal adjunctive diagnostic modality for uncommon pathogens.
Introduction
Salmonella, a Gram-negative bacillus within the Enterobacteriaceae family, is a significant global pathogen responsible for foodborne illnesses mainly transmitted through the fecal-oral route (1). Infections typically manifest as gastroenteritis, bacteremia, or localized infections. Although relatively uncommon, Salmonella enterica-induced arthritis presents clinically with a fever incidence of 0.1%–0.2% in pediatric septic arthritis, predominantly affecting the hip joint (2). This condition often occurs in children with underlying conditions such as sickle cell disease, immunodeficiency, or those undergoing immunosuppressive therapy (3, 4). Septic arthritis stemming from S. enterica infection is rare in healthy children. The advent of metagenomic next-generation sequencing (mNGS) presents a novel approach to pathogen detection. This article presents a case study of septic arthritis caused by S. enterica infection diagnosed via mNGS in an otherwise healthy child.
Case presentation
A 3-year-old boy was admitted with swelling and pain in his right knee joint persisting for 13 days, accompanied by a 3-day fever. Prior to symptom onset, he accidentally scratched the skin over his right knee while playing. The injury resulted in local skin abrasions and bleeding but did not impede his ability to walk. The following day, he experienced pain and restricted mobility in the right knee, along with a limp. Initially, there were no signs of fever, chills, redness, or swelling in the affected joint. He received initial treatment at a local orthopedic hospital, where plain radiographs revealed no abnormalities, and a diagnosis of synovitis was considered. Despite over 10 h of topical traditional Chinese medicine application, there was no improvement in pain or mobility. Two days after the onset of symptoms, the patient exhibited significant redness, swelling, warmth, and pain in the right knee joint, accompanied by worsened mobility and the onset of fever, peaking at 39.5 °C. Magnetic resonance imaging (MRI) revealed a small effusion within the knee joint cavity, confirming synovitis. Intravenous Cefathiamidine was administered for anti-infective therapy. After 48 h, the patient's temperature normalized, and swelling decreased, though pain and limited mobility persisted. The local skin temperature of the right knee remained elevated compared to the healthy side, with symptoms gradually improving over a 10-day treatment period.
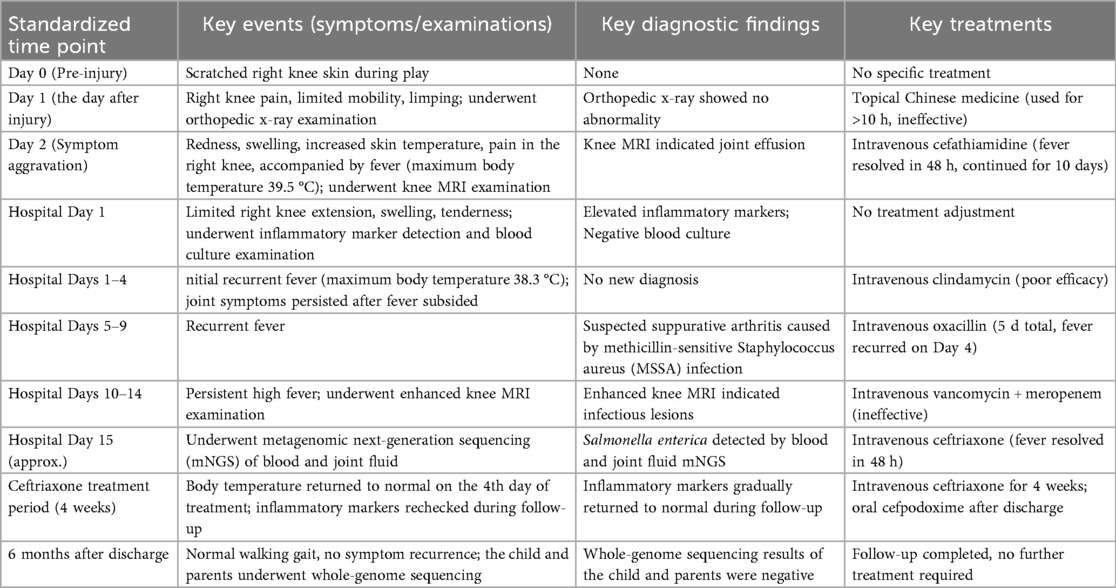
Upon admission, the child presented with vital signs within normal ranges: Temperature, 36.3 °C; heart rate, 97 beats/min; respiratory rate, 19 breaths/min; and blood pressure, 86/53 mmHg. The right knee showed limited extension and was swollen with increased skin temperature and tenderness. The patellar taping test was inconclusive. Laboratory findings indicated: white blood cell count 12.0 × 109/L,hemoglobin,92 g/L, blood platelet count 656 × 109/L, C-reactive protein 26.2 mg/L,and erythrocyte sedimentation rate: 107 