- 1Genomics and Bioanalytics, Los Alamos National Laboratory, Los Alamos, NM, United States
- 2International Livestock Research Institute and ILRI/BMZ One Health Research, Education, Outreach and Awareness Centre, Nairobi, Kenya
- 3National Livestock Resources Research Institute, National Agricultural Research Organization, Kampala, Uganda
- 4Emergency Centre for Transboundary Animal Diseases, Food and Agriculture Organization of the United Nations, Nairobi, Kenya
- 5Department of Veterinary Tropical Diseases, University of Pretoria, Onderstepoort, South Africa
Abattoirs are facilities where livestock are slaughtered and are an important aspect in the food production chain. There are several types of abattoirs, which differ in infrastructure and facilities, sanitation and PPE practices, and adherence to regulations. In each abattoir facility, worker exposure to animals and animal products increases their risk of infection from zoonotic pathogens. Backyard abattoirs and slaughter slabs have the highest risk of pathogen transmission because of substandard hygiene practices and minimal infrastructure. These abattoir conditions can often contribute to environmental contamination and may play a significant role in disease outbreaks within communities. To assess further the risk of disease, we conducted a scoping review of parasites and pathogens among livestock and human workers in abattoirs across 13 Eastern African countries, which are hotspots for zoonoses. Our search results (n = 104 articles) showed the presence of bacteria, viruses, fungi, and macroparasites (nematodes, cestodes, etc.) in cattle, goats, sheep, pigs, camels, and poultry. Most articles reported results from cattle, and the most frequent pathogen detected was Mycobacterium bovis, which causes bovine tuberculosis. Some articles included worker survey and questionnaires that suggested how the use of PPE along with proper worker training and safe animal handling practices could reduce disease risk. Based on these findings, we discuss ways to improve abattoir biosafety and increase biosurveillance for disease control and mitigation. Abattoirs are a ‘catch all’ for pathogens, and by surveying animals at abattoirs, health officials can determine which diseases are prevalent in different regions and which pathogens are most likely transmitted from wildlife to livestock. We suggest a regional approach to biosurveillance, which will improve testing and data gathering for enhanced disease risk mapping and forecasting. Next generation sequencing will be key in identifying a wide range of pathogens, rather than a targeted approach.
1. Introduction
An abattoir, commonly known as a slaughterhouse, is a facility where livestock are slaughtered for food. Abattoirs are key elements in the global food production chain and are found all over the world. Each country has unique protocols for slaughtering animals based on the size of the facility, location within communities, national and subnational regulations, and customs, including the predominant religion of the people (1). In developing countries, the raising and slaughtering of livestock is a common practice in rural areas and abattoirs (including slaughter slabs and sometimes backyard slaughters) are essential to the livelihood of the community (1, 2).
Different stakeholders including butchers, traders, farm gate buyers, transporters, abattoir assistants, and water suppliers often populate abattoirs. These workers are exposed to animals, animal products, and animal waste in all types of abattoirs. There is concern that unregulated abattoirs have higher rates of occupational health problems, including zoonoses, diseases that are transmitted from animals to people, particularly because pre-slaughter and post-slaughter inspection may not be strict (3). There are four general types of abattoirs: export abattoirs, national abattoirs, municipal abattoirs, and backyard slaughter facilities or slaughter slabs (4). Figure 1 shows photos of each type of abattoir while Table 1 provides descriptions, requirements and operationalization, and associated risks. Export abattoirs are the most regulated since they are certified by national regulatory bodies and have in-house official meat inspectors. Meat is generally exported to other countries or continents, and there is low risk of infection due to state-of-the-art infrastructure and hygiene standards. National abattoirs adhere to government regulations to distribute meat within the same country. These locations have low to medium risk of pathogen transmission due to consistent veterinary meat inspections, adequate infrastructure, and sanitation practices (Table 1). Municipal abattoirs supply meat to the sub-national system, but do not comply with all national government regulations and safety requirements. These abattoirs have medium to high risk of infection because of limited infrastructure and inconsistent training, sanitation, and hygiene practices. In rural areas, backyard slaughter facilities are poorly built and often lack fencing, walls, or a roof (13) (Figure 1; Table 1). Butchering can even occur on bare ground. These facilities have a high risk of disease transmission because of minimal supervision, substandard hygiene practices and infrastructure, lack of awareness regarding disease risk, and absence of training programs (2, 13).

Figure 1. Photos showing the different types of abattoirs, including export abattoirs (A–C), national abattoirs (D,E), municipal abattoirs (F–H), and local, backyard slaughter slabs (I–L). Photo credit: Folorunso Fasina and Susan Kerfua.

Table 1. Types of abattoirs in Eastern Africa with descriptions, requirements and operationalization, and risks associated with each one.
Exposure to livestock pathogens, including bacteria, viruses, fungi, and parasites (14) can cause human morbidity and mortality. Infected livestock presents an occupational hazard to workers that encounter blood, placenta, fetuses, and uterine secretions. Human infection with pathogens at abattoirs can then lead to local outbreaks among workers (15) and can spread throughout a community either through ingestion or through direct or indirect contact (16, 17). Furthermore, each infected animal carcass that is condemned decreases the available food output for the community and reduces farmers’ incomes. Animals that are infected with dangerous pathogens can become unthrifty, die, and abort fetuses, thereby directly affecting livelihoods and economic security. For example, brucellosis, a serious bacterial disease (Brucella spp.) that infects livestock, can lead to low birth rates due to abortions and stillbirths (18). The extra resources that are used to prevent and limit human infection from zoonoses are an additional cost (19). Disposal of infected carcasses, for example, is a challenge because they have to be incinerated or buried. To avoid having meat condemned, animals could be immediately taken to the abattoir at the first signs of disease, for the owner to reduce the burden of losses through partial cost recovery. However, when these infected animals end up at an abattoir, workers, other livestock, and the food chain are at risk of zoonoses and trade-sensitive diseases (20).
In developing countries, inadequate veterinary infrastructure, lack of hygiene, improper meat inspection, scarcity of protective clothing, insufficient work practice knowledge, and inadequate number of staff all reduce work efficiencies and increase the likelihood of abattoir workers becoming infected. This can be a major source of foodborne illnesses, blood-borne infections, or physical injuries (21, 22). Unlike developed countries that have large industrial meat processing facilities with mandatory regulations and protocols, developing nations may have several unregulated facilities in rural areas where personal protective equipment and training is minimal or unused. In these locations, workers are more susceptible to adverse health effects, such as diarrhea, skin infections, pneumonia, meningitis, and sepsis. Some common zoonotic bacterial pathogens found in abattoirs, such as Salmonella, Campylobacter, and Pseudomonas (23) are becoming resistant to antibiotics and can lead to hospitalization, longer recovery time, and death. Due to the public health and economic security concerns, there is a need for governments and stakeholders to enhance abattoir infrastructure and workplace safety.
In some abattoirs, the daily supply of animals brought in for slaughter exceeds the production output. Without the organized accommodation, animal carcasses remain outside after slaughter, and are scavenged by wildlife communities. Dependent species, such as vultures and other scavenging birds (e.g., marabou stork), potentially limit infection to humans (24). However, many of these scavengers are endangered. Vulture declines are shown to lead to more feral dogs. Human interaction with these feral companions can increase the risk of zoonotic infection and lead to diseases like rabies (25). Vultures provide a crucial ecosystem service in scavenging carcasses of dead animals, and abattoirs in general have been found to be important for supporting a large number and diversity of scavenger species (26). The dissemination of carcass remains into the environment through vultures may be less risky for additional disease propagation due to scavenging species having evolved to live on decaying meat by having more acidic digestion systems than other animals (27). Understanding the system of abattoirs is coupled with understanding the avian scavenger crisis of looming extinctions and the loss of critical ecosystem functions (26).
Environmental contamination is an added risk since the abattoir wastewater and effluent may contain animal-products, pathogens (including antimicrobial resistant microbes), parasites, and residues (25, 28). Most backyard abattoirs and slaughter slabs described in Table 1 do not have the capacity and infrastructure to treat abattoir liquid waste. Therefore, liquid waste, which consists of urine, blood, and wastewater is released into surrounding areas when it rains via waterways. These pollutants can secrete into landscapes and negatively impact the environment. For example, abattoir runoff can cause harmful algal blooms (29) and result in antimicrobial resistant bacteria (30) if animal remains are not disposed of properly (31). Also, some surface waters such as streams and wells near abattoirs are highly contaminated with microbes and chemicals and become unfit for human consumption (32, 33).
In recent years, there is increased interest in abattoir-related research in Eastern Africa, but to date, we are unaware of a comprehensive review on the association of abattoirs with zoonotic disease infection in this part of the world. This scoping review will explore data collected specifically from abattoirs in Eastern Africa. The main questions for this review are (1): What parasites and pathogens have been reported from livestock and humans working at abattoirs in Eastern Africa? (2) What are other risk factors associated with these abattoirs? (3) What are things to consider for improving abattoir biosecurity and increasing biosurveillance for disease control and mitigation? In this review, we identify common zoonotic pathogens in livestock slaughtered in abattoirs and in abattoir workers, identify the sample types and tests (e.g., PCR, ELISA, culture, gross meat examination, etc.) used for pathogen detection, understand the circumstances surrounding infection risk, and identify the necessary steps to decrease disease risk at these potential hotspots. We highlight some of the growing trepidations associated with abattoirs in developing nations and propose recommendations and solutions to protect abattoir workers from occupational hazards and emerging zoonosis. We also suggest ways to improve biosurveillance at abattoirs in Eastern Africa in particular. Lastly, we cover the ecological feedback of abattoirs and their potential importance to wildlife communities.
2. Methods
The scope of this review was limited to abattoirs in the following Eastern African countries that are common hotspots for zoonoses: Uganda, Tanzania, Kenya, Ethiopia, Rwanda, Mozambique, Somalia, Djibouti, Madagascar, South Sudan, Eritrea, Burundi, and Zambia. We used PRISMA guidelines for scoping reviews and only included scientific peer-reviewed literature in the viable results. Web of Science was the primary database used for the search strategy. The search range consisted of data from January 1, 2010 to June 1, 2022. The following criteria was entered into the advanced search box: TS = (“slaughterhouse” OR “abattoir”) AND TS = (“zoonotic” OR “zoonoses”) AND TS = (“Ethiopia”); TS = (“slaughterhouse” OR “abattoir”) AND TS = (“zoonotic” OR “zoonoses”) AND TS = (“Tanzania”); TS = (“slaughterhouse” OR “abattoir”) AND TS = (“zoonotic” OR “zoonoses”) AND TS = (“Uganda”); TS = (“slaughterhouse” OR “abattoir”) AND TS = (“zoonotic” OR “zoonoses”) AND TS = (“Kenya”); TS = (“slaughterhouse” OR “abattoir”) AND TS = (“zoonotic” OR “zoonoses”) AND TS = (“Kenya” OR “Uganda” OR “Tanzania” OR “Rwanda” OR “Ethiopia”). TS = (“slaughterhouse” OR “abattoir”) AND TS = (“zoonotic” OR “zoonoses”) AND TS = (“Burundi”); TS = (“slaughterhouse” OR “abattoir”) AND TS = (“zoonotic” OR “zoonoses”) AND TS = (“Djibouti”); TS = (“slaughterhouse” OR “abattoir”) AND TS = (“zoonotic” OR “zoonoses”) AND TS = (“Eritrea”); TS = (“slaughterhouse” OR “abattoir”) AND TS = (“zoonotic” OR “zoonoses”) AND TS = (“Madagascar”); TS = (“slaughterhouse” OR “abattoir”) AND TS = (“zoonotic” OR “zoonoses”) AND TS = (“Mozambique”); TS = (“slaughterhouse” OR “abattoir”) AND TS = (“zoonotic” OR “zoonoses”) AND TS = (“Somalia”); TS = (“slaughterhouse” OR “abattoir”) AND TS = (“zoonotic” OR “zoonoses”) AND TS = (“South Sudan”); TS = (“slaughterhouse” OR “abattoir”) AND TS = (“zoonotic” OR “zoonoses”) AND TS = (“Zambia”).
