- 1Department of Environmental Health, College of Medicine and Health Sciences, Wollo University, Dessie, Ethiopia
- 2Department of Pre-clerkship, College of Medicine and Health Sciences, Wollo University, Dessie, Ethiopia
- 3Department of Public Health, College of Health Sciences, Debre Tabor University, Gondar, Ethiopia
Objective: More than half of the 700 million people worldwide who lack access to a safe water supply live in sub-Saharan Africa, including Ethiopia. Globally, approximately 2 billion people use drinking water sources that are contaminated with fecal matter. However, little is known about the relationship between fecal coliforms and determinants in drinking water. Therefore, the objective of this study was to investigate the potential for contamination of drinking water and its associated factors in households with children under 5 years of age in Dessie Zuria district in northeastern Ethiopia.
Methods: The water laboratory was conducted based on the American Public Health Association guidelines for water and wastewater assessment using a membrane filtration technique. A structured and pre-tested questionnaire was used to identify factors associated with the potential for contamination of drinking water in 412 selected households. A binary logistic regression analysis was performed to determine the factors associated with the presence or absence of fecal coliforms in drinking water, with a 95% confidence interval (CI) and a value of p ≤ 0.05. The overall goodness of the model was tested using the Hosmer-Lemeshow test, and the model was fit.
Results: A total of 241 (58.5%) households relied on unimproved water supply sources. In addition, approximately two-thirds 272 (66.0%) of the household water samples were positive for fecal coliform bacteria. Water storage duration ≥3 days (AOR = 4.632; 95% CI: 1.529–14.034), dipping method of water withdrawal from a water storage tank (AOR = 4.377; 95% CI: 1.382–7.171), uncovered water storage tank at control (AOR = 5.700; 95% CI: 2.017–31.189), lack of home-based water treatment (AOR = 4.822; 95% CI: 1.730–13.442), and unsafe household liquid waste disposal methods (AOR = 3.066; 95% CI: 1.706–8.735) were factors significantly associated with the presence of fecal contamination in drinking water.
Conclusion: Fecal contamination of water was high. The duration of water storage, the method of water withdrawal from the storage container, covering of the water storage container, the presence of home-based water treatment, and the method of liquid waste disposal were factors for fecal contamination in drinking water. Therefore, health professionals should continuously educate the public on proper water use and water quality assessment.
Introduction
Water is the second most important resource for the existence of all living things, including humans (1). Access to safe water supply and sanitation is recognized as a basic human right. It also plays a critical role in achieving adequate nutrition, gender equality, education, and poverty eradication (2–6). Water safety depends on a variety of factors, from the quality of the source water to its storage and handling in the home (7). More than half of the 700 million people worldwide who lack access to a safe water supply live in sub-Saharan African countries, including Ethiopia (8). In developing countries, more than 80% of the burden of disease is associated with poor drinking water quality, largely due to contamination from unsanitary conditions (8, 9).
Fecal coliforms are a group of bacteria from the normal flora of human and animal feces that can contaminate soil and water. E. coli in water sources is responsible for disease outbreaks (10). According to a World Health Organization report (WHO), an estimated 1.8 billion people rely on water contaminated with Escherichia coli (E. coli) (8). In rural areas of most developing countries, bacterial contamination of drinking water is a major cause of waterborne disease. Bacterial infections caused by contaminated drinking water remain a serious threat to public health, including fatal diarrhea. The problem is particularly severe in third World countries due to deteriorating environmental conditions caused by high levels of open defecation. Worldwide, 215 million people defecate in the open, which is a major source of diarrheal disease transmission in under five children, usually caused by E. coli (11).
According to WHO, unsafe water, poor sanitation, and inadequate hygiene cause 1.5 million preventable deaths each year, with children under five being the most affected populations. Eight million children die before they turn five each year, and diarrheal diseases cause 250 million missed school days (12). Diarrhea and waterborne diseases are the leading cause of mortality and morbidity in developing countries. Globally, 2 billion people use contaminated drinking water sources that are contaminated with feces (13, 14). More than 1.1 billion people in the world still live without access to safe drinking water sources, two-thirds of whom live in Africa, especially in southern and southern Africa. In addition, 2.4 billion people do not have access to even basic sanitation, resulting in 1.8 billion deaths each year from diarrheal diseases, mostly children under five.
In third world countries, 80% of all illnesses are due to poor quality drinking water, largely due to contamination from unsanitary conditions (8, 15). The major health problem in Ethiopia continues to be communicable diseases, which primarily affect the low socioeconomic status population (16). A lack of basic hygiene affects a child’s ability to survive, grow, and develop. Inadequate water, sanitation, and hygiene (WASH) is directly related to malnutrition and is associated with recurrent diarrheal diseases or intestinal worm infections (2). It still accounts for about 11% of global child mortality, although the number of deaths has decreased by one-third in the last decade, from 1.2 million in 2000 to 0.7 million in 2011 (13, 17).
The highest rates of this problem on the African continent are in Ethiopia, where 20% of the urban population and 80% of the rural population lack access to clean water (1). More than 60% of communicable diseases are primarily due to adverse environmental factors such as unsafe and inadequate water supply and poor hygiene and sanitation practices (1, 6, 17). Sources of drinking water contamination can range from source to fork (1, 6, 18). Despite various studies on water supply in Ethiopia, there is still a large gap in quantifying drinking water contamination and associated factors, especially in rural Ethiopia (12). It has been difficult to obtain published data on drinking water quality contamination potential and associated factors. Therefore, the objective of this study was to assess drinking water contamination potential and associated factors in households with children under 5 years of age in rural areas of Dessie Zuria district in northeastern Ethiopia.
Materials and methods
Study area
Dessie Zuria district is one of the 105 districts in the Amhara regional state of Ethiopia. It is located in the eastern parts of Ethiopian highlands in the southern Wollo zone. It borders Ilu to the south, Legambo to the west, Tenta to the northwest, Kutaber to the north, Tehuledere to the northeast, Kalu to the east, and Oromia zone to the southeast. It consists of 32 neighborhoods with a population density of 168.22, which is higher than the zone average of 147.58 persons per square kilometer (19). Based on the 2014 Ethiopian population projection report, the district has a total population of 176,309, of which 86,217 are males and 90,092 are females (20). A study conducted in Dessie Zuria district revealed that revealed that the prevalence of acute diarrhea among under-five children was 11% (95%CI: 7.8–14.3%) (21) (Figure 1).
Study design and period
A community-based cross-sectional study was employed to assess the potential contamination of drinking water quality in households with children under 5 years of age. The study was conducted from January 1 to February 30, 2021.
Populations of the study
Source population
The source population of the study was all households in the Dessie Zuria district with children under 5 years of age during the data collection.
Study population
The study population for this study was all selected neighborhoods in the Dessie Zuria district and all households in which children under-5 years of age living at the time of data collection.
Eligibility criteria
Inclusion criteria
Households which are used for residential purpose is included in the study. Households that had been living in the study area for more than 6 months were included in the study. Furthermore, respondents who were 18 years and older at the time of data collection were included in the study.
