- 1Linghu People’s Hospital, Huzhou, China
- 2Department of Public Health, Xinjiang Medical University, Ürümqi, China
- 3First Affiliated Hospital of Xinjiang Medical University, Ürümqi, China
Objective: The current status of the occurrence of anxiety, depression, and sleep disorders in mental workers was investigated. The effects of anxiety, depression, and CLOCK, PER2, and RORA gene polymorphisms and their interactions on sleep disorders were further analyzed, to provide scientific references for the reduction of the risk of the occurrence of sleep disorders in mental workers.
Methods: Anxiety, depression, and sleep disorders in the study population were measured by applying the Self-Assessment Scale for Anxiety (SAS), the Self-Assessment Scale for Depression (SDS), and the Pittsburgh Sleep Quality Index (PSQI). The CLOCK, PER2, and RORA genes of 748 mental workers (374 of whom were randomly selected from the sleep disorder group and 374 of whom were randomly selected from the normal sleep group) were genotyped by imLDR™ genotyping technology, and the relationship between CLOCK, PER2, and RORA gene polymorphisms and their interactions with sleep disorders were analyzed.
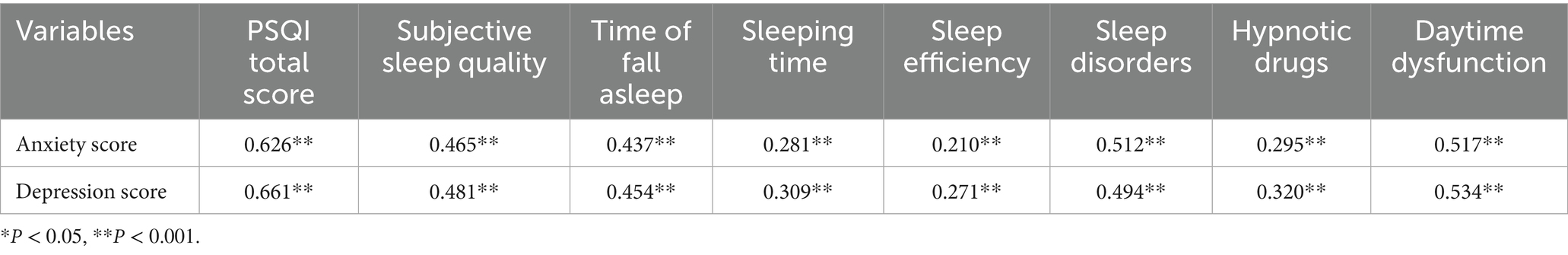
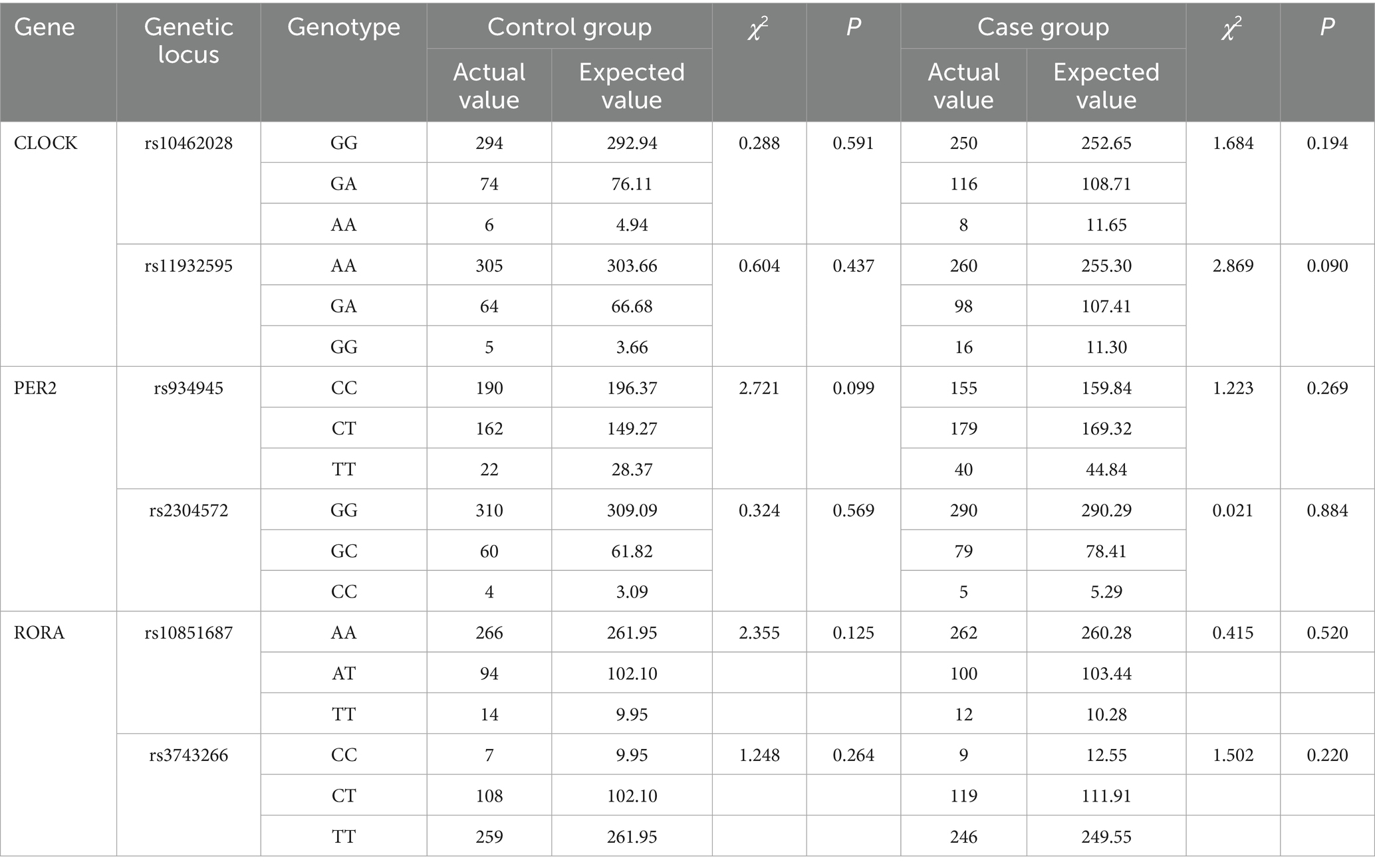
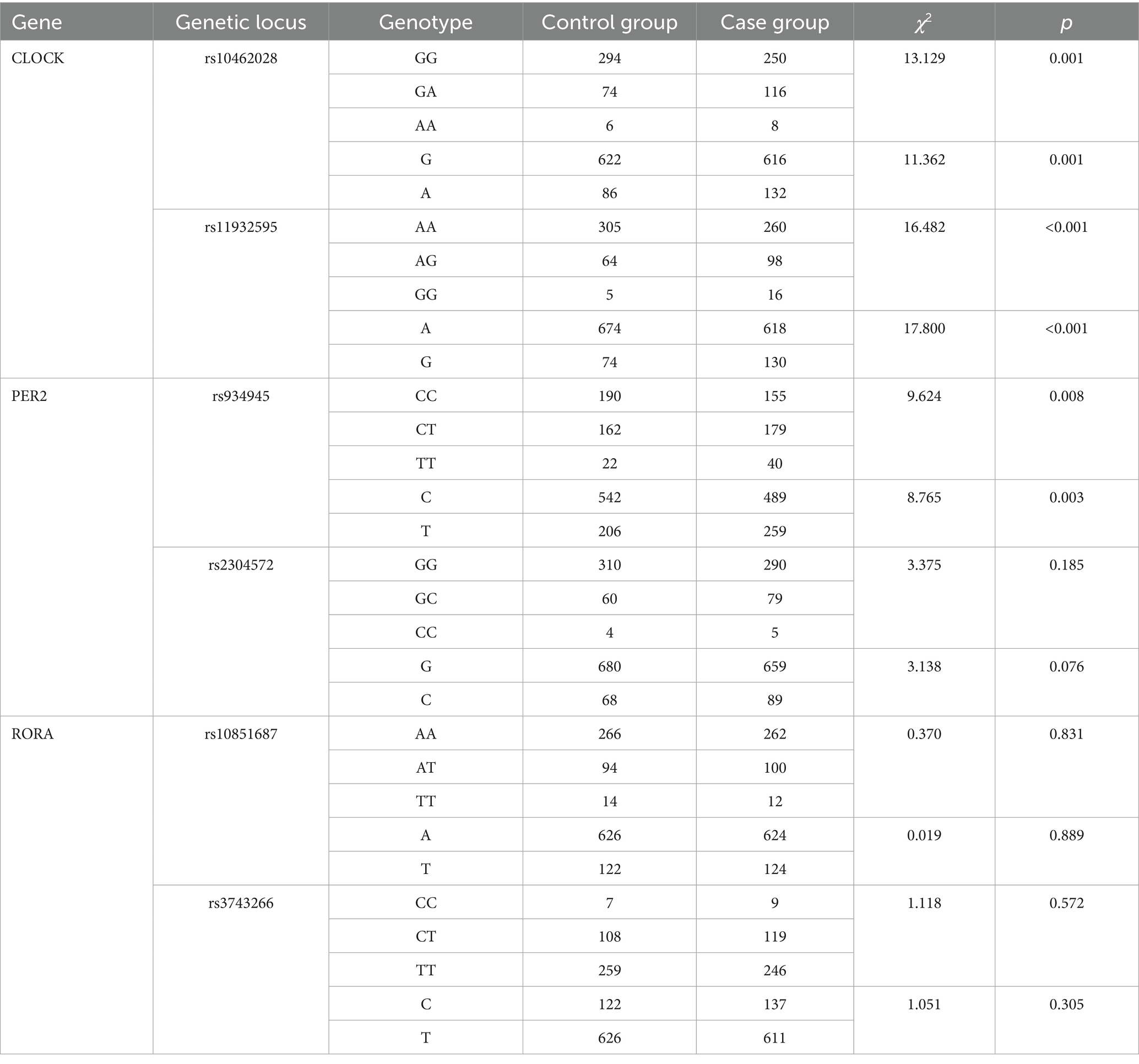
Results: The detection rate of sleep disorders among mental workers was 27.88%. There were significant differences in the rates of sleep disorders among mental workers of different genders, ages, marital status, shifts, education, title, occupation, and monthly income (p < 0.05). There was a difference in the prevalence of sleep disorders between groups with different levels of anxiety and depression (p < 0.001). Anxiety and depression scores were positively related to PSQI scores (rs = 0.626, rs = 0.661, p < 0.001) and their scores in all dimensions. The rs10462028 and rs11932595 of the CLOCK gene, the rs934945 of the PER2 gene, and the distribution of genotypes and allele frequencies of each genotype, as well as allele frequency, were significantly different in the sleep-disordered group and the normal-sleep group (both p < 0.001). The difference in distribution was also significant (all p < 0.05). The interaction of rs934945, anxiety, and depression (OR = 10.461, 95% CI: 3.695–29.621) increased the risk of sleep disorders in mental workers (p < 0.05).
Conclusion: Mental workers experience significant sleep disorders, so effective measures should be taken to reduce anxiety and depression. The interaction of rs934945, anxiety, and depression was associated with a higher prevalence of sleep disorders in mental workers.
1 Background
The rapid development of the social economy, the continuous advancement of globalization, and the rapid changes in emerging technologies have all posed significant challenges to contemporary workers (1). As the main force of productivity, the physical and mental health of workers has a very important impact on the development of the economy and social stability, and poor physical and mental health among workers will lead to a low work capacity, reduced sleep quality, frequent production accidents, and can even cause huge social and economic losses (2). At present, an increasing number of scholars are interested in the negative health effects caused by psychosocial factors, and previous studies have shown that the incidence of occupational stress and psychological disorders in laborers has not only gradually increased but also seriously jeopardized the quality of their sleep and their physical and mental health (3–5). Therefore, it is particularly important to pay attention to the impact of psychosocial factors on the physical and mental health of workers and to improve the current situation.
Anxiety and depression, as the most common disorders resulting from mental health conditions, occur widely in all types of populations and have high lifetime prevalence rates (6). A U. S. Census Bureau survey indicated that the prevalence of anxiety and depression among U. S. adults in 2020 was three times higher than in 2019 (7). Globally, depression has been identified as a leading cause of ill health and disability (8), and the World Health Organization (WHO) has estimated that depression will be the world’s highest-burden disease in the next decade (9).
