- 1Department of Pharmacology and Toxicology, Faculty of Medicine Novi Sad, University of Novi Sad, Novi Sad, Serbia
- 2Department of Gynecology, Clinical Centre of Vojvodina, Novi Sad, Serbia
- 3University of Bijeljina, Dvorovi, Bosnia and Herzegovina
- 4Institute of Radiology, Institute for Child and Youth Health Care, Vojvodina, Novi Sad, Serbia
Introduction
Cancer is a leading cause of death (1)-based on WHO data, 8.2 million people die each year from cancer, an estimated 13% of all deaths worldwide (2). Cancer medicine is an important component of overall health care costs (3, 4). The shift from conventional cytotoxic drugs to targeted cancer therapies (TCT) has caused the cost for cancer treatment to rise substantially with often modest survival benefits and sometimes only for the purpose of palliative treatment (5–8). There is currently little association between therapeutic effects and requested prices, e.g., of the 12 drugs approved by the FDA for cancer in 2012, 9 were priced at more than US$10,000/month with only 3 prolonging survival, two by less than 2 months (5, 9). Drug expenditure is one of the largest components of health spending in Eastern Europe (10, 11), i.e., spending for monoclonal antibodies used in malignancies in Serbia has shown 20-fold increase from 2004 to 2012 (7). Several studies that compared availability and reimbursement of TCTs among different health care settings, regions and countries, demonstrated high rate of variation in number of medications reimbursed between 12–EU and USA (16), as well as among different EU countries (16–20). With large discrepancy between cost and clinical benefit for some TCTs (6, 18, 21), reimbursement of these medication represents an issue even for high income countries. There is limited data about the use and reimbursement of these drugs in former Yugoslavia (22). Countries of former Yugoslavia deal with limited financial resources available for health care spending, based on payroll taxation as a major source of financing and most public expenditure on health flows through the health insurance funds (23). High unemployment rates, and a large share of the active labor force working in the informal sector where contributions to health insurance are not made (22, 24), further complicates the functioning of insurance health funds. Countries of former Yugoslavia, being in the process of harmonization with the EU, try to bridge the gap between the conflicting need to reimburse novel, expensive medication and lack of resources (25, 26).
Financial Burden of Drugs for Malignancies
The cost of delivering high quality cancer care is outstripping the national budgets of countries of former Yugoslavia. Serbia (SRB), Bosnia and Herzegovina (BiH), FYR Macedonia (MKD) and Croatia (HRV) are classified as upper middle income, while Slovenia (SVN) is the only high income country according to the World Bank data. As witnessed by current estimates given in Table 1, health care financing remains under pressure—Serbia and Republic of Srpska (RS) spend the largest share of GDP on healthcare, but actual health expenditure per capita is only around 500 USD. Pharmaceutical expenditure is one of the key components in increased health-care spending, and cancer treatment is important component of pharmaceutical expenditure. Between 10 and 25% of total pharmaceutical expenditure in eastern Europe was related to the procurement of drugs for malignancies. For referencing, Slovakia (SVK), an EU country which shares large number of similarities to the countries of the former Yugoslavia region1, spent almost a quarter of total pharmaceutical expenditure on drugs for malignancies. Aside from the other health costs associated with cancer, pharmacotherapy is an important component of the cancer treatment—recent study conducted in Serbia concluded that pharmacotherapy costs accounted for 42.37% of all cancer related health care expenditure (27). TCTs are the major cancer care cost drivers in Eastern Europe as these drugs are relatively expensive in comparison to the other cancer treatment options. Monoclonal antibodies (Table 2) and protein-kinase inhibitors (Table 3) absolute expenditure is much lower in RS and Serbia compared to Slovakia, Slovenia, and Croatia, but high proportion of pharmaceutical spending stems from TCT procurement. Given the increase in malignancy incidence, aging population, and lack of fully implemented screening programs, the financial pressure novel cancer treatments pose on budgets in countries in transition is expected to increase further in the future. Differences in TCTs spending might be related to differences in level of reimbursement across Eastern Europe, as these large expenses are limiting factor for reimbursement of these medication (26), especially in low resource settings.
