- 1Department of Otorhinolaryngology Head and Neck Surgery, The Second Affiliated Hospital, Jiangxi Medical College, Nanchang University, Nanchang, Jiangxi, China
- 2Department of Hepatobiliary Medicine, Fuzong Clinical Medical College of Fujian Medical University, Fuzhou, Fujian, China
- 3Department of Otolaryngology-Head and Neck Surgery, Jiangdu People’s Hospital Affiliated to Yangzhou University, Yangzhou, Jiangsu, China
The increasing prevalence of microplastics (MPs) in the environment has raised urgent concerns regarding their implications for human health. This comprehensive review integrates recent findings on the sources, classification, and pathways of MPs into the human body, highlighting their potential cellular toxicity and systemic health risks. We discuss the mechanisms by which MPs may induce inflammatory responses, oxidative stress, and cellular damage, thereby contributing to various diseases. Notably, we examine the synergistic effects of MPs in conjunction with other environmental pollutants, which may amplify their adverse health outcomes. This synthesis of current research underscores the critical need for multidisciplinary approaches to investigate the multifaceted interactions between MPs and human health, ultimately guiding future studies and informing public health strategies to mitigate exposure and associated risks.
1 Introduction
Plastics have gained widespread global usage due to their durability, affordability, and various other advantages. However, despite the extensive consumption of plastic materials, only about 20% of plastic waste is recycled or incinerated, leaving a significant portion abandoned in landfills or dispersed throughout the natural environment (121). The generation of global plastic waste is rapidly escalating in tandem with increased plastic production. According to data from Plastics Europe,1 it is projected that by 2050, global plastic waste could reach approximately 2.4 billion tons, more than six times the volume produced in 2020. Due to their resistance to degradation, plastics can persist in the environment for decades or even centuries, breaking down into smaller fragments through processes such as physical abrasion, chemical reactions, and biological degradation. Microplastics (MPs) have emerged as a rapidly expanding category of pollutants, raising significant concerns in both environmental and health contexts due to their toxicological effects.
MPs are defined as water-insoluble synthetic solid particles or polymer matrices derived from both primary and secondary sources, typically characterized as plastic particles with dimensions less than 5 mm (1). They are categorized into primary MPs—those directly released into the environment through human activities, such as plastic particles found in personal care products like cleansers and cosmetics—and secondary MPs, which result from the degradation of larger plastic items such as ropes, clothing, and packaging (2). While plastics exhibit environmental persistence, once they enter ecosystems, they undergo chemical weathering, photodegradation, biodegradation, and mechanical forces that compromise their structural integrity. This process leads to fragmentation into particles ranging from micrometers to nanometers in size (3). Humans can be exposed to MPs present in the air through inhalation or dermal contact. In aquatic and soil environments, MPs can accumulate pollutants and enter the food chain via drinking water and agricultural products, potentially leading to biomagnification at higher trophic levels. This accumulation raises significant concerns regarding human health (4).
Given their pervasive nature, microplastic pollution poses a considerable global challenge. It is essential to continually address the origins of these tiny particles and assess their potential impacts on human health. Although numerous review articles have focused on the distribution of MPs in aquatic ecosystems such as oceans and surface waters, research concerning their implications for human populations remains limited, with conclusive evidence regarding health risks still lacking. In this context, we present a review of recent advancements in understanding the toxicity of MPs. This includes an examination of their definition, classification, sources, pathways into the human body, mechanisms of cytotoxicity, health risks associated with human systems, and interactions with other pollutants. Unlike previous reviews predominantly focused on the environmental distribution of MPs, this study advances the field by elucidating their molecular targets in human systems and dissecting toxicological mechanisms through a multidimensional lens. We systematically synthesize exposure pathways across atmospheric, aquatic, and terrestrial matrices while integrating emerging clinical evidence. By overcoming fragmented approaches in existing literature, this review establishes a cohesive theoretical framework linking mechanistic insights to intervention strategies, thereby offering novel perspectives for mitigating MPs-associated health risks and aims to establish a foundation for further exploration in this critical area of research.
2 MPs in the environment
2.1 Definition of MPs
The persistence of plastics in the environment poses significant challenges, as they can remain intact for decades or even centuries. Over time, these materials fragment into smaller particles due to processes such as physical abrasion, chemical reactions, and biodegradation. In 2004, Thompson et al. identified plastic particles approximately 20 μm in size along British coastlines and in marine environments, leading to the introduction of the term “microplastics” (MPs) (5). MPs are now defined as plastic fragments or particles with diameters less than 5 mm, and their widespread presence in daily life results in unavoidable human exposure (6).
MPs can manifest in various forms, with their properties influenced by their origins and the environmental conditions they encounter. Factors such as residence time, the initial structure of primary plastics, and degradation processes—including photodegradation, mechanical wear, and biological contamination—play crucial roles in shaping these particles (7). Generally, fibrous, granular, and fragmented MPs arise from larger plastic items through mechanisms like photodegradation and mechanical action (8). Microplastic particles are typically spherical and range from a few micrometers (μm) to several millimeters (mm) in size, with most diameters falling between 1 mm and a few mm. In contrast, microplastic fibers are longer and narrower than other types of MPs, measuring from 10 μm to several millimeters in length. Due to their small size and varied forms, MPs can easily contaminate food sources, increasing ingestion risk. Furthermore, they tend to accumulate within the human body more readily and are more challenging to eliminate.
2.2 Classification of MPs
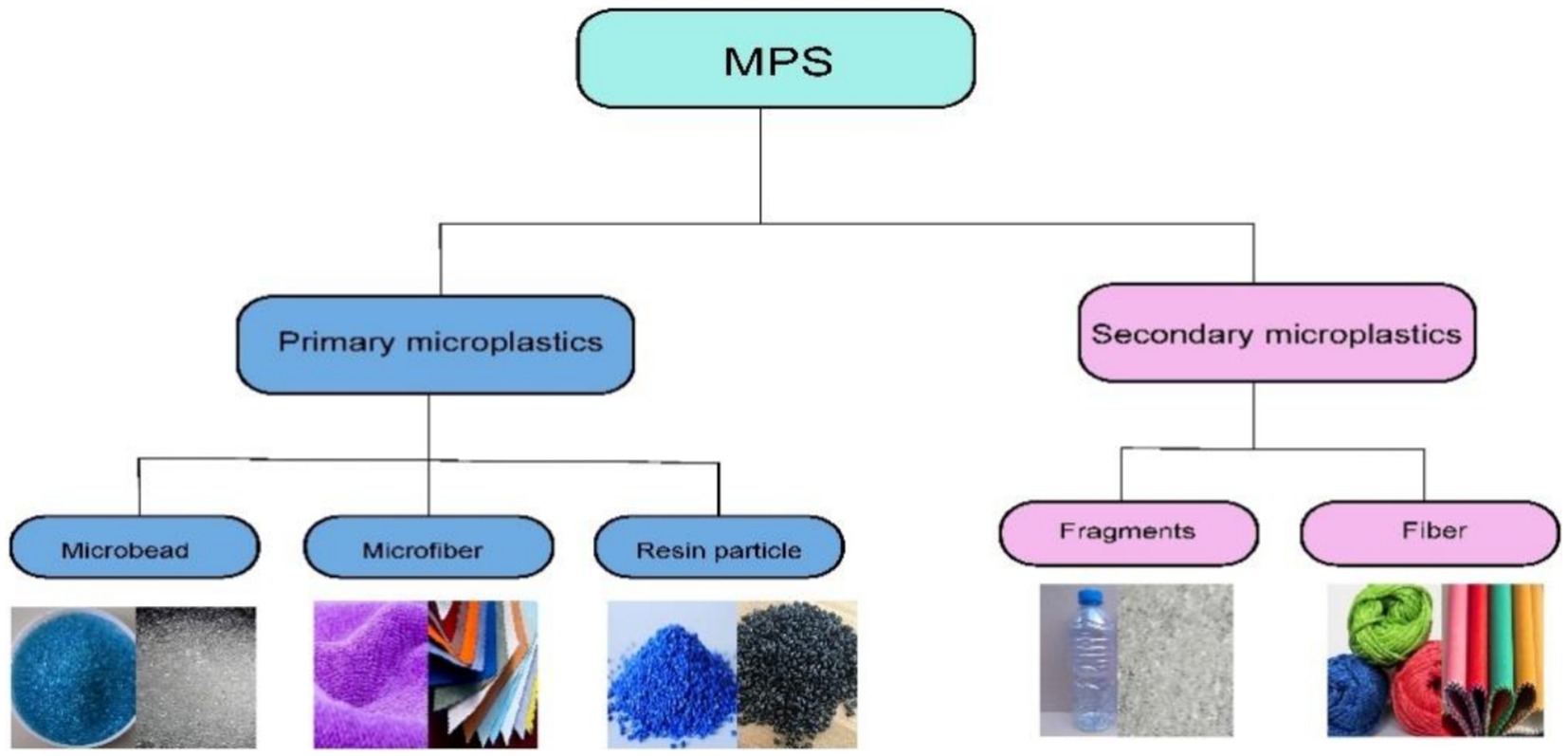
Based on the sources of MPs, they can be divided into two major categories: primary MPs and secondary MPs (2). Figure 1 and Table 1 provides an overview of the classification and sources of MPs.
Primary MPs are defined as small plastic particles that are either manufactured at a microscale or produced as by-products during the production process. These particles are specifically designed for various applications, including their use as injection molding powders, abrasive grains, or resin particles (9). Additionally, primary MPs can arise from the wear and tear of larger plastic items throughout their lifecycle, such as the abrasion of tires during driving or the shedding of synthetic fibers during laundering (10). Common forms of primary MPs include microbeads, microfibers, and resin pellets.
Microbeads are tiny plastic spheres, typically measuring less than 5 mm in diameter, that are often found in personal care and cosmetic items such as exfoliating scrubs and toothpaste. Their primary role in these products is to act as abrasives or to enhance texture. Due to their small size, microbeads can easily enter aquatic systems and often evade filtration processes at wastewater treatment facilities (11). Microfibers are fine plastic filaments that originate from textiles like synthetic clothing, carpets, and home furnishings. These fibers can be released at various stages of a textile’s lifecycle, including during production, usage, washing, and even post-treatment. Microfibers are also present in personal care products, including cigarette filters, wet wipes, and face masks (12). Resin pellets serve as raw materials for plastic product manufacturing and can pose environmental risks to aquatic ecosystems if mishandled or accidentally released during production, transportation, or processing (4). These primary MPs have the potential to adsorb and transport hazardous chemicals, thereby increasing their ecological impact.
Secondary MPs are small plastic particles generated from the degradation and fragmentation of larger plastic products such as bags, bottles and Wrapping materials. This breakdown occurs due to mechanical forces like waves and abrasion, chemical reactions, and exposure to ultraviolet radiation from sunlight (13). Given the frequent release of larger plastic items into the environment, secondary MPs constitute the primary components of microplastic pollution. The predominant forms of secondary MPs include fragments and fibers (14). Plastic fragments are unevenly shaped remnants produced through the breakdown of larger plastic items, whereas fibers are slender strands originating from textiles, including garments, ropes, and fishing nets.
