- 1Eastlake Research Group, Oakville, ON, Canada
- 2Canadian Health Policy Institute, Toronto, ON, Canada
- 3Fraser Institute, Vancouver, BC, Canada
Introduction: Canada may soon see the introduction of a national pharmaceutical insurance system. New Zealand has a government-funded healthcare system used by all residents that operates within a tight cost-containment budget. The objective of this analysis was to compare the main mortality causes in Canada and New Zealand and examine listings in current Canadian provincial public drug plans and the New Zealand national drug formulary.
Materials and Methods: Age-standardized mortality rates from 2000 to 2015 and data on hospital discharges and average length of stay in hospital for Canada and New Zealand were obtained from the Organization for Economic Cooperation and Development's website. Information on insured medications was obtained from Canadian provincial drug plan lists and the New Zealand Pharmaceutical Schedule current in mid-2019.
Results: Hospital discharge rates for cardiovascular disorders, malignancies and respiratory disorders and mortality rates for acute myocardial infarction, ischemic heart disease and cerebrovascular disease were higher, on average over the observation period, in New Zealand than in Canada, but mortality rates for malignancies and respiratory disorders were similar. Reimbursement listing rates for cancer drugs and some cardiovascular medications were lower in New Zealand than in Canada.
Discussion: Higher hospital discharge and mortality rates suggest poorer patient health in New Zealand compared with Canada. This may be due to lower reimbursement listing rates for some medications in New Zealand. New Zealand's drug coverage system has contained costs, but it restricts or denies access to new innovative medicines with the potential to improve patients' lives. Although a New Zealand-style national pharmacare scheme in Canada would offer the opportunity to restrain drug expenditure, it would likely fail to satisfy patients and healthcare providers and could diminish health outcomes, resulting in higher costs in other healthcare sectors.
Introduction
The sustainability of prescription drug insurance coverage is a perennial political issue in Canada. Provincial governments for whom healthcare consumes close to 50% of their overall budgets are concerned about new innovative medications that provide therapies for conditions not previously treatable but have high costs.
The Canadian pharmaceutical environment will soon see significant changes as part of the federal government's focus on “affordability, accessibility and appropriate use of prescription drugs” (1). For example, the government has imposed sweeping changes to the regulations that govern the quasi-judicial tribunal that sets maximum prices for patented medicines sold in Canada (2). The new regulations replace countries that have higher drug prices with lower price countries in the tribunal's international price comparison analysis, enforce a hard, low cost-effectiveness threshold, and impose a reduction in a drug's price if its annual sales exceed a specified amount (3). These changes will reduce Canada's attractiveness to pharmaceutical manufacturers as an important country in which launch their new products (4).
In addition, interest in a national drug insurance program has intensified with the announcement in its 2019 budget that the Liberal federal government is “moving forward” on “foundational elements” of national pharmacare (5). With the re-election of the Liberals in 2019 and support from other like-minded political parties, the process may proceed. Canada is the only country in the world with a universal public health system that covers healthcare providers, hospitalizations and laboratory services but not prescription drugs in the outpatient setting. Reimbursement for drug expenditures is available through government-funded plans and private insurance paid for by individuals or cost-shared with employers or unions. Around two-thirds of Canadians are covered by private insurance, while federal and provincial government drug plans—mainly designed to provide coverage to seniors, social assistance recipients and some special groups, or when costs are deemed to be catastrophic—offer a degree of coverage to about 25% of the population (6, 7). Many private plans provide wider drug coverage than public plans, including brand-name products, but often require deductibles and/or copayments and may have yearly or lifetime financial coverage; in addition, changing employment can lead to a loss of insurance. Government plans have complex systems of deductibles, copayments and premiums and, for many drugs, special or restricted access criteria that results in variation in patient eligibility, out-of-pocket expenses and coverage, which has led to significant inequalities between provinces.
New Zealand has a government-funded universal healthcare system that all residents use. To provide more timely access to medical and dental benefits, about 35% of the adult population has private health insurance most of whom pay for it themselves (8). However, most insurers in the New Zealand market only cover the copayments on prescription medicines that are publicly funded.
New Zealand's government created the Pharmaceutical Management Agency, known as PHARMAC, in 1993 “to make decisions on which medicines and medical devices are funded in order to get the best health outcomes from within the available funding” (9). PHARMAC operates on a fixed budget for pharmaceuticals so that, when assessing a new medicine for inclusion in the Pharmaceutical Schedule (the drug formulary) (10), funding decisions are based on clinical and economic assessments that examine the need and health benefit of a new medicine and its costs and potential savings (11). Consideration is also given to other drugs that must be forgone and price concessions that must be obtained from manufacturers to fund the new product within the budgetary cycle. PHARMAC takes recommendations from its health technology assessment (HTA) expert committee and negotiates with manufacturers to reach a provisional listing agreement. A product is added to the national Pharmaceutical Schedule only if an acceptable proposal is achieved, which can lead to “bundling” deals with manufacturers for multiple medicines (12). As of June 2019, over 100 medications had been recommended for inclusion in the subsidized program by the HTA committee but were unfunded, some of which had been waiting for funding for more than 10 years (13).
The Canadian federal government's Advisory Council on the Implementation of National Pharmacare examined international models of national pharmacare, including New Zealand's system, but did not recommend that Canada should copy any specific country (14). However, some Canadian health policy analysts have suggested that a Canadian national pharmacare scheme should include some components of the system used to control pharmaceutical costs in New Zealand (15–17). The objective of this evaluation was to review mortality and hospital discharge rates in the three disease areas (malignancies and circulatory and respiratory diseases) that account for more than two-thirds of the deaths in Canada and New Zealand in the light of prescription drug coverage by Canadian provincial public drug plans and the New Zealand national system.
Materials and Methods
Mortality data for malignancies and circulatory and respiratory diseases for Canada and New Zealand were obtained from the website of the Organization for Economic Cooperation and Development (OECD) (18) as age-standardized death rates per 100,000 population, standardized to the total OECD population for 2010, for the period between 2000 and 2015 (latest year for which cause of mortality data were available for both countries). Mortality data represent a hard health outcome measure.
Data on hospital discharges, excluding day cases, as a rate per 100,000 population and average length of stay in hospital, calculated by dividing the number of relevant bed-days by the number of discharges during the year, were also obtained for the two countries from 2000 to 2016 (data for both countries were only available for this period) as supplementary information about softer health outcomes.
Information on medications used to treat conditions in the three disease categories covered in Canada was obtained from on-line provincial public drug plan benefit lists current in mid-2019, including any from relevant special or exceptional access product lists maintained by some provinces. Only oncology drug reimbursement information from British Columbia, Alberta, Saskatchewan, Ontario and Quebec was used in the analysis because details of covered cancer drugs are accessible in separate publicly available benefit lists or are included in the provincial formulary for these provinces, whereas the formularies of New Brunswick, Nova Scotia, Prince Edward Island, and Newfoundland and Labrador list some but not all oncology drugs, while the oncology drug formulary for Manitoba is not publicly available. The Pharmaceutical Schedule current at mid-2019 was used to identified relevant subsidized medications, including oncology products, in New Zealand.
