- 1Department of Dermatology, West China Hospital, Sichuan University, Chengdu, China
- 2Laboratory of Dermatology, Clinical Institute of Inflammation and Immunology, Frontiers Science Center for Disease-related Molecular Network, West China Hospital, Sichuan University, Chengdu, China
- 3Laser Research Centre, Faculty of Health Science, University of Johannesburg, Doornfontein, South Africa
- 4Outpatient Department of West China Hospital, Sichuan University, Chengdu, China
Introduction: During the outbreak of Coronavirus disease 2019 (COVID-19), health care workers wore personal protective equipment including masks, gloves and goggles for a long time. In order to reduce the transmission routes of the virus, public places were sprayed with disinfectant. Moreover, the body, hands and clothing were frequently disinfected and washed for hygiene purposes. Studies have shown that these practices could easily irritate the skin and damage the skin barrier. Long-term irritation or exposure to allergens may lead to the occurrence of contact dermatitis (CD).
Methods: Subject headings were searched via the National Library of Medicine (PubMed) and web of science databases: COVID-19; contact dermatitis; adverse skin reaction; PPE; dermatitis; mask; glory; hand hygiene, disinfection; face shield; goggle; protect cloth. A total of 246 and 646 articles were retrieved from the two databases, respectively. 402 articles remained after removing duplicates. Reviews, non-English articles, articles that could not be accessed to read or did not conform to our topic were excluded. Finally, a total of 32 cross-sectional studies, 9 case reports and 2 randomized controlled trials were included.
Discussion: This article reviews reports of CD caused by various prevention and hygiene measures during the COVID-19 pandemic. The amount of skin damage caused by COVID-19 prevention measures could be decreased by improved education about skin management.
1. Introduction
In December 2019,the novel Coronavirus disease 2019 (COVID-19) caused by the SARS-CoV-2 virus first became known to the public and quickly rose to pandemic status all over the world (1, 2). According to the World Health Organization, up to 30 January 2023, the number of people diagnosed with COVID-19 had reached 753,001,888 with 6,807,572 deaths reported (3). The advent of COVID-19 vaccines has dramatically reduced the number of infections in the pandemic, but the emergence of new virus variants has made prevention and control difficult (4). In the last 3 years, to combat the overwhelming COVID-19 pandemic, countless health care workers (HCWs) strived on the front line to protect the health and life of the general public. Considering the origin and various modes of SARS-CoV-2 transmission (5, 6), effective preventive measures have been shown to play a great role in reducing the risk of infection (7). Personal protective equipment (PPE) including gloves, masks, N95 respirators, goggles, face shields and gowns) was widely employed. Moreover, other preventive measures (hand sanitizer and disinfectants) were used by HCWs, while preventive measures and masks were also recommended for non-HCWs (8).
However, the long-time wearing of PPE and frequent hand washing or disinfection could often lead to adverse skin reactions (ASR), such as contact dermatitis (CD), as one of the most common dermatoses (9, 10). CD is an inflammatory reaction in which the skin at the contact site becomes inflamed due to exposure to exogenous substances, it can be divided into irritant contact dermatitis (ICD) and allergic contact dermatitis (ACD) according to the etiology. 80% of CD cases were classified as ICD. ICD is caused by exposure of the skin to the irritants, resulting in changes in the epidermal barrier, with itching, pain, burning as the main symptoms. ACD is mainly caused by the activation of cell-mediated immune response by chemical lipophilic molecules, which is mainly manifested as pruritus (11). In a recent cross-sectional study, 65.3% of participant HCWs were self-diagnosed with skin lesions, 25.8% of which were contact/atopic dermatitis (10). One study reported that during the pandemic, CD accounted for 16.5% of PPE-related occupational skin disorders. The most affected parts of the body were the bridge of the nose (24.7%), cheeks (21.3%), forehead (10.3%) and palms (2.8%) (12). Montero-Vilchez et al. reported that CD was the most common ASR associated with PPE (13). Therefore, this review focuses on the development CD as a result of exposure to COVID-19 prevention measures.
Wearing PPE for more than 6 h per day increases the chances of developing ASRs (14). Eczema, pruritus, erythema, edema, urticaria-like plaques, blisters, erosion, exudative lesions, scaling and desquamation are the main manifestations of CD. CD can not only cause health issues for HCWs in the process of performing their medical duties, but can also affect non-HCWs exposed to preventive measures like masks and disinfectants.
2. Contact dermatitis caused by PPE during the COVID-19 pandemic
2.1. Contact dermatitis caused by mask wearing
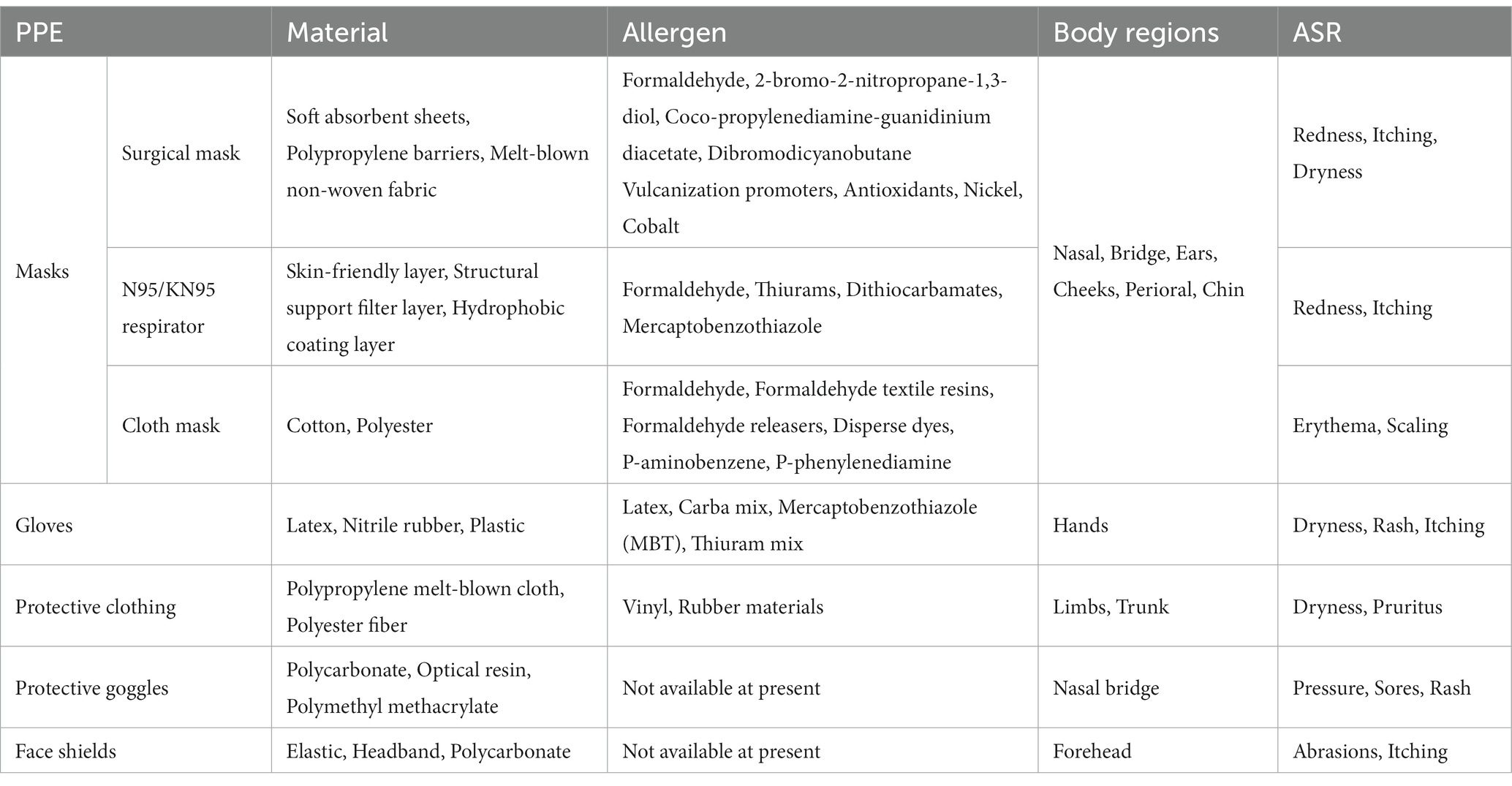
Because SARS-CoV-2 is mainly transmitted by air-borne droplets and according to the previous clinical experience obtained with protection against severe acute respiratory syndrome (SARS), masks were recommended as crucial for preventing the virus from being spread. Masks became daily necessities for HCWs involved with COVID-19 prevention and treatment, as well as being widely used by the general public (5, 15). Research has shown that wearing a mask can greatly cut down the risk of contracting COVID-19 (16). Figure 1 shows several commonly used types of masks. In a cross-sectional study, 37.8% (73/193) of HCWs wore a mask for more than 8 h a day, as compared to 16.5% (313/1897) of non-HCWs. The prolonged skin contact with the mask could cause various ASRs, such as redness (51%), pruritus (49.5%) and acne (43.7%) and of these, 6.2% of HCWs were diagnosed with CD (17). The bridge of the nose, ears, cheeks, perioral area and the chin were the most common sites to be affected (18).
