- 1Department of Pharmacy, Xijing 986 Hospital, Fourth Military Medical University, Xi’an, Shaanxi, China
- 2Department of Dermatology, Xijing Hospital, Fourth Military Medical University, Xi’an, Shaanxi, China
White phosphorus (WP), a highly reactive and toxic substance, has been widely used in military applications. White phosphorus munitions (WPMs) embody a complex intersection of military utility and humanitarian concern, inflicting devastating injuries through their dual destructive mechanisms. These weapons induce severe thermal and chemical damage, penetrating deep into tissues to cause progressive necrosis and life-threatening systemic toxicity even with minimal exposure. This review synthesizes current understanding of WP injury pathophysiology—including hypocalcemia-induced arrhythmias, acute respiratory distress syndrome, and hepatorenal failure—while examining evidence-based interventions spanning battlefield first aid to advanced regenerative therapies. By integrating perspectives from military medicine, toxicology, and global health equity, this review provides a comprehensive foundation for clinicians, and researchers confronting the multidimensional challenges posed by WP injuries in conflict and post-conflict settings.
1 Introduction
White phosphorus munitions (WPMs), historically known for their significant destructive power, have played a complex and contentious role in warfare since their unique chemical properties were first discovered. First isolated through Hennig Brand’s serendipitous 1,669 experiment involving urine distillation, WP exhibits unique thermochemical properties—a low melting point (44.1°C), autoignition at 34–40°C under ambient conditions, and combustion temperatures exceeding 1,300°C (1–4). These attributes drove its rapid militarization, from British forces deploying WP grenades in World War I trenches to modern artillery shells used in asymmetric conflicts (5). Although Protocol III of the Convention on Certain Conventional Weapons (CCW) restricts WP use near civilians, its recurrent deployment in Fallujah, Gaza, Lebanon, and Ukraine (2022–2023) underscores persistent legal ambiguities (6).
However, the deployment of WPMs carries severe consequences, particularly concerning the profound harm they inflict on the human body. Upon ignition, these munitions produce extreme heat and toxic fumes, leading to extensive chemical burns and respiratory injuries. Such injuries cause significant pain, complicating medical treatment and extending recovery periods, often resulting in permanent disabilities or fatalities (7, 8). The pathobiology of WP injuries arises from synergistic thermal and chemical mechanisms. Combustion generates metastable P₄O₁₀ aerosols that hydrolyze into orthophosphoric acid (H₃PO₄) upon mucosal contact, inducing liquefactive necrosis. Crucially, lipid-soluble WP particles permeate fascial planes, causing delayed systemic toxicity through phosphide-induced Ca2+ chelation and mitochondrial dysfunction. Clinical reports confirm fatalities from ventricular arrhythmias even with <10% total body surface area (TBSA) burns, attributable to acute hypocalcemia (serum Ca2+ < 1.8 mmol/L) (9).
Current emergency protocols emphasize three imperatives: (1) immediate oxygen exclusion via immersion or wet dressings to halt combustion; (2) mechanical debridement over copper sulfate application due to nephrotoxicity risks; and (3) continuous cardiac monitoring for arrhythmia prevention. Emerging interventions like chelating hydrogels and extracorporeal phosphorus adsorption show preclinical promise but lack battlefield validation. Post-conflict environmental burdens further complicate recovery—unburned WP oxidizes into persistent phosphates that bioaccumulate in aquatic ecosystems, exemplified by Lebanon’s 2006 conflict where 462 hectares of farmland became non-arable (10).
Given these characteristics, the use of WPMs has attracted substantial international attention, leading to some regulatory restrictions. Despite these limitations, WPMs continues to be employed in certain regional conflicts, resulting in numerous civilian and military casualties. Consequently, raising awareness about WPMs burns and disseminating emergency response measures is of paramount importance. By synthesizing existing literature, we emphasize the severity of WPMs burns, enhance awareness of these specific injuries, and offer practical guidance for medical professionals and military personnel. Through this review, we hope to promote a deeper understanding of WP burns and provide direction and reference for future research and practice.
2 The physicochemical properties of WP
2.1 Chemical structure and stability
WP (chemical formula P₄) is one of the allotropes of phosphorus, typically appearing as a colorless, transparent, or slightly yellow crystalline solid (11). In its solid form, it is classified under the UN numbers 2,447, 1,381, and 1,338 (12). The presence of impurities can alter its color, resulting in what is known as yellow phosphorus. WP is extensively used in various industrial applications, including the production of semiconductors and fireworks, as well as in rodenticides and incendiary devices (12). It possesses a density of 1.82 g/cm3, a melting point of 44.1°C, and a boiling point of 280°C (13). Despite its significant industrial and military applications, WP is highly reactive and toxic, posing substantial risks to both the environment and human health.
The chemical structure of WP consists of a tetrahedron of four phosphorus atoms. Although this structure provides some stability, it places WP in a high-energy state, making it unstable at room temperature. It readily reacts with oxygen in the air to form phosphorus pentoxide (P₄O₁₀), releasing energy and demonstrating spontaneous combustion. This characteristic necessitates stringent safety measures during storage and use, typically requiring an airtight environment (14). Moreover, WP is nearly insoluble in water. Although water can extinguish burning WP, the compound can quickly reignite and produce smoke under dry conditions (13).
