- 1Chinese Center for Disease Control and Prevention, National Institute of Parasitic Diseases, Shanghai, China
- 2Department of Pathogen Biology, the Key Laboratory of Microbiology and Parasitology of Anhui Province, the Key Laboratory of Zoonoses of High Institutions in Anhui, School of Basic Medical Sciences, Anhui Medical University, Hefei, China
- 3Department of Epidemiology and Biostatistics, School of Public Health, Anhui Medical University, Hefei, Anhui, China
- 4Brown School, Washington University, St. Louis, MO, United States
- 5Malaria Elimination Initiative, Institute for Global Health Sciences, University of California, San Francisco, San Francisco, CA, United States
- 6Eck Institute for Global Health, University of Notre Dame, Notre Dame, IN, United States
- 7Research for Implementation Unit, The Special Programme for Research and Training in Tropical Diseases, World Health Organization, Geneva, Switzerland
- 8Chinese Center for Tropical Diseases Research, Shanghai, China
- 9WHO Collaborating Centre for Tropical Diseases, Shanghai, China
- 10National Center for International Research on Tropical Diseases, Ministry of Science and Technology, Shanghai, China
- 11Key Laboratory of Parasite and Vector Biology, Ministry of Health, Shanghai, China
- 12School of Global Health, Chinese Center for Tropical Diseases Research, Shanghai Jiao Tong University School of Medicine, Shanghai, China
Mosquito-borne diseases are major global health problems that threaten nearly half of the world’s population. Conflicting resources and infrastructure required by the coronavirus disease 2019 (COVID-19) global pandemic have resulted in the vector control process being more demanding than ever. Although novel vector control paradigms may have been more applicable and efficacious in these challenging settings, there were virtually no reports of novel strategies being developed or implemented during COVID-19 pandemic. Evidence shows that the COVID-19 pandemic has dramatically impacted the implementation of conventional mosquito vector measures. Varying degrees of disruptions in malaria control and insecticide-treated nets (ITNs) and indoor residual spray (IRS) distributions worldwide from 2020 to 2021 were reported. Control measures such as mosquito net distribution and community education were significantly reduced in sub-Saharan countries. The COVID-19 pandemic has provided an opportunity for innovative vector control technologies currently being developed. Releasing sterile or lethal gene-carrying male mosquitoes and novel biopesticides may have advantages that are not matched by traditional vector measures in the current context. Here, we review the effects of COVID-19 pandemic on current vector control measures from 2020 to 2021 and discuss the future direction of vector control, taking into account probable evolving conditions of the COVID-19 pandemic.
Introduction
Mosquitoes are the most important vectors for disease transmission in terms of morbidity and mortality rates. Malaria, dengue fever, and yellow fever transmitted by mosquitoes have significantly high incidences, posing several public health problems (1). More than 600,000 people died of malaria in 2021 (2). Fifty to one hundred million people were infected with dengue fever annually, which leads to half a million hospitalizations (3). Infections by or continuous transmission of yellow fever, chikungunya, and Zika viruses also have significant public health impact, threatening more than 40% of the global population (4). Since the coronavirus disease 2019 (COVID-19) pandemic some low-income tropical or endemic countries have been unable to sustain funding for mosquito-borne diseases to ensure control (5).
Investment in the knowledge of the pathogenesis of these mosquito-borne diseases and antiviral drugs has increased exponentially over the past 20 years, but progress in the development of effective treatments, with the exception of malaria, remains slow (6). In the same way, the development of vaccines against mosquito-borne diseases has never reached its goals, with the exception of yellow fever. Therefore, for many vector-borne diseases, vector control remains the primary intervention to control mosquito-borne diseases through several techniques classified by physical, biological, chemical, genetic, and environmental aspects. Before the discovery of insecticides in the 1930s and the large-scale use of insecticides and mosquito nets, vector control interventions relied primarily on environmental management (7). The focus is on removing mosquito breeding sites and improving housing by installing screens to prevent mosquitoes from entering through doors and windows. This involves the installation of tight-fitting screened doors, screening or closing eaves, and replacing thatched roofs with solid materials such as metal or tile (8). In the past century, the deployment of insecticide-treated nets (ITNs), long-lasting insecticidal nets (LLINs), and indoor residual spraying (IRS) have become the primary and recommended means of mosquito vector control (7). Significant progress in malaria, dengue fever, and Zika viruses, the most important mosquito-borne diseases, has been achieved through the distribution of treated mosquito nets to at-risk populations and insecticide spraying. However, due to the cost issues, and operational constraints, traditional vector control measures are losing efficiency in controlling mosquito-borne diseases. The benefits from these techniques are gradually plateauing (9). Although multiple strategies are being used and the development of LLINs and IRS with different compounds is accelerating, the global burden of mosquito-borne diseases on public health and economies continues to increase (10).
On March 11, 2020, the World Health Organization (WHO) declared COVID-19 a global pandemic (11). Confirmed cases emerged in more than 200 countries. Along with the long-standing challenges of globalization, climate change, urbanization and insecticide resistance, the mosquito vector control process within the COVID-19 context was facing unprecedented difficulties, further highlighting the need for new technologies and strategies. A series of challenges will prevent the critical goals of the WHO Global Strategy on malaria for 2030 from being met (12). Therefore, we must change our thinking and adopt innovative and transformative approaches to vector control. Biological control, represented by the release of sterile male mosquitoes and new biocides, has excellent potential, but no reports of novel strategies were developed or implemented during COVID-19. In this review, we explore the interaction between COVID-19 and mosquito-borne diseases, and we discuss promising vector control strategies within the current environment. Data for this review were initially identified through a search of PubMed, Web of Science and ScienceDirect. We independently extracted and recorded data from each eligible study. Specific content and reasons for the implementation of mosquito vector measures affecting COVID-19 were manually screened to ensure accuracy. We ended up including 19 articles in Table 1 that describe the impacts from 2020 to 2021.
Effects of COVID-19 on current mosquito vector control measures
In the early days of the COVID-19 pandemic, most countries took measures in the form of lockdowns. In the short term, the lockdown may have had a positive impact on mosquito-borne diseases by preventing regional or inter-country transmission of infected individuals (32). In parallel, the vector will continue to reproduce and host-seek on humans, alongside a reduction in access to health facilities and health professionals combined with supply chain issues. As more countries opened their borders and stopped the lockdown, the need to reconsider how they balanced COVID-19 with other epidemics appeared. For example, the complete closure of health and vector control team activity during the lockdown may have resulted in increased vector populations (33). Common mosquito-borne diseases, such as Zika, dengue fever, and malaria, are at risk of outbreaks (34, 35). Additionally, the co-infection of SARS-CoV-2 and dengue fever viruses have imposed a significant burden on healthcare systems in dengue-endemic regions (36). India imposed its first nationwide lockdown on March 24, 2020. Observations were conducted in two areas of Bangalore, India, comparing data before and after the lockdown (February to April 2020, collected once a month). Compared to February, the Aedes aegypti house index and Breteau index increased from 6.6 and 9.3 to 26.6 and 34.6, respectively. Very significant increase compared to 2017 to 2019 data for this location (37). In addition to India, mosquito larval site monitoring in Sri Lanka, Cuba, Indonesia, and Malaysia demonstrated varying increases (38–41). This suggests that the probability of mosquito-borne disease outbreaks may increase in places where mosquito populations become larger. Within this context, mosquito vector control measures should not be reduced or abandoned but should be given more attention. Furthermore, lockdown measures obliged people to stay much more in their home, where the transmission of arboviral diseases usually occurs. Cavany et al. (42) used a model to predict changes in dengue incidence due to lockdown, with the proportion of people infected in their own homes increasing from 54% in normal conditions to 66% in lockdown conditions, and the rate of secondary household attacks increasing from 0.109 to 0.128, a 17% increase.