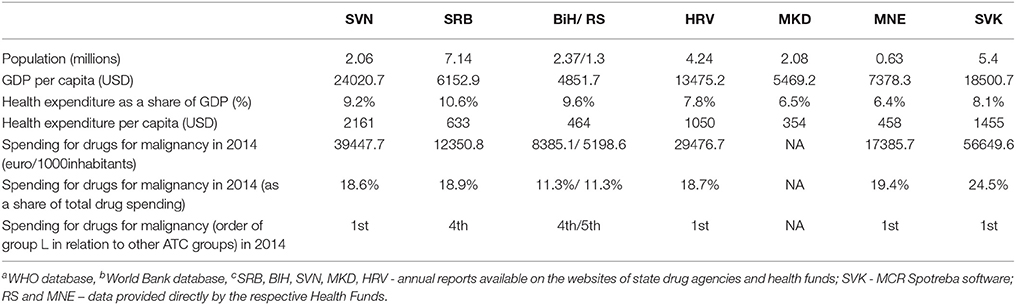
Table 1. Characteristics of countries: basic health care parametersa, health spendingb and spending for drugs for malignanciec.
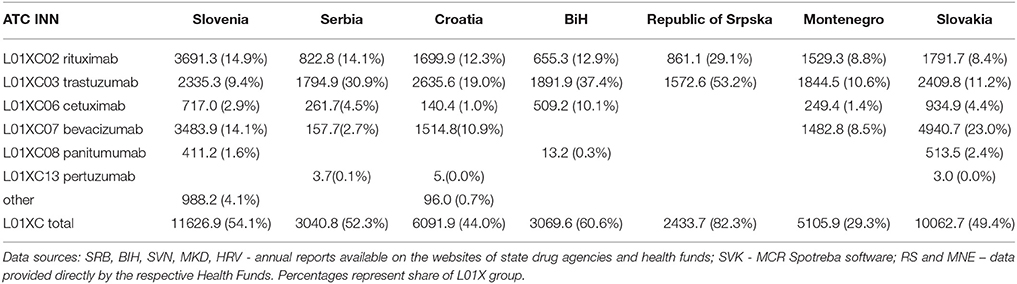
Table 2. Pharmaceutical spending for drugs from group L01XC (monoclonal antibodies) in euro/1000inhabitants.
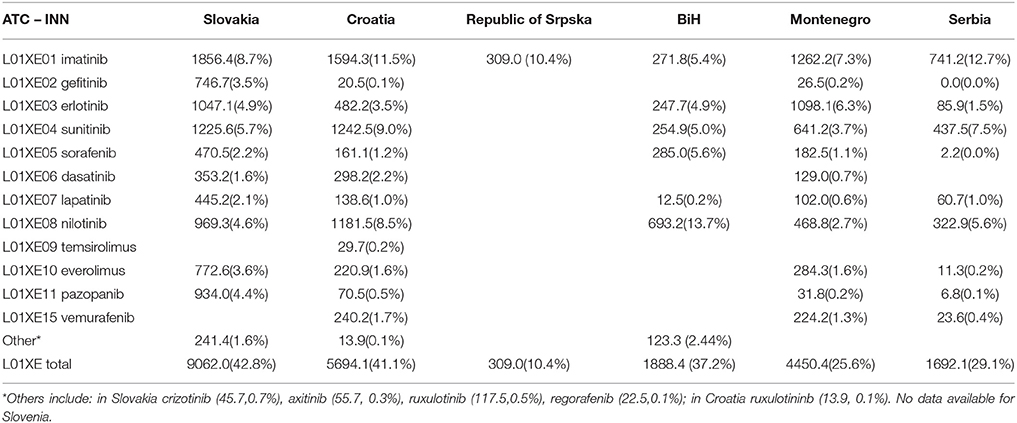
Table 3. Pharmaceutical spending for drugs from group L01XE (TK inhibitors) in euro/1000inhabitants.
