- 1Stanley Ho Centre for Emerging Infectious Diseases, The Chinese University of Hong Kong, Shatin, Hong Kong SAR, China
- 2Special Preventive Programme, Centre for Health Protection, Department of Health, Hong Kong, Hong Kong SAR, China
People living with human immunodeficiency virus (PLHIV) constitute a unique group at higher risk of hepatitis C virus (HCV) co-infection. In light of the diverse profiles of PLHIV, we differentiated between men who have sex with men (MSM) and non-MSM in the characterization of the epidemiologic features of HIV/HCV co-infection. Clinical data of HCV co-infection patients from the HIV specialist clinic in Hong Kong were retrospectively collected in conjunction with their HIV subtypes and HCV genotypes. Logistic regression models were used to identify factors associated with HIV/HCV co-infection in MSM. Survival analysis was performed to compare the time lag between HIV and HCV diagnoses between two groups. Latent class analysis was conducted to describe the features of different classes of co-infections. Four classes of HIV/HCV co-infections were identified: local MSM acquiring HCV after HIV diagnosis, local MSM with HIV/HCV co-diagnoses, local non-MSM, and non-local non-MSM. Accounting for over half of the co-infections, MSM were more likely to be younger, local residents, and associated with HCV genotype 3, compared to genotypes 1 and 6 in non-MSM. Overall, MSM had higher odds of achieving HIV viral suppression and co-diagnosing with a sexually transmitted infection at HCV diagnosis, and having a longer time lag between HIV and HCV diagnoses. Drug injection accounted for a majority of non-MSM HCV infection. There were distinctive epidemiologic differences between MSM and non-MSM co-infected with HIV and HCV, the characteristics of which could inform intervention strategies for achieving HCV micro-elimination.
Introduction
The World Health Organization (WHO) estimated that 1% of the global population were living with hepatitis C virus (HCV) infection (1) and it was estimated that 290,000 deaths were attributed to HCV in a year (2). The HCV burden varied considerably among geographic regions (3). The general population's anti-HCV prevalence was low in Europe (4), but can be up to 13% in Uzbekistan in Central Asia (5). In view of the increasing burden of HCV worldwide, the WHO set out to “eliminate viral hepatitis as a major public health threat by 2030” in 2016 (6).
An understanding of HCV epidemiology is the cornerstone of designing effective public health policies toward the elimination goal. A meta-analysis on global data highlighted that HCV has been prevalent (82%) among people who inject drugs (PWID), and a unique group of men who have sex with men (MSM) living with human immunodeficiency virus (HIV) was identified to be at higher risk of co-infection with HCV (6%), compared to those who were HIV-negative (7). The latter epidemiologic pattern was confirmed in another study that both incidence and prevalence of HCV were higher among MSM living with HIV (8). Over the years, the prevalence of HIV/HCV co-infection among MSM increased while the burden in PWID has become alleviated (9). As the burden of HCV is often concentrated in certain sub-populations (10), the task of elimination could be strategically divided by group for targeted interventions so as to achieve population-wide elimination. Such a divide-and-conquer approach is now widely described as “micro-elimination” (11).
In Hong Kong, as in other places, HIV/HCV co-infections have emerged in MSM in recent years. On the other hand, the HCV prevalence has remained high among PWID (12) despite a low HIV prevalence over the past decades (13). In 2020, the Hong Kong Government has adopted an action plan to implement a hepatitis elimination policy that includes the use of direct-acting antivirals to treat HCV infection for all patients co-infected with HIV, with an aim to achieve micro-elimination (https://www.hepatitis.gov.hk/english/action_plan/intro.html). It is hypothesized that there are multiple sets of profiles of HIV/HCV co-infections in the community. A study has therefore been conducted aiming to differentiate HIV/HCV co-infected patients in Hong Kong into epidemiologic groups, the result of which could support the design of bespoke community-specific micro-elimination strategies.
Materials and Methods
Clinical data were collected from the largest public HIV specialist clinic in Hong Kong which provides care to over half of all HIV patients in the territory. Demographic data fields included gender, ethnicity, residency status, year of birth, and incarceration status. Clinical data fields included death date, HIV route of transmission, HIV diagnosis date, HIV subtype, HCV diagnosis date, HCV genotype, disease stages of HIV and HCV. Longitudinal HIV viral load, CD4 cell count, sexually transmitted infection (STI) diagnoses, and HCV viral load were also retrieved. Data access approval was obtained from the Department of Health (L/M 220/2015 and 140/2017). This study was approved by The Joint Chinese University of Hong Kong—New Territories East Cluster Clinical Research Ethics Committee with waiver of consent (Ref. No. 2015.320).
Univariable and backward Akaike information criterion-based stepwise multivariable binomial logistic regression models were used to differentiate MSM from non-MSM HIV/HCV co-infected patients. The difference in time-to-event between HIV and HCV diagnosis was compared between the two groups with other factors by Cox proportional hazards model. Kaplan-Meier curves were constructed to illustrate the difference in time tag between two diagnoses among groups. Co-infected patients were further characterized by latent class analysis, before distinguishing characteristics associated with each latent class in term of time lag between HIV and HCV diagnoses with univariable binomial logistic regression models and a generalized linear model using one class as reference. All analyses were performed in R.
