- Department of Community and Family Medicine, All India Institute of Medical Sciences, Raebareli, Uttar Pradesh, India
It is still debatable whether all children should receive the COVID-19 vaccine. The comparatively mild cases and low risk of COVID-19 in children compared to adults, as well as the lack of clarity on the relative effects of the disease and vaccine, indicate that the risk-benefit ratio of vaccination in children is more nuanced. To consider and highlight the complexity of policy decisions regarding COVID-19 vaccination in children, we outlined the points regarding for and against vaccination of children against COVID-19 in this systemic review. Using Medical Search Headings (MeSH) terms and keywords, we searched PubMed, PubMed Central, Scopus, and Google Scholar. The primary search term was COVID-19 vaccination (all synonyms), factors (all synonyms), and among children (all synonyms). A total of 367 articles were searched. Finally, 64 articles met the inclusion criteria and were included in the review. The major theme/tone of 28 (43.75%) articles was in favor of children's COVID vaccination, and they were highlighting the positive factors, whereas the major theme/tone of 20 (31.25%) articles was against it. Approximately 16 (25.0%) articles were in a neutral position. Major factors highlighted by articles in favor of childhood COVID vaccination were as follows: the increasing rate of disease burden (29 articles), prevention of interruption of academic activities of children or school reopening (24 articles), and a role in defense against COVID infection (21 articles). Major factors against childhood vaccination were as follows: mild infection among children (27 articles), ethical concerns and legal problems regarding the consent of minors (17 articles), and vaccine hesitancy among parents for childhood vaccination (11 articles). Whereas, factors of uncertainty were the role in the reduction of community transmission (19 articles), protection against MIS-C (10 articles), and defense against long COVID (7 articles). Considering all the factors of COVID-19 disease progression among children, a cautious approach will be essential before proceeding with COVID-19 vaccination in children.
Introduction
Due to its extremely contagious nature, COVID-19 which emerged in late December 2019, quickly spread from person to person, resulting in a global pandemic (1). Wuhan, China, was the site of the disease outbreak's initial notification in December 2019. Later, on 11 March 2020, the World Health Organization (WHO) designated COVID-19 to be a pandemic illness (2). According to the most recent data, as of 02 August 2022, there were more than 6.3 million deaths globally and more than 545 million confirmed cases of COVID-19 (3). The recently discovered variant “Omicron” was classified as a variant of concern on 26 November 2021 (4). It was also reported that a variety named “IHU” with a Cameroonian origin has been found in France. Different COVID-19 vaccines have been produced over time, and vaccination campaigns are currently being conducted in all countries to reduce mortality and morbidity caused by COVID-19 infection. The majority of vaccines available now are for adults. Children account for ~1–3% percent of all confirmed COVID-19 cases. Children have fewer severe diseases and a better prognosis than adults, and deaths are exceedingly uncommon, mostly involving teenagers and those with serious underlying comorbidities. Adults have been the primary target of COVID-19 vaccination trials, and there is currently little information on the vaccine's safety in children (5). There are deficiencies in proven studies regarding the effect of vaccination on preventing the transmission of coronavirus. WHO's position statement on COVID-19 vaccination among children says that it should be country-specific considering the epidemiological and social context (6). Whereas, the CDC suggests that children as young as 6 months to 4 years of age also should get the primary doses of the COVID-19 vaccine (7). Even GAVI has favored childhood COVID vaccination considering the poor healthcare facilities for the most vulnerable kids globally (8). The question of universal childhood COVID vaccination should be evidence-based as well as strong on the social and ethical background. Presently, there is diverse opinion and views among global experts on the issue of universal childhood COVID vaccination. In this context, the study was conducted with the following objective:
(A) To examine current expert opinions or viewpoints on universal childhood COVID vaccination.
(B) Based on expert opinion, quantify the factors associated with childhood COVID vaccination (positive/negative/uncertain).
COVID-19 vaccines for children
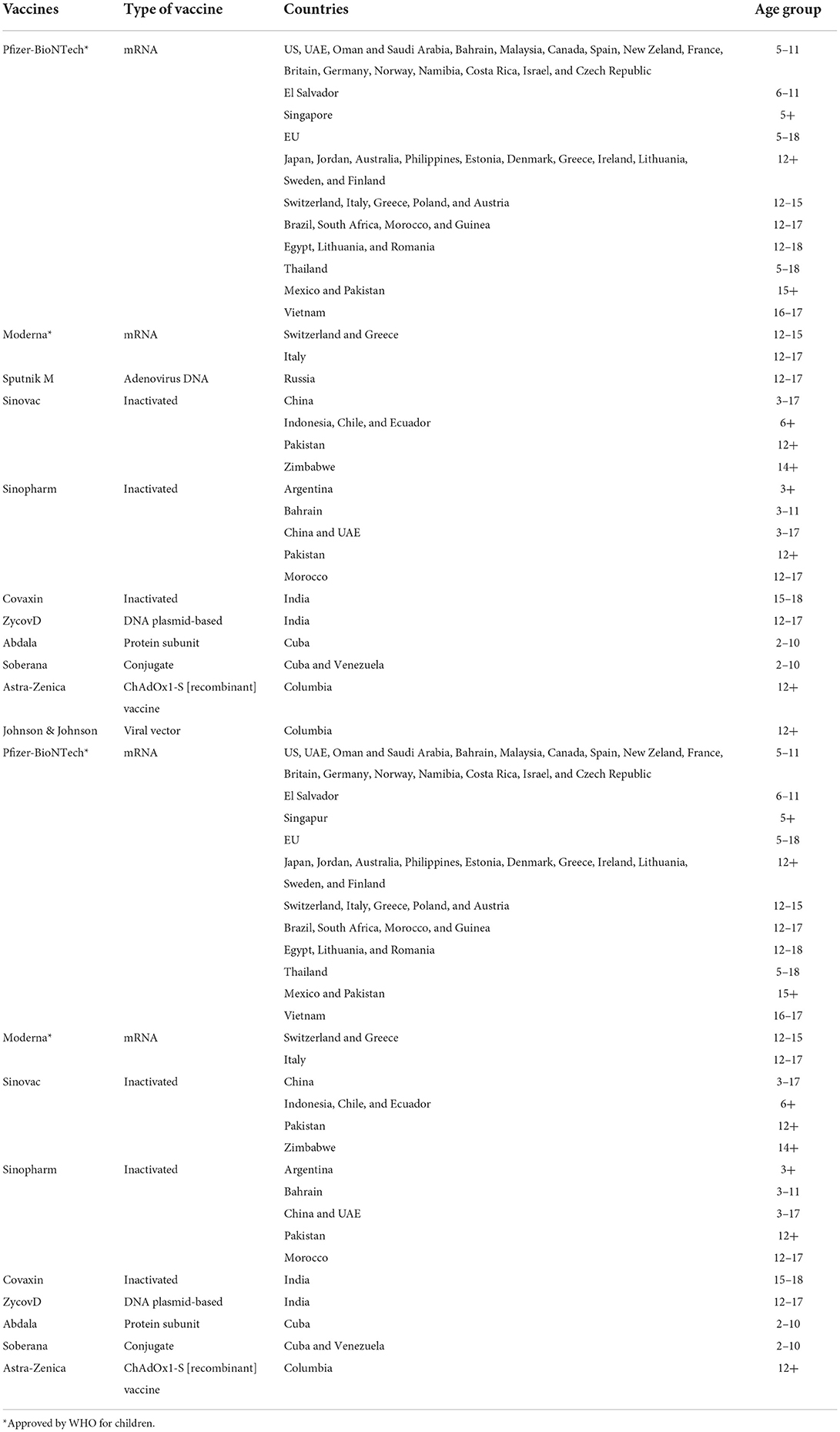
The following vaccines against COVID-19 have been determined by WHO to have met the requirements for safety and efficacy in individuals aged 18 years and over as of 12 January 2022: AstraZeneca/Oxford vaccine, Johnson & Johnson, Moderna, Pfizer/BioNTech, Sinopharm, Sinovac, COVAXIN, Covovax, and Nuvaxovid. The Pfizer vaccine is safe to use for individuals 5 years of age and older, while the Moderna vaccine is safe to use for individuals 12 years of age and older, according to the Strategic Advisory Group of Experts (SAGE) of WHO (9) (Table 1).
Materials and methods
Eligibility criteria
A systematic review of the articles was conducted between January 2020 and March 2022 to identify the articles discussing the factors related to COVID vaccination for children. Any factor which is directly or indirectly related to or affects the decision of COVID-19 universal vaccination among children was considered for inclusion in the study.
Some of the major probable factors considered were:
(a) Scientific knowledge about the COVID vaccine's content, efficacy, utility, and so on in childhood vaccination
(b) Programmatic aspects of childhood COVID vaccination
(c) Ethical and social factors, and so on.
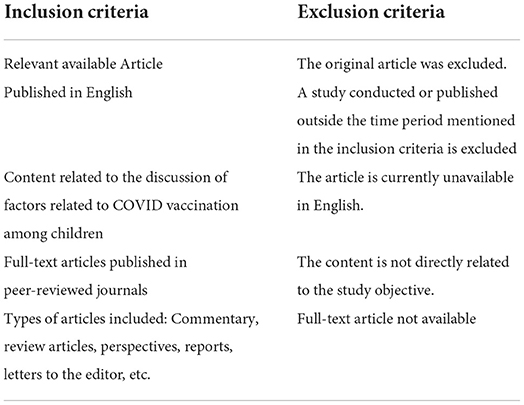
The inclusion and exclusion criteria used for the inclusion and exclusion of the article identified from the search are mentioned in Table 2.
Search strategy and information source
The systematic strategy was used for literature searching using electronic databases and supplemented by hand searching and cross-referencing. A further search was also conducted on the Internet via search engines such as Google Scholar, and so on. We searched Pub Med, Pub Med Central, and Scopus electronic databases using the Medical Search Headings (MeSH) terms and keywords. The primary search terms were COVID-19 vaccination (all synonyms), factors (all synonyms), and among children (all synonyms). We used “text word searching.” In this “text word searching” method, we searched for the above-mentioned words' appearance anywhere in the document, not only in the full text of the article. MeSH searching was (COVID-19 vaccination OR Coronavirus immunization OR SARS-CoV-2) AND (factors OR conditions) AND (related with OR associated with) AND (among Children OR Adolescent OR Child). The articles which were included in the systematic review were cross-checked through their references and citations to confirm that all relevant articles were included.
