Abstract
As the pandemic continues, individuals with re-detectable positive (RP) SARS-CoV-2 viral RNA among recovered COVID-19 patients have raised public health concerns. It is imperative to investigate whether the cases with re-detectable positive (RP) SARS-CoV-2 might cause severe infection to the vulnerable population. In this work, we conducted a systematic review of recent literature to investigate reactivation and reinfection among the discharged COVID-19 patients that are found positive again. Our study, consisting more than a total of 113,715 patients, indicates that the RP-SARS-CoV-2 scenario occurs plausibly due to reactivation, reinfection, viral shedding, or testing errors. Nonetheless, we observe that previously infected individuals have significantly lower risk of being infected for the second time, indicating that reactivation or reinfection of SARS-CoV-2 likely have relatively less impact in the general population than the primary infection.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a respiratory virus from the family coronaviradae and order nidovirales. Other viruses from the same family include the severe acute respiratory syndrome coronavirus (SARS-CoV) and middle east respiratory syndrome coronavirus (MERS-CoV), which are known to infect humans and caused hundreds of thousands of deaths worldwide (mostly in Asia and the middle east) (1, 2). COVID-19 is caused by SARS-CoV-2 and has been considered a devastating public health problem globally since its emergence in China in late 2019. The disease has, as of 29 March 2021 affected about 127 million people with over 2.7 million deaths across the globe (3). Due to effective and timely interventions, more than 70 million people have already recovered from the SARS-CoV-2 infection, indicating the impact of timely interventions and treatment which showed remarkable progress by facilitating the recovery of large number of patients even before the emergence of vaccines against the infection (3–5).
Recently, the issue of reinfection (SARS-CoV-2 subsequent infection after recovery from previous episode of the infection) and reactivation (also known as relapse, a re-detectable positive SARS-CoV-2 viral RNA in recovered patient which occurs within the first 4 weeks of previous infection) have been reported in several studies [see for instance (6–21), and the references therein]. These studies highlighted the possibility of reactivation and reinfection of SARS-CoV-2 which needs urgent attention from the researchers as well the public health policymakers. A re-detectable positive (RP) SARS-CoV-2 infection is ascertained commonly by using reverse transcriptase-polymerase chain reaction (RT-PCR) test from a COVID-19 patient after recovery from the primary infection before confirmation of reactivation or reinfection. Moreover, the positivity of RT-PCR can also be detected due to RNA viral shedding (22) or diagnostic testing errors likely due to technical issues of RT-PCR assays (23). A recent retrospective study by Agarwal et al. (22) who analyzed 851 SARS-CoV-2-positive patients with at least two positive PCR tests found that 99 of them remained SARS-CoV-2-positive after 28 days from their initial diagnosis date. The report showed that the median lower and upper bounds for viral RNA shedding in COVID-19 patients occurred between 2 to 3 weeks (22).
This raises serious concerns on whether a more precautionary measures should be considered in declaring the recovery phase from COVID-19 infection, and the significance of follow-up, especially in the most vulnerable population (24). In this work, we reviewed some primary studies that evaluated the possible reactivation and/or reinfection of SARS-CoV-2 mostly based on clinical or laboratory reports to shed more light on possible reactivation and/or reinfection of SARS-CoV-2 by recovered patients after satisfying the standard discharge criteria. Our study aimed to provide recommendations to help to prevent further spread of the virus since most clinical features, significance, and the potential cause of RP-SARS-CoV-2 patients remain unclear.
Standard Discharge Criteria for SARS-CoV-2 Patients
The standard discharge criteria from the isolation/hospitalization process for a COVID-19 patient who recovered from a primary episode of the infection (4, 5, 9, 10, 25–28) are summarized as follows:
-
Normal temperature (<38°C) for more than 72 h consecutively before the discharge;
-
A notable improvement in respiratory symptoms;
-
Clear acute exudative lesions of chest computed tomography (CT) images must be improved;
-
Two consecutive negative results for RT-PCR carried out at least 24-h apart;
-
Hospital care no longer required;
-
Specific IgG appearance by a serological test.
According to previous studies (9–11), some COVID-19 patients were found positive from RT-PCR results for the second time (usually) within 5–13 days after discharge from the isolation before confirmation of reactivation or relapse (5, 29, 30), while some patients were found to be RP-SAR-CoV-2 at least 4 weeks from the first episode of the infection, indicating the possibility of reinfection (6, 7). Therefore, urgent research is needed to disentangle possible reasons of RP- SAR-CoV-2 after recovery from primary infection to guide policy-making and help in controlling further spared of the virus (9, 31–34).
Currently, there is little knowledge or information about possible reasons for RP-SARS-CoV-2, which might probably be due to reactivation, reinfection, viral shedding, or testing errors. Nonetheless, many reports on possible reactivation and reinfection in recovered COVID-19 patients were asymptomatic or have mild to moderate symptoms (27, 35) which typically recover within 14 days interval. Table 1 summarizes the characteristics of RP-SARS-CoV-2 in recovered patients, which occurs plausibly due to reactivation or reinfection, including the population-based observational study in Denmark consisting of 4 million individuals with possible reinfection of 2.11% (16).
