- Department of Oncology, The Affiliated Huaian No.1 People’s Hospital of Nanjing Medical University, Huai’an, China
Introduction: There is evidence suggesting that Bisphenol A (BPA) is associated with increased all-cause mortality in adults. However, the specific nature of the relationship between BPA exposure and cancer mortality remains relatively unexplored.
Methods: The National Health and Nutrition Examination Survey (NHANES) dataset was used to recruit participants. Urinary BPA was assessed using liquid chromatography-mass spectrum (LC–MS). Through the use of multivariable Cox proportional hazard regressions and constrained cubic splines, the relationships between urine BPA and death from all causes and cancer were investigated.
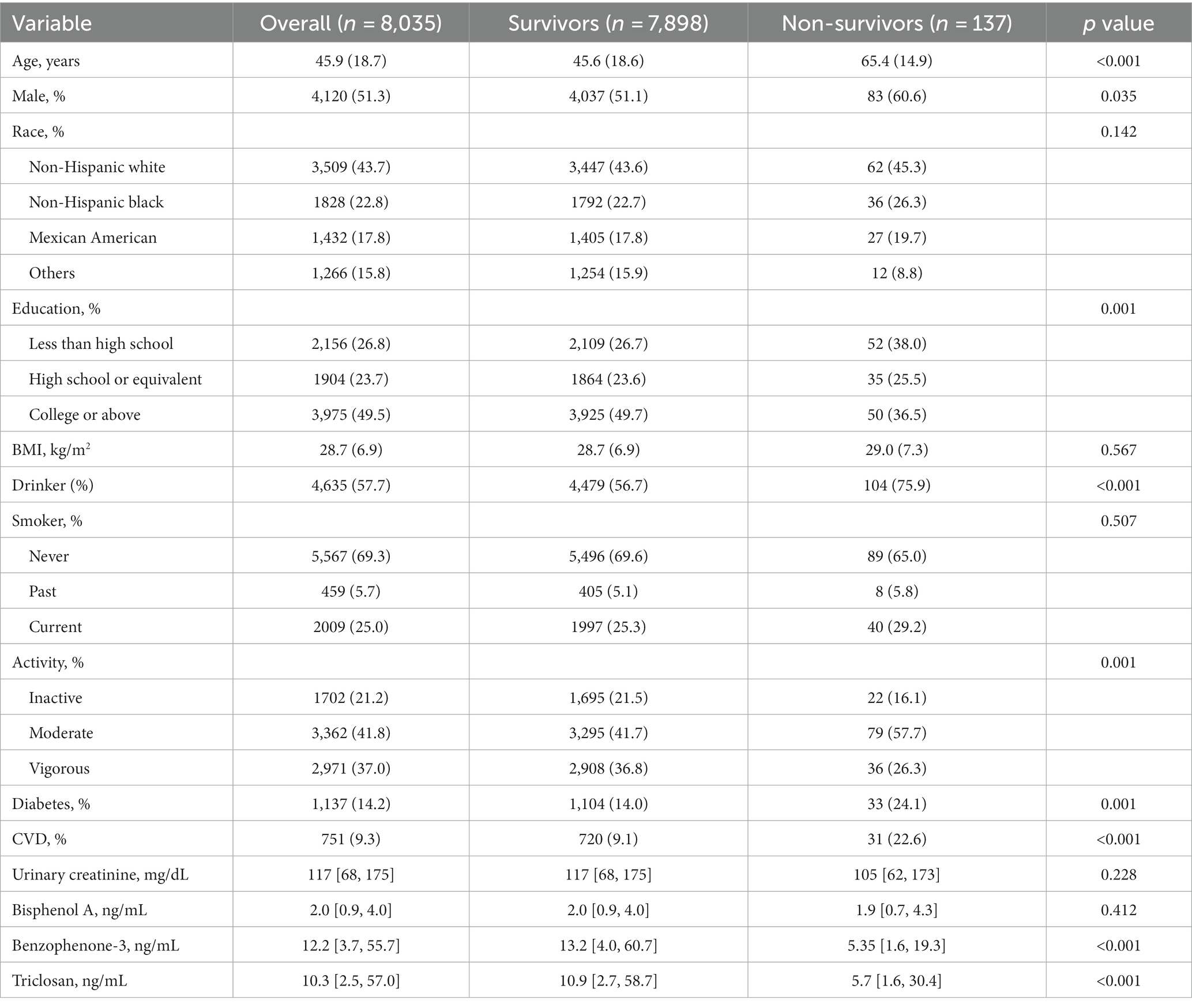
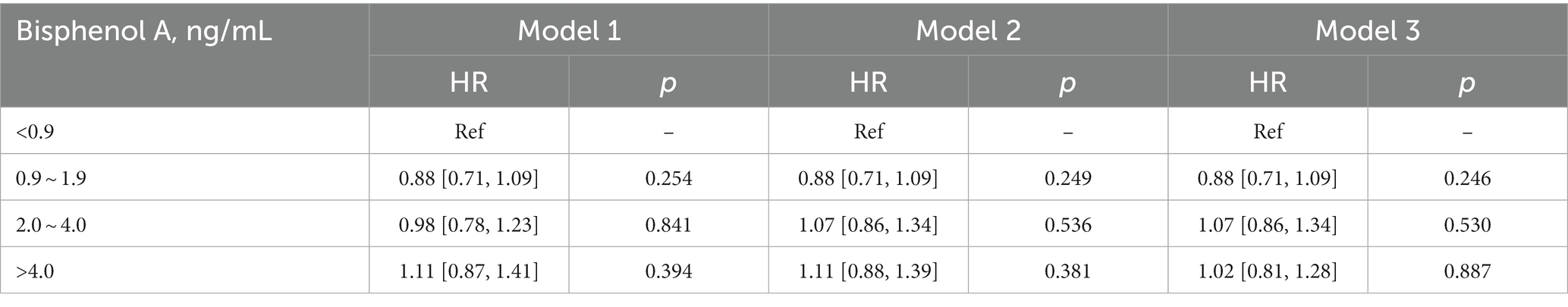
Results: This study has a total of 8,035 participants, and 137 died from cancers after a 7.5-year follow-up. The median level of BPA was 2.0 g/mL. Urinary BPA levels were not independently associated with all-cause mortality. For cancer mortality, the second quartile’s multivariable-adjusted hazard ratio was 0.51 (95% confidence interval: 0.30 to 0.86; p = 0.011) compared to the lowest quartile. The restricted cubic splines showed that the association was nonlinear (p for nonlinearity = 0.028) and the inflection point was 1.99 ng/mL.
Conclusion: Urinary BPA exposure was U-shaped associated with the risk of cancer mortality, and a lower level of BPA less than 1.99 ng/mL was associated with a higher risk of cancer mortality.
Introduction
Bisphenol A (BPA) is a kind of environmental phenols utilized in baby bottles, food containers, and dentistry (1). The exposure of humans to BPA is pervasive, originating from various sources such as consumer products, food, water, and dust (2). National biological monitoring data in the United States reveals that BPA is detectable in more than 90% of urine samples in the general population (3). Currently, 12 states have enforced regulations to restrict the use of BPA in the United States. While BPA is known to undergo rapid metabolism and is primarily eliminated through urine, its cumulative exposure in everyday items could lead to concerns regarding potential long-term health consequences (4). The potential pathways underlying BPA-induced adverse health outcomes include endocrine disruption (5), oxidative stress (6) and inflammation (7).