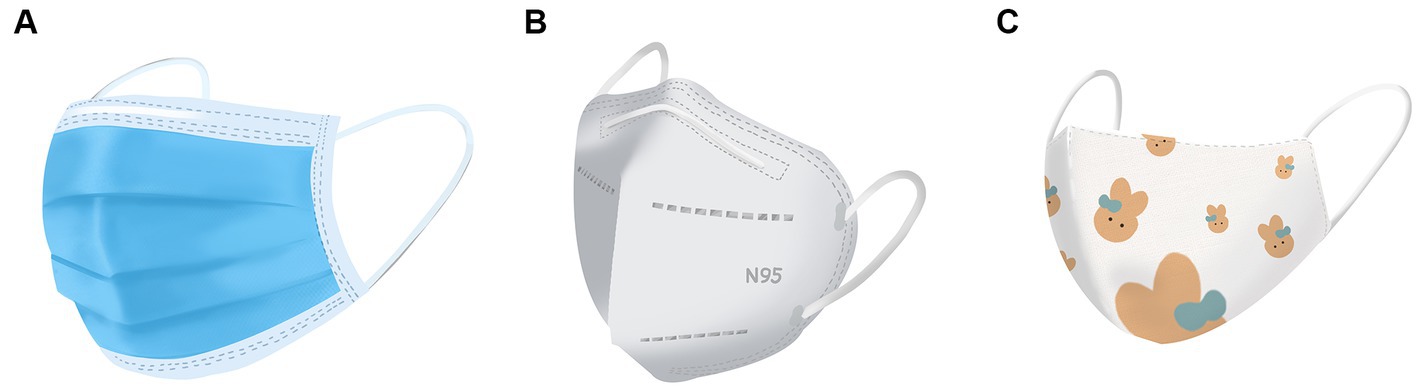
Figure 1. Various masks commonly used during the COVID-19 pandemic (A) surgical mask; (B) N95 mask; (C) cloth mask.
Long-term wearing of masks may cause an increase in the surface temperature of the covered skin (19). The warm, humid and closed environment is beneficial to the growth of microorganisms, while the secretion of pilosebaceous glands can be blocked, resulting in the development of acne. Excessive compression may lead to the occurrence of skin pressure ulcers (20). The occlusive environment makes the skin more permeable and more sensitive to irritation by physical friction/pressure or by exogenous chemicals, eventually leading to CD (21).
In a previous study in the Philippines, the prevalence of mask-induced CD was 34.6%, the variation may due to a large difference in the distribution of HCWs and non-HCWs (22). Skiveren et al. (23) found that the prevalence of ASRs due to face-masks in HCWs was 61.9% using an online questionnaire. Cazzaniga et al. (24) showed that about 18.4% of mask wearers in the community experienced ASRs, including redness, itching, swelling and erosion in the mask contact area.
2.2. Surgical masks
Surgical masks are the predominant type worn by non-HCWs, due to their safety and relative availability. Wear surgical masks correctly can greatly lower the emission of particles associated with aerosol-generating procedures (AGP) by 90% when speaking or coughing. These masks could effectively reduce the number of particles of influenza virus released from the respiratory tract into the environment (25, 26). Nevertheless, the longer the mask is worn even if replaced, the greater the risk of CD (27).
A typical surgical mask usually includes three or four layers. The inner layer is a soft absorbent sheet, the middle one or two layers are polypropylene barriers and the outer layer is melt-blown non-woven fabric with water resistance (28). The main facial lesions caused by surgical masks were redness (55/142), itching (49/142), dryness (20/142), acne (10/142) and rash (8/142) (29). Long-term wearing of polypropylene surgical masks may lead to ACD because formaldehyde, a known ACD sensitizer, is a decomposition product of polypropylene (30, 31). Coco-propylenediamine-guanidinium diacetate and dibromodicyanobutane, which are used in surgical masks are also potential allergens (32). In addition, the elastic bands on surgical masks can also contribute to ACD. The chemical promoters used to accelerate the vulcanization of rubber (including antioxidants) are allergens contained in the elastic bands of surgical masks (33). It is worth noting that although the metal strips (containing nickel and cobalt) on the nose bridge of the masks do not directly contact the skin, they could still contribute to ACD (34). Table 1 shows the main materials, potential allergens, lesion sites and manifestations of CD caused by various types of PPE.
Aerts et al. reported a case of ACD due to a polypropylene surgical mask containing formaldehyde and 2-bromo-2-nitropropane-1,3-diol, along with with recurrent rosacea (35). A systematic review presented three cases of CD caused by surgical masks, with symptoms including erythema, itching and burning sensation on the face (36). In another study, one case of ACD and two cases of ICD (both associated with double-layer surgical masks) were reported with erythema and scaling involving the retroauricular area (37).
2.3. N95/KN95 respirators
N95/KN95 respirators filter out 95% of particles and are more effective than surgical masks in preventing emission of particles from AGPs (sneezing and coughing), making them a good choice for HCWs (28, 29, 38–40). They consist of an inner skin-friendly layer, two structural support filter layers in the middle (mainly made of polyethylene) and an outer hydrophobic coating layer (mostly made of polypropylene) (28, 31). However, because the N95/KN95 respirator fits tighter on the face, it is responsible for more ASRs compared to surgical masks (24). Formaldehyde has been detected in N95/KN95 respirators, accelerating the possible development of ACD (30, 41, 42). In addition, the elastic bands of the N95 respirator along with the sponge strip in the masks have been reported to contribute to the occurrence of ACD. The culprit agents were proposed to be rubber additives in the elastic bands (such as thiurams, dithiocarbamates, or mercaptobenzothiazole), or polyurethane sponge in the respirator (30, 43, 44). The N95/KN95 respirators are attached by ear bands which can cause physical pressure ulcers on the ears (45). Postauricular dermatitis is another form of CD that sometimes occurs after wearing N95/KN95 respirators. Bothra et al. (37) reported four occurrences of ACD (2 cases) or ICD (2 cases) after N95/KN95 respirator use, with symptoms including erythema, desquamation and papules. Most HCWs wear a N95/KN95 respirator for a long time, so the incidence of facial dermatitis could be as high as 81.69%, which mainly consists of redness and itching of the nose and cheeks (29).
Different masks can be selected by the public according to different protection needs. HCWs who caring for COVID-19 patients are recommended to use N95/KN95 respirators, this kind of occupational injury is sometimes unavoidable. In order to reduce skin damage, ordinary surgical masks are the appropriate choice for general people who do not go to public venues or contact with COVID-19 patients in hospitals.
2.4. Cloth masks
Cloth masks are not as efficient as surgical masks or N95 respirators in filtering particulate matter, but have been used during the COVID-19 pandemic when mask supplies were scarce (24, 46–48). In one study, free formaldehyde was found in cotton masks or polyester masks, which may cause ACD (49). In addition, textiles can contain formaldehyde resins or can release formaldehyde, disperse dyes, p-aminobenzene and p-phenylenediamine, which are all potential sensitizing factors (32). However, compared with surgical masks and N95 respirators, cloth masks may cause fewer ASRs (50). In contrast, another study showed no significant difference in skin reactions between cotton masks and surgical masks (51). Bothra reported one case of ACD characterized by erythema and scaling caused by the use of a cloth mask (37). Therefore, although the prevalence rate is low, long-term cloth mask wearers should not ignore the possibility of CD.
To prevent mask-induced CD the following measures can be taken, thin hydrocolloid dressings or thin foam dressings can be applied prophylactically, washing of the face with a mild, scented cleanser at morning and night, wear a head-attached mask instead of an ear-attached mask and take a break every 2 hours after wearing a mask (52).
