- 1Beijing Key Laboratory of Intraocular Tumor Diagnosis and Treatment, Beijing Ophthalmology and Visual Sciences Key Lab, Medical Artificial Intelligence Research and Verification Key Laboratory of the Ministry of Industry and Information Technology, Beijing Tongren Eye Center, Beijing Tongren Hospital, Capital Medical University, Beijing, China
- 2Beijing Key Laboratory of Ophthalmology and Visual Sciences, Beijing Tongren Eye Center, Beijing Institute of Ophthalmology, Beijing Tongren Hospital, Capital Medical University, Beijing, China
- 3Cardiology Department, Kailuan General Hospital, Tangshan, China
- 4Health Care Center, Kailuan Group, Tangshan, China
- 5Department of Ophthalmology, Fuxing Hospital, Capital Medical University, Beijing, China
- 6Department of Ophthalmology, Beijing Liangxiang Hospital, Capital Medical University, Beijing, China
- 7Department of Ophthalmology, The Second Hospital of Anhui Medical University, Hefei, China
- 8Department of Ophthalmology, Shanxi Provincial People's Hospital, Taiyuan, China
- 9Department of Ophthalmology, Medical Faculty Mannheim of the Ruprecht-Karls-University of Heidelberg, Heidelberg, Germany
This study aimed to investigate the prevalence of age-related macular degeneration (AMD) in patients with diabetes mellitus (DM) and diabetic retinopathy (DR) and analyze whether DR is a risk factor for AMD. This population-based epidemiological study included 14,440 people from the Kailuan Eye Study in 2016, of whom 1,618 were patients with type 2 DM aged over 50 years, and 409 had DM with DR. We analyzed whether there were differences in the prevalence of AMD between DM with DR and DM without DR, and conducted a hierarchical statistical analysis according to different stages of DR. Using variable regression analysis, we explored whether DR constituted a risk factor for AMD. In the DM population, the prevalence of wet AMD in patients with DM with and without DR was 0. 3 and 0.2%, respectively, with no significant difference (P = 0.607). Meanwhile, the prevalence of dry AMD in patients with DM with and without DR was 20.8 and 16.0%, respectively, with a significant difference. In the subgroup analysis of dry AMD, the prevalence of early, middle, and late dry AMD in DM with DR was 14.4, 5.9, and 0.5%, respectively. In DM without DR, the prevalence of early, middle, and late dry AMD was 10.5, 4.8, and 0.7%, respectively (P = 0.031). In the subgroup analysis of DR staging, statistical analysis could not be performed because of the limited number of patients with PDR. In the variable regression analysis of risk factors for dry AMD, after adjusting for age, sex, body mass index, hypertension, and dyslipidemia, DR constituted the risk factor for dry AMD. In conclusion, DM did not constitute a risk factor for AMD, and the prevalence of wet AMD and dry AMD in patients with DM and DR was higher than that in patients with DM without DR (among which dry AMD was statistically significant). Multivariate regression analysis confirmed that DR is an independent risk factor for dry AMD. Reasonable control of DM and slowing down the occurrence and development of DR may effectively reduce the prevalence of AMD in patients with DM.
Introduction
Diabetes mellitus (DM), a metabolic disorder, can affect multiple systems of the body. The eye is an important organ that can be affected by DM, and almost every ocular structure may be involved. Diabetic retinopathy (DR), which seriously affects visual function, is associated with the progression of DR and DM. The prevalence of DR increases with the duration of DM. After 10–15 years from the diagnosis of DM, patients develop DR (1). The prevalence of DM has increased in both developing and developed countries (1).
In China, the prevalence of DM increased from <1% in 1980 to 11.6% in 2013, with 114 million people affected (2). As a major complication of DM, DR is the leading cause of blindness in the working-age population. The duration of DM and glycemic control levels have a major effect on the development of DM complications. The prevalence of DR increases with the aging of the Chinese population. In the adult DM population in northern China, the prevalence of DR is as high as 37.1–43.1%, that is, more than one-third of patients with DM have DR, of which the prevalence of DR threatening vision is 5–6.3%. As an irreversible blinding eye disease, DR is a heavy burden on society and families (3–6).
However, more attention should be paid to age-related macular degeneration (AMD). AMD can be dry (non-exudative) or wet (exudative). The late stages of AMD include geographic atrophy (GA) and wet AMD. Globally, AMD is the leading cause of blindness in people over 50 years of age. Moreover, 8.7% of the worldwide population has AMD, and the projected number of people with the disease is approximately 196 million in 2020 and 288 million in 2040 (7, 8). Epidemiological studies on the prevalence of AMD in northern China show that the prevalence of early AMD is 1.4%-3.0%, and that of late AMD is 0.1–0.2% (9–11). The treatment of advanced AMD is difficult and expensive, placing a heavy economic burden on families and society.
As the country with the largest population in the world, China has begun to enter an aging society. Visual quality is an important aspect of improving the sense of acquisition of quality of life in the elderly. DR and AMD, two important blinding eye diseases, seriously affect the quality of life of patients, and their prevention and treatment are urgently needed. Patients with DM will eventually reach the age group of more than 50 years. What are the results when the two diseases are superimposed? The conclusions drawn from different studies were controversial. Some studies have shown that DM and DR can increase the prevalence of AMD (12–21), whereas some have shown that DM and DR are not related to the onset of AMD (22–27) and that DM or DR might be protective factors against AMD (28–32). The research methods adopted, such as epidemiological research, medical insurance data, case-control, sampling population, age setting, disease classification, and staging standards, the number of cases, and race, may have affected the differences in research conclusions.
This population-based epidemiological study in northern China aimed to explore the relationship between the prevalence of AMD in DR and non-DR populations and provide a scientific basis for the comprehensive prevention and control of the two ocular diseases with a high blindness rate.
Materials and Methods
The Kailuan Eye Study was introduced previously (33–36). This retrospective cross-sectional study included 14,440 people who had undergone ophthalmologic and general examinations from the longitudinal Kailuan Study in 2016.
The body mass index (BMI) was calculated for all study participants. BP was assessed with the participants sitting for at least 5 min. Blood samples were collected under fasting conditions to determine the blood glucose, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglyceride (TC), and total cholesterol (TG) concentrations.