An initial search generated 297 results. Upon first review, several of the findings were not affiliated with the location of interest, Eastern Africa, and were removed. Most studies were filtered out because they were duplicates, not conducted in abattoirs, or were done in other countries. After all the search results were adequately screened, we retrieved a total of 104 applicable articles. We kept articles that tested for and/or found parasites and pathogens in livestock and humans, as well as articles that described the results of questionnaires or surveys of abattoir workers. We removed articles that were reviewing specific diseases, but we kept results of meta-analyzes, since they reported the prevalence of pathogens from specific abattoirs. The final tally of relevant articles was 43 for Ethiopia, 20 for Kenya, 14 for Uganda, 12 for Tanzania, 5 for Madagascar, 3 for Zambia, 2 for Rwanda, 2 for South Sudan, 2 for Djibouti, 1 for Eritrea, 0 for Burundi, Mozambique, and Somalia, for a total of 104 articles (Figure 2).
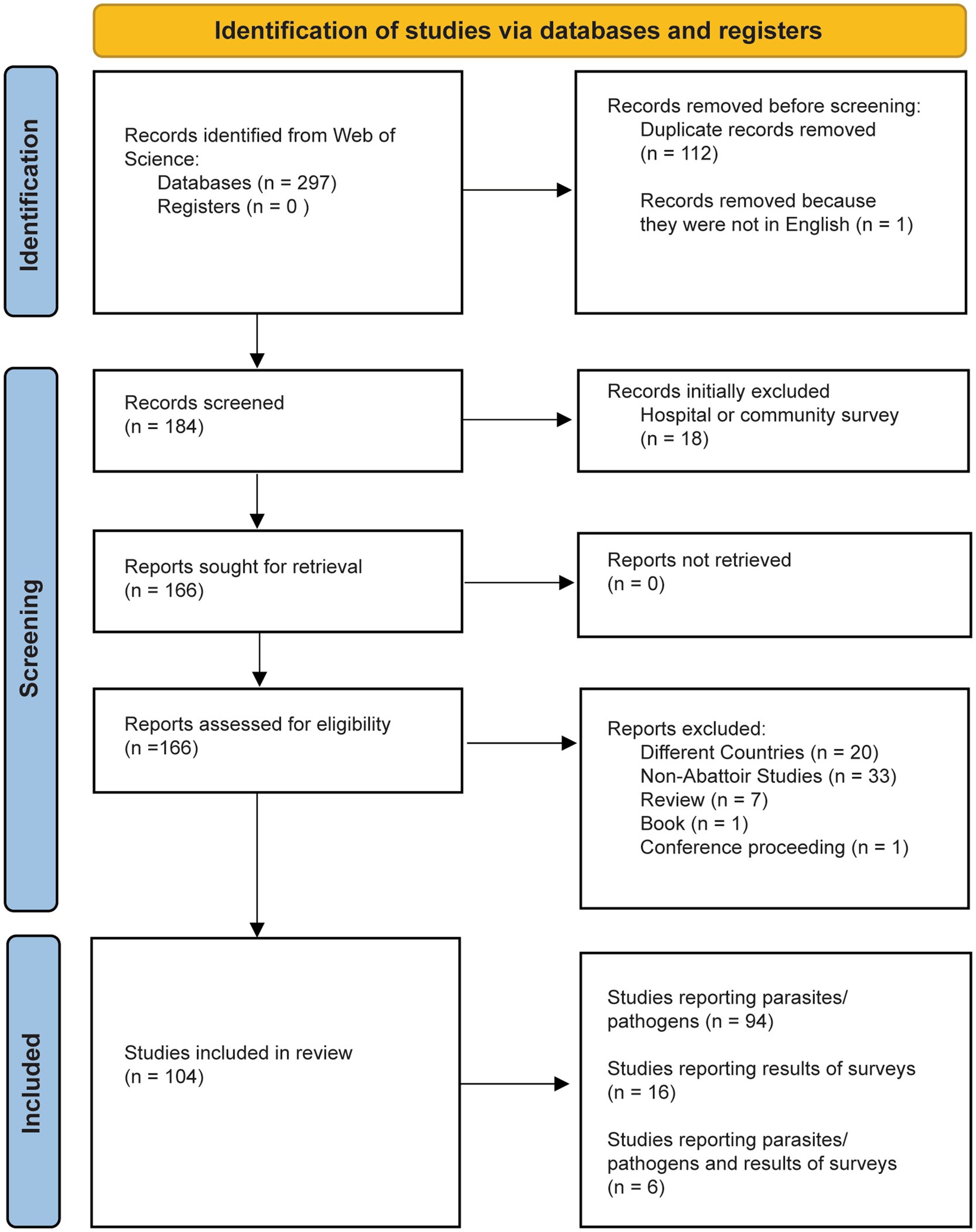
Figure 2. Identification, screening, and inclusion of articles in this review. Our search resulted in 104 articles.
The goal was not to find every pathogen detected, but to find the most common zoonotic pathogens. During the literature search, we recorded all the pathogens that were detected in each article even if they were not zoonotic pathogens. Based on our search criteria, we may have missed a few records of specific zoonotic diseases because the articles did not use the term zoonotic to refer to the specific pathogens. We could have also missed articles that did not use the terms abattoir or slaughterhouse. However, we are confident that we found the most important and common zoonotic pathogens during our search.
The final group of articles were summarized for the following data: disease and pathogens found in abattoir workers and livestock sampled at abattoirs, tests used to determine positive samples, prevalence or seroprevalence of the pathogen in humans and/or livestock, and what species of livestock was tested. We only list diseases which are caused by parasites and pathogens, not those caused by other issues, such as anthracosis (black dust in the lungs caused by breathing in carbon particles in urban areas) and melanosis. We also did not include diseases or conditions for which there could be multiple causes and the specific agent responsible was not determined (e.g., pleurisy). For the articles that reported results of questionnaires and surveys, we summarized this information and present some of the major findings regarding personal protective equipment (PPE), knowledge of disease risk, sanitation, training, and vaccination.
3. Results of literature survey
3.1. Pathogens and diseases identified in abattoirs
There were 104 articles found during the literature search that met the search criteria (Figure 2). Out of these results, 94 directly tested and found parasites and pathogens in livestock of human samples, 16 reported results of worker surveys and questionnaires (Figure 2); and 6 reported the results of both questionnaires and direct detection of the parasite or pathogen being examined. All countries except Burundi, Mozambique, and Somalia had at least one article on zoonotic pathogens in abattoirs.
Both livestock and humans were either tested or visually examined for pathogens in Eastern African abattoirs. Several articles were abattoir surveys in which they reported the results of many meat inspections and many samples taken from individual livestock animals. Therefore, these articles found more than one parasite or pathogen. Because of multiple parasite/pathogen records per article, we found 130 individual parasite/pathogen records out of the 94 articles (Table 2). These parasite/pathogen records were comprised of 42 species groups.
Articles documented bacterial (71 records), viral (14 records), and fungal (2 records) pathogens as well as macroparasites (43 records), such as nematodes, cestodes, and trematodes (Table 2). We found two records of intracellular protozoan parasites, Sarcocystis spp. and Plasmodium falciparum (malaria). The most common pathogen detected in abattoirs was Mycobacterium bovis, causing bovine tuberculosis (BTB) (n = 22 articles), followed by Echinococcus spp. causing Echinococcosis/hydatidosis (n = 16 articles). There were 9 articles that found Taenia saginata/Cysticercus bovis, which causes bovine cysticercosis.
Most animals surveyed were cattle. There were 86 parasite/pathogen records in cattle. Sheep (15 records), goats (18 records), pigs (18 records), and camels (6 records) were also screened (Table 2). There was only one article that tested poultry. In Ethiopia, chickens tested positive for Campylobacter spp. using standard bacteriological techniques and PCR (Table 2). A high proportion of these bacteria were found to be resistant to ampicillin, amoxicillin, and streptomycin (72). There were seven instances of ticks being tested for bacterial and viral pathogens after they were removed from livestock. Rickettsia spp., African swine fever virus, Crimean-Congo hemorrhagic fever virus, and Alkhurma hemorrhagic fever virus were found in ticks feeding on livestock in abattoirs (Table 2). Tick sampling seems to be an overlooked method to track pathogens at abattoirs. Some zoonotic pathogens can be vectored by ticks, and sampling ticks could provide more knowledge about transmission among livestock and humans.
There were 21 instances of parasites and pathogens found in humans (Table 2). There were five articles that tested for Brucella spp., the most common pathogen tested for. In humans, most of the articles used various serology tests to test for past exposure. For example, tests included Rose Bengal plate test and agglutination tests for Brucella spp., ELISA for Leptospira spp., and IgM and IgG serology for Rift Valley fever virus. There was only one article that used PCR, and they tested for Mycobacterium bovis. One article was a meta-analysis of humans with bovine cysticercosis (Taenia saginata), which summarized prevalence using a variety of articles and methods. A second article was a questionnaire to determine past history of having human taeniasis, also caused by T. saginata, but did not directly test them in humans (Table 3). Out of 151 respondents, 71.5% reported having human taeniasis (38).
Most tests used to determine animal or human infection were done using various serology tests, very few used PCR or sequencing. For example, for determining brucellosis infection, most studies used the Rose Bengal test and the complement fixation test. Most of these studies, therefore, give information regarding past exposure. For other parasites and pathogens, meat condemnation was listed, which is usually performed by a meat inspector, which looks for lesions, cysts, changes in color, and abnormal size of meat products as well as organs such as the lungs, liver, kidneys, hearts, and spleen (36). During meat inspection, a number of parasites including, but not limited to, trematodes or nematodes can be observed, and can be subsequently collected. Cysts or lesions can be sampled for further identification using molecular tools, culturing, microscopy (e.g., histopathology), and standard biochemical tests for bacteria. The following parasites and pathogens were typically found using gross examination during routine meat inspection: Taenia saginata/Cysticercus bovis, Mycobacterium bovis, Echinococcus spp., Fasciola spp., and Onchocerca spp. For some, like many bacterial species, further testing involved culturing, microscopy, and PCR tests (Table 2).
Two dimorphic fungal pathogens were detected in cattle slaughtered in a Kenyan abattoir: Paracoccidioides brasiliensis, which causes paracoccidioidomycosis and Blastomyces dermatitidis, which causes blastomycosis (37). Both of these pathogens were found using cellular microscopy. Out of 176 lesions found from 929 cattle examined, 58 tested positive for dimorphic fungi. No other studies tested for fungal pathogens. The true prevalence of these fungal pathogens is unclear and understanding livestock infection can improve surveillance efforts and environmental monitoring, which is important for understanding and tracking emerging fungal pathogens. Both fungal pathogens can infect humans, and blastomycosis has been listed as an emerging risk in both North America and Africa (133).
3.2. Surveys and questionnaires to assess training, sanitation, PPE, etc.
In this literature review, we found 16 articles in which surveys were conducted across Eastern Africa to assess the awareness of abattoir workers in developing nations. In each of the countries of interest in Eastern Africa, testimonials from abattoir workers demonstrated the need for disease control and implementation of prevention strategies. Through questionnaires and interviews with abattoir workers, studies found that there is an essential need for training programs, personal protective equipment, immunization programs for workers, infrastructure improvement, enhanced diagnostics and biosurveillance, and public awareness to minimize zoonosis and protect public health (Table 3).
The top subject evaluated in the surveys and questionnaires was knowledge of pathogen or disease risk from livestock (12/16 articles). Participants knew of common diseases like tuberculosis, anthrax, and brucellosis (123–125), but other diseases were less well known, such as MERS-CoV (124). Personal protective equipment (9/16 articles), consuming raw or undercooked meat (6/16 articles), and sanitation/meat safety (6/16 articles) were also asked about and evaluated in many articles (Table 2). Other subjects evaluated included infrastructure improvements, cross-border movement and trading of animals, training programs, and public awareness.
