- 1Department of Biosciences, COMSATS University Islamabad, Islamabad, Pakistan
- 2Key Laboratory of Parasite and Vector Biology, MOH, Shanghai, China
- 3Department of Parasitology, Faculty of Veterinary Medicine, University of Firat, Elazig, Turkey
- 4Department of Lifesciences, University of Management & Technology, Lahore, Pakistan
- 5WHO Collaborating Centre for Tropical Diseases, Shanghai, China
- 6Chinese Center for Disease Control and Prevention, National Institute of Parasitic Diseases, Shanghai, China
Background: Cystic echinococcosis (CE) is a global zoonotic parasitic disease caused by the larval stage of Echinococcus granulosus and it has been reported from both livestock and humans in Pakistan. The definitive host of E. granulosus is the dog, and the large number of stray dogs in Pakistan contributes to the spread of CE. However, there is little information between stray dogs and CE relation in the country.
Methods: During the study, total 123 butcher's shops and abattoirs were included for collection of data relating to the hydatid cyst prevalence in slaughtered animals (sheep, goat, cattle, and buffaloes). The number of animals slaughtered in each butcher's shop during sampling period was also recorded, and the association of the shop environment with dogs was inspected.
Results: Data was collected for CE from 123 butcher's shops in Rawalpindi and Islamabad, Pakistan. The slaughtering rate the in the butcher's shops was 2–10 animals/day including sheep/goat/cattle and buffaloes. The overall prevalence of CE in all examined animals was 2.77%. In buffaloes the higher prevalence was recorded as compared to other hosts. The findings showed that lung and liver were most affected organs and majority (59%) of the cysts were fertile in infected animals. The presence of a large number of stray dogs were an important factor in the spread of CE. They were rarely vaccinated, have easy access to infected offal at slaughtering site and had insufficient or inappropriate anthelmintic treatment.
Conclusions: The most pressing need is to raise public awareness of this huge problem by considering CE a major ailment and promoting the collection and mapping of epidemiological data. Efficient CE control is required, especially treating dogs with antiparasitic drugs, for which government support and affiliation with the veterinary sector is essential.
Introduction
Echinococcosis is one of the 20 neglected zoonotic diseases (NZD) prioritized by the World Health Organization (WHO) (1). Cystic echinococcosis (CE) is a globally NZD caused by the dog tapeworm Echinococcus granulosus. The global annual infection rate is 1.2 million, the annual death rate is about 2.2%, and an estimated 3.6 million disability-adjusted life years (DALYs) are lost because of this disease per annum (2). In addition, CE is responsible for over US$ 3 billion expenses every year (3). CE is more prevalent in areas where people survive on animal husbandry and agricultural activities (4), and the rate is higher in nomadic and semi-nomadic populations due to this lifestyle (5).
Conditions such as poor hygiene and failure to wash contaminated food facilitate the spread of CE infection in the human population (6). CE transmission from food to humans is common in areas where people usually consume raw vegetables; most are cultivated in open fields where stray dogs roam freely and contaminate the vegetables by dropping feces containing E. granulosus eggs (7). One of the major risk factors for CE infection is open slaughtering of livestock without veterinary supervision. Due to lack of supervision, infected offal is ingested by dogs, which, as the intermediate host, spread infected eggs to the environment. The study aimed to analyze CE prevalence, presence of stray dogs and their association with slaughtering habits in abattoirs /butcher shops in the study area.
Materials and Methods
Study Area
A study was conducted in Islamabad and Rawalpindi districts of Pakistan.
Topography
Islamabad, the capital city of Pakistan, is located in Pothohar Plateau (33.43°N 73.04°E) at 540 m (1,770 ft.) above the sea level. 505 km2 of this area is urban whereas 401 km2 of is rural (8). Adjoining Islamabad is the city of Rawalpindi and both the cities are often referred to as the twin cities, 84% of the population here is Punjabi, 9% Pashto and 7% others. Rawalpindi is located at an elevation of 508 m and spans over an area of 259 km2 (9).
