- 1Center for the Study of Human Health, Emory University Atlanta, Atlanta, GA, USA
- 2Center for Ethics Neuroethics Program, Emory University Atlanta, Atlanta, GA, USA
- 3Department of Neurology, Emory University Atlanta, Atlanta, GA, USA
- 4Department of Psychiatry and Behavioral Sciences, Emory University Atlanta, Atlanta, GA, USA
A commentary on
Attention to eyes is present but in decline in 2–6-month-old infants later diagnosed with autism
by Jones W, Klin A. Nature (2013) 504:427–31. doi: 10.1038/nature12715
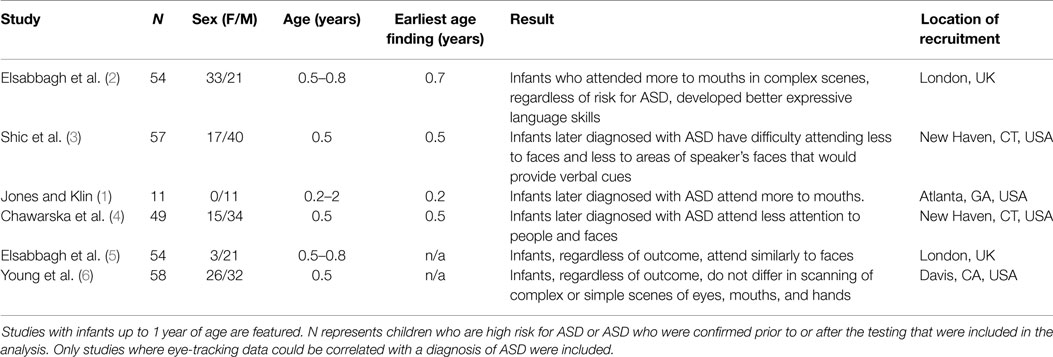
A recent Nature article provided preliminary evidence that infants age 2–6 months old, who were later diagnosed with Autism Spectrum Disorder (ASD), fixated more on the mouth than eyes and more at objects than people when viewing videos of typical childhood social scenes (1). While the sample was small, a reliable pattern of decline in eye fixation accurately predicted their level and classification of symptoms at age three suggesting that – for the first time – an infant could be assessed within the first 6 months of life for their potential of developing ASD (see Table 1 for studies that used eye-tracking with infants 12 months and younger). These eye-tracking devices, which are currently in clinical trials, could provide access to an affordable and objective tool with the potential for extremely early intervention. Detecting ASD risk during the first 6 months of life presents unprecedented opportunities to intervene, providing children opportunities to build critical skills before autistic characteristics fully emerge. Because the eye-tracking device allows for a non-invasive, portable assessment, the device could also enable pediatricians to provide comparable screening services globally. With such promise, a near future where infants are placed into an eye-tracking device at routine pediatric visits is compelling, if not guaranteed.
Autism Spectrum Disorder is characterized by developmental differences and difficulties in social communication and interaction coupled with repetitive behaviors (7) with an estimated prevalence of 1 in 68 children in American populations (Developmental Disabilities Monitoring Network Surveillance Year 2010 Principal Investigators and Centers for Disease Control and Prevention (CDC) 2014) and a range of 1 in 333 to 1 in 86 among European nations. A global mean of 1 in 160 children (8) makes ASD a public health concern more prevalent than juvenile cancers (9) or diabetes (10). Diagnoses are generally made subjectively by assessing behavioral symptoms through parental interview and behavioral observations of the child (8, 9). Presently, clinicians are unable to reliably diagnose ASD until 12 months using the Autism Diagnostic Observation Schedule (ADOS) Toddler module (11); however, diagnosis usually happens much later. A 2014 CDC study estimated age at diagnosis using DSM IV TR classifications, which included several disorders under the category of ASDs rather than the DSM 5 model of ASD as a single diagnosis. It reported 48 months as the mean age of diagnosis for Autistic Disorder, 53 months for ASD or Pervasive Developmental Disorder-Not Otherwise Specified, and 75 months for Asperger’s syndrome (12). Widespread recommendations by professionals for early identification and intervention (8, 13, 14), along with a lack of viable community systems of long-term care (15), have driven priorities for autism research and funding toward reducing the age of diagnosis and intervention (16).
