- 1Department of Pharmacology and Clinical Pharmacy, Faculty of Pharmacy, Universitas Padjadjaran, Jatinangor, Indonesia
- 2Center of Excellence for Pharmaceutical Care Innovation, Faculty of Pharmacy, Universitas Padjadjaran, Jatinangor, Indonesia
- 3Department of Clinical Pharmacy and Pharmacology, University of Groningen, University Medical Centre Groningen, Groningen, Netherlands
- 4Medication Adherence Expertise Centre of The Northern Netherlands (MAECON), Groningen, Netherlands
Introduction: Medication non-adherence is an important public health issue, associated with poor clinical and economic outcomes. Globally, self-reported instruments are the most widely used method to assess medication adherence. However, the majority of these were developed in high-income countries (HICs) with a well-established health care system. Their applicability in low- and middle-income countries (LMICs) remains unclear. The objective of this study is to systematically review the applicability of content and use of self-reported adherence instruments in LMICs.
Method: A scoping review informed by a literature search in Pubmed, EBSCO, and Cochrane databases was conducted to identify studies assessing medication adherence using self-reported instruments for patients with five common chronic diseases [hypertension, diabetes, dyslipidemia, asthma, or Chronic Obstructive Pulmonary Disease (COPD)] in LMICs up to January 2022 with no constraints on publication year. Two reviewers performed the study selection process, data extraction and outcomes assessment independently. Outcomes focused on LMIC applicability of the self-reported adherence instruments assessed by (i) containing LMIC relevant adherence content; (ii) methodological quality and (iii) fees for use.
Findings: We identified 181 studies that used self-reported instruments for assessing medication adherence in LMICs. A total of 32 distinct types of self-reported instruments to assess medication adherence were identified. Of these, 14 self-reported instruments were developed in LMICs, while the remaining ones were adapted from self-reported instruments originally developed in HICs. All self-reported adherence instruments in studies included presented diverse potential challenges regarding their applicability in LMICs, included an underrepresentation of LMIC relevant non-adherence reasons, such as financial issues, use of traditional medicines, religious beliefs, lack of communication with healthcare provider, running out of medicine, and access to care. Almost half of included studies showed that the existing self-reported adherence instruments lack sufficient evidence regarding cross cultural validation and internal consistency. In 70% of the studies, fees applied for using the self-reported instruments in LMICs.
Conclusion: There seems insufficient emphasis on applicability and methodological rigor of self-reported medication adherence instruments used in LMICs. This presents an opportunity for developing a self-reported adherence instrument that is suitable to health systems and resources in LMICs.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier: CRD42022302215.
1. Introduction
Medication adherence is a dynamic process that evolves over time and with prolonged treatment (1). As such, patients with chronic diseases are more likely to have poor medication adherence. Poor adherence to chronic medication is associated with worsened disease control, increased cost, increased hospitalization rates, decreased quality of life, and increased mortality (2–5). Research showed that if 25% of non-adherent people become adherent, this could save $13.7 billion annually and avert 7 million hospitalizations, in the USA alone (6). Moreover, the World Health Organization highlighted adherence as a key indicator for the quality of care (7). Therefore, addressing poor medication adherence is one of the most important factors that contribute to achieving therapy goals in chronic disease management (8). Notably, adherence to chronic medication in low-and-middle-income countries (LMICs) is poorer than in high-income countries (HICs) (9, 10).
Various methods have been used to assess medication adherence, including pill count (11, 12), prescription records and claim-reviewing (13, 14), electronic monitoring devices (15–17), and self-reported instruments (18, 19). Self-reported instruments are cheap, easy to use, and practical because they can highlight the underlying concerns leading to medication non-adherence (20, 21). Particularly, self-reported instruments have become the preferred choice to assess medication adherence in LMICs due to limited resources and logistics (21, 22). However, most of the self-reported medication adherence instruments have been developed in HICs with a well-established health care system. Therefore, these instruments may have several drawbacks when used in LMICs, such as lack of local applicability and the extra costs of obtaining a license (23, 24).
Current self-reported adherence instruments assess different reasons for non-adherence such as patients' behaviors, perceptions, and beliefs that are considered as non-intentional (e.g., forgetfulness) (25, 26) and intentional (e.g., a conscious decision after balancing the pros and cons of a medication) (26, 27), and patients' experiences (e.g., condition-related factors, socioeconomic-related factors, interaction with healthcare professionals, and therapy-related factors) (25, 26, 28). Adherence is a complicated phenomenon affected by the interaction of multiple-factors (7), however there are currently no instruments that capture all potential reasons for non-adherence (28). Importantly, due to the complexities of adherence behavior, accurate self-report instruments should capture particular characteristics and non-adherence barriers (29). Evidence from a previous scoping review showed that low medication adherence across multiple LMICs was driven by the same factors and having similar reasons such as ignorance, unfavorable attitudes, and unfavorable beliefs (30). Notably, information on self-reported adherence instruments' local applicability in LMIC is needed.
Systematic reviews about self-reported instruments on medication adherence have been conducted by a number of previous studies, focusing on their general performance (21, 28, 31, 32). However, to date, there is no comprehensive review that assessed the applicability of self-reported instruments for medication adherence in LMICs. The objective of this study is to systematically review the applicability of content and use of self-reported instruments in LMICs.
2. Method
2.1. Study design
This scoping review was reported in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) guidelines (33) (Supplementary material 1). The protocol was registered at PROSPERO with number registration CRD42022302215.
2.2. Information sources and search strategy
Three electronic databases (PubMed, EBSCO, and Cochrane) were searched up to January 2022 with no constraints on publication year to identify studies assessing medication adherence for five common chronic diseases: hypertension, asthma, COPD, diabetes mellitus, and/or hyperlipidemia. Hand-searching was considered necessary to identify relevant articles that had been unindexed and to ensure that relevant studies were not ignored by a snowballing process which involved checking references of included studies for additional relevant studies. This review adopted the definition of adherence as the process by which patients take their medications as prescribed, composing of initiation (moment when the first dose was taken), implementation (the actual dose of the patient from the start to the last dose), and discontinuation (end of treatment) (34). The PCC mnemonic: participants (chronic disease patients), concepts (the applicability of self-reported instruments for medication adherence), and context (low middle income countries) was used to develop search terms (35). The full search strategy using a combination of medical subject heading terms and text words can be found in the Supplementary material 2.
2.3. Eligibility criteria
Articles, regardless paid (subscription) or free (open access), were eligible if they met the following inclusion criteria: (1) experimental and observational studies focusing on medication adherence as a primary outcome using a self-reported instrument; (2) performed among patients with hypertension, diabetes mellitus, hyperlipidemia, asthma, and/or COPD; (3) published in English, and (4) conducted in LMICs. Of note, we defined LMICs using the World Bank's, 2021 Gross National Income (GNI) per capita by range USD 1,046–4,095 (36).
Articles were excluded if: (1) no peer-reviewed article; (2) reviews, case reports, conference proceedings, opinion pieces, letters to the editor, and commentaries.
2.4. Selection process
One author (QAK) conducted the potential eligibility evaluation based on screening the titles and abstracts. The full texts of potentially eligible articles were retrieved and assessed by QAK. An independent second person (SDA) conducted further independent verification of the abstract and full-text screening. Any disagreements among the reviewers (QAK and SDA) were resolved using consensus.
2.5. Data extraction process
Relevant data from the selected articles were extracted by QAK and verified by SDA. For data extraction, a standardized form with predefined and piloted data extraction criteria was used which was manually extracted in Microsoft Excel 2010 and backed up on Google Drive.
2.6. Data items
In short, the following data items were extracted:
1) Study characteristics: first author, year of publication, country of the study, the aim of the study, study design, type of medication, study period, response rate of self-reported instrument, population (type of chronic disease), sample size, and adherence phases (initiation, implementation, discontinuation).
2) Characteristics of the self-reported medication adherence instruments used: instrument name, number of items, type of scoring, original language, country of development, and psychometric properties (validity or reliability value), and whether a fee for using applies.
3) The applicability of the self-reported medication adherence instruments in LMICs as further defined below.
2.7. Synthesis methods
We first synthesized general information of the LMIC studies that used a self-reported adherence instrument, including type of instrument used, disease area, country and study design.
Secondly, we summarized the use, content (e.g., inclusion of adherence phases) and quality of the self-reported instruments' use (e.g., response rate) in LMIC studies.
Third, the applicability of the self-reported instruments was defined by: (1) incorporating different factors for non-adherence grouped according to the WHO categories: patient related factors, medication related factors, healthcare provider and healthcare system related factors, and societal related factors) (7) taking LMIC relevant medication adherence issues into account, (2) was it formally translated and back-translated in a professional manner, (3) whether the instrument used was validated in their own country considering cultural adaptation, and (4) whether a fee applies for using self-reported instruments in LMICs. One reviewer (QAK) read all the included studies, annotated them, and identified and categorized the applicability, which were further verified by SDA.