Study Duration
The data was collected from January to July, 2017(for 6 months). Butcher shops in different areas of Rawalpindi and Islamabad were visited twice per month to collect the data on prevalence and presence of stray dogs in the slaughterhouses.
Study Design
A cross-sectional survey was designed to get the recent data hydatid cyst incidence. The data was collected from butcher shops of the twin cities. Questionnaire was designed for butcher shops/slaughterhouses among urban and rural areas which was descriptive in nature. The information about stray dogs present along the territory of slaughterhouses/butcher shops were recorded.
Data Collection Methods
The data on presence of stray dogs, CE prevalence in animals, as well as on socio-demographic characteristics was collected using questionnaires. Moreover, data was analyzed to determine the factors associated with the risk of CE. As there is no local specific name of this disease, pictures of cysts in animal organs and of infected humans were shown to the participants to identify the disease better. The knowledge of the participants was measured as binary outcomes (10, 11).
Laboratory Investigations
In order to examine hydatid cysts properly, following parameters were carried out: Types of cysts (sterile, fertile, calcified, or under-developed), organ specificity (lungs and liver), and prevalence of hydatidosis. Presence of cysts in different organs was analyzed by routine post-mortem of the carcass. The cysts were dissected and collected into sterile containers separately on organ basis for further description.
Cyst Characterization
Sterile scalpel blades were used for cyst incision. The fluid present inside these cysts was used to check the existence of protoscoleces either in the form of brood capsule (closes to the germinal layer) or in the cyst fluid considering as a fertility indicative. Viability test was performed on fertile cysts. In viability test a drop of fluid from cyst containing the protoscoleces was observed under microscope to check amoeboid like peristaltic movements. For clear microscopic observations equal volume of 0.1% aqueous eosin solution was also mixed with equal volume of fluid containing the protoscoleces. Sterile hydatid cysts were characterized on the basis of inner lining, generally smooth with a slight turbid enclosed fluid otherwise rough calcified cyst with no or less fluid (12). Calcified cysts were coarse and nodular having an internal chamber with calcified or chalky deposits in the cyst wall. Underdeveloped cysts were small 1–2 mm in size, defined germinal layer are firm in texture with very little fluid but presence of protoscoleces was not observed (13).
Morphology of Protoscoleces
Polyvinyl-lactophenol was used for mounting protoscoleces cysts. Hooks damage was prevented by applying gentle pressure on cover slip. A calibrated eye-piece micrometer was used for all measurements under oil immersion. Morphometric analysis was done as described by Hobbs et al. (14).
Data Analysis
Data was analyzed as described previously (15).
Results
Data was collected for CE from 123 butcher's shops in Rawalpindi and Islamabad, Pakistan. The slaughtering rate in the butcher's shops was 2–10 animals/day including cattle, goat, sheep, and buffaloes. Overall prevalence of CE in the slaughterhouses/butcher shops was 2.77% (300/10,800) according to this survey. Prevalence was higher in buffaloes followed by cattle, sheep, and goat, respectively. The site of infection, number of cysts and kind of cysts are shown in Table 1.
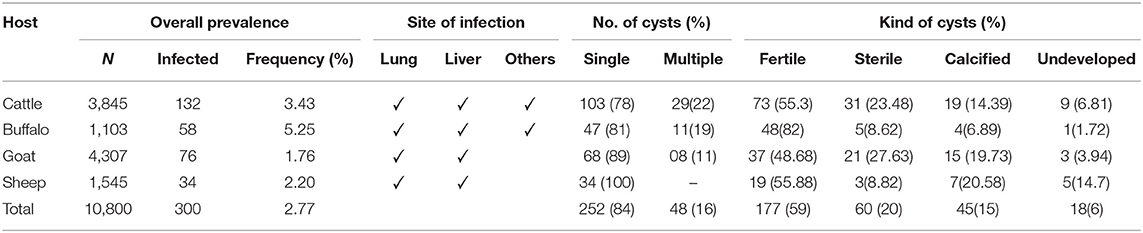
Table 1. Overall prevalence (%) of hydatidosis in various organs of slaughtered Cattle, Buffalo, Goat, and Sheep.