Selection process
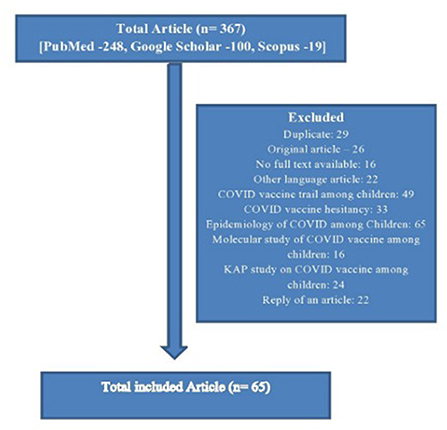
The articles that emerged from the database were screened in a two-stage process. First, the title and abstract of the study were assessed to determine whether they met the inclusion criteria. In the second stage, the full text of the included article was reviewed against the inclusion criteria. When there was any uncertainty about the inclusion or exclusion of an article, the whole text of the article was reviewed separately by two reviewers (SP and CMM) and conciseness was reached through discussion. The flow diagram which has been used for the selection of the studies is given below in Figure 1.
The data extraction process
A standard proforma was used for data extraction.
1. Identification (article title, authors, and year of publication).
2. All the included articles were screened by each researcher individually to find out the probable factors discussed in the article about childhood COVID vaccination, and the factors were listed in the data extraction form. The only mention of a factor in an article does not qualify to include it as a factor discussed in that article. There should be a substantial discussion of that factor in that article to include it in the final data extraction form.
3. Finally, both authors identified the article's major conclusion or major theme or tone (In favor of Childhood COVID-19 vaccinations/against/both). The article's tone was derived from the factors discussed in the article. If there was more than one theme or conclusion, then the article was assigned a positive or negative based on the number of positive and negative factors discussed in it. In cases where there is uncertainty about the number of positive and negative factors, the theme or tone of the article was assigned as an uncertain or neutral category.
Data synthesis
Initial screening of the article according to inclusion and exclusion criteria was conducted by the first author (SP), and the discrepancy was addressed by the first and second authors (SP and CMM). Data extraction of the included studies was done by the second author (CMM), and the random sample was independently checked by all others. All the disagreements were resolved by discussion.
Results
The total number of articles searched was 367. Among them, 64 articles' were finally included according to inclusion criteria. In that, the major tone of 28 (43.75%) articles was in favor of children's COVID vaccination and they were highlighting mostly the positive factors, whereas the major themes of 20 (31.25%) articles were against it, and similarly, they were highlighting major negative factors. Approximately, 16 (25.0%) articles were in the neutral position, highlighting both the pros and cons of COVID-19 vaccination for children. According to the type of the articles, the majority were editorials (14, 21.9%), commentary or viewpoints (18, 28.1%), review articles or perspectives (11, 17.2%), letters to the editor (6, 9.4%), and others (15, 23.4%) (Table 3).
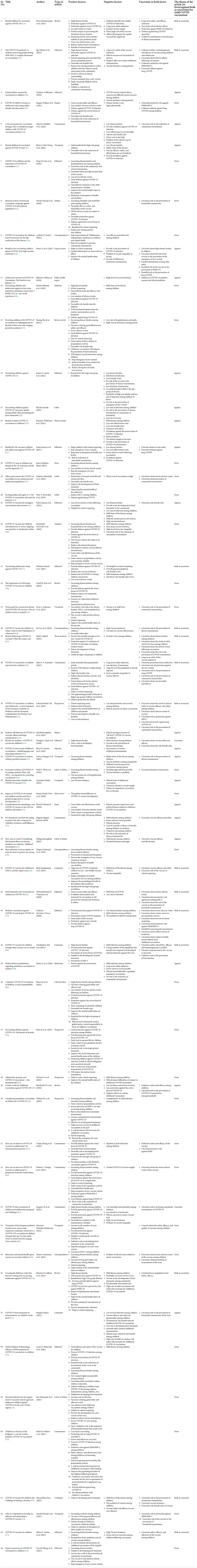
Table 3. Summary of the included study and description of factors discussed related to childhood COVID vaccination.
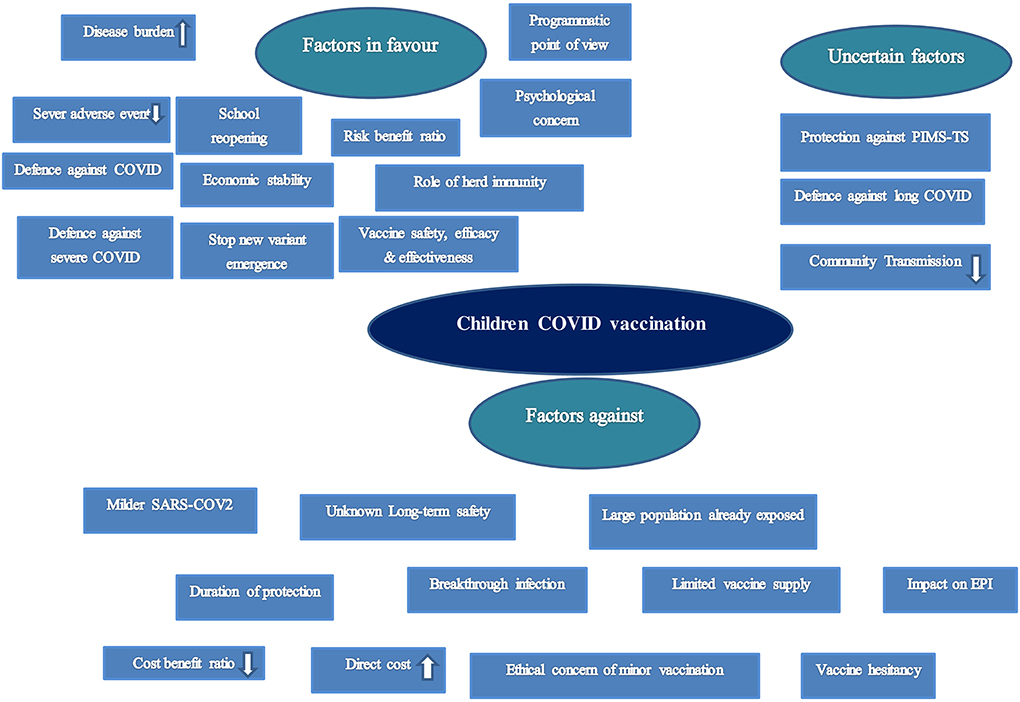
The total number of factors discussed in relation to childhood COVID-19 vaccination was: 41 (Figure 2).
(1) Disease burden among children: 41 articles
(2) Vaccine safety, efficacy, and effectiveness: 40 articles
(3) Level of severe adverse events: 31 articles
(4) Defense against COVID-19 infection: 26 articles
(5) Defense against the severe form of COVID: 23 articles
(6) Back to normal for children (school reopening): 26 articles
(7) Faster return to prepandemic activity and economic stability: 10 articles
(8) Stop the emergence of new variant: 17 articles
(9) Cost-benefit analysis: 5 articles
(10) Risk-benefit analysis: 18 articles
(11) Role of herd immunity: 17 articles
(12) Programmatic point of view: 10 articles
(13) Psychological aspects: 16 articles
(14) Social and ethical responsibility: 2 articles
(15) Role of PMIS/MIS-C: 16 articles
(16) Defense against long COVID-19: 13 articles
(17) Contribution in the community or household transmissions: 41 articles
(18) Children have milder COVID-19 Infection: 30 articles
(19) Long-term safety unknown: 7 articles
(20) The large population already exposed/immune: 2 articles
(21) Questionable duration of protection: 5
(22) Breakthrough infection: 2
(23) Limited vaccine supply: 5 articles
(24) Impact on routine immunization: 5 articles
(25) High direct cost of vaccination: 3 articles
(26) Ethical concern: 20 articles
(27) Vaccine hesitancy: 15 articles
(28) High-risk group of children requires COVID-19 vaccination: 9
(29) Problem of Orphanage, malnutrition among children: 1 article
(30) Decrease requirement of personal protective equipment: 1 article
(31) Indirect harm of lockdown: 5 articles
(32) Indirect benefit of childhood vaccination for elderly: 4 articles
(33) Reduction in disruption of routine healthcare facility: 7 articles
(34) Epidemiological shift in higher age group children: 1
(35) Beneficial for international travel: 1 article
(36) Higher case fatality and low transmission rate in future: 1 article
(37) High rate of adult vaccination will reduce the necessity of childhood vaccination: 2 articles
(38) Failure of compulsory vaccination law: 1 article
(39) Vaccine delivery inequality: 5 articles
(40) Gender-based inequality in the vaccine: 1 article
(41) Socio-economic inequality in vaccination: 1 article
Top three favorable factors for childhood COVID-19 vaccination mentioned by the highest number of articles:
(a) Increasing rate of disease burden (29 articles)
(b) Prevention of interruption of academic activities of children or school reopening: 24 articles
(c) Role in defense against COVID-19 infection (21 articles),
Top three unfavorable/against factors for childhood COVID-19 vaccination mentioned by the highest number of articles:
(a) Mild infection among children (27 articles),
(b) Ethical concerns and legal problems regarding the consent of minors (17 articles),
(c) Vaccine hesitancy among parents for childhood vaccination (11 articles).