Sleep, as a biological process, takes up one-third of the human life cycle, ensures the continuity of human health, and is considered one of the most basic physiological needs of humans (10). Normal healthy sleep is characterized by a long duration, good quality, appropriate timing, regularity, and the absence of sleep disorders (11). Sleep disorders are chronic conditions characterized by the inability to fall asleep normally or to maintain sleep continuity, resulting in poor subjective sleep satisfaction (12). The high prevalence of sleep disorders is a major problem in modern society, and epidemiologic surveys have shown that approximately one-third of the general adult population in Europe reports having a sleep disorder (13, 14). The results of several studies have confirmed that sleep disorders, such as poor sleep quality, insufficient sleep time, sleep disruption, difficulty falling asleep, and insomnia, lead to an increased risk of chronic diseases (e.g., diabetes mellitus, hypertension, and obesity), as well as psychiatric disorders (e.g., anxiety disorders, depression, etc.) (15–17). Sleep disorders can also seriously affect the work efficiency of laborers, leading to a decrease in productivity and an increased occurrence of accidents in the production process (18). Therefore, it is urgently necessary to pay more attention to the occurrence of sleep disorders in occupational groups.
In recent years, due to the rapid development of molecular biology, a large number of scholars have delved into sleep-related studies examining general demographic and psychosocial factors as well as genetic factors. The circadian biological clock network (central and peripheral oscillators) controls circadian rhythms and coordinates the expression of a series of genes that enable the organism to anticipate and adapt to environmental changes (19). Biological rhythms are composed of two parts: the exogenous part, regulated by environmental factors (20), and the endogenous part, related to genetic factors (21). Currently, CLOCK, PER, CRY, and BMAL1 are the major circadian clock genes found in humans, and there are other clock genes involved in circadian rhythm regulation, such as RORA, RORB, and casein kinase-1 (CK1). Circadian clock genes alter 24-h rhythms by affecting endogenous circadian rhythms and desynchronization between sleep–wake cycles, which can increase the risk of developing sleep disorders (22). Polymorphisms (SNPs) in clock genes have been associated with the development of anxiety disorders, depression, and sleep disorders, and it has been found that circadian clock genes have a wide range of physiological effects on cognition, mood, and sleep behavior and that mutations in them can lead to mood disorders, imbalances in the sleep–wake cycle, and changes in the organism’s metabolic levels (23–25). However, research findings regarding the relationship between various circadian clock genes, psychological disorders, and sleep disorders have been inconsistent; therefore, the relationship between clock genes, psychological disorders, and sleep disorders needs to be further explored.
Mental work is a form of labor that involves external information processing, treatment, and integration through the central nervous system, as well as the transformation and output of internal information. The challenges faced by mental workers are gradually increasing due to the rapid development of science and technology. Mental workers, characterized by high cognitive load and work pressure, face a higher risk of anxiety, depression, and sleep disorders (26). Recently, more studies have been conducted on the effects of anxiety, depression, and circadian clock genes on sleep disorders, while fewer studies have been conducted on the effects of anxiety, depression, and the interaction of circadian clock genes on sleep disorders in mental workers. CLOCK and PER2 are core circadian clock genes directly involved in the regulation of the sleep–wake cycle, while RORA has been linked to circadian rhythm disruption and sleep homeostasis via its role in nuclear receptor signaling. Previous studies have primarily focused on CLOCK and PER2 in sleep disorders, but RORA’s role remains underexplored, particularly in mental worker populations. Therefore, this study investigated the current status of anxiety, depression, and sleep disorders in the mental worker population, analyzing the effects of anxiety, depression, and CLOCK, PER2, and RORA gene polymorphisms, as well as their interactions on sleep disorders to provide scientific references for the reduction of the risk of sleep disorders in the mental worker population. This study adheres to the STREGA (STrengthening the Reporting of Genetic Association studies) guidelines for transparent reporting of genetic associations. It is important to acknowledge that genetic studies may face reproducibility challenges due to population heterogeneity and multiple testing, which are addressed here via rigorous quality control and statistical corrections.