Rostellar Hook Morphology
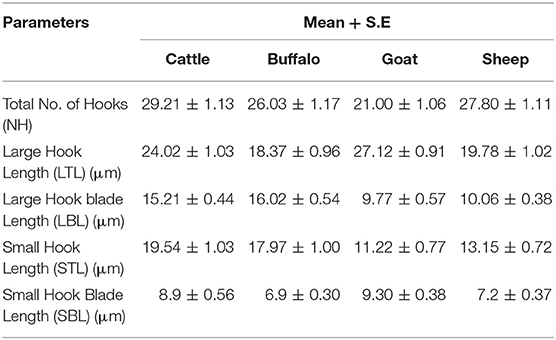
The parameters which were observed to check protoscolex rostellar hook morphology in infected animals were total hooks number, their total length (μm) of hooks and blade length (μm) as shown in Table 2.
Total Number of Hooks (NH)
Protoscoleces hooks number was observed and it was found that total number was 29.21 ± 1.13 in cattle origin, 26.03 ± 1.17 in buffalo origin, 21.0 ± 1.06 in goat origin, and 27.80 ± 1.11 in sheep origin as shown in Table 1. The study results indicated that the maximum number of hooks were observed on protoscoleces of sheep origin and minimum on those of goat origin.
Large Hook Total Length (LTL) (μm)
Protoscoleces large hooks was observed for total length (micrometers, μm) and it was found that it was 24.02 ± 1.03 in cattle origin, 18.37 ± 0.96 in buffalo origin, 27.12 ± 0.91 in goat origin, and 19.78 ± 1.02 in sheep origin. In goat origin large hook length was maximum (27.12 ± 0.91) and in case of buffalo origin it was minimum (18.37 ± 0.96).
Large Hook Blade Length (LBL)(μm)
Protoscoleces blade length of large hooks on was observed as 15.21 ± 0.44 in cattle originated infections, 16.02 ± 0.54 in buffalo originated infections, 9.77 ± 0.57 in goat origin, and 10.06 ± 0.38 in sheep origin as shown in Table 1. It is clear from these values that buffalo originated infection LBL was found maximum (16.02 ± 0.54) and in goat originated it was minimum (9.77 ± 0.57).
Small Hook Total Length (STL) (μm)
Protoscoleces of small hooks total length was observed as 19.54 ± 1.03 in cattle, 17.97 ± 1.00 in buffalo, 11.22 ± 0.77 in goat, and 13.15 ± 0.72 in sheep origin as shown in Table 1. In cattle originated STL was maximum (19.54 ± 1.03) and in case of goat origin it was minimum (11.22 ± 0.77).
Small Hook Blade Length (SBL)(μm)
Protoscoleces small hooks blade length on was recorded as 8.9 ± 0.56 in cattle origin, 6.9 ± 0.30 in buffalo origin, 9.30 ± 0.38 in goat origin, and 7.2 ± 0.37 in sheep origin. In goat origin SBL was maximum (9.30 ± 0.38), while in case of buffalo origin it was minimum (6.9 ± 0.30).