In a questionnaire survey conducted at the Harar Municipal Abattoir in Ethiopia, 300 randomly selected workers self-reported their awareness of taeniasis, caused by Taenia saginata, a zoonotic tapeworm parasite. Questions included awareness of taeniasis risks involved with consuming raw or undercooked meat and if workers have ever observed small tapeworm segments in their feces or clothing (41). Out of the respondents, 65% were conscious of the risks associated with T. saginata, including ingestion of raw or undercooked meat. Another 62% of respondents reported personal infection by the proglottids of T. saginata (41). The high association (p < 0.005) in infected participants was attributed to factors such as eating undercooked beef for religious customs, but more research is needed to determine whether the tapeworms originated from the affiliated abattoir or any of the other factors above (41). In a similar study conducted at the Kombolcha Elfora and Dessie city abattoirs in Ethiopia, 104 workers completed a questionnaire about their food safety knowledge, specifically pertaining to meat hygiene and safety techniques (129). Eighty-nine percent of participants did not know about meat safety and 74% were insufficient in their workplace practices (129).
In Kenya, public health officials offered a questionnaire to 737 participants across 142 slaughterhouses and asked about their knowledge of zoonosis, hygiene practices at the slaughterhouse, and slaughterhouse equipment practices (102). The participants also provided blood samples to check for leptospirosis, which is a pathogenic bacterial disease of the genus Leptospira with animal reservoirs (134). The blood test results showed a high seroprevalence of leptospirosis in 13.4% of the workers (102). Findings from the questionnaire showed that workers who were around urine or infected organs during evisceration of the carcass were at a higher risk for leptospirosis (102). Further analysis of the worker feedback showed that smoking, eating, or having an open wound created a pathway for infection within the abattoir. Slaughterhouses with roofs increased the chance of leptospirosis since the disease can live in cooler-shaded environments for longer periods of time if not adequately cleaned (102). Ingestion of waters from nearby well or spring water increased the chances of exposure due to contamination from slaughter waste or animal urine runoff.
In northern Tanzania, slaughterhouse workers, including meat inspectors, were interviewed to learn about their meat safety perceptions, priorities, and practices in relation to risk (125). The meat inspectors frequently mentioned their concern about anthrax since the disease can affect both animals and people. Several of the professionals recalled personal experiences, such as seeing visible worms in the animal intestines, and felt comfortable identifying anthrax based on the symptoms (125). Other than anthrax, many of the workers were able to identify signs of illness, such as swollen inner organs, but did not always know the name of the disease. The workers often attributed disease to unhealthy livestock keepers that may lack zoonotic awareness or are unwilling to invest in vaccination to prevent disease. When workers were asked how they know the meat is wholesome and fit for human consumption, they reported that they inspected the meat and that it was marked with a government stamp (125). For hygiene practices, all the workers knew the importance of keeping their workstation clean with soap and water but only two workers reported the importance of washing their work slab between each animal rotation. Workers reported the infrequent use of uniforms and discussed how dirty clothes, flip-flops, and lack of bathing can lead to contamination (125). Other reported contamination pathways were from the free roam of dogs, chickens, or wild birds that sometimes entered the slaughterhouse. Frequent handwashing was seldom, and meat knives were often dropped and reused after a brief wipe down (125). The same knives were used to isolate gut contents and explore feces.
In a study conducted in the Kabale District of Uganda, 348 participants from the local communities and abattoir were asked about their awareness surrounding the epidemiological risk of Rift Valley fever (126). Among the participants, 94% of butchers were aware of Rift Valley fever in comparison to 85% from other occupations (126). When the 348 participants were asked how the disease spreads, only 34 knew that Rift Valley fever can spread through mosquitos and infected bodily fluids (126). Butchers were able to identify symptoms, such as nasal discharge in animals. When asked about personal protective equipment, only 29% of participants reported usage. In this finding, butchers used the most PPE, including gumboots and aprons, most likely to reduce blood stains on their bodies and home dress, but rarely wore gloves (126). Based on the findings in Ethiopia, Kenya, Tanzania, and Uganda, there is an apparent need to increase Risk Communication and Community Engagement (RCCE) and utilize appropriate information, education, and communication (IEC) materials to create zoonotic awareness for abattoir workers and improve hygiene practices to protect public health.
4. Need for vaccination
Most countries demonstrated the need for vaccination to protect animals and people. For instance, one of the reasons for the continuation in the foot-and-mouth problem in Africa, includes poor performance of current animal vaccination programs (135). In a review of vaccination on the effectiveness and profitability of preventative veterinary interventions in controlling infectious diseases of ruminant livestock in sub-Saharan Africa, the authors found that vaccination is the most effective and profitable means of controlling infectious livestock diseases in sub-Saharan Africa (136). However, the authors note the challenges for disease control, and vaccination implementation must integrate pathogen surveillance and optimal vaccine delivery tools. Many, if not most, of the important zoonotic or economically devastating viral diseases in sub-Saharan Africa have developed vaccines. Controlling exposure and disease transmission in animals would reduce diseases arriving at a slaughterhouse.
In Ethiopia where there is a high human population that live in rural areas, many livestock are at the risk of brucellosis. Multiple surveys conducted in the Debre Zei and Modjo export abattoirs resulted in serological evidence of brucellosis, 1.76% in small ruminants (1.86% in caprine and 1.63% ovine brucellosis) (64). This relatively low level of seropositivity of antibodies to brucellosis may be underappreciated, and it may encourage silent spread of zoonosis to humans inadvertently. Furthermore, because Brucella spp. is a slow-growing organism, and people in developing countries often have poor hospitalization record (attendance), except in life-threatening situations, Brucella spp. infection may get established in humans, especially abattoir workers before it begins to manifest clinical signs that warrant hospitalization.
In a 9-month study conducted in 2012, a total of 566 slaughterhouse workers from 84 ruminant slaughterhouses in Kenya provided blood samples to screen for Coxiella burnetii antibodies, the cause of Q fever (108). The survey participants ranged from the ages of 18–82 years of age and have worked in the abattoirs or slaughterhouses for an average of 10 years. The various work roles included exsanguination through jugular severance, depilation, and skinning, eviscerating and sectioning the carcass, cleaning the intestines, and cleaning the slaughterhouse among others. An ELISA test showed that 210 workers tested positive for C. burnetti with a prevalence of 37.1%, which demonstrates a need for vaccinations of high-risk occupationally exposed humans (108).
In the southern Kabale district of Uganda, a March 2016 survey with 657 community members, including 117 abattoir workers, investigated participant livestock ownership along with knowledge and behaviors of Rift Valley fever (RVF) (126). Nearly half of the participants reported that they were involved with slaughtering or butchering and 69% believed they were at risk of getting RVF (126). Furthermore, 88% of butchers felt they were at higher risk than farmers or herdsman due to their contact with dead livestock (126). Although 90% of participants were aware of RVF and how people can get sick from animals, there was limited knowledge on the signs and symptoms (126). The lack of a human or animal RVF vaccine in Uganda can cause significant morbidity and mortality in humans and animals. In the four countries, the lack of vaccination remains a common denominator. If abattoir workers were vaccinated against endemic zoonotic diseases in their locality, the burden of associated morbidity and mortality may be reduced.
5. Effects of abattoirs in the community
5.1. Livestock and human infection
Parasites and pathogens can result in high morbidity and mortality in livestock resulting in the loss of animals and animal products, or reduction in the value of goods to be sold. In the case of infected females, several pathogens can cause them to abort fetuses, further limiting the ability to replace animals in the herd. The loss of livestock can impact food security in the community, increase malnutrition, and cause developmental defects like stunting in children (18). In addition, indirect effects of these infections include the fact that workers may experience loss of workdays or loss of productive years due to premature death. To minimize such disease burden in abattoirs and improve occupational health and safety within developing nations, it is essential to increase value-chain associated Risk Communication and Community Engagement (RCCE) starting from the farm and build awareness among workers.
Because abattoir serves as a link between the farm and fork, not only are abattoir workers at risk, but community members are at risk of infection if they consume infected animal products or have direct connections with abattoir workers. For instance, in a group A Streptococcus skin outbreak in Wales, United Kingdom, 21 workers were infected, five of which were found to have tetracycline resistant Streptococcus infection. In addition, four community members developed infections (16). Furthermore, other studies suggest that wind dispersal of Coxiella burnetii, which may be abattoir associated, can cause community outbreaks of Q fever up to 2 km away (137, 138).
5.2. Declines in vultures and a rise in disease
Backyard slaughter facilities face challenges with solid waste disposal (139). Slaughtered animal remains and other waste such as manure, bones, condemned carcasses, among others, are left at the site after a days’ work (33). This allows for vultures to scavenge. Vultures and other scavenging birds play an important role in the ecological process through scavenging and the consumption of carrion (Figure 3A). Since vultures have corrosive digestive tracts, they can quickly consume carcasses and ingest pathogens that would be harmful to other animals or people. By cleaning up animal remains in the environment, vultures help mitigate disease transmission. Declines in vultures are indirectly translating to an increase in some diseases, such as rabies. For instance, from 2014 to 2019, a study of 6 separate abattoirs in Addis Ababa, Ethiopia estimated a 12% decline in carrion consumption by vertebrate scavengers, including vultures (26) and 62% decrease in vulture population in Kenya since the 1970s. Notable species declines over the course of the study include three globally critically endangered birds: white-backed, Ruppell’s, and hooded vultures. Reasons for the decline may be attributed electrocution, poisoning, habitat loss, trapping for food, and/ or improvement in waste management practices or competitive exclusion from the rise of feral dogs (Figure 3B). Two of the abattoirs began processing carrion into animal food or fertilizers, hauling carrion to a dumpsite, or burning carrion. In the other abattoirs, there was still plenty of carrion available at the end of the study period, so food availability was not a factor. To limit feral dogs and still allow vulture access, the study suggested the addition of fences around the carrion disposal sites to reduce competitive exclusion (26). Vultures help limit the spread of pathogens by consuming infected carrion, but the observed recent declines in their population have hindered this ecosystem service.