Top three uncertain factors for childhood COVID-19 vaccination mentioned by the highest number of articles:
(a) Contribution in reduction of community or household transmissions: 19 articles
(b) Protective role against PMIS/MIS-C: 10 articles
(c) Defense against long COVID-19: 7 articles
Discussion
Factors in favor
Scientific evidence
Disease burden among children
Children of all ages may be infected with COVID-19, according to the evidence, although the disease burden is lower in them due to a lower prevalence of exposure and infrequent testing. Regardless of symptoms, studies on acute or previous COVID-19 infections in children and adolescents have shown that the infection incidence is comparable to that of adults. A high disease burden despite mild symptoms favors COVID-19 vaccination in children (21, 26, 27, 32, 36, 38, 48, 72, 77, 78). According to a multicentric surveillance study from many nations, the infection rate (laboratory-confirmed cases) among kids was as high as 18%. According to a CDC report, around 16–17% of laboratory-confirmed COVID-19 cases in the United States involved children. However, this percentage may be an underestimate of the true situation given the significant number of asymptomatic or mild cases among children that have not yet been tested (79). In total, 41 (64.0%) articles were discussing on the trend of COVID-19 in children, among them authors of 29 articles (45.3%) (16, 17, 21, 22, 26, 27, 32, 36, 38–40, 45, 47, 48, 54, 55, 58, 60, 64, 66–70, 73, 74, 76–78) were only highlighting high disease burden among children. Similarly, author of 10 articles (15.6%) (5, 19, 20, 28, 31, 35, 43, 57, 63, 71) was against it. Two of the articles (3.1%) (24, 41) focused on both the positive and negative aspects.
The infection incidence among youngsters could increase globally in future, like in South Africa or the United States, due to the advent of novel variations (e.g., Omicron, IHU, etc.) (48, 80).
Vaccine safety, efficacy, and effectiveness
Similar to the adult COVID-19 vaccine trial for the safety and efficacy of different COVID-19 vaccines, the children's trial, also in different age groups, has been conducted globally and data have been encouraging. Although World Health Organization (WHO) has approved the Pfizer-BioNTech (BNT162b2) vaccine and Moderna for children vaccination with an efficacy of 90.7% (5–11 years) and 100.0% (12–17 years), India's covaxine vaccine shows 95–98% efficacy (2–18 years) and Soberana 02 of Cuba (92.4%), Sinopharma, and Sinovac vaccine also shows a high level of safety and efficacy among children (9, 81–86). There are 18 studies (19, 21, 22, 26, 27, 36, 39, 40, 51, 56, 57, 60, 69, 72–74, 76, 78) which favor COVID vaccination in children in relation to safety, efficacy, and effectiveness, similarly 6 studies (5, 23, 30, 70, 71, 75) were raising concern about vaccine safety, efficacy, and effectiveness. And 16 studies were neutral or uncertain (16, 17, 31, 40–43, 45, 52, 53, 55, 58, 63, 65, 68, 77).
Level of severe adverse events
Among all the articles, 7 articles (30, 31, 40, 52, 59, 70, 77) were highlighting all adverse events as major events related with COVID vaccination, whereas 14 articles (19, 21, 22, 24, 26, 27, 32, 35, 39, 42, 51, 60, 73, 74) focusing all those events as minor or rare events; 10 articles (16, 17, 33, 41–43, 56, 57, 66, 69) were reflecting in a neutral tone about adverse events following COVID vaccination. Myocarditis (6 articles) (16, 17, 24, 31, 42, 58), pericarditis (6 articles) (16, 17, 24, 31, 42, 58). Chest pain (3 articles) [17, 21, 254], Fever (2 articles) (17, 21), Myalgia (2 articles) (17, 21), fatigue (2 articles) (17, 21), cerebral venous thrombosis (2 articles) (16, 17), MIS-C (2 articles) (16, 17), and headache, decreased left ventricular ejection, dysrhythmia, Vaccine-induced thrombocytopenia (VITT), Pediatric nephrotic syndrome, sore throat, and neck pain were reported by one article each. Myocarditis, which was reported and verified in 1,626 cases in the USA, was the most frequent adverse event associated with the COVID-19 vaccination in the 16- to 24-year-old age group (December 2020 to August 2021) (87).
Defense against COVID-19 infection
Evidence suggests that the rates of infection, as well as the severity of symptoms of COVID-19, are comparatively less among children, but the report of WHO has shown that from 30 December 2019 to 25 October 2021, COVID-related reported deaths were 1,797 among under 5 years, 1,328 among 5–14 years, and 7,023 among 15–24 years. It is also a fact that overlapping clinical presentation of COVID-19 with various childhood upper respiratory tract infections leads to misclassification as well as underestimation of COVID-19 in this age group (88). Authors have found that 21 articles (16, 17, 19, 21, 22, 26, 27, 36, 37, 42, 57, 58, 60, 61, 64, 66–68, 70, 72, 74) were pointing toward the role of COVID-19 vaccine in prevention of infection among children, whereas five articles (5, 20, 24, 30, 31) were not finding any significant role. The COVID-19 vaccine is having a definitive role in prevention among high-risk children (37, 89, 90). Countries having a high burden of different communicable diseases (e.g., malnutrition, tuberculosis, anemia, etc.) among children will face a serious impact unless universal COVID-19 vaccination is conducted for the high-risk group (90). Similarly countries having high population rates and a small proportion of infection will have a large number of absolute cases. Globally, primary immunization for different vaccine-preventable diseases (e.g., influenza, rubella, etc.) is conducted despite having a low incidence rate and hospitalization similar to the COVID-19 infection rate among children (88). Even adult emergency COVID-19 prophylactic vaccination was initiated in absence of any conclusive evidence.
Defense against COVID-19 complications (severe form of COVID-19)
The severe form of COVID-19 infection is usually seen among children with comorbidities, for example, malignancy, immunodeficiency, respiratory, cardiac, renal diseases, and so on (89, 91, 92). Even among healthy children also the chances of Multisystem inflammatory Syndrome of Childhood (MIS-C) (16, 43, 74), long COVID (43), severe COVID (43), critical illness (36), and increased duration of disease (43) are more in the absence of vaccination. Among the 64 articles, 19 articles (16, 19, 22, 36–39, 57–61, 65–68, 70, 72, 74) have emphasized the role of the COVID-19 vaccine against the severe form of disease, whereas only four articles (30, 31, 63) have highlighted no role in it including one uncertain article (24). With the emergence of a new variant of concern, the COVID risk of severe infection among healthy children might be many folds high in the future. Recently Omicron variant with more than 50 mutations has infected children under 5 years at a very high rate, especially in African countries (15).
Back to normal for children (e.g., school reopening)
In the first and second waves of COVID-19, school closure (partial/complete) was done as a presumptive approach rather than a risk-based approach (88). School closure had a significant negative impact on the educational services of the children, similarly, it had physical, mental, and emotional distress also (93). Although in the early part of the pandemic it was evident that school settings had a potential role in COVID-19 transmission, and when there is rampant community transmission of the virus, there is hardly any beneficial effect from the school closure (94). Although the decision on COVID-19 vaccination among children should be based on multiple factors (equity, availability, scientific evidence, susceptibility, etc.), universal vaccination of children against COVID-19 will reduce the fear among children as well as parents also (17, 26, 33, 39, 69, 73, 74). Among 64 articles, 24 articles (16, 17, 22, 24, 26, 27, 31, 33, 35, 36, 39, 42, 43, 54, 60–62, 64, 66, 69, 70, 73, 74, 77) had the opinion for childhood COVID-19 vaccination for early school reopening but three articles (37, 55, 57) were uncertain or against it. COVID-19 vaccination might be an essential requirement for international travel also (16).
Faster return to pre-pandemic activity and economic stability
Children are the main victims of COVID-19, which has had a direct impact on childhood education (24 million children are not in school, which is equivalent to a loss of US$10 trillion). In addition, it has left a long-lasting scar on society in the form of poverty, malnutrition, unemployment, food insecurity, and economic instability (88). Worldwide most countries have already vaccinated or started vaccinating their adult population. Many nations have begun immunizing youngsters against COVID. If children in low- and middle-income nations receive vaccinations, it will be simpler for any nation to return to its pre-pandemic status, which calls for the early continuation of all activities (16, 17, 77). This will facilitate the rapid recovery of the economy (57). In the present review, nine articles (16, 27, 36, 42, 51, 61, 64, 66, 74) have highlighted the role of early childhood COVID vaccination for faster return of pre-pandemic activity in society whereas only one article (57) had a different opinion.
Stop the emergence of new variants
Although there is no accepted theory regarding “How to stop the emergence of the new variant,” the best possible way to prevent the emergence of a new variant is to stop or minimize the spread of the virus (95). The generation of new mutant variations of the coronavirus is facilitated by its high rate of community transmission, and new variants (delta and omicron) have been identified to have a significant part in the rise in pediatric cases (17). Roll out of adult COVID vaccination in full swing will make the virus easily transmittable among vulnerable children, giving rise to a new mutant variant (54, 96). In total, 13 articles (17, 27, 31, 32, 36, 41, 54, 57, 64, 66, 68, 73, 78) in the present review have raised concern about the emergence of new variants among children in future in the absence of childhood COVID vaccination whereas four authors (16, 24, 28, 42) were not in full agreement with them.
Risk benefits analysis
The risk-benefit ratio of COVID vaccination is favorable, especially for those having comorbidities. However, a few cases of myocarditis with the mRNA vaccine create some doubt about the favorable risk-benefit ratio, but there is not any sufficient evidence of this (19, 22, 65, 66). Among adolescents, a study conducted in England also highlighted a favorable risk-benefit ratio unless the incidence of the case comes down very low (97). Out of the 64 articles, 8 articles (17, 19, 26, 27, 33, 42, 60, 66) were in favor of positive risk-benefit ratio, whereas 5 articles (20, 30, 37, 70, 71) were against it and 4 authors (35, 45, 65, 75) were uncertain.
Role of herd immunity
Universal youth and children COVID-19 vaccine will not only benefit the recipient directly but also aid in attaining herd immunity among all age groups. It will be especially helpful for people (such as the elderly) for whom direct immunization is occasionally not possible due to a variety of health difficulties. Earlier, it had been evidenced that immunization of children was much more beneficial (e.g., influenza, pneumococcal, and many other diseases) rather than the elderly. If childhood vaccination creates herd immunity, then it will act as a barrier for all age groups (43, 56, 75, 98). In this review, only 14 articles (27, 33, 41, 43, 45, 56, 58, 61, 64, 65, 68, 72, 75, 78) were highlighting the role of herd immunity related to childhood COVID vaccination and 3 articles (35, 70, 71) was against this opinion.