2.2 Combustion characteristics and reactivity of WP
WP is renowned for its distinctive combustion properties, which are central to its physical and chemical characteristics. With an exceptionally low ignition point of around 30°C, it is highly susceptible to spontaneous combustion at room temperature (7, 15–17). This susceptibility is further exacerbated by even minimal friction or impact, making its handling particularly hazardous (11). The combustion process of WP involves complex chemical reactions and significant physical effects, such as the emission of thermal and light radiation. When ignited, it produces a bright yellow flame accompanied by substantial heat and a dense cloud of white smoke. The temperatures generated during this process can reach approximately 1,300°C, far surpassing the typical 500–1,000°C observed in residential fires, thereby underscoring its potential danger (18). In addition to its high-temperature combustion, WP exhibits unique luminescent properties. In dark environments, it glows with a distinctive green light, a phenomenon known as chemiluminescence (19), resulting from its reaction with oxygen in the air. This reaction also produces a garlic-like odor and further white smoke (17). Beyond its reactivity with oxygen, WP is capable of vigorous reactions with various other chemicals, including halogens and sulfur. These reactions can lead to the formation of toxic byproducts such as phosphine (PH₃), which pose additional health and environmental risks (20). WP is highly lipophilic and can penetrate deep into tissues, leading to severe thermal and chemical burns (21). The high reactivity and potential hazards associated with WP necessitate stringent safety measures during its storage and handling. Typically, it is stored underwater or in inert atmospheres to prevent accidental ignition.
2.3 Molecular mechanisms of WP toxicity
WP is highly toxic, posing significant risks to human health (Figure 1). Classified as lethal by the Globally Harmonized System of Classification and Labeling of Chemicals (GHS) for both inhalation (H330) and ingestion (H300), its oral lethal dose (LD50) in humans is extremely low at 1.4 mg/kg (5, 22). Toxicity primarily stems from its combustion products—phosphorus pentoxide (P₄O₁₀) and phosphoric acid (H₃PO₄)—which exert multiple harmful effects at the molecular level (9).
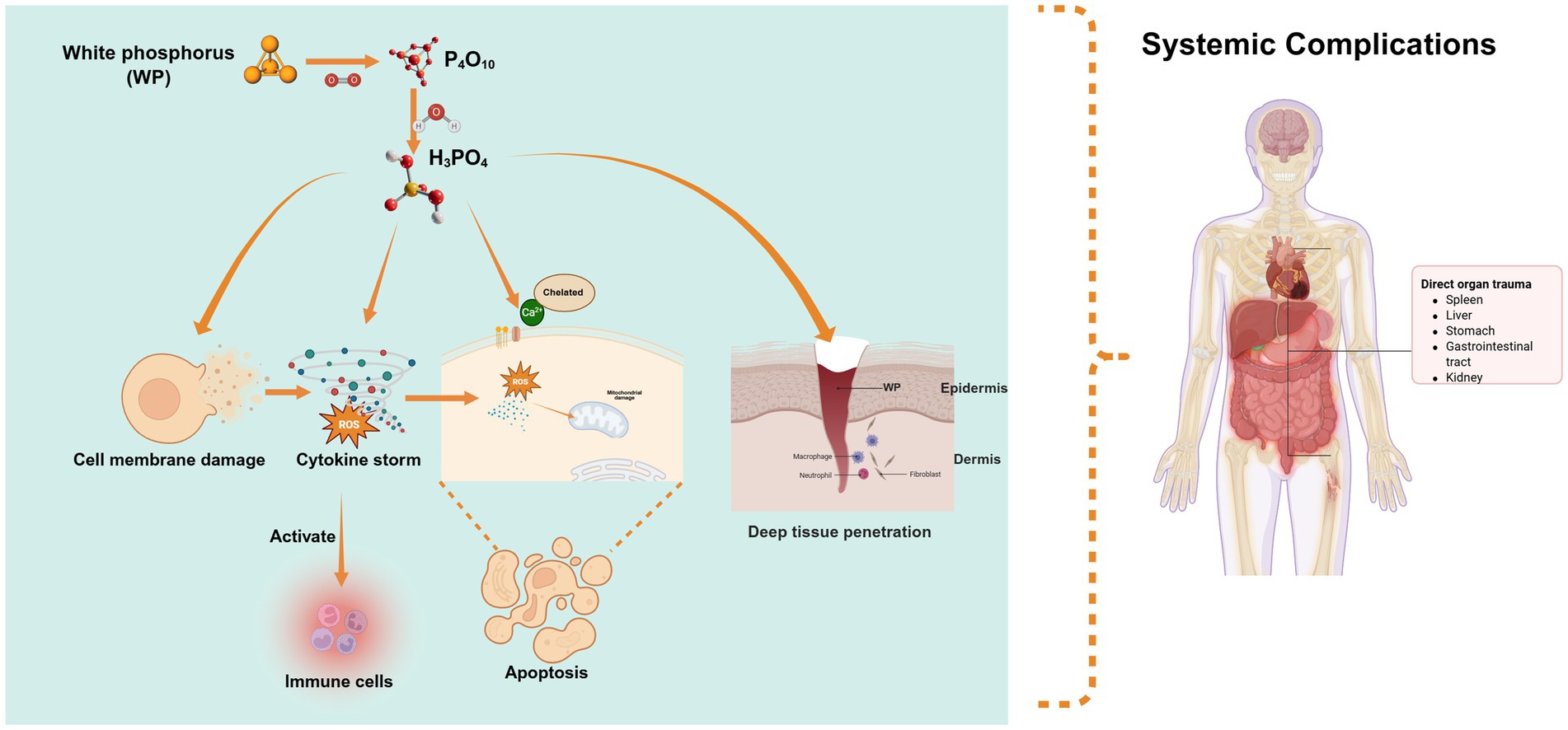
Figure 1. Molecular mechanisms of WP toxicity: the toxicity of white phosphorus stems from its combustion products (P₄O₁₀, H₃PO₄), which, via acidity and corrosivity, damage cell membranes, generate ROS, disrupt calcium homeostasis, and trigger intrinsic apoptotic pathways. Additionally, its lipophilicity enables deep penetration causing systemic toxicity (e.g., metabolic acidosis, multi-organ failure), while combustion products activate immune cells, exacerbate inflammation, and drive chronic complications.
