Abstract
Introduction:
Hydroxysafflor yellow A (HSYA), its primary bioactive metabolite of Carthamus tinctorius L. (safflower), has shown therapeutic potential in various inflammatory diseases. However, its role in alleviating inflammation and oxidative stress in non-alcoholic fatty liver disease (NAFLD) remains unclear. This study investigates the therapeutic effects of HSYA in mice with NAFLD, focusing on its impact on gut microbiota and serum non-targeted metabolomics to elucidate the mechanisms underlying its efficacy.
Methods:
NAFLD was induced in mice using a high-fat diet (HFD), followed by intragastric administration of hydroxysafflor yellow A (HSYA). Serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), total cholesterol (TC), and triglycerides (TG) were quantified to evaluate liver function and lipid metabolism. Oxidative stress markers, including superoxide dismutase (SOD) activity and malondialdehyde (MDA) concentration, were also assessed. The pro-inflammatory cytokines IL-6, TNF-α, and IL-1β in serum were measured using ELISA. The hepatic expression of NLRP3 inflammasome and its downstream effector, Caspase-1, was analyzed by Western blot. Histopathological examination of liver tissues was performed using hematoxylin and eosin (H&E) staining to evaluate structural damage. Furthermore, alterations in the gut microbiota composition were characterized via 16S rDNA sequencing of fecal samples. Untargeted metabolomics was conducted to identify serum metabolic variations and elucidate enriched metabolic pathways associated with HSYA treatment.
Results:
HSYA significantly inhibited HFD-induced weight gain and alleviated liver inflammation. It reduced serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST) and triglycerides (TG) (P < 0.05). HSYA administration decreased hepatic mRNA and protein expression of nucleotide binding oligomerization domain like receptor protein 3 (NLRP3), Caspase-1 and interleukin - 1β (IL-1β) while increasing superoxide dismutase (SOD) activity (P < 0.05). Gut microbiota analysis revealed a significant increase in the abundance of Turicibacter, while a reduction of Ruminococcus. Serum metabolomics identified a reduction in inflammation-associated metabolites, such as phenylalanine and tyrosine, alongside enhanced phenylalanine and tyrosine biosynthesis pathways.
Discussion:
HSYA demonstrates potent anti-inflammatory and antioxidant effects, effectively mitigating liver inflammation and oxidative stress in NAFLD mice. Its therapeutic mechanisms may involve modulating gut microbiota and regulating serum phenylalanine and tyrosine metabolism, offering insights into its potential as a treatment for NAFLD.
1 Introduction
Safflower (Carthamus tinctorius L.), a member of the Asteraceae family, is a traditional Chinese herbal medicine widely used for the treatment of cardiovascular and cerebrovascular diseases (Xue et al., 2021). Its therapeutic properties include scavenging oxygen free radicals and reducing inflammation (Wu et al., 2021). The primary bioactive compounds in safflower are its yellow pigments, including safflower yellow A, safflower yellow B and hydroxysafflower yellow A (HSYA), with HSYA accounting for approximately 85% of the total yellow pigments (Zhao et al., 2020; Wang et al., 2021). HSYA has been extensively investigated for its cardiovascular and cerebrovascular benefits, particularly its ability to eliminate oxygen-free radicals and suppress inflammatory cell infiltration (Mani et al., 2020).
Non-alcoholic fatty liver disease (NAFLD) is the most prevalent chronic liver disease globally (Paternostro and Trauner, 2022), characterised by excessive fat accumulation in more than 5% of liver cells in the absence of alcohol consumption, viral infections, or drug-induced liver damage (Bence and Birnbaum, 2021; Tilg et al., 2021). NAFLD affects approximately 25% of the global population, with obesity being a key risk factor (Riazi et al., 2022; Wong et al., 2023). It is strongly associated with insulin resistance, type 2 diabetes and metabolic syndrome, with a prevalence exceeding 50% among individuals with type 2 diabetes (Muzurović et al., 2021; Kosmalski et al., 2023). Excessive hepatic fat accumulation disrupts metabolic processes, leading to increased reactive oxygen species (ROS) production, mitochondrial endoplasmic reticulum stress and inflammatory damage (Lim et al., 2021; Čolak and Pap, 2021). Patients with NAFLD often exhibit impaired antioxidant defences, characterised by reduced blood levels of antioxidants (e.g., vitamins E and C) and elevated lipid peroxidation products and systemic oxidative stress (Arroyave-Ospina et al., 2021; Yasmin et al., 2021).
Natural dietary antioxidants have shown promise in mitigating NAFLD-related oxidative stress, mitochondrial dysfunction, insulin resistance and inflammation (Sohouli et al., 2020; Mohammadian et al., 2024). While HSYA is recognised as a potent antioxidant, its therapeutic potential for NAFLD remains underexplored. This study aims to investigate the effects and mechanisms of HSYA using a high-fat diet-induced NAFLD mouse model, offering new insights into its potential as a treatment for NAFLD.
2 Materials and methods
2.1 Animals, NAFLD model and treatment
Following the methods reported by Sun et al. (2023), an NAFLD model was established in ICR mice using a high-fat diet (HFD). Detailed experimental procedures are provided in the Supplementary Material S1. Male ICR mice (6 weeks old; 25 g ± 4 g) were obtained from Jiangsu Wukong Biotechnology Co., Ltd. (Nanjing, China) and housed at the Jiangsu University Experimental Animal Centre (Ethics Committee of Jiangsu University approval number: UJS-IACUC-AP-2022032011). Forty mice were randomly assigned to four groups (10 mice per group): the normal control group (NC), the NAFLD group, the low-dose HSYA group (HSYAL) and the high-dose HSYA group (HSYAH). The NC group was fed a standard diet, while the other three groups were fed HFD, with 40% of energy derived from fat. HSYA (purity >98%) was provided by Shanghai Shifeng Biological Co., Ltd. (Shanghai, China). Based on previous studies (Liu et al., 2018; Lee et al., 2020), HSYAL and HSYAH groups received oral doses of 60 mg/kg and 120 mg/kg HSYA, respectively, while the NC and NAFLD groups were administered an equal volume of saline daily. The experiment was concluded at the end of the 12th week, and samples were collected for analysis.
2.2 Serum biochemical analysis, qPCR, Western blotting and ELISA assay
Based on Sun et al.'s method (Sun et al., 2023), serum biochemical indicators, including triglycerides (TG), total cholesterol (TC), alanine aminotransferase (ALT), aspartate aminotransferase (AST), superoxide dismutase (SOD) and malondialdehyde (MDA), were measured. Assay kits were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Detailed procedures are available in the Supplementary Material S2. The expressions of IL-1β, NLRP3 and Caspase-1 in liver tissues were assessed using quantitative PCR (qPCR) and Western blotting, as described by Sun et al. Primer sequences and qPCR protocols are provided in the Supplementary Material S3. Serum concentrations of IL-6, TNF-α and IL-1β were quantified using ELISA kits (Meimian, Yancheng, China), strictly adhering to the manufacturer’s instructions.
2.3 Hematoxylin-eosin (HE) staining
Liver tissue samples (∼2 mm3) were fixed in 4% paraformaldehyde for 16 h. Fixed samples were then sent to Shanghai Labway Medical Testing Co., Ltd. (Shanghai, China) for HE staining. Tissue sections were prepared at a thickness of 4 μm. Stained sections were examined under a microscope to evaluate liver inflammation in each group.
2.4 16S rRNA gene sequencing
Colon contents from the mice were sent to Ekemo Tech Group Co., Ltd. (Shenzhen, China) for bacterial 16S rRNA gene sequencing. The resulting gene sequence data were annotated by species and analysed using bioinformatics methods. Principal component analysis (PCA) and orthogonal partial least squares discriminant analysis (OPLS-DA) were performed to evaluate the model’s effectiveness and predictability. Cluster heatmap analysis revealed that HSYA significantly influenced the gut microbiota composition in mice.
2.5 Non-targeted metabolomic analysis
Mouse serum samples were analysed for untargeted metabolomics by Ekemo Tech Group Co., Ltd. (Shenzhen, China) using the UPLC-Q-TOF-MS/MS platform (Agilent Technologies, Waldbronn, Germany). Multivariate statistical analyses, including PCA and OPLS-DA, were conducted to process the metabolomics data. Variable importance in projection (VIP) values for each metabolite were calculated using the MetaX software. Differentially expressed metabolites were identified based on the criteria VIP >1.0 and P < 0.05. These metabolites were then subjected to the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis and associated network analysis.
2.6 Statistical analysis
Statistical analyses were performed using SPSS 20.0 (SPSS, Chicago, IL, USA), and results are expressed as mean ± standard deviation (SD). Group comparisons were conducted using t-tests and one-way ANOVA to assess statistical significance. A P-value <0.05 was considered statistically significant. Data visualisation and further analyses were carried out using GraphPad Prism software and the Bioincloud online platform (https://bioincloud.tech/).
