- 1Shanghai Municipal Center for Disease Control and Prevention, Shanghai, China
- 2Department of Epidemiology, School of Public Health, Fudan University, Shanghai, China
- 3Shanghai Municipal Center for Health Promotion, Shanghai, China
- 4Department of Epidemiology, School of Public Health, University of Michigan, Ann Arbor, MI, United States
- 5Department of Health Economics, School of Public Health, Fudan University, Shanghai, China
- 6Yangpu District Center for Disease Control and Prevention, Shanghai, China
- 7Chongming District Center for Disease Control and Prevention, Shanghai, China
- 8Songjiang District Center for Disease Control and Prevention, Shanghai, China
Background: Streptococcus pneumoniae infection among adults, especially in adults over 60 years old in China results in a large number of hospitalizations and a substantial financial burden. This study assessed the vaccine effectiveness (VE) of 23-valent pneumococcal polysaccharide vaccine (PPV23) against pneumococcal diseases among the elderly aged 60 years or older in Shanghai, China.
Methods: We conducted a test-negative case–control study among the elderly aged 60 years or older who sought care at hospitals in 13 districts of Shanghai from September 14, 2013 to August 31, 2019. A case was defined as pneumococcal disease and testing positive for Streptococcus pneumoniae. Controls had symptoms congruent with pneumococcal disease but were negative for Streptococcus pneumoniae. We conducted 1:2 matching by gender, age, hospital and admission date. Vaccination status was verified from the immunization system database. VE was calculated with conditional logistic regression according to the formula (1–OR) ×100%.
Results: Overall, 603 adults aged 60 years or older with pneumococcal disease and positive for Streptococcus pneumoniae were included as cases, and 19.6% (118 persons) had a recorded PPV23 vaccination. The controls included 1,206 adults, whose vaccination rate was 23.8% (287 persons). The VE against pneumococcal diseases among the whole population was 24% (95% CI: 2%, 40%) and among women 44% (95% CI: 6%, 67%). After adjusting for multiple variables, the effectiveness of PPV23 against pneumococcal diseases was still statistically significant with VE for all of 25% (95% CI: 3%, 42%) and VE for women of 49% (95% CI: 11%, 71%).
Conclusion: PPV23 was effective against pneumococcal diseases in adults aged 60 years or older in Shanghai, China. Its relatively high effectiveness among women warrants this group to be particularly targeted for vaccination, with further research on why vaccination effectiveness is less among men.
Introduction
Streptococcus pneumoniae (Pneumococcus) is a common opportunistic pathogen which can colonize the nasopharynx asymptomatically (1). Occasionally, it can invade the lower respiratory tract or the bloodstream and cause various diseases, such as pneumonia, meningitis, bacteremia, otitis media and sinusitis (2, 3). Infants, young children under 2 years old and the elderly are particularly susceptible, along with those with chronic diseases (4–6).
There is a large burden of pneumococcal disease, with 827 thousand deaths from pneumococcal pneumonia and 118 thousand deaths from pneumococcal meningitis worldwide in 2010 (7). There is little information about the burden of pneumococcus in China. It could be responsible for 3.0–8.5% of community-acquired pneumonia in China, although studies in neighboring countries have found larger proportions (e.g., 9.1–65.0% in studies in Japan, and 13.5–60.8% in South Korea) (8).
There are currently two categories of pneumococcal vaccine available in China: a 23-valent polysaccharide vaccine (PPV23) and a 13-valent conjugate vaccine (PCV13). In the US, PPV23 is recommended in all adults ≥65 years old, with PCV13 administered 1 year prior to PPV23 in certain high risk groups (9). Experts within China have also recommended PPV23 vaccination for routine use in adults ≥60 years old (10). A review of PPV23 vaccine studies in older adults have found adequate antibody responses, but mixed results in its effectiveness to prevent illness or death from pneumonia (11). Studies have found PPV23 to be cost-effective within China (12), but these analyses are highly reliant on the estimates of PPV23 effectiveness. In order for there to be a universal recommendation for PPV23 from the Chinese government, more studies of vaccine effectiveness within the country are needed.