The immediate effect of COVID-19 on mosquito vector control measures was the massive diversion of medical resources. Budgets for actions, such as IRS and ITNs, have been massively cut (43). ITN and IRS defined by WHO as cornerstones of mosquito vector control. The rapid delivery of ITNs to populations at risk of mosquito-borne diseases in a remarkably short period of time through mass campaigns is currently the primary method of ITN operation (44). Governments, private sectors, and religious and humanitarian organizations have been working on this for the past few decades, and much has been accomplished. Sleeping under ITNs has reduced the incidence rate of malaria by 50% (45). Since the declaration of COVID-19 as a pandemic, attention has shifted to COVID-19, interrupting several intervention programs for equally health-threatening infectious diseases (46). Simultaneously, control activities, such as the distribution of mosquito nets and community education, ceased or were significantly reduced (43). The highest levels of mosquito-borne diseases have been found in sub-Saharan Africa for the past 20 years, and those regions were then the ones suffering most of the consequences of COVID-19 disruptions. Furthermore, after the COVID-19 pandemic, the crowding out of medical resources, diversion of funds, and interruption of logistics caused by the embargo made the original control measures impossible to implement (47). Overall, the impacts of the COVID-19 pandemic during 2020 and 2021 were much larger than envisioned for several mosquito-borne diseases on different continents (Table 1).
Varying degrees of disruptions in malaria control and ITNs and IRS distribution worldwide from 2020 to 2021 were reported (Table 1). According to the WHO, less than half of the 22 million ITNs planned for global distribution in 2020 had been distributed as of November 2020. Meanwhile, less than half of the routine IRS activities in malaria-endemic countries have been completed (48). A majority (58%) of countries (out of 64) report disruptions in the service delivery of their malaria control programs from 2020 to 2021 (5). Reducing IRS and ITN allocation is expected to lead to severe consequences. In the most extreme scenario, conventional malaria control measures, including a 75% reduction in ITN distribution and drug shortages, would increase sub-Saharan malaria morbidity and mortality rates by more than 20 and 50%, respectively (49). Hogan et al. (50) predicted the extent of disruption to healthcare and malaria control services during the COVID-19 pandemic. They estimated that the global malaria mortality rate could increase by 36% over the next 5 years, mainly due to a shortage of ITNs and the scarcity of other essential commodities. In addition, ITNs and IRS are labor-intensive vector control measures, the implementation of which inevitably leads to interaction between communities, in contradiction with the recommendation on COVID-19 (to avoid crowding). For this reason, the budget for COVID-19 personal protective equipment in several activities has been increased (51). Creating more outdoor facilities and improving indoor air circulation in residential and commercial buildings to reduce the risk of COVID-19 may increase exposure to mosquitoes (52).
In addition to the consequences of the COVID-19 pandemic, several challenges are currently faced by conventional vector control activities, such as the costs of implementation, traditional mosquito vector measures, slow operational implementation, and insecticide resistance (53). The increasing trend of insecticide resistance observed in recent years is alarming, and resistance to four classes of insecticides (pyrethroids, organochlorines, carbamates, and organophosphates) was reported in 32% of the countries with mosquito-borne disease transmission. The newest approved ingredient in IRS products, clothianidin, has already been resistant in Central Africa (54). Moreover, 90% of malaria-endemic countries have reported resistance to at least one class of insecticides in Anopheles (9). Although ITN- and IRS-led vector control methods remain valid today against malaria, their lifespan is shortened. When the COVID-19 outbreak became a pandemic, new, transformative, and innovative vector control technologies were already required and are now more strongly necessary to address the current situation.
Innovative vector control strategies in development in the current context
Since early 2020, some countries have combined modern technology with traditional vector interventions to facilitate follow-up. For example, the ITN distribution was monitored through digital technology with a mobile application for timely monitoring and supervisory feedback, allowing more rapid collection of household statistics and ITN distribution data. This technology allowed for avoiding contact with personnel to a certain extent (51, 55).
A prior Mexican study used unmanned aerial vehicles (UAVs) to identify Ae. aegypti breeding sites and spraying to reduce the need for field technicians, achieving a 64.9% agreement between UAVs and ground monitoring. Moreover, UAVs can access breeding sites that cannot be accessed or identified by traditional ground monitoring and disinfection and ensure that routine disinfection and monitoring is conducted during the lockdown (56). In another case, Gabriel Carrasco-Escobar et al. used drones in Peru to identify Anopheles darlingi breeding sites through high-resolution images and multispectral profiles with an overall accuracy of 86.73–96.98% (57). In addition to collecting mosquito habitats and disinfecting them, drones are valuable tools for monitoring the environmental factors that influence disease dynamics. Flaviviruses are primarily maintained by wild, non-human primate hosts, and drones can map the migration patterns of wildlife populations and changes in their habitats. This brings benefits for real-time monitoring of disease dynamics, as well as vector intervention programs (58, 59).
Releasing sterile or lethal gene-carrying male mosquitoes
Releasing male mosquitoes as biological insecticides is a cutting-edge technology with great promise. These technologies are based on gram-negative intracytoplasmic bacteria of the genus Wolbachia, found in 76% of the world’s insect species and is the most widely distributed commensal bacterium worldwide (60). Manipulation of Wolbachia strains can induce anti-RNA viral properties in its hosts, inhibiting the development of pathogens, such as dengue virus and chikungunya virus, in mosquito vectors (61, 62) and is also associated with several reproductive operations in mosquito vectors (63). The result of the CI will be the suppression of the mosquito population. Therefore, CI-based population control is referred to as an incompatible insect technique (IIT). Different from conventional mosquito vector control methods, IIT involves the regular release of Wolbachia-carrying male mosquito populations with appropriate methods to reduce the mosquito population size, thus achieving the goal of disease control. With the study of the principle of CI and the development of embryo microinjection techniques, progress has also been made in important mosquito vectors that do not naturally carry Wolbachia by injecting infected insect cytoplasm or tissue into mosquito embryos (64). Several successful trials have shown positive results of Wolbachia in mosquito vector control (65–67).
The sterile insect technique releases large numbers of sterile male mosquitoes to mate with wild females (68). Sterility methods include chemical, radiation, hybrid sterility, and chromosomal translocation, of which radiation sterility is the most commonly used. SIT has the advantages of being environmentally friendly and controllable on a large factory scale. For decades, SIT has achieved many successes in agricultural control and population suppression, and it is currently widely tested against Culex, Anopheles and Aedes mosquitoes (69–71). An example, among many others, of an SIT field trial, was conducted during the COVID-19 pandemic in southern Germany infested with Ae. albopictus. Continued release of sterile male mosquitoes from May to September 2020 was achieved in the trial areas of Ludwigshafen and Freiburg, with egg sterility reaching 84.7 ± 12.5% and 62.7 ± 25.8%, respectively; in comparison, the natural sterility in the control area was 14.6 ± 7.3% (72).
Genetic sterility was also used with the release of insects with a dominant lethality gene (RIDL). The corresponding gene expression in the target population is introduced by releasing male transgenic mosquitoes carrying the dominant lethal gene. The expression of dominant lethal genes in the currently developed RIDL system is regulated by the Tet-Off system. The lethal gene is under the control of the tetracycline resistance operon (tetO), a response element of the tetracycline transcriptional activator (tTA). In the absence of tetracycline, the tTA activator binds tetO and activates the promoter to induce the expression of dominant lethal genes. In contrast, in the presence of tetracycline, tTA binds to tetracycline and prevents it from binding to the tetO site, thereby inhibiting the system (73). In the wild, the offspring of RIDL mosquitoes express the gene because of the lack of tetracycline in their diet, thus achieving control of population density. Compared to SIT, RIDL does not require manual separation of males and females, the sex-specific promoter separates males from females, and there is no reduction in the competitive ability of males (74). Various RIDL strains have been developed, including A. aegypti, A. albopictus, and Anopheles gambiae, which are conditionally lethal, specifically lethal, and wingless (73, 75–77).