Programmatic point of view
If a benefit-risk assessment of children's vaccination shows that COVID-19 vaccination is beneficial for the country, the already existing infrastructure, logistics, and manpower for routine immunization will be a booster, mainly for developing countries. It has been observed that in many developing countries, lack of enough manpower is a major hindrance in delaying COVID vaccination (99). It will be relatively simpler to vaccinate every child quickly if the government adds COVID-19 vaccination to routine immunization, especially in low- and middle-income nations. In total, 10 articles (16, 21, 26, 36, 38, 55, 64, 67, 74, 77) in the present review were highlighting the programmatic favorable point of COVID vaccine inclusion through routine immunization among children.
Psychosocial point of view
Parents' mental health will always be disrupted in a family where parents are immunized but the children are not. Parents will be free of phobia if all children receive the COVID-19 vaccine (100–102). Similarly, the free movement of children will also improve their social interaction and social health among them. In our present review, 16 articles (17, 21, 22, 24, 27, 33, 37, 39, 43, 55, 60–62, 69, 70, 74) were highlighting the mental health issue of the parents and necessity of social interaction among children in favor of childhood vaccination.
Ethical obligation and practical necessity of children's COVID-19 vaccination
Globally, different countries had given emergency approval for the adult COVID vaccination program at a very early stage in the absence of many unanswered questions of efficacy, effectiveness, and so on (e.g., COVID-19 adult Vaccination was rolled out in India with COVAXINE with the report of Phase II trial data which showed good efficacy and safety as emergency approval, similarly COVAXINE children (2–18 years) vaccine has also got approval for emergency use based on Phase I/II trial) (102, 103). Question can be raised why not the same principle can be used for rolling out children's COVID-19 vaccination, rather than waiting for more evidence on vaccine effectiveness in the reduction of disease transmission or severity among children. It is a social and ethical responsibility and practical necessity to immunize children against COVID-19 (60, 61, 64, 76). Two articles (17, 61) were highlighting the ethical obligations and social responsibility of childhood COVID vaccination in the present review.
Other than these major factors, few of the articles have highlighted some other relevant favorable factors related to childhood COVID vaccination: Indirect harm of lockdown (16, 57, 61, 73), indirect benefit of vaccination (27, 42, 57, 76), reduction in disruption of normal healthcare services (21, 27, 31, 43, 54, 57, 76) protection for high-risk group of children (5, 17, 28, 41, 51, 55, 60, 65, 70), benefit in international travel (16), less children become orphan or malnutrated (72), and decrease requirement of mask or other personal protective equipments (74).
Factors with uncertainty
Protection against PIMS-TS or MIS-C
Multisystem inflammatory syndrome (MIS-C) or Pediatric Inflammatory multisystem syndrome, temporally associated with COVID-19 (PIMS-TS) is one of the life-threatening complications of COVID-19 (104). There is still a lack of substantial evidence about the pathogenesis of the condition that whether it is a complication of the natural process of COVID infection among children or as a result of antigen-antibody reaction following COVID vaccination (16). The USA had reported more than 2,300 cases of MIS-C among children (5–11 years) since the inception of the pandemic. A few isolated cases of MIS-C have been reported in a study conducted in the USA among adolescents following COVID-19 vaccination but most of them were not life-threatening (105, 106). In the present review, 16 articles were discussing the role of MIS-C and COVID-19 whereas 6 articles (23, 34, 58, 66, 70, 74) were favoring its protective role and 10 articles (16, 17, 19, 24, 39, 43, 56, 57, 65, 76) were uncertain about it.
Defense against long COVID-19
If symptoms of COVID-19 in children persist for 4 to 16 weeks or longer, then it is called long COVID-19 (97). Several studies report a prevalence of long COVID-19 ranging from 1.2 to 66%. But most of the studies might have the limitation of overestimation of the risk. Some studies have evidence about the protective effect of COVID-19 vaccination against long COVID-19 among adults but lack evidence in the case of children. So, vaccination for defense against long COVID-19 is a matter of debate (16, 17, 23, 107–109). In the present study, out of the 64 articles, 7 articles (16, 17, 19, 24, 31, 42, 43) were uncertain about the COVID vaccine's role in preventing long COVID-19 whereas 6 articles (26, 34, 57, 66, 70, 74) were completely favoring it.
Contribution to reducing community or household transmissions
Theory about community or household transmission of coronavirus is ever-evolving. In the early part of the infection, adults and the elderly were the sources of infection, whereas later, children were contributing a major role in transmission. Evidence also suggests that though no vaccine can give complete protection, the vaccinated individual has a lower rate of infection compared to the unvaccinated which establishes the efficacy level of the vaccine. But still, there is no conclusive evidence on COVID-19 vaccine's role in reducing transmission (community or household) (17, 28, 31, 42, 56, 58, 67, 74, 110, 111). About 17 articles (5, 17, 19, 21, 23, 31, 33, 38, 47, 61, 66, 67, 69, 70, 72, 73, 78) in present review were in favor of beneficial role of COVID vaccine in reducing childhood transmission, whereas 23 articles (16, 20, 22, 24, 28–30, 36, 39–43, 45, 52, 56–59, 63, 71, 75, 76) were uncertain or against the role of children in COVID transmission or beneficial role of COVID vaccine.
Factors against
Children have milder COVID-19 infection
Studies suggest that children are less frequently infected by the COVID-19 virus and there are multiple factors/theories behind it. Frequent viral infections with other types of coronaviruses in the past, fewer ACE receptors or high activity of the ACE enzyme, well-developed thymus gland that produce very good T-cell and memory cell immunity, lower prevalence of comorbidity, high level of primary immunity by childhood vaccination, or cross-protection with BCG vaccination may be some of the factors behind it (1, 100, 112–115). In the present review, 27 articles (5, 16, 26–30, 35–37, 39, 41, 43, 45, 47, 52, 53, 55–59, 62, 65, 70, 72, 75) were highlighting the mild form of COVID-19 infection among children which prevents it from universal childhood COVID vaccination, whereas only 3 articles (42, 48, 64) were against it. However, Pulmonary embolism, myocarditis and cardiomyopathy, venous thromboembolism, acute or unspecific renal failure, Coagulation or hemorrhagic disorders, cardiac dysrhythmia, encephalopathy, and febrile seizures are some of the notable systemic complications of COVID among children (116–118).
Long-term safety unknown
There is a scarcity of clinical trials among children globally regarding the long-term as well as midterm adverse effects, whereas any kind of adverse event can produce a lifelong impact on children (23, 30, 31, 41, 64). mRNA COVID vaccine can cause genetic changes and anaphylaxis, and damage to vascular endothelial cells can exuberate lung and cardiovascular injury also. There is the possibility of myocardial fibrosis, cardiac dysfunction, and so on, following COVID-19 vaccination (16). In the present review, 4 articles (16, 42, 52, 59) was raising unknown long-term safety of COVID vaccine among children as a major negative factor for childhood COVID-19 vaccination whereas 3 articles (17, 24, 40) were uncertain about it.
A large population already exposed/immune
Globally, most countries have done complete/partial COVID vaccination of a major portion of the adult or elderly population. Seroprevalence studies also highlight that a major proportion of the population in different countries shows a high level of exposure to coronavirus (e.g., ICMR study of servo-prevalence in India during June–July 2021 was 67.6% and among them, 57.2% were 6–9 years age group, 68% in Estonia in mid-June 2021, and 59% among the unvaccinated in Poland in May 2021) (42, 119). So, if a large portion of the population has been already exposed/immunized or developed natural immunity, then there is less chance of infection, transmission, and severity of COVID among children. In the present review, two articles (42, 58) were highlighting it as a major negative factor for childhood COVID vaccination.
Duration of protection
A report by CDC has highlighted that immunological protection of the COVID vaccine declines 6–8 months following vaccination and different studies have also raised concern about the long effectiveness of the COVID vaccine (120). Since there is uncertainty about the long-term protective effect of the COVID-19 vaccine, exposing the children will not be a wise decision. Three articles (28, 42, 68) in this present review have shown negative concern about the duration of protection following COVID vaccination among children, whereas two were uncertain about it (48, 58).
Breakthrough infection
As the number of COVID immunized populations is increasing globally, the number of cases of reinfection or breakthrough infection also will raise because no vaccine can give complete protection. A report from Johns Hopkins has highlighted that 1 in 5,000 was the rate of reinfection/breakthrough infection in Washington state (17 January to 21 August 2021) among the fully vaccinated population. In that study, some areas had a breakthrough infection rate of 1 in 100 (121). Moreover, universal COVID-19 vaccinations for children will be a threat, as a newer variant of COVID-19 increases the chances of more breakthrough infections. In the present review, only two articles (42, 48) have raised concern about the relation between breakthrough infection and COVID vaccination among children.
Limited vaccine supply
One of the critical factors in deciding on universal COVID vaccination is the free supply and availability of the vaccine which is mainly lacking in low- and middle-income countries. Because of this scarcity of vaccines, 1 in 100 in low-income countries and 1 in 10 in lower-middle-income countries have achieved full vaccination status, whereas it is 1 in 2 in high-income countries (122). Therefore, constrain of the COVID-19 vaccine have forced many countries globally to follow a stepwise approach to COVID vaccination considering the risk factors (e.g., disease incidence, comorbidity, mortality, etc.) (30). Five articles (16, 29, 30, 49, 66) have considered limited vaccine supply as one of the important hindrances to universal COVID vaccination for children in the present review.
Impact on routine immunization
The COVID-19 pandemic has caused a huge disruption of the healthcare delivery services since the beginning of the pandemic and COVID vaccination also caused extra stress on the already overburdened healthcare system which lacks sufficient manpower, resources, and logistics, especially for lower economic countries (46, 55, 63, 71, 123). It has been reflected by a report of UNICEF that in 2020 number of missed doses of routine vaccines was the highest globally since 2009 (123). Condition is much worse in developing countries [e.g., In India, BCG and Pentavalent missed doses were 1 lakh and 2 lakh, respectively, in March 2020 (124), and in Pakistan, those who missed a dose of measles and polio were 40 and 50 million, respectively, during the same period] (125). Five articles (16, 17, 46, 63, 71) in the present review have shown the disruption of routine immunization or other health services as one of the negative factors/hindrances for universal childhood COVID vaccination.