2 Measures
2.1 Subjects
In this study, questionnaires were administered, and blood samples were collected from June 2022 to February 2023 using a whole-cluster sampling method by sampling selected teachers, civil servants, medical staff members, and administrators at the Health Management Center of Xinjiang Medical University. In this study, questionnaires were distributed to a total of 2,100 mental workers. Excluding those whose questionnaires were of substandard quality, a total of 1994 questionnaires were returned, with a recovery rate of 94.95%. The inclusion criteria were (1) age ≥18 years; (2) working experience ≥1 year; (3) willingness to cooperate in the collection of blood samples after being informed of the purpose and significance of the study by the investigator. The exclusion criteria were (1) a history of physical diseases that may cause sleep disorders, such as cardiovascular and cerebrovascular diseases, diabetes mellitus, etc.; (2) a history of psychiatric diseases that may cause sleep disorders, such as bipolar disorder, schizophrenia, etc.; (3) having been treated for sleep quality problems with medication or hospitalization in the last 3 months.
Case-control matching was performed at a 1:1 ratio based on age (±5 years), gender, and educational level to minimize confounding by demographic factors. The case flow was as follows: 556 sleep disorder cases were identified from 1994 participants, and 374 cases were randomly selected and matched to 374 normal sleep controls, resulting in a total of 748 subjects for genetic analysis. The sample size for different test efficacies in case-control studies was estimated using the PASS software package; sample size was calculated based on prior studies showing that PER2 polymorphisms are associated with sleep disorders (OR = 1.5, α = 0.05, β = 0.2), which resulted in a sample size of 368, and 374 subjects in each of the case and control groups in this study, which was in line with the requirements of the sample size. The research proposal was approved by the Ethics Committee of Xinjiang Medical University (the ethical NO.20170214–174). Prior to the start of the survey, all respondents voluntarily provided written informed consent forms.
2.2 Self-assessment scale for anxiety
The Self-Assessment Scale for Anxiety (SAS) was developed by Zung (27), and Dunstan and Scott (28) showed that the scale had good reliability and validity in the population. The scale includes 20 items that cover a variety of anxiety symptoms, including psychological symptoms (e.g., “I feel fearful for no reason,” “I feel like I’m falling apart”) and somatic symptoms (e.g., “my arms and legs are shaking,” “I feel my heart beating rapidly”), and it instructs the study subjects to respond according to their condition over the prior week. The scale uses a 4-level scoring system (in which items 5, 9, 13, 17, and 19 are inversely scored), and the score range of each item is 1 ~ 4 points. The total score was multiplied by 1.25 to obtain the final score, and a final score of 50 points, according to the Chinese standard ≥50, indicates the occurrence of anxiety. The Cronbach’s α coefficient of the scale in this study is 0.75, indicating that the SAS has good reliability.
2.3 Self-assessment scale for depression
The Self-Assessment Scale for Depression (SDS), developed by Zung et al. (29), was used, which was shown by Jokelainen (30) and others to have good reliability and validity. The scale consists of 20 items covering a wide range of depressive symptoms that the study participants were instructed to answer based on the prior week. A 4-level scoring system was used (with 2, 5, 6, 11, 12, 14, 16, 17, 18, and 20 being reverse-scored items), with a range of 1 to 4 points assigned for each item. The total score was multiplied by 1.25 to obtain the final score, and a final score of ≥53 points suggested the occurrence of depression according to the Chinese standard. In the present study, the Cronbach’s α coefficient of this scale was 0.86, indicating good reliability.
2.4 Pittsburgh sleep quality index
The Chinese version of the Pittsburgh Sleep Quality Index (PSQI) questionnaire, developed by Buysse et al. (31), was used. This questionnaire includes the seven dimensions of subjective sleep quality, time to sleep, sleep duration, sleep efficiency, sleep disorders, hypnotic drugs, and daytime dysfunction, totaling 19 items. The seven dimensions were scored using a 4-level scoring system, with each item having a score ranging from 0 ~ 3 points. Referring to the national standard, a cumulative score greater than 7 was understood as indicating sleep disorders (32), and the Cronbach’s alpha coefficient of this scale was 0.82 in this study.
2.5 Determination of gene polymorphisms
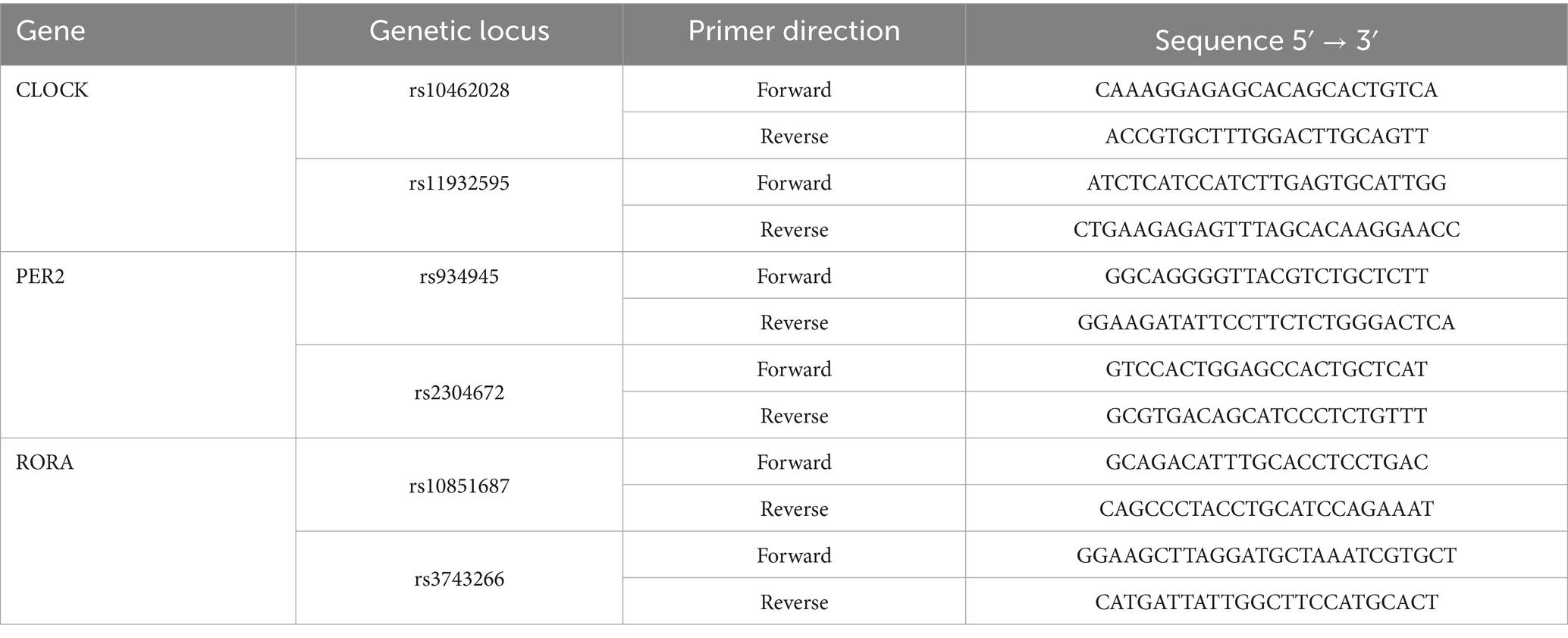
Based on the relevant literature from China and abroad and the HapMap database1, SNP genotype information of the Chinese genome (CHB) and Northwest European ancestry (CEU, for Uyghur participation) was used in this study. The Haploview software package2 was used to set the corresponding running parameters. Minimum allele frequency (MAF) > 0.1 and the chain imbalance parameter, r2 > 0.8 were used to screen genetic locations. The SNP loci of the CLOCK gene (rs11932595 and rs10462028), PER2 gene (rs934945 and rs2304572), and RORA gene (rs10851687 and rs3743266) were screened and analyzed in this study. Information regarding the primers used is listed in Table 1.
2.6 Quality control
2.6.1 Quality control of the epidemiological investigation
Strict quality control was used in both epidemiological field investigations and experimental processes. Before the questionnaires were administered, the investigators were trained on the content of the questionnaire and the method of questionnaire completion. The questionnaire numbers were matched with the same blood sample numbers. During the investigation, the investigator urged each respondent to complete the questionnaire correctly and completely, and unqualified questionnaires were re-filled or discarded.
2.6.2 Laboratory quality control
DNA extraction and PCR were carried out in strict accordance with the experimental procedures. After completion of the daily experiments, the operating table and experimental supplies were sterilized.