Extensive trials have demonstrated the feasibility and unique advantages of releasing sterile or lethal gene-carrying male mosquitoes for mosquito vector control. In addition to the absence of insecticide resistance problems associated with traditional methods, long-term cost-saving benefits will address the current funding shortfall due to COVID-19 (78). In terms of implementation and effectiveness, there are advantages to using new technologies for mosquito control that are difficult to match with traditional methods in the current environment. The biggest challenge for the release of male mosquitoes carrying sterile or lethal genes is transportation. It is crucial that they arrive at the release site within 24 h; otherwise, their survival rate, flight ability, and mating ability can be negatively affected (79). Unfortunately, the absence of a globally common procedure for the transport of male mosquitoes makes it challenging to use these new technologies on a large scale in developing countries. However, combining multiple control tools and methods, such as geographic information systems, spatial analysis, or UAV could potentially improve the current situation and increase sustainability (80, 81). Other than that, most of these innovative technologies also have drawbacks that are not yet fully overcome. Larval rearing, field monitoring, selection of suitable strains, and construction of models with optimal solutions for release frequency and time to achieve the best release strategy have essential effects on control effectiveness (82). To optimize the utilization of resources and ensure the sustainability of the control program, new technologies must be integrated with a risk stratification system. Incorporating efficient predictive models and a centralized monitoring approach will significantly enhance the practicality of adopting these new technologies (83).
Novel biopesticides
In addition, biopesticides have become popular in recent years and have certain advantages in the current context. Fungi and bacteria are the main focus of current biopesticide research. The mechanism of action between the fungus and host is significantly complex and divided into several stages of adhesion, penetration, and colonization. Fungal spores invade the epidermis and break open the body wall by forming infestation structures, interfering with the metabolic function of the host and secreting toxins (84–86). From the perspective of mosquito control mechanisms, the fungus is highly suitable for on-site mosquito control. The fungus can attach to mosquito carcasses to reproduce and create an epidemic within the mosquitoes for continuous power. Mosquitoes with fungal disease can carry fungal spores to other mosquito habitats to infect more mosquitoes (87). Fungal biopesticides have low developmental costs, are convenient to use, and have considerable effects. There are no reports on mosquito resistance to fungal biopesticides (88). These properties make fungal insecticides promising for mosquito control. Metarhizium anisopliae and Beauveria bassiana are more developed than other fungi in fungal mosquito control. They can shorten the lifespan of many mosquitoes, including Anopheles, Aedes, and Culex (89–91). Mosquitoes exposed to M. anisopliae and B. bassiana die within 3–14 days, and their desire to suck blood and reproduce is reduced (87, 92). In addition to affecting survival time and reproductive capacity, M. anisopliae and B. bassiana have inhibitory effects on mosquito pathogens. Fang found that recombinant M. anisopliae can prevent the development of Plasmodium in the vector and can reduce the number of sporozoites by 98%, indicating that M. anisopliae is effective in fighting against malaria (93). Deng found that Zika virus (ZIKV) titer levels in the midgut, head and salivary glands were significantly reduced after feeding ZIKV to Ae. albopictus females (94). Another study found that Ae. aegypti infected with both M. anisopliae and dengue virus type 2 (DENV-2), and the infection rates of DENV-2 in the heads and midguts were significantly reduced (95). As abiotic factors (temperature, humidity, and ultraviolet radiation) affect the effectiveness of fungi in field applications, and the low virulence of the fungus leads to low efficiency of mosquito killing has been an important reason for its popularity is not widespread. Therefore some researchers have inserted some natural and synthetic genes into the fungal genome to improve their virulence and tolerance (96). For example, heat-tolerant genes can be genetically engineered into M. anisopliae to enhance their adaptability (97). Androctonus australis insect toxin is a neurotoxin widely used for recombinant expression in fungi (98). A prior study reported the toxicity of the recombinant M. anisopliae formed by this gene in adult Ae. aegypti increased by nine times (99). In addition, many other genes, such as [SM1]8 and scorpine, were recombined into pathogenic fungi to enhance their control of mosquito-borne infectious diseases (93).
The most mature bacterial biopesticide is Bacillus thuringiensis. It is a gram-positive, rod-shaped, spore-forming bacterium with facultative oxygen demand. Its toxin is mainly present in accompanying spore crystals formed during the development of budding spores (100). B. thuringiensis is active against Lepidoptera, Coleoptera, Diptera, Hymenoptera, Homoptera, Orthoptera, and Nematoda, but it is not toxic to mammals (101, 102). The development of B. thuringiensis var. israelensis enables B. thuringiensis to be invested in mosquito control on a large scale. B. thuringiensis var. israelensis is effective against 72 species of mosquitoes (21 species of Anopheles, 21 species of Aedes, and 17 species of Culex) (103). As a biofriendly insecticide with high mosquito control efficiency, convenient storage, and application forms, B. thuringiensis has been used in many applications worldwide. The primary tool for mosquito control in Hawaii, United States, is B. thuringiensis (104, 105). B. thuringiensis in drinking water is harmless to humans and well suited for use as a household-level biocide, and its resistance is difficult to pass on to mosquitoes (106). These characteristics make the number of B. thuringiensis unlimited in the field of mosquito control, providing a powerful alternative for mosquito control in the current environment. Another bacterium that has been relatively successful in mosquito killing is Bacillus sphericus. B. thuringiensis and B. sphericus have their own advantages and disadvantages; B. thuringiensis has a broad spectrum of insecticides, while B. sphericus has a long shelf life (107).
Biological insecticides are more widely used in practical applications than the release of male mosquitoes for control. They are currently used mainly in combination with traditional chemical insecticides to delay the problem of drug resistance. As an environmentally friendly tool that is also highly specific and can limit the growth of target populations in successive generations after application, its advantages are clear. It is highly suitable for promotion in the current environment to address resistance and cost issues (108). Biological control, represented by the release of male mosquitoes and biopesticides, can solve many of the pain points of traditional vector measures in the current context (Table 2).
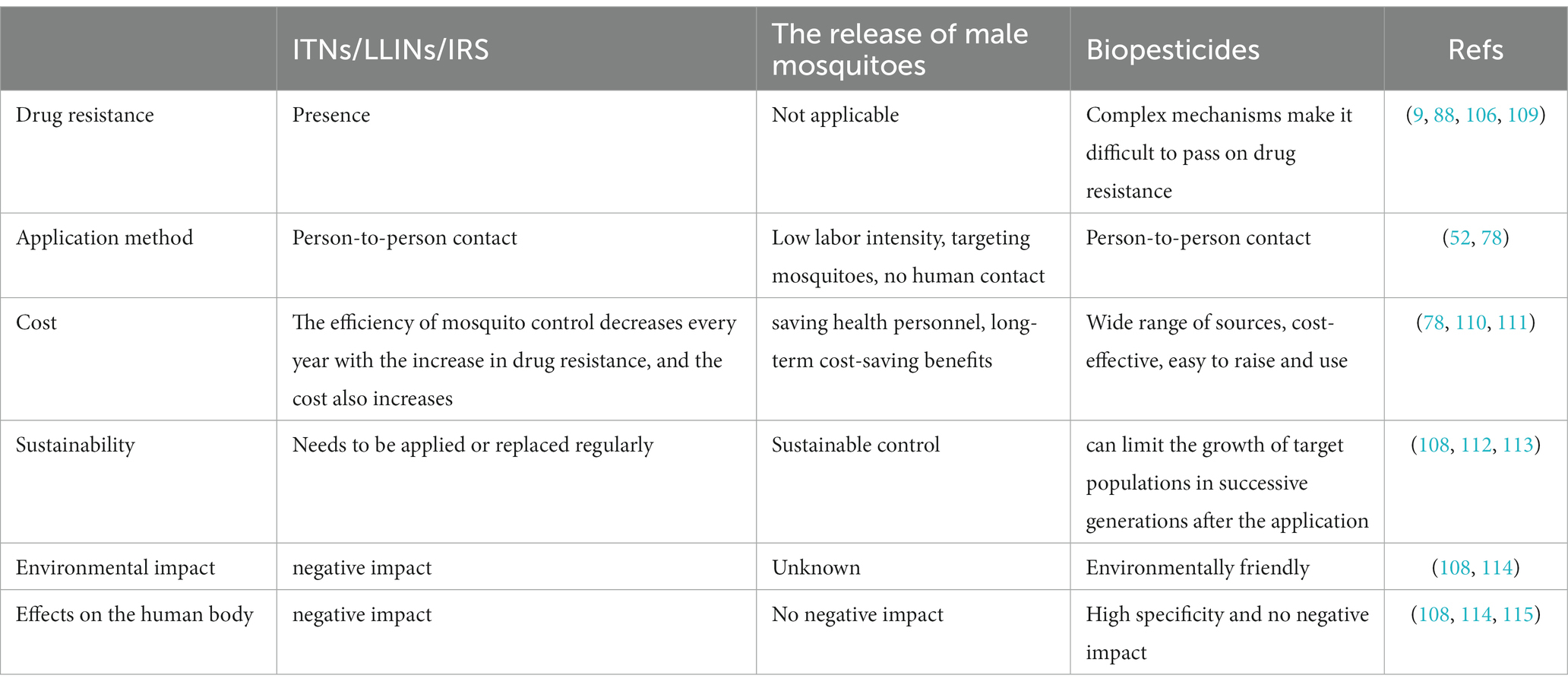
Table 2. Comparison of traditional vector control (ITNs/LLINs/IRS) and new vector control technologies (the release of male Mosquitoes and biopesticides).
