- 1Department of Rheumatology and Immunology, The First People's Hospital of Xiaoshan District, Xiaoshan Affiliated Hospital of Wenzhou Medical University, Hangzhou, China
- 2Department of Scientific Research, The First People's Hospital of Xiaoshan District, Xiaoshan Affiliated Hospital of Wenzhou Medical University, Hangzhou, China
- 3Department of Osteoporosis Care and Control, The First People's Hospital of Xiaoshan District, Xiaoshan Affiliated Hospital of Wenzhou Medical University, Hangzhou, China
Background: Maintaining functional independence in older adults is a critical public health objective. Although skeletal muscle mass is recognized as a key contributor to functional capacity outcomes, the exact relationship between them among community-dwelling older adults requires further investigation.
Methods: This cross-sectional study analyzed data from 13,322 participants aged ≥65 years from the 2018 Chinese Longitudinal Healthy Longevity Survey (CLHLS). Appendicular skeletal muscle mass index (ASMI) was calculated using a validated anthropometric equation, while functional capacity outcomes were assessed using basic and instrumental activities of daily living (BADL/IADL) scores. Multivariable linear regression, generalized additive models, and threshold effect analysis were employed to evaluate associations, with adjustments for demographic, socioeconomic, lifestyle, and comorbidity factors.
Results: In fully adjusted models, ASMI showed significant inverse associations with both BADL score (β = −0.072, 95% CI: −0.103 to −0.042) and IADL score (β = −0.225, 95% CI: −0.290 to −0.159). Threshold effect analysis revealed sex-specific inflection points: below 6.2 kg/m2 in men and 5.2 kg/m2 below in women, ASMI was negatively associated with BADL/IADL scores, whereas above these thresholds, the associations weakened or reversed. Subgroup analyses indicated stronger effects among men, urban residents, and those with stroke or cardiovascular disease.
Conclusion: Muscle mass demonstrates sex-specific, non-linear associations with functional capacity outcomes in older adults, identifying critical thresholds that may inform targeted strategies to preserve independence.
Introduction
The global demographic transition toward population aging represents one of the most significant public health challenges of the 21st century, with China undergoing this transformation at an unprecedented scale and pace (1). Epidemiological data reveal a striking acceleration in aging dynamics: between 1990 and 2022, the proportion of China’s population aged ≥65 years increased 2.7-fold (from 5.57 to 14.9%), while the absolute number grew 3.3-fold (from 64 to 210 million individuals) (2). This demographic shift coincides with substantial gains in life expectancy (from 68.55 years in 1990 to 77.7 years in 2019), which is projected to reach 81.3 years by 2035 (3). Of particular concern are projections indicating that the disabled older adults population (≥65 years) will triple from 11.4 million in 2010 to 34.8 million by 2030, underscoring an urgent need for interventions that preserve functional autonomy to mitigate socioeconomic burdens (4).
Within this context, sarcopenia has emerged as a pivotal geriatric syndrome, characterized by the progressive, generalized loss of skeletal muscle mass and function (5). Contemporary understanding recognizes skeletal muscle not only as a contractile organ, but as a sophisticated endocrine platform that secretes myokines and exerkines capable of systemic metabolic regulation (6, 7). The musculoskeletal system’s dual roles in mechanical support and biochemical signaling make its age-related decline particularly consequential, with sarcopenia directly contributing to disability, frailty, and diminished quality of life (8, 9).
Although skeletal muscle mass is well-established as a contributor to physical capacity, growing evidence highlights the nuanced and complex nature of this relationship, particularly among older adults, where empirical studies have reported considerable inconsistencies (10, 11). For example, one systematic review concluded that the association between muscle mass and physical performance is weak or negligible (11). In contrast, a more recent comprehensive review demonstrated that reduced muscle mass is significantly associated with an increased risk of functional decline in older adults (10). Current clinical models predominantly assume linear relationships, potentially overlooking critical non-linear dynamics such as threshold effects and interactive mechanisms that could substantially improve intervention strategies. To better understand these complex associations, our study was designed to investigate the non-linear relationship between skeletal muscle mass and functional capacity in older adults.
Methods
Study design and population
We conducted a cross-sectional analysis using data from the 2018 wave of the Chinese Longitudinal Healthy Longevity Survey (CLHLS). Trained interviewers collected data through standardized face-to-face interviews. The study protocol was approved by the Biomedical Ethics Committee of Peking University, and all participants provided written informed consent. Publicly available data can be accessed through the CLHLS repository (https://opendata.pku.edu.cn/dataverse/pku/).
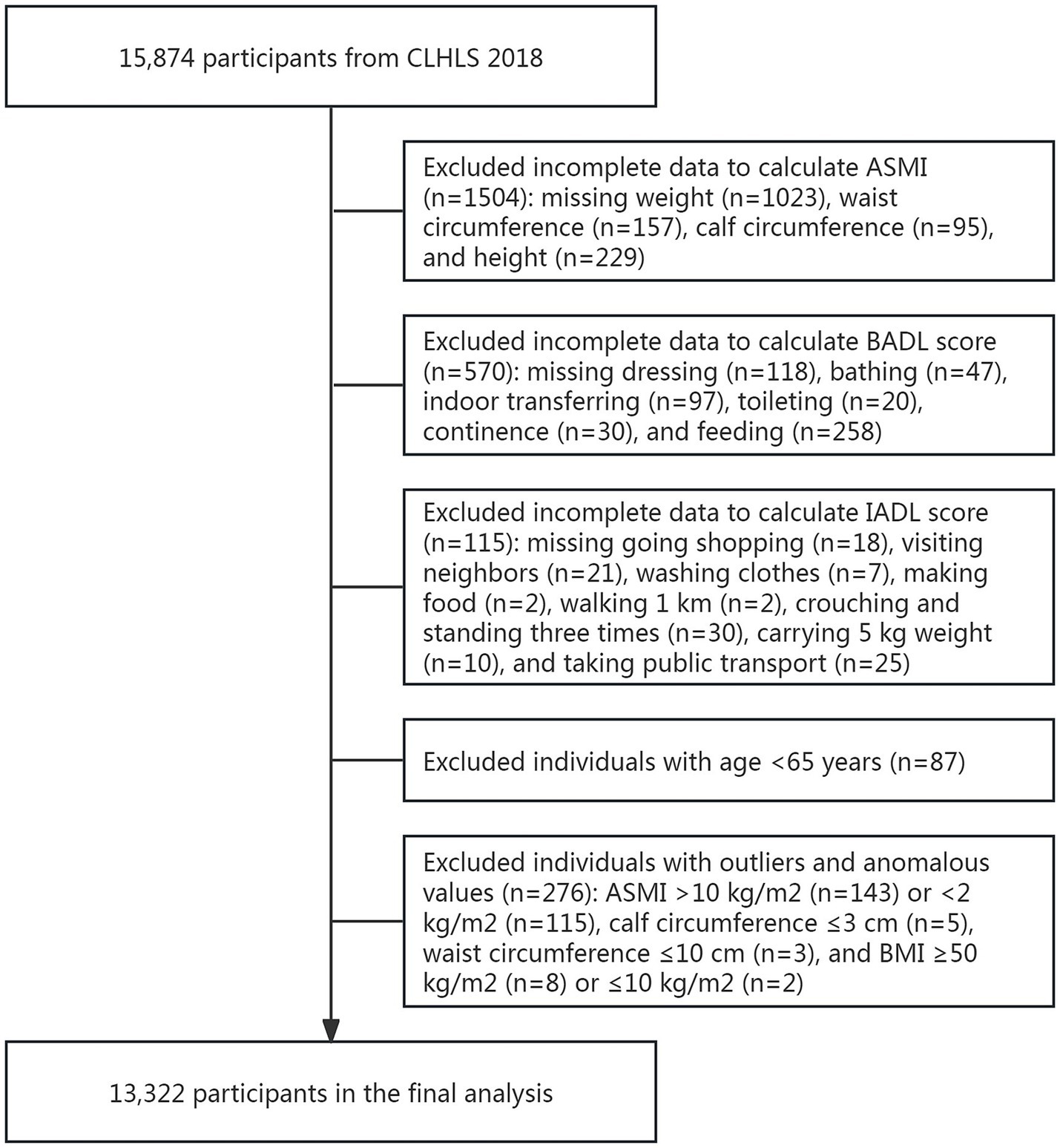
From the initial sample (n = 15,874), we excluded participants with: (1) missing appendicular skeletal muscle index (ASMI, n = 1,504) measurements, (2) incomplete assessments of either basic activities of daily living (BADL, n = 570) or instrumental activities of daily living (IADL, n = 115) scores, (3) age < 65 years (n = 87); and outliers or anomalous values (n = 276). The final analytical sample comprised 13,322 participants (Figure 1).