Currently, the American Academy of Pediatrics (AAP) (17) and the CDC (18) recommend community-wide, routine 18- and 24-month-old screenings for ASD. Early identification can provide the opportunity to facilitate early skill development and reduce the population of children and adults who are reliant on inadequate community resources for long-term care and opportunities for community engagement (15, 19). While this tool is in early phases, it has rapidly moved to clinical trials; ethical concerns must be considered alongside and as an integral part of the research, not only for future patients, but also for current study participants. Importantly, preclinical assessment technologies run the risk of bearing false negatives, i.e., children not identified as autistic but who receive an ASD diagnosis at a later age, and false positives, i.e., children identified as likely to be autistic who are not later diagnosed with ASD. Parents will undoubtedly want to do something with a positive screen. Studies have shown that parent’s uncertainty about their child’s medical diagnosis can lead to poor coping or adaptation to their child’s condition (20). Conversely, parents may be overly reassured by the preclinical assessment. For example, parents whose children receive a negative screen may assume their child will not develop ASD and miss opportunities to intervene.
In the event of a false positive, parents might invest in unnecessary expensive interventions, surveillance, and treatments as well as lead to changes in the life trajectories of the child, caregivers, and entire family. Caregivers may decide to change their financial plans and reallocate time and material resources to a child’s early intervention or care. Even after a false positive is identified, caregivers may be unable to cease looking for signs of ASD as a child ages or perhaps mistake a positive assessment with a diagnosis and although there is public recognition of the importance of ASD identification throughout the world (21), ASD is often considered an unwanted, even alarming, stigmatized condition (22, 23). Healthcare providers will need to be able to advise parents on what parents can do while delivering an appropriately cautious interpretation of such preclinical testing results.
We must also consider how clinicians should respond when parents whose children receive a positive screen inquire about interventions for their infant given the lack of evidenced based interventions for infants. Preclinical screens, that assess a phenotype that might predict ASD, but is not a key trait of the diagnosis, such eye-tracking technologies assess risk and are not diagnostic for ASD. This is important to emphasize to everyone from parents, study participants, and patient schedulers to insurance companies. Risk can be characterized in terms of both severity and susceptibility (24). ASD represents a diverse set of symptoms, abilities, and impairments and a variable timeline of development. It is unclear how much consistent predictive power preclinical testing will have for describing specific risks for severity, behavioral profiles, and age of symptom onset. With this ambiguity, there is significant potential for misunderstanding, resistance to a preclinical assessment, and damage to the therapeutic alliance of the families and clinicians.
Further, using a word like “risk” – understood differently among clinicians and the general public and across cultures – may not be wholly appropriate (25). The word “risk” may fail to communicate the vast range of possible phenotypic outcomes and instead place too much focus on negative outcomes. Adherents of a growing neurodiversity movement – an advocacy position that rejects the ideas that autism is unwanted and should be “cured” and, instead, acknowledges autism as a natural variant of human neurological development (26; See Box 1 for more on neurodiversity) – would resist the use of “risk” in relation to ASD (28). Healthcare providers and parents will need to understand the meaning of “risk” associated with a positive preclinical assessment and be able to weigh the potential benefits of treatment against the consequences of not seeking or participating in recommended interventions. Detailed communication guidelines need to be developed and disseminated with this tool alongside a public health campaign.
Box 1. Neurodiversity and this article’s language.