2.7 Statistical analysis
In this study, Epi Data 3.1 software was used for the database creation, double entry of valid questionnaires, and logical checking. IBM SPSS STATISTICS 25.0 was used for data analysis. The normality test was performed for the measurement data, and data obeying normal distribution were described by ± s. Further, two independent samples t-tests were used for the comparison of the means of the two groups, and an ANOVA was used for the comparison of the means of multiple groups. If there was a difference in the overall total, the SNK-q test was used for the comparison of the two. Count data were described by the rate and constitutive ratio, and the comparison of the rate was performed by the χ2 test. Associations between anxiety, depression, and sleep disorders were analyzed by partial correlation analysis and a logistic regression model was used to analyze the factors influencing sleep disorders. GMDR software was used to fit the gene–environment interaction model (test level α = 0.05). GMDR was chosen for interaction modeling because it effectively captures high-order gene–environment interactions, which is challenging with traditional logistic regression that primarily handles low-order or additive effects. For genetic association analyses, multiple testing corrections were performed using the Bonferroni method. The significance threshold was adjusted to p < 0.008 (0.05/6 SNPs) to account for the six genetic loci tested. GMDR has limitations, including potential sensitivity to sample size and difficulty in interpreting high-order interactions.
3 Results
3.1 Demographic characteristics of mental workers
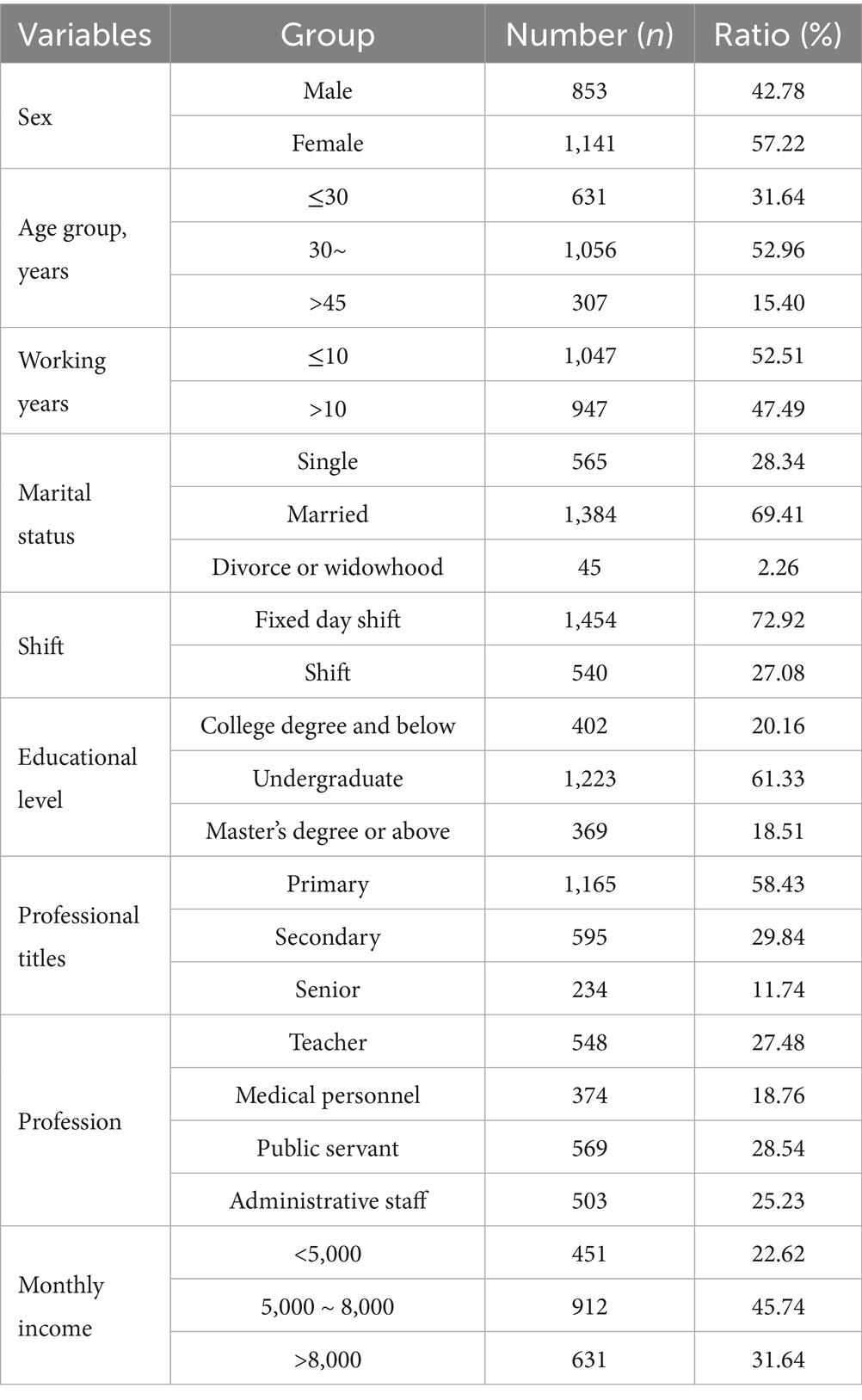
The demographic characteristics of the participants are shown in Table 2.
3.2 Occurrence of sleep disorders according to different population characteristics
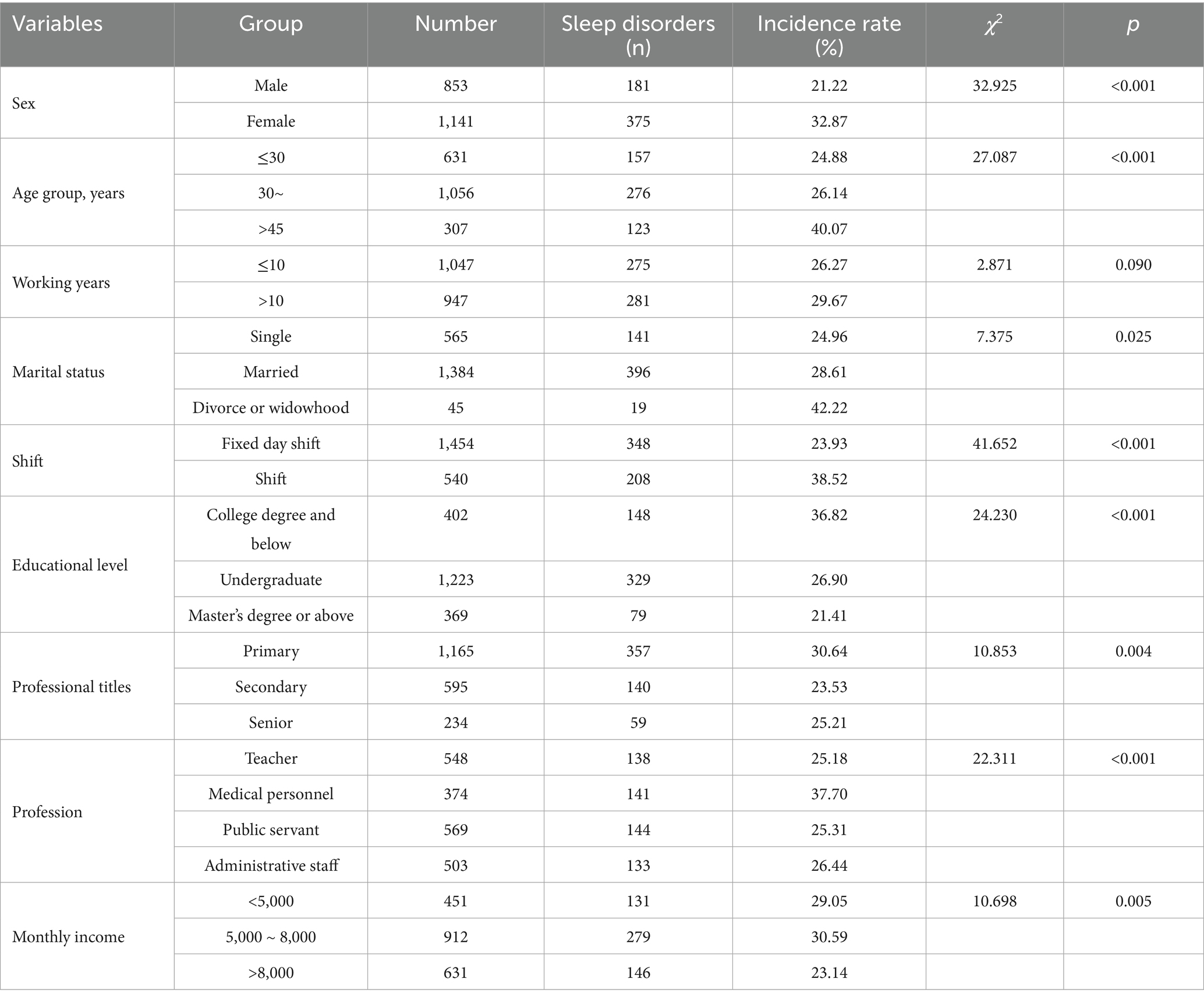
Sleep disorders occurred in 556 (27.88%) of mental workers in this study (p < 0.05). The detection rate of sleep disorders varied widely with statistical significance (p < 0.05) according to gender, age, shift work, education, title, occupation, and monthly income (see Table 3).