Reimbursement of Novel Drugs for Malignancies
Where there are specialized national heath technology assessment (HTA) frameworks present, the process of reimbursement of novel cancer treatments is based on pharmacoeconomic evaluation which is necessary in order to maximize the cost-effectiveness of these expensive medication (25, 28). Within EU, there are large differences in the number of approved TCTs and stringency of levels at which drug is considered cost-effective (29). Also, some of the TCTSs are subjected to special evaluations as they are considered orphan drugs. The EU has created a common procedural framework through the adoption of Transparency Directive (Council Directive 89/105/EEC) to ensure that national pricing and reimbursement decisions are made in a transparent manner. In the Balkans, such information is not readily available. Each country uses different schemes and policies for the pharmaceutical pricing and reimbursement adapted to its own economic and health needs (11, 28, 30, 31). Positive drug lists are available on the websites of the respective health funds, but the information about the assessment guiding the reimbursement decision process is difficult to find. Cost-effectiveness studies on TCT in oncology have been scarcely reported in published literature in the Balkan region (32).
Eastern Europe belongs to the quite a different healthcare milieu compared to the developed Western economies (25). Following the fall of the SFRJ, newly formed countries struggled with remnants of socialistic health policies and the rising expenses of modern healthcare. The systems in place could no longer meet the needs of growing and aging population. While many of the Balkan countries nominally guarantee universal healthcare coverage through compulsory health insurance (26) in reality there are numerous obstacles in providing health care services in the former Yugoslavia region. Countries with lower GDP such as RS, Montenegro, Macedonia, and Serbia, all have good access to classic cytotoxic drugs, but availability of novel cancer therapies is limited. As of 1st September 2017, only rituximab, trastuzumab, and imatinib are reimbursed in all of the countries of former Yugoslavia (Figure 1). However, drugs such as rituximab, trastuzumab, and imatinib, drugs included on the WHO list of essential medicines and considered standard of care for a range of malignancies are reimbursed in all surveyed countries. However, more developed countries such as Slovenia and Croatia have higher number of reimbursed TCTs than other countries of former Yugoslavia. These differences might be related to different mechanisms of reimbursement decision making. In Bosnia and Herzegovina, health decision processes are carried out by the expert boards of the health funds (33). In the RS, drugs for malignancies are listed on the separate list of cytotoxic drugs. The reimbursement decisions are based on the experience of Serbian tertiary health care institutions and recommendations of the European Society for Medical Oncology. In the Federation of BiH, drugs for malignancies are included on the special positive list, so called “solidarity list” which includes drugs for malignancies, multiple sclerosis, hemophilia, HIV and other diseases. Solidarity fund aims to provide universal coverage of the patients with specific condition throughout the 10 cantons in BiH federation. Small number of registered innovative and brand medicines in Macedonia is a result of the generic prescribing policies, the delays, and the strict inclusion rules for the positive list (34). Some novel medicines have been rejected on the justification for limited national financial