- 1Department of Psychaitry, National Clinical Research Center for Mental Disorders, The Second Xiangya Hospital of Central South University, Changsha, China
- 2Department of Psychiatry, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, Hangzhou, China
Background: Several studies had examined the association between brain-derived neurotrophic factor (BDNF) Val66Met polymorphism and methamphetamine (METH) use disorder, whereas the results were conflicting. The aim of this study was to conduct a meta-analysis to achieve a pooled effect size of the association between BDNF Val66Met polymorphism and METH use disorder.
Methods: Literature searches were conducted in PubMed, EMBASE, and Cochrane Library up to July, 2020. All relevant studies on the relationship of BDNF Val66Met polymorphism and METH addiction were retrieved. Pooled odds ratios (ORs) with 95% confidence intervals (95% CIs) were calculated in the dominant, recessive, co-dominant, and allele model to appraise the association.
Results: Seven case–control studies with a total of 2,204 subjects (956 METH-dependent cases and 1,248 healthy controls) were included in this meta-analysis. The results showed a significant correlation between BDNF Val66Met polymorphism and METH dependence in overall population under different genetic models. However, subgroup analysis indicated that the association only existed in Han Chinese but not in other Asian populations.
Conclusion: Although the current data indicate that BDNF Val66Met polymorphism might be a potential genetic factor for METH use disorder, more researches are needed to prove its role in different populations.
Introduction
Methamphetamine (METH) is highly abused throughout the world, which remains an extremely serious public health issue. Although efforts to investigate the biological processes of METH use disorder (i.e., abuse, dependence, and psychosis) have uncovered several potential pathogenic factors, there is still a lack of putative biomarkers to predict its susceptibility. Former family and twins studies indicated that the development of substance use disorders was closely connected with genetic factor (1–3). Kendler et al. (4) found a heritability estimate of 57% for stimulants abuse/dependence in males, while Mcgue et al. (5) found that the heritability of use and abuse of amphetamines was 16% in adolescents. Besides, many genetic animal models supported the effects of genetic factors on METH addiction (6, 7). However, to date, the key genetic variations that increase susceptibility to METH addiction have not been identified. Brain-derived neurotrophic factor (BDNF) belongs to the neurotrophin family, which is widely expressed in the adult brain and plays a key role in the facilitation of development, differentiation, and survival of midbrain dopaminergic neurons as well as the modulation of synaptic transmission. Many reports showed that BDNF was involved in the development of psychiatric and substance use disorders and had a potential role in the treatment of these disorders (8–11). Experiments on animal models showed that increasing BDNF expression could promote the survival and protection of dopaminergic neurons following METH administration (12, 13).
BDNF Val66Met (rs6265; G196A), an early found functional single-nucleotide polymorphism (SNP) in BDNF gene, resulted in a valine (Val)-to-methionine (Met) substitution in the prodomain. BDNF Val66Met not only affected the secretion of BDNF but also changed the structure of BDNF and affected its interaction with HAP1 and Sortilin1 (14–16). BDNF Val66Met was involved in the modulation of neuronal morphology and synaptic plasticity as well as the development of neuronal, psychiatric, and drug use disorders (17–23). Researches suggested that BDNF Val66Met polymorphism was a potential predisposing genetic factor for different substance use disorders, such as cigarette, alcohol, cocaine, heroin, and METH (24–28). Significant differences in BDNF Val66Met genotype distribution were found between METH addicts and controls, and higher 66Val allele frequencies were found in both Asian and Caucasian drug addiction cases (28, 29). Excessive use of METH can induce psychotic episodes. Greening et al. (30) found that METH differentially altered dopamine signaling markers between mice with different BDNF Val66Met genotypes (Val/Val vs. Met/Met), implicating involvement of BDNF in Meth-induced reprogramming of the mesolimbic proteome, while the research by Sim et al. (31) suggested that the BDNF Val66Met polymorphism might contribute to METH dependence and psychosis in the Chinese population but not in other Malaysian ethnicities.
As mentioned above, though more and more studies have found that METH addiction is associated with BDNF, there are still many contradictions about the association between Val66Met polymorphism and METH addiction. The reason for the conflicting results might be ethnic specificity, limited samples, participant heterogeneity, and insufficient statistical ability. Therefore, the meta-analysis aimed at synthetizing current studies on the association of BDNF Val66Met polymorphism and susceptibility to METH use disorder.
Methods
Literature Search
Any literature concerning the association between BDNF polymorphisms and METH addiction was sought by using the Medical Subject Headings terms “methamphetamine,” “polymorphism,” “variant,” “BDNF,” “brain-derived neurotropic factor,” “rs6265,” “rs6265 G>A,” “G rs6265A,” and “Val66Met” in databases (PubMed, EMBASE, and Cochrane Library) up to July, 2020. To find more appropriate studies, the references of the key studies were hand-searched. When detailed information for calculating effect size was lacking, we contacted the authors directly. The study was registered at PROSPERO (CRD42020193010).
Inclusion and Exclusion Criteria
Case–control and cross-sectional studies were included irrespective of publication status or language. In this meta-analysis, all included studies met the following criteria: (a) studies focused on the association between BDNF Val66Met polymorphism and METH use disorder susceptibility, (b) patients were diagnosed using standard diagnostic criteria by a psychiatrist, (c) genotypes distribution of the control group should be in Hardy–Weinberg Equilibrium (HWE), and (d) there were sufficient data of the genotypes in the case and control group to compute the odds ratios (ORs) and 95% confidence intervals (CIs). The exclusion criteria were as follows: (a) METH-dependent subjects involving other substance use disorders, (b) publications with patients with other neuropsychiatric disorders, and (c) no genotypic data available.
