- 1Department of Military Psychology, School of Psychology, Third Military Medical University,Chongqing, China
- 2Xiamen Cancer Hospital, The First Affiliated Hospital of Xiamen University, Xiamen, China
- 3Department of Fundamental, Army Logistical University of PLA, Chongqing, China
- 4Breast Center of Southwest Hospital, Third Military Medical University, Chongqing, China
- 5Psychiatry Department, No.991 Hospital of Chinese Liberation Army, Xiangyang, China
Objective: Currently, fear of cancer recurrence (FCR) is emerging as an important issue for long-term breast cancer survivors and is associated with lower quality of life and functional impairment. Given that there is a dearth of research regarding the FCR of Chinese breast cancer survivors, this study investigated whether the short form of the Fear of Cancer Recurrence Inventory (FCRI) could detect high FCR and explored the level and characteristics of FCR in breast cancer survivors.
Methods: Two hundred forty patients who had undergone successful breast cancer surgery in China submitted their survey through a website. The participants’ demographic and medical data, level of FCR, anxiety, depression, and quality of life were assessed.
Results: Two hundred seven patients with ages ranging from 19 to 60 years completed the questionnaires. The mean FCR score of the total sample was 18.39. A cutoff score of 12 or higher on the short form of the FCRI was optimal for the detection of high FCR with a sensitivity of 98.6% and a specificity of 35%, and the PPV (positive predictive values) and NPV (negative predictive values) were 44% and 98%, respectively. The area under the curve of the receiver operating characteristics (ROC) analysis was 83%. A total of 159 breast cancer survivors (76.81%) experienced high FCR levels (FCR score > 12), characterized by lower functional and overall health than survivors with a low FCR (P < 0.01).
Conclusions: The short form of the FCRI is capable of detecting high FCR and is therefore able to assist Chinese breast cancer survivors in receiving appropriate care for reducing FCR.
Background
Breast cancer is the most common cancer among women worldwide (1). In China, breast cancer has been the leading cause of cancer incidence in women (2). According to the GLOBOCAN statistics, the incidence of breast cancer is up to 21.6/10,000 in Chinese females (3). Rapid advancements in cancer treatments, early cancer diagnosis, and better treatment strategies have led to continuous increases in survival rates (4), but breast cancer and its treatment are often accompanied by physical and psychological impairment and reduction in quality of life (5). With more attention paid to the life-after-treatment phase, many breast cancer survivors experience multiple physical and psychosocial long-term and delayed effects of treatment (e.g., fatigue, pain, and anxiety) (6–8).
Emerging evidence has demonstrated that fear of cancer recurrence (FCR) is a problematic long-term and delayed effect experienced by cancer survivors. Cancer survivors report an unmet need for help dealing with FCR. FCR is defined as worry or anxiety that cancer could recur or develop in the same place or spread to another part of the body (9). Previous researchers report prevalence rates of FCR ranging from 37% to 99% among breast cancer survivors (10–12). Moderate FCR is expected and adaptive (e.g., holding an appropriate vigilance for signs of recurrence, maintaining medical follow-up, and engaging in healthy lifestyle). However, excessive FCR may adversely impact survivors’ emotions and social activities (12, 13). It was found that cancer survivors with high FCR often experience more self-focus and excessive psychological distress (14). In addition, from a public health standpoint, FCR is related to increased health-care utilization and costs (15, 16). In a recently published study, FCR was the most distressful and commonly reported problem by patients with breast cancer (17, 18).
Currently, researchers have developed many tools to assess cancer survivors’ FCR, such as the Fear of Recurrence Questionnaire (FRQ) (19), the Fear of Progression Questionnaire (FoP-Q) (20), and the Concerns about Recurrence Scale (CARS) (21). Among these scales, the Fear of Cancer Recurrence Inventory (FCRI) has been verified to possess good specificity and sensitivity and is best suited for diverse cancer populations (22). This 42-item inventory is a multidimensional measure that evaluates seven components of FCR: triggers (nine items), severity (nine items), psychological distress (four items), coping strategies (nine items), functional impairments (six items), insight (three items), and reassurance (three items) (23). The scale was initially validated in 600 cancer patients with different tumor sites, and it was found that all subscales had good internal consistency and reliability, and the 1-month retest reliability ranged from medium to high (24). In China, the Chinese version of the FCRI has been assessed in a sample of 240 cancer survivors; the scale possessed good reliability and validity and could be used to measure fear of cancer recurrence (25). Subsequently, researchers found in clinical application that the severity factor score of the scale was highly correlated with the total score. Therefore, the severity factor of the FCRI (nine items in total) was recommended as a short scale for rapid screening of cancer recurrence fear (25). However, the cutoff values were also different in different populations. A study of 60 French-Canadian cancer survivors showed that the nine-item short scale of fear of cancer recurrence showed good psychometric characteristics, and the study considered that the cutoff had high sensitivity and specificity when divided into 13 points (18). More specifically, a receiver operating characteristic analysis showed that this cutoff score was associated with a sensitivity of 88% and a specificity of 75%. Additionally, Fardell and his colleagues examined 240 Australian cancer survivors using the short scale of fear of cancer recurrence and found that 22 was the optimal cutoff score for screening high and low cancer recurrence fear (26). However, the capacity of the short scale to screen patients for FCR and identify the presence of clinically significant levels of FCR among a large sample of Chinese breast cancer survivors has not yet been demonstrated.
