Abstract
A growing body of evidence demonstrates the importance of forests and wild animal-based foods for diets within tropical environments. However, deforestation and associated land-use changes can have competing effects on nutrition and food security as communities reorient from wild food use and subsistence-based agriculture to import/export markets. This research examines dietary differences and associated changes in food security during intermediate stages of deforestation and market integration in the agriculture-forest frontier of Cross River State, Nigeria. We used participant responses to mixed-methods interviews (n = 528) in six communities to measure individual dietary diversity, household food access, and short-term nutritional status, with specific attention to animal-based foods and the cultural and economic values attached to them, in two interior forest (n = 177) and four forest-edge (n = 351) communities. Multivariate analysis of dietary compositions revealed differences in food categories and types of meat consumed between forest environments. People in forest-edge communities reported consuming less bushmeat and dark green leafy vegetables, and more pulses, domestic meat, fish, eggs, dairy, other vegetables, sweets, condiments, and non-red palm oil compared to interior forest communities. Bushmeat was highly preferred and had more economic value than other animal-based foods, regardless of location. Forest-edge communities had fewer households involved in bushmeat related activities, and fewer hunters per household. However, traders in forest-edge communities sold a larger proportion of meat to people outside of the community than did traders in interior forest communities. Measures of nutrition and food security, but not wealth, improved in relation to dietary patterns in forest-edge communities compared to interior forest communities. Our results may reflect a “best of both worlds” scenario during the intermediate stages of deforestation and agricultural expansion near forested areas, where people have access to forest resources, increased ability to capitalize on forest goods, and access to market goods as they become integrated into market economies. Understanding the dietary consequences of environmental change is important, as food-related experiences may shape the trajectories of livelihood practices and landscape changes in tropical forests of biodiversity significance.
Introduction
Food provisioning is an important ecosystem service of forests, contributing to improved dietary diversity, nutrition, and food security in rural areas (Powell et al., 2011; Johnson et al., 2013; Vinceti et al., 2013; Ickowitz et al., 2014; Vira et al., 2015; Galway et al., 2018; Rasolofoson et al., 2018). Consumption of wild animals (colloquially known as “bushmeat”) is considered particularly valuable, as it improves access to bioavailable nutrients that can be difficult to obtain from plants alone (Fa et al., 2003, 2015; Murphy and Allen, 2003; Sirén and Machoa, 2008; Cawthorn and Hoffman, 2015). Mounting evidence for nutritional benefits of forests suggests that forest conservation itself may offer benefits on par with nutrition-sensitive interventions (Ruel et al., 2013; Rasolofoson et al., 2018). For example, forest proximity causes children to have 25% greater dietary diversity (Rasolofoson et al., 2018), and removing access to wildlife is projected to induce a 29% increase in the prevalence of childhood anemia and a tripling of cases among those in the poorest households in Madagascar (Golden et al., 2011).
There are multiple interrelated pathways by which food systems may respond to tropical land use changes, including interactions between agricultural expansion, market integration, and conservation policies. Agricultural expansion is the leading cause of tropical deforestation, altering local ecologies and contributing to biodiversity losses (Geist and Lambin, 2002; van Vliet et al., 2012). Conservation policies aimed, in part, at reducing agricultural expansion and deforestation often restrict use of remnant forests thereby also limiting access to wild foods and new agricultural land (Ribot et al., 2006; Sandbrook et al., 2010). Limited access to wild foods can have negative consequences for nutrition and food security in local communities, especially in low income areas (Myers et al., 2013; van Noordwijk et al., 2014). Limited access to land from agricultural expansion and/or conservation policies further alters food systems by encouraging intensive agriculture when space is limited and forest clearing is prohibited (van Vliet et al., 2012). Land use intensification and monocropping can in turn create new agricultural challenges; for example from pests, weeds, and reduced soil quality (Geist and Lambin, 2002; van Vliet et al., 2012). Overall, declining diversity in agricultural production is associated with lower household and individual dietary diversity (Jones, 2017).
Land use change can have additional effects on food systems when deforestation results in reorientation to import/export markets. Markets can negatively affect dietary diversity (Reyes-García et al., 2019), as communities shift away from locally collected and produced foods toward processed foods high in fat, sugar, and salt (Kuhnlein and Receveur, 1996; Popkin, 2004; Kuhnlein et al., 2009; Piperata et al., 2011; Van Vliet et al., 2015; Reyes-García et al., 2019). Market access is also associated with decreased use of shifting cultivation strategies and increased reliance on intensive and commercial agriculture (van Vliet et al., 2012). However, market access and integration can also help redistribute food, increase dietary diversity, and shape food preferences (Bowles, 1998; Sibhatu et al., 2015; Clary et al., 2017; Koppmair et al., 2017; Ickowitz et al., 2019). Thus, with market integration, commercialization of agricultural, and forest products can provide new food and income opportunities that may improve nutritional outcomes and purchasing power. However, this may lead to trade-offs when income does not translate into improved nutrition (Herforth and Ahmed, 2015).
Bushmeat provides a clear example of the trade-off between nutrition and income. Bushmeat is a nutritionally significant component of local diets, providing an important source of protein (Fa et al., 2003), fat (Sirén and Machoa, 2008), and iron (Golden et al., 2011). There are demonstrated links between bushmeat consumption and improved nutritional status in rural hunting communities (Golden et al., 2011; Fa et al., 2015; Sarti et al., 2015). However, large profit margins incentivize trade in local, national, and international markets, thereby diverting nutritionally important resources outside of communities (Fa et al., 2002, 2006). Widespread exploitation and commercialization of bushmeat across West and Central Africa may therefore threaten food security as well as biodiversity (Fa et al., 2002, 2015; Ripple et al., 2016; Wilkie et al., 2016). For example, projected declines in availability of bushmeat protein over the next 50 years is expected to leave very few countries in the Congo Basin able to meet daily protein requirements (Fa et al., 2003).
Dietary transitions, and their associated health consequences, are primarily understood from studies of hunter-gatherer populations that provide a baseline for measuring the effects of market integration and increased reliance on agriculture (e.g., Reyes-García et al., 2019), and large panel studies that offer insights into the global trends and causal pathways by which forests impact nutrition (e.g., Rasolofoson et al., 2018). However, these approaches can systematically miss important variation at intermediate stages of deforestation and/ or market integration, when communities are lumped together using low stringency criteria, or when sites are ignored because they do not align within well-defined categories (e.g., forested vs. not forested; hunter-gatherer vs. farmer). Furthermore, forest communities with limited deforestation and market integration are often remote, making data collection resource intensive (Reyes-García et al., 2019). As a result, we know very little about the diets of people who live in marginal environments or who exist within the unexamined spaces of these gradients (i.e., semi-forested; hunter-farmers). Understanding these contexts is important, in that they reflect intermediate stages of dietary transitions, where people have access to forest, agricultural, and market foods, as well as the ability to capitalize on these resources via increased market vicinity. Furthermore, the food experiences in these intermediate stages contribute to the trajectory of dietary transitions within landscapes of change, and their consequent effects on health of humans and the environment.