Quality Assessment
The methodological quality assessment of inclusion studies was performed with the Newcastle-Ottawa scale (NOS) (32). A “star system” has been used to assess data quality based on three aspects: the selection, comparability, and exposure or outcome of interest. Two independent researchers identified the literatures, and divergences between reviewers were resolved through discussion or with the assistance of a third researcher. The power of meta-analysis was estimated by STATA 12.0 software (Stata Corporation, College Station, Texas, USA). When the power of test was higher than 0.8, the result was considered acceptable.
Data Extraction
Data extraction from an individual study included the following: first author, publication year, site of study, ethnicity, sample size, age, gender distribution, diagnosis criteria, genotyping method, HWE of cases and controls, and distribution of genotypes. Two researchers extracted the data independently, and disagreements were settled through discussion or with the assistance of a third researcher.
Statistical Analysis
Statistical analysis was performed by STATA 12.0 software (Stata Corporation, College Station, Texas, USA). The relationship between BDNF Val66Met polymorphism and METH use disorder was appraised by pooled ORs and 95% CIs in dominant model (Met/Met + Val/Met vs. Val/Val), recessive model (Met/Met vs. Val/Val + Val/Met), co-dominant model (Met/Met vs. Val/Val; Val/Met vs. Val/Val), and allele model (Met vs. Val). Summary OR results and publication bias were evaluated by Z test and Egger's test, respectively. P < 0.05 was considered statistically significant. I2 and Q test statistics were employed to ascertain the heterogeneity among studies. When the heterogeneity was high (I2 > 50% or P < 0.1), a random-effect model was selected. Otherwise, the fixed effects model was chosen. Subgroup analyses were conducted according to ethnicity. Sensitivity analysis was performed with leave-one-out approach to examine the stability of analysis.
Results
Literature Search
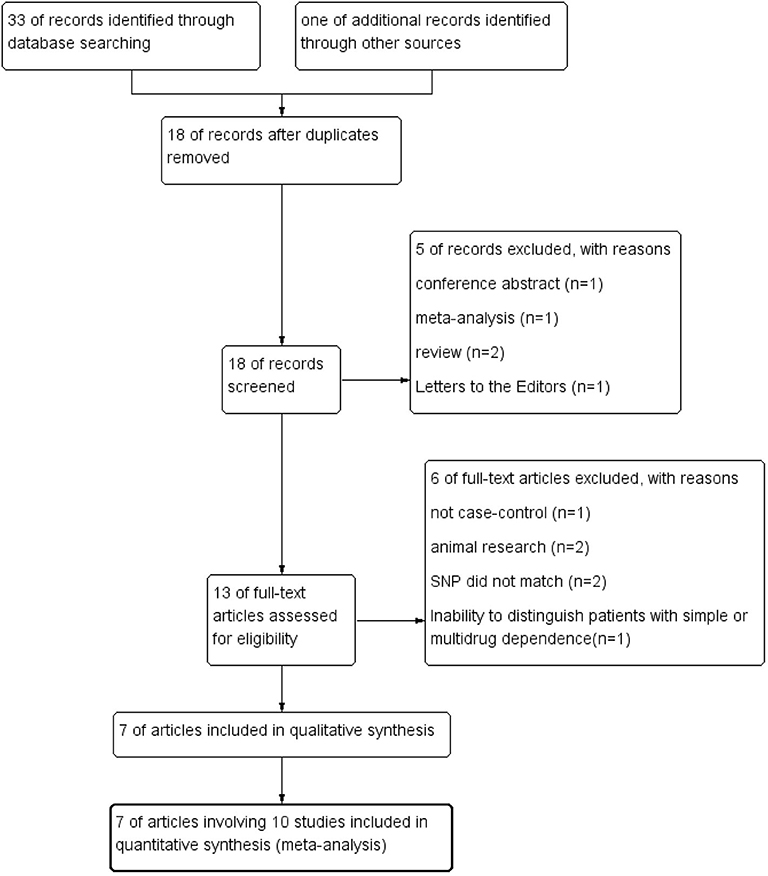
The process of systematic literature search and selection was displayed in Figure 1. We identified 33 articles through database searching and 1 additional article through reference lists searching. After excluding duplicates, 18 articles remained. Screening the titles and abstracts of these 18 articles resulted in 13 relevant studies. After the full-text review of the remaining 13 articles, 7 studies were qualified for final data analysis.
