- School of Basic Medical Sciences, Chengdu University of Traditional Chinese Medicine, Chengdu, China
Background: Non-small-cell lung cancer (NSCLC), a leading cause of global cancer mortality, often presents at advanced stages with limited efficacy from standard chemotherapy. This study evaluated the efficacy and safety of six oral traditional Chinese medicines (TCMs) combined with chemotherapy for NSCLC.
Methods: Following PRISMA-NMA guidelines, a systematic review and network meta-analysis of 36 randomized controlled trials (RCTs, n = 2,846 patients) was conducted. Databases including PubMed, China National Knowledge Infrastructure (CNKI), and Web of Science were searched. Outcomes assessed included objective response rate (ORR), immune markers (CD4–CD8 ratio and natural killer (NK) cells), tumor markers (CA125, carcinoembryonic antigen (CEA), and CYFRA21-1), and adverse events. Data were synthesized using STATA 14.0 and R software, with risk of bias evaluated via the Cochrane RoB 2.0 tool.
Results: Compared to chemotherapy alone, Tongguanteng (TGT, Marsdenia tenacissima) demonstrated superior improvement in the ORR [OR = 1.88, 95% CI: 1.25–2.83]. This effect may be attributable to its vincristine content, which modulates apoptosis through cell-cycle regulation pathways. Huisheng (HS) ranked second in efficacy [OR = 1.34, 95% CI: 1.10–1.61], with its emodin component suppressing NSCLC proliferation via NF-κB pathway inhibition. HS was also associated with improvements in immune markers, including CD4+/CD8+ ratio and NK cell activity. Conversely, TGT significantly reduced tumor markers: CA125, CEA, and cytokeratin-19 fragment (CYFRA21-1). This latter observation may be explained by tenacissoside’s inhibition of cytochrome P450 enzymes (CYP2D6/CYP3A4), which alter drug metabolism. Although TCM–chemotherapy combinations exhibited improved safety profiles compared to chemotherapy alone, the analysis revealed potential publication bias and moderate heterogeneity.
Conclusion: HS and TGT, potentially through their bioactive components, may enhance chemotherapy efficacy in NSCLC by targeting immune and metabolic pathways. However, these conclusions need further verification. Findings should be interpreted cautiously due to potential bias, limited RCT numbers, and the geographical concentration of studies. Future research should isolate compound-specific effects and validate mechanisms in global trials.
1 Introduction
Non-small-cell lung cancer (NSCLC) remains a leading cause of global mortality and significantly compromises patient quality of life (Kong et al., 2023; Sung et al., 2021). NSCLC—comprising adenocarcinoma, squamous cell carcinoma, and large cell carcinoma—accounts for approximately 85% of all lung cancer cases. The 5-year survival rate for advanced-stage (III–IV) NSCLC is less than 15% (Qin et al., 2024). Current treatment modalities include radiotherapy, chemotherapy, molecularly targeted therapies, and immune checkpoint inhibitors (Cronin et al., 2022). Due to the lack of effective early screening methods, most patients are diagnosed at advanced stages, contributing to poor prognosis (Ettinger et al., 2021). The 2021 National Comprehensive Cancer Network (NCCN) guidelines recommend high-dose cisplatin or paclitaxel–platinum (TP) combination therapy for advanced NSCLC. However, emerging evidence suggests limited efficacy of these regimens (Herbst et al., 2018; Han et al., 2005; de Jong et al., 2023). Additionally, resistance to immune checkpoint inhibitors (e.g., PD-1/PD-L1 inhibitors) and targeted therapies poses a significant clinical challenge (Guisier et al., 2020; Wang et al., 2022). These limitations underscore the urgent need for integrated therapeutic strategies to improve patient outcomes.
The Guidelines for the Diagnosis and Treatment of Tumors issued by the Chinese Society of TCM highlight TCM’s role in oncology, including the use of oral Chinese medicines as adjunctive therapies (Wang et al., 2022). Evidence suggests that oral Chinese medicines can significantly improve survival rates and prognosis in lung cancer patients (Zhao et al., 2019a). Tumor markers (e.g., CEA and CYFRA21-1) and inflammatory biomarkers (e.g., IL-6 and CRP) are recognized as key prognostic indicators for NSCLC (Huai et al., 2023). In clinical practice, oral Chinese medicines are increasingly employed alongside conventional treatments to modulate these biomarkers, demonstrating the potential to enhance therapeutic outcomes (Wang et al., 2024; Zhao, 2023a). In TCM theory, NSCLC is classified as pulmonary retention (肺积), a condition linked to blood stasis and qi stagnation. Oral TCM therapies aim to resolve stasis and restore physiological balance through compounds with bioactive properties (Song et al., 2023). For example, astragaloside IV (derived from Astragalus membranaceus) reverses paclitaxel resistance in NSCLC by suppressing the EREG/ErbB/ERK signaling pathway, while Celastrus orbiculatus extracts induce ROS-mediated pyroptosis and autophagy to inhibit tumor progression (Xiu et al., 2024; Luo et al., 2023). Synergistic interactions among TCM components further amplify their antitumor efficacy (Qin et al., 2020). Currently, multiple standardized oral Chinese medicines are commercially available. This study evaluates the efficacy and safety of six oral traditional Chinese medicines (TCMs) combined with chemotherapy in NSCLC treatment.
2 Methods
This study has been registered in the PROSPERO international prospective register of systematic reviews (registration ID: CRD42024580740). The methodology and reporting adhere rigorously to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Network Meta-Analyses (PRISMA-NMA) guidelines and meta-analysis standards (Hutton et al., 2015).
2.1 Literature retrieval
We searched seven databases, namely, China National Knowledge Infrastructure (CNKI), Wanfang Database, China Science and Technology Journal Database (VIP), China Biomedical Literature Database (CBM), PubMed, Embase, and Web of Science, from inception to 17 September 2024. The search approach is explained in Supplementary Material S1A. In addition, the ingredients of the oral Chinese medicine are listed in Supplementary Material S2.
2.2 Inclusion criteria
2.2.1 Types of studies
This study includes RCTs published in English or Chinese that examined oral Chinese medicine in conjunction with chemotherapy drugs, namely, cisplatin or cisplatin plus paclitaxel.
2.2.2 Types of participants
Participants were diagnosed in accordance with the Chinese Guidelines for the Diagnosis and Treatment of Common Malignant Tumors and confirmed to have NSCLC by pathology or cytology (Association, 2022). Participants’ ages varied from 35 to 85 years. The treatment duration ranged from 3 to 24 weeks, irrespective of nationality or gender.
2.2.3 Interventions and comparison
The intervention group received treatment with a TCM oral solution in combination with cisplatin or cisplatin plus paclitaxel, whereas the control group was solely treated with cisplatin or cisplatin plus paclitaxel.
2.2.4 Outcome
The primary indicator is the objective response rate (OOR), while the secondary indicators include the TCM syndrome score, CD4–CD8 ratio, NK cells, CYFRA21-1, platelets, CA125, and CEA.
2.3 Exclusion criteria
Exclusion criteria were as follows: 1. duplicated literature; 2. literature that used multiple herbal oral medicines; 3. literature that used chemotherapeutic agents other than cisplatin and paclitaxel; and 4. studies employing non-standardized or modified formulations (e.g., decoctions with variable ingredients).
2.4 Study selection
EndNote X9 is used to manage all articles. Following the removal of duplicates, two reviewers (YQM and JL) evaluated the papers according to the qualifying criteria. The complete text was subsequently reviewed for evaluation. Disputes were settled by team deliberation or LYL and TS.
