Abstract
We investigated the effects of walking in a forest environment on salivary cortisol concentrations. Seventy-four young male participants walked for 15 min in forested and urban environments, and saliva was collected before and after walking. Our previous study reported salivary cortisol concentrations after walking only. This study was aimed at clarifying the combined effects of walking and environment by comparing post-walking data with pre-walking data. Walking in a forest environment decreased mean cortisol concentration from 9.70 to 8.37 nmol/L, whereas walking in an urban environment barely changed mean cortisol concentration, from 10.28 to 10.01 nmol/L. Two-way repeated analysis of variance revealed a significant interaction effect between the environment and walking (p < 0.001) in addition to the main effects of each (p < 0.001 and p = 0.001, for walking and environment, respectively). For further analysis, the proportion of participants who exhibited decreased cortisol after forest-walking was compared with the previously reported proportion of participants who exhibited decreased cortisol after viewing forest landscapes. Although the proportion of positive responders was slightly higher after walking (69%) than it was after viewing (60%), this difference was not statistically significant (p = 0.093). The present study revealed a significant combined effect of walking and the environment on cortisol concentrations.
Introduction
Recent years have seen growing interest in the therapeutic effects of forest environments on human health. “Shinrin-yoku,” a Japanese term that means “forest-bathing,” is now used internationally to refer to forest exposure for therapeutic or preventive health purposes (1–4). As Bowler et al. (5) reported, 13 studies on the effects of walking in natural environments were published between 1991 and 2008, most of which reported that nature therapy decreased negative emotions, such as anger, fatigue, and sadness, and enhanced positive emotions, such as energy. The psychological benefits of forest-walking have been supported by many subsequent studies (6, 7). However, the benefits of forest-walking are not only psychological. Other studies have shown that it has desirable effects on physiological functions as well, including decreased blood pressure (8–11), increased high-frequency component (HF) of heart rate variability (HRV) (12–15), decreased cerebral activity (16), and enhanced immune functions (17, 18).
Cortisol level is another metric that has been used in studies on the therapeutic effects of forest exposure (16, 19–24). Cortisol is a steroid hormone produced by the zona fasciculata of the adrenal cortex to regulate carbohydrate, fat, and protein metabolism. Cortisol level is considered to be an indicator of the activity of the hypothalamic-pituitary-adrenal (HPA) axis, which is responsible for corticotropin-releasing hormone (CRH) and adrenocorticotropin (ACTH) secretion. The HPA system is among the major stress response systems in humans. ACTH-mediated cortisol secretion increases blood pressure and glucose levels while suppressing the immune system; this set of physiological reactions is called the “fight-or-flight” response. Cortisol level has accordingly been used as a biological indicator of stress.
The authors have previously reported that walking in a forested environment significantly reduced salivary cortisol concentrations in 74 young male participants (25). In that study, post-walking data were compared between subjects who had walked in forest environments and subjects who had walked in urban environments, but pre-walking data was not included in the analysis. Given that salivary cortisol is affected not only by environmental stress but also by physical exercise such as walking, however, it is desirable to separate the effect of walking and that of environment in studies of nature therapy. Several previous studies have reported that walking in a forested environment reduces serum (9, 19) or salivary (20, 21, 23) cortisol concentrations; however, no study has yet clearly demonstrated the interaction between the effects of walking and environment.
In this study, salivary cortisol data collected before walking was analyzed along with our previously published results. Two-way analysis of variance (ANOVA) was performed to examine the combined effects of walking and forest exposure. In addition, we compared the results of forest-walking with those of forest-viewing reported in our previous study (24) to determine whether forest-walking has a stronger beneficial effect than forest-viewing has.
Materials and Methods
Study Sites and Participants
The study areas were seven forests and seven urban areas across Japan. Urban areas were selected near city centers or railway stations. Seventy-five Japanese male university students (aged 20–29 years) participated in the experiments. As one participant's pre-walking cortisol concentration data was missing, however, salivary cortisol concentrations of 74 participants were analyzed.