Study Characteristics
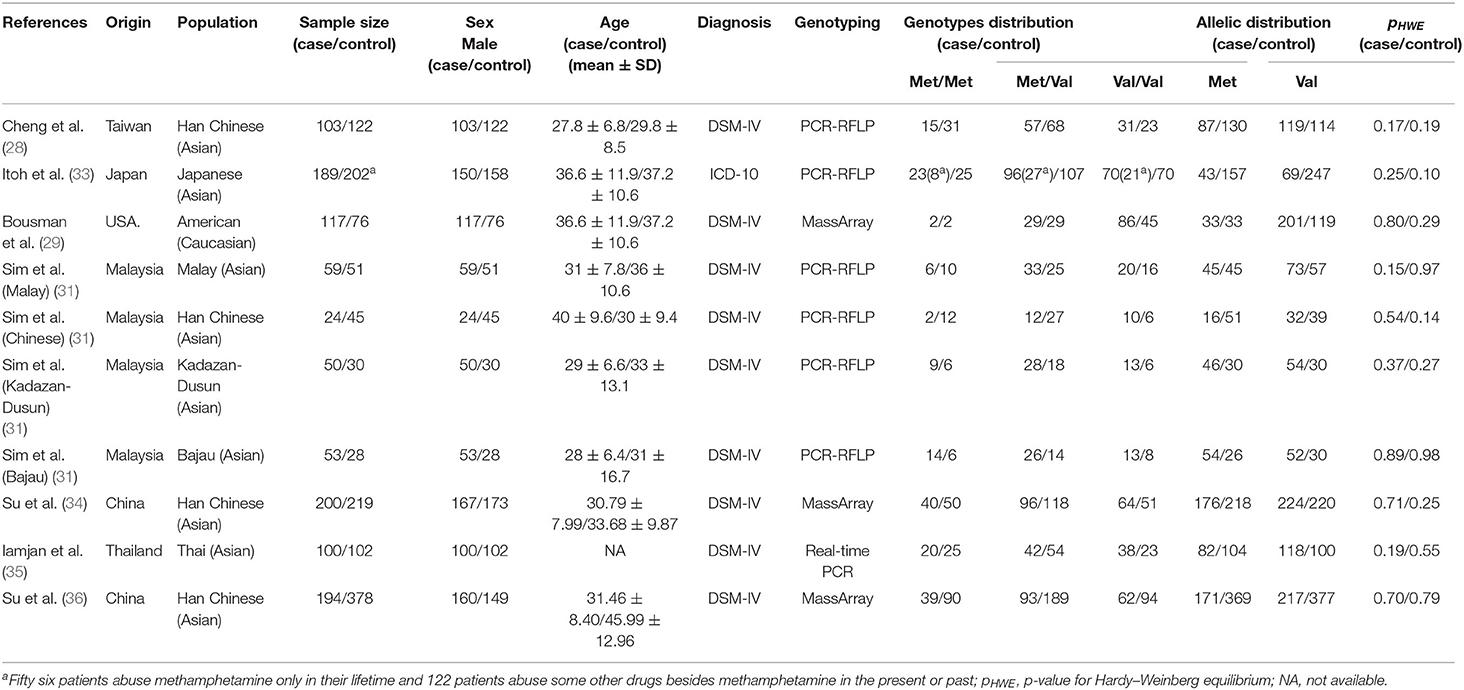
Seven case–control studies (28, 29, 31, 33–36) with a total of 2,204 subjects (956 METH-dependent cases and 1,248 healthy controls) were included in this meta-analysis. Table 1 summarized the general characteristic of inclusion studies. All included studies were in accordance with HWE. The NOS scores of all studies were >6, indicating that they had good methodological quality (Supplementary Table 1). Except for one (33) employing ICD-10 criteria, all studies used DSM-IV criteria to identify METH-dependent subjects. One study from Malaysia were carried out in four different ethnic groups (31). After classification of this study into four subgroups, the number of studies was raised to 10.
Of the 10 different cohorts included in our study, 9 were from Asia (28, 31, 33–36). Two studies from China, one study from Taiwan, and one study from Malaysia were carried out in the Han Chinese population (28, 31, 34, 36). Cheng et al. (28) studied BDNF Val66Met polymorphism in both METH- and heroin-dependent patients, and only the former was included in the study. In the study by Itoh et al. (33), 56 patients abused MTEH only, while 122 patients abused other drugs besides METH. According to the predetermined inclusion criteria, the 56 subjects with METH dependence only were included in the data analysis. Four of the included (28, 29, 31, 35) studies were carried out in male only. Itoh et al. (33) analyzed the association of two BDNF gene SNPs with METH abuse in Japan. Bousman et al. (29) investigated association of six putative SNPs in six genes (AKT1, ARRB2, BDNF, COMT, GSTP1, and OPRM1) with METH abuse in Caucasian.
Meta-Analysis
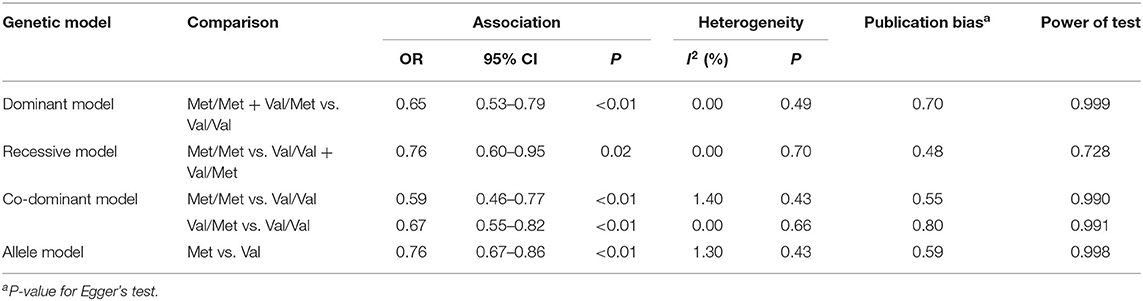
The summary data of different genetic models indicated a significant association between BDNF Val66Met polymorphism and METH addiction in overall population, and no publication bias and significant heterogeneity were identified (Table 2 and Figure 2). Except for the recessive model, the power of meta-analyses was acceptable (Table 2). Figure 3 displayed the results of sensitivity analysis for different genetic models in the overall population. When the study by Cheng et al. was removed, the pooled OR changed a lot (from OR = 0.76, 95% CI: 0.60–0.95 to OR = 0.80, 95% CI: 0.63–1.02, Figure 3E) under the recessive model. While for the dominant, co-dominant, and allele model, the overall effect was not reversed after the deletion of study by Cheng et al. (Figures 3A–D). Therefore, this study was retained.