2.5. Contact dermatitis caused by gloves
During the COVID-19 pandemic, hand to mouth contact is another major transmission route of SARS-CoV-2, so gloves are essential for medical staff (5). As the duration of glove wearing increased, it was found that the risk of ASR also increased (53). HCWs with skin lesions wore PPE for more than 8 h per day on average (54). Figure 2 shows several types of PPE in addition to masks. In order to increase their own safety, some HCWs choose to wear multi-layered gloves. One survey showed that 25.7% of HCWs liked to wear double-layer gloves, among which 62% had complained of hand skin irritation (55). In another survey, among all participants with hand skin lesions, 69.9% of HCWs wore double gloves and 24.3% wore triple gloves (56). However, there is no evidence that increasing the number of glove layers worn could provide better protection against COVID-19 infection. On the contrary, multiple layer gloves actually increased the likelihood of hand CD (21). Therefore, it is recommended to reduce the number of layers of latex gloves consistent with appropriate and safe protection, so as to reduce the risk of hand skin damage. If wearers are allergic to latex, cotton or plastic gloves can be worn inside the latex gloves, while patch testing for specific allergens is needed to know which materials to avoid.
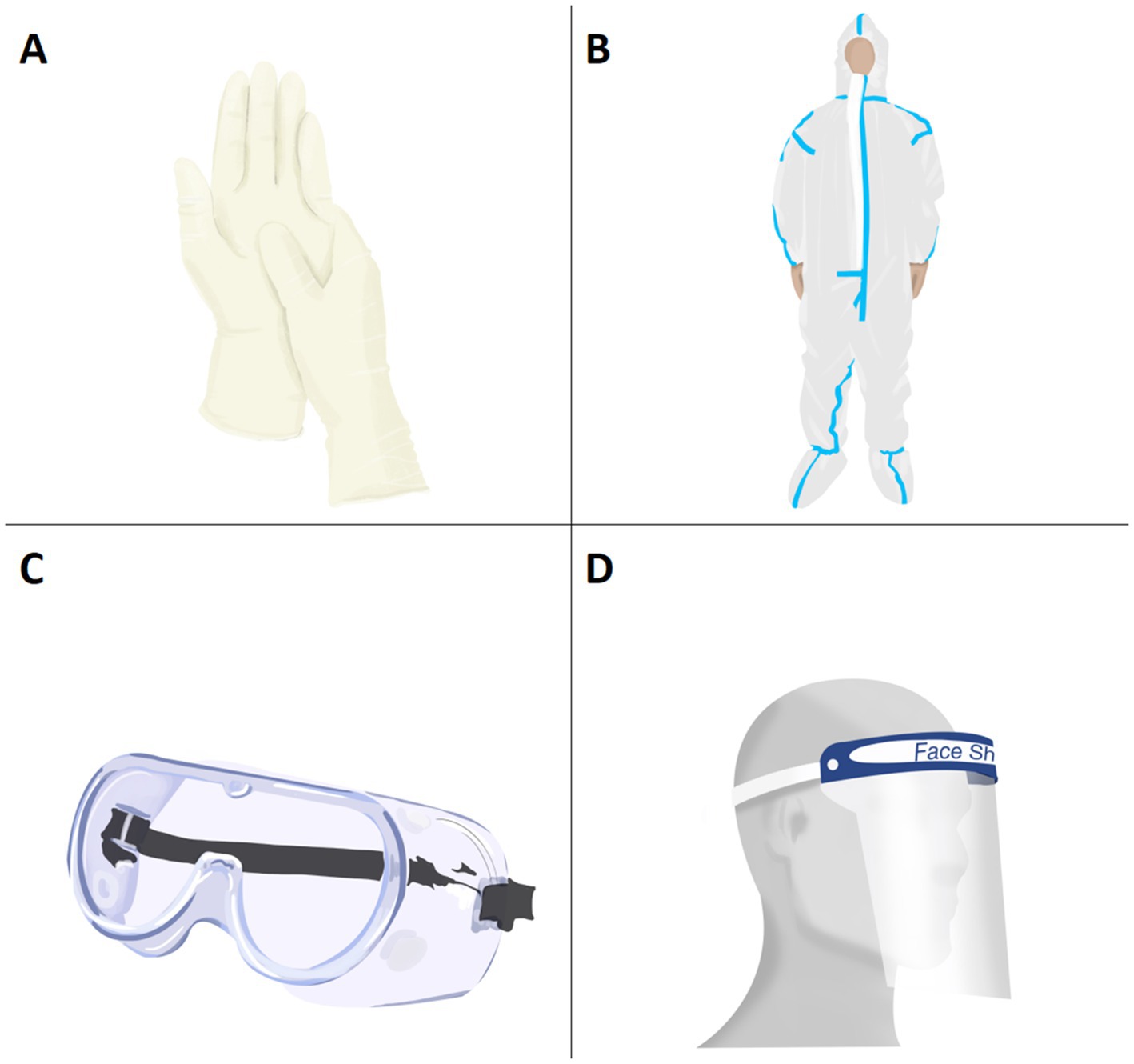
Figure 2. Various PPE in addition to masks (A) glove; (B) protective clothing; (C) protective goggle; (D) face shield.
The components of medical gloves are variable. Due to their strong durability, latex and nitrile rubber are often the preferred materials for gloves, while plastic polyvinylchloride (PVC) gloves, have also won the favor of some HCWs, because of their hypoallergenic properties. However, additives, such as carba mix (diphenylguanidine, zinc-dibutyldithiocarbamate and zinc-diethyldithiocarbamate), mercaptobenzothiazole (MBT), or thiuram mix (tetramethylthiuram monosulfide, disulfiram, tetramethylthiuram disulfide and dipentamethylenethiuram disulfide) which could be contained in PVC could also contribute to ACD (57). Alves et al. (58) reported one case of ACD caused by an allergy to MBT during the COVID-19 pandemic. Several hours after wearing latex gloves, vesicular erythema with itching developed on the hands and wrists. The powder in gloves may promote the development of hand itching and eczema, so powder free gloves could be an option for some individuals, while hands should be dried after washing to reduce the risk of maceration (59).
One study found that among the complications caused by glove wearing, dryness (75%) and rash/itching (72.2%) were the main complaints (60), which was consistent with previous studies by Sliva et al. (61) and Xia et al. (62). In addition, sweating and redness could be also hand symptoms (63). In another study of ASRs related to the use of latex gloves, after using latex gloves for an average of 10 h per day for about 3.5 months, 54 (88.5%) of the surveyed HCWs, complained of some dermatitis symptoms, such as dry skin (55.7%), itching (31.2%), rash (23.0%) and chapped skin (21.3%) (64).
Overhydration of the stratum corneum is a possible consequence of prolonged glove wearing, further accelerating the development of maceration and erosion, the chemical materials in latex glove may contribute to the development of CD (21). Hand cream can improve the maceration, but if irreversible erosion and exudation occurs, 3% boric acid solution aqueous dressings or topical application of zinc oxide ointment may be necessary. In patients with CD, topical glucocorticoids can be used (21).
2.6. Contact dermatitis caused by protective clothing
Protective clothing is generally used by HCWs in high-risk areas, but long working hours, (especially in areas with high ambient temperatures in summer) will undoubtedly cause some discomfort for HCWs (65). This could promote long-term contact of the skin with sweat and heat, which could eventually become a causative factor of CD (66, 67).
The incidence of occupational CD involving protective clothing is rare, with only 143 (3.6%) reported in a recent global systematic review with a total cohort size of 3,958 individuals (12). In a previous study by Hu et al. (64) the occurence of ASRs due to protective clothing was 60.7%, with dry skin (36.1%) and pruritus (34.4%) being the most common complaints. Among the dermatoses associated with the use of protective clothing, ICD stands out (61), with itching and rash the main symptoms caused by prolonged close friction and pressure irritation (60). In another study, ASRs were reported by 11% of 175 HCWs who regularly wore protective clothing, with the most common symptoms being pruritus and erythema and one case of rash (68). These numbers were quite different from the sturdy by Hu et al. (64). The reason may be that the Hu et al. study took place early in the outbreak of COVID-19. The increase in the number of cases has contributed to work intensity and pressure on medical staff. In high pressure situations, HCWs tend to wear protective clothing for longer and have less chance to take it off.
To reduce the incidence of ICD caused by protective clothing, moisturizers or emollients can be used in the pressure areas on the body (69). If conditions permit, HCWs can control the wearing time and regularly remove PPE to prevent or mitigate excessive skin temperatures and sweating.