Demographic parameters of the 74 male participants are shown in Table 1. According to the Japanese National Health and Nutrition Survey 2016 (26), mean height and weight of Japanese males aged 20–29 years were reported as 171.5 cm and 67.6 kg, respectively. These reference values were almost identical to those of our participants. Thus, the participants in this study can be regarded as a representative sample of the Japanese young male population.
Table 1
| Age (year) | Height (cm) | Weight (kg) | BMI | |
|---|---|---|---|---|
| Mean | 22.4 | 172.4 | 65.4 | 22.0 |
| SD | 1.8 | 5.8 | 10.3 | 3.1 |
| Max | 29 | 187.5 | 110.0 | 33.6 |
| Min | 20 | 155.0 | 50.0 | 17.4 |
Demographics of the male participants (n = 74).
BMI, Body mass index.
None of the participants reported a history of physical or psychiatric disorders. During the study period, alcohol and tobacco consumption was prohibited and caffeine consumption was limited.
Experimental Design
The experiment was performed over two consecutive days at each of the seven study areas. Prior to the experiment, the aim of this study and the experimental protocol were explained and general instructions were provided to the participants. After this orientation, the participants visited and previewed the two experimental sites in their study area. The participants in each study area were randomly divided into two groups, one of which performed the experiment in the forest area prior to the urban area, while the other group performed the experiment in the urban area prior to the forest area.
On the experiment day, participants woke up between 6:30 and 7:30 a.m. After arriving at experimental site, the participants rested in a chair for 5 min and then saliva was sampled before they began walking. Each participant walked in his assigned urban or forested environments for 15 min. Walking paths were nearly level in all environments. In this study, participants were instructed to walk relatively slowly and to walk at the same speed in both the environments. We confirmed that no significant difference was observed regarding walking speed between forest and urban environments. Second saliva sample was collected immediately after the walking.
After this first experiment, all participants spent the night in identical single rooms at the same hotel. On the second day, the participants switched field sites. The experimental protocol for the second day was the same as that for the first day.
Salivary Cortisol Measurements
Salivary samples were collected before lunch. The time of saliva collection varied from 9:30 a.m. to 12:00 p.m. Although the time of saliva collection varied among the participants, each participant was measured at approximately the same time on the two experimental days.
Saliva was collected using the Salivette® system (No. 51.1534; Sarstedt, Nümbrecht, Germany). Each participant held two pieces of absorbent cotton in his mouth for 2 min, and the saliva in these cotton wads was later extracted by centrifugation. The samples were immediately frozen and transported to the laboratories of SRL, Inc., (Tokyo, Japan). Each sample consisted of a 0.25-mL aliquot of saliva, and its cortisol concentration was analyzed by radioimmunoassay.
In addition to salivary cortisol, heart rate, blood pressure, salivary immunoglobulin A concentration, and Profile of Mood States (POMS) were also measured in this experiment; the present study, however, deals only with the salivary cortisol results. Post-walking data for the other physiological metrics were reported in our previous paper (25).
Statistical Analysis
The combined effect of environment (urban or forest) and walking (before or after) on salivary cortisol concentration was statistically tested by two-way repeated measure analysis of variance (ANOVA). Both environment and walking were analyzed as within-subject factors. In addition to the p-value, partial eta-squared (η2) was calculated as an effect size for the factor. The simple main effect was then analyzed as a post-hoc test. The above statistical tests were performed on natural-log-transformed data rather than raw cortisol concentrations because cortisol concentrations exhibit a non-normal distribution except in the early morning (27, 28).
For further analysis, the proportions of participants who exhibited positive and negative responses were compared. Positive response was defined as having a lower cortisol concentration immediately before walking in the forest environments than immediately before walking in the urban environments. Negative response was defined as the opposite. The results were compared with those of our previous study (24) in which we investigated salivary cortisol concentrations in 348 young male participants after they viewed forest landscapes. The difference between the present and previous studies with respect to the ratio of negative to positive responders was compared by the Chi-squared test. A p < 0.05 was considered to indicate statistical significance for the ANOVA and Chi-squared tests. These statistical analyses were performed using R ver. 3.5.3.