Table 1
| Country | Age (years) | Gender (among RP) | Number of patient involve in study | Symptoms on first infection | Symptoms on second infection | Time interval between discharged and RP (days)* | Rate of infection | Remark | References |
|---|---|---|---|---|---|---|---|---|---|
| Hong Kong, China | 33 | M | 1 | Symptomatic | Asymptomatic | 123 | Reinfection | (6) | |
| USA | 25 | M | 1 | Symptomatic | Symptomatic with hospitalization | 48 | Reinfection | (36) | |
| Belgium | 52 | F | 1 | Symptomatic | Symptomatic | 93 | Reinfection | (37) | |
| Ecuador | 46 | M | 1 | Symptomatic | Symptomatic | 63 | Reinfection | (38) | |
| India | 25% 28 | F = 1, M = 1 | 2 | Asymptomatic | Asymptomatic | 100 and 101 | Reinfection | (39) | |
| China | 46 | F = 1 | 1 | Mild | Mild | 6 | Reactivation | (40) | |
| Mexico | At least 20 | F = 53.9% among reinfection | 100,432 | Asymptomatic or mild to severe | Mild to severe | 28 | 258/100432 = 0.26% | Reinfection | (7) |
| China | 30–36 | F = 2; M = 2 | 4 | 3 Mild to moderate, and 1 asymptomatic | Asymptomatic | 5–13 | Reactivation | (9) | |
| China | 27–89 (Median age = 56) | F = 12; M = 11 | 651 | Mild to moderate | 12 = moderate, 9 = severe, and 2 = critical | Median = 15 | 23/651 = 3% | Reactivation | (10) |
| China | F>M | 209 | Mild to moderate | Mild to moderate | 2–13 | 22/209 = 10.5% | Reactivation | (11) | |
| China | <60 | 262 | Mild to moderate and severe | Mild to moderate | 14 | 38/262 = 14.5% | Reactivation | (12) | |
| USA | 82 | M | 1 | Mild to moderate | Mild to moderate | 10 | Reactivation | (31) | |
| USA | 1 | 28 | Reinfection | (22) | |||||
| China | 47.0 (40.5–55.5) | F = 9 (35%), M = 16 (64%) | 51 | Mild to moderate and severe | Mild to moderate and severe | 12–26 | Reactivation | (41) | |
| Turkey | 46 and 47 | M | 2 | Mild | Mild | 100 and 104 | Reinfection | (42) | |
| China | 12–49 | F = 2, M = 2 | 17 | Mild to moderate | Mild | 3 | Reactivation | (43) | |
| USA | 55 | F | 1 | Mild | Mild to moderate | 18 | Reactivation | (44) | |
| China | 50 | M | 1 | Mild to moderate | Mild to moderate | 40 | Reinfection | (45) | |
| China | 1–73 | F = 7, M = 6 | 13 | Mild to moderate | Mild to moderate | 5–14 | 31% (4/13) | Reactivation | (46) |
| China | 57 | F = 1 | 1 | Mild to moderate | Mild to moderate | 4 | Reactivation | (47) | |
| China | 0.92–86 | 92 | Mild to moderate and severe | Mild to moderate and severe | 2–48 | (48) | |||
| China | 0.25–69 | F = 42,M = 45 | Mild to moderate | Mild to moderate | 2–19 | Reactivation | (23) | ||
| China | 2.5–12.7 | F = 13, M = 11 | Mild to moderate | Mild to moderate | 2.4–12 | Reactivation | (49) | ||
| China | 4–80 (Median age = 37.2) | F = 8,M = 12 | 147 | Mild to moderate | Mild to moderate | Median = 17.25 | Reactivation | (50) | |
| China | 33.5–58.5 (Median age = 46.5) | F = 26,M = 34 | Mild to moderate | Mild to moderate | 4–24 | Reactivation | (51) | ||
| China | Median age = 34 | F = 57,M = 36 | 7–14 | Reactivation | (52) | ||||
| China | Mild to moderate | Mild to moderate | 7–11 | Reactivation | (29) | ||||
| China | 2–7 | 14 | 7–17 | Reactivation | (53) | ||||
| China | 18–90 (Median age = 48) | F = 157,M = 128 | 285 | Mild to moderate and severe | Mild to moderate and severe | 5–8 | F = 65.6, M = 44.4 | Reactivation | (54) |
| China | 40 | M | 1 | Mild to moderate and severe | Mild to moderate | 5 | Reactivation | (55) | |
| China | Median age = 54 | F = 70.6%,M = 29.4% | 98 | Mild to moderate | Mild to moderate | <17 | F = 5/32,M = 12/66 | Reactivation | (56) |
| France | 19–91 | F = 45.5%,M = 54.5% | Mild to severe | Mild to severe | 4–27 | Reactivation | (57) | ||
| China | 60–76 | M = 33.3% | 126 | Asymptomatic | 10–18 | Reactivation | (58) | ||
| Korea | 0–>80 | 8922 | Asymptomatic to mild | Median = 19 | Reactivation | (59, 60) | |||
| China | <29–79 | F = 59%,M = 41% | 576 | Median = 14 | Reactivation | (61) | |||
| China | 1–72 | M = 13(65%) | 182 | Mild to moderate | 7–14 | Reactivation | (62) | ||
| China | <12–60 | M = 25 (14.5%) | 172 | Mild to severe | Mild to moderate | 3.46–11.18 | Reactivation | (63) | |
| China | Range = 23–68 | M = 4 (26.7%) | 85 | Mild to moderate | Mild to moderate | 9–30 | (64) | ||
| Iran | Median = 52 | M = 5 (55.6%) | 13 | Mild to moderate | Mild to moderate | 15–48 | (65) | ||
| China | 26–72 | M = 3 (37.5%) | 108 | Mild to severe | Asymptomatic | 6–28 | Reactivation | (66) | |
| China | 36–66 | M = 4 (66.7%) | 11 | Mild to moderate | 6–27 | Reactivation | (67) | ||
| China | M = 10 (40%) | 68 | Mild to moderate | <7 | Reactivation | (68) | |||
| China | M = 9 (17.6%) | 51 | Mild to moderate | 7–14 | Reactivation | (69) | |||
| China | 9–62 | M = 8 (53.3%) | 15 | Moderate | Mild | 15 | Reactivation | (70) | |
| Brunei Darussalam | Median = 47 | M = 12 (57.1%) | 106 | Asymptomatic and mild | 11–17 | Reactivation | (71) | ||
| China | M = 1 (50%) | 62 | Mild | Asymptomatic | 6–14 | Reactivation | (72) | ||
| China | 23–57 | M = 14 (70%) | 20 | Mild | Asymptomatic | 7 | Reactivation | (73) | |
| China | 29–87 | M = 23 (43.4%) | 257 | Mild to severe | Asymptomatic and mild | 1–12 | Reactivation | (74) | |
| China | M = 12 (54.5%) | 161 | 1–14 | Reactivation | (75) | ||||
| China | 37 | 1–6 | Reactivation | (76) | |||||
| China | 27–42 | M = 2 (40%) | 55 | Mild to moderate | 4–17 | Reactivation | (77) | ||
| Italy | 37–78 | M = 3 (50%) | 29 | Mild to moderate | Asymptomatic | 13–24 | Reactivation | (78) | |
| China | 18–71 | M = 12 (63.2%) | 71 | Mild to severe | Mild to severe | 1–17 | Reactivation | (79) | |
| China | 19–79 | M = 12 (44.4%) | 285 | Asymptomatic | 15 | Reactivation | (73) | ||
| China | 34 | Severe | Asymptomatic | 15 | Reactivation | (80) | |||
| China | 34–74 | M = 1 (33.3%) | Mild | Asymptomatic | 1–5 | Reactivation | (81) | ||
| China | 70 | M = 1 | 1 | Moderate to severe | Asymptomatic | 13 | Reactivation | (82) | |
| China | 35 | M = 1 | 1 | Mild | Mild | 14 | (83) | ||
| Italy | 48 | M = 1 | 1 | Severe | Moderate | 30 | Reinfection | (84) | |
| China | <67 | M = 4 (57.1%) | Mild to moderate | Asymptomatic | 7–13 | Reactivation | (85) | ||
| China | 54 | M = 1 | 1 | Moderate | Asymptomatic | 4 | Reactivation | (86) | |
| Brazil | 26 | M = 1 | 1 | Mild | Severe | 30 | Reinfection | (87) | |
| China | 21 and 55 | M = 2 | 2 | Moderate | 17 | Reactivation | (88) | ||
| China | 30–56 | Mild to moderate | 3–14 | Reactivation | (89) | ||||
| China | 8 | M = 1 | 1 | Mild | Mild | 15 | Reactivation | (90) | |
| Korea | 8 | M = 1 | 1 | Mild | Mild | 14 | Reactivation | (91) | |
| Switzerland | 77 and 81 | F = 2 | 2 | Moderate | Moderate and severe | 14–21 | Reactivation | (34) | |
| Italy | 69 | F = 1 | 1 | Mild to moderate | Asymptomatic | 23 | Reactivation | (92) | |
| Korea | 72 | F = 1 | 1 | Moderate | 6 | Reactivation | (93) |
Recurrence of SARS-CoV-2 in recovered patients.