Results
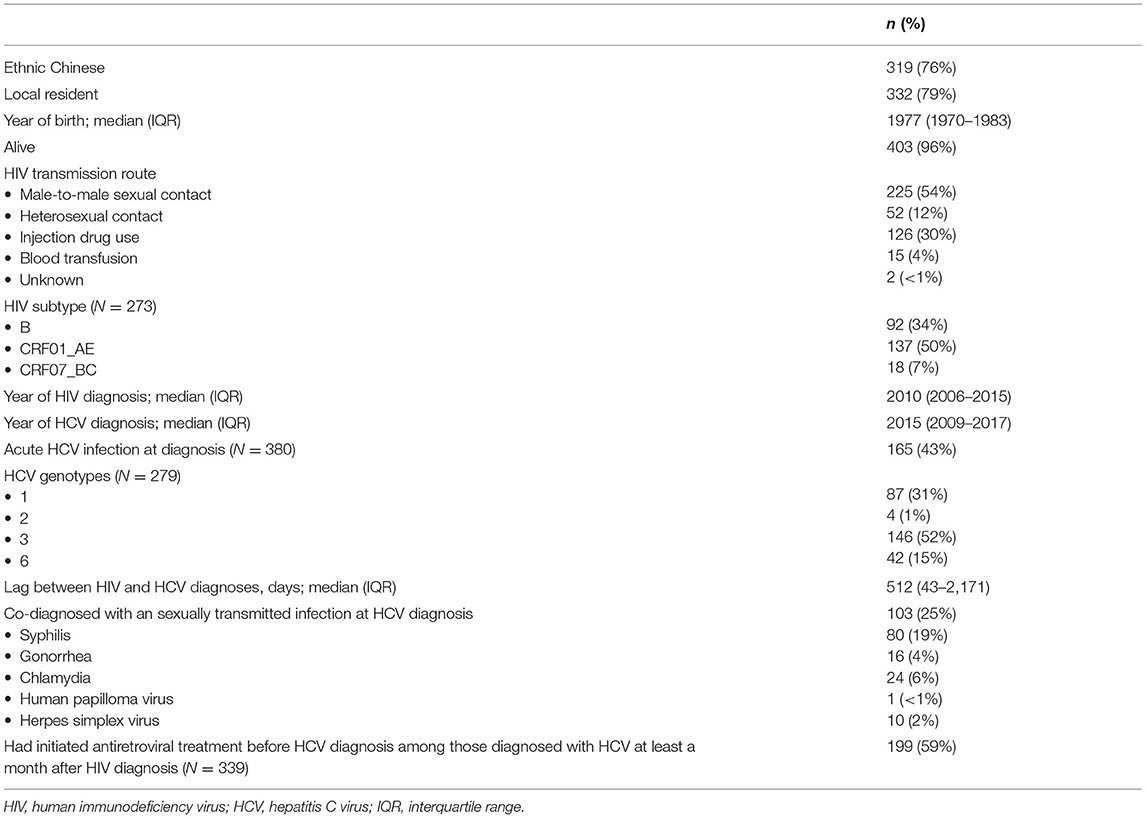
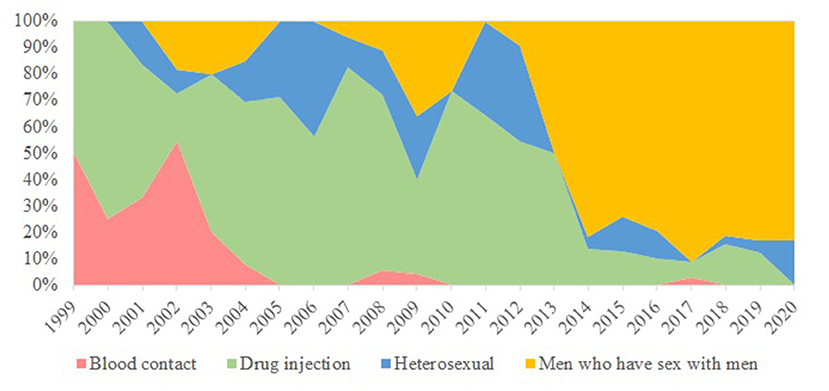
Totally 420 records of HIV/HCV co-infections were identified from the database comprising 5,135 HIV patients, as of February 2021. The earliest year of first HIV and HCV diagnosis was 1985 and 1999, respectively. A large proportion was alive (96%), ethnic Chinese (76%), and local residents (79%) (Table 1). About half (54%) of the HIV/HCV co-infections were diagnosed in MSM. Other transmission routes included injection drug use (30%) and heterosexual contact (12%). The temporal trend is illustrated in Figure 1. Their median year of birth was 1977 [interquartile range (IQR) 1970–1983]. The most prevalent HIV subtypes (N = 273) were CRF01_AE (50%), B (34%), and CRF07_BC (7%). About 43% of the HCV infections were diagnosed as acute infections (N = 380). Of 279 records with available HCV genotypes, genotypes 3 contributed to over half (52%) while the remainders were genotypes 1 (31%), 6 (15%), and 2 (1%). The median time lag between HIV and HCV diagnoses was 512 days (IQR 43–2,171 days).
