- 1Department of Nephrology, Xiyuan Hospital, China Academy of Chinese Medical Sciences, Beijing, China
- 2Beijing University of Chinese Medicine, Beijing, China
- 3Department of Statistics, Purdue University, West Lafayette, IN, America
- 4Xiyuan Hospital, China Academy of Chinese Medical Sciences, Beijing, China
- 5NMPA Key Laboratory for Clinical Research and Evaluation of Traditional Chinese Medicine, Beijing, China
- 6National Clinical Research Center for Chinese Medicine Cardiology, Beijing, China
Background: The purpose of this meta-analysis was to evaluate the controversy of angiotensin-converting enzyme inhibitor (ACEI) in combination with angiotensin-receptor blocker (ARB) in the treatment of chronic kidney disease (CKD) based on dose.
Methods: PubMed, EMBASE, and Cochrane Library were searched to identify randomized controlled trials (RCTs) from inception to March 2020. The random effects model was used to calculate the effect sizes. Potential sources of heterogeneity were detected using sensitivity analysis and meta-regression.
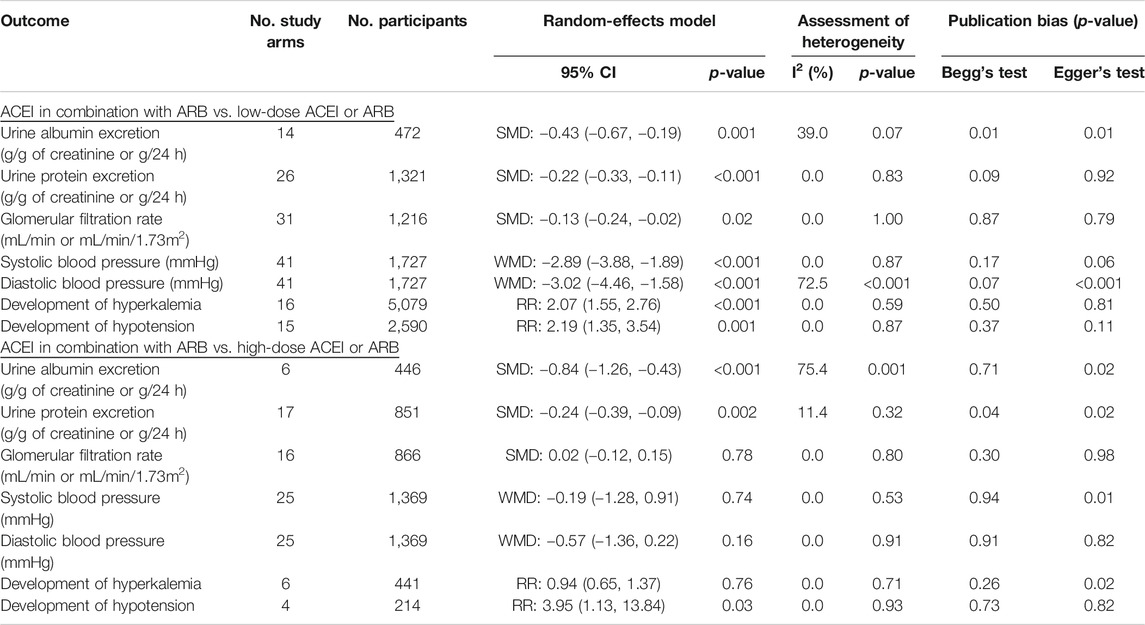
Results: This meta-analysis of 53 RCTs with 6,375 patients demonstrated that in patients with CKD, ACEI in combination with ARB was superior to low-dose ACEI or ARB in reducing urine albumin excretion (SMD, −0.43; 95% CI, −0.67 to −0.19; p = 0.001), urine protein excretion (SMD, −0.22; 95% CI, −0.33 to −0.11; p < 0.001), and blood pressure (BP), including systolic BP (WMD, −2.89; 95% CI, −3.88 to −1.89; p < 0.001) and diastolic BP (WMD, −3.02; 95% CI, −4.46 to −1.58; p < 0.001). However, it was associated with decreased glomerular filtration rate (GFR) (SMD, −0.13; 95% CI, −0.24 to −0.02; p = 0.02) and increased rates of hyperkalemia (RR, 2.07; 95% CI, 1.55 to 2.76; p < 0.001) and hypotension (RR, 2.19; 95% CI, 1.35 to 3.54; p = 0.001). ACEI in combination with ARB was more effective than high-dose ACEI or ARB in reducing urine albumin excretion (SMD, −0.84; 95% CI, −1.26 to −0.43; p < 0.001) and urine protein excretion (SMD, −0.24; 95% CI, −0.39 to −0.09; p = 0.002), without decrease in GFR (SMD, 0.02; 95% CI, −0.12 to 0.15; p = 0.78) and increase in rate of hyperkalemia (RR, 0.94; 95% CI, 0.65 to 1.37; p = 0.76). Nonetheless, the combination did not decrease the BP and increased the rate of hypotension (RR, 3.95; 95% CI, 1.13 to 13.84; p = 0.03) compared with high-dose ACEI or ARB.
Conclusion: ACEI in combination with ARB is superior in reducing urine albumin excretion and urine protein excretion. The combination is more effective than high-dose ACEI or ARB without decreasing GFR and increasing the incidence of hyperkalemia. Despite the risk of hypotension, ACEI in combination with ARB is a better choice for CKD patients who need to increase the dose of ACEI or ARB (PROSPERO CRD42020179398).
Introduction
Chronic kidney disease, characterized by a reduced glomerular filtration rate (GFR) and/or increased urinary albumin excretion, is an increasing public health issue owing to its high prevalence and increased risk of end-stage renal disease, cardiovascular disease, and premature death (Matsushita et al., 2010). The prevalence of CKD is estimated to be 8–16% worldwide (Jha et al., 2013). CKD is a great global-health challenge, especially in low- and middle-income countries (Mills et al., 2015). National and international efforts for the prevention, detection, and treatment of CKD are needed to reduce its morbidity and mortality worldwide.
Hypertension commonly coexists with CKD, and its prevalence progressively increases with decline in kidney function (Muntner et al., 2010; Egan et al., 2014). According to recent guidelines, angiotensin-converting enzyme inhibitor (ACEI) or angiotensin-receptor blocker (ARB) should be the drugs of first choice for CKD (Kalaitzidis and Elisaf, 2018). The 2020 Kidney disease: Improving Global Outcomes (KDIGO) guideline recommends that treatment with an ACEI or an ARB be initiated in patients with diabetes, hypertension, and albuminuria and that these medications be titrated to the highest approved dose that is tolerated. The 2012 KDIGO guideline on IgA nephropathy recommends long-term ACEI or ARB treatment when proteinuria is >1 g/d, with up-titration of the drug depending on blood pressure (BP), and to achieve proteinuria <1 g/day. However, some CKD patients still have proteinuria after ACEI or ARB treatment (Igarashi et al., 2006; Slagman et al., 2011). Previous studies have suggested that the additive antiproteinuric and hypotensive effects of combined renin–angiotensin–aldosterone system (RAAS) blockade were superior to single RAAS blockade in CKD (Susantitaphong et al., 2013). Nonetheless, the use of ACEI in combination with ARB is not supported by all recent guidelines owing to concerns regarding adverse events such as renal dysfunction, hyperkalemia, and symptomatic hypotension in high-risk CKD patients (Esteras et al., 2015). Whether ACEI in combination with ARB or increasing the dose of ACEI or ARB is more effective in the treatment of CKD remains controversial. Therefore, the present meta-analysis of randomized controlled trials (RCTs) was designed to assess the efficacy and safety of ACEI in combination with ARB in patients with CKD based on the dose.
Methods
Data Sources and Searches
We searched PubMed, EMBASE, and Cochrane Library from inception to March 2020 to retrieve relevant articles. Two reviewers (Mingming Zhao and Rumeng Wang) independently screened the titles and abstracts of all electronic citations and full-text articles were retrieved for comprehensive review and independently rescreened. If a disagreement occurred between them, it was resolved by consulting with a third investigator (Yu Zhang). Medical Subject Headings and free-text terms were used in each database with the following relevant keywords: “diabetic nephropathy,” “hypertensive nephropathy,” “glomerular disease,” “proteinuria,” “renal insufficiency,” “kidney disease,” “chronic renal failure,” “chronic kidney disease,” “drug therapy combination,” “renin–angiotensin system,” “angiotensin-converting enzyme inhibitor,” and “angiotensin receptor blocker” (Supplementary Material S1).
Study Selection
We included studies that met the following inclusion criteria: 1) patients (>18 years old) with CKD (KDIGO: CKD is defined as abnormalities of kidney structure or function, present for >3 months, with implications for health); 2) the intervention group received ACEI in combination with ARB (dual therapy), and the comparison group received ACEI or ARB (single therapy); 3) the outcomes involved albuminuria, proteinuria, GFR (creatinine clearance or estimated GFR), BP, hyperkalemia (>5.5 mmol/L or as defined in the individual studies), or hypotension (as defined in the individual studies); 4) randomized, controlled, crossover, or parallel trials; 5) the articles were published in English language.
Data Extraction and Quality Assessment
Two reviewers (Mingming Zhao and Rumeng Wang) extracted data independently and disagreements were resolved by consulting with a third investigator (Yu Zhang). The following data were extracted from each of the published studies included in our review: the first author’s name, publication year, study design, intervention, dose of ACEI or ARB (low-dose: single dose compared with the same RAAS blockade in ACEI in combination with ARB group; high-dose: more than single dose compared with the same RAAS blockade in ACEI in combination with ARB group), sample size, percentage of men, mean age of subjects, duration of intervention, GFR, urine albumin or protein excretion rate, systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure, hyperkalemia, and hypotension. The methodological quality of the included studies was evaluated according to the recommendation of the Cochrane Handbook, including random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. Marked 1 point when the risk was low.
Data Synthesis and Analysis
The random effects model was used to calculate the effect sizes of eligible studies. For continuous outcomes, we calculated a weighted mean difference (WMD) or standard mean difference (SMD) with a 95% confidence interval (CI). For dichotomous outcomes, we estimated the relative risk (RR) with a 95% CI.