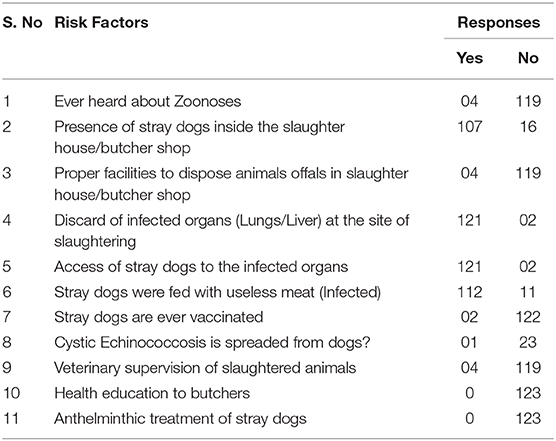
In the present study the number of stray dogs were recorded in all 123 slaughterhouse/butcher shops. It ranged from 1 to 5 dogs/site. The main contributing factor to the spread of CE was the large number of stray dogs (Table 3); they were rarely vaccinated, have easy access to infected offal in rural areas (Figures 1A–C), and had insufficient or inappropriate anthelmintic treatment.

Figure 1. (A-C) Showing the association and access of stray dogs to infected offal's at butcher shops.
In addition, there were few municipal slaughterhouses, limited veterinary supervision and inspection of slaughterhouses, few facilities for the disposal of infected offal, and there was home or illegal livestock slaughtering, and lack of health education. It was observed that stray dogs have a close association with the slaughtering sites and increase the chances to get infected with CE. The finding of this study has showed that stray dogs (range 1–5) were present in the territories of all the butcher shops/slaughter houses that has an open access to infected offal of the slaughtered livestock. These stray dogs are not treated with any antiparasitic drug.
Discussion
Cystic echinococcosis (CE) is a chronic larval cestode infection caused by E. granulosus in humans and domestic livestock, principally transmitted by an intermediate host (16). CE is recognized as a neglected disease of public health significance worldwide, particularly in low-income countries (17). Pakistan is a country with low socioeconomic development and the hygiene conditions are poor. Poor hygiene conditions such as no proper hand washing, no water boiling, lack of proper cleanliness of shops and surrounding areas, eating of contaminated food and raw vegetables, and feeding dogs meat infected with cysts are involved in the prevalence of CE in humans. The epidemiological studies showed that CE is highly prevalent r in third world countries (18). The higher prevalence of CE in Pakistan might be due to inappropriate waste dumping, poor social-economic condition of the country, very poor sanitary system, and unorganized slaughtering. In addition to these factors personal unhygienic situation is also playing a crucial role (19).
The findings showed that overall prevalence of CE was 2.77%. The prevalence was higher in buffaloes followed by cattle, sheep, and goat, respectively. In Pakistan the first incidence of CE in intermediate hosts was explored in 1968. The prevalence of E. granulosus was 35% (52/148) in buffaloes and 27% (17/62) in cattle (10).
In current study, lung and liver was most affected organ as compared to others. The lung wise prevalence was 30.9, 22.8, and 58.8%, in cattle, buffaloes, and camels, respectively while in liver it was 21.42, 17.47, and 26.4% in cattle, buffaloes, and camels, respectively (11). The prevalence of hydatid cyst in liver, lung, spleen, heart, and kidneys was 25.31, 47.31, 1.83, 0.06, and 0.51%, respectively (15). In sheep and goat, the prevalence was 8.25 and 8.05%, respectively (20). In a comprehensive survey, the overall prevalence of hydatidosis was 6.67% in livestocks (21). Mustafa et al. (22), reported that the prevalence of hydatid cysts as 3.24, 2.44, and 2.44% in sheep, goats and cattle, respectively while Tasawar et al. (23) reported the prevalence of 7.39% in sheep and 10.69% in buffaloes of Multan, Punjab, Pakistan. Previously it was shown that hydatid cyst prevalence was between 5 and 46% in livestock species (24).
A report from Lahore showed that hydatidosis is prevalent in sheep (8.85%) and in goats (6.21%). This survey was conducted to determine the organ specificity of hydatidosis, organ wise distribution of hydatidosis showed that in goats 40.56% in liver, followed by 34.38% in lungs, 16.95% in lungs and liver together, and 0.49% in spleen. In sheep, highly infected organ was lungs whereas liver was most infected organ in goats (20). Sheep and goat liver hydatid cyst prevalences were 46.74 and 23.28% and the rates in lungs were 17.37 and 13.68%, respectively (25).