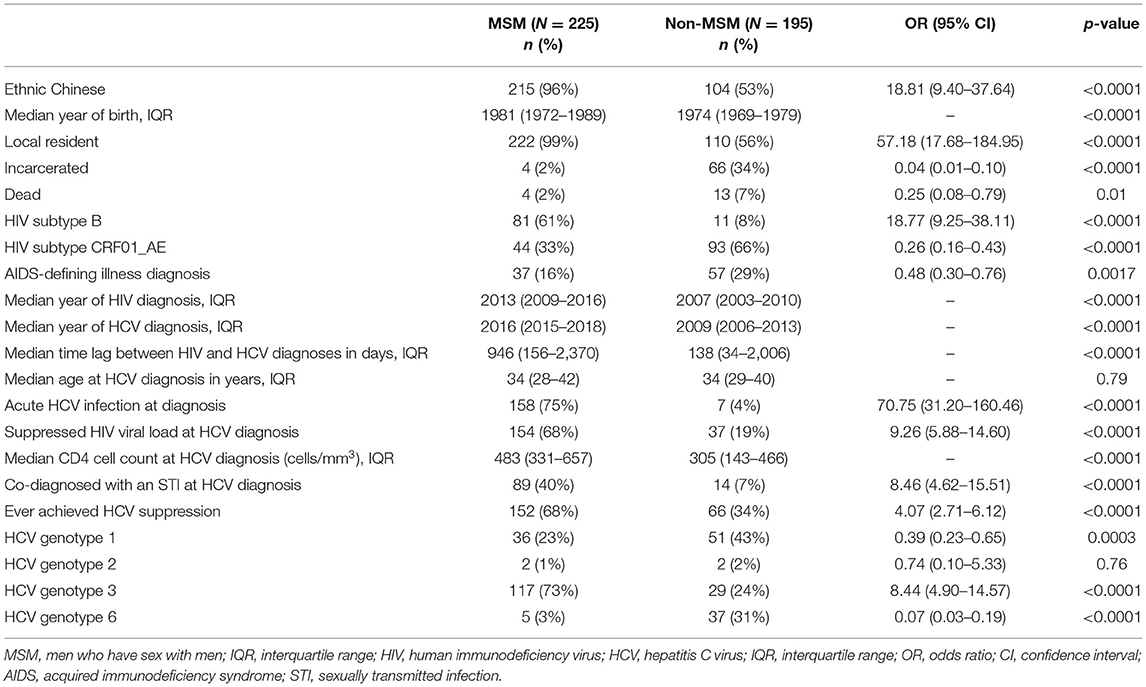
In this cohort, HIV/HCV co-infected MSM were more likely to be ethnic Chinese [odds ratio (OR) 18.81, 95% confidence interval (95% CI) 9.40–37.64, p < 0.0001], younger (p < 0.0001), local residents (OR 57.18, 95% CI 17.68–184.95, p < 0.0001), and less likely to have been incarcerated (OR 0.04, 95% CI 0.01–0.10, p < 0.0001) (Table 2). They were also more likely to be diagnosed with HIV recently (p < 0.0001), and of HIV subtype B (OR 18.77, 95% CI 9.25–38.11, p < 0.0001). MSM had a higher baseline HIV viral load (p = 0.0026), and were less likely to have progressed to AIDS (OR 0.48, 95% CI 0.30–0.76, p = 0.0017). The time lag between HIV and HCV diagnosis was longer among MSM (p < 0.0001) with only 31% having been diagnosed HCV within a year after HIV diagnosis (OR 5.69, 95% CI 3.74–8.66, p < 0.0001). The median lag between HIV and HCV diagnosis in MSM was 2.59 years, while non-MSM had a significantly shorter period of 4.52 months (p < 0.0001). The distribution of the two diagnoses is illustrated in Figure 2. The age of HCV diagnoses was similar between MSM and non-MSM (p = 0.79), but the year of first HCV diagnosis was later in MSM with a median year of 2016 compared with 2009 in non-MSM (p < 0.0001).
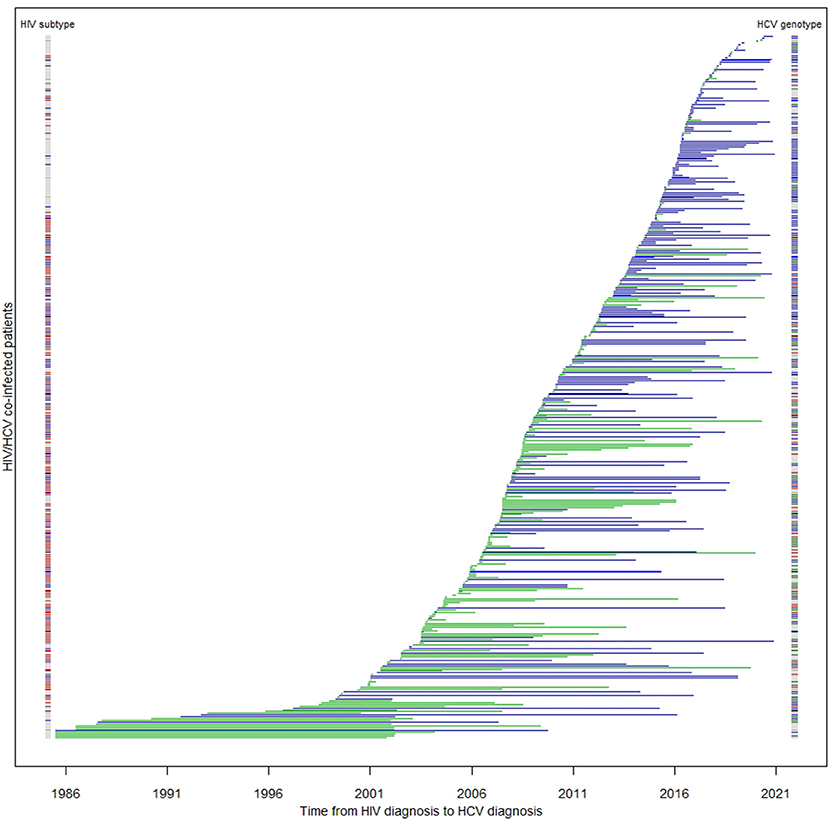
Figure 2. Duration between HIV and HCV diagnoses among co-infected patients (N = 413). Each horizontal line represents a patient's time to HCV serconversion since HIV diagnosis. Blue lines are MSM; green lines are non-MSM. The color band on the left denotes HIV subtype of the corresponding patient: blue: subtype B; red: subtype CRF01_AE; other subtypes: brown; unknown: gray. The color band on the right denotes their HCV genotype: green: 1; brown: 2; blue: 3; red: 6.