3.3 The occurrence of sleep disorders in mental workers with different levels of anxiety
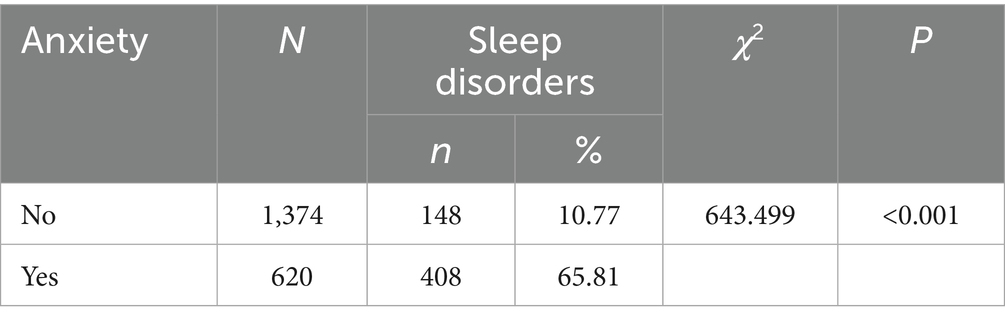
The results of the study showed that there was a statistically significant difference in the occurrence of sleep disorders between groups with different levels of anxiety (χ2 = 643.499, p < 0.001), suggesting that the occurrence of sleep disorders is associated with anxiety (see Table 4).
3.4 The occurrence of sleep disorders in mental workers with different level of depression
The results of the study showed that there was a significant difference in the occurrence of sleep disorders between groups with different levels of depression (χ2 = 810.588, p < 0.001), suggesting that the occurrence of sleep disorders is related to depression (see Table 5).
3.5 Partial correlation analysis of anxiety, depression, and sleep disorders in mental workers
Anxiety scores were positively correlated with the scores of all dimensions of sleep disorders (p < 0.05), and depression scores were positively correlated with the scores of all dimensions of sleep disorders (p < 0.05), suggesting that there is a positive correlation between the degree of anxiety and depression and sleep disorders and that anxiety, depression, and sleep affect each other (see Table 6).
3.6 Genetic susceptibility to sleep disorders in mental workers
3.6.1 Hardy–Weinberg genetic balance test
The results of the Hardy–Weinberg genetic balance test show that the actual values of the genotypes of the rs10462028 and rs11932595 of the CLOCK gene, the rs934945 and rs2304572 of the PER2 gene, and the rs10851687 and rs3743266 of the RORA gene are in good agreement with the expected values, and none of the differences were statistically significant (p > 0.05). It is suggested that the mental workers in the case and control groups have biological clock gene frequencies that are in accordance with the law of genetic equilibrium and that are in a state of genetic equilibrium (see Table 7).
3.6.2 Correlation study of CLOCK, PER2, and RORA genes with sleep disorders
The distribution frequencies of each genotype and allele of the rs10462028 and rs11932595 of the CLOCK gene and the rs934945 of the PER2 gene were different in the distributions of case and control groups (p < 0.05). The rs2304572 of the PER2 gene, the rs10851687 of the RORA gene, and the rs3743266 showed no difference in the distribution frequency of each genotype and allele (p > 0.05) (see Table 8).
3.7 Gene–environment interactions for sleep disorders
3.7.1 GMDR model of gene–environment interaction
The GMDR software was used to build the interaction model for analyzing the effect of the one-way statistically significant interaction between rs10462028, rs11932595, rs934945, anxiety, and depression on sleep disorders. As shown in Table 9, the fitted interaction model between rs934945, anxiety, and depression was optimal with p = 0.0010, a cross-validation consistency of 10/10, and a test sample accuracy of 0.8535.
3.7.2 Gene–environment interaction on sleep disorders — logistic regression analysis
Logistic regression model fit was evaluated via Hosmer-Lemeshow test (χ2 = 5.32, p = 0.72), indicating good agreement between observed and predicted values. The results of the gene–environment interaction regression analysis showed that compared with the CC genotype at rs934945, the CT/TT genotype (OR = 1.442, 95% CI: 1.077 ~ 1.931) increased the risk of sleep disorders occurring in mental workers, and compared with those who had no anxiety and those who had no depression, those who were anxious (OR = 4.630, 95% CI: 1.912 ~ 11.211) or depressed (OR = 10.160, 95% CI: 7.245 ~ 14.248) had a higher risk of sleep disorders. The interaction of rs934945, anxiety, and depression (OR = 10.461, 95% CI: 3.695 ~ 29.621) increased the risk of sleep disorders in mental workers (see Table 10).
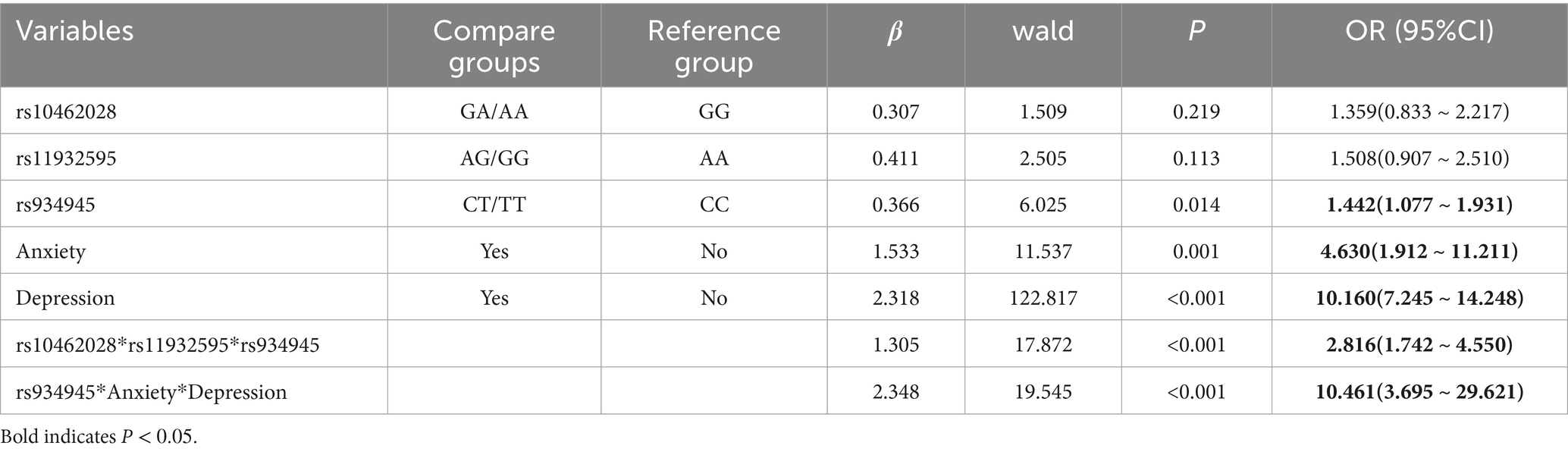
Table 10. Logistic regression analysis of gene–gene and gene–environment interactions on sleep disorders.
4 Discussion
The detection rate of sleep disorders was 27.88% in this study including 1944 mental workers. Niu et al.’s (33) study on the relationship between burnout and sleep quality of teachers in undergraduate colleges and universities in Inner Mongolia found that the positive rate of sleep disorders was 12.4%, and Lv et al.’s (34) study on the sleep quality of workers in the railroad vehicle system showed that the detection rate of sleep disorders was 20.47%. When compared with the above results of the studies on the sleep disorders of these occupational groups, the sleep disorders of mental workers in Xinjiang were more serious. However, with the rapid development of the economy, mental workers, as the main force of productivity, face an increasingly fast-paced life and work environment, and various kinds of overtime work, shift work, and increasing work pressure have seriously affected their sleep time and quality (35, 36).