mm/h. Liver and kidney function, autoantibodies, Interferon Gamma Release Assay, tuberculin skin test, and blood cultures were negative. Empiric antibacterial therapy with Clindamycin was administered intravenously for 4 days, resulting in recurrent fever for the initial 2 days, peaking at 38.3 °C. Fever subsided after 2 days, but joint symptoms persisted. Due to suspected methicillin-sensitive Staphylococcus aureus (MSSA) infection-induced purulent arthritis, treatment shifted to intravenous oxacillin for 5 days. However, fever recurred after 4 days, prompting consideration of septic arthritis from methicillin-resistant Staphylococcus aureus (MRSA) or other gram-negative bacteria. Antibiotics were escalated to intravenous oxacillin for 5 days. Therefore, the antibiotic regimen was upgraded to intravenous vancomycin and meropenem. Despite this, the child continued to experience a recurrent high fever. Enhanced MRI of the right knee joint revealed abnormal signals in the distal femoral epiphysis involving the adjacent epiphyseal cartilage with local penetration of the epiphyseal cartilage into the joint cavity, lateral collateral ligament, and anterior cruciate ligament. Multiple areas of synovial thickening and enhancement were observed, with slight swelling of the soft tissue around the right knee joint and effusion in the suprapatellar bursa and joint cavity, suggesting infectious lesions (Figures 1, 2). Chest, abdominal, and cardiac ultrasonography revealed no evidence of migratory lesions or thrombosis in the limbs. As blood cultures were negative, blood was sent for mNGS to confirm the pathogen, which detected Salmonella enterica (Figure 3). Similarly, mNGS testing of joint cavity effusion aspirates yielded Salmonella enterica (Figure 4), whereas cultures of the joint cavity effusion were negative. Metagenomic next-generation sequencing (mNGS) analysis showed that Salmonella enterica was detected in both blood and joint fluid samples. The genome coverage of Salmonella enterica was 0.0061% (2/4,094) in blood and 0.0285% (14/103) in joint fluid, with 2 and 14 microbial reads identified respectively (The mNGS workflow and bioinformatics description are provided in the Supplementary Materials). Following the mNGS results, the treatment was adjusted to ceftriaxone as an anti-infective therapy,considering its excellent tissue penetration. After 48 h of ceftriaxone treatment, the child's fever improved, and the swelling and pain in the right knee joint were gradually alleviated. Four days later, the patient's body temperature normalized. The child received intravenous ceftriaxone for 4 weeks and remained stable during follow-up visits. The CRP and ESR returned to normal, and the child was discharged on oral cefpodoxime during outpatient follow-up. The child's condition stabilized and recovered with a normal walking gait. Whole-genome sequencing revealed negative results for the child and his parents during the follow-up visit after six months, with no recurrence of symptoms.The detailed diagnosis and treatment process are shown in Table 1.