In recent years, natural repellents have gained increasing attention due to their pure plant ingredients which are low in residue and easy to degrade. They are also known for being low or non-toxic, having minimal skin irritation effects and being environmentally friendly (116). Natural repellents are primarily derived from various plant parts such as stems, roots, leaves, flowers, and fruits, among others. The active ingredients of these repellents are mostly esters, ketones, and alcohols of terpenoids, and they often contain flavonoids and alkaloids, among others. Currently, the focus on the development of natural repellents involves the extraction of natural plant products and the analysis of their active ingredients. This analysis is considered a hot issue in natural repellent research (117). In addition to the technologies described above, several mosquito vector control technologies are still being developed, including acoustic larvicides, RNAi-based biocides, and nanotechnology, which are equally desirable. In general, the situation of mosquito-borne diseases during the COVID-19 pandemic is serious, and the application and promotion of new vector control strategies should be strengthened to effectively reduce the spread of mosquito-borne infectious diseases.
Conclusion
Although the new mosquito vector control technologies introduced above have significant advantages over traditional strategies, the market and practical applications are still dominated by traditional methods. There are several reasons for this phenomenon.
i. The market for new technologies is difficult to guarantee, resulting in insufficient motivation and funding for research and development, forming a vicious circle.
ii. Alternative and novel ways of approaching the issue may combat historical and habitual thinking, allowing new paradigms to combat the current transmission.
iii. Previous mosquito vector control focused more on quick results, especially chemical insecticide-led vector control, which ignored the long-term benefits and environmental and ecological effects. A scale-down of these control measures invariably leads to an immediate increase in vectors.
iv. The standardization process is slow, and larger-scale field trials cannot be conducted based on the funding needed.
The COVID-19 pandemic has exposed weaknesses in some countries’ preparedness and response capacity for public health crises and the inadequacy of existing mosquito vector control systems. The COVID-19 pandemic has had a significant effect on mosquito vector control and, in its aftermath, brings a huge opportunity to improve old strategies or develop more efficient and resilient ones. At the national level, epidemics have received unprecedented attention and some tilt of resources to public health. Systems and structures established as a result of pandemics may also present new opportunities for mosquito-borne disease control; for example, the strengthening of community infrastructure and the system established by the state for monitoring the spread of COVID-19 also facilitate mosquito-borne disease projects. Simultaneously, laboratory capacity has improved in many countries, and the increased power of sequencing technology and increased level of testing can also be used to strengthen mosquito vector surveillance activities. At the individual level, more people are willing to learn about this aspect and pay more attention to epidemic prevention in their daily lives, which will be helpful for future health education campaigns on mosquito-borne diseases and the promotion and implementation of mosquito-borne measures. To alleviate the current dilemma of mosquito-borne disease in the context of the COVID-19 and to prevent mosquito-borne disease from becoming the successive COVID-19, a change in concept, the development of new technology research, and the accelerated operation of field trials are needed.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This paper was funded by China–Africa cooperation project on malaria control under the Project (No. 2020-C4-0002-3), the program of the Chinese Center for Tropical Diseases Research (no.131031104000160004) as well as UNICEF/UNDP/World Bank/WHO Special Program for Research and Training in Tropical Diseases (TDR) Small Grant (WHO Reference 2021/1104003–0) to WDQ, and National Natural Science Foundation of China (NO. 8210082025) and Anhui Provincial Natural Science Foundation Project (no. 2108085QH347) to DSQ.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CI, cytoplasmic incompatibility; COVID-19, coronavirus disease 2019; DENV-2, dengue virus type 2; IIT, incompatible insect technique; IRS, indoor residual spraying; ITNs, insecticide-treated nets; LLINs, long-lasting insecticidal nets; RIDL, the release of insects with a dominant lethality gene; SIT, sterile insect technique; tetO: tetracycline resistance operon; tTA, tetracycline transcriptional activator; UAVs, unmanned aerial vehicles; WHO, World Health Organization; ZIKV, Zika virus.
References
1. Jones, RT , Ant, TH , Cameron, MM , and Logan, JG . Novel control strategies for mosquito-borne diseases, vol. 376. London: The Royal Society (2021). 20190802 p.
2. World Health Organization . World malaria report 2022. Geneva: World Health Organization. (2022). Available at: https://www.who.int/multi-media/details/introducing-the-world-malaria-report-2022. (Accessed January 21, 2022).
3. World Health Organization . Dengue and severe dengue. Geneva: World Health Organization (2022). Available at: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue. (Accessed January 10, 2022).
4. Wilder-Smith, A , Ooi, E-E , Horstick, O , and Wills, B . Dengue. Lancet. (2019) 393:350–63. doi: 10.1016/S0140-6736(18)32560-1
5. Chanda-Kapata, P , Ntoumi, F , Kapata, N , Lungu, P , Mucheleng'anga, LA , Chakaya, J, et al. Tuberculosis, HIV/AIDS and malaria health services in sub-Saharan Africa–a situation analysis of the disruptions and impact of the COVID-19 pandemic. Int J Inf Secur. (2022) 124:S41–6. doi: 10.1016/j.ijid.2022.03.033
6. Nasar, S , Rashid, N , and Iftikhar, S . Dengue proteins with their role in pathogenesis, and strategies for developing an effective anti-dengue treatment: a review. J Med Virol. (2020) 92:941–55. doi: 10.1002/jmv.25646
7. Wilson, AL , Courtenay, O , Kelly-Hope, LA , Scott, TW , Takken, W , Torr, SJ, et al. The importance of vector control for the control and elimination of vector-borne diseases. PLoS Negl Trop Dis. (2020) 14:e0007831. doi: 10.1371/journal.pntd.0007831
8. Lindsay, SW , Davies, M , Alabaster, G , Altamirano, H , Jatta, E , Jawara, M, et al. Recommendations for building out mosquito-transmitted diseases in sub-Saharan Africa: the DELIVER mnemonic. Philos Trans R Soc Lond B Biol Sci. (1818) 376:20190814. doi: 10.1098/rstb.2019.0814
9. Namias, A , Jobe, NB , Paaijmans, KP , and Huijben, S . The need for practical insecticide-resistance guidelines to effectively inform mosquito-borne disease control programs. elife. (2021) 10:e65655. doi: 10.7554/eLife.65655
10. Franklinos, LH , Jones, KE , Redding, DW , and Abubakar, I . The effect of global change on mosquito-borne disease. Lancet Infect Dis. (2019) 19:e302–12. doi: 10.1016/S1473-3099(19)30161-6
11. Harapan, H , Itoh, N , Yufika, A , Winardi, W , Keam, S , Te, H, et al. Coronavirus disease 2019 (COVID-19): a literature review. J Infect Public Health. (2020) 13:667–73. doi: 10.1016/j.jiph.2020.03.019
12. Monroe, A , Williams, NA , Ogoma, S , Karema, C , and Okumu, F . Reflections on the 2021 world malaria report and the future of malaria control, vol. 21. Berlin: Springer (2022).