Results
Statistics for cortisol concentration are presented in Table 2. Note that the post-walking cortisol concentrations are the data reported in our previous paper (25).
Table 2
| Salivary cortisol concentration (nmol/L) | ||||
|---|---|---|---|---|
| Urban | Forest | |||
| Before | After | Before | After | |
| Mean | 10.28 | 10.01 | 9.70 | 8.37 |
| SD | 4.58 | 3.98 | 4.04 | 2.90 |
| Median | 8.83 | 8.97 | 8.69 | 7.73 |
| Skewness* | 0.30 | 0.14 | 0.24 | −0.13 |
| Kurtosis* | −0.39 | −0.46 | 0.41 | 0.21 |
Statistics for salivary cortisol concentrations in forest and urban environments (n = 74).
Skewness and kurtosis were calculated based on logarithmic-transformed cortisol concentration data.
Walking in a forest environment decreased mean cortisol concentration from 9.70 to 8.37 nmol/L. Walking in an urban environment, on the other hand, barely changed mean cortisol concentration from 10.28 to 10.01 nmol/L. Similar tendencies were also observed in the median values, which changed from 8.69 to 7.73 nmol/L for forest-walking, and from 8.83 to 8.97 nmol/L for urban-walking.
The skewness and kurtosis presented in Table 2 were calculated based on log-transformed cortisol concentrations whereas the other statistics were calculated based on raw cortisol concentrations. The skewness and kurtosis values were nearly zero, suggesting that the log-transformed cortisol concentrations indicated almost normal distribution.
The results of ANOVA are summarized in Table 3. Both environment (urban or forest) and walking (before or after) were statistically significant factors (p = 0.001 and p < 0.001, respectively). In addition to the main effects, the interaction effect between environment and walking was statistically significant (p < 0.001).
Table 3
| Factor | F(1, 73) | p | Partial η2 |
|---|---|---|---|
| Walking (before/after) | 12.59 | <0.001 | 0.147 |
| Environment (urban/forest) | 10.97 | 0.001 | 0.131 |
| Interaction (environment × walking) | 15.17 | <0.001 | 0.172 |
Results of two-way repeated measure ANOVA (n = 74).
ANOVA (analysis of variance) was performed on log-transformed cortisol concentration data.
The results of the post-hoc comparisons are shown in Figure 1. The post-hoc tests revealed that mean cortisol concentration after walking was significantly lower in forest environments than in urban environments (p < 0.001), whereas mean cortisol concentration before walking was not significantly different between forest and urban environments (p = 0.207). Comparison of the data taken before and after walking revealed that walking in a forest environment significantly decreased salivary cortisol (p < 0.001), whereas walking in an urban environment did not significantly change salivary cortisol (p = 0.658).