Direct cost
Children's hospital admission and treatment costs for COVID-19 will be lower because hospitalization rates are so low, but their universal COVID vaccine will come at a significant direct cost. About three articles have highlighted it as a major negative factor for COVID vaccination among children (16, 20, 33).
Cost-benefit analysis
If we analyze direct cost vs. vaccine cost, then vaccine cost is cheaper than direct cost, for example, hospital admission and treatment cost, similarly if we consider the total cost (direct and indirect cost) vs. vaccine cost, then vaccine cost is much cheaper than the total cost that includes hospital admission and treatment cost, physical and mental health problems of children, mental health problems of parents, and so on (126).
A combined report of the World Bank, UNESCO, and UNICEF (December 2021) highlighted that the disruption of education among students will cause a $17 trillion loss of lifetime earnings, whereas a revised report has shown that the impact will be much higher (127). Therefore, if we talk about cost, then it is beneficial to vaccinate children against COVID-19. Vaccine cost is very less but the overall cost that will be paid in comparison to vaccination is much higher. In this review, one article (16) was highlighting a positive cost-benefit ratio for childhood vaccination, whereas four articles' (20, 28, 30, 31) opinions were vice-versa probably considering the caseload among children which will impact the cost-effectiveness of the program.
Ethical issues and concerns
Several ethical factors or concerns related to childhood COVID-19 vaccination have been highlighted by the articles included in the present review. A total of 17 articles were raising the direct ethical problem of minor or adolescent vaccination (17, 18, 20, 35, 43, 44, 47, 49, 51, 52, 57, 59, 62, 67, 69, 71, 75), whereas 3 articles (25, 65, 74) were either against it or uncertain. The difficult process of consent was highlighted by two articles (19, 35), violation of four principles of ethics was highlighted by one article (60), and the ethical problem of “off level vaccine use” (44), the legal problem of childhood vaccination (51), ethical problems of mandatory COVID vaccination (59), ethical problems of vaccine trail among minor (67), and ethical concern about risk-benefit ratio (71) were highlighted in one article each.
Vaccine hesitancy
According to WHO, vaccine hesitancy is among top 10 threats to global health. Vaccine hesitancy is a universal phenomenon. For children, especially with the new vaccine, it is very difficult to assure and obtain consent from parents. Sometimes one parent may agree, but others not. Parents are worried about side effects, the necessity of vaccination for their children, and the safety and efficacy of vaccines. Since only few vaccines are approved by the WHO while others are in the trial phase, on ethical grounds also, it is very difficult to vaccinate children against COVID-19 (19, 25, 35, 40, 44, 47, 49, 51–53, 59, 62, 68). In total, 11 articles (17, 18, 25, 27, 35, 36, 47, 48, 53, 67, 77) were highlighting the problem of vaccine hesitancy in this review.
Problems that need more concern as compared to COVID-19 vaccination
Developing and underdeveloped countries have the double burden of different communicable (e.g., malaria, tuberculosis, ARI, etc.) as well as non-communicable diseases (e.g., malnutrition) where the effect of these conditions may be more devastating than COVID. If a large number of resources (money, manpower) have been utilized for universal COVID vaccination, then it raises a serious question on the principle of equity and justice (128). However, only one article (71) was addressing this important issue of judicial distribution of scarce resources.
Similarly, an epidemiological shift in the age group following childhood vaccination (28, 42), vaccine inequality (24, 36, 55, 67), gender-based inequality in COVID vaccination (50), higher case fatality and low infection rate among children in future (28), high rates of adult vaccination can reduce the requirement of childhood vaccination (70, 71), failure of compulsory vaccination law in past (49), and socio-economic status based inequility (42) are some of the minor factors against childhood COVID vaccination highlighted by a few of the articles.
Age-related difference in the immune response to COVID-19 infection between children and adult
Moreover, a weak “Immunosenescence,” a mechanism developed from “inflamm-aging,” is associated with an age-related reduction in innate and adaptive immune response which leads to a reduced ability to fight with novel COVID-19 virus (129). Children have a high level of innate immunity, adaptive immunity, heterogeneous immunity, and off-target effect of vaccine which in turn develop a difference in the age-related immune response to COVID-19 infection (130).
Age-related difference in the immune response to COVID vaccine between children and adult
Similar to age-related decline in immune response following COVID infection, vaccine-induced immunity also followed the same trend and it had been found in the case of earlier vaccines for Hepatitis B, pneumococcal vaccine, and influenza (131). BioNTech/Pfizer BNT162b2 also found an inverse relationship between age- and vaccine-induced immunity (132). Moreover, Children's immune response varies significantly with age. The level of antigen, as well as dose, can vary for infants and toddlers compared to school-age children or adolescents. It also holds for booster dose which requires more clinical evidence (76).
Conclusion
Regardless of whether children should receive the COVID-19 vaccine, many nations have already started immunizing kids in stages. Although there is a lack of detailed information regarding hospitalization, severity, and child COVID mortality, little study has been done on the COVID-19 vaccine's safety and long-term efficacy in children. The vaccination studies for COVID-19 aim to promote immunity and offer individual disease protection. Only a selected few COVID-19 vaccines have received emergency approval for use in children's vaccination, but they still need to demonstrate long-term efficacy in stopping or reducing coronavirus transmission. The role of COVID-19 vaccines in the prevention of disease or suppression of the severity of the disease is a question of debate.
Several criteria, including the role of children in transmission, vaccine safety, efficacy, and length of protection, will determine whether all children will eventually receive vaccinations. Most significantly, it will rely on whether such vaccines stop the spread of the disease, resulting in herd immunity for the community. Therefore, before being given to children, each vaccination should be properly evaluated and shown to be safe. The effectiveness of vaccination in preventing transmission has not yet been studied extensively (63). However, recently a study conducted in Singapore has highlighted the role of the BNT162b2 Vaccine in preventing the transmission of COVID-19 among children (133).
Nevertheless, the beneficial effect (e.g., good efficacy, reduced adverse events, safety, etc.) demonstrated by a few of the clinical trials on childhood COVID vaccination should be interpreted cautiously because of many methodological limitations (e.g., limited sample size) which might have drastically changed when large-scale mass children vaccination starts.
The present systematic review has found that there was a diverse opinion among the experts on the matter of childhood COVID vaccination. In this regard, two-fifth of the article's major or prominent tone/theme was in favor of COVID vaccination among children, whereas three-fifth of the tone of the article was against, uncertain, or inconclusive. Among all the factors discussed/highlighted by the different authors, major factors which were mostly in favor of childhood COVID vaccination was the increasing rate of disease burden, school reopening, and defense against COVID infection. Similarly, major factors against childhood vaccination highlighted by the majority of articles were the mild nature of infection among children, ethical concerns and legal problems regarding the consent of minors' vaccination, and vaccine hesitancy among parents for childhood vaccination. Whereas, the vaccine's role in the reduction of community transmission, the protective role against PMIS/MIS-C, and the defense against long COVID was a major factor of uncertainty among them.
According to WHO, when creating their COVID-19 immunization policies and programs, nations should take into account the individual and population advantages of immunizing children in their unique epidemiological and socioeconomic environment.
Therefore, before moving further with COVID-19 immunization for children, a careful approach will be necessary due to the difference in disease progression rate among various age clusters.
Strengths
The present review article may be probably one of the few studies that have tried to generate evidence using a systematic review format based on the opinion of different experts/reviewers on the topic of childhood COVID-19 vaccination considering all the possible positive and negative factors. The finding may be helpful for decision/policymakers to develop policy on evidence-based research for COVID vaccination among children. It will also encourage the generation of new research ideas/horizons for addressing the uncertain factors related to childhood COVID vaccination in the future.
Limitations
All the articles included in the present systematic review were either review articles or opinions or viewpoints and so on. There is not a single original article that has covered all the possible factors for consideration in childhood COVID-19 vaccination. Authors have not tried to identify the interrater agreement between them which can be a potential source of bias in the case of theme generation of an article. Several articles were excluded from the review because of the unavailability of full text which might have influenced the analysis and conclusion. Since all the articles were review articles or expert opinions, the assessment of the quality of the included articles was not possible using any standard systematic review assessment tool.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
SP conceptualized the study, data analysis, manuscript writing, and editing. CM contributed in the data extraction process, reviewing of articles, and manuscript editing. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Aygun D, Onal P, Apaydin G, Çokugraş H. Coronavirus infections in childhood and vaccine studies. Turk Arch Pediatr. (2021) 56:10–14. doi: 10.5152/TurkArchPediatr.2020.20255
2. Sidiq KR, Sabir DK, Ali SM, Kodzius R. Does early childhood vaccination protect against COVID-19? Front Mol Biosci. (2020) 7:120. doi: 10.3389/fmolb.2020.00120
3. WHO, Coronavirus (COVID-19) dashboard. Who.int. Available online at: https://COVID19.who.int/ (accessed August 02, 2021).
4. Tracking SARS-CoV-2 variants. Who.int. Available online at: https://www.who.int/activities/tracking-SARS-CoV-2-variants (accessed January 04, 2022).
5. Wong BLH, Ramsay ME, Ladhani SN. Should children be vaccinated against COVID-19 now? Arch Dis Child. (2021) 106:1147–8. doi: 10.1136/archdischild-2020-321225
6. Interim statement on COVID-19 vaccination for children. Who.int. Available online at: https://www.who.int/news/item/11–08-2022-interim-statement-on-COVID-19-vaccination-for-children (accessed September 05, 2022).
7. Stay Up to Date with COVID-19 Vaccines Including Boosters. Centre for Disease Control and Prevention (CDC). Available online at: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/stay-up-to-date.html?CDC_AA_refVal=https%3A%2F%2F (accessed September 04, 2022).
8. Why kids in low-income countries could face a higher risk of dying of COVID-19. GAVI. Available online at: https://www.gavi.org/vaccineswork/why-kids-low-income-countries-could-face-higher-risk-dying-COVID-19 (accessed September 03, 2022).
9. COVID-19 vaccines advice. Who.int. Available online at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/COVID-19-vaccines/advice (accessed February 03, 2022).