Variables
Appendicular skeletal muscle mass (ASM) was calculated using the validated Kawakami equation: ASM (kg) = 2.955 × sex (men = 1, women = 0) + 0.255 × weight (kg) - 0.130 × waist circumference (cm) + 0.308 × calf circumference (cm) + 0.081 × height (cm) - 11.897 (12). To account for body size, ASM was normalized by height squared to derive the ASMI (kg/m2).
Functional capacity outcomes were assessed using two validated scales. The BADL scale evaluated six domains: (1) dressing, (2) bathing, (3) indoor transferring, (4), toileting, (5) continence, and (6) feeding. The IADL scale assessed eight higher-level functional domains: (1) going shopping, (2) visiting neighbors, (3) washing clothes, (4) making food, (5) walking one kilometer, (6) crouching and standing three times, (7) carryng five kg weight, and (8) taking public transportation. In the present study, all items were scored on a 3-point scale (1 = fully independent to 3 = completely dependent), generating total scores ranging 6–18 for BADL and 8–24 for IADL, where higher scores indicated greater functional dependence.
The analysis adjusted for multiple potential confounders across four categories: (1) demographic factors including age (65–79, 80–94, and ≥95 years), sex, years of schooling (0, 1 to 6, or ≥ years), marital status (married, separated/divorced/widowed/never married), and residence (city, town, rural); (2) socioeconomic status measured by annual household income (<10,000; 10,000 to 30,000; 30,001 to 60,000; or >60,000 yuan); (3) lifestyle factors including status of exercise, smoke and drink; and (4) comorbidities including hypertension, diabetes, stroke or cardiovascular disease, cancer, and dementia.
Statistical analyses
Participants were stratified into quartiles based on ASMI for baseline comparisons. Continuous variables were expressed as mean ± standard deviation, while categorical variables were presented as percentages. Between-group comparisons were performed using ANOVA for normally distributed continuous variables, Kruskal-Wallis tests for non-normally distributed continuous variables, and χ2 tests for categorical variables. The association between ASMI and functional capacity was examined using a series of progressively adjusted linear regression models: (1) an unadjusted model assessing crude associations; (2) a model adjusted for age and sex; and (3) a fully adjusted model incorporating demographic, socioeconomic, lifestyle, and comorbidity factors. To assess potential nonlinear associations, generalized additive models with smoothing splines were used for exploratory analysis. Where significant nonlinearity was indicated, piecewise linear regression was employed to identify and quantify threshold effects. Subgroup analyses were conducted to examine potential effect modifications by testing relevant interaction terms. All analyses were performed using R version 3.4.3 and EmpowerStats software. A two-tailed p-value < 0.05 was considered statistically significant.
Results
Study population characteristics
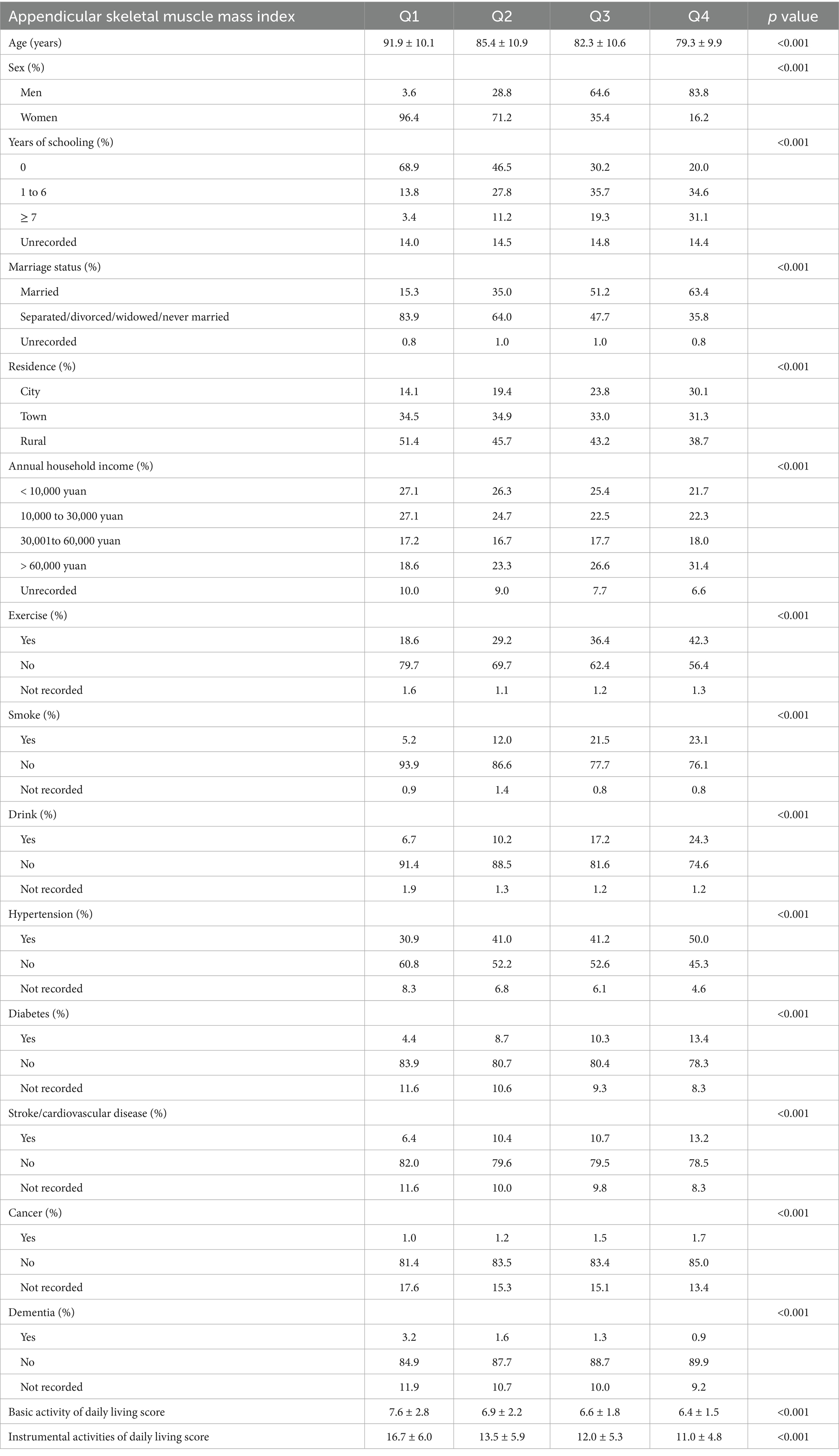
The characteristics of participants stratified by ASMI quartiles are presented in Table 1. Age exhibited a significant inverse trend, declining from 91.9 ± 10.1 years in Q1 to 79.3 ± 9.9 years in Q4. Sex distribution varied markedly, with men representing only 3.6% of Q1 but 83.8% of Q4. Higher ASMI was associated with greater educational level and elevated household income. Lifestyle behaviors also differed: regular exercise increased from 18.6% (Q1) to 42.3% (Q4), while smoking (Q1: 5.2%; Q4: 23.1%) and drinking (Q1: 6.7%; Q4: 24.3%) were more prevalent in higher quartiles. Comorbidities, including hypertension, diabetes, and stroke/cardiovascular disease, were more frequent in participants with higher ASMI. In contrast, dementia prevalence decreased from 3.2% (Q1) to 0.9% (Q4). Functional capacity outcomes showed poorer scores in both BADL (7.6 ± 2.8 in Q1 vs. 6.4 ± 1.5 in Q4) and IADL (16.7 ± 6.0 in Q1 vs. 11.0 ± 4.8 in Q4) among higher ASMI groups.