There is a lack of a gold standard measure for FCR and no unified definition of what constitutes a clinical level of FCR. Previous studies showed that a higher FCR was generally associated with increased anxiety in cancer survivors. It is possible to use the subscale of the Hospital Depression and Anxiety Scale as the gold standard measure of the short form of the FCRI for the evaluation of FCR. Determining the cutoff point using the short form of the FCRI is beneficial for screening high-risk patients, and these patients could receive interventions to lower their fear of cancer recurrence.
Previous studies have revealed that FCR has detrimental impacts on cancer survivors’ quality of life. Weert and colleagues found that approximately 30% of survivors reported decreased quality of life due to physical concerns after their diagnosis and treatment (27). There is evidence that higher levels of FCR are associated with poorer quality of life among all types of cancer survivors, such as breast, colorectal, lung, pancreatic and periampullary, urogynecologic, and testicular cancer survivors (28). However, until recently, few studies have explored the characteristics related to Chinese breast cancer survivors’ FCR and the differences in quality of life between patients with low FCR and high FCR based on the cutoff point.
Therefore, the aims of this study were a) to assess the capacity of the short form of the Fear of Cancer Recurrence Inventory to screen the clinical levels of FCR in Chinese breast cancer survivors and b) to compare the quality of life between breast cancer survivors with high FCR and low FCR.
Methods
Participants
This study utilized a cross-sectional observational research design. Patients were recruited from a website over a recruitment period of 3 months (from May to July 2017). The inclusion criteria were as follows: a) confirmed first diagnosis of stage 0–III breast cancer; b) age greater than or equal to 18 years; c) no recurrence or metastases; d) completed treatment (i.e., lumpectomy, mastectomy, and radiation but not hormone therapy); and e) able to read and understand Chinese. The exclusion criteria were diagnoses of stage IV cancer, severe cognitive impairments, and psychiatric disorders. A total of 240 breast cancer survivors submitted the survey. Among those, 33 participants were excluded because 21 were currently receiving treatment and 12 experienced cancer recurrence. Finally, 207 participants were eligible for this study.
Procedure
Ethical approval was obtained from the Ethics Committee of Third Military Medical University of China prior to the start of the study (ref. no. ChiCTR-OOC-17012132). The survey was uploaded to https://www.wjx.cn/jq/13794312.aspx, a website that allows surveys to be taken confidentially online. The researchers posted this survey to a patient network group. These participants logged into the website and read the purpose of the study, and then they needed to complete a booklet containing questionnaires about both demographic and medical information. By returning the booklet, the participants gave their written informed consent to take part in the study.
Measures
Demographic and medical information was assessed with questions regarding age, marital status, education level, employment, family yearly income, years since diagnosis, cancer stage, and cancer treatments received. With the use of Brislin’s two-way translation model, the translation of all the English versions of the scales was carried out independently by two psychology professionals (29). Then, a psychology expert modified the two translated versions and reached a consensus with the two translators.
Fear of Recurrence
The nine-item Fear of Cancer Recurrence Inventory is a short form of the FCRI (nine-item FCRI) and corresponds to the severity subscale of the FCRI (42 items). The nine-item FCRI evaluates the presence and severity of intrusive thoughts associated with FCR. Each item is rated on a Likert scale ranging from 0 (“not at all” or “never”) to 4 (“a great deal’ or “all the time”). A higher score indicates higher levels of FCR. In this study, the reliability coefficient of the Chinese version of the short form of the FCRI was 0.912 (30).
Depression and Anxiety
The Hospital Anxiety and Depression Scale (HADS) includes 14 items divided into two subscales (depression and anxiety), each with seven items. Scores obtained for each subscale range from 0 to 21. The more anxiety, depression, and psychological distress the patients suffer from, the higher the scores are. The HADS includes no somatic items that could be confused by signs related to physical illness. In this study, we utilized the scores of the anxiety subscale as the gold standard to differentiate high and low fear of cancer recurrence. A total score of 8 or higher indicated high distress (31). The reliability coefficient of the anxiety subscale was 0.732, and that of the depression subscale was 0.734.