2.5 Data extraction
YQM and JL, two reviewers, used a pre-made extraction form to extract data. The author’s name, sample size, stage, age, gender (M/F), length of therapy, and outcome indicators were among the information that was retrieved. Two reviewers cross-checked the extracted data. During the study selection process, disagreements between the two investigators were resolved by LYL and TS.
2.6 Assessment of risk of bias
RoB 2.0 (Sterne et al., 2019) was used to assess the risk of bias for the included studies. RoB 2.0 evaluates the risk of bias in five areas, namely, bias from outcome measures, bias from missing outcome data, bias from divergence from established interventions, bias from the randomization method, and bias from selective reporting of outcomes.
2.7 Statistical analysis
Statistical analyses were performed using STATA 14.0, R software, and Microsoft Excel 2019. R software (version 4.3.0) was used for data synthesis. MD and 95% CI were measured for continuous variables, and relative risk (RR) was calculated for the 95% CI of dichotomous data. For dichotomous variables, RR was used as an effect size indicator based on the OOR rate of patients with a 95% CI for reporting (Dias et al., 2013; Mills et al., 2013). For continuous variables, such as NK cells, CA125, CEA, CYFRA21-1, and CD4–CD8, MD and 95% CI were calculated. Comparisons were made using the SUCRA for TCM oral liquids. SUCRA curves indicated the most effective and least effective treatments in terms of 100% and 0% percentages, respectively. SUCRA curves and consistency tests were performed using Stata 14.0 software (Rücker and Schwarzer, 2015; Trinquart et al., 2016). Funnel plots were drawn and compared to determine whether there was a publication bias in this network meta-analysis.
Subsequently, time series analysis (TSA) was implemented to evaluate both data robustness and meta-analytic conclusions. This methodology employs sequential monitoring boundaries through the calculation of the accrued sample size (information size, IS) from included studies. The Z-curve represents the temporal accumulation of trial evidence, where intersection with alpha-spending boundaries indicates conclusive evidence for intervention efficacy, while crossing futility boundaries demonstrates therapeutic ineffectiveness. TSA boundaries mitigate type I (α = 5%) and type II (β = 20%) error risks through predefined monitoring criteria. Our analysis preserved a two-sided α = 5% significance level and calculated the required IS based on 80% power to detect a 20% relative risk reduction in mechanical compression. Analytical procedures used Copenhagen Trial Unit’s TSA software (version 0.9.5.10 Beta9).
3 Results
3.1 Study selection and characteristics
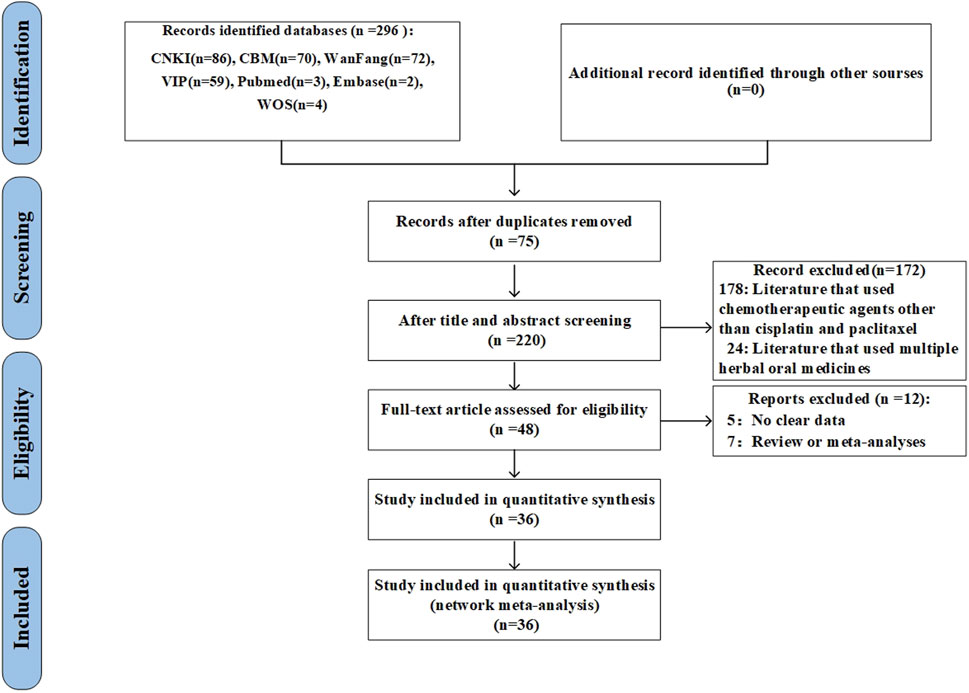
The preliminary analysis revealed 296 studies through the search approach. Following the elimination of duplicates, 220 studies were evaluated based on their titles and abstracts. At this juncture, 172 extraneous citations were eliminated, allowing for the selection of 48 studies for comprehensive review. Following the exclusion of reviews, meta-analyses, and studies lacking outcome data, 36 studies were incorporated (Figure 1).
3.2 Characteristics of included studies
A total of 36 studies involving 2,846 patients were included in this study, covering six oral Chinese medicines, namely, JinFuKang (JFK) oral liquid, Huisheng (HS) oral liquid, Xuekang (XK) oral liquid, Tianfoshen (TFS) oral liquid, Fuzheng (FZ) oral liquid, and Tongguanteng (TGT) oral liquid (Lin, 2003; Chen, 2011; Ding et al., 2011; Liu et al., 2011a; Liu et al., 2011b; Xu et al., 2011; Yu, 2011; Jia, 2013a; Jia, 2013b; Zhang, 2013; Jia, 2014; Wei, 2014; Song, 2015; Li, 2016; Pu, 2016; Yu, 2016; Li, 2017; Liu, 2017; Ranbai, 2017; Wu, 2017; Zhang, 2017; Yang L., 2018; Yang W., 2018; Yang X., 2018; Li, 2019; Mu, 2019; Tang, 2019; Zhai, 2019; Zhang, 2019; Zhao et al., 2019b; Xiao, 2021; Zhang, 2021; Lu, 2022; Liu, 2023; Song, 2023; Xie, 2023; Zhao, 2023b). The retrieval strategies of the included studies can be found in Supplementary Material S1B.
3.3 Quality assessment
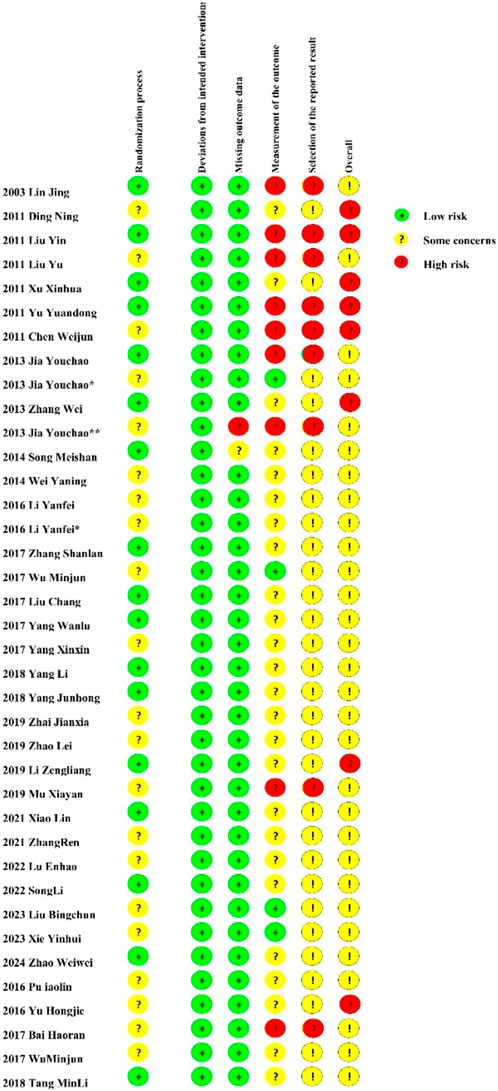
Out of the 36 randomized controlled trials examined, 16 disclosed their randomization methodologies. Nevertheless, the methodology for randomized sequence creation was ambiguous or inadequately documented in 20 investigations. 9 studies were assessed as high-risk for incompleteness. Detailed quality judgments are presented in Figure 2. In addition, each study includes a ConPhyMP tool sheet (Supplementary Material S4).