Exposure to bisphenol A starts very early in life, causing adverse health outcomes not only in children but also later in life (8, 9). The BPA exposure has been linked to disruptions in endocrine function and metabolism (10), which can contribute to the development of metabolic disorders (11). Some studies showed that BPA exacerbated inflammation by regulating gut physiology (12) and was involved in the development of type 2 diabetes mellitus (13), obesity (14), hypertension (15) and cardiovascular disease (16). Despite mounting evidence indicating potential toxic effects of BPA on various human cancers (17, 18), the relationship between BPA exposure and mortality remains unclear.
A study reported that BPA exposure was positively related to all-cause mortality in adults. However, nonsignificant association between BPA exposure and cancer mortality was found (19). Even so, the previous study had a lower number of sample and lacked a nonlinear analysis. Therefore, we conducted a study utilizing a comprehensive database to examine the correlation between urinary BPA levels and mortality rates related to all causes and cancer.
Methods
Study participants
Our study utilized data from NHANES, a program specifically designed to evaluate the health and nutritional status of individuals, both adults and children, in the United States. The data covered the period from 2003 to 2012. Individuals with missing data on urinary creatinine (n = 2) or mortality (n = 13) as well as those who were diagnosed with cancer (n = 68) were excluded from a pool of adult participants with complete records of urinary BPA (n = 8,118). Ultimately, a total of 8,035 participants were included in the study. The study was approved by the institutional review board of National Center of Health Statistics and all participants provided written informed consent.
Covariates collection
The method for measuring baseline urinary BPA levels in NHANES has been previously described (20). In brief, spot urine samples were collected from each participant and promptly transferred to specimen containers within 4 hours of collection. The determination of urinary BPA levels involved the use of online solid phase extraction, advanced liquid chromatography, and tandem mass spectrometry. It’s worth noting that the limit of detection (LOD) for urinary BPA levels was 0.20 ng/mL. Urinary BPA levels that fell below the LOD were recorded as the LOD value divided by the square root of two. Creatinine levels were measured using the Jaffe rate reaction assay.