Figure 1
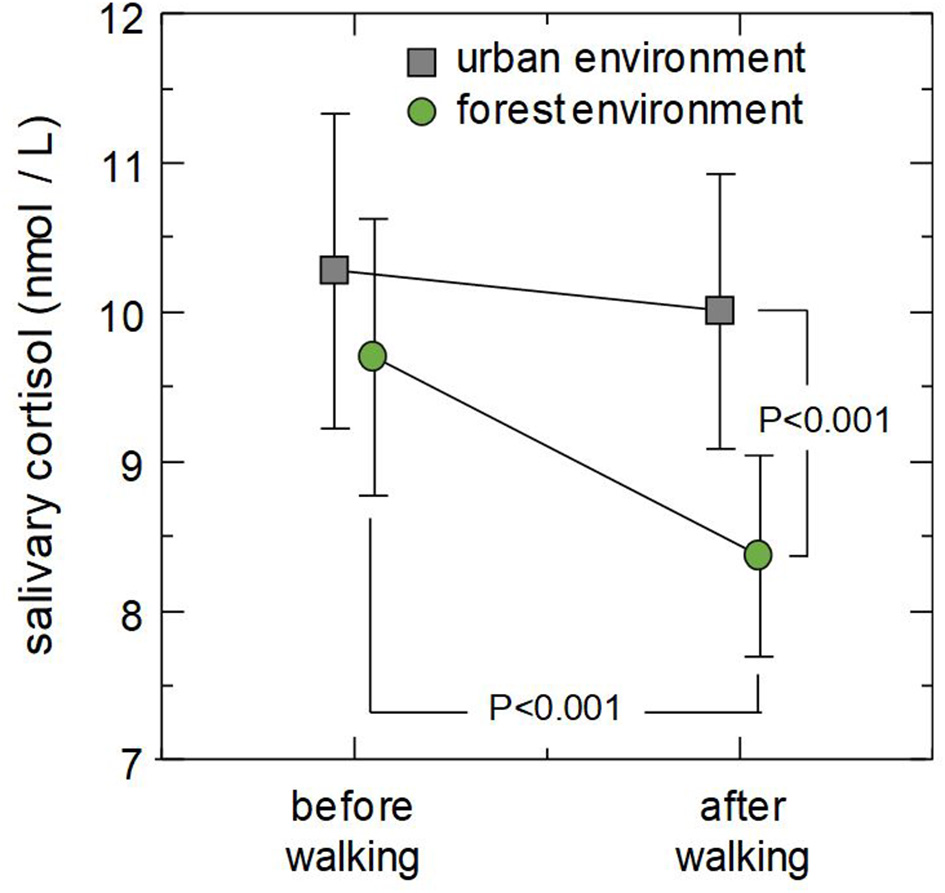
Combined effect of environment and walking on salivary cortisol concentrations. Markers indicate mean cortisol concentrations in urban (square) and forest (circle) environments and error bars designate ±2SE (standard error). P-values are the results of post-hoc analysis (simple main effect test).
Fifty-one (69%) of the 74 participants exhibited a positive response (i.e., a decrease in salivary cortisol) to walking in a forest environment. The remaining participants exhibited a negative response (n = 16, 22%) or unchanged cortisol levels (n = 7, 9%). A comparison between the ratio of positive to negative responders in the present walking study and that in our previous viewing study (24) is summarized in Table 4. In the previous viewing study, 209 (60%) of 348 participants were positive responders while the remaining participants exhibited a negative response (n = 118, 34%) or unchanged cortisol levels (n = 21, 6%). A Chi-square test revealed that the difference in the distribution of positive and negative responders between the walking and viewing studies was not statistically significant (p = 0.093), although the percentage of positive responders was considerably larger in the walking study than in the viewing study (24).
Table 4
| Negative response | Unchanged | Positive response | |
|---|---|---|---|
| Walking (n = 74) | 16 (22%) | 7 (9%) | 51 (69%) |
| Viewing (n = 348)* | 118 (34%) | 21 (6%) | 209 (60%) |
| Chi squared = 4.76, p = 0.093 | |||
Number of participants who exhibited positive/negative responses in salivary cortisol after forest exposure.
Results on cortisol levels after viewing urban or forest landscapes were presented in our previous report (24).
Discussion
Effect of Walking in a Forest Environment on Salivary Cortisol Concentration
This study aimed to investigate and to distinguish between the effect of walking and that of forest exposure on salivary cortisol concentration. Walking in a forest environment significantly reduced salivary cortisol concentrations, whereas walking in an urban area had little effect on cortisol. Moreover, cortisol concentrations before walking were not significantly different between the forest and urban environments, whereas cortisol concentrations after walking were significantly lower in the forest environments than in the urban environments. In short, an interaction effect between walking and environment was clearly demonstrated in this study.