13. Suiyanka, L , Alegana, VA , and Snow, RW . Insecticide-treated net distribution in Western Kenya: impacts related to COVID-19 and health worker strikes. Int Health. (2022) 14:537–9. doi: 10.1093/inthealth/ihab051
14. Likwela, JL , Ngwala, PL , Ntumba, AK , Ntale, DC , Sompwe, EM, et al. Digitalized long-lasting insecticidal nets mass distribution campaign in the context of Covid-19 pandemic in Kongo central, Democratic Republic of Congo: challenges and lessons learned. Malar J. (2022) 21:1–15. doi: 10.1186/s12936-022-04258-8
15. World Health Organization . The potential impact of health service disruptions on the burden of malaria: a modelling analysis for countries in sub-Saharan Africa. Geneva: World Health Organization (2020). Available at: https://apps.who.int/iris/bitstream/handle/10665/331845/9789240004641-eng.pdf. (Accessed January 21, 2022).
16. Guerra, C , Donfack, OT , Vaz, LM , Nlang, JM , Nchama, LON , Eyono, JNM, et al. Malaria vector control in sub-Saharan Africa in the time of COVID-19: no room for complacency BMJ glob. Health. (2020) 5:1–4. doi: 10.1136/bmjgh-2020-003880
17. Brooke, BD , Raman, J , Frean, J , Rundle, J , Maartens, F , Misiani, E, et al. Implementing malaria control in South Africa, Eswatini and southern Mozambique during the COVID-19 pandemic. S Afr Med J. (2020) 110:1072–6. doi: 10.7196/SAMJ.2020.v110i11.15286
18. Heuschen, A-K , Abdul-Mumin, A , Adokiya, M , Lu, G , Jahn, A , Razum, O, et al. Impact of the COVID-19 pandemic on malaria cases in health facilities in northern Ghana: a retrospective analysis of routine surveillance data. Malar J. (2022) 21:1–8. doi: 10.1186/s12936-022-04154-1
19. Gavi, S , Tapera, O , Mberikunashe, J , and Kanyangarara, M . Malaria incidence and mortality in Zimbabwe during the COVID-19 pandemic: analysis of routine surveillance data. Malar J. (2021) 20:1–9. doi: 10.1186/s12936-021-03770-7
20. Lorenz, C , Azevedo, TS , and Chiaravalloti-Neto, F . COVID-19 and dengue fever: a dangerous combination for the health system in Brazil. Travel Med Infect Dis. (2020) 35:101659. doi: 10.1016/j.tmaid.2020.101659
21. Rabiu, AT , Mohan, A , Çavdaroğlu, S , Xenophontos, E , Costa, ACS , Tsagkaris, C, et al. Dengue and COVID-19: a double burden to Brazil. J Med Virol. (2021) 93:4092–3. doi: 10.1002/jmv.26955
22. Morales, DO , Quinatoa, PA , and Cagua, JC . Characterization of an outbreak of malaria in a non-endemic zone on the coastal region of Ecuador. Biomedica. (2021) 41:100–12. doi: 10.7705/biomedica.5816
23. Navarro, JC , Arrivillaga-Henríquez, J , Salazar-Loor, J , and Rodriguez-Morales, AJ . COVID-19 and dengue, co-epidemics in Ecuador and other countries in Latin America: pushing strained health care systems over the edge. Travel Med Infect Di. (2020) 37:101656. doi: 10.1016/j.tmaid.2020.101656
24. Wilder-Smith, A , Tissera, H , Ooi, EE , Coloma, J , Scott, TW , and Gubler, DJ . Preventing dengue epidemics during the COVID-19 pandemic. Am J Trop Med Hyg. (2020) 103:570–1. doi: 10.4269/ajtmh.20-0480
25. Durón, RM , Sánchez, E , Choi, JN , Peralta, G , Ventura, SG , Soto, RJ, et al. Honduras: two hurricanes, COVID-19, dengue and the need for a new digital health surveillance system. J Public Health. (2021) 43:e297–8. doi: 10.1093/pubmed/fdaa266
26. Siddiqui, JA , Aamar, H , Siddiqui, A , Essar, MY , Khalid, MA , and Mousavi, SH . Malaria in Afghanistan: challenges, efforts and recommendations. Ann Med Surg. (2022) 81:104424. doi: 10.1016/j.amsu.2022.104424
27. Penjor, K , Zangpo, T , Clements, AC , Gray, DJ , and Wangdi, K . Has COVID19 derailed Bhutan’s national malaria elimination goal? A commentary. Malaria J. (2021) 20:1–3. doi: 10.1186/s12936-020-03562-5
28. Passah, M , Nengnong, CB , Wilson, ML , Carlton, JM , Kharbamon, L , and Albert, S . Implementation and acceptance of government-sponsored malaria control interventions in Meghalaya. India Malaria J. (2022) 21:1–14. doi: 10.1186/s12936-022-04223-5
29. Tahir, MJ , Siddiqi, AR , Ullah, I , Ahmed, A , Dujaili, J , and Saqlain, M . Devastating urban flooding and dengue outbreak during the COVID-19 pandemic in Pakistan. Med J Islam Repub Iran. (2020) 34:169. doi: 10.47176/mjiri.34.169
30. Olive, M-M , Baldet, T , Devillers, J , Fite, J , Paty, M-C , Paupy, C, et al. The COVID-19 pandemic should not jeopardize dengue control. Plos Negl Trop D. (2020) 14:e0008716. doi: 10.1371/journal.pntd.0008716
31. ANSES . AVIS de l’Agence nationale de se’curite´ sanitaire de l’alimentation, de l’environnement et du travail relatif à l’e’valuation du rapport be’ne’fice risque des pratiques de lutte anti-vectorielle habituellement mises en œuvre pour lutter contre la dengue, dans le contexte actuel de confinement global. (2022). Available at: https://www.anses.fr/fr/system/files/VECTEURS2020SA0057.pdf. (Accessed January 21, 2022).