Vaccination is an important means of preventing disease and limiting the spread of antibiotic resistance within pneumococcal strains (13). In Shanghai, vaccination of children with PPV23 or PCV13 is not mandatory and requires out-of-pocket payment (14). Since September 14, 2013, Shanghai has launched a major public health service project where residents of 60 years and above can receive a PPV23 vaccination for free. In order to further explore the protective effectiveness of PPV23, a matched test negative case–control study was carried out in Shanghai.
Methods
Study Site
This study was conducted in 33 hospitals carrying out the laboratory test project of Streptococcus pneumoniae in 13 districts of Shanghai, including Huangpu District, Xuhui District, Putuo District, Jing'an District, Yangpu District, Minhang District, Baoshan District, Jiading District, Songjiang District, Qingpu District, Fengxian District, Pudong New District and Chongming District. Shanghai is one of the most economically developed cities with the highest population density in China, with a population of over 23 million (15).
Study Population
A matched case-control survey was conducted using the test negative design. We limited our analysis to Shanghai residents aged ≥60 years old because people in this age group are eligible to be given one dose of PPV23 free of charge (16). Patients were eligible to be included if they were hospitalized for diseases congruent with pneumococcal infection, such as pneumonia (including imaging diagnosis of pneumonia), meningitis, bacteremia, otitis media, other lower respiratory tract infections, acute episode of chronic bronchitis, and other lung infection between September 14, 2013 and August 31, 2019. Positive cases were those patients with a positive pneumococcal test by routine culture or PCR test from blood, sputum, cerebrospinal fluid, throat swab, pleural effusion or other samples by urine antigen detection. Those with a negative pneumococcal test from all sources were selected as controls. The final study subjects of the control group were randomly matched by gender (same), age (±5 years), hospital (same) and date of admission (±60 days) in a 1:2 case-control ratio.
Data Collection
The questionnaire was designed by Shanghai Municipal Center for Disease Control and Prevention (SCDC), and the information of the subjects were retrospectively collected by the project staff who had been trained by SCDC. The investigators collected information related to the patient's demographics (name, gender, age), lifestyle information (smoking status, drinking status), disease history (respiratory related diseases, chronic underlying diseases, immune system diseases), hospitalization information (admission date, antibiotic use, discharge diagnosis, outcomes), Streptococcus pneumoniae testing (result of test, samples, and date of sampling), and coinfection (Mycoplasma pneumoniae, Haemophilus influenzae, etc.) from different departments in the chosen site hospitals.
Furthermore, we collected vaccination history through the electronic immunization information system maintained by SCDC, which recorded immunization information on all registered residents including date of vaccination, dose and type of vaccine. From this system we abstracted PPV23 and influenza vaccination (within the last year before admission). Subjects without any vaccination records or who received a PPV23 vaccination after discharge were considered as having no PPV23 vaccination.
Definitions
We determined the types of diseases suffered by the subjects according to ICD codes (International Classification of Diseases, Tenth revision, Clinical Modification) as follows: the ICD codes of pneumonia were J12-J18 and J69, the ICD code for meningitis was G00.1, the ICD code of bacteremia was A40.3, the ICD code of otitis media was H66, the ICD code of other lower respiratory tract infections were J20-22, the ICD codes of acute episode of chronic bronchitis were J44.0 and J44.1, the ICD codes of other lung infection were J98.414 and J98.414A. In addition, in order to include as many cases as possible in the case group, patients with imaging diagnosis of pneumonia (chest radiographs, CT and other ancillary tests indicating pulmonary inflammation) and positive for pneumococcal tests were also included in the case group, which was not applicable in the control group.
Statistical Analysis
In this test-negative case-control study, the basic characteristics of case and control groups were compared using Chi-square test or Fisher's exact test. Unadjusted and adjusted ORs were estimated using conditional logistic regression models, which included a matching stratum based on gender, age, hospital, and date of admission. We include three sets of models to adjust for possible confounders. Model 1 adjusted for age, medical history (respiratory related diseases, chronic underlying diseases and immune system diseases) and smoking status. Model 2 adjusted for the same inputs as model 1, but also co-infection with other pathogens. Model 3 adjusted for the same inputs as model 2, but also included antibiotics using before sampling and influenza vaccination within previous year.
Vaccine effectiveness (VE) against pneumococcal diseases was estimated as one minus the OR multiplied by 100%. We stratified VE estimates by gender. Statistical analyses were performed in SAS9.4 and two-sided tests were used at a level of 0.05.