MSM had a higher odds to have achieved HIV viral suppression at HCV diagnosis (OR 9.26, 95% CI 5.88–14.60, p < 0.0001), and be co-diagnosed with an STI (OR 8.46, 95% CI 4.62–15.51, p < 0.0001), particularly syphilis (p < 0.0001), gonorrhea (p = 0.0001), and chlamydia (p = 0.0006). MSM were more likely to be of HCV genotype 3 (OR 8.44, 95% CI 4.90–14.57, p < 0.0001), while non-MSM were likely to have genotypes 1 (OR 2.58, 95% CI 1.54–4.34, p = 0.0003), or 6 (OR 13.99, 95% CI 5.29–36.90, p < 0.0001). Overall, the clinical outcome of HCV infection was also different between two groups with MSM more likely to have attained undetectable viral load (OR 4.07, 95% CI 2.71–6.12, p < 0.0001). The difference was significant only for genotypes 1 (p = 0.021) and 3 (p = 0.043), but not 6 (p = 0.18).
Factors associated with HCV co-infection in MSM were examined by applying the multivariable logistic regression model. The significant factors included Chinese ethnicity [adjusted odds ratio (aOR) 3.11, 95% CI 1.03–10.20], local residency status (aOR 6.73, 95% CI 1.86–32.83), and other clinical factors at HCV diagnosis: latter year of HCV diagnosis (p = 0.0007), diagnosis as an acute HCV infection (p < 0.0001), co-diagnosis with another STI (p < 0.0001), and having achieved suppressed HIV viral load (p = 0.035). The probability of HCV infection after HIV diagnosis was evaluated with survival analysis. Being MSM [hazard ratio (HR) 0.76, 95% CI 0.62–0.92, p = 0.0052], having achieved HIV viral suppression at HCV diagnosis (HR 0.43, 95% CI 0.35–0.52, p < 0.0001), earlier year of HCV diagnosis (HR 0.98, 95% CI 0.96–0.999, p = 0.034), and diagnosis during acute stage HCV infection (HR 0.75, 95% CI 0.61–0.92, p = 0.0051) had lower hazards, hence, a longer lag between HIV and HCV diagnoses. Figure 3 shows the survival curves of HCV detection post-HIV diagnosis stratified by MSM/non-MSM and HIV viral suppression status at HCV diagnosis.
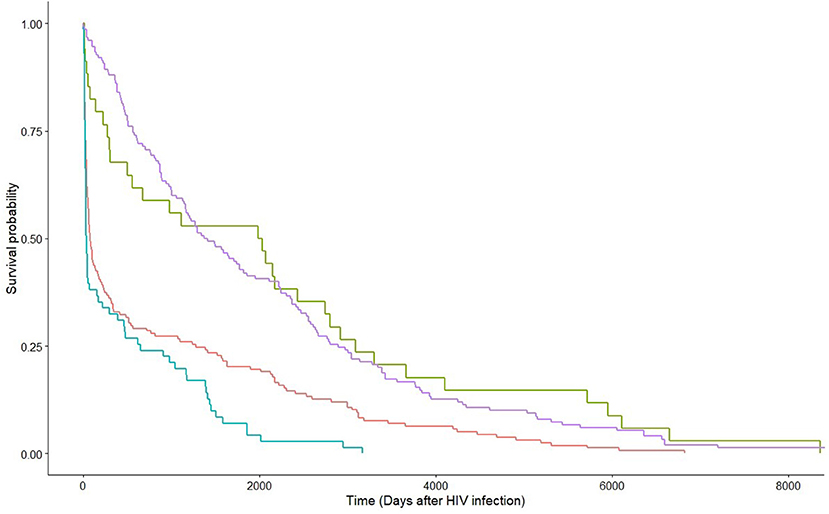
Figure 3. Kaplan-Meier estimates by HIV viral suppression status at HCV diagnosis within strata of route of transmission. Green and red lines are non-MSM with and without HIV viral suppression at HCV diagnosis, respectively; whereas purple and blue lines are MSM with and without HIV viral suppression at HCV diagnosis, respectively.