2.8. Methodological quality properties assessment methods
One author (QAK) independently reviewed qualifying studies for methodological quality based on their study design. An independent second author (SDA) conducted further independent verification. Any disagreements among the reviewers (QAK and SDA) were resolved using consensus.
For observational studies, we used the Newcastle-Ottawa Scale (NOS) quality assessment for cohort (37) and cross-sectional studies (38) using the star rating system, where each study was evaluated for sample selection, comparability of the groups and the outcome assessment. The scores translate into an overall rating of good, fair or poor study quality using the Agency for Health care Research and Quality (AHRQ)-developed thresholds. Studies were considered as good quality if they have 3 or 4 stars in the selection domain AND 1 or 2 stars in the comparability domain AND 2 or 3 stars in the outcome/exposure domain, they were considered as fair quality if they have 2 stars in selection domain AND 1 or 2 stars in comparability domain AND 2 or 3 stars in outcome/exposure domain, and they were considered as poor quality if they have 0 or 1 star in selection domain OR 0 stars in comparability domain OR 0 or 1 stars in outcome/exposure domain (39). For cross-sectional studies, studies that scored a total of 9–10 points were considered as very good studies, those with 7–8 points were considered as good studies, those with 5–6 points were considered as moderate studies, and those with 4 points or less were considered as unsatisfactory studies.
The Joanna Briggs Institute Critical Appraisal Tools was used to assess the quality of randomized controlled studies (40) and quasi experimental studies (41). Studies were categorized as “high quality” if they met at least 75% of these standards, “moderate” if they met between 50 and 75% of relevant standards, and “low” if <50%.
The 5-point Mixed Method Appraisal Tool was also used to assess the quality of mixed method studies (42). Studies were categorized as “high quality” if they met at least 75% of these standards, “moderate” if they met between 50 and 75% of relevant standards, and “low” if <50%.
Furthermore, we evaluated the quality of standard measurement properties using the Consensus-Based Standards for the Selection of Health Measurement Instruments (COSMIN) checklist (43). Since the COSMIN checklist is a modular tool, it may not be necessary to complete the whole checklist when evaluating the quality of studies (44). Therefore, we only assessed internal consistency and cross-cultural validity. In the COSMIN checklist assessment, we followed all translation and validation that might be done previously in small studies before these included studies. The total score is obtained by taking the lowest response option of any item per measurement property, with possible scores on a four-point scale of inadequate, doubtful, adequate, or very good.
3. Results
3.1. Studies identified
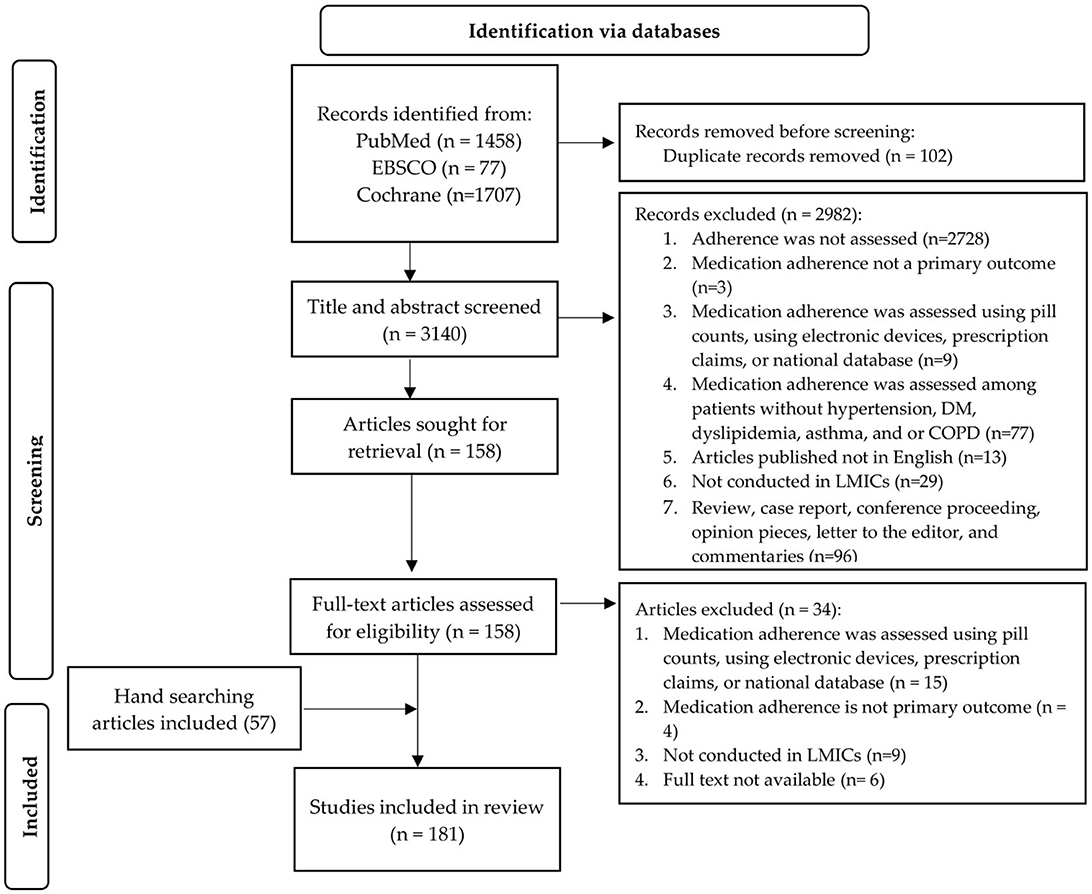
A total of 3,242 records were identified through the systematic search, and 102 duplicates were removed. After screening the abstracts and titles, 2,982 articles were excluded, and 158 full text articles were assessed for eligibility. Then, 57 articles were added as a result of hand-searching the literature. A total of 34 full-text articles were excluded because adherence was not assessed by self-reported instruments (n = 15), were not conducted in LMIC (n = 9), full text was not available (n = 6), and adherence was not the primary outcome (n = 4). Finally, 181 articles met the selection criteria, and were included in this scoping review. The study selection process is illustrated in a PRISMA flow chart (Figure 1).
3.2. Studies' general characteristics
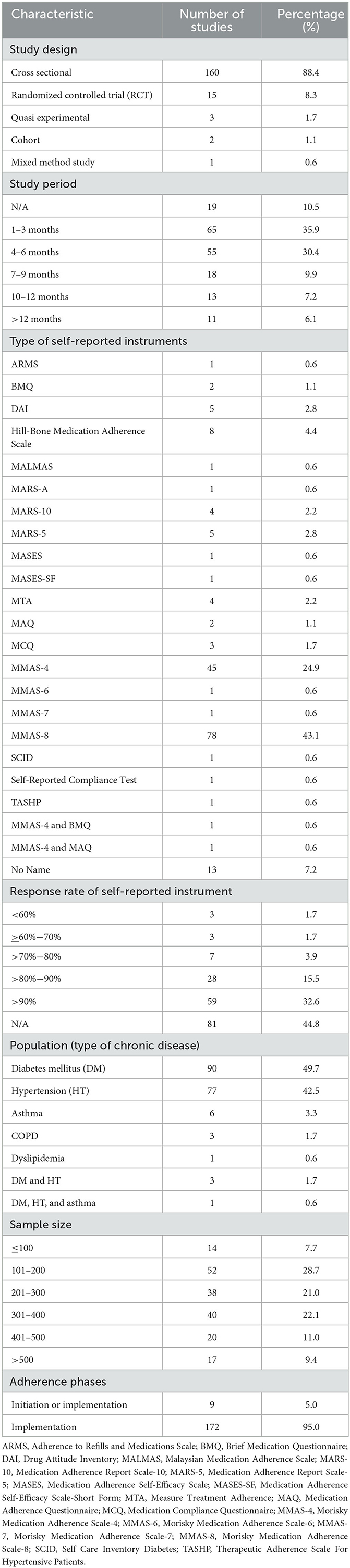
Table 1 summarizes the general characteristics of the included studies published from 1995 (45) to 2022 (46). Half of the studies included (90/181; 49.7%) assessed medication adherence in patients with diabetes mellitus and patients with hypertension (77/181; 42.5%). Six studies assessed adherence in asthma patients, three studies assessed in COPD patients, and one study assessed it in dyslipidemia patients. Four studies assessed multiple types of chronic diseases. While most instruments were disease agnostic, three self-reported instruments were disease specific, such as the Adherence Report Scale for Asthma (MARS-A) for asthma, Self-Care Inventory (SCI) for diabetes mellitus, and Therapeutic Adherence Scale for Hypertensive Patients (TASHP) for hypertension.
The majority of studies were conducted in Ethiopia (27/181), India (17/181), and Nigeria (16/181) (Supplementary material 3). Two studies were conducted in two countries simultaneously, such as in Lebanon and Jordan or Ghana and Nigeria. Figure 2 shows the country coverage of the included studies as well as the number of studies. A pink gradient color represents the number of studies, a light color suggests a small number of studies, and the darker the color gradation indicates the greater the number of studies.
