- 1State Key Laboratory of Oral Diseases and National Clinical Research Center for Oral Diseases, Department of Oral and Maxillofacial Surgery, West China Hospital of Stomatology, Sichuan University, Chengdu, Sichuan, China
- 2State Key Laboratory of Oral Diseases and National Clinical Research Center for Oral Diseases and Eastern Clinic, West China Hospital of Stomatology, Sichuan University, Chengdu, Sichuan, China
Cleft lip and palate (CLP) is a prevalent congenital anomaly of the maxillofacial region, characterized by abnormal openings in the lip or palate. This condition, affecting approximately 1 in 700 newborns globally, can manifest as cleft lip only, cleft palate only, or both. The etiology of CLP remains multifactorial, involving genetic and environmental influences, with maternal systemic diseases during pregnancy emerging as significant risk factors. Conditions such as circulatory disorders, endocrine and metabolic disorders, infectious diseases, and autoimmune diseases have been associated with increased CLP incidence. These maternal health issues can disrupt normal embryonic development, leading to cleft formation and affecting the child’s overall wellbeing, including feeding, speech, dental health, and psychological state. This review explores the relationship between maternal systemic diseases, including circulatory, endocrine and metabolic, infectious, and autoimmune disorders, and the occurrence of CLP in newborns. Understanding these connections is crucial for improving maternal health during pregnancy and reducing the risk of CLP, highlighting the importance of early monitoring and intervention.
1 Introduction
CLP is a common congenital defect of the maxillofacial region and is the most common craniofacial malformation in humans. The global average incidence rate among newborns is 1/700 (1). It is characterized by an abnormal opening or gap in the lip or the upper palate. Depending on the area involved, it can be classified into three types: cleft lip only, cleft palate only, and CLP (1). CLP can occur independently, which is more common and referred to as non-syndromic CLP (2). It can also occur in conjunction with other congenital diseases, known as syndromic CLP, with congenital heart disease being the most common associated condition (3). From an embryonic development perspective, during the sixth week of normal embryonic development, the upper lip forms as the globular process fuses with the maxillary processes on both sides (4). In this process, the globular process grows toward the oral cavity, forming the primary palate, and by the end of the seventh week, the lateral palatine processes, which develop from the maxillary processes, fuse to form the upper palate (4, 5). Any disruption in the fusion process of the globular process and the palate during embryonic development can lead to the occurrence of cleft lip, cleft palate, or CLP (6). CLP not only affect aesthetics and oral function but also result in related complications such as feeding difficulties, speech problems, dental defects, malocclusion, abnormal facial growth, and middle ear infections (7). These issues can lead to lifelong psychosocial problems, significantly impacting the mental health of the affected child (8).
The exact cause of CLP is not fully understood. It is currently believed to result from a combination of genetic and environmental factors (9). Genetic mutations or variations, along with parental lifestyle and living environment, such as excessive alcohol consumption, smoking, inadequate intake of vitamins or minerals during pregnancy, and the use of certain medications during pregnancy (such as painkillers, antibiotics, and antihypertensive drugs), can all increase the likelihood of a newborn developing CLP (10). Additionally, factors such as parental consanguinity, educational level, and health status may also be associated with the occurrence of CLP in children (1, 9, 11–14).
In recent years, an increasing number of studies have begun focusing on the relationship between maternal health conditions and the occurrence of CLP in newborns. Maternal systemic diseases may negatively impact pregnancy outcomes. Conditions such as cardiovascular diseases (e.g., hypertension, atherosclerosis), diabetes, obesity, and autoimmune diseases (e.g., lupus) have been shown to increase the risk of complications during pregnancy (15–20). Metabolic disorders during pregnancy can also raise the risk of adverse outcomes and significantly affect fetal development, including the formation and fusion of oral and facial structures (21, 22). Therefore, maternal systemic diseases during fetal development may be closely related to the occurrence of CLP in newborns, increasing the risk of this congenital condition (16, 23, 24). This review will explore the relationship between maternal systemic diseases and the occurrence of congenital CLP in children, focusing on conditions such as circulatory system diseases, endocrine and metabolic disorders, infectious diseases, and autoimmune diseases (Table 1). The aim is to review and synthesize available evidence linking maternal systemic diseases with CLP in newborns, to highlight areas where further basic and clinical research are needed.
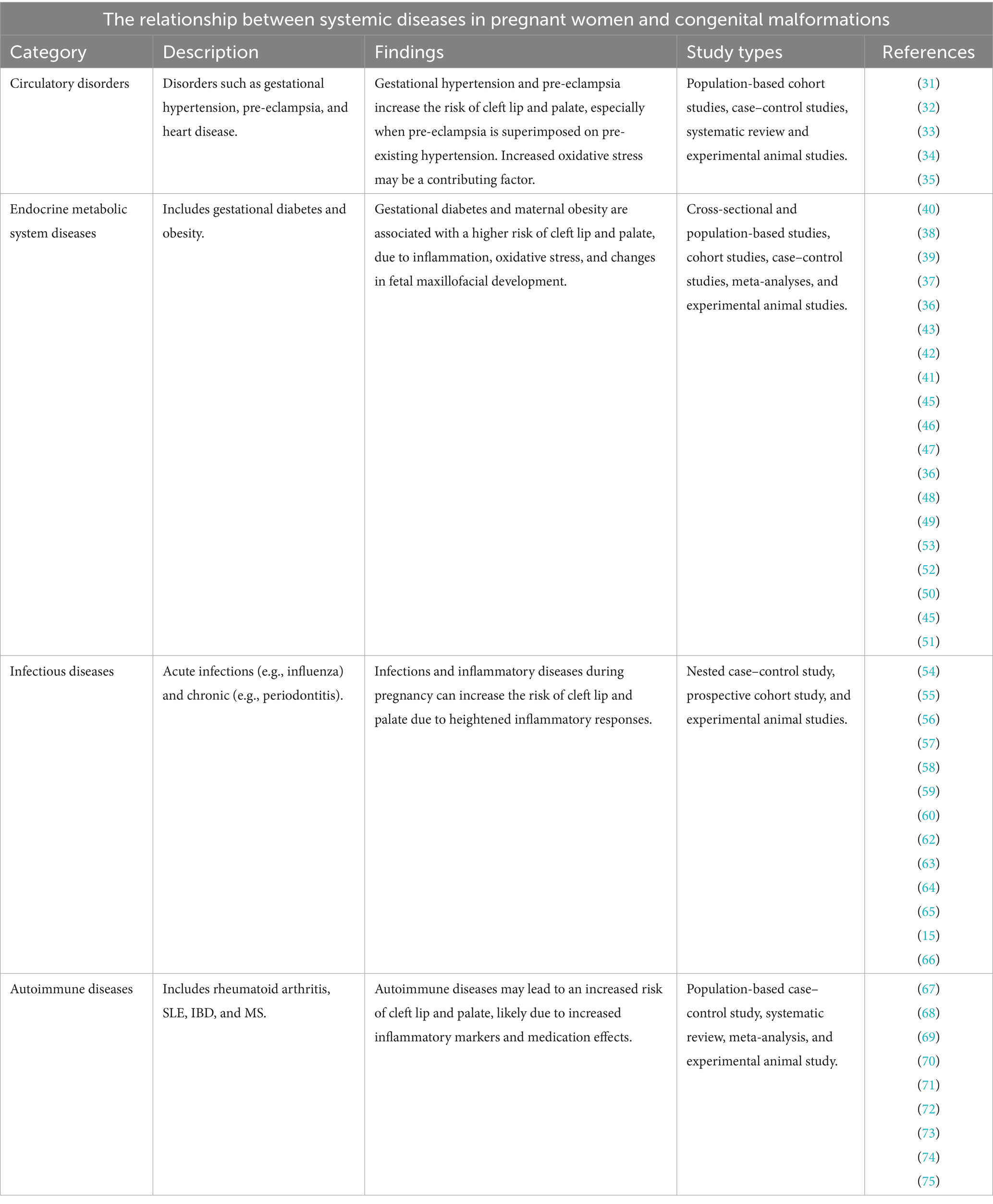
Table 1. The relationship between systemic diseases in pregnant women and congenital malformations, and relationship between drug exposure and congenital malformations.