These compounds directly damage cell membranes through their acidity and corrosivity, increasing permeability and triggering cell death, while releasing intracellular contents and activating inflammation. Simultaneously, the combustion process generates reactive oxygen species (ROS), causing oxidative stress that damages cellular components, impairs mitochondrial function, and induces apoptosis. Phosphoric acid also disrupts calcium homeostasis by binding to calcium channels, leading to abnormal signaling and cellular dysfunction. These combined effects—membrane damage, ROS generation, and calcium dysregulation—ultimately trigger intrinsic apoptosis pathways (7, 23–25). Moreover, WP’s lipophilicity enables deep tissue penetration and systemic toxicity, manifesting as metabolic acidosis, hypocalcemia, hyperphosphatemia, and multi-organ failure. Additionally, phosphoric acid and combustion products activate immune cells, fueling inflammation and contributing to chronic complications (4, 26, 27). Given its corrosive nature and far-reaching impacts, strict safety protocols are essential to prevent exposure and mitigate risks to both human health.
3 Symptoms of exposure to WP from explosive burns
WPMs, characterized by their pyrophoric properties and high chemical reactivity, inflict multifaceted trauma through combined thermal, chemical, and systemic toxic effects (28). Data from modern conflicts, such as the 2020 Nagorno-Karabakh war, reveal alarming injury patterns: 79.3% of victims sustained head injuries, 90.2% upper limb burns, and 46.3% lower limb involvement, with 37.9% exhibiting multiple shrapnel wounds. Notably, 28.7% required intensive care, and 10.3% succumbed to complications like acute respiratory distress syndrome (ARDS) and multi-organ failure within 30 days of hospitalization (29, 30).
3.1 Initial symptoms of exposure to WP
As the body’s largest organ, the skin is the first to bear the brunt when it comes into contact with WP. Upon contact, WP rapidly oxidizes at temperatures ranging from 34 to 40°C, generating extreme heat exceeding 800°C. This intense heat causes full-thickness burns, which are marked by yellow necrotic eschars and emit a pungent garlic-like odor (17, 31). The lipid-soluble P₄ molecules penetrate deep into subcutaneous tissues, continuing to cause progressive liquefactive necrosis even after the surface flames are extinguished.
Initial symptoms of WP burns include erythema, excruciating pain (more severe than sulfuric acid burns), and vesicular eruptions. In the extremities, these symptoms often progress to compartment syndrome. These burns are a combination of thermal and chemical injuries. The corrosive action of phosphoric acid, the heat generated by the reaction with phosphorus pentoxide, and the latter’s hygroscopic properties all contribute to extensive tissue damage (7, 13, 28). WP burns have a distinctive waxy yellow appearance in natural light and fluoresce under ultraviolet light. Compared to typical thermal burns, the healing process for WP burns is significantly slower. Full-thickness burns caused by WP are usually necrotic, necessitating immediate and effective medical treatment. If phosphorus particles are not completely removed from the burn site, they will keep reacting and generating heat until they are eliminated, the phosphorus is exhausted, or the oxygen supply is cut off (32).
In addition, the smoke produced by burning WP contains highly toxic and corrosive components like phosphorus pentoxide and phosphoric acid. Inhalation of this smoke irritates the respiratory system, triggering symptoms such as coughing, chest tightness, and breathing difficulties (33). Severe inhalation can lead to chemical pneumonia, pulmonary edema, or even respiratory failure. The irritants in the smoke also affect the eyes and mucous membranes, causing eye pain, tearing, and conjunctival congestion (34). Even in outdoor settings, low concentrations of WP smoke can immediately irritate the eyes, mucous membranes, and upper respiratory tract. In enclosed spaces, high concentrations can cause severe, irreversible damage to both the upper and lower respiratory tracts. WP particles can directly damage the cornea, leading to burns and potentially corneal perforation. Exposure to the smoke can also result in eye irritation, blepharospasm, photophobia, tearing, and conjunctivitis (35).
3.2 Subsequent symptoms of contamination by WP explosion
WP contamination from burns initiates a cascade of severe physiological disruptions upon entering the body. Inhalation can directly burn the lungs, heart, and other organs, and may also lead to systemic absorption (27, 28). Skin or mucous membrane exposure can similarly result in systemic uptake, triggering multi-organ dysfunction. This manifests as liver, kidney, and cardiac toxicity, alongside metabolic imbalances like hypocalcemia and elevated serum phosphorus levels. Blood abnormalities, including thrombocytopenia and coagulopathy, have also been observed. The central nervous system is affected, causing delirium, confusion, hallucinations, and coma. Moreover, the traumatic scene of a WP explosion—characterized by searing flames and acrid smoke—can inflict profound psychological trauma, often leading survivors to develop post-traumatic stress disorder (PTSD) with symptoms of persistent fear, anxiety, nightmares, and flashbacks (17, 23, 26, 36–38).