Heterogeneity of the included studies was described with the I2 index and the chi-square test. I2 ≥ 50% and p < 0.05 were used to indicate medium-to-high heterogeneity. We detected the potential sources of heterogeneity using meta-regression based on a priori selected study characteristics, including baseline of GFR, duration of intervention, mean age of subjects, and quality of included studies. Sensitivity analysis was performed to assess the robustness of the pooled results. The publication bias was evaluated using Begg’s test and Egger’s test. Statistical analysis was performed using Stata (version 15.1). The methodological quality of the included studies was assessed using RevMan5.3. We have registered the protocol for the present systematic review and meta-analysis, and the registration number in PROSPERO is CRD42020179398.
Results
Characteristics and Quality of the Studies
A total of 24,880 studies (18,664 from PubMed, 4,034 from EMBASE, and 2,182 from the Cochrane Library) were identified, of which 53 studies met the inclusion criteria (Figure 1A).
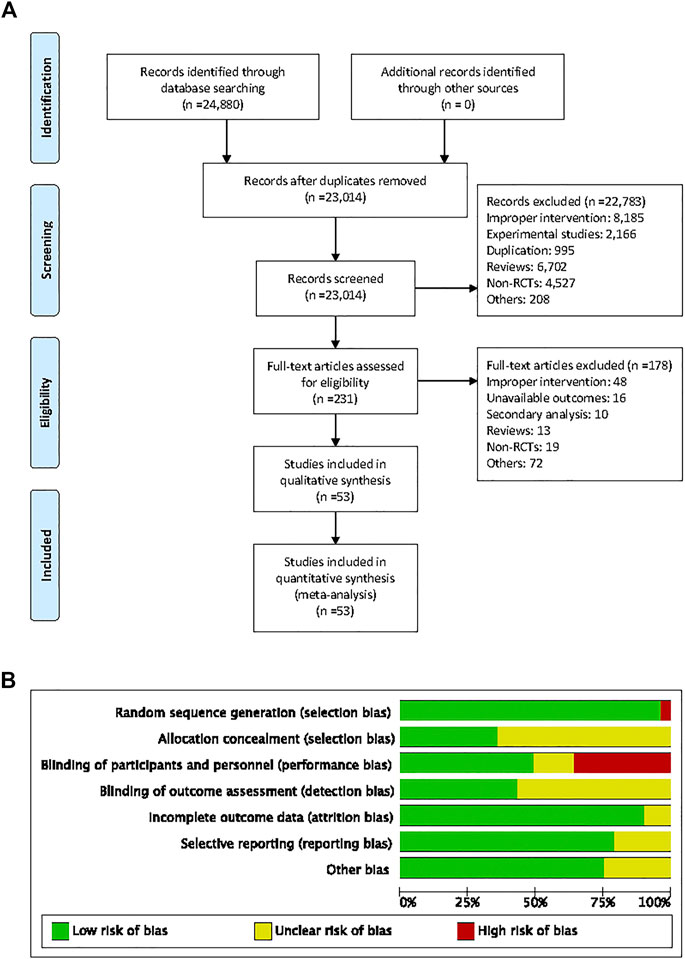
FIGURE 1. Flow diagram of searching for eligible studies and risk of bias summary. (A) Flow diagram of searching for eligible studies, (B) risk of bias summary.
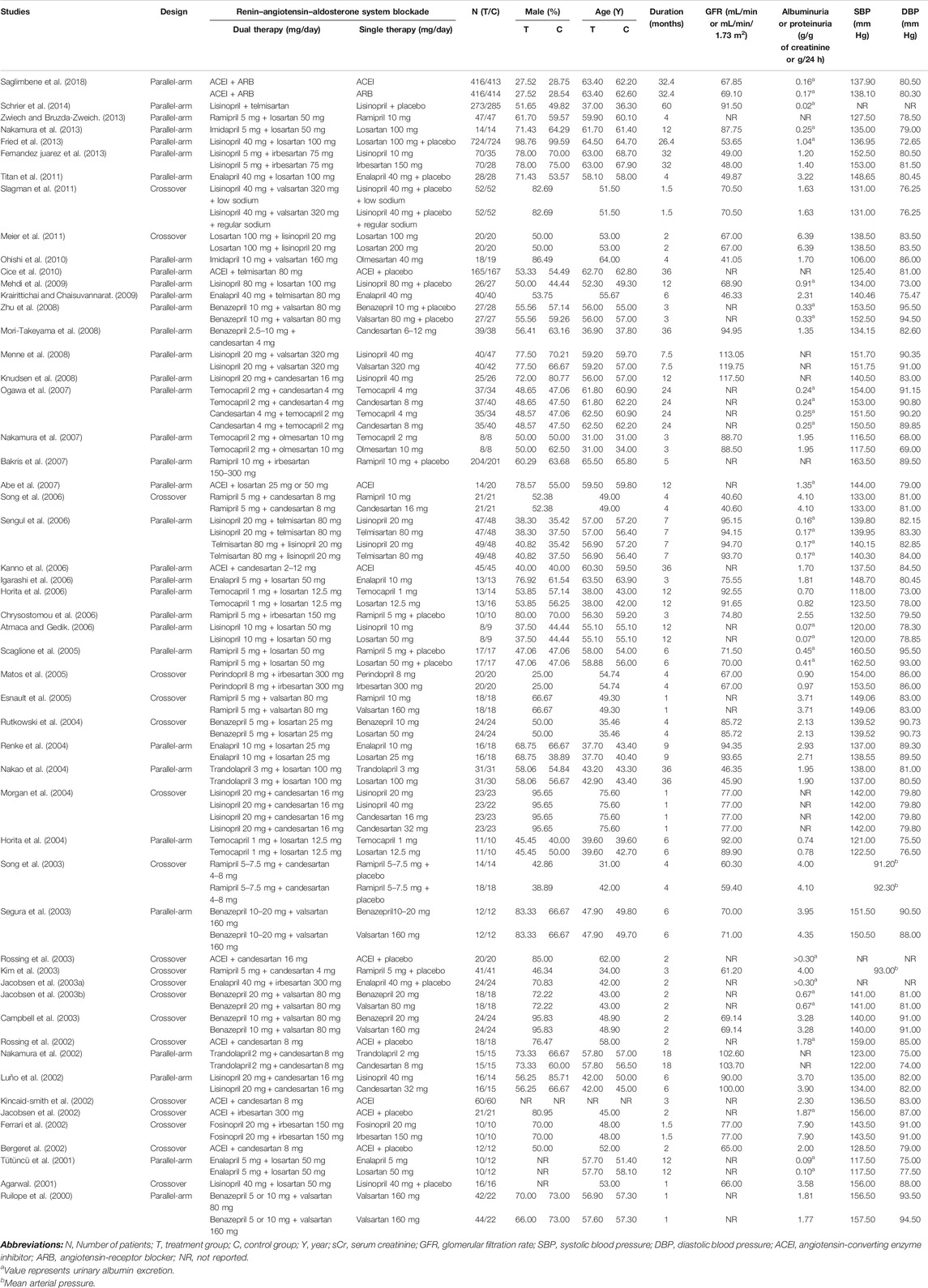
The characteristics of the individual trials are presented in Table 1. Fifty-three studies with 6,375 patients consisted of 19 crossover and 34 parallel-arm RCTs. The sample size varied from 10 to 1,448. The mean age of the subjects of the trials ranged from 31 to 76 years, and the duration of intervention ranged from 1 to 60 months. Twenty-eight studies enrolled patients with GFR ≥60 mL/min or mL/min/1.73 m2 and eight studies enrolled patients with GFR <60 mL/min or mL/min/1.73 m2. Seventeen studies did not report the subjects’ baseline kidney function. Fourteen studies were of fair quality (score 1–3) and 39 were of good quality (score 4–7) (Figure 1B).
Efficacy and Safety of ACEI in Combination with ARB vs. Low-Dose ACEI or ARB
Compared with low-dose ACEI or ARB, ACEI in combination with ARB significantly reduced urine albumin excretion (SMD, −0.43; 95% CI, −0.67 to −0.19; p = 0.001), urine protein excretion (SMD, −0.22; 95% CI, −0.33 to −0.11; p < 0.001), and BP (SBP: WMD, −2.89; 95% CI, −3.88 to −1.89; p < 0.001; DBP: WMD, −3.02; 95% CI, −4.46 to −1.58; p < 0.001) (Table 2; Figures 2–4). However, dual therapy was associated with decreased GFR (SMD, −0.13; 95% CI, −0.24 to −0.02; p = 0.02), increased rates of hyperkalemia (RR, 2.07; 95% CI, 1.55 to 2.76; p < 0.001) and hypotension (RR, 2.19; 95% CI, 1.35 to 3.54; p = 0.001) compared with low-dose ACEI or ARB (Table 2; Figures 5–7).
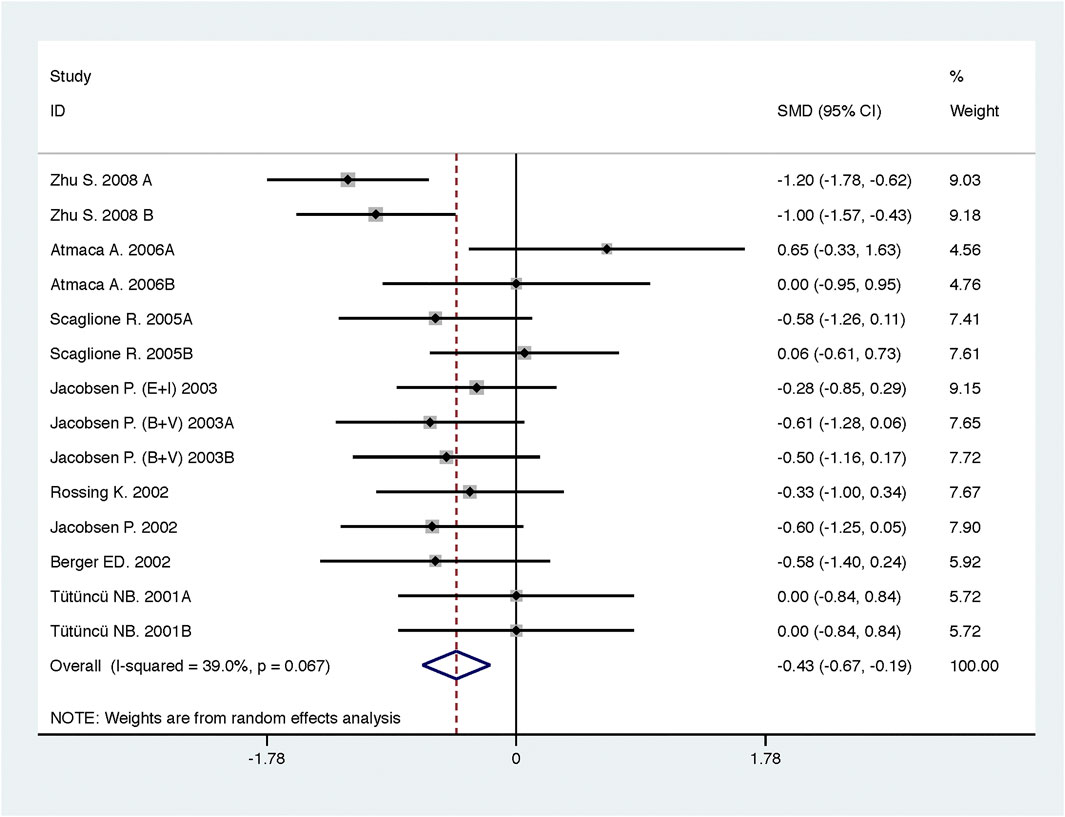
FIGURE 2. Comparison of ACEI in combination with ARB vs. low-dose ACEI or ARB for urine albumin excretion (g/g of creatinine or g/24 h).