In this study, we examine the effects of tropical deforestation and land use change on diets and food security in an agricultural-forest frontier in West Africa. Our research is focused in a highly relevant system within Cross River State in the South-South geopolitical zone of Nigeria, where expansion of subsistence and commercial agriculture and regional conservation efforts have altered the landscape that provides food and livelihoods. Cross River State contains the largest tract of contiguous forest left in Nigeria and is one of Africa's most important biodiversity reserves (Oates, 1999; Myers et al., 2000; Kamden-Toham et al., 2006). Diverse faunal assemblages within Cross River provide bushmeat to rural communities and urban markets throughout Nigeria and into Cameroon (Fa et al., 2014; Friant et al., 2015; Lameed et al., 2015; Abere et al., 2016). Communities in Cross River vary in their proximity and access to forests and their degree of market integration, in part, because of the long and complicated history of the formation of Cross River National Park and the more recent expansion of the agricultural frontier (Oates, 1999; Ite and Adams, 2000; Schoneveld, 2014). Here we examine how these landscape changes (i.e., the combined impacts of deforestation, agricultural expansion, and forest protection) affect diets and food security within this agriculture-forest frontier. We use a concept of food security that extends beyond caloric sufficiency and dietary staples, toward a more balanced view that reflects access to sufficient quantities of nutritious food for an active and healthy life (USDA, 1996; Ickowitz et al., 2014; Pingali, 2015). Using this framework, we examine how land use changes and market integration at the agriculture-forest frontier affect: (1) diets, (2) bushmeat consumption and trade, (3) food values, and (4) nutrition and food security outcomes. Finally, we consider the implications of our results for human and ecosystem health within landscapes of change.
The forests in Cross River are part of the Cross-Sanaga-Bioko coastal forest, which contains primary and secondary growth forest and unusually high species richness and diversity (Myers et al., 2000; Oates et al., 2004; WWF, 2016). The southern forests of Nigeria cover <2% of Nigeria's landmass, with deforestation in this region dating back to colonial rule in the 1800s and continuing beyond independence (1960s) at an estimated annual rate of 3.7% (FAO, 2010; Enuoh and Ogogo, 2018). Cross River National Park was established in 1991 with an initial plan to extend park boundaries to protect most nearby intact forest and bring rural development projects and guaranteed support for communities that would lose access to agricultural land and non-timber forest products (Ite, 1998; Oates, 1999; Ite and Adams, 2000). However, these plans were never fully implemented due to disputes over funds that were prioritized over conservation objectives, and the withdrawal of support from international donors in response to the execution of environmental activists in Nigeria at the time (Oates, 1999; Ite and Adams, 2000). As a result, Cross River National Park was never fully established, and limited funds have resulted in a support zone consisting of uncompensated and resentful communities on the periphery and interior of protected areas. Meanwhile, population growth and limited access to land contributed to early refusals to grant land for re-settlement of interior forest communities (Ewah, 2013). These communities now exist as designated enclaves within both divisions of the park, where they are allocated forest for farming and hunting. Communities outside of the designated park boundaries are classified as support zone communities. The South is one of Nigeria's largest producers of export crops, including cocoa, rubber, and palm oil, with rapid expansion of large new privatized areas of land allocated to “high-capacity” agricultural investors that are encroaching on both protected and indigenous lands (Schoneveld, 2014).
Local inhabitants of this region do not easily fall within the “hunter-gatherer”—“farmer” dichotomy, and are perhaps best characterized as hunter-agriculturalist societies that depend mainly on agriculture for staple food items and use a combination of wild and cultivated vegetables and animals (Rupp, 2003; Ewah, 2013; Friant et al., 2015; Lameed et al., 2015; Abere et al., 2016). Rural communities in this area depend on the forest for cooking fuel, farmland, and for non-timber forest products, including bushmeat. Forest protection prohibits, to some extent, agricultural expansion into protected areas. However, lax enforcement of laws has resulted in exploitation of forests for timber, construction of roads, agricultural land, and non-timber forest products. The ecological integrity of the forest is now severely threatened by a myriad of human activities, exacerbated by population explosion and high levels of poverty and unemployment (Mahmoud et al., 2017; Enuoh and Ogogo, 2018).
Methods
Study Site and Participants
Our study included six out of 105 (est.) communities near the Oban (~3,000 km2) and Okwangwo (~640 km2) divisions of Cross River National Park (CRNP) in Nigeria (Figure 1). To increase the generalizability of our results, we included communities that represent the three predominate cultural groups living near the park: Boki, Ejagham, and Ayo (Chrisomalis, 2006). Communities were selected to ensure both divisions of the park were represented and to maximize sampling across cultural groups and local government areas. Within these criteria, we selected communities where we had previous research experience or the ability to establish contact with people who could facilitate our entry into potentially resentful communities.