10. TIMESOFINDIA.COM. Coronavirus: List of COVID-19 vaccines available for kids around the world. Times of India. (2022). Available online at: https://timesofindia.indiatimes.com/life-style/health-fitness/health-news/coronavirus-list-of-COVID-19-vaccines-available-for-kids-around-the-world/photostory/88713112.cms (accessed January 19, 2022).
11. Chughtai A. Infographic: Vaccinating children against COVID. Al Jazeera (2021). Available online at: https://www.aljazeera.com/news/2021/11/24/infographic-which-countries-are-vaccinating-children (accessed February 02, 2022).
12. Reuters. Which countries are vaccinating children against COVID-19? Deccan Herald (2021). Available online at: https://www.deccanherald.com/international/world-news-politics/which-countries-are-vaccinating-children-against-COVID-19–1047030.html (accessed February 02, 2022).
13. Live. Countries already vaccinating children against COVID-19. Full list here. mint (2021). Available online at: https://www.livemint.com/news/india/list-of-countries-already-vaccinating-children-against-COVID-11640511929281.html (accessed February 02, 2022).
14. Reuters.com. Available online at: https://www.reuters.com/business/healthcare-pharmaceuticals/countries-vaccinating-children-against-COVID-19–2021-06–29/ (accessed February 03, 2022).
15. Reuters. Explained: A list of countries that are vaccinating children against COVID-19. The Indian Express (2021). Available online at: https://indianexpress.com/article/explained/global-COVID-vaccination-drive-for-children-7340799/ (accessed February 04, 2022).
16. Zimmermann P, Pittet LF, Finn A, Pollard AJ, Curtis N. Should children be vaccinated against COVID-19? Arch Dis Child. (2021) 107:e1. doi: 10.1136/archdischild-2021-323040
17. Rudan I, Adeloye D, Katikireddi V, Murray J, Simpson C, Shah SA, et al. The COVID-19 pandemic in children and young people during 2020–2021: a complex discussion on vaccination. J Glob Health. (2021) 11:01011. doi: 10.7189/jogh.11.01011
18. Wilkinson D, McBride AKS. Clinical ethics: consent for vaccination in children. Arch Dis Child. (2022) 107:3–4. doi: 10.1136/archdischild-2021-322981
19. Wallace M, Oliver S. COVID-19 mRNA Vaccines in Adolescents and Young Adults: Benefit-risk Discussion. New York, NY: Centers for Disease Control (2021).
20. Giubilini A, Gupta S, Heneghan C. A focused protection vaccination strategy: why we should not target children with COVID-19 vaccination policies. J Med Ethics. (2021) 47:565–6. doi: 10.1136/medethics-2021-107700
21. Xue F-X, Shen K-L. COVID-19 in children and the importance of COVID-19 vaccination. World J Pediatr. (2021) 17:462–6. doi: 10.1007/s12519-021-00466-5
22. Principi N, Esposito S. Reasons in favour of universal vaccination campaign against COVID-19 in the pediatric population. Ital J Pediatr. (2022) 48:4. doi: 10.1186/s13052-021-01192-4
23. Praticò AD, Ruggieri M. COVID-19 vaccination for children: may be necessary for the full eradication of the disease. Pediatr Res. (2021) 90:1102–3. doi: 10.1038/s41390-021-01643-y
24. Li G, Finn A, Pollard AJ. Should we be vaccinating children against COVID-19 in high-income countries? Expert Rev Vaccines. (2021) 20:1043–6. doi: 10.1080/14760584.2021.1951245
25. Olick RS, Yang YT, Shaw J. Adolescent consent to COVID-19 vaccination: the need for law reform: the need for law reform. Public Health Rep. (2022) 137:163–7. doi: 10.1177/00333549211048784
26. Glikman D, Stein M, Shinwell ES. Vaccinating children and adolescents against severe acute respiratory syndrome coronavirus 2 (COVID 19)-The Israeli experience. Acta Paediatr. (2021) 110:2496–8. doi: 10.1111/apa.15982
27. She J, Liu L, Liu W. Providing children with COVID-19 vaccinations is challenging due to lack of data and wide-ranging parental acceptance. Acta Paediatr. (2022) 111:35–44. doi: 10.1111/apa.16137
28. Lavine JS, Bjornstad O, Antia R. Vaccinating children against SARS-CoV-2. Hard to justify right now for most children in most countries. BMJ. (2021) 373:n1197. doi: 10.1136/bmj.n1197
29. Abi-Jaoude E. Vaccinating children against SARS-CoV-2: maximise uptake among adults while prioritising the most vulnerable. BMJ. (2021) 373:n1533. doi: 10.1136/bmj.n1533
30. Wilkinson D, Finlay I, Pollard AJ, Forsberg L, Skelton A. Should we delay COVID-19 vaccination in children? BMJ. (2021) 374:n1687. doi: 10.1136/bmj.n1687
31. Saxena S, Skirrow H, Wighton K. Should the UK vaccinate children and adolescients against COVID-19? BMJ. (2021) 374. doi: 10.1136/bmj.n1866
32. Tanne JH. COVID-19: cases in children rise sharply in US as doctors call for vaccine approval. BMJ. (2021) 374:n2030. doi: 10.1136/bmj.n2030
33. Marchetti F, Tamburlini G. Other good reasons for COVID-19 vaccination in pre-adolescent and adolescent populations. BMJ. (2021) 374:n2052. doi: 10.1136/bmj.n2052
34. Beer P, Abbeele KV. Inviting adolescents aged 12–17 for COVID-19 vaccination: the need for patience. BMJ. (2021) 374:n2172. doi: 10.1136/bmj.n2172
35. Saxena S, Skirrow H, Bedford H, Wighton K. COVID-19 vaccines for teenagers: conversations and consent. BMJ. (2021) 374:n2312. doi: 10.1136/bmj.n2312
36. Govender K, Nyamaruze P, McKerrow N, et al. COVID-19 vaccines for children and adolescents in Africa: aligning our priorities to situational realities. BMJ Global Health. (2022) 7:e007839. doi: 10.1136/bmjgh-2021-007839
37. Bonati M, Benelli E. Vaccinating adolescents wisely against COVID-19. BMJ Paediatr Open. (2021) 5:e001191. doi: 10.1136/bmjpo-2021-001191
38. Kao CM, Orenstein WA, Anderson EJ. The importance of advancing severe acute respiratory syndrome coronavirus 2 vaccines in children. Clin Infect Dis. (2021) 72:515–8. doi: 10.1093/cid/ciaa712
39. Anderson EJ, Campbell JD, Creech CB, Frenck R, Kamidani S, Munoz FM, et al. Warp speed for Coronavirus disease 2019 (COVID-19) vaccines: why are children stuck in neutral? Clin Infect Dis. (2021) 73:336–40. doi: 10.1093/cid/ciaa1425
40. Liu F, Fu H-D, Mao J-H. Coronavirus disease 2019 vaccine for children in China: when to start? Mandatory or voluntary?: when to start? Mandatory or voluntary? Chin Med J. (2021) 134:3015–6. doi: 10.1097/CM9.0000000000001779
41. Leidford H. Should children get COVID vaccines? What the science says. Nature. (2021) 595–638. doi: 10.1038/d41586-021-01898-9
42. Ioannidis JPA. COVID-19 vaccination in children and university students. Eur J Clin Invest. (2021) 51:e13678. doi: 10.1111/eci.13678
43. Dembiński Ł, Vieira Martins M, Huss G, Grossman Z, Barak S, Magendie C, et al. SARS-CoV-2 vaccination in children and adolescents-a joint statement of the European Academy of Paediatrics and the European Confederation for primary care paediatricians. Front Pediatr. (2021) 9:721257. doi: 10.3389/fped.2021.721257
44. Lanphier E, Fyfe S. Pediatric off-label use of COVID-19 vaccines: ethical and legal considerations. Hastings Cent Rep. (2021) 51:27–32. doi: 10.1002/hast.1296
45. Opel DJ, Diekema DS, Ross LF. Should we mandate a COVID-19 vaccine for children? JAMA Pediatr. (2021) 175:125–6. doi: 10.1001/jamapediatrics.2020.3019
46. Jenssen BP, Fiks AG. COVID-19 and routine childhood vaccinations-identifying gaps and informing solutions. JAMA Pediatr. (2022) 176:21–3. doi: 10.1001/jamapediatrics.2021.4248
47. Morgan L, Schwartz JL, Sisti DA. COVID-19 vaccination of minors without parental consent: respecting emerging autonomy and advancing public health. JAMA Pediatr. (2021) 175:995–6. doi: 10.1001/jamapediatrics.2021.1855
48. Bird PW, Riff R, Folwell A, Holmes CW, Tang JW. Increased incidence of COVID-19 in younger patients (May-July 2021)-An argument for extending vaccination? J Med Virol. (2022) 94:811–3. doi: 10.1002/jmv.27363
49. Burke C. Should universities mandate the COVID-19 vaccine? J Physician Assist Educ. (2021) 32:189–91. doi: 10.1097/JPA.0000000000000376
50. Vora KS, Sundararajan A, Saiyed S, Dhama K, Natesan S. Impact of COVID-19 on women and children and the need for a gendered approach in vaccine development. Hum Vaccin Immunother. (2020) 16:2932–7. doi: 10.1080/21645515.2020.1826249
51. Reiss DR, Caplan AL. Considerations in mandating a new COVID-19 vaccine in the USA for children and adults. J Law Biosci. (2020) 7:lsaa025. doi: 10.1093/jlb/lsaa025
52. de Miguel Beriain I. We should not vaccinate the young to protect the old: a response to Giubilini, Savulescu, and Wilkinson. J Law Biosci. (2021) 8:lsab015. doi: 10.1093/jlb/lsab015
53. Sprengholz P, Betsch C. Zero-sum or worse? Considering detrimental effects of selective mandates on voluntary childhood vaccinations. J Pediatr. (2022) 240:318–9. doi: 10.1016/j.jpeds.2021.08.018
54. Gurdasani D, Drury J, Greenhalgh T, Griffin S, Haque Z, Hyde Z, et al. Mass infection is not an option: we must do more to protect our young. Lancet. (2021) 398:297–8. doi: 10.1016/S0140-6736(21)01589-0