Where a medicine was only listed in Canadian formularies, information on regulatory applications approved by the Medicines and Medical Devices Safety Authority (19) was used to ascertain whether the product was approved in New Zealand. Similarly, if a medication was in the New Zealand Pharmaceutical Schedule but not in any Canadian provincial formulary, Health Canada's Drug Product Database (20) was used to determine whether it had regulatory approval in Canada.
Fewer drugs receive regulatory approval in New Zealand (21), which makes comparisons of the comprehensiveness of reimbursement listing challenging. For example, if there are 20 drugs in a class of which only 10 have regulatory approval in New Zealand and all are listed in the Pharmaceutical Schedule, the reimbursement listing rate is 100%. On the other hand, if all 20 drugs have regulatory approval in Canada, but provincial drug plans only cover 10, the reimbursement listing rate is 50%, although the same number of products receive reimbursement in both countries. However, drug reimbursement systems can only include medications that have regulatory approval in their country. Consequently, only reimbursement listing rates (the number of drugs with listing as a percentage of the number with regulatory approval) are reported for each country and compared using Fisher's exact test.
Results
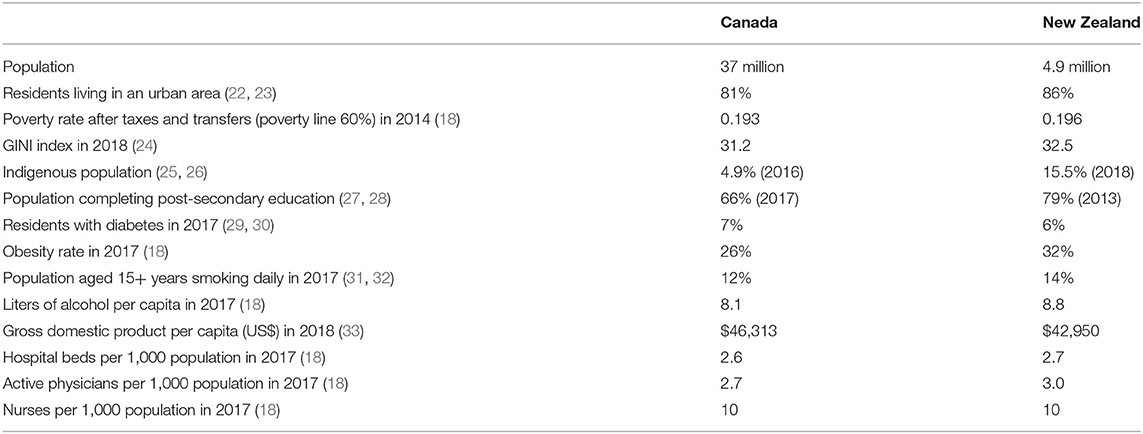
Although large differences exist between the two countries' geographic areas and populations, over 80% of their residents live in an urban area with a similar population density (Table 1). Poverty rates in the two countries are similar. Rates of residents with diabetes and obesity or who are regular smokers and the per capita consumption of alcohol, which are risk factors for cardiovascular disease and some cancers, are also similar in the two countries. Both countries have diverse economies, with international trade being a significant component of both economies, although New Zealand has a 7% lower per capita gross domestic product. In addition, both countries provide hospital and physician services without payment at the point of service, with the numbers of hospital beds and active physicians and nurses per 1,000 population in 2017 being virtually the same. These similarities and differences between the two countries should be borne in mind when considering the results of the analysis.
Hospital discharge rates for neoplasms and circulatory diseases in New Zealand between 2000 and 2016 were consistently higher than the rates in Canada by 25% on average, while the rate for respiratory diseases was consistently higher by 67% on average (Figure 1). Figure 2 shows the length of stay in hospital averaged over the 2000–2016 period. Although consistency exists between the two countries in the overall average length of stay and that for respiratory diseases, the New Zealand averages for disorders of the cardiovascular system and heart failure are approximately twice those in Canada and, remarkably, the New Zealand averages for cerebrovascular diseases, ischemic heart disease other than acute myocardial infarction, and hypertensive disease are three to seven times longer than in Canada.
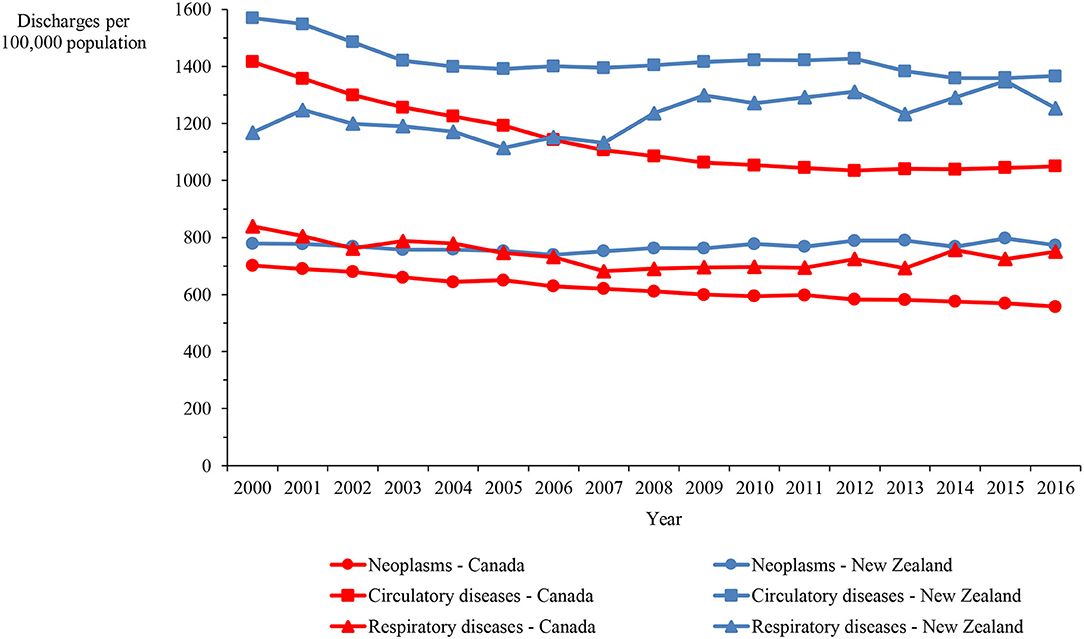
Figure 1. Hospital discharge rate in Canada and New Zealand for neoplasms and circulatory and respiratory diseases, 2000–2016.