The number of female mental workers in this study who developed sleep disorders was 357 (32.78%), and the detection rate of sleep disorders among them was higher than among male workers, a result that is consistent with the findings of Fu et al. (37). This may be due to the fact that compared with men, working women need to balance family and work, and under this dual pressure, they are prone to experiencing work–family conflicts, which may further lead to psychological disorders that can, in turn, have an impact on sleep quality (38). In addition, differences in the functioning of the endocrine system between men and women also influence gender differences in sleep quality outcomes. Estrogen modulates GABA receptor sensitivity to maintain sleep. Pre-menstrual and menopausal estrogen declines disrupt sleep continuity (39). Females exhibit stronger stress-induced cortisol responses, which interfere with circadian gene (e.g., PER2) expression, creating a “hormone-psychology-gene” feedback loop. The present study also found that the prevalence of sleep disorders and the related scores on all dimensions were higher among mental workers working shifts than among those working regular day shifts. A cross-sectional study on the relationship between shift work and sleep disorders among Chinese workers by Zhang et al. (40) found that the prevalence of sleep disorders was significantly higher among workers engaged in shift work than among workers working regular day shifts. The reason for this may be that shift work causes occupational groups to reverse day and night, as well as work under bright lights at night, resulting in an imbalance of their circadian rhythms that affects their wake–sleep cycle and leads to sleep disorders (41). In this study, we found that the detection rate of poor sleep quality among divorced or widowed mental workers was higher than that of unmarried and married people. The reason may be that they not only lack social support but also suffer from emotional shock, leading to a higher prevalence of sleep disorders. Regarding the effects of different marital statuses on sleep quality, the findings of different studies vary. Some scholars have found that unmarried workers have a higher prevalence of sleep disorders due to their younger age, lack of work experience, and lower social support (42); other studies have found a higher detection rate of sleep disorders among married workers (43).
In this study, we investigated the occurrence of different anxiety conditions and sleep disorders in 1994 mental workers and found that the detection rate of sleep disorders in the anxiety group was much higher than that in mental workers without anxiety, and studies, such as that by Guo et al. (44), have also shown that the presence of negative emotions, such as anxiety, increases the incidence of sleep disorders. Further analysis of the partial correlation between the anxiety scores and sleep quality scores of mental workers showed a positive correlation between anxiety scores and scores on all dimensions of the sleep quality scores, as well as Korkmaz et al.’s (45) study on healthcare workers. It is suggested that the more severe the level of anxiety, the higher the likelihood of sleep disorders, and that the level of sleep quality can be improved by reducing the level of anxiety and other adverse emotions in mental workers, thereby improving their sleep quality. The present study investigated the occurrence of different depressive conditions and sleep disorders in 1994 mental workers and found that the incidence of sleep disorders in depressed patients was 76.21%, which was much higher than the incidence of sleep disorders in the non-depressed population (10.97%). The results of the current study also showed a positive correlation between the depression scores and sleep disorder scores of mental workers, which is consistent with the findings of other scholars (46). Given these results, alleviating depression among mental workers through reasonable ways may reduce the incidence of their sleep disorders to a greater extent. It is important to note that the cross-sectional design of this study limits our ability to establish causal relationships. The observed associations between genetic polymorphisms, anxiety, depression, and sleep disorders reflect correlations rather than direct causation. Longitudinal studies are needed to confirm the temporal sequence of these associations.
In recent years, as scholars have gradually delved deeper into the study of sleep, it has been found that the optimal quality of sleep required for the organism to maintain physiological homeostasis is affected by demographic characteristics, in addition to genetic and environmental factors. Studies have shown that the percentage of sleep quality determined by genetic factors ranges from 31 to 55%, which suggests that genetic factors have a significant influence on sleep quality (47) and that the occurrence of sleep disorders may be caused by gene–environment interactions (48). In this study, we found that anxiety, depression, and polymorphism at the rs934945 of the PER2 gene were risk factors for the development of sleep disorders. The PER2 gene is a core component of the circadian clock, encoding a key protein that regulates the sleep–wake cycle. The rs934945 polymorphism in PER2 may affect the transcriptional regulation or protein stability of PER2, thereby disrupting circadian rhythmicity. Previous studies have shown that variants in PER2 are associated with delayed sleep phase disorder and altered sleep homeostasis (49). The rs934945 SNP might influence the interaction between PER2 and other clock genes (e.g., CLOCK, BMAL1), leading to dysregulation of the circadian network and increased susceptibility to sleep disorders when combined with psychological stressors like anxiety and depression. The results of this study also suggest that changes in PER2 gene expression can affect sleep. Gene–environment interactions arise from different responses of individuals to environmental stimuli, depending on their genotype, or from different genetic effects between individuals due to differences in their living and working environments. Further exploration of the possible gene–environment interactions on the effects of sleep disorders and the identification of gene–environment interactions could potentially ameliorate the risk of the development of sleep disorders and help unravel the underlying biological pathways.
The results of the gene–environment modeling showed that the model constructed by rs934945 with anxiety and depression was optimal and that there may be an interaction between rs934945, anxiety, and depression on the generation of sleep disorders. Loci rs10462028, rs11932595, and rs934945, as well as anxiety and depression, were included as dependent variables in the logistic regression equations, and the results showed that the polymorphism of the rs934945 of the PER2 gene, anxiety, and depression was associated with a higher prevalence of sleep disorders in mental workers. The polymorphism of rs10462028 and rs11932595 of CLOCK gene had no effect on sleep disorders, which is different from the findings of Vanderlind et al. (50). Therefore, the effects of the rs10462028 and rs11932595 of the CL OCK gene on sleep disorders need to be demonstrated by further research. The interaction of rs934945, anxiety, and depression in the logistic regression results showed that the interaction of rs934945, anxiety, and depression significantly increased the prevalence of sleep disorders (OR = 10.461, 95% CI: 3.695–29.621), and the results were the same as those of the model constructed by GMDR software, which may be attributed to the fact that individuals with susceptibility genes are more likely to be adversely affected by negative environmental factors (51). Recent research has elaborated on the neurobiological links between sleep disturbances and mood disorders, highlighting that circadian gene dysregulation (e.g., PER2 variants) may disrupt serotonin and cortisol pathways, which are also implicated in anxiety and depression (52). This aligns with our finding that rs934945 interacts with anxiety/depression, potentially via shared neuroendocrine pathways. Realizing that the existence of interactions between biological clock genes and anxiety and depression can lead to an increased risk of sleep disorders in mental workers, suggests that, as a potential biological pathway, gene–environment interactions should be paid attention to in terms of their impact on sleep disorders.
These findings have practical implications for targeted interventions. For mental workers carrying the PER2 rs934945 CT/TT genotype, prioritizing psychological support (e.g., cognitive-behavioral therapy for anxiety and depression) may mitigate sleep disorder risk by addressing the gene–environment interaction. Workplace policies could also include circadian-aligned shift schedules (avoiding frequent night shifts) for this subgroup, as their genetic susceptibility may amplify circadian disruption from irregular work hours. Additionally, regular screening combining psychological assessments (SAS/SDS) and genetic testing could identify high-risk individuals for early intervention.
This study offers three key advantages. First, it is among the first to systematically investigate the interactive effects of anxiety, depression, and circadian clock gene polymorphisms (CLOCK, PER2, RORA) on sleep disorders in mental workers. Unlike prior studies that analyzed psychological or genetic factors in isolation, we confirmed via GMDR modeling and logistic regression that the interaction between rs934945 (PER2) and anxiety/depression significantly elevates sleep disorder risk (OR = 10.461, 95% CI: 3.695–29.621), highlighting a “gene-psychology” synergistic effect. Second, the large sample size (n = 1994) and multi-dimensional assessments (SAS, SDS, PSQI scales plus genotyping) enhance result reliability compared to single-scale or small-sample studies. The reliability of our assessments is supported by recent validation studies confirming that SAS, SDS, and PSQI are robust tools for evaluating mental health and sleep quality in occupational populations, with consistent psychometric properties across diverse cultural contexts (53). Third, while RORA gene variants showed no independent association, this study introduces a novel focus on RORA’s role in circadian-sleep networks, paving the way for future research.