Ophthalmological examinations included measurement of visual acuity, intraocular pressure, and slit-lamp-assisted biomicroscopy of the eye. Using a non-mydriatic fundus camera (CR6-45 NM; Canon, Inc, Osta, Tokyo, Japan), we obtained two 45° fundus photographs centered on the optic nerve head and macula. If the pupil diameter did not allow fundus photography with sufficient photographic quality, the pupil was dilated medically by applying eye drops containing 0.5% tropicamide and 0.5% phenylephrine hydrochloride. Using fundus photographs, DR was assessed in a masked manner without knowledge of other ocular or systemic parameters of the study participants. Both eyes were evaluated.
The diagnosis of DM was based on any of the following three criteria: measurement of the fasting blood glucose concentration of 7.0 mM, a self-reported history of DM, or a history of medication with hypoglycemic agents.
The diagnostic criteria for hypertension were blood pressure of ≥140/90 mmHg, positive history of hypertension, or the use of antihypertensive drugs.
The clinical classification of hyperlipidemia included hypercholesterolemia (TC≥6.2 mmol/L), hyperglycemia (TG≥2.3 mmol/L), mixed hyperlipidemia (TC ≥6.2 mmol /L and TG≥2.3 mmol/L), and low HDL-C levels (<1.0 mmol/L).
The diagnostic criteria for BMI were based on the guidelines for prevention and control of overweight and obesity in Chinese adults as follows: underweight (BMI, <18.5 kg/m2), normal (BMI, 18.5–23.9 kg/m2), overweight (BMI, 24.0–27.9 kg/m2), obese (BMI, ≥28 kg/m2).
The diagnostic criteria for AMD were based on the Beckman Macular Research Classification System. The lesions were assessed within two optic disc diameters of the fovea of either eye. AMD was classified as follows: no apparent aging changes (no drusen and no AMD pigmentary abnormalities), normal aging changes (small drusen of ≤ 63γm and no AMD pigmentary abnormalities), early AMD (medium drusen of > 63 γm, ≤ 125 γm, and no AMD pigmentary abnormalities), intermediate AMD (large drusen of >125 γm or any AMD pigmentary abnormalities), and late AMD (neovascular AMD or any geographic atrophy).
DR grading was performed according to the Early Treatment of Diabetic Retinopathy Study (ETDRS) criteria. DR severity was graded as mild non-proliferative DR (20 ETDRS levels of <43 with at least one microaneurysm), moderate non-proliferative DR (43 ETDRS levels of <53), severe non-proliferative DR (53 ETDRS levels of <61), and proliferative DR (ETDRS level of 61). An experienced and trained ophthalmologist assessed the photographs. In cases of doubt, photographs were reassessed by a panel that included several ophthalmologists.
Statistical analysis was performed using the R software. The results were expressed as mean and standard deviation or as mean and 95% CI. Logistic regression models were used to estimate the odds ratios (ORs) and their 95% CIs for each risk factor for DR. Statistical significance was set at P < 0.05.
Ethics Statement
This study adhered to the tenets of the Declaration of Helsinki. The Medical Ethics Committee of Beijing Tongren Hospital approved the study protocol, and informed consent was obtained from all patients after an explanation of the nature and possible consequences of the study.
Results
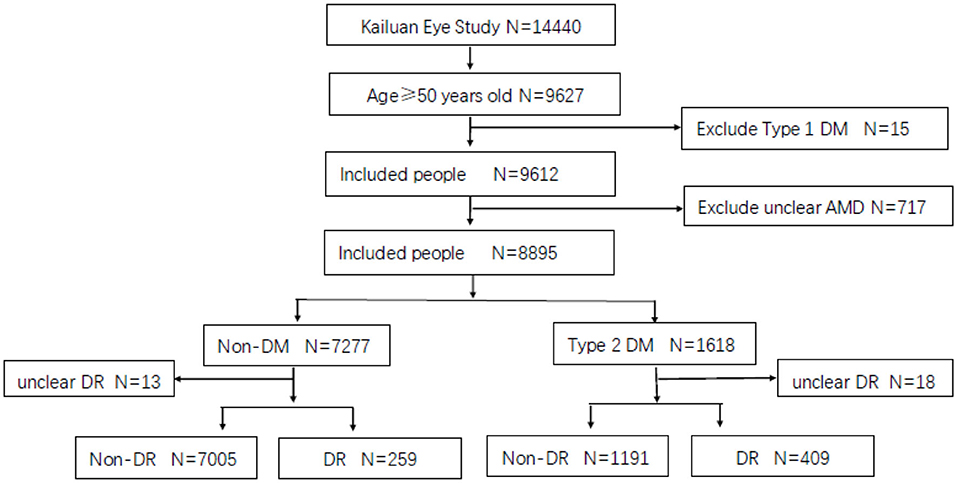
A total of 14,440 participants were enrolled in the Kailuan Eye Study, of whom 9,627 were older than 50 years old. Fifteen were excluded for having type 1 DM and 717 for an unclear diagnosis of AMD. Finally, 8,895 were included in the analysis group. Among them, the number of patients with type 2 DM was 1,618, 409 of whom had DM combined with DR, and 1,191 had DM without DR, excluding 18 people diagnosed with unclear DR. The prevalence of DM aged 50 years was 16.83%. The prevalence of DR was 25.28% in the population with DM aged > 50 years (Figure 1).
Notably, among the 7,277 non-DM participants, 13 were excluded from image unrecognition, and 259 people were found to have DR image characteristics, including microaneurysm, retinal hemorrhage, hard exudation, cotton–wool, and retinal neovascularization, in the non-DM group, with a prevalence of 3.56%. There are two possibilities; one is that in epidemiological field screening, although the random blood glucose level is normal, there may be abnormal glucose tolerance or low insulin function; and another reason is that the current diagnostic criteria for DM are not suitable for this group of people; that is, the blood glucose level for the normal population is already high.