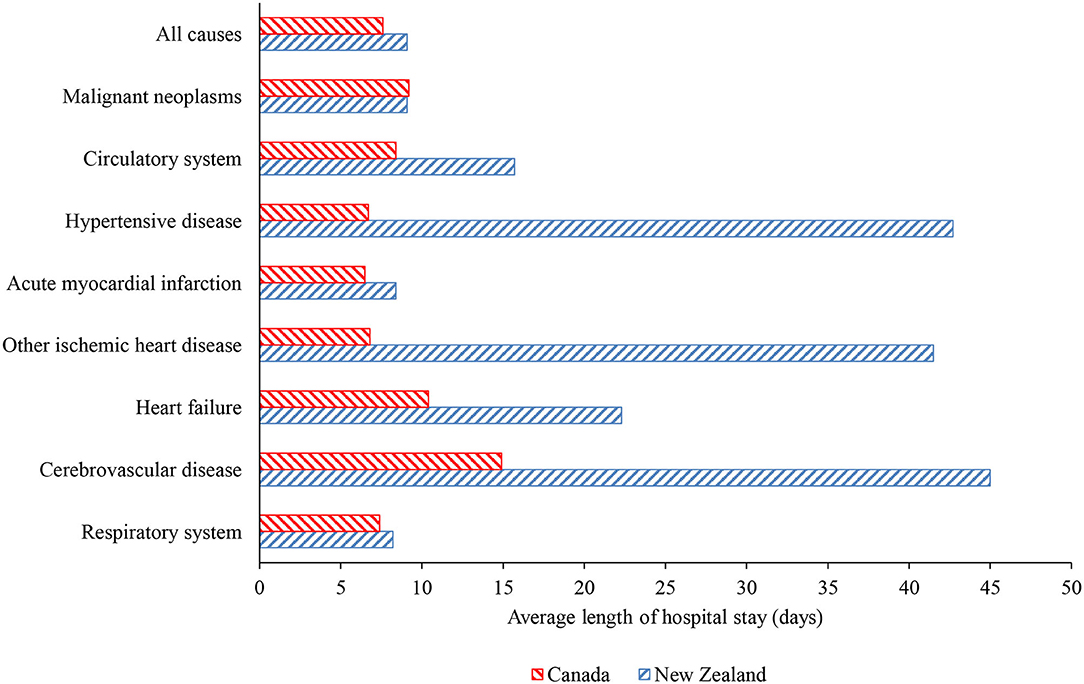
Figure 2. Average length of hospital stay over 2000–2016 period in Canada and New Zealand for all causes, malignancies, respiratory diseases, and selected cardiovascular conditions.
Mortality rates for Canada and New Zealand show that the main causes of death in both countries are malignancies and circulatory and respiratory diseases, accounting for 65.1% of the deaths in Canada and 71.4% of the deaths in New Zealand in 2015. The age-standardized mortality rate for circulatory diseases was higher in New Zealand than Canada by 31.1% on average, ranging between 21.4 and 37.8%, over the 16-year period. In contrast, the rates for malignancies and respiratory diseases were only marginally higher in New Zealand by 2.6% on average (varying between −0.3% and 7.8%) and 5.7% (varying between −9.8% and 15.7%), respectively (Figure 3).
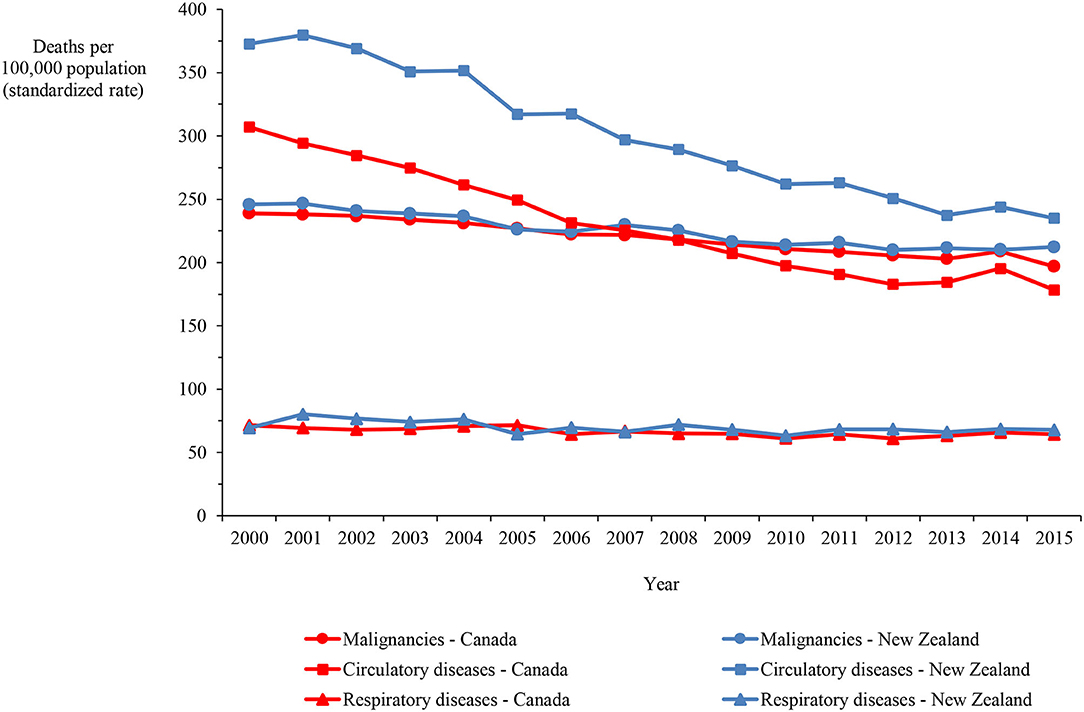
Figure 3. Mortality in Canada and New Zealand from malignancies and circulatory and respiratory diseases, 2000–2015.
Mortality data on ischemic heart disease and cerebrovascular disease, which account for the majority of circulatory disease deaths, demonstrate that, although the rate more than halved in both categories in both countries over the 16-years, the rate in New Zealand exceeded the Canadian rate by 33.6% and 60.9%, on average, for ischemic heart disease and cerebrovascular disease, respectively (Figure 4). Figures 5A,B show mortality rates for the four major cancers: breast, colorectal, lung and prostate. The New Zealand rate was higher than the Canadian rate for breast, colorectal and prostate cancer by 9.7%, 37.2%, and 32.7% on average, respectively. However, the New Zealand lung cancer rate was lower than the Canadian rate by 27.4%, on average over the 16-year period.