Figure 1
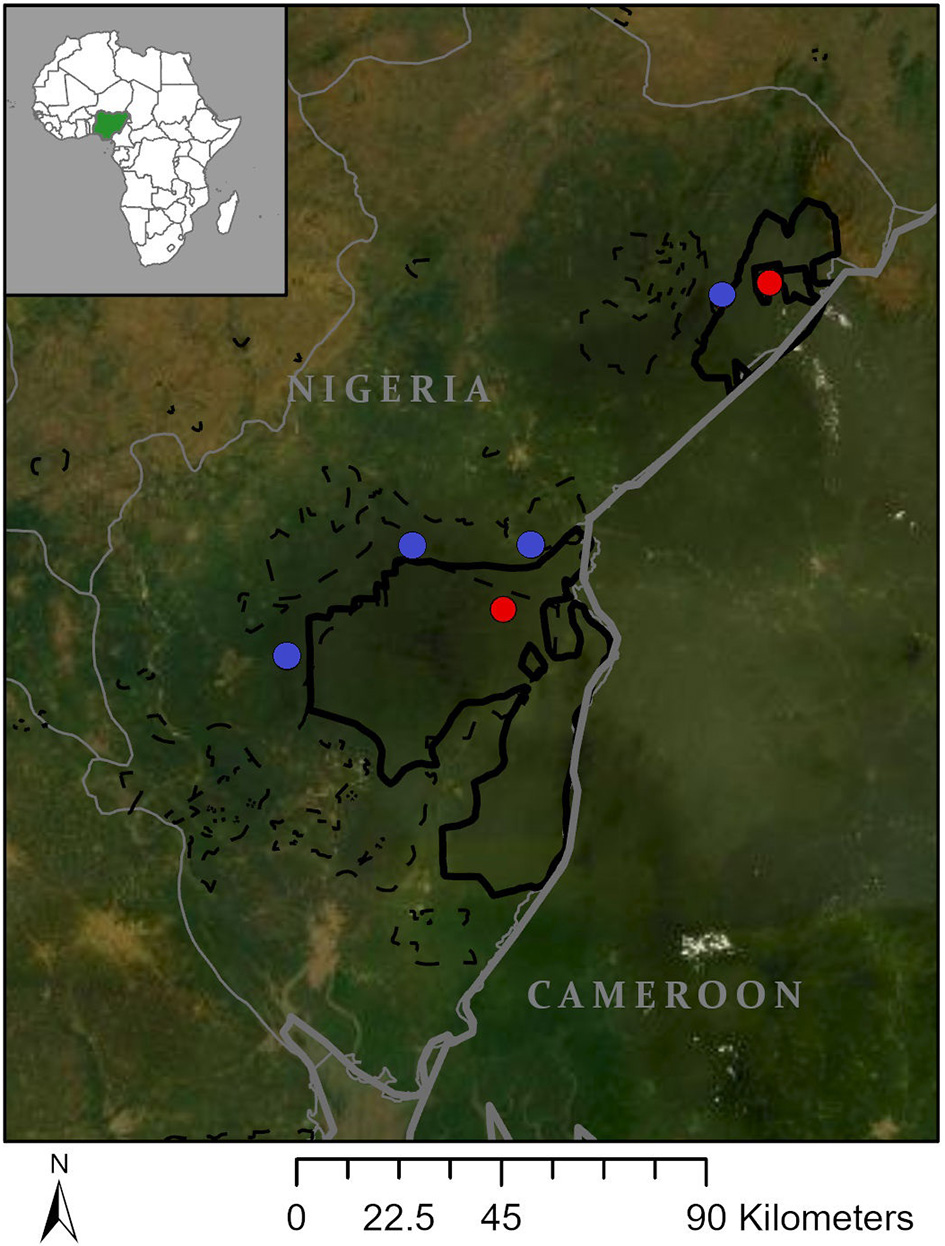
Study communities. Map showing location of deep (red) and marginal (blue) forest communities relative to Cross River National Park (green) in Nigeria.
We selected two communities designated as enclaves within the interior of CRNP (“interior forest”) and four communities designated as the support zone on the periphery of CRNP (“forest-edge”). Interior and forest-edge communities differed in: (1) proximity and access to forests, (2) road access, and (3) access to markets for selling and purchasing food. Due to their location within CRNP, interior forest communities are surrounded by forest, lack motorable roads, and are typically accessed by foot or motorbike via forest trails and partially graded dirt roads that cut through protected areas representing a mosaic of forest and agricultural land. Depending on the mode of travel, it took inhabitants of interior forest communities between half and a full day to reach the nearest markets, and their loads were limited to what they could fit on the back of a motorbike on poor roads or what could be carried on their heads (~20–50 kg). Forest-edge communities are typically accessed by motorbike, vehicles, or motorboat via grated dirt roads, paved roads, or rivers that connect communities to major roads. It took people between 30 min and 2 h to reach major roads and markets, and goods were transported via motorbikes (with heavier loads), vehicles, or boats. Due to their location on the periphery of CRNP, forest-edge communities had access to remnant community forest areas between communities and the park, but most of the surrounding landscapes were heavily deforested from expansion of farmlands, timber business, and/or private commercial agriculture industries (e.g., palm oil plantations).
Data Collection
We restricted data collection to the wet/lean season (June–August 2017) to limit effects of seasonal variation in food availability and road access across sites. We combined individual questionnaires and anthropometric measurements with key informant interviews and participant observations to obtain data on individual diets and nutritional status, household food security, and cultural and economic values attached to food items. All interviews were conducted in Nigerian Pidgin English, which is the lingua franca of the region, to limit differences in interpretation of questionnaires across cultures. However, translations to local languages were made ad hoc when specific words or phrases were not well-understood. Questionnaire instruments were translated into Nigerian Pidgin English, back translated, piloted, and adapted in a neighboring village where no study activities took place. During this pilot phase, we developed initial food lists from observations in households, farms, and local markets and shops. We then worked with key informants who added foods, information on edible parts, and food sources. They also provided locally relevant phrases and examples for evaluating food insecurity (e.g., lists of undesirable foods and local phrasing for “lack of resources”) (Coates et al., 2007; Kennedy et al., 2011). Within each study community, we piloted the questionnaire, asking key informants to answer and then explain the meaning of each question to help ensure it was understood locally. However, because we did not undergo the full adaptation process in each community, we caution that biases could have been introduced where we missed more optimal phrases and locally relevant examples.
Within communities, we randomly selected households from a drawn village map. Households were defined as people who regularly shared food from the same pot. From the questionnaires, we obtained demographic, livelihood, and socioeconomic information, including household participation in the bushmeat trade and household food insecurity, alongside information on individual dietary diversity and meat consumption. Questionnaires were implemented with the head of household responsible for food production (n = 323), representing an average of 48% of households per village (range: 14–84%). This person was typically female (n = 318), unless there was no female present in the household (n = 5). We then randomly re-sampled ~50% of those households to obtain dietary information from men within the same household (n = 155) for a total of 478 individuals (interior forest n = 158, forest-edge n = 320). To evaluate undernutrition, we recorded the mid-upper arm circumference of all respondents (Godoy et al., 2006; USAID et al., 2018). Questionnaire responses and anthropometric measures were recorded by one of four Nigerian research assistants who were accompanied by local translators who verbally translated into local dialects as needed. Answers to closed-ended questions were recorded using ODK® software on a tablet, and open-ended questions were transcribed in real time. All households were offered soap as an incentive gift for participation. We then purposively selected men and women involved in hunting, cooking, and trading in meat as key informants to obtain information on meat preferences and economic values attached to different types of meat (interior forest n = 19, forest-edge n = 31).