Ethics Statement
This study was approved by the institutional review board of Shanghai Municipal Center for Disease Control and Prevention. We did not obtain informed consent for each study subject because the data were obtained from medical or vaccination registries. Electronic registries included some personal information like name, ID number, cell phone number and address, but these were not included in the datasets made available for analysis.
Results
A total of 640 pneumococcal patients aged 60 years and above with a positive pneumococcal test who were admitted to the investigation site hospitals for pneumococcal diseases during September 14, 2013 and August 31, 2019 were selected into this study as cases (Figure 1). A total of 33 cases were removed after further review indicated they did not meet the inclusion criteria. We removed an additional 4 cases for whom there were not a sufficient number of control subjects to match. The final sample included 603 cases and 1,206 controls.
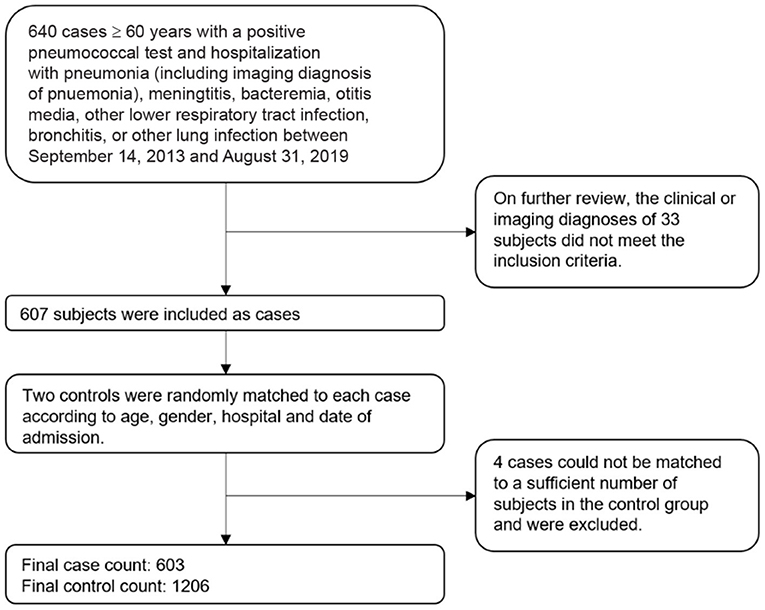
Figure 1. Sampling schematic of cases and controls in the study of pneumococcal vaccine effectiveness in Shanghai.
Demographic Characteristics
Cases and controls had similar distribution by age, gender, district distribution, drinking status, and history of respiratory related diseases, chronic underlying diseases and immune system diseases, but differed in the other characteristics (Table 1). About 22.1% were women in both of the case group and control group; the ratio of urban and suburban areas in the case group and control group was both about 1:1.8. Of the 603 cases, 492 (81.6%) cases were tested positive in sputum culture, 31 (5.1%) in blood culture, 1 (0.2%) in pleural effusion, 4 (0.7%) in CSF, 4 (0.7%) in throat swab, 46 (7.6%) in urine antigen, 9 (1.5%) in other samples, 8 (1.3%) in both sputum culture and blood culture, 6 (1.0%) in both sputum culture and throat swab, 2 (0.4%) in both sputum culture and urine antigen.