Exclusion criteria
Respondents who were seriously ill or had other mental health problems that prevented us from obtaining reliable information were excluded from the study.
Sample size determination
The sample size for the household survey was determined using the single population proportion formula (22) taking the assumptions that p (50%) of the household-level water samples are positive for fecal-contaminated drinking water, with a 95% confidence interval (CI) and a 5% margin of error.
Hence, the sample size was 384 and considering a non-response rate of 10%, the final sample size of the study was 422. Before collecting the water samples, the survey data were collected from the selected households.
Sampling procedure
Eight neighborhoods were selected from the total 32 neighborhoods by a simple random sampling technique using a lottery method. Then, the lists of households with children under 5 years of age from each selected neighborhood were obtained from the health extension workers of the selected neighborhoods. Then, the participant household from each neighborhoods was selected using systematic random sampling, using the kth value determined by dividing the study household by the total sample size. The number of participant from each neighborhood was determined by proportional allocation. K = the total number of households’/sample size 3,380/422 = 8.01 = 8.
Data collection tools and techniques
The questionnaire was developed from the literature that had been published in an international reputable journal (23, 24) and modified according to the study setting. Water samples for fecal coliform determination were collected from household drinking water storage tanks. The water sample from each household was collected based on the American Public Health Association (APHA) guidelines for water and wastewater assessment (25). The sample was collected in a sterile 100-mL bottle container. To ensure the quality of the sample collected, the bottles containing the water samples were placed in a cooler with cold packs immediately after collection to maintain a temperature of 4°C and then transported to the laboratory for analysis within 24 h of sample collection (11, 25).
For the household survey, the data were collected using structured, pretested questionnaires. Initially, the questionnaire was prepared in English and translated into the local language (Amharic) and back-translated into English to assure its consistency. The data were collected through a face-to-face interview and observational. The questionnaire consists of the socio-demographic characteristics of the respondents, water handling practices, hygiene practices, sanitation, and waste management. A total of four Environmental Health professionals and two laboratory technicians were recruited to collect household data and water samples from January 1 to February 30, 2021. The supervision of the data collection process was done by two master’s holders of Environmental Health experts.
Study variables
Dependent variable
The outcome variable of the study was the presence of fecal coliform contamination in a drinking water sample, with either (a yes/no) option.
Independent variables
The independent variables of the study were Sociodemographic characteristics (age, sex, marital status, family size, education level of the household head/spouse, occupational status of the household head/spouse, and monthly income); water handling practice; sanitation practices in the immediate area [safe disposal of human excreta (feces and urine) and disposal of household wastewater, proper segregation, collection, and disposal of solid waste, type of latrine, location of latrine, and ownership of latrine].
Operational definitions
Fecal coliforms
Fecal coliforms are groups of thermos-tolerant, rod-shaped, non-spore-forming, Gram-negative, oxidase-negative, aerobic or facultative anaerobic bacteria capable of growing in the presence of bile salts or other surface-active substitutes with analogous growth-inhibitory activity and fermenting lactose with gas and acid (or aldehyde) production within 48 h at 44 ± 0.5°C (26).
Improved water source
Tap water in home, yard, or property, public tap or standpipe, tube well or borehole, protected dug well, protected springs, and rainwater collection (27).
Improved sanitation
Flush or drain to the sewer, septic tank or pit latrine, ventilated improved pit latrine (VIP), pit latrine with slab, and composting toilet (27).
Unimproved water source
Unprotected dug wells, unprotected springs, carts with small tanks or barrels, water supplied by tanker trucks, surface water (river, dam, lake, pond, stream, canal, and irrigation canal), and bottled water (27).
Unimproved sanitation
Flush or drain elsewhere (i.e., no piped sewer system, septic tank, or pit latrine), pit latrine without slab/open pit, bucket, hanging toilet or hanging latrine, communal facilities of any kind, and no facilities, bush or field (28).
Proper latrine use
Households with functioning latrines and at least no discernible feces on the premises, discernible fresh feces through the squat hole, and the footpath to the latrine were not covered with grasses (18).
Proper waste disposal
A method of disposal that included burning, burying in a pit, or keeping in a container and disposing of in a designated place (18).
Unsafe disposal of children’s feces
Unsafe disposal of children’s feces: disposal of children’s feces in open areas or not at all; feces is considered unsafe if left in the open, thrown in the trash, placed/washed/flushed down open drains, or buried (29).
Solid waste disposal
Disposing of waste by burning, burying it in a pit or storing it in a container, composting, and disposing of it in a designated location is considered “proper” disposal while disposing of waste in an open field is considered “improper” disposal (30).
Safe human excreta disposal
It is the practice of disposing excreta through better sanitation technologies such as pit latrine with slab, VIP latrine flush latrine and avoid of open defecation in the family members of the households (31).
Unsafe disposal of human excreta
It is the practice of disposing human excreta in unsanitary conditions like a pit latrine without slab, bucket, hanging toilet or hanging latrine, the absence of latrine facilities, or having the practice of excreting in bush or field (31).
Data quality control
Data collectors and supervisors received 2 days training on the general aim of the study, the data collection process, and other relevant topics. The questionnaire was pretested on 5% of the sample size in Kalu district, who were excluded from the final study results. Necessary modifications were made based on the feedback from the pre-test before starting the final data collection. Water samples from each household were collected in sterilized glass bottles. All water samples were collected by trained laboratory technicians. Sample bottles were clearly labeled prior to sample collection. Samples were collected according to standardized procedures for collecting drinking water samples. All collected water samples were stored at 4°C prior to analysis. All water samples were analyzed in the laboratory within 4 h of collection. Prior to analysis, the required laboratory equipment and culture media were sterilized. All analytical procedures were carefully performed, and high-quality agar medium was used (17). Water samples were transported on ice in a cooler and analyzed within 2–4 h. In addition, the water samples were analyzed in triplicate according to the standard methods for the analysis of water and wastewater of the APHA guideline. The instruments were calibrated before use and throughout the process (25).
Determination of fecal coliforms
A 100 mL volume of a water sample was drawn through a membrane filter (45-micron pore size) through the use of a vacuum pump. The filter was placed on a petri (culture) dish on a pad with lauryl sulfate broth media, which feeds coliform bacteria and inhibits the growth of other bacteria and incubated for 24 h at 44.50°C. This elevated temperature heat-shocks non-fecal bacteria and suppresses their growth. As the fecal coliform colonies grow, they produced an acid (by fermenting lactose) that reacts with the aniline dye in the agar, thus gave the colonies their blue color, and making them easier to count. After 22–26 h, the agar plates were removed from the 44.5°C incubator and counted the colonies that have any blue color. These were taken as positive for fecal coliform bacteria in the investigated water samples (25, 32).