Associations between ASMI and BADL/IADL scores
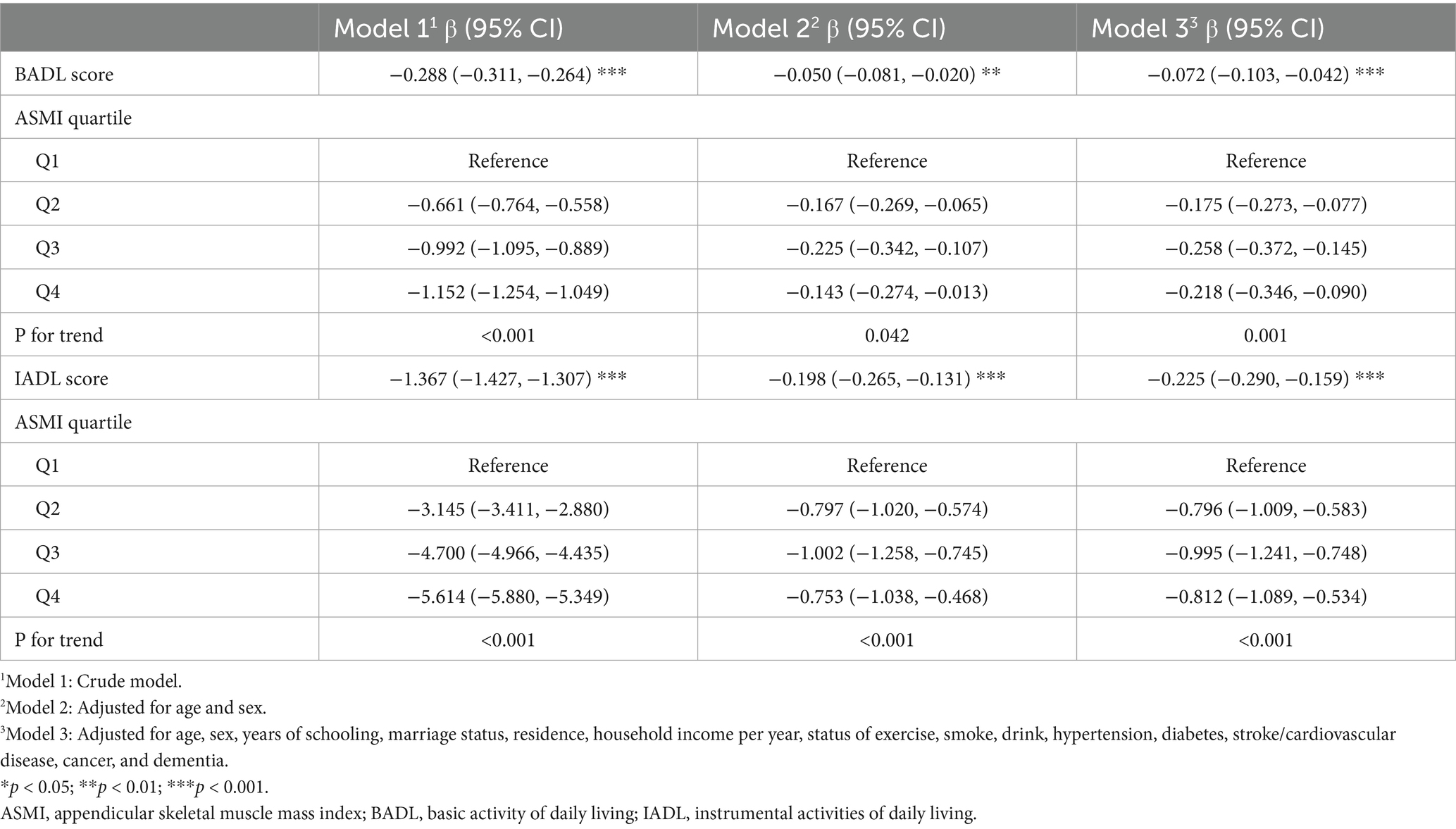
As shown in Table 2, multivariable regression analyses demonstrated significant inverse associations between ASMI and functional capacity outcomes, for both BADL (Model 3: β = −0.072, 95% CI: −0.103 to −0.042) and IADL scores (Model 3: β = −0.225, 95% CI: −0.290 to −0.159) across all models. Trend analyses confirmed these patterns, showing significant dose–response relationships, with Q3 demonstrating the strongest effects for both BADL and IADL scores in the fully adjusted model. These nonlinear relationships were further supported by the patterns illustrated in Figure 2.