Household and Sociodemographic Information
We collected information to identify demographic and socioeconomic factors that may influence diets and food security status, including: age (years); marital status (yes/no); children (number); education (primary school or less/ beyond primary school); and primary occupations (top 3; open). We collected more detailed information from households that participated in hunting or trading bushmeat, including: hunters per households (number), household participation in trading meat (yes/no), destination of meat sold (inside/outside of community), and average proportion of meat sold within (vs. outside of) communities (none [0%], little [5%], some [25%], half [50%], most [75%], all [100%]). We created a wealth index by scoring household assets, including: house ownership, material of roof and walls, number of rooms, type of toilet, household items, and hired farm laborer (Malleson et al., 2008).
Individual Dietary Diversity
We recorded dietary diversity data for 478 participants using 24-h open recalls followed by a second round of probing for additional food items (Kennedy et al., 2011). We categorized food items into 15 food categories−10 main food categories ([1] grains, white roots and tubers, and plantains, [2] pulses (beans, peas, and lentils), [3] nuts and seeds, [4] dairy, [5] meat, poultry and fish, [6] eggs, [7] dark green leafy vegetables, [8] other vitamin-A fruits and vegetables, [9] other vegetables, and [10] other fruits) and five “other” categories ([1] insects and other small protein foods, [2] red palm oil, [3] other oils and fats, [4] sweets, [5] condiments, other beverages, and seasonings) (FAO and FHI 360, 2016) (Table S1). Large invertebrates (e.g., African giant snails and land crabs) were incorporated into the initial meat, fish and seafood category, whereas smaller invertebrates (e.g., small snails, shrimp, and crayfish) were incorporated into the insects and other small proteins category. We added an expanded 30-day recall for animal-based foods where meat, fish, and large invertebrates were disaggregated (Table S2). Within each category we further categorized food sources as either imported or produced within the community or collected from the forest. We calculated dietary diversity scores by first summing the 10 main food categories into a score ranging from 0 to 10. We calculated proportion of the respondents reporting consumption of food items from each group, comparing interior and forest-edge communities. We then calculated Minimum Dietary Diversity for Women of Reproductive Age (MDD-W) by sub-setting women of reproductive age (15–49; n = 232) and categorizing them as achieving minimum dietary diversity (score ≥ 5; more likely to have adequate micronutrient intakes) or not achieving minimum dietary diversity (score <5) (FAO and FHI 360, 2016). Mid-upper arm circumference (MUAC), an indicator of short-term nutritional status, was measured to the nearest millimeter (mm) using MUAC tape (Frisancho, 2008). We used standard MUAC cutoffs to further categorize participants as overweight (MUAC ≥ 25 cm) or underweight (MUAC ≤ 24 cm) (Tang et al., 2013), however pregnancy status was not known for females.
Household Food Security
We ranked 323 households on a Household Food Insecurity Access Scale (HFIAS) based on the prevalence and frequency of experiences of food insecurity (Coates et al., 2007). In each household, we interviewed the individual most involved in food preparation and meals and asked them to respond on behalf of the household. Interview responses were used to quantify experiences of nine household food insecurity access-related conditions within three domains (i.e., anxiety, insufficient quality, and insufficient quantity and physical consequences). We ranked households on the food insecurity access scale by combing prevalence and frequency-of-occurrence to create a score ranging from 0 (secure) to 27 (insecure) (Coates et al., 2007).
Cultural Salience of Bushmeat
To measure the cultural salience of different meat items, we asked key informants to free list animals across multiple domains (e.g., taste preferences and economic value). Following free listing exercises, we used images of wild animals from Kingdon's Pocket Guide of African Mammals (Kingdon, 2005) and standard images of domestic animals and fish sourced from the internet, to ask participants to rank their listed animals.
Data Analysis
We used descriptive statistics to analyze sociodemographic, dietary, and nutritional characteristics of our study population. From dietary recall data, we categorized each food item into food categories (FAO and FHI 360, 2016) (Table S1) and calculated the percentage of diets that included at least one food item in each food category. We also calculated the percentage of diets that included food items from each category that were produced or imported and food items that were harvested from the wild (in either forest or farm). We used mixed-effects linear and logistic regression models, in which we incorporated village as a random effect to account for community clustering of non-independent samples, to compare our samples between interior forest and forest-edge communities. For models containing more than one predictor variable, we used backwards elimination of variables and retained only significant variables (at the alpha = 0.05 level) and first-order interactions among significant main effects in the final model. All analyses were performed in RGui 3.4.4 and statistical significance was determined at the alpha = 0.05 level.
Multivariate Analysis of Diet Composition
We examined the multivariate composition of diets, and bushmeat specifically, in deep and forest-edge communities via non-metric multidimensional scaling analysis (NMDS) with Jaccard dissimilarity matrices. We removed unidentified bushmeat and collapsed categories for animals that were not regularly differentiated (e.g., pangolin, monkey, and nocturnal primate species). We tested for differences in compositional dissimilarity (position of the group centroid) using Permutational Multivariate Analysis of Variance (PERMANOVA) and analysis of multivariate homogeneity of group dispersion (average distance of group members to the group centroid) (PERMADISP), both with 999 permutations. To identify the specific food items that characterized deep and forest-edge communities, we used an indicator species analysis. Indicator values (IV) range from 0 to 1, with higher values for stronger indicators. Only food items with IV > 0.3 and p < 0.05 were considered good indicators (Dufrene and Legendre, 1997). We performed analyses using the metaMDS, adonis2, and betadisp functions within the vegan and indval function within labdsv package in RGui 3.4.4.
Cultural Domain Analysis
We calculated cultural salience (Smith's S) from ranked free lists produced during key informant interviews, where:
We constructed salience plots to visualize taste preferences and economic values of animals by relating the frequency that each animal was mentioned to the average rank assigned to it. We performed analysis using the AnthroTools package in RGui 3.4.4.
Results
Demographics
The primary occupations of our respondents were farming, harvesting of non-timber forest products, and trading in goods (Table 1). We found no differences between deep and forest-edge communities with respect to demographics, livelihoods, or household size (Table 1).