Systemic toxicity from WP unfolds in three distinct stages. The first stage is marked by gastrointestinal symptoms: upper abdominal pain, nausea, vomiting, loss of appetite, and occasionally jaundice (9, 20). These may be accompanied by headache, convulsions, coma, and cardiovascular failure; affected individuals’ breath, saliva, and vomit often carry a characteristic garlic-like odor. The second stage appears asymptomatic but shows early signs of toxic hepatitis upon liver histology examination (23, 24). By the third stage, occurring 4 to 8 days post-exposure, multi-organ failure sets in, encompassing neurotoxicity, bleeding tendencies, liver and kidney failure, and shock.
WP’s lipid solubility enables deep subcutaneous penetration, causing extensive necrosis. These burns are far more painful than typical thermal injuries and rapidly induce critical physiological changes. Within an hour of exposure, hypocalcemia, hyperphosphatemia, and calcium-phosphorus imbalances can occur (39, 40). Phosphoric acid, formed when phosphorus pentoxide reacts with water, consumes calcium (and potentially magnesium) during neutralization, forming insoluble calcium (magnesium) phosphate (19). This process depletes blood calcium and magnesium levels, with the degree of imbalance reflecting the amount of phosphorus “burned” in the body. In some patients, life-threatening hypocalcemia and hyperphosphatemia develop within the first hour, risking arrhythmias like QT interval prolongation, ST-T wave changes, bradycardia, or sudden death (9, 17, 26, 41).
The consequences extend beyond physical harm. Approximately 68% of survivors develop long-term neuropsychiatric disorders, including insomnia, hypervigilance, and PTSD, worsened by the weapon’s luminous smoke and recurring combustion events (29). Research by Khurshid et al. found that 42% of cases suffered memory impairment and executive dysfunction, likely due to lipid-soluble phosphorus metabolites crossing the blood–brain barrier. The psychological toll is compounded by disfigurement: 20.7% of victims sustain facial burns that impede social reintegration (32).
Environmental contamination further exacerbates the risk. In conflict zones, soil phosphorus levels can reach 14.1%, posing threats through residual particle ignition and groundwater contamination by phosphoric acid. This persistence turns acute burns into chronic wounds; 31% of cases develop Marjolin’s ulcers within 5 years. The ongoing cycle of harm underscores the need for integrated environmental decontamination in post-conflict medical strategies (42).
The complex interplay of immediate tissue damage, progressive systemic toxicity, and lasting psychosocial disability demands comprehensive, multidisciplinary approaches in conflict medicine.
4 Battlefield first aid for WP burns
WPMs impose catastrophic injuries through combined thermal-chemical damage and systemic toxicity. These characteristics mandate time-critical interventions to limit tissue destruction and systemic toxicity.
4.1 Core principles of emergency management
Effective battlefield care requires sequential implementation of five critical actions: (1) rapid removal of contaminated clothing to prevent secondary ignition; (2) oxygen deprivation through water immersion or moist dressings; (3) mechanical debridement of particles under low-light conditions; (4) continuous hypothermic irrigation (1–4°C) to suppress residual combustion; and (5) systemic monitoring for electrolyte abnormalities.
4.2 First aid for WP contamination in battlefield conditions
WP, a highly hazardous chemical with self-igniting properties in air, demands immediate isolation from oxygen sources upon exposure. The initial response includes prompt removal of all contaminated clothing and accessories to mitigate further injury (24, 43). Emergency Treatment Protocol is as follows:
4.2.1 Oxygen deprivation and fire extinguishment
The first-line intervention involves extinguishing the burning WP by immersing the affected area in cold water (<25°C) or applying water-saturated dressings, which should be replaced every 5–7 min. Given its low melting point of 44°C, warm or hot water must be strictly avoided, as it can liquefy the phosphorus, increasing its surface contact and exacerbating tissue damage (28). Additionally, aggressive water flushing risks dispersing burning particles onto uninjured skin or rescuers, which may reignite upon drying (13).
4.2.2 Particle decontamination
Mechanical removal of unburned phosphorus particles is a critical step. Visible particles should be carefully extracted using blunt forceps under the illumination of a Wood’s lamp, which causes WP to fluoresce. In the absence of a Wood’s lamp, Ultraviolet (UV) flashlights or fluorescent detection devices may substitute, leveraging WP’s fluorescent properties for particle localization (7, 31, 44). Gentle compression with moist gauze for 3–5 min can dislodge embedded particles from subcutaneous tissues (43, 45). Copper sulfate, traditionally used for particle visualization, is contraindicated due to its potential to cause glucose-6-phosphate dehydrogenase (G6PD) inhibition, leading to hemolytic anemia and hemoglobinuria. Instead, 1–3% silver nitrate solutions offer a safer alternative for identifying deeply embedded particles (29, 46).
4.2.3 Wound management and stabilization
Following particle removal, continuous irrigation with cold saline is essential to ensure complete decontamination. High-pressure water jets, such as those from syringes, can dislodge tenacious embedded particles while maintaining a cold temperature to prevent phosphorus liquefaction. Before definitive treatment, cold saline-soaked gauze dressings provide interim protection against re-ignition. Pain control should be administered according to standard analgesic protocols (29, 47, 48).
4.2.4 Transport and post-treatment care
Burned areas should be covered with moist, cool gauze to prevent re-exposure to air and subsequent reignition during transport. In cases where maintaining gauze moisture is challenging, hydrogel dressings like Water-Jel WJ110, composed of 96% water and containing antibacterial tea tree oil, offer effective cooling and infection prevention (49, 50). As alternatives, Moist Exposed Burn Ointments can be applied; in extreme emergencies (51), urine-soaked bandages may serve as a last-resort measure.