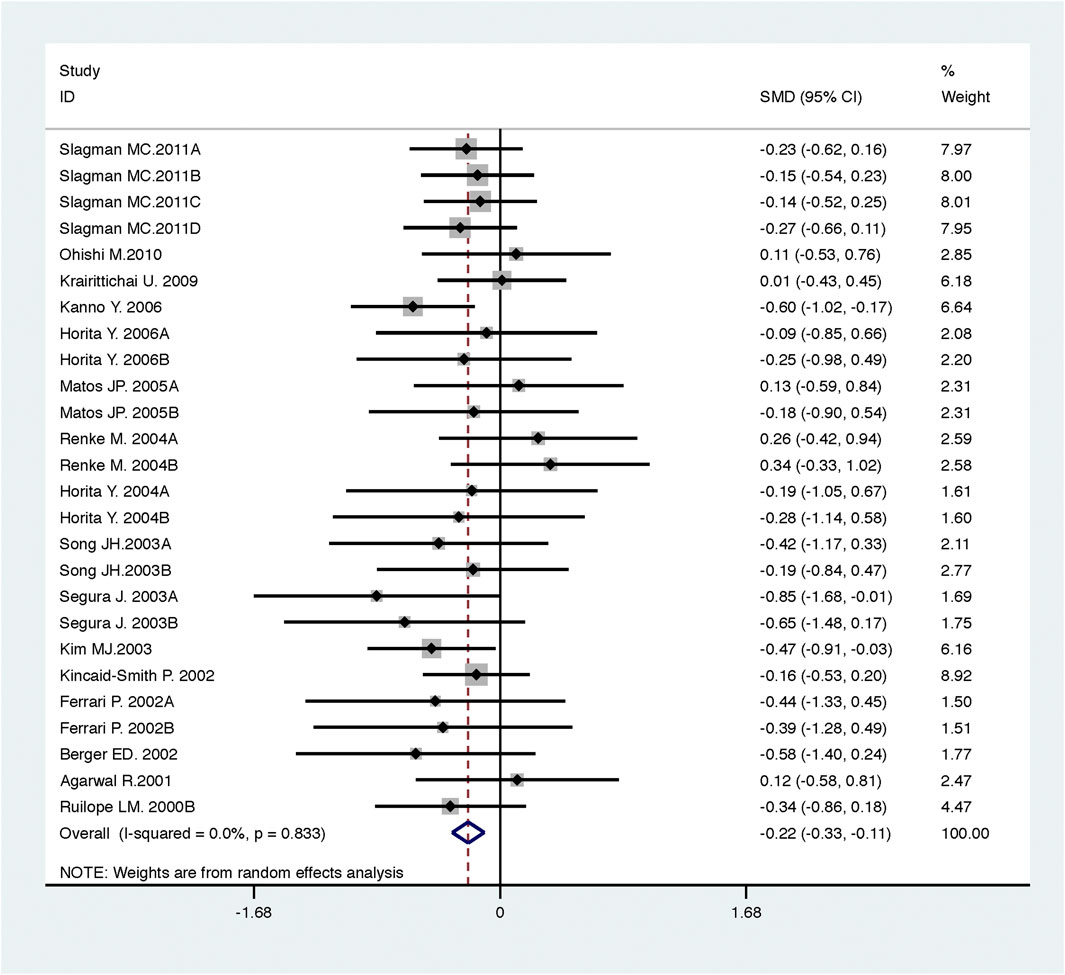
FIGURE 3. Comparison of ACEI in combination with ARB vs. low-dose ACEI or ARB for urine protein excretion (g/g of creatinine or g/24 h).
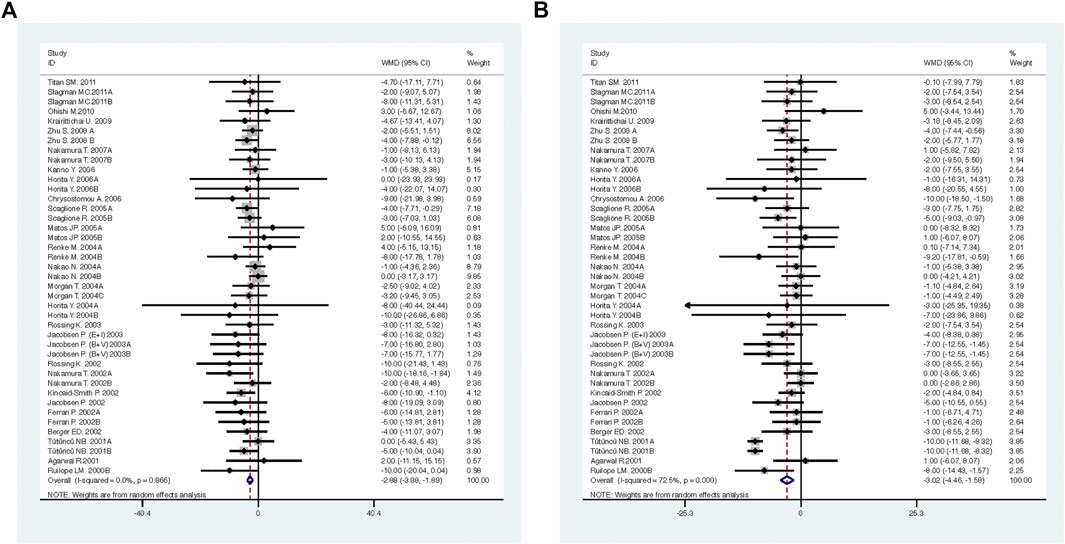
FIGURE 4. Comparison of ACEI in combination with ARB vs. low-dose ACEI or ARB for blood pressure. (A) systolic blood pressure (mmHg), (B) diastolic blood pressure (mmHg).
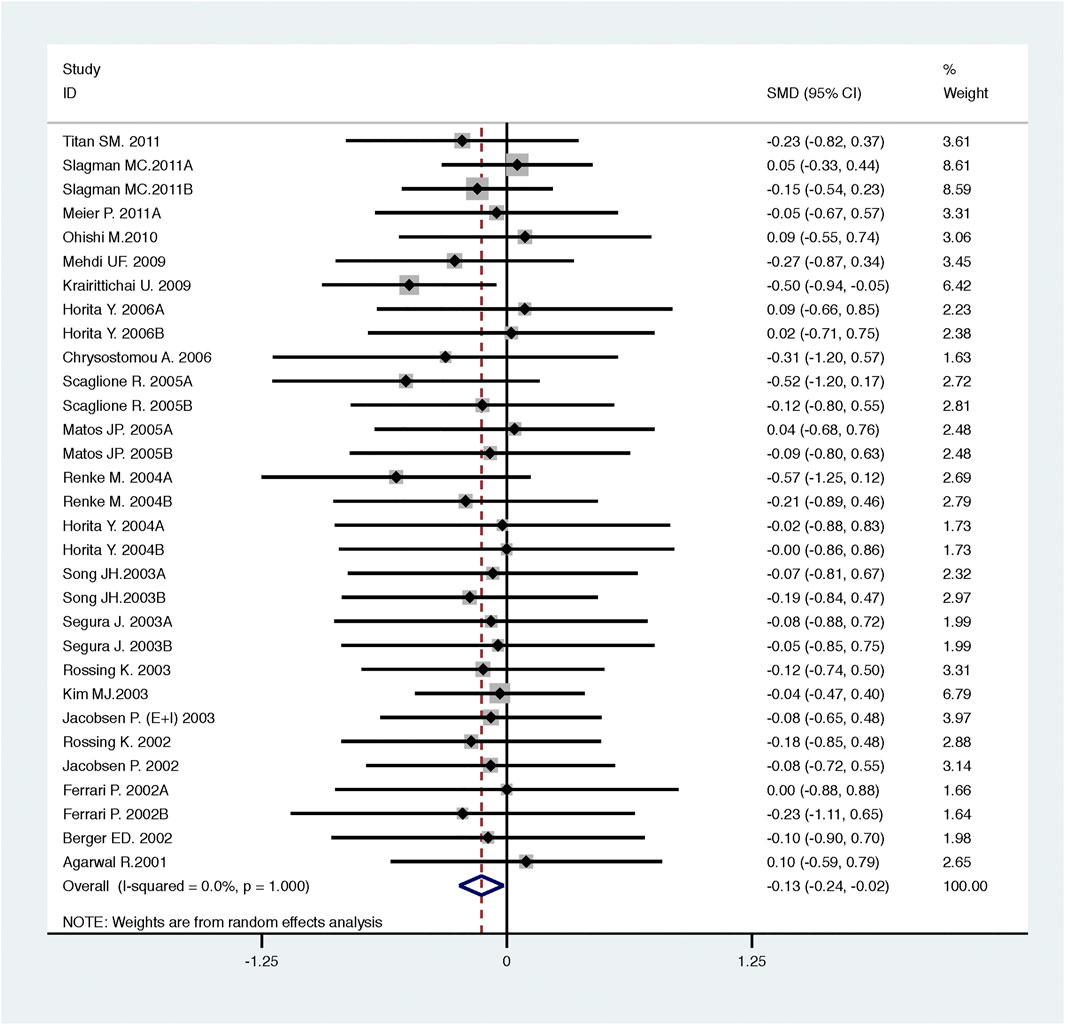
FIGURE 5. Comparison of ACEI in combination with ARB vs. low-dose ACEI or ARB for glomerular filtration rate (mL/min or mL/min/1.73m2).