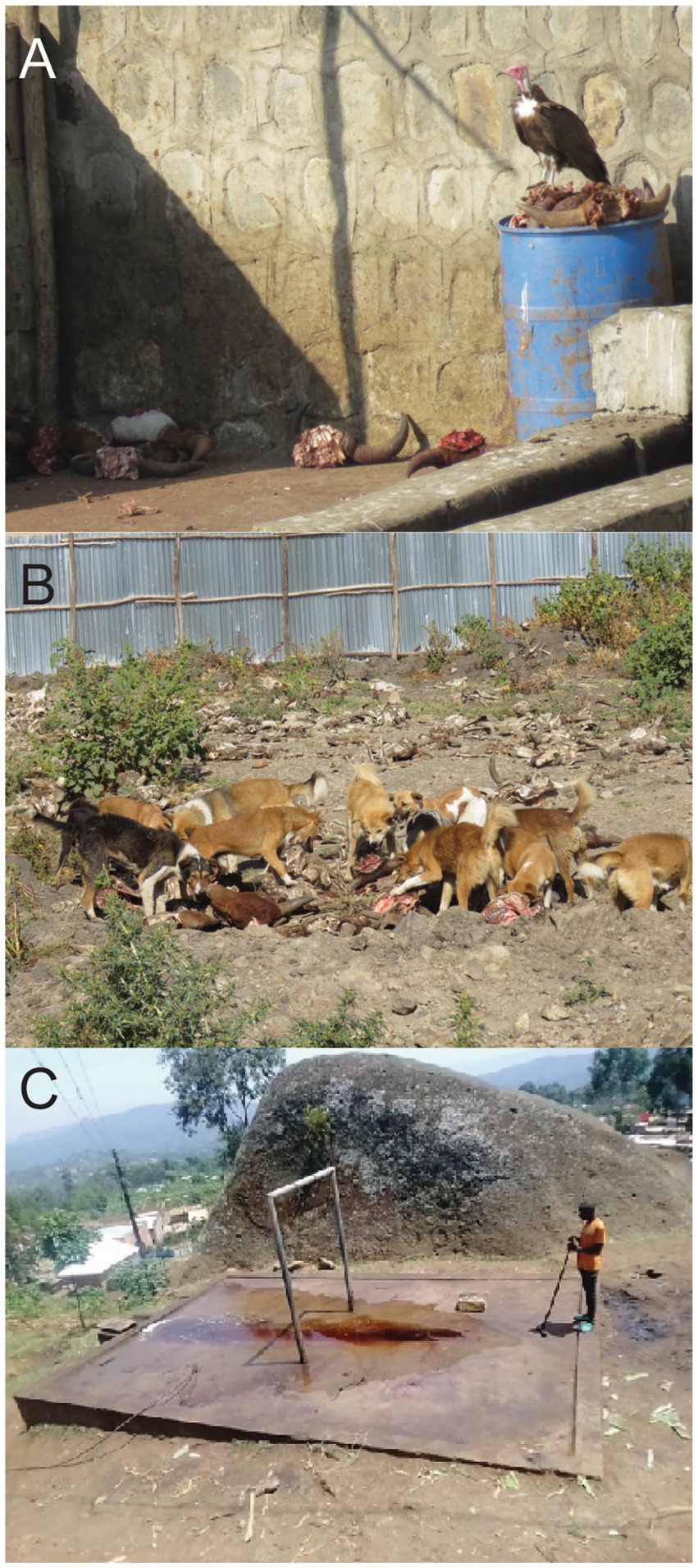
Figure 3. (A) A hooded vulture (Necrosyrtes monachus) and (B) feral dogs at an abattoir consuming slaughtered animal remains in Ethiopia. (C) Photo of backyard slaughter slab showing liquid waste runoff that can cause environmental contamination. Photo credits: Jeanne Fair, Susan Kerfua, Philip Wakimwere.
In Eastern Africa, Marabou storks (Leptoptilos crumeniferus) and other scavenging birds such as piedcrow, piapiac, spur-winged lapwing, or cattle egret have become a common sight at abattoirs (140). Because some scavengers, particularly those with longer and more pointed beaks like the Marabou stork, carry their food away from slaughter sites (141), there is a risk for environmental pollution when infected animal remains drop off the stork’s beak as its moving. Nevertheless, marabou storks also play a critical role in abattoir waste management (140).
5.3. Environmental contamination
Environmental contamination of animal products remains a problem, even in developed countries, such as the United States and many countries in Europe. Abattoir processes result in high volumes of waste products, which need to be disposed of properly. Not only does the waste contain pathogens, but also high amounts of carbon, nitrogen, and phosphorous, which further impacts the environment. The runoff with high amounts of nitrogen and phosphorous can cause harmful algal blooms (29). In some smaller, local abattoirs, the left-over blood and animal products are left to flow into the environment and contaminate the landscape (Figure 3C). Previous studies have used wastewater to monitor infectious diseases from abattoirs including but not limited to one that identified multi-resistant Pseudomonas aeruginosa (23).
6. Designing an effective surveillance strategy at abattoirs
Due to the fact that abattoirs are the intersection between humans, livestock, and the environment, they remain potential hotspots for emerging and re-emerging diseases. Designing a targeted surveillance strategy for abattoirs is important for limiting infection and decreasing the chances of local and regional outbreaks, for both zoonotic and transboundary trade-sensitive diseases. Parasites and pathogens that abattoir workers are exposed to may be harder to control because sometimes, reservoir wildlife hosts may constantly infect cattle, sheep, and goats during grazing. Abattoirs are a ‘catch all’ for these pathogens and can potentially serve as sources of multiple infection to abattoir workers. By surveying animals in abattoirs, health officials can determine many things including, which diseases are prevalent in the different geographical locations where the animals are sourced from, whether there are changing trends and patterns, and which pathogens are most likely to be transmitted from wildlife to livestock, especially for those who grazed principally in the wildlife areas. They can ask questions like, are there regional differences in quality of meat sourced from different locations, and what are the potential roles of transport facilities along roads to the abattoirs, just to mention a few.
Abattoir-sourced epidemiological information may be beneficial. Rather than sporadic testing of people and livestock at the abattoirs, a regional approach, possibly covering areas where all animals arriving at the abattoirs are sourced from, will likely yield the best results. This includes surveying abattoirs in countries that are currently understudied like Burundi, Mozambique, and Somalia, among others. Surveillance at a regional level will allow workers and health officials to understand when and where infections are occurring to inform anticipatory actions and informed decisions. One question that relates to wildlife reservoirs and transmission to livestock is identifying the ecological factors that correlate with higher livestock diseases. For example, knowing which seasons, climate conditions, and land use types promote infection could allow abattoir workers and health officials to be more vigilant at certain times of the year, after certain climate events, or in certain habitats. Expanding this to a regional level will increase the power to effectively monitor livestock and human health for limiting disease spread throughout a community. Since these are One Health issues, having a regional network for establishing and sustaining communication, coordination, and collaboration is essential for achieving the best health outcomes.
Pathogen testing is required for early detection and successful biosurveillance. Serology is a common strategy for determining past exposure to pathogen infections or measuring antibodies to vaccination and can give a broad picture of disease risk in each area, but may not be specific for a current infection, except partially when paired serum sampling is done. PCR is a much better approach because it can determine genetic markers of pathogens and who is infected at the time of sampling. More precise conditions can be recorded as well, and aid in more accurate disease forecasting and modeling predictions. There are still drawbacks of PCR, however, which includes testing for targeted pathogens (exclusivity) that health officials are worried about or are specifically tracking, giving an opportunity to miss out on potential incidental findings. Other methods may include traditional culture for pathogens, and several versions of microscopy. There are a lot of pathogens that infect livestock and could impact the food chain and ultimately humans, but there may not be the capability or capacity to test for all things.
Intense efforts have been put over the last decade to introduce next generation sequencing and other tools in developing countries to improve biosecurity, biosafety, and biosurveillance to mitigate diseases (142). By increasing the capacity to test and do biosurveillance on more pathogens, we can get a more complete picture of the infectious disease risk in an area. We highlight the need to continue surveying abattoirs, surrounding areas, and community members who may be at the greatest risk for infection. It is likely that there are many other parasites and pathogens present at abattoirs than are currently known because of lack of testing, and higher prevalence of those currently being tested for. For example, malaria may mask other febrile infections (111) or having a fever may be diagnosed as malaria without any diagnostic test (143). Shotgun metagenomic sequencing can be done on a variety of sample types and find all important bacteria and viruses infecting livestock or humans at time of sampling.
During our literature review, we found that only three articles collected ticks and tested them for tick-borne pathogens. Tick collection can be done as livestock are brought in for slaughter. They can also be collected after slaughter as animals are being processed. Storage of ticks does not require freezing; ticks can be stored in ethanol before being processed for pathogens. Because ticks only vector certain species of bacteria and viruses, they can be screened using PCR, including new multiplex PCR panels [(e.g., 144)], or sequenced using shotgun metagenomic sequencing. We suggest including tick collection in pathogen surveillance studies to better understand the circumstances surrounding transmission of tick-borne pathogens.
7. General abattoir recommendations
In developing countries, the governments and stakeholders should consider several proposed efforts to enhance abattoir worker safety. The first step is to improve abattoir infrastructure. In Tanzania, workers reported that scavengers, like stray dogs, were frequent visitors of the abattoir (42). Livestock remains and visceral organs are often left outside after slaughter and these carcasses become potential hotbeds for disease. Free roaming and scavenging dogs consume the infected meat and can develop parasites like tapeworms. The dogs then shed the tapeworm eggs in their feces. When grazing sheep consume the tapeworm eggs on pastures, they develop cysts in their organs from the parasites. If livestock are infected, the carcasses are condemned at the abattoir and place workers at risk. To address the problem, there needs to be a surrounding barrier to keep animals from entering the slaughterhouse perimeters.
Training programs should also be developed and implemented to educate workers about proper meat safety practices and workplace hygiene. The surveys conducted in Eastern Africa showed that most workers had little to no understanding of the implications associated with the diseases that animals can carry and transmit. Workers need to be aware that a greater proportion of emerging infectious diseases have an animal origin, and that 60% of existing human infectious diseases are zoonotic (145). Frequent handwashing, equipment sterilization, and proper cleaning of meat tables after each use will be an effective control measure against dangerous pathogens. Although there are hundreds of diseases around the world, workers should know how to identify syndromes, symptoms, and signs of infection in the animals that they work with and how to safely process infected carcasses. Once workers gain the necessary knowledge and skills surrounding meat handling, they need to have access to personal protective equipment (PPE) to safeguard from workplace injuries and illnesses. All workers should be required to wear PPE before the start of their shift. PPE should include a hard hat, safety glasses, facemask, coveralls, hard/steel toed boots, and cut resistant gloves. Earplugs should be offered to workers that use loud power tools. Other than a need for training and PPE, abattoir workers should receive vaccinations.
Vaccination could be beneficial to people that live in disease hotspots. Some vaccines that are available to humans include influenza, Q fever. Based on the work of Cook et al. (108), which screened blood samples for Coxiella burnetii antibodies, and another study showing 2.5% seroprevalence in community members (146), the slaughterhouse workers have a seroprevalence of 37.1%, an indication of higher risk of occupational exposure’. Although proper hand washing and the use of protective equipment can help minimize the risk of exposure, a vaccine-based approach could be an efficient means to prevent and control zoonotic infectious diseases to humans. Greater vaccine accessibility for slaughterhouse workers may be an investment but the preventative program is cheaper than the emergency response cost associated with an epidemic.
However, supplying vaccines to the appropriate places and convincing farmers and abattoir workers to get vaccinated can be difficult. Vaccine availability and supply chains are often the limiting factor for lack of vaccine use. There is also considerable hesitancy for vaccine use in smallholder farmers, which can supply abattoirs (147), including those makeshift abattoirs that have the greatest risk of transmission. One reason is that for some zoonotic diseases, which overall do not seem to significantly impact livestock before they are sold, they do not see the point of vaccinating their animals, even though they risk infection themselves (147).
Overall, the worker feedback received from surveys across several developing nations in this study demonstrates the need to protect abattoir workers from emerging zoonosis. There is an essential need for governments and stakeholders to allocate funding to increase abattoir worker awareness, require training programs, provide personal protective equipment, and encourage vaccination to minimize zoonosis and protect public health. Additional biosurveillance, including quarterly human serology testing within abattoirs, could help mitigate disease outbreaks. Although the investment could be costly, the occupational risks and emerging threat of zoonotic diseases are far too important to overlook.
8. Conclusion
In this scoping review, our goal was to understand the role of abattoirs for zoonotic disease risk in Eastern Africa. We identified common parasites and pathogens found in abattoirs, reviewed occupational risk factors associated with abattoirs, and provided recommendations to improve abattoir worker safety to reduce disease risk. Based on these data, we provided recommendations on how to improve biosecurity and develop a biosurveillance network in Eastern Africa. Our search results identified 42 species of parasites and pathogens in abattoir workers and livestock slaughtered at facilities found in 13 Eastern African countries The most reported pathogen was Mycobacterium bovis, which causes bovine tuberculosis. Recommendations to reduce disease risk include enhancing abattoir safety for workers, requiring the use of PPE, offering proper occupational training, and enforcing safe animal handling practices.
Limitations of this review include biases in the literature search process. One source of bias is not finding articles that reference zoonotic pathogens but do not refer to them as zoonotic. Another source of bias is the limitations of the articles themselves. Most articles were focused on one or two pathogens in livestock or human samples, potentially missing other important zoonotic pathogens. Additionally, many articles used serology to test for past exposure to pathogens. For livestock this is limiting for determining risk of infection to abattoir workers and communities. For abattoir workers, this information may mean that they were not infected at an abattoir; they could have been infected elsewhere. For determining risk, it is important to determine infection status at time of sampling, which can be done using PCR or next generation sequencing.