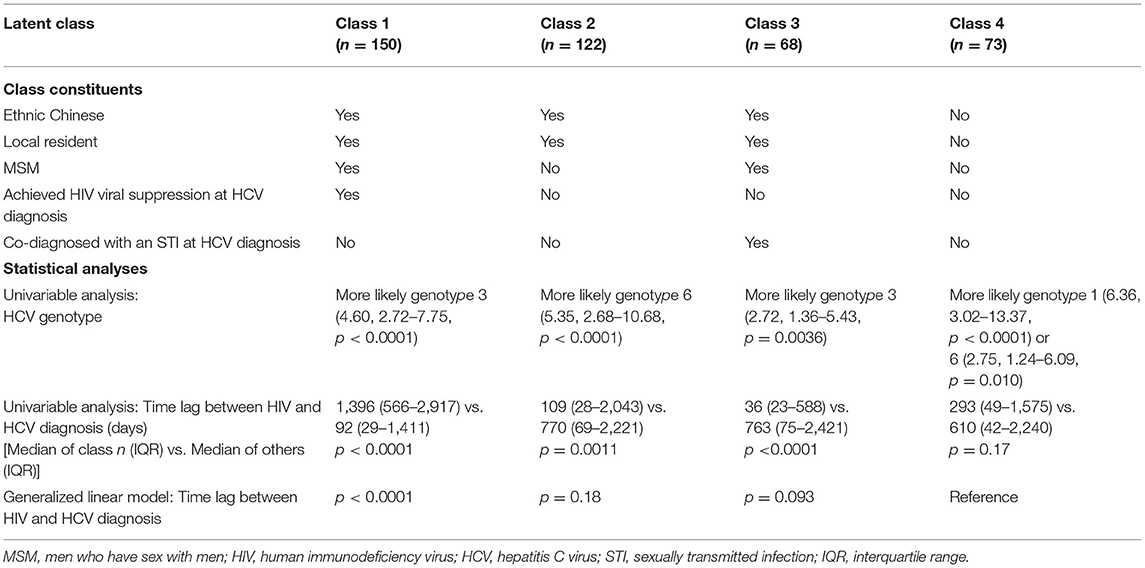
To characterize the HIV/HCV co-infection, five factors were selected for latent class analysis: Chinese ethnicity, local residency, MSM, suppressed HIV viral load at HCV diagnosis, and co-diagnosed with an STI at HCV diagnosis. The goodness of fit test showed that either the two-class or the three-class model fitted the data poorly, with a significant bootstrap p-value < 0.01. A four-class model fitted the data and was therefore chosen for further analysis (p = 0.20). The first class, constituting 35% of the patients, was featured by local Chinese MSM who had achieved HIV viral suppression at HCV diagnosis without an STI co-diagnosis (Table 3). One-third (33%) belonged to the second class who were local Chinese non-MSM without an STI co-diagnosis or HIV viral suppression at HCV diagnosis. Both the first and third classes were more likely to be associated with HCV genotype 3 (p < 0.01). The third class was the smallest (14%), who were local Chinese MSM who were co-diagnosed with an STI but yet to achieve suppressed HIV viral load at the time of HCV diagnosis. The last class (17%) were non-Chinese non-resident non-MSM who had not achieved HIV viral suppression and were not co-diagnosed with an STI at HCV diagnosis. These two non-MSM classes were more likely to be associated with HCV genotype 6 (p <= 0.01), while Class 4 was additionally associated with genotype 1 (p < 0.0001). Class 1 was uniquely associated with a longer time lag between HIV and HCV diagnoses (p < 0.0001), whereas Classes 2 and 3 had shorter lags (p = 0.0011 and p < 0.0001, respectively). No significant association was found in relation to the time lag of Class 4. With reference to Class 4, Class 1 had a significantly longer duration between HIV and HCV diagnoses (p < 0.0001).
Discussion
In Hong Kong, over half of the HIV/HCV co-infections occurred in MSM, the proportion of which was particularly higher in recent years. Emergence of HCV, not just HIV, in the MSM community in the past decade is a cause for concern. The detection of unique HIV subtypes and HCV genotypes associated with MSM suggested the presence of a founder effect in the community (14). From the results of latent class analysis, HIV/HCV co-infected patients could be classified into four classes, two of which were composed of MSM. The two MSM classes were distinguishable by their HIV viral suppression status and STI co-diagnosis at HCV diagnosis. The longer lag between HIV and HCV diagnoses in MSM who had achieved HIV viral suppression at HCV diagnosis without a co-diagnosed STI signified that they were infected with HCV long after HIV infection, and that HCV was not co-transmitting with bacterial STI. They may have formed a subgroup of MSM living with HIV after diagnosis and continued to engage in condomless sex which predisposed them to the risk of HCV (15). But networking factor only contributed to a part of the observation, while sexual behavioral factors remained crucial in facilitating HCV transmission (16).
Separately, MSM who had not achieved HIV viral suppression and were co-diagnosed with an STI at HCV diagnosis had a shorter time lag between HIV and HCV diagnoses, implying that their HCV and STI were acquired before HIV diagnosis, and that the HIV and HCV diagnoses were made concurrently. This echoed with the results in another study that anal mucosal damage by ulcerative STI and HIV co-infection could increase risk of incident HCV infection (16). These were the members in the community who were engaged in higher sexual risk activities, such as chemsex, which heightened their risk of contracting STI, HIV, and HCV. They were often targeted in HIV/STI prevention programmes (17), the effectiveness of which would need to be evaluated.
In the past two decades, advances in antiretroviral therapy have enabled HIV infected MSM to not just become free from HIV disease progression, but also help maintaining daily, including sexual, activities, which placed them at risk of STI and HCV infections (18). Some MSM living with HIV have continued engaging in condomless sex and drug injection (slamming) which poses an infection risk of HCV and bacterial STI (19). The positive association between longer lags and acute HCV diagnosis confirmed with this observation. As they also served at important positions in a transmission network (20), more frequent HCV testing among this group of patients is warranted for detecting the infection early to control the spread of HCV among MSM living with HIV.