2.7. Contact dermatitis caused by protective goggles
The use of goggles can effectively prevent SARS-CoV-2 from entering the eyes through small droplets (70, 71). During the COVID-19 pandemic, 67% of HCWs used goggles for more than 4 h a day (18). The most common complications were physical stress-related skin lesions and CD may result from damage to skin integrity caused by prolonged mechanical friction (21, 60). The skin lesions may progress from erythema and depression to erosion and ulceration (12). Pressure sores and rashes are the most common dermatoses caused by goggles (61). Irritation by excessive or prolonged sweating can act as an accelerator (54).
One survey showed that 58% of skin problems were related to goggles (68), which agreed with the previous findings of Lan J et al. and the bridge of the nose was the most susceptible area after wearing goggles for more than 6 h per day (72). Goggle wearing could be a cause of ICD, so in order to reduce the skin pressure injury around the eyes and nasal bridge of HCWs, they are advised to take them off regularly, wipe off sweat, avoid using latex bands that can cause allergies and apply skin cream before wearing (54).
2.8. Contact dermatitis caused by face shields
The full-length PPE face mask typically consists of an elastic headband and a clear polycarbonate, sheet to protect the face from direct contact with aerosols or fluid splashes (73). It carries the risk of causing pressure sores and rashes (61). Abrasions, itching, pain and other changes in skin properties may also occur as a result of prolonged wearing of face shields (74). One study showed that of all the skin problems caused by PPE, face shields accounted for 23% and the forehead was the most affected site (68). In a previous study, the most common skin problems encountered in the management of COVID-19 was CD and 17.31% of these cases were caused by face shields of all types of PPE (54).
3. Contact dermatitis caused by disinfectant products during the COVID-19 pandemic
3.1. Contact dermatitis caused by hand hygiene products
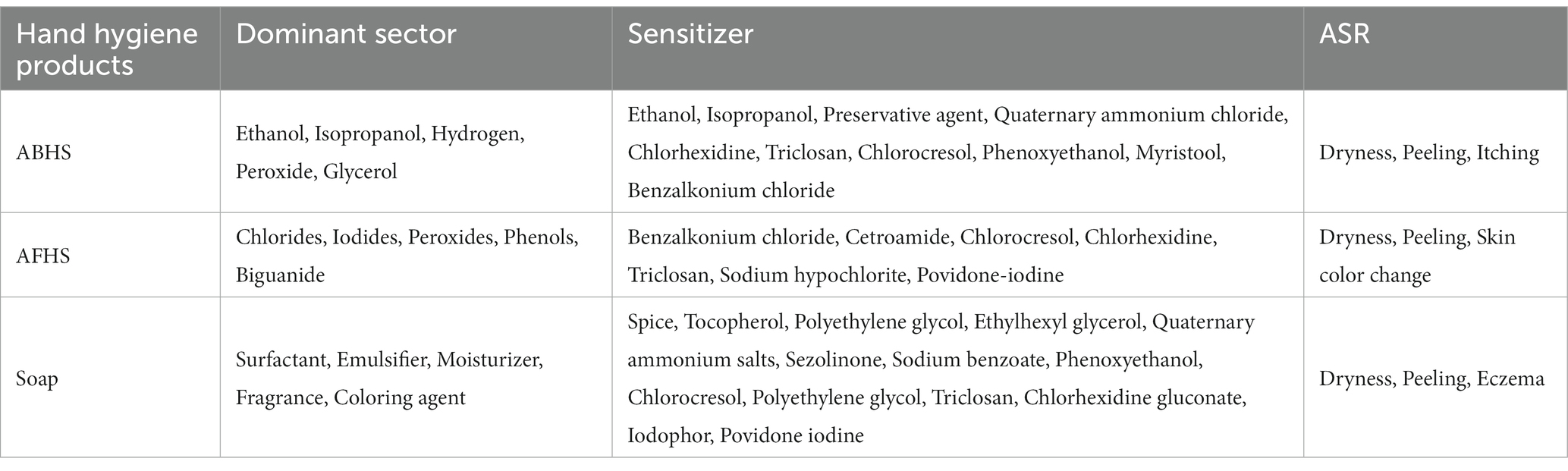
Hand hygiene is particularly important in order to protect individuals from being infected during contact with infected people or in public places. The WHO and the China CDC recommend using alcohol-based hand sanitizer (ABHS), regular soap, or alcohol-free hand sanitizer (AFHS) to combat COVID-19 transmission (75, 76). The main ingredients of ABHS formulations are, ethanol, isopropanol, hydrogen peroxide, glycerol and water. The alcohol concentration ranges between 60 and 95% as the standard for optimal bactericidal and virucidal activity (77). The active ingredients of AFHS are quaternary ammonium compounds, iodophor and chloride. In addition, additives such as excipients and preservatives are commonly used in hand sanitizers (78). Soap contains surfactants, moisturizers, emulsifiers, perfume and various additives that are used to lower the risk of viral transmission (79). Table 2 lists the active ingredients, allergenic substances and resulting ASRs of some hand hygiene products.
However, frequent hand disinfection with alcohol and hand washing with soap or hand sanitizer during the COVID-19 pandemic can result in the development of ICD and ACD (80–82). Various chemical additives (including disinfectants and fragrances) present in hand hygiene products may be responsible for the increase of hand ACD. Typical allergens or irritants include ethanol, isopropanol, perfumes, quaternary ammonium salts, iodine, chlorhexidine, triclosan, chlorocresol, sodium benzoate, phenoxylethanol and stearols (32). Isopropanol disrupts the lipid bilayer structure between cells, leading to denaturation of proteins (83). Triclosan, chlorhexidine and quaternary ammonium compounds have been suggested to contribute to the development of dermatitis (79, 84).
The surfactants contained in hand hygiene products can remove the natural oils of the hands, disrupt the skin barrier and frequent exposure of the hands to water can also lead to increased skin permeability and separation of the stratum corneum, enhancing the irritating effects of surfactants on the skin and leading to ICD (74, 85–87).
It is clear that hand-washing for greater than 10 s 8–10 times per day using hand hygiene products significantly raises the risk of eczema and dryness on the hands (88, 89). Hand hygiene and disinfection can also exacerbate existing eczema and induce new skin problems (90). Most HCWs washed their hands more than 10 times a day for an average of 20 s while managing the COVID-19 pandemic (18, 72, 91). Hand skin damage is also common for workers in the general public who need to maintain regular hand hygiene (92). A cross-sectional study in Bangladesh showed that 41.8% of participants experienced ASRs due to long-term use of hand cleaning products. Most people used ABHS (75.53%) and/or soapy water (69.35%), while fewer used only AFHS (1.22%). Dry skin (34.39%) and peeling (11.71%) were the most common symptoms of ASRs. ABHS users were more easily to experience pruritus (8.13%), while soapy water users were more easily to experience peeling (12.9%), rashes (7.46%) and AFSH users were more easily to experience skin color changes (13.33%) (9). These numbers were analogical to the findings reported by Dash et al. (93), Abdi et al. (29) and Cebeci et al. (94).
Of the body parts affected by wearing PPE, hands are the most common, yet in practice only a few people use hand creams or moisturizers (94, 95). For prevention, rinsing of hands with warm water, the use of moisturizers or hand creams can replenish the moisture and lipids on the skin surface, restoring the skin barrier (79). While ABHS can also dissolve natural lipids in the epidermis, studies have found that ABHS are less damaging to the epidermal barrier compared to soap (96). Therefore, individuals who are not allergic to alcohol are advised to choose ABHS instead of soap. To reduce the occurrence of allergies, the choice of non-perfumed hand sanitizers and soaps is also recommended.
3.2. Contact dermatitis caused by environmental disinfectants
In some countries, coastal areas and densely populated cities have a higher risk of virus transmission (97). Recognizing the potential risk of airborne transmission of SARS-CoV-2, the use of environmental disinfection can help reduce any residual virus on indoor surfaces and in the air, especially in hospitals, classrooms, shopping malls and other places where crowds gather (98, 99). Sodium hypochlorite, ethanol, isopropanol and glutaraldehyde are examples of environmental disinfectants in common use (100). Extensive spraying of disinfectants throughout the environment may cause disinfectants to remain on the skin of people who come into contact with surfaces. Because many disinfectants are fat soluble, they can penetrate the surface of the skin, leading to the occurrence of ASRs. For example, alcohol causes dryness/itching/burning, chloride causes burning/pain/redness/blisters, aldehydes cause yellow-brown discoloration, while disinfectants are regarded as potential causes of for irritations and allergic skin diseases (101, 102). In a survey of household disinfectant use, ASRs occurred in 8% of respondents who used cleansers or disinfectants (103). Avoiding the overuse of excessive concentrations of environmental disinfectants is probably worth considering.