Immediately after injury, effervescent calcium and magnesium tablets should be administered both orally and topically (dissolved in water) to counteract potential hypocalcemia and hyperphosphatemia. In the subsequent 12 h, close monitoring of serum calcium and phosphorus levels, electrocardiogram changes, and signs of multi-organ failure is imperative, with assessments conducted hourly to detect and manage complications promptly (19).
4.3 Systemic treatment
Upon admission to the rear hospital’s emergency department, a Wood’s lamp examination is essential to identify and completely remove any residual WP on the skin. Post-particle removal, burn wounds require immediate cooling and cleansing. Cooling not only decelerates burn progression and alleviates pain but also minimizes deep-tissue damage. Rinsing with copious amounts of clean water is recommended, while avoiding forceful flows that may exacerbate wound injury. The cleansing process targets the elimination of remaining particles and contaminants, thereby reducing the risk of infection. To safeguard wound integrity, substances like soap and alcohol should be strictly avoided during cleaning (28).
Debridement, a cornerstone of treatment, involves excising necrotic and non-viable tissue. Immediate surgical debridement is often necessary and may need reiteration until all phosphorus particles are eradicated. Wounds should be inspected at least twice daily for new particles or areas of smoldering, which signal the need for further intervention. Covering the debrided area with a 5% sulfamylon solution between surgeries aids monitoring. Definitive wound closure should be deferred until thorough debridement is confirmed, at which point split-thickness skin grafting can proceed (29, 44, 50).
Medication and life-support measures are integral to first-aid management. Analgesics such as morphine or fentanyl are administered to control pain, while timely fluid and electrolyte replacement is crucial for preventing and treating shock, maintaining hemodynamic stability. In severe burns, antibiotics may be prescribed to combat infection, and corticosteroids can mitigate the inflammatory response (52, 53).
Clinicians must also assess the risk of systemic absorption in WP burn patients. Significant hypocalcemia can trigger arrhythmias, while inhalation of combustion smoke may lead to respiratory distress or renal failure. In such cases, fluid resuscitation combined with antibiotic therapy and dexamethasone administration may be warranted (33).
For critically ill patients, standard care includes appropriate fluid replacement and vigilant monitoring of electrolyte levels (especially calcium and phosphorus) and electrocardiograms (ECGs). These measures enable early detection and mitigation of complications like hypocalcemia, hyperphosphatemia, and arrhythmias (47). Fluid administration is titrated based on hourly urine output, aiming for 0.5–1 mL/kg/h (17, 54).
Topical treatments are key to burn management. Sulfamylon, a sulfonamide antibiotic, is commonly used for severe burns, serving as an adjunct for second- and third-degree injuries with efficacy against various Gram-positive and -negative bacteria, including Pseudomonas aeruginosa (52). Flaminal Forte, composed of a hydrous alginate polymer and a glucose oxidase–peroxidase bio-enzyme system stabilized by guaiacol, offers antibacterial action and continuous debridement (49, 55).
Innovative approaches enhance treatment efficacy. Pulsed lavage systems using 4°C saline reduce particle retention compared to static irrigation (56), While quantum dot-embedded dressings show potential in wound management, there is currently a lack of research on their ability to enable real-time phosphorus detection with a sensitivity of 0.1 μg/cm2, and further investigation is needed to explore their feasibility and practical applications in this regard (57).
Once the initial treatment phase concludes, patients transition to outpatient follow-up. This comprehensive care plan includes ongoing wound management, pain control, physical therapy, rehabilitation, and psychological support. Some may require skin grafting and interventions to prevent scarring and contractures (58). Emerging therapies like allogeneic mesenchymal stem cell transplantation show promise in accelerating wound healing (Table 1) (59, 60).
5 Conclusion
WPMs pose a significant and enduring threat in contemporary warfare. Their dual thermal and chemical action inflicts devastating multi-system injuries, inducing liquefactive tissue necrosis and systemic toxicity simultaneously. Despite international regulations under the Convention on Certain Conventional Weapons (CCW), definitional ambiguities—especially the exemptions for multipurpose munitions—enable their continued use in conflict zones.
The lipid solubility of WP allows it to penetrate deep into tissues and be absorbed systemically. Even minor burns (affecting ≤10% of the TBSA) can trigger fatal hypocalcemia and multi-organ failure. Prompt intervention is crucial. Immediate removal of particles, thorough saline irrigation, and application of hypoxia-inducing dressings like saline-soaked gauze can stop the combustion process. However, traditional treatments such as using copper sulfate are controversial due to the risks of hemolysis and nephrotoxicity. Systemic complications, including electrolyte imbalances (hypocalcemia, hyperphosphatemia) and coagulopathy, necessitate strict monitoring and calcium gluconate supplementation. Long-term management should also address multiple organ failure, psychological trauma, and socioeconomic reintegration for survivors.
To address these clinical and logistical challenges, future strategies should prioritize three critical advancements: first, advancing portable battlefield decontamination technologies to improve WP particle removal under resource-limited conditions; second, developing biodegradable alternatives (e.g., hexachloroethane) for smoke-screen applications to reduce reliance on harmful incendiary munitions; and third, integrating stem cell therapy and pH-neutral hydrogels into standardized battlefield protocols to enhance wound repair and mitigate systemic toxicity.
Notably, translating these strategies into practice requires robust evidence, yet conducting randomized controlled trials (RCTs) in conflict settings presents unique hurdles. These include ethical constraints related to enrolling combat casualties, variable environmental conditions, and limited access to standardized data collection infrastructure. Such barriers underscore the need for structured observational studies and multicenter registries to generate evidence for battlefield protocols—particularly for novel interventions like mesenchymal stem cell therapy.