Neurodiversity is a growing advocacy movement that aims to decrease autism-related stigma by encouraging tolerance of neurological difference (27, 28). Various strategies are encouraged towards this aim, including increasing the visibility of autistic individuals throughout communities, more funding allocation for research focussed on improving the quality of life of autistic individuals (rather than “curing autism”), and inclusion of autistic voices in research, policy, and advocacy. Language is an important tool for neurodiversity. This includes using the phrase “autistic person” rather than “person with autism” to demonstrate that autism is important to identity (27). Linguistic models of neurodiveristy also includes a shift from a “language of deficit” to a “language of difference.” This is done through subtle shifts in language, such as that in the current article. These linguistic choices serve to present ASDs or core attributes of ASD as a particular state of being that is not necessarily considered unwanted or in need of a cure. A discussion of preclinical detection can imply that preventative interventions must be used because autism itself is problemantic. However, in our discussion we advocate that early intervention can ultimately lead to promoting greater community involvement of autistic individuals rather than “normalizing” or eradicating autistic individuals. In both the clinical and research domains the goals and rationale of developing and implementing such technologies will need to be clearly and thoughtfully described.
While the 2014 US Patient Protection and Affordable Care Act (ACA) protects against discrimination of pre-existing conditions (29), it remains unclear how such a preclinical “diagnosis” or “assessment,” which is not a clinically confirmed diagnosis, would impact life- or long-term-care insurance policies. The US ACA states that all Marketplace insurance plans, must cover ASD screening, which usually consists of a behavioral checklist, for children at 18 and 24 months (30), yet it is unclear how the ACA would address the much more costly eye-tracking screening. In addition, only 41 states in the US have passed legislation requiring some level of insurance coverage for ASD services and therapies, but these policies rarely cover screenings (31). Many EU countries follow the recommendations the Royal College of Paediatrics and Child Health, the European Commission of Public Health, and the UK National Screening Committee (32, 33), which discourage community-wide screening (34). The World Health Organization’s resolution on ASD and recent meeting reports do not prioritize screening, focusing instead on increasing resources for autistic individuals and reducing stigma (21, 35). Even with national health care, most countries are not currently prepared to reimburse for infant or preclinical screens.
Stakeholders in the ASD community, including professionals, families, and diagnosed individuals, need to work with public policy makers, researchers, and clinicians to formulate strategies and regulations to determine when preclinical assessment should be performed and who is qualified to interpret and deliver such assessments (e.g., preclinical counselors, clinical psychologists, or primary care doctors). Because information about one’s brain health often feels especially identity forming (36), there must be safeguards for maintaining privacy of preclinical information. Without addressing these concerns, these tools, despite their enormous potential for providing opportunities for early intervention and substantial reduction of individual and societal healthcare costs, risk losing resources and public support to be fully developed and advanced.
Author Contributions
Both JS and KR contributed to the research and writing of this article. JS focused on the areas concerning autism research and practice while KR focused on the neuroethical implications of the article. Both authors were equally involved in the development of this article.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Jones W, Klin A. Attention to eyes is present but in decline in 2-6-month-old infants later diagnosed with autism. Nature (2013) 504:427–31. doi:10.1038/nature12715
2. Elsabbagh M, Bedford R, Senju A, Charman T, Pickles A, Johnson MH, et al. What you see is what you get: contextual modulation of face scanning in typical and atypical development. Soc Cogn Affect Neurosci (2014) 9:538–43. doi:10.1093/scan/nst012
3. Shic F, Macari S, Chawarska K. Speech disturbs face scanning in 6-month-old infants who develop autism spectrum disorder. Biol Psychiatry (2014) 75:231–7. doi:10.1016/j.biopsych.2013.07.009
4. Chawarska K, Macari S, Shic F. Decreased spontaneous attention to social scenes in 6-month-old infants later diagnosed with autism spectrum disorders. Biol Psychiatry (2013) 74:195–203. doi:10.1016/j.biopsych.2012.11.022
5. Elsabbagh M, Gliga T, Pickles A, Hudry K, Charman T, Johnson MH, et al. The development of face orienting mechanisms in infants at-risk for autism. Behav Brain Res (2013) 251:147–54. doi:10.1016/j.bbr.2012.07.030
6. Young GS, Merin N, Rogers SJ, Ozonoff S. Gaze behavior and affect at 6 months: predicting clinical outcomes and language development in typically developing infants and infants at risk for autism. Dev Sci (2009) 12:798–814. doi:10.1111/j.1467-7687.2009.00833.x
7. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association (2013).