3.3. Contact dermatitis caused by disinfectants for clothing
Common disinfectants for clothing include phenolic compounds, quaternary ammonium salts and chlorine-producing disinfectants (104). The occurrence of the COVID-19 increased the frequency of disinfectant use in laundry and clothes washing. Benzalkonium chloride, a kind of quaternary ammonium cationic detergent was recommended in the cleaners for clothing of AIDS patients, but has been reported several times to cause ACD and children were even more susceptible (84, 105–108). Children who wore clothing treated with benzalkonium chloride in the laundry process, experienced symptoms such as erythema, tenderness, itching, rash and scaling (106). However, CD caused by clothing disinfection is still rare and understanding its mechanism of self-sensitization is the way to prevent it.
4. Conclusion
For the purpose of dealing with the overwhelming COVID-19 pandemic, everyone especially medical workers, have been faced with great challenges. PPE has become a weapon in the fight against the virus, while hand cleansers and disinfectants used appropriately are also important to reduce the transmission routes of this disease. However, the skin damage caused by COVID preventative measures deserves some attention. Many studies have reported the skin damage caused by it and CD is one of the common diseases. In order to have a comprehensive understanding of CD caused by protective measures, the review discussed the CD caused by masks, disinfectants and other PPE to the general public, especially HCWs during the pandemic. Involving a relatively complete set of protective equipment. Masks, gloves, protective clothing, goggles, face masks and disinfectants that may appear in various occasions are comprehensively summarized. The possible skin lesions were discussed, the components of various protective equipment and the potential allergens were also listed.
In particular, the CD related damage to the skin caused by environmental and clothing disinfectants mentioned in this article tends to be ignored in the previous study. The public needs to be educated about the choice of appropriate prevention measures and avoid skin damage with excessive protection. Moisturizers and mild skin care products can effectively reduce skin damage and prevent the occurrence of CD. At the same time, attention should be paid to reducing the frequency and duration of PPE wearing, especially when epidemiologically unnecessary. In cases of serious skin damage, it is recommended to seek the help of dermatologists. This review aims to summarize the contact dermatitis caused by various protective measures against COVID-19 and makes targeted recommendations on ways to reduce the associated lesions, we also propose future research directions for how to decrease the occurrence of skin lesions in the face of a pandemic with effective protection.
Author contributions
HT and HW performed the literature search and wrote the first draft of the article. MH, LJ, YZ, and YX critically revised the manuscript for content and meaning. XW had the original idea for the article and critically revised the work. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by National Natural Science Foundation of China (no. 81903226).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Cucinotta, D, and Vanelli, M. Who declares Covid-19 a pandemic. Acta Biomed. (2020) 91:157–60. doi: 10.23750/abm.v91i1.9397
2. World Health Organization. Director-General’s opening remarks at the media briefing on Covid-19–30 March 2020. Geneva: World Health Organization (2020).
3. World Health Organization. Who coronavirus (Covid-19) dashboard. Geneva: World Health Organization. (2022). Available at: https://covid19.who.int/ (Accessed December 27, 2022).
4. Zhang, HP, Sun, YL, Wang, YF, Yazici, D, Azkur, D, Ogulur, I, et al. Recent developments in the immunopathology of Covid-19. Allergy. (2023) 78:369–88. doi: 10.1111/all.15593
5. Abd, EW, Eassa, SM, Metwally, M, Al-Hraishawi, H, and Omar, SR. Sars-Cov-2 transmission channels: a review of the literature. MEDICC Rev. (2020) 22:51–69. doi: 10.37757/MR2020.V22.N4.3
6. Umakanthan, S, Sahu, P, Ranade, AV, Bukelo, MM, Rao, JS, Abrahao-Machado, LF, et al. Origin, transmission, diagnosis and Management of Coronavirus Disease 2019 (Covid-19). Postgrad Med J. (2020) 96:753–8. doi: 10.1136/postgradmedj-2020-138234
7. Hamimes, A, Aouissi, HA, Ababsa, M, Lounis, M, Jayarajah, U, Napoli, C, et al. The effect of preventive measures and vaccination against Sars-Cov-2 on the infection risk, treatment, and hospitalization: a cross-sectional study of Algeria. Viruses. (2022) 14:2771. doi: 10.3390/v14122771
8. World Health Organization. Rational use of personal protective equipment for coronavirus disease 2019 (Covid-19). Geneva World Health Organization. (2020). doi: 10.1093/tropej/fmab011 Available at: https://apps.who.int/iris/bitstream/handle/10665/331215/WHO-2019-nCov-IPCPPE_use-2020.1-eng.pdf.
9. Roy, S, Iktidar, MA, Saha, AD, Chowdhury, S, Tabassum Hridi, ST, Sayeem Tanvir, SM, et al. Hand hygiene products and adverse skin reactions: a cross-sectional comparison between healthcare and non-healthcare Workers of Bangladesh during Covid-19 pandemic. Heliyon. (2022) 8:e12295. doi: 10.1016/j.heliyon.2022.e12295
10. Da Silva, BE, Sant'Ana Mandelbaum, MH, Lanzillotti, RS, Granja, PD, da Silva, LF, and Tonole, R. Skin lesions resulting from use of personal protective equipment in the context of Covid-19: a cross-sectional study. J Wound Care. (2022) 31:S22–8. doi: 10.12968/jowc.2022.31.Sup12.S22
11. Sasseville, D. Occupational contact dermatitis. Allergy Asthma Clin Immunol. (2008) 4:59–65. doi: 10.1186/1710-1492-4-2-59
12. Keng, BMH, Gan, WH, Tam, YC, and Oh, CC. Personal protective equipment-related occupational dermatoses during Covid-19 among health care workers: a worldwide systematic review. JAAD Int. (2021) 5:85–95. doi: 10.1016/j.jdin.2021.08.004
13. Montero-Vilchez, T, Cuenca-Barrales, C, Martinez-Lopez, A, Molina-Leyva, A, and Arias-Santiago, S. Skin adverse events related to personal protective equipment: a systematic review and Meta-analysis. J Eur Acad Dermatol Venereol. (2021) 35:1994–2006. doi: 10.1111/jdv.17436
14. Etgu, F, and Onder, S. Skin problems related to personal protective equipment among healthcare workers during the Covid-19 pandemic (online research). Cutan Ocul Toxicol. (2021) 40:207–13. doi: 10.1080/15569527.2021.1902340
15. Seto, WH, Tsang, D, Yung, RW, Ching, TY, Ng, TK, Ho, M, et al. Effectiveness of precautions against droplets and contact in prevention of nosocomial transmission of severe acute respiratory syndrome (Sars). Lancet. (2003) 361:1519–20. doi: 10.1016/s0140-6736(03)13168-6
16. Sugimura, M, Chimed-Ochir, O, Yumiya, Y, Ohge, H, Shime, N, Sakaguchi, T, et al. The association between wearing a mask and Covid-19. Int J Environ Res Public Health. (2021) 18:9131. doi: 10.3390/ijerph18179131
17. Alamawi, HO, Alruwaili, MS, Alswayed, SK, Alhumaidi, WA, Aldabali, SO, and Alfalah, HA. Mask-induced facial dermatoses in the Saudi Arabian population during the Covid-19 pandemic: a cross-sectional study. Cureus. (2022) 14:e31151. doi: 10.7759/cureus.31151
18. Daye, M, Cihan, FG, and Durduran, Y. Evaluation of skin problems and dermatology life quality index in health care workers who use personal protection measures during Covid-19 pandemic. Dermatol Ther. (2020) 33:e14346. doi: 10.1111/dth.14346