3 Results
3.1 Weight and liver changes in NAFLD mice
Both low and high doses of HSYA demonstrated significant therapeutic effects on body weight and liver pathology in mice with NAFLD. The HFD led to marked weight gain and the development of fatty liver disease in the mice. By week 8, the body weight of the HSYAH group was significantly lower than that of the NAFLD group (P < 0.05), while no significant change was observed in the HSYAL group (P < 0.05) (Figures 1A,B).
FIGURE 1
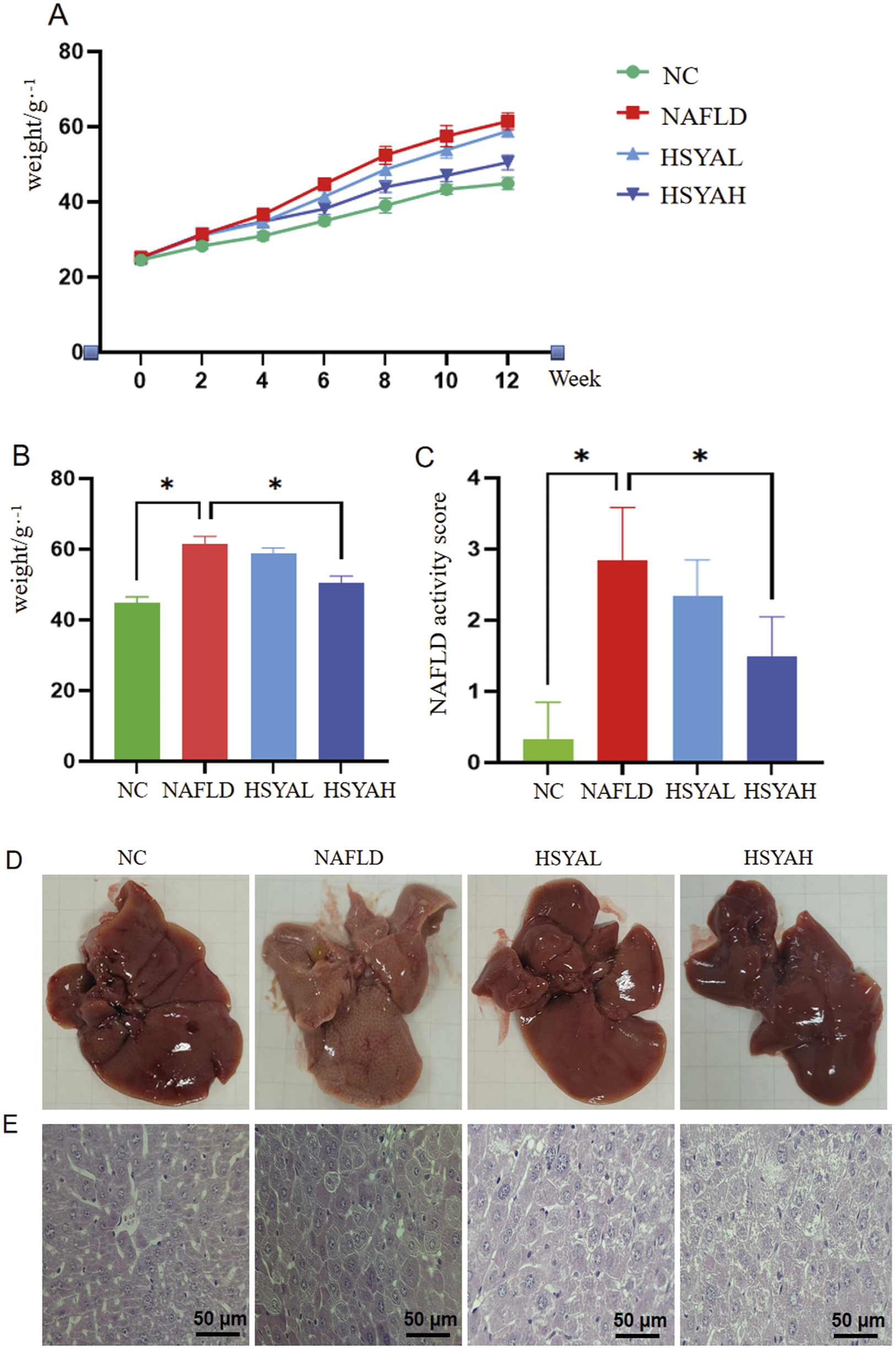
Changes in body weight and liver damage in mice. ICR mice were induced with NAFLD by high-fat diet and treated with high and low concentrations of HYSA (60 mg/kg and 120 mg/kg). Changes in body weight (A,B) and NAFLD activity score (C) of each group of mice were monitored during the experiment, and the color (D) and structural changes (E) of the liver in each group of mice were observed at the end of the experiment (n = 10).
Mice in the NAFLD group exhibited pale, greasy livers, whereas both low and high doses of HSYA significantly improved liver damage, with the HSYAH group showing more pronounced improvement (Figures 1C,D). Staining revealed that liver cells in the NAFLD group exhibited prominent lipid droplets, inflammatory cell infiltration and cell necrosis. Following HSYA intervention, liver pathology in both the HSYAH and HSYAL groups showed significant recovery, with a marked reduction in necrotic cells (Figure 1E).
3.2 HSYA improves oxidative stress and blood lipid levels in NAFLD mice
HFD significantly elevated the expressions of NLRP3, Caspase-1 and IL-1β in the liver (P < 0.05) (Figure 2). Serum levels of IL-6, IL-1β and TNF-α were also significantly increased (P < 0 0.05) (Figure 2), while serum SOD levels were significantly reduced and MDA levels were significantly elevated, indicating increased oxidative stress (P < 0.05) (Figure 3). Moreover, serum TG, TC, ALT and AST levels were significantly elevated (P < 0.05), indicating that the HFD induced lipid metabolism disorders, leading to fat deposition in the liver and the onset of fatty liver disease (Figure 3).
FIGURE 2
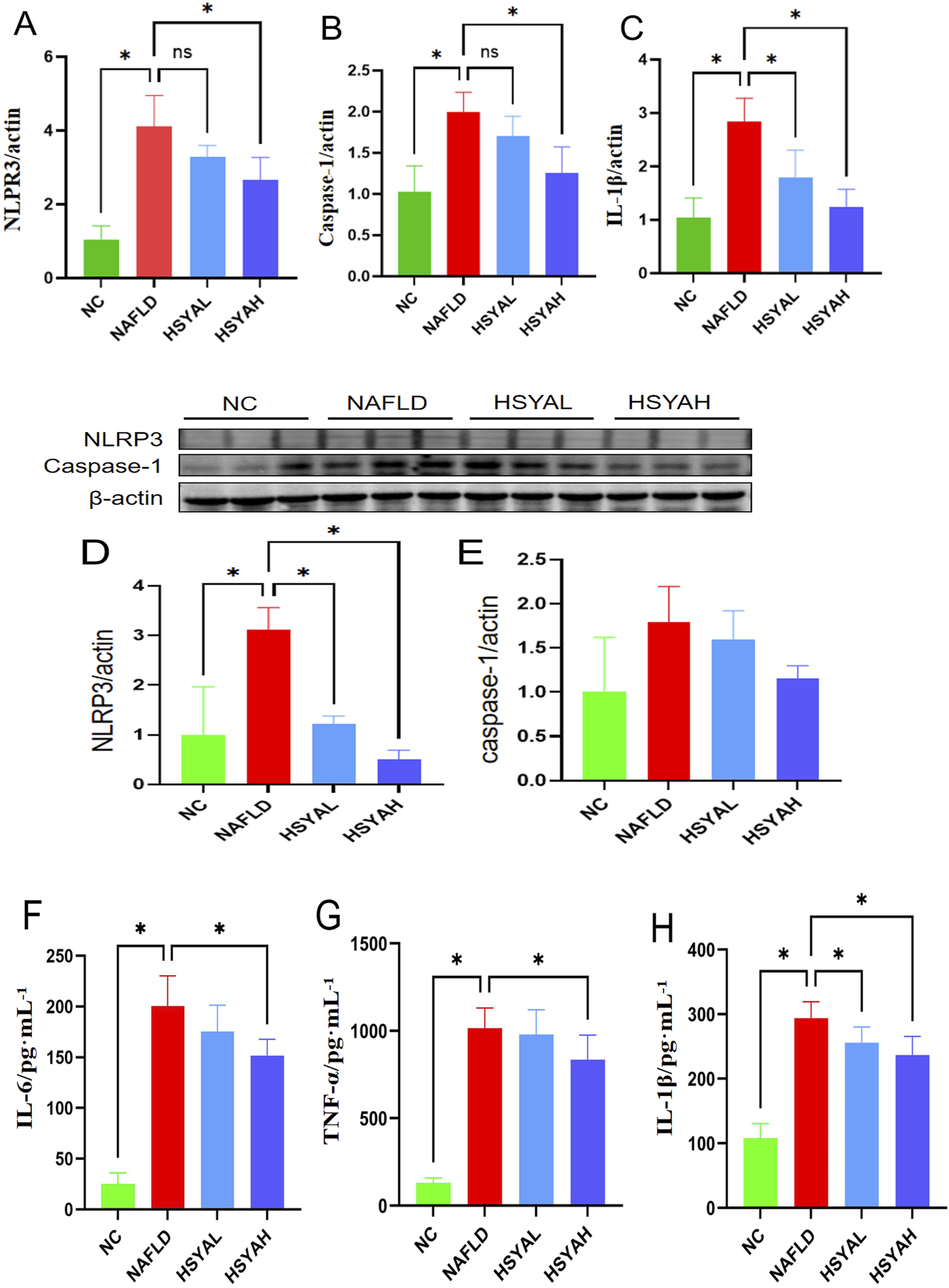
The inflammatory factors in mice were detected using qPCR (A–C), Western blotting (D,E), and ELISA methods (F–H). The qPCR method was used to detect the mRNA expression levels of NLRP3, Caspase-1, and IL-1β in the liver (n = 6). The Western blotting method was used to detect the expression levels of NLRP3 and Caspase-1 in the liver (n = 3). The ELISA method was used to detect the expression levels of IL-6, TNF-α, and IL-1β in the serum (n = 3). *: P < 0.05; ns: P > 0.05.
