- 1Department of Disease Control, School of Veterinary Medicine, University of Zambia, Lusaka, Zambia
- 2Department of Environmental Health, School of Medicine and Health Sciences, Eden University, Lusaka, Zambia
- 3Department of Public Health, Michael Chilufya Sata School of Medicine, Copperbelt University, Kitwe, Zambia
- 4Department of Animal Health and Livestock Development, Blantyre Agriculture Development Division (BLADD), Mpemba, Malawi
- 5Faculty of Veterinary Medicine, Red Sea University, Galkaio, Somalia
- 6Infectious Diseases Unit, Department of Internal Medicine, The University Teaching Hospital, Lusaka, Zambia
- 7Animal and Plant Health Agency Woodham Lane, New Haw Surrey, United Kingdom
- 8Department of Disease Control and Prevention, School of Medicine and Health Sciences, Eden University, Lusaka, Zambia
- 9Department of Paraclinical Studies, School of Veterinary Medicine, University of Zambia, Lusaka, Zambia
- 10Africa Center of Excellence for Infectious Diseases of Humans and Animals, The University of Zambia, Lusaka, Zambia
- 11Department of Arctic and Marine Biology, Faculty of Biosciences, Fisheries and Economics, UiT the Arctic University of Norway, Tromsø, Norway
Background: Brucellosis is a neglected debilitating zoonosis widely recognized as an occupational health hazard. The seroprevalence of human anti-Brucella antibodies in high-risk populations, as well as their risk factors, have not been well-documented in Zambia. This study aimed at estimating the Brucella seroprevalence in herdsmen and abattoir workers and assess the associated risk factors.
Methods: A cross-sectional seroepidemiological study was carried out between May and December 2020 among abattoir workers and herdsmen in Namwala, Monze and Choma districts of Southern Province in Zambia. Seroprevalence was assessed by indirect enzyme-linked immunosorbent assay (i-ELISA) or competitive enzyme-linked immunosorbent assay (c-ELISA) while a questionnaire was administered to obtain epidemiological data.
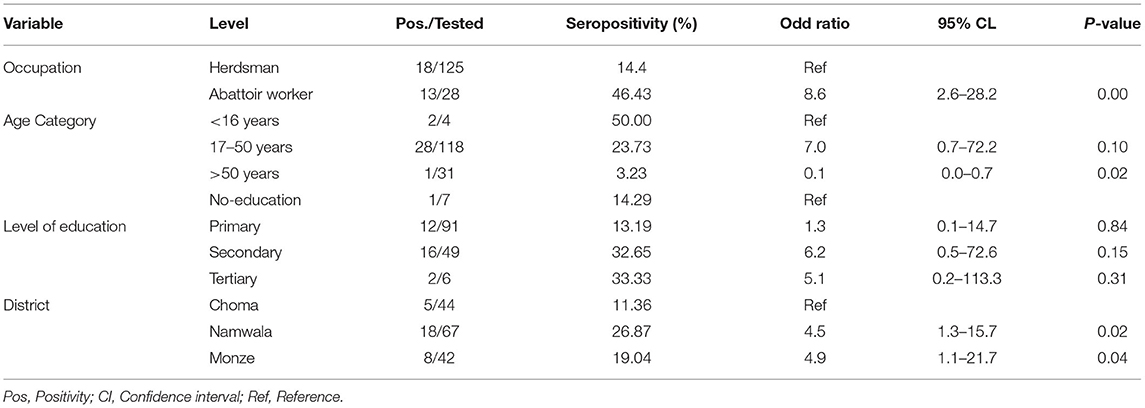
Results: A total of 153 individuals were recruited in the study. The overall Brucella seroprevalence was 20.3% (95% CI: 14.6–27.5). Seropositivity among herdsmen and abattoir workers was 14.4% (95% CI: 9.2–21.8) and 46.4%, (95% CI: 28.8–65.0), respectively. Comparable seropositive results among districts showed Namwala with 26.9%, which was the highest, seconded by Monze 19.0%, and the least was Choma with 11.36%, seropositivity. The multivariate logistic regression model showed that occupation, age category, and district of residence were predictors of being seropositive to Brucella spp. antibodies. The odds of abattoir workers being seropositive to Brucella antibodies were 8.6 (95% CI: 2.6–28.2) higher than that of herdsmen being the reference group. The odds of age category 17–50 years being seropositive to Brucella antibodies were 7.0 (95% CI: 0.7–72.2) higher than being <16 years as the reference group. The odds of one having attained primary level of education being seropositive to Brucella were 1.3 (95% CI: 0.1–14.7) or secondary level of education were 6.2 (95% CI: 0.5–72.6) or tertiary level of education were 5.1 (95% CI: 0.2, 113.3) higher than that of no level of education as the reference group. Furthermore, the odds of a respondent being seropositive to Brucella antibodies were 4.5 (95% CI: 1.3–15.7) for Namwala and 4.9 (95% CI: 1.1–21.7) for Monze higher than that of Choma as the reference group.
Conclusion: Anti-Brucella antibodies are prevalent among herdsmen and abattoir workers in the study areas of Zambia (20.26%), a sign of exposure to Brucella pathogens. Type of profession, age and level of education seem to influence the exposure to Brucella pathogens. This zoonosis should be considered as one of the differential diagnosis in humans presenting intermittent fever, malaria-like signs and general pain in humans.