means and the existence of therapeutic alternatives. Serbia made effort to increase the availability of cutting edge cancer treatment, and in the 2016 revision of positive list included large number of TCTs not formerly available, making the reimbursement status similar to Croatia and Slovenia. The impact of this change on patient outcomes and expenditure need to be assessed in the future. There is no national HTA agency in Serbia, but the National Health Insurance Fund is involved in pharmacoeconomic assessment and health ministry includes an HTA Committee to support reimbursement decisions. As mentioned before, no information about pharmacoeonomic assessment guiding the reimbursement decisions can be found on the respective websites. This is the consequence of the fact that comprehensive and consistent systems for HTA are non-existent or underdeveloped in most of the region (33, 35). Despite some progress, pharmaceutical market is still inadequately responsive to population needs (8). On the contrary, Slovakia has national HTA agency, and assessment of added therapeutic value in Slovakia is conducted during the decision making process on the reimbursement of medicines. The pharmacoeconomic analysis is conducted by a specialist working group and these reports are mandatory during the reimbursement process (28). Out of countries of former Yugoslavia, only Slovenia and Croatia have made steps toward full HTA implementation (3, 30, 36, 37). In 2006, Croatia developed strategy for the development of the Croatian health care system which included formation of the independent, non-profit institution called Agency for Quality and Accreditation in Health. This is the basis for the HTA procedures in Croatia. Furthermore, Croatia has a special Fund for very expensive drugs 400.000.000,00 HRK (53 million €) (38). In Slovenia, HTA-related processes are carried out by the Agency for medicinal products and medical devices of the Republic of Slovenia (JAZMP) and National Institute of Public Health (NIJZ) and autonomous research organizations (39). No national HTA organization has been established, but the results of HTAs have to be considered in decision making process for planning, budgeting, pricing and reimbursement of health products. However, it is still not obligatory for the conclusion and policy outcomes of the HTA to be publicly available. In other countries, currently there is no formal HTA system in place (40). Deficiency of a HTA system and therefore inefficient procurement processes mean that the Balkan countries often invest in less cost-effective medicines and sometimes pay more than west European countries (3, 11, 26, 28). Several of the monoclonal antibodies were deemed not cost-effective for all of the recommended indications according to the NICE National Institute for Health and Care Excellence (NICE), one of the most important HTA agencies in Europe, criteria (11). Technologies not cost-effective by NICE appraisal are probably not going to be worth the resources in current setting. In order to make the most of the funds available, countries of former Yugoslavia need to develop mechanisms for development and implementation of HTA systems in the drug reimbursement processes. These strategies could help with overcoming difficulties in funding and delivering medical care in emerging markets with a rapidly growing demand for health services (23).
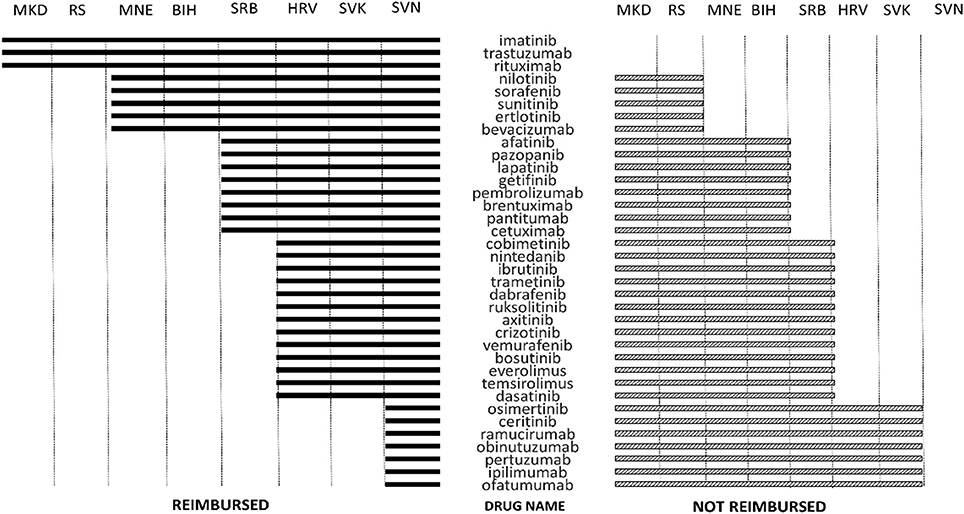
Figure 1. Reimbursement status of innovative oncological medicines in countries of former Yugoslavia on September 1st, 2017. Source websites of the respective websites with the last available positive drug list on the date of data extraction.