3.4 Network meta-analysis
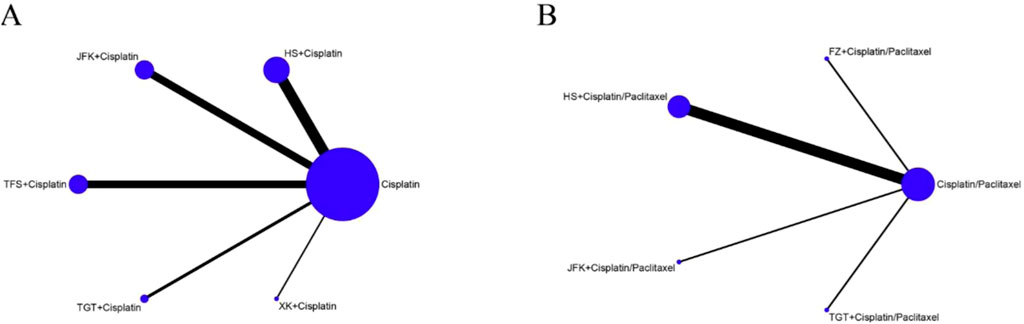
The network diagram is shown in Figure 3, where the lines in the graphic represent the interventions of direct comparison, the line thickness indicates the number of studies, and the dot size indicates the sample size of the intervention. We performed a statistical analysis of all indicators using a random-effects model, with a total of 50,000 iterations, beginning with the 20,001st simulation.
3.5 Primary indicators
3.5.1 Objective response rate
A total of 30 trials revealed the OOR, encompassing 5 interventions and 2,167 patients. As indicated in Figures 3A, B, the network diagram indicates the relative quantity of available evidence for various therapies, which included JFK, HS, XK, TFS, and TGT oral liquids and chemotherapy. The graphic illustrates 30 direct comparisons of the effects of these therapies on the OOR.
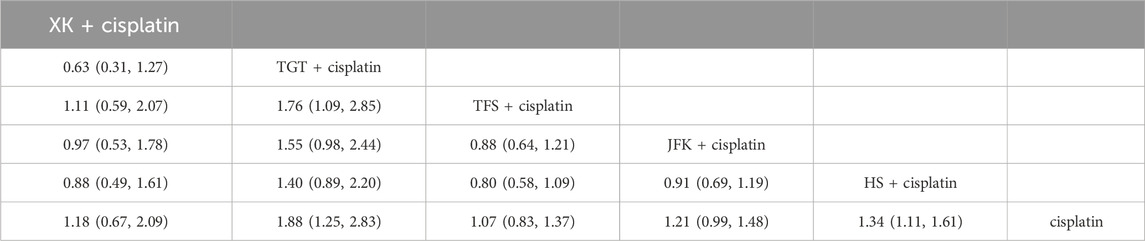
Compared with cisplatin alone, the values were as follows: TGT (OR = 1.88, 95% CI [1.25, 2.83]), HS + cisplatin (OR = 1.34, 95% CI [1.11, 1.61]), JFK + cisplatin (OR = 1.21, 95% CI [0.99, 1.48]), XK + cisplatin (OR = 1.18, 95% CI [0.67, 2.09]), and TFS + cisplatin (OR = 1.07, 95% CI [0.83, 1.37]) (Table 1). The ranking results can be found in Supplementary Material S4A.
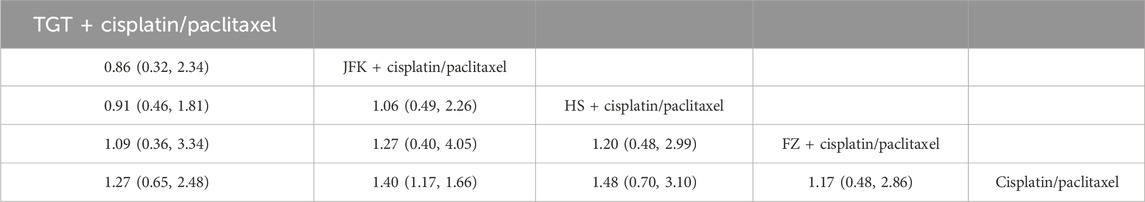
We also evaluated the effectiveness of adding four oral Chinese medicines to TP therapy. Compared with cisplatin + paclitaxel, the values were as follows: HS + cisplatin/paclitaxel (OR = 1.48, 95% CI [0.70, 3.10]), JFK + cisplatin/paclitaxel (OR = 1.40, 95% CI [1.17, 1.66]), TGT + cisplatin/paclitaxel (OR = 1.27, 95% CI [0.65, 2.48]), and FZ + cisplatin/paclitaxel (OR = 1.17, 95% CI [0.48, 2.86]) (Table 2). The ranking results can be found in Supplementary Material S4B.
3.5.2 Subgroup analysis
We evaluated the ORR across varying treatment durations (Supplementary Material S5). In subgroup analyses of cisplatin combined with oral Chinese medicines for NSCLC, inter-subgroup heterogeneity was low (I2 < 50%, p > 0.05), supporting the application of a fixed-effects model. The results suggest a positive correlation between treatment duration and efficacy, with TGT + cisplatin demonstrating the highest efficacy at 24 weeks (OR = 3.33, 95% CI [1.14, 9.75]).
Similarly, in subgroup analyses of TP therapy combined with TCM oral formulations for NSCLC, heterogeneity remained low (I2 < 50%, p > 0.05), justifying the use of a fixed-effects model. The analysis identified 3 weeks as the optimal treatment duration, with statistically significant differences (p < 0.05) observed, particularly for HS + cisplatin, which showed the highest efficacy (OR = 3.25, 95% CI [1.18, 8.96]). No significant differences in total treatment efficacy were observed among other subgroups (p > 0.05).
3.5.3 Time series analysis
The application of time series analysis (TSA) boundaries to favorable ORR outcomes revealed a required information size (IS) of 4,011 to achieve 80% statistical power. Furthermore, ORR analysis for cisplatin–paclitaxel combination therapy indicated that an IS of 6,429 was necessary to attain 80% power. However, the Z-curve crossed the futility boundary even though it failed to cross TSA boundaries (Supplementary Material S6). These pieces of evidence suggested some correlation between interventions and outcomes.