Data management and analysis
Epi Data version 3.1 and SPSS 25.0 were used for data entry and analysis, respectively. The results are presented in tables using frequencies and percentages. The outcome of the study was to measure the presence or absence of fecal coliforms in households stored for drinking water. A binary logistic regression analysis was performed to determine the factors associated with the potential for contamination of drinking water at the 95% confidence interval. Initially, a bivariable analysis was performed using a crude odd ratio (COR), and variables with a cutoff value of less than 0.25 were retained for multivariate analysis with a 95% confidence interval. In the multivariable analysis, the adjusted odds ratio (AOR) with the corresponding 95% confidence interval (CI) was then used to quantify the association between the dependent and independent variables with a 95% confidence interval. Therefore, variables with a value of p less than 0.05 at 95% CI were taken as factors significantly associated with the potential for contamination of drinking water by fecal coliforms. Analysis of fecal coliforms in drinking water was based on standard methods for water and wastewater testing adapted from APHA using the membrane filtration technique.
Results
Socio-demographic characteristics of the participants
In this study, 412 participants took part, with a response rate of 97.6%. The majority of participants were between 31 and 40 years old, Muslim, had primary education (1–8), were married, and were farmers with 178 (43.2%), 286 (69.4%), 123 (29.9%), 213 (51.7%), and 268 (65%), respectively. Nearly, half of the 186 (45.1%) households had an average family size of ≥5. Finally, half of the 213 participants (51.7%) had an average monthly household income of less than 1,000 Ethiopian Birr (Table 1).
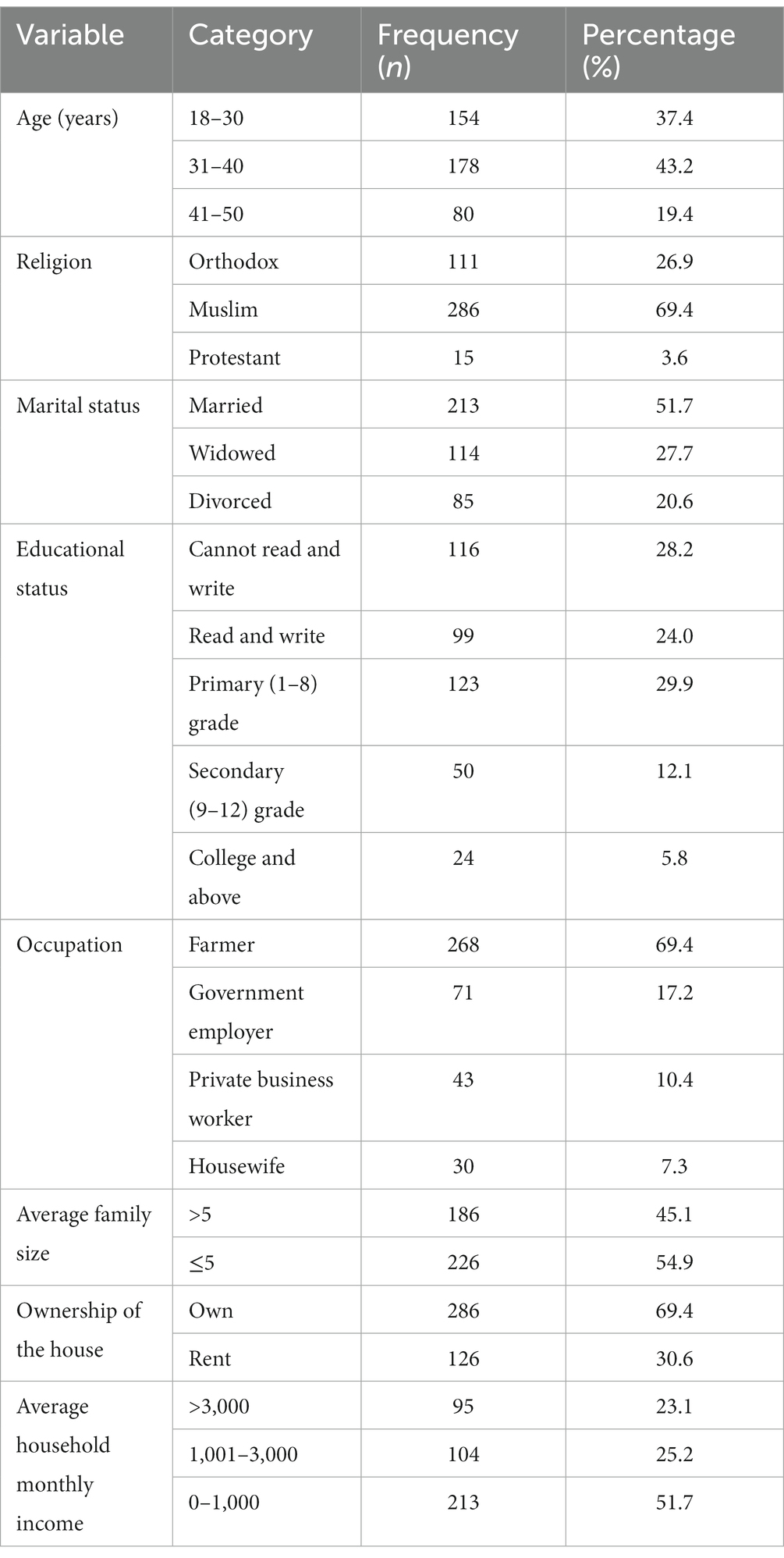
Table 1. Socio-demographic characteristics of the respondents in Dessie Zuria district Northeastern Ethiopia from January to February 2021.
Water supply condition
The results of this study revealed that a quarter 103 (25.0%) of households used river water as their primary source of drinking water. Generally, unimproved water sources accounted for more than half of 241 (58.5%) of the households. More than half of 234 (56.8%) of the households’ primary sources of water supply water located at a distance of at least 1 km. The study also revealed that nearly two-thirds of 252 (61.2%) households placed their drinking water storage containers on the floor. Additionally, more than one-third [152 (36.9%)] of the households used the dipping method of water withdrawal from storage containers. Furthermore, one-third 162 (39.3%) of the households practiced home-based water treatment. Finally, this study also indicated that less than half of the 194 (47.1%) households had covered their household water storage containers during the inspection period (Table 2).