Understanding hotspots of infectious diseases should be a global priority to limit infection and prevent outbreaks. Abattoirs, particularly in developing countries, can be important tools for biosurveillance, helping to detect disease risk in a community and mitigate local outbreaks, but they are not currently being used in this capacity. It should be emphasized that abattoirs are important One Health interfaces with frequent interactions between humans, animals, and the environment, and each facility is a unique source of transmission potential that can be exploited for identifying livestock and wildlife pathogens in a community or region and aid in outbreak control and mitigation. Future work in relatively understudied countries like Burundi, Mozambique, and Somalia will improve zoonotic disease risk assessment by providing more data on important parasites and pathogens in the region. Thus, we suggest a regional biosurveillance network centered around abattoirs, which will improve testing and data gathering for enhanced risk mapping and forecasting. Next generation sequencing will be key in the ability to identify a wide range of pathogens, rather than a targeted approach that is limited in scope.
Author contributions
JF and AB contributed to conception and design of the review. KR completed the literature search and summarized all articles. KR and AB wrote the first draft of the manuscript. JF, BB, SK, and FF wrote sections of the manuscript. All authors provided comments and edits to the manuscript, read, and approved the submitted version.
Funding
This work was funded by the US Defense Threat Reduction Agency Biological Threat Reduction Program R-00716- 19-0 (DTRA) administered by the US Department of Defense.
Acknowledgments
We would like to thank the entire Kenya Coinfection Project team.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Mamo, Y. Livestock trade in COMESA: assessment of livestock market and mapping of Enterprises in Exporting and Importing Countries to establish basic data on import and export of live animal (beef cattle and small ruminants) and meat. COMESA Common Mark East South Afr Lusaka Zamb (2019).
2. Kariuki, S, Onsare, R, Mwituria, J, Ngetich, R, Nafula, C, Karimi, K, et al. FAO/WHO project report: improving food safety in meat value chains in Kenya. Food Prot Trends. (2013) 33:172–9.
3. Elelu, N, Aiyedun, JO, Mohammed, IG, Oludairo, OO, Odetokun, IA, Mohammed, KM, et al. Neglected zoonotic diseases in Nigeria: role of the public health veterinarian. Pan Afr Med J. (2019) 32:15659. doi: 10.11604/pamj.2019.32.36.15659
4. Clottey, SJ. “Premises for slaughter.,” Manual for the slaughter of small ruminants in developing countries. FAO Animal Production and Health Paper. (1985) Available at: https://www.fao.org/3/X6552E/X6552E00.htm#TOC.
5. Swai, ES, and Schoonman, L. Human brucellosis: Seroprevalence and risk factors related to high risk occupational groups in Tanga municipality, Tanzania. Zoonoses Public Health. (2009) 56:183–7. doi: 10.1111/j.1863-2378.2008.01175.x
6. Mohamed, M, Mosha, F, Mghamba, J, Zaki, SR, Shieh, W-J, Paweska, J, et al. Epidemiologic and clinical aspects of a Rift Valley fever outbreak in humans in Tanzania, 2007. Am J Trop Med Hyg. (2010) 83:22–7. doi: 10.4269/ajtmh.2010.09-0318
7. Biffa, D, Skjerve, E, Oloya, J, Bogale, A, Abebe, F, Dahle, U, et al. Molecular characterization of Mycobacterium bovis isolates from Ethiopian cattle. BMC Vet Res. (2010) 6:28. doi: 10.1186/1746-6148-6-28
8. Muwonge, A, Johansen, TB, Vigdis, E, Godfroid, J, Olea-Popelka, F, Biffa, D, et al. Mycobacterium bovis infections in slaughter pigs in Mubende district, Uganda: a public health concern. BMC Vet Res. (2012) 8:168. doi: 10.1186/1746-6148-8-168
9. Nabukenya, I, Kaddu-Mulindwa, D, and Nasinyama, GW. Survey of Brucellainfection and malaria among abattoir workers in Kampala and Mbarara districts, Uganda. BMC Public Health. (2013) 13:901. doi: 10.1186/1471-2458-13-901
10. Gebremedhin, EZ, Abdurahaman, M, Hadush, T, and Tessema, TS. Seroprevalence and risk factors of toxoplasma gondii infection in sheep and goats slaughtered for human consumption in Central Ethiopia. BMC Res Notes. (2014) 7:696. doi: 10.1186/1756-0500-7-696
11. Thomas, LF, Harrison, LJS, Toye, P, de Glanville, WA, Cook, EAJ, Wamae, CN, et al. Prevalence of Taenia solium cysticercosis in pigs entering the food chain in western Kenya. Trop Anim Health Prod. (2016) 48:233–8. doi: 10.1007/s11250-015-0949-6
12. Cook, EAJ, de Glanville, WA, Thomas, LF, Kariuki, S, De, BBM, and Fèvre, EM. Working conditions and public health risks in slaughterhouses in western Kenya. BMC Public Health. (2017) 17:14. doi: 10.1186/s12889-016-3923-y
13. Swai, ES, and Schoonman, L. A survey of zoonotic diseases in trade cattle slaughtered at Tanga city abattoir: a cause of public health concern. Asian Pac J Trop Biomed. (2012) 2:55–60. doi: 10.1016/S2221-1691(11)60190-1
14. Acke, S, Couvreur, S, Bramer, WM, Schmickler, M-N, De Schryver, A, and Haagsma, JA. Global infectious disease risks associated with occupational exposure among non-healthcare workers: a systematic review of the literature. Occup Environ Med. (2022) 79:63–71. doi: 10.1136/oemed-2020-107164
15. Paton, NI, Leo, YS, Zaki, SR, Auchus, AP, Lee, KE, Ling, AE, et al. Outbreak of Nipah-virus infection among abattoir workers in Singapore. Lancet. (1999) 354:1253–6. doi: 10.1016/S0140-6736(99)04379-2
16. Humphreys, CP, Morgan, SJ, Walapu, M, Harrison, GAJ, Keen, AP, Efstratiou, A, et al. Group a streptococcal skin infection outbreak in an abattoir: lessons for prevention. Epidemiol Infect. (2007) 135:321–7. doi: 10.1017/S0950268806006819
17. Paphitis, K, Pearl, DL, Berke, O, McEwen, SA, and Trotz-Williams, L. Detection of spatial and spatio-temporal Salmonella Heidelberg and Salmonella Typhimurium human case clusters focused around licensed abattoirs in Ontario in 2015, and their potential relation to known outbreaks. Zoonoses Public Health. (2021) 68:609–21. doi: 10.1111/zph.12849
18. Franc, KA, Krecek, RC, Häsler, BN, and Arenas-Gamboa, AM. Brucellosis remains a neglected disease in the developing world: a call for interdisciplinary action. BMC Public Health. (2018) 18:125. doi: 10.1186/s12889-017-5016-y
19. Bennett, R. The ‘direct Costs’of livestock disease: the development of a system of models for the analysis of 30 endemic livestock diseases in Great Britain. J Agric Econ. (2003) 54:55–71. doi: 10.1111/j.1477-9552.2003.tb00048.x
20. Adonu, RE, Dzokoto, L, and Salifu, SI. Sanitary and hygiene conditions of slaughterhouses and its effect on the health of residents (a case study of Amasaman slaughterhouse in the Ga west municipality, Ghana). (2017).
21. Banjo, TA, Onilude, AA, Busari, A, Ogundahunsi, OA, Olooto, WE, Familoni, OB, et al. Occupational health hazards among abattoir workers in Abeokuta. Acad Arena. (2013) 5:29–36.
22. Harmse, J, Engelbrecht, J, and Bekker, J. The impact of physical and ergonomic hazards on poultry abattoir processing workers: a review. Int J Environ Res Public Health. (2016) 13:197. doi: 10.3390/ijerph13020197
23. Igbinosa, IH, Beshiru, A, and Igbinosa, EO. Antibiotic resistance profile of Pseudomonas aeruginosa isolated from aquaculture and abattoir environments in urban communities. Asian Pac J Trop Dis. (2017) 7:47–52. doi: 10.12980/apjtd.7.2017D6-363
24. Plaza, PI, Blanco, G, and Lambertucci, SA. Implications of bacterial, viral and mycotic microorganisms in vultures for wildlife conservation, ecosystem services and public health. Ibis. (2020) 162:1109–24. doi: 10.1111/ibi.12865
25. Adamu, M, and Dahiru, M. A review on abattoir wastewater treatment for environmental health improvement. J Environ Bioremed Toxicol. (2020) 3:26–31. doi: 10.54987/jebat.v3i2.548
26. Buechley, ER, Murgatroyd, M, Ruffo, AD, Bishop, RC, Christensen, T, Marra, PP, et al. Declines in scavenging by endangered vultures in the horn of Africa. J Wildl Manag. (2022) 86:e22194. doi: 10.1002/jwmg.22194
27. Jacobs, L, McMahon, BH, Berendzen, J, Longmire, J, Gleasner, C, Hengartner, NW, et al. California condor microbiomes: bacterial variety and functional properties in captive-bred individuals. PLoS One. (2019) 14:e0225858. doi: 10.1371/journal.pone.0225858
28. Al-Gheethi, A, Ma, NL, Rupani, PF, Sultana, N, Yaakob, MA, Mohamed, RMSR, et al. Biowastes of slaughterhouses and wet markets: an overview of waste management for disease prevention. Environ Sci Pollut Res. (2021) 30:71780–93. doi: 10.1007/s11356-021-16629-w
29. Shukri, AA, Kyambadde, J, and Hawumba, JF. The impact of Kalerwe abattoir wastewater effluent on the water quality of the Nsooba Channel. Agric Res Technol Open Access J. (2017) 6:555677. doi: 10.19080/ARTOAJ.2017.06.555677
30. Alam, M-U, Rahman, M, Abdullah-Al-Masud, IMA, Asaduzzaman, M, Sarker, S, Rousham, E, et al. Human exposure to antimicrobial resistance from poultry production: assessing hygiene and waste-disposal practices in Bangladesh. Int J Hyg Environ Health. (2019) 222:1068–76. doi: 10.1016/j.ijheh.2019.07.007
31. Omole, DO, and Longe, EO. An assessment of the impact of abattoir effluents on river Illo, Ota, Nigeria. J Environ Sci Technol. (2008) 1:56–64. doi: 10.3923/jest.2008.56.64
32. Sangodoyin, AY, and Agbawhe, OM. Environmental study on surface and groundwater pollutants from abattoir effluents. Bioresour Technol. (1992) 41:193–200. doi: 10.1016/0960-8524(92)90001-E