Implications
Large variations and inconsistencies in the decision and processes of assessing and determining the reimbursement of novel cancer treatments was noted in the present study. This extent of the regional disparities in availabilty of TCTs should motivate the decision makers in the region to identify and implement innovative financing mechanisms to expand financial resources available for reimburesemt of novel cancer medication. National systems need to be regularly reviewed and adapted in order to take into account market evolutions and patients' needs. Comprehensive system rooted in responsible reimbursement policy based on cost–effectiveness principles is needed for assessing both new and existing healthcare technologies. The optimum strategy to achieve value in the provision of cancer care in the Balkans needs to be developed.
Author Contributions
AS and ZT Study conception and design. LT, IV, and ZB Acquisition of data. AT, OH, and MPK Analysis and interpretation of data. AT and ZB Drafting of manuscript. AS and ZT Critical revision.
Funding
This work was supported by the Ministry of Science and Technological Development, Republic of Serbia (project No. 41012) and by the Provincial Secretariat for Science and Technological Development, Autonomous Province of Vojvodina (project No.114-451-2178/2016-03).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
1. ^Historical background, healthcare system with compulsory public health insurance as a main pillar of health financing, largely centralized health-care system with the overall power trusted to the ministries of health and social welfare and the health insurance funds and remnants of the old system based on the principle of free treatment with the proportion of health funding from private insurance or direct payments still marginal in comparison to the western Europe average.
References
1. Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. (2017) 3:524–48. doi: 10.1001/jamaoncol.2016.5688
2. World Health Organization. Cancer Factsheet. Available online at: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx (2017).
3. Pejcic A, Jakovljevic M. Pharmaceutical expenditure dynamics in the Balkan countries. J Med Econ. (2017) 20:1013–7. doi: 10.1080/13696998.2017.1333514
5. Etzioni R, Gulati R, Lin DW (ed). Measures of survival benefit in cancer drug development and their limitations. Urol Oncol. (2015) 33:122–7. doi: 10.1016/j.urolonc.2014.11.002
6. Jakovljevic M, Malmose-Stapelfeldt C, Milovanovic O, Rancic N, Bokonjic D. Disability, Work absenteeism, sickness Benefits, and cancer in selected european OecD countries—Forecasts to 2020. Front Public Health (2017) 5:23. doi: 10.3389/fpubh.2017.00023
7. Jakovljevic MB. Oncology monoclonal antibodies expenditure trends and reimbursement projections in the emerging Balkan market. Farmeconomia Health Econ Therapeut Pathways (2014) 15:27–32. doi: 10.7175/fe.v15i1.909
8. Stojkovic M, Milovanovic O. Health spending follows pace of population aging: challenges lying ahead of the largest Western Balkan market. Front Public Health (2015) 3:58. doi: 10.3389/fpubh.2015.00058
9. Stegmeier F, Warmuth M, Sellers W, Dorsch M. Targeted cancer therapies in the twenty-first century: lessons from imatinib. Clin Pharmacol Therapeut. (2010) 87:543–52. doi: 10.1038/clpt.2009.297
10. Iskrov G, Miteva-Katrandzhieva T, Stefanov R. Challenges to orphan drugs access in Eastern Europe: the case of Bulgaria. Health Policy (2012) 108:10–8. doi: 10.1016/j.healthpol.2012.08.013
11. Jakovljević M, Jovanović M, Lazić Z, Jakovljević V, Ðukić A, Velicković R, et al. Current efforts and proposals to reduce healthcare costs in Serbia. Serb J Exp Clin Res. (2011) 12:161–3.