Similarly, frequency of fertile cysts was higher as compared to sterile, calcified, and underdeveloped cysts, respectively. Hydatid cysts can be categorized as non-viable, viable, and fertile (26). Only the fertile cysts carry the active form of the parasite protoscoleces (27). The cysts diameter was 2–30 cm and it is as the inner layer from where larvae grow (28).
Zoonotic helminthes (Toxocara spp. and Echinococcus spp.) can transmit to humans by dogs and cats (29). Globally, human and dog interaction cause significant social, economic and public health issue mainly the zoonotic diseases (30). Dogs play crucial role in spread of many zoonotic infectious diseases (31). Higher population of stray dogs is one of the main contributing factors in spread of CE in Pakistan. They are infrequently vaccinated and easy access to infected offal. Poor hygienic conditions, lack of veterinary supervision and inspection of slaughterhouses, home or illegal livestock slaughtering occurs, and there is a lack of health education due to poverty (6).
The dog population depends on the accessibility of resources (for example, shelter, food, and water) (32). Although the actual number of stray dogs worldwide is not known, of the 500 million dogs in the world, around 75% are thought to be stray (33). Stray dogs survive consists of edible debris and contributions from human beings (34). Dog populations is directly linked with the size of the local human population (35).
Stray dogs are one of the important reservoirs for the transmission of zoonotic helminthes that are of public health concern especially Echinococcus specie. A study from Karachi (the biggest city of Pakistan), shows that among selected dogs presence of intestinal helminthes was confirmed 99% dogs and 7% carried E. granulosus (36).
To attain effective control of CE, it is essential to raise knowledge and awareness regarding hazardous practices and defensive measures against the disease within the community. Pakistan, being a developing country, is densely populated and socioeconomically poor. Overall poor sanitary system in Pakistan is very poor and majority of the inhabitants lives in crowded area. Rural inhabitants mainly survive on small-scale agriculture and farming. Laborers working in the fields often interact with animals and, due to illiteracy, have limited knowledge of health and hygiene and therefore are often infected by Echinococcus spp. (37).
In the early years of the twenty-first century, CE contributed a major global disease burden; it is one of the 12 commonest NZDs (38). It is very difficult to regulate the control of NZDs, particularly when curing humans does not prevent transmission; moreover, treatment of livestock is perceived as a low priority because the livestock hosts are usually asymptomatic (39). Since 1960, several intervention programs have demonstrated effective control of E. granulosus transmission, leading to a significant reduction of CE and improved public health (40). Despite these intervention programs, further work is still necessary. Thus, at present, we recommend increasing the awareness of the seriousness of CE and promoting the collection and mapping of epidemiological data. Efficient CE control requires government support and affiliation with the veterinary sector.
Conclusion
In countries with a high number of stray dogs, such as Pakistan, and where the public education level is low, the first task for CE control should be to raise public awareness and try to prevent infected offal from being fed to dogs. Field studies should be conducted on this subject, training seminars should be given, information should be given to children in primary schools, butchers should be trained, the community should be informed by imams in mosques, and informative TV and radio programs should be broadcast.
Data Availability Statement
The data used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Statement
The animal study was reviewed and approved by the Departmental Ethics Review Board (ERB) at the COMSATS University Islamabad (CUI), Pakistan, under ERB/18/72. This study was carried out in strict accordance with the recommendations of the guide for the care and use of laboratory animals.
Author Contributions
AK and MA collected the data and wrote the paper following discussions with HA and SS. SS and JC also revised the paper and improved the technical quality of the manuscript. AK and JC contributed reagents and materials. All authors approved the final version of the paper.
Funding
This study was supported by the Laboratory of Parasite and Vector Biology, MOH, China (grant no. WSBKFKT2017-01 to AK) and the National Natural Science Foundation of China (grant no. 81772225 to JC). The funding was used for the survey purpose and storage of samples.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors are thankful to AM and SI for their input which aided the completion of this study.