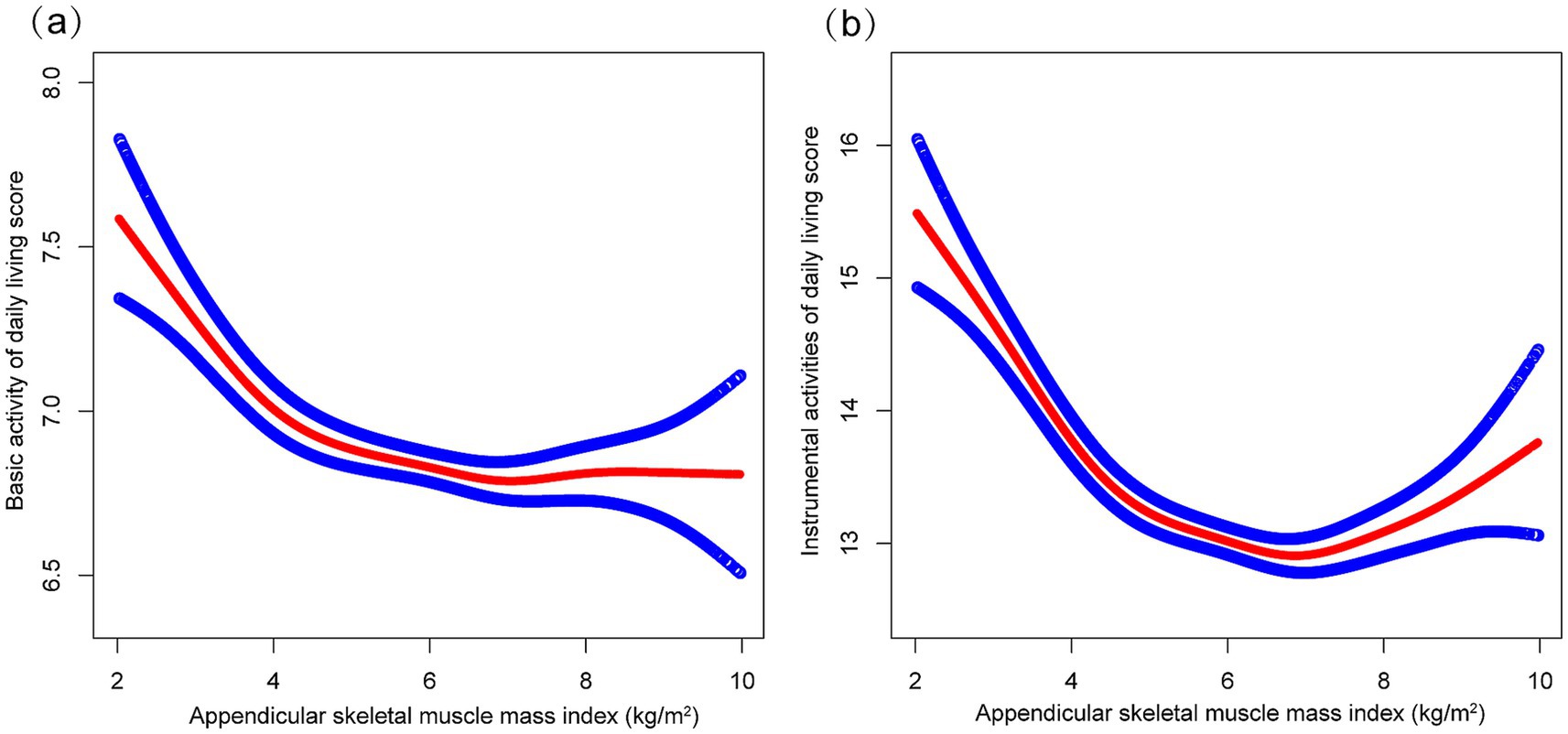
Figure 2. The correrations of appendicular skeletal muscle index with basic activities of daily living score (a) and instrumental activities of daily living score (b). Higher scores indicate greater functional dependence. Age, sex, years of schooling, marriage status, residence, household income per year, status of exercise, smoke, drink, hypertension, diabetes, stroke/cardiovascular disease, cancer, and dementia were adjusted.
Subgroup analysis
Subgroup analyses indicated differential associations between ASMI and functional decline (BADL/IADL) across demographic and clinical factors (Figure 3). For BADL (Figure 3a), significant interactions were observed for sex, residence, exercise, diabetes, and stroke/cardiovascular disease, with stronger negative effects in men (β = −0.118, 95% CI: −0.160 to −0.076), city residents (β = −0.198, 95% CI: −0.268 to −0.128), non-exercisers (β = −0.119, 95% CI: −0.161 to −0.078), diabetics (β = −0.158, 95% CI: −0.248 to −0.067), and stroke/cardiovascular patients (β = −0.244, 95% CI: −0.366 to −0.121). IADL (Figure 3b) showed significant interactions with residence, hypertension, and those with stroke/cardiovascular disease, with urban residents (β = −0.331, 95% CI: −0.466 to −0.197) and those with stroke/cardiovascular diseases (β = −0.529, 95% CI: −0.754 to −0.303) exhibiting steeper declines. Potential U-shaped nonlinear relationships stratified by age and sex are further illustrated in Figures 4, 5.
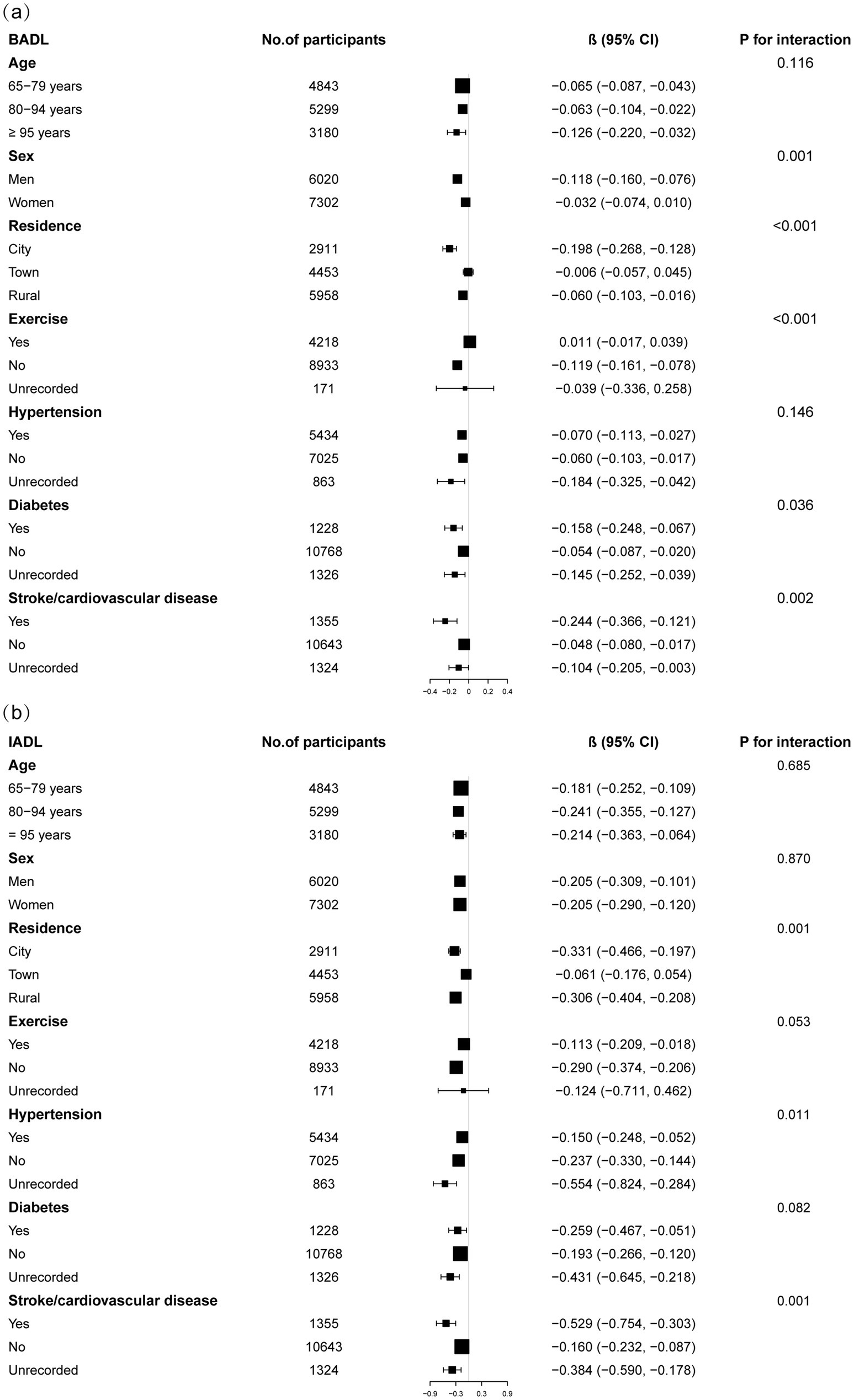
Figure 3. Subgroup analysis of the correrations of appendicular skeletal muscle index with basic activities of daily living score (a) and instrumental activities of daily living score (b). Higher scores indicate greater functional dependence. Age, sex, years of schooling, marriage status, residence, household income per year, status of exercise, smoke, drink, hypertension, diabetes, stroke/cardiovascular disease, cancer, and dementia were adjusted. In the subgroup analysis, the model is not adjusted for the stratification variable itself.