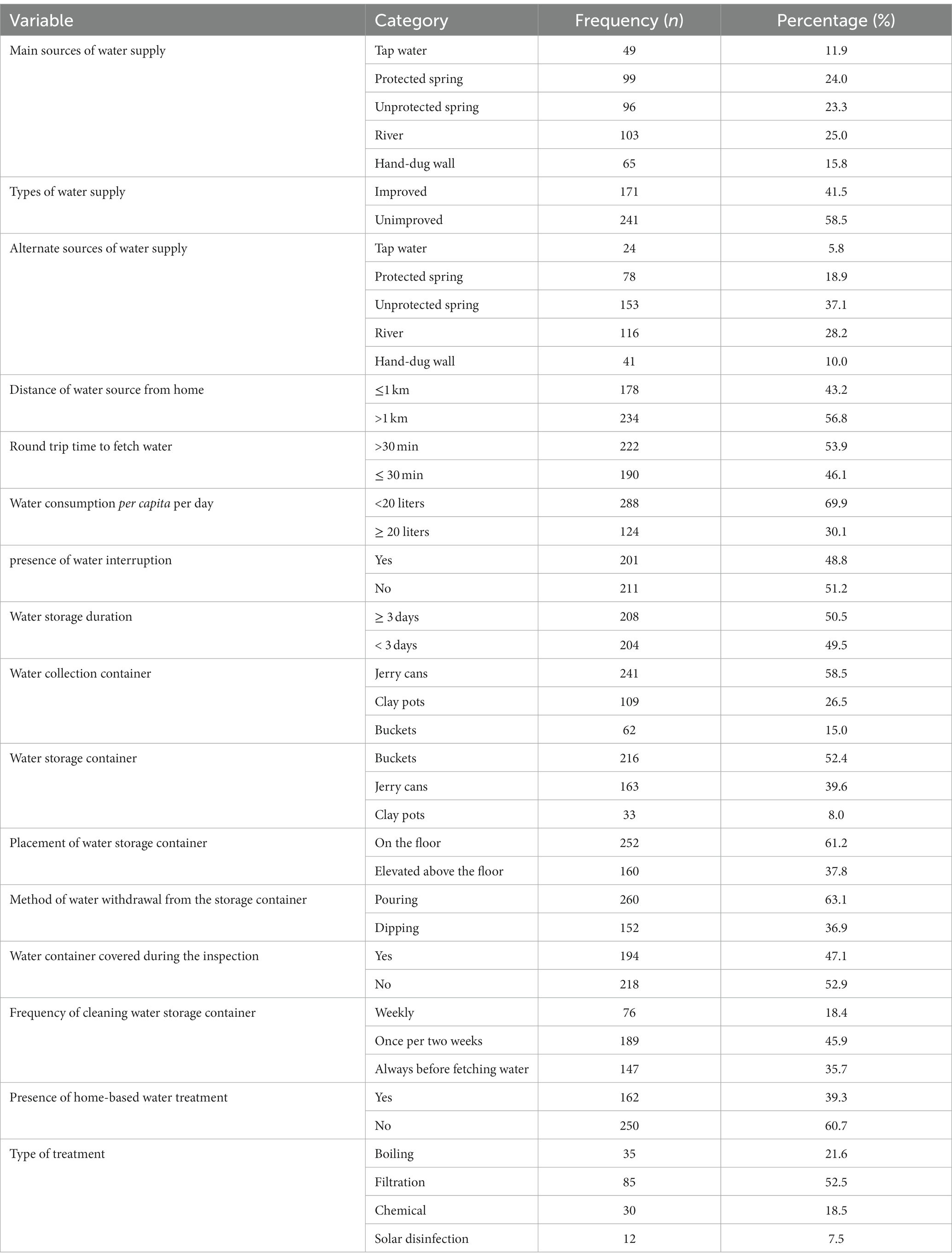
Table 2. Water supply conditions and handling practice in Dessie Zuria district Northeastern Ethiopia, from January to February 2021.
Sanitation facilities and related condition of the households
Less than three-quarters 285 (69.2%) of the households had latrines during the survey time, of whom only 208 (73.0%) households used the latrine properly. Of the households which had latrines, half 143 (50.2%) of the households used pit latrines without slabs. Regarding the cleanliness of latrines, less than half of 129 (45.3%) were clean. This finding also indicated that less than half of 177 (43.0%) households had hand-washing facilities at home, and 127 (30.8%) households used only water for hand washing. Finally, this finding revealed that about two-thirds of 272 (66.0%) of the sampled drinking water was positive for fecal coliform (Table 3).
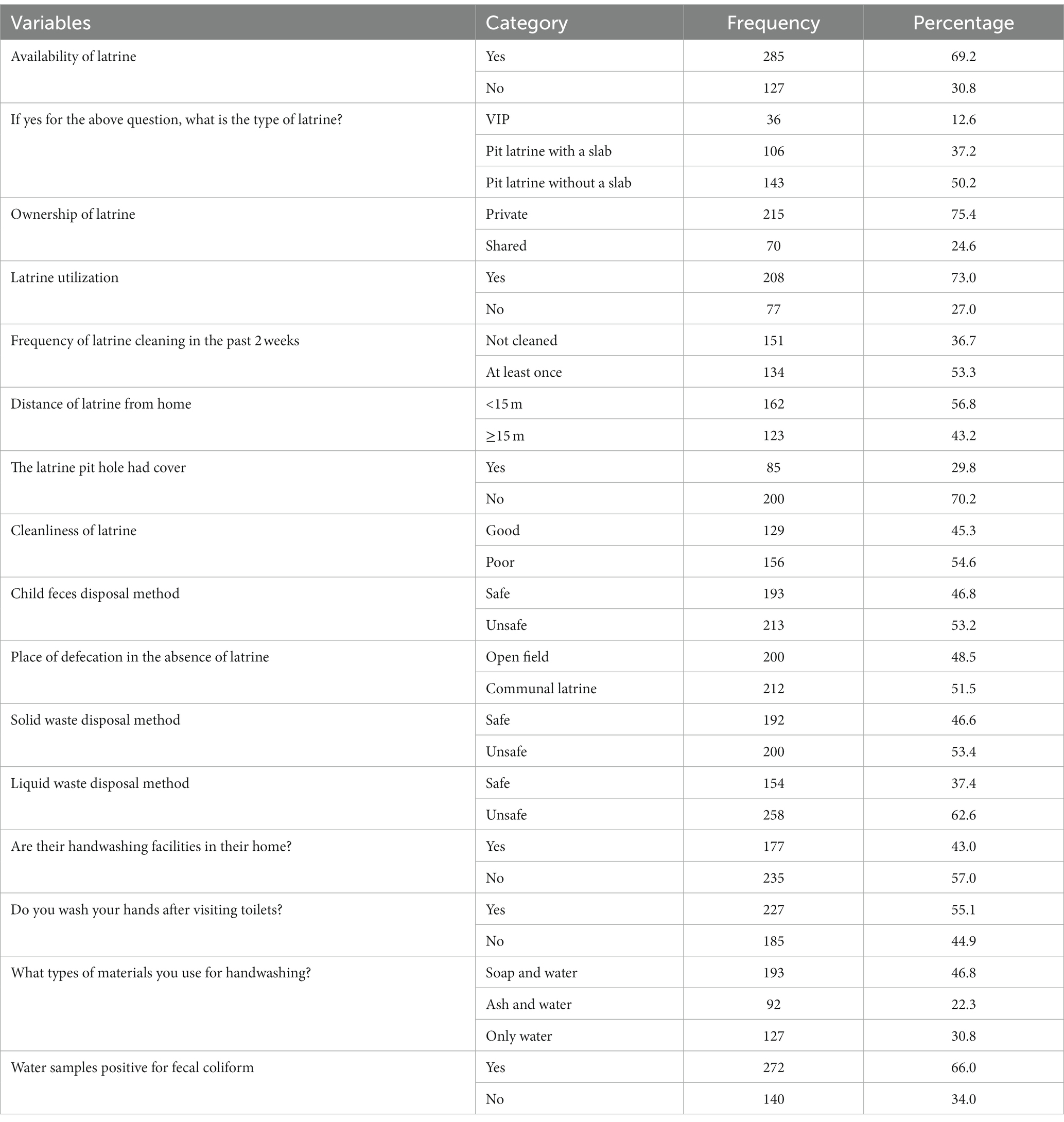
Table 3. Sanitation facilities conditions and related variables in Dessie Zuria district, northeastern Ethiopia, from January to February 2021.