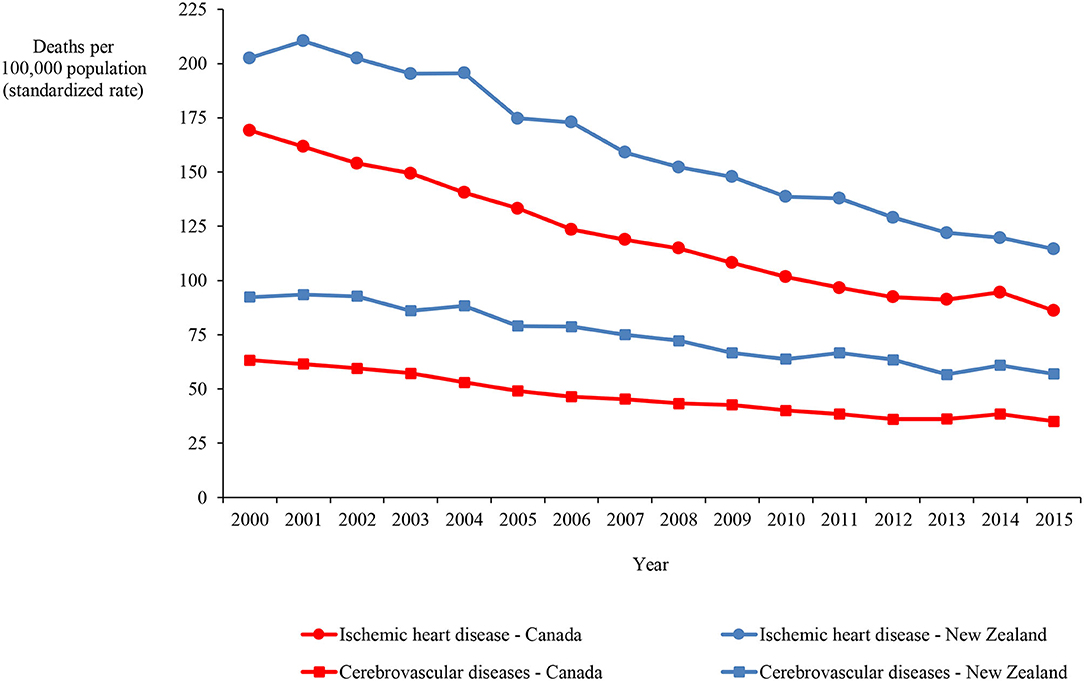
Figure 4. Mortality in Canada and New Zealand for ischemic heart and cerebrovascular diseases, 2000–2015.
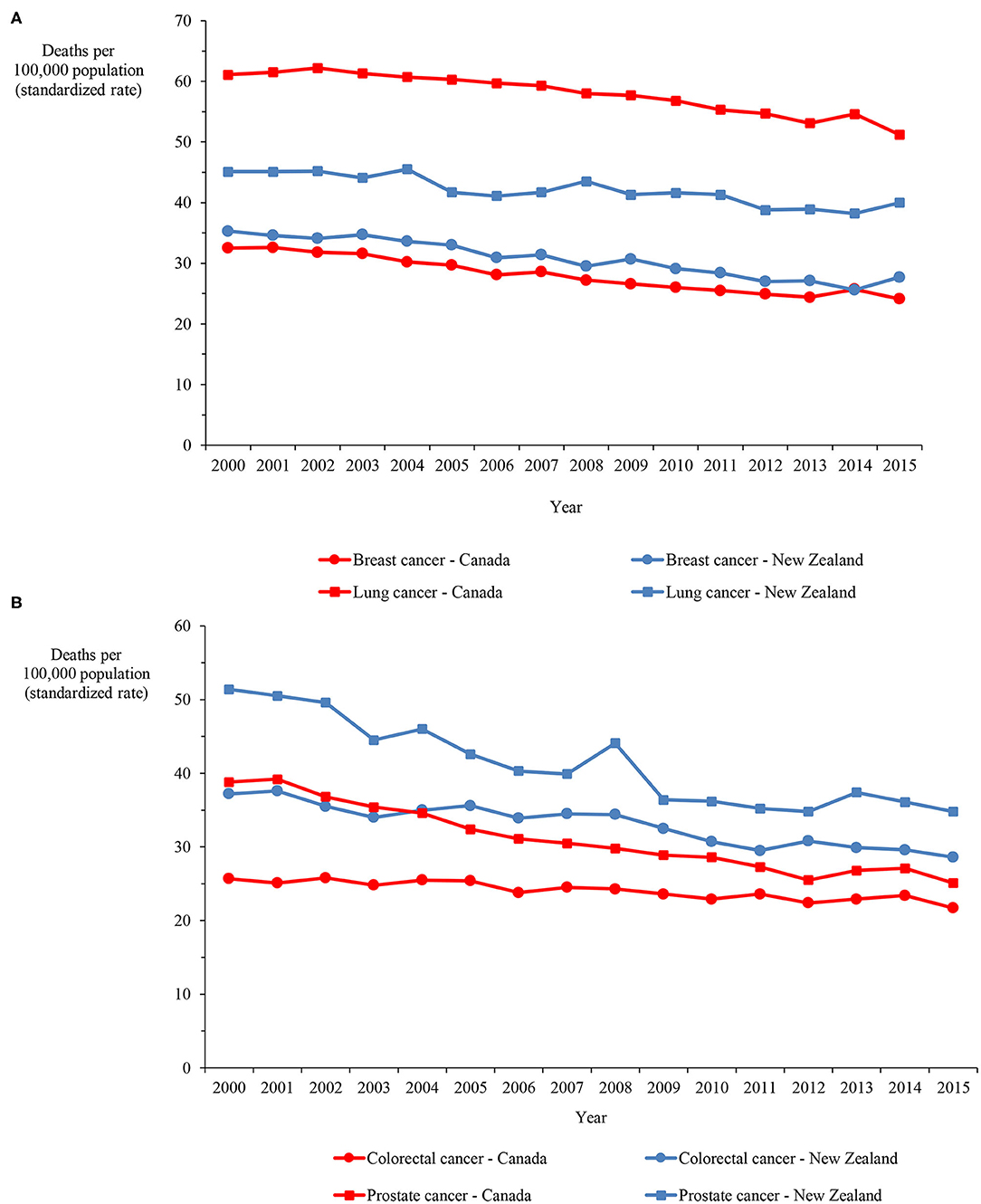
Figure 5. (A) Mortality in Canada and New Zealand for breast and lung cancers, 2000–2015. (B) Mortality in Canada and New Zealand for colorectal and prostate cancers, 2000–2015.
A total of 263 medications from the three system categories (cardiovascular, anti-cancers and respiratory) were identified for which the regulatory approval rate was 85.9% in New Zealand (226 drugs) compared with 97.0% in Canada (255 drugs). Of the medicines with regulatory approval in New Zealand, 127 (56.7%) were listed in the Pharmaceutical Schedule compared with a median number of 232 drugs (91.0%) with regulatory approval in Canada that are listed in provincial drug plans (p < 0.0001).