55. Kampmann B, Okomo U. COVID-19 vaccines for children in LMICs: another equity issue. Lancet. (2021) 398:731–2. doi: 10.1016/S0140-6736(21)01748-7
56. Velavan TP, Pollard AJ, Kremsner PG. Herd immunity and vaccination of children for COVID-19. Int J Infect Dis. (2020) 98:14–5. doi: 10.1016/j.ijid.2020.06.065
57. Martinin-Torres F. Pediatric vaccination against COVID-19 and despite COVID-19. Anal Pediatría. (2022) 96:4–7. doi: 10.1016/j.anpede.2021.11.001
58. Zou X, Cao B. COVID-19 vaccines for children younger than 12 years: are we ready? Lancet Infect Dis. (2021) 21:1614–5. doi: 10.1016/S1473-3099(21)00384-4
59. Savulescu J, Giubilini A, Danchin M. Global ethical considerations regarding mandatory vaccination in children. J Pediatr. (2021) 231:10–6. doi: 10.1016/j.jpeds.2021.01.021
60. Brusa M, Barilan YM. Voluntary COVID-19 vaccination of children: a social responsibility. J Med Ethics. (2021) 47:543–6. doi: 10.1136/medethics-2021-107370
61. Klass P, Ratner AJ. Vaccinating children against COVID-19 - the lessons of measles. N Engl J Med. (2021) 384:589–91. doi: 10.1056/NEJMp2034765
62. McGrew S, Taylor HA. Adolescents, parents, and COVID-19 vaccination - who should decide? N Engl J Med. (2022) 386:e2. doi: 10.1056/NEJMp2116771
63. Eberhardt CS, Siegrist C-A. Is there a role for childhood vaccination against COVID-19? Pediatr Allergy Immunol. (2021) 32:9–16. doi: 10.1111/pai.13401
64. Plotkin SA, Levy O. Considering mandatory vaccination of children for COVID-19. Pediatrics. (2021) 147:e2021050531. doi: 10.1542/peds.2021-050531
65. Zhong Y, Lee LY, Tambyah PA, Liew WK, Lee BW. How can we best use COVID-19 vaccines in adolescents? An international perspective. J Adolesc Health. (2021) 69:878–80. doi: 10.1016/j.jadohealth.2021.08.014
66. Tyungu DL, O'Leary ST, Middleman AB. How can we best use COVID-19 vaccines in adolescents? A perspective from the United States. J Adolesc Health. (2021) 69:881–3. doi: 10.1016/j.jadohealth.2021.09.008
67. Zimet GD, Silverman RD, Fortenberry JD. Coronavirus disease 2019 and vaccination of children and adolescents: prospects and challenges. J Pediatr. (2021) 231:254–8. doi: 10.1016/j.jpeds.2020.11.002
68. Mantovani MP, Sanz AC, Huss G, Mestrovic J, Vural M, Pop TL. Viewpoint of the European pediatric societies over severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination in children younger than age 12 years amid return to school and the surging virus variants. J Pediatr. (2021) 239:250–1. doi: 10.1016/j.jpeds.2021.09.013
69. Cauchemez S, Bosetti P, Kiem CT, Mouro V, Consoli A, Fontanet A. Education and mental health: good reasons to vaccinate children. Lancet. (2021) 398:387. doi: 10.1016/S0140-6736(21)01453-7
70. Ladhani SN. Crossing the rubicon: a fine line between waiting and vaccinating adolescents against COVID-19. J Infect. (2021) 83:294–7. doi: 10.1016/j.jinf.2021.07.015
71. Obaro S. COVID-19 herd immunity by immunisation: are children in the herd? Lancet Infect Dis. (2021) 21:758–9. doi: 10.1016/S1473-3099(21)00212-7
72. Rodewald LE, Shen K-L, Yang Y-H, Wong GW-K, Namazova-Baranova L, Rosenwasser LJ, et al. Global Pediatric Pulmonology Alliance (GPPA) proposal for COVID-19 vaccination in children. World J Pediatr. (2021) 17:458–61. doi: 10.1007/s12519-021-00459-4
73. Kobayashi J, Takeuchi R, Shibuya F, Murata Y, Takahashi K. Recommendations for the urgent need to vaccinate school-aged and adolescent children against COVID-19 in the Asia-Pacific region. Trop Med Health. (2021) 49:74. doi: 10.1186/s41182-021-00365-5
74. Schleiss MR, John CC, Permar SR. Children are the key to the Endgame:a case for routine pediatric COVID vaccination. Vaccine. (2021) 39:5333–6. doi: 10.1016/j.vaccine.2021.08.005
75. Serra ME. COVID-19 vaccine for children: the challenge of making a decision. Arch Argent Pediatr. (2021) 119:294–5. doi: 10.5546/aap.2021.eng.294
76. Principi N, Esposito S. Why it is important to develop an effective and safe pediatric COVID-19 vaccine. Vaccines. (2021) 9:127. doi: 10.3390/vaccines9020127
77. Gerber JS, Offit PA. COVID-19 vaccines for children. Science. (2021) 374:913. doi: 10.1126/science.abn2566
78. Zheng Y-J, Wang X-C, Feng L-Z, Xie Z-D, Jiang Y, Lu G, et al. Expert consensus on COVID-19 vaccination in children. World J Pediatr. (2021) 17:449–57. doi: 10.1007/s12519-021-00465-6
79. Deville JG, Song E, Ouellette CP. COVID-19: Clinical manifestations and diagnosis in children. UpToDate. Uptodate.com. Available online at: https://www.uptodate.com/contents/COVID-19-clinical-manifestations-and-diagnosis-in-children (accessed March 04, 2022).
80. Page M, Le. Why omicron isn't more severe in kids despite rise in hospitalizations. New Scientist (2022). Available online at: https://www.newscientist.com/article/2304688-why-omicron-isnt-more-severe-in-kids-despite-rise-in-hospitalisations/ (accessed February 07, 2022).
81. Weise E, Weintraub K. Moderna says its COVID-19 vaccine found to be 100% effective in children 12 to 17 two weeks after second dose. USA today (2021). Available online at: https://www.usatoday.com/story/news/health/2021/05/25/moderna-COVID-19-vaccine-safe-children-study/7422896002/ (accessed February 07, 2022).
82. Vadrevu KM, Reddy S, Jogdand H, Ganneru B, Mirza N, Tripathy VN, et al. Immunogenicity and safety of an inactivated SARS-CoV-2 vaccine (BBV152) in children from 2 to 18 years of age: an open-label, age-de-escalation phase 2/3 study. Lancet Infect Dis. (2022) 22:1303–12. doi: 10.1016/S1473-3099(22)00307-3
83. Walter EB, Talaat KR, Sabharwal C, Gurtman A, Lockhart S, Paulsen GC, et al. Evaluation of the BNT162b2 COVID-19 vaccine in children 5 to 11 years of age. N Engl J Med. (2022) 386:35–46. doi: 10.1056/NEJMoa2116298
84. Xiaoyu W. Sinopharm vaccine approved to treat children, teenagers. China Daily (2021). Available online at: https://www.chinadaily.com.cn/a/202107/20/WS60f60b13a310efa1bd662ea4.html (accessed February 07, 2022).
85. Reardon S. Cuba's bet on home-grown COVID vaccines is paying off. Nature. (2021) 600:15–6. doi: 10.1038/d41586-021-03470-x
86. Priyan V. Sinovac's COVID-19 vaccine elicits robust immune responses in children. Clinical Trials Arena (2021). Available online at: https://www.clinicaltrialsarena.com/news/sinovac-vaccine-immune-children/ (accessed February 07, 2022).
87. Oster ME, Shay DK, Su JR, Gee J, Creech CB, Broder KR, et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from December 2020 to August 2021. JAMA. (2022) 327:331–40. doi: 10.1001/jama.2021.24110
88. Interim statement on COVID-19 vaccination for children adolescents. Who.int. Available online at: https://www.who.int/news/item/24–11-2021-interim-statement-on-COVID-19-vaccination-for-children-and-adolescents (acce4ssed February 07, 2022).
89. Fernandes DM, Oliveira CR, Guerguis S, Eisenberg R, Choi J, Kim M, et al. Severe acute respiratory syndrome Coronavirus 2 clinical syndromes and predictors of disease severity in hospitalized children and youth. J Pediatr. (2021) 230:23–31.e10. doi: 10.1016/j.jpeds.2020.11.016
90. Frenck RW Jr, Klein NP, Kitchin N, Gurtman A, Absalon J, Lockhart S, et al. Safety, immunogenicity, and efficacy of the BNT162b2 COVID-19 vaccine in adolescents. N Engl J Med. (2021) 385:239–50. doi: 10.1056/NEJMoa2107456
91. Gotzinger F, Santiago-García B, Noguera-Julian A, Lanaspa M, Lancella L, Calò Carducci FI, et al. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health. (2020) 4:653–61. doi: 10.1016/S2352-4642(20)30177-2
92. Castagnoli R, Votto M, Licari A, Brambilla I, Bruno R, Perlini S, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: a systematic review. JAMA Pediatr. (2020) 174:882–9. doi: 10.1001/jamapediatrics.2020.1467
93. Coronavirus disease (COVID-19): Schools,. Who.int. Available online at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/question-and-answers-hub/q-a-detail/coronavirus-disease-COVID-19-schools (accessed February 07, 2022).
94. COVID-19 in children the role of school settings in transmission -second update. Europa.eu. (2021). Available online at: https://www.ecdc.europa.eu/sites/default/files/documents/COVID-19-in-children-and-the-role-of-school-settings-in-transmission-second-update.pdf (accessed February 07, 2022).
95. Bajaj V Gadi N Spihlman AP Wu SC Choi CH and Moulton VR. Aging, immunity, and COVID-19: how age influences the host immune response to coronavirus infections? Front Physiol. (2021) 11:571416. doi: 10.3389/fphys.2020.571416
96. COVID vaccine: What parents need to know. Hopkinsmedicine.org (2022). Available online at: https://www.hopkinsmedicine.org/health/conditions-and-diseases/coronavirus/COVID19-vaccine-what-parents-need-to-know (accessed March 04, 2022).