FIGURE 3
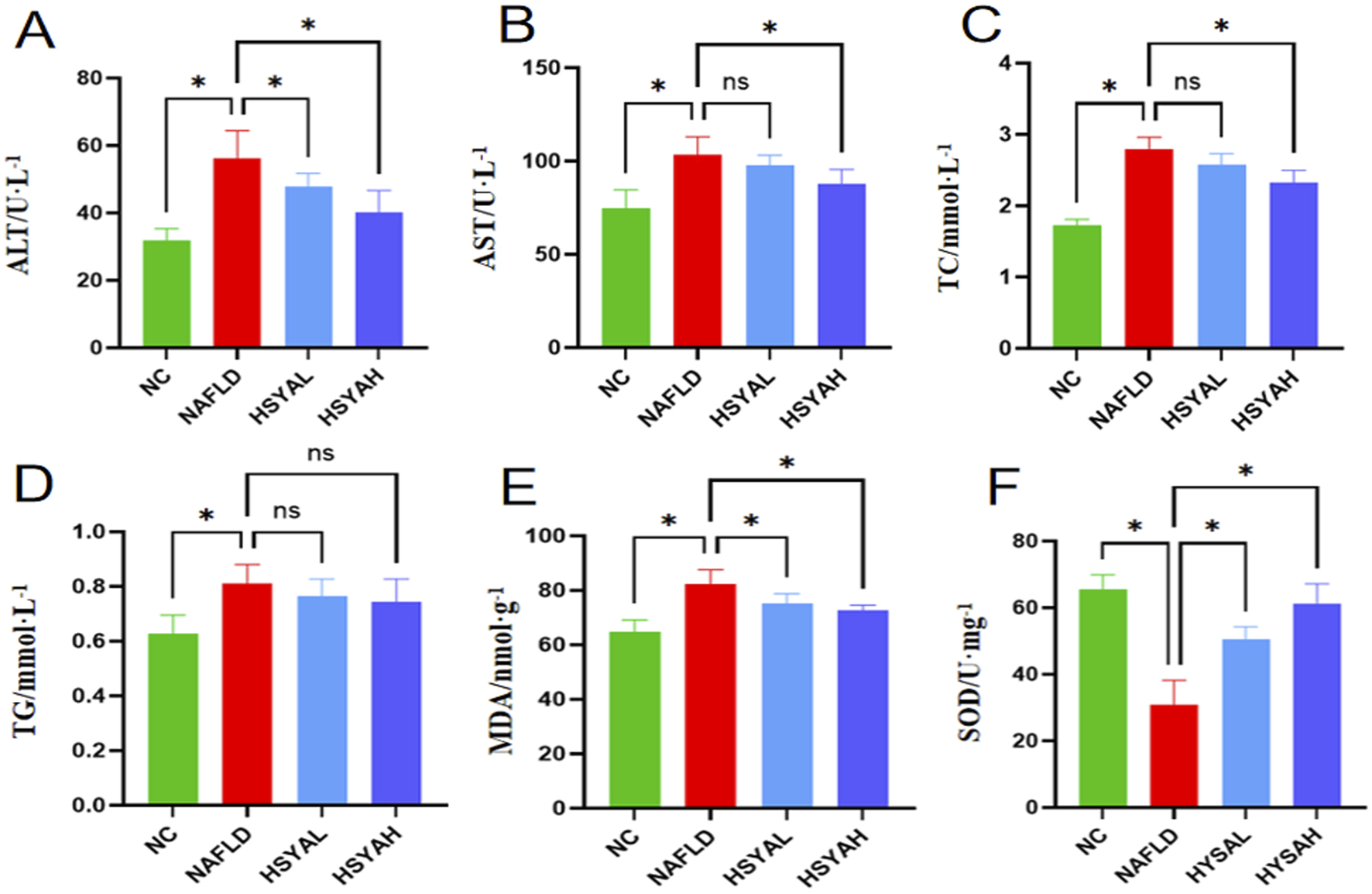
Liver function indicators ALT and AST levels in serum (A,B), n = 6. The blood lipid related indicators TC and TG levels (C,D), n = 6. The oxidative stress response indicators MDA and SOD levels (E,F), n = 6, and. *: P < 0.05; ns: P > 0.05.
Following HSYA intervention, the mRNA expression levels of NLRP3, Caspase-1 and IL-1β in the liver were significantly reduced (P < 0.05), and protein expression levels of NLRP3 and Caspase-1 were also significantly decreased (P < 0.05). Serum levels of IL-6, IL-1β and TNF-α were also significantly decreased (P < 0.05) (Figure 2), indicating that high-dose HSYA significantly inhibits the inflammatory response in the liver. Furthermore, oxidative stress markers SOD and MDA showed significant improvement, indicating that both low and high doses of HSYA effectively reduced oxidative stress induced by the HFD (P < 0.05) (Figure 3). After HSYA intervention, there was no significant change in TG levels, but high-dose HSYA significantly reduced TC levels, and ALT and AST levels showed significant improvement (P < 0.05), suggesting that high-dose HSYA effectively alleviated liver damage and oxidative stress in NAFLD mice (Figure 3).
3.3 HSYA reshapes intestinal microbiota and serum metabolomics in NAFLD mice
Given that high-dose HSYA (120 mg/kg) effectively suppresses oxidative stress and exhibits a pronounced hepatoprotective effect in NAFLD mice, we further investigated its impact on gut microbiota and serum metabolomics.
The results indicate that HSYA does not significantly influence the α-diversity of the intestinal microbiota in NAFLD mice, as measured by the Shannon index (P > 0.05). However, PCA revealed distinct separation among the three sample groups (Figures 4A,B).