2 Methods
2.1 Search strategy
This study was conducted as a narrative review of the literature focusing on the association between maternal systemic diseases and orofacial clefts in newborns. Relevant articles were identified by searching PubMed, Web of Science, and Embase databases for studies published in English from 2000 to 2024.
Search strategy utilizes the following key terms: Maternal Condition Terms: Pregnancy-related: “pregnant women, ““maternal, ““pregnancy complications”; Circulatory: “hypertension, ““preeclampsia, ““hypertensive disorders of pregnancy”; Metabolic: “diabetes mellitus, ““gestational diabetes, ““obesity, ““maternal obesity, ““metabolic syndrome”; Infectious: “infection, ““viral infection, ““bacterial infection, ““periodontal disease, ““periodontitis, ““fever”; Autoimmune: “autoimmune disease, ““lupus, ““systemic lupus erythematosus, ““inflammatory bowel disease”; Medication exposure: “antiepileptic drugs, ““anticonvulsants, ““beta-blockers,” “angiotensin converting enzyme inhibitors,” “antibiotics,” “corticosteroids.” Outcome Terms: “cleft lip,” “cleft palate,” “orofacial cleft,” “cleft,” “congenital anomaly,” “congenital malformation,” “birth defect.”
Titles and abstracts identified through the search were independently screened by two reviewers for relevance. Full-text articles were then assessed by eligibility criteria. Disagreements regarding article inclusion were resolved by discussion or consultation with a third reviewer.
2.2 Eligibility criteria
We specifically included case–control studies, prospective and retrospective cohort studies, comprehensive cross-sectional analyses, and methodologically sound meta-analyses that provided quantitative assessments of these relationships.
We excluded studies focused exclusively on genetic factors without consideration of maternal systemic conditions. Similarly, animal studies lacking human translational components were omitted from the primary analysis. Case reports and small case series with insufficient statistical power were also excluded from our main analysis, though they were occasionally referenced to provide mechanistic insights or to highlight potential pathophysiological pathways warranting further investigation.
3 Systemic diseases and their impact
Systemic diseases encompass a broad spectrum of disorders that affect multiple organs and body systems simultaneously. These conditions can be classified into several major categories, including circulatory disorders, endocrine and metabolic disorders, infectious diseases, and autoimmune diseases. Research has shown that inflammation plays a fundamental role in the etiology of these diseases across the life span (25). The inflammatory response to cellular injury or pathogen-associated signals—particularly involving damage-associated molecular patterns (DAMPs) such as high-mobility group box 1 (HMGB1), S100 proteins, heat shock proteins (HSPs), and nucleic acids—serves as both an initiating factor and a perpetuating mechanism in disease progression (26, 27). Immune system dysregulation represents a central feature in systemic diseases, manifesting through various mechanisms, including altered immune cell function and inflammatory mediator production (28). This dysregulation can lead to a state of chronic systemic inflammation, which has been linked to multiple pathological conditions and organ dysfunction (29, 30).
3.1 Circulatory disorders
Circulatory disorders during pregnancy encompass heart disease, gestational hypertension, and pre-eclampsia (31). Although heart disease in pregnancy is considered a circulatory disorder, current evidence does not support a direct association between maternal heart disease and non-syndromic CLP. But it has been reported that hypertension in pregnancy and pre-eclampsia are associated with the development of non-syndromic CLP (32). A cohort study comprising 2.49 million newborns demonstrated that gestational hypertension and pre-eclampsia elevated the likelihood of non-syndromic CLP in newborns (33). Furthermore, the risk of having a child with non-syndromic CLP in a woman with pre-eclampsia superimposed on pre-existing hypertension was more than twice as high as the risk in a woman without hypertension (33). A further cross-sectional study, based on data from 29 countries, demonstrated that chronic maternal hypertension was associated with an increase in the prevalence of CLP of more than four-fold, with an eight-fold increase observed in cases where the mother had pre-eclampsia (32). The levels of circulating oxidative stress markers, such as malondialdehyde (MDA) and superoxide dismutase, were significantly elevated in mothers with gestational hypertension or pre-eclampsia compared to those with a normal pregnancy (34). Furthermore, MDA levels correlated with the severity of pre-eclampsia, suggesting that gestational hypertension and pre-eclampsia place mothers in a state of oxidative stress imbalance (35); this imbalance may be a probable cause of CLP in the fetus.
3.2 Endocrine and metabolic disorders
Disorders of the endocrine and metabolic system that predispose mothers during pregnancy include gestational diabetes and gestational obesity (36–40). Gestational diabetes mellitus (GDM), defined as abnormal glucose tolerance initiated or first detected during pregnancy, has been demonstrated to significantly affect fetal development, with an increased risk of developing CLP (37–40). The hyperglycemic environment of pregnancy in diabetic rats has been demonstrated to result in alterations in inositol and prostaglandin metabolism and increased levels of reactive oxygen species (ROS), which in turn affects the development of neural crest-derived organs, leading to a variety of craniofacial malformations, including CLP (41–44).
In addition, GDM has been shown to induce a chronic inflammatory state in the mother (45). Women with GDM have been observed to exhibit elevated levels of several blood inflammatory markers, including the neutrophil-to-lymphocyte ratio (NLR), the platelet-to-lymphocyte ratio (PLR), the white blood cell (WBC) count, and the neutrophil count, in comparison to healthy pregnant women (46). Plasma protein profiling has revealed the presence of a heightened abundance of pro-inflammatory proteins, which include pigment epithelium-derived factor (PEDF), proteoglycan (PRG4), and fibronectin 1 (FN1), in the plasma of women prior to the diagnosis of gestational diabetes, suggesting that the inflammatory state was present long before the diagnosis of diabetes mellitus (47). It may affect the maxillofacial development of the fetus from early pregnancy onwards.