Author contributions
MW: Investigation, Writing – original draft, Conceptualization. YB: Conceptualization, Writing – original draft, Data curation. XZ: Writing – review & editing, Supervision. JZ: Conceptualization, Writing – review & editing. AK: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We thank all of the authors for their efforts in this work and all of the participants involved in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Correction note
A correction has been made to this article. Details can be found at: 10.3389/fpubh.2025.1673762.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ARDS, Acute respiratory distress syndrome; CCW, Certain Conventional Weapons; ECGs, Electrocardiograms; GHS, Globally Harmonized System of Classification and Labeling of Chemicals; G6PD, Glucose-6-phosphate dehydrogenase; ICRC, International Committee of the Red Cross; PTSD, Post-traumatic stress disorder; ROS, Reactive oxygen species; TBSA, Total body surface area; TGF-β3, Transforming growth factor-β3; UV, Ultraviolet; WP, White phosphorus; WPMs, White phosphorus munitions.
References
1. Krafft, F. Phosphorus. From elemental light to chemical element. Angew Chem Int Ed Eng. (1969) 8:660–71.
2. Wiesner, A, Steinhauer, S, Beckers, H, Muller, C, and Riedel, S. [P4H]+[Al(OTeF5)4]−: protonation of white phosphorus with the brønsted superacid H[Al(OTeF5)4](solv). Chem Sci. (2018) 9:7169–73. doi: 10.1039/c8sc03023e
3. Barton, DHR, and Zhu, J. Elemental white phosphorus as a radical trap: a new and general route to phosphonic acids. J Am Chem Soc. (1993) 115:2071–2.
4. Aviv, U, Kornhaber, R, Harats, M, and Haik, J. The burning issue of white phosphorus: a case report and review of the literature. Disaster Mil Med. (2017) 3:6. doi: 10.1186/s40696-017-0034-y
5. Preuß-Wössner, J, Wernicke, M, Gerling, I, Zimak, N, and Klückmann, S. Altlasten des Krieges – Verbrennungen durch weißen Phosphor. Rechtsmedizin. (2020) 30:31–7. doi: 10.1007/s00194-019-00361-4
6. Youvan, D. The controversial use of white phosphorus in modern conflicts: A critical analysis. ResearchGate [Preprint]. (2023). doi: 10.13140/RG.2.2.34122.54726
7. Palao, R, Monge, I, Ruiz, M, and Barret, JP. Chemical burns: pathophysiology and treatment. Burns. (2010) 36:295–304. doi: 10.1016/j.burns.2009.07.009
8. Hulse, EJ, Davies, JO, Simpson, AJ, Sciuto, AM, and Eddleston, M. Respiratory complications of organophosphorus nerve agent and insecticide poisoning. Implications for respiratory and critical care. Am J Respir Crit Care Med. (2014) 190:1342–54. doi: 10.1164/rccm.201406-1150CI
9. Bowen, TE, Whelan, TJ Jr, and Nelson, TG. Sudden death after phosphorus burns: experimental observations of hypocalcemia, hyperphosphatemia and electrocardiographic abnormalities following production of a standard white phosphorus burn. Ann Surg. (1971) 174:779–84.
10. Takshe, AA, Van der Molen, I, and Lovett, JC. Examining the lack of legal remedies for environmental damage in the 2006 Lebanon–Israel war. Environ Policy Gov. (2012) 22:27–41. doi: 10.1002/eet.594
11. Al Barqouni, LN, Skaik, SI, Shaban, NR, and Barqouni, N. White phosphorus burn. Lancet. (2010) 376:68. doi: 10.1016/S0140-6736(10)60812-4
12. Karunadasa, KP, Abeywickrama, Y, and Perera, C. White phosphorus burns managed without copper sulfate: lessons from war. J Burn Care Res. (2010) 31:503. doi: 10.1097/BCR.0b013e3181db52be
13. Frank, M, Schmucker, U, Nowotny, T, Ekkernkamp, A, and Hinz, P. Not all that glistens is gold: civilian white phosphorus burn injuries. Am J Emerg Med. (2008) 26:e3-5:974. doi: 10.1016/j.ajem.2008.03.011
14. Scheer, M, Balázs, G, and Seitz, A. P 4 activation by Main group elements and compounds. Chem Rev. (2010) 110:4236–56. doi: 10.1021/cr100010e
15. Robinett, DA, Shelton, B, and Dyer, KS. Special considerations in hazardous materials burns. J Emerg Med. (2010) 39:544–53. doi: 10.1016/j.jemermed.2007.10.045
16. Eldad, A. War burns: the blow and the cure. Clin Dermatol. (2002) 20:388–95. doi: 10.1016/s0738-081x(02)00239-0
17. Chou, TD, Lee, TW, Chen, SL, Tung, YM, Dai, NT, Chen, SG, et al. The management of white phosphorus burns. Burns. (2001) 27:492–7. doi: 10.1016/s0305-4179(01)00003-1
18. Holleman, AF. Lehrbuch der anorganischen Chemie. Chemie, Berlin, Boston: De Gruyter (1985). doi: 10.1515/9783110838176
19. Lakota, J. Medical consequences and treatment of injuries caused by white phosphorus munitions. J NBC Protection Corps. (2024) 7:276–85. doi: 10.35825/2587-5728-2023-7-4-276-285
20. Simon, FA, and Pickering, LK. Acute yellow phosphorus poisoning. "smoking stool syndrome". JAMA. (1976) 235:1343–4.
21. Lukey, BJ, Romano, JAJr., Romano, JA, Salem, H, Lukey, BJ, and Salem, H.(Eds.). Chemical warfare agents: Chemistry, pharmacology, toxicology, and therapeutics, Second Edition (2nd ed.). CRC Press. (2007). doi: 10.1201/9781420046625
22. Toxicological Profile for White Phosphorus. Atlanta (GA). Agency for Toxic Substances and Disease Registry (ATSDR) toxicological profiles. Atlanta (GA). (1997).