Time interval between discharged and RP represents the period between the discharge and the time during which a patient tested positive again. Gender represents the RP of each gender.
Methods
Searching Strategy and Study Screening Process
We conducted a systematic review on the possibilities of reactivation and reinfection of SARS-CoV-2 in recovered patients that covered published peer-reviewed articles in the literature from Nov 1, 2019, to Mar 29, 2021. Following the guidelines by the “Preferred Reporting Items for Systematic reviews and meta-Analyses” (PRISMA) (34), we searched the following databases: MEDLINE; PubMed; and Embase for papers published in English, among which only human participants were studied. Our search strategy includes (severe acute respiratory syndrome coronavirus 2 OR SARS-CoV-2 OR 2019-nCoV OR coronavirus disease 2019 OR COVID-19) AND (reinfection OR reactivation OR relapse OR RNA shedding OR viral shedding OR re-detectable positive) AND (recovered patients OR discharge patients OR post-COVID-19 patients). Related references were also searched through preprint servers (bioRxiv and medRxiv) and general google search, and reviewing the reference list of the included articles. Letters to editors and commentaries were also included to ensure robust coverage of the existing literature. All retrieved records were imported into the ENDNOTE citation software and duplicates were removed using the ENDNOTE built-in “Find Duplicates” feature. Finally, the titles, abstracts, and full text of the generated studies were sequentially screened to ascertain the studies that met the inclusion criteria of the review.
Study Selection and Eligibility Criteria
The following inclusion criteria were used in study selection: (i) articles published in peer-reviewed journals, case reports, letters to editors, and commentaries; (ii) articles studying the COVID-19 reactivation or reinfection in recovered patients; as well as SARS-CoV-2 RNA shedding in recovered COVID-19 patients; and (iii) articles published in English or at least with an abstract in the English language. A flow chart of the search strategy and study selection process is presented in Figure 1 using PRISMA guidelines (94). Studies that reported the possibility of reactivation and/or reinfection of SARS-CoV-2 for patients with other comorbid conditions such as asthma, old age, and type 2 diabetes were also included in this study. It is important to note that the following exclusion criteria were used in this study: (i) studies with irrelevant topics; (ii) lack of information (data) or ineligible article types; (iii) review studies; and (iv) review protocol. Similarly, research articles reported SARS-CoV-2 reactivation or reinfection in recovered patients published in a non-English language, or have no accessible full-text access were also excluded. Study search and screening processes were conducted independently by two reviewers/authors (SSM and SZ).
Figure 1
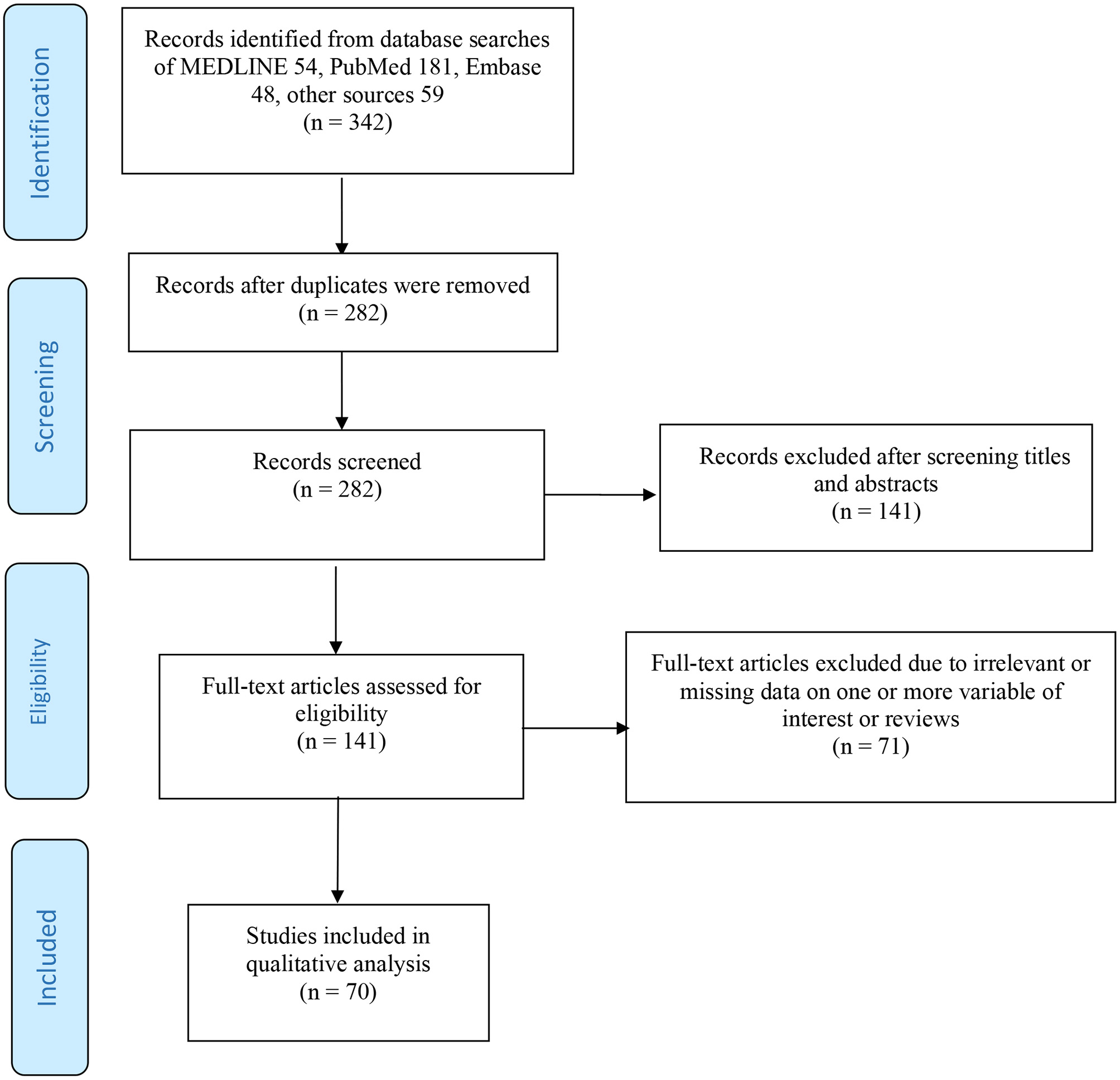
Flow diagram of the searching strategy and article selection process.
Data Extraction and Analysis
Relevant data were extracted independently by SSM and SZ to ensure accurate reporting. The generated results were compared, and any inconsistency in the data was resolved by further discussions among the authors. The generated results were then further synthesized. The data analyzed included the incidence of SARS-Cov-2 reactivation and reinfection in recovered COVID-19 patients.
Results
Search Findings
In total, 342 articles were identified in total (54, MEDLINE; 181, PubMed; 48, Embase; and 59, other sources). There were 282 studies left after removing the duplicates. After 141 articles were excluded by screening the titles and abstracts, we retrieved 141 articles eligible for the full-text screening. We excluded 71 articles based on the aforementioned exclusion criteria. Eventually, 70 studies that satisfied the eligibility criteria were included in this review for further analyses, and they include primary research studies, letters to editors, commentaries, and case reports. Overall, the included studies recruited more than 113,715 patients.