Abbreviations
WHO, World Health Organization; NZDs, Neglected Zoonotic Diseases; CE, Cystic Echinococcosis; DALYs, Disability Adjusted Life Years
References
1. The World Health Organization. Working to Overcome the Global Impact of Neglected Tropical Diseases: First WHO Report on Neglected Tropical Diseases. Geneva: World Health Organization (2010).
2. Craig PS, Budke CM, Schantz PM, Tiaoyin L, Jiamin Q, Yurong Y, et al. Human echinococcosis: a neglected disease. Trop Med Health. (2007) 35:283–92. doi: 10.2149/tmh.35.283
3. Agudelo Higuita NI, Brunetti E, Mccloskey C. Cystic echinococcosis. J Clin Microbiol. (2006) 54:518–23. doi: 10.1128/JCM.02420-15
4. Romig T, Deplazes P, Jenkins D, Giraudoux P, Massolo A, Craig PS, et al. Ecology and life cycle patterns of echinococcus species. Adv Parasitol. (2017) 95:213–314. doi: 10.1016/bs.apar.2016.11.002
5. Harandi MF, Moazezi SS, Saba M, Grimm F, Kamyabi H, Sheikhzadeh F, et al. Sonographical and serological survey of human cystic echinococcosis and analysis of risk factors associated with seroconversion in rural communities of Kerman, Iran. Zoonoses Public Health. (2011) 58:582–8. doi: 10.1111/j.1863-2378.2011.01407.x
6. Possenti A, Manzano-Romá R, Sánchez-Ovejero C, Boufana B, La Torre G, Siles-LucasM, et al. Potential risk factors associated with human cystic echinococcosis: systematic review and meta-analysis. PLoS Neglect Trop Dis. (2016) 10:e0005114. doi: 10.1371/journal.pntd.0005114
7. Moshfe A, Sarkari B, Arefkhah N, Nikbakht R, Shahriarirad R, Rezae Z, et al. Seroepidemiological study of cystic echinococcosis in nomadic communities in the southwest of Iran: a population-based study. J Immunoassay Immunochem. (2018) 40:183–92. doi: 10.1080/15321819.2018.1547974
9. Sheikh IS, Pasha MK, Williams VS, Raza SQ, Khan KSA. Environmental geology of the Islamabad-Rawalpindi Area, Northern Pakistan. In: Warwick PD, Wardlaw BR, editors. Regional Studies of the Potwar Plateau Area, Northern Pakistan. U.S. Geological Survey (2007). p. 1–8. doi: 10.3133/b2078
10. Shiekh SA, Hussain MZ. Incidence of hydatidosis in livestock in Lahore. Pak J Sci. (1968) 19:239–42.
11. Khan MQ, Afzal M, Ali S. Prevalence and serology of hydatidosis in large ruminants of Pakistan. Vet Parasitol. (1990) 37:163–8. doi: 10.1016/0304-4017(90)90071-I
12. Parija SC. Medical Parasitology, Protozoology and Helminthology Text and Atlas, Vol. 2. New Delhi: Chennai Medical Book Publisher (2004). p. 221–9.
13. Simsek S, Koroglu E. Evaluation of enzyme-linked immunosorbent assay (ELISA) and enzyme-linked immunoelectrotransfer blot (EITB) for immunodiagnosis of hydatid diseases in sheep. Acta Trop. (2004) 92:17–24. doi: 10.1016/j.actatropica.2004.04.006
14. Hobbs RP, Lymbery AJ, Thompson RCA. Rostellar host morphology of Echinococcus granulosus (Batsch, 1786) from natural and experimental Australian hosts, and its implication for strain recognition. Parasitology. (1990) 101:273–81. doi: 10.1017/S0031182000063332
15. Anwar AH, Shamim H, Rana MN, Khan A. Qudoos. Hydatidosis: prevalence and biometrical studies in cattle (Bob indicub) in Pakistan. J Agric Sci. (2000) 37:29–32.