Figure 2. Forest plot for the association between BDNF Val66Met polymorphism and methamphetamine dependence susceptibility in five genetic models. (A) Allelic model (Met vs. Val). (B) Co-dominant model (Met/Met vs. Val/Val). (C) Co-dominant model (Val/Met vs. Val/Val). (D) Dominant model (Met/Met + Val/Met vs. Val/Val). (E) Recessive model (Met/Met vs. Val/Val + Val/Met).
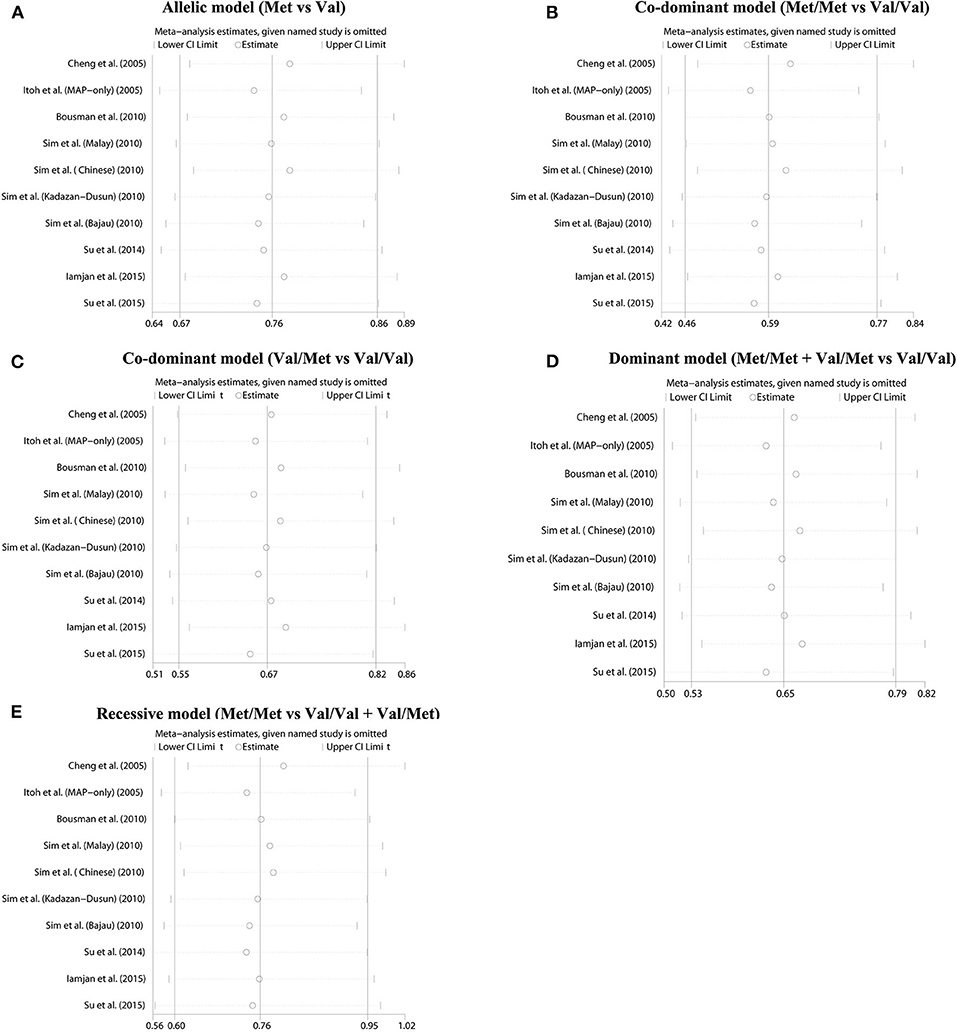
Figure 3. Sensitivity analysis results. (A) Allelic model (Met vs. Val). (B) Co-dominant model (Met/Met vs. Val/Val). (C) Co-dominant model (Val/Met vs. Val/Val). (D) Dominant model (Met/Met + Val/Met vs. Val/Val). (E) Recessive model (Met/Met vs. Val/Val + Val/Met).