Introduction
Human brucellosis is an infectious occupational disease, prevalent in Sub-Saharan African countries and typically caused by B. abortus, B. melitensis, B. canis and B. suis (1). The disease is listed as one of the seven neglected zoonotic diseases by the World Health Organization (WHO) (2). In humans, it usually originates from an animal reservoir (3). Brucellosis mainly affects high-risk occupational groups such as veterinarians, laboratory personnel, abattoir workers, slaughterhouse personnel, livestock keepers and farmers (4). These individuals get infected through inhalation of infectious aerosols, direct contact with infected animals/carcasses, or their products (raw milk, cheese and unpasteurized milk) (5). Brucellosis displays non-specific acute symptoms such as intermittent fever, backache, headaches, anorexia, weight loss, weakness and arthralgia (6). These symptoms are also seen in other diseases such as Malaria and Typhoid leading, therefore, to misdiagnosis and wrong therapy (7). Before the discovery of antibiotics, human brucellosis was described as “the disease rarely kills anybody, but it often makes a patient wish he were dead” (TIME magazine 1943). Human Brucella seroprevalence has been documented in different parts of the world among highly occupational groups which are comparatively mentioned as follows: China 15.5% (8); India 4.96% (9); Pakistan 18% (10); Malaysia 5.4% (11); Saudi Arabia 33.9% (12); Greece 3% (13); Egypt 31.3% (14); South Sudan 33.3% (15); Nigeria 24.1% (16); Cameroon 5.6% (17); Kenya 5.7% and 31.8% (18); Uganda 17% (19) and Tanzania 1.41% (20). In Zambia, there is a scarcity of data on human Brucella infections although seroprevalence has previously been estimated to be at about 5.03 % among livestock farmers in rural communities (21). Most health facilities in developing countries, including Zambia, rarely carry out routine brucellosis screening, therefore, the disease may be misdiagnosed and mistreated as other febrile diseases such as malaria (22) and underreported (23).
The seroprevalence and the associated risk factors of human infection in high-risk populations have not been well-understood and documented in the Southern Province of Zambia. Yet, a recent brucellosis study conducted in cattle in the same province found a herd seroprevalence of 28.5% (24). Considering that more than a third of Africa's population depend solely on livestock and livestock products for their livelihoods (25), the likelihood of human infection is therefore high. This is because most infected animals with brucellosis in Africa are not culled due to the economic consequences (26). This has led to the endemicity of human brucellosis in Africa (27) since livestock are the main source of infection to humans (22).
Although brucellosis is of great public health and economic concern, there has never been a brucellosis livestock mass vaccination campaign, making the current epidemiological situation in Zambia uncertain. Furthermore, laboratory diagnostic capacity is very weak mainly relying on rapid agglutination tests for diagnosis (28). These serological screening tests and results are insufficient in providing satisfactory evidence to attract any policies that would direct and reinforce control strategies both in human and livestock. Exploration of this health problem could give evidence-based data that would guide interventions since brucellosis requires multidisciplinary control approach. This study was therefore carried out to determine the Brucella seroprevalence among abattoir workers and herdsmen of Namwala, Monze and Choma districts as well as to find out factors associated with Brucella seropositivity.
Materials and Methods
Study Areas
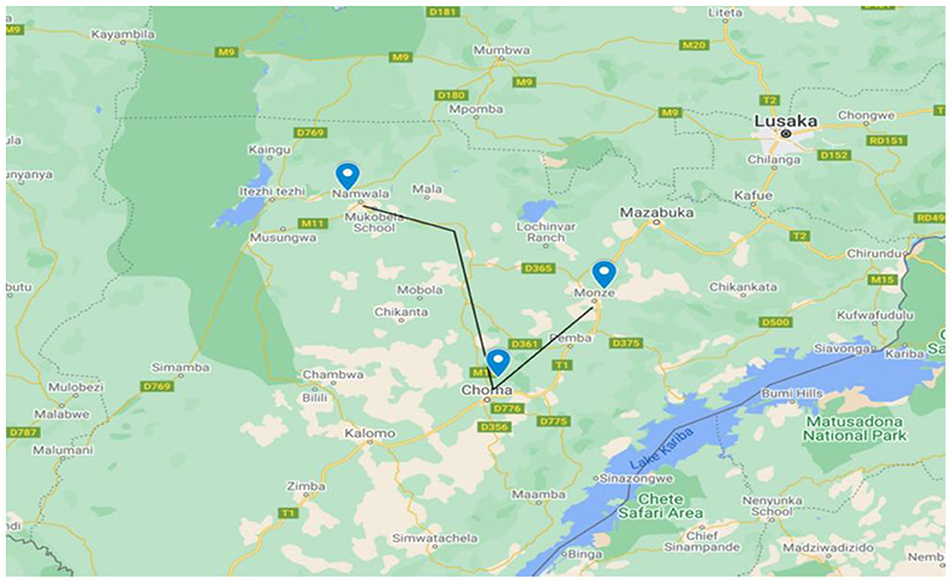
A cross-sectional study was carried out in Namwala, Choma and Monze districts of Southern province of Zambia (Figure 1). The province and study districts were purposively selected because they are the top cattle producers in Zambia (29). Furthermore, these areas are endemic for bovine brucellosis as documented by several serological surveys (24, 29, 30). Southern Province lies between latitudes 15°14′ S and 17°42′ S and longitudes 25°E and 28° S. It has a total land surface area of 85,283 Km2 with an estimated human population of 1,907,784 and a cattle population of 2,105,891 (31). In these districts, a pastoral or nomadic cattle-grazing system is practiced, where animals are grazed in the Kafue flats/floodplains in dry seasons and moved to the upper areas during the wet season (30).
Study Population
The study population comprised of persons occupationally at risk of exposure to Brucella infections in Namwala, Choma and Monze districts of Southern province of Zambia. The individuals were grouped into two categories (herdsmen and abattoir workers), depending on their level of daily activities that could lead to direct contact with either suspected Brucella infected animals or infected animal carcass.