12. Faden RR, Chalkidou K, Appleby J, Waters HR, Leider JP. Expensive cancer drugs: a comparison between the United States and the United Kingdom. Milbank Q. (2009) 87:789–819. doi: 10.1111/j.1468-0009.2009.00579.x
13. Philipson T, Eber M, Lakdawalla DN, Corral M, Conti R, Goldman DP. An analysis of whether higher health care spending in the United States versus Europe is ‘worth it’ in the case of cancer. Health Aff. (2012) 31:667–75. doi: 10.1377/hlthaff.2011.1298
14. Aggarwal A, Ginsburg O, Fojo T. Cancer economics, policy and politics: What informs the debate? Perspectives from the EU, Canada and US. J Cancer Policy (2014) 2:1–11. doi: 10.1093/annonc/mdx110
15. Aggarwal A, Sullivan R. Affordability of cancer care in the United Kingdom – Is it time to introduce user charges? J Cancer Policy (2014) 2:31–9. doi: 10.1016/j.jcpo.2013.11.001
16. Luengo-Fernandez R, Leal J, Gray A, Sullivan R. Economic burden of cancer across the European Union: a population-based cost analysis. Lancet Oncol. (2013) 14:1165–74. doi: 10.1016/S1470-2045(13)70442-X
17. Cheema PK, Gavura S, Migus M, Godman B, Yeung L, Trudeau ME. International variability in the reimbursement of cancer drugs by publically funded drug programs. Curr Oncol. (2012) 19:e165–76. doi: 10.3747/co.19.946
18. Drummond M, de Pouvourville G, Jones E, Haig J, Saba G, Cawston H. A comparative analysis of two contrasting European approaches for rewarding the value added by drugs for cancer: England versus France. PharmacoEconomics (2014) 32:509–20. doi: 10.1007/s40273-014-0144-z
19. Chamberlain C, Collin SM, Hounsome L, Owen-Smith A, Donovan JL, Hollingworth W. Equity of access to treatment on the Cancer Drugs Fund: A missed opportunity for cancer research? J Cancer Policy (2015) 5:25–30. doi: 10.1016/j.jcpo.2015.06.003
20. Mihajlović J, Dolk C, Tolley K, Simoens S, Postma MJ. Reimbursement of Targeted cancer therapies within 3 different european health care systems. Clin Therapeut. (2015) 37:474–80. doi: 10.1016/j.clinthera.2014.12.005
21. Sharma P, Wagner K, Wolchok JD, Allison JP. Novel cancer immunotherapy agents with survival benefit: recent successes and next steps. Nat Rev Cancer (2011) 11:805–12. doi: 10.1038/nrc3153
22. Petrusic T, Jakovljevic M. Budget impact of publicly reimbursed prescription medicines in the Republic of Srpska. Front Public Health (2015) 3:213. doi: 10.3389/fpubh.2015.00213
23. Jakovljevic M, Lazarevic M, Milovanovic O, Kanjevac T. The new and old Europe: east-west split in pharmaceutical spending. Front Pharmacol. (2016) 7:18. doi: 10.3389/fphar.2016.00018
24. Bredenkamp C, Gragnolati M. Sustainability of Healthcare Financing in the Western Balkans: An Overview Of Progress and Challeages. Washington, DC: World Bank Publications (2007).
25. Srivastava D, McGuire A. Analysis of prices paid by low-income countries-how price sensitive is government demand for medicines? BMC Public Health (2014) 14:767. doi: 10.1186/1471-2458-14-767
26. Jakovljevic MB. Resource allocation strategies in Southeastern European health policy. Eur J Health Econ. (2013) 14:153–9. doi: 10.1007/s10198-012-0439-y
27. Kovačević A, Dragojević-Simić V, Rančić N, Jurišević M, Gutzwiller F, Matter-Walstra K, et al. End-of-life costs of medical care for advanced stage cancer patients. Vojnosanit Pregl. (2015) 72:334–41. doi: 10.2298/VSP1504334K
28. Sorenson C, Kanavos P, Karamalis M. HTA in central and Eastern Europe: current status, challenges and opportunities. J Med Dev Reg. (2009) 6:34–45.