Quality of Life
The European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire C30 (EORTC-QLQ-C30) is widely used to assess the quality of life of cancer patients. The scale comprises 30 items covering five function subscales (physical, role, emotional, cognitive, and social), nine common cancer symptoms (fatigue, nausea/vomiting, pain, dyspnea, insomnia, appetite loss, constipation, diarrhea, and financial difficulties), and an overall health status (32). Respondents rate the frequency of occurrence of the former 28 items from 1 (not at all) to 4 (often) and the 29th and 30th items from 1 (worst) to 7 (best). The higher the scores in the functional domain and overall quality of life, the better the health status and quality of life. The higher the score in the symptom domain, the worse the quality of life. Symptoms were not examined in this study. Cronbach’s alpha coefficient of the scale is 0.962.
Data Analyses
Before the data were analyzed using SPSS (version 18.0), the normality of the relevant data was verified, and the distribution was found to be normal. Patients were categorized into two groups according to their anxiety scores: patients with clinical levels of anxiety (i.e., anxiety score ≥ 8) and patients with nonclinical levels of anxiety (i.e., anxiety score < 8). Receiver operating characteristics (ROC) analysis was conducted to evaluate the performance of the short form of the FCRI. The accuracy properties of sensitivity, specificity, and positive and negative predictive values were assessed at each cutoff point of the short form of the FCRI. Furthermore, the area under the ROC curve and its 95% confidence interval were examined. To differentiate high FCR from low FCR, an optimal cutoff point should have high sensitivity and specificity, which maximizes the proportion of patients whose test results are accurate. Independent t tests were performed for anxiety and depression to assess differences between individuals with high and low FCR based on the cutoff score.
Results
Participant Characteristics
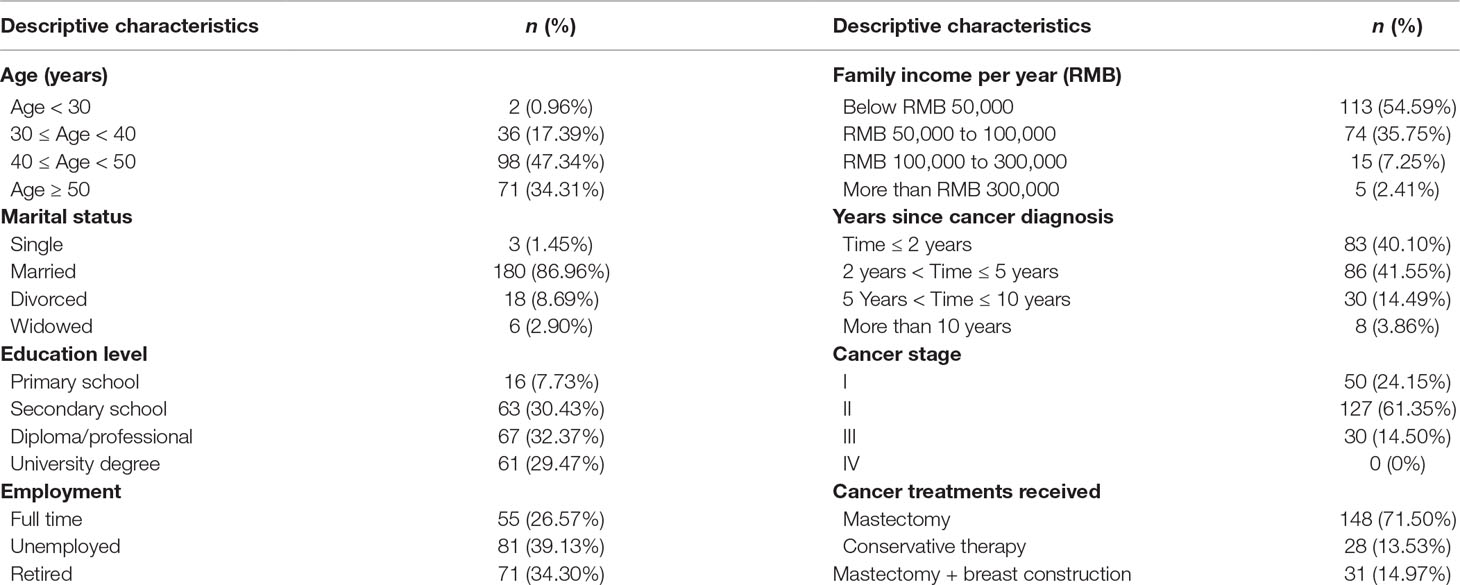
The age of the participants ranged from 19 to 60 years. Approximately 47.34% of the participants belonged to the 40–50-year age group. The majority of the participants (86.96%) were married. In terms of education level, 29.47% of the subjects had a tertiary education, while 7.73% had a primary education. Most participants were unemployed (39.13%), and 54.59% of them earned less than 50,000 RMB ($7,908) as their family yearly income. In terms of the clinical characteristics of the participants, 81.65% of the participants reported that it was less than 5 years since their breast cancer diagnosis. The sociodemographic characteristics and clinical features of the participants are presented in Table 1.