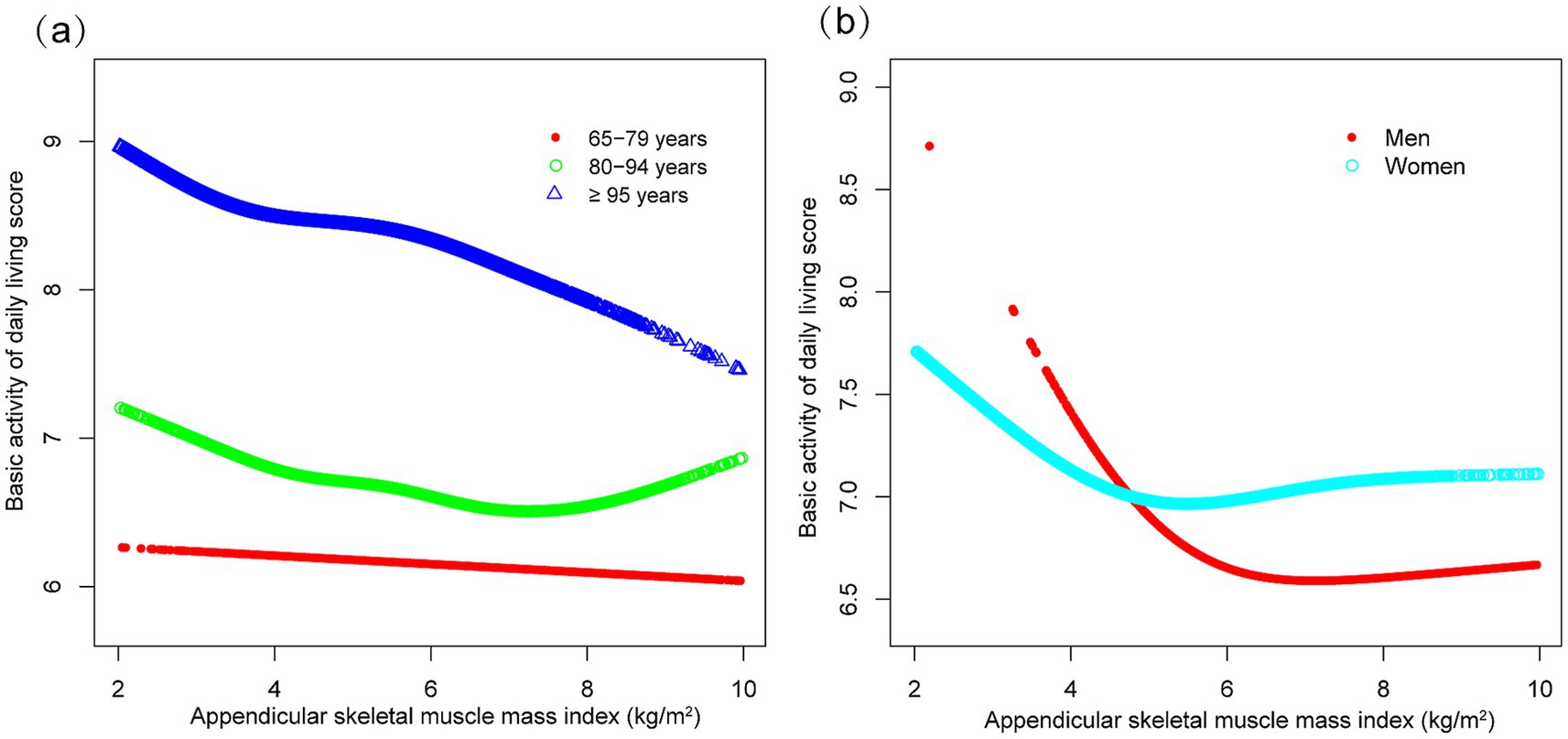
Figure 4. Curve associations between appendicular skeletal muscle index and basic activities of daily living score, stratified by age (a) and sex (b). Higher scores indicate greater functional dependence. Age, sex, years of schooling, marriage status, residence, household income per year, status of exercise, smoke, drink, hypertension, diabetes, stroke/cardiovascular disease, cancer, and dementia were adjusted. In the subgroup analysis, the model is not adjusted for the stratification variable itself.