Furthermore, essential participant information through questionnaires, which encompassed sociodemographic details and lifestyle factors were collected. Sociodemographic variables included age, gender, race, and educational level. Race was categorized into four groups: Mexican-American, non-Hispanic white, non-Hispanic black, and other races. Educational level was divided into three categories: college or higher education, high school or equivalent, and less than high school. Lifestyle factors encompassed smoking, alcohol consumption, and physical activity level. Smoking status was determined by whether participants had smoked at least 100 cigarettes in their lifetime. Alcohol consumption was assessed based on the daily or yearly number of drinks consumed. Physical activity level was calculated using total metabolic equivalent of task minutes per week and classified into three groups: inactive, moderate, and vigorous. Body Mass Index (BMI) was computed by dividing weight (in kilograms) by the square of height (in meters). Diabetes was defined as a previously diagnosis, fasting glucose levels of ≥7.0 mmol/L, glycated hemoglobin levels of ≥6.5%, or the use of antidiabetic medication. Participants were also queried about their history of congestive heart failure, coronary artery disease, angina, heart attack, or stroke, and those who reported such conditions were identified as having a history of cardiovascular disease (CVD).
The study outcomes consisted of all-cause mortality and cancer mortality. Mortality status of the study participants was determined by linking to the National Death Index until 31st December 2015. Cancer diagnosis was according to ICD-10 codes C00-C97, which specifically identify malignant neoplasms.
Statistical analysis
To account for selection variations, oversampling and adjustments for non-responses, the sampling weights common to NHANES data were incorporated in our study. To evaluate the variances between groups, either Student’s t-test for continuous variables or Chi-square tests for categorical variables was used. For the analysis of survival rates, univariate analysis was conducted using Kaplan–Meier analysis with the Log-rank test. Multivariable survival analysis, on the other hand, was performed using Cox proportional hazards analysis. It presented the cumulative incidence function of cancer mortality with non-cancer mortality as a competing risk. Model 1 was adjusted for urinary creatinine, Model 2 was adjusted for urinary creatinine, age, gender, and race. Model 3 was additionally adjusted for education level, BMI, drinker, smoker, activity, diabetes, and cardiovascular diseases (CVD). Restricted cubic splines with knots placed at the 5th, 50th, and 95th percentiles were used to assess potential nonlinear relationships. All statistical analyzes were carried out using R software, specifically version 3.6.
Results
The study included a sizable cohort of 8,035 individuals, predominantly middle-aged, with a slight majority being male. According to Table 1, 137 cases of cancer mortality were documented over a 7.5-year follow-up period. The measurement of urinary bisphenol A (BPA) levels revealed a median concentration of 2.0 ng/mL. Notably, individuals who succumbed to cancer during the follow-up period tended to be older (p = 0.001), male (p = 0.035), and had higher incidences of diabetes (p = 0.001) and cardiovascular disease (p < 0.001). These observations underline the importance of considering demographic and health-related factors when assessing mortality risk.
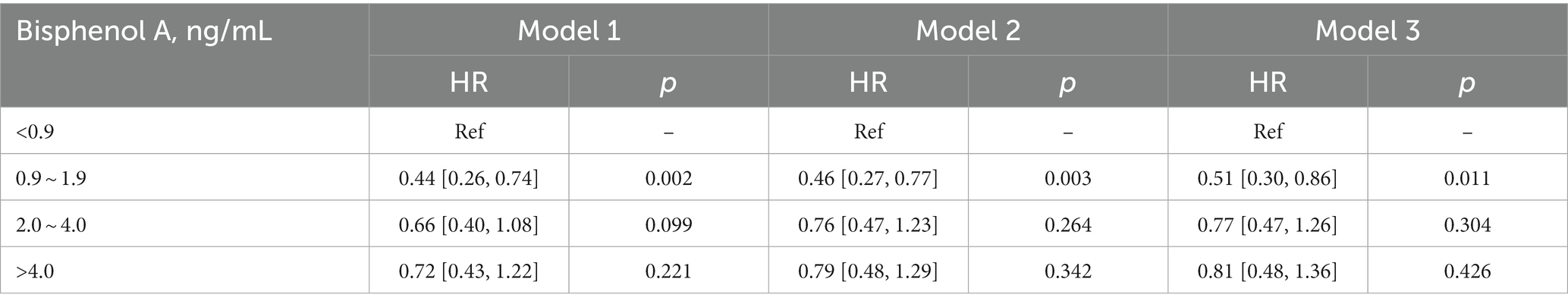
Kaplan–Meier analysis indicated significant associations between urinary BPA levels and both all-cause mortality and cancer mortality. The analysis suggests that lower urinary BPA levels are associated with an increased risk of all-cause mortality (log-rank p = 0.01) and cancer mortality (log-rank p = 0.007) (Figure 1). However, compared with the lowest quartile of BPA, no association of all-cause mortality was observed in any quartiles across models, which suggested that BPA was not independently associated with all-cause mortality (Table 2).