2.3 Origin of MPs
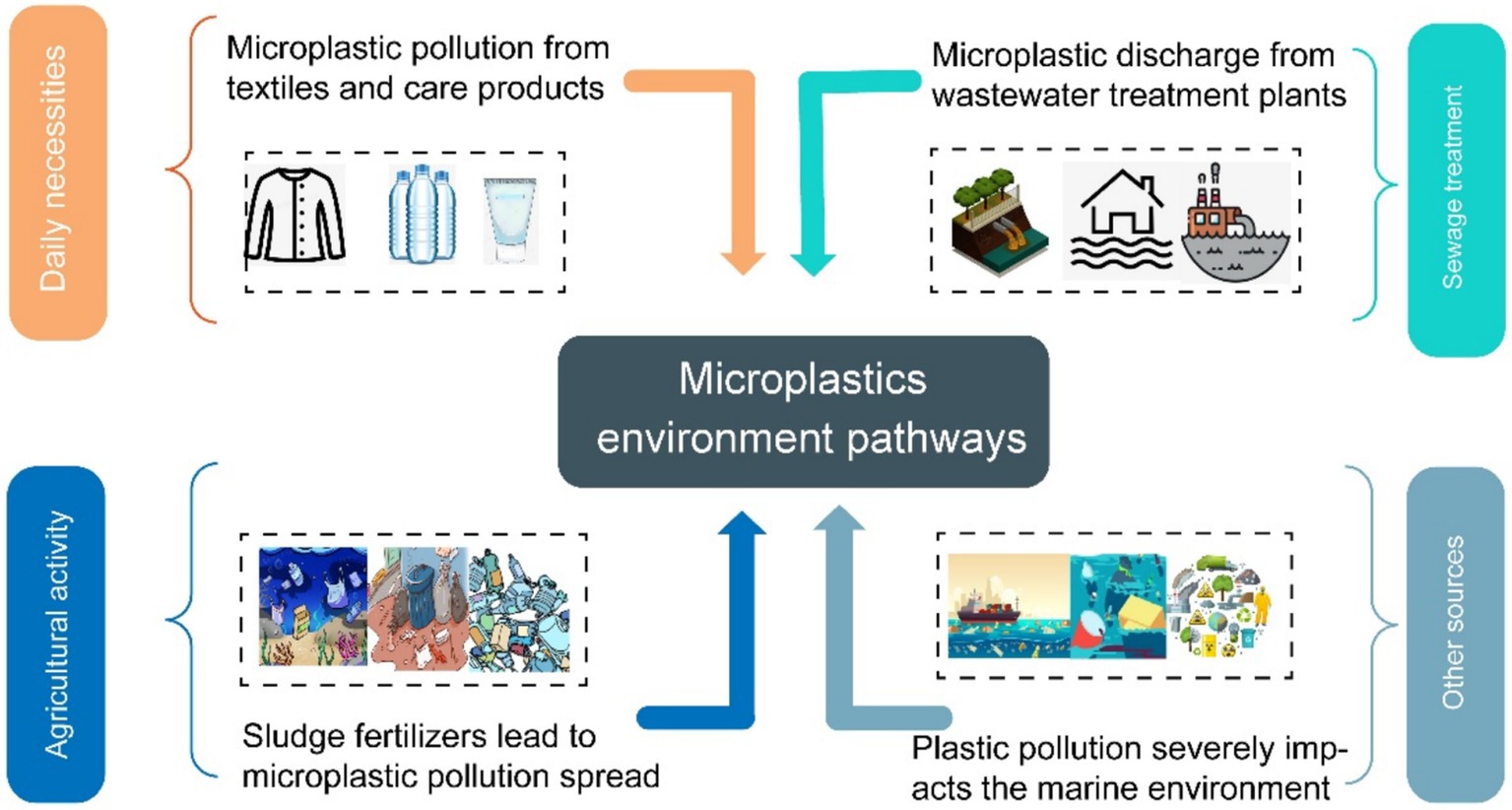
The classification of microplastics is closely intertwined with their environmental origins. MPs have numerous sources. Major sources include the use of everyday products (such as detergents, cosmetics), agricultural activities, wastewater discharge, and more (Figure 2).
2.3.1 Daily supplies
Textiles, especially those made from synthetic materials like nylon and polyester, as well as natural fibers such as wool, significantly contribute to microplastic pollution (15). The laundering of these textile products subjects them to mechanical and chemical stresses, resulting in the release of tiny microfibers. Due to their diminutive size, these microfibers are often unable to be effectively filtered out by wastewater treatment facilities, leading to their eventual discharge into aquatic ecosystems (16). Studies have shown that on average, washing a single piece of clothing can release between 1,900 and 1 million microfibers, while washing textiles made primarily of polyester can result in the release of over 6 million fibers. These findings highlight the significant contribution of laundry activities to microplastic pollution in the environment (17).
In personal care products, primary MPs are predominantly found, including microbeads, microfibers, and other forms. These products typically include soaps, shampoos, conditioners, body washes, cosmetics, and skin care products. The exfoliating microbeads are found in care products such as facial cleansers and toothpaste (18). The ingredients labeled in personal care products, such as polyethylene, polypropylene, polyethylene terephthalate, and polymethyl methacrylate, may be primary components of microplastics. When used in everyday life, the primary MPs they generate can bypass wastewater treatment plants and enter aquatic environments through wastewater. Simultaneously, they can also be ingested by aquatic organisms. Microbeads are often added to products as exfoliants or abrasives, and they are intentionally manufactured as small plastic particles. When these items are laundered, the microbeads may be released into aquatic environments, thereby exacerbating microplastic pollution. The ingestion of these MPs by aquatic life can lead to a range of ecological issues, including potential harm to the health of the organisms and the transfer of pollutants up the food chain (19). Studies have shown that a single use of toothpaste during brushing can release approximately 4,000 microbeads (17). A survey conducted across various supermarkets in China revealed that 7.1% of facial cleansers and 2.2% of body washes were found to contain microplastics, with polyethylene identified as the primary material comprising these MPs (20).
2.3.2 Wastewater treatment plant
Wastewater treatment facilities are primarily engineered to eliminate organic matter, nutrients, and various pollutants from wastewater; however, they are not particularly effective in removing MPs (21). Treated wastewater can enter aquatic environments through multiple routes, including the direct discharge of effluent, overflow during storm events, and the use of sewage sludge as fertilizer (22). Furthermore, there is a notable connection between wastewater treatment plants and the textiles and personal care products that contribute to MP pollution. Addressing this relationship could provide a viable strategy for mitigating the presence of MPs. A study conducted on two wastewater treatment facilities in Turkey found that influent water contained between 1 million and 6.5 million microplastic particles daily, while the effluent water ranged from 220,000 and 1.5 million particles per day. The research identified seven distinct types of polymers, with polyester comprising the majority of those detected (23).
2.3.3 Rural activity
Agricultural land fertilizers often originate from sludge produced by industrial wastewater treatment, aiming to recycle organic matter and provide nutrients (24). Consequently, this can lead to a considerable buildup of MPs in agricultural lands, from which these particles can subsequently enter aquatic ecosystems through multiple pathways, including rainfall, leaching, and irrigation practices (25). A study evaluated MPs in 124 organic compost samples, including those derived from single feedstocks (e.g., livestock/poultry manure, crop straw, solid waste) and composite materials. Results revealed significant variations in MPs abundance, with solid waste compost exhibiting the highest concentration (6,615 items/kg), whereas crop straw compost contained the lowest (1,500 items/kg). Annual MPs input to agricultural soils via compost application was estimated at 6.96 × 107–1.88 × 108 items/ha (26). Analysis of soil MPs in China’s Weishan Irrigation District demonstrated significant variations in MP abundance across land-use types, with mean concentrations of 900, 512,615, and 633 items/kg detected in vegetable fields, croplands, orchards, and woodlands, respectively. MPs were predominantly characterized by small particle sizes (0.2–1 mm, >78% of total), blue/purple or transparent coloration (>60%), and film-, fiber-, or fragment-like morphologies (>78%). Polyethylene (>36%) was the most prevalent polymer type. Notably, organic fertilizers were identified as a major contributor to MP contamination (27).
2.3.4 Other sources
Additionally, plastics enter water systems through casual disposal and fishing gear (28). These materials undergo degradation when exposed to sunlight, resulting in the formation of various microplastic fragments. Notably, beach litter constitutes roughly 80% of the plastic waste present in ocean environments. Furthermore, practices such as recreational pursuits, and unregulated fishing, combined with the growing trend of human migration to coastal areas, suggest that the introduction of plastic waste into marine ecosystems is expected to increase in the future. The wide distribution of microplastics in the environment enables them to enter the human body through a variety of ways and affect human cells and organs.
3 The mechanisms of pollutant adsorption on MPs
MPs are widely distributed in the environment and their unique surface properties make them ideal carriers for various harmful microorganisms, forming “contaminant-microplastics complexes” with potential transmission risks (2). Current studies confirm that microplastic surfaces exhibit significant adsorption capacity for various environmental contaminants, including organic pollutants, heavy metals, and pathogenic microorganisms (29, 30). Due to their substantial surface area and hydrophobic properties, MPs serve as significant carriers for contaminants. Additionally, the mechanisms of contaminant adsorption onto MPs also involve electrostatic repulsion and attraction, pore blockage, and site competition (31). For instance, studies have demonstrated that arsenic can adsorb onto microplastics through non-covalent bonding and electrostatic interactions (32, 33), resulting in enhanced arsenic accumulation and synergistic toxic effects (34). In a study, the inhibitory effect of organic matter on activated carbon fibers was reported. The research demonstrated that there was no significant adsorption of organic matter itself, while the concentration of organic matter markedly increased (35). These findings suggest a competitive adsorption between organic matter and perfluorooctanoic acid, as well as pore blockage of activated carbon fibers by organic matter.
4 Pathways of MPs into human cells
MPs primarily enter the bodies of organisms, including humans, through consumption, inhalation, and skin contact. Traces of MPs have been detected in feces, meconium, placentas, lung tissue, breast milk, saliva, blood, facial tissue, liver, kidneys, and the colon (36).