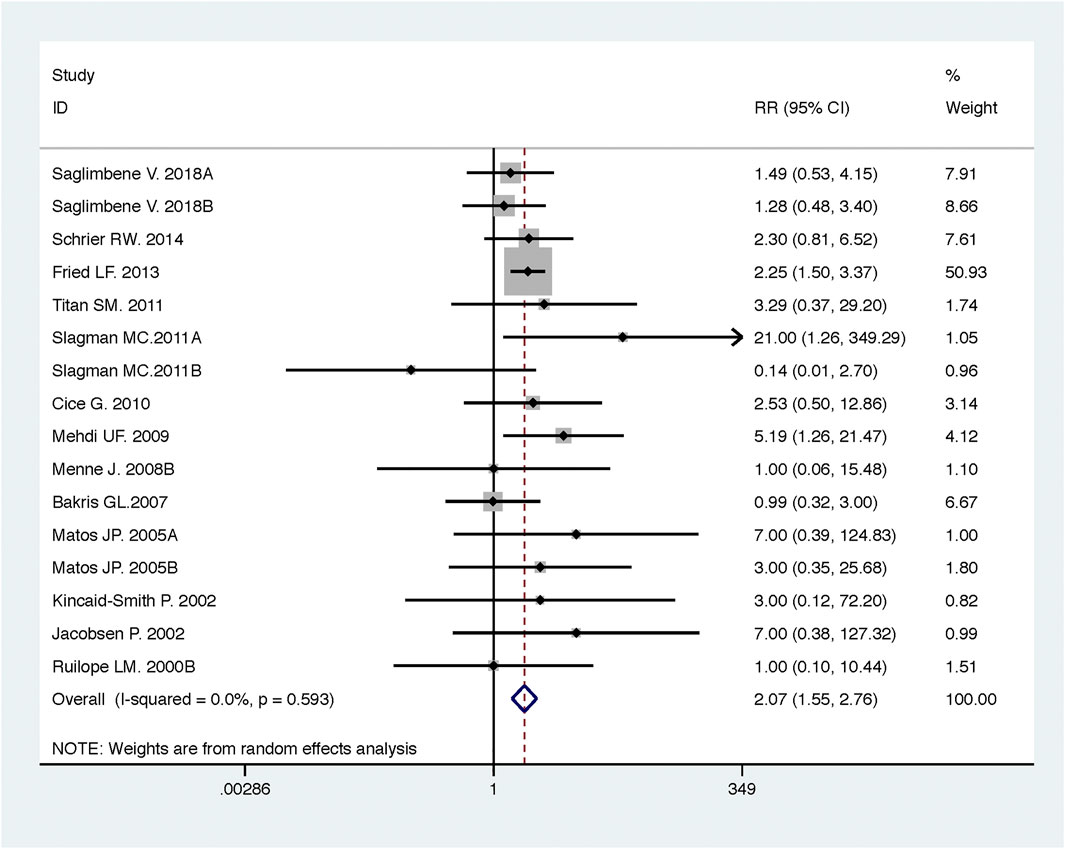
FIGURE 6. Comparison of ACEI in combination with ARB vs. low-dose ACEI or ARB for development of hyperkalemia.
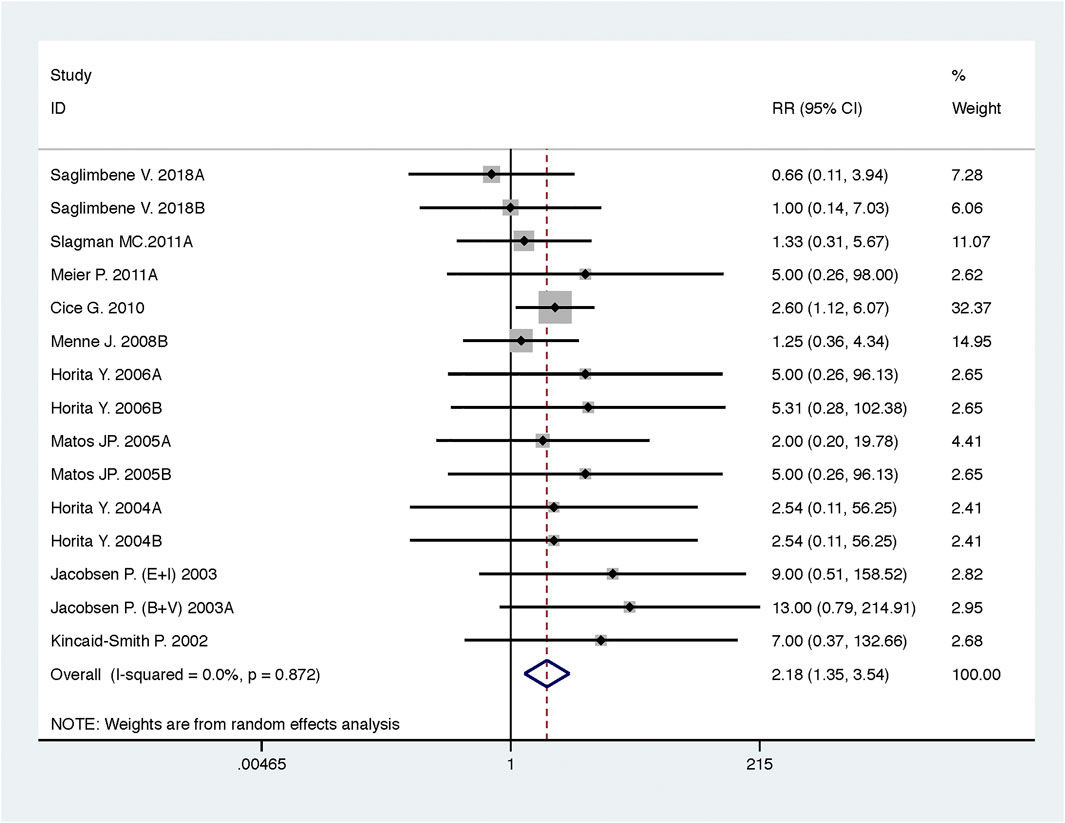
FIGURE 7. Comparison of ACEI in combination with ARB vs. low-dose ACEI or ARB for development of hypotension.
Efficacy and Safety of ACEI in Combination with ARB vs. High-Dose ACEI or ARB
Compared with high-dose ACEI or ARB, ACEI in combination with ARB significantly reduced urine albumin excretion (SMD, −0.84; 95% CI, −1.26 to −0.43; p < 0.001) and urine protein excretion (SMD, −0.24; 95% CI, −0.39 to −0.09; p = 0.002) (Table 2; Figures 8, 9). The combination did not decrease SBP (WMD, −0.19; 95% CI, −1.28 to 0.91; p = 0.74) and DBP (WMD, −0.57; 95% CI, −1.36 to 0.22; p = 0.16) (Table 2; Figure 10). ACEI in combination with ARB was not associated with decreased GFR (SMD, 0.02; 95% CI, −0.12 to 0.15; p = 0.78) and an increased rate of hyperkalemia (RR, 0.94; 95% CI, 0.65 to 1.37; p = 0.76) compared with high-dose ACEI or ARB (Table 2; Figures 11, 12). However, dual therapy was associated with an increased rate of hypotension (RR, 3.95; 95% CI, 1.13 to 13.84; p = 0.03) (Table 2; Figure 13).
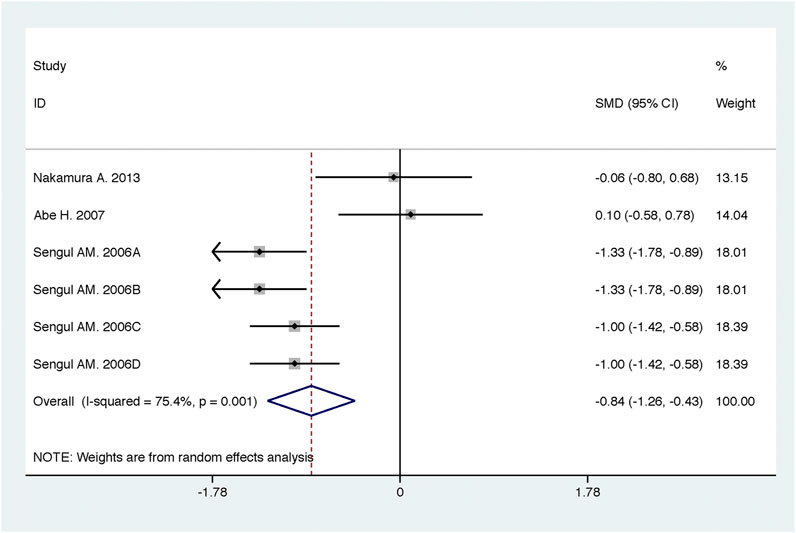
FIGURE 8. Comparison of ACEI in combination with ARB vs. high-dose ACEI or ARB for urine albumin excretion (g/g of creatinine or g/24 h).