16. Larrieu E, Gavidia CM, Lightowlers MW. Control of cystic echinococcosis: background and prospects. Zoonoses Public Health. (2019) 66:889–99. doi: 10.1111/zph.12649
17. Abdulhameed MF, Robertson ID, Al-Azizz SA, Habib I. Neglected zoonoses and the missing opportunities for one health education: the case of cystic echinococcosis among surgically operated patients in Basrah, Southern Iraq. MDPI. (2019) 7:4. doi: 10.3390/diseases7010004
18. Fatimi SH, Sajjad N, Muzaffar M. Ruptured hydatid cyst presenting as pneumothorax. J Infect Dis Dev Countries. (2010) 4:256–8. doi: 10.3855/jidc.538
19. Hussain A, Maqbool A, Tanveer A, Anees A. Studies on morphology of Echinococcus granulosus from different animal-dog origin. Punjab Univ J Zool. (2005) 20:151–7.
20. Iqbal HJ, Maqbool A, Lateef M, Khan MA, Riaz A, Mahmood A, et al. Studies on hydatidosis in sheep and goats at Lahore, Pakistan. J Anim Plant Sci. (2012) 22:894–7.
21. Latif AA, Tanveer A, Maqbool A, Siddiqi N, Kyaw-tanner M, Traub RJ. Morphological and molecular characterization of Echinococcus granulosus in livestock and humans in Punjab, Pakistan. Vet Parasitol. (2010) 170:44–49. doi: 10.1016/j.vetpar.2010.02.003
22. Mustafa I, Shahbaz M, Asif S, Khan MR, Saeed U, Sadiq F, et al. Availability, cyst characteristics and hook morphology of Echinococcus granulosus isolates from livestock (cattle, sheep and goats) in Central Punjab, Pakistan. Kafkas Univ Vet Fak Derg. (2015) 21:849–54.
23. Tasawar Z, Naz F, Lashari MH. The prevalence of hydatidosis in sheep and buffaloes at Multan, Punjab, Pakistan. Global Vet. (2014) 12:332–5. doi: 10.5829/idosi.gv.2014.12.03.82272
24. Shafiq M, Tanveer A, Athar M. Epidemiology and economical aspects of hydatidosis/echinococcosis in different animals and man (Ph.D. Thesis). University of the Punjab, Lahore, Pakistan (2005). p. 415.
25. Ahmed S, Nawaz M, Gul R, Zakir M, Razzaq A. Some epidemiological aspects of hydatidosis of lungs and livers of sheep and goats in Quetta, Pakistan. Pak J Zool. (2006) 38:1.
26. Larrieu EJ, Frider B. Human cystic echinococcosis: contributions to the natural history of the disease. Ann Trop Med Parasitol. (2001) 95:679–87. doi: 10.1080/00034980120094730
27. Kamenetzky L, Gutierrez AM, Canova SG, Haag KL, Guarnera EA, Parra A, et al. Several strains of Echinococcus granulosus infect livestock and humans in Argentina. Infect Genet Evol. (2002) 2:129–36. doi: 10.1016/S1567-1348(02)00131-4
28. Harandi MF, Hobbs RP, Adams PJ, Mobedi I, Morgan-Ryan UM, Thompson RCA. Molecular and morphological characterization of Echinococcus granulosus of human and animal origin in Iran. Parasitology. (2002) 125:367–73. doi: 10.1017/S0031182002002172
29. Kardjadj M, Ben-Mahdi MH. Epidemiology of dog-mediated zoonotic diseases in Algeria: a One Health control approach. New Microbes New Infect. (2019) 28:17–20. doi: 10.1016/j.nmni.2019.01.001
30. Strube C, Neubert A, Springer A, von Samson-Himmelstjerna G. Survey of German pet owners quantifying endoparasitic infection risk and implications for deworming recommendations. Parasit Vectors. (2019) 12:203. doi: 10.1186/s13071-019-3410-2
31. Anonymous. Leading Edge. Rabies Has Its Day. Available online at: http://infection.thelancet.com; www.rabiescontrol.net/Lancet.pdf (accessed September 20, 2010).