Factors associated with fecal contamination of drinking water
In multivariable logistic regression analysis, duration of water storage in the house, method of water withdrawal from storage containers, covering of drinking water storage containers during the inspection, practice of household water treatment, and type of human excreta disposal were factors significantly associated with the potential for contamination drinking water at (95% CI). Drinking water stored for more than 3 days was 4,632 times more likely to be positive for fecal coliform than the corresponding groups. Households with the practice of dipping method of water withdrawal from storage containers were 4.377 times more likely to have fecal coliform bacteria than the corresponding groups. Furthermore, households which did not cover the water storage containers during the inspection time were 5.700 times more likely to have fecal coliform bacteria than the corresponding groups. Additionally, households which did not practice home-based water treatment were 4,822 times more likely to test positive for fecal coliform than the corresponding groups. Finally, households which had poor human excreta disposal practice were 3.066 times more likely to have positive for fecal coliform bacteria than the corresponding groups (Table 4).
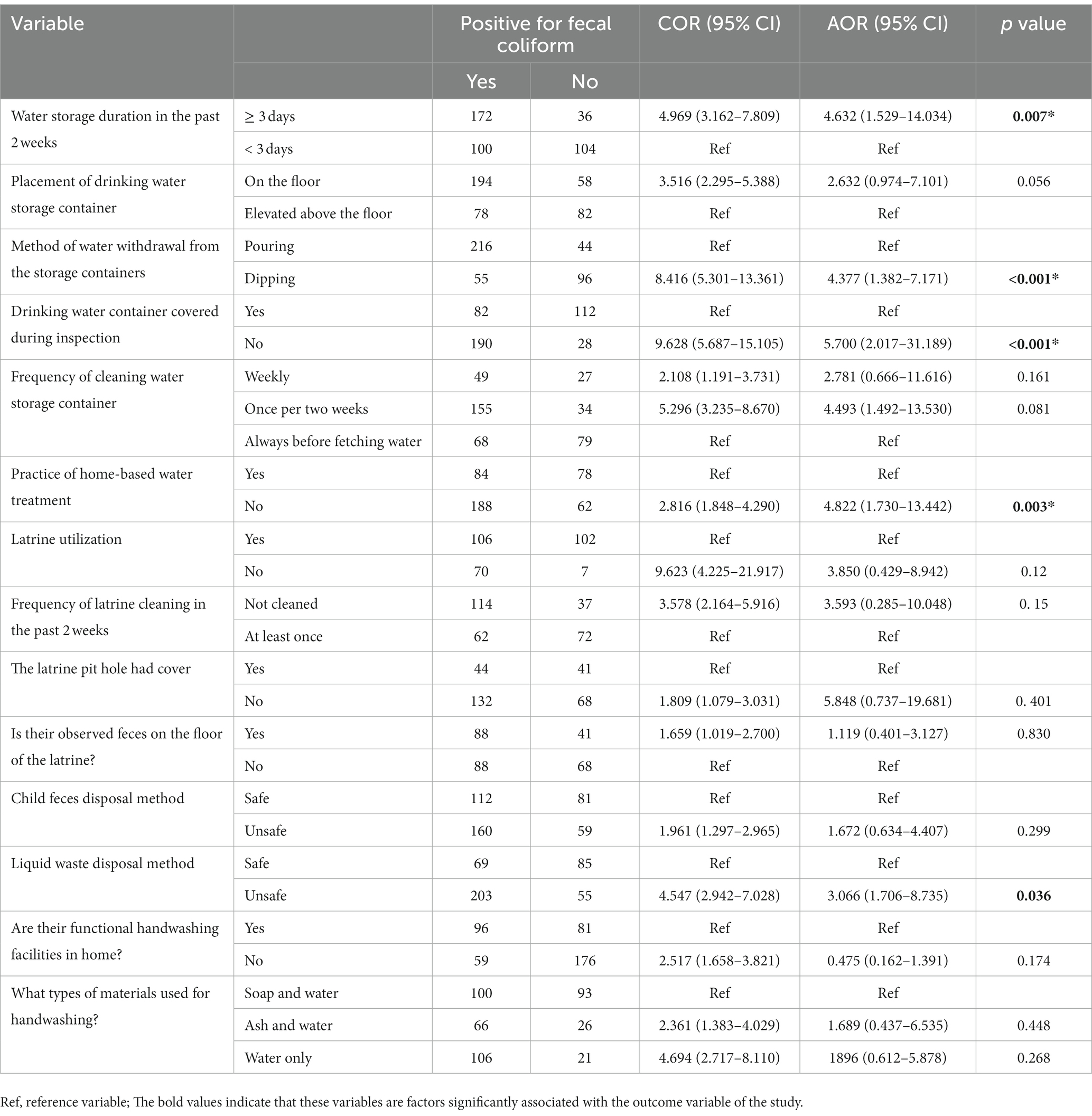
Table 4. Factors associated with the contamination potential of drinking water and associated factors among households with under-five children in rural areas of Dessie Zuria, District, Northeast Ethiopia, from January to February 2021.
Discussion
Access to safe water supply, adequate sanitation, and hygiene facilities are essential necessities of life. Yet, the problem is severe in rural parts of the developing countries, including Ethiopia (33). More than 60% of communicable diseases burden in Ethiopia are highly associated with poor environmental conditions. Hence, people continue to rely on unimproved water supply sources, which are highly susceptible to various types of contaminants (17). The use of microbial-contaminated water is thought to be the cause of between 10 and 20 million deaths annually and 250 million cases of sickness worldwide. This finding revealed that more than half (58.5%) of the households relied on unimproved sources of drinking water supply, which was higher than the findings in Ethiopia (43%) (34) and Kenya (17.2%) (35). Poor access to improved water sources leads to frequent disease outbreaks, which may account for up to 80% of health burdens mainly in developing countries (34, 36) which creates a significant financial and social burden such as school absenteeism and loss of productivity (34).
The finding of the current study showed that about two-thirds (66.0%) of the water samples taken at the household level indicated the contamination of drinking water were tested positive for fecal coliform, which was matched with the finding in Pakistan (37). On the other hand, the current finding was higher than the findings in Ethiopia (50.2%) (38), (39%) (34), (56.5%) (17), (33%) (39), (40%) (40), (37%) (41), Uganda (8.7%) (8), and Kenya (17.3%) (42). On the contrary, this finding was less than the findings in Ethiopia (72.6%) (17), (80%) (43), and (83.3%) (44). This high prevalence of fecal coliform bacteria in drinking water samples at the household level may not only originated from poor handling practice but also initially from the sources of water supply, mainly for households who relied on unimproved sources of water supply. Based on the WHO guidelines, drinking water must be free from fecal coliform bacteria to be fit for consumption (38). Therefore, the quality of water and its associated factors, its quality should be assessed not only from the household level but also at the sources for identifying key points of contamination and designing an appropriate intervention.