97. Lopez F, Catala M, Prats C, Estrada O, Oliva I, Prat N, et al. A cost-benefit analysis of COVID-19 vaccination in Catalonia. Vaccines. (2021) 10:59. doi: 10.3390/vaccines10010059
98. Learning losses from COVID-19 school closures could impoverish a whole generation. Unesco.org (2021). Available online at: https://en.unesco.org/news/learning-losses-COVID-19-school-closures-could-impoverish-whole-generation (accessed January 27, 2022).
99. Gurdasani D, Bhatt S, Costello A, Denaxas S, Flaxman S, Greenhalgh T, et al. Vaccinating adolescents against SARS-CoV-2 in England: a risk-benefit analysis. J R Soc Med. (2021) 114:513–24. doi: 10.1177/01410768211052589
100. Nikolopoulou GB, Maltezou HC. COVID-19 in children: where do we stand? Arch Med Res. (2022) 53:1–8. doi: 10.1016/j.arcmed.2021.07.002
101. Tagoe ET, Sheikh N, Morton A, Nonvignon J, Sarker AR, Williams L, Megiddo I. COVID-19 vaccination in lower-middle income countries: national stakeholder views on challenges, barriers, and potential solutions. Front Public Health. (2021) 9:709127. doi: 10.3389/fpubh.2021.709127
102. Reinberg S. HealthDay. COVID has most parents nervous about kids vaccines. WebMD (2020). Available online at: https://www.webmd.com/lung/news/20200812/2-in-3-parents-nervous-about-childhood-vaccines-during-pandemic-survey (accessed February 10, 2022).
103. McBride L. Fear of COVID-19 in kids is getting ahead of the data. Atlantic monthly (Boston, Mass: 1993). (2021). Available online at: https://www.theatlantic.com/ideas/archive/2021/08/children-delta-COVID-19-risk-adults-overreact/619728/ (accessed February 10, 2022).
104. Restricted Use of COVAXINTM Under Clinical Trial Mode. Gov.in. Available online at: https://www.mohfw.gov.in/pdf/Version4PDFCOVAXINImplementationPlan11Jan2021.pdf (accessed July 12, 2022).
105. Mordani S. Covaxin gets emergency use approval for kids aged 2–18 years. India Today (2021). Available online at: https://www.indiatoday.in/coronavirus-outbreak/story/covaxin-gets-emergency-approval-kids-2-18-years-1863866–2021-10-12 (accessed February 10, 2022).
106. CDC. COVID-19 Vaccines for Children and Teens. (2022). Available online at: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/children-teens.html?CDC_AA_refVal=https%3A%2F%2F (accessed February 10, 2022).
107. Zimmermann P, Curtis N. Why does the severity of COVID-19 differ with age? Understanding the mechanisms underlying the age gradient in outcome following SARS-CoV-2 infection. Pediatr Infect Dis J. (2022) 41:36–45 doi: 10.1097/INF.0000000000003413
108. Müller L, Andrée M, Moskorz W, Drexler I, Walotka L, Grothmann R, et al. Age-dependent immune response to the biontech/Pfizer BNT162b2 coronavirus disease 2019 vaccination. Clin Infect Dis. (2021) 73:2065–72. doi: 10.1093/cid/ciab381
109. Venkatesan P. Do vaccines protect from long COVID? Lancet Infect Dis. (2022) 22:43–55. doi: 10.1016/S2213-2600(22)00020-0
110. Zimmermann P, Pittet LF, Curtis N. How common is long COVID in children and adolescents? Pediatr Infect Dis J. (2021) 40:e482–7. doi: 10.1097/INF.0000000000003328
111. Zimmermann P, Pittet L F, Curtis N. Long COVID in children and adolescents. BMJ. (2022) 376:o143 doi: 10.1136/bmj.o143
112. Li F, Li YY, Liu MJ, Fang LQ, Dean NE, Wong GWK, et al. Household transmission of SARS-CoV-2 and risk factors for susceptibility and infectivity in Wuhan: a retrospective observational study. Lancet Infect Dis. (2021) 21:617–28. doi: 10.1016/S1473-3099(20)30981-6
113. Franco-Paredes C. Transmissibility of SARS-CoV-2 among fully vaccinated individuals. Lancet Infect Dis. (2022) 22:16. doi: 10.1016/S1473-3099(21)00768-4
114. Lahariya C. COVID-19 vaccine for high-risk kids should be prioritized. The Tribune (2021). Available online at: https://www.tribuneindia.com/news/comment/COVID-19-vaccine-for-kids-should-be-prioritised-325096 (accessed December 14, 2021).
115. COVID-19 Vaccine For Kids? Five Reasons Why Experts Think It's A Bad Idea. Outlook. (2021). Available online at: https://www.outlookindia.com/website/story/india-news-COVID-19-five-reasons-why-experts-think-kids-dont-need-vaccines/401249 (accessed December 14, 2021).
116. Sinaei R, Pezeshki S, Parvaresh S, Sinaei R. Why COVID-19 is less frequent and severe in children: a narrative review. World J Pediatr. (2021) 17:10–20. doi: 10.1007/s12519-020-00392-y
117. Dhochak N, Singhal T, Kabra SK, Lodha R. Pathophysiology of COVID-19: Why children fare better than adults? Indian J Pediatr. (2020) 87:537–46. doi: 10.1007/s12098-020-03322-y
118. Collier DA, Ferreira IATM, Kotagiri P, Datir RP, Lim EY, Touizer E, et al. Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature. (2021) 596:417–22. doi: 10.1038/s41586-021-03739-1
119. Coronavirus disease (COVID-19): Variants of SARS-CoV-2. Who.int. Available online at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/question-and-answers-hub/q-a-detail/coronavirus-disease-%28COVID-19%29-variants-of-SARS-CoV-2?gclid=Cj0KCQjwguGYBhDRARIsAHgRm49vPohYHSOWGSWm1hZa_pPxpk0YR0eyFkDr3jPPxww0v2VA3cqmOUkaApvpEALw_wcB (accessed September 07, 2022).
120. Consolini R, Costagliola G, Spada E, Colombatto P, Orsini A, Bonuccelli A, et al. (2022) Case report: MIS-C with prominent hepatic and pancreatic involvement in a vaccinated adolescent – a critical reasoning. Front Pediatr. (2022) 10:896903. doi: 10.3389/fped.2022.896903
121. 2 of 3 Indians have COVID-19 antibodies: ICMR serosurvey findings explained. The Indian Express (2021). Available online at: https://indianexpress.com/article/explained/explained-icmr-COVID-fourth-serosurvey-findings-7413949/ (accessed December 30, 2021).
122. CDC. Science brief: COVID-19 vaccines and vaccination. Centers for Disease Control and Prevention (2022). Available online at: https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/fully-vaccinated-people.html (accessed March 16, 2022).
123. Breakthrough infections: Coronavirus after vaccination. Hopkinsmedicine.org (2022). Available online at: https://www.hopkinsmedicine.org/health/conditions-and-diseases/coronavirus/breakthrough-infections-coronavirus-after-vaccination (accessed March 16, 2022).
124. Maxman A. The fight to manufacture COVID vaccines in lower-income countries. Nature. (2021) 597:455–7. doi: 10.1038/d41586-021-02383-z
125. COVID-19 pandemic leads to major backsliding on childhood vaccinations, new WHO, UNICEF data shows. Unicef.org. Available online at: https://www.unicef.org/press-releases/COVID-19-pandemic-leads-major-backsliding-childhood-vaccinations-new-who-unicef-data (accessed July 12, 2022).
126. Shet A, Dhaliwal B, Banerjee P, DeLuca A, Carr K, Britto C, et al. Childhood immunisations in India during the COVID-19 pandemic. BMJ Paediatr Open. (2021) 5:e001061. doi: 10.1136/bmjpo-2021-001061
127. Rana MS, Ikram A, Salman M, Usman M, Umair M. Negative impact of the COVID-19 pandemic on routine childhood immunization: experience from Pakistan. Nat Rev Immunol. (2021) 21:689–90. doi: 10.1038/s41577-021-00627-7
128. Citizen across India write to the Prime Minister. Registration Number: PMOPG/E/2021/0607117 (2021).
129. Zambrano LD, Newhams MM, Olson SM, Halasa NB, Price AM, Boom JA, et al. Effectiveness of BNT162b2 (Pfizer-BioNTech) mRNA vaccination against multisystem inflammatory syndrome in children among persons aged 12–18 years—United States, July–December 2021. MMWR Morb Mortal Wkly Rep. (2022) 71:52–58. doi: 10.15585/mmwr.mm7102e1
130. Kompaniyets L, Bull-Otterson L, Boehmer TK, Boehmer T K, Baca S, Alvarez P, et al. Post-COVID-19 symptoms and conditions among children and adolescents - United States, March 1, 2020-January 31, 2022. MMWR Morb Mortal Wkly Rep. (2022) 71:993. doi: 10.15585/mmwr.mm7131a3
131. Liguoro I, Pilotto C, Bonanni M, Ferrari ME, Pusiol A, Nocerino A, et al. SARS-CoV-2 infection in children and newborns: a systematic review. Eur J Pediatr. (2020) 179:1029–46. doi: 10.1007/s00431-020-03684-7
132. Antoon JW, Hall M, Howard LM, Herndon A, Freundlich KL, Grijalva CG, et al. COVID-19 and acute neurologic complications in children. Pediatrics. (2022) 150:e2022058167. doi: 10.1542/peds.2022-058167
Keywords: COVID-19, SARS-CoV-2, children, adverse events, vaccine safety, COVID vaccine, immunization
Citation: Paul S and Mishra CM (2022) Do we need to vaccinate every child against COVID-19: What evidence suggests—A systematic review of opinions. Front. Public Health 10:1002992. doi: 10.3389/fpubh.2022.1002992
Received: 25 July 2022; Accepted: 12 October 2022;
Published: 08 November 2022.
Edited by:
Alessandro Orsini, Pisana University Hospital, ItalyReviewed by:
Giorgio Costagliola, University of Pisa, ItalyMangla Sood, Indira Gandhi Medical College, Shimla, India
Copyright © 2022 Paul and Mishra. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chandra Mauli Mishra, bWF1bGljb29sMjJAZ21haWwuY29t
 Sourabh Paul
Sourabh Paul Chandra Mauli Mishra
Chandra Mauli Mishra