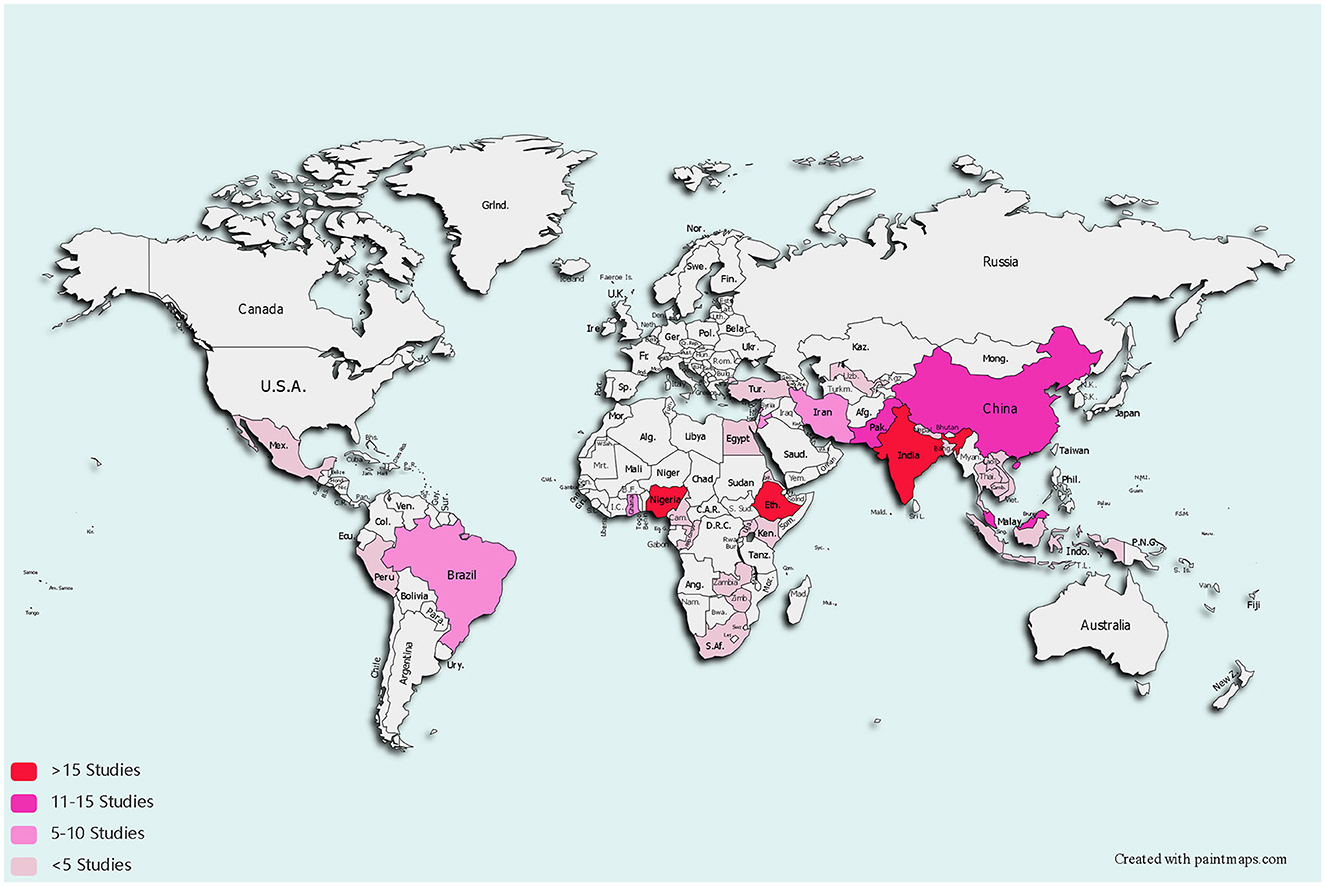
Figure 2. Low middle income countries where self-reported medication adherence instruments were studied.
3.3. Self-reported instruments' use, content, and quality in LMIC
We reviewed 181 eligible studies that focused on self-reported instruments to assess medication adherence in patients living in LMIC with hypertension, diabetes mellitus, dyslipidemia, asthma, or COPD. The sample sizes of the studies ranged from 29 (47) to 1,698 (48). The study period of the studies varied widely, ranging from 1 month (49–52) to 3 years (48). Almost half of the studies included (81/181; 44.8%) did not report a response rate. Of the reported response rate, the rate ranged from 50.8% (49) to 100% (46, 50, 53–59).
Nine studies included people in the initiation and implementation phase of medication adherence (60–68) and the remaining part only included the implementation phase of medication adherence. No studies assessed discontinuation of medication (Supplementary material 4).
Of the 181 studies included, 15 studies performed across 10 countries used LMIC developed self-reported instruments, and the remaining 166 studies (across 32 countries) in LMICs applied existing self-reported instruments. A total of 32 distinct types of self-reported instruments to assess medication adherence were identified (Supplementary material 5). The most common self-reported adherence instruments to assess medication adherence were the MMAS-8 (78/181; 43.1%) and the MMAS-4 (45/181; 24.9%) (Supplementary material 6). Two studies were conducted using a combination of self-reported adherence instruments, such as a combination of the MMAS-4 and BMQ or a combination of MMAS-4 and MAQ. Of the 32 self-reported instruments identified, there were 14 self-reported instruments developed by LMICs (there are two studies utilizing the same instrument), while the other 18 self-reported instruments were adapted from original self-reported instruments developed in HICs (Supplementary material 8).
3.4. Self-reported adherence instruments' applicability in LMIC
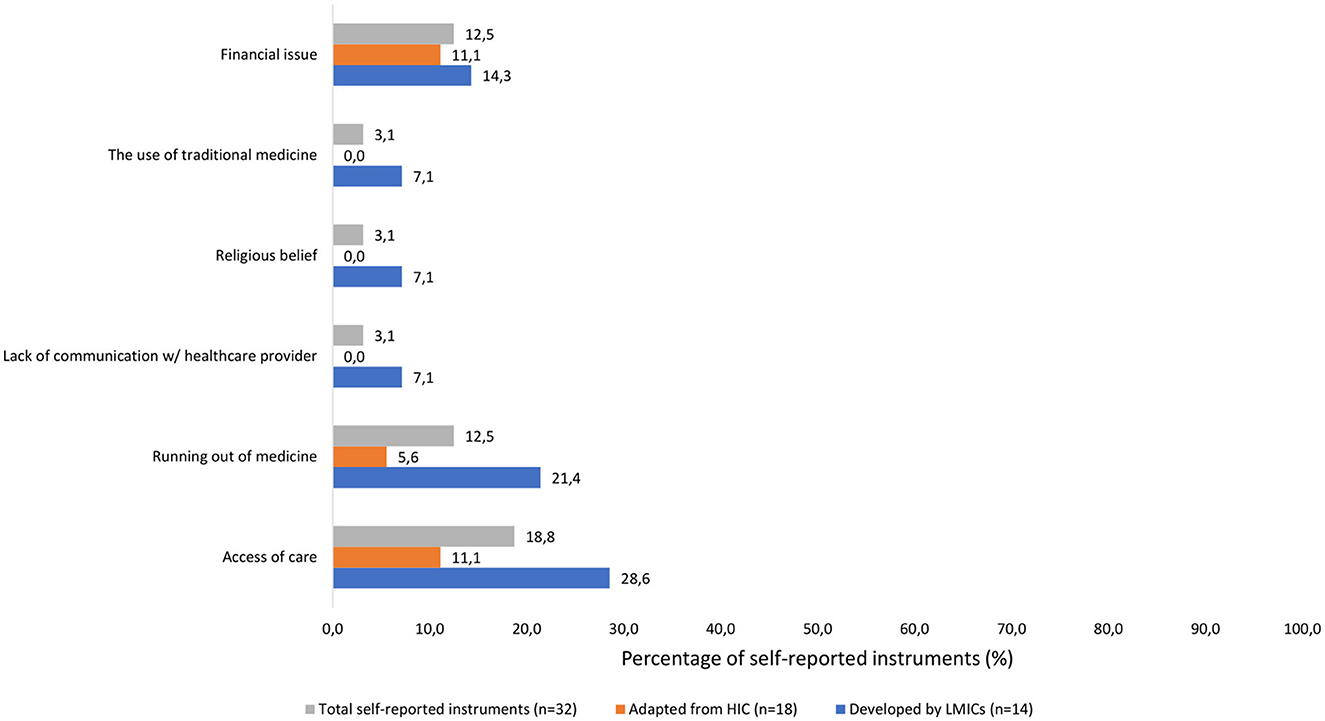
A significant challenge regarding the self-reported instrument applicability in LMICs was that only five studies developed self-reported instruments with modifications for the local context or for the native population in LMICs or patients with low literacy in LMICs (69–73). Additionally, there were six studies using adapted self-reported instruments from HIC to address issues in LMIC such as financial barriers and access to care (64, 74–78) (Supplementary material 7).