3.6 Secondary indicators
3.6.1 TCM syndrome
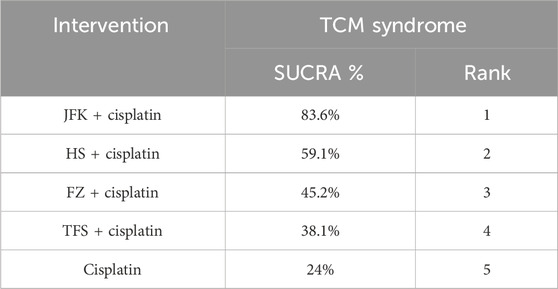
Ten articles documented TCM syndrome, with a total of 827 cases. The ranking results for TCM evidence frequency ratings were as follows: JFK + cisplatin (83.6%) > HS + cisplatin (59.1%) > FZ + cisplatin (45.2%) > TFS + cisplatin (38.1%) > cisplatin (24%) (Figure 4A; Table 3).
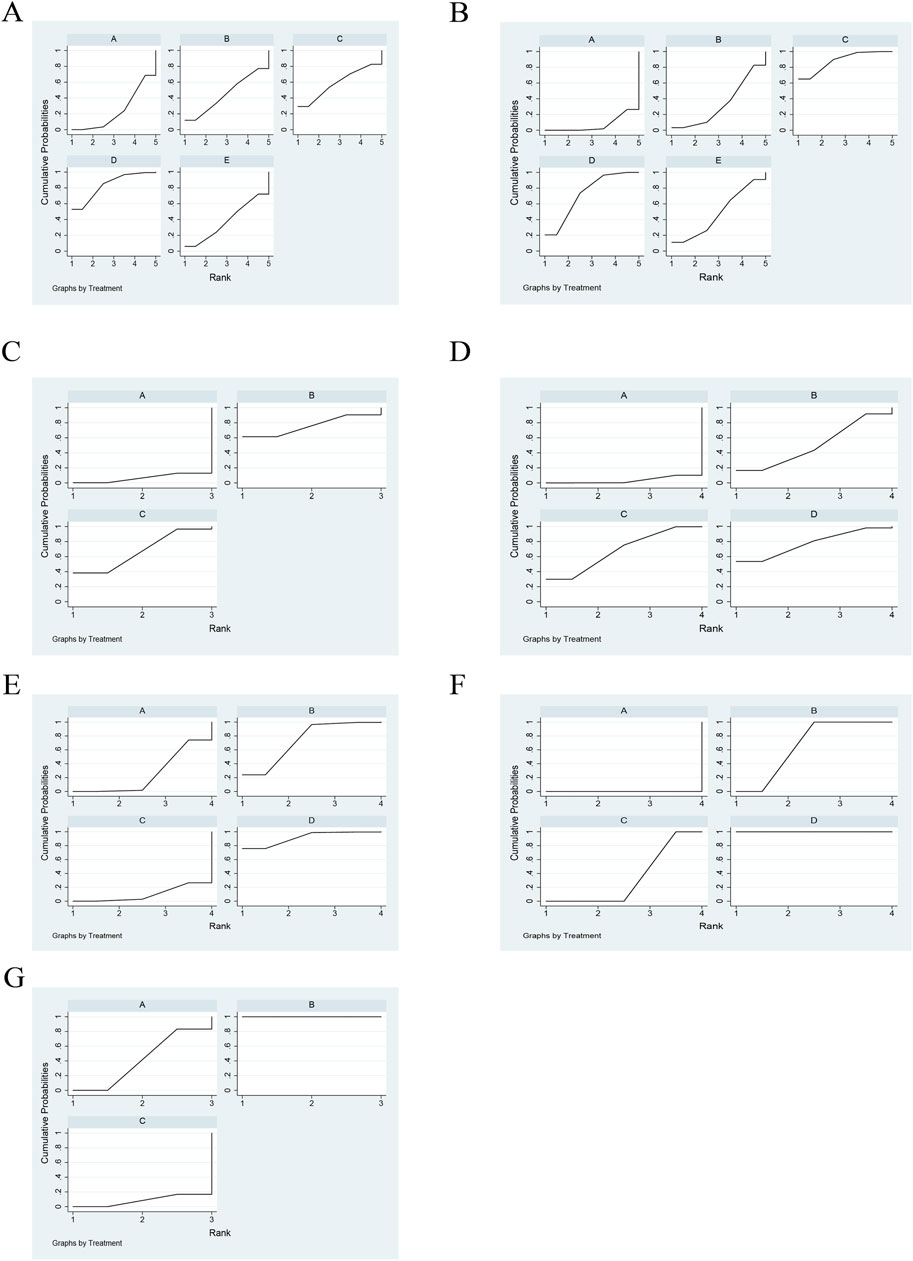
Figure 4. Rank of cumulative probabilities for outcomes. (A) TCM syndrome. (B) CD4–CD8. (C) NK cells. (D) CA125. (E) CEA. (F) CYFRA21-1. (G) Platelets.
3.6.2 Immune markers
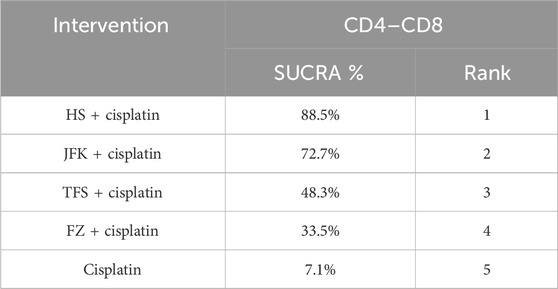
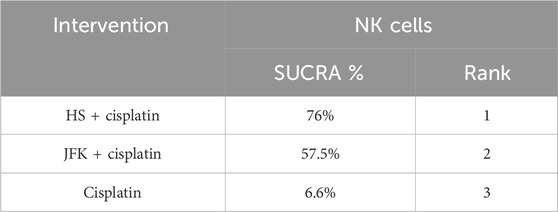
Immune markers were reported in 14 articles, 10 of which reported CD4–CD8 and four reported NK cells, for a total of 997 patients. The ranking results of CD4–-CD8 were HS + cisplatin (88.5%) > JFK + cisplatin (72.7%) > TFS + cisplatin (48.3%) > FZ + cisplatin (33.5%) > cisplatin (7.1%) (Figure 4B; Table 4). In addition, the ranking results of NK cells were HS + cisplatin (76%) > JFK + cisplatin (67.5%) > cisplatin (6.6%) (Figure 4C; Table 5).
3.6.3 Tumor markers
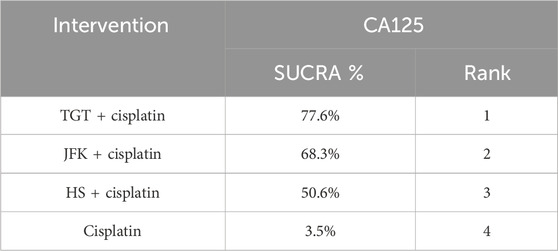
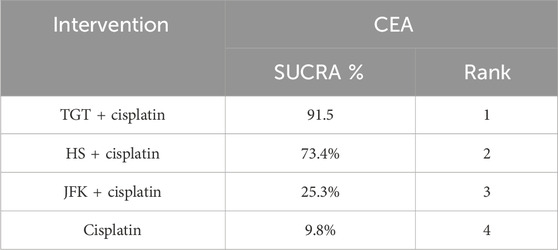
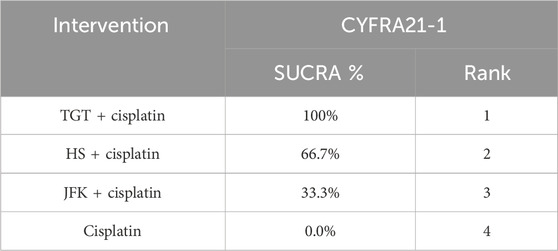
Nine articles reported tumor markers, five of which reported CA125, five of which reported CEA, and four of which reported CYFRA21-1, for a total of 804 patients. The ranking results of CA125 were cisplatin + TGT (77.6%) > cisplatin + JFK (68.3%) > cisplatin + HS (50.6%) > cisplatin (3.5%) (Figure 4D; Table 6). The ranking results of CEA were cisplatin + TGT (91.5%) > cisplatin + HS (73.4%) > cisplatin + JFK (25.3%) > cisplatin (9.8%) (Figure 4E; Table 7). Additionally, the results of CYFRA21-1 were ranked as cisplatin + TGT (100%) > cisplatin + HS (66.7%) > cisplatin + JFK (33.3%) > cisplatin (0%) (Figure 4F; Table 8).