4.1 Inhalation by respiration
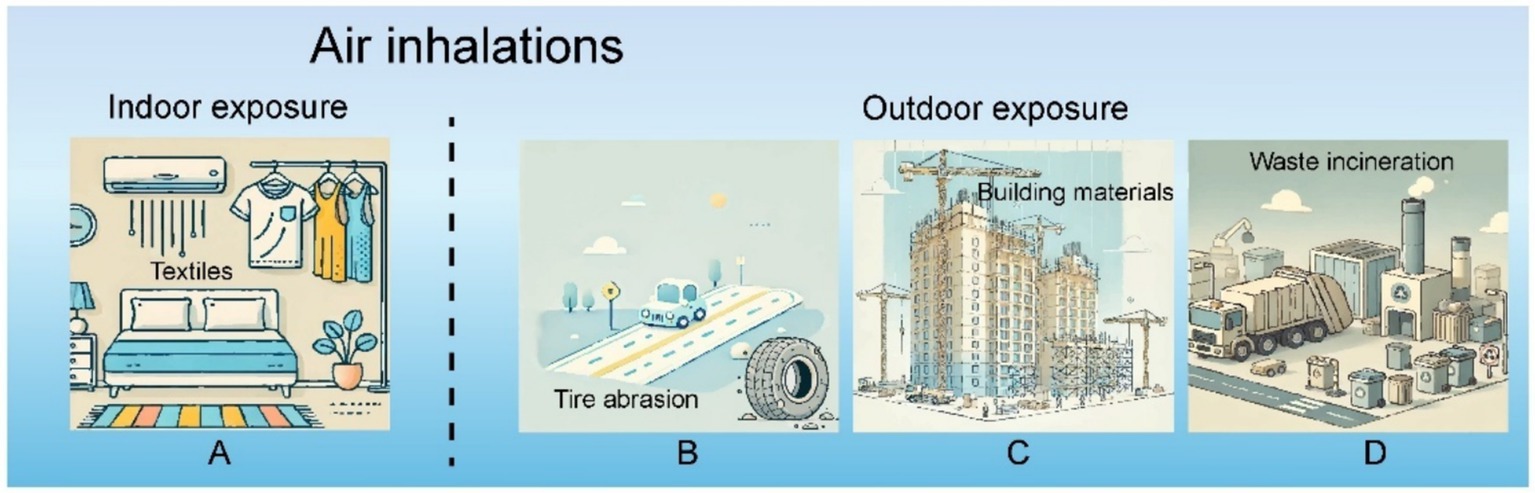
Inhalation via the respiratory system represents a significant route for the introduction of MPs into the human body (Figure 3), with prior research validating their presence in ambient air (37). Notably, MPs measuring less than 5 μm in length and under 3 μm in diameter are particularly susceptible to inhalation, as they can often evade the mucociliary clearance mechanisms of the upper respiratory tract. Once inhaled, these MPs can disseminate throughout the body via the upper digestive system, ultimately accumulating in various organs (38).
Tire wear, construction materials, and waste incineration are primary contributors to the presence of MPs in outdoor environments (39). Research indicates that approximately 3–7% of particulate matter measuring less than 2.5 μm in the atmosphere originates from tire wear, with an average annual emission rate of 0.81 kg of tire dust per person (40). Additionally, polyethylene and polystyrene are frequently utilized as insulating and molding materials in construction, and the expansion of large-scale architectural projects has led to increased MP emissions (41). A study on emissions from incineration reveals that between 1.9 and 565 microplastic items per kilogram of Municipal Solid Waste (MSW) accumulate in the incinerator’s bottom ash, with the incineration process facilitating their release into the atmosphere (42, 43).
Factors like wind direction, altitude, and population density significantly influence the concentration of MPs in outdoor settings. One study found that urban areas exhibit a higher atmospheric concentration of MPs (13.9 items/m3) compared to rural regions (1.5 items/m3) (44). Another investigation in German cities reported no detectable plastic concentrations in less populated areas (0 particles/m3) (45). Furthermore, this research highlighted elevated MP concentrations near the ground, peaking at a height of 1.7 m—nearly three times greater than those measured at 33 and 80 m (45). Wind direction plays a crucial role in the dispersion of plastic particles; the distribution of MPs is significantly correlated with prevailing wind patterns (45). Consequently, atmospheric MPs tend to accumulate in urban locales, from which they can be transported over considerable distances by wind.
For students and white-collar workers, a significant portion of their time is spent indoors. A recent study found that the concentration of indoor MPs ranges between 1.0 and 60.0 fibers/m3, its main component is polysulfated mucopolysaccharides (a type of MPs). Textiles are a common source of polysulfated mucopolysaccharides in indoor environments, primarily composed of fiber particles such as polyamide, acrylic, and polyester (46, 47). Fiber MPs are the most common type of MPs found in indoor environments. Research has shown that the level of indoor MPs (ranging from 1.0 to 60.0 fibers/m3) is significantly higher than the level of outdoor MPs (ranging from 0.3 to 1.5 fibers/m3) (46). The concentration of polyethylene terephthalate (PET) is between 1,550 and 120,000 mg per kilogram (mg/kg) indoors, which is higher than the range of 212–9,020 mg/kg outdoors (48).
The direction and strength of the airflow generated by air conditioners can affect the migration of polysulfated mucopolysaccharides (a type of MPs) into indoor spaces. A study conducted in student dormitories on airflow testing showed that turbulent airflow can lead to the resuspension of MPs, MPs particles are prone to depositing in open food and drinking water indoors. Moreover, activities such as walking, closing doors, or engaging in some indoor exercises can potentially lead to the resuspension of MPs within indoor spaces (49).
4.2 Oral ingestion
The intake of seafood, such as fish, shrimp, and shellfish, constitutes a major route for MPs to infiltrate the human body. Studies indicate that MPs are commonly found in the tissues and organs of marine species, thereby enabling their transmission to humans via the food chain (50). A survey of the Chinese fishery market revealed that various types of MPs—such as fibers, fragments, and particles—were present in all samples of commercially harvested bivalve shellfish, with particles averaging below 250 μm accounting for 33–84% of the total MPs detected (51). In Indonesia, Rochman et al. discovered that 55% of fish samples were contaminated with MPs, with the majority being less than 500 μm in size and mainly consisting of polyethylene and polypropylene (52).
Bottled drinking water is another significant source of MPs, originating from both the plastic bottles and caps. Over time, degradation of these materials can introduce MPs into the water supply (53). Research indicates that consuming tap water leads to an estimated intake of 4,000 MPs annually, while bottled water consumption can result in an additional intake of approximately 90,000 MPs per year (54). A study by Sherri A. Mason and colleagues analyzed 259 bottled water products across nine countries and found that 93% contained MPs. The average concentration of microplastic particles larger than 100 μm was recorded at 10.4 per liter, with fragments and fibers being the predominant forms. Polypropylene was identified as the most common polymer, comprising 54% of the total (55). In Germany, all bottled water samples tested positive for MPs, with 80% of particles ranging from 5 to 20 mm. Recyclable plastic bottles exhibited the highest average microplastic content at 118 particles per liter, primarily composed of polyester (PET) and polypropylene (PP), while single-use bottles contained an average of 14 particles per liter and beverage cartons had 11 particles per liter, with polyethylene being the common material (56).
Moreover, MPs are found in a variety of food sources such as drinking water, beverages, milk, canned goods, sugar, and salt (57). Studies confirm that low-nutrition organisms are more susceptible to environmental MP contamination. Recent research employing two complementary analytical techniques identified that 32% of MPs smaller than 20 μm were present in drinking water samples. This underscores the need for high-performance filtration systems to enhance purification processes (58, 59). Additionally, a study indicated that the risk of ingesting plastic from mussels is lower compared to exposure to fibers from dust during meals, estimating an annual range of ingestion between 13,731 and 68,415 particles per person (60). Yinan Li and colleagues have also reported that commonly consumed beverages worldwide—including beer, tea, and honey—contain MPs in various forms such as particles, fragments, and fibers. These contaminants are primarily attributed to raw materials as well as environmental exposure during processing and packaging (61).
4.3 Skin contact and dermal absorption
MPs can enter the human body and other organisms through dermal exposure to a range of topical products, such as cosmetics, body washes, topical medications, and surgical or prosthetic devices. They can also be absorbed through occupational interactions in both indoor and outdoor environments. Under typical circumstances, MPs do not breach the subcutaneous barrier; however, their capacity to penetrate this barrier is primarily determined by their size and chemical characteristics. Particles measuring less than 100 nm are more likely to penetrate the skin due to their smaller size and increased surface reactivity. Furthermore, larger particles may be absorbed via hair follicles, sweat glands, or damaged areas of the skin (62, 63).
5 Mechanism of cytotoxicity induced by MPs
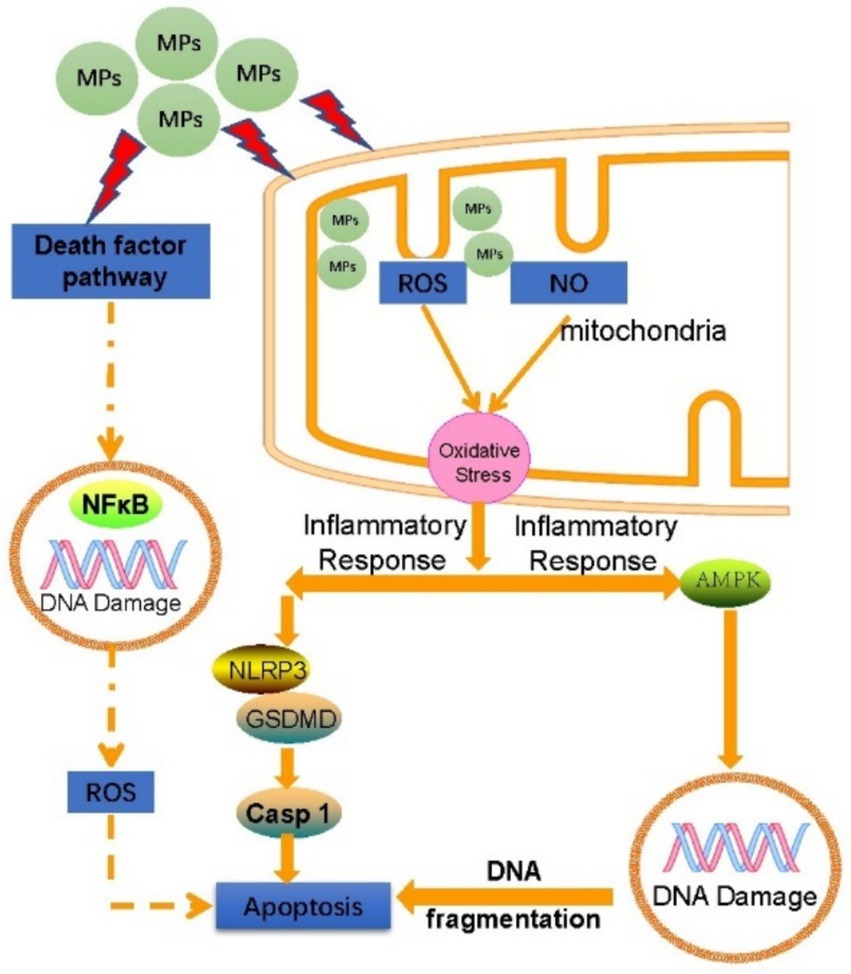
Upon entering mammalian cells, MPs can engage in interactions that elicit a range of cellular responses, including Reactive oxygen species (ROS) stress, Inflammatory responses, Cellular apoptosis, and genomic instability characterized by DNA damage (Figure 4). Once MPs enter mammalian cells, they are enclosed by lysosomes for degradation before being released into the cytoplasm. This release may lead to mitochondrial dysfunction, which in turn increases the production of ROS. Elevated ROS levels can undermine the cell’s antioxidant defense systems, resulting in protein oxidation, lipid peroxidation, and DNA damage (64).