Table 1
| Interior (n = 158) | Edge (n = 320) | Total (N = 478) | |
|---|---|---|---|
| Women: men (%) | 63: 36 | 68: 32 | 66: 33 |
| Average age | 41.1 ± 14.23 | 41.6 ± 15.52 | 41.3 ± 15.09 |
| Family size | 5.22 ± 2.75 | 4.86 ± 2.67 | 4.97 ± 2.73 |
| Education beyond primary school (%) | 60.1 | 69.1 | 66.1 |
| Occupation (%) | |||
| Farmer | 93.7 | 90.6 | 91.6 |
| Harvest NTFPs | 39.9 | 31.6 | 34.3 |
| Trade goods | 12.6 | 20.3 | 17.8 |
| Wealth Index | 7.41 ± 2.35 (n = 97) | 7.76 ± 2.41 (n = 203) | 7.65 ± 2.39 (n = 300) |
Sociodemographic characteristics of respondents.
Dietary Diversity
Dietary diversity was significantly related to village location (X = 9.7, df = 1, p < 0.01) and wealth (X = 6.4, df = 1, p < 0.05), with a marginally significant interactive effect (X = 3.8, df = 1, p = 0.05) such that individuals from wealthier households had marginally higher dietary diversity in forest-edge communities but lower dietary diversity in interior forest communities (Figure 2).
Figure 2
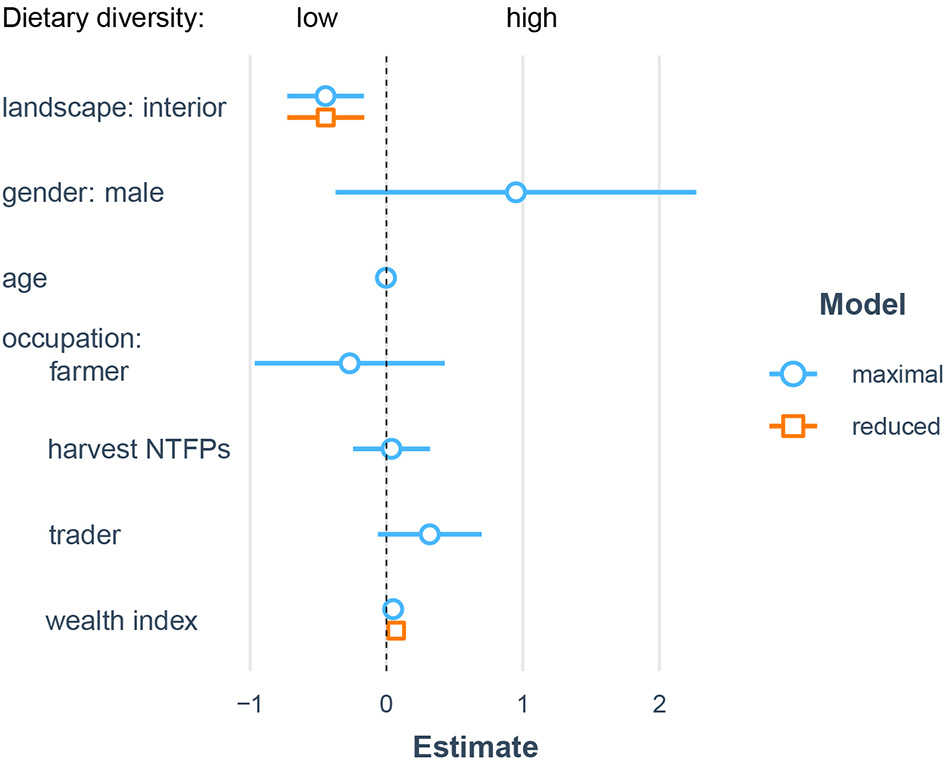
Socioecological predictors of dietary diversity. Results from linear mixed model predicting individual dietary diversity from sociodemographic and landscape differences. Coefficient estimates from full models are shown in blue and coefficients from reduced models retiaing only significant predictors are shown in orange.
Overall, a larger proportion of individuals from interior forest communities reported consuming dark green leafy vegetables (Table 2). Individuals living in forest-edge communities reported consuming more pulses (i.e., beans), dairy, fish, eggs, other vegetables, other oils and fats (i.e., non-red palm oil), sweets, and condiments, other beverages, and seasoning (Table 2). Interior and forest-edge communities differed in where they sourced food items from each category. Specifically, individuals from interior forest communities reported consuming more meat, poultry, and fish (including skin) collected from the wild, and more cultivated vitamin A-rich fruits and vegetables compared to forest-edge communities (Table 2). The opposite trend was true for forest-edge communities, who had a larger proportion of individuals who consumed produced or imported meat, poultry, and fish (including flesh, internal organs, and skin), and more vitamin A-rich fruits and vegetables collected from the forest).
Table 2
| % of diets including food items | % food items produced or imported | % food item collected | ||||
|---|---|---|---|---|---|---|
| Interior | Edge | Interior | Edge | Interior | Edge | |
| Grains, white roots and tubers, and plantains | 99.4 | 99.7 | 100 | 100 | 0 | 0 |
| Pulses (beans, peas, and lentils) | 10.1 | 24.4*** | 100 | 100 | 0 | 0 |
| Nuts and seeds | 82.3 | 80 | 64.6 | 89.1 | 73.1 | 57 |
| Dairy | 3.2 | 12.2** | 100 | 100 | 0 | 0 |
| Meat, poultry, and fish | 87.3 | 92.8 | 43.4 | 73.4** | 89.8* | 64 |
| Flesh meat | 70.9 | 54.7 | 0.8 | 23.3* | 100 | 83.4 |
| Internal organs | 14.5 | 9.4 | 4.3 | 27.6* | 95.6 | 73.3 |
| Skin | 28.5 | 25 | 40 | 76*** | 64.4*** | 25 |
| Fish | 50.6 | 75*** | 60.0 | 78.7 | 47.5 | 29.1 |
| Eggs | 1.3 | 8.1* | 100 | 100 | 0 | 0 |
| Dark green leafy vegetables | 77.8* | 61.2 | 77.2 | 66.7 | 47.1 | 46 |
| Other vitamin A-rich fruits and vegetables | 10.1 | 6.6 | 75** | 28.6 | 25 | 81.0** |
| Other vegetables | 62.5 | 74.7* | 83.8 | 88.3 | 17.2 | 13.8 |
| Other fruit | 43.7 | 62.2 | 100 | 100 | 0 | 0 |
| Insects and other small protein | 67.1 | 81.6 | 97.2 | 99.6 | 13.2 | 12.6 |
| Red palm oil | 99.4 | 96.6 | 100 | 100 | 0 | 0 |
| Other oils and fats | 10.7 | 24.1*** | 100 | 100 | 0 | 0 |
| Sweets | 13.3 | 25.6* | 100 | 100 | 0 | 0 |
| Condiments, other beverages, and seasonings | 94.9 | 99.1* | 100 | 100 | 5.3 | 2.2 |
Consumption of food items and the sources of those foods in diets of deep and forest-edge communities based on 24-h recall data.
p < 0.05,
p < 0.01,
p < 0.001 in linear and logistic mixed-effects regression models comparing deep and forest-edge communities with village incorporated as a random effect.