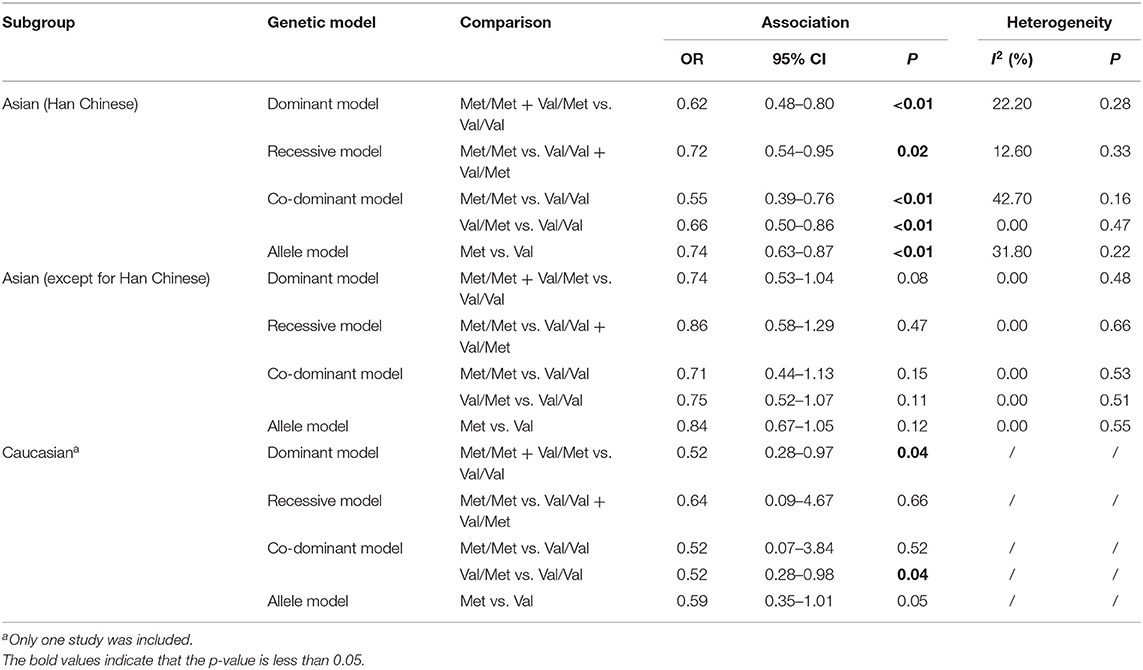
Considering the conflicting results between different populations, the further subgroup analysis by ethnicity was employed. Four studies of Han Chinese origin and five studies of East and Southeast Asian origin (except for Han Chinese) were enrolled in the subgroup analysis (Table 3 and Figure 2). Due to insufficient data, we were unable to perform a subgroup analysis of Caucasian population, but we listed a study of Caucasian origin as a reference in Table 3 and Figure 2. In the subgroup analyses, we found a statistically significant association between BDNF Val66Met polymorphism and METH addiction in Han Chinese, but not in other Asian populations. Heterogeneities were increased in the Han Chinese group under different genetic models.
Discussion
BDNF Val66Met, a non-synonymous SNP of BDNF gene, is related to a variety of neuropsychiatric diseases, such as Parkinson's disease, Alzheimer's disease, schizophrenia, and posttraumatic stress disorder (19–21, 37). Egan et al. (14) firstly examined the effects of BDNF Val66Met polymorphism and demonstrated a role for the variants in memory and hippocampal function. Subsequently, Greening et al. (30) found that BDNF Val66Met involved in METH-induced reprogramming of the mesolimbic proteome. The reward system of the midbrain and memory function of the hippocampus plays an important role in the formation of METH addiction. Therefore, due to the role of BDNF Val66Met in these processes, its relationship with METH addiction has attracted more and more attention. Many studies have examined the association between BDNF Val66Met polymorphism and METH abuse, but the results of these studies were not entirely consistent with each other. In this meta-analysis, we found that there was an association between BDNF Val66Met polymorphism and METH addiction in the overall population, suggesting that BDNF Val66Met polymorphism might be a potential marker for METH-dependent susceptibility. METH is a psychostimulant and has obvious neurotoxicity to dopamine neurons. In the animal METH-treated model, increased BDNF resulted in preservation of corpus striatal dopamine levels, indicating that the BDNF expression had a certain effect on antagonizing the nerve damage induced by METH (38). BDNF expression was increased in METH-dependent patients, and the BDNF Val66Met genotypes can affect the secretion of BDNF (36, 39). Therefore, the effects of BDNF Val66Met polymorphism on BDNF expression might be relevant to METH addiction.
As the use of METH penetrated the geographic regions of different ethnicities in the world, racial differences among METH abusers were observed (40). In the subgroup meta-analysis by ethnicity, we found a significant association between BDNF Val66Met polymorphism and METH addiction in Han Chinese, but not in other Asian populations. This result was different from that of Haerian et al., who found that the BDNF Val66Met polymorphism may be a risk factor for addiction to METH in a South Asian population (17). The difference might result from the differences in the included trials and subgroup analysis. In the study by Haerian et al., five inclusion studies investigated the association between BDNF Val66Met polymorphism and METH addiction, and one of them recruited patients with simple or multidrug dependence. In this meta-analysis, we only included studies that explored the relationship between simple METH addiction and BDNF Val66Met polymorphism. Therefore, seven trials were included in this meta-analysis, including four trials the same as the inclusion studies by Haerian et al. and three additional trials. Haerian et al. conducted subgroup analysis only according to Asian and Caucasian ethnicity. Our study further separated Han Chinese from Asian ethnicity; for many studies, it was found that the 66Val allele frequency in Han Chinese was significantly different from the controls and the weight of Han Chinese in the overall analysis was relatively high. This meta-analysis found that BDNF Val66Met might be a genetic factor for METH use disorder among the Han Chinese population. The allele frequency of BDNF Val66Met varied between different geographical areas. The frequency of 66Val allele in East Asians is 0.510, while that in South Asians is 0.807 (41). The distribution of BDNF Val66Met genotypes was also different among the inclusion studies, implying that there might be differences in susceptibility to METH among people with diverse genetic backgrounds. In the subgroup analysis, heterogeneities were increased in the Han Chinese group. This heterogeneity might result from the participants' heterogeneity among the inclusion studies. Of the four studies carried out in Han Chinese, half (28, 31) only included METH-dependent male as research subjects. Research has reported the gender differences in socio-demographic and clinical characteristics of METH abusers (42). Furthermore, Heinzerling et al. (43) found that there was a significant interaction between sex and BDNF Val66Met among METH-dependent patients. Although the biological mechanism contributing to these differences is unclear, additional researches on the different biological basis of response to METH among male and female are warranted.