Maternal obesity in the early stages of pregnancy is positively correlated with the risk of CLP in the offspring (36), and the incidence of CLP is positively correlated with maternal obesity (37). Maternal obesity is associated with chronic metabolic inflammation (48); maternal obesity prior to pregnancy results in the accumulation of macrophages in the placenta (49), which in turn leads to an increase in the levels of reactive oxygen species and pro-inflammatory cytokines in maternal plasma, including IL-8, IL-6, CRP, TNF-α and IFN-γ (45, 50–53), which in turn affects fetal maxillofacial development, leading to the development of CLP.
3.3 Infectious diseases
Common infectious diseases in pregnant mothers include a variety of acute infections and chronic inflammatory diseases (54). Acute infections such as influenza, acute bronchitis, and urinary tract infections have been shown to be associated with the risk of developing CLP (55). These acute infections typically present with sudden onset of symptoms and can cause maternal fever, which is particularly concerning during early pregnancy. Research has shown that maternal fever above 38.5°C during the first trimester significantly increases the risk of orofacial clefts (56). Studies showed that untreated maternal fever was associated with a higher risk of oral clefts, particularly non-isolated clefts, although the risk did not significantly differ by fever severity (57). These acute infections can affect fetal development through various mechanisms, including direct pathogen effects and maternal inflammatory responses (58).
Chronic maternal infections have been identified as significant risk factors for orofacial clefts. Common chronic infections during pregnancy include periodontitis, chronic cytomegalovirus (CMV) and hepatitis B virus (HBV) infection. Among these, periodontitis is characterized by periodontal tissue inflammation caused by gram-negative, anaerobic bacteria in the subgingival region (59). These pathogenic microorganisms trigger systemic inflammatory responses, elevating pro-inflammatory cytokines including IL-1, TNF-α, IL-6, and prostaglandin E2 (60–62). Similarly, maternal CMV infection has been associated with increased inflammatory markers and adverse fetal outcomes (63). Chronic HBV infection during pregnancy can lead to persistent inflammation and elevated cytokine levels (64). These chronic infections share common pathogenic mechanisms whereby inflammatory mediators can enter systemic circulation, potentially crossing the placental barrier and interfering with normal craniofacial development during the critical embryonic period (15, 65, 66).
3.4 Autoimmune diseases
Autoimmune diseases are characterized by a female predominance and first manifest during the reproductive phase (67). Autoimmune diseases such as rheumatoid arthritis, systemic lupus erythematosus (SLE), inflammatory bowel disease (IBD), and multiple sclerosis (MS) are common autoimmune diseases during pregnancy, and their prevalence has increased in recent years (68, 69). Changes in physiology and hormone levels associated with pregnancy can lead to SLE flares and an increased inflammatory response in the body (70). Mothers with SLE have elevated serum levels of the pro-inflammatory cytokine IL-10, accompanied by elevated levels of several chemokines (71). Although it has been reported that mothers with SLE have a higher risk of giving birth to a child with CLP, the reason for this is unclear and may be related to the use of glucocorticoids (72).
IBD, comprising Crohn’s disease and ulcerative colitis, is a chronic immune-mediated disorder frequently diagnosed during reproductive years (73). The disease is characterized by dysregulated immune responses and elevated pro-inflammatory cytokines, particularly TNF-α, IL-1β, and IL-6, which contribute to persistent intestinal inflammation (74). Studies have shown that patients with active IBD have a significantly higher risk of adverse pregnancy outcomes, including preterm birth, spontaneous abortion, and infants small for gestational age (75). However, the specific relationship between maternal IBD and the development of CLP requires further investigation, as current evidence is limited, and mechanisms remain unclear.
3.5 Intrauterine exposure to drugs
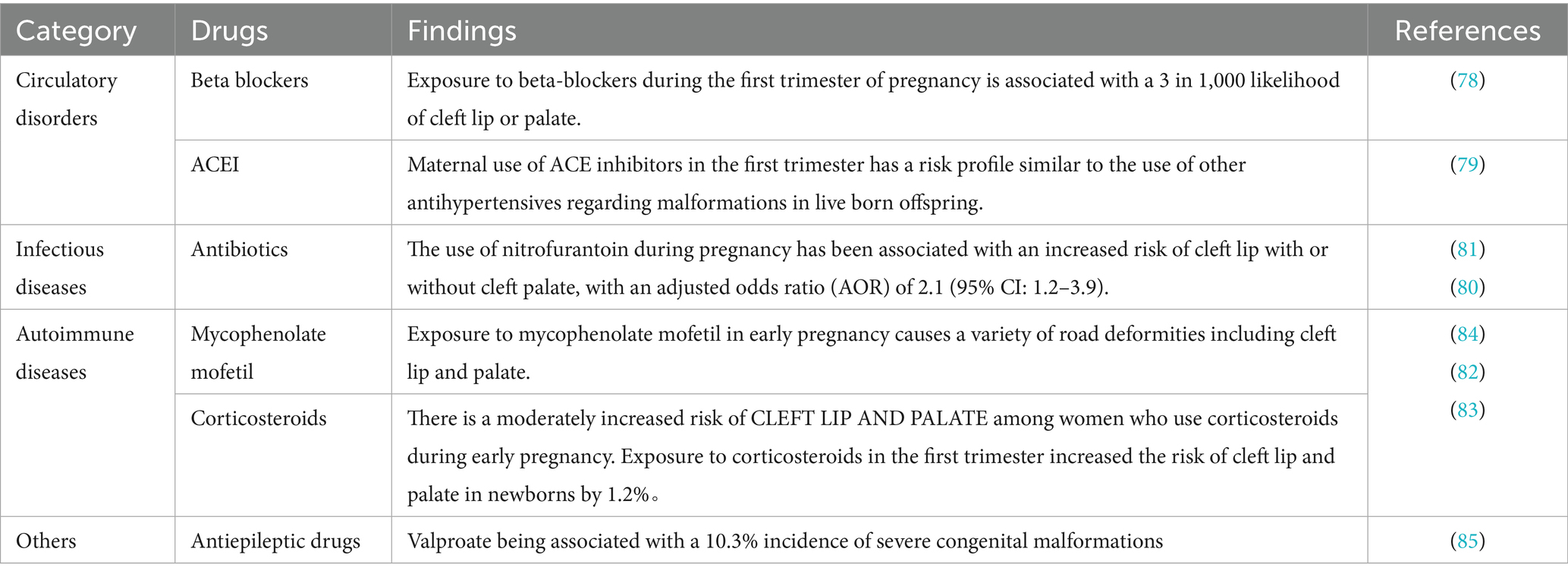
During pregnancy, women with systemic diseases often need medication to manage their conditions and prevent acute flare-ups. It is important to recognize that the potential teratogenic effects of these drugs may also contribute to the overall risk of congenital anomalies in the offspring. Despite the presence of the placental barrier, nearly all drugs taken by pregnant women can enter the fetal circulation to some extent through passive diffusion (76). Some medications used to treat systemic illnesses are linked to an increased risk of birth defects such as CLP (77). For example, beta blockers and angiotensin converting enzyme inhibitors (ACEI) for hypertension (78, 79), antibiotics for infections (80, 81), and immunosuppressant drugs for autoimmune disorders have been associated with these outcomes (82–84). Antiepileptic drugs pose a high risk of birth defects, with valproate being associated with a 10.3% incidence of severe congenital malformations (85) (Table 2). Therefore, it is crucial to carefully weigh the benefits of controlling systemic conditions during pregnancy against the potential risks these medications pose to the fetus. Given that many drugs have dose-dependent teratogenic effects (86), it is advisable to use the lowest effective doses when treating systemic diseases in pregnant women.