33. Chukwu, O. Analysis of ground water pollution from abattoir waste in Minna, Nigeria. Res J Dairy Sci. (2008) 2:74–7.
34. Gallardo, C, Okoth, E, Pelayo, V, Anchuelo, R, Martin, E, Simon, A, et al. African swine fever viruses with two different genotypes, both of which occur in domestic pigs, are associated with ticks and adult warthogs, respectively, at a single geographical site. J Gen Virol. (2011) 92:432–44. doi: 10.1099/vir.0.025874-0
35. Horton, KC, Fahmy, NT, Watany, N, Zayed, A, Mohamed, A, Ahmed, AA, et al. Crimean Congo hemorrhagic fever virus and Alkhurma (Alkhumra) virus in ticks in Djibouti. Vector Borne Zoonotic Dis. (2016) 16:680–2. doi: 10.1089/vbz.2016.1951
36. Mellau, BL, Nonga, HE, and Karimuribo, ED. Slaughter stock abattoir survey of carcasses and organ/offal condemnations in Arusha region, northern Tanzania. Trop Anim Health Prod. (2011) 43:857–64. doi: 10.1007/s11250-010-9773-1
37. Kuria, JN, and Gathogo, SM. Concomitant fungal and Mycobacterium bovis infections in beef cattle in Kenya. Onderstepoort J Vet Res. (2013) 80:4. doi: 10.4102/ojvr.v80i1.585
38. Gutema, FD, Shiberu, T, Agga, GE, Abdi, RD, Hiko, A, Tufa, TB, et al. Bovine cysticercosis and human taeniasis in a rural community in Ethiopia. Zoonoses Public Health. (2020) 67:525–33. doi: 10.1111/zph.12716
39. Jorga, E, Van Damme, I, Mideksa, B, and Gabriël, S. Identification of risk areas and practices for Taenia saginata taeniosis/cysticercosis in Ethiopia: a systematic review and meta-analysis. Parasit Vectors. (2020) 13:375. doi: 10.1186/s13071-020-04222-y
40. Assefa, A, and Bihon, A. Bovine cysticercosis in Ethiopia_ a systematic review and meta-analysis of prevalence from abattoir-based surveys|Elsevier enhanced reader. Prev Vet Med. 169:104707. doi: 10.1016/j.prevetmed.2019.104707
41. Terefe, Y, Redwan, F, and Zewdu, E. Bovine cysticercosis and its food safety implications in Harari People’s National Regional State, Eastern Ethiopia. Onderstepoort J Vet Res. (2014) 81:01–6. doi: 10.4102/ojvr.v81i1.676
42. Komba, EVG, Komba, EV, Mkupasi, EM, Mbyuzi, AO, Mshamu, S, Luwumba, D, et al. Sanitary practices and occurrence of zoonotic conditions in cattle at slaughter in Morogoro municipality, Tanzania: implications for public health. Tanzan J Health Res. (2012) 14:131–8. doi: 10.4314/thrb.v14i2.6
43. Tembo, W, and Nonga, HE. A survey of the causes of cattle organs and/or carcass condemnation, financial losses and magnitude of foetal wastage at an abattoir in Dodoma, Tanzania. Onderstepoort J Vet Res. (2015) 82:855. doi: 10.4102/ojvr.v82i1.855
44. Beyene, T, and Hiko, A. Zoonotic metacestodes and associated financial loss from cattle slaughtered at Yabello municipal abattoir, Borana-Oromia, Ethiopia. Parasite Epidemiol Control. (2019) 5:e00096. doi: 10.1016/j.parepi.2019.e00096
45. Swai, E, Mwezimpya, I, Ulicky, E, Mbise, A, and Moshy, W. An abattoir survey of contagious bovine pleuropneumonia lesions in slaughtered cattle in selected districts in northern Tanzania. Asian Pac J Trop Biomed. (2013) 3:303–6. doi: 10.1016/S2221-1691(13)60067-2
46. Noah, EY, Kimera, SI, Kusiluka, LJM, and Wambura, P. Abattoir surveillance demonstrates contagious bovine pleuropneumonia is widespread in Tanzania. Trop Anim Health Prod. (2015) 47:1607–13. doi: 10.1007/s11250-015-0907-3
47. Bahiru, G, Jena, PK, Addissie, A, Behera, MR, Fromsa, A, and Gumi, B. Zoonotic tuberculosis in occupationally exposed groups in the Adama municipal abattoir, Central Ethiopia. Ethiop J Health Dev. (2022) 36:5001. doi: 10.20372/ejhd.v36i1.5001
48. Zerom, K, Tessema, TS, Mamo, G, Bayu, Y, and Ameni, G. Tuberculosis in dromedaries in eastern Ethiopia: abattoir-based prevalence and molecular typing of its causative agents. Small Rumin Res. (2013) 109:188–92. doi: 10.1016/j.smallrumres.2012.07.030
49. Regassa, A, Tassew, A, Amenu, K, Megersa, B, Abunna, F, Mekibib, B, et al. A cross-sectional study on bovine tuberculosis in Hawassa town and its surroundings, southern Ethiopia. Trop Anim Health Prod. (2010) 42:915–20. doi: 10.1007/s11250-009-9507-4
50. Hiko, A, and Agga, GE. First-time detection of mycobacterium species from goats in Ethiopia. Trop Anim Health Prod. (2011) 43:133–9. doi: 10.1007/s11250-010-9665-4
51. Biffa, D, Bogale, A, and Skjerve, E. Diagnostic efficiency of abattoir meat inspection service in Ethiopia to detect carcasses infected with Mycobacterium bovis: implications for public health. BMC Public Health. (2010) 10:462. doi: 10.1186/1471-2458-10-462
52. Biffa, D, Inangolet, F, Bogale, A, Oloya, J, Djonne, B, and Skjerve, E. Risk factors associated with prevalence of tuberculosis-like lesions and associated mycobacteria in cattle slaughtered at public and export abattoirs in Ethiopia. Trop Anim Health Prod. (2011) 43:529–38. doi: 10.1007/s11250-010-9729-5
53. Biffa, D, Bogale, A, Godfroid, J, and Skjerve, E. Factors associated with severity of bovine tuberculosis in Ethiopian cattle. Trop Anim Health Prod. (2012) 44:991–8. doi: 10.1007/s11250-011-0031-y
54. Aylate, A, Shah, SN, Aleme, H, and Gizaw, TT. Bovine tuberculosis: prevalence and diagnostic efficacy of routine meat inspection procedure in Woldiya municipality abattoir north Wollo zone, Ethiopia. Trop Anim Health Prod. (2013) 45:855–64. doi: 10.1007/s11250-012-0298-7
55. Ejo, M, Haile, B, Tariku, T, Nigatu, S, Kebede, E, Bitew, AB, et al. Bacteriological and molecular characterization of Mycobacterium bovis isolates from tuberculous lesions collected among slaughtered cattle, Northwest Ethiopia. BMC Microbiol. (2021) 21:286. doi: 10.1186/s12866-021-02349-1
56. Belete, A, Tilahun, S, Haile, B, Demessie, Y, Nigatu, S, Getachew, A, et al. Prevalence of bovine tuberculosis and distribution of tuberculous lesions in cattle slaughtered at Gondar, Northwest Ethiopia. Infect Ecol Epidemiol. (2021) 11:1986919-Article No.: 1986919. doi: 10.1080/20008686.2021.1986919
57. Ghebremariam, MK, Hlokwe, T, Rutten, VPMG, Allepuz, A, Cadmus, S, Muwonge, A, et al. Genetic profiling of Mycobacterium bovis strains from slaughtered cattle in Eritrea. PLoS Negl Trop Dis. (2018) 12:6406. doi: 10.1371/journal.pntd.0006406
58. Gathogo, SM, Kuria, JKN, and Ombui, JN. Prevalence of bovine tuberculosis in slaughter cattle in Kenya: a postmortem, microbiological and DNA molecular study. Trop Anim Health Prod. (2012) 44:1739–44. doi: 10.1007/s11250-012-0131-3
59. Kuria, JKN, Akwalu, SK, and Muema, LM. The etiology and public health significance of mycobacteriosis of cattle in Kenya. Int J Mycobacteriol. (2018) 7:251–6. doi: 10.4103/ijmy.ijmy_80_18
60. Mellau, LSB, Nonga, HE, and Karimuribo, ED. A slaughterhouse survey of lung lesions in slaughtered stocks at Arusha, Tanzania. Prev Vet Med. (2010) 97:77–82. doi: 10.1016/j.prevetmed.2010.08.008
61. Habarugira, G, Rukelibuga, J, Nanyingi, MO, and Mushonga, B. Bovine tuberculosis in Rwanda: prevalence and economic impact evaluation by meat inspection at Societe des abattoirs de Nyabugogo-Nyabugogo abattoir, Kigali. J S Afr Vet Assoc. (2014) 85:1062. doi: 10.4102/jsava.v85i1.1062
62. Tembo, NFP, Muma, JB, Hangombe, MB, and Munyeme, M. Clustering and spatial heterogeneity of bovine tuberculosis at the livestock/wildlife interface areas in Namwala District of Zambia. Vet World. (2020) 13:478–88. doi: 10.14202/vetworld.2020.478-488
63. Tsegay, A, Tuli, G, Kassa, T, and Kebede, N. Seroprevalence and risk factors of brucellosis in abattoir workers at Debre Zeit and Modjo export abattoir, Central Ethiopia. BMC Infect Dis. (2017) 17:2208. doi: 10.1186/s12879-017-2208-0
64. Tsegay, A, Tuli, G, Kassa, T, and Kebede, N. Seroprevalence and risk factors of brucellosis in small ruminants slaughtered at Debre Ziet and Modjo export abattoirs, Ethiopia. J Infect Dev Ctries. (2015) 9:373–80. doi: 10.3855/jidc.4993
65. Mirambo, MM, Mgode, GF, Malima, ZO, John, M, Mngumi, EB, Mhamphi, GG, et al. Seropositivity of Brucella spp. and Leptospira spp. antibodies among abattoir workers and meat vendors in the city of Mwanza, Tanzania: a call for one health approach control strategies. PLoS Negl Trop Dis. (2018) 12:e0006600. doi: 10.1371/journal.pntd.0006600
66. Erume, J, Roesel, K, Dione, MM, Ejobi, F, Mboowa, G, Kungu, JM, et al. Serological and molecular investigation for brucellosis in swine in selected districts of Uganda. Trop Anim Health Prod. (2016) 48:1147–55. doi: 10.1007/s11250-016-1067-9
67. Boone, I, Henning, K, Hilbert, A, Neubauer, H, Von Kalckreuth, V, Dekker, DM, et al. Are brucellosis, Q fever and melioidosis potential causes of febrile illness in Madagascar? Acta Trop. (2017) 172:255–62. doi: 10.1016/j.actatropica.2017.05.013
68. Madut, NA, Muleme, J, Kankya, C, Nasinyama, GW, Muma, JB, Godfroid, J, et al. The epidemiology of zoonotic brucellosis in Bahr el Ghazal region of South Sudan. Front Public Health. (2019) 7:156. doi: 10.3389/fpubh.2019.00156
69. Madut, NA, Ocan, M, Muwonge, A, Muma, JB, Nasinyama, GW, Godfroid, J, et al. Sero-prevalence of brucellosis among slaughterhouse workers in Bahr el Ghazal region, South Sudan. BMC Infect Dis. (2019) 19:450:450. doi: 10.1186/s12879-019-4066-4
70. Nonga, H, Sells, P, and Karimuribo, ED. Occurrences of thermophilic Campylobacter in cattle slaughtered at Morogoro municipal abattoir, Tanzania. Trop Anim Health Prod. (2010) 42:73–8. doi: 10.1007/s11250-009-9387-7
71. Tegegne, HA, Berhanu, A, Getachew, Y, Serda, B, Nölkes, D, Tilahun, S, et al. Microbiological safety and hygienic quality of camel meat at abattoir and retail houses in Jigjiga city, Ethiopia. J Infect Dev Ctries. (2019) 13:188–94. doi: 10.3855/jidc.9686
72. Hagos, Y, Gugsa, G, Awol, N, Ahmed, M, Tsegaye, Y, Abebe, N, et al. Isolation, identification, and antimicrobial susceptibility pattern of Campylobacter jejuni and Campylobacter coli from cattle, goat, and chicken meats in Mekelle, Ethiopia. PLoS One. (2021) 16:e0246755. doi: 10.1371/journal.pone.0246755