FIGURE 4
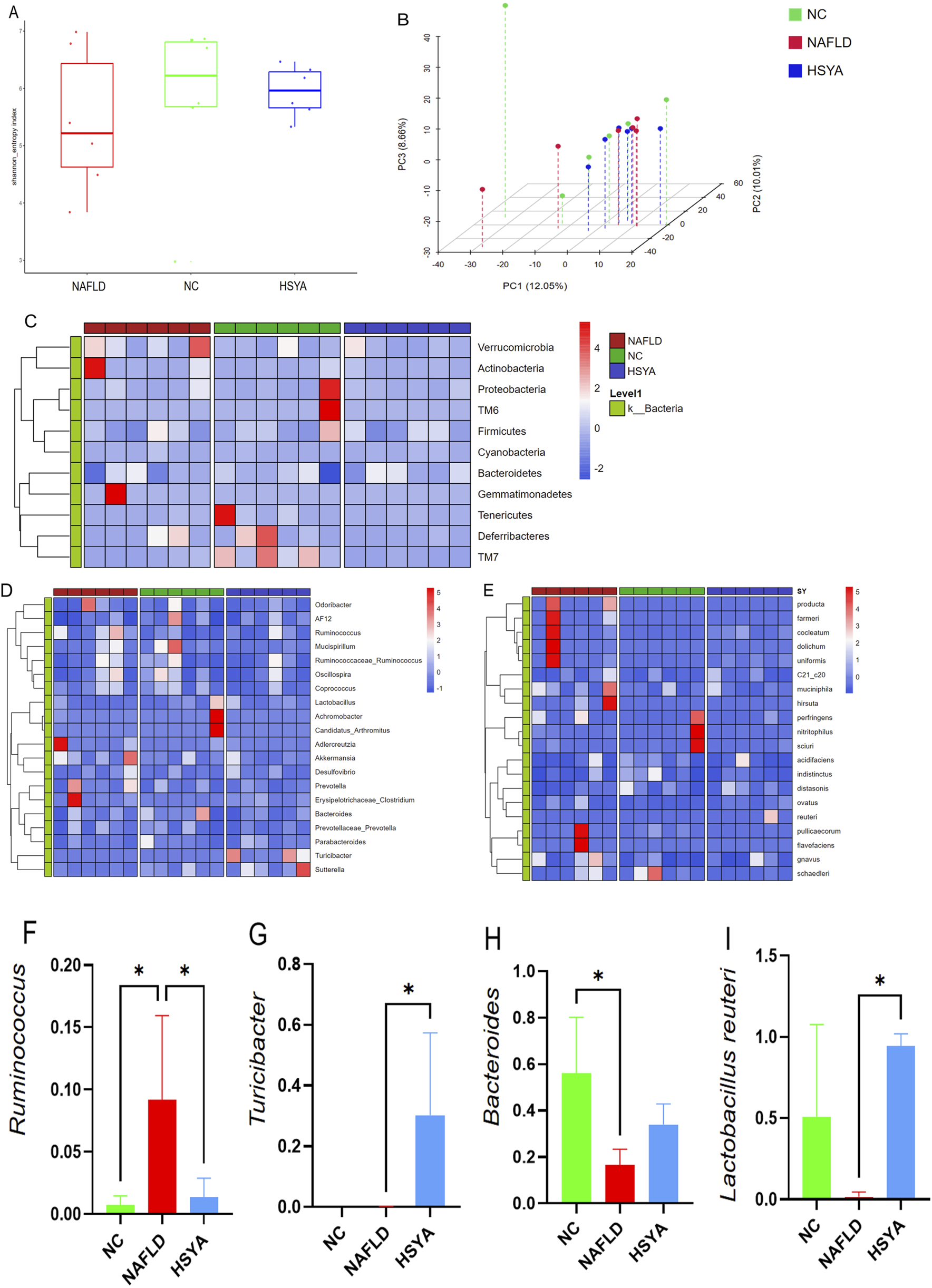
Using 16S rDNA technology to detect the gut microbiota of mice (n = 6). The α diversity (A) and β diversity (B) of gut microbiota in each group of mice. Further cluster heatmap analysis revealed significant changes in the abundance of bacteria at the phylum (C), genus (D), and species (E) levels in the gut microbiota of mice. Intestinal microbes with significant differences in abundance (F–I). *: P < 0.05.
Analyze the composition of mouse gut microbiota at the phylum, genus, and species levels using cluster heatmap method (Figures 4C–E). At the genus level, NAFLD mice demonstrated increased relative abundances of Ruminococcus, with concurrent reductions in Bacteroides; HSYA treatment significantly increased the abundance of Turicibacter levels, with concurrent reduction in Ruminococcus (P < 0.05). At the species level, HSYA treatment significantly increased the abundance of Lactobacillus reuteri (P < 0.05) (Figures 4F-I).
Metabolomic analyses using electrospray ionisation in both positive (ESI+) and negative (ESI−) modes revealed distinct clustering and separation among the NC, NAFLD and HSYA groups. This confirmed the successful establishment of the NAFLD model. In the ESI− mode, the HSYA group exhibited closer alignment with the NC group, highlighting HSYA’s significant regulatory effects on metabolic disturbances in NAFLD mice (Figures 5A,B). OPLS-DA identified numerous differential metabolites based on VIP > 1 and P < 0.05 criteria (Figures 5C–H; Tables 1,2). Pathway enrichment analysis revealed that HSYA modulated several metabolic pathways, including phenylalanine, tyrosine and tryptophan biosynthesis, starch and sucrose metabolism and phenylalanine metabolism (Figures 5I,J).
FIGURE 5
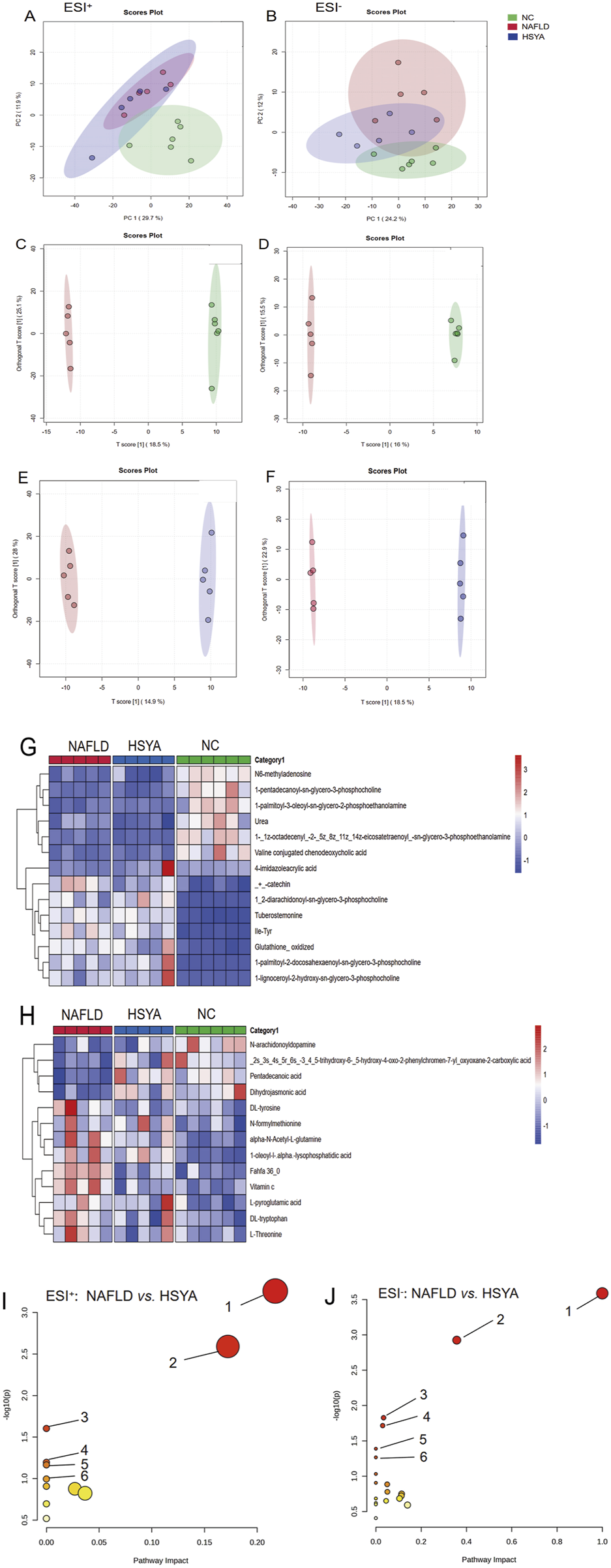
Using non-targeted metabolomics technology to detect the serum metabolomics of mice (n = 6). In ESI+ mode, the mouse serum PCA plot shows that samples from each group were clearly clustered (A); in ESI− mode, samples from each group were clustered and separated (B). Further analysis using OPLS-DA (C-F) identified different metabolites in the ESI+ mode (G) and the ESI− mode (H) based on VIP>1 and P < 0.05 criteria. Enrichment of the above different metabolites obtained the metabolic pathways related to NAFLD in mice regulated by HSYA. ESI+(I): ① biosynthesis of phenylalanine, tyrosine, and tryptophan; ② phenylalanine metabolism; ③ niacin and niacinamide metabolism; ④ starch and sucrose metabolism; ⑤ glycine, serine, and threonine metabolism; ⑥ tryptophan metabolism. ESI−(J): ① biosynthesis of phenylalanine, tyrosine, and tryptophan; ② pyrimidine metabolism; ③ phenylalanine metabolism; ④ ascorbic acid and aldaric acid metabolism; ⑤ biotin metabolism; ⑥ histidine metabolism.