4 Conclusion
CLP are relatively common birth defects, with varying prevalence rates observed in different populations (1). The impact of CLP on an individual may vary depending on the severity and extent of the condition, affecting the overall quality of life and emotional wellbeing of the affected child, as well as their appearance, feeding, articulation, dentition, hearing, and psychosocial wellbeing. The treatment of CLP requires a multidisciplinary approach, involving a sequence of timely and age-appropriate interventions. These include surgeries for the lip and palate, followed by postoperative orthodontics, orthognathic surgery, and essential psychological support to ensure the patient’s overall physiological and psychological wellbeing (9). Nevertheless, further investigation is required to elucidate the impact of maternal health on the occurrence of CLP.
This review aims to introduce the relationship between maternal circulatory, endocrine and metabolic, infectious, and autoimmune diseases during pregnancy and the development of CLP in newborns (Figure 1). The presence of the disease before or during mid-pregnancy may elevate the risk of fetal birth defects (87). Large-scale cohort studies have shown that gestational hypertension and pre-eclampsia notably increase the risk of non-syndromic CLP, possibly through oxidative stress and impaired placental function, highlighting the importance of early detection and blood pressure management in clinical practice. In terms of metabolic factors, conditions such as gestational diabetes mellitus and maternal obesity contribute to CLP risk by inducing chronic inflammatory processes and disrupting critical signaling pathways in neural crest development; recent research points to a continuum of risk that scales with the severity of these metabolic disturbances. For infectious diseases, the presence of acute maternal fever or persistent infections—such as influenza, hepatitis B, or periodontitis—during early pregnancy significantly raises the likelihood of CLP, likely via direct teratogenicity and heightened systemic inflammation, though more mechanistic studies and randomized trials are needed. The influence of autoimmune diseases (like SLE and IBD) is increasingly recognized, with both disease activity and treatment playing roles in mediating risk, yet the distinction between drug effect and underlying immune dysfunction requires further study. Medication exposures, especially certain antihypertensives and antiepileptics, also warrant individualized risk–benefit analysis, as both drug type and timing can influence CLP occurrence. Collectively, these findings underscore the necessity of comprehensive, condition-specific research to clarify the molecular mechanisms and gene–environment interactions underlying each systemic disease. Additionally, many other factors increase the risk of a newborn having a CLP, such as high maternal exposure to alcohol during pregnancy, smoking, stress during pregnancy, and the use of assisted reproduction techniques, all of which are significantly associated with the birth of a child with a CLP (37, 88–90).
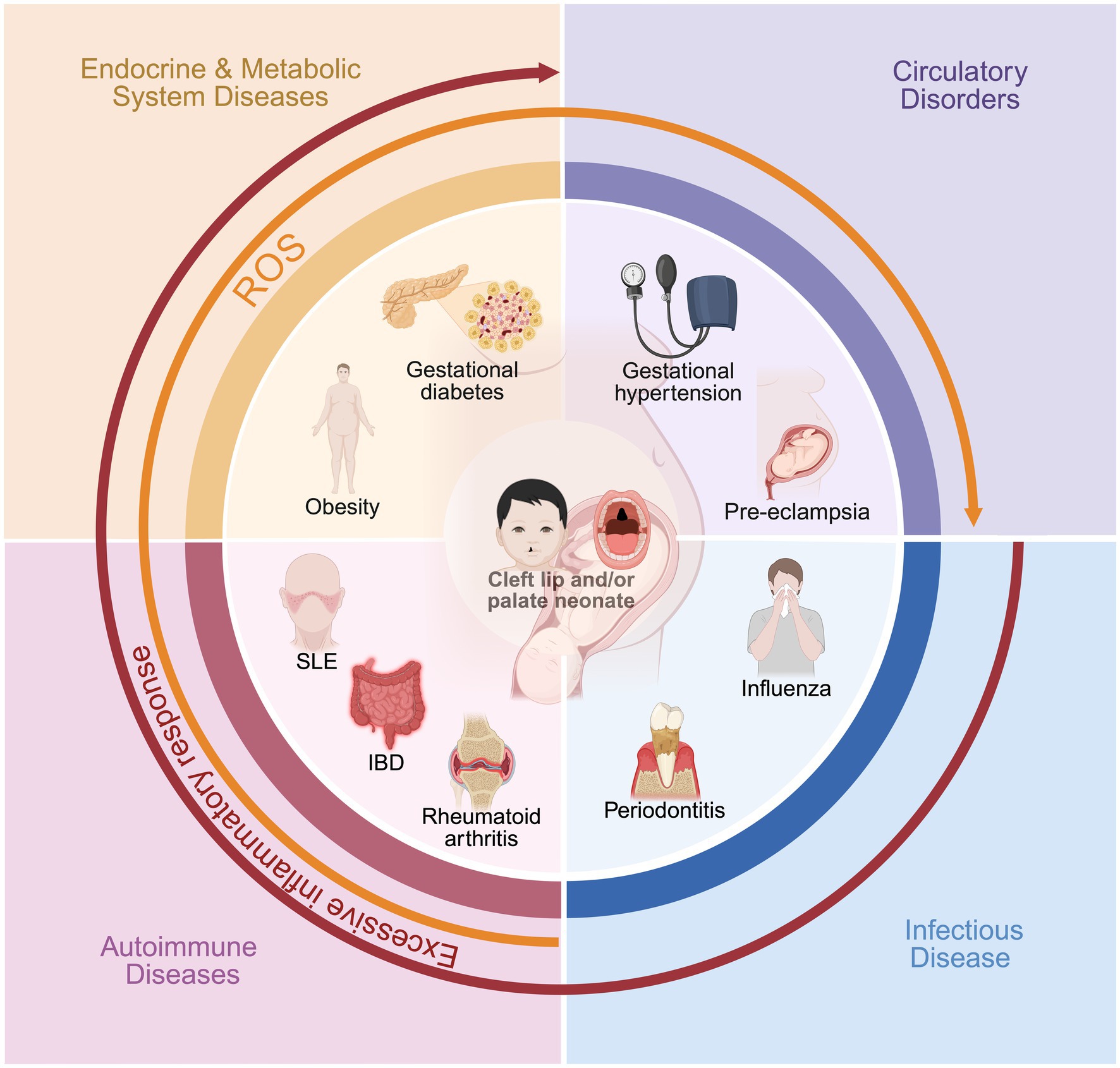
Figure 1. The impact of maternal systemic diseases on the occurrence of cleft lip and palate in newborns.