3.6.4 Platelet
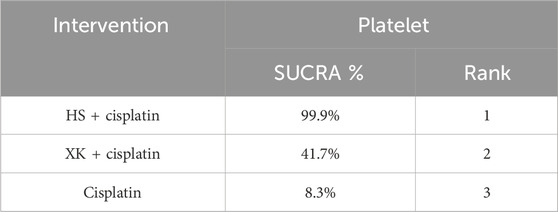
Seven articles reported platelets for a total of 415 patients. The ranking results of platelets were HS + cisplatin (100%) > XK + cisplatin (41.7%) > cisplatin (8.3%) (Figure 4G; Table 9).
3.7 Consistency test
Due to the network diagrams not forming closed loops, we conducted a consistency test on the relevant literature. The results reveal that the p-values for all indicators were below 0.05 (p < 0.05), indicating significant inconsistencies, with the exceptions of TGT in OOR and TP therapy; HS and JFK in CD4–CD8; JFK in TCM syndrome; JFK, TGT, and HS in tumor markers; and HS and XK in platelets (p > 0.05) (Supplementary Material S7).
3.8 Safety
Eleven publications reported adverse reactions to oral Chinese medicine + cisplatin, and seven publications reported adverse reactions to oral Chinese medicine + TP therapy, all of which were categorized into four classes. Among these, seven publications reported adverse reactions to HS, five to JFK, two to TFS, two to FZ, and one to TGT (Supplementary Material S8A,B).
3.8.1 Publication bias
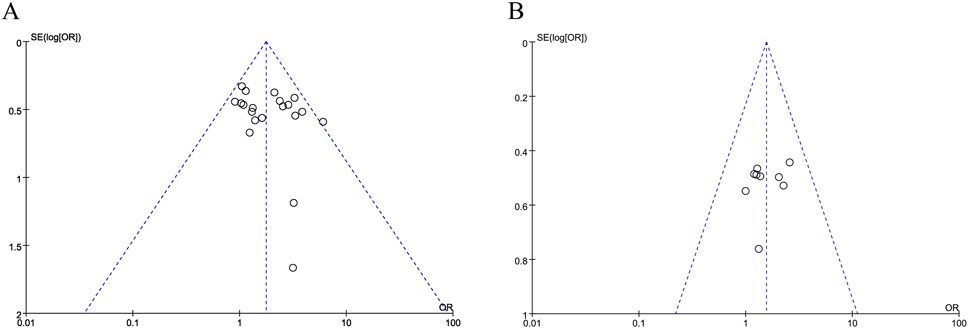
The funnel plot of the objective response rates is visibly lopsided, showing publication bias in these data (Figures 5A,B).
3.8.2 Sensitivity analysis
To address potential bias, sensitivity analyses excluded studies with a high risk of bias in randomization or outcome completeness. Subgroup analyses further stratified results by treatment duration, and fixed-effects models were applied to account for heterogeneity.
4 Discussion
This network meta-analysis constitutes the first systematic assessment of six oral TCMs combined with chemotherapy for NSCLC. Our findings indicate that adjunctive TCM therapy significantly improves the ORR, modulates immune and tumor biomarkers, reduces TCM syndrome scores, and mitigates chemotherapy-related adverse events. Among the evaluated TCMs, HS and TGT demonstrated the highest therapeutic efficacy. However, the mechanistic foundations, clinical relevance, and limitations of these findings require further investigation.
Regarding the ORR, immune markers (e.g., CD4+/CD8+ ratio and NK cell activity), and platelet regulation, HS and TGT exhibited the most pronounced therapeutic effects. Existing evidence highlights the significant role of HS and TGT components in NSCLC pathogenesis (Wang et al., 2014; Luo et al., 2024; Que et al., 2021). HS primarily contains emodin and eugenol (Xiang and Ay, 2014), both demonstrating mechanistic relevance: emodin suppresses KRAS-mutant NSCLC proliferation by downregulating sPLA2-IIa and NF-κB pathway expression, positioning it as a potential therapeutic target (Zhang et al., 2022), while eugenol exerts antitumor activity via TRIM59-mediated inhibition of NF-κB signaling (Cui et al., 2019). These mechanisms correlate with observed immunomodulatory improvements, suggesting that HS enhances chemotherapy through dual anti-proliferative and immune-enhancing pathways. TGT, enriched with vincristine and tenacissoside, significantly reduced tumor markers (CA125, CEA, and CYFRA21-1) (Wei et al., 2024; M, 2023). Vincristine impedes NSCLC progression by disrupting cell-cycle dynamics and inducing apoptosis (AbouEl et al., 2006; Ahn et al., 2013; Proia et al., 2012), whereas tenacissoside inhibits gefitinib metabolites (M523595, M608236, and M537194) within 24 h post-administration and suppresses hepatic CYP2D6/CYP3A4 activity within 6 h (Zhao et al., 2020). This dual-action mechanism—direct cytotoxicity and metabolic interference—likely explains TGT’s superior tumor marker suppression. To elucidate TCMs’ adjuvant role, future studies should delineate compound-specific mechanisms and validate findings in multinational cohorts.
Safety analyses revealed that TCM–chemotherapy combinations were better tolerated than chemotherapy alone. However, due to limited data, chemotherapy-associated toxicities remain a critical concern requiring vigilant monitoring (Huang et al., 2020).
Despite these insights, our study has notable limitations. First, geographical bias arises from the predominance of Chinese trials, which may limit generalizability to global populations. Second, the observed inconsistencies likely stem from clinical and methodological heterogeneity, particularly sparse direct comparisons and variability in chemotherapy protocols. Although sensitivity analyses support the primary conclusions, these limitations necessitate cautious interpretation. Third, the lack of pharmacokinetic data prevents definitive conclusions about compound-specific interactions. Fourth, the relatively small number of trials for certain interventions, particularly in subgroup analyses (e.g., TP therapy combinations), may limit the robustness of indirect comparisons in the network meta-analysis.
5 Conclusion
This study investigated the efficacy and safety of six oral Chinese medicines in conjunction with chemotherapy drugs. HS and TGT may be better oral Chinese medicines for the adjuvant therapy of NSCLC. The results need to be considered with caution due to the potential risk of bias and the small number of RCTs.