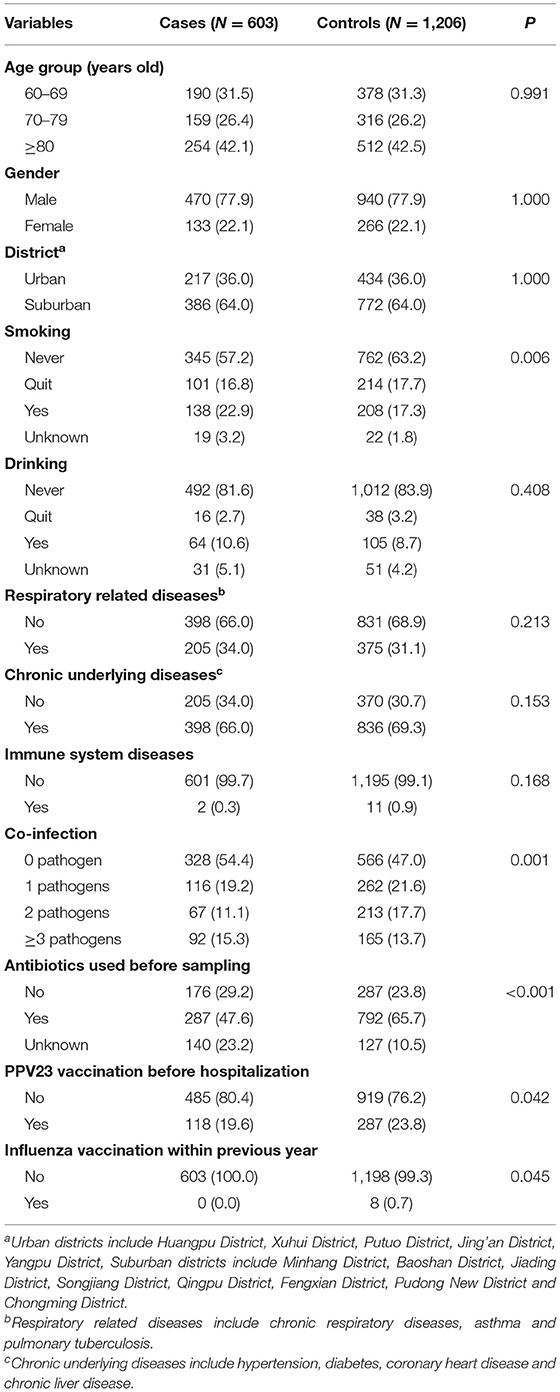
Table 1. Basic characteristics of research objects by pneumococcus positive patients (cases) and pneumococcus negative patients (controls).
Figure 2 shows the clinical presentation within cases and controls. A plurality of cases had the other lung infection (34.3%), although acute episode of chronic bronchitis (31.0%) and pneumonia (23.4%) were also common. For controls, a plurality had pneumonia (45.7%).
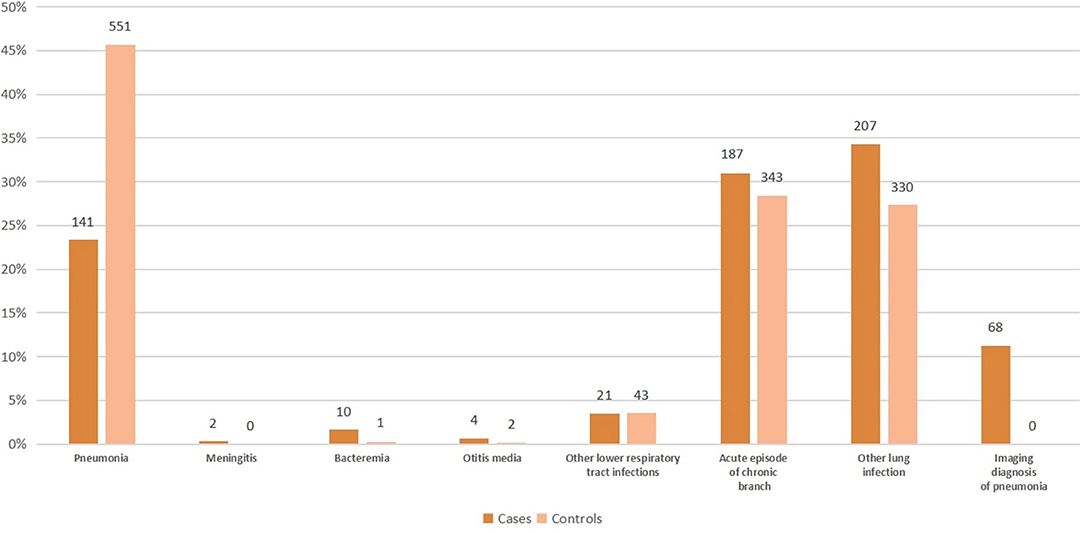
Figure 2. Clinical presentation within cases and controls in the study of pneumococcal vaccine effectiveness in Shanghai.
Unadjusted VE of PPV23 Vaccination
Through a 1:2 matched analysis, 70 pairs of in the case and control groups were both vaccinated, 753 pairs were not vaccinated in either group, 166 pairs were vaccinated in the case group but not in the control group, while 217 pairs were vaccinated in the control group but not in the case group. The overall unadjusted VE of PPV23 vaccination against pneumococcal diseases was 24% (95% CI: 2%, 40%). The point estimate of VE for women (44%, 95%CI: 6%,67%) was higher than that for men (16%, 95% CI: −12%, 37%) (Table 2).