The use of improved source of water supply may not be guarantee for safe water quality at the consumption level. Therefore, good water handling practice should be done in addition to utilizing improved sources of water supply to overcome the burden of water related diseases, especially for children under the age of 5 (33). Less than half (47.1%) of the households covered the drinking water storage container during the survey, which was lower than the findings in Ethiopia (90.9%) (45), (92.5%) (18, 35), and, Sudan (91.7%) (46). The covering of water storage containers was one of the factors, which affect the potential for contamination of drinking water for fecal coliforms, which was consistent with the findings in Kenya (42).
Poor water handling practices are highly associated with post-contamination of drinking water (28, 47). The practice of dipping method of water withdrawal from storage containers may cause contamination of water despite using water sources from contamination at the sources of water supply (48). In addition, utensils used to withdraw water from storage containers and the hands of people handling the water are also common sources of water contamination (49). More than one-third (36.7%) of households had practiced the dipping method of withdrawing water from storage containers, which was lower than the results in Ethiopia (53.9%) (45) and Nigeria (58.8%) (50). In contrast, this result was higher than the results in Ethiopia (27%) (18). The method of water withdrawal from the storage container is one of the determining factors affecting fecal coliforms in water, which was confirmed by the study conducted in Kenya (42, 48). Contaminated drinking water at the point of collection may be attributed to various factors that may be at the source, transport, storage, or at the household handling practice (42). The possible rationale for this finding could be the fact that those who withdraw water from the storage containers by the dipping increase the risk of contamination. Hands of water handler may harbor various types of pathogenic microorganisms due to their poor hygienic practice.
Household water treatment plays an important role in improving drinking water quality. This type of treatment plays a particularly important role for households that depend on unimproved water supply sources, especially in developing countries. It has the potential to reduce the risk of diarrheal diseases by up to 61%. Boiling is the most common method of household water treatment in low-and middle-income countries but is not always practiced effectively (36). The results of the current study showed that only about one-third (39.3%) of the sampled households practiced household water treatment, which is consistent with studies from Ethiopia (44.1%) (45) and (34.3%) (51). On the other hand, the result of this study was lower than the studies conducted in Ethiopia 60% (30) but higher than the study conducted in Ethiopia (14%) (36), 2.8% (18, 52), 25.4% in Kenya (42), and Sudan (19.8%) (46). Based on the results of the current study, the presence of home-based water treatment is one of the factors affecting fecal coliform contamination in drinking water, which is consistent with the studies conducted in eastern Ethiopia (44) and Kenya (48).
Improper disposal of human excreta, such as construction of latrines near water sources and inadequate protection of water at the source, are considered major causes of fecal contamination. In most cases, households with exposed feces have high levels of microbial contamination. Human feces may contain a variety of pathogenic microorganisms, which cause diseases outbreaks such as typhoid fever, dysentery, cholera, and gastroenteritis (42). The results indicate that unsafe disposal of human excreta is another factor affecting fecal contamination of drinking water, which is consistent with studies conducted in Kenya (42) and Ethiopia (17).
Limitations of the study
This study has certain limitations. Some of the data were collected through interviews and self-report, which means that some of the responses may have social desirability bias. The study used a cross-sectional design, which may make it difficult to establish a cause-and-effect relationship between the dependent and independent variables of the study. Due to the hotspot nature of this study, there may be seasonal variations in the bacteriological quality of the drinking water. Furthermore, since the water samples were taken only from household level, the status of fecal coliform bacterial contamination of drinking water at the sources were ignored which may be a confounding factor for the fecal coliform bacteria at the household level.
Conclusion
The results indicate that household drinking water is significantly contaminated with fecal matter. Fecal coliform bacteria were detected in more than two-thirds of the water samples. The length of time water was stored in the household, the method of water withdrawal from storage tanks, the presence of domestic water treatment, and the method of disposal of human excreta were factors significantly related to the potential for contamination of drinking water. Therefore, the district health department should work to improve the quality of drinking water through hygiene education by promoting simple, acceptable, and cost-effective treatment methods and the use of narrow-mouth containers such as jerricans and bottles. In addition, the health and water supply authorities should regularly monitor the bacteriological quality of drinking water at the source, in distribution, and in households. Finally, future research should focus on the quality of drinking water from source to consumption, using other types of bacteria in addition to indicator bacteria.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethics approval was obtained from the institutional ethics committee of Wollo University, University of Medicine and Health Sciences, under ethical reference number CMHS/675/13. The relevant letters were also requested from the district administration. Before the start of data collection, participants were informed of the purpose of the study and confirmed the confidentiality of their information, which would be used exclusively for scientific research purposes. Participation in the study was completely voluntary, and the autonomy of the participants was respected. Participants were also informed that they had the unrestricted right to withdraw from the study at any time.
Author contributions
GB, MA, SH, AG, and LB contributed to the conception and design of the study. GB, SH, DT, ZW, and BD conducted the investigation. GB, LB, MA, AK, BD, ZW, BS, and AG performed data management and analysis. GB, MA, DT, AG, LB, ZW, and BS wrote and edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research project was sponsored by Wollo University with Grant No.: WU/6879 n-05/13.
Acknowledgments
We would like to express our deepest gratitude to the University of Wollo for funding this research project. We would also like to thank our friends for their encouragement and constructive comments from the development of the proposal to this stage. Finally, we would like to thank the data collectors, supervisors, and study participants who sacrificed their valuable time for the success of this research project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1199314/full#supplementary-material
Abbreviations
AOR, Adjusted odd ratio; APHA, American public health association; CI, Confidence interval; COR, Crude odd ratio; E. coli , Escherichia coli ; SSA, Sub Saharan Africa; WHO, World Health Organization.
References
1. Abdi, A.M. Assessment of knowledge and hygienic practice of bacteriological quality of drinking water in house hold level in Jigjiga City, Eastern Ethiopia. (2018)
2. Kaniambady, S, Vasu, DPGS, and Kulkarni, AG. A community-based cross-sectional study to assess the drinking water handling and management practices, and sanitary practices at the household level in Sullia taluk, Karnataka. Int J Community Med Public Health. (2017) 4:1678. doi: 10.18203/2394-6040.ijcmph20171783
3. Oladele, O. I., and Mudhara, M.. Empowerment of women in rural areas through water use security and agricultural skills training for gender equity and poverty reduction in Kwa Zulu-Natal and North West Province: Report to the Water Research Commission. (2016) Water Research Commission.
4. Caruso, BA, Conrad, A, Patrick, M, Owens, A, Kviten, K, Zarella, O, et al. Water, sanitation, and women's empowerment: a systematic review and qualitative meta-synthesis. PLOS Water. (2022) 1:e0000026. doi: 10.1371/journal.pwat.0000026
5. De Guzman, K., Stone, G., Yang, A.R., Schaffer, K.E., Lo, S., Kojok, R., et al. Drinking water and the implication for gender equity and empowerment: A systematic review of qualitative and quantitative evidence. Int J Hyg Environ Health. doi: 10.1016/j.ijheh.2022.114044 (Epub ahead of print).