29. Dagovic A, Matter Walstra K, Gutzwiller S, Djordjevic N, Rankovic A, Djordjevic G, et al. Resource use and costs of newly diagnosed cancer initial medical care. Eur J Oncol. (2014) 19:166–84. Available online at: http://mattioli1885journals.com/index.php/Europeanjournalofoncology/article/view/3640
30. Jakovljevic M, Vukovic M, Chen C-C, Antunovic M, Dragojevic-Simic V, Velickovic-Radovanovic R, et al. Do health reforms impact cost consciousness of health care professionals? Results from a nation-wide survey in the Balkans. Balkan Med J. (2016) 33:8–17. doi: 10.5152/balkanmedj.2015.15869
31. Jakovljevic MB, Djordjevic N, Jurisevic M, Jankovic S. Evolution of the Serbian pharmaceutical market alongside socioeconomic transition. Exp Rev Pharmacoeconom Outcomes Res. (2015) 15:521–30. doi: 10.1586/14737167.2015.1003044
32. Mihajlović J, Pechlivanoglou P, Sabo A, Tomić Z, Postma MJ. Cost-effectiveness of everolimus for second-line treatment of metastatic renal cell carcinoma in Serbia. Clin Therapeut. (2013) 35:1909–22. doi: 10.1016/j.clinthera.2013.10.004
33. Catic T, Begovic B. PHP126 Overview of HTA Process and implementation among health stakeholders in bosnia and herzegovina–survey based research. Value Health (2011) 14:A356. doi: 10.1016/j.jval.2011.08.677
34. Angelovska B, Ivanovska V, Drakalska E. The Climate for the Innovative Medicines in the Republic of Macedonia. (2014). Available online at: http://eprints.ugd.edu.mk/10151/17/Innovative%20medicines%20-%20Eng.pdf
35. Van WIlder P, Mabilia V, Kuipred Cavaco Y, Mc Guinn J. Towards a Harmonised EU Assessment of the Added Therapeutic Value of Medicines. Brussels: European Parliament (2015). p. 115.
36. Dankó D, Petrova G. Health technology assessment in the Balkans: opportunities for a balanced drug assessment system. Biotechnol Biotechnol Equip. (2014) 28:1181–9. doi: 10.1080/13102818.2014.978636
37. Dagovic A, Zugic A, Jakovljevic MB. Macroeconomic policy impact on oncology-related public expenditure in an emerging European market–signs of early recovery. Serbian J Exp Clin Res. (2015) 16:43–50. doi: 10.1515/sjecr-2015-0007
38. Huic M editor. Health Technology Assessment (HTA) in Croatia: First five years. ADVANCE-HTA Capacity Building Workshop, Warsaw. Available online at: http://www.advance-hta.eu/PDF/WarsawWorkshop/Presentations/7-HTA-Croatia.pdf (2014).
39. Turk E, Albreht T. HTA in Slovenia-New Developments: Institute of Public Health of the Republic of Slovenia Available online at: http://hpm.org/si/a11/4.pdf (2008).
Keywords: targeted therapy, monoclonal antibodies, protein kinase inhibitors, HTA, balkans
Citation: Tomić Z, Tomas A, Benšova Z, Tomić L, Horvat O, Varga I, Paut Kusturica M and Sabo A (2018) Challenges of Providing Access to Cutting-Edge Cancer Medicines in the Countries of Eastern Europe. Front. Public Health 6:193. doi: 10.3389/fpubh.2018.00193
Received: 18 April 2018; Accepted: 25 June 2018;
Published: 24 July 2018.
Edited by:
Nemanja Rancic, Military Medical Academy, SerbiaReviewed by:
Georgi Iskrov, Plovdiv Medical University, BulgariaMaja Račić, University of East Sarajevo, Bosnia and Herzegovina
Copyright © 2018 Tomić, Tomas, Benšova, Tomić, Horvat, Varga, Paut Kusturica and Sabo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ana Tomas, YWFuYWFfdG9tYXNAeWFob28uY29t; YW5hLnRvbWFzQG1mLnVucy5hYy5ycw==
 Zdenko Tomić1
Zdenko Tomić1 Ana Tomas
Ana Tomas Ana Sabo
Ana Sabo