In contrast with the present results, several previous studies have reported finding no cortisol-reducing effect of forest-walking. In a study on the effects of walking for 45 min along a mountain path (29), salivary cortisol was repeatedly measured before, immediately after, 20 min after and 40 min after each participant's walk but did not significantly vary with time. In a study conducted in Finland (22), subjects were exposed to three different environments (forest, park and urban). Their exposure to these environments consisted of 15 min of viewing and 30 min of walking. An ANOVA on salivary cortisol revealed that only time factor (before and after exposure) was significant, whereas environment type and interaction effect were insignificant. Komori et al. (30) investigated the effects of walking in urban and forest environments on salivary cortisol concentration and found that, as in the present study, walking in a forest environment significantly reduced cortisol concentration, yet urban-walking also reduced cortisol concentration considerably but not significantly, and cortisol concentrations after walking were not significantly different between forest and urban environments. In short, the interaction between walking and environment was unclear in their results. Accordingly, the significant interaction demonstrated in the present study is considered to be a novel finding in the field of nature therapy.
These other studies' negative results regarding the interaction between walking and forest exposure might be attributed to the high intensity of walking as an exercise. Physical exercise itself is known to increase cortisol concentrations: for example, Cook et al. (31) reported an extremely high cortisol concentration (100 nmol/L) in a marathon runner just after completion of the race. Jacks et al. (32) also reported higher salivary cortisol levels after 1 h of high-intensity exercise (76% VO2max); after less than 20 min of exercise, on the other hand, salivary cortisol concentrations were not affected regardless of the intensity. To achieve an observable increase in salivary cortisol, therefore, both high exercise intensity (more than 70% VO2max) and an exercise duration of at least 20 min might be required (33). As some of the forest-walking experiments in previous studies were more intense and longer in duration than our own, the walking in these experiments may have increased subjects' cortisol concentrations, which would obscure the cortisol-reducing effect of the forest environment.
Longer walking duration is linked to another difficulty related to the interpretation of cortisol responses. It is well known that cortisol exhibits clear diurnal variation. Cortisol shows a maximum concentration at 30–40 min after waking and then declines throughout the day. This phenomenon is known as the cortisol awakening response (CAR) (34). Therefore, if two measurements of salivary cortisol are taken more than an hour or two apart and then compared, the second measurement will be lower than the first simply due to the passage of time. Taking all of these points into account, we consider that high-intensity walking for long periods (e.g., mountain climbing) is an inappropriate experimental design for studying the effects of forest-walking on cortisol levels.
Forest-Walking vs. Forest-Viewing
The biophilia hypothesis was proposed by Edward O. Wilson in 1984 (35). Biophilia is defined as the “innate tendency to focus on life and life-like processes”(36). In primitive environment, natural features, including trees or forests, provided food, water, or shelter to our ancestors. Thus, biophilia may have been an adaptive characteristic. However, certain people strongly dislike natural settings. This tendency is called biophobia (37). Considering that various types of dangers also exist in the natural environment (e.g., predators and poisonous animals), biophobia is additionally an adaptive psychological trait. Therefore, the effect of the natural environment on humans is dual-faced. Hence, we considered that exploring both the positive and negative aspects of the natural environment is important.
In another of our previous studies (38), the effect of walking in a forest environment and the effect of viewing a forest landscape on HRV indices was investigated. In the study, a positive responder was defined as a participant who exhibited an increase in log-transformed high-frequency component (lnHF). Regarding the lnHF of HRV, the proportion of positive responders was ~65% in the walking study and ~79% in the viewing study; this difference is statistically significant (p < 0.01).
A similar analysis was conducted on salivary cortisol concentrations. In the present results, ~69% of the participants exhibited a positive response to forest-walking. In our previous study on the effect of viewing a forest landscape (24), the proportion of positive responders was ~60%. Contrary to the lnHF of HRV, with regard to cortisol levels, the proportion of positive responders was considerably higher in the walking study than in the viewing study, though this difference did not reach significance. One possible reason for this result might have been an insufficient sample size. The sample size in the viewing study was 348, while the sample size in the walking study was only 74; thus the statistical power might have been insufficient to detect the difference. Therefore, we cannot conclude from this result that there is no difference between the effects of forest-walking and those of forest-viewing on salivary cortisol concentrations.