5.1 Oxidative stress
Mitochondria serve as the primary source of reactive oxygen species (ROS), and oxidative stress arises from an imbalance between ROS production and the capacity of the antioxidant defense system. MPs can disrupt mitochondrial membrane potential and hinder mitochondrial energy production, contributing to this imbalance (65, 66). Research by Hao Wu et al. demonstrated that polystyrene MPs can induce DNA damage, cell cycle arrest, and necrotic apoptosis in mouse ovarian granulosa cells by enhancing ROS production (67). Furthermore, MPs have been shown to diminish the activity of antioxidant enzymes in vitro, thereby triggering oxidative stress. For instance, polystyrene MPs can lead to hepatocyte apoptosis and alter glycolytic flux through ROS-mediated calcium overload (68, 69).
5.2 Inflammatory reaction
MPs can stimulate the release of cytokines such as IL-6, IL-8, and IL-10, leading to immune-mediated inflammatory responses that may ultimately result in necrosis of cellular or tissue structures (70). In a murine study, MPs were shown to enhance the expression of inflammatory proteins cPLA2 and COX-1, which subsequently caused renal damage in the mice (71). Research involving chicken cardiomyocytes revealed that MPs could alter the NF-κB-NLRP3-GSDMD and AMPK-PGC-1α signaling pathways due to ROS overload, thereby inducing oxidative stress, pyroptosis in cardiomyocytes, inflammation, and impairments in mitochondrial function and energy metabolism (72). Furthermore, a study conducted on rats suggested that MPs could induce both pyroptosis and apoptosis in ovarian granulosa cells through the NLRP3/Caspase-1 signaling pathway, potentially triggered by ROS (73).
5.3 Apoptosis
Research has demonstrated that exposure to MPs in zebrafish models leads to a significant upregulation of apoptosis-related genes, including p53, gadd45ba, and casp3b, ultimately resulting in the degradation of gill tissue structure (74). Similarly, another study reported that MPs markedly activated NF-κB, pro-inflammatory cytokines, and apoptosis markers in human microglial cells as well as in mouse brains. This activation included increased levels of BAX, cleavage of PARP, and the activation of caspases 3 and 8, culminating in the apoptosis of microglial cells in both species (75). Additionally, Siwen Li and colleagues found that MPs can induce hepatocyte apoptosis through calcium overload driven by reactive oxygen species (ROS) (68).
5.4 Gene damage
In a study measuring various stress indices in zebrafish exposed to MPs, including Lipid oxidative damage, DNA lesions, autophagic process, caspase activation, metabolite alterations, and changes in ventricular contraction frequency and force—parameters that correlate with the fish’s swimming speed—it was determined that DNA damage was the most pronounced effect observed (76). Research involving human peripheral blood lymphocytes revealed that exposure to MPs significantly elevated the frequency of micronuclei (MN), nuclear bridge formation (NPB), and nuclear bud formation (NBUD). Notably, even at lower concentrations, MPs contributed to increased genomic instability. The mechanical interactions between MPs and cells, along with the release of additives from MPs, are potential mechanisms underlying this enhanced genomic instability (77). A further investigation revealed that contact with MPs can lead to DNA lesions in both the mitochondria and nucleus, which results in the movement of double-stranded DNA fragments into the cytoplasm. This event activates the DNA-sensing adaptor protein STING, subsequently initiating the cGAS/STING signaling pathway. This cascade of reactions promotes the translocation of NFκB into the nucleus, where it enhances the expression of pro-inflammatory cytokines, ultimately contributing to liver fibrosis (78). Additionally, a study on fish demonstrated that even at low concentrations, nanoparticles (NPs) can penetrate the nucleus and cause DNA damage, as evidenced by an increased incidence of abnormal erythrocyte nuclei (79). The above cytotoxic mechanisms can further cause multiple organ dysfunction such as nervous system and cardiovascular system.
6 Health risk of MPs to different organ
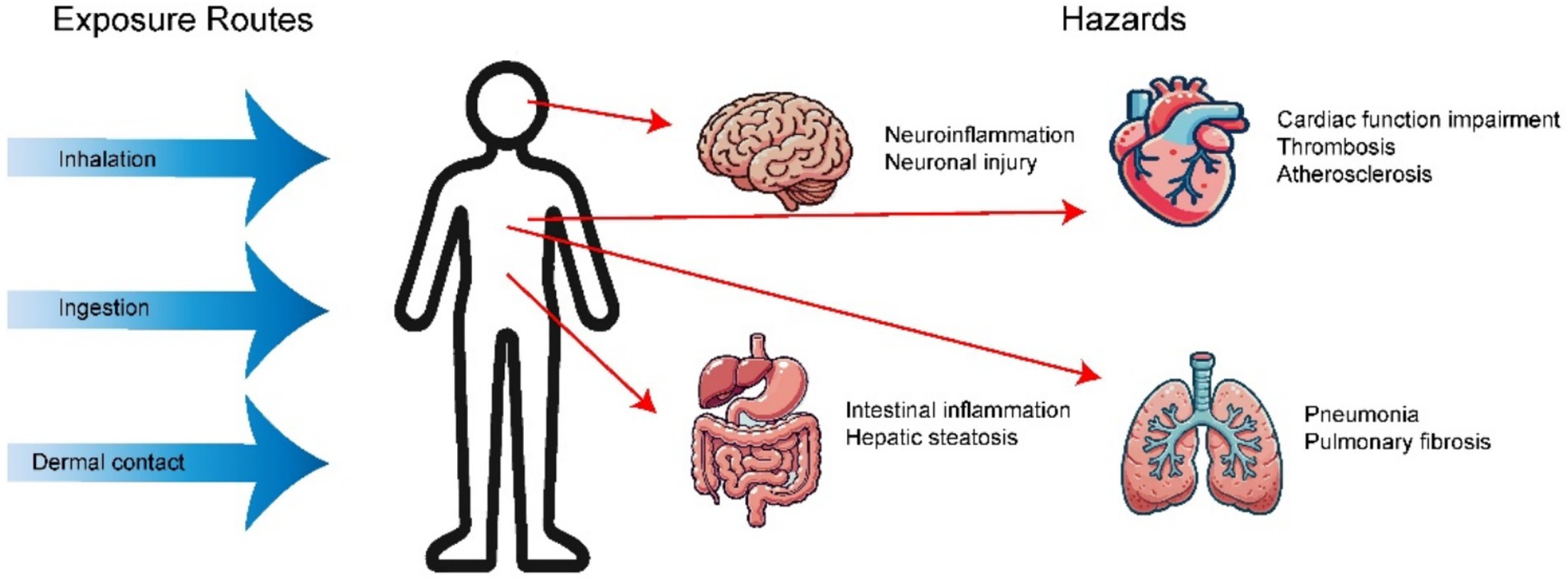
Research suggests that exposure to these microscopic plastic particles can lead to a range of detrimental effects on human health. (Figure 5 and Table 2).
6.1 Neurotoxicity: damage to the blood–brain barrier
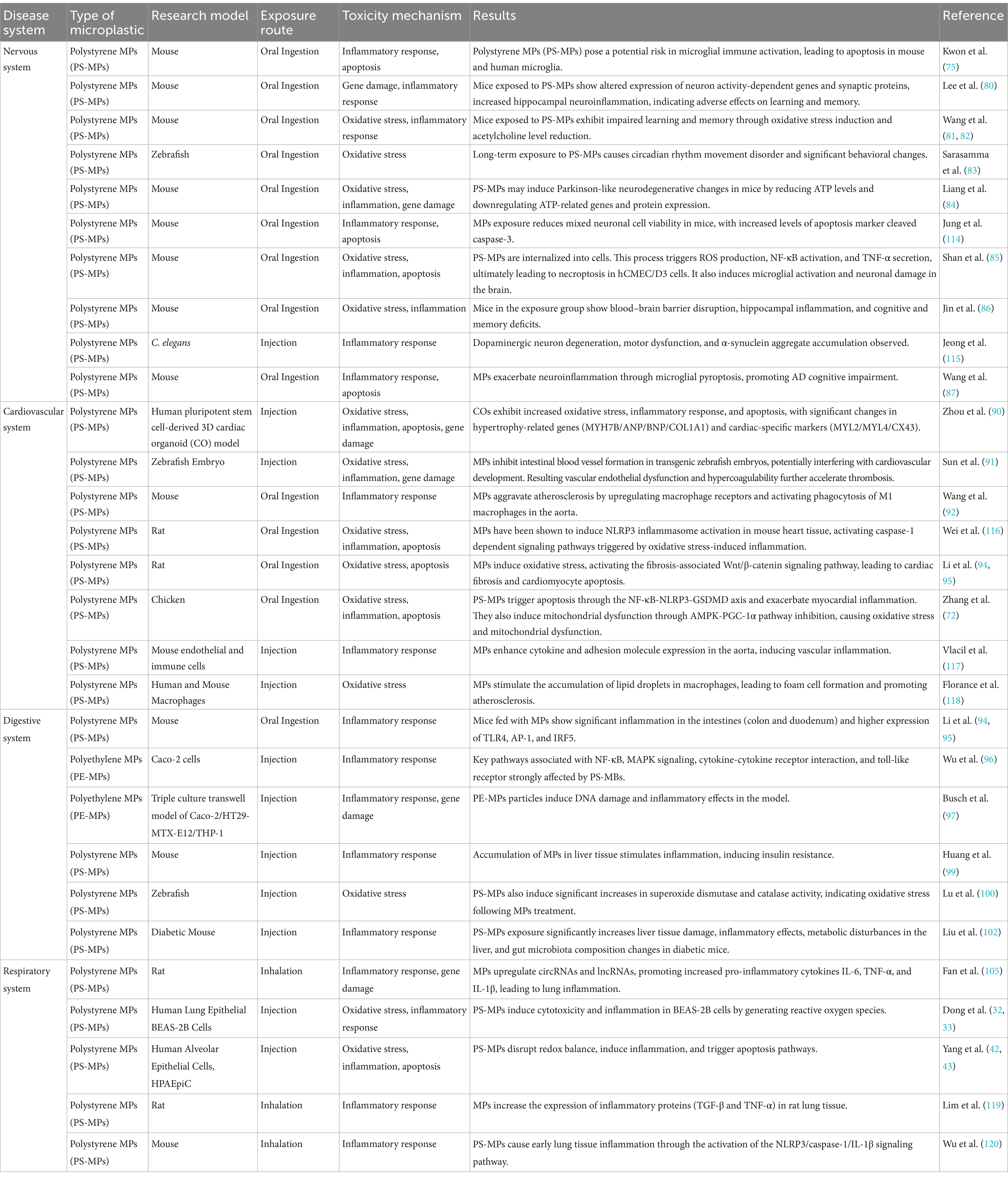
MPs have the capability to traverse the blood–brain barrier and accumulate within brain tissue. Research indicates that smaller MPs are more likely to penetrate this barrier. For instance, polystyrene microplastic particles (PS-MPs) measuring 50 nm can cross into the mouse brain, where they activate microglial cells and inflict significant neuronal damage (75). Additionally, PS-MPs have been shown to reduce levels of synaptic proteins, disrupt neurotransmitter function, induce neuroinflammation, and lead to deficits in learning and memory in mice (80–82). The neurotoxic effects of MPs are further evidenced by declines in motor abilities across certain species, which manifest as behavioral changes (83). This indicates that MPs could play a role in the onset of neurodegenerative disorders, such as Parkinson’s disease, Huntington’s disease, and Alzheimer’s disease (84–87).