ROC Analysis
The area under the curve of the ROC analysis was 83% (P < 0.001; 95% CI = 0.773–0.887), suggesting a good level of diagnostic accuracy (see Figure 1). Table 2 presents the sensitivity and specificity rates obtained for a sample of scores from the short form of the FCRI. According to our goal to validate a rapid screening tool of FCR, more emphasis was placed on the capacity of the instrument to detect the largest possible number of survivors with clinical levels of FCR (i.e., sensitivity) and to maximize the proportion of negative test results corresponding to nonclinical FCR survivors (i.e., NPV) (30). Based on these criteria, a score of 12 or higher appeared to constitute the optimal clinical cutoff score to differentiate between high FCR and low FCR, with a sensitivity of 98.6% and a specificity of 35%. The PPV (positive predictive values) and NPV (negative predictive values) were 44% and 98%, respectively.
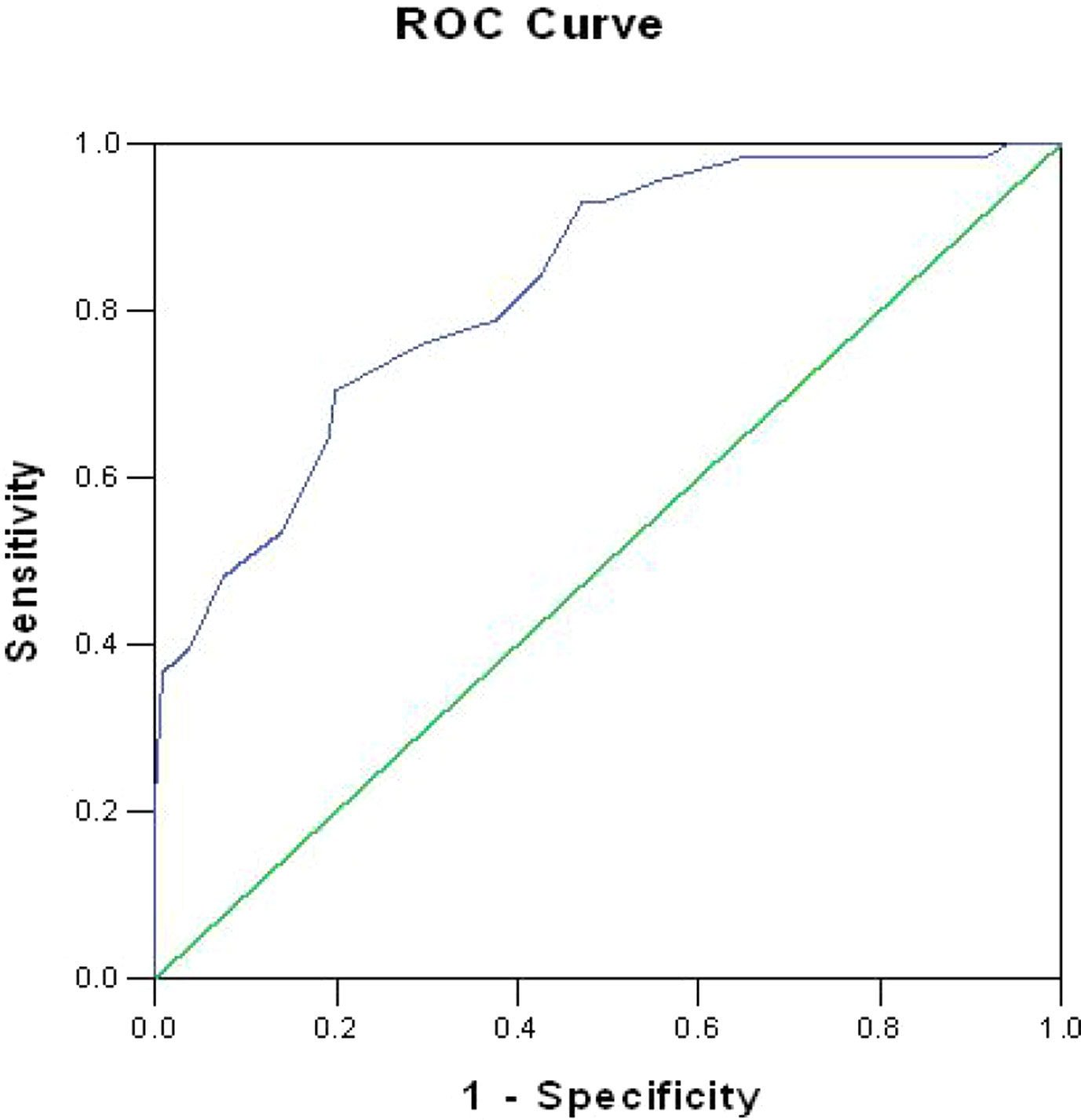
Figure 1 Receiver operating characteristics (ROC) curve for the short form of the Fear of Cancer Recurrence Inventory.