TABLE 1
| NO | Metabolite | Formula | HMDB | m/z | rt (s) | Which-max |
|---|---|---|---|---|---|---|
| 1 | L-Anserine | C10H16N4O3 | HMDB0000194 | 282.15447 | 431.497 | ↑ |
| 2 | Urea | CH4N2O | HMDB0000294 | 61.03924 | 93.3845 | ↓ |
| 3 | 4-imidazoleacrylic acid | C6H6N2O2 | HMDB0000301 | 139.05004 | 304.984 | ↓ |
| 4 | 1-Stearoyl-2-oleoyl-sn-glycerol 3-phosphocholine (SOPC) | C44H86NO8P | HMDB0000564 | 832.58196 | 121.96 | ↑ |
| 5 | Deoxycholic acid | C24H40O4 | HMDB0000626 | 785.61096 | 42.913 | ↑ |
| 6 | Trimethylamine n-oxide | C3H9NO | HMDB0000925 | 76.07505 | 338.9335 | ↓ |
| 7 | DL-2-Aminooctanoic acid | C8H17NO2 | HMDB0000991 | 201.15814 | 213.152 | ↓ |
| 8 | Folinic acid | C20H23N7O7 | HMDB0001562 | 474.17015 | 432.069 | ↑ |
| 9 | (+)-catechin | C15H14O6 | HMDB0002780 | 291.10577 | 418.518 | ↑ |
| 11 | Glutathione, oxidized | C20H32N6O12S2 | HMDB0003337 | 613.15574 | 515.461 | ↑ |
| 12 | N6-methyladenosine | C11H15N5O4 | HMDB0004044 | 282.11625 | 400.027 | ↓ |
| 14 | 1-(1z-octadecenyl)-2-(5z,8z,11z,14z-eicosatetraenoyl)-sn-glycero-3-phosphoethanolamine | C43H78NO7P | HMDB0005779 | 734.53258 | 39.3875 | ↓ |
| 15 | Linoleoylcarnitine | C25H45NO4 | HMDB0006469 | 424.33891 | 43.565 | ↓ |
| 16 | Sialyl lewis x | C31H52N2O23 | HMDB0006565 | 430.14241 | 308.297 | ↓ |
| 17 | 1-palmitoyl-2-docosahexaenoyl-sn-glycero-3-phosphocholine | C46H80NO8P | HMDB0007991 | 806.56728 | 40.5845 | ↑ |
| 18 | 1,2-diarachidonoyl-sn-glycero-3-phosphocholine | C48H81NO8P+ | HMDB0008443 | 830.56344 | 122.992 | ↑ |
| 20 | 1-lignoceroyl-2-hydroxy-sn-glycero-3-phosphocholine | C32H66NO7P | HMDB0010405 | 608.45903 | 184.41 | ↑ |
Differential metabolites in mouse serum in the ESI+ mode (NAFLD group vs. HSYA group).
TABLE 2
| NO | Metabolite | Formula | HMDB | m/z | rt(s) | Which-max |
|---|---|---|---|---|---|---|
| 1 | Vitamin c | C6H8O6 | HMDB0000044 | 351.05442 | 431.348 | ↑ |
| 2 | cis-Aconitate | C6H6O6 | HMDB0000072 | 173.00803 | 467.6255 | ↑ |
| 3 | DL-tyrosine | C9H11NO3 | HMDB0000158 | 180.06549 | 333.17 | ↑ |
| 4 | Phenylalanine | C9H11NO2 | HMDB0000159 | 164.07081 | 298.4045 | ↑ |
| 5 | L-Threonine | C4H9NO3 | HMDB0000167 | 118.05035 | 403.657 | ↑ |
| 6 | Indoleacetic acid | C10H9NO2 | HMDB0000197 | 130.05026 | 397.1245 | ↑ |
| 7 | L-pyroglutamic acid | C5H7NO3 | HMDB0000267 | 128.03588 | 311.1905 | ↑ |
| 8 | 3-hydroxy-3-methylglutaric acid | C6H10O5 | HMDB0000355 | 161.04463 | 403.563 | ↑ |
| 9 | 2-aminoadipic acid | C6H11NO4 | HMDB0000510 | 160.06063 | 436.143 | ↑ |
| 10 | Glutamine | C5H10N2O3 | HMDB0000641 | 145.06161 | 424.263 | ↑ |
| 11 | L-methionine | C5H11NO2S | HMDB0000696 | 148.04287 | 319.019 | ↑ |
| 12 | Pentadecanoic acid | C15H30O2 | HMDB0000826 | 241.21581 | 54.627 | ↑ |
| 13 | N-formylmethionine | C6H11NO3S | HMDB0001015 | 176.03728 | 207.8135 | ↑ |
| 15 | 5-methoxytryptophan | C12H14N2O3 | HMDB0002339 | 467.17127 | 35.727 | ↓ |
| 16 | N-acetylserine | C5H9NO4 | HMDB0002931 | 146.04511 | 312.919 | ↑ |
| 17 | Glutathione disulfide | C20H32N6O12S2 | HMDB0003337 | 611.1419 | 538.491 | ↓ |
| 18 | 2-oleoyl-1-stearoyl-sn-glycero-3-phosphoserine | C42H80NO10P | HMDB0010163 | 788.52802 | 41.042 | ↓ |
| 19 | N-palmitoyl-d-erythro-dihydroceramide-1-phosphate | C34H70NO6P | HMDB0010698 | 618.47279 | 51.437 | ↓ |
| 20 | 1-palmitoyl-2-hydroxy-sn-glycero-3-phosphoethanolamine | C21H44NO7P | HMDB0011503 | 452.27493 | 185.345 | ↓ |
| 23 | 2-oleoyl-1-palmitoyl-sn-glycero-3-phosphoserine | C40H76NO10P | HMDB0012357 | 760.51583 | 65.53 | ↓ |
| 24 | 1,2-distearoyl-sn-glycero-3-phospho-l-serine | C42H82NO10P | HMDB0012378 | 773.52958 | 94.835 | ↓ |
| 26 | DL-tryptophan | C11H12N2O2 | HMDB0030396 | 203.08143 | 295.0945 | ↑ |
| 28 | D-Mannose | C6H12O6 | HMDB0062473 | 179.05691 | 313.3165 | ↑ |
Differential metabolites in mouse serum in the ESI− mode (NAFLD group vs. HSYA group).
3.4 Analysis of the correlation between gut microbiota and serum differential metabolites
Pearson correlation analysis revealed significant associations between the relative abundance of various gut microbiota and serum metabolites (Figure 6). The relative abundance of Turicibacter showed a positive correlation with phosphatidylcholine levels in mouse serum and a negative correlation with DL-tyrosine, sulfanilamide and urea levels. Similarly, the abundance of Verrucomicrobia was positively correlated with Fahfa:36.0 levels and negatively correlated with leucine and deoxycholic acid levels. Additionally, Erysipelotrichaceae-Clostridium abundance exhibited a positive correlation with N-formylmethionine levels and a negative correlation with trehalose and phosphatidylcholine levels.
FIGURE 6
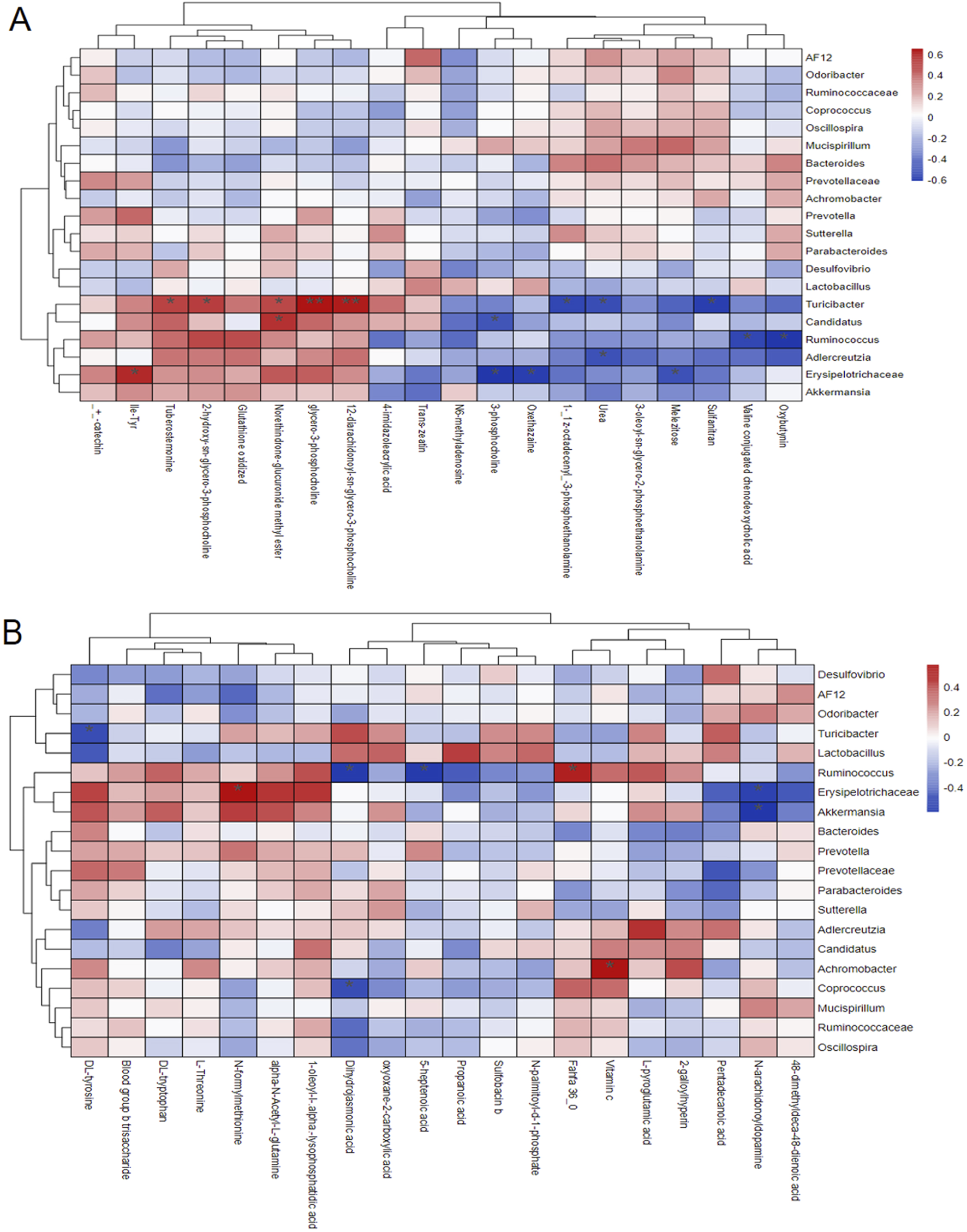
Pearson correlation analysis results of differences in gut microbiota and serum metabolites in mice under ESI+ mode (A) and ESI− mode (B).