Study Design and Sample Size Calculation
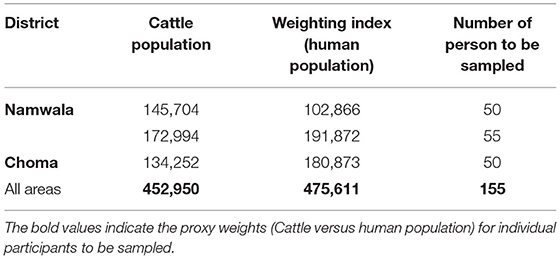
A sample size of 153 was calculated using the Ausvet EpiTools software (http://epitools.ausvet.com.au/) based on the following assumptions: Assumed prevalence estimation of 5.03% (21), desired absolute precision of +/−2% and confidence level of 95%. Sampling was stratified according to the study districts and weighted using the cattle population as proxy weighting value for the persons to be sampled (Table 1).
Sampling Technique and Sample Collection
A total of 153 individuals (125 herdsmen and 28 abattoir workers) were sampled from three purposively selected districts, namely Namwala, Monze, and Choma. Furthermore, these districts are subdivided into villages namely; Simaunbi, Mbabala, Mapanza, Katengwa, Maala, Batoka, Nakeempe, Kayuni, Muyoba, Siakasenke, Chitonga, Nakamboma, Nteme, Baambwe and Hakunkula. The herdsmen were selected based on their proximity to the abattoir, from locations adjacent to the abattoir to locations approximately 100 km from the abattoir. There were five active abattoirs in Namwala, five in Monze, and one in Choma. A list of all abattoir workers both in contact with the meat or slaughter of animals or both from an abattoir in each of the three districts, was obtained and used to randomly select individuals to screen. An informed consent was obtained from all study participants before blood collection. Four (4) ml of blood was collected via the median cubital vein by a clinical officer and stored in sterile plain tubes at +4°C. A semi-structured pre-tested questionnaire was then administered to collect information on the knowledge, attitudes, and practices of abattoir workers and herdsmen. The questionnaire was first developed in the English language and translated to the local language for better understanding of the questions by the participants.
Laboratory Analysis
Serum samples from 153 participants were tested in parallel to detect anti-Brucella antibodies using the i-ELISA (Ingezim Brucella Compac 2.0, Madrid, Spain) and c-ELISA (SVANOVIR®Brucella-Ab Boehringer Ingelheim Svanova, Uppsala, Sweden) test kits according to manufacturer's instructions. On i-ELISA and c-ELISA tests, the result of the sample was compared with the mean cut-off value which was >40% mean optical density and >30% mean optical density of the four conjugate control wells, respectively. Any test sample giving an optical density equal to or below this value was recorded as being positive. In this study, parallel interpretation of results was used. Therefore, any sera testing positive either on i-ELISA or c-ELISA was regarded as positive.
Data Analysis
The data obtained were coded and entered in the Microsoft Excel 2016®, exported, cleaned and analyzed using STATA version 14® (Stata Corp., College Station, TX, USA). Categorical data were expressed in percentage, and seroprevalence was calculated by dividing the number of positive sera samples by the total samples analyzed. Using the cut-off of mean optical density ≥30% and mean optical density ≥40%, for c-Elisa and i-ELISA, respectively, the independent effects of categorical risk factors on anti-Brucella spp. seropositivity were assessed using Fisher's exact test. Variables with a p-value ≤ 0.25 from univariate analysis were used as candidate variables in the logistic model. Multivariable logistic regression model was used to calculate odds ratio at 95% confidence interval to see the degree of association between Brucella seropositivity and the risk factors. The validity of the model to the observed data was assessed by computing the Pearson chi-square goodness-of-fit test.
Ethical Consideration
Ethical clearance was obtained from Excellence in Research Ethics and Science (ERES) before commencing the study (Ref No. 2018-Dec-004). Permission to conduct the study was obtained from the Ministry of Health and the National Health Research Authority. The aim and brief background of the study were explained to the study participants in the local language, thereafter written informed consents were obtained for blood sample collection and questionnaire interview. Participants were assured of confidentiality, anonymity and were free to withdraw from the study whenever they chose to do so without incurring any consequence.
Results
An overall Brucella seroprevalence of 20.3% (95% CI: 14.6–27.5) was estimated, based on parallel interpretation. The abattoir workers' category (n = 28) had a high Brucella seroprevalence (46.4%, n = 13, p < 0.001) as compared to the herdsmen (n = 125) category (14.4%, n = 18). Comparable seropositive results among districts showed Namwala with 26.9% (n = 18), which was the highest, seconded by Monze 19.0% (n = 8), and the least was Choma with 11.4% (n = 5) seropositive. Brucella seroprevalence of 5.2% (95% CI: 2.6–10.2) and 17.0% (95% CI: 11.8–23.9) were determined using c-ELISA and i-ELISA, respectively (Table 2). Namwala recorded high seroprevalences of 6.0 % (95% CI: 2.2–15.1) and 26.9% (95% CI: 17.5–38.9) for c-ELISA and i-ELISA; followed by Monze 4.8% (95% CI: 1.2–17.6) and 14.3% (95% CI: 6.5–28.7) for c-ELISA and i-ELISA, respectively. The least seroprevalence was recorded in Choma which was a duplicate value of 4.5% (95% CI: 1.1–16.8) for c-ELISA and i-ELISA, respectively. The difference between the groups (herdsmen and abattoir workers) was statistically significant (p < 0.001).