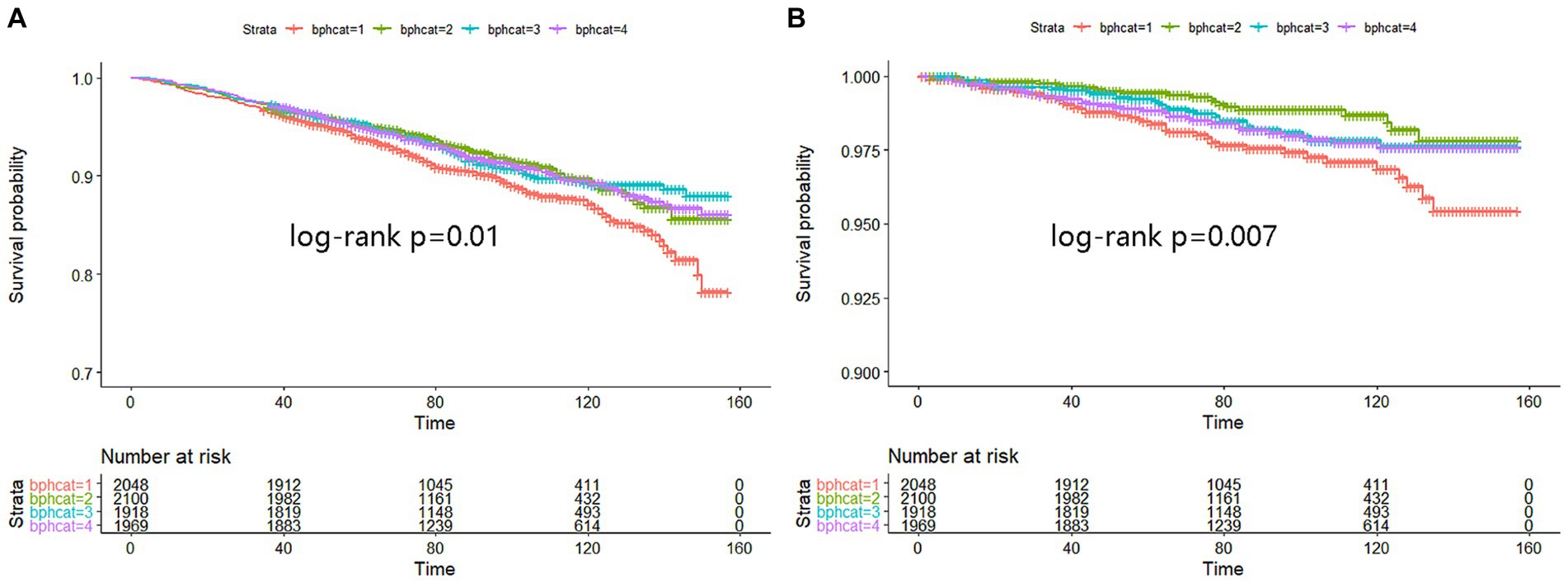
Figure 1. Kaplan–Meier analysis was performed to assess the relationship between urinary BPA levels and both all-cause mortality (A) and cancer mortality (B). The X-axis represented months while Y-axis represented survival probability. Strata 1: <0.9; Strata 2: 0.9 ~ 1.9; Strata 3: 2.0 ~ 4.0; Strata 4: >4.0.
On the contrary with expectations, the risk of cancer mortality was reduced with the increase of BPA levels both in the unadjusted model and adjusted models (Table 3). Most importantly, the second quartile’s multivariable-adjusted hazards ratio (HR) was 0.51 (95% CI: 0.30 to 0.86; p = 0.011) compared to the lowest quartile of BPA. However, these associations were not consistent across all quartiles of BPA levels, indicating a more nonlinear relationship. In order to confirm the nonlinear relationship, we used restricted cubic splines (Figure 2). We found that urinary BPA was U-shaped associated with cancer mortality (p for nonlinearity = 0.028). This suggests that the relationship is not purely linear, but rather exhibits a threshold effect. Specifically, lower levels of BPA (below approximately 1.99 ng/mL) were associated with an increased risk of cancer mortality. Beyond this threshold, higher BPA levels appeared to confer protection against cancer mortality. This nonlinear relationship highlights the complexity of BPA’s impact on health outcomes and underscores the need for careful consideration of dose–response relationships. Future research should focus on elucidating the mechanisms underlying these observed associations and conducting longitudinal studies to validate these findings across diverse populations and settings.