In summary, considering the race specificity, limited samples, participant heterogeneity, and insufficient statistical ability of the current studies on the association between BDNF Val66Met polymorphism and simple METH use disorder, we applied a meta-analysis to get a pooled effect size, illustrating the potential effect of BDNF Val66Met on METH addiction. Besides, in the subgroup analysis according to the ethnicity, we found that the effect of BDNF Val66Met on METH addiction had ethnically specific differences. However, there were still several limitations in this meta-analysis. First of all, the majority of the inclusion studies were conducted on men, so the relationship between BDNF Val66Met polymorphism and gender cannot be observed among METH abusers. Second, the limitation of sample size made a relative low power of the recessive model. Besides, too few Caucasian studies were carried out, and a stratified analysis by Caucasians could not be done under different genetic models because of the insufficient frequency data. Finally, this study only focuses on the association between BDNF Val66Met polymorphism and METH-dependent susceptibility without considering gene–gene or gene–environment interactions. Therefore, this conclusion still needs to be supported by more researches carried out in different populations, and more researches are needed to be conducted.
Conclusions
This is the first meta-analysis to study the relationship between simple METH addiction and BDNF Val66Met polymorphism. In this meta-analysis, we found that the expression of BDNF 66Met in METH-dependent patients was lower than that in the controls, indicating that BDNF 66Met might be a protective factor for METH addiction. Besides, in the subgroup analysis, we found that BDNF 66Met was differentially expressed only in the Han population, suggesting that the protective effect of BDNF 66Met might be ethnic-specific. The mechanism of BDNF Val66Met gene polymorphism on METH addiction in the Han population remains to be further studied. The current studies on association between METH addiction and BDNF Val66Met polymorphism are mainly from Asia, while few are from other districts. Therefore, the current results might be modified with the increase of studies in other regions. Considering these limitations, large-sample studies with different ethnicities should be conducted to determine the role of the BDNF Val66Met polymorphism in different populations.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
LH and TL: study design. LH, QW, and YL data collection, analysis, and interpretation. LH: drafting of the manuscript. TL and YL: critical revision of the manuscript. All authors approval of the final version for publication.
Funding
The study was supported by the National Key R & D Program of China (2017YFC1310400), the National Natural Science of China (Grant No. 81671324), and the provincial Natural Science Foundation of Hunan (Grant No. 2020JJ4795).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2020.585852/full#supplementary-material
References
1. Otowa T, Gardner CO, Kendler KS, Hettema JM. Parenting and risk for mood, anxiety and substance use disorders: a study in population-based male twins. Soc Psychiatry & Psychiatr Epidemiol. (2013) 48:1841–9. doi: 10.1007/s00127-013-0656-4
2. Kendler KS, Gardner CO. Twin studies of adult psychiatric and substance dependence disorders: are they biased by differences in the environmental experiences of monozygotic and dizygotic twins in childhood and adolescence? Psychol Med. (1998) 28:625–33. doi: 10.1017/S0033291798006643
3. Kendler KS, Davis CG, Kessler RC. The familial aggregation of common psychiatric and substance use disorders in the national comorbidity survey: a family history study. Br J Psychiatry. (1997) 170:541–48. doi: 10.1192/bjp.170.6.541
4. Kendler KS, Jacobson KC, Prescott CA, Neale MC. Specificity of genetic and environmental risk factors for use and abuse/dependence of cannabis, cocaine, hallucinogens, sedatives, stimulants, and opiates in male twins. Am J Psychiatry. (2003) 160:687. doi: 10.1176/appi.ajp.160.4.687
5. Mcgue M, Elkins I, Iacono WG. Genetic and environmental influences on adolescent substance use and abuse. Am J Med Genet. (2000) 96:671–77. doi: 10.1002/1096-8628(20001009)96:5<671::aid-ajmg14>3.0.co;2-w
6. Phillips TJ, Shabani S. An animal model of differential genetic risk for methamphetamine intake. Front Neurosci. (2015) 9:327. doi: 10.3389/fnins.2015.00327
7. Shabani S, Dobbs LK, Ford MM, Mark GP, Finn DA, Phillips TJ. A genetic animal model of differential sensitivity to methamphetamine reinforcement. Neuropharmacology. (2012) 62:2169–77. doi: 10.1016/j.neuropharm.2012.01.002
8. Nagahara AH, Tuszynski MH. Potential therapeutic uses of bdnf in neurological and psychiatric disorders. Nat Rev Drug Discov. (2011) 10:209–19. doi: 10.1038/nrd3366
9. Manning EE, Halberstadt AL, van den Buuse M. Bdnf-deficient mice show reduced psychosis-related behaviors following chronic methamphetamine. Int J Neuropsychopharmacol. (2015) 19:pyv116. doi: 10.1093/ijnp/pyv116
10. Ren Q, Ma M, Yang C, Zhang JC, Yao W, Hashimoto K. Bdnf–trkb signaling in the nucleus accumbens shell of mice has key role in methamphetamine withdrawal symptoms. Transl Psychiatry. (2015) 5:e666. doi: 10.1038/tp.2015.157