By comprehending the detrimental effects of CLP and employing preventative measures, it is conceivable that the prevalence of this condition could be diminished, consequently enhancing the overall quality of life for those affected (91, 92). Furthermore, routine antenatal assessments and the prompt identification of potential issues can facilitate the management and planning of CLP treatment (93). Therefore, it is recommended to emphasize the need for early pregnancy follow-up in women with systemic diseases, receive appropriate medical care and close monitoring during pregnancy to control identified risk factors and receive appropriate protective measures (92), in order to reduce the chances of neonates developing CLP occurrence and to ensure the best possible pregnancy outcome for both mother and baby.
Author contributions
HS: Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. MD: Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. JC: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. RY: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. BS: Conceptualization, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing. HH: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. YW: Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (82301148), China Postdoctoral Science Foundation (2024T170605), Sichuan Province Science and Technology Support Program (2025ZNSFSC0758), Sichuan Postdoctoral Science Foundation (TB2022005), Health Commission of Sichuan Province Medical Science and Technology Program (24QNMP060), and Research Funding from West China School/Hospital of Stomatology Sichuan University (RCDWJS2024-7).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Nasreddine, G, El Hajj, J, and Ghassibe-Sabbagh, M. Orofacial clefts embryology, classification, epidemiology, and genetics. Mut Res-Rev Mutation Res. (2021) 787:108373. doi: 10.1016/j.mrrev.2021.108373
2. Martinelli, M, Palmieri, A, Carinci, F, and Scapoli, L. Non-syndromic cleft palate: an overview on human genetic and environmental risk factors. Front Cell Dev Biol. (2020) 8:592271. doi: 10.3389/fcell.2020.592271
3. Azadgoli, B, Munabi, NCO, Fahradyan, A, Auslander, A, Mccullough, M, Aflatooni, N, et al. Congenital heart disease in patients with cleft lip/palate and its impact on cleft management. Cleft Palate Craniofac J. (2020) 57:957–66. doi: 10.1177/1055665620924915
4. Hammond, NL, and Dixon, MJ. Revisiting the embryogenesis of lip and palate development. Oral Dis. (2022) 28:1306–26. doi: 10.1111/odi.14174
5. Deshpande, AS, and Goudy, SL. Cellular and molecular mechanisms of cleft palate development. Laryngoscope Inv Otolaryngol. (2019) 4:160–4. doi: 10.1002/lio2.214
6. Dixon, MJ, Marazita, ML, Beaty, TH, and Murray, JC. Cleft lip and palate: understanding genetic and environmental influences. Nat Rev Genet. (2011) 12:167–78. doi: 10.1038/nrg2933
7. Zhao, Y-J, Xiong, Y-X, and Wang, Y. Three-dimensional accuracy of facial scan for facial deformities in clinics: a new evaluation method for facial scanner accuracy. PLoS One. (2017) 12:9402. doi: 10.1371/journal.pone.0169402
8. Ardouin, K, Hare, J, and Stock, NM. Emotional well-being in adults born with cleft lip and/or palate: a whole of life survey in the United Kingdom. Cleft Palate Craniofac J. (2020) 57:877–85. doi: 10.1177/1055665619896681
9. Vyas, T, Gupta, P, Kumar, S, Gupta, R, Gupta, T, and Singh, HP. Cleft of lip and palate: a review. J Family Med Prim Care. (2020) 9:2621–5. doi: 10.4103/jfmpc.jfmpc_472_20
10. Kulesa-Mrowiecka, M, Lipowicz, A, Marszałek-Kruk, BA, Kania, D, Wolański, W, Myśliwiec, A, et al. Characteristics of factors influencing the occurrence of cleft lip and/or palate: a case analysis and literature review. Children. (2024) 11:399. doi: 10.3390/children11040399
11. Saleem, K, Zaib, T, Sun, W, and Fu, S. Assessment of candidate genes and genetic heterogeneity in human non syndromic orofacial clefts specifically non syndromic cleft lip with or without palate. Heliyon. (2019) 5:e03019. doi: 10.1016/j.heliyon.2019.e03019
12. Silva, CM, Pereira, MCM, Queiroz, TB, and Neves, LTD. Can parental consanguinity be a risk factor for the occurrence of nonsyndromic oral cleft? Early Hum Dev. (2019) 135:23–6. doi: 10.1016/j.earlhumdev.2019.06.005
13. Slah-Ud-Din, S, Ali, K, Mahd, SM, Nisar, S, and Nisar, O. Factors associated with an increased risk of facial malformations. Cureus. (2023) 15:e41641. doi: 10.7759/cureus.41641
14. Vital Da Silva, HP, De Medeiros Oliveira, GH, Galvao Ururahy, MA, Bezerra, JF, Costa De Souza, KS, Bortolin, RH, et al. Application of high-resolution array platform for genome-wide copy number variation analysis in patients with nonsyndromic cleft lip and palate. J Clin Lab Anal. (2018) 32:e22428
15. Bánhidy, F, Acs, N, Puhó, EH, and Czeizel, AE. A possible association of periodontal infectious diseases in pregnant women with isolated orofacial clefts in their children: a population-based case-control study. Birth Defects Res A Clin Mol Teratol. (2010) 88:466–73. doi: 10.1002/bdra.20664
16. García-Ríos, P, Pecci-Lloret, MP, and Oñate-Sánchez, RE. Oral manifestations of systemic lupus erythematosus: a systematic review. Int J Environ Res Public Health. (2022) 19:910. doi: 10.3390/ijerph191911910
17. Helle, E, and Priest, JR. Maternal obesity and diabetes mellitus as risk factors for congenital heart disease in the offspring. J Am Heart Assoc. (2020) 9:e011541. doi: 10.1161/JAHA.119.011541
18. Langley-Evans, SC, Pearce, J, and Ellis, S. Overweight, obesity and excessive weight gain in pregnancy as risk factors for adverse pregnancy outcomes: a narrative review. J Hum Nutr Diet. (2022) 35:250–64. doi: 10.1111/jhn.12999