73. Asmare, K, Abayneh, T, Mekuria, S, Ayelet, G, Sibhat, B, Skjerve, E, et al. A meta-analysis of contagious caprine pleuropneumonia (CCPP) in Ethiopia. Acta Trop. (2016) 158:231–9. doi: 10.1016/j.actatropica.2016.02.023
74. Abebe, D, and Sisay, TT. Determination of Corynebacterium pseudotuberculosis prevalence and antimicrobial susceptibility pattern of isolates from lymph nodes of sheep and goats at an organic export abattoir, Modjo, Ethiopia. Lett Appl Microbiol. (2015) 61:469–76. doi: 10.1111/lam.12482
75. Wampande, EM, Waiswa, P, Allen, DJ, Hewson, R, Frost, SDW, and Stubbs, SCB. Phylogenetic characterization of Crimean-Congo hemorrhagic fever virus detected in African blue ticks feeding on cattle in a Ugandan abattoir. Microorganisms. (2021) 9:438. doi: 10.3390/microorganisms9020438
76. Andriamandimby, SF, Marianneau, P, Rafisandratantsoa, J-T, Rollin, PE, Heraud, J-M, Tordo, N, et al. Crimean-Congo hemorrhagic fever serosurvey in at-risk professionals, Madagascar, 2008 and 2009. J Clin Virol. (2011) 52:370–2. doi: 10.1016/j.jcv.2011.08.008
77. Samuel, W, and Zewde, GG. Prevalence, risk factors, and distribution of Cysticercus tenuicollis in visceral organs of slaughtered sheep and goats in Central Ethiopia. Trop Anim Health Prod. (2010) 42:1049–51. doi: 10.1007/s11250-010-9537-y
78. Badoul, NA, Kagira, J, Ngonga, F, and Dinka, H. Development of loop-mediated isothermal amplification combined with lateral flow dipstick assay for a rapid and sensitive detection of cystic echinococcosis in livestock in Kenya. J Trop Med. (2022) 2022:1–9. doi: 10.1155/2022/4928009
79. Omondi, HA, Gitau, G, Gathura, P, Mulinge, E, Zeyhle, E, Kimeli, P, et al. Prevalence and genotyping of Echinococcus granulosussensu lato from livestock in North-Eastern Kenya. J Helminthol. (2020) 94:e205:e205. doi: 10.1017/S0022149X20000899
80. Dini, FM, Poglayen, G, Benazzi, C, Gentile, A, Morandi, B, Mwinuka, NT, et al. Laboratory analysis as support to slaughterhouse inspection in Songea cattle abattoir (Tanzania): a public health perspective. Vet Parasitol Reg Stud Rep. (2022) 27:100672–2. doi: 10.1016/j.vprsr.2021.100672
81. Kebede, N, Gebre-Egziabher, Z, Tilahun, G, and Wossene, A. Prevalence and financial effects of Hydatidosis in cattle slaughtered in Birre-Sheleko and Dangila abattoirs, northwestern Ethiopia. Zoonoses Public Health. (2011) 58:41–6. doi: 10.1111/j.1863-2378.2009.01250.x
82. Negash, K, Beyene, D, and Kumsa, B. Cystic echinococcosis in cattle slaughtered at Shashemanne municipal abattoir, south Central Oromia, Ethiopia: prevalence, cyst distribution and fertility. Trans R Soc Trop Med Hyg. (2013) 107:229–34. doi: 10.1093/trstmh/trt003
83. Kumsa, B. Cystic echinococcosis in slaughtered cattle at Addis Ababa abattoir enterprise, Ethiopia|Elsevier enhanced reader. Vet Anim Sci. (2019) 7:100050. doi: 10.1016/j.vas.2019.100050
84. Guduro, GG, and Desta, AH. Cyst viability and economic significance of Hydatidosis in southern Ethiopia. J Parasitol Res. (2019) 2019:1–7. doi: 10.1155/2019/2038628
85. Golassa, L, Abebe, T, and Hailu, A. Evaluation of crude hydatid cyst fluid antigens for the serological diagnosis of hydatidosis in cattle. J Helminthol. (2011) 85:100–8. doi: 10.1017/S0022149X10000349
86. Getahun, D, Van Henten, S, Abera, A, Senkoro, M, Owiti, P, Lombamo, F, et al. Cysts and parasites in an abattoir in Northwest Ethiopia; an urgent call for action on “one health.”. J Infect Dev Ctries. (2020) 14:53S–7S. doi: 10.3855/jidc.11713
87. Dana, D, Suleman, S, Workneh, N, Massa, D, Levecke, B, Kifleyohannes, T, et al. Cystic echinococcosis, a food-borne zoonotic neglected tropical disease in slaughtered cattle at Jimma town municipal abattoir, Southwest Ethiopia. Ann Parasitol. (2021) 67:627–35. doi: 10.17420/ap6704.379
88. Erbeto, K, Zewde, G, and Kumsa, B. Hydatidosis of sheep and goats slaughtered at Addis Ababa abattoir: prevalence and risk factors. Trop Anim Health Prod. (2010) 42:803–5. doi: 10.1007/s11250-009-9495-4
89. Atherstone, C, Diederich, S, Pickering, B, Smith, G, Casey, G, Fischer, K, et al. Investigation of ebolavirus exposure in pigs presented for slaughter in Uganda. Transbound Emerg Dis. (2020) 68:1521–30. doi: 10.1111/tbed.13822
90. Wambui, J, Tasara, T, Njage, PMK, and Stephan, R. Species distribution and antimicrobial profiles of Enterococcus spp. isolates from Kenyan small and medium Enterprise slaughterhouses. J Food Prot. (2018) 81:1445–9. doi: 10.4315/0362-028X.JFP-18-130
91. Habarugira, G, Mbasinga, G, Mushonga, B, Teedzai, C, Kandiwa, E, and Ojok, L. Pathological findings of condemned bovine liver specimens and associated economic loss at Nyabugogo abattoir, Kigali, Rwanda. Acta Trop. (2016) 164:27–32. doi: 10.1016/j.actatropica.2016.07.020
92. Opio, LG, Abdelfattah, EM, Terry, J, Odongo, S, and Okello, E. Prevalence of fascioliasis and associated economic losses in cattle slaughtered at lira municipality abattoir in northern Uganda. Animals. (2021) 11:681. doi: 10.3390/ani11030681
93. Bersisa, A, Tulu, D, and Negera, C. Investigation of bacteriological quality of meat from abattoir and butcher shops in Bishoftu, Central Ethiopia. Int J Microbiol. (2019) 2019:1–8. doi: 10.1155/2019/6416803
94. Mersha, G, Asrat, D, Zewde, BM, and Kyule, M. Occurrence of Escherichia coli O157:H7 in faeces, skin and carcasses from sheep and goats in Ethiopia. Lett Appl Microbiol. (2010) 50:71–6. doi: 10.1111/j.1472-765X.2009.02757.x
95. Dulo, F, Feleke, A, Szonyi, B, Fries, R, Baumann, MPO, and Grace, D. Isolation of multidrug-resistant Escherichia coli O157 from goats in the Somali region of Ethiopia: a cross-sectional, abattoir-based study. PLoS One. (2015) 10:e0142905. doi: 10.1371/journal.pone.0142905
96. Ayenew, HY, Mitiku, BA, and Tesema, TS. Occurrence of virulence genes and antimicrobial resistance of E. coli O157:H7 isolated from the beef carcass of Bahir Dar City, Ethiopia. Vet Med Int. (2021) 2021:1–8. doi: 10.1155/2021/8046680
97. Balinda, SN, Belsham, GJ, Masembe, C, Sangula, AK, Siegismund, HR, and Muwanika, VB. Molecular characterization of SAT 2 foot-and-mouth disease virus from post-outbreak slaughtered animals: implications for disease control in Uganda. Epidemiol Infect. (2010) 138:1204–10. doi: 10.1017/S0950268809991427
98. Ukuli, AQ, and Mugimba, KK. Seroprevalence of hepatitis E in swine abattoir workers. Afr Health Sci. (2017) 17:1022–8. doi: 10.4314/ahs.v17i4.9
99. Chambaro, HM, Sasaki, M, Muleya, W, Kajihara, M, Shawa, M, Mwape, KE, et al. Hepatitis E virus infection in pigs: a first report from Zambia. Emerg Microbes Infect. (2021) 10:2169–72. doi: 10.1080/22221751.2021.2002669
100. Temmam, S, Besnard, L, Andriamandimby, SF, Foray, C, Rasamoelina-Andriamanivo, H, Heraud, J-M, et al. High prevalence of hepatitis E in humans and pigs and evidence of Genotype-3 virus in swine, Madagascar. Am J Trop Med Hyg. (2013) 88:329–38. doi: 10.4269/ajtmh.2012.12-0615
101. Muwonge, A, Kankya, C, Godfroid, J, Djonne, B, Opuda-Asibo, J, Biffa, D, et al. Prevalence and associated risk factors of mycobacterial infections in slaughter pigs from Mubende district in Uganda. Trop Anim Health Prod. (2010) 42:905–13. doi: 10.1007/s11250-009-9506-5
102. Cook, EAJ, de Glanville, WA, Thomas, LF, Kariuki, S, De Clare Bronsvoort, BM, and Fèvre, EM. Risk factors for leptospirosis seropositivity in slaughterhouse workers in western Kenya. Occup Environ Med. (2017) 74:357–65. doi: 10.1136/oemed-2016-103895
103. Ngugi, JN, Fèvre, EM, Mgode, GF, Obonyo, M, Mhamphi, GG, Otieno, CA, et al. Seroprevalence and associated risk factors of leptospirosis in slaughter pigs; a neglected public health risk, western Kenya. BMC Vet Res. (2019) 15:403. doi: 10.1186/s12917-019-2159-3
104. Alinaitwe, L, Kankya, C, Allan, KJ, Rodriguez-Campos, S, Torgerson, P, and Dreyfus, A. Bovine leptospirosis in abattoirs in Uganda: molecular detection and risk of exposure among workers. Zoonoses Public Health. (2019) 66:636–46. doi: 10.1111/zph.12616
105. Atherstone, C, Mgode, GF, Dhand, NK, Alonso, S, Grace, D, Ward, MP, et al. Selected endemic Zoonoses in pigs presenting for slaughter in Kampala, Uganda. Am J Trop Med Hyg. (2020) 103:2552–60. doi: 10.4269/ajtmh.20-0033
106. Rahelinirina, S, Moseley, MH, Allan, KJ, Ramanohizakandrainy, E, Ravaoarinoro, S, Rajerison, M, et al. Leptospira in livestock in Madagascar: uncultured strains, mixed infections and small mammal-livestock transmission highlight challenges in controlling and diagnosing leptospirosis in the developing world. Parasitology. (2019) 146:1707–13. doi: 10.1017/S0031182019001252
107. Kiyonga, AN, EAJ, C, NMA, O, Kivali, V, Reusken, C, Haagmans, BL, et al. Middle East respiratory syndrome coronavirus (MERS-CoV) seropositive camel handlers in Kenya. Viruses. (2020) 12:396. doi: 10.3390/v12040396
108. Cook, EAJ, de Glanville, WA, Thomas, LF, Kiyonga, A, Kivali, V, Kariuki, S, et al. Evidence of exposure to C. burnetii among slaughterhouse workers in western Kenya. One Health. (2021) 13:100305. doi: 10.1016/j.onehlt.2021.100305
109. Mwololo, D, Nthiwa, D, Kitala, P, Abuom, T, Wainaina, M, Kairu-Wanyoike, S, et al. Sero-epidemiological survey of Coxiella burnetii in livestock and humans in Tana River and Garissa counties in Kenya. PLoS Negl Trop Dis. (2022) 16:e0010214. doi: 10.1371/journal.pntd.0010214
110. Deressa, FB, Kal, DO, Gelalcha, BD, and Magalhães, RJS. Seroprevalence of and risk factors for Q fever in dairy and slaughterhouse cattle of Jimma town, South Western Ethiopia. BMC Vet Res. (2020) 16:385. doi: 10.1186/s12917-020-02598-8
111. Mutai, BK, Wainaina, JM, Magiri, CG, Nganga, JK, Ithondeka, PM, Njagi, ON, et al. Zoonotic surveillance for Rickettsiae in domestic animals in Kenya. Vector Borne Zoonotic Dis. (2013) 13:360–6. doi: 10.1089/vbz.2012.0977
112. Horton, KC, Jiang, J, Maina, A, Dueger, E, Zayed, A, Ahmed, AA, et al. Evidence of Rickettsia and Orientia infections among abattoir Workers in Djibouti. Am J Trop Med Hyg. (2016) 95:462–5. doi: 10.4269/ajtmh.15-0775