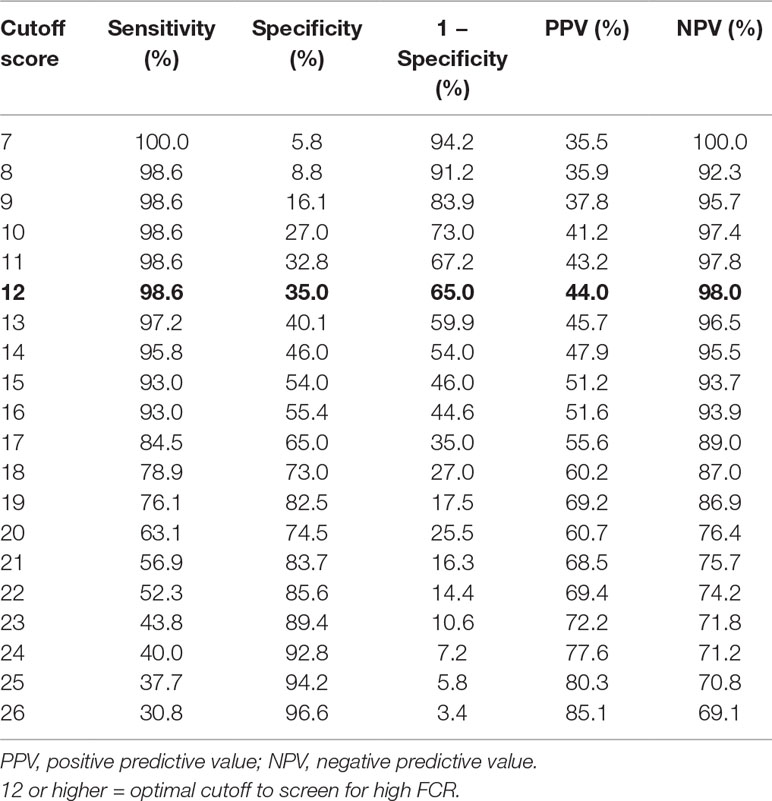
Table 2 Performance of the short form of the Fear of Cancer Recurrence Inventory (FCRI) as a screening tool to detect clinical FCR (n = 207).
Comparisons of Quality of Life Between Breast Cancer Survivors With High FCR and Low FCR
The mean score of FCR was 18.39 (7.14). Among the 207 breast cancer survivors, 159 (76.81%) were considered to display high FCR levels (FCR score > 12), while 48 patients (23.19%) showed low fear of cancer recurrence. Table 3 demonstrates that breast cancer survivors with high FCR have significantly lower functional and overall health than have survivors with low fear (P < 0.01).
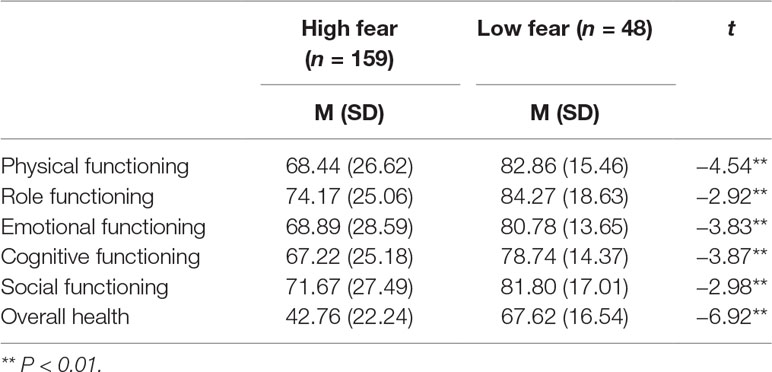
Table 3 Differences in quality of life between survivors with high fear of cancer recurrence (FCR) and low FCR.
Discussion
The aims of our study were to assess the capacity of the short form of the FCRI to screen for clinical levels of FCR among Chinese breast cancer survivors and to compare the quality of life between breast cancer survivors with high FCR and those with low FCR. Our results demonstrated that a cutoff score of 12 or higher on the short form of the FCRI had optimal sensitivity and specificity values for screening clinical FCR. Additionally, our results revealed that breast cancer survivors with high FCR were significantly more likely to have lower quality of life than cancer survivors with low FCR.