This study has several limitations. First, its cross-sectional design precludes causal inference, necessitating longitudinal cohort studies to validate the temporal sequence of “gene-psychology-sleep” associations. Second, the sample primarily comprises mental workers from select Xinjiang enterprises, with insufficient ethnic minority representation, limiting generalizability to broader populations. Third, unadjusted lifestyle factors (e.g., physical activity, caffeine intake, medication use) may confound the observed relationships. Fourth, self-reported questionnaires are susceptible to recall bias. Future research should include multi-ethnic samples, objective sleep monitoring (e.g., polysomnography), and longitudinal designs to clarify mechanisms.
Again, the cross-sectional design precludes conclusions about causality. For example, we cannot determine whether anxiety/depression precedes sleep disorders or vice versa, nor can we confirm that rs934945 directly modulates this relationship-longitudinal cohort studies tracking genetic, psychological, and sleep parameters over time are needed to clarify temporal sequences.
5 Conclusion
In summary, this study found that the occurrence of anxiety, depression, and sleep disorders is serious among mental workers in Xinjiang and that the polymorphisms of anxiety, depression, and PER2 genes and their interactions have an impact on the occurrence of sleep disorders. The situation of sleep disorders among mental workers in Xinjiang is very serious, so the relevant departments and workplaces should develop corresponding policies and measures to rationally arrange work tasks and shift systems, while at the same time carrying out targeted psychological counseling and lectures to alleviate mental workers’ anxiety, depression, and other negative emotions. Simultaneously, their self-confidence and ability to positively cope with problems should be cultivated, so as to reduce the effects of anxiety and depression on sleep disorders and further promote the development of occupational groups to reduce sleep disorders and improve physical and mental health in the working population. The scales used in this study are internationally used scales with good reliability and validity, thus minimizing bias.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The research proposal was approved by the Ethics Committee of Xinjiang Medical University (the ethical No. 20170214–174). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
XY: Data curation, Writing – original draft. XM: Data curation, Writing – review & editing. LS: Writing – review & editing. XL: Conceptualization, Project administration, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was funded by the National Natural Science Foundation of China (grant number: 81760581), the 14-th Five-Year Plan Distinctive Program of Public Health and Preventive Medicine in Higher Education Institutions of Xinjiang Uygur Autonomous Region and the Xinjiang Uygur Autonomous Region Second Batch of “Tianchi Talents” Introduction Program Projects.
Acknowledgments
The authors thank the study participants.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
1. Min, J, Kim, Y, Lee, S, Jang, TW, Kim, I, and Song, J. The fourth industrial revolution and its impact on occupational health and safety, worker’s compensation and labor conditions. Saf Health Work. (2019) 10:400–8. doi: 10.1016/j.shaw.2019.09.005
2. Torrance, I, and Heron, R. Occupational health should be part of the NHS. BMJ. (2017) 357:j2334. doi: 10.1136/bmj.j2334
3. Chirico, F, Heponiemi, T, Pavlova, M, Zaffina, S, and Magnavita, N. Psychosocial risk prevention in a global occupational health perspective. A descriptive analysis. Int J Environ Res Public Health. (2019) 16:2470. doi: 10.3390/ijerph16142470
4. Desouky, D, and Allam, H. Occupational stress, anxiety and depression among egyptian teachers. J Epidemiol Global Health. (2017) 7:191–8. doi: 10.1016/j.jegh.2017.06.002
5. Booker, LA, Sletten, TL, Alvaro, PK, Barnes, M, Collins, A, Chai-Coetzer, CL, et al. Exploring the associations between shift work disorder, depression, anxiety and sick leave taken amongst nurses. J Sleep Res. (2020) 29:e12872. doi: 10.1111/jsr.12872
6. Brondolo, E, Brady ver Halen, N, Pencille, M, Beatty, D, and Contrada, RJ. Coping with racism: a selective review of the literature and a theoretical and methodological critique. J Behav Med. (2009) 32:64–88. doi: 10.1007/s10865-008-9193-0
7. Twenge, JM, and Joiner, TE. Us census bureau-assessed prevalence of anxiety and depressive symptoms in 2019 and during the 2020 covid-19 pandemic. Depress Anxiety. (2020) 37:954–6. doi: 10.1002/da.23077
8. Stringaris, A. What is depression? J Child Psychol Psychiatry. (2017) 58:1287–9. doi: 10.1111/jcpp.12844
9. Depression W H O. Other common mental disorders: Global health estimates. Geneva: World Health Organization (2017). 24 p.
10. Perez-Pozuelo, I, Zhai, B, Palotti, J, Mall, R, Aupetit, M, Garcia-Gomez, JM, et al. The future of sleep health: a data-driven revolution in sleep science and medicine. NPJ Digit Med. (2020) 3:1–15. doi: 10.1038/s41746-020-0244-4
11. Panel, CC, Watson, NF, Badr, MS, et al. Recommended amount of sleep for a healthy adult: a joint consensus statement of the american academy of sleep medicine and sleep research society. J Clin Sleep Med. (2015) 11:591–2. doi: 10.5664/jcsm.4758
12. Medic, G, Wille, M, and Hemels, ME. Short-and long-term health consequences of sleep disruption. Nat Sci Sleep. (2017) 9:151–61. doi: 10.2147/NSS.S134864
13. Kerkhof, GA. Epidemiology of sleep and sleep disorders in the Netherlands. Sleep Med. (2017) 30:229–39. doi: 10.1016/j.sleep.2016.09.015
14. Ohayon, MM, and Bader, G. Prevalence and correlates of insomnia in the swedish population aged 19–75 years. Sleep Med. (2010) 11:980–6. doi: 10.1016/j.sleep.2010.07.012
15. Jike, M, Itani, O, Watanabe, N, Buysse, DJ, and Kaneita, Y. Long sleep duration and health outcomes: a systematic review, meta-analysis and meta-regression. Sleep Med Rev. (2018) 39:25–36. doi: 10.1016/j.smrv.2017.06.011
16. Sakamoto, K, Higo-Yamamoto, S, Egi, Y, Miyazaki, K, and Oishi, K. Memory dysfunction and anxiety-like behavior in a mouse model of chronic sleep disorders. Biochem Biophys Res Commun. (2020) 529:175–9. doi: 10.1016/j.bbrc.2020.05.218
17. Srivali, N, Thongprayoon, C, Tangpanithandee, S, Krisanapan, P, Mao, MA, Zinchuk, A, et al. Periodic limb movements during sleep and risk of hypertension: a systematic review. Sleep Med. (2023) 102:173–9. doi: 10.1016/j.sleep.2023.01.008
18. Wiegelmann, M, Völker, J, and Sonnentag, S. Sleep has many faces: the interplay of sleep and work in predicting employees’ energetic state over the course of the day. J Occup Health Psychol. (2023) 28:52–63. doi: 10.1037/ocp0000345
19. Franken, P, Thomason, R, Heller, HC, O’Hara, BF, et al. A non-circadian role for clock-genes in sleep homeostasis: a strain comparison. BMC Neurosci. (2007) 8:1–11. doi: 10.1186/1471-2202-8-87
20. Tähkämö, L, Partonen, T, and Pesonen, A-K. Systematic review of light exposure impact on human circadian rhythm. Chronobiol Int. (2019) 36:151–70. doi: 10.1080/07420528.2018.1527773
21. Ashbrook, LH, Krystal, AD, Fu, Y-H, and Ptáček, LJ. Genetics of the human circadian clock and sleep homeostat. Neuropsychopharmacology. (2020) 45:45–54. doi: 10.1038/s41386-019-0476-7
22. Weissová, K, Škrabalová, J, Skálová, K, Červená, K, Bendová, Z, Miletínová, E, et al. Circadian rhythms of melatonin and peripheral clock gene expression in idiopathic rem sleep behavior disorder. Sleep Med. (2018) 52:1–6. doi: 10.1016/j.sleep.2018.07.019
23. Tsao, C-H, Flint, J, and Huang, G-J. Influence of diurnal phase on behavioral tests of sensorimotor performance, anxiety, learning and memory in mice. Sci Rep. (2022) 12:1–10. doi: 10.1038/s41598-021-03155-5
24. Shi, S, White, M, Borsetti, H, Shi, SQ, White, MJ, Borsetti, HM, et al. Molecular analyses of circadian gene variants reveal sex-dependent links between depression and clocks. Transl Psychiatry. (2016) 6:e748–8. doi: 10.1038/tp.2016.9
25. Shi, L, Liu, Y, Jiang, T, Yan, P, Cao, F, Chen, Y, et al. Relationship between mental health, the clock gene, and sleep quality in surgical nurses: a cross-sectional study. Biomed Res Int. (2020) 2020:5763. doi: 10.1155/2020/4795763
26. Crespo-Ruiz, B, Rivas-Galan, S, Fernandez-Vega, C, Crespo-Ruiz, C, and Maicas-Perez, L. Executive stress management: physiological load of stress and recovery in executives on workdays. Int J Environ Res Public Health. (2018) 15:2847. doi: 10.3390/ijerph15122847
27. Zung, W. The self-rating anxiety scale (sas). Instruments for measuring nursing practice and other health care variables. Maryland: US Dept of Health, Education and Welfare, (1979): 196–199.