Comparison of Jaccard dissimilarity matrices, built from binary responses to 24-h dietary recalls assessing dietary diversity (n = 15 food categories), showed that individuals from interior forest communities had a different dietary composition than forest-edge communities (PERMANOVA: F = 12.1, df = 1, p < 0.001). Intragroup variability did not differ between sites (PERMADISP: F = 0.39, df = 1, p = 0.52). A non-metric multidimensional scaling plot shows a degree of dietary similarity (overlapping dietary compositions) but also dietary differences (different group centroids) between interior and forest-edge communities (Figure 3A). Dark green leafy vegetables were a significant indicator category characteristic of interior forest community diets (IV = 0.43, p < 0.001). Other vegetables (IV = 0.40, p < 0.05), fruits (IV = 0.54, p < 0.01), insects and other small proteins (IV = 0.51, p < 0.001), and condiments (IV = 0.50, p < 0.05) were all indicator categories of forest-edge diets (Figure 3B).
Figure 3
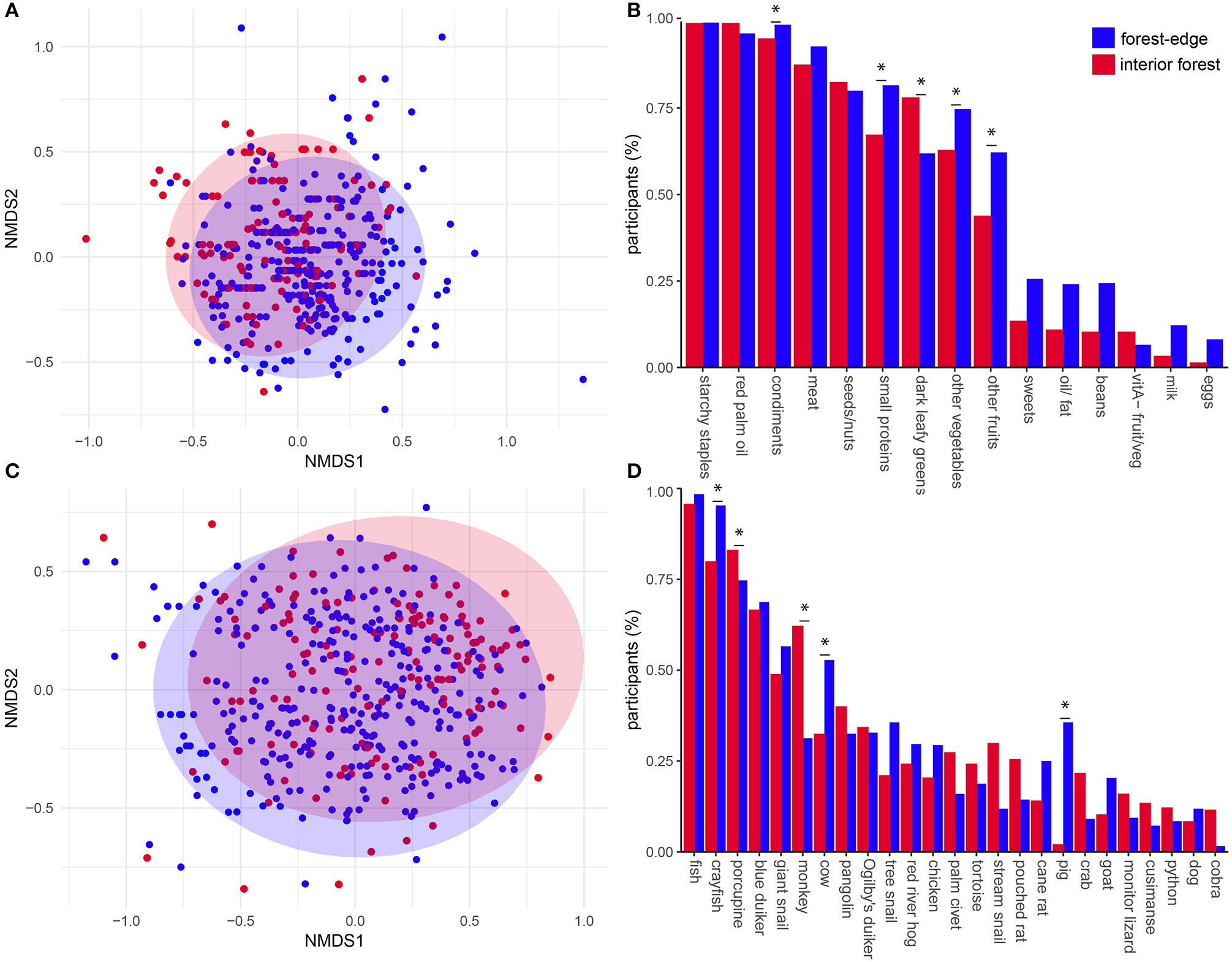
Diet compositions of deep and forest-edge communities. Nonmetric multidimensional scaling (NMDS) plot of the first two axes of Jaccard distance matrices describing dietary composition from 24-h recall data (stress = 0.11, k = 4) (A) and bushmeat composition from 30-day recall data (stress = 0.16, k = 3) (C). Plots are comparing dietary compositions of individuals in interior (red) and forest-edge (blue) forest communities. Barplots compare relative frequency of consumption of food items from each food category (B) and different animals (D) between locations (items with relative frequency < 0.05 not shown). Asterisks indicate indicator foods with IV > 0.3 and p < 0.05 of deep and forest-edge diets.
Comparison of Jaccard dissimilarity matrices, built from animal-source foods reported during 30-day dietary recalls, showed that individuals from interior and forest-edge communities consumed different compositions of meat (PERMANOVA: F = 9.33, df = 1, p < 0.001) and had different intragroup variability (PERMDISP: F = 4.86, df = 1, p < 0.05). The non-metric multidimensional scaling plot shows a degree of similarity in meat consumed (overlapping dietary compositions) but also dietary differences (different group centroids) between deep and forest-edge communities, with the latter showing higher dispersion (Figure 3C). Together, these results show that the core composition of consumed meat was similar in interior and forest-edge communities, and that individuals from forest-edge communities consumed on average a higher diversity of animals. Monkeys (Cercopithecus sp.) (IV = 0.41, p < 0.001) and porcupine (Atherurus africanus) (IV = 0.43, p < 0.05) were significant indicator species of interior forest diets, whereas crayfish (IV = 0.52, p < 0.001), pigs (IV = 0.34, p < 0.001), and cows (IV = 0.33, p < 0.001) were indicators of forest-edge diets (Figure 3D).