The overall reimbursement coverage rate in New Zealand was considerably lower than the median listing rate in Canadian provincial drug plans for angiotensin-converting-enzyme inhibitors (ACEIs; 60.0% vs. 100.0%; p = 0.007), angiotensin receptor blocking (ARB) drugs (30.8% vs. 100.0%; p < 0.0001), statins (71.4% vs. 100.0%; p = 0.19), drugs for cancers other than the four major cancer types of breast, colorectal, lung and prostate (56.2% vs. 95.2%; p = 0.012), and drugs used to treat multiple types of cancer (75.0% vs. 96.9%; p = 0.026) (Table 2). The listing rates in the two countries for other cardiovascular drugs and respiratory drugs were reasonably consistent.
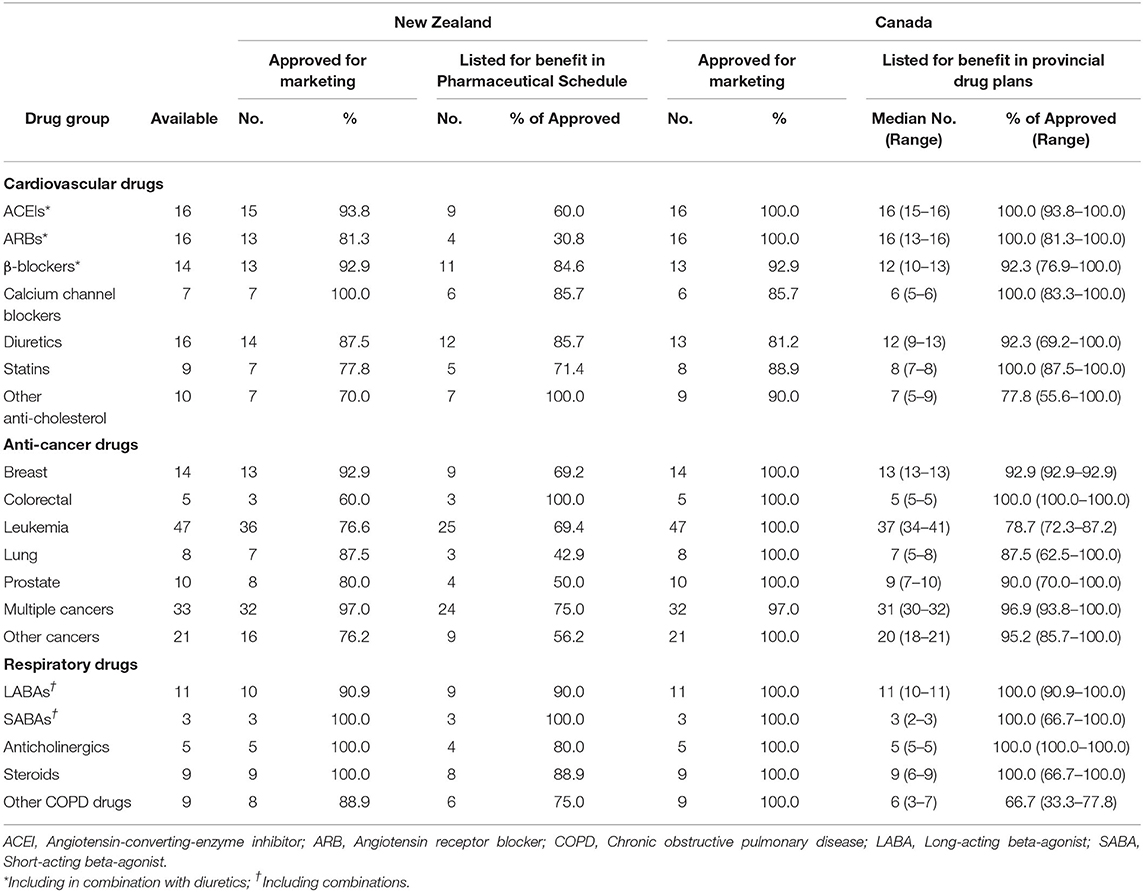
Table 2. Summary results of the comparison of selected drugs approved and listed for benefit in New Zealand and Canada, June 2019.
Discussion
It has been suggested that a Canadian national pharmacare program should adopt some aspects of the national system used in New Zealand (15–17), making a comparison between the two countries pertinent. While there are, as Table 1 demonstrates, similarities in several social determinants of health, health care access and factors that are risk factors for cardiovascular disease and some cancers, New Zealand has a higher proportion of indigenous residents, who have poorer levels of education, income and access to health care services. Moreover, New Zealand's much smaller population may make the country a less attractive market to pharmaceutical manufacturers.
Interesting differences in health outcomes can be seen between Canada and New Zealand. In particular, mortality and hospital discharge rates in New Zealand were generally higher and the average length of stay in hospital longer for cardiovascular disorders than in Canada. This is not the result of differences in the prevalence rates of these disorders, which are similar in both countries (34, 35). Nevertheless, increased length of stay may be due to other reasons, such as differences in treatment [e.g., Canadians admitted with acute coronary syndrome may be more likely to receive coronary angiography, which is associated with shorter lengths of stay, than New Zealanders (36)], the type of hospital, or differences in demographic characteristics (37).
Any impact of drug access on cardiovascular mortality is likely to take many years to be seen since many cardiovascular drugs are prescribed for the treatment of conditions such as hypertension and high cholesterol levels, which are risk factors for cardiovascular mortality. A period of 25–30-years is a reasonable length of time to make such an assessment for the ACEI and statin drugs and their impact should be observable by 2015, but the ARB drugs were introduced more recently. However, mortality from ischemic heart and cerebrovascular diseases and the rate of hospital discharges remain higher and the length of hospital stay for these health conditions is longer in New Zealand compared with Canada. The fewer ACEI and statin drugs and perhaps the ARB drugs funded and available in New Zealand may have contributed to these differences. More drugs in a class may make little difference, but patients are variable biologic entities so that a one-size-fits-all approach does not necessarily work.
Unlike cardiovascular drugs, oncology drugs are not prescribed as preventative therapy but to try to alleviate an existing disorder. Despite the proliferation of cancer therapies over the past 25 years, the lack of oncology drugs in the New Zealand formulary does not appear to have negatively impacted overall cancer mortality. Although mortality from three of the four major cancers (breast, colorectal, and prostate) is higher in New Zealand and fewer drugs specifically targeting these cancers are covered in New Zealand than in Canada, several new oncology drugs approved for marketing are not approved in New Zealand and others have either immature efficacy data or the efficacy results fail to demonstrate an improvement over existing medicines. For four cancers, including breast cancer, PHARMAC staff participated in the development of American Society of Clinical Oncology Cancer Research Committee (ASCO-CRC) targets for clinically meaningful progression-free survival (PFS) and overall survival (OS) and uses them in the evaluation of new drugs (38). For example, trastuzumab emtansine and palbociclib for metastatic breast cancer were not listed in New Zealand at the time of this analysis (although approved for marketing in New Zealand soon after approval in Canada) but were covered by almost all Canadian provincial plans. Trastuzumab emtansine has an improvement in OS and palbociclib has an improvement in PFS in the ASCO-CRC acceptable ranges for clinical value and, after an HTA of more than 2 years, both drugs are now listed in the PHARMAC formulary.