32. Wandeler AI, Matter HC, Kappeler A, Budde A. The ecology of dogs and canine rabies: a selective review. Rev Sci Tech. (1993) 12:51–71. doi: 10.20506/rst.12.1.663
33. WSPA. Suffering in Slums: The Global Stray Dog Problem. (2010). Available online at: www.wspa-usa.org (accessed September 22, 2010).
34. Smith R. How to Solve Romanian Street Dog Problem Effectively, Humanely and Forever. (2005). Available online at: www.actionagainstpoisoning.com (accessed August 2, 2013).
35. Butler JRA, Du Toit JT, Bingham J. Free-ranging domestic dogs (Canis familiaris) as predators and prey in rural Zimbabwe: threats of competition and disease to large wild carnivores. Biol Conserv. (2004) 115:369–78. doi: 10.1016/S0006-3207(03)00152-6
36. Ahmed N, Riaz A, Zubair Z, Saqib M, Ijaz S, Nawaz-Ul-Rehman MS, et al. Molecular analysis of partial VP-2 gene amplified from rectal swab samples of diarrheic dogs in Pakistan confirms the circulation of canine parvovirus genetic variant CPV-2a and detects sequences of feline panleukopenia virus (FPV). Virol J. (2018) 15:45. doi: 10.1186/s12985-018-0958-y
37. Ahmed H, Ali S, Afzal MS, Khan AA, Raza H, Shah ZH, et al. Why more research needs to be done on echinococcosis in Pakistan. Infect Dis Poverty. (2017) 6:90. doi: 10.1186/s40249-017-0309-z
38. WHO. The Control of Neglected Zoonotic Diseases: Community-based Interventions for Prevention and Control. Geneva: World Health Organization (2010).
39. Craig PS, McManus DP, Lightowlers MW, Chabalgoity JA, Garcia HH, Gavidia CM, et al. Prevention and control of cystic echinococcosis. Lancet Infect Dis. (2007) 7:385–94. doi: 10.1016/S1473-3099(07)70134-2
40. Gemmell MA, Roberts MG, Beard TC, Campano Diaz S, Lawson JR, Nonnemaker JM. Chapter 6: Control of echinococcosis. In: Eckert J, Gemmell MA, Meslin FX, Pawlowski ZS, editors. WHO/OIE Manual on Echinococcosis in Humans and Animals: A Public Health Problem of Global Concern (Paris: WHO/OIE) (2001).
Keywords: cystic echinococcosis, Echinococcus granulosus, livestock, dog, public health, Pakistan
Citation: Khan A, Ahmed H, Simsek S, Afzal MS and Cao J (2020) Spread of Cystic Echinococcosis in Pakistan Due to Stray Dogs and Livestock Slaughtering Habits: Research Priorities and Public Health Importance. Front. Public Health 7:412. doi: 10.3389/fpubh.2019.00412
Received: 19 September 2019; Accepted: 23 December 2019;
Published: 29 January 2020.
Edited by:
Alfonso J. Rodriguez-Morales, Technological University of Pereira, ColombiaReviewed by:
Ana Afonso, University of São Paulo, BrazilMario Santoro, Stazione Zoologica Anton Dohrn, Italy
Copyright © 2020 Khan, Ahmed, Simsek, Afzal and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haroon Ahmed, aGFyb29uYWhtYWQxMkB5YWhvby5jb20=; Jianping Cao, Y2FvanBAeWFob28uY29t
 Aisha Khan1,2
Aisha Khan1,2 Haroon Ahmed
Haroon Ahmed Sami Simsek
Sami Simsek Jianping Cao
Jianping Cao