8. Lai MC, Lombardo MV, Baron-Cohen S. Autism. Lancet (2014) 383:896–910. doi:10.1016/S0140-6736(13)61539-1
9. Committee on Children with Disabilities. Developmental surveillance and screening of infants and young children. Pediatrics (2001) 108:192–5. doi:10.1542/peds.108.1.192
10. Dabelea D, Zuk AM, Rosenbaum M, Desvarieux M. Prevalence of diagnosed and undiagnosed type 2 diabetes mellitus among US adolescents: results from the continuous NHANES, 1999-2010. Am J Epidemiol (2014) 179:396–7. doi:10.1093/aje/kwt277
11. Luyster R, Gotham K, Guthrie W, Coffing M, Petrak R, Pierce K, et al. The Autism Diagnostic Observation Schedule – toddler module: a new module of a standardized diagnostic measure for autism spectrum disorders. J Autism Dev Disord (2009) 39(9):1305–20. doi:10.1007/s10803-009-0746-z
12. Daniels AM, Halladay AK, Shih A, Elder LM, Dawson G. Approaches to enhancing the early detection of autism spectrum disorders: a systematic review of the literature. J Am Acad Child Adolesc Psychiatry (2014) 53:141–52. doi:10.1016/j.jaac.2013.11.002
13. Allely CS, Gillberg C, Wilson P. Neurobiological abnormalities in the first few years of life in individuals later diagnosed with autism spectrum disorder: a review of recent data. Behav Neurol (2014) 2014:210780. doi:10.1155/2014/210780
14. Buescher AV, Cidav Z, Knapp M, Mandell DS. Costs of autism spectrum disorders in the United Kingdom and the United States. JAMA Pediatr (2014) 168:721–8. doi:10.1001/jamapediatrics.2014.210
15. Iadarola S, Hetherington S, Clinton C, Dean M, Reisinger E, Huynh L, et al. Services for children with autism spectrum disorder in three, large urban school districts: perspectives of parents and educators. Autism (2015) 19:694–703. doi:10.1177/1362361314548078
16. Tomlinson M, Yasamy MT, Emerson E, Officer A, Richler D, Saxena S. Setting global research priorities for developmental disabilities, including intellectual disabilities and autism. J Intellect Disabil Res (2014) 58:1121–30. doi:10.1111/jir.12106
17. Johnson CP, Myers SM, American Academy of Pediatrics Council on Children With, Disabilities. Identification and evaluation of children with autism spectrum disorders. Pediatrics (2007) 120:1183–215. doi:10.1542/peds.2007-2361
18. Screening and Diagnosis. Centers for Disease Control and Prevention. (2015). Available from: http://www.cdc.gov/ncbddd/autism/screening.html
19. Kogan MD, Strickland BB, Blumber SJ, Singh GK, Perrin JM, van Dyck PC. A national profile of the health care experiences and family impact of autism spectrum disorder among children in the United States, 2005-2006. Pediatrics (2008) 122:e1149–58. doi:10.1542/peds.2008-1057
20. Madeo AC, O’Brien KE, Bernhardt BA, Biesecker BB. Factors associated with perceived uncertainty among parents of children with undiagnosed medical conditions. Am J Med Genet A (2012) 158A:1877–84. doi:10.1002/ajmg.a.35425