19. Lawesson, SS, Swahn, E, Pihlsgård, M, Andersson, T, Angerås, O, Brolin, EB, et al. Association between history of adverse pregnancy outcomes and coronary artery disease assessed by coronary computed tomography angiography. JAMA. (2023) 329:393–404. doi: 10.1001/jama.2022.24093
20. Ye, WR, Luo, C, Huang, J, Li, CL, Liu, ZX, and Liu, FK. Gestational diabetes mellitus and adverse pregnancy outcomes: systematic review and meta-analysis. BMJ. (2022) 377:946. doi: 10.1136/bmj-2021-067946
21. Malaza, N, Masete, M, Adam, S, Dias, S, Nyawo, T, and Pheiffer, C. A systematic review to compare adverse pregnancy outcomes in women with Pregestational diabetes and gestational diabetes. Int J Environ Res Public Health. (2022) 19:846. doi: 10.3390/ijerph191710846
22. Purohit, AM, Oyeka, CP, Khan, SS, Toscano, M, Nayak, S, Lawson, SM, et al. Preventing adverse cardiovascular outcomes in pregnancy complicated by obesity. Curr Obstet Gynecol Rep. (2023) 12:129–37. doi: 10.1007/s13669-023-00356-9
23. Ács, L, Bányai, D, Nemes, B, Nagy, K, Ács, N, Bánhidy, F, et al. Maternal-related factors in the origin of isolated cleft palate-a population-based case-control study. Orthod Craniofac Res. (2020) 23:174–80. doi: 10.1111/ocr.12361
24. Regina Altoé, S, Borges, ÁH, Neves, A, Aranha, AMF, Borba, AM, Espinosa, MM, et al. Influence of parental exposure to risk factors in the occurrence of Oral clefts. J Dent. (2020) 21:119–26. doi: 10.30476/DENTJODS.2019.77620.0
25. Huang, X, He, D, Pan, Z, Luo, G, and Deng, J. Reactive-oxygen-species-scavenging nanomaterials for resolving inflammation. Mater Today Bio. (2021) 11:100124. doi: 10.1016/j.mtbio.2021.100124
26. Guan, G, Polonowita, A, Sun, Q, and Mei, L. Immune-mediated conditions and cellular biomarkers for early diagnosis of oral diseases. Nano Trans Med. (2023) 2:100001. doi: 10.1016/j.ntm.2023.100001
27. Roh, JS, and Sohn, DH. Damage-associated molecular patterns in inflammatory diseases. Immune Netw. (2018) 18:e27. doi: 10.4110/in.2018.18.e27
28. Wigerblad, G, and Kaplan, MJ. Neutrophil extracellular traps in systemic autoimmune and autoinflammatory diseases. Nat Rev Immunol. (2023) 23:274–88. doi: 10.1038/s41577-022-00787-0
29. Chen, Y, Li, Y, Liu, M, Xu, W, Tong, S, and Liu, K. Association between systemic immunity-inflammation index and hypertension in us adults from Nhanes 1999–2018. Sci Rep. (2024) 14:5677. doi: 10.1038/s41598-024-56387-6
30. Hu, W, Wang, Z-M, Feng, Y, Schizas, M, Hoyos, BE, Van Der Veeken, J, et al. Regulatory T cells function in established systemic inflammation and reverse fatal autoimmunity. Nat Immunol. (2021) 22:1163–74. doi: 10.1038/s41590-021-01001-4
31. Wedlund, F, Von Wowern, E, and Hlebowicz, J. Increased cesarean section rate and premature birth according to modified who maternal cardiovascular risk in pregnant women with congenital heart disease. PLoS One. (2023) 18:e0294323. doi: 10.1371/journal.pone.0294323
32. Bellizzi, S, Ali, MM, Abalos, E, Betran, AP, Kapila, J, Pileggi-Castro, C, et al. Are hypertensive disorders in pregnancy associated with congenital malformations in offspring? Evidence from the who multicountry cross sectional survey on maternal and newborn health. BMC Pregnancy Childbirth. (2016) 16:198. doi: 10.1186/s12884-016-0987-8
33. Weber, KA, Mayo, JA, Carmichael, SL, Stevenson, DK, Winn, VD, and Shaw, GM. Occurrence of selected structural birth defects among women with preeclampsia and other hypertensive disorders. Am J Epidemiol. (2018) 187:668–76. doi: 10.1093/aje/kwx269
34. Ibrahim, A, Khoo, MI, Ismail, EHE, Hussain, NHN, Zin, AAM, Noordin, L, et al. Oxidative stress biomarkers in pregnancy: a systematic review. Reprod Biol Endocrinol. (2024) 22:93. doi: 10.1186/s12958-024-01259-x
35. Afrose, D, Chen, H, Ranashinghe, A, Liu, CC, Henessy, A, Hansbro, PM, et al. The diagnostic potential of oxidative stress biomarkers for preeclampsia: systematic review and meta-analysis. Biol Sex Differ. (2022) 13:26. doi: 10.1186/s13293-022-00436-0
36. Cedergren, M, and Källén, B. Maternal obesity and the risk for orofacial clefts in the offspring. Cleft Palate Craniofac J. (2005) 42:367–71. doi: 10.1597/04-012.1
37. Heydari, M-H, Sadeghian, A, Khadivi, G, Mustafa, HJ, Javinani, A, Nadjmi, N, et al. Prevalence, trend, and associated risk factors for cleft lip with/without cleft palate: a national study on live births from 2016 to 2021. BMC Oral Health. (2024) 24:3797. doi: 10.1186/s12903-023-03797-z
38. Spilson, SV, Kim, HJ, and Chung, KC. Association between maternal diabetes mellitus and newborn oral cleft. Ann Plast Surg. (2001) 47:477–81. doi: 10.1097/00000637-200111000-00001
39. Sun, B, Reynolds, KS, Garland, MA, Mcmahon, M, Saha, SK, and Zhou, CJ. Epigenetic implications in maternal diabetes and metabolic syndrome-associated risk of orofacial clefts. Birth Defects Res. (2023) 115:1835–50. doi: 10.1002/bdr2.2226
40. Sweeting, A, Wong, J, Murphy, HR, and Ross, GP. A clinical update on gestational diabetes mellitus. Endocr Rev. (2022) 43:763–93. doi: 10.1210/endrev/bnac003
41. Cederberg, J, Picard, JJ, and Eriksson, UJ. Maternal diabetes in the rat impairs the formation of neural-crest derived cranial nerve ganglia in the offspring. Diabetologia. (2003) 46:1245–51. doi: 10.1007/s00125-003-1100-1
42. Chang, TI, Horal, M, Jain, SK, Wang, F, Patel, R, and Loeken, MR. Oxidant regulation of gene expression and neural tube development: insights gained from diabetic pregnancy on molecular causes of neural tube defects. Diabetologia. (2003) 46:538–45. doi: 10.1007/s00125-003-1063-2
43. Eriksson, UJ, Borg, LA, Cederberg, J, Nordstrand, H, Simán, CM, Wentzel, C, et al. Pathogenesis of diabetes-induced congenital malformations. Ups J Med Sci. (2000) 105:53–84. doi: 10.1517/03009734000000055
44. Goshtasbi, H, Hashemzadeh, N, Fathi, M, Movafeghi, A, Barar, J, and Omidi, Y. Mitigating oxidative stress toxicities of environmental pollutants by antioxidant nanoformulations. Nano Trans Med. (2025) 4:100087. doi: 10.1016/j.ntm.2025.100087