Although mortality rates from lung cancer are higher in Canada in the OECD data for 2000–2015, a recent report comparing survival, mortality and incidence of seven cancers (colon, lung, esophagus, ovary, pancreas, rectum and stomach) in seven countries, including Canada and New Zealand, between 1995 and 2014 demonstrated that the 5-years survival rate for lung cancer in Canada was 40–55% higher between 2000 and 2014 than the New Zealand rate (39). New Zealand had lower survival rates than Canada for all the cancers in the report between 2000 and 2014, except esophageal cancer.
This analysis has limitations. First and foremost, it is an associative assessment so that it is not possible to prove that lower drug listing rates lead to increased mortality and higher hospital discharge rates. Second, despite the OECD data being age-standardized making comparisons valid, other factors may have affected the health outcomes. Third, mortality rates by cause were only available to 2015 in the OECD data and, consequently, are not current. A further limitation is that listing of medications does not necessarily equate with access. Copayments, deductibles and premiums required by Canadian public drug plans, can place drugs, especially costly new ones, beyond reach. Once a drug is covered in New Zealand, this issue may be less of a problem because, in most cases, patients pay a copayment of only NZ$5 (about CAN$4.50) (40), which is less than half the dispensing fee in most provinces before any copayments or deductibles are added. Clinical criteria for coverage of a drug also frequently restrict access.
No evaluation of the therapeutic value of each drug was performed in this analysis. Some authors have reported that new drugs offer little benefit over existing medicines. For instance, fewer than 50% of the drugs entering the German health care system were reported to add benefit (41)—a re-analysis showed that <40% had minor or “non-quantifiable” benefit (41). Other authors have judged only 10–15% of new drugs to be “therapeutically innovative” (42). However, there is no agreement on the evaluation of therapeutic benefit. Different agencies within and between countries with differing agendas may have conflicting assessments. In some cases, the evaluation is restricted to meaningful clinical efficacy, while others take a wider perspective and include an assessment of adverse effects and convenience of use (43). Patients frequently take an even broader view about what constitutes therapeutic value.
With 41.8% of new regulatory-approved medications reimbursed in 2011–2016, Canada ranks 18th out of 20 OECD countries, while New Zealand ranks 20th with just 21.8% of new medications reimbursed in the same period (44). Access to new drugs, including anti-hypertensives and statins, has been demonstrated in Canada and several other countries to have a beneficial impact on health outcomes that outweigh the increased cost (45–49), although these findings are not universally accepted (50, 51). The more limited access to anti-hypertensives in New Zealand is surprising because hypertension is “a worldwide problem of enormous consequence” (52). The PHARMAC system has contained costs in New Zealand, but it restricts or denies access to important new medicines with the potential to improve patients' lives.
The attractiveness of a country as a priority jurisdiction in which pharmaceutical companies seek regulatory approval for their new products is based on several factors, such as the potential number of patients with the disorder for which the drug is indicated, the likelihood of obtaining a profitable price for the product and gaining drug reimbursement coverage, and the country's overall investment climate. The ability to progress innovative treatments through regulatory, reimbursement and pricing processes in a timely manner with government officials and politicians having a comprehensive understanding of costs and system savings (not simply focusing on price) is a critical element. Canada's complex pricing and reimbursement environment already places the country's attractiveness for new innovative medications, especially costly ones, at a lower level than the United States and Europe because innovation is not seen to be valued in Canadian governments' pricing decisions (53). For example, the manufacturer of elexacaftor/tezacaftor/ivacaftor has decided not apply for marketing approval for the drug until the uncertainty surrounding Canada's current regulatory pricing policy is resolved to its satisfaction.
National pharmacare and medication affordability are the current focuses of Canadian politicians, government officials and health policy analysts. Changes in the regulations that govern the tribunal that sets maximum prices for patented medicines sold in Canada (3) will decrease the attractiveness of Canada as a jurisdiction in which global pharmaceutical companies seek regulatory approval for their new products (54, 55). This has the potential to decrease the number of new drugs brought to Canada and, thus, reduce the number covered; early evidence supports this prediction (4, 56). Including tight price controls similar to those used in New Zealand in a national pharmacare scheme in Canada would offer public payers the opportunity to restrain drug expenditure but would likely fail to satisfy patients and healthcare providers and could result in higher costs in other healthcare sectors. More research is required to understand the impact of tighter cost-containment on prescription drug coverage and health outcomes before making radical changes to the Canadian pharmaceutical environment.
Data Availability Statement
Publicly available datasets were analyzed in this study. These data can be found here: http://stats.oecd.org/.
Author Contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Conflict of Interest
In the past 3 years, the author has received consultant fees from 3Sixty Public Affairs Inc. and Fasken, research and publication fees from Advocacy Solutions, the Canadian Health Policy Institute, the Fraser Institute, Macdonald-Laurier Institute, Merck Sharp & Dohme (New Zealand) Ltd., RAREi (a collaboration of innovative pharmaceutical companies focused on the development of medicines for rare disorders) and Ward Health, and publication processing expenses from Canadian PKU and Allied Disorders Inc. and Shire Pharma Canada ULC. No conflict of interest exists between these activities and the present work.
Acknowledgments
I gratefully acknowledge research funding for this work from Medicines New Zealand, including the article processing fee. I also thank Graeme Jarvis, Phyllida Duncan, and Tanya Baker from Medicines New Zealand and Lisa Woods from Victoria University of Wellington for statistical comments on earlier drafts of this article. Although Medicines New Zealand staff had the opportunity to review the manuscript, they had no control over the design, analysis or reporting of this work.
References
1. Government of Canada. Remarks from the Honourable Jane Philpott, Minister of Health, to the Economic Club of Canada. Ottawa: Government of Canada (2017). Available online at: https://www.canada.ca/en/health-canada/news/2017/05/economic_club_ofcanada-may162017.html (accessed October 12, 2020).
2. Patented Medicine Prices Review Board. Mandate and Jurisdiction. Ottawa: Government of Canada. (2018). Available online at: http://pmprb-cepmb.gc.ca/about-us/mandate-and-jurisdiction.
3. Patented Medicine Prices Review Board. PMPRB Draft Guidelines. Ottawa: Patented Medicine Prices Review Board. (2020). Available online at: https://www.canada.ca/content/dam/pmprb-cepmb/documents/consultations/draft-guidelines/2020/PMPRB-Guidelines2020-en.pdf.
4. Rawson NSB, Adams J. Access to New Medicines: What Ottawa should Learn from COVID-19. Ottawa: Macdonald-Laurier Institute for Public Policy. (2020).Available online at: https://www.macdonaldlaurier.ca/covid19-weakness-new-medicines/.