32. Been, JV , Ochoa, LB , Bertens, LC , Schoenmakers, S , Steegers, EA , and Reiss, IK . Impact of COVID-19 mitigation measures on the incidence of preterm birth: a national quasi-experimental study. Lancet Public Health. (2020) 5:e604–11. doi: 10.1016/S2468-2667(20)30223-1
33. Wilder-Smith, A , Bar-Yam, Y , and Fisher, D . Lockdown to contain COVID-19 is a window of opportunity to prevent the second wave. J Travel Med. (2020) 27:taaa091. doi: 10.1093/jtm/taaa091
34. Harapan, H , Ryan, M , Yohan, B , Abidin, RS , Nainu, F , Rakib, A, et al. Covid-19 and dengue: double punches for dengue-endemic countries in Asia. Rev Med Virol. (2021) 31:e2161. doi: 10.1002/rmv.2161
35. Pergolizzi, J , LeQuang, JA , Umeda-Raffa, S , Fleischer, C , Pergolizzi, J III, Pergolizzi, C, et al. The Zika virus: lurking behind the COVID-19 pandemic? Int J Clin Pharm. (2021) 46:267–76. doi: 10.1111/jcpt.13310
36. Wu, D , Lu, J , Liu, Q , Ma, X , and He, W . To alert coinfection of COVID-19 and dengue virus in developing countries in the dengue-endemic area. Infect Control Hosp Epidemiol. (2020) 41:1482. doi: 10.1017/ice.2020.187
37. Reegan, AD , Gandhi, MR , Asharaja, AC , Devi, C , and Shanthakumar, SP . COVID-19 lockdown: impact assessment on Aedes larval indices, breeding habitats, effects on vector control programme and prevention of dengue outbreaks. Heliyon. (2020) 6:e05181. doi: 10.1016/j.heliyon.2020.e05181
38. Liyanage, P , Tozan, Y , Tissera, HA , Overgaard, HJ , and Rocklöv, J . Assessing the associations between Aedes larval indices and dengue risk in Kalutara district, Sri Lanka: a hierarchical time series analysis from 2010 to 2019. Parasit Vectors. (2022) 15:1–15. doi: 10.1186/s13071-022-05377-6
39. Del Carmen, Marquetti M , Castillo, M , Peraza, I , Milian, M , Molina, R , Leyva, M, et al. Surveillance of Aedes aegypti using a reduction sampling size for its application during the COVID-19 pandemic in Havana, Cuba. (2022). Available at: https://meddocsonline.org/journal-of-veterinary-medicine-and-animal-sciences/Surveillance-of-aedes-aegypti-using-a-reduction-sampling-size-for-its-application-during-the-covid-19-pandemic-in-havana-cuba.pdf. (Accessed January 21, 2022).
40. Sinarpi, TT . Identifikasi dan pengukuran kepadatan larva nyamuk aedes di wilayah kerja uptd puskesmas pontianak barat. Jumantik. (2022) 9:27–35. doi: 10.29406/jjum.v9i1.4117
41. Ong, S-Q , Ahmad, H , and Mohd Ngesom, AM . Implications of the COVID-19 lockdown on dengue transmission in Malaysia. Infect Dis Rep. (2021) 13:148–60. doi: 10.3390/idr13010016
42. Cavany, SM , España, G , Vazquez-Prokopec, GM , Scott, TW , and Perkins, TA . Pandemic-associated mobility restrictions could cause increases in dengue virus transmission. PLoS Negl Trop Dis. (2021) 15:e0009603. doi: 10.1371/journal.pntd.0009603
43. Diptyanusa, A , and Zablon, KN . Addressing budget reduction and reallocation on health-related resources during COVID-19 pandemic in malaria-endemic countries. Malar J. (2020) 19:411. doi: 10.1186/s12936-020-03488-y
44. Ranson, H , N’Guessan, R , Lines, J , Moiroux, N , Nkuni, Z , and Corbel, V . Pyrethroid resistance in African anopheline mosquitoes: what are the implications for malaria control? Trends Parasitol. (2011) 27:91–8. doi: 10.1016/j.pt.2010.08.004
45. Pryce, J , Richardson, M , and Lengeler, C . Insecticide-treated nets for preventing malaria. Cochrane Database Syst Rev. (2018) 11:CD000363. doi: 10.1002/14651858.CD000363.pub3
46. Osei, SA , Biney, RP , Anning, AS , Nortey, LN , and Ghartey-Kwansah, G . Low incidence of COVID-19 case severity and mortality in Africa; could malaria co-infection provide the missing link? BMC Infect Dis. (2022) 22:1–11. doi: 10.1186/s12879-022-07064-4
47. Jindal, A , and Rao, S . Lockdowns to contain COVID-19 increase risk and severity of mosquito-borne disease outbreaks. MedRxiv. (2020) 2020:20061143. doi: 10.1101/2020.04.11.20061143
48. World Health Organization . World malaria report. 20 years of global progress and challenges. (2020) Available at: https://www.who.int/publications/i/item/9789240015791. (Accessed January 21, 2022).
49. Weiss, DJ , Bertozzi-Villa, A , Rumisha, SF , Amratia, P , Arambepola, R , Battle, KE, et al. Indirect effects of the COVID-19 pandemic on malaria intervention coverage, morbidity, and mortality in Africa: a geospatial modelling analysis. Lancet Infect Dis. (2021) 21:59–69. doi: 10.1016/S1473-3099(20)30700-3
50. Hogan, AB , Jewell, BL , Sherrard-Smith, E , Vesga, JF , Watson, OJ , Whittaker, C, et al. Potential impact of the COVID-19 pandemic on HIV, tuberculosis, and malaria in low-income and middle-income countries: a modelling study. Lancet Glob Health. (2020) 8:e1132–41. doi: 10.1016/S2214-109X(20)30288-6
51. Aïkpon, R , Affoukou, C , Hounpkatin, B , Eclou, D-D , Cyaka, Y , Egwu, E, et al. Digitalized mass distribution campaign of insecticide-treated nets (ITNs) in the particular context of Covid-19 pandemic in Benin: challenges and lessons learned. Malar J. (2020) 19:1–10. doi: 10.1186/s12936-020-03508-x
52. Khan, SA , Webb, CE , and Kassim, NFA . Prioritizing mosquito-borne diseases during and after the COVID-19 pandemic. Western Pac Surveill Response J. (2021) 12:40–1. doi: 10.5365/wpsar.2020.11.3.017
53. Weetman, D , Kamgang, B , Badolo, A , Moyes, CL , Shearer, FM , Coulibaly, M, et al. Aedes mosquitoes and Aedes-borne arboviruses in Africa: current and future threats. Int J Environ Res Public Health. (2018) 15:220. doi: 10.3390/ijerph15020220
54. Makoni, M . Malaria fighters' latest chemical weapon may not last long. Science. (2020) 369:1153. doi: 10.1126/science.369.6508.1153
55. WHO . Planning for safe ITN distribution in the context of COVID-19 transmission. (2022). Available at: https://allianceformalariaprevention.com/wp-content/uploads/2021/02/Planning-safe-ITN-distribution-EN.pdf. (Accessed 21 January 2022).