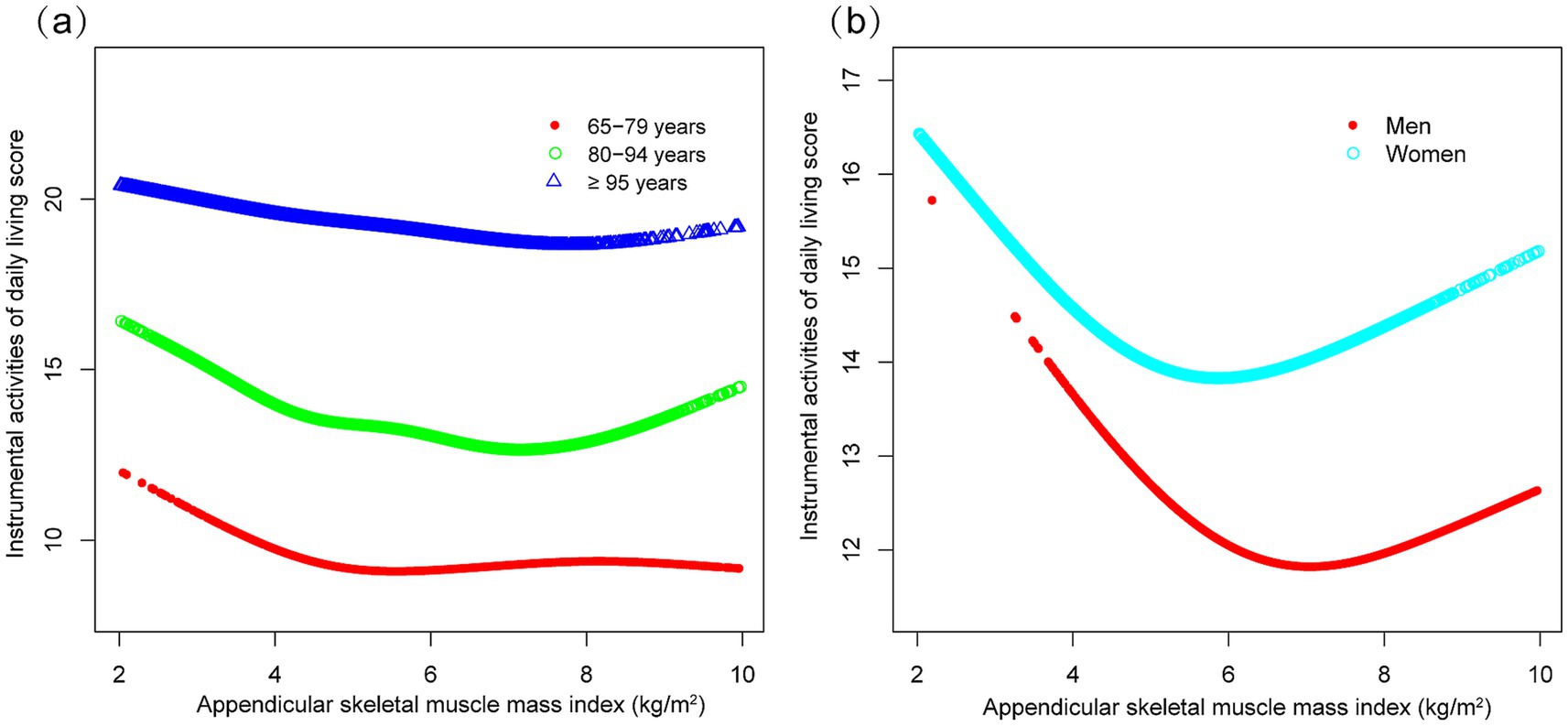
Figure 5. Curve associations between appendicular skeletal muscle index and instrumental activities of daily living score, stratified by age (a) and sex (b). Higher scores indicate greater functional dependence. Age, sex, years of schooling, marriage status, residence, household income per year, status of exercise, smoke, drink, hypertension, diabetes, stroke/cardiovascular disease, cancer, and dementia were adjusted. In the subgroup analysis, the model is not adjusted for the stratification variable itself.
Threshold effect analysis
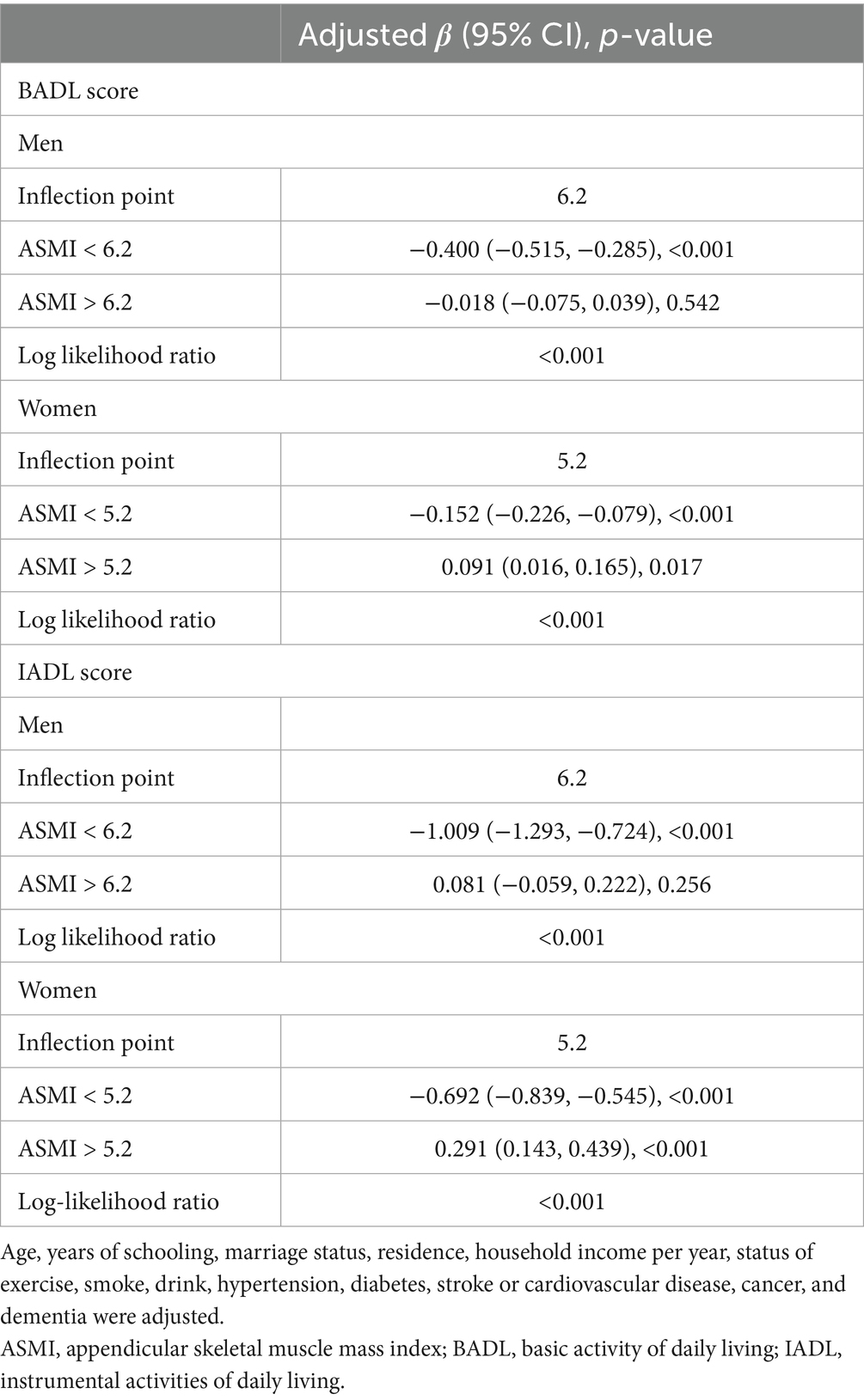
Threshold effect analysis revealed significant sex-specific associations between ASMI and functional capacity measures (Table 3). Distinct inflection points were observed at 6.2 kg/m2 for men and 5.2 kg/m2 for women. In men, ASMI demonstrated strong inverse associations with both BADL (β = −0.400, 95% CI: −0.515 to −0.285) and IADL scores (β = −1.009, 95% CI: −1.293 to −0.724) below the 6.2 kg/m2 threshold. Above this threshold, these associations became non-significant (BADL: β = −0.018, 95% CI: −0.075 to 0.039; IADL: β = 0.081, 95% CI: −0.059 to 0.222). Women exhibited a comparable pattern below the 5.2 kg/m2 threshold, with negative associations for both BADL (β = −0.152, 95% CI: −0.226 to −0.079) and IADL (β = −0.692, 95% CI: −0.839 to −0.545). Above this threshold, the relationships became positive (BADL: β = 0.091, 95% CI: 0.016 to 0.165; IADL: β = 0.291, 95% CI: 0.143 to 0.439). The robustness of these threshold effects was confirmed by log-likelihood ratio tests (all p < 0.001).