23. Ben-Hur, N, Giladi, A, Neuman, Z, Shugerman, B, and Applebaum, J. Phosphorus burns--a pathophysiological study. Br J Plast Surg. (1972) 25:238–44.
25. Chorna, V, Shkondin, S, Lypkan, VM, Tomashevskyi, AV, Kolomiets, VV, and Zavodiak, AY. Post-traumatic effects of phosphorus weapons: from pathogenesis to treatment. Environ Health. (2024):28–35. doi: 10.32402/dovkil2024.02.028
26. Konjoyan, TR. White phosphorus burns: case report and literature review. Mil Med. (1983) 148:881–4.
27. Berndtson, AE, Fagin, A, Sen, S, Greenhalgh, DG, and Palmieri, TL. White phosphorus burns and arsenic inhalation: a toxic combination. J Burn Care Res. (2014) 35:e128–31. doi: 10.1097/BCR.0b013e31828c73dd
28. Barillo, DJ, Cancio, LC, and Goodwin, CW. Treatment of white phosphorus and other chemical burn injuries at one burn center over a 51-year period. Burns. (2004) 30:448–52. doi: 10.1016/j.burns.2004.01.032
29. Barqouni, L, Abu Shaaban, N, and Elessi, K. Interventions for treating phosphorus burns. Cochrane Database Syst Rev. (2014) 2014:CD008805. doi: 10.1002/14651858.CD008805.pub3
30. Brutyan, S, Babayan, K, Barseghyan, N, Petrosyan, V, Knipper, P, Begue, T, et al. Evidence for chemical burns by white phosphorus in Armenian soldiers during the 2020 Nagorno-Karabakh war. Injury. (2021) 52:1100–1. doi: 10.1016/j.injury.2021.02.072
31. Mozingo, DW, Smith, AA, McManus, WF, Pruitt, BA Jr, and Mason, AD Jr. Chemical burns. J Trauma. (1988) 28:642–7.
32. Khurshid, R, Sajid, H, Ashraf, H, Majeed, S, Hanif, F, and Rashid, S. The human suffering caused by bomb containing white phosphorus: health effects. Pak J Med Health Sci. (2022) 16:172–4. doi: 10.53350/pjmhs22163172
33. Okazaki, A, Takeda, Y, Matsuda, Y, Shibata, K, and Kasahara, K. Chemical pneumonitis caused by inhalation of white phosphorus fumes. Am J Respir Crit Care Med. (2020) 201:e12. doi: 10.1164/rccm.201904-0734IM
34. Daly, M, Tuft, SJ, and Munro, PM. Acute corneal calcification following chemical injury. Cornea. (2005) 24:761–5. doi: 10.1097/01.ico.0000154040.80442.8b
35. Schmidt, I, Friedel, R, Schmitz, H, Marx, F, and Markgraf, E. The Marjolin's ulcer: a malignant and rarely complication after burn trauma of the upper extremity - a case report. Unfallchirurg. (2000) 103:68–72. doi: 10.1007/s001130050010
36. Summerlin, WT, Walder, AI, and Moncrief, JA. White phosphorus burns and massive hemolysis. J Trauma. (1967) 7:476–84.
37. Conner, JC, and Bebarta, VS. Images in clinical medicine. White phosphorus dermal burns. N Engl J Med. (2007) 357:1530. doi: 10.1056/NEJMicm061897
38. Eldad, A, and Simon, GA. The phosphorous burn--a preliminary comparative experimental study of various forms of treatment. Burns. (1991) 17:198–200.
39. Hu, AJ. Intravenous drop of calcium gluconate for phosphorus burns. Zhonghua Wai Ke Za Zhi. (1993) 31:421–4.
40. Dai, NT, Chen, TM, Cheng, TY, Chen, SL, Chen, SG, Chou, GH, et al. The comparison of early fluid therapy in extensive flame burns between inhalation and noninhalation injuries. Burns. (1998) 24:671–5.
41. Davis, KG. Acute management of white phosphorus burn. Mil Med. (2002) 167:83–4. doi: 10.1093/milmed/167.1.83
42. Voie, OA, Johnsen, A, Stromseng, A, and Longva, KS. Environmental risk assessment of white phosphorus from the use of munitions - a probabilistic approach. Sci Total Environ. (2010) 408:1833–41. doi: 10.1016/j.scitotenv.2010.01.002
43. Mendelson, JA. Some principles of protection against burns from flame and incendiary munitions. J Trauma. (1971) 11:286–94.
44. Kaufman, T, Ullmann, Y, and Har-Shai, Y. Phosphorus burns: a practical approach to local treatment. J Burn Care Rehabil. (1988) 9:474–5.
45. Curreri, PW, Asch, MJ, and Pruitt, BA. The treatment of chemical burns: specialized diagnostic, therapeutic, and prognostic considerations. J Trauma. (1970) 10:634–42.
46. Song, ZY, Lu, YP, and Gu, XQ. Treatment of yellow phosphorus skin burns with silver nitrate instead of copper sulfate. Scand J Work Environ Health. (1985) 11:33.