Author contributions
YM: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Writing – original draft, Writing – review and editing. JL: Methodology, Project administration, Resources, Software, Writing – review and editing. LL: Writing – review and editing, Formal analysis. TS: Validation, Visualization, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Correction note
A correction has been made to this article. Details can be found at: 10.3389/fphar.2025.1646824.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1579613/full#supplementary-material
References
AbouEl, H., Braam, S. R., and Kruyt, F. A. (2006). Paclitaxel and vincristine potentiate adenoviral oncolysis that is associated with cell cycle and apoptosis modulation, whereas they differentially affect the viral life cycle in non-small-cell lung cancer cells. Cancer gene Ther. 13 (12), 1105–1114. doi:10.1038/sj.cgt.7700984
Ahn, K. C., Choi, J. Y., Kim, J. S., Hwang, S. G., Kim, W. J., Park, J. K., et al. (2013). ICAM-3 endows anticancer drug resistance against microtubule-damaging agents via activation of the ICAM-3-AKT/ERK-CREB-2 pathway and blockage of apoptosis. Biochem. Biophys. Res. Commun. 441 (2), 507–513. doi:10.1016/j.bbrc.2013.10.096
Association, C. A. C. (2022). Chinese guidelines for the Diagnosis and treatment of Common malignant tumors.
Chen, W. (2011). The effect of Huisheng oral liquid on Fib and PLT levels in patients with advanced non-small cell lung cancer. Shandong Med. J. 51 (51), 104–105. doi:10.3969/j.issn.1002-266X.2011.51.064
Cronin, K. A., Scott, S., Firth, A. U., Sung, H., Henley, S. J., Sherman, R. L., et al. (2022). Annual report to the nation on the status of cancer, part 1: National cancer statistics. Cancer 128 (24), 4251–4284. doi:10.1002/cncr.34479
Cui, Z., Liu, Z., Zeng, J., Chen, L., Wu, Q., Mo, J., et al. (2019). Eugenol inhibits non-small cell lung cancer by repressing expression of NF-κB-regulated TRIM59. Phytotherapy Res. PTR 33 (5), 1562–1569. doi:10.1002/ptr.6352
de Jong, C., Herder, G. J. M., van Haarlem, S. W. A., van der Meer, F. S., van Lindert, A. S. R., Ten Heuvel, A., et al. (2023). Association between genetic variants and peripheral neuropathy in patients with NSCLC treated with first-line platinum-based therapy. Genes 14 (1), 170. doi:10.3390/genes14010170
Dias, S., Welton, N. J., Sutton, A. J., Caldwell, D. M., Lu, G., and Ades, A. E. (2013). Evidence synthesis for decision making 4: inconsistency in networks of evidence based on randomized controlled trials. Med. Decis. Mak. Int. J. Soc. Med. Decis. Mak. 33 (5), 641–656. doi:10.1177/0272989X12455847
Ding, N., Song, Y., and Zhang, F. (2011). Observation of the therapeutic effect of Huisheng oral liquid as adjuvant therapy for 35 cases of advanced NSCLC. Shandong Med. J. 51, 118–119. doi:10.3969/j.issn.1002-266X.2011.51.073
Ettinger, D. S., Wood, D. E., Aisner, D. L., Akerley, W., Bauman, J. R., Bharat, A., et al. (2021). NCCN guidelines insights: non-small cell lung cancer, version 2.2021. J. Natl. Compr. Cancer Netw. JNCCN 19 (3), 254–266. doi:10.6004/jnccn.2021.0013
Guisier, F., Piton, N., Bellefleur, M., Delberghe, N., Avenel, G., Angot, E., et al. (2020). Crizotinib-induced osteitis mimicking bone metastasis in a stage IV ALK-rearranged NSCLC patient: a case report. BMC Cancer 20 (1), 14. doi:10.1186/s12885-019-6486-3
Han, J. Y., Hong, E. K., Lee, S. Y., Yoon, S. M., Lee, D. H., and Lee, J. S. (2005). Thymidine phosphorylase expression in tumour cells and tumour response to capecitabine plus docetaxel chemotherapy in non-small cell lung cancer. J. Clin. Pathol. 58 (6), 650–654. doi:10.1136/jcp.2004.022764
Herbst, R. S., Morgensztern, D., and Boshoff, C. (2018). The biology and management of non-small cell lung cancer. Nature 553 (7689), 446–454. doi:10.1038/nature25183
Huai, Q., Luo, C., Song, P., Bie, F., Bai, G., Li, Y., et al. (2023). Peripheral blood inflammatory biomarkers dynamics reflect treatment response and predict prognosis in non-small cell lung cancer patients with neoadjuvant immunotherapy. Cancer Sci. 114 (12), 4484–4498. doi:10.1111/cas.15964
Huang, J., Wang, Z., Xue, H., Cao, A., Turner, C., Wang, J., et al. (2020). Huisheng oral solution adjunct with platinum-based chemotherapy for the treatment of advanced non-small cell lung cancer: a meta-analysis and systematic review. Front. Pharmacol. 11, 476165. doi:10.3389/fphar.2020.476165
Hutton, B., Salanti, G., Caldwell, D. M., Chaimani, A., Schmid, C. H., Cameron, C., et al. (2015). The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann. Intern. Med. 162 (11), 777–784. doi:10.7326/M14-2385
Jia, Y. (2013a). The effect of Huisheng oral liquid on serum interleukin-2 and tumor necrosis factor alpha in patients with advanced non-small cell lung cancer. Chin. J. Clin. Ration. Drug Use 6 (36), 148–149. doi:10.15887/j.cnki.13-1389/r.2013.36.138
Jia, Y. (2013b). Effects of hui-sheng oral liquid on the expression of interleukin-6 and interleukin-18 of patients with advanced non-small-cell lung cancer. Anti-tumor Pharm. 3 (06), 447–450. doi:10.3969/j.issn.2095-1264.2013.109
Jia, Y. (2014). Analysis of the effect of hui sheng oral liquid combined with cisplatin chemotherapy on the immune function of lung cancer patients. Chin. J. Front. Med. Sci. 06 (11), 54–56. doi:10.3969/j.issn.1674-7372.2014.11.022
Kong, F., Wang, C., Zhao, L., Liao, D., Wang, X., Sun, B., et al. (2023). Traditional Chinese medicines for non-small cell lung cancer: therapies and mechanisms. Chin. Herb. Med. 15 (4), 509–515. doi:10.1016/j.chmed.2023.05.004
Li, Y. (2016). Observation of the clinical efficacy of Xuekang oral liquid in preventing chemotherapy-induced thrombocytopenia in NSCLC patients. J. Clin. Psychosomatic Dis. z1 (22), 100–101. doi:10.3969/j.issn.1672-187X.2016.z1.130
Li, Y. (2017). Observation of the therapeutic effect of Xuekang oral liquid on preventing thrombocytopenia caused by chemotherapy in malignant tumors: Liaoning university of traditional Chinese medicine.
Li, Z. (2019). Clinical study on the combination of Jinfukang oral solution and pemetrexed in the treatment of non-small cell lung cancer. Drugs Clin. 34 (06), 1827–1830. doi:10.7501/j.issn.1674-5515.2019.06.049
Lin, J. (2003). Clinical study on the treatment of non small cell lung cancer with jinfukang oral liquid. Jiangxi J. Traditional Chin. Med. 02, 19–21. doi:10.3969/j.issn.0411-9584.2003.02.010
Liu, B. (2023). Clinical study on the combination of Tongguan Teng oral solution and chemotherapy for the treatment of non-small cell lung cancer. Liaoning J. Traditional Chin. Med. 50 (03), 82–84. doi:10.13192/j.issn.1000-1719.2023.03.024
Liu, C. (2017). Observation of the short term efficacy of tianfoshen oral liquid combined with GP scheme in the treatment of advanced non small cell lung cancer in elderly patients. Oncol. Prog. 15 (03), 294–296. doi:10.11877/j.issn.1672-1535.2017.15.03.21
Liu, Y., Fan, L., Qu, F., and Zhu, Q. (2011a). Observation of the therapeutic effect of Xuekang oral liquid in preventing and treating thrombocytopenia caused by GP regimen chemotherapy in non-small cell lung cancer. Chin. J. Misdiagnostics 11 (09), 2096. doi:10.3969/j.issn.1002-266X.2011.51.065
Liu, Y., Hong, H., and Liu, X. (2011b). Observation of the recent efficacy of Huisheng oral liquid combined with TP protocol in the treatment of 32 cases of advanced non small cell lung cancer. Shandong Med. J. 106.