Discussion
Our cross-sectional study reveals a non-linear association between ASMI and functional capacity in older adults, characterized by sex-specific inflection points at 6.2 kg/m2 for men and 5.2 kg/m2 for women. These findings challenge the prevailing linear paradigm of muscle mass-function relationships and support the development of threshold-based, sex-specific interventions to mitigate functional decline in aging populations.
Skeletal muscle mass is a well-established determinant of physical function in older adults, and its decline is linked to adverse health outcomes, including disability and reduced quality of life (13, 14). The loss of muscle mass reflects not only structural deterioration but also functional impairment, manifesting as diminished muscle strength and power—critical factors for maintaining independence (15, 16). However, inconsistencies persist in the literature, with some studies reporting no significant association between muscle mass and functional decline (17). These discrepancies may stem from methodological heterogeneity, including variations in muscle mass quantification, differences in body size normalization, and divergent definitions of functional outcomes.
The Asian Working Group for Sarcopenia recommends calf circumference as a practical screening tool for muscle mass loss in community settings (18). Although anthropometric proxies such as calf circumference and predictive algorithms provide convenient estimates, their accuracy in determining total-body skeletal muscle mass remains limited, prompting ongoing investigations into more sophisticated measurement techniques (19, 20). Our use of a validated equation that incorporates calf circumference as a key variable, aligns with previous studies in Asian populations (12, 21, 22). Notably, skeletal muscle mass plays a critical role in functional prognosis across diverse clinical settings, including acute stroke recovery (23), and demonstrates sex- and location-dependent associations with disability risk (24) —findings that corroborate our stratified analyses.
Our identified thresholds (6.2 kg/m2 for men and 5.2 kg/m2 for women) provide important context when compared with established sarcopenia diagnostic criteria. The Asian Working Group for Sarcopenia 2019 consensus recommends ASMI cut-offs of <7.0 kg/m2 for men and <5.4 kg/m2 for women (18), while the European Working Group on Sarcopenia suggests <7.0 kg/m2 for men and <5.5 kg/m2 for women (25). Our anthropometrically derived thresholds are notably lower, which may reflect methodological differences between anthropometric estimation and direct measurement techniques, as well as our focus on functional outcomes rather than sarcopenia diagnosis. Importantly, our thresholds represent inflection points for functional capacity rather than diagnostic cut-offs, suggesting that functional decline may begin at muscle mass levels below current diagnostic thresholds. For screening applications, the Kawakami equation used in this study provides a practical and cost-efficient tool for estimating muscle mass in community settings. In contrast to resource-intensive techniques such as dual-energy X-ray absorptiometry or bioelectrical impedance analysis, this approach utilizes readily obtainable anthropometric parameters, enabling scalable deployment in primary care, community health programs, and large-scale public health initiatives. For intervention strategies, older adults with muscle mass below the identified sex-specific thresholds may benefit from intensive, multimodal interventions incorporating progressive resistance training, dietary protein optimization, and task-specific functional exercises.
The age-related decline in skeletal muscle mass and function is driven by multifaceted biological mechanisms, including reductions in muscle fiber number and size, as well as qualitative alterations in muscle proteins (26). Myokines, which are secreted by muscle cells during contraction, play a pivotal role in regulating muscle metabolism and systemic physical function through autocrine and endocrine signaling (27). Concurrently, mitochondrial dysfunction in aging muscle contributes to deficits in exercise performance, gait stability, and insulin sensitivity, even among physically active older adults (28). Interventions such as combined strength training and tai chi have demonstrated efficacy in augmenting muscle mass in sarcopenic individuals (29), while high-protein dietary strategies can mitigate muscle loss during energy deficit (30). These findings underscore the potential for targeted lifestyle interventions to ameliorate functional decline.
Our study leverages a large sample and rigorous adjustment for confounders. However, several limitations warrant consideration. First, the cross-sectional design precludes causal inference, as the observed associations may reflect reverse causation or bidirectional relationships between muscle mass and functional capacity. Longitudinal studies with repeated measures are needed to clarify temporal sequences, establish causality, and address these concerns. Second, our reliance on anthropometric equations rather than direct measurement methods introduces potential measurement error and may lead to overestimation or underestimation of true muscle mass in individual cases. Therefore, the identified thresholds should be interpreted with considerable caution in clinical settings and require validation using gold-standard measurement techniques. Third, our subgroup analyses, while informative, were exploratory and not adjusted for multiple comparisons. These findings should therefore be interpreted as generating hypotheses for future validation rather than confirming definitive subgroup differences. Fourth, although the BADL/IADL scales are validated, they may not fully capture the multidimensional nature of functional decline in older adults. These scales primarily assess basic tasks and may miss important domains such as balance, gait speed, endurance, and cognitive-motor integration. Fifth, our decision not to independently adjust for BMI or waist circumference as covariates—guided by the validated Kawakami equation methodology—may introduce potential confounding by adiposity in the muscle mass–function relationship, particularly given the positive associations observed above the sex-specific thresholds.
Conclusion
Our findings identify critical inflection points in the muscle mass-function relationship, suggesting that maintaining ASMI above sex-specific values (6.2 kg/m2 for men; 5.2 kg/m2 for women) is correlated with preserved autonomy in older adults. These observations highlight the potential value of moving beyond uniform recommendations toward precision strategies tailored to individual biomechanical and metabolic profiles. The results offer actionable insights for clinical practice and public health policy focused on supporting functional independence in aging populations.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: data are publicly accessible through the CLHLS open-access repository (https://opendata.pku.edu.cn/dataverse/pku/).