113. Cook, EAJ, Grossi-Soyster, EN, De Glanville, WA, Thomas, LF, Kariuki, S, De Clare Bronsvoort, BM, et al. The sero-epidemiology of Rift Valley fever in people in the Lake Victoria Basin of western Kenya. PLoS Negl Trop Dis. (2017) 11:e0005731. doi: 10.1371/journal.pntd.0005731
114. Shoemaker, TR, Nyakarahuka, L, Balinandi, S, Ojwang, J, Tumusiime, A, Mulei, S, et al. First laboratory-confirmed outbreak of human and animal Rift Valley fever virus in Uganda in 48 years. Am J Trop Med Hyg. (2019) 100:659–71. doi: 10.4269/ajtmh.18-0732
115. Andriamandimby, SF, Randrianarivo-Solofoniaina, AE, Jeanmaire, EM, Ravololomanana, L, Razafimanantsoa, LT, Rakotojoelinandrasana, T, et al. Rift Valley fever during rainy seasons, Madagascar, 2008 and 2009. Emerg Infect Dis. (2010) 16:963–70. doi: 10.3201/eid1606.091266
116. Awol, N, Ayelet, G, Jenberie, S, Gelaye, E, Sisay, T, and Nigussie, H. Bacteriological studies on pulmonary lesions of camel (Camelus dromedarius) slaughtered at Addis Ababa abattoir, Ethiopia. Afr J Microbiol Res. (2011) 5:522–7. doi: 10.5897/AJMR10.532
117. Sibhat, B, Molla Zewde, B, Zerihun, A, Muckle, A, Cole, L, Boerlin, P, et al. Salmonella Serovars and antimicrobial resistance profiles in beef cattle, slaughterhouse personnel and slaughterhouse environment in Ethiopia. Zoonoses Public Health. (2011) 58:102–9. doi: 10.1111/j.1863-2378.2009.01305.x
118. Wabeto, W, Abraham, Y, and Anjulo, AA. Detection and identification of antimicrobial-resistant Salmonella in raw beef at Wolaita Sodo municipal abattoir, southern Ethiopia. J Health Popul Nutr. (2017) 36:52. doi: 10.1186/s41043-017-0131-z
119. Kikuvi, GM, Ombui, JN, and Mitema, ES. Serotypes and antimicrobial resistance profiles of Salmonella isolates from pigs at slaughter in Kenya. J Infect Dev Ctries. (2010) 4:243–8. doi: 10.3855/jidc.446
120. Geresu, MA, Assefa Wayuo, B, and Mamo, KG. Occurrence and antimicrobial susceptibility profile of Salmonella isolates from animal origin food items in selected areas of Arsi zone, southeastern Ethiopia, 2018/19. Int J Microbiol. (2021) 2021:1–13. doi: 10.1155/2021/6633522
121. Mekibib, B, Abdisa, D, Denbarga, Y, and Abebe, R. Muscular Sarcocystis infection in ruminants slaughtered at municipality abattoir and selected Hotels in Hawassa city, southern Ethiopia: prevalence and associated risk factors. Vet Parasitol Reg Stud Rep. (2019) 18:100333. doi: 10.1016/j.vprsr.2019.100333
122. Cook, EAJ, Gitahi, N, de Glanville, WA, Thomas, LF, Kariuki, S, Kangethe, E, et al. Prevalence and risk factors for exposure to Toxoplasma gondii in slaughterhouse workers in western Kenya. BMC Infect Dis. (2021) 21:944. doi: 10.1186/s12879-021-06658-8
123. Fekadu, F, Beyene, TJ, Beyi, AF, Edao, BM, Tufa, TB, Woldemariyam, FT, et al. Risk perceptions and protective behaviors toward bovine tuberculosis among abattoir and butcher Workers in Ethiopia. Front Vet Sci. (2018) 5:169. doi: 10.3389/fvets.2018.00169
124. Kamau, E, Ongus, J, Gitau, G, Galgalo, T, Lowther, SA, Bitek, A, et al. Knowledge and practices regarding Middle East respiratory syndrome coronavirus among camel handlers in a slaughterhouse, Kenya, 2015. Zoonoses Public Health. (2019) 66:169–73. doi: 10.1111/zph.12524
125. Waldman, L, Hrynick, TA, Benschop, J, Cleaveland, S, Crump, JA, Davis, MA, et al. Meat safety in northern Tanzania: inspectors’ and slaughter workers’ risk perceptions and management. Front Vet Sci. (2020) 7:309. doi: 10.3389/fvets.2020.00309
126. De St Maurice, A, Nyakarahuka, L, Purpura, L, Ervin, E, Tumusiime, A, Balinandi, S, et al. Rift Valley fever: a survey of knowledge, attitudes, and practice of slaughterhouse workers and community members in Kabale District, Uganda. PLoS Negl Trop Dis. (2018) 12:e0006460. doi: 10.1371/journal.pntd.0006460
127. Nyokabi, S, Birner, R, Bett, B, Isuyi, L, Grace, D, Güttler, D, et al. Informal value chain actors’ knowledge and perceptions about zoonotic diseases and biosecurity in Kenya and the importance for food safety and public health. Trop Anim Health Prod. (2018) 50:509–18. doi: 10.1007/s11250-017-1460-z
128. Lysholm, S, Fischer, K, Lindahl, JF, Munyeme, M, and Wensman, JJ. Seropositivity rates of zoonotic pathogens in small ruminants and associated public health risks at informal urban markets in Zambia. Acta Trop. (2022) 225:106217. doi: 10.1016/j.actatropica.2021.106217
129. Gebeyehu, DT, and Tsegaye, H. Food safety knowledge and practice of abattoir and butcher shop workers: a health risk management perspective. One Health Outlook. (2022) 4:14. doi: 10.1186/s42522-022-00070-1
130. Prinsen, G, Benschop, J, Cleaveland, S, Crump, JA, French, NP, Hrynick, TA, et al. Meat safety in Tanzania’s value chain: experiences, explanations and expectations in butcheries and eateries. Int J Environ Res Public Health. (2020) 17:2833. doi: 10.3390/ijerph17082833
131. Atherstone, C, Galiwango, RG, Grace, D, Alonso, S, Dhand, NK, Ward, MP, et al. Analysis of pig trading networks and practices in Uganda. Trop Anim Health Prod. (2019) 51:137–47. doi: 10.1007/s11250-018-1668-6
132. Medley, AM, Gasanani, J, Nyolimati, CA, McIntyre, E, Ward, S, Okuyo, B, et al. Preventing the cross-border spread of zoonotic diseases: multisectoral community engagement to characterize animal mobility-Uganda, 2020. Zoonoses Public Health. (2021) 68:747–59. doi: 10.1111/zph.12823
133. Friedman, S. Emerging fungal infections: new patients, new patterns, and new pathogens. J Fungi. (2019) 5:67. doi: 10.3390/jof5030067
134. Cordonin, C, Turpin, M, Bringart, M, Bascands, J-L, Flores, O, Dellagi, K, et al. Pathogenic Leptospira and their animal reservoirs: testing host specificity through experimental infection. Sci Rep. (2020) 10:7239. doi: 10.1038/s41598-020-64172-4
135. Thomson, GR, Penrith, M-L, Atkinson, MW, Atkinson, SJ, Cassidy, D, and Osofsky, SA. Balancing livestock production and wildlife conservation in and around Southern Africa’s Transfrontier conservation areas. Transbound Emerg Dis. (2013) 60:492–506. doi: 10.1111/tbed.12175
136. Nuvey, FS, Arkoazi, J, Hattendorf, J, Mensah, GI, Addo, KK, Fink, G, et al. Effectiveness and profitability of preventive veterinary interventions in controlling infectious diseases of ruminant livestock in sub-Saharan Africa: a scoping review. BMC Vet Res. (2022) 18:332. doi: 10.1186/s12917-022-03428-9
137. Brouqui, P, Badiaga, S, and Raoult, D. Q Fever Outbreak in Homeless Shelter. Emerg Infect Dis. (2004) 10:1297–9. doi: 10.3201/eid1007.031020
138. Clark, NJ, and Soares Magalhães, RJ. Airborne geographical dispersal of Q fever from livestock holdings to human communities: a systematic review and critical appraisal of evidence. BMC Infect Dis. (2018) 18:218. doi: 10.1186/s12879-018-3135-4
139. Bandaw, T, and Herago, T. Review on abattoir waste management. Glob Vet. (2017) 19:517–24. doi: 10.5829/idosi.gv.2017.517.524
140. Arroway, E. Factors affecting presence and occupancy of marabou storks (Leptoptilos crumeniferus) at abattoirs and slaughter slabs near Jinja, Uganda. Univ Denver Undergrad Res J. (2022) 2022:1–9.
141. Moreno-Opo, R, Trujillano, A, and Margalida, A. Behavioral coexistence and feeding efficiency drive niche partitioning in European avian scavengers. Behav Ecol. (2016) 27:1041–52. doi: 10.1093/beheco/arw010
142. Bartlow, AW. How cooperative engagement programs strengthen sequencing capabilities for biosurveillance and outbreak response. Front Public Health. (2021) 9:6. doi: 10.3389/fpubh.2021.648424
143. Chipwaza, B, Mugasa, JP, Mayumana, I, Amuri, M, Makungu, C, and Gwakisa, PS. Community knowledge and attitudes and health workers’ practices regarding non-malaria febrile illnesses in eastern Tanzania. PLoS Negl Trop Dis. (2014) 8:2896. doi: 10.1371/journal.pntd.0002896
144. Buchan, BW, Jobe, DA, Mashock, M, Gerstbrein, D, Faron, ML, Ledeboer, NA, et al. Evaluation of a novel multiplex high-definition PCR assay for detection of tick-borne pathogens in whole-blood specimens. J Clin Microbiol. (2019) 57:e00513–9. doi: 10.1128/JCM.00513-19
145. Salyer, SJ, Silver, R, Simone, K, and Barton, BC. Prioritizing Zoonoses for Global Health capacity building–themes from one health zoonotic disease workshops in 7 countries, 2014–2016. Emerg Infect Dis. (2017) 23:S55–64. doi: 10.3201/eid2313.170418
146. Wardrop, NA, Thomas, LF, Cook, EAJ, De Glanville, WA, Atkinson, PM, Wamae, CN, et al. The sero-epidemiology of Coxiella burnetii in humans and cattle, western Kenya: evidence from a cross-sectional study. PLoS Negl Trop Dis. (2016) 10:e0005032. doi: 10.1371/journal.pntd.0005032
147. Donadeu, M, Nwankpa, N, Abela-Ridder, B, and Dungu, B. Strategies to increase adoption of animal vaccines by smallholder farmers with focus on neglected diseases and marginalized populations. PLoS Negl Trop Dis. (2019) 13:e0006989. doi: 10.1371/journal.pntd.0006989
Keywords: abattoir, slaughterhouses, livestock, zoonotic disease, one health
Citation: Rodarte KA, Fair JM, Bett BK, Kerfua SD, Fasina FO and Bartlow AW (2023) A scoping review of zoonotic parasites and pathogens associated with abattoirs in Eastern Africa and recommendations for abattoirs as disease surveillance sites. Front. Public Health 11:1194964. doi: 10.3389/fpubh.2023.1194964
Edited by:
Abdelaziz Ed-Dra, Sultan Moulay Slimane University, MoroccoReviewed by:
Ana Cláudia Coelho, University of Trás-os-Montes and Alto Douro, PortugalHassan El-Abid, Ministry of Health (Morocco), Morocco
Copyright © 2023 Rodarte, Fair, Bett, Kerfua, Fasina and Bartlow. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrew W. Bartlow, YWJhcnRsb3dAbGFubC5nb3Y=
 Katie A. Rodarte1
Katie A. Rodarte1 Jeanne M. Fair
Jeanne M. Fair Bernard K. Bett
Bernard K. Bett Susan D. Kerfua
Susan D. Kerfua Folorunso O. Fasina
Folorunso O. Fasina Andrew W. Bartlow
Andrew W. Bartlow