Lu, E. (2022). Clinical study on the combination of Jinfukang oral liquid and adjuvant chemotherapy for early postoperative intervention of non-small cell lung cancer. J. Beijing Univ. Traditional Chin. Med. 45 (11), 1103–1109. doi:10.3969/j.issn.1006-2157.2022.11.004
Luo, B., Wang, P., Tian, J., Chu, X., Lu, X., Yang, Y., et al. (2024). Jinfukang inhibits lung cancer metastasis by regulating T cell receptors. J. Ethnopharmacol. 318 (Pt A), 116885. doi:10.1016/j.jep.2023.116885
Luo, D., Dai, X., Tian, H., Fan, C., Xie, H., Chen, N., et al. (2023). Sophflarine A, a novel matrine-derived alkaloid from Sophora flavescens with therapeutic potential for non-small cell lung cancer through ROS-mediated pyroptosis and autophagy. Phytomedicine Int. J. phytotherapy Phytopharm. 116, 154909. doi:10.1016/j.phymed.2023.154909
Mills, E. J., Thorlund, K., and Ioannidis, J. P. (2013). Demystifying trial networks and network meta-analysis. BMJ Clin. Res. 346, f2914. doi:10.1136/bmj.f2914
Mu, X. (2019). Jinfukang oral liquid combined with softening decoction and chemotherapy for the treatment of stage IIIb-IV non small cell lung cancer of qi yin deficiency type. Chin. J. Clin. Res. 32 (09), 1271–1274. doi:10.13429/j.cnki.cjcr.2019.09.030
M, W. (2023). Molecular mechanism of Tongguanteng Oral Liquid and its active ingredient kaempferol inhibiting gastric cancer cell proliferation via PI3K/AKT pathway.
Proia, D. A., Sang, J., He, S., Smith, D. L., Sequeira, M., Zhang, C., et al. (2012). Synergistic activity of the Hsp90 inhibitor ganetespib with taxanes in non-small cell lung cancer models. Investig. New Drugs 30 (6), 2201–2209. doi:10.1007/s10637-011-9790-6
Pu, X. (2016). Analysis of the therapeutic effect of Tianfoshan oral solution combined with pemetrexed on elderly patients with advanced lung adenocarcinoma. Pract. Geriatr. 30 (12), 987–989. doi:10.3969/j.issn.1003-9198.2016.12.006
Qin, P., Li, Q., Zu, Q., Dong, R., and Qi, Y. (2024). Natural products targeting autophagy and apoptosis in NSCLC: a novel therapeutic strategy. Front. Oncol. 14, 1379698. doi:10.3389/fonc.2024.1379698
Qin, Z., Jia, M., Yang, J., Xing, H., Yin, Z., Yao, Z., et al. (2020). Multiple circulating alkaloids and saponins from intravenous Kang-Ai injection inhibit human cytochrome P450 and UDP-glucuronosyltransferase isozymes: potential drug-drug interactions. Chin. Med. 15, 69. doi:10.1186/s13020-020-00349-3
Que, Z. J., Yao, J. L., Zhou, Z. Y., Yu, P., Luo, B., Li, H. G., et al. (2021). Jinfukang inhibits lung cancer metastasis by upregulating CX3CL1 to recruit NK cells to kill CTCs. J. Ethnopharmacol. 275, 114175. doi:10.1016/j.jep.2021.114175
Ranbai, H. (2017). Clinical study on the combination of Yiqi Yangyin Ruanjian Jiedu Tang and chemotherapy in the treatment of advanced non-small cell lung cancer. Shanghai: Shanghai University of Traditional Chinese Medicine.
Rücker, G., and Schwarzer, G. (2015). Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med. Res. Methodol. 15, 58. doi:10.1186/s12874-015-0060-8
Song, L. (2023). Liaoning journal of traditional Chinese medicine. Liaoning J. Traditional Chin. Med. 50 (06), 138–140. doi:10.13192/j.issn.1000-1719.2023.06.037
Song, L., Zhang, C., Xiao, Y., and Zhou, X. (2023). Clinical observation of Tongguanteng oral solution for non-small cell lung cancer. Liaoning J. Traditional Chin. Med. 50 (06), 138–140.
Song, M. (2015). The efficacy of Xuekang oral liquid in preventing and treating thrombocytopenia caused by GP regimen chemotherapy in non-small cell lung cancer: Liaoning university of traditional Chinese medicine.
Sterne, J. A. C., Savović, J., Page, M. J., Elbers, R. G., Blencowe, N. S., Boutron, I., et al. (2019). RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ Clin. Res. 366, l4898. doi:10.1136/bmj.l4898
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 71 (3), 209–249. doi:10.3322/caac.21660
Tang, M. (2019). Clinical study on the treatment of bone metastasis in non-small cell lung cancer with spleen and kidney deficiency syndrome using Fuzheng oral liquid. Changsha: Hunan University of Chinese Medicine.
Trinquart, L., Attiche, N., Bafeta, A., Porcher, R., and Ravaud, P. (2016). Uncertainty in treatment rankings: reanalysis of network meta-analyses of randomized trials. Ann. Intern. Med. 164 (10), 666–673. doi:10.7326/M15-2521
Wang, J., Han, X., Jiang, M., and Guo, H. (2022). Overview of clinical research evidence of oral traditional Chinese patent medicines and simple preparations in the treatment of lung cancer. Chin. J. Exp. Traditional Med. Formulae 28 (08), 204–213.