The Prevalence of AMD in Diabetic and Non-DM Participants
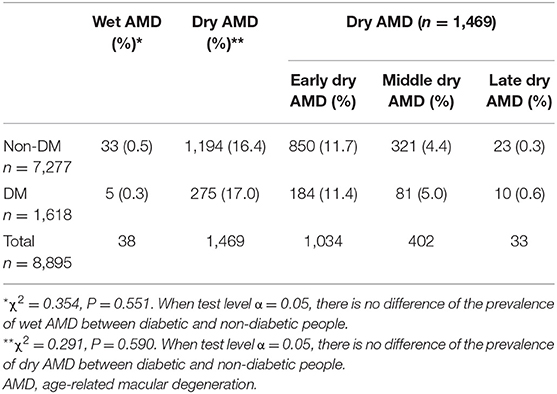
The prevalence of wet and dry AMD was 0.5% and 16.4%, respectively, in the non-DM population (7,277 people). In the patients with type 2 DM (1,618 people), the prevalence of wet AMD and dry AMD was 0.3 and 17%, respectively. There was no significant difference in the prevalence of wet and dry AMD between the diabetic and non-diabetic participants.
In a staging study of dry AMD, the prevalence of dry AMD in the early, middle, and late stages without DM was 11.7, 4.4, and 0.3%, respectively. The prevalence of dry AMD in the early, moderate, and late stages of DM was 11.4, 5, and 0.6%, respectively, with no significant differences (Table 1).
The Prevalence of AMD in DM With DR and DM Without DR
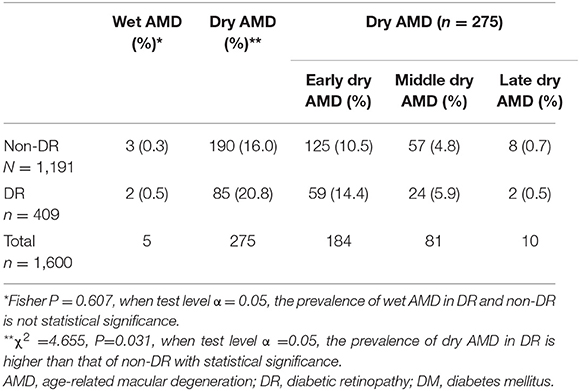
In the population with DM without DR (1191 people), the prevalence of wet AMD and dry AMD was 0.3 and 16.0%, respectively. In the population of patients with DM and DR (409 people), the prevalence of wet and dry AMD was 0.5 and 20.8%, respectively. The Fisher's exact probability method showed that there was no significant difference in the prevalence of wet AMD between the DR and non-DR groups (P = 0.607 and α = 0.05), whereas there was a significant difference in the prevalence of dry AMD between the DR and non-DR groups when α = 0.05 (χ2 = 4.655 and P = 0.031). The prevalence of dry AMD was higher in the DR group than in the non-DR group.
In a staging study of dry AMD in the non-DM population, the prevalence of early, middle, and late dry AMD was 10.5, 4.8, and 0.7%, respectively, in the DM population, the prevalence of early, middle, and late dry AMD was 14.4, 5.9, and 0.5%, respectively, with significant differences (Table 2).
The Prevalence of AMD in Different DR Stages of DM
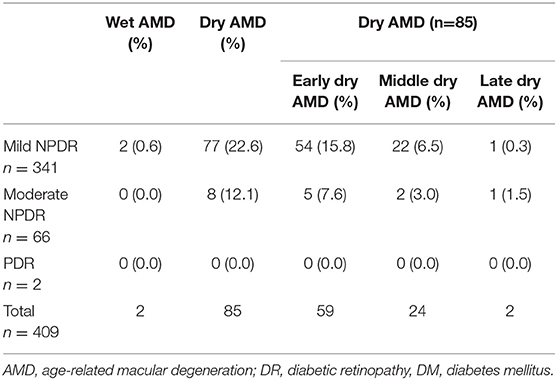
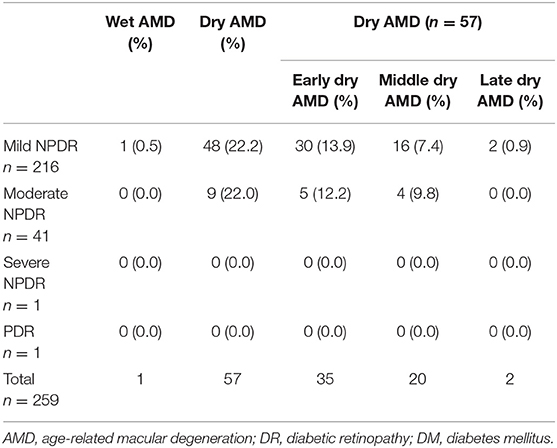
In different stages of DM, because wet AMD did not combine with moderate NPDR (n = 66) and severe NPDR (n = 2), it was impossible to calculate the difference in the prevalence of dry and wet AMD (Table 3).
The Prevalence of AMD in Different “DR” Stages of Non-DM
In non-DM participants, some patients with “DR” were also staged according to DR. Because moderate NPDR (n = 44), severe NPDR (n = 1), and PDR (n = 1) were not combined with wet AMD, it is impossible to count the difference in the prevalence of dry and wet AMD in the different “DR” stages of non-DM (Table 4).
Multivariate Analysis of Influencing Factors of Dry AMD
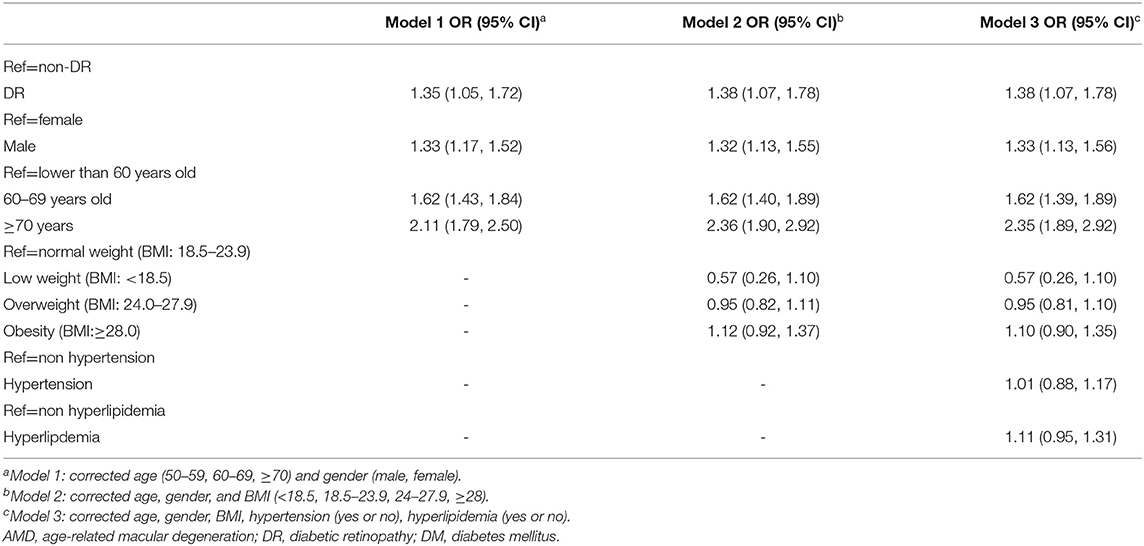
In the analysis of risk factors for dry AMD, the following three models were established: Model 1, corrected age; Model 2, corrected for age, sex, and BMI (< 8.5, 18.5–23.9, 24–27.9, ≥ 28); and Model 3, adjusted for age, sex, BMI, hypertension (yes or no), and dyslipidemia (yes or no).