Regarding patient related factors, it was shown that just under 25% of self-reported instruments assessed traveling and financial issues. There was no adapted self-reported from HIC that assessed the use of traditional medicine and only 7.1% of developed self-reported instruments from LMICs assessed the use of traditional medicine.
Healthcare provider and system related factors demonstrated a low percentage of self-reported instruments considering lack of communication with healthcare provider, access to care, and running out of medication.
Similarly, social factors represented < 25%. None of the adapted self-reported measures from HICs examined religious beliefs as a reason for non-adherence, and only 7.1% of the developed self-reported instruments from LMICs did so (Figure 3).
3.5. Methodological quality properties
All studies included that used cohort, quasi, and mixed-method design studies were considered as good category according to the quality appraisal checklist (Supplementary material 9). Twelve randomized controlled trials were considered as high quality while three other RCTs were considered as moderate quality. The majority of the cross-sectional studies were considered as moderate quality (65 studies) and good quality (58 studies). There were 37 cross sectional studies that were considered as unsatisfactory quality since there were no descriptions of sampling strategy, no justification for the sample size, no description of the response rate, and no validated measurement tool.
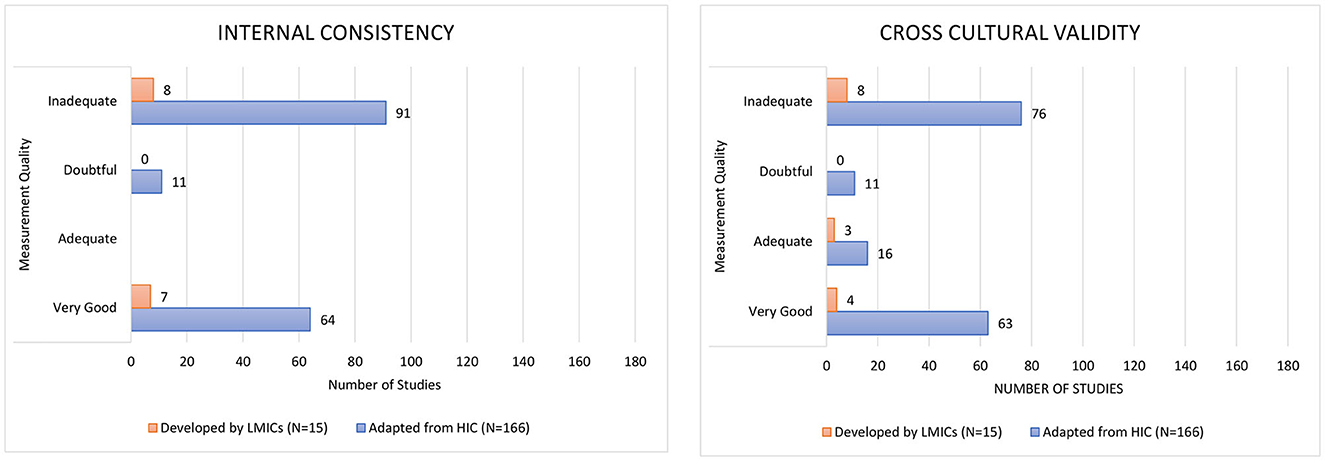
According to the COSMIN checklist, half of the studies were rated as inadequate for internal consistency (54.7%) since there were no reported Cronbach alpha values or item-total correlation calculated (Figure 4). Nearly half of the studies were rated inadequate for cross-cultural validation (46.4%) as samples were not similar regarding relevant characteristics across groups or the approach was not appropriate, i.e., they did not follow the guidelines developed for translation and cross-cultural adaptation questionnaires (79, 80). There were four studies which verbally translated the instrument into the local language for some of the participants, when needed (Supplementary material 10).
3.6. Fee applied for using self-reported instruments in LMICs
One hundred twenty-six studies (70%) identified the license fee requirement of the self-reported instrument as a challenge. Among the self-reported instruments that request extra fees for license are the MMAS-8 (78/181, 43.1%), MMAS-4, (47/181; 26.0%), and ARMS (1/181; 0.6%).
4. Discussion
4.1. Main findings
Most of the 32 self-reported adherence instruments applied in 181 LMIC studies include some patient, medication, healthcare provider and system, and societal factors related to non-adherence. HICs and LMICs both face a wide range of challenges, though the specific issues they encounter can differ quite significantly. Some of the challenges that are common in both HICs and LMICs include being busy, memory difficulties, traveling, lack of knowledge, low necessity, high concern, polypharmacy, and experiencing adverse effects. In LMICs, there is often a greater emphasis on the use of traditional medicine, religious belief, and lack of communication with the healthcare provider. Additionally, financial issues, access to care, and running out of medicines may act as barrier to medication adherence in LMICs. However, these LMIC specific issues are only represented in the 14 identified self-reported instruments developed in LMICs. Notably, still 166 out of the 181 studies (91.7%) in LMICs applied an instrument developed in a HIC with just over half of studies (99/181 studies, 54.7%) having inadequate internal consistency and almost half (84/181 studies, 46.4%) having inadequate cross cultural validation. Around 70% of the studies indicated that a fee applied for using the instrument.
4.2. Interpretation
Our findings highlight the differences in applicability of self-reported adherence instruments in HIC vs. LMIC. Indeed, some problems, such as financial hardship, usually occur more often in LMICs than in HICs, and do significantly contribute to medication non-adherence (81, 82). Despite the fact that these were among the most frequent risk factors for non-adherence in LMICs, only a few instruments have incorporated this issue. Financial issues relate to transportation costs and other basic living expenses like housing, food, and school cost, all competing with the cost of medical treatment (83). In several previous studies it has been reported that patients with low income are more at risk for non-adherence than those with high income (84–86). Even though patients with low income in HICs may have similar relative risk, its absolute impact is higher in LMICs given the proportion of people live in poverty in LMICs is higher than in HICs and social security systems are less developed (87).
Another LMIC specific issue involved traditional medicines that were used by a large portion of the population in LMICs (88), and this has contributed to medication non-adherence (81). Using traditional medicine has become a growing phenomenon because most people believe contemporary medication is ineffective and harmful to their kidneys (89, 90). As a result, people tend to refuse conventional medication and prefer using traditional herbals. Indeed, traditional medicine use is prevalent in LMICs as well as in HICs, with 80% of people in the world utilizing traditional medicine (91). However, health-seeking behaviors regarding traditional medicine use differ between HICs and LMICs (92, 93). In HICs, traditional medicines are utilized as a supplemental therapy to conventional medicine, whereas in LMICs, traditional medicines are often the primary treatment (94). Moreover, multiple studies in LMIC settings have demonstrated that using traditional medicine might be a barrier to seek for treatment in the conventional healthcare system (95–97).
In LMICs, religion plays a significant role in daily life and it could be a challenging issue regarding non-adherence to medication due to the beliefs about the super power of God (98). People who place a high value on religion tend to share the view that God can and does perform miracles and has absolute control over everything in their lives (99). Misinterpretation of religious beliefs has generated the belief that religion-related healing is superior to conventional medication. Several studies in LMICs have found that religiosity can be a barrier to medication adherence. For example, patients with chronic diseases stop taking their medication because they believe their pastors' prayers will cure them (100, 101).
LMIC studies also showed that poor communication of healthcare providers with patients was linked to low medication adherence (102, 103). Inadequate and infrequent patient-provider contact and patient education regarding the medication affected the understanding of many patients. These patients claimed that during consultations, their doctors did not inquire about medication adherence or provided insufficiently detailed instructions on how to take their prescriptions (104). Therefore, communication between the health professional and the patient during the course of the medical encounter should play a critical role in improving medication adherence.
Several studies conducted in LMICs reported “running out of medicine” as reason for non-adherence (105–107). In LMICs, it is often found that lack of access and affordability to medicines is a barrier to good health. Several gaps in local health systems impede the delivery of medicines to millions of people including purchasing procedures, tax and tariff laws, markups along the supply chain, and the poor effectiveness of national drug regulatory agencies (108). A systematic review has shown the scarce availability and affordability of essential medicines for chronic diseases in some LMIC with many not reaching the WHO target of 80% availability (109). A possible explanation for running out of medicines was caused by the limited availability of medication in primary health care (105). Patients were supposed to get all their prescription drugs from primary care and most of them refused to go to pharmacies to pay out-of-pocket (107). Lack of access to healthcare is frequently used as an euphemism for low utilization of available services, which is often found in LMICs (110). Patients who do not have easy access to healthcare may be less likely to follow their treatment plan and less likely to adhere to their medications as prescribed (111).
While content of many self-reported instruments, i.e., the inclusion of LMIC specific barriers, was already deemed a shortcoming, also several contextual and process factors that require attention were identified. For example, only half of the studies reported a translation process when relevant, whereas a translation process based on guidelines is required for those translated to a local language (112). The translation process is critical in order to maintain conceptual, content, semantic, and construct equivalences between the two languages and cultures, which is required to get credible measurement results (113). Translation and cultural adaptation would guarantee that the questionnaire's responses are reflected and analyzed in a consistent manner (79). This is particularly relevant for LMICs since the self-reported instruments are often the only source of adherence information.