Antonelli et al. (3) performed a meta-analysis on the effects of forest exposure on salivary cortisol concentrations. They analyzed six studies on forest-viewing (including our previous results) and four studies on forest-walking using a “forest plot” to represent their results. The effect of forest-viewing (i.e., the mean difference in post-viewing cortisol levels between forest and urban environments) was estimated at −0.05 μg/dl with a 95% confidence interval (CI) of −0.07 to −0.04 μg/dl. The effect of forest-walking, on the other hand, was estimated at −0.04 μg/dl with a 95% CI of −0.07 to −0.01 μg/dl. In short, they found that forest-walking had a slightly smaller effect on salivary cortisol than forest-viewing had. This finding is discrepant with the results of this study. At present, we cannot clearly conclude that either viewing or walking is more effective at reducing salivary cortisol concentrations. Further investigations will be needed on the difference between viewing and walking regarding the therapeutic effect of the forest environment.
Conclusion
The present study explored the interaction effect between walking (before and after) and environment (forest and urban) on salivary cortisol concentrations, although previous studies have reported that this interaction is unclear. Furthermore, a comparison of walking outcomes with viewing outcomes showed that the proportion of positive responders was somewhat larger after walking than after viewing, although this difference was not significant. It therefore remains unclear whether forest-walking is more therapeutic than viewing a forest landscape. This is an important question in the field of forest therapy, and future meta-analyses on cortisol are expected to answer it.
Statements
Data availability statement
The datasets generated for this study are available on request to the corresponding author.
Ethics statement
The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of the Forestry and Forest Products Research Institute, Japan (project identification code number: 16-558), and the Center for Environment, Health and Field Sciences, Chiba University, Japan (project identification code number: 5). Participants were informed about the purposes and procedures of the study and provided written informed consent prior to enrollment. They were free not to attend or to cease participation in the program at any time.
Author contributions
HK contributed to statistical analysis, interpretation of the results, and manuscript preparation. CS and HI were involved with data acquisition and initial analysis of the results. B-JP and TK participated in data acquisition and study design. YM had an important role in the research, particularly in experimental design, data acquisition, and manuscript preparation. All authors contributed to the preparation of the manuscript and are responsible for the final editing and approval.
Funding
This study was partially supported by a JSPS KAKENHI grant, number JP16107007.
Acknowledgments
The authors thank Yuko Tsunetsugu of the University of Tokyo for her assistance in the experiments.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1.
SongCIkeiHMiyazakY. Physiological effects of nature therapy: a review of the research in Japan. Int J Environ Res Public Health. (2016) 13:781. 10.3390/ijerph13080781
2.
HansenMMJonesRTocchiniK. Shinrin-yoku (forest bathing) and nature therapy: a state-of-the-art review. Int J Environ Res Public Health. (2017) 14:851. 10.3390/ijerph14080851
3.
MiyazakiY. Shinrin-yoku: The Japanese Way of Forest Bathing for Health and Relaxation. London: Octopus Publishing Group Ltd. (2018).
4.
AntonelliMBarbieriGDonellD. Effects of forest bathing (shinrin-yoku) on levels of cortisol as a stress biomarker: a systematic review and meta-analysis. Int J Biometeorol. (2019) 63:1117–34. 10.1007/s00484-019-01717-x
5.
BowlerDEBuyung-AliLMKnightTMPullinAS. A systematic review of evidence for the added benefits to health of exposure to natural environments. BMC Public Health. (2010) 10:456. 10.1186/1471-2458-10-456
6.
SongCIkeiHParkBJLeeJKagawaTMiyazakiY. Psychological benefits of walking through forest areas. Int J Environ Res Public Health. (2018) 15:2804. 10.3390/ijerph15122804
7.
SongCIkeiHKagawaTMiyazakiY. Effects of walking in a forest on young women. Int J Environ Res Public Health. (2019) 16:229. 10.3390/ijerph16020229
8.
LiQOtsukaTKobayashiMWakayamaYInagakiHKatsumataMet al. Acute effects of walking in forest environments on cardiovascular and metabolic parameters. Eur J Appl Physiol. (2011) 111:2845–53. 10.1007/s00421-011-1918-z
9.