56. Valdez-Delgado, KM , Moo-Llanes, DA , Danis-Lozano, R , Cisneros-Vázquez, LA , Flores-Suarez, AE , Ponce-García, G, et al. Field effectiveness of drones to identify potential Aedes aegypti breeding sites in household environments from Tapachula, a dengue-endemic city in southern Mexico. Insects. (2021) 12:663. doi: 10.3390/insects12080663
57. Carrasco-Escobar, G , Manrique, E , Ruiz-Cabrejos, J , Saavedra, M , Alava, F , Bickersmith, S, et al. High-accuracy detection of malaria vector larval habitats using drone-based multispectral imagery. PLoS Negl Trop Dis. (2019) 13:e0007105. doi: 10.1371/journal.pntd.0007105
58. Stark, DJ , Fornace, KM , Brock, PM , Abidin, TR , Gilhooly, L , Jalius, C, et al. Long-tailed macaque response to deforestation in a plasmodium knowlesi-endemic area. EcoHealth. (2019) 16:638–46. doi: 10.1007/s10393-019-01403-9
59. Jumail, A , Liew, T-S , Salgado-Lynn, M , Fornace, KM , and Stark, DJ . A comparative evaluation of thermal camera and visual counting methods for primate census in a riparian forest at the lower Kinabatangan wildlife sanctuary (LKWS). Malaysian Borneo Primates. (2021) 62:143–51. doi: 10.1007/s10329-020-00837-y
60. Hilgenboecker, K , Hammerstein, P , Schlattmann, P , Telschow, A , and Werren, JH . How many species are infected with Wolbachia? A statistical analysis of current data. FEMS Microbiol Lett. (2008) 281:215–20. doi: 10.1111/j.1574-6968.2008.01110.x
61. Bian, G , Xu, Y , Lu, P , Xie, Y , and Xi, Z . The endosymbiotic bacterium Wolbachia induces resistance to dengue virus in Aedes aegypti. PLoS Pathog. (2010) 6:e1000833. doi: 10.1371/journal.ppat.1000833
62. Moreira, LA , Iturbe-Ormaetxe, I , Jeffery, JA , Lu, G , Pyke, AT , Hedges, LM, et al. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and plasmodium. Cells. (2009) 139:1268–78. doi: 10.1016/j.cell.2009.11.042
63. Bourtzis, K , Dobson, SL , Xi, Z , Rasgon, JL , Calvitti, M , Moreira, LA, et al. Harnessing mosquito–Wolbachia symbiosis for vector and disease control. Acta Trop. (2014) 132:S150–63. doi: 10.1016/j.actatropica.2013.11.004
64. LePage, D , and Bordenstein, SR . Wolbachia: can we save lives with a great pandemic? Trends Parasitol. (2013) 29:385–93. doi: 10.1016/j.pt.2013.06.003
65. Hoffmann, AA , Montgomery, B , Popovici, J , Iturbe-Ormaetxe, I , Johnson, P , Muzzi, F, et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature. (2011) 476:454–7. doi: 10.1038/nature10356
66. Mains, JW , Brelsfoard, CL , Rose, RI , and Dobson, SL . Female adult Aedes albopictus suppression by Wolbachia-infected male mosquitoes. Sci Rep. (2016) 6:1–7. doi: 10.1038/srep33846
67. Utarini, A , Indriani, C , Ahmad, RA , Tantowijoyo, W , Arguni, E , Ansari, MR, et al. Efficacy of Wolbachia-infected mosquito deployments for the control of dengue. N Engl J Med. (2021) 384:2177–86. doi: 10.1056/NEJMoa2030243
68. Lees, RS , Gilles, JR , Hendrichs, J , Vreysen, MJ , and Bourtzis, K . Back to the future: the sterile insect technique against mosquito disease vectors. Curr Opin Insect Sci. (2015) 10:156–62. doi: 10.1016/j.cois.2015.05.011
69. Patterson, R , Weidhaas, D , Ford, H , and Lofgren, C . Suppression and elimination of an island population of Culex pipiens quinquefasciatus with sterile males. Science. (1970) 168:1368–70. doi: 10.1126/science.168.3937.1368
70. Weidhaas, D , Breeland, S , Loforen, C , Dame, D , and Kaiser, R . Release of chemosterilized males for the control of Anopheles albimanus in El Salvador. IV. Dynamics of the test population. Am J Trop Med Hyg. (1974) 23:298–308. doi: 10.4269/ajtmh.1974.23.298
71. Petersen, J , Lounibos, L , and Lorimer, N . Field trials of double translocation heterozygote males for genetic control of Aedes aegypti (L.)(Diptera: Culicidae). Bull Entomol Res. (1977) 67:313–24. doi: 10.1017/S0007485300011135
72. Becker, N , Langentepe-Kong, SM , Tokatlian Rodriguez, A , Oo, TT , Reichle, D , Lühken, R, et al. Integrated control of Aedes albopictus in Southwest Germany supported by the sterile insect technique. Parasit Vectors. (2022) 15:1–19. doi: 10.1186/s13071-022-05177-y
73. Labbé, GM , Scaife, S , Morgan, SA , Curtis, ZH , and Alphey, L . Female-specific flightless (fsRIDL) phenotype for control of Aedes albopictus. PLoS Negl Trop Dis. (2012) 6:e1724. doi: 10.1371/journal.pntd.0001724
74. Huang, Y-JS , Higgs, S , and Vanlandingham, DL . Biological control strategies for mosquito vectors of arboviruses. Insects. (2017) 8:21. doi: 10.3390/insects8010021
75. Fu, G , Lees, RS , Nimmo, D , Aw, D , Jin, L , Gray, P, et al. Female-specific flightless phenotype for mosquito control. Proc Natl Acad Sci USA. (2010) 107:4550–4. doi: 10.1073/pnas.1000251107
76. Carvalho, DO , McKemey, AR , Garziera, L , Lacroix, R , Donnelly, CA , Alphey, L, et al. Suppression of a field population of Aedes aegypti in Brazil by sustained release of transgenic male mosquitoes. PLoS Negl Trop Dis. (2015) 9:e0003864. doi: 10.1371/journal.pntd.0003864
77. Galizi, R , Doyle, LA , Menichelli, M , Bernardini, F , Deredec, A , Burt, A, et al. A synthetic sex ratio distortion system for the control of the human malaria mosquito. Nat Commun. (2014) 5:1–8. doi: 10.1038/ncomms4977
78. Robinson, A , Cayol, J , and Hendrichs, J . Recent findings on medfly sexual behavior: implications for SIT. Fla Entomol. (2002) 85:171–81. doi: 10.1653/0015-4040(2002)085[0171:RFOMSB]2.0.CO;2
79. Guo, J , Zheng, X , Zhang, D , and Wu, Y . Current status of mosquito handling, transporting and releasing in frame of the sterile insect technique. Insects. (2022) 13:532. doi: 10.3390/insects13060532
80. Dyck, VA , Hendrichs, J , and Robinson, AS . Sterile insect technique: Principles and practice in area-wide integrated pest management. Abingdon: Taylor & Francis (2021).
81. Carrasco-Escobar, G , Moreno, M , Fornace, K , Herrera-Varela, M , Manrique, E , and Conn, JE . The use of drones for mosquito surveillance and control. Parasit Vectors. (2022) 15:473. doi: 10.1186/s13071-022-05580-5
82. Harris, AF , Nimmo, D , McKemey, AR , Kelly, N , Scaife, S , Donnelly, CA, et al. Field performance of engineered male mosquitoes. Nat Biotechnol. (2011) 29:1034–7. doi: 10.1038/nbt.2019
83. Näslund, J , Ahlm, C , Islam, K , Evander, M , Bucht, G , and Lwande, OW . Emerging mosquito-borne viruses linked to Aedes aegypti and Aedes albopictus: global status and preventive strategies. Vector Borne Zoonotic Dis. (2021) 21:731–46. doi: 10.1089/vbz.2020.2762
84. Clarkson, JM , and Charnley, AK . New insights into the mechanisms of fungal pathogenesis in insects. Trends Microbiol. (1996) 4:197–203. doi: 10.1016/0966-842X(96)10022-6
85. Wang, C , Hu, G , and Leger, RJS . Differential gene expression by Metarhizium anisopliae growing in root exudate and host (Manduca sexta) cuticle or hemolymph reveals mechanisms of physiological adaptation. Fungal Genet Biol. (2005) 42:704–18. doi: 10.1016/j.fgb.2005.04.006
86. Scholte, EJ , Takken, W , and Knols, BG . Pathogenicity of five east African entomopathogenic fungi against adult Anopheles gambiae ss mosquitoes (Diptera, Culicidae). In: Proceedings of the Netherlands Entomological Society meeting, pp. 25–29. (2003).