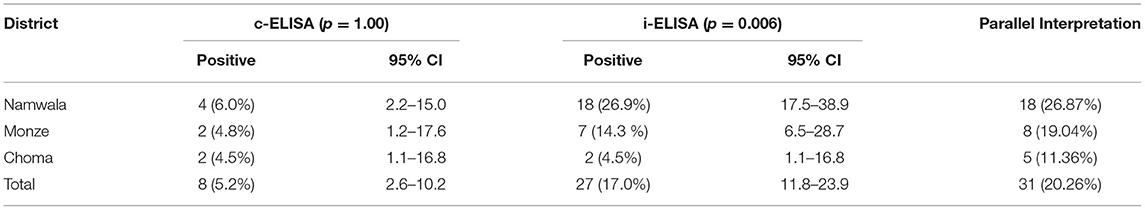
Table 2. Seroprevalence of human anti-brucella antibodies in Southern province by district among herdsmen and abattoir workers using c-ELISA or i-ELISA in parallel interpretation.
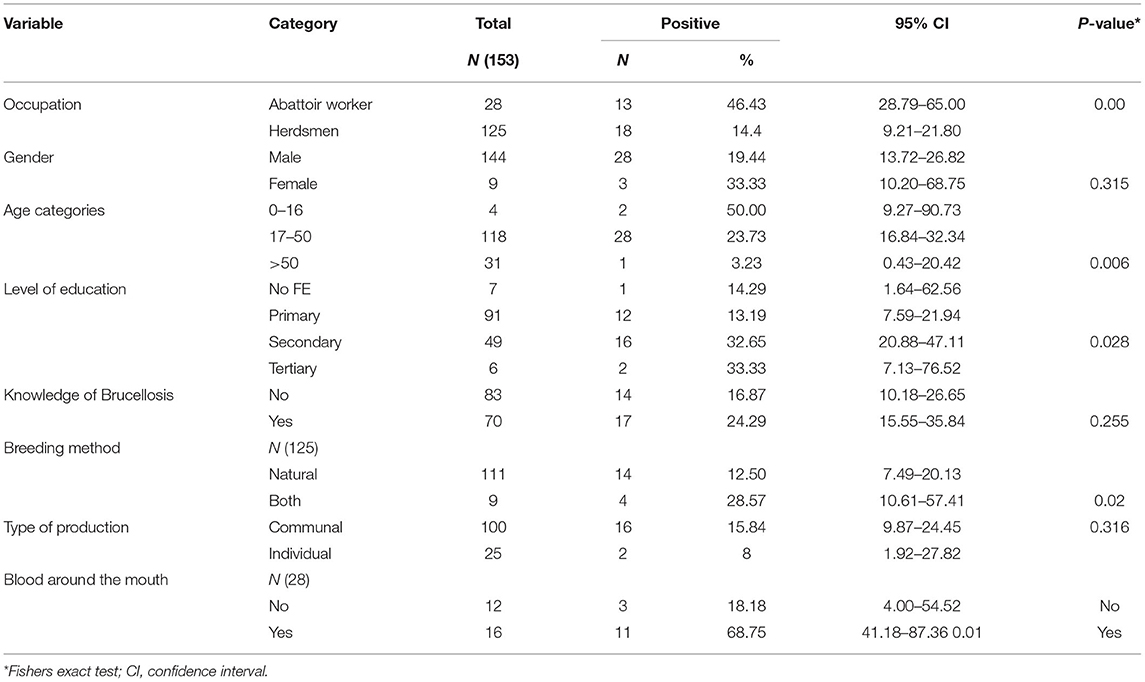
Seropositive reactions among the abattoir workers and herdsmen varied according to the lifestyle and activities of the different categories of respondents. Brucella IgG seropositive respondents were 28 males and 3 females. The majority of the respondents (54.3%; n = 83), reported that they had never heard about brucellosis. Less than half (45.8 %; n = 70) of the participants were knowledgeable about the disease (Table 3). There was no association between Brucella seropositivity and some factors such as gender, knowledge and type of production among herdsmen and abattoir workers (Table 3). In contrast, there was a statistically significant association between Brucella seropositivity for herdsmen and type of breeding method (natural/both) (P = 0.02) as well as abattoir workers who, during their line of duty, had blood splashed around their mouth (P = 0.01).
Logistic Regression Model and Validation
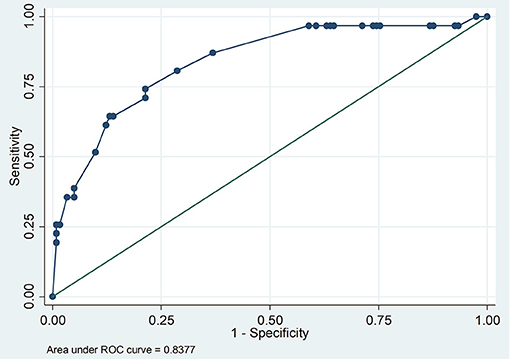
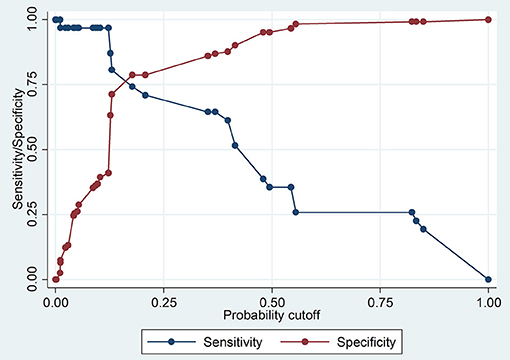
The Pearson chi-square goodness-of- fit test (p = 0.1908) showed that the model fitted the data thus increasing its reliability in predicting. The Receiver-operating Characteristic curve ROC analysis demonstrated that the model was good in prediction (the area under the curve was 0.8377) (Figure 2). The model had rather high sensitivity and low specificity in classifying individuals as seropositive or seronegative (Figure 3).