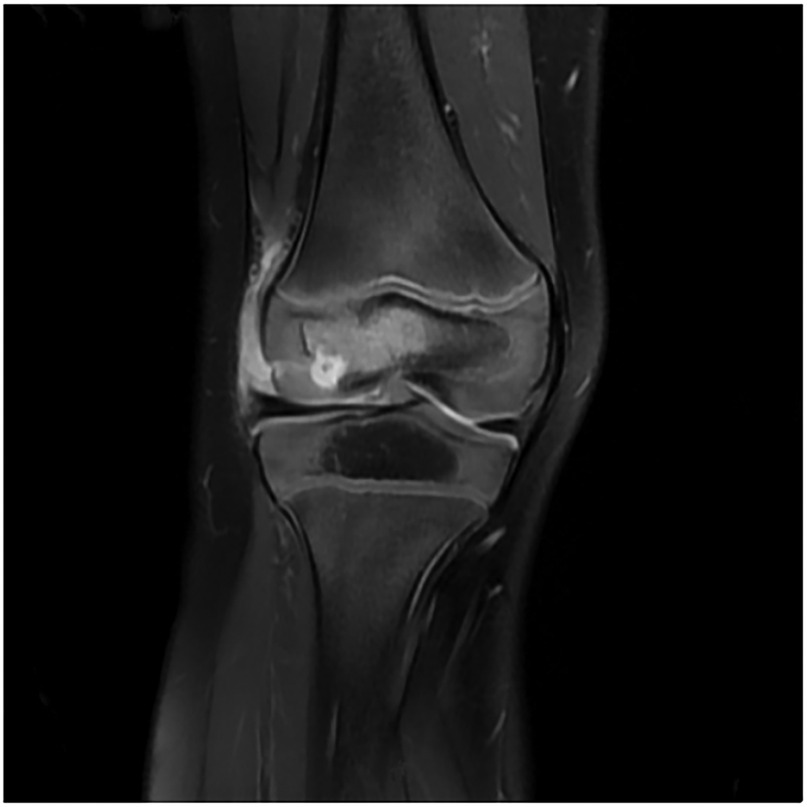
Figure 1. The image demonstrates abnormal hyperintense signals with rim enhancement in the epiphysis of the left distal femur, consistent with septic arthritis-related bone marrow edema and inflammatory changes.
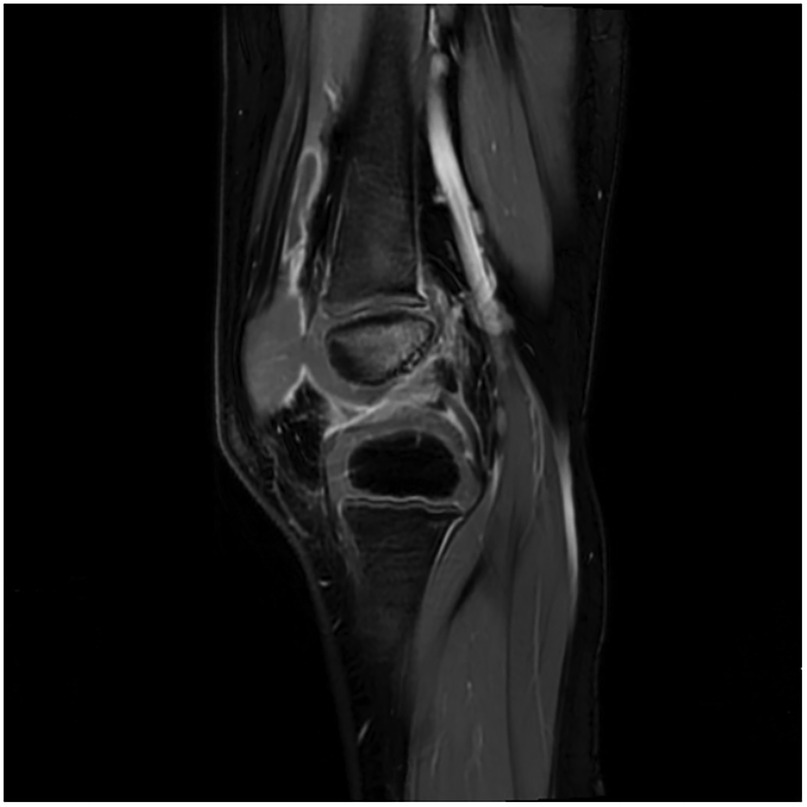
Figure 2. The image reveals prominent joint effusion, synovial enhancement, and surrounding soft tissue edema, consistent with septic arthritis-related inflammatory changes.