11. Haile CN, Kosten TR, Kosten TA. Pharmacogenetic treatments for drug addiction: cocaine, amphetamine and methamphetamine. Am J Drug Alcohol Abuse. (2009) 35:161–77. doi: 10.1080/00952990902825447
12. Joyce JN, Renish L, Osredkar T, Walro JM, Kucera J, Dluzen DE. Methamphetamine-induced loss of striatal dopamine innervation in bdnf heterozygote mice does not further reduce d3 receptor concentrations. Synapse. (2004) 52:11–9. doi: 10.1002/syn.10309
13. Shahidi S, Mehrpour O, Sadeghian R, Soleimani Asl S, Komaki A. Alteration level of hippocampus bdnf expression and long-term potentiation upon microinjection of brl15572 hydrochloride in a rat model of methamphetamine relapse. Brain Res Bull. (2019) 148:18–24. doi: 10.1016/j.brainresbull.2019.03.008
14. Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, et al. The bdnf val66met polymorphism affects activity-dependent secretion of bdnf and human memory and hippocampal function. Cell. (2003) 112:257–69. doi: 10.1016/S0092-8674(03)00035-7
15. Zamani M, Eslami M, Nezafat N, Hosseini SV, Ghasemi Y. Evaluating the effect of bdnf val66met polymorphism on complex formation with hap1 and sortilin1 via structural modeling. Comput Biol Chem. (2018) 78:282–9. doi: 10.1016/j.compbiolchem.2018.12.010
16. Lohia R, Salari R, Brannigan G. Sequence specificity despite intrinsic disorder: how a disease-associated val/met polymorphism rearranges tertiary interactions in a long disordered protein. PLoS Comput Biol. (2019) 15:e1007390. doi: 10.1371/journal.pcbi.1007390
17. Haerian BS. Bdnf rs6265 polymorphism and drug addiction: a systematic review and meta-analysis. Pharmacogenomics. (2013) 14:2055–65. doi: 10.2217/pgs.13.217
18. McGregor CE, English AW. The role of bdnf in peripheral nerve regeneration: activity-dependent treatments and val66met. Front Cell Neurosci. (2018) 12:522. doi: 10.3389/fncel.2018.00522
19. Pitts BL, Whealin JM, Harpaz-Rotem I, Duman RS, Krystal JH, Southwick SM, et al. Bdnf val66met polymorphism and posttraumatic stress symptoms in U.S. Military veterans: protective effect of physical exercise. Psychoneuroendocrinology. (2018) 100:198–202. doi: 10.1016/j.psyneuen.2018.10.011
20. Schweiger JI, Bilek E, Schäfer A, Braun U, Moessnang C, Harneit A, et al. Effects of bdnf val66met genotype and schizophrenia familial risk on a neural functional network for cognitive control in humans. Neuropsychopharmacology. (2018) 44:590–7. doi: 10.1038/s41386-018-0248-9
21. Pietzuch M, King AE, Ward DD, Vickers JC. The influence of genetic factors and cognitive reserve on structural and functional resting-state brain networks in aging and alzheimer's disease. Front Aging Neurosci. (2019) 11:30. doi: 10.3389/fnagi.2019.00030
22. Ninan I, Bath KG, Dagar K, Perez-Castro R, Plummer MR, Lee FS, et al. The bdnf val66met polymorphism impairs nmda receptor-dependent synaptic plasticity in the hippocampus. J Neurosci. (2010) 30:8866–70. doi: 10.1523/JNEUROSCI.1405-10.2010
23. Anastasia A, Deinhardt K, Chao MV, Will NE, Bracken C. Val66met polymorphism of bdnf alters prodomain structure to induce neuronal growth cone retraction. Nat Commun. (2013) 4:2490. doi: 10.1038/ncomms3490
24. Korhonen T, Loukola A, Hällfors J, Salomaa V, Kaprio J. Is brain derived neurotrophic factor (bdnf) associated with smoking initiation? Replication using a large finnish population sample. Nicotine Tob Res. (2018) 22:293–6. doi: 10.1093/ntr/nty218
25. Wojnar M, Jakubczyk A, Wnorowska A, Klimkiewicz A, Sliwerska E, Burmeister M, et al. An association between val66met brain-derived neurotrophic factor (bdnf) gene polymorphism and post-treatment relapse in alcohol dependence. Alcohol Clin Exp Res. (2010) 33:693–702. doi: 10.1111/j.1530-0277.2008.00886.x
26. Briand LA, Lee FS, Blendy JA, Pierce RC. Enhanced extinction of cocaine seeking in brain-derived neurotrophic factor val66met knock-in mice. Eur J Neurosci. (2012) 35:932–9. doi: 10.1111/j.1460-9568.2012.08021.x
27. Chen SL, Lee SY, Chang YH, Wang TY, Chen SH, Chu CH, et al. The bdnf val66met polymorphism and plasma brain-derived neurotrophic factor levels in han chinese heroin-dependent patients. Sci Rep. (2015) 5:8148. doi: 10.1038/srep08148
28. Cheng CY, Hong CJ, Yu YWY, Chen TJ, Wu HC, Tsai SJ. Brain-derived neu factor (val66met) genetic polymorphism is associated with substance abuse in males. Mol Brain Res. (2005) 140:86–90. doi: 10.1016/j.molbrainres.2005.07.008
29. Bousman CA, Glatt SJ, Cherner M, Atkinson JH, Grant I, Tsuang MT, et al. Preliminary evidence of ethnic divergence in associations of putative genetic vari for methamphetamine dependence. Psychiatry Res. (2010) 178:295–8. doi: 10.1016/j.psychres.2009.07.019
30. Greening DW, Notaras M, Chen M, Xu R, Smith JD, Cheng L, et al. Chronic methamphetamine interacts with bdnf val66met to remodel psychosis pathways in the mesocorticolimbic proteome. Mol Psychiatry. (2019). doi: 10.1038/s41380-019-0617-8
31. Sim MS, Mohamed Z, Hatim A, Rajagopal VL, Habil MH. Association of brain-derived neurotrophic factor (val66met) genetic polymorphism with methamphetamine dependence in a malaysian population. Brain Res. (2010) 1357:91–6. doi: 10.1016/j.brainres.2010.08.053
32. Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The newcastle-ottawa scale (nos) for assessing the quality of nonrandomised studies in meta-analyses (2019). http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed July15 2020).