Studies have explored the cutoff value of cancer survivors’ clinical levels of FCR using an FCR questionnaire in many countries. Simard and Savard found that the short form of FCRI was a rapid and effective tool to screen clinical levels of FCR, and a cutoff score of 13 or higher on the FCRI-SF was found in 60 French-Canadian mixed cancer survivors (21). Custers and his colleagues explored whether the Cancer Worry Scale (CWS) could be used as a tool to identify high levels of fear of recurrence in breast cancer survivors in the Netherlands, and the results suggested that a score of 14 or higher on the CWS was optimal for detecting severe levels of FCR (33). Similarly, they found that a cutoff score greater than or equal to 14 on the CWS was optimal for the detection of high FCR in colorectal cancer survivors (34). However, to our knowledge, few studies have explored the screening capacity of the FCRI and a cutoff point among Chinese breast cancer survivors. As already mentioned, obtaining a cutoff point is quite challenging given the absence of a “gold standard” measure and definition of FCR. Our study revealed a cutoff score of 12 or higher on the short form of the FCRI, which was lower than the cutoff scores in other countries. A possible reason for this difference is that our study was conducted with only females and breast cancer survivors. Additionally, Chinese breast cancer survivors may lack cancer knowledge and may be more likely to misunderstand the association between cancer and death. Moreover, many cancer survivors have negative beliefs that cancer is incurable, which may increase their FCR (35–37).
This study revealed that FCR is very prevalent in Chinese breast cancer survivors. In accordance with previous studies, cancer survivors with a high level of FCR reported lower functional and overall health. To our knowledge, once diagnosed, breast cancer survivors may experience psychological distress to some degree, which could negatively affect quality of life and well-being. Many studies have found that increased FCR was related to emotional distress and poor mental health in cancer survivors, which has a detrimental effect on their quality of life, including physical and psychological functioning (38, 39). These findings suggest that FCR is a common experience among cancer survivors and that some survivors report more fear than do others. Hence, cancer survivors with high levels of FCR need to receive better psychological treatment.
Study Limitations
This study has several possible limitations. First, although the sample size can meet the requirements of the study, there may still be some selection bias. Therefore, it is necessary to further expand the sample size for testing because the relatively small sample size of our study may reduce the power of between-group comparisons and would limit the generalization of the prevalence rates of FCR among Chinese breast cancer survivors. In the future, our findings need to be replicated with a larger sample of Chinese breast cancer survivors. Second, the cutoff score is limited by the absence of gold standard criteria to diagnose FCR, and the specificity of our study was very low because our goal was to validate a rapid screening tool for FCR to detect the largest possible number of survivors with clinical levels of FCR (i.e., sensitivity) and to maximize the proportion of negative test results corresponding to nonclinical FCR survivors (i.e., NPV). Additionally, the cross-sectional design of our study does not make it possible to determine whether anxiety and depression were risk factors or consequences of FCR.
Clinical Implications
The study provides empirical support for the short form of the FCRI to be used as an optimal tool to detect high FCR and determined a cutoff point. This finding enables researchers to screen breast cancer survivors with high FCR and could guide doctors to pay attention to patients’ FCR. Future research needs to develop psychological interventions that decrease FCR and improve quality of life among Chinese breast cancer survivors.
Conclusion
In summary, the short form of the FCRI is able to detect high levels of FCR among breast cancer survivors. The high prevalence of FCR indicated that particular attention should be paid to Chinese females with breast cancer. Early psycho-education and management focusing on FCR specifically need to be provided for breast cancer survivors.
Ethics Statement
Ethical approval was obtained from the Ethics Committee of Third Military Medical University of China prior to the start of the study (ref. no.: ChiCTR-OOC-17012132).
Author Contributions
YX, FL, LZ, XC, SX and WC collected the data. WH, WZ and LP analyzed the data, LP and ML designed the experiment and wrote this paper.
Funding
This work was supported by the National Natural Science Fund (No. 31700958) and Chongqing Technology Innovation and Application Demonstration Project (No. cstc2018jscx-msybX0119).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors are grateful for the generous contributions of the research participants and the staff who assisted with data collection during the study.
References
1. Desanti CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin (2014) 64(4):252–71. doi: 10.3322/caac.21235
2. Fan L, Strasser-Weippl K, Li JJ, St Louis J, Finkelstein DM, Yu KD, et al. Breast cancer in China. Lancet Oncol (2014) 15(7):279–89. doi: 10.1016/S1470-2045(13)70567-9
3. World Health Organization. GLOBOCAN 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012. Lyon, France: International Agency for Research on Cancer (2014).