45. Pantham, P, Aye, IL, and Powell, TL. Inflammation in maternal obesity and gestational diabetes mellitus. Placenta. (2015) 36:709–15. doi: 10.1016/j.placenta.2015.04.006
46. Wang, L, Yao, H, Shen, W, Wang, X, Huang, C, Yu, X, et al. Gestational diabetes mellitus is associated with blood inflammatory indicators in a Chinese pregnant women population. Gynecol Endocrinol. (2022) 38:153–7. doi: 10.1080/09513590.2021.2015762
47. Van, JAD, Luo, Y, Danska, JS, Dai, F, Alexeeff, SE, Gunderson, EP, et al. Postpartum defects in inflammatory response after gestational diabetes precede progression to type 2 diabetes: a nested case-control study within the swift study. Metabolism. (2023) 149:155695. doi: 10.1016/j.metabol.2023.155695
48. Denizli, M, Capitano, ML, and Kua, KL. Maternal obesity and the impact of associated early-life inflammation on long-term health of offspring. Front Cell Infect Microbiol. (2022) 12:940937. doi: 10.3389/fcimb.2022.940937
49. Challier, JC, Basu, S, Bintein, T, Minium, J, Hotmire, K, Catalano, PM, et al. Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta. Placenta. (2008) 29:274–81. doi: 10.1016/j.placenta.2007.12.010
50. Englich, B, Herberth, G, Rolle-Kampczyk, U, Trump, S, Röder, S, Borte, M, et al. Maternal cytokine status may prime the metabolic profile and increase risk of obesity in children. Int J Obes. (2017) 41:1440–6. doi: 10.1038/ijo.2017.113
51. Fernández-Sánchez, A, Madrigal-Santillán, E, Bautista, M, Esquivel-Soto, J, Morales-González, A, Esquivel-Chirino, C, et al. Inflammation, oxidative stress, and obesity. Int J Mol Sci. (2011) 12:3117–32. doi: 10.3390/ijms12053117
52. Kretschmer, T, Schulze-Edinghausen, M, Turnwald, EM, Janoschek, R, Bae-Gartz, I, Zentis, P, et al. Effect of maternal obesity in mice on Il-6 levels and placental endothelial cell homeostasis. Nutrients. (2020) 12:296. doi: 10.3390/nu12020296
53. Maguire, RL, House, JS, Lloyd, DT, Skinner, HG, Allen, TK, Raffi, AM, et al. Associations between maternal obesity, gestational cytokine levels and child obesity in the Nest cohort. Pediatr Obes. (2021) 16:e12763. doi: 10.1111/ijpo.12763
54. Megli, CJ, and Coyne, CB. Infections at the maternal–fetal interface: an overview of pathogenesis and defence. Nat Rev Microbiol. (2022) 20:67–82. doi: 10.1038/s41579-021-00610-y
55. Ács, L, Nemes, B, Nagy, K, Ács, M, Bánhidy, F, and Rózsa, N. Maternal factors in the origin of cleft lip/cleft palate: a population-based case-control study. Orthod Craniofac Res. (2024) 27:6–13. doi: 10.1111/ocr.12738
56. Shi, F-P, Huang, Y-Y, Dai, Q-Q, Chen, Y-L, Jiang, H-Y, and Liang, S-Y. Maternal common cold or fever during pregnancy and the risk of orofacial clefts in the offspring: a systematic review and meta-analysis. Cleft Palate Craniofac J. (2021) 60:446–53. doi: 10.1177/10556656211067695
57. Shahrukh Hashmi, S, Gallaway, MS, Waller, DK, Langlois, PH, and Hecht, JT. Maternal fever during early pregnancy and the risk of oral clefts. Birth Defects Res A Clin Mol Teratol. (2010) 88:186–94. doi: 10.1002/bdra.20646
58. Fitzgerald, E, Hor, K, and Drake, AJ. Maternal influences on fetal brain development: the role of nutrition, infection and stress, and the potential for intergenerational consequences. Early Hum Dev. (2020) 150:105190. doi: 10.1016/j.earlhumdev.2020.105190
59. Fujihara, C, Murakami, K, Magi, S, Motooka, D, Nantakeeratipat, T, Canela, A, et al. Omics-based mathematical modeling unveils pathogenesis of periodontitis in an experimental murine model. J Dent Res. (2023) 102:1468–77. doi: 10.1177/00220345231196530
60. Figuero, E, Han, YW, and Furuichi, Y. Periodontal diseases and adverse pregnancy outcomes: mechanisms. Periodontol. (2020) 83:175–88. doi: 10.1111/prd.12295
61. Gou, Y, Qi, K, Wei, Y, Gu, Z, and Xie, H. Advances of calcium phosphate nanoceramics for the osteoinductive potential and mechanistic pathways in maxillofacial bone defect repair. Nano Trans Med. (2024) 3:100033. doi: 10.1016/j.ntm.2024.100033
62. Pax, K, Buduneli, N, Alan, M, Meric, P, Gurlek, O, Dabdoub, SM, et al. Placental Tlr recognition of salivary and subgingival microbiota is associated with pregnancy complications. Microbiome. (2024) 12:64. doi: 10.1186/s40168-024-01761-9
63. Pass, RF, and Arav-Boger, R. Maternal and fetal cytomegalovirus infection: diagnosis, management, and prevention. F1000Res. (2018) 7:255. doi: 10.12688/f1000research.12517.1
64. Zhang, L, Jiang, T, Yang, Y, Deng, W, Lu, H, Wang, S, et al. Postpartum hepatitis and host immunity in pregnant women with chronic Hbv infection. Front Immunol. (2022) 13:1112234. doi: 10.3389/fimmu.2022.1112234
65. Bobetsis, YA, Graziani, F, Gürsoy, M, and Madianos, PN. Periodontal disease and adverse pregnancy outcomes. Periodontol. (2020) 83:154–74. doi: 10.1111/prd.12294
66. Zi, MY, Longo, PL, Bueno-Silva, B, and Mayer, MP. Mechanisms involved in the association between periodontitis and complications in pregnancy. Front Public Health. (2014) 2:290. doi: 10.3389/fpubh.2014.00290
67. Merz, WM, Fischer-Betz, R, Hellwig, K, Lamprecht, G, and Gembruch, U. Pregnancy and autoimmune disease. Dtsch Arztebl Int. (2022) 119:145–56. doi: 10.3238/arztebl.m2021.0353
68. Jølving, LR, Nielsen, J, Kesmodel, US, Nielsen, RG, Beck-Nielsen, SS, and Nørgård, BM. Prevalence of maternal chronic diseases during pregnancy - a nationwide population based study from 1989 to 2013. Acta Obstet Gynecol Scand. (2016) 95:1295–304. doi: 10.1111/aogs.13007
69. Kersten, I, Lange, AE, Haas, JP, Fusch, C, Lode, H, Hoffmann, W, et al. Chronic diseases in pregnant women: prevalence and birth outcomes based on the SniP-study. BMC Pregnancy Childbirth. (2014) 14:75. doi: 10.1016/j.rdc.2007.01.002