The results showed that DR is a risk factor for dry AMD, and male sex and age were risk factors for dry AMD. Meanwhile, BMI, hypertension, and hyperlipidemia were not identified as risk factors for dry AMD (Table 5).
Discussion
The global crude prevalence of avoidable vision impairment and blindness in adults aged ≥ 50 years did not change between 2010 and 2019 (37). The leading global cause of blindness in those aged 50 years and older in 2020 was cataracts, followed by glaucoma, under-corrected refractive error, AMD, and DR (37). Although DR was the fifth leading cause of blindness in 2020, it was the only cause of blindness that showed a global increase in age-standardized prevalence between 1990 and 2020. Furthermore, more than 600 million people are projected to live with diabetes by 2040 (38). Because people with DM live increasingly longer with improvements in medical conditions, the number of people with DR and vision impairment is expected to increase rapidly, especially in China. DR management requires a disproportionate amount of social and economic resources. The prevalence of blindness due to AMD has declined by almost 30% from 1990 to 2020. This decrease is probably associated with the widespread clinical introduction of anti-VEGF therapy for wet AMD. However, most patients with AMD have untreatable dry AMD that can progress to GA (37). DR and AMD remain the most important ocular public health problems in China and worldwide.
International Diabetes Federation (IDF) estimated the global population with DM to be 463 million in 2019 and projected it to be 700 million by 2045 (2). As the most common and specific complication of DM, DR is one of the leading causes of preventable blindness in the adult working population (39). A declining trend in DR prevalence has been suggested, particularly in developed countries (40–42). This is the result of systemic control in patients with DM. It has been estimated that 51% of all the patients with blindness due to DR come from the Asia-Pacific region. The prevalence of DR among patients with DM ranges from 10% in India to 43% in Indonesia within the Asia-Pacific (43, 44). The highest number of people with DM is in China (116 million), which reflects the rapid economic growth and urbanization in China with significant lifestyle and dietary changes (39). In China, a large-scale population-based study conducted on 46,239 adults reported a 14-fold increase in DM prevalence over 30 years (0.67% in 1980 vs. 9.7% in 2008) (45).
Globally, AMD is the leading cause of blindness among people over 50 years old (7, 8). Epidemiological studies on the prevalence of AMD in northern China show that the prevalence of early AMD is 1.4–3.0%, and that of late AMD is 0.1–0.2% (9–11). The prevalence of early AMD in the Chinese sample was similar to that in white individuals in the Blue Mountains Eye Study (BMES) and other studies (10).
Today's working-age group will become tomorrow's elderly group. What is the relationship between DM, DR, and AMD in the Chinese population? Some research has been conducted on this topic, but the conclusion is contradictory.
The research methods on the relationship between DM or DR and AMD include longitudinal studies (prospective cohort study and retrospective cohort study), cross-sectional studies (cross-sectional population-based study and observational analysis of randomized clinical trial), case-control studies (retrospective descriptive observational case-control and case-control studies), and systematic reviews and meta-analyses. The study design that we used was cross-sectional population-based epidemiology. Every method has its advantages but also has limitations. In this study, AMD included early, middle, and late stages, and late-stage AMD included GA and wet AMD. DR includes NPDR and PDR, whereas NPDR includes mild, moderate, and severe NPDR. It is difficult to correlate each stage of DR to each stage and type of AMD individually, which requires epidemiological research with a large sample size and long-term longitudinal follow-up.
The relationship between DR and AMD remains inconsistent because of different research methods and sample sizes; that is, some studies have shown that DM and DR can increase the prevalence of AMD (12–21), some have shown that DM and DR are not related to AMD (22–27), and some have shown that DM or DR might protect against AMD (28–32).
Early studies did not find a relationship between DM and AMD (12–21). Later, the Beaver Dam Eye Study (BDES) (17) and BMES (15) found that DM and AMD were correlated, but their conclusions were contradictory. The BDES showed that DM was not associated with early AMD or GA. However, the prevalence of exudative AMD among people with DM was higher (9.4%) than that of those without DM (4.7%) who were older than 75 years. The BMES showed that DM was only associated with GA but not with wet AMD or early AMD. In 2013, Medicare data from 6,621 patients over the age of 69 years with newly diagnosed DM (1995–2005) in the United States showed that NPDR significantly increased the risk of dry AMD and wet AMD, whereas PDR can only increase the risk of wet AMD. The risk of dry AMD and wet AMD did not increase in patients with DM, but not without DR. There was no difference in the risk of wet AMD between PDR and NPDR (12). A retrospective study of the longitudinal health insurance database of Taiwan (1997–2012) showed that patients with DM had a 1.4-fold increase in dry and wet AMD compared to matched patients without DM, although the difference was not significant. In contrast, patients with DM and DR had a 4 and 3.9-fold increased incidence of dry and wet AMD, respectively. The HR for the development of dry AMD and wet AMD was 3.89 and 3.42 for patients with DM and DR and those without DR, respectively (P < 0.001) (14). This is consistent with the conclusion of our study that DM may not be a risk factor for increasing the prevalence of AMD, but DR does.
However, studies have shown that the incidence rate of AMD in the DM population is lower than that in the general population (28–31). Recent research has shown that the prevalence of neovascular AMD of the eyes in people with DR is low (0.04%). Moreover, the prevalence of choroidal neovascularization (CNV) in eyes with DR is low, with a lower prevalence of AMD. Diabetic choroidopathy plays a significant role in CNV formation in eyes with DR (32).