Figure 3. mNGS results from joint fluid. Genome coverage and the number of specific reads detected for Salmonella enterica are shown. Two unique reads mapped to the S. enterica genome out of 4,094 microbial reads, with a genome coverage of 0.0061%.
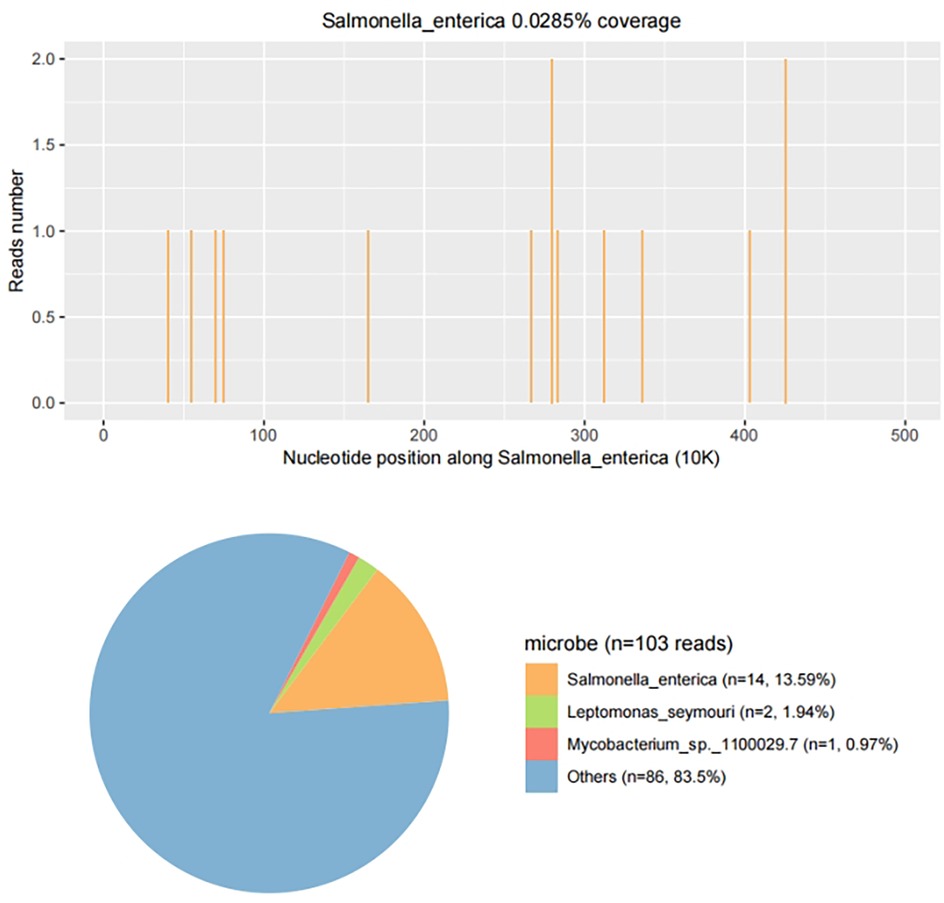
Figure 4. mNGS results from joint fluid. Genome coverage and the number of specific reads detected for Salmonella enterica are shown. A total of 14 reads mapped to the S. enterica genome out of 103 microbial reads, with a genome coverage of 0.0285%.