4. Spronk I, Schellevis FG, Burgers JS, De GB, Korevaar JC. Incidence of isolated local breast cancer recurrence and contralateral breast cancer: a systematic review. Breast (2018) 39:70–9. doi: 10.1016/j.breast.2018.03.011
5. Abrahams HJG, Gielissen MFM, Verhagen CAHHVM, Knoop H. The relationship of fatigue in breast cancer survivors with quality of life and factors to address in psychological interventions: a systematic review. Clin Psychol Rev (2018) 63:1–11. doi: 10.1016/j.cpr.2018.05.004
6. Zhou Y, Irwin ML, Ferrucci LM, Mccorkle R, Ercolano EA, Li F, et al. Health-related quality of life in ovarian cancer survivors: results from the American cancer society’s study of cancer survivors—I. Gynecol Oncol (2016) 141(3):543–49. doi: 10.1016/j.ygyno.2016.04.006
7. Simard S, Savard J, Ivers H. Fear of cancer recurrence: specific profiles and nature of intrusive thoughts. J Cancer Surviv (2010) 4(4):361–71. doi: 10.1007/s11764-010-0136-8
8. Lim JW, Shon EJ, Paek M, Daly B. The dyadic effects of coping and resilience on psychological distress for cancer survivor couples. Support Care Cancer (2014) 22(12):3209–17. doi: 10.1007/s00520-014-2334-9
9. LeeJones C, Humphris G, Dixon R, Hatcher MB. Fear of cancer recurrence—a literature review and proposed cognitive formulation to explain exacerbation of recurrence fears. Psychooncology (1997) 6(2):95–105. doi: 10.1002/(SICI)1099-1611(199706)6:2<95::AID-PON250>3.0.CO;2-B
10. Simard S, Thewes B, Humphris G, Dixon M, Hayden C, Mireskandari S, et al. Fear of cancer recurrence in adult cancer survivors: a systematic review of quantitative studies. J Cancer Surviv (2013) 7(3):300–22. doi: 10.1007/s11764-013-0272-z
11. Thewes B, Lebel S, Sequin Leclair C, Butow P. A qualitative exploration of fear of cancer recurrence (FCR) amongst Australian and Canadian breast cancer survivors. Support Care Cancer (2016) 24(5):2269–76. doi: 10.1007/s00520-015-3025-x
12. Stanton AL, Ganz PA, Kwan L, Meyerowitz BE, Bower JE, Krupnick JL, et al. Outcomes from the moving beyond cancer psychoeducational, randomized, controlled trial with breast cancer patients. J Clin Oncol (2005) 23(25):6009–18. doi: 10.1200/JCO.2005.09.101
13. Crist JV, Grunfeld EA. Factors reported to influence fear of recurrence in cancer patients: a systematic review. Psychooncology (2013) 22(5):978–86. doi: 10.1002/pon.3114
14. Thewes B, Bell ML, Butow P. Fear of cancer recurrence in young early-stage breast cancer survivors: the role of metacognitive style and disease-related factors. Psychooncology (2013) 22(9):2059–63. doi: 10.1002/pon.3252
15. Lebel S, Rosberger Z, Edgar L, Devins GM. Emotional distress impacts fear of the future among breast cancer survivors not the reverse. J Cancer Surviv (2009) 3(2):117–27. doi: 10.1007/s11764-009-0082-5
16. Sarkar S, Sautier L, Schilling G, Bokemeyer C, Koch U, Mehnert A. Anxiety and fear of cancer recurrence and its association with supportive care needs and health-care service utilization in cancer patients. J Cancer Surviv (2015) 9(4):567–75. doi: 10.1007/s11764-015-0434-2
17. Champagne A, Ivers H, Savard J. Utilization of health care services in cancer patients with elevated fear of cancer recurrence. Psychooncology (2018) 27(8):1958–64. doi: 10.1002/pon.4748
18. Custers JA, Gielissen MF, de Wilt JH, Honkoop A, Smilde TJ, van Spronsen DJ, et al. Towards an evidence-based model of fear of cancer recurrence for breast cancer survivors. J Cancer Surviv (2017) 11(1):41–7. doi: 10.1007/s11764-016-0558-z
19. Northouse LL. Mastectomy patients and the fear of cancer recurrence. Cancer Nurs (1981) 4(3):213–20. doi: 10.1097/00002820-198106000-00004
20. Herschbach P, Berg P, Dankert A, Duran G, Engsthastreiter U, Waadt S, et al. Fear of progression in chronic diseases: psychometric properties of the Fear of Progression Questionnaire. Psychother Psychosom Med Psychol (2011) 58(6):505–11. doi: 10.1016/j.jpsychores.2005.02.007
21. Vickberg SM. The concerns about recurrence scale (CARS): a systematic measure of women’s fears about the possibility of breast cancer recurrence. Ann Behav Med (2003) 25(1):16–24. doi: 10.1207/S15324796ABM2501_03
22. Ellegaard MB, Grau C, Zachariae R, Bonde JA. Fear of cancer recurrence and unmet needs among breast cancer survivors in the first five years. A cross-sectional study. Acta Oncol (2017) 56(2):314–20. doi: 10.1080/0284186X.2016.1268714
23. Simonelli LE, Siegel SD, Duffy NM. Fear of cancer recurrence: a theoretical review and its relevance for clinical presentation and management. Psychooncology (2016) 26(10):1444. doi: 10.1002/pon.4168
24. Simard S, Savard J. Fear of Cancer Recurrence Inventory: development and initial validation of a multidimensional measure of fear of cancer recurrence. Support Care Cancer (2009) 17(3):241–51. doi: 10.1007/s00520-008-0444-y
25. Su T, Liu H-X, Tian J, Deng Z-Z, Wang Z-L. Revision and reliability and validity test of Chinese version of cancer recurrence fear scale. Chinese J Pract Nursing (2018) 34(20):1571–76.