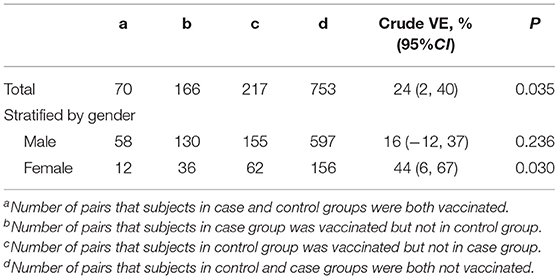
Table 2. Unadjusted VE of PPV23 vaccination against pneumococcal diseases among people aged 60 years or older.
Adjusted VE of PPV23 Vaccination
We provide three sets of models to adjust for various confounders, but the estimates of VE remained stable across these models. For model 1, which adjusted for age, medical history (respiratory related diseases, chronic underlying diseases, and immune system diseases) and smoking status, overall VE was 24% (95% CI: 3%, 41%), and was lower in men (17%, 95% CI: −11%, 37%) than women (47%, 95% CI: 9%, 70%). In models 2 and 3, the estimates of VE did not change by more than a few percentage points (Table 3).
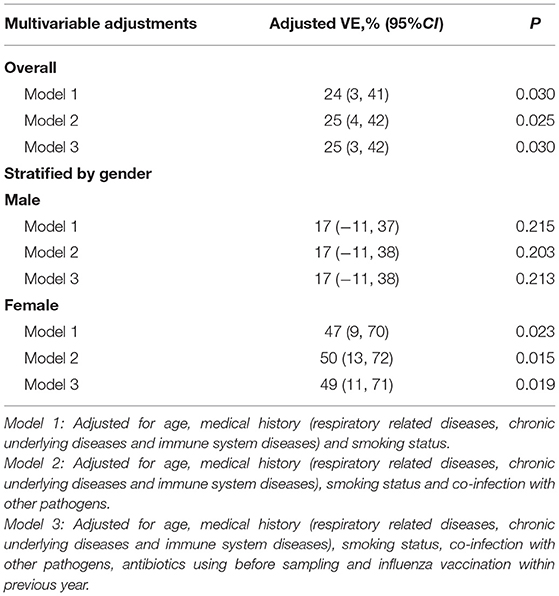
Table 3. Adjusted VE of PPV23 vaccination against pneumococcal diseases among people aged 60 years or older.
Discussion
Widespread pneumococcal vaccination in adults could protect against Streptococcus pneumoniae infected diseases. However, there is less information on the effectiveness of the vaccine from observational studies of older adults (17–19). For example, our previous cohort study in Shanghai has found some evidence of a protective effect against community-acquired pneumonia especially among elderly population of lower age (16). But further information about its potential to protect against a wider variety of diseases is needed to support further implementation of pneumococcal vaccination programs in China. In this matched test negative case-control study in China, we examined the effectiveness of PPV23 to protect against pneumococcal disease in older adults, stratified by gender.
This study found PPV23 to be effective in adults aged 60 years or older in Shanghai. Our estimate of vaccine effectiveness is lower than some other studies which examined invasive pneumococcal disease and pneumococcal pneumonia, but our estimate is higher than other studies which examined all-cause pneumonia. For example, the VE of the PPV23 against all-cause pneumonia in a systematic review from 2020 ranged from 3 to 16% (20), overlapping with the results from a systematic review by Kraicer-Melamed et al., where the VE was 17% in cohort studies and 7% in case–control studies in 2016 (21). The VE against pneumococcal pneumonia ranged from 5 to 53% in previous systematic reviews but varied across study designs: VE ranged from 48 to 53% in case–control study, and 37% in a test-negative study reported by Falkenhorst et al. (22), 5–45% in cohort studies reported by Kraicer-Melamed et al. (21) and VE were 45–53% in observational studies by Tin et al. (23). VE against IPD was 29% in a case–control study in South Korea (21); VE against vaccine serotype invasive pneumococcal disease was 42% in the case–control study but 50% in another case–control study, and it was 72.8% with the screening method and 44.5% with the indirect cohort method by Rodriguez et al. (24).