The findings of our study showed that DM had no effect on the prevalence of AMD, but DR could promote the development of dry AMD. The prevalence of wet AMD and dry AMD was 0.5 and 16.4%, respectively, in the non-DM population and 0.3 and 17%, respectively, in the DM population. There was no significant difference in the prevalence of wet and dry AMD between the diabetic and non-diabetic individuals.
The prevalence of wet AMD and dry AMD was 0.3% and 16.0%, respectively, in the population with DM without DR and 0.5 and 20.8%, respectively, in the population with DM and DR. There was no significant difference in the prevalence of wet AMD between the DR and non-DR groups. The prevalence of dry AMD was significantly higher in the DR group than in the non-DR group.
In the Kailuan Eye Study, we also found another finding of “DR” in the non-DM population. Among the 7,277 non-DM subjects, 259 people were found to have “DR” image characteristics, including microaneurysm, retinal hemorrhage, hard exudation, cotton-wool, and retinal neovascularization in no-DM, and the prevalence was 3.56%. There have been reports of retinopathy in persons without DM in the Handan Eye Study (46). The prevalence of retinopathy among participants without DM was 13.6%, and the age and sex-standardized prevalence of retinopathy in the Chinese adult population (aged 30 years) without DM was estimated to be 12.1%. Its association with fasting plasma glucose and BP suggests that early microvascular damage occurs at “high normal” levels of blood.
Why can DR accelerate the onset of AMD? DR may share common pathogenic pathways with AMD. The biological interplay between DR and AMD is complicated and not fully understood (13). First, DM may lead to the accumulation of highly stable advanced glycation end products (AGEs) in multiple tissues, including the retinal pigment epithelium (RPE) cell layers and photoreceptors, which are implicated in the pathogenesis of AMD (47–50). Second, hyperglycemia and dyslipidemia in patients with disturbed homeostasis of the retina by inducing inflammatory responses, which might play a role in AMD (51–53). Third, VEGF plays an important role in both DR and AMD, and both benefit from anti-VEGF treatment (13, 14). Fourth, swept-source optical coherence tomography demonstrated a significant reduction in central macular thickness in PDR compared to controls (54). Understanding diabetic choroidopathy may improve our knowledge of the mechanisms underlying DR and AMD. Finally, structural retinal changes occur with aging. Retinal thickness decreases with age, especially in the inner nuclear layer. The macular blood flow was reduced by an average of 20%. Microglia are less motile and respond more slowly to injury. RPE decreases melanin production and increases lipofuscin accumulation. Müller cells are more susceptible to oxidative stress (55). These anatomical changes may contribute to the pathogenesis of DR combined with AMD in the elderly population.
DR is a major complication of DM. Globally, DR is the leading cause of preventable blindness in adults aged 20–74 years. Because it is avoidable blindness, slowing down the occurrence of DR after the occurrence of DM should be the key point. In most patients, retinopathy develops 10–15 years after the diagnosis of DM (1). The most common modifiable risk factors for DR in the Asia–Pacific region are hyperglycemia, BP, dyslipidemia, and obesity (43). Data from the UK Prospective Diabetes Study (UPDS) and the Diabetes Control and Complications Trial (DCCT) showed that controlling blood glucose (glycated hemoglobin A1c, HbA1c <7%) and BP levels slowed the onset and progression of DR (56–59). In China, a 5-year community-based prospective study on patients with type 2 DM showed DR regression in 24% of patients, which occurred mostly in patients with lower glucose levels (60). In Hong Kong, the regression rate was 13.2% and was also associated with lower HbA1c levels and the absence of albuminuria (61). These data suggest that the onset and progression of DR may be slowed down by reasonably controlling DM to reduce the prevalence of AMD in patients with DM.
The limitation of this study is its cross-sectional design rather than a longitudinal study. Therefore, the prevalence can only be discussed, and its incidence cannot be analyzed. Although our study was a population-based epidemiological study, the sample size was limited. Some diseases require hierarchical research, and some studies cannot be conducted owing to the limited number of cases. For example, in the study of DM combined with different DR stages, there were only two cases of PDR, and neither was combined with dry nor wet AMD; therefore, the study of PDR combined with dry and wet AMD cannot be counted. The stages of AMD include early, middle, and late stages, such as GA and wet AMD. The stages of DR included NPDR and PDR, and NPDR included mild, moderate, and severe NPDR. It is very difficult to correlate each stage of DR to each stage and type of AMD individually; therefore, epidemiological research or meta-analysis with a larger sample size is needed.
In conclusion, to our knowledge, this is the first epidemiological population-based study to discuss the relationship between DR and AMD in the northern mainland China. This study confirmed that the prevalence of dry AMD in DM complicated with DR was increased, and DR was a risk factor for dry AMD.
For patients with DM, how to screen for DR early and how to reduce or delay the occurrence of DR to effectively reduce the prevalence of AMD is an important topic. Future research aims to use new screening techniques combined with the in-depth learning artificial intelligence technique, to screen for early DR (62–65). The precise changes in choroidal thickness and structure of DR and AMD may provide evidence for the common pathogenesis of DR and AMD, although it is complex and unclear and needs to be discovered. An animal model of diabetic choroidopathy and the construction of animal models for DR combined with CNV are needed in the future (66). Whether PRP and anti-VEGF therapy can delay AMD occurrence in patients with DR should be investigated. Larger and longitudinal population-based epidemiological studies are needed to determine the exact relationship between the different stages of DR and AMD.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics Statement
The studies involving human participants were reviewed and approved by Beijing Tongren Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
ZY, ZJinq, and WW contributed to the interpretation of data and drafting of the report. WW contributed to the study design and review. All authors contributed to the article and approved the submitted version.
Conflict of Interest
CS was employed by Kailuan Group.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank Xiuhua Guo and Haiping Zhang for statistical assistance.