Beyond translation, we observed that only a small number of self-reported adherence instruments have been validated. Furthermore, psychometric properties when adapting or developing self-reported instruments in LMICs were often not reported. When a study does not have sufficient psychometric properties, the results of the study cannot be trusted, and the quality of the self-reported adherence instrument remains unclear (44). It has been noted in a recent systematic review that in almost half of included studies, the existing self-reported adherence instruments lack sufficient evidence to meet validity criteria (114). Moreover, no reporting of response rates or having a low response rate were observed in almost a half of included studies, which could increase the likelihood of selection bias and decrease the external validity (115). Indeed, the true prevalence of non-adherence may be underestimated if there is a high rate of non-response, especially if the non-response is associated with the outcome or if non-responders significantly differ from responders (116).
Finally, many self-reported adherence instruments require fees in exchange for licenses to use them in research. This hampers more adherence research in low-resource settings where these fees are unaffordable. At the same time, LMIC heavily rely on self-reported instruments in absence of for example electronic monitoring of adherence using more advanced technologies. Having self-reported instruments available free of charge is therefore essential.
4.3. Strengths and limitations
Some strengths and limitations of our review should be mentioned. This is the first systematic literature review analyzing the use of self-reported adherence instruments and their applicability in LMICs. It could guide selection of adherence self-reported instruments most relevant for measuring medication adherence in LMICs. This review extends previous literature on adherence and emphasizes the challenges of implementing these self-reported instruments in LMICs. A limitation is that potential publication bias may exist due to the exclusion of studies not published in English and limited inclusion of gray literature. Also, while three databases were extensively searched, some studies may have not been included in these three databases. Furthermore, due to the broad inclusion criteria, significant heterogeneity in study design, duration and sample size was found, making direct comparisons between studies challenging.
4.4. Recommendations for future research and practice
Our findings highlight that it is necessary to develop an adherence self-reported instrument that can be adapted to the local context, the health systems and resources available in LMIC in order to precisely and accurately capture medication non-adherence (117). As such, healthcare providers can obtain better insight into potential non-adherence issues and address them during patient counseling to avoid complications of diseases and unnecessary costs.
5. Conclusion
The applicability of self-reported instruments in LMICs was deemed suboptimal. Main issues that need improvement: the inclusion of LMICs relevant issues should be increased, proper translation into the local language and formal cross-cultural validation should be performed, and fees applied for using self-reported instruments in LMICs should be lowered or removed. Nonetheless, because of the methodological shortcomings observed in some included studies, the findings of this study call for the development of a well-validated self-reported adherence instrument that can be universally applied to context, health systems and resources in LMICs.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
QAK conducted the literature searches and wrote the first draft of this manuscript. QAK and SDA conducted screening and data extraction. QAK, SDA, JvB, and RA contributed to the revision of the manuscript. All authors contributed to development of the review protocol, interpretation of findings, revising the manuscript, and approved the final manuscript.
Funding
This study was supported by a grant-in-aid from Universitas Padjadjaran. QAK was supported by a postgraduate scholarship (PMDSU Scholarship) from the Indonesian Ministry of Education, Culture, Research, and Technology.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1104510/full#supplementary-material
References
1. Basu S, Garg S, Sharma N, Singh MM. Improving the assessment of medication adherence: challenges and considerations with a focus on low-resource settings. Tzu Chi Med J. (2019) 31:73–80. doi: 10.4103/tcmj.tcmj_177_18
2. Alfian SD, Sukandar H, Lestari K, Abdulah R. Medication adherence contributes to an improved quality of life in type 2 diabetes mellitus patients: a cross-sectional study. Diabetes Ther. (2016) 7:755–64. doi: 10.1007/s13300-016-0203-x
3. Cutler RL, Fernandez-Llimos F, Frommer M, Benrimoj C, Garcia-Cardenas V. Economic impact of medication non-adherence by disease groups: a systematic review. BMJ Open. (2018) 8:e016982. doi: 10.1136/bmjopen-2017-016982
4. Ip Q, Malone DC, Chong J, Harris RB, Labiner DM. Economic impact of epilepsy and the cost of nonadherence to antiepileptic drugs in older Medicare beneficiaries. Epilepsy Behav. (2018) 80:208–14. doi: 10.1016/j.yebeh.2018.01.009
5. Desai R, Nayak R. Effects of medication nonadherence and comorbidity on health resource utilization in schizophrenia. J Manag Care Spec Pharm. (2019) 25:37–44A. doi: 10.18553/jmcp.2019.25.1.037
6. Lloyd JT, Maresh S, Powers CA, Shrank WH, Alley DE. How much does medication nonadherence cost the medicare fee-for-service program? Med Care. (2019) 57:218–24. doi: 10.1097/MLR.0000000000001067
7. WHO. Adherence to Long Term Therapies: Evidence For Action. Geneva: World Health Organization (2003).
8. Lötsch F, Auer-Hackenberg L, Groger M, Rehman K, Morrison V, Holmes E, et al. Adherence of patients to long-term medication: a cross-sectional study of antihypertensive regimens in Austria. Wien Klin Wochenschr. (2015) 127:379–84. doi: 10.1007/s00508-015-0782-y
9. Nielsen JØ, Shrestha AD, Neupane D, Kallestrup P. Non-adherence to anti-hypertensive medication in low- and middle-income countries: a systematic review and meta-analysis of 92443 subjects. J Hum Hypertens. (2017) 31:14–21. doi: 10.1038/jhh.2016.31
10. Mogre V, Johnson NA, Tzelepis F, Shaw JE, Paul C. A systematic review of adherence to diabetes self-care behaviours: evidence from low- and middle-income countries. J Adv Nurs. (2019) 75:3374–89. doi: 10.1111/jan.14190
11. Bucek A, Raymond J, Leu C-S, Warne P, Abrams EJ, Dolezal C, et al. Preliminary validation of an unannounced telephone pill count protocol to measure medication adherence among young adults with perinatal HIV infection. J Assoc Nurses AIDS Care. (2020) 31:35–41. doi: 10.1097/JNC.0000000000000082
12. Hartman L, Cutolo M, Bos R, Opris-Belinski D, Kok MR, Griep-Wentink HJRM, et al. Medication adherence in older people with rheumatoid arthritis is lower according to electronic monitoring than according to pill count. Rheumatology. (2021) 60:5239–46. doi: 10.1093/rheumatology/keab207
13. Andrade SE, Kahler KH, Frech F, Chan KA. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf. (2006) 15:565–74. doi: 10.1002/pds.1230
14. Sutherland JJ, Morrison RD, McNaughton CD, Daly TM, Milne SB, Daniels JS, et al. Assessment of patient medication adherence, medical record accuracy, and medication blood concentrations for prescription and over-the-counter medications. JAMA Netw Open. (2018) 1:1–10. doi: 10.1001/jamanetworkopen.2018.4196
15. Márquez Contreras EM, Rivero SM, García ER, López-García-Ramos L, Vilas JCP, Suárez AB, et al. Specific hypertension smartphone application to improve medication adherence in hypertension: a cluster-randomized trial. Curr Med Res Opin. (2019) 35:167–73. doi: 10.1080/03007995.2018.1549026
16. McGrady ME, Ramsey RR. Using electronic monitoring devices to assess medication adherence: a research methods framework. J Gen Intern Med. (2020) 35:2707–14. doi: 10.1007/s11606-020-05905-z
17. Tay TR, van Boven JFM, Chan A, Hew M. Electronic inhaler monitoring (EIM) for chronic airways disease: development and application of a Multi-Dimensional Efficacy Framework. J Allergy Clin Immunol Pract. (2021) 10:1189–201.e1. doi: 10.1016/j.jaip.2021.11.027
18. Mannheimer S, Thackeray L, Hullsiek KH, Chesney M, Gardner EM, Wu AW, et al. A randomized comparison of two instruments for measuring self-reported antiretroviral adherence. AIDS Care HIV. (2008) 20:161–9. doi: 10.1080/09540120701534699
19. Stirratt MJ, Dunbar-Jacob J, Crane HM, Simoni JM, Czajkowski S, Hilliard ME, et al. Self-report measures of medication adherence behavior: recommendations on optimal use. Transl Behav Med. (2015) 5:470–82. doi: 10.1007/s13142-015-0315-2
20. Simoni JM, Kurth AE, Pearson CR, Pantalone DW, Merrill JO, Frick PA. Self-report measures of antiretroviral therapy adherence: a review with recommendations for HIV research and clinical management. AIDS Behav. (2006) 10:227–45. doi: 10.1007/s10461-006-9078-6
21. Garfield S, Clifford S, Eliasson L, Barber N, Willson A. Suitability of measures of self-reported medication adherence for routine clinical use: a systematic review. BMC Med Res Methodol. (2011) 11:149. doi: 10.1186/1471-2288-11-149
22. Tandon S, Chew M, Eklu-Gadegbeku CK, Shermock KM, Morisky DE. Validation and psychometric properties of the 8-item Morisky Medication Adherence Scale (MMAS-8) in Type 2 diabetes patients in sub-Saharan Africa. Diabetes Res Clin Pract. (2015) 110:129–36. doi: 10.1016/j.diabres.2015.10.001