4 Discussion
The pathogenesis of NAFLD is multifaceted, involving oxidative stress, lipid metabolism dysregulation and gut microbiota imbalance (Tilg et al., 2021). Although the progression of simple NAFLD is reversible, untreated chronic inflammation and oxidative stress in the liver can escalate the condition into non-alcoholic steatohepatitis (NASH) and eventually liver fibrosis, significantly worsening patient prognosis (Ramai et al., 2021; Tacke and Weiskirchen, 2021; Li J. et al., 2022).
HSYA, the primary bioactive metabolite of safflower (C. tinctorius), belongs to the flavonoid class of compounds (Zhao et al., 2020). Modern pharmacological research has demonstrated that HSYA exhibits anti-inflammatory, antioxidant, anti-apoptotic and protective effects on the cardiovascular and nervous systems (Kong et al., 2022; Yu et al., 2022; Wang et al., 2024). Animal studies have shown that HSYA enhances the expression of antioxidant enzymes, such as superoxide dismutase, in the liver (Lee et al., 2020; Yan et al., 2020; Yao et al., 2025). Furthermore, research using H2O2-induced HepG2 cells and oxidative stress models in adipocytes has indicated that HSYA promotes the expression of cellular antioxidant factors and enzymes, improving cellular resilience against oxidative stress (Yan et al., 2020). Our findings corroborate these results, demonstrating that oral administration of HSYA significantly reduces inflammatory responses and oxidative stress in high-fat diet-induced NAFLD mice. Notably, HSYA significantly decreased serum TC levels in these mice, although it had no significant effect on triglyceride (TG) levels. This discrepancy may be attributed to HSYA altering cholesterol distribution within the body. However, the current study utilised HE staining to evaluate liver pathology, which did not allow for a detailed analysis of lipid content. Future studies will employ Oil Red O staining to assess liver lipid profiles and investigate HSYA’s specific effects on cholesterol and other lipid distributions.
To further explore the mechanisms by which HSYA mitigates inflammation and liver damage in NAFLD, we analysed gut microbiota and serum non-targeted metabolomics in the experimental mice. HFD significantly increased the abundance of Ruminococcus in the intestines, consistent with previous findings of elevated Ruminococcus levels in the gut of patients with NAFLD (Lee et al., 2020; Pettinelli et al., 2022). HSYA treatment significantly reduced Ruminococcus abundance in the intestines of high-fat diet-fed mice while significantly increasing the abundance of beneficial Turicibacter.
Existing research indicates that both Ruminococcus and Turicibacter in the gut are involved in tryptophan metabolism. The abundance of Ruminococcus is positively correlated with the production of indoleamine 2,3-dioxygenase (IDO1) in the gut (Li et al., 2022b). It can activate macrophage IDO1 through the metabolite succinate, thereby activating macrophages and consuming tryptophan to produce a large amount of the pro-inflammatory metabolite kynurenine (Riazati et al., 2022; Ma et al., 2024). Turicibacter carries a homologous gene for tryptophan hydroxylase, which can catalyze the conversion of tryptophan into 5-hydroxytryptophan and inhibit the production of kynurenine, thereby reducing the host’s inflammatory response levels (Gonçalves et al., 2022; Devereaux et al., 2024). These findings suggest that HSYA ameliorates gut microbiota dysbiosis in NAFLD and indicate that its therapeutic effects may be mediated, at least in part, through modulation of the gut microbiota.
Further serum non-targeted metabolomics analysis revealed that HSYA modulates the metabolism of phenylalanine and the biosynthetic pathways of phenylalanine, tyrosine and tryptophan in NAFLD mice. Phenylalanine, an aromatic amino acid with physiological activity, is converted into tyrosine in the body. Both phenylalanine and tryptophan are essential amino acids that must be obtained through dietary intake. A study involving over 2,000 subjects reported significantly higher levels of phenylalanine, tyrosine and other amino acids in NAFLD patients compared to healthy individuals (Hasegawa et al., 2020). These findings suggest that the biosynthetic metabolism of phenylalanine, tyrosine and tryptophan plays a critical role in the pathogenesis of NAFLD, with their metabolic pathways closely linked to lipid metabolism in the liver (Chen and Vitetta, 2020; Masoodi et al., 2021; Teunis et al., 2022). In this study, HSYA treatment significantly reduced tyrosine levels in NAFLD mice, indicating its ability to regulate metabolic disturbances involving tyrosine and related amino acids. This regulatory effect may represent a potential mechanism by which HSYA exerts its therapeutic effects on NAFLD. However, since this study did not include faecal microbiota transplantation experiments, we cannot conclusively confirm that HSYA improves serum metabolomic disturbances in NAFLD mice via modulation of gut microbiota. Future research will incorporate faecal microbiota transplantation to provide a more comprehensive understanding of the relationship between HSYA, gut microbiota and metabolomic regulation.
Pearson correlation analysis revealed a significant negative correlation between DL-tryptophan levels and the relative abundance of Turicibacter. Previous studies have demonstrated that Turicibacter in the mammalian gut reduces serum cholesterol, triglycerides and adipose tissue weight (Lynch et al., 2023; Yin et al., 2023; Zhao et al., 2023). In our study, HSYA treatment significantly increased the abundance of Turicibacter in the intestines of NAFLD mice, which was accompanied by significant improvements in oxidative stress and blood lipid profiles. Additionally, we observed a significant increase in serum phosphatidylcholine levels in the HSYA-treated group. Phosphatidylcholine plays a key role in phospholipid synthesis, promotes fat metabolism and inhibits hepatic fat deposition, thereby protecting the liver (Maev et al., 2020; Li et al., 2022c; Lu et al., 2022). These findings are consistent with previous research, further supporting the beneficial effects of HSYA on lipid metabolism and liver health in NAFLD mice.
This study revealed a significant positive correlation between serum N-formylmethionine levels and the abundance of Erysipelotrichaceae in the intestine. NAFLD mice exhibited elevated serum N-formylmethionine levels, which were significantly reduced following HSYA treatment. Previous research by Sigurdsson et al. (2022) reported that increased N-formylmethionine is often associated with activation of the pentose phosphate pathway and suppression of mitochondrial fatty acid β-oxidation. Erysipelotrichaceae are known to proliferate in the intestines of hosts experiencing high levels of inflammation (Chen and Vitetta, 2020), during which pro-inflammatory cytokine IL-1β expression and oxidative stress levels are significantly elevated (Bennett et al., 2020; Li et al., 2020). In this study, HSYA treatment significantly reduced the abundance of Erysipelotrichaceae in the intestines of NAFLD mice, coinciding with a decrease in serum N-formylmethionine levels. These findings suggest that Erysipelotrichaceae may play a pivotal role in the inflammatory processes of NAFLD and that HSYA mitigates inflammation by decreasing the abundance of this microorganism. The reduction in N-formylmethionine levels further indicates a decrease in oxidative stress and inflammation. However, the precise mechanisms by which Erysipelotrichaceae contribute to NAFLD pathogenesis remain to be elucidated through further investigation.