Food and Nutrition Security
Households from interior forest communities exhibited significantly higher household food insecurity access scores, fewer women of reproductive age who achieved minimum dietary diversity scores, and lower average mean upper arm circumference (MUAC) in men (Table 3). However, differences in MUAC were not associated with significant differences in the proportion of adults who were categorized as over or underweight (as designated using standard MUAC cutoffs) in interior and forest-edge communities (Table 3).
Table 3
| Interior | Forest-edge | |
|---|---|---|
| Household food insecurity access score (M ± SD) | 13.50 ± 5.70 (n = 103)* | 8.85 ± 5.84 (n = 220) |
| Achieved minimum dietary diversity (%) | 57% (n = 73) | 75% (n = 158)** |
| Male MUAC (M ± SD) | 27.03 ± 2.54 (n = 58) | 28.19 ± 3.14 (n = 102)* |
| Female MUAC (M ± SD) | 26.81 ± 3.52 (n = 99) | 27.60 ± 2.86 (n = 218) |
| Underweight (%) | 14.0 (n = 158) | 12.8 (n = 320) |
| Overweight (%) | 7.6 (n = 158) | 7.2 (n = 320) |
Food security and nutritional status, by forest proximity.
p < 0.05,
p < 0.01 in linear and logistic mixed-effects regression models comparing deep and forest-edge communities with village incorporated as a random effect.
Bushmeat Hunting and Trade
Interior forest communities had a significantly higher proportion of households with bushmeat hunters and/or traders and a higher number of hunters and/or trappers per hunting household compared to forest-edge communities (Table 4). Respondents from both locations reported selling meat that they hunted to people within and outside of the community. However, traders from interior forest communities reported selling a relatively larger proportion of meat to people within the communities (Table 4).
Table 4
| Interior | Forest-edge | |
|---|---|---|
| Household involvement in bushmeat trade (%) | 66.0 (n = 103)* | 44.5 (n = 220) |
| Bushmeat hunters per household (M ± SD) | 2.41 ±1.64 (n = 49)** | 1.67 ± 0.87 (n = 92) |
| Hunter households selling bushmeat (%) | 93.9 (n = 46) | 92.4 (n = 85) |
| Sell bushmeat inside communities (%) | 76.1 (n = 42) | 85.7 (n = 70) |
| Sell bushmeat outside communities (%) | 38.8 (n = 42) | 59.8 (n = 92) |
| Proportion of meat sold within communities (%) | 41.2 (n = 32)** | 23.4 (n = 58) |
Participation in bushmeat hunting and trade, by forest proximity.
p < 0.05,
p < 0.01 in linear and logistic mixed-effects regression models comparing deep and forest-edge communities with village incorporated as a random effect.
Cultural Salience of Animals as Food
Seventy-four percent of participants reported a preference for bushmeat, compared to 19% who preferred fish, and 7% who preferred domestic animal meat. Salience scores (Smith's S) for specific animals revealed preferences for similar species across sites. The top five preferred animals in each landscape type were: African brush-tailed porcupine (Atherus africanus) (interior forest: S = 0.35; forest-edge: S = 0.20), pangolin (Manis spp.) (interior forest: S = 0.20; forest-edge: S = 0.13), red river hog (Potamochoerus porcus) (interior forest: S = 0.09; forest-edge: S = 0.09), monkeys (Cercopithecus sp.) (interior forest: S = 0.17; forest-edge: S = 0.07), Ogilby's duiker (Cephalophus ogilbyi) (interior forest: S = 0.04), and blue duiker (Cephalophus monticola) (forest-edge S = 0.04) (Figures 4A,B).
Figure 4
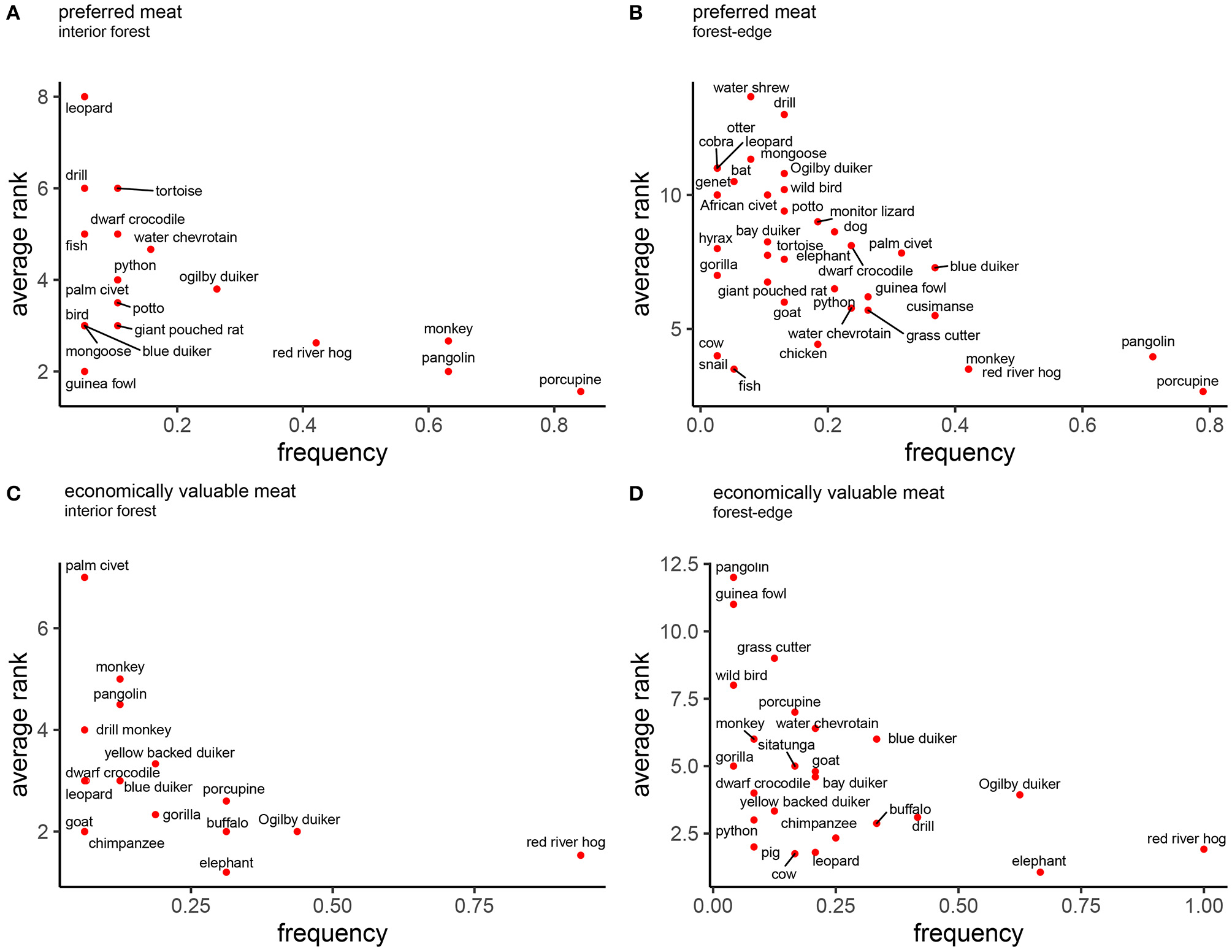
Salience of animals as food and economic resource, by forest proximity. Salience plots of the frequency an animal was mentioned (x-axis) and the average rank assigned to each animal (y-axis) during free-listing exercises with key informants. Plots show preferred animals (A,B) and most economically important (C,D) animals in the lower right-hand corner, comparing deep (A,C) and marginal (B,D) forest communities.