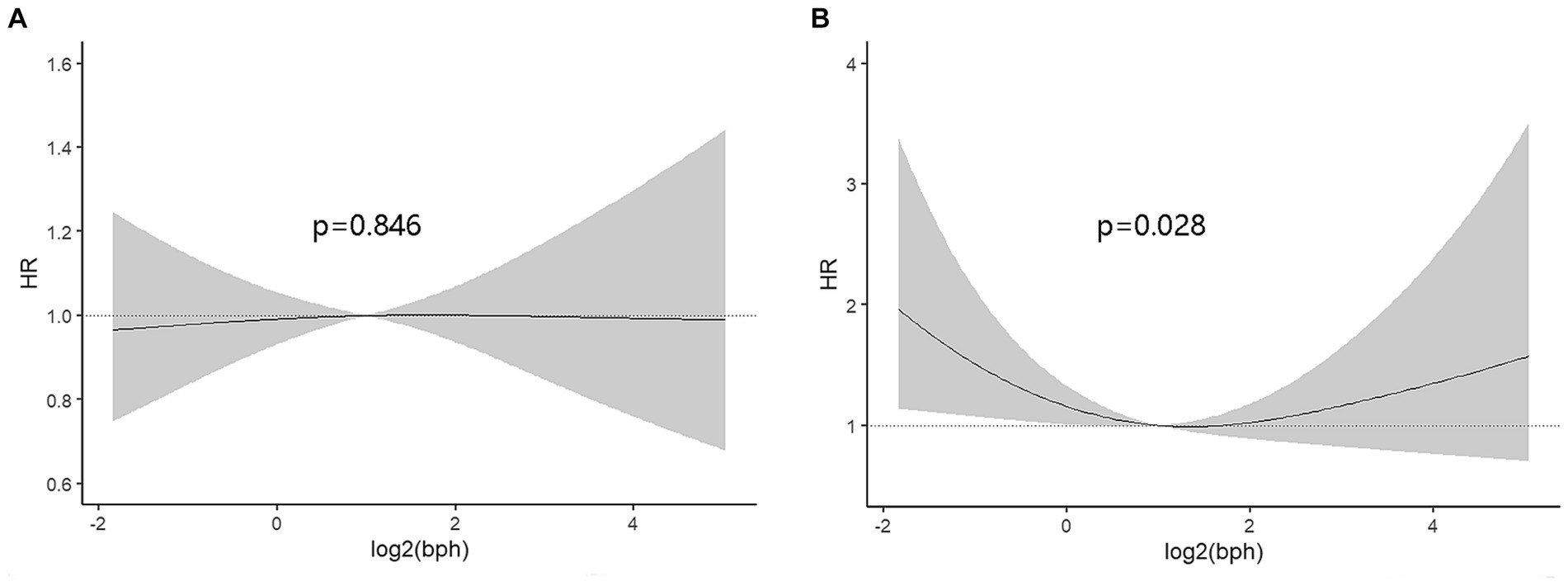
Figure 2. The dose–response analysis was performed to assess the relationship between urinary BPA and all-cause mortality (A) or cancer mortality (B). The X-axis represented log-transformed BPA concentrations while Y-axis represented hazard ratio.
Discussion
In this study, we found that urinary BPA was U-shaped associated with the risk of cancer mortality and a lower level less than 1.99 ng/mL increasing the risk of cancer mortality. This study provides valuable insights into the complex relationship between urinary BPA levels and cancer mortality, emphasizing the need for a nuanced understanding of dose–response dynamics and the consideration of confounding factors in epidemiological research.
BPA is a widely used raw material and is involved in the initiation and development of hormone-dependent cancers. A comparable study discovered that higher exposure to BPA was independently related with an elevated risk of all-cause mortality but with no significant association with cancer mortality (19). They divided BPA into tertiles and did not explore the nonlinear relationship. A recent study showed that the highest tertile of urinary BPA levels corresponded to a 36% increase in all-cause mortality and a 62% increase in CVD mortality compared to the lowest tertile (21). Different from previous study, we demonstrated a U-shaped association between BPA exposure and cancer mortality using dose–response analysis and adjusting for more variables. A study also found that BPA was not significantly associated with all-cause mortality in overall population, but in the obesity, diabetes, and hypertension subgroups (22). Variations in the characteristics of the study populations, especially comorbidity, may account for the discrepancy.