Epidemiological Findings
The reviewed studies covered SARS-CoV-2 incidence of possible reactivation, reinfection, or viral shedding worldwide. The majority of the studies were from China. Subsequently, the included studies (Table 1) estimated the time interval for RP-SARS-CoV-2 in recovered patients after follow-up, following discharge from isolation, or hospitalization after satisfying standard discharge criteria.
RP-SARS-CoV-2 Reinfection Possibilities
Following previous reports (6, 7, 12, 32), we re-examined some of the clinical features, infection ratios, recovery, and potential reasons of possible reactivation and/or reinfection of SARS-CoV-2 to shade more light on the current issue of RP-SARS-CoV-2 in recovered patients, and provide suggestions for public health policy-makers to guide effective control of SARS-CoV-2 transmission. Our study would be valuable to policy-makers since there was until recently no clear epidemiological underpinning explanation for the resurgence of COVID-19 infection among patients that tested positive on a retest.
Here, we reported some scenarios that analyze possible reinfection of SARS-CoV-2 in recovered patients. A recent retrospective cohort study in Mexico by Murillo-Zamora et al. (7) revealed some possible factors that predict severe symptomatic SARS-COV-2 reinfection, which suggested that reinfection occurs when the time lag between discharge and RP is at least 28 days (that is, a second-time infection after a patient satisfied the standard discharge criteria). Moreover, they found that the risk of previously infected patients being infected for the second time was 258/100,432 = 0.26%, with a case fatality rate of 11/258 = 4.3%, while the overall infection attack rate in Mexico, as of 18 January 2021, was 1.273% with a case fatality rate as 8.572%. Note that, as of November 17, 2020, Mexico has 1,641,428 COVID-19 cases, including 140,704 associated deaths (3, 95). Their results also revealed some multiple factors related to an increased risk of severe symptomatic SARS-COV-2 reinfection, which was asthma 1.26 (95% CI: 1.06–1.50), older age 1.007 (95% CI: 1.003–1.010), obesity 1.12 (95% CI: 1.01–1.24), type 2 diabetes mellitus 1.22 (95% CI: 1.07–1.38), and previous severe SARS-CoV-2 infection 1.20 (95% CI: 1.03–1.39).
Another recent clinical study by Duggan et al. (31) examined an 82-year-old COVID-19 patient who has been identified with some underline health conditions (including a history of advanced Parkinson's disease, insulin-dependent diabetes, chronic kidney disease, and hypertension). After recovery, the patient tested positive to SARS-CoV-2 on a re-test at least 48 days after the first infection, indicating that the RP was likely due to reinfection. Also, a study by To et al. (6) in Hong Kong reported a situation of RP-SARS-CoV-2 by a 33-year-old man that was detected 123 days after the previous episode of the infection (following discharge). During the period of the first infection, the symptoms were mostly mild, which was resolved/improved during the isolation or hospitalization process. A total of 2 weeks later, the patient satisfied the standard discharge criteria and was discharged from the hospital, following two consecutive negative SARS-CoV-2 results carried out by RT-PCR test at least 24-h apart. The second infection was detected and found to be asymptomatic but a different strain from the previous episode. This showed that the RP differs from the first infection (strain) which was verified by whole-genome analysis. The two strains belonged to different origin or clades with 24 nucleotide differences, which was of high quantity considering the relatively slow mutation rate detected for SARS-CoV-2 up-to-date. The first strain identified has a similar origin to the viruses that originated from Hong Kong, while the second strain identified has a similar origin to viruses from Spain. Consequently, another useful way to detect the positivity of RT-PCR is due to viral shedding from previous infections (22).
Possible Relapse Rather Than Reinfection of SARS-CoV-2
In this section, we reported scenarios that analyzed the possible reactivation of SARS-CoV-2 in recovered patients. According to a clinical report by Lan et al. (9), four medical workers aged 30–36 years old were found to be RP-SARS-CoV-2 within 5–13 days from recovery from the first episode of the infection. The patients were discharged from the isolation following the standard discharge procedure (9). This highlighted that some recovered COVID-19 patients can still be positive (or carriers) on a retest. This draws wide attention and raises a lot of public health concerns. Moreover, a study by Mei et al. (10) showed that 23 of 651 patients (about 3%) who satisfied the standard discharge criteria tested positive on a retest during the follow-up processes after recovery from the first infection. The median age of the RP group was 56 years, and there were slightly more women than men. Thus, we observed that the average duration from discharge to subsequent infection within 15 days is more likely to be reactivation.
Furthermore, a study by Tang et al. (11) carried out in Shenzhen, China, re-examined 209 patients that recovered from COVID-19 infection following the standard discharge criteria. After follow-up, they found that 22 of the patients (about 10.5%) were RP for SARS-CoV-2 on a retest, highlighting a possibility of relapse, as the second time (RT-PCR) results were found to be positive at the interval of 2–13 days between discharge and subsequent infection (re-positive on re-test).
Discussion
It has been more than a year since the COVID-19 pandemic started in Wuhan, China, and rapidly spread across the globe. Although a large portion of COVID-19 patients has gradually recovered, it is imperative to follow up with recovered patients to investigate possible reasons for RP-SARS-CoV-2. There are still a lot of unknown clinical features related to COVID-19 epidemiology, especially in recovered patients. In this regard, it is necessary to understand the epidemiological features of RP-SARS-CoV-2 in recovered patients and to examine whether they are potential threats to public health (96). Several studies that reported the situations of RP-SARS-CoV-2 suggested that subsequent infection mostly occurs due to reactivation or reinfection rather than testing errors or prolonged viral shedding (16). However, this issue needs urgent attention to investigate whether RP-SARS-CoV-2 patients could be a serious public health problem. However, some studies reported that a small proportion (about 1%) of the population can be RP for SARS-CoV-2, and possibly due to reactivation or reinfection (16, 17). Furthermore, previous reports (7, 34, 35) highlighted that RP-SARS-CoV-2 is less likely to cause serious problems to public health since the rate of RP seems low (about 1%), and new infections declining after recovery from the first episode of the infection (16, 35), which is likely due to the suspected herd immunity (97, 98). This suggests that previously infected individuals have a significantly lower risk of being infected for the second time. Consequently, the aforementioned studies highlighted the possibility of reactivation and/or reinfection of SARS-CoV-2, which is less likely to cause a serious public health problem. However, we argue that these issues of RP-SARS-CoV-2 need further investigation, even though a small proportion has been reported to be RP after discharge. This is due to the fact that, despite numerous studies on COVID-19 as part of the efforts to curtail the spread of the virus, up to date, a lot of its epidemiological features remained unknown.
Overall, we observed that (i) if the time lag between discharge and RP of SRAS-CoV-2 is at most 28 days, these might be reinfection or relapse of previous infection; (ii) if the time lag is 2 months, it is more likely to be reinfection; and (iii) if the time lag is 3 months or above, it is very likely to be true reinfection (17). However, the most reliable way is to perform sequencing twice and get two different strains of the virus. Also, a possible reactivation usually occurs when the time lag is at most 15 days following discharge from the first episode of the infection (9, 11). It is worth mentioning that the reactivation of SARS-CoV-2 by RT-PCR found at least 28 days were associated with substantial genetic differences. We also observed that few infected individuals were able to generate second-time infection following the RP-SARS-CoV-2, which is regarded as possible reactivation or reinfection (depending on the period for subsequent infection), and this can be identified using a RT-PCR test.