28. Dunstan, DA, and Scott, N. Norms for zung’s self-rating anxiety scale. BMC Psychiatry. (2020) 20:1–8. doi: 10.1186/s12888-019-2427-6
29. Zung, WW, Richards, CB, and Short, MJ. Self-rating depression scale in an outpatient clinic: further validation of the sds. Arch Gen Psychiatry. (1965) 13:508–15. doi: 10.1001/archpsyc.1965.01730060026004
30. Jokelainen, J, Timonen, M, Keinänen-Kiukaanniemi, S, Härkönen, P, Jurvelin, H, and Suija, K. Validation of the zung self-rating depression scale (sds) in older adults. Scand J Prim Health Care. (2019) 37:353–7. doi: 10.1080/02813432.2019.1639923
31. Buysse, DJ, Reynolds, CF, Monk, TH, Berman, SR, and Kupfer, DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. (1989) 28:193–213. doi: 10.1016/0165-1781(89)90047-4
32. Tsai, P-S, Wang, S-Y, Wang, M-Y, Su, CT, Yang, TT, Huang, CJ, et al. Psychometric evaluation of the chinese version of the Pittsburgh sleep quality index (cpsqi) in primary insomnia and control subjects. Qual Life Res. (2005) 14:1943–52. doi: 10.1007/s11136-005-4346-x
33. Niu, WL, Hou, RL, Wei, LQ, Luo, X, Zhang, X, et al. Relationship between teacher burnout and sleep quality in Inner Mongolia undergraduate colleges. J Baotou Med Coll. (2020) 36:43–6. doi: 10.16833/j.cnki.jbmc.2020.05.015
34. Lv, YP, Zhang, GF, and Li, M. The effects of cell phone dependence on sleep quality and the mediating role of anxiety among railroad vehicle system workers. China Prim Health Care. (2022) 36:110–2. doi: 10.3969/j.issn.1001-568X.2022.08.0033
35. Li, X, Gao, X, and Liu, J. Cross-sectional survey on the relationship between occupational stress, hormone levels, and the sleep quality of oilfield workers in Xinjiang, China. Int J Environ Res Public Health. (2019) 16:3316. doi: 10.3390/ijerph16183316
36. Hicklin, D, and Schwander, J. Shift work and sleep. Praxis. (2019) 108:119–24. doi: 10.1024/1661-8157/a003163
37. Fu, W, Wang, C, Zou, L, Guo, Y, Lu, Z, Yan, S, et al. Psychological health, sleep quality, and coping styles to stress facing the covid-19 in Wuhan, China. Transl Psychiatry. (2020) 10:1–9. doi: 10.1038/s41398-020-00913-3
38. Yi, X, Yang, J, Gao, X, and Li, F. The relationship between occupational stress, mental health and work ability of coal chemical workers in Xinjiang. Front Psych. (2022) 13:903534. doi: 10.3389/fpsyt.2022.903534
39. Morssinkhof, MWL, Van Wylick, DW, Priester-Vink, S, Van Der Werf, YD, Den Heijer, M, Van Den Heuvel, OA, et al. Associations between sex hormones, sleep disorders and depression: a systematic review. Neurosci Biobehav Rev. (2020) 118:669–80. doi: 10.1016/j.neubiorev.2020.08.006
40. Zhang, Y, Shen, J, Zhou, Z, Sang, L, Zhuang, X, Chu, M, et al. Relationships among shift work, hair cortisol concentration and sleep disorders: a cross-sectional study in China. BMJ Open. (2020) 10:e038786. doi: 10.1136/bmjopen-2020-038786
41. Chen, J, Du, L, Wang, P, Wei, H, Han, Z, et al. Effects of circadian rhythm disruption on sleep due to ambient light. Adv Mod Biomed. (2015) 15:6046–9. doi: 10.13241/j.cnki.pmb.2015.31.013
42. Wang, J, Liu, J, Xie, H, and Gao, X. Effects of work stress and period3 gene polymorphism and their interaction on sleep quality of non-manual workers in Xinjiang, China: a cross-sectional study. Int J Environ Res Public Health. (2022) 19:6843. doi: 10.3390/ijerph19116843
43. Park, H, and Suh, B. Association between sleep quality and physical activity according to gender and shift work. J Sleep Res. (2020) 29:e12924. doi: 10.1111/jsr.12924
44. Guo, Y, Deng, X, Tang, C, et al. Analysis of the correlation between anxiety and depression with tinnitus and sleep quality in occupational noise deafness patients. China Occupat Med. (2021) 48:407–11.
45. Korkmaz, S, Kazgan, A, Çekiç, S, Tartar, AS, Balcı, HN, and Atmaca, M. The anxiety levels, quality of sleep and life and problem-solving skills in healthcare workers employed in covid-19 services. J Clin Neurosci. (2020) 80:131–6. doi: 10.1016/j.jocn.2020.07.073
46. Sandberg, JC, Grzywacz, JG, Talton, JW, Quandt, SA, Chen, H, Chatterjee, AB, et al. A cross-sectional exploration of excessive daytime sleepiness, depression, and musculoskeletal pain among migrant farmworkers. J Agromedicine. (2012) 17:70–80. doi: 10.1080/1059924X.2012.626750
47. Watson, NF, Harden, KP, Buchwald, D, Vitiello, MV, Pack, AI, Weigle, DS, et al. Sleep duration and body mass index in twins: a gene-environment interaction. Sleep. (2012) 35:597–603. doi: 10.5665/sleep.1810
48. Lane, JM, Qian, J, Mignot, E, Redline, S, Scheer, FAJL, and Saxena, R. Genetics of circadian rhythms and sleep in human health and disease. Nat Rev Genet. (2023) 24:4–20. doi: 10.1038/s41576-022-00519-z
49. Ballester-Navarro, P, Martínez-Madrid, MJ, Javaloyes-Sanchís, A, Belda-Canto, C, Aguilar, V, Inda, MDM, et al. Interplay of circadian clock and melatonin pathway gene variants in adults with autism, intellectual disability and sleep disorders. Res Autism Spectr Disord. (2021) 81:101715. doi: 10.1016/j.rasd.2020.101715
50. Vanderlind, WM, Beevers, CG, Sherman, SM, Trujillo, LT, McGeary, JE, Matthews, MD, et al. Sleep and sadness: exploring the relation among sleep, cognitive control, and depressive symptoms in young adults. Sleep Med. (2014) 15:144–9. doi: 10.1016/j.sleep.2013.10.006
51. He, SC, Wu, S, Du, XD, Jia, Q, Wang, C, Wu, F, et al. Interactive effects of corticotropin-releasing hormone receptor 1 gene and work stress on burnout in medical professionals in a chinese han population. J Affect Disord. (2019) 252:1–8. doi: 10.1016/j.jad.2019.03.084
52. Francis, TC, and Porcu, A. Emotionally clocked out: cell-type specific regulation of mood and anxiety by the circadian clock system in the brain. Front Mol Neurosci. (2023) 16:1188184. doi: 10.3389/fnmol.2023.1188184
Keywords: mental workers, anxiety, depression, sleep disorders, circadian clock genes
Citation: Yi X, Ma X, Shi L and Li X (2025) A matched case-control study on the interaction of anxiety, depression, and circadian CLOCK genes (CLOCK, PER2, RORA) in sleep disorders among mental workers. Front. Public Health. 13:1579151. doi: 10.3389/fpubh.2025.1579151
Edited by:
Yibo Wu, Zhejiang University, ChinaReviewed by:
Dina Keumala Sari, Universitas Sumatera Utara, IndonesiaAhmad Mohammad Shaddad, Assiut University, Egypt
Copyright © 2025 Yi, Ma, Shi and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xue Li, eGp5a2R4OTc5N0AxMjYuY29t
†These authors have contributed equally to this work
 Xiaoting Yi1†
Xiaoting Yi1† Xiaofan Ma
Xiaofan Ma Lingyun Shi
Lingyun Shi Xue Li
Xue Li