BPA is an endocrine disruptor with multiple effects. BPA exhibits estrogenic properties by binding to estrogen receptors and interfering with the regular functioning of the endocrine system (23). Additionally, BPA has the potential to influence biological processes, including cell signaling, gene expression, and apoptosis, which can contribute to a range of health issues affecting the reproductive, immune, metabolic, and nervous systems (24, 25). Several studies found a U-shaped association between BPA levels and the risk of diabetes (26) and obesity (27). A higher level of BPA impacted the production of ROS (6), cancer metabolites (28), and tumoral immune microenvironment (29), contributing to the migration and invasion of cancer cells. Conversely, a lower level may be the reflection of an imbalance of endocrine-related pathways. BPA could inhibit DNA replication and cell proliferation in tumor cells (30) by modulating cell cycle-and apoptosis-related proteins and genes in cancerous cells (31). Therefore, a lower and higher level of BPA both influenced the cancer mortality. More studies are warranted to explain the dose-repose relationship.
There may be various underlying mechanisms driving the positive correlation between BPA and all-cause mortality (Figure 3). Firstly, BPA caused endocrine disruption through agonistic or antagonistic behavior at various nuclear receptors such as estrogen (ER), androgen (AR) and glucocorticoid (GR) (32). Besides, BPA exposure resulted in a strong induction of oxidative stress and inflammatory response (33, 34). However, more research is necessary to elucidate the biological mechanisms underlying this association.

Figure 3. The pathophysiological mechanisms between BPA and cancer mortality. ER, estrogen; AR, androgen; GR, glucocorticoid; ROS, reactive oxidative stress.
This study boasts several notable strengths, including its use of a nationally representative cohort from the United States and rigorous quality control measures. Nonetheless, this study does come with certain constraints. To begin with, BPA levels were assessed solely through spot urine samples at the baseline, offering no insight into long-term BPA exposure or fluctuations in BPA concentrations within the body. Lastly, it’s important to note that the generalizability of this study could be limited due to the exclusion of participants with incomplete covariate data, potentially introducing selection bias. BPA exposure is more related to hormone-associated cancers such as breast, prostate and ovarian cancers. However, the number of specific cancer type was few in the original database, which need to be verified by further large-scale cancer epidemiological investigations.
Conclusion
In conclusion, we found BPA exposure was U-shaped associated with the risk of cancer mortality, and a lower level of BPA less than 1.99 ng/mL was associated with a higher risk of cancer mortality. Our results can serve as valuable information for guiding policies related to enhanced monitoring of chemical exposures and risk assessment in cancer prevention field.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by National Center of Health Statistics. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YY: Writing – original draft. QC: Data curation, Writing – original draft. XD: Supervision, Writing – original draft. QZ: Project administration, Writing – review & editing. XZ: Project administration, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Jamal, A, Rastkari, N, Dehghaniathar, R, Nodehi, RN, Nasseri, S, Kashani, H, et al. Prenatal urinary concentrations of environmental phenols and birth outcomes in the mother-infant pairs of Tehran environment and neurodevelopmental disorders (TEND) cohort study. Environ Res. (2020) 184:109331. doi: 10.1016/j.envres.2020.109331
2. Dekant, W, and Volkel, W. Human exposure to bisphenol a by biomonitoring: methods, results and assessment of environmental exposures. Toxicol Appl Pharmacol. (2008) 228:114–34. doi: 10.1016/j.taap.2007.12.008
3. Lehmler, HJ, Liu, B, Gadogbe, M, and Bao, W. Exposure to bisphenol a, bisphenol F, and bisphenol S in U.S. adults and children: the National Health and nutrition examination survey 2013-2014. ACS Omega. (2018) 3:6523–32. doi: 10.1021/acsomega.8b00824