References
1. Jampol LM, Glassman AR, Sun J. Evaluation and care of patients with diabetic retinopathy. N Engl J Med. (2020) 382:1629–37. doi: 10.1056/NEJMra1909637
2. Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. (2019) 157:107843. doi: 10.1016/j.diabres.2019.107843
3. Xie XW, Xu L, Jonas JB, Wang YX. Prevalence of diabetic retinopathy among subjects with known diabetes in China: the Beijing Eye Study. Eur J Ophthalmol. (2009) 19:91–9. doi: 10.1177/112067210901900114
4. Wang FH, Liang YB, Zhang F, Wang JJ, Wei WB, Tao QS, et al. Prevalence of diabetic retinopathy in rural China: the Handan Eye Study. Ophthalmology. (2009)116:461–7. doi: 10.1016/j.ophtha.2008.10.003
5. Cao K, Wang B, Friedman DS, Hao J, Zhang Y, Hu A, et al. Handan Eye Study Group. Diabetic retinopathy, visual impairment, and the risk of six-year death: a cohort study of a rural population in China. Ophthalmic Res. (2021) 64:983–90. doi: 10.1159/000512667
6. Wang FH, Liang YB, Peng XY, Wang JJ, Zhang F, Wei WB, et al. Handan Eye Study Group. Risk factors for diabetic retinopathy in a rural Chinese population with type 2 diabetes: the Handan Eye Study. Acta Ophthalmol. (2011) 89:e336–343. doi: 10.1111/j.1755-3768.2010.02062.x
7. Wong WL, Su X, Li X. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. (2014) 2:e106–116. doi: 10.1016/S2214-109X(13)70145-1
8. Jonas JB. Global prevalence of age-related macular degeneration. Lancet Glob Health. (2014) 2:e65–66. doi: 10.1016/S2214-109X(13)70163-3
9. You QS, Xu L, Yang H, Li YB, Wang S, Wang JD, et al. Five-year incidence of age-related macular degeneration: the Beijing Eye Study. Ophthalmology. (2012) 119:2519–25. doi: 10.1016/j.ophtha.2012.06.043
10. Liang YB, Gao LQ, Peng Y, Shen R, Duan XR, Friedman DS, et al. Prevalence of age-related macular degeneration in a rural Chinese population: the Handan Eye Study. Ophthalmology. (2011) 118:1395–401. doi: 10.1016/j.ophtha.2010.12.030
11. Xu L, Wang S, Li Y, Jonas JB. Retinal vascular abnormalities and prevalence of age-related macular degeneration in adult Chinese: the Beijing Eye Study. Am J Ophthalmol. (2006) 142:688–9. doi: 10.1016/j.ajo.2006.05.028
12. Cousins SW, Lee PP, Sloan FA. Ten-year incidence of age-related macular degeneration according to diabetic retinopathy classification among medicare beneficiaries. Retina. (2013) 33:911–9. doi: 10.1097/IAE.0b013e3182831248
13. Chen X, Rong SS, Xu Q, Tang FY, Liu Y, Gu H, et al. Diabetes mellitus and risk of age-related macular degeneration: a systematic review and meta-analysis. PLoS ONE. (2014) 19:e108196. doi: 10.1371/journal.pone.0108196
14. He MS, Chang FL, Lin HZ, Wu JL, Hsieh TC, Lee YC. The association between diabetes and age-related macular degeneration among the elderly in Taiwan. Diabetes Care. (2018) 41:2202–11. doi: 10.2337/dc18-0707
15. Mitchell P, Wang JJ. Diabetes, fasting blood glucose and age-related maculopathy: the Blue Mountains Eye Study. Aust N Z J Ophthalmol. (1999) 27:197–9. doi: 10.1046/j.1440-1606.1999.00211.x
16. Clemons TE, Milton RC, Klein R, Seddon JM, Ferris FL 3rd. Age-Related Eye Disease Study Research Group Risk factors for the incidence of advanced age-related macular degeneration in the Age-Related Eye Disease Study (AREDS): AREDS report no 19. Ophthalmology. (2005) 112:533–9. doi: 10.1016/j.ophtha.2004.10.047
17. Klein R, Klein BE, Moss SE. Diabetes, hyperglycemia, and age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology. (1992) 99:1527–34. doi: 10.1016/S0161-6420(92)31770-1
18. Leske MC, Wu SY, Hennis A, Nemesure B, Yang L, Hyman L, et al. Barbados Eye Studies Group Nine-year incidence of age-related macular degeneration in the Barbados Eye Studies. Ophthalmology. (2006) 113:29–35. doi: 10.1016/j.ophtha.2005.08.012
19. Topouzis F, Anastasopoulos E, Augood C, Bentham GC, Chakravarthy U, de Jong PT, et al. Association of diabetes with age-related macular degeneration in the EUREYE study. Br J Ophthalmol. (2009) 93:1037–41. doi: 10.1136/bjo.2008.146316
20. Choi JK, Lym YL, Moon JW, Shin HJ, Cho B. Diabetes mellitus and early age-related macular degeneration. Arch Ophthalmol. (2011) 129:196–9. doi: 10.1001/archophthalmol.2010.355
21. Klein R, Deng Y, Klein BE, Hyman L, Seddon J, Frank RN, et al. Cardiovascular disease, its risk factors and treatment,and age-related macular degeneration: Women's Health Initiative Sight Exam ancillary study. Am J Ophthalmol. (2007) 143:473–83. doi: 10.1016/j.ajo.2006.11.058
22. Maltzman BA, Mulvihill MN, Greenbaum A. Senile macular degeneration and risk factors: a case-control study. Ann Ophthalmol. (1979) 11:1197–201.