The multivariate logistic regression model showed that occupation, age category, and district of residence were predictors of being seropositive to Brucella spp (Table 4). The odds of abattoir worker being seropositive to Brucella antibodies were 8.6 (95% CI: 2.6–28.2) higher than that of herdsmen being the reference group. The odds of age category 17–50 years being seropositive to Brucella antibodies were 7.0 (95% CI: 0.7–72.2) higher than being <16 years as the reference group. The odds of one having attained primary level of education being seropositive to Brucella were 1.3 (95% CI: 0.1–14.7) or secondary level of education were 6.2 (95% CI: 0.5–72.6) or tertiary level of education were 5.1 (95% CI: 0.2, 113.3) higher than that of no level of education as the reference group. Furthermore, the odds of a respondent being seropositive to Brucella antibodies were 4.5 (95% CI: 1.315.7) for Namwala and 4.9 (95% CI: 1.1–21.7) Monze higher than that of Choma as the reference group.
Discussion
Seroprevalence of Human Brucella Antibodies
The estimated seroprevalence of Brucella antibodies was 20.3% (14.4% in herdsmen and 46.4% in abattoir workers). This is higher than that reported in a previous studies undertaken between August 2004 and July 2005 in the Southern province of Zambia, which found a 5.03% seroprevalence in humans (21). However, the cited study did not include abattoir workers, but rather focused only on livestock farmers. Abattoir workers were at an increased risk of exposure to Brucella because farmers in the study area tend to cull animals that are old or have reproductive challenges by slaughtering them. Furthermore, during the slaughtering process, abattoir workers are constantly exposed to aerosols and animal parts i.e., blood, tissues, fluids, with inadequate or poor use of personal protective equipment. Further, these workers were at a high risk of injury (knife-cuts) as compared to herdsmen, which increases the risk of exposure to the Brucella pathogen (20). A Brucella cattle herd seroprevalence of 28.5% has been previously been estimated in this study area (24). Comparable seropositive results among the study districts showed that Namwala had the highest (26.8%), followed by Monze (19.4%), while Choma had the lowest (11.4%). The high seropositivity in Namwala district can be attributed to its close proximity to the Kafue flood plains where most of the livestock come into close contact with wild animals. Migration of livestock from the sampled districts to the Kafue plains in search of pasture during the dry season is regular and usual phenomenon due to drastic change in the weather conditions thus increasing co-mingling of animals and transmission of infectious diseases such as brucellosis. Herdsmen tend to drink raw milk, hence the increased likelihood of exposure (5). The difference observed may also be due to the use of different diagnostic test and also the difference in the environmental condition of the region.
Our findings are higher than the 1.41% reported among butcher men, abattoir workers and herdsmen in Tanzania (20) and the 5.6 and 4.7% among abattoir worker in Cameroon (17) and Ethiopia (32), respectively. Handling of fetus and uterine contents was associated with increased risk of human brucellosis in Cameroon whereas in Ethiopia this was attributed to low levels of disease awareness and one working in the abattoir.
Similarly, our findings are higher than the reported seroprevalences among slaughterhouse workers (9.6%) in Ghana (33) and livestock farmers (4.4%) in Uganda (34) but lower than 33.3% observed among cattle herders in South Sudan (15). In contrast, our findings are comparable to 18.5% by (35), higher than the 33.5% reported among abattoir workers in Nigeria (36) and higher than the 7.9% among butcher and slaughterhouse workers in Iran (37).
One of the limitations of this study was that formal random sampling was not achieved as inclusion in the study was voluntary. Further, the participants in this study were not clinically examined for evidence of brucellosis. Therefore, the estimated seroprevalence does not imply persons were suffering from brucellosis, but that the persons had anti-Brucella antibodies, thus likely to be infected. Despite this, the results give a good indication of brucellosis situation in the sampled districts. In endemic areas, as prevailing in the study areas, cases of sub-clinical and self-limiting episodes of brucellosis are likely to show anti-Brucella spp. antibodies thus reducing assay specificity (38). However, the seroprevalence found in the current study parallels with bovine herd Brucella seroprevalence that has been reported to be 28.5% (24) and 20.7% by (29). It is most probable that the Brucella infected animals in our study area were likely to act as reservoirs for human brucellosis (39). Thus, high bovine Brucella seroprevalence in the region could explain the possible reason for the high seroprevalence in humans.
Risk Factors Associated With Brucella Seropositivity
In this study, occupation, age category and district of residence were identified as risk factors for human brucellosis. Our current study revealed that seroprevalence was highest among abattoir workers 46.43% (n = 13). The odds of disease were 8.6 times higher in abattoir workers than in herdsmen, implying the former were more at risk.
The 17–50 years' age category (considered to be actively working with livestock) was the most commonly affected with Brucella in the study area although this was not statistically significant. Furthermore, a respondent who was in the 17–50 years' age category was 7 times more likely to be infected with brucellosis than those who were <16 years old (school going children). These did most of the physical work requiring direct contact with the livestock and livestock products. Similarly (17), reported an association between age and an increased seroprevalence. Similar findings have been reported in Pakistan where age was statistically associated to Brucella seropositivity (40).
Namwala district had 4-fold higher risk of brucellosis than Choma. This might be due to the fact that Namwala has the highest cattle density in the Country and animals are communally grazed on the Kafue plains (30). Furthermore, Mfune et al. (24) established that the odds of testing positive for Brucella in animals were high in Namwala district (OR = 8.55, CI: 2.66–27.44) compared to those from other districts.