On the other hand, HCV transmissions in non-MSM had occurred more commonly through injection drug use rather than sexual activities, who were therefore less likely to have been co-diagnosed with an STI (21). Needle sharing poses a transmission risk of both HIV and HCV that they may have acquired both infections at the same time, hence the short diagnosis lag between the two infections. Given the HIV prevalence remained low in the PWID community, acquisition of HIV through sexual transmission cannot be ruled out. Compared with MSM, individuals with heterosexual HIV risk were more likely to have late presentation at HIV diagnosis (22) as only a small proportion had regular HIV testing (23). If the infections were co-diagnosed, the HIV viral load would not be suppressed at HCV diagnosis before receiving antiretroviral therapy. The two non-MSM classes were differentiated by ethnicity and residency status. A considerable proportion of HIV/HCV co-infected patients belonged to ethnic minorities who had acquired both infections through injection drug use outside Hong Kong (24). The time lags between non-local and local non-MSM were not significantly different and they both had a wide interquartile range, from about a month to 4 or 5 years, showing high intra-class heterogeneity.
It could be argued that drug-injecting MSM were the bridge between the two viral communities (14). However, the distinct genotype distributions among risk groups could only be explained by founder effect (25, 26). It is likely that only a fraction of the HCV co-infections could be attributable to their needle sharing behavior (27). Some MSM denied any drug injection behavior, prompting the possibility of sexual transmission of HCV (28) as supported by results from phylogenetic analysis (29). Separately, non-drug-injecting MSM, compared with PWID and drug-injecting MSM, were more phylogenetically clustered, suggesting that sexual transmission per se could be the main driving force of HCV transmission among MSM (30). The differential profile by HIV subtypes and HCV genotypes indicated that transmission events of HIV and HCV could be independent in co-infected individuals (29, 31–33), while HIV infection might have fuelled the later acquisition of HCV (34). In our study, more than half of the incident HCV infections were diagnosed at least half a year after HIV diagnosis (35); this showed that active HCV transmission chains were present in a cluster of MSM living with HIV who were sexually active therewithin (36).
Unlike the situation in other places where HIV subtypes (29) and HCV genotypes (31) could be used to differentiate one's transmission chain, the four classes of HIV/HCV co-infections in Hong Kong could not be clearly distinguished with molecular data alone. By integrating latent class analysis with molecular epidemiology, the results have shown that each class has its distinct features and requires unique strategies to prevent and control HCV infection to achieve micro-elimination.
This study carries several limitations. Not all HIV/HCV co-infected patients in the territory were analyzed, but the samples in this study constituted the majority of patients in Hong Kong. Behavioral factors, such as practice of chemsex and slamming (19), were not included in the analysis, which limited the interpretation of some findings. This highlights the importance of collecting co-infected patients' recent sexual activity histories and drug injection behaviors at HCV diagnosis to better understand their relationship with HCV infection. As a high-intermediate endemicity city for hepatitis B, a majority of HBV infections in Hong Kong was acquired perinatally and as such, HBsAg status has not been specifically requested for this study. Separately, this study aimed to understand the epidemiology of HCV transmission dynamics only, treatment associated factors, including HIV and HCV regimens, were not transcribed for analysis. As only co-infection patients' data were used, it was not possible to conduct a survival analysis to determine the hazard ratio of acquiring HCV after HIV diagnosis. Separately, HIV patients who were diagnosed with HCV after the follow-up period were not included in this analysis. Our approach was therefore to differentiate the time lag between two diagnoses between two groups, that is, a higher hazard is equivalent to a shorter lag.
Our results have characterized the syndemic of HIV and HCV in the population level and identified four sui generis groups of patients requiring tailored interventions in order to be able to end both epidemics. In view of the growing concern of HCV transmission among MSM living with HIV, the complex epidemiological pattern revealed is essential for planning multifaceted public health policies to implement local viral hepatitis elimination plan.
Data Availability Statement
The data analyzed in this study is subject to the following licenses/restrictions. The data that support the findings of this study are available from Department of Health. Restrictions apply to the availability of these data, which were used under license for this study. Data are available from the authors with the permission of the Department of Health and the Ethics Committee. Requests to access these datasets should be directed to Department of Health, https://www.dh.gov.hk/english/contactus/contactus.html.
Ethics Statement
The studies involving human participants were reviewed and approved by The Joint Chinese University of Hong Kong—New Territories East Cluster Clinical Research Ethics Committee. The Ethics Committee waived the requirement of written informed consent for participation.
Author Contributions
SSL: conceptualization, writing—original draft, writing—review and editing, supervision, project administration, and funding acquisition. BW and KW: investigation, data curation, and writing—review and editing. TK: data curation, methodology, formal analysis, visualization, and writing—original draft. All authors contributed to the article and approved the submitted version.
Funding
Health and Medical Research Fund of Food and Health Bureau (Ref: 18170282).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Li Ka Shing Institute of Health Sciences is thanked for providing technical support.
References
1. World Health Organization. Global Hepatitis Report, 2017. (2017). Available online at: https://www.who.int/publications/i/item/global-hepatitis-report-2017 (accessed December 1, 2021).
2. World Health Organization. Hepatitis C. (2021). Available online at: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c (accessed December 1, 2021).