However, we emphasized that caution should be exercised especially for vulnerable populations even after recovery from SARS-CoV-2. Also, close monitoring on an outpatient basis appears crucial, since the clinical features and potential reasons for possible reactivation and reinfection remained unclear. Like other studies, our work is not free from limitations; for instance, the time interval to remark on a possible reason for RP of SARS-CoV-2 is short considering the emergence of the new COVID-19 strain in some parts of the world, and this may cause exclusion in some reinfected group of individuals. Thus, further studies should be done as more COVID-19 data is being collected worldwide.
Conclusion
In this work, we reported the plausibility of SARS-CoV-2 reactivation and reinfection in the context of the growing body of literature surrounding the dynamic of SARS-CoV-2 using RT-PCR test results. Our findings suggested the importance of dynamic surveillance of SARS-CoV-2 viral RNA for infectivity examination or assessment. Although there is currently no clear evidence that the RP-SARS-CoV-2 patient causes severe infection in a vulnerable population, more precautionary measures should be taken in declaring recovery from COVID-19 infections. Our study also emphasized the importance of follow-up in recovered patients to prevent further spread of the virus. Finally, we found that previously infected individuals have a significantly lower risk of being infected again than the first time infection.
Statements
Author contributions
XT, SSM, and DH: conceptualization. XT, SSM, SZ, and DH: formal analysis and writing - original draft. DH: writing - review & editing. All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
This work is partly supported by Shenzhen Key Medical Discipline Construction Fund (No. SZXK064); Sanming Project of Medicine in Shenzhen (SZSM202011008); and The Science and Technology plan project of Shenzhen (No. JSGG20200225152848007).
Acknowledgments
We are grateful to the handling editor and reviewers for the useful comments that helped to strengthen the initial draft of our manuscript.
Conflict of interest
DH was supported by an Alibaba (China)-Hong Kong Polytechnic University Collaborative Research Project. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1.
Hu B Guo H Zhou P Shi ZL . Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. (2020) 19:141–54. 10.1038/s41579-020-00459-7
2.
Li Q Guan X Wu P Wang X Zhou L Tong Y et al . Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. (2020) 382:1199–207. 10.1056/NEJMoa2001316
3.
WHO . World Health Organization (WHO), Coronavirus Disease (COVID-19) Dashboard. (2020). Available online at: https://covid19.who.int/ (accessed November 15, 2020).
4.
World Health Organization (WHO) . COVID-19 Vaccines. (2021). Available online at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines (accessed April 1, 2021).
5.
Gao Z Xu Y Guo Y Xu D Zhang L Wang X et al . A systematic review of re-detectable positive virus nucleic acid among COVID-19 patients in recovery phase. Infect Genet Evolut. (2020) 85:104494. 10.1016/j.meegid.2020.104494
6.
To KK-W Hung IF-N Ip JD Chu AW-H Chan W-M Tam AR et al . COVID-19 re-infection by a phylogenetically distinct SARS-coronavirus-2 strain confirmed by whole genome sequencing. Clin Infect Dis. (2020) ciaa1275. 10.1093/cid/ciaa1275
7.
Murillo-Zamora E Mendoza-Cano O Delgado-Enciso I Hernandez-Suarez CM . Predictors of severe symptomatic laboratory-confirmed SARS-COV-2 reinfection. Public Health. (2020) 193:113–5. 10.1016/j.puhe.2021.01.021
8.
European Centre for Disease Prevention and Control (ECDC) . Reinfection With SARS-CoV: Considerations for Public Health Response. (2020). Available online at: https://www.ecdc.europa.eu/sites/default/files/documents/Re-infection-and-viral-shedding-threat-assessment-brief.pdf (accessed January 1, 2021).
9.
Lan L Xu D Ye G Xia C Wang S Li Y et al . Positive RT-PCR test results in patients recovered from COVID-19. JAMA. (2020) 323:1502–3. 10.1001/jama.2020.2783
10.
Mei Q Li J Du R Yuan X Li M Li J . Assessment of patients who tested positive for COVID-19 after recovery. Lancet Infect Dis. (2020) 20:1004–5. 10.1016/S1473-3099(20)30433-3
11.
Tang X Zhao S He D Yang L Wang MH Li Y et al . Positive RT-PCR tests among discharged COVID-19 patients in Shenzhen, China. Infect Contr Hospit Epidemiol. (2020) 41:1110–2. 10.1017/ice.2020.134
12.
An J Liao X Xiao T Qian S Yuan J Ye H et al . Clinical characteristics of recovered COVID-19 patients with re-detectable positive RNA test. Ann Translat Med. (2020) 8:1084. 10.21037/atm-20-5602
13.
Hanrath AT Payne BA Duncan CJ . Prior SARS-CoV-2 infection is associated with protection against symptomatic reinfection. J Infect. (2020) 82:e29–e30. 10.1016/j.jinf.2020.12.023
14.
Dos Santos LA de Góis Filho PG Silva AM Santos JV Santos DS Aquino MM . Recurrent COVID-19 including evidence of reinfection and enhanced severity in thirty Brazilian healthcare workers. J Infect. (2021) 82:399–406. 10.1016/j.jinf.2021.01.020
15.
Harvey RA Rassen JA Kabelac CA Turenne W Leonard S Klesh R et al . Association of SARS-CoV-2 seropositive antibody test with risk of future infection. JAMA Inter Med. (2021) 181:672–9. 10.1001/jamainternmed.2021.0366
16.
Hansen CH Michlmayr D Gubbels SM Mølbak K Ethelberg S . Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: a population-level observational study. Lancet. (2021) 397:1204–12. 10.1016/S0140-6736(21)00575-4
17.
Perez G Banon T Gazit S Moshe SB Wortsman J Grupel D et al . A 1 to 1000 SARS-CoV-2 reinfection proportion in members of a large healthcare provider in Israel: a preliminary report. medRxiv. (2021) 1–16. 10.1101/2021.03.06.21253051
18.
BBC News . COVID-19 Immunity: Can You Catch it Twice? (2021). Available online at: https://www.bbc.com/news/health-52446965 (accessed January 14, 2021).
19.
Bloomberg . South Africa Has Found About 4000 COVID-19 Reinfections. (2021). Available online at: https://www.bloomberg.com/news/articles/2021-02-24/south-africa-has-found-about-4-000-covid-19-re-infections (accessed on 25 February 2021).
20.
Advisory Board . Just How likely are You to Catch the Coronavirus Twice? Here's What New Research Reveals. (2021). Available online at: https://www.advisory.com/daily-briefing/2021/03/02/reinfection (accessed March 02, 2021).
21.
Pilz S Chakeri A Ioannidis JP Richter L Theiler-Schwetz V Trummer C et al . SARS-CoV-2 re-infection risk in Austria. Eur J Clin Invest. (2021) 51:e13520. 10.1101/2021.02.08.21251362
22.