A noteworthy association is the type of husbandry practices (Communal/individual) coupled with contact with the Kafue lechwe which have been documented to harbor brucellosis on the flood plains of the Kafue River in this ecosystem (30, 41). Assenga et al. (39) demonstrated the presence of anti-Brucella antibodies in humans, livestock, and wildlife in the Katavi- Rukwa ecosystem in Tanzania. Transmission of the infection between wildlife, livestock and humans is likely to continue due to increasing human activities in the human wildlife interface.
However, the spread out large odds ratios, with wide confidence intervals obtained in this study should be cautiously interpreted, given that the distribution of the individuals within the two occupational categories of the risk factors was not even.
Although this study implicated livestock breeding method (natural breeding) (p = 0.02) as a probable risk factor associated to Brucella seropositivity, Nguna et al. (34) reported that livestock breeding method was not an important risk factor among the herdsmen. Communal grazing coupled with natural breeding is widely practiced hence the risk of transmission of disease to susceptible animals. Similar studies were done by Kubuafor et al. (42) where a significant association between antibodies against Brucella and a history of abortions and retained placenta was observed.
History of blood splashes around the mouth was observed to be associated with seropositivity among abattoir workers (p = 0.01). Similar findings have been documented in Southwest Nigeria (43), Tanzania (44) and Egypt (45). Noteworthy difference is that breeding method and blood around the mouth were potential risk factors and statistically significant at univariate analysis but were dropped in the final multivariable logistic regression model. This was done to increase the predictability of the final model.
The odds of disease in participants older than 50 years was <1, implying old-age was a protective factor. In spite of the high seroprevalence of Brucella infection in humans, it was not considered for routine laboratory diagnosis in cases of acute febrile illness.
Conclusion
Anti-Brucella antibodies in herdsmen and abattoir workers was prevalent in Southern province of Zambia (20.3%), an indication of exposure to Brucella pathogens. The seroprevalence was higher than that observed in similar studies in Zambia. The majority of the respondents, 54.25% (n = 83) reported having not heard of brucellosis. Less than half 45.75 of % (n = 70), of the participants were knowledgeable of the disease, thus it can be concluded that community knowledge about the risk factors of human brucellosis was poor. The important predicators of Brucella seropositivity were occupation, age category and district. This zoonosis should always be one of the differential diagnosis in humans when intermittent fever, malaria like signs and general pain are observed in humans.
Study Limitations
The Covid-19 pandemic, outbreak of Foot and Mouth Disease in the study areas and the instability in some parts of the country which was attributed to terrorist gas-attacks during the sampling period made it difficult to collect human and cattle blood samples. Most individuals were not willing to participate in the study, alleging that it was against their religious and cultural beliefs for blood to be drawn from them. Thirdly, since our study did not present the bacteriological evidence or molecular-based tests, the seropositivity results might be caused by previous exposure to infection or cross-reactivity. However, c-ELISA used in this study is known to have high specificity.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by Excellence in Research Ethics and Science (ERES). Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author Contributions
MM and RM: conceptualization and validation. MM, JBM, and RM: methodology. MM, JBM, MS, and FB: software. MM, JBM, and JG: formal analysis. MM, RM, and CC: investigation. RM: resources and project administration. MM, JBM, and JK: data curation. MM: writing—original draft preparation and visualization. RM, JK, AM, JM, BH, JG, and JBM: writing—review and editing. JBM, RM, and JG: supervision. JBM: funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by the African Centre for Infectious Diseases in Humans and Animals UNZA (ACEIDHA-UNZA).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We acknowledge the Provincial health officer, District health officers, clinical officers and nurses for their support during sampling; the laboratory staff at the department of Disease Control, School of Veterinary Medicine, The University of Zambia, and ACEIDHA-UNZA.
References
1. Dean AS, Crump L, Greter H, Schelling E, Zinsstag J. Global burden of human brucellosis: a systematic review of disease frequency. PLoS Negl Trop Dis. (2012) 6:1–8. doi: 10.1371/journal.pntd.0001865
2. Seleem MN, Boyle SM, Sriranganathan N. Brucellosis: a re-emerging zoonosis. Vet Microbiol. (2010) 140:392–8. doi: 10.1016/j.vetmic.2009.06.021
3. Godfroid J, Saegerman C, Blasco JM. Brucellosis in terrestrial wildlife. Rev Sci Tech Off Int Epiz. (2013) 32:27–42. doi: 10.20506/rst.32.1.2180
4. Ducrotoy M, Bertu WJ, Matope G, Cadmus S, Conde-álvarez R, Gusi AM, et al. Acta tropica brucellosis in sub-saharan africa: current challenges for management, diagnosis and control. Acta Tropica. (2017) 165:179–93. doi: 10.1016/j.actatropica.2015.10.023
5. Godfroid J. Brucellosis in livestock and wildlife: Zoonotic diseases without pandemic potential in need of innovative one health approaches. Arch Public Health. (2017) 75:1–6. doi: 10.1186/s13690-017-0207-7
6. Pandit DP, Pandit PT. Human brucellosis: are we neglecting an enemy at the backyard? Med J DY Patil Vidyapeeth. (2013) 6. doi: 10.4103/0975-2870.118265
7. Asakura S, Makingi G, Kazwala R, Makita K, Ntirandekura JB, Matemba LE, et al. Brucellosis awareness and knowledge in communities worldwide: a systematic review and meta-analysis of 79 observational studies. Infec Ecol Epidemiol. (2019) 9:1–20. doi: 10.1080/20008686.2018.1556548
8. Hu J, Zhang X, Yang H, Zhang S, Wang T, An S, et al. Brucellosis screening and follow-up of seropositive asymptomatic subjects among household members of shepherds in China. Eur J Clin Microbiol Infect Dis. (2021) 40:1325–28. doi: 10.1007/s10096-020-04115-z
9. Sharma HK, Kotwal SK, Singh DK, Malik MA, Kumar A, Singh M. Seroprevalence of human brucellosis in and around Jammu, India, using different serological tests. Vet World. (2016) 9:742–6. doi: 10.14202/vetworld.2016.742-746
10. Waheed U, Butt ZN, Ashraf WO, Khan QM. Seroprevalence and molecular epidemiosurveillance of brucellosis in Pakistan. Online J. Public Health Inform. (2018) 10:2579. doi: 10.5210/ojphi.v10i,1.8647
11. Jama'ayah MZ, Heu JY, Norazah A. Seroprevalance of brucellosis among suspected cases in Malaysia. Malays J Pathol. (2011) 33:31–4.