6. Andualem, Z, Dagne, H, Azene, ZN, Taddese, AA, Dagnew, B, Fisseha, R, et al. Households access to improved drinking water sources and toilet facilities in Ethiopia: a multilevel analysis based on 2016 Ethiopian demographic and health survey. BMJ Open. (2021) 11:e042071. doi: 10.1136/bmjopen-2020-042071
7. Banda, K, Sarkar, R, Gopal, S, Govindarajan, J, Harijan, BB, Jeyakumar, MB, et al. Water handling, sanitation and defecation practices in rural southern India: a knowledge, attitudes and practices study. Trans R Soc Trop Med Hyg. (2007) 101:1124–30. doi: 10.1016/j.trstmh.2007.05.004
8. Agensi, A, Tibyangye, J, Tamale, A, Agwu, E, and Amongi, C. Contamination potentials of household water handling and storage practices in Kirundo sub-county, Kisoro District. Uganda J Environ Public Health. (2019) 2019:1–8. doi: 10.1155/2019/7932193
9. Garba, I, Tijjani, MB, Aliyu, MS, Yakubu, SE, Wada-Kura, A, and Olonitola, OS. Prevalence of Escherichia Coli in some public water sources in Gusau municipal, North-Western Nigeria. Bayero J Pure Appl Sci. (2009) 2:134–7. doi: 10.4314/bajopas.v2i2.63800
10. Nidhi, K, Sood, NK, Patel, PC, Patel, SM, and Mandalia, AH. A study of the prevalence of pathogenic bacteria, particularly, fecal coliforms and their antibiotic resistance pattern in environmental water samples of a tertiary-care hospital, Ahmedabad. Int J Med Sci Public Health. (2015) 4:1739–43. doi: 10.5455/ijmsph.2015.10042015359
11. Gwimbi, P, George, M, and Ramphalile, M. Bacterial contamination of drinking water sources in rural villages of Mohale Basin, Lesotho: exposures through neighborhoods’ sanitation and hygiene practices. Environ Health Prev Med. (2019) 24:33. doi: 10.1186/s12199-019-0790-z
12. Biniyam, S, Fessahaye, A, and Tefera, B. Sanitation practice and associated factors among slum dwellers residing in urban slums of Addis Ababa, Ethiopia: a community-based cross-sectional study. J Public Health Epidemiol. (2018) 10:370–9. doi: 10.5897/JPHE2018.1064
13. Nelson, SB, Panicker, PR, Nandakumar, L, and Malaichamy, K. Knowledge and practice regarding diarrheal diseases and drinking water usage in Kanyakumari district, South India. Nat J Res Commun Med. (2017) 6:217–22.
14. Hothur, R, Arepalli, S, and Bhadreshwara, ADV. A KAP study on water sanitation and hygiene among residents of Parla Village, Kurnool District, Andhra Pradesh. Int J Community Med Public Health. (2019) 6:2081–5. doi: 10.18203/2394-6040.ijcmph20191823
15. Gebrekiros, G, Desta, H, Desalegn, A, and Genet, G. Water handling and low-cost treatment practice of people living with human immunodeficiency virus (HIV) in Arba Minch town, southern Ethiopia, 2016. J AIDS HIV Res. (2017) 9:171–8. doi: 10.5897/JAHR2017.0432
16. Kumie, A, and Ali, A. An overview of environmental health status in Ethiopia with particular emphasis on its organization, drinking water and sanitation. Ethiop J Health Dev. (2005) 19:89–103. doi: 10.4314/ejhd.v19i2.9977
17. Getachew, A, Tadie, A, Chercos, DH, and Guadu, T. Level of fecal coliform contamination of drinking water sources and its associated risk factors in rural settings of North Gondar zone, Ethiopia: a cross-sectional community-based study. Ethiop J Health Sci. (2018) 28:227–34. doi: 10.4314/ejhs.v28i2.14
18. Kassie, GG, and Hayelom, D. Assessment of water handling and sanitation practices among rural communities of Farta Woreda, Northwest Ethiopia. Am J Health Res. (2017) 5:119. doi: 10.11648/j.ajhr.20170505.11
19. Endalew, B. Determinants of households saving capacity and Bank account holding experience in Ethiopia: the case of Dessie Zuria district. J Econom Sustain Dev. (2019) 10:1–16. doi: 10.7176/JESD
20. Abebe, A. (2013). The Federal Democratic Republic of Ethiopia central statistical agency population projection of Ethiopia for all regions: Wereda level from 2014–2017.
21. Natnael, T, Lingerew, M, and Adane, M. Prevalence of acute diarrhea and associated factors among children under five in semi-urban areas of northeastern Ethiopia. BMC Pediatr. (2021) 21:290. doi: 10.1186/s12887-021-02762-5
22. Kelsey, JL, Whittemore, AS, Evans, AS, and Thompson, WD. Methods in Observational Epidemiology: Monographs in Epidemiology and Biostatistics. New York, Oxford: Oxford University Press (1996).
23. Rameck, M. Determining the association between household drinking water handling practices and bacteriological quality of drinking water at the point-of-use in the rural communities of Murewa district, Zimbabwe. (2018) MSc thesis. University of the Western Cape, Zimbabwe.
24. Washington State Department of Health Environmental Public Health Office of Drinking Water Field guide sanitary surveys special purpose investigations technical assistance well site inspections. (2018)
25. Rice, E.W., Baird, R.B., Eaton, A.D., and Clesceri, L.S. Standard methods for the examination of water and wastewater. 22nd Edition. (2012) American Public Health Association, American Water Works Association, Water Environment Federation, United States of America.