Overall, estimates of VE could vary for a number of reasons. These reasons include how the outcome is defined, serotype distribution, different study designs, characteristics of included participants, time since vaccination, and follow-up time. The range of VE from previous studies highlights the need for local epidemiological work, which can best inform local vaccine policies. The novelty of our study was to look across all pneumococcal disease, ranging from pneumonia, meningitis, bacteremia, and other respiratory infections. We did not find another study which was as comprehensive as ours.
Our study found VE was varied by gender. Similarly, a previous test-negative design study found women have higher protection against pneumococcal pneumonia after PPV23 vaccination in Japan in 2017 (25). Wiemken et al. (26) also reported that effectiveness of PPV23 against hospital admission for pneumococcal pneumonia was higher in women than in men. Reviews of evidence suggest that women are more likely to develop a type III hypersensitivity reaction after PPV23 administration (27), and that serotype-specific pneumococcal antibody concentrations after PCV administration are greater in girls than in boys (28). Our findings support the hypothesis that vaccine effect could be more efficacious in women (29).
Limitations
Our study has some limitations. Firstly, we used different detection methods (blood culture, sputum culture, sputum PCR, and urinary antigen tests) to identify Streptococcus pneumoniae in different hospitals, and the detection limit of each method was different, for example, the PCR-based method can effectively identify pneumococcus in patients who are on antibiotic treatment, but might increase the chance of detection of pneumococcal carriage from respiratory specimens and the use of a low specificity test is known to underestimate true vaccine effectiveness in the test-negative design. The different sensitivities and specificities across these tests could impact our estimates of VE. However, it was difficult to include a sufficient number of cases during the retrospective investigation since no unified standard pneumococcal detection has been carried out in various hospitals. So in order to include more participants in the case group, patients positive for different test methods were included in this study. Furthermore, to include as many cases as possible in the case group, patients with imaging diagnosis of pneumonia (chest radiographs, CT and other ancillary tests indicating pulmonary inflammation) and positive for pneumococcal tests were also included in the case group but not in the control group, which would be a selection bias. Secondly, almost all the studies on PPV23 including the cohort study we conducted before have reported VE stratified by age groups all found decreasing VE with increasing age (20), but this study did not adopt stratified analysis on vaccine effect of different age groups, and also did not perform a stratified analysis of different disease types due to the 1:2 paired design. In addition, we did not analyze the time effect on the relationship between PPV23 vaccination and pneumococcal diseases in our study. Finally, as this was a retrospective case- control study, there was a selection bias in our study, for example: (1) A larger proportion of patients in the control group have pneumonia and other lower respiratory tract infections. (2) In the matching process, the subjects in the control group who have pneumococcal diseases but haven't been tested for pneumococcal test were also matched for the subjects in the case group. However, our study also has some advantages: although it is a case-control study, the recall bias is greatly reduced because the records are directly derived from the hospital information and vaccine information system.
Conclusion
In summary, the 23-valent pneumococcal polysaccharide vaccine was effective against pneumococcal diseases in adults aged 60 years or older in Shanghai, and it should be recommended, in particularly, for women. These findings can influence future promotion of PPV23 vaccination in other regions in China.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
XS and XG contributed to the conception and design of the study under the supervision of GZ. XL, JQ, XM, YY, and JW performed the data collection. JQ and XX conducted statistical analysis, wrote the manuscript and revised as needed under the guidance of YL, AW, HJ, XG, and XL. ZH and JR supervised the overall study and provided feedback throughout all stages. All authors contributed to the article and approved the submitted version.
Funding
Research support funding for this project was received from National Technology Key Research Program of China (2016ZX09101091).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank Shao Zhujun and Wu Weishou and staffs from 33 project hospitals and 13 district centers for Disease Control and Prevention in Shanghai.
References
1. Simell B, Auranen K, Kayhty H, Goldblatt D, Dagan R, O'Brien KL. The fundamental link between pneumococcal carriage and disease. Expert Rev Vaccines. (2012) 11:841–55. doi: 10.1586/erv.12.53
2. Bogaert D, De Groot R, Hermans PW. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis. (2004) 4:144–54. doi: 10.1016/S1473-3099(04)00938-7
3. Weiser JN, Ferreira DM, Paton JC. Streptococcus pneumoniae: transmission, colonization and invasion. Nat Rev Microbiol. (2018) 16:355–67. doi: 10.1038/s41579-018-0001-8
4. CDC. Pneumococcal disease. Epidemiology and Prevention of Vaccine-Preventable Diseases. Washington DC: The Public Health Foundation (2012). 233–48 p.