Agarwal V Venkatakrishnan AJ Puranik A Kirkup C Lopez-Marquez A Challener DW et al . Long-term SARS-CoV-2 RNA shedding and its temporal association to IgG seropositivity. Cell Death Discov. (2020) 6:138. 10.1038/s41420-020-00375-y
23.
Lu J Peng J Xiong Q Liu Z Lin H Tan X et al . Clinical, immunological and virological characterization of COVID-19 patients that test re-positive for SARS-CoV-2 by RT-PCR. EBio Med. (2020) 59:102960. 10.1016/j.ebiom.2020.102960
24.
Center for Disease Prevention and Control (CDC) . Re-infection With COVID-19. (2021). Available online at: https://www.cdc.gov/coronavirus/2019-ncov/your-health/reinfection.html (accessed March 16, 2021).
25.
China National Health Commission (CNHC) Diagnosis and Treatment of 2019-nCoV pneumonia in China . (2020). Available online at: http://www.nhc.gov.cn/yzygj/s7653p/202002/d4b895337e19445f8d728fcaf1e3e13a.shtml (accessed February 19, 2020).
26.
European Centre for Disease Prevention and Control (ECDC) . Guidance for Discharge and Ending Isolation in the Context of Widespread Com-munity Transmission of COVID-19–First Update. (2020). Available online at: https://www.ecdc.europa.eu/sites/default/files/documents/covid-19-guidance-discharge-and-ending-isolation-firstupdate.pdf (accessed March 30, 2021).
27.
Dao TL Gautret P . Recurrence of SARS-CoV-2 viral RNA in recovered COVID-19 patients: a narrative review. Eur J Clin Micro Infect Dis. (2020) 40:13–25. 10.1007/s10096-020-04088-z
28.
World Health Organization (WHO) Criteria for Releasing COVID-19 Patients From Isolation. (2020). Available online at: https://www.who.int/news-room/commentaries/detail/criteria-for-releasing-covid-19-patients-from-isolation (accessed November 15, 2020).
29.
Zhang B Liu S Dong Y Zhang L Zhong Q Zou Y et al . Positive rectal swabs in young patients recovered from coronavirus disease 2019 (COVID-19). J Infect. (2020) 81:e49–52. 10.1016/j.jinf.2020.04.023
30.
Zhang W Du RH Li B Zheng XS Yang XL Hu B et al . Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microb Infect. (2020) 9:386–9. 10.1080/22221751.2020.1729071
31.
Duggan NM Ludy SM Shannon BC Reisner AT Wilcox SR . Is novel coronavirus 2019 reinfection possible? Interpreting dynamic SARS-CoV-2 test results through a case report. Am J Emerg Med. (2020) 39:256.e1–3. 10.1016/j.ajem.2020.06.079
32.
Chen D Xu W Lei Z Huang Z Liu J Gao Z et al . Recurrence of positive SARS-CoV-2 RNA in COVID-19: a case report. Int J Infect Dis. (2020) 93:297–9. 10.1016/j.ijid.2020.03.003
33.
Xiao AT Tong YX Gao C Zhu L Zhang YJ Zhang S et al . Dynamic profile of RT-PCR findings from 301 COVID-19 patients in Wuhan, China: a descriptive study. J Clin Virol. (2020) 127:104346. 10.1016/j.jcv.2020.104346
34.
Ravioli S Ochsner H Lindner G . Reactivation of COVID-19 pneumonia: a report of two cases. J Infect. (2020) 81:e72–3. 10.1016/j.jinf.2020.05.008
35.
He Y Dong YC . A perspective on re-detectable positive SARS-CoV-2 nucleic acid results in recovered COVID-19 patients. Disast Med Publ Health Prepar. (2020) 1–9. 10.1017/dmp.2020.392
36.
Tillett RL Sevinsky JR Hartley PD Kerwin H Crawford N Gorzalski A et al . Genomic evidence for reinfection with SARS-CoV-2: a case study. Lancet Infect Dis. (2021) 21:52–8. 10.1016/S1473-3099(20)30764-7
37.
Van Elslande J Houben E Depypere M Brackenier A Desmet S André E et al . Diagnostic performance of seven rapid IgG/IgM antibody tests and the Euroimmun IgA/IgG ELISA in COVID-19 patients. Clin Microbiol Infect. (2020) 26:1082–7. 10.1016/j.cmi.2020.05.023
38.
Prado-Vivar B Becerra-Wong M Guadalupe JJ Márquez S Gutierrez B Rojas-Silva P et al . A case of SARS-CoV-2 reinfection in Ecuador. Lancet Infect Dis. (2020) 21:e142. 10.1016/S1473-3099(20)30910-5
39.
Gupta V Bhoyar RC Jain A Srivastava S Upadhayay R Imran M et al . Asymptomatic reinfection in 2 healthcare workers from India with genetically distinct severe acute respiratory syndrome Coronavirus 2. Clin Infect Dis. (2020): ciaa1451. 10.1093/cid/ciaa1451
40.
Wu F Zhang W Zhang L Wang D Wan Y . Discontinuation of antiviral drugs may be the reason for recovered COVID-19 patients testing positive again. Br J Hosp Med. (2020) 81:1–2. 10.12968/hmed.2020.0156
41.
Ao Z Li Y Wei J Jiang J Wang X Zhang P et al . Clinical characteristics and potential factors for recurrence of positive SARS-CoV-2 RNA in convalescent patients: a retrospective cohort study. Clin Exp Med. (2021) 271:1–7. 10.1007/s10238-021-00687-y
42.
Atici S Ek ÖF Yildiz MS Sikgenç MM Güzel E Soysal A . Symptomatic recurrence of SARS-CoV-2 infection in healthcare workers recovered from COVID-19. J Infect Dev Ctries. (2021) 15:69–72. 10.3855/jidc.14305
43.
Chen Y Bai W Liu B Huang J Laurent I Chen F et al . Re-evaluation of retested nucleic acid-positive cases in recovered COVID-19 patients: report from a designated transfer hospital in Chongqing, China. J Infect Public Health. (2020) 13:932–4. 10.1016/j.jiph.2020.06.008
44.
Lancman G Mascarenhas J Bar-Natan M . Severe COVID-19 virus reactivation following treatment for B cell acute lymphoblastic leukemia. J Hematol Oncol. (2020) 13:131. 10.1186/s13045-020-00968-1
45.
Li J Long X Fang X Zhang Q Hu S Lin Z et al . SARS-CoV-2 positivity in a discharged COVID-19 patient: a case report. Clin Microbiol Infect. (2020) 26:1115–7. 10.1016/j.cmi.2020.04.032
46.
Li Y Hu Y Yu Y Zhang X Li B Wu J et al . Positive result of Sars-Cov-2 in faeces and sputum from discharged patients with COVID-19 in Yiwu, China. J Med Virol. (2020) 92:1938–47. 10.1002/jmv.25905
47.
Liu F Cai ZB Huang JS Niu HY Yu WY Zhang Y et al . Repeated COVID-19 relapse during post-discharge surveillance with viral shedding lasting for 67 days in a recovered patient infected with SARS-CoV-2. J Microbiol Immunol Infect. (2021) 54:101–4. 10.1016/j.jmii.2020.07.017
48.