3. Stanaway JD, Flaxman AD, Naghavi M, Fitzmaurice C, Vos T, Abubakar I, et al. The global burden of viral hepatitis from 1990 to 2013: findings from the global burden of disease study 2013. Lancet. (2016) 388:1081–8. doi: 10.1016/S0140-6736(16)30579-7
4. Han R, Zhou J, François C, Toumi M. Prevalence of hepatitis C infection among the general population and high-risk groups in the EU/EEA: a systematic review update. BMC Infect Dis. (2019) 19:655. doi: 10.1186/s12879-019-4284-9
5. Botheju WSP, Zghyer F, Mahmud S, Terlikbayeva A, El-Bassel N, Abu-Raddad LJ. The epidemiology of hepatitis C virus in Central Asia: systematic review, meta-analyses, and meta-regression analyses. Sci Rep. (2019) 9:2090. doi: 10.1038/s41598-019-38853-8
6. World Health Organization. Global Health Sector Strategy on Viral Hepatitis 2016-2021. (2016). Available online at: https://apps.who.int/iris/bitstream/handle/10665/246177/WHO-HIV-2016.06-eng.pdf (accessed December 1, 2021).
7. Platt L, Easterbrook P, Gower E, McDonald B, Sabin K, McGowan C, et al. Prevalence and burden of HCV co-infection in people living with HIV: a global systematic review and meta-analysis. Lancet Infect Dis. (2016) 16:797–808. doi: 10.1016/S1473-3099(15)00485-5
8. Cuomo G, Digaetano M, Menozzi M, Tagliazucchi S, Guaraldi G, Borghi V, et al. Incidence of HCV infection amongst HIV positive men who had sex with men and prevalence data from patients followed at the infectious diseases clinic of modena, Italy. Dig Liver Dis. (2018) 50:1334–8. doi: 10.1016/j.dld.2018.05.021
9. Cacoub P, Dabis F, Costagliola D, Almeida K, Lert F, Piroth L, et al. Burden of HIV and hepatitis C co-infection: the changing epidemiology of hepatitis C in HIV-infected patients in France. Liver Int. (2015) 35:65–70. doi: 10.1111/liv.12639
10. Li CW, Yang CJ, Sun HY, Tsai MS, Lin SP, Lin TY, et al. Changing seroprevalence of hepatitis C virus infection among HIV-positive patients in Taiwan. PLoS ONE. (2018) 13:e0194149. doi: 10.1371/journal.pone.0194149
11. Lazarus JV, Wiktor S, Colombo M, Thursz M. EASL International Liver Foundation. Micro-elimination—a path to global elimination of hepatitis C. J Hepatol. (2017) 67:665–6. doi: 10.1016/j.jhep.2017.06.033
12. Chan DPC, Lee KCK, Lee SS, Tan TY. Community-based molecular epidemiology study of hepatitis C virus infection in injection drug users. Hong Kong Med J. (2017) 23:S27–30.
13. Kwan TH, Wong NS, Lee SS. Participation pattern of methadone users and its association with social connection and HIV status: analyses of electronic health records data. PLoS ONE. (2019) 14:e0216727. doi: 10.1371/journal.pone.0216727
14. Chan CP, Uemura H, Kwan TH, Wong NS, Oka S, Chan DPC, et al. Review on the molecular epidemiology of sexually acquired hepatitis C virus infection in the Asia-Pacific region. J Int AIDS Soc. (2020) 23:e25618. doi: 10.1002/jia2.25618
15. Poon CM, Wong NS, Kwan TH, Wong HTH, Chan KCW, Lee SS. Changes of sexual risk behaviors and sexual connections among HIV-positive men who have sex with men along their HIV care continuum. PLoS ONE. (2018) 13:e0209008. doi: 10.1371/journal.pone.0209008
16. Ghisla V, Scherrer AU, Nicca D, Braun DL, Fehr JS. Incidence of hepatitis C in HIV positive and negative men who have sex with men 2000-2016: a systematic review and meta-analysis. Infection. (2017) 45:309–21. doi: 10.1007/s15010-016-0975-y
17. Kwan TH, Lee SS. Bridging awareness and acceptance of pre-exposure prophylaxis among men who have sex with men and the need for targeting chemsex and hiv testing: cross-sectional survey. JMIR Public Health Surveill. (2019) 5:e13083. doi: 10.2196/13083
18. Daskalopoulou M, Rodger AJ, Phillips AN, Sherr L, Elford J, McDonnell J, et al. Condomless sex in HIV-diagnosed men who have sex with men in the UK: prevalence, correlates, and implications for HIV transmission. Sex Transm Infect. (2017) 93:590–8. doi: 10.1136/sextrans-2016-053029
19. Jin F, Dore GJ, Matthews G, Luhmann N, Macdonald V, Bajis S, et al. Prevalence and incidence of hepatitis C virus infection in men who have sex with men: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. (2021) 6:39–56. doi: 10.1016/S2468-1253(20)30303-4
20. Kwan TH, Wong NS, Lui GCY, Chan KCW, Tsang OTY, Leung WS, et al. Incorporation of information diffusion model for enhancing analyses in HIV molecular surveillance. Emerg Microbes Infect. (2020) 9:256–62. doi: 10.1080/22221751.2020.1718554
21. Viral Hepatitis Control Office,. Surveillance of Viral Hepatitis in Hong Kong: 2019 Report. (2020). Available online at: https://www.chp.gov.hk/files/pdf/viral_hep_sur_report_2019.pdf (accessed December 1, 2021).