5. Morneau WF. Investing in the Middle Class: Budget 2019. Ottawa: Her Majesty the Queen in Right of Canada. (2019). Available online at: https://budget.gc.ca/2019/docs/plan/budget-2019-en.pdf.
6. Sutherland G, Dinh T. Understanding the Gap: a Pan-Canadian Analysis of Prescription Drug Insurance Coverage. Toronto: Conference Board of Canada (2017). Available online at: https://www.conferenceboard.ca/e-library/abstract.aspx?did=9326.
7. Skinner BJ. Prescription Drug Plan Coverage 2016: How Many Canadians were Insured, Under-Insured or Uninsured? Can Health Policy. Toronto: Canadian Health Policy Institute. (2018). Available online at: https://www.canadianhealthpolicy.com/products/prescription-drug-plan-coverage-2016–how-many-canadians-were-insured–under-insured-or-uninsured-.html
8. Ministry of Health. Private Health Insurance Coverage 2011-15: New Zealand Health Survey. Wellington: New Zealand Government. (2016). Available online at: https://www.health.govt.nz/publication/private-health-insurance-coverage-2011-15-new-zealand-health-survey
9. PHARMAC. Our Place in the Health System. Wellington: New Zealand Government. (2020). Available online at: https://www.pharmac.govt.nz/about/your-guide-to-pharmac/factsheet-03-our-place-in-the-health-system
10. PHARMAC. Pharmaceutical Schedule. Wellington: New Zealand Government. (2020). Available online at: https://www.pharmac.govt.nz/tools-resources/pharmaceutical-schedule
11. PHARMAC. How Medicines are Funded. Wellington: New Zealand Government. (2020). Available online at: https://www.pharmac.govt.nz/medicines/how-medicines-are-funded/.
12. Coleman C, Wonder M. Exploring the implications of a fixed budget for new medicines: a study of reimbursement of new medicines in Australia and New Zealand. Aust Health Rev. (2015) 39:455–61. doi: 10.1071/AH14122
13. Della Barca C. Funding Medicines in New Zealand: Revision of the Medicines Waiting List to 30 June 2019. Auckland: Subscripts Ltd. (2019). Available online at: https://www.medicinesnz.co.nz/fileadmin/user_upload/Medicines_Waiting_List_to_30_June_2019.pdf
14. Advisory Council on the Implementation of National Pharmacare. A Prescription for Canada: Achieving Pharmacare for All. Ottawa: Government of Canada. (2019). Available online at: https://www.canada.ca/content/dam/hc-sc/images/corporate/about-health-canada/public-engagement/external-advisory-bodies/implementation-national-pharmacare/final-report/final-report.pdf.
15. Morgan S, Hanley G, McMahon M, Barer M. Influencing drug prices through formulary-based policies: lessons from New Zealand. Healthc Policy. (2007) 3:e121–40. doi: 10.12927/hcpol.2007.19097
16. Gagnon MA. A Roadmap to a Rational Pharmacare Policy in Canada. Ottawa: Canadian Federation of Nurses Unions. (2014). Available online at: https://nursesunions.ca/wp-content/uploads/2017/05/Pharmacare_FINAL.pdf
17. Kelley LT, Tenbensel T, Johnson A. Ontario and New Zealand pharmaceuticals: cost and coverage. Healthc Policy. (2018) 13:23–34. doi: 10.12927/hcpol.2018.25496
18. Organization for Economic Cooperation and Development. OECD.stat. Paris: Organization for Economic Cooperation and Development. (2019). Available online at: http://stats.oecd.org
19. Medsafe. Medicines Product/Application Search. Wellington: New Zealand Government, (2019). Available online at: http://www.medsafe.govt.nz/regulatory/DbSearch.asp
20. Government of Canada. Drug Product Database Online Query. Ottawa: Government of Canada. (2019). Available online at: https://health-products.canada.ca/dpd-bdpp/index-eng.jsp
21. Rawson NSB. Comparison of numbers and timing of new medication regulatory approvals in Canada and New Zealand. Regul Toxicol Pharmacol. (2019) 101:24–8. doi: 10.1016/j.yrtph.2018.10.019
22. Statista. Canada: Degree of Urbanization from 2008 to 2018 Statista. (2020). Available online at: https://www.statista.com/statistics/271208/urbanization-in-canada/
23. Statista. New Zealand: Urbanization from 2008 to 2018 Statista. (2020). Available online at: https://www.statista.com/statistics/455899/urbanization-in-new-zealand/
24. World data atlas. Knoema. (2020). Available online at: https://knoema.com/atlas
25. Statistics Canada. Focus on Geography Series, 2016 Census. Ottawa: Statistics Canada (2019). Available online at: https://www12.statcan.gc.ca/census-recensement/2016/as-sa/fogs-spg/Facts-CAN-eng.cfm?Lang=Eng&GK=CAN&GC=01&TOPIC=9
26. StatsNZ. Maori Population Estimates: at 30 June 2018. Wellington: New Zealand Government. (2018) Available online at: https://www.stats.govt.nz/information-releases/maori-population-estimates-at-30-june-2018
27. Statistics Canada. Education Indicators in Canada: an International Perspective. Ottawa: Statistics Canada. (2018). Available online at: https://www150.statcan.gc.ca/n1/en/pub/81-604-x/81-604-x2018001-eng.pdf?st=IGin3Zeu
28. StatsNZ. 2013 Census Quickstats about National Highlights. Wellington: New Zealand Government. (2013). Available online at: http://archive.stats.govt.nz/Census/2013-census/profile-and-summary-reports/quickstats-about-national-highlights/education.aspx#gsc.tab=0
29. Statistics Canada. Diabetes. (2017). Ottawa: Government of Canada. (2018). Available online at: https://www150.statcan.gc.ca/n1/pub/82-625-x/2018001/article/54982-eng.htm
30. Best Practice Advocacy Centre New Zealand. A Rising Tide of Type 2 Diabetes in Younger People: What can Primary Care do? Dunedin: Best Practice Advocacy Centre New Zealand, (2018). Available online at: https://bpac.org.nz/2018/diabetes.aspx#main-nav
31. Statistics Canada. Smoking. (2016). Ottawa: Statistics Canada. (2017). Available online at: https://www150.statcan.gc.ca/n1/pub/82-625-x/2017001/article/54864-eng.htm
32. Health Promotion Agency. Facts and Figures. Wellington: Health Promotion Agency. (2019). Available online at: https://www.smokefree.org.nz/smoking-its-effects/facts-figures
33. World Bank. GDP per capita (current US$). Washington, DC: World Bank. (2019). Available online at: https://data.worldbank.org/indicator/NY.GDP.PCAP.CD?locations=NZ
34. Government of Canada. Canadian Chronic Disease Surveillance System. Ottawa: Government of Canada. (2018). Available online at: https://health-infobase.canada.ca/ccdss/data-tool.