23. Hatah E, Rahim N, Makmor-Bakry M, Shah NM, Mohamad N, Ahmad M, et al. Development and validation of Malaysia Medication Adherence Assessment Tool (MyMAAT) for diabetic patients. PLoS ONE. (2020) 15:1–17. doi: 10.1371/journal.pone.0241909
24. Tesfaye W, Peterson G. Self-reported medication adherence measurement tools: some options to avoid a legal minefield. J Clin Pharm Ther. (2021) 47:363–8. doi: 10.1111/jcpt.13515
25. Griva K, Neo HLM, Vathsala A. Unintentional and intentional non-adherence to immunosuppressive medications in renal transplant recipients. Int J Clin Pharm. (2018) 40:1234–41. doi: 10.1007/s11096-018-0652-6
26. Cea-Calvo L, Marín-Jiménez I, de Toro J, Fuster-RuizdeApodaca MJ, Fernández G, Sánchez-Vega N, et al. Different associations of intentional and non-intentional non-adherence behaviors with patient experience with healthcare and patient beliefs in medications: a survey of patients with chronic conditions. Patient Prefer Adherence. (2020) 14:2439–50. doi: 10.2147/PPA.S281985
27. Weinman J, Graham S, Canfield M, Kleinstäuber M, Perera AI, Dalbeth N, et al. The Intentional Non-Adherence Scale (INAS): initial development and validation. J Psychosom Res. (2018) 115:110–6. doi: 10.1016/j.jpsychores.2018.10.010
28. Nguyen TMU, La Caze A, Cottrell, N. What are validated self-report adherence scales really measuring?: a systematic review. Br J Clin Pharmacol. (2014) 77:427–45. doi: 10.1111/bcp.12194
29. AlGhurair SA, Hughes CA, Simpson SH, Guirguis LM. A systematic review of patient self-reported barriers of adherence to antihypertensive medications using the world health organization multidimensional adherence model. J Clin Hypertens. (2012) 14:877–86. doi: 10.1111/j.1751-7176.2012.00699.x
30. Chauke GD, Nakwafila O, Chibi B, Sartorius B, Mashamba-Thompson T. Factors influencing poor medication adherence amongst patients with chronic disease in low-and-middle-income countries: a systematic scoping review. Heliyon. (2022) 8:e09716. doi: 10.1016/j.heliyon.2022.e09716
31. Kwan YH, Weng SD, Loh DHF, Phang JK, Oo LJY, Blalock DV, et al. Measurement properties of existing patient-reported outcome measures on medication adherence: systematic review. J Med Internet Res. (2020) 22:1–26. doi: 10.2196/19179
32. Plevinsky JM, Gutierrez-Colina AM, Carmody JK, Hommel KA, Crosby LE, McGrady ME, et al. Patient-reported outcomes for pediatric adherence and self-management: a systematic review. J Pediatr Psychol. (2020) 45:340–57. doi: 10.1093/jpepsy/jsz096
33. Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. (2018) 169:467–73. doi: 10.7326/M18-0850
34. Vrijens B, De Geest S, Hughes DA, Przemyslaw K, Demonceau J, Ruppar T, et al. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol. (2012) 73:691–705. doi: 10.1111/j.1365-2125.2012.04167.x
35. Peters MDJ. In no uncertain terms: the importance of a defined objective in scoping reviews. JBI Database System Rev Implement Rep. (2016) 14:1–4. doi: 10.11124/jbisrir-2016-2838
36. The World Bank. New World Bank Country Classifications by Income Level: 2021-2022. (2021). Available online at: https://blogs.worldbank.org/opendata/new-world-bank-country-classifications-income-level-2021-2022 (accessed November 5, 2021).
37. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. (2013). Available online at: https://www.ohri.ca/programs/clinical_epidemiology/nos_manual.pdf (accessed April 5, 2023).
38. Herzog R, Álvarez-Pasquin MJ, Díaz C, Del Barrio JL, Estrada JM, Gil Á. Are healthcare workers' intentions to vaccinate related to their knowledge, beliefs and attitudes? a systematic review. BMC Public Health. (2013) 13:154. doi: 10.1186/1471-2458-13-154
39. Shamsrizi P, Gladstone BP, Carrara E, Luise D, Cona A, Bovo C, et al. Variation of effect estimates in the analysis of mortality and length of hospital stay in patients with infections caused by bacteria-producing extended-spectrum beta-lactamases: a systematic review and meta-analysis. BMJ Open. (2020) 10:e030266. doi: 10.1136/bmjopen-2019-030266
40. Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, et al. The Joanna Briggs Institute Critical Appraisal Checklist for Randomized Controlled Trials (Aromataris E, Munn Z, editors). Adelaide, SA: Joanna Briggs Institute (2017), p. 1–9.
41. Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, et al. The Joanna Briggs Institute Critical Appraisal Checklist for Quasi Experimental Studies. Adelaide, SA: Joanna Briggs Institute. (2017).
42. Hong QN, Pluye P, Fàbregues S, Bartlett G, Boardman F, Cargo M, et al. Mixed Methods Appraisal Tool (MMAT), Version 2018 User Guide. Montreal, QC: McGill (2018), p. 1–11.
43. Mokkink LB, de Vet HCW, Prinsen CAC, Patrick DL, Alonso J, Bouter LM, et al. Consensus-based standards for the selection of health measurement instruments (COSMIN) risk of bias checklist for systematic review of patient-reported outcome measures. Qual Life Res. (2018) 27:1171–9. doi: 10.1007/s11136-017-1765-4
44. Mokkink LB, Prinsen CAC, Patrick DL, Alonso J, Bouter LM, de Vet HCW, et al. COSMIN methodology for systematic reviews of Patient-Reported Outcome Measures (PROMs) User Manual. Cosmin Manual for Systematic Reviews of Proms Cosmin, (February). (2018), p. 1–78. Available online at: https://www.cosmin.nl/wp-content/uploads/COSMIN-syst-review-for-PROMs-manual_version-1_feb-2018.pdf (accessed March 31, 2023).
45. Garay-Sevilla ME, Nava LE, Malacara JM, Huerta R, de León JD, Mena A, et al. Adherence to treatment and social support in patients with non-insulin dependent diabetes mellitus. J Diabetes Complications. (1995) 9:81–6. doi: 10.1016/1056-8727(94)00021-F
46. Faisal K, Tusiimire J, Yadesa TM. Prevalence and factors associated with non-adherence to antidiabetic medication among patients at mbarara regional referral Hospital, Mbarara, Uganda. Patient Prefer Adherence. (2022) 16:479–91. doi: 10.2147/PPA.S343736
47. Lerman I, Díaz JPM, Ibarguengoitia MER, Pérez FJG, Villa AR, Velasco ML, et al. Nonadherence to insulin therapy in low-income, type 2 diabetic patients. Endocr Pract. (2009) 15:41–6. doi: 10.4158/EP.15.1.41
48. Gomes MB, Negrato CA. Adherence to insulin therapeutic regimens in patients with type 1 diabetes. A nationwide survey in Brazil. Diabetes Res Clin Pract. (2016) 120:47–55. doi: 10.1016/j.diabres.2016.07.011
49. Balasubramanian A, Nair SS, Rakesh PS, Leelamoni K. Adherence to treatment among hypertensives of rural Kerala, India. J Fam Med Prim Care. (2018) 7:64–9. doi: 10.4103/jfmpc.jfmpc_423_16
50. Tefera YG, Gebresillassie BM, Emiru YK, Yilma R, Hafiz F, Akalu H, et al. Diabetic health literacy and its association with glycemic control among adult patients with type 2 diabetes mellitus attending the outpatient clinic of a university hospital in Ethiopia. PLoS ONE. (2020) 15:1–15. doi: 10.1371/journal.pone.0231291
51. Mohamad M, Moussally K, Lakis C, El-Hajj M, Bahous S, Peruzzo C, et al. Self-reported medication adherence among patients with diabetes or hypertension, Médecins Sans Frontières Shatila refugee camp, Beirut, Lebanon: a mixed-methods study. PLoS ONE. (2021) 16:e0251316. doi: 10.1371/journal.pone.0251316
52. Shimels T, Kassu RA, Bogale G, Bekele M, Getnet M, Getachew A, et al. Magnitude and associated factors of poor medication adherence among diabetic and hypertensive patients visiting public health facilities in Ethiopia during the COVID-19 pandemic. PLoS ONE. (2021) 16:1–13. doi: 10.1371/journal.pone.0249222
53. Ambaw AD, Alemie GA, W/Yohannes SM, Mengesha ZB. Adherence to antihypertensive treatment and associated factors among patients on follow up at University of Gondar Hospital, Northwest Ethiopia. BMC Public Health. (2012)12:282–7. doi: 10.1186/1471-2458-12-282
54. Dego TR, Bobasa EM. Adherence to anti-hypertensive medication and contributing factors among non-comorbid hypertensive patients in two hospitals of Jimma town, South West Ethiopia. Gulhane Med J. (2016) 58:60–6. doi: 10.5455/gulhane.1286
55. Yassine M, Al-Hajje A, Awada S, Rachidi S, Zein S, Bawab W, et al. Evaluation of medication adherence in Lebanese hypertensive patients. J Epidemiol Glob Health. (2016) 6:157–67. doi: 10.1016/j.jegh.2015.07.002
56. Akoko BM, Fon PN, Ngu RC, Ngu KB. Knowledge of hypertension and compliance with therapy among hypertensive patients in the bamenda health district of cameroon: a cross-sectional study. Cardiol Ther. (2017) 6:53–67. doi: 10.1007/s40119-016-0079-x
57. Mekonnen HS, Gebrie MH, Eyasu KH, Gelagay AA. Drug adherence for antihypertensive medications and its determinants among adult hypertensive patients attending in chronic clinics of referral hospitals in Northwest Ethiopia. BMC Pharmacol Toxicol. (2017) 18:27. doi: 10.1186/s40360-017-0134-9
58. Mariye T, Girmay A, Birhanu T, Tasew H, Teklay G, Baraki Z, et al. Adherence to insulin therapy and associated factors among patients with diabetes mellitus in public hospitals of Central Zone of Tigray, Ethiopia, 2018: a cross-sectional study. Pan Afr Med J. (2019) 33:309. doi: 10.11604/pamj.2019.33.309.17547
59. Mathur D, Deora S, Kaushik A, Bhardwaj P, Singh K. Awareness, medication adherence, and diet pattern among hypertensive patients attending teaching institution in western Rajasthan, India. J Fam Med Prim Care. (2020) 9:2342–9. doi: 10.4103/jfmpc.jfmpc_193_20
60. Atulomah NO, Florence OM, Oluwatosin A. Treatment adherence and risk of non-compliance among hypertensives at a Teaching Hospital in Ogun state, southwest Nigeria. J Life Phys Sci. (2010) 3:143–9. doi: 10.4314/ejhs.v30i2.12
61. Malik A, Yoshida Y, Erkin T, Salim D, Hamajima N. Hypertension-related knowledge, practice and drug adherence among inpatients of a hospital in samarkand, uzbekistan. Nagoya J Med Sci. (2014) 76:255–63.