5. Kyaw MH, Rose CJ, Fry AM, Singleton JA, Moore Z, Zell ER, et al. The influence of chronic illnesses on the incidence of invasive pneumococcal disease in adults. J Infect Dis. (2005) 192:377–86. doi: 10.1086/431521
6. Pneumococcal conjugate vaccine for childhood immunization–WHO position paper. Wkly Epidemiol Rec. (2007) 82:93–104
7. Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. (2012) 380:2095–128. doi: 10.1016/S0140-6736(12)61728-0
8. Hung IF, Tantawichien T, Tsai YH, Patil S, Zotomayor R. Regional epidemiology of invasive pneumococcal disease in Asian adults: epidemiology, disease burden, serotype distribution, and antimicrobial resistance patterns and prevention. Int J Infect Dis. (2013) 17:e364–73. doi: 10.1016/j.ijid.2013.01.004
9. Matanock A, Lee G, Gierke R, Kobayashi M, Leidner A, Pilishvili T. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged >/=65 years: updated recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. (2019) 68:1069–75. doi: 10.15585/mmwr.mm6846a5
10. Chen Q, Wang L, Xie M, Li X. Recommendations for influenza and Streptococcus pneumoniae vaccination in elderly people in China. Aging Med. (2020) 3:1–11. doi: 10.1002/agm2.12102
11. Assaad U, El-Masri I, Porhomayon J, El-Solh AA. Pneumonia immunization in older adults: review of vaccine effectiveness and strategies. Clin Interv Aging. (2012) 7:453–61. doi: 10.2147/CIA.S29675
12. Zhao D, Gai TR, Cui M, He J, Wu B. Cost-effectiveness of a 23-valent pneumococcal polysaccharide vaccine immunization programme for the elderly in Shanghai, China. Vaccine. (2016) 34:6158–65. doi: 10.1016/j.vaccine.2016.11.003
13. Hu J, Sun X, Huang Z, Wagner AL, Carlson B, Yang J, et al. Streptococcus pneumoniae and Haemophilus influenzae type b carriage in Chinese children aged 12–18 months in Shanghai, China: a cross-sectional study. BMC Infect Dis. (2016) 16:149. doi: 10.1186/s12879-016-1485-3
14. Zhuang JL, Wagner AL, Laffoon M, Lu YH, Jiang QW. Procurement of category 2 vaccines in China. Vaccines. (2019) 7:97. doi: 10.3390/vaccines7030097
15. Chenyan Z. Shanghai's Population to Hit 23 Million. (2011). Available online at: http://www.chinadaily.com.cn/china/2011–02/22/content_12059643.htm (accessed: Feb 22, 2011).
16. Guo X, Qiu J, Ren J. Evaluation of the efficacy of 23-valent pneumococcal polysaccharide vaccine for the elderly aged 60 and above in Shanghai during 2013–2018 for 5 years. Chin J Prev Med. (2020) 54:923–8. doi: 10.3760/cma.j.cn112150-20200306-00262
17. Tomczyk S, Bennett NM, Stoecker C, Gierke R, Moore MR, Whitney CG, et al. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. (2014) 63:822–5.