Discussion
Salmonella, a zoonotic intestinal pathogen, is primarily categorized into Salmonella typhi, Salmonella paratyphi, and non-typhoidal Salmonella (NTS) (5). The former two primarily cause typhoid fever, while the latter mainly induces non-specific enteritis and invasive extraintestinal infections. Salmonella enterica falls under the NTS bacteria family. Children typically contract NTS infection through ingestion or contact with contaminated substances, presenting symptoms such as diarrhea (6). NTS infections can disseminate via the bloodstream, leading to localized infections, with septic arthritis being a rare complication (7). Most cases of Salmonella arthritis affect single joints, while others arise post-trauma or after prosthetic joint replacement (8, 9). Notably, the absence of a prior unclean diet, diarrheal symptoms, and similar manifestations in cohabiting family members is a unique clinical feature of our case. This differs from the typical clinical profile of Salmonella arthritis—where gastrointestinal symptoms or epidemiological links (e.g., family exposure) are commonly reported (5, 10), further expanding the phenotypic spectrum of this rare infection. Initial trauma management involved topical traditional Chinese medicine, but the possibility of infection through the wound cannot be dismissed.
This case underscores the clinical challenge of diagnosing culture-negative Salmonella arthritis in children. As timely diagnosis of pediatric septic arthritis is critical to prevent permanent disability, reliance solely on traditional culture methods may lead to delays—especially given the low blood culture positive rate (10%–15%) for Salmonella arthritis (11). Although microbial culture remains the gold standard for pathogen identification, mNGS offers distinct advantages: shorter turnaround time and higher sensitivity (61.9% vs. 45.2% in a 42-patient prospective study) (12, 13). Consistent with previous suggestions that genetic sequencing is optimal for suspected infections with negative etiological tests (14). A notion supported by our case, where mNGS confirmed Salmonella enterica infection despite negative blood, bone marrow, and joint fluid cultures. Importantly, to our knowledge, this is the first case report documenting the diagnosis of Salmonella arthritis using mNGS, expanding the utility of this technology for rare, culture-negative pediatric osteoarticular infections.
Third-generation cephalosporins are the preferred initial antibiotics for treating NTS infection in children. Quinolones are generally not recommended for pediatric use, though they might be considered in severe infections where other antibiotics are unavailable (15). Carbapenems are effective against multidrug-resistant Salmonella infections. However, meropenem proved ineffective in this case, possibly due to inadequate local concentration in the joints. Initial treatment with clindamycin and cefotiam did not significantly alleviate joint swelling and pain, likely due to poor bacterial sensitivity. Ceftriaxone was chosen for its superior tissue penetration and efficacy.
Septic arthritis caused by NTS generally carries a more favorable prognosis compared to that caused by other Gram-negative bacteria. When empirical anti-infective treatment proves ineffective, consideration should be given to the possibility of Salmonella arthritis, highlighting the necessity of pathogen detection. Salmonella infections are typically identified through fluid or stool cultures; however, in cases where conventional microbial cultures yield negative results, mNGS can be employed to confirm the presence of the pathogen. Although susceptibility test results may not be readily available, third-generation cephalosporins are currently favored as treatment options. In similar scenarios, ceftriaxone, in alignment with local epidemiology and bacterial resistance rates, may be contemplated to avert delays in achieving effective treatment in future. Ensuring adequate and complete therapy courses is paramount for effectively treating lesions and preventing recurrence.