26. Fardell JE, Jones G, Smith AB, Lebel S, Thewes B, Costa D, et al. Exploring the screening capacity of the fear of cancer recurrence inventory-short form for clinical levels of fear of cancer recurrence. Psychooncology (2018) 27(2):492–99. doi: 10.1002/pon.4516
27. Weert EV, Hoekstra-Weebers JEHM, May AM, Korstjens I, Ros WJG, Schans CPVD. The development of an evidence-based physical self-management rehabilitation programme for cancer survivors. Patient Educ Couns (2008) 71(2):169–90. doi: 10.1016/j.pec.2007.11.027
28. Koch L, Jansen L, Brenner H, Arndt V. Fear of recurrence and disease progression in long-term (≥5 years) cancer survivors—a systematic review of quantitative studies. Psychooncology (2013) 22(1):1–11. doi: 10.1002/pon.3022
29. Brislin RW. Back-translation for cross-cultural research. J Cross Cult Psychol (1970) 1(3):185–216. doi: 10.1177/135910457000100301
30. Simard S, Savard J. Screening and comorbidity of clinical levels of fear of cancer recurrence. J Cancer Surviv (2015) 9(3):481–91. doi: 10.1007/s11764-015-0424-4
31. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiat Scand (1983) 67(6):361. doi: 10.1111/j.1600-0447.1983.tb09716.x
32. Fayers P, Bottomley A. Quality of life research within the EORTC—the EORTC QLQ-C30. Eur J Cancer (2002) 38(supp-S4):133. doi: 10.1016/S0959-8049(01)00448-8
33. Custers JA, van den Berg SW, van Laarhoven HW, Bleiker EM, Gielissen MF, Prins JB. The cancer worry scale: detecting fear of recurrence in breast cancer survivors. Cancer Nurs (2014) 37(1):44–50. doi: 10.1097/NCC.0b013e3182813a17
34. Custers JAE, Gielissen MFM, Janssen SHV, De WJHW, Prins JB. Fear of cancer recurrence in colorectal cancer survivors. Support Care Cancer (2016) 24(2):555–62. doi: 10.1007/s00520-015-2808-4
35. Ho RT, Chan CL, Ho SM. Emotional control in Chinese female cancer survivors. Psychooncology (2010) 13(11):808–17. doi: 10.1002/pon.799
36. Zhong BL, Li SH, Lv SY, Tian SL, Liu ZD, Li XB, et al. Suicidal ideation among Chinese cancer inpatients of general hospitals: prevalence and correlates. Oncotarget (2017) 8(15):25141–50. doi: 10.18632/oncotarget.15350
37. Lu Q, Yeung NCY, You J, Dai J. Using expressive writing to explore thoughts and beliefs about cancer and treatment among Chinese American immigrant breast cancer survivors. Psychooncology (2016) 25(11):1371. doi: 10.1002/pon.3991
38. Kim Y, Carver CS, Spillers RL, Kaw LGK. Dyadic effects of fear of recurrence on the quality of life of cancer survivors and their caregivers. Qual Life Res (2012) 21(3):517–25. doi: 10.1007/s11136-011-9953-0
Keywords: cancer, oncology, fear of cancer recurrence, anxiety, depression, breast cancer survivors
Citation: Peng L, Huang W, Zhang W, Xu Y, Lu F, Zhong L, Chen X, Xu S, Chen W and Li M (2019) Psychometric Properties of the Short Form of the Fear of Cancer Recurrence Inventory (FCRI) in Chinese Breast Cancer Survivors. Front. Psychiatry 10:537. doi: 10.3389/fpsyt.2019.00537
Received: 07 February 2019; Accepted: 10 July 2019;
Published: 07 August 2019.
Edited by:
Josef Jenewein, Psychiatric Clinic Zugersee, SwitzerlandReviewed by:
Anja Mehnert, University Hospital Leipzig, GermanyAlexandre Berney, Lausanne University Hospital (CHUV), Switzerland
Copyright © 2019 Peng, Huang, Zhang, Xu, Lu, Zhong, Chen, Xu, Chen and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Min Li, bGltaW41MjI2N0B0bW11LmVkdS5jbg==
 Li Peng1
Li Peng1 Fang Lu
Fang Lu Min Li
Min Li