Turicibacter in the gut can also affect host glycerophospholipid metabolism and tryptophan/tyrosine synthesis metabolism (Chen et al., 2024). The results of this study suggest that the abundance of Turicibacter in the gut is positively correlated with the concentration of phosphatidylcholine in the serum and negatively correlated with the concentration of tyrosine. Turicibacter can express specific bile salt hydrolases (BSH) that participate in bile acid synthesis and metabolism, thereby affecting the host’s absorption and metabolism of lipids, including the metabolism of cholesterol, triglycerides, and phospholipids (Lynch et al., 2023). The study results indicate that the proliferation of Turicibacter may enhance lipid metabolic activity by promoting bile acid dissociation. Changes in the abundance of Turicibacter may affect tyrosine-related metabolic pathways through the “microbe-host co-metabolism axis”. The potential mechanism might be that the massive proliferation of Turicibacter inhibits the host’s tryptophan metabolism (kynurenine pathway) or phenylalanine metabolism, leading to a reduction in tyrosine synthesis precursors, thereby lowering the serum tyrosine concentration.
In conclusion, HSYA is a potent antioxidant capable of mitigating oxidative stress and inflammatory damage in the liver by modulating the gut microbiota and regulating metabolic pathways, including those of phenylalanine, tyrosine and tryptophan. These effects collectively contribute to the therapeutic potential of HSYA in treating NAFLD.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The animal studies were approved by the Ethics Committee of the Jiangsu University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
LW: Data curation, Investigation, Methodology, Writing – original draft, Writing – review and editing. XD: Investigation, Methodology, Validation, Writing – original draft. WS: Investigation, Validation, Writing – original draft. TJ: Investigation, Methodology, Writing – original draft. AA: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review and editing. JH: Conceptualization, Funding acquisition, Investigation, Project administration, Resources, Writing – original draft. PW: Conceptualization, Funding acquisition, Investigation, Project administration, Supervision, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Zhenjiang Key R&D Program - Social Development Project (SH2023073) and Jiangsu Province Traditional Chinese Medicine Science and Technology Development Project (MS2022126).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1568608/full#supplementary-material
References
1
Arroyave-OspinaJ. C.WuZ.GengY.MoshageH. (2021). Role of oxidative stress in the pathogenesis of non-alcoholic fatty liver disease: implications for prevention and therapy. Antioxidants Basel, Switz.10, 174. 10.3390/antiox10020174
2
BenceK. K.BirnbaumM. J. (2021). Metabolic drivers of non-alcoholic fatty liver disease. Mol. Metab.50, 101143. 10.1016/j.molmet.2020.101143
3
BennettC. J.HenryR.SnipeR.CostaR. (2020). Is the gut microbiota bacterial abundance and composition associated with intestinal epithelial injury, systemic inflammatory profile, and gastrointestinal symptoms in response to exertional-heat stress. J. Sci. Med. Sport23, 1141–1153. 10.1016/j.jsams.2020.06.002
4
ChenJ.VitettaL. (2020). Gut microbiota metabolites in NAFLD pathogenesis and therapeutic implications. Int. J. Mol. Sci.21, 5214. 10.3390/ijms21155214
5
ChenM.ZhaoY.LiS.ChangZ.LiuH.ZhangD.et al (2024). Maternal malic acid may ameliorate oxidative stress and inflammation in sows through modulating gut microbiota and host metabolic profiles during late pregnancy. Antioxidants Basel, Switz.13, 253. 10.3390/antiox13020253
6
ČolakE.PapD. (2021). The role of oxidative stress in the development of obesity and obesity-related metabolic disorders. J. Med. Biochem.40, 1–9. 10.5937/jomb0-24652
7
DevereauxJ.RobinsonA. M.StavelyR.DavidsonM.DargahiN.EphraimR.et al (2024). Alterations in tryptophan metabolism and de novo NAD(+) biosynthesis within the microbiota-gut-brain axis in chronic intestinal inflammation. Front. Med. (Lausanne)11, 1379335. 10.3389/fmed.2024.1379335
8
GonçalvesS.Nunes-CostaD.CardosoS. M.EmpadinhasN.MaruggJ. D. (2022). Enzyme promiscuity in serotonin biosynthesis, from bacteria to plants and humans. Front. Microbiol.13, 873555. 10.3389/fmicb.2022.873555
9
HasegawaT.IinoC.EndoT.MikamiK.KimuraM.SawadaN.et al (2020). Changed amino acids in NAFLD and liver fibrosis: a large cross-sectional study without influence of insulin resistance. Nutrients12, 1450. 10.3390/nu12051450
10
KongJ.SunS.MinF.HuX.ZhangY.ChengY.et al (2022). Integrating network pharmacology and transcriptomic strategies to explore the pharmacological mechanism of hydroxysafflor yellow A in delaying liver aging. Int. J. Mol. Sci.23, 14281. 10.3390/ijms232214281
11
KosmalskiM.ŚliwińskaA.DrzewoskiJ. (2023). Non-alcoholic fatty liver disease or type 2 diabetes mellitus-the chicken or the egg dilemma. Biomedicines11, 1097. 10.3390/biomedicines11041097
12
LeeM.ZhaoH.LiuX.LiuD.ChenJ.LiZ.et al (2020). Protective effect of hydroxysafflor yellow A on nephropathy by attenuating oxidative stress and inhibiting apoptosis in induced type 2 diabetes in rat. Oxid. Med. Cell. Longev.2020, 7805393. 10.1155/2020/7805393
13
LiJ.JiaS.YuanC.YuB.ZhangZ.ZhaoM.et al (2022a). Jerusalem artichoke inulin supplementation ameliorates hepatic lipid metabolism in type 2 diabetes mellitus mice by modulating the gut microbiota and fecal metabolome. Food Funct.13, 11503–11517. 10.1039/d2fo02051c
14
LiL. L.WangY. T.ZhuL. M.LiuZ. Y.YeC. Q.QinS. (2020). Inulin with different degrees of polymerization protects against diet-induced endotoxemia and inflammation in association with gut microbiota regulation in mice. Sci. Rep.10, 978. 10.1038/s41598-020-58048-w
15
LiY.AdenijiN. T.FanW.KunimotoK.TörökN. J. (2022b). Non-alcoholic fatty liver disease and liver fibrosis during aging. Aging Dis.13, 1239–1251. 10.14336/AD.2022.0318
16
LiY.LiuN.GeY.YangY.RenF.WuZ. (2022c). Tryptophan and the innate intestinal immunity: crosstalk between metabolites, host innate immune cells, and microbiota. Eur. J. Immunol.52, 856–868. 10.1002/eji.202149401
17
LimS.KimJ. W.TargherG. (2021). Links between metabolic syndrome and metabolic dysfunction-associated fatty liver disease. Trends Endocrinol. metabolism TEM32, 500–514. 10.1016/j.tem.2021.04.008
18
LiuJ.YueS.YangZ.FengW.MengX.WangA.et al (2018). Oral hydroxysafflor yellow A reduces obesity in mice by modulating the gut microbiota and serum metabolism. Pharmacol. Res.134, 40–50. 10.1016/j.phrs.2018.05.012
19
LuY.FengT.ZhaoJ.JiangP.XuD.ZhouM.et al (2022). Polyene phosphatidylcholine ameliorates high fat diet-induced non-alcoholic fatty liver disease via remodeling metabolism and inflammation. Front. Physiol.13, 810143. 10.3389/fphys.2022.810143
20
LynchJ. B.GonzalezE. L.ChoyK.FaullK. F.JewellT.ArellanoA.et al (2023). Gut microbiota Turicibacter strains differentially modify bile acids and host lipids. Nat. Commun.14, 3669. 10.1038/s41467-023-39403-7
21
MaS.LiJ.YeH.WuC.ZhangJ.XuS.et al (2024). Indoleamine 2, 3-dioxygenase 1 activation in macrophage exacerbates hepatic ischemia-reperfusion injury by triggering hepatocyte ferroptosis. Int. Immunopharmacol.130, 111692. 10.1016/j.intimp.2024.111692
22