6.2 Cardiopulmonary toxicity
Recent research has identified various types of MPs in different cardiac tissues, with the largest diameter recorded at 469 μm (88). The direct cardiotoxic effects of MPs encompass arrhythmias, compromised cardiac function, pericardial edema, and myocardial fibrosis. At the microvascular level, MPs can induce hemolysis, thrombosis, blood coagulation, and damage to vascular endothelium, primarily through mechanisms involving oxidative stress, inflammation, and apoptosis (89). In an in vitro study utilizing a three-dimensional cardiac organoid model derived from human pluripotent stem cells, exposure to MPs significantly altered the expression of genes associated with cardiac hypertrophy (MYH7B/ANP/BNP/COL1A1), while cardiac-specific markers such as MYL2, MYL4, and CX43 were also notably elevated (90). Additionally, in a zebrafish embryo model, exposure to MPs increased the incidence of thrombosis and exacerbated atherosclerosis by activating and upregulating macrophage receptors. Toxic effects of MPs on the cardiovascular system have also been documented in zebrafish, mouse, and chicken models (Table 1) (91, 92).
6.3 Hepatotoxicity
The gastrointestinal tract serves as the primary entry point for MPs. Research has demonstrated that the accumulation of MPs in the intestines can activate immune and inflammatory responses in the intestinal mucosa, potentially leading to damage to the mucosal lining (93). Studies indicate that once MPs enter the intestines, they can trigger inflammatory reactions in murine models (94, 95). Additionally, a series of in vitro investigations have revealed that exposure to MPs can result in oxidative stress, DNA damage, inflammatory responses, cell membrane injury, and apoptosis of intestinal epithelial cells, ultimately compromising intestinal barrier function (96–98).
Given that the liver is the main organ responsible for detoxifying exogenous substances, the effects of MPs on liver function are varied. Research involving mice has shown that polystyrene microplastics (PS-MPs) accumulate in the liver and contribute to insulin resistance by promoting inflammation and inhibiting insulin signaling pathways (99). In zebrafish, MPs have been found to induce lipid accumulation and potentially cause histological damage, including necrosis and hemorrhage (100). In mice, liver damage from PS-MPs is indicated by elevated levels of alkaline phosphatase (ALP) aspartate, alanine aminotransferase (ALT), aminotransferase (AST), and lactate dehydrogenase (LDH), alongside hepatotoxicity and dysbiosis of gut microbiota (101). Notably, one study reported that PS-MPs exacerbated lipid metabolism abnormalities in diabetic mice, leading to heightened inflammatory responses. This suggests that individuals with chronic conditions may exhibit increased sensitivity to plastic pollution (102).
6.4 Respiratory toxicity
Numerous studies have indicated that inhalation of MPs present in the atmosphere can lead to significant pulmonary toxicity and respiratory conditions, including asthma, pneumonia, emphysema, and allergic rhinitis. Animal research has consistently demonstrated that inhaling MPs can trigger pulmonary inflammatory responses, intensify oxidative stress, and even result in pulmonary fibrosis (103, 104). For instance, studies involving the intratracheal administration of amino-polystyrene nanoplastics (APS-NPs) in mice have revealed inflammatory infiltration in lung tissue (105). Moreover, a variety of in vitro investigations have shown that MPs can induce oxidative stress, inflammatory responses, genetic damage, and apoptosis in lung cells (32, 33, 42, 43, 106, 107). Recent findings also suggest that respiratory Ingestion of PS-MPs can disrupt the balance of nasal and pulmonary microbiota (108). Additionally, a clinical cohort study identified a higher concentration of MPs in patients diagnosed with allergic rhinitis (109).
7 Hazards of MPs interacting with other pollutants
MPs have a significant surface area and exhibit hydrophobic properties, allowing them to function as carriers for various environmental pollutants, such as organic materials, heavy metals, and pathogenic microorganisms. This adsorption can result in exacerbated effects on biological organisms and human health (1).
7.1 MPs interact with organic matter
The interplay between MPs and environmental organic compounds can facilitate bioaccumulation and intensify toxic effects in living organisms. For instance, the interaction between F-53B and PS-MPs significantly increased the transcription of pro-inflammatory genes such as cxcl-clc and il-1β, while also elevating the levels of inducible nitric oxide synthase (iNOS) protein in zebrafish larvae, thereby inducing inflammatory stress in these fry (110). Additionally, another study demonstrated that the combination of MPs with polybrominated diphenyl ethers (PBDEs) worsened developmental and thyroid toxicity in zebrafish (81, 82).
7.2 MPs interact with heavy metal
MPs can serve as carriers for various environmental heavy metals, such as arsenic (As), cadmium (Cd), and lead (Pb). When these MPs, laden with heavy metals, are introduced into organisms or the human body, they can pose significant health risks. Research involving oysters demonstrated that the presence of MPs combined with arsenic resulted in synergistic effects on gene expression, which in turn led to heightened oxidative stress and increased apoptosis (34, 111).
7.3 MPs interact with causative agent
MPs can also carry potential pathogenic microorganisms, leading to human infections with pathogens such as pathogenic Vibrio species (112). A study has shown that MPs can carry the novel coronavirus, potentially accelerating its spread and infection (113).
In summary, as a carrier of environmental pollutants, microplastics can amplify their health risks by adsorbing organic pollutants, heavy metals and pathogens. This further highlights the need to study the synergistic toxicity of microplastics.
8 Conclusion
The pervasive presence of MPs in various environmental matrices poses significant challenges to public health, necessitating urgent attention from the scientific community and policymakers alike. This review elucidates the complex interactions between MPs and human health, revealing their potential to disrupt cellular functions, induce inflammatory pathways, and exacerbate oxidative stress. The evidence presented underscores the multifactorial nature of MP toxicity, particularly when considered alongside other environmental contaminants, which may synergistically enhance health risks.
Moving forward, it is imperative to adopt a multidisciplinary approach to further investigate the biological mechanisms underlying MP-induced toxicity. Future research should prioritize longitudinal studies that assess the long-term health impacts of chronic MP exposure, as well as the identification of vulnerable populations who may be disproportionately affected. Additionally, the development of standardized methodologies for the detection and quantification of MPs in biological samples will be crucial for advancing our understanding of their bioaccumulation and systemic effects.
To address the escalating health risks posed by microplastics (MPs), a multi-tiered strategy integrating policy, research, and technological innovation is imperative. Governments must strengthen regulations to phase out single-use plastics and microbeads while mandating the adoption of advanced filtration technologies in wastewater treatment systems to intercept MPs. Concurrently, research priorities should focus on establishing standardized toxicity assessment protocols, investigating long-term bioaccumulation effects, and elucidating the synergistic interactions between MPs and co-pollutants (e.g., heavy metals, pathogens), with targeted studies on vulnerable populations. Technological advancements in biodegradable materials and enhanced recycling methodologies are critical to reducing plastic dependency. Furthermore, public awareness campaigns should promote behavioral modifications, such as minimizing bottled water consumption and prioritizing natural textiles. These measures aim to mitigate the environmental and health threats posed by MPs and drive societal transitions toward sustainability. This comprehensive approach not only addresses current challenges but also provides a framework for future research and practice to foster a greener and healthier ecological environment. Ultimately, addressing the public health implications of microplastics requires collaborative efforts across disciplines, including toxicology, epidemiology, and environmental science. By fostering a comprehensive understanding of MPs’ health impacts, we can inform regulatory frameworks and public health strategies aimed at reducing exposure and mitigating the risks associated with this emerging environmental threat. The urgency of this issue calls for immediate action to safeguard human health and preserve the integrity of our ecosystems.
Author contributions
XZ: Data curation, Formal analysis, Writing – original draft. CYu: Data curation, Formal analysis, Writing – original draft. PW: Methodology, Writing – original draft. CYa: Methodology, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the National Natural Science Foundation of China (No. 82160211) and the Jiangxi Provincial Nature Science Foundation (No. 2021BAG70031).