62. Omar MS, San KL. Diabetes knowledge and medication adherence among geriatric patient with type 2 diabetes mellitus. Int J Pharm Pharm Sci. (2014) 6:103–6.
63. Sajith M, Panjak M, Pawar A, Modi A, Sumariya R. Medication adherence to antidiabetic therapy in patients with type 2 diabetes mellitus. Int J Pharm Pharm Sci. (2014) 6:564–70.
64. Heissam K, Abuamer Z, El-Dahshan N. Patterns and obstacles to oral antidiabetic medications adherence among type 2 diabetics in Ismailia, Egypt: a cross section study. Pan Afr Med J. (2015) 20:1–7. doi: 10.11604/pamj.2015.20.177.4025
65. Istilli PT, Alves Pereira MC, de Souza Teixeira CR, Zanetti ML, Canata Becker TA, Paschoalin Marques JV. Treatment adherence to oral glucose-lowering agents in people with diabetes: using the brief medication questionnaire. J Diabetes Nurs. (2015) 19:340–8.
66. Sankar UV, Lipska K, Mini GK, Sarma PS, Thankappan KR. The adherence to medications in diabetic patients in rural Kerala, India. Asia Pac J Public Health. (2015) 27:NP513–23. doi: 10.1177/1010539513475651
67. Nazir SR, Hassali MA, Saleem F, Bashir S, Aljadhey H. Does adherence to the therapeutic regimen associate with health related quality of life: findings from an observational study of type 2 diabetes mellitus patients in Pakistan. Pak J Pharm Sci. (2017) 30:2159–65.
68. Akrom A, Anggitasari W. Adherence and quality of life among diabetic patients with hypertension. Int J Public Health Sci. (2018) 8:14. doi: 10.11591/ijphs.v8i1.15240
69. Hassan NB, Hasanah CI, Foong K, Naing L, Awang R, Ismail SB, et al. Identification of psychosocial factors of noncompliance in hypertensive patients. J Hum Hypertens. (2006) 20:23–9. doi: 10.1038/sj.jhh.1001930
70. Karakurt P, Kaşikçi M. Factors affecting medication adherence in patients with hypertension. J Vasc Nurs. (2012) 30:118–26. doi: 10.1016/j.jvn.2012.04.002
71. Ramli A, Ahmad NS, Paraidathathu T. Medication adherence among hypertensive patients of primary health clinics in Malaysia. Patient Prefer Adherence. (2012) 6:613–22. doi: 10.2147/PPA.S34704
72. Gelaw BK, Mohammed A, Tegegne GT, Defersha AD, Fromsa M, Tadesse E, et al. Nonadherence and contributing factors among ambulatory patients with antidiabetic medications in Adama Referral Hospital. J Diabetes Res. (2014) 2014:617041. doi: 10.1155/2014/617041
73. Hossain A, Mithila O. Sleep duration and treatment compliance: a population-based cross-sectional study of hypertensive patients in Bangladesh. BMC Res Notes. (2016) 9:271. doi: 10.1186/s13104-016-2075-6
74. Gimenes HT, Zanetti ML, Haas VJ. Factors related to patient adherence to antidiabetic drug therapy. Rev Lat Am Enfermagem. (2009) 17:46–51. doi: 10.1590/S0104-11692009000100008
75. Shams MEE, Barakat EAME. Measuring the rate of therapeutic adherence among outpatients with T2DM in Egypt. Saudi Pharm J. (2010) 18:225–32. doi: 10.1016/j.jsps.2010.07.004
76. Faria HTG, Rodrigues FFL, Zanetti ML, de Araújo MFM, Damasceno MMC. Factors associated with adherence to treatment of patients with diabetes mellitus. ACTA Paul Enfer. (2013) 26:231–7. doi: 10.1590/S0103-21002013000300005
77. Turan GB, Aksoy M, Çiftçi B. Effect of social support on the treatment adherence of hypertension patients. J Vasc Nurs. (2019) 37:46–51. doi: 10.1016/j.jvn.2018.10.005
78. Abu Khudair S, Khader YS, Morrissey H, El-Khatib Z, Sandor J. Factors associated with suboptimal adherence to hypertensive medications among syrian refugees - Cross-sectional study at the Zaatari camp, Jordan. Patient Preference Adherence. (2021) 15:2125–35. doi: 10.2147/PPA.S327903
79. Beaton DE, Bombardier C, Guillemin F, Ferraz MB. Guidelines for the process of cross-cultural adaptation of self-report measures. Spine. (2000) 25:3186–91. doi: 10.1097/00007632-200012150-00014
80. Tsang S, Royse CF, Terkawi AS. Guidelines for developing, translating, and validating a questionnaire in perioperative and pain medicine. Saudi J Anaesth. (2017) 11:S80–9. doi: 10.4103/sja.SJA_203_17
81. Macquart de Terline DM, Kane A, Kramoh KE, Toure IA, Mipinda JB, Diop IB, et al. Factors associated with poor adherence to medication among hypertensive patients in twelve low and middle income Sub-Saharan countries. PLoS ONE. (2019) 14:e0219266. doi: 10.1371/journal.pone.0219266
82. Tabyshova A, Sooronbaev T, Akylbekov A, Mademilov M, Isakova A, Erkinbaeva A, et al. Medication availability and economic barriers to adherence in asthma and COPD patients in low-resource settings. Prim Care Respir Med. (2022) 32:20. doi: 10.1038/s41533-022-00281-z
83. Tuller DM, Bangsberg DR, Senkungu J, Ware NC, Emenyonu N, Weiser SD. Transportation costs impede sustained adherence and access to HAART in a clinic population in Southwestern Uganda: a qualitative study. AIDS Behav. (2010) 14:778–84. doi: 10.1007/s10461-009-9533-2
84. Fernandez-Lazaro CI, Adams DP, Fernandez-Lazaro D, Garcia-González JM, Caballero-Garcia A, Miron-Canelo JA. Medication adherence and barriers among low-income, uninsured patients with multiple chronic conditions. Res Social Adm Pharm. (2019) 15:744–53. doi: 10.1016/j.sapharm.2018.09.006
85. Lee H, Park JH, Floyd JS, Park S, Kim HC. Combined effect of income and medication adherence on mortality in newly treated hypertension: nationwide study of 16 million person-years. J Am Heart Assoc. (2019) 8:e013148. doi: 10.1161/JAHA.119.013148
86. Ji NJ, Hong YP. Effect of income level on adherence to antidepressant treatment in first onset depression outpatients. PLoS ONE. (2020) 15:e0238623. doi: 10.1371/journal.pone.0238623
87. Mahembe E, Odhiambo NM. The dynamics of extreme poverty in developing countries. Stud. Univ. “Vasile Goldis” Arad – Econ. Ser. (2018) 28:18–35. doi: 10.2478/sues-2018-0007
88. Lassale C, Gaye B, Diop IB, Mipinda JB, Kramoh KE, Kouam CK, et al. Use of traditional medicine and control of hypertension in 12 African countries. BMJ Global Health. (2022) 7:1–7. doi: 10.1136/bmjgh-2021-008138
89. Sato A. Revealing the popularity of traditional medicine in light of multiple recourses and outcome measurements from a user's perspective in Ghana. Health Policy Plan. (2012) 27:625–37. doi: 10.1093/heapol/czs010
90. Atinga RA, Yarney L, Gavu NM. Factors influencing long-term medication non-adherence among diabetes and hypertensive patients in Ghana: a qualitative investigation. PLoS ONE. (2018) 13:1–15. doi: 10.1371/journal.pone.0193995
91. Oyebode O, Kandala N-B, Chilton PJ, Lilford RJ. Use of traditional medicine in middle-income countries: a WHO-SAGE study. Health Policy Plan. (2016) 31:984–91. doi: 10.1093/heapol/czw022
92. Gureje O, Nortje G, Makanjuola V, Oladeji BD, Seedat S, Jenkins R. The role of global traditional and complementary systems of medicine in the treatment of mental health disorders. Lancet Psychiatry. (2015) 2:168–77. doi: 10.1016/S2215-0366(15)00013-9
93. Street R, Falkenberg T, Sundberg T, Balakrishna Y, Abrams A, Kredo T. Participation of traditional, complementary and alternative health practitioners in conventional health systems in low and middle-income countries. Cochrane Database Syst Rev. (2019) 2019:CD013391. doi: 10.1002/14651858.CD013391