71. Björkander, S, Bremme, K, Persson, JO, Van Vollenhoven, RF, Sverremark-Ekström, E, and Holmlund, U. Pregnancy-associated inflammatory markers are elevated in pregnant women with systemic lupus erythematosus. Cytokine. (2012) 59:392–9. doi: 10.1016/j.cyto.2012.04.046
72. Gladman, DD, Tandon, A, Ibañez, D, and Urowitz, MB. The effect of lupus nephritis on pregnancy outcome and fetal and maternal complications. J Rheumatol. (2010) 37:754–8. doi: 10.3899/jrheum.090872
73. Ali, MF, He, H, and Friedel, D. Inflammatory bowel disease and pregnancy: fertility, complications and treatment. Ann Gastroenterol. (2020) 33:579–90. doi: 10.20524/aog.2020.0536
74. Guan, Q, and Zhang, J. Recent advances: the imbalance of cytokines in the pathogenesis of inflammatory bowel disease. Mediat Inflamm. (2017) 2017:4810258. doi: 10.1155/2017/4810258
75. Brondfield, MN, and Mahadevan, U. Inflammatory bowel disease in pregnancy and breastfeeding. Nat Rev Gastroenterol Hepatol. (2023) 20:504–23. doi: 10.1038/s41575-023-00758-3
76. Syme, MR, Paxton, JW, and Keelan, JA. Drug transfer and metabolism by the human placenta. Clin Pharmacokinet. (2004) 43:487–514. doi: 10.2165/00003088-200443080-00001
77. Laspro, M, Brydges, HT, Verzella, AN, Schechter, J, Alcon, A, Roman, AS, et al. Association of commonly prescribed antepartum medications and incidence of orofacial clefting. Cleft Palate Craniofac J. (2024) 62:10556656241237679. doi: 10.1177/10556656241237679
78. Bateman, BT, Heide-Jørgensen, U, Einarsdóttir, K, Engeland, A, Furu, K, Gissler, M, et al. Β-blocker use in pregnancy and the risk for congenital malformations: an international cohort study. Ann Intern Med. (2018) 169:665–73. doi: 10.7326/M18-0338
79. Li, DK, Yang, C, Andrade, S, Tavares, V, and Ferber, JR. Maternal exposure to angiotensin converting enzyme inhibitors in the first trimester and risk of malformations in offspring: a retrospective cohort study. BMJ. (2011) 343:d5931. doi: 10.1136/bmj.d5931
80. Crider, KS, Cleves, MA, Reefhuis, J, Berry, RJ, Hobbs, CA, and Hu, DJ. Antibacterial medication use during pregnancy and risk of birth defects: national birth defects prevention study. Arch Pediatr Adolesc Med. (2009) 163:978–85. doi: 10.1001/archpediatrics.2009.188
81. Mølgaard-Nielsen, D, and Hviid, A. Maternal use of antibiotics and the risk of orofacial clefts: a nationwide cohort study. Pharmacoepidemiol Drug Saf. (2012) 21:246–53. doi: 10.1002/pds.2179
82. Anderka, MT, Lin, AE, Abuelo, DN, Mitchell, AA, and Rasmussen, SA. Reviewing the evidence for mycophenolate mofetil as a new teratogen: case report and review of the literature. Am J Med Genet A. (2009) 149a:1241–8. doi: 10.1002/ajmg.a.32685
83. Carmichael, SL, Shaw, GM, Ma, C, Werler, MM, Rasmussen, SA, and Lammer, EJ. Maternal corticosteroid use and orofacial clefts. Am J Obstet Gynecol. (2007) 197:585–683. doi: 10.1016/j.ajog.2007.05.046
84. Källén, B. Maternal drug use and infant cleft lip/palate with special reference to corticoids. Cleft Palate Craniofac J. (2003) 40:624–8. doi: 10.1597/02-077
85. Tomson, T, Battino, D, Bonizzoni, E, Craig, J, Lindhout, D, Perucca, E, et al. Comparative risk of major congenital malformations with eight different antiepileptic drugs: a prospective cohort study of the Eurap registry. Lancet Neurol. (2018) 17:530–8. doi: 10.1016/S1474-4422(18)30107-8
86. Tomson, T, Battino, D, Bonizzoni, E, Craig, J, Lindhout, D, Sabers, A, et al. Dose-dependent risk of malformations with antiepileptic drugs: an analysis of data from the Eurap epilepsy and pregnancy registry. Lancet Neurol. (2011) 10:609–17. doi: 10.1016/S1474-4422(11)70107-7
87. Zhou, S, Zhou, N, Zhang, H, Yang, W, Liu, Q, Zheng, L, et al. A prospective multicenter birth cohort in China: pregnancy health atlas. Eur J Epidemiol. (2024) 39:1297–310. doi: 10.1007/s10654-024-01157-x
88. Alsharif, MT, Alamoudi, RA, and Sabbagh, HJ. Maternal stress as a risk factor for non-syndromic orofacial clefts: systematic review and meta-analysis. Saudi Dental J. (2023) 35:207–19. doi: 10.1016/j.sdentj.2023.02.004
89. Chung, KC, Kowalski, CP, Kim, HM, and Buchman, SR. Maternal cigarette smoking during pregnancy and the risk of having a child with cleft lip/palate. Plast Reconstr Surg. (2000) 105:485–91. doi: 10.1097/00006534-200002000-00001
90. Silva, H, Arruda, TTS, Souza, KSC, Bezerra, JF, Leite, GCP, Brito, MEF, et al. Risk factors and comorbidities in Brazilian patients with orofacial clefts. Braz Oral Res. (2018) 32:e24. doi: 10.1590/1807-3107bor-2018.vol32.0024
91. Alade, A, Ismail, W, Nair, R, Schweizer, M, Awotoye, W, Oladayo, A, et al. Periconceptional use of vitamin a and the risk of giving birth to a child with nonsyndromic orofacial clefts-a meta-analysis. Birth Defects Res. (2022) 114:467–77. doi: 10.1002/bdr2.2005
92. Ferrazzi, E, Tiso, G, and Di Martino, D. Folic acid versus 5- methyl tetrahydrofolate supplementation in pregnancy. Eur J Obstet Gynecol Reprod Biol. (2020) 253:312–9. doi: 10.1016/j.ejogrb.2020.06.012
Keywords: CLP, maternal, pregnancy, systemic diseases, risk factors
Citation: Sui H, Du M, Chen J, Yang R, Shi B, Huang H and Wang Y (2025) The impact of maternal systemic diseases on the occurrence of cleft lip and palate in newborns: a narrative review. Front. Public Health. 13:1568140. doi: 10.3389/fpubh.2025.1568140
Edited by:
Karin Windsperger, Medical University of Vienna, AustriaReviewed by:
Rosa Helena Wanderley Lacerda, Federal University of Paraíba, BrazilCopyright © 2025 Sui, Du, Chen, Yang, Shi, Huang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hanyao Huang, aHVhbmdoYW55YW9fY25Ac2N1LmVkdS5jbg==; Yan Wang, MTA1NTQ1MzIzNUBxcS5jb20=
†These authors have contributed equally to this work
 Hao Sui
Hao Sui Meijun Du
Meijun Du Jiali Chen
Jiali Chen Renjie Yang
Renjie Yang Bing Shi1
Bing Shi1 Hanyao Huang
Hanyao Huang