12. Al-hakami AM, Alqahtani AJ, Moosa RA, Kadasah SK, Gofashe Y, Binzafrah AF, et al. Seroprevalence of brucellosis among exposed agro-pastoral communities in southern Saudi Arabia. Asian Pac J Trop Med. (2019) 12:545–51. doi: 10.4103/1995-7645.272484
13. Andriopoulos P, Floros D, Gioti N, Mariolis A, Paola A, Gil R, et al. Brucella seroprevalence in a high-risk population in greece: a cross-sectional study. Interdiscip Perspect Infect Dis. (2018) 2018:8751921. doi: 10.1155/2018/8751921
14. El-Moselhy EA, Zayet H, El-Khateeb AS, Mohammed AS, El-Tiby DM, et al. Human brucellosis: seroprevalence, risk factors, and barriers of protection among slaughterhouses' workers in El-Menia Governorate, Egypt. Arch Clin Pathol J. (2018) 1:1.
15. Madut NA, Muwonge A, Nasinyama GW, Muma B, Godfroid J, Jubara AS, et al. The sero-prevalence of brucellosis in cattle and their herders in Bahr el Ghazal region, South Sudan. PLOS Negl Trop Dis. (2018) 1–14. doi: 10.1371/journal.pntd.0006456
16. Aworh MK, Okolocha E, Kwaga J, Fasina F, Lazarus D, Suleman I, et al. Human brucellosis: seroprevalence and associated exposure factors among abattoir workers in Abuja, Nigeria - 2011. Pan Afr Med J. (2013) 8688:1–9. doi: 10.11604/pamj.2013.16.103.2143
17. Awah-ndukum J, Moctar M, Mouiche M, Kouonmo-ngnoyum L, Bayang HN, Manchang TK, et al. Seroprevalence and risk factors of brucellosis among slaughtered indigenous cattle, abattoir personnel and pregnant women in Ngaoundéré, Cameroon. (2018) 18:1–13. doi: 10.1186/s12879-018-3522-x
18. Ogola E, Thumbi S, Osoro E, Osoro E, Munyua P, Omulo S, et al. Sero-prevalence of brucellosis in humans and their animals: a linked cross-sectional study in two selected counties in Kenya. Online J Public Health Inform. (2014) 6:2579. doi: 10.5210/ojphi.v6i1.5166
19. Tumwine G, Matovu E, Kabasa JD, Owiny DO, Majalija S. Human brucellosis: sero-prevalence and associated risk factors in agro-pastoral communities of Kiboga District, Central Uganda. BMC Public Health. (2015) 15:1–8. doi: 10.1186/s12889-015-2242-z
20. Sagamiko FD, Mfune RL, Hang BM, Karimuribo ED, Mwanza AM, Sindato C, et al. Seroprevalence of human brucellosis and associated risk factors among high-risk occupations in mbeya region of tanzania. J Epidemiol Res. (2020) 6. doi: 10.5430/jer.v6n1p1
21. Muma JB, Samui K, Munyeme M, Lund A, Ielsen K, Chimana H, et al. Brucellosis in rural communities in Zambia and factors associated with increased anti-Brucella spp. antibody presence. UNZA J Sci Technol. (2008) 12:9–18.
23. Godfroid J, Cloeckaert A, Liautard JP, Kohler S, Fretin D, Walravens K, et al. A review article from the discovery of the Malta fever's agent to the discovery of a marine mammal reservoir, brucellosis has continuously been a re-emerging zoonosis. Vet. Res. (2005) 36:313–26. doi: 10.1051/vetres:2005003
24. Mfune RL, Mubanga M, Silwamba I, Sagamiko F, Mudenda S, Daka V, et al. Seroprevalence of bovine brucellosis in selected districts of Zambia. Int J Environ Res Public Health. (2021) 18:1436. doi: 10.3390/ijerph18041436
25. Mufinda Franco Cazembe F, Nunes CB. Prevalence and factors associated with human brucellosis in livestock professionals. Rev Saúde Pública. (2017) 1–10. doi: 10.1590/s1518-8787.2017051006051
26. McDermott J, Grace D, Zinsstag J. Economics of brucellosis impact and control in lowincome countries. OIE Rev Sci Tech. (2013) 32:249–61. doi: 10.20506/rst.32.1.2197
28. Georg N, Loderstaedt U, Hahn A, Hinz R, Erich A, Eibach D, et al. Acta tropica microbiological laboratory diagnostics of neglected zoonotic diseases (NZDs). Acta Tropica. (2017) 165:40–65. doi: 10.1016/j.actatropica.2015.09.003
29. Muma JB, Syakalima M, Munyeme M, Zulu VC, Simuunza M, Kurata M. Bovine tuberculosis and brucellosis in traditionally managed livestock in selected districts of Southern Province of Zambia. Vet Med Int. (2013) 2013:730367. doi: 10.1155/2013/730367
30. Muma JB, Samui KL, Siamudaala VM. Prevalence of antibodies to Brucella spp. And individual risk factors of infection in traditional cattle, goats and sheep reared in livestock – wildlife interface areas of Zambia. Trop Anim Health Prod. (2006) 38:195–206. doi: 10.1007/s11250-006-4320-9
31. Fisheries MOF, Office CS. Republic of Zambia Central Statsistical Office the 2017/18 Livestock and Aquaculture Census Report Summary Report. Chikankata (2017).