Acknowledgments
We would like to thank Chunping Yang and Xu Zhang for reviewing and providing advice on the final search strategy. We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript. We thank Adobe Illustrator 2024 for its Figures assistance during the preparation of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
1. Li, W, Zu, B, Yang, Q, Guo, J, and Li, J. Sources, distribution, and environmental effects of microplastics: a systematic review. RSC Adv. (2023) 13:15566–74. doi: 10.1039/D3RA02169F
2. Zhao, B, Rehati, P, Yang, Z, Cai, Z, Guo, C, and Li, Y. The potential toxicity of microplastics on human health. Sci Total Environ. (2024) 912:168946. doi: 10.1016/j.scitotenv.2023.168946
3. Amato-Lourenco, LF, Dos, SGL, de Weger, LA, Hiemstra, PS, Vijver, MG, and Mauad, T. An emerging class of air pollutants: potential effects of microplastics to respiratory human health? Sci Total Environ. (2020) 749:141676. doi: 10.1016/j.scitotenv.2020.141676
4. Mortensen, NP, Fennell, TR, and Johnson, LM. Unintended human ingestion of nanoplastics and small microplastics through drinking water, beverages, and food sources. Nanoimpact. (2021) 21:100302. doi: 10.1016/j.impact.2021.100302
5. Thompson, RC, Olsen, Y, Mitchell, RP, Davis, A, Rowland, SJ, John, AW, et al. Lost at sea: where is all the plastic? Science. (2004) 304:838. doi: 10.1126/science.1094559
6. Wang, C, Zhao, J, and Xing, B. Environmental source, fate, and toxicity of microplastics. J Hazard Mater. (2021) 407:124357. doi: 10.1016/j.jhazmat.2020.124357
7. Barnes, DK, Galgani, F, Thompson, RC, and Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos Trans R Soc Lond Ser B Biol Sci. (2009) 364:1985–98. doi: 10.1098/rstb.2008.0205
8. Xue, B, Zhang, L, Li, R, Wang, Y, Guo, J, Yu, K, et al. Underestimated microplastic pollution derived from fishery activities and “hidden” in deep sediment. Environ Sci Technol. (2020) 54:2210–7. doi: 10.1021/acs.est.9b04850
9. Laskar, N, and Kumar, U. Plastics and microplastics: a threat to environment. Environ Technol Innov. (2019) 14:100352. doi: 10.1016/j.eti.2019.100352
10. Sharma, S, and Chatterjee, S. Microplastic pollution, a threat to marine ecosystem and human health: a short review. Environ Sci Pollut R. (2017) 24:21530–47. doi: 10.1007/s11356-017-9910-8
11. Priya, A, Anusha, G, Thanigaivel, S, Karthick, A, Mohanavel, V, Velmurugan, P, et al. Removing microplastics from wastewater using leading-edge treatment technologies: a solution to microplastic pollution-a review. Bioprocess Biosyst Eng. (2023) 46:309–21. doi: 10.1007/s00449-022-02715-x
12. Athey, SN, and Erdle, LM. Are we underestimating anthropogenic microfiber pollution? A critical review of occurrence, methods, and reporting. Environ Toxicol Chem. (2022) 41:822–37. doi: 10.1002/etc.5173
13. Schmid, C, Cozzarini, L, and Zambello, E. Microplastic’s story. Mar Pollut Bull. (2021) 162:111820. doi: 10.1016/j.marpolbul.2020.111820
14. Emenike, EC, Okorie, CJ, Ojeyemi, T, Egbemhenghe, A, Iwuozor, KO, Saliu, OD, et al. From oceans to dinner plates: the impact of microplastics on human health. Heliyon. (2023) 9:e20440. doi: 10.1016/j.heliyon.2023.e20440
15. Mahbub, MS, and Shams, M. Acrylic fabrics as a source of microplastics from portable washer and dryer: impact of washing and drying parameters. Sci Total Environ. (2022) 834:155429. doi: 10.1016/j.scitotenv.2022.155429
16. Le, LT, Nguyen, KN, Nguyen, PT, Duong, HC, Bui, XT, Hoang, NB, et al. Microfibers in laundry wastewater: problem and solution. Sci Total Environ. (2022) 852:158412. doi: 10.1016/j.scitotenv.2022.158412
17. Prata, JC. Microplastics in wastewater: state of the knowledge on sources, fate and solutions. Mar Pollut Bull. (2018) 129:262–5. doi: 10.1016/j.marpolbul.2018.02.046
18. Nawalage, NSK, and Bellanthudawa, BKA. Synthetic polymers in personal care and cosmetics products (PCCPs) as a source of microplastic (MP) pollution. Mar Pollut Bull. (2022) 182:113927. doi: 10.1016/j.marpolbul.2022.113927
19. Cortes-Arriagada, D, Miranda-Rojas, S, Camarada, MB, Ortega, DE, and Alarcon-Palacio, VB. The interaction mechanism of polystyrene microplastics with pharmaceuticals and personal care products. Sci Total Environ. (2023) 861:160632. doi: 10.1016/j.scitotenv.2022.160632
20. Lei, K, Qiao, F, Liu, Q, Wei, Z, Qi, H, Cui, S, et al. Microplastics releasing from personal care and cosmetic products in China. Mar Pollut Bull. (2017) 123:122–6. doi: 10.1016/j.marpolbul.2017.09.016
21. Magni, S, Binelli, A, Pittura, L, Avio, CG, Della, TC, Parenti, CC, et al. The fate of microplastics in an Italian wastewater treatment plant. Sci Total Environ. (2019) 652:602–10. doi: 10.1016/j.scitotenv.2018.10.269
22. Azeem, I, Shakoor, N, Chaudhary, S, Adeel, M, Zain, M, Ahmad, MA, et al. Analytical challenges in detecting microplastics and nanoplastics in soil-plant systems. Plant Physiol Biochem. (2023) 204:108132. doi: 10.1016/j.plaphy.2023.108132
23. Gundogdu, S, Cevik, C, Guzel, E, and Kilercioglu, S. Microplastics in municipal wastewater treatment plants in Turkey: a comparison of the influent and secondary effluent concentrations. Environ Monit Assess. (2018) 190:626. doi: 10.1007/s10661-018-7010-y
24. Okoffo, ED, Tscharke, BJ, O’Brien, JW, O’Brien, S, Ribeiro, F, Burrows, SD, et al. Release of plastics to Australian land from biosolids end-use. Environ Sci Technol. (2020) 54:15132–41. doi: 10.1021/acs.est.0c05867
25. Harley-Nyang, D, Memon, FA, Jones, N, and Galloway, T. Investigation and analysis of microplastics in sewage sludge and biosolids: a case study from one wastewater treatment works in the UK. Sci Total Environ. (2022) 823:153735. doi: 10.1016/j.scitotenv.2022.153735
26. Zhang, J, Guo, N, Ding, W, Han, B, Zhao, M, Wang, X, et al. Microplastic pollution and the related ecological risks of organic composts from different raw materials. J Hazard Mater. (2023) 458:131911. doi: 10.1016/j.jhazmat.2023.131911
27. Xu, G, Feng, W, Yang, Y, Xu, Z, Liu, Y, and Li, H. Occurrence, sources, and risks of microplastics in agricultural soils of Weishan Irrigation District in the lower reaches of the Yellow River, China. J Hazard Mater. (2025) 491:137849. doi: 10.1016/j.jhazmat.2025.137849
28. Song, X, Zhuang, W, Cui, H, Liu, M, Gao, T, Li, A, et al. Interactions of microplastics with organic, inorganic and bio-pollutants and the ecotoxicological effects on terrestrial and aquatic organisms. Sci Total Environ. (2022) 838:156068. doi: 10.1016/j.scitotenv.2022.156068
29. Sun, J, Dai, X, Wang, Q, van Loosdrecht, MCM, and Ni, BJ. Microplastics in wastewater treatment plants: detection, occurrence and removal. Water Res. (2019) 152:21–37. doi: 10.1016/j.watres.2018.12.050
30. Kumar, M, Mazumder, P, Silori, R, Manna, S, Panday, DP, Das, N, et al. Prevalence of pharmaceuticals and personal care products, microplastics and co-infecting microbes in the post-COVID-19 era and its implications on antimicrobial resistance and potential endocrine disruptive effects. Sci Total Environ. (2023) 904:166419. doi: 10.1016/j.scitotenv.2023.166419
31. Joo, SH, Liang, Y, Kim, M, Byun, J, and Choi, H. Microplastics with adsorbed contaminants: mechanisms and treatment. Environ Chall (Amst). (2021) 3:100042. doi: 10.1016/j.envc.2021.100042
32. Dong, Y, Gao, M, Song, Z, and Qiu, W. As(III) adsorption onto different-sized polystyrene microplastic particles and its mechanism. Chemosphere. (2020) 239:124792. doi: 10.1016/j.chemosphere.2019.124792
33. Dong, CD, Chen, CW, Chen, YC, Chen, HH, Lee, JS, and Lin, CH. Polystyrene microplastic particles: in vitro pulmonary toxicity assessment. J Hazard Mater. (2020) 385:121575. doi: 10.1016/j.jhazmat.2019.121575
34. Lebordais, M, Gutierrez-Villagomez, JM, Gigault, J, Baudrimont, M, and Langlois, VS. Molecular impacts of dietary exposure to nanoplastics combined with arsenic in Canadian oysters (Crassostrea virginica) and bioaccumulation comparison with Caribbean oysters (Isognomon alatus). Chemosphere. (2021) 277:130331. doi: 10.1016/j.chemosphere.2021.130331
35. Wang, Y, Niu, J, Li, Y, Zheng, T, Xu, Y, and Liu, Y. Performance and mechanisms for removal of perfluorooctanoate (PFOA) from aqueous solution by activated carbon fiber. RSC Adv. (2015) 5:86927–33. doi: 10.1039/C5RA15853B
36. Winiarska, E, Jutel, M, and Zemelka-Wiacek, M. The potential impact of nano- and microplastics on human health: understanding human health risks. Environ Res. (2024) 251:118535. doi: 10.1016/j.envres.2024.118535
37. O’Brien, S, Okoffo, ED, O’Brien, JW, Ribeiro, F, Wang, X, Wright, SL, et al. Airborne emissions of microplastic fibres from domestic laundry dryers. Sci Total Environ. (2020) 747:141175. doi: 10.1016/j.scitotenv.2020.141175
38. Wieland, S, Balmes, A, Bender, J, Kitzinger, J, Meyer, F, Ramsperger, AF, et al. From properties to toxicity: comparing microplastics to other airborne microparticles. J Hazard Mater. (2022) 428:128151. doi: 10.1016/j.jhazmat.2021.128151
39. Evangeliou, N, Grythe, H, Klimont, Z, Heyes, C, Eckhardt, S, Lopez-Aparicio, S, et al. Atmospheric transport is a major pathway of microplastics to remote regions. Nat Commun. (2020) 11:3381. doi: 10.1038/s41467-020-17201-9
40. Kole, PJ, Lohr, AJ, Van Belleghem, F, and Ragas, A. Wear and tear of tyres: a stealthy source of microplastics in the environment. Int J Environ Res Public Health. (2017) 14:1265. doi: 10.3390/ijerph14101265
41. Hadei, M, Mesdaghinia, A, Nabizadeh, R, Mahvi, AH, Rabbani, S, and Naddafi, K. A comprehensive systematic review of photocatalytic degradation of pesticides using nano TiO(2). Environ Sci Pollut R. (2021) 28:13055–71. doi: 10.1007/s11356-021-12576-8
42. Yang, Z, Lu, F, Zhang, H, Wang, W, Shao, L, Ye, J, et al. Is incineration the terminator of plastics and microplastics? J Hazard Mater. (2021) 401:123429. doi: 10.1016/j.jhazmat.2020.123429