35. National Health Committee. Strategic Overview: Cardiovascular Disease in New Zealand. Wellington: New Zealand Government. (2013). Available online at: https://www.moh.govt.nz/NoteBook/nbbooks.nsf/0/FAC55041FD6DBDADCC257F7F006CDC16/$file/strategic-verview-cardiovascular-disease-in-nz.pdf
36. Wang TKM, Grey C, Jiang Y, Jackson R, Kerr A. Trends in length of stay following acute coronary syndrome hospitalization in New Zealand 2006-2016: ANZACS-QI 32 study. NZ J Med. (2020) 133:29–42. doi: 10.1016/j.hlc.2019.11.015
37. Rumball-Smith J, Sarfati D, Hider P, Blakely T. Ethnic disparities in the quality of hospital in New Zealand, as measured by 30-day rate of unplanned readmission/death. Int J Qual Health Care. (2013) 25:248–54. doi: 10.1093/intqhc/mzt012
38. Evans J, Laking G, Strother M, Wang T, Metcalfe S, Blick G, et al. Mind the gap: an analysis of foregone health gains from unfunded cancer medicines in New Zealand. Semin Oncol. (2016) 43:625–37. doi: 10.1053/j.seminoncol.2016.10.004
39. Arnold M, Rutherford MJ, Bardot A, Ferlay J, Andersson TML, Myklebust TA, et al. Progress in cancer survival, mortality and incidence in seven high-income countries 1995-2014 (ICBP SURVMARK-2): a population-based study. Lancet Oncol. (2019) 20:1493–505. doi: 10.1016/S1470-2045(19)30456-5
40. Ministry of Health. Pharmaceutical Copayments. Wellington: New Zealand Government, (2014). Available online at: https://www.health.govt.nz/our-work/primary-health-care/primary-health-care-subsidies-and-services/pharmaceutical-co-payments
41. Wieseler B, McGauran N, Kaiser T. New drugs: where did we go wrong and what can we do better? BMJ. (2019) 366:14837. doi: 10.1136/bmj.14837
42. Lexchin J. Health Canada's use of expedited review pathways and therapeutic innovation, 1995-2016: cross-sectional analysis. BMJ Open. (2018) 8:e023605. doi: 10.1136/bmjopen-2018-023605
43. Patented Medicine Prices Review Board. Compendium of Policies, Guidelines and Procedures – updated February 2017. Ottawa: Government of Canada. (2017). Available online at: http://www.pmprb-cepmb.gc.ca/view.asp?ccid=492#c6
44. Medicines Australia. Comparison of Access and Reimbursement Environments. 3rd Edn. Deakin: Medicines Australia. (2017). Available online at: https://medicinesaustralia.com.au/wp-content/uploads/sites/52/2015/03/MA-Compare-Edition-3-October-2017.pdf
45. Lichtenberg FR, Grootendorst P, Van Audenrode M, Latremouille-Viau D, Lefebvre P. The impact of drug vintage on patient survival: a patient-level analysis using Quebec's provincial health plan data. Value Health. (2009) 12:847–56. doi: 10.1111/j.1524-4733.2009.00532.x
46. Lichtenberg FR. The impact of pharmaceutical innovation on premature cancer mortality in Canada, 2000-2011. Int J Health Econ Manag. (2015) 15:339–59. doi: 10.1007/s10754-015-9172-2
47. Hostenkamp G, Lichtenberg FR. The impact of recent chemotherapy innovation on the longevity of myeloma patients: US and international evidence. Soc Sci Med. (2015) 130:162–71. doi: 10.1016/j.socscimed.2015.02.003
48. Cutler DM, Long G, Berndt ER, Royer J, Fournier AA, Sasser A, et al. The value of antihypertensive drugs: a perspective on medical innovation. Health Aff. (2007) 26:97–110. doi: 10.1377/hlthaff.26.1.97
49. Grabowski DC, Lakdawalla DN, Goldman DP, Eber M, Liu LZ, Abdelgawad T, et al. The large social value resulting from use of statins warrants steps to improve adherence and broaden treatment. Health Aff. (2012) 31:2276–85. doi: 10.1377/hlthaff.2011.1120
50. Grootendorst P, Piérard E, Shim M. Life-expectancy gains from pharmaceutical drugs: a critical appraisal of the literature. Expert Rev Pharmacoecon Outcomes Res. (2009) 9:353–64. doi: 10.1586/erp.09.35
51. Zhang Y, Soumerai SB. Do newer prescription drugs pay for themselves? A reassessment of the evidence. Health Aff. (2007) 26:880–6. doi: 10.1377/hlthaff.26.3.880
52. Fisher NDL, Curfman G. Hypertension – a public health challenge of global proportions. JAMA. (2018) 320:1757–9. doi: 10.1001/jama.2018.16760
53. Pugatch Consilium. Ascending the Peak of Pharmaceutical Innovation. Biopharmaceutical Competitiveness and Investment (BCI) Survey. 4th edition. Washington, DC: Pugatch Consilium. (2017). Available online at: http://www.pugatch-consilium.com/reports/BCI_2017_Report.pdf
54. Rawson NSB, Lawrence D. New Patented Medicine Regulations In Canada: Updated Case Study of a Manufacturer's Decision-Making about a Regulatory Submission for a Rare Disorder Treatment. Can Health Policy. Toronto: Canadian Health Policy Institute. (2020). Available online at: https://www.canadianhealthpolicy.com/products/new-patented-medicine-regulations-in-canada–updated-case-study—en-fr-.html
55. Life Sciences Ontario. Impact of PMPRB Pricing Changes: Final Research Report. Toronto: Life Sciences Ontario (2020). Available online at: https://lifesciencesontario.ca/wp-content/uploads/2020/02/Research-Etc.-PMPRB-Survey-02-03-20.pdf
56. Life Sciences Ontario. New Medicine Launches: Canada in a Global Context. Toronto: Life Sciences Ontario. (2020). Available online at: https://lifesciencesontario.ca/wp-content/uploads/2020/06/EN_LSO_Global-Launch-Benchmarking_Webinar-June22-20_Final.pdf
Keywords: mortality, health outcomes, prescription drugs, Canada, New Zealand, drug insurance
Citation: Rawson NSB (2020) Leading Causes of Mortality and Prescription Drug Coverage in Canada and New Zealand. Front. Public Health 8:544835. doi: 10.3389/fpubh.2020.544835
Received: 22 March 2020; Accepted: 22 September 2020;
Published: 30 October 2020.
Edited by:
Amelia Kekeletso Ranotsi, Maluti Adventist College, LesothoCopyright © 2020 Rawson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nigel S. B. Rawson, ZWFzdGxha2VyZ0BnbWFpbC5jb20=
 Nigel S. B. Rawson
Nigel S. B. Rawson