23. Hyman LG, Lilienfeld AM, Ferris FL 3rd, Fine SL. Senile macular degeneration: a case-control study. Am J Epidemiol. (1983) 118:213–27. doi: 10.1093/oxfordjournals.aje.a113629
24. Blumenkranz MS, Russell SR, Robey MG, Kott-Blumenkranz R, Penneys N. Risk factors in age-related maculopathy complicated by choroidal neovascularization. Ophthalmology. (1986) 93:552–8. doi: 10.1016/S0161-6420(86)33702-3
25. Voutilainen-Kaunisto RM, Teräsvirta ME, Uusitupa MI, Niskanen LK. Age-related macular degeneration in newly diagnosed type 2 diabetic patients and control subjects: a 10-year follow-up on evolution, risk factors, and prognostic significance. Diabetes Care. (2000) 23:1672–8. doi: 10.2337/diacare.23.11.1672
26. Kahn HA, Leibowitz HM, Ganley JP, Kini MM, Colton T, Nickerson RS, et al. The Framingham Eye Study II Association of ophthalmic pathology with single variables previously measured in the Framingham Heart Study. Am J Epidemiol. (1977) 106:33–41. doi: 10.1093/oxfordjournals.aje.a112429
27. Cackett P, Yeo I, Cheung CM, Vithana EN, Wong D, Tay WT, et al. Relationship of smoking and cardiovascular risk factors with polypoidal choroidal vasculopathy and age-related macular degeneration in Chinese persons. Ophthalmology. (2011) 118:846–52. doi: 10.1016/j.ophtha.2010.09.026
28. Borrone R, Saravia M, Bar D. Age related maculopathy and diabetes. Eur J Ophthalmol. (2008) 18:949–54. doi: 10.1177/112067210801800615
29. Proctor B, Ambati J. Age-related macular degeneration and diabetic retinopathy: is diabetic retinopathy protective against ARMD? ARVO. (2007) 48:2149.
30. Zylbermann R, Landau D, Rozenman Y, Abrahami S, Pollack A. Exudative age related macular degeneration in patients with diabetic retinopathy and its relation to retinal laser photocoagulation. Eye. (1997) 11(Pt 3):872–5. doi: 10.1038/eye.1997.224
31. Chakravarthy U, Wong TY, Fletcher A, Piault E, Evans C, Zlateva G, et al. Clinical risk factors for age-related macular degeneration: a systematic review and meta-analysis. BMC Ophthalmol. (2010) 10:31. doi: 10.1186/1471-2415-10-31
32. Singh SR, Parameswarappa DC, Govindahari V, Lupidi M, Chhablani J. Clinical and angiographic characterization of choroidal neovascularization in diabetic retinopathy. Eur J Ophthalmol. (2021) 31:584–91. doi: 10.1177/1120672120902027
33. Wang Q, Wang YX, Wu SL, Chen SH, Yan YN, Yang MC, et al. Ocular axial length and diabetic retinopathy: the Kailuan Eye Study. Invest Ophthalmol Vis Sci. (2019) 60:3689–95. doi: 10.1167/iovs.19-27531
34. Wang Q, Liu L, Jonas JB, Gao B, Wu SL, Chen SH, et al. Albuminuria and retinal vessel density in diabetes without diabetic retinopathy: the Kailuan Eye Study. Acta Ophthalmol. (2021) 99:669–78. doi: 10.1111/aos.14670
35. Yang MC, Zhu XB, Wang YX, Wu SL, Wang Q, Yan YN, et al. Influencing factors for peripheral and posterior lesions in mild non-proliferative diabetic retinopathy-the Kailuan Eye Study. Int J Ophthalmol. (2020) 13:1467–6. doi: 10.18240/ijo.2020.09.20
36. Zhou W, Yang J, Wang Q, Wang Y, Yan Y, Wu S, et al. Systemic Stressors and retinal microvascular alterations in people without diabetes: the Kailuan Eye Study. Invest Ophthalmol Vis Sci. (2021) 62:20. doi: 10.1167/iovs.62.2.20
37. GBD 2019 Blindness and Vision Impairment Collaborators; Vision Loss Expert Group of the Global Burden of Disease Study. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the Right to Sight: an analysis for the Global Burden of Disease Study. Lancet Glob Health. (2021) 9:144–60.
38. Ogurtsova K, da Rocha Fernandes JD, Huang Y, Linnenkamp U, Guariguata L, Cho NH, et al. IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. (2017) 128: 40–50. doi: 10.1016/j.diabres.2017.03.024
39. Teo ZL, Tham YC, Yu M, Chee ML, Rim TH, Cheung N, et al. Global Prevalence of Diabetic Retinopathy and Projection of Burden through 2045: Systematic Review and Meta-analysis. Ophthalmology. (2021) 128:1580–91. doi: 10.1016/j.ophtha.2021.04.027
40. Claessen H, Kvitkina T, Narres M, Trautner C, Zöllner I, Bertram B, et al. Markedly decreasing incidence of blindness in people with and without diabetes in southern Germany. Diabetes Care. (2018) 41:478–84. doi: 10.2337/dc17-2031
41. Zhang X, Saaddine JB, Chou CF, Cotch MF, Cheng YJ, Geiss LS, et al. Prevalence of diabetic retinopathy in the United States, 2005-2008. JAMA. (2010) 304:649–56. doi: 10.1001/jama.2010.1111
42. Cheung N, Wong TY. Changing trends of blindness: the initial harvest from translational public health and clinical research in ophthalmology. Am J Ophthalmol. (2012) 153:193–5. doi: 10.1016/j.ajo.2011.11.022
43. Chua J, Lim CXY, Wong TY, Sabanayagam C. Diabetic retinopathy in the Asia-Pacific. Asia Pac J Ophthalmol (Phila). (2018) 7:3–16. doi: 10.22608/APO.2017511
44. Rizwan A, Sufyan A, Asghar A, Khan H, Ahmad B, Rabbani MH. Awareness of diabetic retinopathy among diabetic patients. J Pak Med Assoc. (2021) 71:651–5. doi: 10.47391/JPMA.897
45. Yang SH, Dou KF, Song WJ. Prevalence of diabetes among men and women in China. N Engl J Med. (2010) 362:2425–6. doi: 10.1056/NEJMoa0908292
46. Peng XY, Wang FH, Liang YB, Wang JJ, Sun LP, Peng Y, et al. Retinopathy in persons without diabetes: the Handan Eye Study. Ophthalmology. (2010) 117:531–7. doi: 10.1016/j.ophtha.2009.07.045
47. Stitt AW. AGEs and diabetic retinopathy. Invest Ophthalmol Vis Sci. (2010) 51:4867–74. doi: 10.1167/iovs.10-5881
48. Hammes HP, Hoerauf H, Alt A, Schleicher E, Clausen JT, Bretzel RG, et al. N(epsilon) (carboxymethyl)lysin and the AGE receptor RAGE colocalize in age-related macular degeneration. Invest Ophthalmol Vis Sci. (1999) 40:1855–9.