Liu HQ Yuan B An YW Chen KJ Hu Q Hu XP et al . Clinical characteristics and follow-up analysis of 324 discharged COVID-19 patients in Shenzhen during the recovery period. Int J Med Sci. (2021) 18:347–55. 10.7150/ijms.50873
49.
Peng D Zhang J Ji Y Pan D . Risk factors for redetectable positivity in recovered COVID-19 children. Pediatr Pulmonol. (2020) 55:3602–9. 10.1002/ppul.25116
50.
Tian M Long Y Hong Y Zhang X Zha Y . The treatment and follow-up of 'recurrence' with discharged COVID-19 patients: data from Guizhou, China. Environ Microbiol. (2020) 22:3588–92. 10.1111/1462-2920.15156
51.
Wu J Liu X Liu J Liao H Long S Zhou N . Coronavirus disease 2019 test results after clinical recovery and hospital discharge among patients in China. JAMA Netw Open. (2020) 3:e209759. 10.1001/jamanetworkopen.2020.9759
52.
Yang C Jiang M Wang X Tang X Fang S Li H et al . Viral RNA level, serum antibody responses, and transmission risk in recovered COVID-19 patients with recurrent positive SARS-CoV-2 RNA test results: a population-based observational cohort study. Emerg Microb Infect. (2020) 9:2368–78. 10.1080/22221751.2020.1837018
53.
Zhao W Wang Y Tang Y Zhao W Fan Y Liu G et al . Characteristics of children with reactivation of SARS-CoV-2 infection after hospital discharge. Clin Pediatr. (2020) 59:929–32. 10.1177/0009922820928057
54.
Zheng J Zhou R Chen F Tang G Wu K Li F et al . Incidence, clinical course and risk factor for recurrent PCR positivity in discharged COVID-19 patients in Guangzhou, China: a prospective cohort study. PLoS Negl Trop Dis. (2020) 14:e0008648. 10.1371/journal.pntd.0008648
55.
Zhou X Zhou J Zhao J . Recurrent pneumonia in a patient with new coronavirus infection after discharge from hospital for insufficient antibody production: a case report. BMC Infect Dis. (2020) 20:500. 10.1186/s12879-020-05231-z
56.
Zhu H Fu L Jin Y Shao J Zhang S Zheng N et al . Clinical features of COVID-19 convalescent patients with re-positive nucleic acid detection. J Clin Lab Anal. (2020) 34:e23392. 10.1002/jcla.23392
57.
Gousseff M Penot P Gallay L Batisse D Benech N Bouiller K et al . Clinical recurrences of COVID-19 symptoms after recovery: viral relapse, reinfection or inflammatory rebound?. J Infect. (2020) 81:816–46. 10.1016/j.jinf.2020.06.073
58.
Du HW Chen JN Pan XB Chen XL Yixian-Zhang Fang SF et al . Prevalence and outcomes of re-positive nucleic acid tests in discharged COVID-19 patients. Eur J Clin Microbiol Infect Dis. (2021) 40:413–7. 10.1007/s10096-020-04024-1
59.
Kang Y-J . South Korea's COVID-19 infection status: from the perspective of re-positive test results after viral clearance evidenced by negative test results. Disaster Med Public Health. (2020) 14:762–64. 10.1017/dmp.2020.168
60.
KCDA . Findings From Investigation and Analysis of Re-positive Cases. (2020). Available online at: https://www.cdc.go.kr/board/board.es?mid=a30402000000&bid=0030 (accessed September 7, 2020).
61.
Deng W Guang T-W Yang M Li J-R Jiang D-P Li C-Y et al . Positive results for patients with COVID-19 discharged form hospital in Chongqing, China. BMC Infect Dis. (2020) 20:429. 10.1186/s12879-020-05151-y
62.
Yuan B Liu H-Q Yang Z-R Chen Y-X Liu Z-Y Zhang K et al . Recurrence of positive SARS-CoV-2 viral RNA in recovered COVID-19 patients during medical isolation observation. Sci Rep. (2020) 10:11887. 10.1038/s41598-020-68782-w
63.
Yuan J Kou S Liang Y Zeng J Pan Y Liu L . PCR assays turned positive in 25 discharged COVID-19 patients. Clin Infect Dis. (2020) 71:2230–2. 10.1093/cid/ciaa398
64.
Li C Luo F Xie L Gao Y Zhang N Wu B . Chest CT study of fifteen COVID-19 patients with positive RT-PCR retest results after discharge. Quant Imaging Med Surg. (2020) 10:1318–24. 10.21037/qims-20-530
65.
Habibzadeh P Sajadi MM Emami A Karimi MH Yadollahie M Kucheki M et al . Rate of re-positive RT-PCR test among patients recovered from COVID-19. Biochem Med. (2020) 30:355–6. 10.11613/BM.2020.030401
66.
Cao H Ruan L Liu J Liao W . The clinical characteristic of eight patients of COVID-19 with positive RT-PCR test after discharge. J Medic Virol. (2020) 92:2159–64. 10.1002/jmv.26017
67.
Chen M An W Xia F Yang P Li K Zhou Q et al . Clinical characteristics of rehospitalized patients with COVID-19 in China. J Medic Virol. (2020) 92:2146–51. 10.1002/jmv.26002
68.
Liu BM Yang QQ Zhao LY Xie W Si XY . Epidemiological characteristics of COVID-19 patients in convalescence period. Epidemiol Infect. (2020) 148:e108. 10.1017/S0950268820001181
69.
Liu C Ye L Xia R Zheng X Yuan C Wang Z et al . Chest computed tomography and clinical follow-up of discharged patients with COVID-19 in Wenzhou City, Zhejiang, China. Ann Am Thorac Soc. (2020) 17:1231–7. 10.1513/AnnalsATS.202004-324OC
70.
Qiao XM Xu XF Zi H Liu GX Li BH et al . Re-positive cases of nucleic acid tests in discharged patients with COVID-19: a follow-up study. Front Med. (2020) 7:349. 10.3389/fmed.2020.00349
71.
Wong J Koh WC Momin RN Alikhan MF Fadillah N Naing L . Probable causes and risk factors for positive SARS-CoV-2 test in recovered patients: evidence from Brunei Darussalam. J Medic Virol. (2020) 92:2847–51. 10.1002/jmv.26199
72.
Xing Y Mo P Xiao Y Zhao O Zhang Y Wang F . Post-discharge surveillance and positive virus detection in two medical staff recovered from coronavirus disease 2019 (COVID-19), China, January to February 2020. Eurosurveillance. (2020) 25:2000191. 10.2807/1560-7917.ES.2020.25.10.2000191
73.
Zheng KI Wang X-B Jin X-H Liu W-Y Gao F Chen Y-P et al . A case series of recurrent viral RNA positivity in recoveredCOVID-19 Chinese patients. J Gen Intern Med. (2020) 35:2205–6. 10.1007/s11606-020-05822-1
74.