47. Eldad, A, Wisoki, M, Cohen, H, Breiterman, S, Chaouat, M, Wexler, MR, et al. Phosphorous burns: evaluation of various modalities for primary treatment. J Burn Care Rehabil. (1995) 16:49–55.
48. Ben-Hur, N, Giladi, A, Applebaum, J, and Neuman, Z. Phosphorus burns: the antidote: a new approach. Br J Plast Surg. (1972) 25:245–9.
49. Li, M, Li, H, Li, X, Zhu, H, Xu, Z, Liu, L, et al. A bioinspired alginate-gum arabic hydrogel with micro−/nanoscale structures for controlled drug release in chronic wound healing. ACS Appl Mater Interfaces. (2017) 9:22160–75. doi: 10.1021/acsami.7b04428
50. Witkowski, W, Surowiecka-Pastewka, A, Biesaga, M, and Gierczak, T. Experimental comparison of efficiency of first aid dressings in burning white phosphorus on bacon model. Med Sci Monit. (2015) 21:2361–6. doi: 10.12659/MSM.894991
51. Mabvuure, NT, Brewer, CF, Gervin, K, and Duffy, S. The use of moist exposed burn ointment (MEBO) for the treatment of burn wounds: a systematic review. J Plast Surg Hand Surg. (2020) 54:337–43. doi: 10.1080/2000656X.2020.1813148
52. Avni, T, Levcovich, A, Ad-El, DD, Leibovici, L, and Paul, M. Prophylactic antibiotics for burns patients: systematic review and meta-analysis. BMJ. (2010) 340:c241. doi: 10.1136/bmj.c241
53. Doi, N, Nakatani, Y, Inaba, Y, Kondo, T, Furukawa, F, and Kanazawa, N. Acute-phase effects of single-time topical or systemic corticosteroid application immediately after hot water-induced burn injury of various grades. Trends Immunotherapy. (2017) 1:19–27. doi: 10.24294/ti.v1.i1.14
54. Blumetti, J, Hunt, JL, Arnoldo, BD, Parks, JK, and Purdue, GF. The parkland formula under fire: is the criticism justified? J Burn Care Res. (2008) 29:180–6. doi: 10.1097/BCR.0b013e31815f5a62
55. Spanholtz, TA, Theodorou, P, Amini, P, and Spilker, G. Severe burn injuries: acute and long-term treatment. Dtsch Arztebl Int. (2009) 106:607–13. doi: 10.3238/arztebl.2009.0607
56. Knappe, K, Lunz, A, Bülhoff, M, Schonhoff, M, Renkawitz, T, Kretzer, JP, et al. Pulsatile lavage systems and their potential to penetrate soft tissue. Eur J Trauma Emerg Surg. (2023) 49:327–33. doi: 10.1007/s00068-022-02067-x
57. Pang, Q, Lou, D, Li, S, Wang, G, Qiao, B, Dong, S, et al. Smart flexible electronics-integrated wound dressing for real-time monitoring and on-demand treatment of infected wounds. Adv Sci (Weinh). (2020) 7:1902673. doi: 10.1002/advs.201902673
59. Jo, H, Brito, S, Kwak, BM, Park, S, Lee, MG, and Bin, BH. Applications of mesenchymal stem cells in skin regeneration and rejuvenation. Int J Mol Sci. (2021) 22:2410. doi: 10.3390/ijms22052410
60. Bian, D, Wu, Y, Song, G, Azizi, R, and Zamani, A. The application of mesenchymal stromal cells (MSCs) and their derivative exosome in skin wound healing: a comprehensive review. Stem Cell Res Ther. (2022) 13:24. doi: 10.1186/s13287-021-02697-9
61. Hu, C, and Wang, L. Advances in the treatment of liver injury based on mesenchymal stem cell-derived exosomes. Stem Cell Res Ther. (2024) 15:474. doi: 10.1186/s13287-024-04087-3
62. Lee, AJ, Dale, JJ, Ruckley, CV, Gibson, B, Prescott, RJ, and Brown, D. Compression therapy: effects of posture and application techniques on initial pressures delivered by bandages of different physical properties. Eur J Vasc Endovasc Surg. (2006) 31:542–52. doi: 10.1016/j.ejvs.2005.10.023
63. Vanderstichele, S, and Vranckx, JJ. Anti-fibrotic effect of adipose-derived stem cells on fibrotic scars. World J Stem Cells. (2022) 14:200–13. doi: 10.4252/wjsc.v14.i2.200
Keywords: battlefield medicine, tactical medicine, white phosphorus munitions, chemical burns, battlefield first aid
Citation: Wang M, Bai Y, Zhang X, Zhang J and Kang A (2025) White phosphorus munitions: pathophysiology, clinical management, and multidisciplinary perspectives on burn injuries and humanitarian challenges. Front. Public Health. 13:1632840. doi: 10.3389/fpubh.2025.1632840
Edited by:
Tammy Angaline Butterick, United States Department of Veterans Affairs, United StatesReviewed by:
Jakub Zachaj, Klinika Psychiatryczna NOZ Warszawski Uniwersytet Medyczny, PolandKelly Ribeiro Moura Barboza, Vila Velha University, Brazil
Copyright © 2025 Wang, Bai, Zhang, Zhang and Kang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Along Kang, a2Fsb25nMTkwMEAxNjMuY29t
†These authors have contributed equally to this work
 Mingchan Wang
Mingchan Wang Yaxing Bai
Yaxing Bai Xiaorui Zhang1
Xiaorui Zhang1