33. Itoh K, Hashimoto K, Shimizu E, Sekine Y, Ozaki N, Inada T, et al. Association study between brain-derived neurotrophic factor gene polymorphisms and methamphetamine abusers in Japan. Am J Med Genet B Neuropsychiatr Genet. (2005) 132B:70–3. doi: 10.1002/ajmg.b.30097
34. Su H, Tao J, Zhang J, Xie Y, Sun Y, Li L, et al. An association between bdnf val66met polymorphism and impulsivity in methamphetamine abusers. Neurosci Lett. (2014) 582:16–20. doi: 10.1016/j.neulet.2014.08.030
35. Iamjan SA, Thanoi S, Watiktinkorn P, Nudmamud-Thanoi S, Reynolds GP. Bdnf (val66met) genetic polymorphism is associated with vulnerability for methamphetamine dependence. Pharmacogenomics. (2015) 16:1541–45. doi: 10.2217/pgs.15.96
36. Su H, Tao J, Zhang J, Xie Y, Wang Y, Zhang Y, et al. The effects of bdnf val66met gene polymorphism on serum bdnf and cognitive function in methamphetamine-dependent patients and normal controls: a case-control study. J Clin Psychopharmacol. (2015) 35:517–24. doi: 10.1097/JCP.0000000000000390
37. Sampedro F, Marín-Lahoz J, Martínez-Horta S, Pagonabarraga J, Kulisevsky J. Pattern of cortical thinning associated with the bdnf val66met polymorphism in parkinson's disease. Behav Brain Res. (2019) 372:112039. doi: 10.1016/j.bbr.2019.112039
38. Dluzen DE. The effect of gender and the neurotrophin, bdnf, upon methamphetamine-induced neurotoxicity of the nigrostriatal dopaminergic system in mice. Neurosci Lett. (2004) 359:135–38. doi: 10.1016/j.neulet.2004.01.061
39. Lang UE, Hellweg R, Sander T, Gallinat J. The met allele of the bdnf val66met polymorphism is associated with increased bdnf serum concentrations. Mol Psychiatry. (2009) 14:120–2. doi: 10.1038/mp.2008.80
40. Herbeck DM, Brecht ML, Pham AZ. Racial/ethnic differences in health status and morbidity among adults who use methamphetamine. Psychol Health Med. (2013) 18:262–74. doi: 10.1080/13548506.2012.701754
41. Phan L, Jin Y, Zhang H, Qiang W, Shekhtman E, Shao D, et al. Alfa: allele frequency aggregator (2020). www.ncbi.nlm.nih.gov/snp/docs/gsr/alfa/ (accessed July 15, 2020).
42. He J, Xie Y, Tao J, Su H, Wu W, Zou S, et al. Gender differences in socio-demographic and clinical characteristics of methamphetamine inpatients in a Chinese population. Drug Alcohol Depend. (2013) 130:94–100. doi: 10.1016/j.drugalcdep.2012.10.014
Keywords: BDNF Val66Met, polymorphism, methamphetamine, addiction, meta-analysis
Citation: He L, Liao Y, Wu Q and Liu T (2020) Association Between Brain-Derived Neurotrophic Factor Val66Met Polymorphism and Methamphetamine Use Disorder: A Meta-Analysis. Front. Psychiatry 11:585852. doi: 10.3389/fpsyt.2020.585852
Received: 21 July 2020; Accepted: 19 October 2020;
Published: 19 November 2020.
Edited by:
Xiaochu Zhang, University of Science and Technology of China, ChinaReviewed by:
Masoud Sadeghi, Kermanshah University of Medical Sciences, IranYazmín Hernández-Díaz, Universidad Juárez Autónoma de Tabasco, Mexico
Copyright © 2020 He, Liao, Wu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tieqiao Q. Liu, bGl1dGllcWlhbzEyM0Bjc3UuZWR1LmNu
 Li He1
Li He1 Yanhui Liao
Yanhui Liao Qiuxia Wu
Qiuxia Wu Tieqiao Liu
Tieqiao Liu