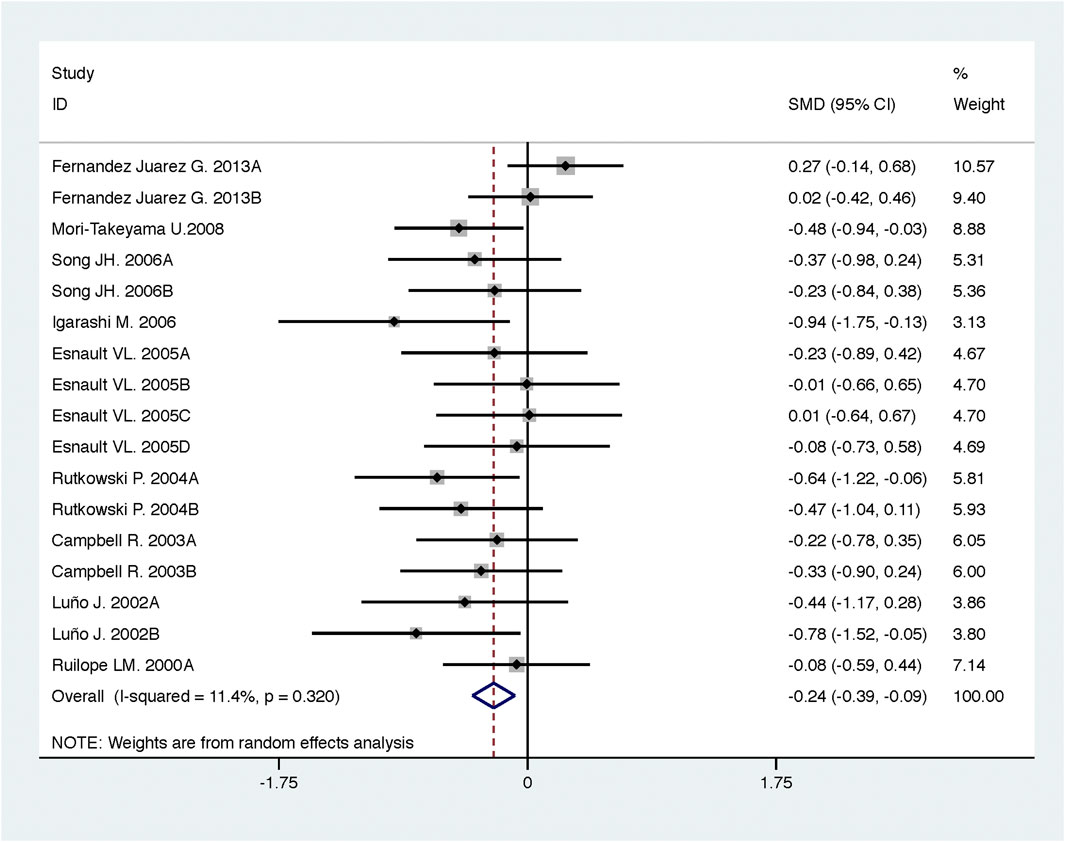
FIGURE 9. Comparison of ACEI in combination with ARB vs. high-dose ACEI or ARB for urine protein excretion (g/g of creatinine or g/24 h).
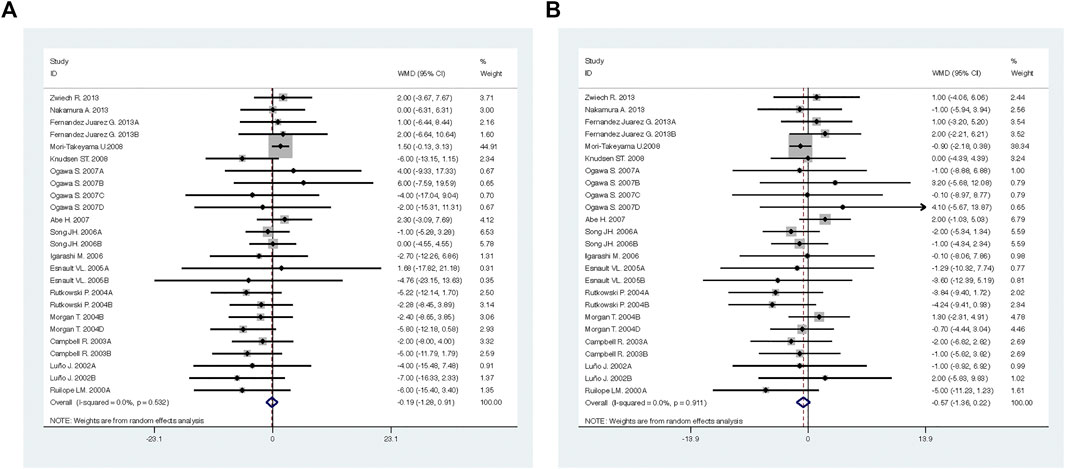
FIGURE 10. Comparison of ACEI in combination with ARB vs. high-dose ACEI or ARB for blood pressure. (A) systolic blood pressure (mmHg), (B) diastolic blood pressure (mmHg).
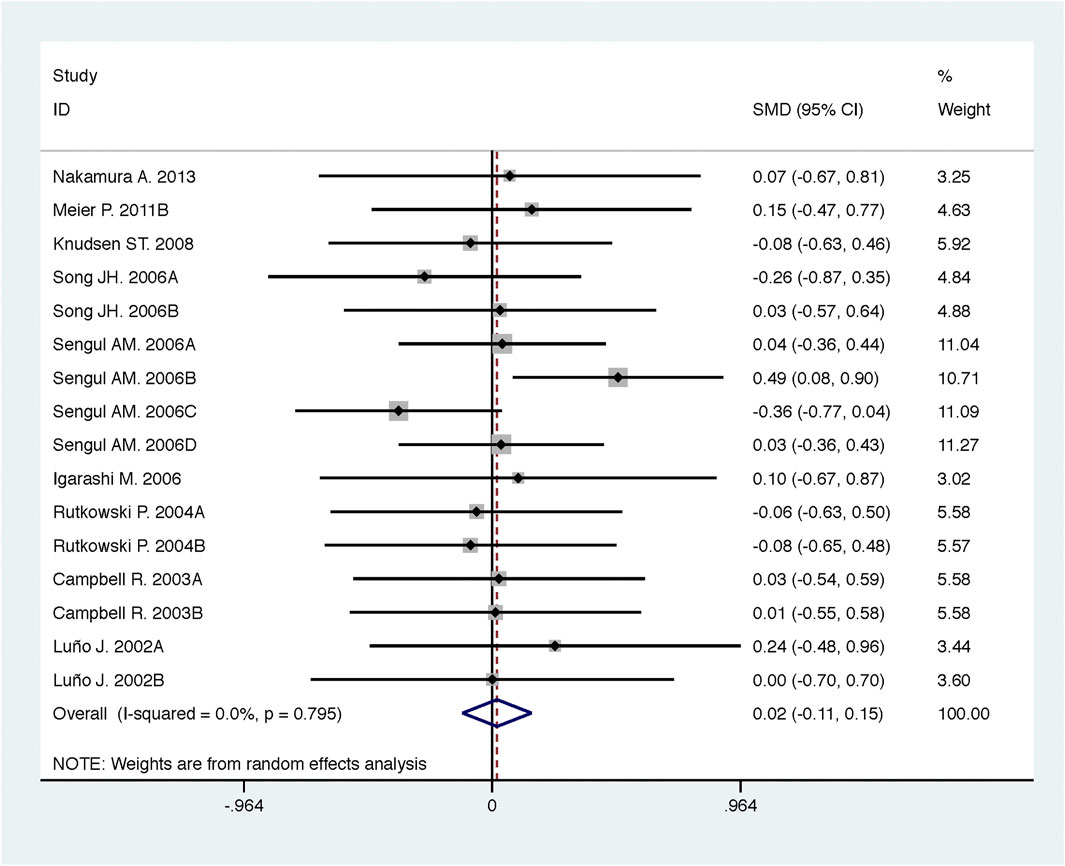
FIGURE 11. Comparison of ACEI in combination with ARB vs. high-dose ACEI or ARB for glomerular filtration rate (mL/min or mL/min/1.73m2).
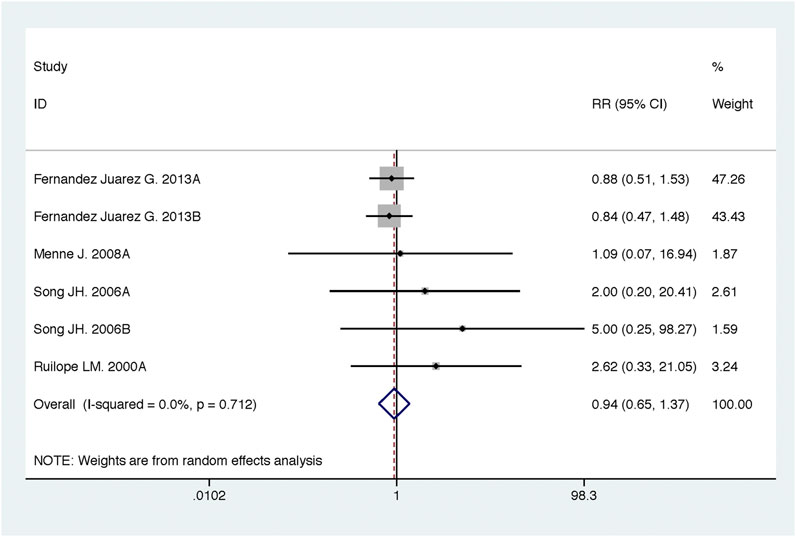
FIGURE 12. Comparison of ACEI in combination with ARB vs. high-dose ACEI or ARB for development of hyperkalemia.
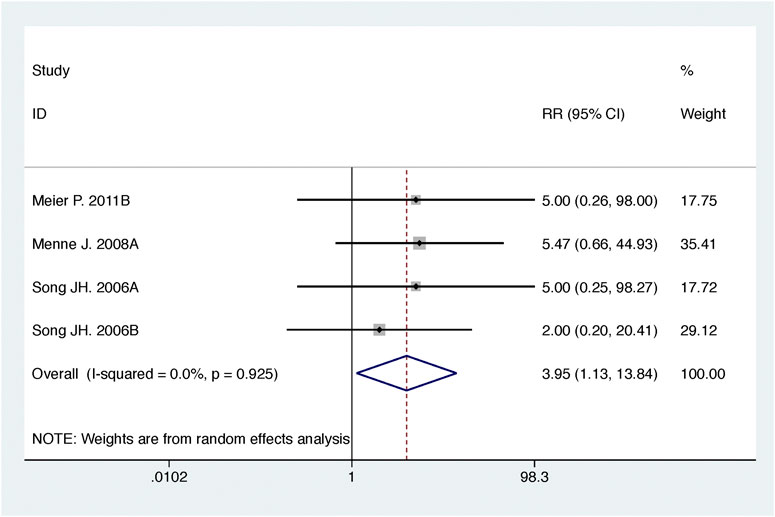
FIGURE 13. Comparison of ACEI in combination with ARB vs. high-dose ACEI or ARB for development of hypotension.