MaevI. V.SamsonovA. A.PalgovaL. K.PavlovC. S.ShirokovaE. N.VovkE. I.et al (2020). Effectiveness of phosphatidylcholine as adjunctive therapy in improving liver function tests in patients with non-alcoholic fatty liver disease and metabolic comorbidities: real-life observational study from Russia. BMJ Open Gastroenterol.7, e000368. 10.1136/bmjgast-2019-000368
23
ManiV.LeeS. K.YeoY.HahnB. S. (2020). A metabolic perspective and opportunities in pharmacologically important safflower. Metabolites10, 253. 10.3390/metabo10060253
24
MasoodiM.GastaldelliA.HyötyläinenT.ArretxeE.AlonsoC.GagginiM.et al (2021). Metabolomics and lipidomics in NAFLD: biomarkers and non-invasive diagnostic tests. Nat. Rev. Gastroenterol. Hepatol.18, 835–856. 10.1038/s41575-021-00502-9
25
MohammadianK.FakharF.KeramatS.StanekA. (2024). The role of antioxidants in the treatment of metabolic dysfunction-associated fatty liver disease: a systematic review. Antioxidants Basel, Switz.13, 797. 10.3390/antiox13070797
26
MuzurovićE.MikhailidisD. P.MantzorosC. (2021). Non-alcoholic fatty liver disease, insulin resistance, metabolic syndrome and their association with vascular risk. Metabolism119, 154770. 10.1016/j.metabol.2021.154770
27
PaternostroR.TraunerM. (2022). Current treatment of non-alcoholic fatty liver disease. J. Intern. Med.292, 190–204. 10.1111/joim.13531
28
PettinelliP.ArendtB. M.SchwengerK.SivarajS.BhatM.ComelliE. M.et al (2022). Relationship between hepatic gene expression, intestinal microbiota, and inferred functional metagenomic analysis in NAFLD. Clin. Transl. Gastroenterol.13, e00466. 10.14309/ctg.0000000000000466
29
RamaiD.FacciorussoA.VigandtE.SchafB.SaadedeenW.ChauhanA.et al (2021). Progressive liver fibrosis in non-alcoholic fatty liver disease. Cells10, 3401. 10.3390/cells10123401
30
RiazatiN.KableM. E.NewmanJ. W.AdkinsY.FreytagT.JiangX.et al (2022). Associations of microbial and indoleamine-2,3-dioxygenase-derived tryptophan metabolites with immune activation in healthy adults. Front. Immunol.13, 917966. 10.3389/fimmu.2022.917966
31
RiaziK.AzhariH.CharetteJ. H.UnderwoodF. E.KingJ. A.AfsharE. E.et al (2022). The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterology and Hepatology7, 851–861. 10.1016/S2468-1253(22)00165-0
32
SigurdssonM. I.KobayashiH.AmreinK.NakahiraK.RogersA. J.Pinilla-VeraM.et al (2022). Circulating N-formylmethionine and metabolic shift in critical illness: a multicohort metabolomics study. Crit. care London, Engl.26, 321. 10.1186/s13054-022-04174-y
33
SohouliM. H.FatahiS.SayyariA.OlangB.ShidfarF. (2020). Associations between dietary total antioxidant capacity and odds of non-alcoholic fatty liver disease (NAFLD) in adults: a case-control study. J. Nutr. Sci.9, e48. 10.1017/jns.2020.39
34
SunC.QiuC.ZhangY.YanM.TanJ.HeJ.et al (2023). Lactiplantibacillus plantarum NKK20 alleviates high-fat-diet-induced nonalcoholic fatty liver disease in mice through regulating bile acid anabolism. Molecules28, 4042. 10.3390/molecules28104042
35
TackeF.WeiskirchenR. (2021). Non-alcoholic fatty liver disease (NAFLD)/non-alcoholic steatohepatitis (NASH)-related liver fibrosis: mechanisms, treatment and prevention. Ann. Transl. Med.9, 729. 10.21037/atm-20-4354
36
TeunisC.NieuwdorpM.HanssenN. (2022). Interactions between tryptophan metabolism, the gut microbiome and the immune system as potential drivers of non-alcoholic fatty liver disease (NAFLD) and metabolic diseases. Metabolites12, 514. 10.3390/metabo12060514
37
TilgH.AdolphT. E.DudekM.KnolleP. (2021). Non-alcoholic fatty liver disease: the interplay between metabolism, microbes and immunity. Nat. Metab.3, 1596–1607. 10.1038/s42255-021-00501-9
38
WangY.AnJ.ZhouJ.ChangL.ZhangQ.PengF. (2024). Hydroxysafflor yellow A: a natural pigment with potential anticancer therapeutic effect. Front. Pharmacol.15, 1495393. 10.3389/fphar.2024.1495393
39
WangY.LiX.DengF.YinR. (2021). Hydroxy-safflower yellow A alleviates osteoporosis in ovariectomized rat model by inhibiting carbonic anhydrase 2 activity. Front. Pharmacol.12, 734539. 10.3389/fphar.2021.734539
40
WongV. W.EkstedtM.WongG. L.HagströmH. (2023). Changing epidemiology, global trends and implications for outcomes of NAFLD. J. Hepatol.79, 842–852. 10.1016/j.jhep.2023.04.036
41
WuX.CaiX.AiJ.ZhangC.LiuN.GaoW. (2021). Extraction, structures, bioactivities and structure-function analysis of the polysaccharides from Safflower (Carthamus tinctorius L.). Front. Pharmacol.12, 767947. 10.3389/fphar.2021.767947
42
XueX.DengY.WangJ.ZhouM.LiaoL.WangC.et al (2021). Hydroxysafflor yellow A, a natural compound from Carthamus tinctorius L with good effect of alleviating atherosclerosis. Phytomedicine91, 153694. 10.1016/j.phymed.2021.153694
43
YanK.WangX.PanH.WangL.YangH.LiuM.et al (2020). Safflower yellow and its main component HSYA alleviate diet-induced obesity in mice: possible involvement of the increased antioxidant enzymes in liver and adipose tissue. Front. Pharmacol.11, 482. 10.3389/fphar.2020.00482
44
YaoM.LiuY.MengD.ZhouX.ChangD.LiL.et al (2025). Hydroxysafflor yellow A attenuates the inflammatory response in cerebral ischemia-reperfusion injured mice by regulating microglia polarization per SIRT1-mediated HMGB1/NF-κB signaling pathway. Int. Immunopharmacol.147, 114040. 10.1016/j.intimp.2025.114040
45
YasminT.RahmanM. M.KhanF.KabirF.NaharK.LaskerS.et al (2021). Metformin treatment reverses high fat diet-induced non-alcoholic fatty liver diseases and dyslipidemia by stimulating multiple antioxidant and anti-inflammatory pathways. Biochem. Biophys. Rep.28, 101168. 10.1016/j.bbrep.2021.101168
46
YinH.HuangJ.GuoX.XiaJ.HuM. (2023). Romboutsia lituseburensis JCM1404 supplementation ameliorated endothelial function via gut microbiota modulation and lipid metabolisms alterations in obese rats. FEMS Microbiol. Lett.370, fnad016. 10.1093/femsle/fnad016
47
YuL.JinZ.LiM.LiuH.TaoJ.XuC.et al (2022). Protective potential of hydroxysafflor yellow A in cerebral ischemia and reperfusion injury: an overview of evidence from experimental studies. Front. Pharmacol.13, 1063035. 10.3389/fphar.2022.1063035
48
ZhaoF.WangP.JiaoY.ZhangX.ChenD.XuH. (2020). Hydroxysafflor yellow A: a systematical review on botanical Resources, physicochemical properties, drug delivery system, pharmacokinetics, and pharmacological effects. Front. Pharmacol.11, 579332. 10.3389/fphar.2020.579332
49
ZhaoR.JiY.ChenX.MaG.YaoH.LiJ.et al (2023). Flammulina velutipes polysaccharides regulate lipid metabolism disorders in HFD-fed mice via bile acids metabolism. Int. J. Biol. Macromol.253, 127308. 10.1016/j.ijbiomac.2023.127308
Summary
Keywords
hydroxysafflor yellow A, non-alcoholic fatty liver, oxidative stress, gut microbiota, amino acid anabolism
Citation
Wu L, Dong X, Sun W, Jiajun T, Ali A, He J and Wang P (2025) Hydroxysafflor yellow A alleviates oxidative stress and inflammatory damage in the livers of mice with nonalcoholic fatty liver disease and modulates gut microbiota. Front. Pharmacol. 16:1568608. doi: 10.3389/fphar.2025.1568608
Received
30 January 2025
Accepted
23 May 2025
Published
06 June 2025
Volume
16 - 2025
Edited by
Chao Wang, Dalian Medical University, China
Reviewed by
Fu Peng, Sichuan University, China
Annalisa Chiavaroli, University of Studies G. d’Annunzio Chieti and Pescara, Italy
Shi Xue Dai, Guangdong Provincial People’s Hospital, China
Updates
Copyright
© 2025 Wu, Dong, Sun, Jiajun, Ali, He and Wang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liang Wu, wlujs@ujs.edu.cn; Pingping Wang, Wangpp0229@qq.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.