OchiaiHIkeiHSongCKobayashiMTakamatsuAMiuraTet al. Physiological and psychological effects of forest therapy on middle-aged males with high-normal blood pressure. Int J Environ Res Public Health. (2015) 12:2532–42. 10.3390/ijerph120302532
10.
SongCIkeiHMiyazakiY. Sustained effects of a forest therapy program on the blood pressure of office workers. Urban Urban Green. (2017) 27:246–52. 10.1016/j.ufug.2017.08.015
11.
OheYIkeiHSongCMiyazakiY. Evaluating the relaxation effects of emerging forest-therapy tourism: a multidisciplinary approach. Tourism Manage. (2017) 62:322–34. 10.1016/j.tourman.2017.04.010
12.
SongCJoungDIkeiHIgarashiMAgaMParkBJet al. Physiological and psychological effects of walking on young males in urban parks in winter. J Physiol Anthropol. (2013) 32:18. 10.1186/1880-6805-32-18
13.
LeeJTsunetsuguYTakayamaNParkBJLiQSongCet al. Influence of forest therapy on cardiovascular relaxation in young adults. Evid Based Complement Alternat Med. (2014) 2014:834360. 10.1155/2014/834360
14.
KobayashiHSongCIkeiHKagawaTMiyazakiY. Analysis of individual variations in autonomic responses to urban and forest environments. Evid Based Complement Alternat Med. (2015) 2017:671094. 10.1155/2015/671094
15.
SongCIkeiHKobayashiMMiuraTTaueMKagawaTet al. Effect of forest walking on autonomic nervous system activity in middle-aged hypertensive individuals. Int J Environ Res Public Health. (2015) 12:2687–99. 10.3390/ijerph120302687
16.
ParkBJTsunetsuguYKasetaniTHiranoHKagawaTSatoMet al. Physiological effects of Shinrin-yoku (taking in the atmosphere of the forest)—Using salivary cortisol and cerebral activity as indicators. J Physiol Anthropol. (2007) 26:123–8. 10.2114/jpa2.26.123
17.
LiQMorimotoKKobayashiMInagakiHKatsumataMHirataYet al. A forest bathing trip increases human natural killer activity and expression of anti-cancer proteins in female subjects. J Biol Regul Homeost Agents. (2008) 22:45–55.
18.
LiQ. Effect of forest bathing trips on human immune function. Environ Health Prev Med. (2010) 15:9–17. 10.1007/s12199-008-0068-3
19.
MaoGXLanXGCaoYBChenZMHeZHLvYDet al. Effects of short-term forest bathing on human health in a broad-leaved evergreen forest in Zhejiang Province, China. Biomed Environ Sci. (2012) 25:317–24. 10.3967/0895-3988.2012.03.010
20.
SungJWooJMKimWLimSKChungEJ. The effect of cognitive behavior therapy-based “forest therapy” program on blood pressure, salivary cortisol level, and quality of life in elderly hypertensive patients. Clin Exp Hypertens. (2012) 34:1–7. 10.3109/10641963.2011.618195
21.
OchiaiHIkeiHSongCKobayashiMMiuraTKagawaTet al. Physiological and psychological effects of a forest therapy program on middle-aged females. Int J Environ Res Public Health. (2015) 12:15222–32. 10.3390/ijerph121214984
22.
TyrväinenLOjalaAKorpelaKLankiTTsunetsuguYKagawaT. The influence of urban green environments on stress relief measures: a field experiment. J Environ Psychol. (2014) 38:1–9. 10.1016/j.jenvp.2013.12.005
23.
GidlowCJJonesMVHurstGMastersonDClark-CarterDTarvainenMPet al. Where to put your best foot forward: psycho-physiological responses to walking in natural and urban environments. J Environ Psychol. (2016) 45:22–9. 10.1016/j.jenvp.2015.11.003
24.
KobayashiHSongCIkeiHParkBJLeeJKagawaTet al. Population-based study on the effect of forest environment on salivary cortisol concentration. Int J Environ Res Public Health. (2017) 14:931. 10.3390/ijerph14080931
25.