43. Yang, S, Cheng, Y, Chen, Z, Liu, T, Yin, L, Pu, Y, et al. In vitro evaluation of nanoplastics using human lung epithelial cells, microarray analysis and co-culture model. Ecotoxicol Environ Saf. (2021) 226:112837. doi: 10.1016/j.ecoenv.2021.112837
44. Gonzalez-Pleiter, M, Edo, C, Aguilera, A, Viudez-Moreiras, D, Pulido-Reyes, G, Gonzalez-Toril, E, et al. Occurrence and transport of microplastics sampled within and above the planetary boundary layer. Sci Total Environ. (2021) 761:143213. doi: 10.1016/j.scitotenv.2020.143213
45. Klein, M, and Fischer, EK. Microplastic abundance in atmospheric deposition within the metropolitan area of Hamburg, Germany. Sci Total Environ. (2019) 685:96–103. doi: 10.1016/j.scitotenv.2019.05.405
46. Dris, R, Gasperi, J, Saad, M, Mirande, C, and Tassin, B. Synthetic fibers in atmospheric fallout: a source of microplastics in the environment? Mar Pollut Bull. (2016) 104:290–3. doi: 10.1016/j.marpolbul.2016.01.006
47. Sait, S, Sorensen, L, Kubowicz, S, Vike-Jonas, K, Gonzalez, SV, Asimakopoulos, AG, et al. Microplastic fibres from synthetic textiles: environmental degradation and additive chemical content. Environ Pollut. (2021) 268:115745. doi: 10.1016/j.envpol.2020.115745
48. Liu, C, Li, J, Zhang, Y, Wang, L, Deng, J, Gao, Y, et al. Widespread distribution of PET and PC microplastics in dust in urban China and their estimated human exposure. Environ Int. (2019) 128:116–24. doi: 10.1016/j.envint.2019.04.024
49. Zhang, Q, Zhao, Y, Du, F, Cai, H, Wang, G, and Shi, H. Microplastic fallout in different indoor environments. Environ Sci Technol. (2020) 54:6530–9. doi: 10.1021/acs.est.0c00087
50. Pedersen, AF, Meyer, DN, Petriv, AV, Soto, AL, Shields, JN, Akemann, C, et al. Nanoplastics impact the zebrafish (Danio rerio) transcriptome: associated developmental and neurobehavioral consequences. Environ Pollut. (2020) 266:115090. doi: 10.1016/j.envpol.2020.115090
51. Li, J, Yang, D, Li, L, Jabeen, K, and Shi, H. Microplastics in commercial bivalves from China. Environ Pollut. (2015) 207:190–5. doi: 10.1016/j.envpol.2015.09.018
52. Rochman, CM, Tahir, A, Williams, SL, Baxa, DV, Lam, R, Miller, JT, et al. Anthropogenic debris in seafood: plastic debris and fibers from textiles in fish and bivalves sold for human consumption. Sci Rep. (2015) 5:14340. doi: 10.1038/srep14340
53. Yang, L, Kang, S, Luo, X, and Wang, Z. Microplastics in drinking water: a review on methods, occurrence, sources, and potential risks assessment. Environ Pollut. (2024) 348:123857. doi: 10.1016/j.envpol.2024.123857
54. Danopoulos, E, Twiddy, M, and Rotchell, JM. Microplastic contamination of drinking water: a systematic review. PLoS One. (2020) 15:e0236838. doi: 10.1371/journal.pone.0236838
55. Mason, SA, Welch, VG, and Neratko, J. Synthetic polymer contamination in bottled water. Front Chem. (2018) 6:407. doi: 10.3389/fchem.2018.00407
56. Schymanski, D, Goldbeck, C, Humpf, HU, and Furst, P. Analysis of microplastics in water by micro-Raman spectroscopy: release of plastic particles from different packaging into mineral water. Water Res. (2018) 129:154–62. doi: 10.1016/j.watres.2017.11.011
57. Yang, T, and Wang, J. Exposure sources and pathways of micro- and nanoplastics in the environment, with emphasis on potential effects in humans: a systematic review. Integr Environ Assess Manag. (2023) 19:1422–32. doi: 10.1002/ieam.4742
58. Walkinshaw, C, Lindeque, PK, Thompson, R, Tolhurst, T, and Cole, M. Microplastics and seafood: lower trophic organisms at highest risk of contamination. Ecotoxicol Environ Saf. (2020) 190:110066. doi: 10.1016/j.ecoenv.2019.110066
59. Kirstein, IV, Hensel, F, Gomiero, A, Iordachescu, L, Vianello, A, Wittgren, HB, et al. Drinking plastics? - quantification and qualification of microplastics in drinking water distribution systems by microFTIR and Py-GCMS. Water Res. (2021) 188:116519. doi: 10.1016/j.watres.2020.116519
60. Catarino, AI, Macchia, V, Sanderson, WG, Thompson, RC, and Henry, TB. Low levels of microplastics (MP) in wild mussels indicate that MP ingestion by humans is minimal compared to exposure via household fibres fallout during a meal. Environ Pollut. (2018) 237:675–84. doi: 10.1016/j.envpol.2018.02.069
61. Li, Y, Peng, L, Fu, J, Dai, X, and Wang, G. A microscopic survey on microplastics in beverages: the case of beer, mineral water and tea. Analyst. (2022) 147:1099–105. doi: 10.1039/D2AN00083K
62. Schneider, M, Stracke, F, Hansen, S, and Schaefer, UF. Nanoparticles and their interactions with the dermal barrier. Dermatoendocrinol. (2009) 1:197–206. doi: 10.4161/derm.1.4.9501
63. Bastyans, S, Jackson, S, and Fejer, G. Micro and nano-plastics, a threat to human health? Emerg Top Life Sci. (2022) 6:411–22. doi: 10.1042/ETLS20220024
64. Subramanian, D, Ponnusamy, MG, and Dharmadurai, D. A systematic review on the impact of micro-nanoplastics on human health: potential modulation of epigenetic mechanisms and identification of biomarkers. Chemosphere. (2024) 363:142986. doi: 10.1016/j.chemosphere.2024.142986
65. Juranek, I, and Bezek, S. Controversy of free radical hypothesis: reactive oxygen species--cause or consequence of tissue injury? Gen Physiol Biophys. (2005) 24:263–78.
66. Lin, S, Zhang, H, Wang, C, Su, XL, Song, Y, Wu, P, et al. Metabolomics reveal nanoplastic-induced mitochondrial damage in human liver and lung cells. Environ Sci Technol. (2022) 56:12483–93. doi: 10.1021/acs.est.2c03980
67. Wu, H, Liu, Q, Yang, N, and Xu, S. Polystyrene-microplastics and DEHP co-exposure induced DNA damage, cell cycle arrest and necroptosis of ovarian granulosa cells in mice by promoting ROS production. Sci Total Environ. (2023) 871:161962. doi: 10.1016/j.scitotenv.2023.161962
68. Li, S, Ma, Y, Ye, S, Tang, S, Liang, N, Liang, Y, et al. Polystyrene microplastics trigger hepatocyte apoptosis and abnormal glycolytic flux via ROS-driven calcium overload. J Hazard Mater. (2021) 417:126025. doi: 10.1016/j.jhazmat.2021.126025
69. Palaniappan, S, Sadacharan, CM, and Rostama, B. Polystyrene and polyethylene microplastics decrease cell viability and dysregulate inflammatory and oxidative stress markers of MDCK and L929 cells in vitro. Expo Health. (2022) 14:75–85. doi: 10.1007/s12403-021-00419-3
70. Hu, M, and Palic, D. Micro- and nano-plastics activation of oxidative and inflammatory adverse outcome pathways. Redox Biol. (2020) 37:101620. doi: 10.1016/j.redox.2020.101620
71. Wang, YL, Lee, YH, Hsu, YH, Chiu, IJ, Huang, CC, Huang, CC, et al. The kidney-related effects of polystyrene microplastics on human kidney proximal tubular epithelial cells HK-2 and male C57BL/6 mice. Environ Health Perspect. (2021) 129:57003. doi: 10.1289/EHP7612
72. Zhang, Y, Yin, K, Wang, D, Wang, Y, Lu, H, Zhao, H, et al. Polystyrene microplastics-induced cardiotoxicity in chickens via the ROS-driven NF-kappaB-NLRP3-GSDMD and AMPK-PGC-1alpha axes. Sci Total Environ. (2022) 840:156727. doi: 10.1016/j.scitotenv.2022.156727
73. Hou, J, Lei, Z, Cui, L, Hou, Y, Yang, L, An, R, et al. Polystyrene microplastics lead to pyroptosis and apoptosis of ovarian granulosa cells via NLRP3/caspase-1 signaling pathway in rats. Ecotoxicol Environ Saf. (2021) 212:112012. doi: 10.1016/j.ecoenv.2021.112012
74. Umamaheswari, S, Priyadarshinee, S, Kadirvelu, K, and Ramesh, M. Polystyrene microplastics induce apoptosis via ROS-mediated p53 signaling pathway in zebrafish. Chem Biol Interact. (2021) 345:109550. doi: 10.1016/j.cbi.2021.109550
75. Kwon, W, Kim, D, Kim, HY, Jeong, SW, Lee, SG, Kim, HC, et al. Microglial phagocytosis of polystyrene microplastics results in immune alteration and apoptosis in vitro and in vivo. Sci Total Environ. (2022) 807:150817. doi: 10.1016/j.scitotenv.2021.150817
76. Dimitriadi, A, Papaefthimiou, C, Genizegkini, E, Sampsonidis, I, Kalogiannis, S, Feidantsis, K, et al. Adverse effects polystyrene microplastics exert on zebrafish heart - molecular to individual level. J Hazard Mater. (2021) 416:125969. doi: 10.1016/j.jhazmat.2021.125969
77. Cobanoglu, H, Belivermis, M, Sikdokur, E, Kilic, O, and Cayir, A. Genotoxic and cytotoxic effects of polyethylene microplastics on human peripheral blood lymphocytes. Chemosphere. (2021) 272:129805. doi: 10.1016/j.chemosphere.2021.129805
78. Shen, R, Yang, K, Cheng, X, Guo, C, Xing, X, Sun, H, et al. Accumulation of polystyrene microplastics induces liver fibrosis by activating cGAS/STING pathway. Environ Pollut. (2022) 300:118986. doi: 10.1016/j.envpol.2022.118986
79. Brandts, I, Canovas, M, Tvarijonaviciute, A, Llorca, M, Vega, A, Farre, M, et al. Nanoplastics are bioaccumulated in fish liver and muscle and cause DNA damage after a chronic exposure. Environ Res. (2022) 212:113433. doi: 10.1016/j.envres.2022.113433
80. Lee, CW, Hsu, LF, Wu, IL, Wang, YL, Chen, WC, Liu, YJ, et al. Exposure to polystyrene microplastics impairs hippocampus-dependent learning and memory in mice. J Hazard Mater. (2022) 430:128431. doi: 10.1016/j.jhazmat.2022.128431
81. Wang, S, Han, Q, Wei, Z, Wang, Y, Xie, J, and Chen, M. Polystyrene microplastics affect learning and memory in mice by inducing oxidative stress and decreasing the level of acetylcholine. Food Chem Toxicol. (2022) 162:112904. doi: 10.1016/j.fct.2022.112904
82. Wang, Q, Li, Y, Chen, Y, Tian, L, Gao, D, Liao, H, et al. Toxic effects of polystyrene nanoplastics and polybrominated diphenyl ethers to zebrafish (Danio rerio). Fish Shellfish Immunol. (2022) 126:21–33. doi: 10.1016/j.fsi.2022.05.025
83. Sarasamma, S, Audira, G, Siregar, P, Malhotra, N, Lai, YH, Liang, ST, et al. Nanoplastics cause neurobehavioral impairments, reproductive and oxidative damages, and biomarker responses in zebrafish: throwing up alarms of wide spread health risk of exposure. Int J Mol Sci. (2020) 21:1410. doi: 10.3390/ijms21041410