49. Soulis T, Thallas V, Youssef S, Gilbert RE, McWilliam BG, Murray-McIntosh RP, et al. Advanced glycation end products and their receptors co-localise in rat organs susceptible to diabetic microvascular injury. Diabetologia. (1997) 40:619–28. doi: 10.1007/s001250050725
50. Howes KA, Liu Y, Dunaief JL, Milam A, Frederick JM, Marks A, et al. Receptor for advanced glycation end products and age-related macular degeneration. Invest Ophthalmol Vis Sci. (2004) 45:3713–20. doi: 10.1167/iovs.04-0404
51. Zhang W, Liu H, Al-Shabrawey M, Caldwell RW, Caldwell RB. Inflammation and diabetic retinal microvascular complications. J Cardiovasc Dis Res. (2011) 2: 96–103. doi: 10.4103/0975-3583.83035
52. Totan Y, Yagci R, Bardak Y, Ozyurt H, Kendir F, Yilmaz G, et al. Oxidative macromolecular damage in age-related macular degeneration. Curr Eye Res. (2009) 34:1089–93. doi: 10.3109/02713680903353772
53. Venza I, Visalli M, Cucinotta M, Teti D, Venza M. Association between oxidative stress and macromolecular damage in elderly patients with age-related macular degeneration. Aging Clin Exp Res. (2012) 24:21–7. doi: 10.3275/7659
54. Laíns I, Talcott KE, Santos AR, Marques JH, Gil P, Gil J, et al. Choroidal thickness in diabetic retinopathy assessed with swept-source optical coherence tomography. Retina. (2018) 38:173–82. doi: 10.1097/IAE.0000000000001516
55. Leley SP, Ciulla TA, Bhatwadekar AD. Diabetic retinopathy in the aging population: a perspective of pathogenesis and treatment. Clin Interv Aging. (2021) 16:1367–78. doi: 10.2147/CIA.S297494
56. Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy: a systematic review. JAMA. (2007) 298:902–16. doi: 10.1001/jama.298.8.902
57. Diabetes Control and Complications Trial Research Group, Nathan DM, Genuth S, Lachin J. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. (1993) 329:977–86. doi: 10.1056/NEJM199309303291401
58. Matthews DR, Stratton IM, Aldington SJ, Holman RR, Kohner EM. Risks of progression of retinopathy and vision loss related to tight blood pressure control in type 2 diabetes mellitus: UKPDS 69. Arch Ophthalmol. (2004) 122:1631–40. doi: 10.1001/archopht.122.11.1631
59. Estacio RO, Jeffers BW, Gifford N, Schrier RW. Effect of blood pressure control on diabetic microvascular complications in patients with hypertension and type 2 diabetes. Diabetes Care. (2000) 23(Suppl 2): B54–B64.
60. Jin P, Peng J, Zou H, Wang W, Fu J, Shen B, et al. The 5-year onset and regression of diabetic retinopathy in Chinese type 2 diabetes patients. PLoS ONE. (2014) 9:e113359. doi: 10.1371/journal.pone.0113359
61. Tam VH, Lam EP, Chu BC, Tse KK, Fung LM. Incidence and progression of diabetic retinopathy in Hong Kong Chinese with type 2 diabetes mellitus. J Diabetes Complications. (2009) 23:185–93. doi: 10.1016/j.jdiacomp.2008.03.001
62. Vujosevic S, Aldington SJ, Silva P, Hernández C, Scanlon P, Peto T, et al. Screening for diabetic retinopathy: new perspectives and challenges. Lancet Diabetes Endocrinol. (2020) 8:337–47. doi: 10.1016/S2213-8587(19)30411-5
63. Xie Y, Nguyen QD, Hamzah H, Lim G, Bellemo V, Gunasekeran DV, et al. Artificial intelligence for teleophthalmology-based diabetic retinopathy screening in a national programme: an economic analysis modelling study. Lancet Digit Health. (2020) 2:e240–9. doi: 10.1016/S2589-7500(20)30060-1
64. Bora A, Balasubramanian S, Babenko B, Virmani S, Venugopalan S, Mitani A, et al. Predicting the risk of developing diabetic retinopathy using deep learning. Lancet Digit Health. (2021)3:e10–e19. doi: 10.1016/S2589-7500(20)30250-8
65. Bandello F, Cicinelli MV. 19th EURETINA Congress Keynote Lecture: Diabetic Retinopathy Today. Ophthalmologica. (2020) 243:163–71. doi: 10.1159/000506312
Keywords: diabetes mellitus, diabetic retinopathy, age-related macular degeneration, prevalence, epidemiology
Citation: Yongpeng Z, Yaxing W, Jinqiong Z, Qian W, Yanni Y, Xuan Y, Jingyan Y, Wenjia Z, Ping W, Chang S, Ming Y, Yanan L, Jinyuan W, Shouling W, Shuohua C, Haiwei W, Lijian F, Qianqian W, Jingyuan Z, Zihan N, Yuning C, Ying X, Jonas JB and Wenbin W (2022) The Association Between Diabetic Retinopathy and the Prevalence of Age-Related Macular Degeneration—The Kailuan Eye Study. Front. Public Health 10:922289. doi: 10.3389/fpubh.2022.922289
Received: 17 April 2022; Accepted: 10 June 2022;
Published: 18 July 2022.
Edited by:
Khalid Siddiqui, King Saud University, Saudi ArabiaReviewed by:
Hu Bojie, Tianjin Medical University Eye Hospital, ChinaQisheng You, Wayne State University, United States
Copyright © 2022 Yongpeng, Yaxing, Jinqiong, Qian, Yanni, Xuan, Jingyan, Wenjia, Ping, Chang, Ming, Yanan, Jinyuan, Shouling, Shuohua, Haiwei, Lijian, Qianqian, Jingyuan, Zihan, Yuning, Ying, Jonas and Wenbin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Wenbin, d2Vpd2VuYmludHJAMTYzLmNvbQ==
 Zhang Yongpeng
Zhang Yongpeng Wang Yaxing
Wang Yaxing Zhou Jinqiong1
Zhou Jinqiong1 Yang Ming
Yang Ming Luan Yanan
Luan Yanan Wu Shouling
Wu Shouling Chen Shuohua
Chen Shuohua Wang Haiwei
Wang Haiwei Chen Yuning
Chen Yuning Jost B. Jonas
Jost B. Jonas Wei Wenbin
Wei Wenbin