Sensitivity Analysis and Meta-Regression
To ensure reliability of the present meta-analysis, we evaluated the robustness of the results (Table 2) using sensitivity analysis, which indicated that the results of the meta-analysis were robust.
Significant heterogeneities were observed for DBP and urine albumin excretion (Table 2). We detected the potential sources of heterogeneity using meta-regression based on a priori selected study characteristics, including the mean age of subjects, duration of intervention, baseline of GFR, and quality of included studies.
A significant heterogeneity was observed for the outcome of urine albumin excretion (Table 2, summary effect of ACEI in combination with ARB vs. high-dose ACEI or ARB, I2 = 75.4%, p = 0.001), which was dependent on the mean age of subjects (exp, 1.30; 95% CI, 1.04 to 1.63; adjusted R2 = 89.09%; p = 0.03) and duration of intervention (exp, 1.27; 95% CI, 1.09 to 1.48; adjusted R2 = 100.00%; p = 0.01). Using meta-regression, it was found that the heterogeneity of DBP (Table 2, summary effect of ACEI in combination with ARB vs. low-dose ACEI or ARB) was not associated with a priori selected study characteristics.
Publication Bias
Begg’s test and Egger’s test were used to evaluate publication bias based on the key outcomes of the trials included in the meta-analysis. The results suggested less susceptibility to publication bias, except for urine albumin excretion and urine protein excretion (Table 2).
Discussion
In the present meta-analysis of 53 RCTs encompassing 6,375 participants, we aimed to compare the efficacy and safety of ACEI in combination with ARB vs. low-dose and high-dose ACEI or ARB. We demonstrated that ACEI in combination with ARB was superior to low-dose ACEI or ARB in reducing urine albumin excretion, urine protein excretion, and BP, including SBP and DBP. However, the combination was associated with a decreased GFR and increased rates of hyperkalemia and hypotension. ACEI in combination with ARB was more effective in reducing urine albumin excretion and urine protein excretion than high-dose ACEI or ARB, without decreased GFR and increased rate of hyperkalemia. Nonetheless, the combination did not decrease the BP and increased the rate of hypotension compared with the high-dose ACEI or ARB.
Proteinuria and hypertension are risk factors for CKD progression (Liu and Lv, 2019; Nagai et al., 2019). Proteinuria is also an independent predictor of all-cause mortality. A combination of severely decreased GFR and proteinuria further increases the risk of all-cause mortality (Wu et al., 2018). For CKD patients with proteinuria, the updated hypertension guidelines recommend a BP goal of <130/80 mmHg (Hamrahian, 2017). More-intensive BP control is associated with a reduced risk of all-cause mortality compared with less-intensive BP goals in this high-risk population (Juraschek and Appel, 2018). Nevertheless, proportions with uncontrolled BP were greater in those with CKD than in those without CKD, and multiple medications and ACEI/ARB were associated with less uncontrolled BP (Plantinga et al., 2009). It should be emphasized that to lower albuminuria and achieve BP goals, moderate to high doses of ACEI or ARB are often required. However, ACEI or ARB may only reduce proteinuria by up to 40–50% in a dose-dependent manner, particularly if the patient complies with dietary salt restriction (Nakamura et al., 2000). This leads to a recommendation to use a more complete RAAS blockade to maximize kidney protection and improve outcomes. In order to study the effect of dose on ACEI in combination with ARB, we defined low-dose and high-dose as relative values. Compared with the same RAAS blocker in ACEI in combination with ARB group, a low-dose was defined as single dose, and the high-dose was defined as greater than single dose. According to our meta-analysis, ACEI in combination with ARB was superior to low-dose and high-dose ACEI or ARB in reducing urine albumin excretion and urine protein excretion. It is more effective to use ACEI in combination with ARB than to increase the dose of ACEI or ARB.
Although experimental and clinical studies have demonstrated that dual RAAS blockade therapy is more effective in reducing proteinuria and preventing structural lesions than either drug alone (Susantitaphong et al., 2013; Zhang et al., 2017), it is associated with higher incidences of adverse effects than monotherapy. The key safety issues associated with ACEI in combination with ARB are hypotension, which may lead to syncope, and impaired kidney function, which may lead to hyperkalemia (Oktaviono and Kusumawardhani, 2020). In this meta-analysis, although ACEI in combination with ARB was associated with a decrease in GFR and increased incidences of hyperkalemia and hypotension relative to low-dose ACEI or ARB, dual therapy did not decrease GFR nor increase the incidence of hyperkalemia compared with high-dose ACEI or ARB. Except for hypotension, the safety of ACEI in combination with ARB was equivalent to that of high-dose ACEI or ARB, and hypotension in some patients is temporary and mild (Song et al., 2006; Meier et al., 2011).
In recent years, the use of ACEI in combination with ARB has raised controversies, and no systemic review and meta-analysis have analyzed the efficacy and safety of the use of ACEI in combination with ARB in patients with CKD. This meta-analysis evaluated the effect of ACEI in combination with ARB on kidney-related endpoints, BP, and adverse events based on the dose. However, there are certain limitations to this study. First, only a few RCTs have evaluated the efficacy and safety of ACEI in combination with ARB vs. high-dose ACEI or ARB. More large-scale studies are needed to further clarify the application prospect of ACEI in combination with ARB in CKD. Second, some of the studies included in the present analysis were of a fair quality. Third, the included studies were heterogeneous; we performed sensitivity analysis and meta-regression to warrant the reliability of the present meta-analysis. Fourth, most of the included studies were aimed at CKD patients with a normal GFR or only a mildly reduced GFR. There are few with moderately reduced renal function and none with severely reduced renal function. The results of this meta-analysis are only applicable to CKD patients with a fairly maintained kidney function.
Conclusion
In conclusion, ACEI in combination with ARB is superior to low-dose and high-dose ACEI or ARB in reducing urine albumin excretion and urine protein excretion. Although ACEI in combination with ARB is associated with a decreased GFR and increased rates of hyperkalemia and hypotension compared with low-dose ACEI or ARB, the combination is more effective than high-dose ACEI or ARB without decreasing GFR and increasing the incidence of hyperkalemia. Despite the risk of hypotension, ACEI in combination with ARB is a better choice for CKD patients who need to increase the dose of ACEI or ARB. The results of this meta-analysis are only applicable to CKD patients with a fairly maintained kidney function.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
Author Contributions
Research idea and study design: MZ, RW, HQ, and YZ; data acquisition: MZ, RW, YY, MC, and SM; data analysis/interpretation: MZ, RW, YY, HZ, HQ, and YZ; statistical analysis: MZ, RW, MC, SM, and HZ; supervision or mentorship: MZ, RW, HQ, and YZ. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (grant number 81873300); Capital Health Research and Development of Special (grant number 2018-2-4173); and Fundamental Research Funds for the Central public welfare research institutes (grant number ZZ11-023).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.638611/full#supplementary-material
References
Abe, H., Minatoguchi, S., Ohashi, H., Murata, I., Minagawa, T., Okuma, T., et al. (2007). Renoprotective effect of the addition of losartan to ongoing treatment with an angiotensin converting enzyme inhibitor in type-2 diabetic patients with nephropathy. Hypertens. Res. 30 (10), 929–935. doi:10.1291/hypres.30.929
Agarwal, R. (2001). Add-on angiotensin receptor blockade with maximized ACE inhibition. Kidney Int. 59 (6), 2282. doi:10.1046/j.1523-1755.2001.00745.x10.1046/j.1523-1755.2001.0590062282.x
Atmaca, A., and Gedik, O. (2006). Effects of angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and their combination on microalbuminuria in normotensive patients with type 2 diabetes. Adv. Ther. 23 (4), 615. doi:10.1007/bf02850049
Bakris, G. L., Ruilope, L., Locatelli, F., Ptaszynska, A., Pieske, B., de Champlain, J., et al. (2007). Treatment of microalbuminuria in hypertensive subjects with elevated cardiovascular risk: results of the IMPROVE trial. Kidney Int. 72 (7), 879. doi:10.1038/sj.ki.5002455
Berger, E. D., Bader, B. D., Ebert, C., Risler, T., and Erley, C. M. (2002). Reduction of proteinuria; combined effects of receptor blockade and low dose angiotensin-converting enzyme inhibition. J. Hypertens. 20 (4), 739. doi:10.1097/00004872-200204000-00033
Campbell, R., Sangalli, F., Perticucci, E., Aros, C., Viscarra, C., Perna, A., et al. (2003). Effects of combined ACE inhibitor and angiotensin II antagonist treatment in human chronic nephropathies. Kidney Int. 63 (3), 1094. doi:10.1046/j.1523-1755.2003.00832.x
Chrysostomou, A., Pedagogos, E., MacGregor, L., and Becker, G. J. (2006). Double-blind, placebo-controlled study on the effect of the aldosterone receptor antagonist spironolactone in patients who have persistent proteinuria and are on long-term angiotensin-converting enzyme inhibitor therapy, with or without an angiotensin II receptor blocker. Cjasn 1 (2), 256. doi:10.2215/CJN.01040905
Cice, G., Di Benedetto, A., D'Isa, S., D'Andrea, A., Marcelli, D., Gatti, E., et al. (2010). Effects of telmisartan added to angiotensin-converting enzyme inhibitors on mortality and morbidity in hemodialysis patients with chronic heart failure. J. Am. Coll. Cardiol. 56 (21), 1701–1708. doi:10.1016/j.jacc.2010.03.105
Egan, B. M., Li, J., Hutchison, F. N., and Ferdinand, K. C. (2014). Hypertension in the United States, 1999 to 2012. Circulation 130 (19), 1692–1699. doi:10.1161/circulationaha.114.010676
Esnault, V. L. M., Ekhlas, A., Delcroix, C., Moutel, M.-G., and Nguyen, J.-M. (2005). Diuretic and enhanced sodium restriction results in improved antiproteinuric response to RAS blocking agents. Jasn 16 (2), 474. doi:10.1681/ASN.2004060505