19. Scarano, A, Inchingolo, F, and Lorusso, F. Facial skin temperature and discomfort when wearing protective face masks: thermal infrared imaging evaluation and hands moving the mask. Int J Environ Res Public Health. (2020) 17:4624. doi: 10.3390/ijerph17134624
20. Tan, KT, and Greaves, MW. N95 Acne. Int J Dermatol. (2004) 43:522–3. doi: 10.1111/j.1365-4632.2004.02338.x
21. Yan, Y, Chen, H, Chen, L, Cheng, B, Diao, P, Dong, L, et al. Consensus of Chinese experts on protection of skin and mucous membrane barrier for health-care workers fighting against coronavirus disease 2019. Dermatol Ther. (2020) 33:e13310. doi: 10.1111/dth.13310
22. Resuello, TEM, and Puyat, MCA. Mask-induced facial dermatoses during the Covid-19 pandemic: a cross-sectional study in a tertiary medical Center in the Philippines. JAAD Int. (2022) 7:121–3. doi: 10.1016/j.jdin.2022.03.002
23. Skiveren, JG, Ryborg, MF, Nilausen, B, Bermark, S, and Philipsen, PA. Adverse skin reactions among health care workers using face personal protective equipment during the coronavirus disease 2019 pandemic: a cross-sectional survey of six hospitals in Denmark. Contact Dermatitis. (2022) 86:266–75. doi: 10.1111/cod.14022
24. Cazzaniga, S, Pezzolo, E, Colombo, P, and Naldi, L. Face mask use in the community and cutaneous reactions to them during the Covid-19 pandemic: results of a National Survey in Italy. Dermatol Rep. (2022) 14:9394. doi: 10.4081/dr.2022.9394
25. Asadi, S, Cappa, CD, Barreda, S, Wexler, AS, Bouvier, NM, and Ristenpart, WD. Efficacy of masks and face coverings in controlling outward aerosol particle emission from expiratory activities. Sci Rep. (2020) 10:15665. doi: 10.1038/s41598-020-72798-7
26. Leung, NHL, Chu, DKW, Shiu, EYC, Chan, KH, McDevitt, JJ, Hau, BJP, et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med. (2020) 26:676–80. doi: 10.1038/s41591-020-0843-2
27. Wan, X, Lu, Q, Sun, D, Wu, H, and Jiang, G. Skin barrier damage due to prolonged mask use among healthcare workers and the general population during the Covid-19 pandemic: a prospective cross-sectional survey in China. Dermatology. (2022) 238:218–25. doi: 10.1159/000517219
28. Das, S, Sarkar, S, Das, A, Das, S, Chakraborty, P, and Sarkar, J. A comprehensive review of various categories of face masks resistant to Covid-19. Clin Epidemiol Glob Health. (2021) 12:100835. doi: 10.1016/j.cegh.2021.100835
29. Abdi, M, Falahi, B, Ebrahimzadeh, F, Karami-Zadeh, K, Lakzadeh, L, and Rezaei-Nasab, Z. Investigating the prevalence of contact dermatitis and its related factors among hospital staff during the outbreak of the Covid-19 epidemic: a cross-sectional study. Iran J Nurs Midwifery Res. (2022) 27:236–42. doi: 10.4103/ijnmr.IJNMR_373_20
30. Donovan, J, and Skotnicki-Grant, S. Allergic contact dermatitis from formaldehyde textile resins in surgical uniforms and nonwoven textile masks. Dermatitis. (2007) 18:40–4. doi: 10.2310/6620.2007.05003
31. Yu, J, Goldminz, A, Chisolm, S, Jacob, SE, Zippin, JH, Wu, PA, et al. Facial personal protective equipment: materials, resterilization methods, and management of occupation-related dermatoses. Dermatitis. (2021) 32:78–85. doi: 10.1097/DER.0000000000000699
32. Bhatia, R, Sindhuja, T, Bhatia, S, Dev, T, Gupta, A, Bajpai, M, et al. Iatrogenic dermatitis in times of Covid-19: a pandemic within a pandemic. J Eur Acad Dermatol Venereol. (2020) 34:e563–6. doi: 10.1111/jdv.16710
33. Bouabdella, S, Dikhaye, S, and Zizi, N. Contact dermatitis caused by elastic bands from surgical mask. Rev Fr Allergol 2009. (2022) 62:497–9. doi: 10.1016/j.reval.2021.10.011
34. Warshaw, EM, Schlarbaum, JP, Silverberg, JI, DeKoven, JG, Maibach, HI, Sasseville, D, et al. Safety equipment: when protection becomes a problem. Contact Dermatitis. (2019) 81:130–2. doi: 10.1111/cod.13254
35. Aerts, O, Dendooven, E, Foubert, K, Stappers, S, Ulicki, M, and Lambert, J. Surgical mask dermatitis caused by formaldehyde (releasers) during the Covid-19 pandemic. Contact Dermatitis. (2020) 83:172–3. doi: 10.1111/cod.13626
36. Sarfraz, Z, Sarfraz, A, Sarfraz, M, Felix, M, Bernstein, JA, Fonacier, L, et al. Contact dermatitis due to personal protective equipment use and hygiene practices during the Covid-19 pandemic: a systematic review of case reports. Ann Med Surg (Lond). (2022) 74:103254. doi: 10.1016/j.amsu.2022.103254
37. Bothra, A, Das, S, Singh, M, Pawar, M, and Maheswari, A. Retroauricular dermatitis with vehement use of ear loop face masks during Covid-19 pandemic. J Eur Acad Dermatol Venereol. (2020) 34:e549–52. doi: 10.1111/jdv.16692
38. Bartoszko, JJ, Farooqi, MAM, Alhazzani, W, and Loeb, M. Medical masks vs N95 respirators for preventing Covid-19 in healthcare workers: a systematic review and Meta-analysis of randomized trials. Influenza Other Respir Viruses. (2020) 14:365–73. doi: 10.1111/irv.12745
39. Hui, DS, Chow, BK, Chu, L, Ng, SS, Lee, N, Gin, T, et al. Exhaled air dispersion during coughing with and without wearing a surgical or N95 mask. PLoS One. (2012) 7:e50845. doi: 10.1371/journal.pone.0050845
40. Haller, S, Güsewell, S, Egger, T, Scanferla, G, Thoma, R, Leal-Neto, OB, et al. Impact of respirator versus surgical masks on Sars-Cov-2 Acquisition in Healthcare Workers: a prospective multicentre cohort. Antimicrob Resist Infect Control. (2022) 11:27. doi: 10.1186/s13756-022-01070-6
41. Arrington, KC, Glass, LD, and Scheinman, PL. Do not lose sleep over mask allergic contact dermatitis. JAAD Case Rep. (2022) 19:100–2. doi: 10.1016/j.jdcr.2021.11.013
42. Clawson, RC, and Pariser, R. Formaldehyde-induced contact dermatitis from an N95 respirator mask. Cutis. (2021) 108:E11–4. doi: 10.12788/cutis.0305
43. Garrido, PM, Alpalhao, M, Correia, T, and Filipe, P. Allergic contact dermatitis caused by elastic bands of an N95 mask. Contact Dermatitis. (2022) 87:94–5. doi: 10.1111/cod.14100
44. Xie, Z, Yang, YX, and Zhang, H. Mask-induced contact dermatitis in handling Covid-19 outbreak. Contact Dermatitis. (2020) 83:166–7. doi: 10.1111/cod.13599
45. Jiang, W, Cao, W, and Liu, Q. Wearing the N95 mask with a plastic handle reduces pressure injury. J Am Acad Dermatol. (2020) 82:e191–2. doi: 10.1016/j.jaad.2020.04.001
46. Chaiyabutr, C, Sukakul, T, Pruksaeakanan, C, Thumrongtharadol, J, and Boonchai, W. Adverse skin reactions following different types of mask usage during the Covid-19 pandemic. J Eur Acad Dermatol Venereol. (2021) 35:e176–8. doi: 10.1111/jdv.17039
47. Matusiak, L, Szepietowska, M, Krajewski, PK, Bialynicki-Birula, R, and Szepietowski, JC. The use of face masks during the Covid-19 pandemic in Poland: a survey study of 2315 young adults. Dermatol Ther. (2020) 33:e13909. doi: 10.1111/dth.13909
48. Konda, A, Prakash, A, Moss, GA, Schmoldt, M, Grant, GD, and Guha, S. Aerosol filtration efficiency of common fabrics used in respiratory cloth masks. ACS Nano. (2020) 14:6339–47. doi: 10.1021/acsnano.0c03252
49. Kawakami, T, Obama, T, Sakai, S, Takagi, M, Takahashi, N, Oshima, N, et al. Free formaldehyde in non-medical face masks purchased from the Japanese market since the Covid-19 outbreak. J Environ Sci Health A Tox Hazard Subst Environ Eng. (2022) 57:193–7. doi: 10.1080/10934529.2022.2047560
50. de Wijs, LEM, Joustra, MM, Olydam, JI, Nijsten, T, and Hijnen, DJ. Covid-19 in patients with cutaneous immune-mediated diseases in the Netherlands: real-world observational data. J Eur Acad Dermatol Venereol. (2021) 35:e173–6. doi: 10.1111/jdv.17025