Wang, W., Wang, H., Wang, C. M., Gou, S., Chen, Z. H., and Guo, J. (2014). Treatment with Huisheng oral solution inhibits the development of pulmonary thromboembolism and metastasis in mice with Lewis lung carcinoma. Oncol. Lett. 7 (1), 87–94. doi:10.3892/ol.2013.1661
Wang, W., Ye, L., Pan, Y., Li, Y., Li, S. X., Yang, B., et al. (2024). Effect of simplified formula of Jinfukang Oral Liquid on apoptosis of renal tubular epithelial cells induced by cisplatin. China J. Chin. Materia Medica 49 (19), 5315–5326. doi:10.19540/j.cnki.cjcmm.20240712.701
Wei, L., Meng, J., Xiang, D., Yang, Q., Zhou, Y., Xu, L., et al. (2024). Network pharmacology and experimental validation to study the potential mechanism of Tongguanteng injection in regulating apoptosis in osteosarcoma. BMC Complementary Med. Ther. 24 (1), 67. doi:10.1186/s12906-024-04354-z
Wei, Y. (2014). The effect of Huisheng oral liquid on cellular immune function in elderly patients with advanced non-small cell lung cancer after chemotherapy. Med. Inf. 5, 64–65. doi:10.3969/j.issn.1006-1959.2014.05.075
Wu, M. (2017). The effect of Huisheng oral liquid combined with first-line chemotherapy regimen on serum VEGF, IL-6, and MMP-9 levels in patients with advanced non-small cell lung cancer. J. Clin. Pathological Res. 37 (12), 2605–2610. doi:10.3978/j.issn.2095-6959.2017.12.016
Xiang, W. J., and Ay, C. (2014). Identification of main components in Huisheng oral liquid. Shandong Chem. Ind. Shandong Chem. Ind. 12 (43), 70–74. doi:10.3969/j.issn.1008-021X.2014.12.024
Xiao, L. (2021). The effect of Jinfukang oral solution combined with pemetrexed on immune function, tumor markers, and serum VEGF and MMP-9 levels in patients with non-small cell lung cancer. Prog. Mod. Biomed. 21 (13), 2503–2507. doi:10.13241/j.cnki.pmb.2021.13.021
Xie, Y. (2023). The efficacy and coagulation function of Huisheng oral liquid combined with gefitinib in the treatment of advanced EGFR mutation positive non-small cell lung cancer. J. China Prescr. Drug 21 (03), 142–145. doi:10.3969/j.issn.1671-945X.2023.03.042
Xiu, W., Zhang, Y., Tang, D., Lee, S. H., Zeng, R., Ye, T., et al. (2024). Inhibition of EREG/ErbB/ERK by Astragaloside IV reversed taxol-resistance of non-small cell lung cancer through attenuation of stemness via TGFβ and Hedgehog signal pathway. Cell. Oncol. Dordr. Neth. 47 (6), 2201–2215. doi:10.1007/s13402-024-00999-7
Xu, X., Su, J., and Huang, Q. (2011). Evaluation of the efficacy of Huisheng oral liquid combined with chemotherapy in first-line treatment of advanced non-small cell lung cancer. Shandong Med. J. 51 (04), 80–81. doi:10.3969/j.issn.1002-266X.2011.04.039
Yang, L. (2018a). Observation of the therapeutic effect of TP regimen combined with Hui Sheng oral liquid in the treatment of advanced non-small cell lung cancer. J. Med. Inf. 31 (17), 17–19. doi:10.3969/j.issn.1006-1959.2018.17.005
Yang, W. (2018b). Clinical study on the combination of Fuzheng oral liquid and chemotherapy in the treatment of stage IIIB-IV non-small cell lung cancer with qi and blood deficiency. Hunan University of Chinese Medicine. Changsha:
Yang, X. (2018c). Observation of the therapeutic effect of Huisheng oral liquid combined with synchronous radiotherapy and chemotherapy in the treatment of locally advanced NSCLC. Hefei: Anhui Medical University.
Yu, H. (2016). A randomized parallel controlled study on the combination of Tianfoshan oral solution and chemotherapy for the treatment of non-small cell lung cancer with qi and yin deficiency. J. Pract. Traditional Chin. Intern. Med. 30 (06), 59–61. doi:10.13729/j.issn.1671-7813.2016.06.25
Yu, Y. (2011). Observation of the efficacy of Huisheng oral liquid in patients with advanced non-small cell lung cancer. Shandong Med. J. 51 (51), 101–102. doi:10.3969/j.issn.1002-266X.2011.51.062
Zhai, J. (2019). Clinical study on the treatment of advanced non-small cell lung cancer with Huisheng oral liquid combined with recombinant human endostatin and oxaliplatin. Drugs Clin. 34 (05), 1515–1519. doi:10.7501/j.issn.1674-5515.2019.05.054
Zhang, F. Y., Li, R. Z., Xu, C., Fan, X. X., Li, J. X., Meng, W. Y., et al. (2022). Emodin induces apoptosis and suppresses non-small-cell lung cancer growth via downregulation of sPLA2-IIa. Phytomedicine Int. J. Phytotherapy Phytopharm. 95, 153786. doi:10.1016/j.phymed.2021.153786
Zhang, J. (2019). The therapeutic effect of Jinfukang oral liquid on non-small cell lung cancer patients with qi and yin deficiency and its impact on serum MMP-9 levels. Inf. Traditional Chin. Med. 39 (02), 65–68. doi:10.19656/j.cnki.1002-2406.190048
Zhang, R. (2021). Study on the efficacy of Jinfukang oral solution combined with adjuvant chemotherapy in postoperative patients with stage IB-IIB non-small cell lung cancer. Shanghai: Shanghai University of Traditional Chinese Medicine.
Zhang, S. (2017). Observation of the therapeutic effect of Tianfoshen on advanced non-small cell lung cancer of Qi Yin deficiency type. J. Mod. Oncol. 25 (16), 2592–2594. doi:10.3969/j.issn.1672-4992.2017.16.016
Zhang, W. (2013). Clinical efficacy of Huisheng oral liquid as adjuvant therapy for advanced non-small cell lung cancer. World Latest Med. Inf. 14, 207–208. doi:10.3969/j.issn.1671-3141.2013.14.158
Zhao, C., Hao, H., Zhao, H., Ren, W., Jiao, Y., An, G., et al. (2020). Marsdenia tenacissima extract promotes gefitinib accumulation in tumor tissues of lung cancer xenograft mice via inhibiting ABCG2 activity. J. Ethnopharmacol. 255, 112770. doi:10.1016/j.jep.2020.112770
Zhao, L., Wang, J., Li, H., Che, J., Ma, N., and Cao, B. (2019a). Safety and efficacy of tianfoshen oral liquid in non-small cell lung cancer patients as an adjuvant therapy. Evidence-Based Complementary Altern. Med. eCAM. 2019, 1375439. doi:10.1155/2019/1375439
Zhao, L., Wang, J., Li, H., Che, J., Ma, N., Cao, B. A.-O., et al. (2019b). Safety and efficacy of tianfoshen oral liquid in non-small cell lung cancer patients as an adjuvant therapy establishment of a nomogram-based prognostic model (LASSO-COX regression) for predicting progression-free survival of primary non-small cell lung cancer patients treated with adjuvant Chinese herbal medicines therapy: a retrospective study of case series Tianfoshen oral liquid: a CFDA approved clinical traditional Chinese medicine, normalizes major cellular pathways disordered during colorectal carcinogenesis.
Zhao, W. (2023a). Efficacy and safety of Huisheng oral liquid combined with chemotherapy and immunotherapy on advanced non-small cell lung cancer by regulating the PD-1/PD-L1 signaling pathway. Hebei Med. J. 45 (23), 3535–3544. doi:10.3969/j.issn.1002-7386.2023.23.003
Keywords: non-small-cell lung cancer, traditional Chinese medicine, network meta-analysis, oral Chinese medicine, safety
Citation: Ma Y, Li J, Liu L and Shen T (2025) Efficacy and safety of oral Chinese medicine combined with chemotherapy: a systematic review and network meta-analysis. Front. Pharmacol. 16:1579613. doi: 10.3389/fphar.2025.1579613
Received: 19 February 2025; Accepted: 15 May 2025;
Published: 12 June 2025; Corrected: 29 August 2025.
Edited by:
Johnson O. Oladele, Royal Scientific Research Institute, NigeriaReviewed by:
Yuxiang Fei, China Pharmaceutical University, ChinaOluwaseyi Okoro, Ministry of Health and Wellness, Jamaica
Copyright © 2025 Ma, Li, Liu and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liyun Liu, bWF5dXFpMTk5OEBwcm90b24ubWU=; Tao Shen, bWF5dXFpMTk5ODA5MjZAb3V0bG9vay5jb20=
†These authors have contributed equally to this work
 Yuqi Ma
Yuqi Ma Jia Li
Jia Li