84. Liang, B, Huang, Y, Zhong, Y, Li, Z, Ye, R, Wang, B, et al. Brain single-nucleus transcriptomics highlights that polystyrene nanoplastics potentially induce Parkinson’s disease-like neurodegeneration by causing energy metabolism disorders in mice. J Hazard Mater. (2022) 430:128459. doi: 10.1016/j.jhazmat.2022.128459
85. Shan, S, Zhang, Y, Zhao, H, Zeng, T, and Zhao, X. Polystyrene nanoplastics penetrate across the blood-brain barrier and induce activation of microglia in the brain of mice. Chemosphere. (2022) 298:134261. doi: 10.1016/j.chemosphere.2022.134261
86. Jin, H, Yang, C, Jiang, C, Li, L, Pan, M, Li, D, et al. Evaluation of neurotoxicity in BALB/c mice following chronic exposure to polystyrene microplastics. Environ Health Persp. (2022). Erratum) 130:129001. doi: 10.1289/EHP10255
87. Wang, G, Lin, Y, and Shen, H. Exposure to polystyrene microplastics promotes the progression of cognitive impairment in Alzheimer’s disease: association with induction of microglial pyroptosis. Mol Neurobiol. (2024) 61:900–7. doi: 10.1007/s12035-023-03625-z
88. Yang, Y, Xie, E, Du, Z, Peng, Z, Han, Z, Li, L, et al. Detection of various microplastics in patients undergoing cardiac surgery. Environ Sci Technol. (2023) 57:10911–8. doi: 10.1021/acs.est.2c07179
89. Zhu, X, Wang, C, Duan, X, Liang, B, Genbo, XE, and Huang, Z. Micro- and nanoplastics: a new cardiovascular risk factor? Environ Int. (2023) 171:107662. doi: 10.1016/j.envint.2022.107662
90. Zhou, Y, Wu, Q, Li, Y, Feng, Y, Wang, Y, and Cheng, W. Low-dose of polystyrene microplastics induce cardiotoxicity in mice and human-originated cardiac organoids. Environ Int. (2023) 179:108171. doi: 10.1016/j.envint.2023.108171
91. Sun, M, Ding, R, Ma, Y, Sun, Q, Ren, X, Sun, Z, et al. Cardiovascular toxicity assessment of polyethylene nanoplastics on developing zebrafish embryos. Chemosphere. (2021) 282:131124. doi: 10.1016/j.chemosphere.2021.131124
92. Wang, B, Liang, B, Huang, Y, Li, Z, Zhang, B, Du, J, et al. Long-chain acyl carnitines aggravate polystyrene nanoplastics-induced atherosclerosis by upregulating MARCO. Adv Sci. (2023) 10:e2205876. doi: 10.1002/advs.202205876
93. Salim, SY, Kaplan, GG, and Madsen, KL. Air pollution effects on the gut microbiota: a link between exposure and inflammatory disease. Gut Microbes. (2014) 5:215–9. doi: 10.4161/gmic.27251
94. Li, Z, Zhu, S, Liu, Q, Wei, J, Jin, Y, Wang, X, et al. Polystyrene microplastics cause cardiac fibrosis by activating Wnt/beta-catenin signaling pathway and promoting cardiomyocyte apoptosis in rats. Environ Pollut. (2020) 265:115025. doi: 10.1016/j.envpol.2020.115025
95. Li, B, Ding, Y, Cheng, X, Sheng, D, Xu, Z, Rong, Q, et al. Polyethylene microplastics affect the distribution of gut microbiota and inflammation development in mice. Chemosphere. (2020) 244:125492. doi: 10.1016/j.chemosphere.2019.125492
96. Wu, S, Wu, M, Tian, D, Qiu, L, and Li, T. Effects of polystyrene microbeads on cytotoxicity and transcriptomic profiles in human Caco-2 cells. Environ Toxicol. (2020) 35:495–506. doi: 10.1002/tox.22885
97. Busch, M, Kampfer, A, and Schins, R. An inverted in vitro triple culture model of the healthy and inflamed intestine: adverse effects of polyethylene particles. Chemosphere. (2021) 284:131345. doi: 10.1016/j.chemosphere.2021.131345
98. Stock, V, Bohmert, L, Coban, G, Tyra, G, Vollbrecht, ML, Voss, L, et al. Microplastics and nanoplastics: size, surface and dispersant - what causes the effect? Toxicol In Vitro. (2022) 80:105314. doi: 10.1016/j.tiv.2022.105314
99. Huang, D, Zhang, Y, Long, J, Yang, X, Bao, L, Yang, Z, et al. Polystyrene microplastic exposure induces insulin resistance in mice via dysbacteriosis and pro-inflammation. Sci Total Environ. (2022) 838:155937. doi: 10.1016/j.scitotenv.2022.155937
100. Lu, Y, Zhang, Y, Deng, Y, Jiang, W, Zhao, Y, Geng, J, et al. Uptake and accumulation of polystyrene microplastics in zebrafish (Danio rerio) and toxic effects in liver. Environ Sci Technol. (2016) 50:4054–60. doi: 10.1021/acs.est.6b00183
101. Lu, L, Wan, Z, Luo, T, Fu, Z, and Jin, Y. Polystyrene microplastics induce gut microbiota dysbiosis and hepatic lipid metabolism disorder in mice. Sci Total Environ. (2018) 631-632:449–58. doi: 10.1016/j.scitotenv.2018.03.051
102. Liu, S, Wang, Z, Xiang, Q, Wu, B, Lv, W, and Xu, S. A comparative study in healthy and diabetic mice followed the exposure of polystyrene microplastics: differential lipid metabolism and inflammation reaction. Ecotoxicol Environ Saf. (2022) 244:114031. doi: 10.1016/j.ecoenv.2022.114031
103. Prata, JC. Airborne microplastics: consequences to human health? Environ Pollut. (2018) 234:115–26. doi: 10.1016/j.envpol.2017.11.043
104. Lu, K, Lai, KP, Stoeger, T, Ji, S, Lin, Z, Lin, X, et al. Detrimental effects of microplastic exposure on normal and asthmatic pulmonary physiology. J Hazard Mater. (2021) 416:126069. doi: 10.1016/j.jhazmat.2021.126069
105. Fan, Z, Xiao, T, Luo, H, Chen, D, Lu, K, Shi, W, et al. A study on the roles of long non-coding RNA and circular RNA in the pulmonary injuries induced by polystyrene microplastics. Environ Int. (2022) 163:107223. doi: 10.1016/j.envint.2022.107223
106. Goodman, KE, Hare, JT, Khamis, ZI, Hua, T, and Sang, QA. Exposure of human lung cells to polystyrene microplastics significantly retards cell proliferation and triggers morphological changes. Chem Res Toxicol. (2021) 34:1069–81. doi: 10.1021/acs.chemrestox.0c00486
107. Bengalli, R, Zerboni, A, Bonfanti, P, Saibene, M, Mehn, D, Cella, C, et al. Characterization of microparticles derived from waste plastics and their bio-interaction with human lung A549 cells. J Appl Toxicol. (2022) 42:2030–44. doi: 10.1002/jat.4372
108. Zha, H, Xia, J, Li, S, Lv, J, Zhuge, A, Tang, R, et al. Airborne polystyrene microplastics and nanoplastics induce nasal and lung microbial dysbiosis in mice. Chemosphere. (2023) 310:136764. doi: 10.1016/j.chemosphere.2022.136764
109. Tuna, A, Tas, BM, Basaran, KG, Kocak, FM, Sencan, Z, Comert, E, et al. Detection of microplastics in patients with allergic rhinitis. Eur Arch Oto-Rhino-L. (2023) 280:5363–7. doi: 10.1007/s00405-023-08105-7
110. Yang, H, Lai, H, Huang, J, Sun, L, Mennigen, JA, Wang, Q, et al. Polystyrene microplastics decrease F-53B bioaccumulation but induce inflammatory stress in larval zebrafish. Chemosphere. (2020) 255:127040. doi: 10.1016/j.chemosphere.2020.127040
111. Selvam, S, Jesuraja, K, Venkatramanan, S, Roy, PD, and Jeyanthi, KV. Hazardous microplastic characteristics and its role as a vector of heavy metal in groundwater and surface water of coastal South India. J Hazard Mater. (2021) 402:123786. doi: 10.1016/j.jhazmat.2020.123786
112. Kirstein, IV, Kirmizi, S, Wichels, A, Garin-Fernandez, A, Erler, R, Loder, M, et al. Dangerous hitchhikers? Evidence for potentially pathogenic Vibrio spp. on microplastic particles. Mar Environ Res. (2016) 120:1–8. doi: 10.1016/j.marenvres.2016.07.004
113. Amato-Lourenco, LF, de Souza, XCN, Dantas, KC, Dos, SGL, Moralles, FN, Lombardi, S, et al. Airborne microplastics and SARS-CoV-2 in total suspended particles in the area surrounding the largest medical Centre in Latin America. Environ Pollut. (2022) 292:118299. doi: 10.1016/j.envpol.2021.118299
114. Jung, BK, Han, SW, Park, SH, Bae, JS, Choi, J, and Ryu, KY. Neurotoxic potential of polystyrene nanoplastics in primary cells originating from mouse brain. Neurotoxicology. (2020) 81:189–96. doi: 10.1016/j.neuro.2020.10.008
115. Jeong, A, Park, SJ, Lee, EJ, and Kim, KW. Nanoplastics exacerbate Parkinson’s disease symptoms in C. Elegans and human cells. J Hazard Mater. (2024) 465:133289. doi: 10.1016/j.jhazmat.2023.133289
116. Wei, J, Wang, X, Liu, Q, Zhou, N, Zhu, S, Li, Z, et al. The impact of polystyrene microplastics on cardiomyocytes pyroptosis through NLRP3/Caspase-1 signaling pathway and oxidative stress in Wistar rats. Environ Toxicol. (2021) 36:935–44. doi: 10.1002/tox.23095
117. Vlacil, AK, Banfer, S, Jacob, R, Trippel, N, Kuzu, I, Schieffer, B, et al. Polystyrene microplastic particles induce endothelial activation. PLoS One. (2021) 16:e0260181. doi: 10.1371/journal.pone.0260181
118. Florance, I, Chandrasekaran, N, Gopinath, PM, and Mukherjee, A. Exposure to polystyrene nanoplastics impairs lipid metabolism in human and murine macrophages in vitro. Ecotoxicol Environ Saf. (2022) 238:113612. doi: 10.1016/j.ecoenv.2022.113612
119. Lim, D, Jeong, J, Song, KS, Sung, JH, Oh, SM, and Choi, J. Inhalation toxicity of polystyrene micro(nano)plastics using modified OECD TG 412. Chemosphere. (2021) 262:128330. doi: 10.1016/j.chemosphere.2020.128330
120. Wu, Y, Yao, Y, Bai, H, Shimizu, K, Li, R, and Zhang, C. Investigation of pulmonary toxicity evaluation on mice exposed to polystyrene nanoplastics: the potential protective role of the antioxidant N-acetylcysteine. Sci Total Environ. (2023) 855:158851. doi: 10.1016/j.scitotenv.2022.158851
Keywords: microplastics, human health, cellular toxicity, environmental pollutants, systemic health risk
Citation: Zhang X, Yu C, Wang P and Yang C (2025) Microplastics and human health: unraveling the toxicological pathways and implications for public health. Front. Public Health. 13:1567200. doi: 10.3389/fpubh.2025.1567200
Edited by:
Lai Xuefeng, Huazhong University of Science and Technology, ChinaReviewed by:
Niti B. Jadeja, University of Virginia, United StatesOrazio Valerio Giannico, Local Health Authority of Taranto, Italy
Copyright © 2025 Zhang, Yu, Wang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xu Zhang, MTg5ODAxMTA0NzlAMTYzLmNvbQ==; Chunping Yang, NTI0Nzg5MEBxcS5jb20=
†These authors have contributed equally to this work
 Xu Zhang
Xu Zhang Chunhong Yu2†
Chunhong Yu2†