51. Tasic-Kostov, M, Martinovic, M, Ilic, D, and Cvetkovic, M. Cotton versus medical face mask influence on skin characteristics during Covid-19 pandemic: a short-term study. Skin Res Technol. (2022) 28:66–70. doi: 10.1111/srt.13091
52. Desai, SR, Kovarik, C, Brod, B, James, W, Fitzgerald, ME, Preston, A, et al. Covid-19 and personal protective equipment: treatment and prevention of skin conditions related to the occupational use of personal protective equipment. J Am Acad Dermatol. (2020) 83:675–7. doi: 10.1016/j.jaad.2020.05.032
53. Proietti, I, Borrelli, I, Skroza, N, Santoro, PE, Gualano, MR, Bernardini, N, et al. Adverse skin reactions to personal protective equipment during Covid-19 pandemic in Italian health care workers. Dermatol Ther. (2022) 35:e15460. doi: 10.1111/dth.15460
54. Singh, M, Pawar, M, Bothra, A, Maheshwari, A, Dubey, V, Tiwari, A, et al. Personal protective equipment induced facial dermatoses in healthcare workers managing coronavirus disease 2019. J Eur Acad Dermatol Venereol. (2020) 34:e378–80. doi: 10.1111/jdv.16628
55. Altunisik Toplu, S, Altunisik, N, Turkmen, D, and Ersoy, Y. Relationship between hand hygiene and cutaneous findings during Covid-19 pandemic. J Cosmet Dermatol. (2020) 19:2468–73. doi: 10.1111/jocd.13656
56. Yuan, X, Xi, H, le, Y, Xu, H, Wang, J, Meng, X, et al. Online survey on healthcare skin reactions for wearing medical-grade protective equipment against Covid-19 in Hubei Province, China. PLoS One. (2021) 16:e0250869. doi: 10.1371/journal.pone.0250869
57. Tabary, M, Araghi, F, Nasiri, S, and Dadkhahfar, S. Dealing with skin reactions to gloves during the Covid-19 pandemic. Infect Control Hosp Epidemiol. (2021) 42:247–8. doi: 10.1017/ice.2020.212
58. Alves, PB, Alves, MP, Todo-Bom, A, and Regateiro, FS. Concomitant allergic contact dermatitis and Aquagenic Urticaria caused by personal protective equipment in a healthcare worker during the Covid-19 pandemic. Contact Dermatitis. (2021) 85:471–2. doi: 10.1111/cod.13897
59. Edelstam, G, Arvanius, L, and Karlsson, G. Glove powder in the hospital environment -- consequences for healthcare workers. Int Arch Occup Environ Health. (2002) 75:267–71. doi: 10.1007/s00420-001-0296-y
60. Ho, WYB, Tan, LYC, Zhao, X, Wang, D, and Lim, HLJ. Epidemiology of occupational dermatoses associated with personal protective equipment use in the Covid-19 pandemic: risk factors and mitigation strategies for frontline health care workers. JAAD Int. (2022) 8:34–44. doi: 10.1016/j.jdin.2022.03.013
61. Silva, L, Almeida, AGA, Pascoal, LM, Santos Neto, M, Lima, FET, and Santos, FS. Skin injuries due to personal protective equipment and preventive measures in the Covid-19 context: An integrative review. Rev Lat Am Enfermagem. (2022) 30:e3551. doi: 10.1590/1518-8345.5636.3551
62. Xia, W, Fu, L, Liao, H, Yang, C, Guo, H, and Bian, Z. The physical and psychological effects of personal protective equipment on health Care Workers in Wuhan, China: a cross-sectional survey study. J Emerg Nurs. (2020) 46:791–801.e7. doi: 10.1016/j.jen.2020.08.004
63. Atay, S, and Cura, SU. Problems encountered by nurses due to the use of personal protective equipment during the coronavirus pandemic: results of a survey. Wound Manag Prev. (2020) 66:12–6. doi: 10.25270/wmp.2020.10.1216
64. Hu, K, Fan, J, Li, X, Gou, X, Li, X, and Zhou, X. The adverse skin reactions of health care workers using personal protective equipment for Covid-19. Medicine (Baltimore). (2020) 99:e20603. doi: 10.1097/MD.0000000000020603
65. Agarwal, A, Agarwal, S, and Motiani, P. Difficulties encountered while using Ppe kits and how to overcome them: An Indian perspective. Cureus. (2020) 12:e11652. doi: 10.7759/cureus.11652
66. Mehta, RD, and Bumb, RA. Sweat dermatitis. Int J Dermatol. (2000) 39:872. doi: 10.1046/j.1365-4362.2000.00914.x
67. Jose, S, Cyriac, MC, and Dhandapani, M. Health problems and skin damages caused by personal protective equipment: experience of frontline nurses caring for critical Covid-19 patients in intensive care units. Indian J Crit Care Med. (2021) 25:134–9. doi: 10.5005/jp-journals-10071-23713
68. Marraha, F, Al Faker, I, Charif, F, Chahoub, H, Benyamna, Y, Rahmani, N, et al. Skin reactions to personal protective equipment among first-line Covid-19 healthcare workers: a survey in northern Morocco. Ann Work Expo Health. (2021) 65:998–1003. doi: 10.1093/annweh/wxab018
69. Silva, LFM, Almeida, AGA, Pascoal, LM, Santos Neto, M, Lima, FET, and Santos, FS. Skin injuries due to personal protective equipment and preventive measures in the Covid-19 context: an integrative review. Rev Lat Am Enfermagem. (2022) 30:30. doi: 10.1590/1518-8345.5636.3522
70. Yang, W, Chen, T, Wang, H, and He, R. Simulation of medical goggles to stop airborne transmission of viruses: computational fluid dynamics in ergonomics. Ergonomics. (2022) 66:350–65. doi: 10.1080/00140139.2022.2084565
71. Byambasuren, O, Beller, E, Clark, J, Collignon, P, and Glasziou, P. The effect of eye protection on Sars-Cov-2 transmission: a systematic review. Antimicrob Resist Infect Control. (2021) 10:156. doi: 10.1186/s13756-021-01025-3
72. Lan, J, Song, Z, Miao, X, Li, H, Li, Y, Dong, L, et al. Skin damage among health care workers managing coronavirus Disease-2019. J Am Acad Dermatol. (2020) 82:1215–6. doi: 10.1016/j.jaad.2020.03.014
73. Tcharkhtchi, A, Abbasnezhad, N, Zarbini Seydani, M, Zirak, N, Farzaneh, S, and Shirinbayan, M. An overview of filtration efficiency through the masks: mechanisms of the aerosols penetration. Bioact Mater. (2021) 6:106–22. doi: 10.1016/j.bioactmat.2020.08.002
74. Dowdle, TS, Thompson, M, Alkul, M, Nguyen, JM, and Sturgeon, ALE. Covid-19 and dermatological personal protective equipment considerations. Proc (Bayl Univ Med Cent). (2021) 34:469–72. doi: 10.1080/08998280.2021.1899730
75. Prevention CCfDCa. Chinese Center for Disease Control and Prevention: How to prevent the public? (general prevention section). (2020). Available at: https://www.chinacdc.cn/jkzt/crb/zl/szkb_11803/jszl_2275/202001/t20200125_211447.html (Accessed January 25, 2020).
76. World Health Organization. Who guidelines on hand hygiene in health care. Geneva: World Health Organization (2009). Available at: https://www.who.int/publications/i/item/9789241597906 (Accessed January 13, 2023).
77. Saha, T, Khadka, P, and Das, SC. Alcohol-based hand sanitizer–composition, proper use and precautions. Germs. (2021) 11:408–17. doi: 10.18683/germs.2021.1278
78. Villa, C, and Russo, E. Hydrogels in Hand Sanitizers. Materials (Basel). (2021) 14:7. doi: 10.3390/ma14071577
79. Graca, A, Martins, AM, Ribeiro, HM, and Marques, MJ. Indirect consequences of coronavirus disease 2019: skin lesions caused by the frequent hand sanitation and use of personal protective equipment and strategies for their prevention. J Dermatol. (2022) 49:805–17. doi: 10.1111/1346-8138.16431
80. Al Zaabi, A, Abdelhadi, S, and Ruszczak, Z. Personal protective equipment-related dermatoses in Covid-19 frontline health workers. A lesson learned from 1-year single Center in the Uae. Dermatol Ther. (2022) 35:e15624. doi: 10.1111/dth.15624
81. Mirali, S, Fleming, P, and Lynde, CW. Moisturizers and cleansers in the Management of Skin Conditions Caused by personal protective equipment and frequent handwashing. Skin Therapy Lett. (2021) 26:9–13.