4. Stahlhut, RW, Welshons, WV, and Swan, SH. Bisphenol a data in NHANES suggest longer than expected half-life, substantial nonfood exposure, or both. Environ Health Perspect. (2009) 117:784–9. doi: 10.1289/ehp.0800376
5. Cimmino, I, Fiory, F, Perruolo, G, Miele, C, Beguinot, F, Formisano, P, et al. Potential mechanisms of bisphenol a (BPA) contributing to human disease. Int J Mol Sci. (2020) 21:5761. doi: 10.3390/ijms21165761
6. Xia, T, Guo, J, Zhang, B, Song, C, Zhao, Q, Cui, B, et al. Bisphenol a promotes the progression of Colon Cancer through dual-targeting of NADPH oxidase and mitochondrial Electron-transport chain to produce ROS and Activating HIF-1alpha/VEGF/PI3K/AKT Axis. Front Endocrinol (Lausanne). (2022) 13:933051. doi: 10.3389/fendo.2022.933051
7. Hong, T, Jiang, X, Zou, J, Yang, J, Zhang, H, Mai, H, et al. Hepatoprotective effect of curcumin against bisphenol A-induced hepatic steatosis via modulating gut microbiota dysbiosis and related gut-liver axis activation in CD-1 mice. J Nutr Biochem. (2022) 109:109103. doi: 10.1016/j.jnutbio.2022.109103
8. Urbano, T, Zagnoli, F, Malavolti, M, Halldorsson, TI, Vinceti, M, and Filippini, T. Dietary intake of potentially toxic elements and children’s chemical exposure. Curr. Opin. Environ. Sci. Health. (2022) 30:100393. doi: 10.1016/j.coesh.2022.100393
9. Rochester, JR. Bisphenol a and human health: a review of the literature. Reprod Toxicol. (2013) 42:132–55. doi: 10.1016/j.reprotox.2013.08.008
10. Gore, AC. Endocrine-disrupting chemicals. JAMA Intern Med. (2016) 176:1705–6. doi: 10.1001/jamainternmed.2016.5766
11. Heindel, JJ, Blumberg, B, Cave, M, Machtinger, R, Mantovani, A, Mendez, MA, et al. Metabolism disrupting chemicals and metabolic disorders. Reprod Toxicol. (2017) 68:3–33. doi: 10.1016/j.reprotox.2016.10.001
12. DeLuca, JA, Allred, KF, Menon, R, Riordan, R, Weeks, BR, Jayaraman, A, et al. Bisphenol-a alters microbiota metabolites derived from aromatic amino acids and worsens disease activity during colitis. Exp Biol Med (Maywood). (2018) 243:864–75. doi: 10.1177/1535370218782139
13. Dallio, M, Masarone, M, Errico, S, Gravina, AG, Nicolucci, C, Di Sarno, R, et al. Role of bisphenol a as environmental factor in the promotion of non-alcoholic fatty liver disease: in vitro and clinical study. Aliment Pharmacol Ther. (2018) 47:826–37. doi: 10.1111/apt.14499
14. Liu, B, Lehmler, HJ, Sun, Y, Xu, G, Liu, Y, Zong, G, et al. Bisphenol a substitutes and obesity in US adults: analysis of a population-based, cross-sectional study. Lancet Planet Health. (2017) 1:e114–22. doi: 10.1016/S2542-5196(17)30049-9
15. Bae, S, Kim, JH, Lim, YH, Park, HY, and Hong, YC. Associations of bisphenol a exposure with heart rate variability and blood pressure. Hypertension. (2012) 60:786–93. doi: 10.1161/HYPERTENSIONAHA.112.197715
16. Melzer, D, Osborne, NJ, Henley, WE, Cipelli, R, Young, A, Money, C, et al. Urinary bisphenol a concentration and risk of future coronary artery disease in apparently healthy men and women. Circulation. (2012) 125:1482–90. doi: 10.1161/CIRCULATIONAHA.111.069153
17. Hafezi, SA, and Abdel-Rahman, WM. The endocrine disruptor bisphenol a (BPA) exerts a wide range of effects in carcinogenesis and response to therapy. Curr Mol Pharmacol. (2019) 12:230–8. doi: 10.2174/1874467212666190306164507
18. Pellerin, E, Caneparo, C, Chabaud, S, Bolduc, S, and Pelletier, M. Endocrine-disrupting effects of bisphenols on urological cancers. Environ Res. (2021) 195:110485. doi: 10.1016/j.envres.2020.110485
19. Bao, W, Liu, B, Rong, S, Dai, SY, Trasande, L, and Lehmler, HJ. Association between bisphenol a exposure and risk of all-cause and cause-specific mortality in US adults. JAMA Netw Open. (2020) 3:e2011620. doi: 10.1001/jamanetworkopen.2020.11620
20. Granic, A, Sayer, AA, and Robinson, SM. Dietary patterns, skeletal muscle health, and sarcopenia in older adults. Nutrients. (2019) 11:745. doi: 10.3390/nu11040745
21. Chen, YM, Liu, ZY, Chen, S, Lu, XT, Huang, ZH, Wusiman, M, et al. Mitigating the impact of bisphenol a exposure on mortality: is diet the key? A cohort study based on NHANES. Ecotoxicol Environ Saf. (2023) 267:115629. doi: 10.1016/j.ecoenv.2023.115629