94. WHO. WHO Traditional Medicine Strategy 2014-2023. Geneva: World Health Organization (WHO) (2013), p. 1–76.
95. Audet CM, Blevins M, Rosenberg C, Farnsworth S, Salato J, Fernandez J, et al. Symptomatic HIV-positive persons in rural mozambique who first consult a traditional healer have delays in HIV testing: a cross-sectional study. J Acquir Immune Defic Syndr. (2014) 66:5–12. doi: 10.1097/QAI.0000000000000194
96. Mosa M, Thembelihle Z, Bernhard G. Bridging the gap between biomedical and traditional health practitioners in South Africa. S Afr Health Rev. (2016) 2016:83–92.
97. Khursheda A, Akhtar K, Rahman MM. Use of alternative medicine is delaying health-seeking behavior by bangladeshi breast cancer patients. Eur J Breast Health. (2018) 14:166–172. doi: 10.5152/ejbh.2018.3929
98. Kasahun AE, Sendekie AK, Mekonnen GA, Sema FD, Kemal LK, Abebe RB. Impact of personal, cultural and religious beliefs on medication adherence among patients with chronic diseases at university hospital in Northwest Ethiopia. Patient Prefer Adherence. (2022) 16:1787–803. doi: 10.2147/PPA.S370178
99. Wahab NAA, Bakry MM, Ahmad M, Noor ZM, Ali AM. Exploring culture, religiosity and spirituality influence on antihypertensive medication adherence among specialised population: a qualitative ethnographic approach. Patient Prefer Adherence. (2021) 15:2249–65. doi: 10.2147/PPA.S319469
100. Wanyama J, Castelnuovo B, Wandera B, Mwebaze P, Kambugu A, Bangsberg DR, et al. Belief in divine healing can be a barrier to antiretroviral therapy adherence in Uganda. Age Ageing. (2006) 36:98–101. doi: 10.1097/QAD.0b013e32823ecf7f
101. Hess RF, McKinney D. Fatalism and HIV/AIDS beliefs in rural Mali, West Africa. J Nurs Scholarsh. (2007) 39:113–8. doi: 10.1111/j.1547-5069.2007.00155.x
102. Ratanawongsa N, Karter AJ, Parker MM, Lyles CR, Heisler M, Moffet HH, et al. Communication and medication refill adherence the diabetes study of Northern California. JAMA Intern Med. (2013) 173:210–8. doi: 10.1001/jamainternmed.2013.1216
103. Tesfaye WH, Erku D, Mekonnen A, Tefera YG, Castelino R, Sud K, et al. Medication non-adherence in chronic kidney disease: a mixed-methods review and synthesis using the theoretical domains framework and the behavioural change wheel. J Nephrol. (2021) 34:1091–125. doi: 10.1007/s40620-020-00895-x
104. Tiwary A, Rimal A, Paudyal B, Sigdel KR, Basnyat B. Poor communication by health care professionals may lead to life-threatening complications: examples from two case reports. Wellcome Open Res. (2019) 4:1–8. doi: 10.12688/wellcomeopenres.15042.1
105. Mickelson R, Holden RJ. Medication adherence: staying within the boundaries of safety. Ergonomics. (2018) 6:82–103. doi: 10.1080/00140139.2017.1301574
106. Subbaraman R, Thomas BE, Kumar JV, Thiruvengadam K, Khandewale A, Kokila S, et al. Understanding nonadherence to tuberculosis medications in india using urine drug metabolite testing: a cohort study. Open Forum Infect Dis. (2021) 8:1–9. doi: 10.1093/ofid/ofab190
107. Basu S, Engtipi K, Kumar R. Determinants of adherence to antihypertensive treatment among patients attending a primary care clinic with limited medical armamentarium in Delhi, India: a qualitative study. Chronic Illn. (2022) 18:295–305. doi: 10.1177/1742395320959418
108. WHO. Access to Medicines: Making Market Forces Serve the Poor. Geneva: World Health Organization (WHO) (2017).
109. Stolbrink M, Thomson H, Hadfield RM, Ozoh OB, Nantanda R, Jayasooriya S, et al. The availability, cost, and affordability of essential medicines for asthma and COPD in low-income and middle-income countries: a systematic review. Lancet Glob Health. (2022) 10:e1423–42. doi: 10.1016/S2214-109X(22)00330-8
110. Bitton A, Fifield J, Ratcliffe H, Karlage A, Wang H, Veillard JH, et al. Primary healthcare system performance in low-income and middle-income countries: a scoping review of the evidence from 2010 to 2017. BMJ Glob Health. (2019) 4:e001551. doi: 10.1136/bmjgh-2019-001551
111. Kardas P, Lewek P, Matyjaszczyk M. Determinants of patient adherence: a review of systematic reviews. Front Pharmacol. (2013) 4:1–16. doi: 10.3389/fphar.2013.00091
112. Sousa VD, Rojjanasrirat W. Translation, adaptation and validation of instruments or scales for use in cross-cultural health care research: a clear and user-friendly guideline. J Eval Clin Pract. (2011) 17:268–74. doi: 10.1111/j.1365-2753.2010.01434.x
113. Phongphanngam S, Lach HW. Cross-cultural instrument translation and adaptation: challenges and strategies. Pac Rim Int J Nurs Res. (2019) 23:170–9.
114. Wells J, Crilly P, Kayyali R. A systematic analysis of reviews exploring the scope, validity, and reporting of patient-reported outcomes measures of medication adherence in type 2 diabetes. Patient Prefer Adherence. (2022) 2022:1941–54. doi: 10.2147/PPA.S375745
115. Johnson TP, Wislar JS. Response rates and nonresponse errors in surveys. JAMA. (2012) 307:1805–6. doi: 10.1001/jama.2012.3532
116. Story DA, Trait AR. Survey research. Anesthesiology. (2019) 130:192–202. doi: 10.1097/ALN.0000000000002436
Keywords: self-reported instruments, patient-reported medication, medication adherence, chronic diseases, low-middle income countries
Citation: Khoiry QA, Alfian SD, van Boven JFM and Abdulah R (2023) Self-reported medication adherence instruments and their applicability in low-middle income countries: a scoping review. Front. Public Health 11:1104510. doi: 10.3389/fpubh.2023.1104510
Received: 22 November 2022; Accepted: 23 June 2023;
Published: 13 July 2023.
Edited by:
Jeffrey Birk, Columbia University, United StatesReviewed by:
Mohamed Hassan Elnaem, Ulster University, United KingdomMeiry Fernanda Pinto Okuno, Universidade Federal de São Paulo, Brazil
Francis Kalemeera, Independent Researcher, Kampala, Uganda
Copyright © 2023 Khoiry, Alfian, van Boven and Abdulah. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sofa D. Alfian, c29mYS5hbGZpYW5AdW5wYWQuYWMuaWQ=
†ORCID: Qisty A. Khoiry orcid.org/0000-0002-4920-2434
Sofa D. Alfian orcid.org/0000-0001-5419-8938
Job F. M. van Boven orcid.org/0000-0003-2368-2262
Rizky Abdulah orcid.org/0000-0002-8779-6421
 Qisty A. Khoiry
Qisty A. Khoiry Sofa D. Alfian
Sofa D. Alfian Job F. M. van Boven
Job F. M. van Boven Rizky Abdulah
Rizky Abdulah