82. Rundle, CW, Presley, CL, Militello, M, Barber, C, Powell, DL, Jacob, SE, et al. Hand hygiene during Covid-19: recommendations from the American contact dermatitis society. J Am Acad Dermatol. (2020) 83:1730–7. doi: 10.1016/j.jaad.2020.07.057
83. Brinkmann, I, and Muller-Goymann, CC. Role of isopropyl Myristate, isopropyl alcohol and a combination of both in hydrocortisone permeation across the human stratum Corneum. Skin Pharmacol Appl Ski Physiol. (2003) 16:393–404. doi: 10.1159/000072935
84. Dear, K, Palmer, A, and Nixon, R. Contact allergy and allergic contact dermatitis from Benzalkonium chloride in a tertiary dermatology Center in Melbourne, Australia. Contact Dermatitis. (2021) 2021:13826. doi: 10.1111/cod.13826
85. Warner, RR, Boissy, YL, Lilly, NA, Spears, MJ, McKillop, K, Marshall, JL, et al. Water disrupts stratum Corneum lipid lamellae: damage is similar to surfactants. J Invest Dermatol. (1999) 113:960–6. doi: 10.1046/j.1523-1747.1999.00774.x
86. Khosrowpour, Z, Ahmad Nasrollahi, S, Ayatollahi, A, Samadi, A, and Firooz, A. Effects of four soaps on skin trans-epidermal water loss and erythema index. J Cosmet Dermatol. (2019) 18:857–61. doi: 10.1111/jocd.12758
87. Panda, PK, and Sharawat, IK. Fluctuating palmar erythema in a toddler during Covid-19 pandemic: do you know the offender? J Trop Pediatr. (2021) 67:11. doi: 10.1093/tropej/fmab011
88. Loh, EW, and Yew, YW. Hand hygiene and hand eczema: a systematic review and Meta-analysis. Contact Dermatitis. (2022) 87:303–14. doi: 10.1111/cod.14133
89. Metin, N, Turan, C, and Utlu, Z. Changes in dermatological complaints among healthcare professionals during the Covid-19 outbreak in Turkey. Acta Dermatol Alp Pannonica Adriat. (2020) 29:115–22. doi: 10.15570/actaapa.2020.25
90. Polecka, A, Owsianko, N, Awchimkow, A, Baran, A, Hermanowicz, JM, and Flisiak, I. Questionnaire-based study evaluating the hand hygiene practices and the impact of disinfection in the Covid-19 pandemic on hand skin conditions in Poland. J Clin Med. (2022) 12:195. doi: 10.3390/jcm12010195
91. O’Neill, H, Narang, I, Buckley, DA, Phillips, TA, Bertram, CG, Bleiker, TO, et al. Occupational dermatoses during the Covid-19 pandemic: a multicentre audit in the Uk and Ireland. Br J Dermatol. (2021) 184:575–7. doi: 10.1111/bjd.19632
92. Alsaidan, MS, Abuyassin, AH, Alsaeed, ZH, Alshmmari, SH, Bindaaj, TF, and Alhababi, AA. The prevalence and determinants of hand and face dermatitis during Covid-19 pandemic: a population-based survey. Dermatol Res Pract. (2020) 2020:6627472. doi: 10.1155/2020/6627472
93. Dash, G, Patro, N, Dwari, BC, and Abhishek, K. Dermatological impact of hand hygiene practices during Covid-19: a cross-sectional web-based survey among doctors in a tertiary Care Hospital in Eastern India. J Cosmet Dermatol. (2022) 21:1804–8. doi: 10.1111/jocd.15508
94. Cebeci, D, Karasel, S, Rifki, D, Yesildagli, H, and Kalfaoglu, M. The effect of personal protective equipment (Ppe) and disinfectants on skin health during Covid 19 Pandemia. Med Arch. (2021) 75:361–5. doi: 10.5455/medarh.2021.75.361-365
95. Lin, P, Zhu, S, Huang, Y, Li, L, Tao, J, Lei, T, et al. Adverse skin reactions among healthcare workers during the coronavirus disease 2019 outbreak: a survey in Wuhan and its surrounding regions. Br J Dermatol. (2020) 183:190–2. doi: 10.1111/bjd.19089
96. Montero-Vilchez, T, Martinez-Lopez, A, Cuenca-Barrales, C, Quiñones-Vico, MI, Sierra-Sanchez, A, Molina-Leyva, A, et al. Assessment of hand hygiene strategies on skin barrier function during Covid-19 pandemic: a randomized clinical trial. Contact Dermatitis. (2022) 86:276–85. doi: 10.1111/cod.14034
97. Leveau, CM, Aouissi, HA, and Kebaili, FK. Spatial diffusion of Covid-19 in Algeria during the third wave. GeoJournal. (2022) 88:1175–80. doi: 10.1007/s10708-022-10608-5
98. Hardison, RL, Nelson, SW, Limmer, R, Marx, J, Taylor, BM, James, RR, et al. Efficacy of chemical disinfectants against Sars-Cov-2 on high-touch surface materials. J Appl Microbiol. (2022) 2022:20. doi: 10.1093/jambio/lxac020
99. Morawska, L, Tang, JW, Bahnfleth, W, Bluyssen, PM, Boerstra, A, Buonanno, G, et al. How can airborne transmission of Covid-19 indoors be minimised? Environ Int. (2020) 142:105832. doi: 10.1016/j.envint.2020.105832
100. Viana Martins, CP, Xavier, CSF, and Cobrado, L. Disinfection methods against Sars-Cov-2: a systematic review. J Hosp Infect. (2022) 119:84–117. doi: 10.1016/j.jhin.2021.07.014
101. Goh, CF, Ming, LC, and Wong, LC. Dermatologic reactions to disinfectant use during the Covid-19 pandemic. Clin Dermatol. (2021) 39:314–22. doi: 10.1016/j.clindermatol.2020.09.005
102. Dindarloo, K, Aghamolaei, T, Ghanbarnejad, A, Turki, H, Hoseinvandtabar, S, Pasalari, H, et al. Pattern of disinfectants use and their adverse effects on the consumers after Covid-19 outbreak. J Environ Health Sci Eng. (2020) 18:1301–10. doi: 10.1007/s40201-020-00548-y
103. Gharpure, R, Hunter, CM, Schnall, AH, Barrett, CE, Kirby, AE, Kunz, J, et al. Knowledge and practices regarding safe household cleaning and disinfection for Covid-19 prevention–United States, May 2020. MMWR Morb Mortal Wkly Rep. (2020) 69:705–9. doi: 10.15585/mmwr.mm6923e2
104. Rutala, WA, and Weber, DJ. Disinfection, sterilization, and antisepsis: An overview. Am J Infect Control. (2016) 44:e1–6. doi: 10.1016/j.ajic.2015.10.038
105. Tartari, F, Vincenzi, C, Di Altobrando, A, Bruni, F, and Neri, I. Allergic contact dermatitis to Benzalkonium chloride with erythema Multiforme-like reaction in a child. Contact Dermatitis. (2020) 82:397–9. doi: 10.1111/cod.13481
106. Robinson, AJ, Foster, RS, Halbert, AR, King, E, and Orchard, D. Granular parakeratosis induced by Benzalkonium chloride exposure from laundry rinse Aids. Australas J Dermatol. (2017) 58:e138–40. doi: 10.1111/ajd.12551
107. Tian, CJ, Purvis, D, and Cheng, HS. Granular parakeratosis secondary to Benzalkonium chloride exposure from common household laundry rinse Aids. N Z Med J. (2021) 134:128–42.
Keywords: COVID-19, contact dermatitis, personal protective equipment, dermatology, hygiene
Citation: Tang H, Wang H, Hamblin MR, Jiang L, Zhou Y, Xu Y and Wen X (2023) Contact dermatitis caused by prevention measures during the COVID-19 pandemic: a narrative review. Front. Public Health. 11:1189190. doi: 10.3389/fpubh.2023.1189190
Edited by:
Russell Kabir, Anglia Ruskin University, United KingdomReviewed by:
Hani Amir Aouissi, Scientific and Technical Research Center on Arid Regions (CRSTRA), AlgeriaMuhammad Mainuddin Patwary, Environment and Sustainability Research Initiative, Bangladesh
Copyright © 2023 Tang, Wang, Hamblin, Jiang, Zhou, Xu and Wen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiang Wen, eGlhbmd3ZW5fd2N1bXNAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Huimiao Tang
Huimiao Tang Hao Wang2†
Hao Wang2† Michael R. Hamblin
Michael R. Hamblin Yanjun Zhou
Yanjun Zhou Yidan Xu
Yidan Xu Xiang Wen
Xiang Wen