ParkBJTsunetsuguYKasetaniTKagawaTMiyazakiY. The physiological effects of Shinrin-yoku (taking in the forest atmosphere or forest bathing): evidence from field experiments in 24 forests across Japan. Environ Health Prev Med. (2010) 15:18. 10.1007/s12199-009-0086-9
26.
Ministry of Health Labor and Welfare Japan. Japanese National Health and Nutrition Survey (2016). Available online at: https://www.e-stat.go.jp/stat-search/file-download?statInfId=000031666263&fileKind=0
27.
KobayashiHMiyazakiY. Distribution characteristics of salivary cortisol measurements in a healthy young male population. J Physiol Anthropol. (2015) 34:30. 10.1186/s40101-015-0068-0
28.
KobayashiHSongCIkeiHParkBJKagawaTMiyazakiY. Diurnal changes in distribution characteristics of salivary cortisol and immunoglobulin A concentrations. Int J Environ Res Public Health. (2017) 14:987. 10.3390/ijerph14090987
29.
TodaMDenRHasegawa-OhiraMMorimotoK. Effects of woodland walking on salivary stress markers cortisol and chromogranin A. Complement Ther Med. (2013) 21:29–34. 10.1016/j.ctim.2012.11.004
30.
KomoriTMitsuiMTogashiKMatsuiJKatoTUeiDet al. Relaxation effect of a 2-hour walk in Kumano-Kodo forest. J Neurol Neurosci. (2017) 8:1. 10.21767/2171-6625.1000174
31.
CookNJNgAReadGFHarrisBRiad-FahmyD. Salivary cortisol for monitoring adrenal activity during marathon runs. Hormone Res Paediatrics. (1987) 25:18–23. 10.1159/000180628
32.
JacksDESowashJAnningJMcgloughlinTAndresF. Effect of exercise at three exercise intensities on salivary cortisol. J Strength Cond Res. (2002) 16:286–9. 10.1519/00124278-200205000-00018
33.
KirschbaumCHellhammerDH. Salivary cortisol in psychoneuroendocrine research: recent developments and applications. Psychoneuroendocrinology. (1994) 19:313–33. 10.1016/0306-4530(94)90013-2
34.
FriesEDettenbornLKirschbaumC. The cortisol awakening response (CAR): facts and future directions. Int J Psychophysiol. (2009) 72:67–73. 10.1016/j.ijpsycho.2008.03.014
35.
WilsonEO. Biophilia: The Human Bond With Other Species. Cambridge, MA: Harvard University Press (1984).
36.
GulloneE. The biophilia hypothesis and life in the 21st century: increasing mental health or increasing pathology?J Happiness Stud. (2000) 1:293–322. 10.1023/A:1010043827986
37.
UlrichRS. Biophilia, biophobia, and natural landscapes. In: KellertSRWilsonEO, editors. The Biophilia Hypothesis. Washington, DC: Island Press (1993). p. 73–137.
38.
KobayashiHSongCIkeiHParkBJLeeJKagawaTet al. Forest walking affects autonomic nervous activity: a population-based study. Front Public Health. (2018) 6:278. 10.3389/fpubh.2018.00278
Summary
Keywords
shinrin-yoku, forest therapy, walking, salivary cortisol, interaction
Citation
Kobayashi H, Song C, Ikei H, Park B-J, Kagawa T and Miyazaki Y (2019) Combined Effect of Walking and Forest Environment on Salivary Cortisol Concentration. Front. Public Health 7:376. doi: 10.3389/fpubh.2019.00376
Received
25 June 2019
Accepted
25 November 2019
Published
12 December 2019
Volume
7 - 2019
Edited by
Mohiuddin Md. Taimur Khan, Washington State University Tri-Cities, United States
Reviewed by
Jean Challacombe, Colorado State University, United States; Åse Marie Hansen, University of Copenhagen, Denmark
Updates
Copyright
© 2019 Kobayashi, Song, Ikei, Park, Kagawa and Miyazaki.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yoshifumi Miyazaki ymiyazaki@faculty.chiba-u.jp
This article was submitted to Environmental Health, a section of the journal Frontiers in Public Health
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.