Zou Y Wang BR Sun L Xu S Kong YG Shen LJ et al . The issue of recurrently positive patients who recovered from COVID-19 according to the current discharge criteria: investigation of patients from multiple medical institutions in Wuhan, China. J Infect Dis. (2020) 222:1784–8. 10.1093/infdis/jiaa301
75.
Xie C Lu J Di Wu LZ Zhao H Rao B Yang Z . False negative rate of COVID-19 is eliminated by using nasal swab test. Travel Med Infect Dis. (2020) 37:101668. 10.1016/j.tmaid.2020.101668
76.
Wang G Yu N Xiao W Zhao C Wang Z . Consecutive false-negative rRT-PCR test results for SARS-CoV-2 in patients after clinical recovery from COVID-19. J Med Virol. (2020) 92:2887–90. 10.1002/jmv.26192
77.
Ye G Pan Z Pan Y Deng Q Chen L Li J et al . Clinical characteristics of severe acute respiratory syndrome coronavirus 2 reactivation. J Infect. (2020) 80:e14–7. 10.1016/j.jinf.2020.03.001
78.
Landi F Gremese E Rota E Benvenuto F Ciciarello F Monaco MR et al . Positive RT-PCR nasopharyngeal swab in patients recovered from COVID-19 disease: when does quarantine really end?. J Infect. (2020) 81:e1–3. 10.1016/j.jinf.2020.08.034
79.
Li J Wei X Tian W Zou J Wang Y Xue W et al . Clinical features of discharged COVID-19 patients with an extended SARS-CoV-2 RNA positive signal in respiratory samples. Virus Res. (2020) 286:198047. 10.1016/j.virusres.2020.198047
80.
Dou C Xie X Peng Z Tang H Jiang Z Zhong Z et al . A case presentation for positive SARS-CoV-2 RNA recurrence in a patient with a history of type 2 diabetes that had recovered from severe COVID-19. Diabetes Res Clin Pract. (2020) 166:108300. 10.1016/j.diabres.2020.108300
81.
Fu W Chen Q Wang T . Letter to the editor: three cases of redetectable positive SARS-CoV-2 RNA in recovered COVID-19 patients with antibodies. J Medic Virol. (2020) 92:2298–301. 10.1002/jmv.25968
82.
Gao G Zhu Z Fan L Ye S Huang Z Shi Q et al . Absent immune response to SARS-CoV-2 in a 3-month recurrence of coronavirus disease 2019 (COVID-19) case. Infect. (2021) 49:57–61. 10.1007/s15010-020-01485-6
83.
Liu F Cai ZB Huang JS Yu WY Niu HY Zhang Y et al . Positive SARS-CoV-2 RNA recurs repeatedly in a case recovered from COVID-19: dynamic results from 108 days of follow-up. Pathogens Dis. (2020) 78:ftaa031. 10.1093/femspd/ftaa031
84.
Loconsole D Passerini F Palmieri VO Centrone F Sallustio A Pugliese S et al . Recurrence of COVID-19 after recovery: a case report from Italy. Infect. (2020) 16:1–3. 10.1007/s15010-020-01444-1
85.
Peng J Wang M Zhang G Lu E . Seven discharged patientsturning positive again for SARS-CoV-2 on quantitative RT-PCR. Am J Infect Contr. (2020) 48:725–6. 10.1016/j.ajic.2020.03.017
86.
Zhang J-F Yan K Ye H-H Lin J Zheng J-J Cai T . SARS-CoV-2 turned positive in a discharged patient with COVID-19 arouses concern regarding the present standards for discharge. Int J Infect Dis. (2020) 97:212–4. 10.1016/j.ijid.2020.03.007
87.
Alonso FO Sabino BD Guimarães MA Varella RB . Recurrence of SARS-CoV-2 infection with a more severe case after mild COVID-19, reversion of RT-qPCR for positive and late antibody response: case report. J Medic Virol. (2021) 93:655–6. 10.1002/jmv.26432
88.
Dou P Zhang S Wang C Cai L Liu Z Xu Q et al . Serial CT features in discharged COVID-19 patients with positive RT-PCR re-test. Eur J Radiol. (2020) 127:109010. 10.1016/j.ejrad.2020.109010
89.
Jiang M Li Y Han M Wang Z Zhang Y Du X . Recurrent PCR positivity after hospital discharge of people with coronavirus disease 2019 (COVID-19). J Infect. (2020) 81:147–78. 10.1016/j.jinf.2020.03.024
90.
Wang H Li Y Wang F Du H Lu X . Rehospitalization of arecovered coronavirus disease 19 (COVID-19) child with positivenucleic acid detection. Pediatr Infect Dis J. (2020) 39:e69. 10.1097/INF.0000000000002690
91.
Yoo SY Lee Y Lee GH Kim DH . Reactivation of SARS-CoV-2 after recovery. Pediatr Intl. (2020) 62:879–81. 10.1111/ped.14312
92.
Bentivegna E Sentimentale A Luciani M Speranza ML Guerritore L Martelletti P . New IgM seroconversion and positive RT-PCR test after exposure to the virus in recovered COVID-19 patient. J Med Virol. (2021) 93:97–8. 10.1002/jmv.26160
93.
Chae KJ Jin GY Lee CS Lee HB Lee JH Kwon KS . Positive conversion of COVID-19 after two consecutive negative RT-PCR results: a role of low-dose CT. Eur J Radiol. (2020) 129:109122. 10.1016/j.ejrad.2020.109122
94.
Moher D Shamseer L Clarke M Ghersi D Liberati A Petticrew M et al . Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-p) 2015 statement. Syst Rev. (2015) 4:1–9. 10.1186/2046-4053-4-1
95.
World Meter (WM) . Mexico Population. (2020). Available online at: https://www.worldometers.info/world-population/mexico-population/ (accessed November 17, 2020).
96.
Bongiovanni M Basile F . Re-infection by COVID-19: a real threat for the future management of pandemia?Infect Dis. (2020) 52:581–2. 10.1080/23744235.2020.1769177
97.
Britton T Ball F Trapman P . A mathematical model reveals the influence of population heterogeneity on herd immunity to SARS-CoV-2. Science. (2020) 369:846–9. 10.1126/science.abc6810
98.
He D Artzy-Randrup Y Musa SS Stone L . The unexpected dynamics of COVID-19 in Manaus, Brazil: Herd immunity versus interventions. medRxiv. (2021) 1–23. 10.1101/2021.02.18.21251809
Summary
Keywords
COVID-19, pandemic, re-detectable positive, reactivation, reinfection
Citation
Tang X, Musa SS, Zhao S and He D (2021) Reinfection or Reactivation of Severe Acute Respiratory Syndrome Coronavirus 2: A Systematic Review. Front. Public Health 9:663045. doi: 10.3389/fpubh.2021.663045
Received
02 February 2021
Accepted
26 April 2021
Published
11 June 2021
Volume
9 - 2021
Edited by
Bathri Narayan Vajravelu, MCPHS University, United States
Reviewed by
Marat Slessarev, Western University, Canada; Shigui Yang, Zhejiang University, China
Updates
Copyright
© 2021 Tang, Musa, Zhao and He.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daihai He daihai.he@polyu.edu.hk
This article was submitted to Infectious Diseases - Surveillance, Prevention and Treatment, a section of the journal Frontiers in Public Health
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.