22. Raffetti E, Postorino MC, Castelli F, Casari S, Castelnuovo F, Maggiolo F, et al. The risk of late or advanced presentation of HIV infected patients is still high, associated factors evolve but impact on overall mortality is vanishing over calendar years: results from the Italian MASTER Cohort. BMC Public Health. (2016) 16:878. doi: 10.1186/s12889-016-3477-z
23. Gwadz M, Cleland CM, Kutnick A, Leonard NR, Ritchie AS, Lynch L, et al. Factors associated with recent HIV testing among heterosexuals at high risk for HIV infection in New York city. Front Public Health. (2016) 4:76. doi: 10.3389/fpubh.2016.00076
24. Hong Kong Advisory Council on AIDS. Review of Epidemiology for Ethnic Minorities (Em) in Hong Kong. (2011). Available online at: http://www.aca.gov.hk/english/strategies/pdf/EM%20Eng.pdf (accessed December 1, 2021).
25. Zhang AM, Yang M, Gao L, Zhang M, Jiao L, Feng Y, et al. The distinct epidemic characteristics of HCV co-infection among HIV-1-infected population caused by drug injection and sexual transmission in Yunnan, China. Epidemiol Infect. (2019) 147:e261. doi: 10.1017/S0950268819001365
26. Kartashev V, Döring M, Nieto L, Coletta E, Kaiser R, Sierra S, et al. New findings in HCV genotype distribution in selected West European, Russian and Israeli regions. J Clin Virol. (2016) 81:82–9. doi: 10.1016/j.jcv.2016.05.010
27. Morano JP, Gibson BA, Altice FL. The burgeoning HIV/HCV syndemic in the urban Northeast: HCV, HIV, and HIV/HCV coinfection in an urban setting. PLoS ONE. (2013) 8:e64321. doi: 10.1371/journal.pone.0064321
28. Lin AW, Wong KH, Chan K. More safer sex intervention needed for HIV-positive MSM with higher education level for prevention of sexually transmitted hepatitis C. J Int AIDS Soc. (2014) 17:19663. doi: 10.7448/IAS.17.4.19663
29. Kouyos RD, Rauch A, Böni J, Yerly S, Shah C, Aubert V, et al. Clustering of HCV coinfections on HIV phylogeny indicates domestic and sexual transmission of HCV. Int J Epidemiol. (2014) 43:887–96. doi: 10.1093/ije/dyt276
30. Bartlett SR, Applegate TL, Jacka BP, Martinello M, Lamoury FM, Danta M, et al. A latent class approach to identify multi-risk profiles associated with phylogenetic clustering of recent hepatitis C virus infection in Australia and New Zealand from 2004 to 2015. J Int AIDS Soc. (2019) 22:e25222. doi: 10.1002/jia2.25222
31. Le Ngoc C, Tran Thi Thanh T, Tran Thi Lan P, Nguyen Mai T, Nguyen Hoa T, Nghiem My N, et al. Differential prevalence and geographic distribution of hepatitis C virus genotypes in acute and chronic hepatitis C patients in Vietnam. PLoS ONE. (2019) 14:e0212734. doi: 10.1371/journal.pone.0212734
32. Du L, Wu J, Qian P, Xin R, Ni Y, Han R, et al. Phylogeographical analysis reveals distinct sources of HIV-1 and HCV transmitted to former blood donors in China. AIDS Res Hum Retroviruses. (2017) 33:284–9. doi: 10.1089/aid.2016.0147
33. Ishida Y, Hayashida T, Sugiyama M, Tsuchiya K, Kikuchi Y, Mizokami M, et al. Full-genome analysis of hepatitis C virus in Japanese and Non-Japanese patients coinfected with HIV-1 in Tokyo. J Acquir Immune Defic Syndr. (2019) 80:350–7. doi: 10.1097/QAI.0000000000001919
34. Janjua NZ, Yu A, Kuo M, Alvarez M, Cook D, Wong J, et al. Twin epidemics of new and prevalent hepatitis C infections in Canada: BC hepatitis testers cohort. BMC Infect Dis. (2016) 16:334. doi: 10.1186/s12879-016-1683-z
35. Ireland G, Delpech V, Kirwan P, Croxford S, Lattimore S, Sabin C, et al. Prevalence of diagnosed HIV infection among persons with hepatitis C virus infection: England, 2008–2014. HIV Med. (2018) 19:708–15. doi: 10.1111/hiv.12662
36. Bartlett SR, Jacka B, Bull RA, Luciani F, Matthews GV, Lamoury FM, et al. HIV infection and hepatitis C virus genotype 1a are associated with phylogenetic clustering among people with recently acquired hepatitis C virus infection. Infect Genet Evol. (2016) 37:252–8. doi: 10.1016/j.meegid.2015.11.028
Keywords: HIV/HCV co-infection, people living with HIV, men who have sex with men, people who inject drugs, molecular epidemiology
Citation: Kwan TH, Wong BCK, Wong KH and Lee SS (2022) Hepatitis C Co-infection in People Living With HIV—Epidemiologic Differences Between Men Who Have Sex With Men MSM and Non-MSM. Front. Public Health 10:925600. doi: 10.3389/fpubh.2022.925600
Received: 21 April 2022; Accepted: 16 May 2022;
Published: 03 June 2022.
Edited by:
Diego Ripamonti, Papa Giovanni XXIII Hospital, ItalyReviewed by:
Silvia Nozza, San Raffaele Hospital (IRCCS), ItalyStefano Rusconi, University of Milan, Italy
Copyright © 2022 Kwan, Wong, Wong and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shui Shan Lee, c3NsZWVAY3Voay5lZHUuaGs=
 Tsz Ho Kwan
Tsz Ho Kwan Bonnie Chun Kwan Wong
Bonnie Chun Kwan Wong Ka Hing Wong2
Ka Hing Wong2 Shui Shan Lee
Shui Shan Lee