Ethics statement
The studies involving humans were approved by the study protocol received ethical approval from Peking University’s Biomedical Ethics Committee (IRB00001052-13074). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
LC: Writing – original draft. XL: Writing – original draft. ZZ: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors appreciate the time and effort given by participants during the data collection phase of the CLHLS project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Gruber, J, Lin, M, Yang, H, and Yi, J. China's social health Insurance in the era of rapid population aging. JAMA Health Forum. (2025) 6:e251105. doi: 10.1001/jamahealthforum.2025.1105
2. Wang, H, Qin, D, Fang, L, Liu, H, and Song, P. Addressing healthy aging in China: practices and prospects. Biosci Trends. (2024) 18:212–8. doi: 10.5582/bst.2024.01180
3. Bai, R, Liu, Y, Zhang, L, Dong, W, Bai, Z, and Zhou, M. Projections of future life expectancy in China up to 2035: a modelling study. Lancet Public Health. (2023) 8:e915–22. doi: 10.1016/S2468-2667(22)00338-3
4. Han, Y, Hu, K, Wu, Y, and Fang, Y. Future life expectancy with disability among elderly Chinese individuals: a forecast based on trends in stroke and dementia. Public Health. (2021) 198:62–8. doi: 10.1016/j.puhe.2021.06.013
5. Sayer, AA, Cooper, R, Arai, H, Cawthon, PM, Ntsama Essomba, MJ, Fielding, RA, et al. Sarcopenia. Nat Rev Dis Primers. (2024) 10:68. doi: 10.1038/s41572-024-00550-w
6. Saponaro, F, Bertolini, A, Baragatti, R, Galfo, L, Chiellini, G, Saba, A, et al. Myokines and microbiota: new perspectives in the endocrine muscle-gut Axis. Nutrients. (2024) 16:4032. doi: 10.3390/nu16234032
7. Nunes, EA, Stokes, T, McKendry, J, Currier, BS, and Phillips, SM. Disuse-induced skeletal muscle atrophy in disease and nondisease states in humans: mechanisms, prevention, and recovery strategies. Am J Physiol Cell Physiol. (2022) 322:C1068–c1084. doi: 10.1152/ajpcell.00425.2021
8. Gauvain, JB, Mandigout, S, Pambet, M, Monseu, M, Gillain, P, Gautier, J, et al. Correlation between muscle mass and physical activity level in older adults at risk of falling: the FITNESS study. J Frailty Aging. (2024) 13:240–7. doi: 10.14283/jfa.2024.53
9. Petnehazy, N, Barnes, HN, Newman, AB, Kritchevsky, SB, Cummings, SR, Hepplen, RT, et al. Muscle mass, strength, power and physical performance and their association with quality of life in older adults, the study of muscle, mobility and aging (SOMMA). J Frailty Aging. (2024) 13:384–90. doi: 10.14283/jfa.2024.45
10. Visser, M, Sääksjärvi, K, Burchell, GL, and Schaap, LA. The association between muscle mass and change in physical functioning in older adults: a systematic review and meta-analysis of prospective studies. Eur Geriatr Med. (2025). doi: 10.1007/s41999-025-01230-y
11. Riviati, N, and Indra, B. Relationship between muscle mass and muscle strength with physical performance in older adults: a systematic review. SAGE Open Med. (2023) 11:20503121231214650. doi: 10.1177/20503121231214650
12. Kawakami, R, Miyachi, M, Tanisawa, K, Ito, T, Usui, C, Midorikawa, T, et al. Development and validation of a simple anthropometric equation to predict appendicular skeletal muscle mass. Clin Nutr. (2021) 40:5523–30. doi: 10.1016/j.clnu.2021.09.032
13. Distefano, G, and Goodpaster, BH. Effects of exercise and aging on skeletal muscle. Cold Spring Harb Perspect Med. (2018) 8:a029785. doi: 10.1101/cshperspect.a029785
14. Hanna, L, Nguo, K, Furness, K, Porter, J, and Huggins, CE. Association between skeletal muscle mass and quality of life in adults with cancer: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. (2022) 13:839–57. doi: 10.1002/jcsm.12928
15. Reid, KF, and Fielding, RA. Skeletal muscle power: a critical determinant of physical functioning in older adults. Exerc Sport Sci Rev. (2012) 40:4–12. doi: 10.1097/JES.0b013e31823b5f13
16. Mitchell, WK, Williams, J, Atherton, P, Larvin, M, Lund, J, and Narici, M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front Physiol. (2012) 3:260.
17. Schaap, LA, Koster, A, and Visser, M. Adiposity, muscle mass, and muscle strength in relation to functional decline in older persons. Epidemiol Rev. (2013) 35:51–65. doi: 10.1093/epirev/mxs006
18. Chen, LK, Woo, J, Assantachai, P, Auyeung, TW, Chou, MY, Iijima, K, et al. Asian working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. (2020) 21:300–307.e2. doi: 10.1016/j.jamda.2019.12.012
19. Evans, WJ, Guralnik, J, Cawthon, P, Appleby, J, Landi, F, Clarke, L, et al. Sarcopenia: no consensus, no diagnostic criteria, and no approved indication-how did we get here? Geroscience. (2024) 46:183–90. doi: 10.1007/s11357-023-01016-9
20. McCarthy, C, Schoeller, D, Brown, JC, Gonzalez, MC, Varanoske, AN, Cataldi, D, et al. D(3) -creatine dilution for skeletal muscle mass measurement: historical development and current status. J Cachexia Sarcopenia Muscle. (2022) 13:2595–607. doi: 10.1002/jcsm.13083
21. Ren, L, Tang, Y, Yang, R, Hu, Y, Wang, J, Li, S, et al. Plant-based dietary pattern and low muscle mass: a nation-wide cohort analysis of Chinese older adults. BMC Geriatr. (2023) 23:569. doi: 10.1186/s12877-023-04265-7
22. Yang, Y, Wang, Y, Chen, Q, Li, L, and Jia, W. The association between low muscle mass and the risk of depressive symptoms: a cross-sectional study based on the Chinese longitudinal health longevity survey (CLHLS). Brain Behav. (2025) 15:e70267. doi: 10.1002/brb3.70267
23. Honma, K, Honda, Y, Nagase, M, Nakao, Y, Harada, T, Sasanuma, N, et al. Impact of skeletal muscle mass on functional prognosis in acute stroke: a cohort study. J Clin Neurosci. (2023) 112:43–7. doi: 10.1016/j.jocn.2023.04.006
24. Zhou, H, Ding, X, and Luo, M. The association between sarcopenia and functional disability in older adults. J Nutr Health Aging. (2024) 28:100016. doi: 10.1016/j.jnha.2023.100016
25. Cruz-Jentoft, AJ, Bahat, G, Bauer, J, Boirie, Y, Bruyère, O, Cederholm, T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. (2019) 48:601. doi: 10.1093/ageing/afz046
26. Larsson, L, Degens, H, Li, M, Salviati, L, Lee, YI, Thompson, W, et al. Sarcopenia: aging-related loss of muscle mass and function. Physiol Rev. (2019) 99:427–511. doi: 10.1152/physrev.00061.2017
27. Lee, JH, and Jun, HS. Role of Myokines in regulating skeletal muscle mass and function. Front Physiol. (2019) 10:42. doi: 10.3389/fphys.2019.00042
28. Grevendonk, L, Connell, NJ, McCrum, C, Fealy, CE, Bilet, L, Bruls, YMH, et al. Impact of aging and exercise on skeletal muscle mitochondrial capacity, energy metabolism, and physical function. Nat Commun. (2021) 12:4773. doi: 10.1038/s41467-021-24956-2
29. Guo, H, Cao, J, He, S, Wei, M, Meng, D, Yu, I, et al. Quantifying the enhancement of Sarcopenic skeletal muscle preservation through a hybrid exercise program: randomized controlled trial. JMIR Aging. (2024) 7:e58175. doi: 10.2196/58175
Keywords: aging, sarcopenia, skeletal muscle mass, functional capacity, sex differences, CLHLS
Citation: Chen L, Lu X and Zhu Z (2025) The association between skeletal muscle mass and functional capacity outcomes in Chinese older adults: a national community-based study. Front. Public Health. 13:1645850. doi: 10.3389/fpubh.2025.1645850
Edited by:
Giuseppe Caminiti, Università telematica San Raffaele, ItalyReviewed by:
Alessandra Feraco, Università telematica San Raffaele, ItalyShixing Feng, International Academy of Food and Phytomedicine Homology, France
Copyright © 2025 Chen, Lu and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhongxin Zhu, b3J0aG96enhAd211LmVkdS5jbg==
†These authors have contributed equally to this work
 Lin Chen1†
Lin Chen1† Zhongxin Zhu
Zhongxin Zhu