87. Kanzok, SM , and Jacobs-Lorena, M . Entomopathogenic fungi as biological insecticides to control malaria. Trends Parasitol. (2006) 22:49–51. doi: 10.1016/j.pt.2005.12.008
88. Bukhari, T , Takken, W , and Koenraadt, CJ . Development of Metarhizium anisopliae and Beauveria bassiana formulations for control of malaria mosquito larvae. Parasit Vectors. (2011) 4:1–14. doi: 10.1186/1756-3305-4-23
89. Bukhari, T , Middelman, A , Koenraadt, CJ , Takken, W , and Knols, BG . Factors affecting fungus-induced larval mortality in Anopheles gambiae and Anopheles stephensi. Malar J. (2010) 9:1–15. doi: 10.1186/1475-2875-9-22
90. Scholte, E-J , Takken, W , and Knols, BG . Infection of adult Aedes aegypti and Ae. Albopictus mosquitoes with the entomopathogenic fungus Metarhizium anisopliae. Acta Trop. (2007) 102:151–8. doi: 10.1016/j.actatropica.2007.04.011
91. Choi, CJ , Lee, JY , Woo, RM , Shin, TY , Gwak, WS , and Woo, SD . An effective entomopathogenic fungus Metarhizium anisopliae for the simultaneous control of Aedes albopictus and Culex pipiens mosquito adults. J Asia Pac Entomol. (2020) 23:585–90. doi: 10.1016/j.aspen.2020.04.007
92. Blanford, S , Chan, BH , Jenkins, N , Sim, D , Turner, RJ , Read, AF, et al. Fungal pathogen reduces potential for malaria transmission. Science. (2005) 308:1638–41. doi: 10.1126/science.1108423
93. Fang, W , Vega-Rodríguez, J , Ghosh, AK , Jacobs-Lorena, M , Kang, A , and St Leger, RJ . Development of transgenic fungi that kill human malaria parasites in mosquitoes. Science. (2011) 331:1074–7. doi: 10.1126/science.1199115
94. Deng, S , Huang, Q , Wei, H , Zhou, L , Yao, L , Li, D, et al. Beauveria bassiana infection reduces the vectorial capacity of Aedes albopictus for the Zika virus. J Pest Sci. (2019) 92:781–9. doi: 10.1007/s10340-019-01081-0
95. Garza-Hernández, JA , Rodríguez-Pérez, MA , Salazar, MI , Russell, TL , Adeleke, MA , de Luna-Santillana, EDJ, et al. Vectorial capacity of Aedes aegypti for dengue virus type 2 is reduced with co-infection of Metarhizium anisopliae. PLoS Negl Trop Dis. (2013) 7:e2013. doi: 10.1371/journal.pntd.0002013
96. Ortiz-Urquiza, A , Luo, Z , and Keyhani, NO . Improving mycoinsecticides for insect biological control. Appl Microbiol Biotechnol. (2015) 99:1057–68. doi: 10.1007/s00253-014-6270-x
97. Keppanan, R , Sivaperumal, S , Kanta, DC , Akutse, KS , and Wang, L . Molecular docking of protease from Metarhizium anisopliae and their toxic effect against model insect Galleria mellonella. Pestic Biochem Phys. (2017) 138:8–14. doi: 10.1016/j.pestbp.2017.01.013
98. Deng, SQ , Chen, JT , Li, WW , Chen, M , and Peng, HJ . Application of the scorpion neurotoxin AaIT against insect pests. Int J Mol Sci. (2019) 20:3467. doi: 10.3390/ijms20143467
99. Hancock, PA , Thomas, MB , and Godfray, HCJ . An age-structured model to evaluate the potential of novel malaria-control interventions: a case study of fungal biopesticide sprays. Proc Biol Sci. (2009) 276:71–80. doi: 10.1098/rspb.2008.0689
100. Palma, L , Muñoz, D , Berry, C , Murillo, J , and Caballero, P . Bacillus thuringiensis toxins: an overview of their biocidal activity. Toxins. (2014) 6:3296–325. doi: 10.3390/toxins6123296
101. De Barjac, H . International Entomopathogenic Bacillus Centre. Collection of Bacillus thuringiensis and Bacillus sphaericus. (n.d.).
102. De la Fuente-Salcido, NM , Casados-Vázquez, LE , and Barboza-Corona, JE . Bacteriocins of Bacillus thuringiensis can expand the potential of this bacterium to other areas rather than limit its use only as microbial insecticide. Can J Microbiol. (2013) 59:515–22. doi: 10.1139/cjm-2013-0284
103. Margalit, J , and Dean, D . Survey of Bacillus thuringiensis israel variety. Foreign Med. (1986) 3:105–10.
104. Balaraman, K . Occurrence and diversity of mosquitocidal strains of Bacillus thuringiensis. J Vector Dis. (2005) 42:81.
105. Afrane, YA , Mweresa, NG , Wanjala, CL , Gilbreath Iii, TM , Zhou, G , Lee, M-C, et al. Evaluation of long-lasting microbial larvicide for malaria vector control in Kenya. Malar J. (2016) 15:1–9. doi: 10.1186/s12936-016-1626-6
106. World Health Organization . Microbial pest control agent: Bacillus thuringiensis. Geneva: World Health Organization (1999).
107. Nascimento, NA , Torres-Quintero, MC , Molina, SL , Pacheco, S , Romão, TP , Pereira-Neves, A, et al. Functional Bacillus thuringiensis Cyt1Aa is necessary to synergize Lysinibacillus sphaericus binary toxin (bin) against bin-resistant and-refractory mosquito species. Appl Environ Microb. (2020) 86:e02770–19. doi: 10.1128/AEM.02770-19
108. Usta, C . Microorganisms in biological pest control–a review (bacterial toxin application and effect of environmental factors). Curr Pro Bio Res. (2013) 13:287–317. doi: 10.5772/55786
109. Paris, M , David, J-P , and Despres, L . Fitness costs of resistance to Bti toxins in the dengue vector Aedes aegypti. Ecotoxicology. (2011) 20:1184–94. doi: 10.1007/s10646-011-0663-8
110. Barik, TK . Molecular identification of mosquito vectors and their management. Berlin: Springer (2020).
111. Walia, S , Saha, S , Tripathi, V , and Sharma, K . Phytochemical biopesticides: some recent developments. Phytochem Rev. (2017) 16:989–1007. doi: 10.1007/s11101-017-9512-6
112. World Health Organization . Manual on environmental management for mosquito control, with special emphasis on malaria vectors. Geneva: World Health Organization (1982).
113. Benedict, MQ . Sterile insect technique: lessons from the past. J Med Entomol. (2021) 58:1974–9. doi: 10.1093/jme/tjab024
114. Killeen, GF , Tatarsky, A , Diabate, A , Chaccour, CJ , Marshall, JM , Okumu, FO, et al. Developing an expanded vector control toolbox for malaria elimination. BMJ Glob Heal. (2017) 2:e000211. doi: 10.1136/bmjgh-2016-000211
115. Hendrichs, J , Robinson, A , Cayol, J , and Enkerlin, W . Medfly areawide sterile insect technique programmes for prevention, suppression or eradication: the importance of mating behavior studies. Fla Entomol. (2002) 85:1–13. doi: 10.1653/0015-4040(2002)085[0001:MASITP]2.0.CO;2
116. Bekele, D . Review on insecticidal and repellent activity of plant products for malaria mosquito control. Biomed Res Rev. (2018) 2:2–7. doi: 10.15761/BRR.1000114
Keywords: COVID-19, mosquito-borne diseases, vector control, mosquito biological control, mosquito novel control
Citation: Lu H-Z, Sui Y, Lobo NF, Fouque F, Gao C, Lu S, Lv S, Deng S-Q and Wang D-Q (2023) Challenge and opportunity for vector control strategies on key mosquito-borne diseases during the COVID-19 pandemic. Front. Public Health. 11:1207293. doi: 10.3389/fpubh.2023.1207293
Edited by:
Nuno Sepulveda, Warsaw University of Technology, PolandReviewed by:
Lara Ferrero Gómez, Universidade Jean Piaget de Cabo, Cabo VerdeMarta Moreno, University of London, United Kingdom
Copyright © 2023 Lu, Sui, Lobo, Fouque, Gao, Lu, Lv, Deng and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Duo-Quan Wang, d2FuZ2RxQG5pcGQuY2hpbmFjZGMuY24=; Sheng-Qun Deng, ZGVuZ3NoZW5ncXVuQDE2My5jb20=
 Hong-Zheng Lu1,2,3
Hong-Zheng Lu1,2,3 Shenning Lu
Shenning Lu Shan Lv
Shan Lv Sheng-Qun Deng
Sheng-Qun Deng Duo-Quan Wang
Duo-Quan Wang