Conclusion
Septic arthritis caused by Salmonella enteritidis is extremely rare in healthy children, with atypical clinical manifestations. Its diagnosis is challenging when conventional culture results are negative. For culture-negative septic arthritis in children, metagenomic next-generation sequencing (mNGS) should be regarded as an important auxiliary diagnostic tool. Ceftriaxone may be an effective therapeutic option for Salmonella enteritidis-associated arthritis in children. When empirical treatment fails and culture results are negative, clinicians should consider utilizing mNGS even for healthy children without typical epidemiological clues.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
MR: Conceptualization, Writing – original draft. QL: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2025.1704234/full#supplementary-material
References
1. MacKenzie KD, Palmer MB, Köster WL, White AP. Examining the link between biofilm formation and the ability of pathogenic Salmonella strains to colonize multiple host species. Front Vet Sci. (2017) 4:138. doi: 10.3389/fvets.2017.00138
2. Tassinari AM, Romaneli MT, Das N, Pereira RM, Tresoldi AT. Septic arthritis caused by Salmonella enterica serotype rubislaw: a case report. Rev Soc Bras Med Trop. (2019) 52:e20180253. doi: 10.1590/0037-8682-0253-2018
3. Punpanich W, Netsawang S, Thippated C. Invasive salmonellosis in urban Thai children—a ten-year review. Pediatr Infect Dis J. (2012) 31(8):e105–10. doi: 10.1097/INF.0b013e3182569994
4. Wen SCH, Best E, Nourse C. Non-typhoidal Salmonella infections in children: review of literature and recommendations of management. J Paediatr Child Health. (2017) 53(10):936–41. doi: 10.1111/jpc.13485
5. Gharib MH, Alebbi S, Rashid F. Salmonella-Related septic arthritis in an immunocompetent adult: a case report. Cureus. (2022) 14(1):e21030. doi: 10.7759/cureus.21030
6. Chang KM, Karkenny G, Koshy R. Salmonella septic arthritis and bacteremia in a patient with poorly controlled diabetes. Cureus. (2021) 13(12):e20465. doi: 10.7759/cureus.20465
8. Huang JL, Hung JJ, Wu KC, Lee WI, Chan CK, Ou LS. Septic arthritis in patients with systemic lupus erythematosus: salmonella and nonsalmonella infections compared. Semin Arthritis Rheum. (2006) 36:61–7. doi: 10.1016/j.semarthrit.2006.04.003
9. Casado-Castillo F, Kobayashi T, Sekar P, Streit J, De Pena I M. Prosthetic hip infection due to Salmonella enterica serovar enteritidis. IDCases. (2021) 25:e01170. doi: 10.1016/j.idcr.2021.e01170
10. Tirta M, Ampelas D, Tsintavis P, Pilichou A, Krallis P. Salmonella septic hip arthritis in immunocompetent children: three case reports and literature review. Cureus. (2022) 14(8):e27701. doi: 10.7759/cureus.27701
11. Al Nafeesah AS. Nontyphoidal Salmonella septic arthritis of the elbow in a healthy infant. Pan Afr Med J. (2015) 22:357. doi: 10.11604/pamj.2015.22.357.7680
12. Zhao Y, Zhang W, Zhang X. Application of metagenomic next-generation sequencing in the diagnosis of infectious diseases. Front Cell Infect Microbiol. (2024) 14:1458316. doi: 10.3389/fcimb.2024.1458316
13. Ramchandar N, Burns J, Coufal NG, Pennock A, Briggs B, Stinnett R, et al. Use of metagenomic next-generation sequencing to identify pathogens in pediatric osteoarticular infections. Open Forum Infect Dis. (2021) 8(7):ofab346. doi: 10.1093/ofid/ofab346
14. Chen X, Zhu J, Liu Z, Ye J, Yang L, Zhang Z. Mixed infection of three nontuberculous mycobacteria species identified by metagenomic next-generation sequencing in a patient with peritoneal dialysis-associated peritonitis: a rare case report and literature review. BMC Nephrol. (2023) 24(1):95. doi: 10.1186/s12882-023-03156-8
Keywords: mNGS, salmonella enterica, septic arthritis, healthy child, case report
Citation: Ren M and Liao Q (2025) Diagnosis of Salmonella enterica-induced septic arthritis in a healthy child using metagenomic next-generation sequencing: a case report. Front. Pediatr. 13:1704234. doi: 10.3389/fped.2025.1704234
Received: 12 September 2025; Accepted: 6 November 2025;
Published: 19 November 2025.
Edited by:
George A. Syrogiannopoulos, University of Thessaly, GreeceReviewed by:
Vladimir Gostev, Children’s Scientific Clinical Centre for Infectious Diseases, RussiaXuming Li, Hugo Biotechnologies Co., Ltd., China
Copyright: © 2025 Ren and Liao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiong Liao, MzM3NjU0MTBAcXEuY29t
 Min Ren
Min Ren Qiong Liao
Qiong Liao