21. World Health Organization. Autism Spectrum Disorders & Other Developmental Disorders. Geneva: WHO Press (2013).
22. Daley TC. From symptom recognition to diagnosis: children with autism in urban India. Soc Sci Med (2004) 58:1323–35. doi:10.1016/S0277-9536(03)00330-7
23. Gray DE. Everybody just freezes. Everybody is just embarrassed: felt and enacted stigma among parents of children with high functioning autism. Sociol Health Illness (2002) 24:734–49. doi:10.1111/1467-9566.00316
24. Parrott R, Kahl ML, Ndiaye K, Traeder T. Health communication, genetic determinism, and perceived control: the roles of beliefs about susceptibility and severity versus disease essentialism. J Health Commun (2012) 17:762–78. doi:10.1080/10810730.2012.677301
25. Yudell M, Tabok HK, Dawson G, Rossi J, Newschaffer C, Working Group in Autism Risk and Communication and Ethics. Priorities for autism spectrum disorder risk communication and ethics. Autism (2013) 17:701–22. doi:10.1177/1362361312453511
26. Pellicano E, Stears M. Bridging autism, science and society: moving toward an ethically informed approach to autism research. Autism Res (2011) 4:271–82. doi:10.1002/aur.201
27. Kapp S, Gillespie-Lynch K, Sherman L, Hutman T. Deficit, difference or both? Autism and neurodiveristy. Dev Psychol (2013) 49:59–71.
28. Jaarsma P, Welin S. Autism as a natural human variation: reflections on the claims of the neurodiversity movement. Health Care Anal (2012) 20:20–30. doi:10.1007/s10728-011-0169-9
29. Arias JJ, Karlawish J. Confidentiality in preclinical Alzheimer disease studies: when research and medical records meet. Neurology (2014) 82:725–9. doi:10.1212/WNL.0000000000000153
30. Mauch D, Pfefferle S, Booker C, Levin J. Report on State Services to Individuals with Autism Spectrum Disorders. Final Report for Centers for Medicare & Medicaid Services (CMS) ASD Services Project. Cambridge, MA: Abt Associations Inc (2011).
31. State Initiatives. Autism Speaks. (2015). Available from: https://www.autismspeaks.org/state-initiatives
32. Le Couteur A, Core Working Group. National Autism Plan for Children (NAPC): Plan for the Identification, Assessment, Diagnosis and Access to Early Interventions for Pre-School and Primary School Aged Children with Autism Spectrum Disorders (ASDs). London: The National Autistic Society (2003).
33. Allaby M, Sharma M. Screening for Autism Spectrum Disorders in Children Below the Age of 5 Years: A Draft Report for the UK National Screening Committee. Oxford: Solutions for Public Health (2011).
34. García-Primo P, Hellendoorn A, Charman T, Roeyers H, Dereu M, Roge B, et al. Screening for autism spectrum disorders: state of the art in Europe. Eur Child Adolesc Psychiatry (2014) 23:1–17. doi:10.1007/s00787-014-0555-6
35. World Health Organization. Comprehensive and Coordinated Efforts for the Management of Autism Spectrum Disorders. Geneva: WHO Press (2014).
Keywords: pediatric ethics, neuroethics, autism spectrum disorder, eye-tracking, preclinical detection, early detection technology
Citation: Sarrett JC and Rommelfanger KS (2015) Commentary: Attention to Eyes Is Present but in Decline in 2–6-Month-Old Infants Later Diagnosed with Autism. Front. Public Health 3:272. doi: 10.3389/fpubh.2015.00272
Received: 03 September 2015; Accepted: 23 November 2015;
Published: 08 December 2015
Edited by:
Gerry Leisman, National Institute for Brain & Rehabilitation Sciences, Israel; Universidad de Ciencias Médicas de La Habana, CubaReviewed by:
Corrado Romano, IRCCS Associazione Oasi Maria Santissima, ItalyEsther Ben-Itzchak, Ariel University, Israel
Copyright: © 2015 Sarrett and Rommelfanger. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jennifer C. Sarrett, anNhcnJldHRAZW1vcnkuZWR1;
Karen S. Rommelfanger, a3JvbW1lbEBlbW9yeS5lZHU=
 Jennifer C. Sarrett
Jennifer C. Sarrett Karen S. Rommelfanger
Karen S. Rommelfanger