32. Tsegay A, Tuli G, Kassa T, Kebede N. Seroprevalence and risk factors of brucellosis in abattoir workers at debre zeit and modjo export abattoir, central ethiopia. BMC Infect Dis. (2017) 17:1–8. doi: 10.1186/s12879-017-2208-0
33. Tasiame W, Emikpe BO, Folitse RD, Fofie CO, Burimuah V, Johnson S, et al. The prevalence of brucellosis in cattle and their handlers in north tongu school of veterinary medicine, college of health sciences, kwame nkrumah university of science. Afr J Infect Dis. (2016) 10:111–7. doi: 10.21010/ajid.v10i2.6
34. Nguna J, Dione M, Apamaku M, Majalija S, Mugizi DR, Odoch T, et al. Seropositivity in cattle, goats and humans in Iganga District, Uganda. Pan Afr Med J. (2019) 33:99. doi: 10.11604/pamj.2019.33.99.16960
35. Ali S, Ali Q, Neubauer H, Melzer F, Elschner M, Khan I, et al. Seroprevalence and risk factors associated with brucellosis as a professional hazard in Pakistan. Foodborne Pathog Dis. (2013) 10:500–5. doi: 10.1089/fpd.2012.1360
36. Igawe PB, Okolocha E, Kia GS, Irmiya IB, Balogun MS, Nguku P. Seroprevalence of brucellosis and associated risk factors among abattoir workers in Bauchi State, Nigeria. Pan Afr Med J. (2020) 8688:1–10. doi: 10.11604/pamj.2020.35.33.18134
37. Esmaeili S, Naddaf SR, Pourhossein B, Hashemi A. Seroprevalence of brucellosis, leptospirosis, and q fever among butchers and slaughterhouse workers in south-eastern. PLoS ONE. (2016) 11:1–12. doi: 10.1371/journal.pone.0144953
38. Dahouk S. Implications of Laboratory Diagnosis on Brucellosis Therapy. Berlin: Expert Reviews (2011). p. 833–46.
39. Assenga JA, Matemba LE, Muller SK, Malakalinga JJ, Kazwala RR. Epidemiology of Brucella infection in the human, livestock and wildlife interface in the. BMC Vet Res. (2015) 11:189. doi: 10.1186/s12917-015-0504-8
40. Niaz S, Raqeeb A, Khan A, Amir S, Zhu L, Kumar S. Status of human brucellosis in district Malakand, Khyber Pakhtunkhwa, Pakistan. J Infec Public Health. (2020) 12:8–13. doi: 10.1016/j.jiph.2019
41. Munyeme M, Muma JB, Skjerve E, Nambota AM, Phiri IGK, Samui KL, et al. Risk factors associated with bovine tuberculosis in traditional cattle of the livestock/wildlife interface areas in the Kafue basin of Zambia. Prevent Vet Med. (2008) 85:317–28.
42. Kubuafor DK, Awumbila B, Akanmori BD. Seroprevalence of brucellosis in cattle and humans in the Akwapim-South district of Ghana: public health implications. Acta Tropica. (2000) 76:45–8. doi: 10.1016/S0001-706X(00)00088-7
43. Cadmus SIB, Ijagbone IF, Oputa HE, Adesokan HK, Stack JA. Serological survey of brucellosis in livestock animals and workers in Ibadan, Nigeria. Afr J Biomed Res. (2006) 9:163–8. doi: 10.4314/ajbr.v9i3.48900
44. Swai ES, Schoonman L. Human brucellosis: seroprevalence and risk factors related to high risk occupational groups in Tanga Municipality, Tanzania. Zoonoses Public Health. (2009) 56:183–7. doi: 10.1111/j.1863-2
Keywords: anti-bodies, human Brucella, risk factors, seroprvalence, Zambia
Citation: Mubanga M, Mfune RL, Kothowa J, Mohamud AS, Chanda C, Mcgiven J, Bumbangi FN, Hang'ombe BM, Godfroid J, Simuunza M and Muma JB (2021) Brucella Seroprevalence and Associated Risk Factors in Occupationally Exposed Humans in Selected Districts of Southern Province, Zambia. Front. Public Health 9:745244. doi: 10.3389/fpubh.2021.745244
Received: 21 July 2021; Accepted: 29 September 2021;
Published: 17 November 2021.
Edited by:
Susan Christina Welburn, University of Edinburgh, United KingdomReviewed by:
Maryam Dadar, Razi Vaccine and Serum Research Institute, IranBahador Sarkari, Shiraz University of Medical Sciences, Iran
Aman Ullah Khan, University of Veterinary and Animal Sciences, Pakistan
Copyright © 2021 Mubanga, Mfune, Kothowa, Mohamud, Chanda, Mcgiven, Bumbangi, Hang'ombe, Godfroid, Simuunza and Muma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Melai Mubanga, bWVsYWltdWJhbmdhQGdtYWlsLmNvbQ==
 Melai Mubanga
Melai Mubanga Ruth L. Mfune
Ruth L. Mfune John Kothowa1,4
John Kothowa1,4 Ahmed S. Mohamud
Ahmed S. Mohamud Bernard M. Hang'ombe
Bernard M. Hang'ombe Jacques Godfroid
Jacques Godfroid Martin Simuunza
Martin Simuunza John B. Muma
John B. Muma