Esteras, R., Perez-Gomez, M. V., Rodriguez-Osorio, L., Ortiz, A., and Fernandez-Fernandez, B. (2015). Combination use of medicines from two classes of renin-angiotensin system blocking agents: risk of hyperkalemia, hypotension, and impaired renal function. Ther. Adv. Drug Saf. 6 (4), 166–176. doi:10.1177/2042098615589905
Fernandez Juarez, G., Luño, J., Barrio, V., de Vinuesa, S. G., Praga, M., Goicoechea, M., et al. (2013). Effect of dual blockade of the renin-angiotensin system on the progression of type 2 diabetic nephropathy: a randomized trial. Am. J. kidney Dis. 61 (2), 211. doi:10.1053/j.ajkd.2012.07.011
Ferrari, P., Marti, H.-P., Pfister, M., and Frey, F. J. (2002). Additive antiproteinuric effect of combined ACE inhibition and angiotensin II receptor blockade. J. Hypertens. 20 (1), 125–130. doi:10.1097/00004872-200201000-00018
Fried, L. F., Emanuele, N., Zhang, J. H., Brophy, M., Conner, T. A., Duckworth, W., et al. (2013). Combined angiotensin inhibition for the treatment of diabetic nephropathy. N. Engl. J. Med. 369 (20), 1892. doi:10.1056/NEJMoa1303154
Hamrahian, S. M. (2017). Management of hypertension in patients with chronic kidney disease. Curr. Hypertens. Rep. 19 (5), 43. doi:10.1007/s11906-017-0739-9
Horita, Y., Tadokoro, M., Taura, K., Suyama, N., Taguchi, T., Miyazaki, M., et al. (2004). Low-dose combination therapy with temocapril and losartan reduces proteinuria in normotensive patients with immunoglobulin a nephropathy. Hypertens. Res. 27 (12), 963. doi:10.1291/hypres.27.963
Horita, Y., Taura, K., Taguchi, T., Furusu, A., and Kohno, S. (2006). Aldosterone breakthrough during therapy with angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers in proteinuric patients with immunoglobulin A nephropathy. Nephrology 11 (5), 462. doi:10.1111/j.1440-1797.2006.00665.x
Igarashi, M., Hirata, A., Kadomoto, Y., and Tominaga, M. (2006). Dual blockade of angiotensin II with enalapril and losartan reduces proteinuria in hypertensive patients with type 2 diabetes. Endocr. J. 53 (4), 493. doi:10.1507/endocrj.k06-025
Jacobsen, P., Andersen, S., Rossing, K., Hansen, B. V., and Parving, H.-H. (2002).Dual blockade of the renin-angiotensin system in type 1 patients with diabetic nephropathyNephrol. Dial. Transplant. 17 (6), 1019–1024. doi:10.1093/ndt/17.6.1019
Jacobsen, P., Andersen, S., Jensen, B. R., and Parving, H. H. (2003a). Additive effect of ACE inhibition and angiotensin II receptor blockade in type I diabetic patients with diabetic nephropathy. J. Am. Soc. 14, 992–999. doi:10.1097/01.asn.0000054495.96193
Jacobsen, P., Andersen, S., Rossing, K., Jensen, B. R., and Parving, H.-H. (2003b). Dual blockade of the renin-angiotensin system versus maximal recommended dose of ACE inhibition in diabetic nephropathy. Kidney Int. 63 (5), 1874–1880. doi:10.1046/j.1523-1755.2003.00940.x
Jha, V., Garcia-Garcia, G., Iseki, K., Li, Z., Naicker, S., Plattner, B., et al. (2013). Chronic kidney disease: global dimension and perspectives. The Lancet 382 (9888), 260–272. doi:10.1016/s0140-6736(13)60687-x
Juraschek, S. P., and Appel, L. J. (2018). Intensive blood pressure reduction lowers mortality in CKD. Nat. Rev. Nephrol. 14 (1), 5–6. doi:10.1038/nrneph.2017.154
Kalaitzidis, R. G., and Elisaf, M. S. (2018). Treatment of hypertension in chronic kidney disease. Curr. Hypertens. Rep. 20 (8), 64. doi:10.1007/s11906-018-0864-0
Kanno, Y., Takenaka, T., Nakamura, T., and Suzuki, H. (2006). Add-on angiotensin receptor blocker in patients who have proteinuric chronic kidney diseases and are treated with angiotensin-converting enzyme inhibitors. Cjasn 1 (4), 730. doi:10.2215/CJN.01110905
Kim, M. J., Song, J. H., Suh, J. H., Lee, S. W., and Kim, G. A. (2003). Additive antiproteinuric effect of combination therapy with ACE inhibitor and angiotensin II receptor antagonist: differential short-term response between IgA nephropathy and diabetic nephropathy. Yonsei Med. J. 44 (3), 463. doi:10.3349/ymj.2003.44.3.463
Kincaid-Smith, P., Fairley, K., and Packham, D. (2002). Randomized controlled crossover study of the effect on proteinuria and blood pressure of adding an angiotensin II receptor antagonist to an angiotensin converting enzyme inhibitor in normotensive patients with chronic renal disease and proteinuria. Nephrol. Dial. Transplant. 17 (4), 597–601. doi:10.1093/ndt/17.4.597
Knudsen, S. T., Andersen, N. H., Poulsen, S. H., Eiskjaer, H., Hansen, K. W., Helleberg, K., et al. (2008). Pulse pressure lowering effect of dual blockade with candesartan and lisinopril vs. high-dose ACE inhibition in hypertensive type 2 diabetic subjects: a CALM II study post-hoc analysis. Am. J. Hypertens. 21 (2), 172. doi:10.1038/ajh.2007.2
Krairittichai, U., and Chaisuvannarat, V. (2009). Effects of dual blockade of renin-angiotensin system in type 2 diabetes mellitus patients with diabetic nephropathy. J. Med. Assoc. Thai 92 (5), 611.
Liu, D., and Lv, L.-L. (2019). New understanding on the role of proteinuria in progression of chronic kidney disease. Adv. Exp. Med. Biol. 1165, 487–500. doi:10.1007/978-981-13-8871-2_24
Luño, J., Barrio, V., Goicoechea, M. Á., González, C., de Vinuesa, S. G., Gómez, F., et al. (2002). Effects of dual blockade of the renin-angiotensin system in primary proteinuric nephropathies. Kidney Int. 62 (82), S47–S52. doi:10.1046/j.1523-1755.62.s82.10.x
Matos, J. P. S., Rodrigues, M. d. L., Ismerim, V. L., Boasquevisque, E. M., Genelhu, V., and Francischetti, E. A. (2005). Effects of dual blockade of the renin angiotensin system in hypertensive type 2 diabetic patients with nephropathy. Cn 64 (3), 180. doi:10.5414/cnp64180
Matsushita, K., Matsushita, K., van der Velde, M., Astor, B. C., Woodward, M., Levey, A. S., et al. (2010). Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 375 (9731), 2073–2081. doi:10.1016/s0140-6736(10)60674-5
Mehdi, U. F., Adams-Huet, B., Raskin, P., Vega, G. L., and Toto, R. D. (2009). Addition of angiotensin receptor blockade or mineralocorticoid antagonism to maximal angiotensin-converting enzyme inhibition in diabetic nephropathy. Jasn 20 (12), 2641. doi:10.1681/ASN.2009070737
Meier, P., Maillard, M. P., Meier, J. R., Tremblay, S., Gauthier, T., and Burnier, M. (2011). Combining blockers of the renin-angiotensin system or increasing the dose of an angiotensin II receptor antagonist in proteinuric patients: a randomized triple-crossover study. J. Hypertens. 29 (6), 1228. doi:10.1097/HJH.0b013e328346d5dc
Menne, J., Farsang, C., Deák, L., Klebs, S., Meier, M., Handrock, R., et al. (2008). Valsartan in combination with lisinopril versus the respective high dose monotherapies in hypertensive patients with microalbuminuria: the VALERIA trial. J. Hypertens. 26 (9), 1860. doi:10.1097/HJH.0b013e32830508aa
Mills, K. T., Xu, Y., Zhang, W., Bundy, J. D., Chen, C.-S., Kelly, T. N., et al. (2015). A systematic analysis of worldwide population-based data on the global burden of chronic kidney disease in 2010. Kidney Int. 88 (5), 950–957. doi:10.1038/ki.2015.230
Morgan, T., Anderson, A., Bertram, D., and MacInnis, R. J. (2004). Effect of candesartan and lisinopril alone and in combination on blood pressure and microalbuminuria. J. Renin Angiotensin Aldosterone Syst. 5 (2), 64. doi:10.3317/jraas.2004.012
Mori-Takeyama, U., Minatoguchi, S., Murata, I., Fujiwara, H., Ozaki, Y., Ohno, M., et al. (2008). Dual blockade of the rennin-angiotensin system versus maximal recommended dose of angiotensin II receptor blockade in chronic glomerulonephritis. Clin. Exp. Nephrol. 12 (1), 33. doi:10.1007/s10157-007-0013-6
Muntner, P., Anderson, A., Charleston, J., Chen, Z., Ford, V., Makos, G., et al. (2010). Hypertension awareness, treatment, and control in adults with CKD: results from the chronic renal insufficiency cohort (CRIC) study. Am. J. Kidney Dis. 55 (3), 441–451. doi:10.1053/j.ajkd.2009.09.014
Nagai, K., Yamagata, K., Iseki, K., Moriyama, T., Tsuruya, K., Fujimoto, S., et al. (2019). Antihypertensive treatment and risk of cardiovascular mortality in patients with chronic kidney disease diagnosed based on the presence of proteinuria and renal function: a large longitudinal study in Japan. PLoS One 14 (12), e0225812. doi:10.1371/journal.pone.0225812
Nakamura, A., Shikata, K., Nakatou, T., Kitamura, T., Kajitani, N., Ogawa, D., et al. (2013). Combination therapy with an angiotensin-converting-enzyme inhibitor and an angiotensin II receptor antagonist ameliorates microinflammation and oxidative stress in patients with diabetic nephropathy. J. Diabetes Invest. 4 (2), 195–201. doi:10.1111/jdi.12004
Nakamura, T., Inoue, T., Sugaya, T., Kawagoe, Y., Suzuki, T., Ueda, Y., et al. (2007). Beneficial effects of olmesartan and temocapril on urinary liver-type fatty acid-binding protein levels in normotensive patients with immunoglobin A nephropathy. Am. J. Hypertens. 20 (11), 1195. doi:10.1016/j.amjhyper.2007.06.003
Nakamura, T., Ushiyama, C., Osada, S., Takahashi, Y., Shimada, N., Ebihara, I., et al. (2002). Combination therapy of trandolapril and candesartan cilexetil reduces microalbuminuria and urinary endothelin-1 excretion in patients with type 2 diabetes. Clin. Exp. Nephrol. 6 (3), 135–139. doi:10.1007/s101570200023
Nakamura, T., Ushiyama, C., Suzuki, S., Hara, M., Shimada, N., Sekizuka, K., et al. (2000). Effects of angiotensin-converting enzyme inhibitor, angiotensin II receptor antagonist and calcium antagonist on urinary podocytes in patients with IgA nephropathy. Am. J. Nephrol. 20 (5), 373–379. doi:10.1159/000013619
Nakao, N., Seno, H., Kasuga, H., Toriyama, T., Kawahara, H., and Fukagawa, M. (2004). Effects of combination treatment with losartan and trandolapril on office and ambulatory blood pressures in non-diabetic renal disease: a COOPERATE-ABP substudy. Am. J. Nephrol. 24 (5), 543–548. doi:10.1159/000081953
Ogawa, S., Takeuchi, K., Mori, T., Nako, K., Tsubono, Y., and Ito, S. (2007). Effects of monotherapy of temocapril or candesartan with dose increments or combination therapy with both drugs on the suppression of diabetic nephropathy. Hypertens. Res. 30 (4), 325. doi:10.1291/hypres.30.325
Ohishi, M., Takeya, Y., Tatara, Y., Yamamoto, K., Onishi, M., Maekawa, Y., et al. (2010). Strong suppression of the renin-angiotensin system has a renal-protective effect in hypertensive patients: high-dose ARB with ACE inhibitor (Hawaii) study. Hypertens. Res. 33 (11), 1150. doi:10.1038/hr.2010.145
Oktaviono, Y. H., and Kusumawardhani, N. (2020). Hyperkalemia associated with angiotensin converting enzyme inhibitor or angiotensin receptor blockers in chronic kidney disease. Acta Med. Indones 52 (1), 74–79.