18. Moberley S, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev. (2013) 2013:CD000422. doi: 10.1002/14651858.CD000422.pub3
19. Huss A, Scott P, Stuck AE, Trotter C, Egger M. Efficacy of pneumococcal vaccination in adults: a meta-analysis. CMAJ. (2009) 180:48–58. doi: 10.1503/cmaj.080734
20. Berild JD, Winje BA, Vestrheim DF, Slotved H, Valentiner-Branth P, Roth A, et al. A systematic review of studies published between 2016 and 2019 on the effectiveness and efficacy of pneumococcal vaccination on pneumonia and invasive pneumococcal disease in an elderly population. Pathogens. (2020) 9:259. doi: 10.3390/pathogens9040259
21. Kraicer-Melamed H, O'Donnell S, Quach C. The effectiveness of pneumococcal polysaccharide vaccine 23 (PPV23) in the general population of 50 years of age and older: a systematic review and meta-analysis. Vaccine. (2016) 34:1540–50. doi: 10.1016/j.vaccine.2016.02.024
22. Falkenhorst G, Remschmidt C, Harder T, Hummers-Pradier E, Wichmann O, Bogdan C. Effectiveness of the 23-valent pneumococcal polysaccharide vaccine (PPV23) against pneumococcal disease in the elderly: systematic review and meta-analysis. PLoS ONE. (2017) 12:e169368. doi: 10.1371/journal.pone.0169368
23. Tin THM, Stuurman AL, Ferreira G, Alicino C, Bollaerts K, Paganino C , et al. Effectiveness of pneumococcal vaccines in preventing pneumonia in adults, a systematic review and meta-analyses of observational studies. PLoS ONE. (2017) 12:e177985. doi: 10.1371/journal.pone.0177985
24. Gutierrez RM, Ordobas GM, Garcia-Comas L, Sanz MJ, Cordoba DE, Lasheras CM, et al. Effectiveness of 23-valent pneumococcal polysaccharide vaccine in adults aged 60 years and over in the Region of Madrid, Spain, 2008–2011. Euro Surveill. (2014) 19:20922. doi: 10.2807/1560-7917.ES2014.19.40.20922
25. Suzuki M, Dhoubhadel BG, Ishifuji T, Yasunami M, Yaegashi M, Asoh N, et al. Serotype-specific effectiveness of 23-valent pneumococcal polysaccharide vaccine against pneumococcal pneumonia in adults aged 65 years or older: a multicentre, prospective, test-negative design study. Lancet Infect Dis. (2017) 17:313–21. doi: 10.1016/S1473-3099(17)30049-X
26. Wiemken TL, Carrico RM, Klein SL, Jonsson CB, Peyrani P, Kelley RR, et al. The effectiveness of the polysaccharide pneumococcal vaccine for the prevention of hospitalizations due to Streptococcus pneumoniae community-acquired pneumonia in the elderly differs between the sexes: results from the Community-Acquired Pneumonia Organization (CAPO) international cohort study. Vaccine. (2014) 32:2198–203. doi: 10.1016/j.vaccine.2014.02.048
27. Cook IF. Sex differences in injection site reactions with human vaccines. Hum Vaccine. (2009) 5:441–9. doi: 10.4161/hv.8476
28. Voysey M, Barker CI, Snape MD, Kelly DF, Truck J, Pollard AJ. Sex-dependent immune responses to infant vaccination: an individual participant data meta-analysis of antibody and memory B cells. Vaccine. (2016) 34:1657–64. doi: 10.1016/j.vaccine.2016.02.036
Keywords: effectiveness, PPV23, pneumococcal diseases, elderly, test-negative design
Citation: Sun X, Guo X, Qiu J, Zhao G, Xu X, Wagner AL, Jiang H, Huang Z, Ren J, Ma X, Liang X, Yao Y, Wu J and Lu Y (2021) Effectiveness of 23-Valent Pneumococcal Polysaccharide Vaccine Against Pneumococcal Diseases Among the Elderly Aged 60 Years or Older: A Matched Test Negative Case-Control Study in Shanghai, China. Front. Public Health 9:620531. doi: 10.3389/fpubh.2021.620531
Received: 23 October 2020; Accepted: 23 August 2021;
Published: 20 September 2021.
Edited by:
Margaret Ip, The Chinese University of Hong Kong, Hong Kong, SAR ChinaReviewed by:
Chun-Quan Ou, Southern Medical University, ChinaKaifeng Wu, Zunyi Medical University Third Affiliated Hospital, China
Copyright © 2021 Sun, Guo, Qiu, Zhao, Xu, Wagner, Jiang, Huang, Ren, Ma, Liang, Yao, Wu and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaodong Sun, c3VueGlhb2RvbmdAc2NkYy5zaC5jbg==
 Xiaodong Sun1*
Xiaodong Sun1* Xiang Guo
Xiang Guo Jing Qiu
Jing Qiu Genming Zhao
Genming Zhao Abram L. Wagner
Abram L. Wagner Hongli Jiang
Hongli Jiang Zhuoying Huang
Zhuoying Huang Jia Ren
Jia Ren Xiaoying Ma
Xiaoying Ma Yihan Lu
Yihan Lu