22. Chen, S, Tao, Y, Wang, P, Li, D, Shen, R, Fu, G, et al. Association of urinary bisphenol a with cardiovascular and all-cause mortality: National Health and nutrition examination survey (NHANES) 2003-2016. Environ Sci Pollut Res Int. (2023) 30:51217–27. doi: 10.1007/s11356-023-25924-7
23. Wang, Z, Liu, H, and Liu, S. Low-dose bisphenol a exposure: a seemingly instigating carcinogenic effect on breast Cancer. Adv Sci (Weinh). (2017) 4:1600248. doi: 10.1002/advs.201600248
24. Aftabsavad, S, Noormohammadi, Z, Moini, A, and Karimipoor, M. Effect of bisphenol a on alterations of ICAM-1 and HLA-G genes expression and DNA methylation profiles in cumulus cells of infertile women with poor response to ovarian stimulation. Sci Rep. (2021) 11:9595. doi: 10.1038/s41598-021-87175-1
25. Thongkorn, S, Kanlayaprasit, S, Panjabud, P, Saeliw, T, Jantheang, T, Kasitipradit, K, et al. Sex differences in the effects of prenatal bisphenol a exposure on autism-related genes and their relationships with the hippocampus functions. Sci Rep. (2021) 11:1241. doi: 10.1038/s41598-020-80390-2
26. Bi, J, Wang, F, Wei, Y, Zhang, Y, Jia, C, He, J, et al. Association of serum bisphenol a levels with incident overweight and obesity risk and the mediating effect of adiponectin. Chemosphere. (2022) 308:136287. doi: 10.1016/j.chemosphere.2022.136287
27. Wang, F, Zhang, Y, Zhang, S, Han, X, Wei, Y, Guo, H, et al. Combined effects of bisphenol a and diabetes genetic risk score on incident type 2 diabetes: a nested case-control study. Environ Pollut. (2022) 307:119581. doi: 10.1016/j.envpol.2022.119581
28. Hong, X, Wang, G, Liu, X, Wu, M, Zhang, X, Hua, X, et al. Lipidomic biomarkers: potential mediators of associations between urinary bisphenol a exposure and colorectal cancer. J Hazard Mater. (2022) 427:127863. doi: 10.1016/j.jhazmat.2021.127863
29. Palacios-Arreola, MI, Moreno-Mendoza, NA, Nava-Castro, KE, Segovia-Mendoza, M, Perez-Torres, A, Garay-Canales, CA, et al. The endocrine disruptor compound bisphenol-a (BPA) regulates the intra-Tumoral immune microenvironment and increases lung metastasis in an experimental model of breast Cancer. Int J Mol Sci. (2022) 23:2523. doi: 10.3390/ijms23052523
30. Kidani, T, Yasuda, R, Miyawaki, J, Oshima, Y, Miura, H, and Masuno, H. Bisphenol a inhibits cell proliferation and reduces the motile potential of murine LM8 osteosarcoma cells. Anticancer Res. (2017) 37:1711–22. doi: 10.21873/anticanres.11503
31. Mlynarcikova, A, Macho, L, and Fickova, M. Bisphenol a alone or in combination with estradiol modulates cell cycle-and apoptosis-related proteins and genes in MCF7 cells. Endocr Regul. (2013) 47:189–99. doi: 10.4149/endo_2013_04_189
32. Kodila, A, Franko, N, and Sollner, DM. A review on immunomodulatory effects of BPA analogues. Arch Toxicol. (2023) 97:1831–46. doi: 10.1007/s00204-023-03519-y
33. Nagarajan, M, Maadurshni, GB, and Manivannan, J. Exposure to low dose of bisphenol a (BPA) intensifies kidney oxidative stress, inflammatory factors expression and modulates angiotensin II signaling under hypertensive milieu. J Biochem Mol Toxicol. (2023) 38:e23533. doi: 10.1002/jbt.23533
34. Nagarajan, M, Maadurshni, GB, and Manivannan, J. Bisphenol a (BPA) exposure aggravates hepatic oxidative stress and inflammatory response under hypertensive milieu – impact of low dose on hepatocytes and influence of MAPK and ER stress pathways. Food Chem Toxicol. (2023) 183:114197. doi: 10.1016/j.fct.2023.114197
Keywords: environmental phenols, Bisphenol A, cancer mortality, all-cause mortality, NHANES
Citation: Yuan Y, Chen Q, Ding X, Zhong Q and Zhong X (2024) Endocrine disrupting chemical Bisphenol A and its association with cancer mortality: a prospective cohort study of NHANES. Front. Public Health. 12:1341789. doi: 10.3389/fpubh.2024.1341789
Edited by:
Chitra Thakur, Stony Brook University, United StatesReviewed by:
Ronak Loonawat, Wuxi Advanced Therapeutics, Inc., United StatesPriya Wadgaonkar, City of Hope National Medical Center, United States
Copyright © 2024 Yuan, Chen, Ding, Zhong and Zhong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaomin Zhong, MTM5MTIwNzQ5NzFAMTYzLmNvbQ==
 Ying Yuan
Ying Yuan Qian Chen
Qian Chen