Plantinga, L. C., Miller, E. R., Stevens, L. A., Saran, R., Messer, K., Flowers, N., et al. (2009). Blood pressure control among persons without and with chronic kidney disease. Hypertension 54 (1), 47–56. doi:10.1161/hypertensionaha.109.129841
Renke, M., Tylicki, L., Rutkowski, P., and Rutkowski, B. (2004). Low-dose angiotensin II receptor antagonists and angiotensin II-converting enzyme inhibitors alone or in combination for treatment of primary glomerulonephritis. Scand. J. Urol. Nephrol. 38 (5), 427. doi:10.1080/00365590410015687
Rossing, K., Christensen, P. K., Jensen, B. R., and Parving, H.-H. (2002). Dual blockade of the renin-angiotensin system in diabetic nephropathy: a randomized double-blind crossover study. Diabetes care 25 (1), 95–100. doi:10.2337/diacare.25.1.95
Rossing, K., Jacobsen, P., Pietraszek, L., and Parving, H.-H. (2003). Renoprotective effects of adding angiotensin II receptor blocker to maximal recommended doses of ACE inhibitor in diabetic nephropathy: a randomized double-blind crossover trial. Diabetes care 26 (8), 2268–2274. doi:10.2337/diacare.26.8.2268
Ruilope, L. M., Aldigier, J. C., Ponticelli, C., Oddou-Stock, P., Botteri, F., and Mann, J. F. (2000). Safety of the combination of valsartan and benazepril in patients with chronic renal disease. J. Hypertens. 18 (1), 89–95. doi:10.1097/00004872-200018010-00013
Rutkowski, P., Tylicki, L., Renke, M., Korejwo, G., Zdrojewski, Z., and Rutkowski, B. (2004). Low-dose dual blockade of the renin-angiotensin system in patients with primary glomerulonephritis. Am. J. kidney Dis. 43 (2), 260. doi:10.1053/j.ajkd.2003.10.032
Saglimbene, V., Palmer, S. C., Ruospo, M., Natale, P., Maione, A., Nicolucci, A., et al. (2018). The long-term impact of renin-angiotensin system (RAS) inhibition on cardiorenal outcomes (LIRICO): a randomized, controlled trial. Jasn 29 (12), 2890. doi:10.1681/ASN.2018040443
Scaglione, R., Argano, C., Corrao, S., Chiara, T. D., Licata, A., and Licata, G. (2005). Transforming growth factor β1 and additional renoprotective effect of combination ACE inhibitor and angiotensin II receptor blocker in hypertensive subjects with minor renal abnormalities: a 24-week randomized controlled trial. J. Hypertens. 23 (3), 657. doi:10.1097/01.hjh.0000160225.01845.26
Schrier, R. W., Abebe, K. Z., Perrone, R. D., Torres, V. E., Braun, W. E., Steinman, T. I., et al. (2014). Blood pressure in early autosomal dominant polycystic kidney disease. N. Engl. J. Med. 371 (24), 2255. doi:10.1056/NEJMoa1402685
Segura, J., Praga, M., Campo, C., Rodicio, J. L., and Ruilope, L. M. (2003). Combination is better than monotherapy with ACE inhibitor or angiotensin receptor antagonist at recommended doses. J. Renin Angiotensin Aldosterone Syst. 4 (1), 43. doi:10.3317/jraas.2003.007
Sengul, A. M., Altuntas, Y., Kürklü, A., and Aydın, L. (2006). Beneficial effect of lisinopril plus telmisartan in patients with type 2 diabetes, microalbuminuria and hypertension. Diabetes Res. Clin. Pract. 71 (2), 210. doi:10.1016/j.diabres.2005.06.010
Slagman, M. C. J., Waanders, F., Hemmelder, M. H., Woittiez, A.-J., Janssen, W. M. T., Lambers Heerspink, H. J., et al. (2011). Moderate dietary sodium restriction added to angiotensin converting enzyme inhibition compared with dual blockade in lowering proteinuria and blood pressure: randomised controlled trial. Bmj 343, d4366. doi:10.1136/bmj.d4366
Song, J. H., Cha, S. H., Lee, H. J., Lee, S. W., Park, G. H., Lee, S. W., et al. (2006).Effect of low-dose dual blockade of renin-angiotensin system on urinary TGF-β in type 2 diabetic patients with advanced kidney disease. Nephrol. Dial. Transplant. 21 (3), 683–689. doi:10.1093/ndt/gfi310
Song, J. H., Lee, S. W., Suh, J. H., Kim, E. S., Hong, S. B., Kim, K. A., et al. (2003). The effects of dual blockade of the renin- angiotensin system on urinary protein and transforming growth factor-b excretion in 2 groups of patients with IgA and diabetic nephropathy. Cn 60 (5), 318. doi:10.5414/cnp60318
Susantitaphong, P., Sewaralthahab, K., Balk, E. M., Eiam-ong, S., Madias, N. E., and Jaber, B. L. (2013). Efficacy and safety of combined vs. single renin-angiotensin-aldosterone system blockade in chronic kidney disease: a meta-analysis. Am. J. Hypertens. 26 (3), 424–441. doi:10.1093/ajh/hps038
Titan, S. M., Jr., J. M. V., Dominguez, W. V., Barros, R. T., and Zatz, R. (2011). ACEI and ARB combination therapy in patients with macroalbuminuric diabetic nephropathy and low socioeconomic level: a double-blind randomized clinical trial. Cn 76 (4), 273. doi:10.5414/cn107013
Tütüncü, N. B., Gürlek, A., and Gedik, O. (2001). Efficacy of ACE inhibitors and ATII receptor blockers in patients with microalbuminuria: a prospective study. Acta Diabetol. 38 (4), 157. doi:10.1007/s592-001-8073-2
Wu, J., Jia, J., Li, Z., Pan, H., Wang, A., Guo, X., et al. (2018). Association of estimated glomerular filtration rate and proteinuria with all-cause mortality in community-based population in China: a Result from Kailuan Study. Sci. Rep. 8 (1), 2157. doi:10.1038/s41598-018-20554-3
Zhang, F., Liu, H., Liu, D., Liu, Y., Li, H., Tan, X., et al. (2017). Effects of RAAS inhibitors in patients with kidney disease. Curr. Hypertens. Rep. 19 (9), 72. doi:10.1007/s11906-017-0771-9
Zhu, S., Liu, Y., Wang, L., and Meng, Q. H. (2008). Transforming growth factor- 1 is associated with kidney damage in patients with essential hypertension: renoprotective effect of ACE inhibitor and/or angiotensin II receptor blocker. Nephrol. Dial. Transplant. 23 (9), 2841–2846. doi:10.1093/ndt/gfn159
Keywords: ACEI in combination with ARB, dose, chronic kidney disease, urine albumin excretion, urine protein excretion, glomerular filtration rate, hyperkalemia, hypotension
Citation: Zhao M, Wang R, Yu Y, Chang M, Ma S, Zhang H, Qu H and Zhang Y (2021) Efficacy and Safety of Angiotensin-Converting Enzyme Inhibitor in Combination with Angiotensin-Receptor Blocker in Chronic Kidney Disease Based on Dose: A Systematic Review and Meta-Analysis. Front. Pharmacol. 12:638611. doi: 10.3389/fphar.2021.638611
Received: 07 December 2020; Accepted: 09 March 2021;
Published: 06 May 2021.
Edited by:
Norberto Perico, Istituto di Ricerche Farmacologiche Mario Negri (IRCCS), ItalyReviewed by:
Niels Henrik Buus, Aarhus University, DenmarkFrancesca Becherucci, Meyer Children’s Hospital, Italy
Copyright © 2021 Zhao, Wang, Yu, Chang, Ma, Zhang, Qu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hua Qu, aHVhX3F1QHllYWgubmV0; Yu Zhang, emhhbmd5dTgyMjVAMTI2LmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Mingming Zhao
Mingming Zhao Rumeng Wang
Rumeng Wang Yi Yu1,2
Yi Yu1,2 Sijia Ma
Sijia Ma Hanwen Zhang
Hanwen Zhang Hua Qu
Hua Qu Yu Zhang
Yu Zhang