26. Shoaib, M, Asad, MJ, Aziz, S, Usman, M, Rehman, A, Zafar, MM, et al. Prevalence of pathogenic microorganisms in drinking water of Rawalpindi and Islamabad. World J Fish Mar Sci. (2016) 8:14–21. doi: 10.5829/idosi.wjfms.2016.8.1.10288
27. Akoteyon, IS. Factors affecting household access to water supply in residential areas in parts of Lagos metropolis, Nigeria. Bull Geogr Soc Econom Ser. (2019) 43:7–24. doi: 10.2478/bog-2019-0001
28. Usman, MA, Gerber, N, and Pangaribowo, EH. Determinants of household drinking water quality in rural Ethiopia. SSRN Electron J. (2016) 1–33. doi: 10.2139/ssrn.2809564
29. Asnake, D, and Adane, M. Household latrine utilization and associated factors in semi-urban areas of northeastern Ethiopia. PLoS One. (2020) 15:e0241270. doi: 10.1371/journal.pone.0241270
30. Bekele, D, Merdassa, E, Desalegn, M, Mosisa, G, and Turi, E. Determinants of diarrhea in under-five children among health extension model and non-model families in Wama Hagelo District, West Ethiopia: community-based comparative cross-sectional study. J Multidiscip Healthc. (2021) 14:2803. doi: 10.2147/JMDH.S324846
31. UNICEF What do safely manage sanitation services mean for UNICEF programs? WASH discussion paper DP/03/2020. (2020)
32. American Society for Microbiology Bacteriological examination of waters: Membrane filtration protocol. (2015)
33. Willis, AB, Addo, M, Yoder, LE, Johnson, CK, Resler, SL, Leticia, J, et al. Individual and community level factors related to sanitation, water quality, treatment and Management in Rural Communities in Accra, Ghana: a field study report. Int J Transl Med Res Public Health. (2022) 6:e395. doi: 10.21106/ijtmrph.395
34. Gebrewahd, A, Adhanom, G, Gebremichail, G, Kahsay, T, Berhe, B, Asfaw, Z, et al. Tropical diseases. Travel Med Vaccines. (2020) 6:15. doi: 10.1186/s40794-020-00116-0
35. Merga, CA, Tadesse, F, Baye, D, and Seleman, A. Assessment of drinking water accessibility, handling and treatment practice in Assosa Woreda, Benishangul Gumuz region, North West Ethiopia. IRJPH. (2022) 6:66. doi: 10.28933/irjph-2022-01-2205
36. Tsega, N, Sahile, S, Kibret, M, and Abera, B. Bacteriological and physicochemical quality of drinking water sources in a rural community of Ethiopia. Afr Health Sci. (2013) 13:1156–61. doi: 10.4314/ahs.v13i4.42
37. Abbas, N, Wasimi, SA, Al-Ansari, N, and Sultana, N. Water resources problems of Iraq: climate change adaptation and mitigation. J Environ Hydrol. (2018) 26:1–11.
38. Ashuro, Z, Aregu, MB, Kanno, GG, Negassa, B, Soboksa, NE, and Alembo, A. Bacteriological quality of drinking water and associated factors at the internally displaced people sites, Gedeo zone, southern Ethiopia: a cross-sectional study. Environ Health Insights SAGE. (2021) 15:117863022110264–6. doi: 10.1177/11786302211026469
39. Sharma, HR, Worku, W, Hassen, M, Tadesse, Y, Zewdu, M, Kibret, D, et al. Water handling practices and level of contamination between source and point-of-use in Kolladiba town, Ethiopia. Environ We Int J Sci Tech. (2013) 8:25–35.
40. Feleke, H, Medhin, G, Kloos, H, Gangathulasi, J, and Asrat, D. Household-stored drinking water quality among households of under-five children with and without acute diarrhoea in towns of Wegera District, in North Gondar, Northwest Ethiopia. Environ Monit Assess. (2018) 190:669. doi: 10.1007/s10661-018-7033-4
41. Duressa, G, Assefa, F, and Jida, M. Assessment of bacteriological and physicochemical quality of drinking water from source to household tap connection in Nekemte, Oromia, Ethiopia. Hindawi J Environ Public Health. (2019) 7:2129792. doi: 10.1155/2019/2129792
42. Ondieki, JK, Akunga, DN, Warutere, PN, and Kenyanya, O. Bacteriological and physicochemical quality of household drinking water in Kisii town, Kisii County. Kenya Heliyon. (2021) 7:e06937. doi: 10.1016/j.heliyon.2021.e06937
43. Yasin, M, Ketema, T, and Bacha, K. Physico-chemical and bacteriological quality of drinking water of different sources, Jimma zone, Southwest Ethiopia. BMC Res Notes. (2015) 8:541. doi: 10.1186/s13104-015-1376-5
44. Alemeshet Asefa, Y, Alemu, BM, Baraki, N, Mekbib, D, and Mengistu, DA. Bacteriological quality of drinking water from source and point of use and associated factors among households in eastern Ethiopia. PLoS One. (2021) 16:e0258806. doi: 10.1371/journal.pone.0258806
45. Admasie, A, Abera, K, and Feleke, FW. Household water treatment practice and associated factors in rural households of Sodo Zuria District, southern Ethiopia: community-based cross-sectional study. Environ Health Insights. (2022) 16:117863022210950–7. doi: 10.1177/11786302221095036
46. Ibrahim, MMS, ElSayed, ASA, and Osman, FFA. Assessment of water handling practices and prevalence of water borne diseases, East Nile locality, Khartoum state. EAS J Nurs Midwifery. (2022) 4:24–33. doi: 10.36349/easjnm.2022.v04i01.005
47. Gizachew, M, Admasie, A, Wegi, C, and Assefa, E. Bacteriological contamination of drinking water supply from protected water sources to point of use and water handling practices among beneficiary households of Boloso sore Woreda, Wolaita zone, Ethiopia. Hindawi Int J Microbiol. (2020) 10:5340202. doi: 10.1155/2020/5340202
48. Kirianki, PR, Othira, JO, and Kiruki, S. Analysis of microbial quality of drinking water in Njoro sub-county, Kenya. J Environ Pollut Hum Health. (2017) 5:15–21. doi: 10.12691/jephh-5-1-3
49. WHO (2017). Guidelines for drinking-water quality: The fourth edition incorporating the first addendum. Geneva: World Health Organization.
50. Oloruntoba, EO, and Olannye, DU. Drinking water quality and handling practices among women in rural households of Oshimili north local government area of Delta state, Nigeria. E Top J Sci Technol. (2019) 12:249–66. doi: 10.4314/ejst.v12i3.5
51. Tafesse, B, Gobena, T, Baraki, N, Asefa, YA, and Mengistu, DA. Household water treatment practice and associated factors in Gibe District southern Ethiopia: a community-based cross-sectional study. Environ Health Insights. (2021) 15:1–8. doi: 10.1177/11786302211060150
Keywords: water, contamination, fecal coliform, under-five children, Dessie Zuria district, Ethiopia
Citation: Berihun G, Abebe M, Hassen S, Gizeyatu A, Berhanu L, Teshome D, Walle Z, Desye B, Sewunet B and Keleb A (2023) Drinking water contamination potential and associated factors among households with under-five children in rural areas of Dessie Zuria District, Northeast Ethiopia. Front. Public Health. 11:1199314. doi: 10.3389/fpubh.2023.1199314
Edited by:
Mohiuddin Md. Taimur Khan, Washington State University Tri-Cities, United StatesReviewed by:
Keith Dana Thomsen, Lawrence Livermore National Laboratory (DOE), United StatesTanmoy Roy Tusher, Mawlana Bhashani Science and Technology University, Bangladesh
Copyright © 2023 Berihun, Abebe, Hassen, Gizeyatu, Berhanu, Teshome, Walle, Desye, Sewunet and Keleb. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gete Berihun, Z2V0ZWJlcmlodW5AZ21haWwuY29t
 Gete Berihun
Gete Berihun Masresha Abebe1
Masresha Abebe1 Leykun Berhanu
Leykun Berhanu Daniel Teshome
Daniel Teshome Zebader Walle
Zebader Walle Belay Desye
Belay Desye Birhanu Sewunet
Birhanu Sewunet Awoke Keleb
Awoke Keleb