Similarly, the economic salience of different animals was comparable across landscape types, with large bodied wild animals listed as most valuable: red river hog (interior forest: S = 0.38; marginal: S = 0.26), Ogilby duiker (interior forest S = 0.13; forest-edge S = 0.08), African buffalo (Syncerus caffer) (interior forest: S = 0.10; forest-edge: S = 0.06), African forest elephant (Loxodonta cyclotis) (interior forest: S = 0.13; forest-edge S = 0.21), African brush-tailed porcupine (interior forest: S = 0.06), and drill monkey (Mandrillus leucophaeus) (forest-edge: S = 0.07) seen as most valuable (Figures 4C,D).
Domestic animals appeared only in salience plots as preferred foods in forest-edge communities, and included, chicken, dog, goat, and cow (Figure 4B). Similarly, more domestic animals appeared in economic salience plots of forest-edge communities (goat, cow, and pig). Goat appeared in plots derived from both locations, but it was listed more frequently and was assigned higher average rank in forest-edge communities.
Discussion
Across the tropics, forests are being converted to land for subsistence and commercial agriculture, altering local food systems and diets in ways that are currently not well-understood. Our comparison of interior and forest-edge diets highlight the effects of tropical land use changes on local food systems, with implications for understanding the changes occurring at intermediate stages of ecological and dietary transitions at the agricultural-forest frontier. Our results show a high degree of dietary overlap coupled with dietary differences that are associated with better nutrition and food security in forest edges. We argue that nutritional benefits may accrue during intermediate phases of dietary transitions in the tropics—where people retain access to forest resources, obtain access to more agricultural and market goods, and gain the ability to commercialize their food resources. Understanding people's dietary experiences during the early and intermediate stages of deforestation and market integration will be critical, as these early experiences inform dietary and livelihood strategies that further shape ecological and nutritional transitions.
Although forest foods contributed to diets across all sites, we observed fewer forest foods in diets of people living in areas with more deforestation and increased market access. In contrast to forest-edge communities, interior forest communities consumed more dark green leafy vegetables and bushmeat. These observed dietary changes are, to a degree, similar to what has been described during dietary transitions following integration into market economies. Similar to conservation zones with rapid commercial agricultural expansion in Laos (Broegaard et al., 2017), we found that more people in forest-edge communities consumed animal-based foods that were not sourced from the wild. We also observed integration of more processed foods, sweets, and fats, which is similar to dietary transitions described in contemporary hunter-gatherers (Popkin, 2004; Kuhnlein et al., 2009; Crittenden and Schnorr, 2017; Reyes-García et al., 2019). However, we found that interior and forest-edge zone communities were equally likely to consume animal-based foods overall (e.g., meat, protein, and fish), but that forest-edge diets included more beans, dairy, fish, eggs, and other vegetables. Small proteins were an indicator food of forest-edge communities, which can be best explained by high consumption of dried crustaceans, locally referred to as “crayfish,” that are obtained from markets and imported into communities. These findings contrast with dietary transitions described in hunter-gatherer groups, which are characterized by decreased availability of nutritionally important foods (e.g., fruits, vegetables and animal foods) with integration into market economies (Popkin, 2004; Kuhnlein et al., 2009; Crittenden and Schnorr, 2017; Reyes-García et al., 2019). The differences between our study and “typical” hunter-gatherer transitions could be reflective of differences in livelihood strategies (e.g., hunter-agriculturalist) and/or the degree of market integration already present in interior forest communities, while also indicative of non-linear dietary responses to land use change and market integration.
Dietary differences between locations were associated with higher dietary diversity, increased measures of protein, energy, and micronutrient status (e.g., MUAC in men and MDDS-W), and improved food access (i.e., low HFIAS) in forest-edge communities. These results are contrary to previous studies, which found increased dietary diversity in isolated hunter-gatherers compared to close communities with increased market integration (Reyes-García et al., 2019), positive associations between forest use, tree cover, and dietary diversity (Powell et al., 2011), and negative effects of land use change on quality of nutrition in areas adjacent to conservation zones (Broegaard et al., 2017). However, our results are similar to other studies showing improved dietary diversity associated with market access (Sibhatu et al., 2015; Koppmair et al., 2017), and support the notion that market access may be more important for dietary diversity than forest proximity, at least in early and intermediate stages of deforestation. We also note however, that we did not measure differences in agricultural diversity between these sites, which is shown to have a positive effect on dietary diversity (Jones, 2017). Interestingly, while we found no systematic differences between sociodemographic composition of our study samples, we did find a marginal interactive effect of wealth on the relationship between dietary diversity, such that wealth appeared to only contribute to improved dietary diversity in forest-edge communities. This further highlights the importance of market access in the translation of wealth to improved nutrition.
Our results revealed some additional and unexpected trends. For example, we found that vitamin-A rich fruits and vegetables (e.g., bush mango [Irvingiaceae]) were wild-sourced more in forest-edge than interior forest communities. Although contrary to our expectations, this finding may reflect higher availability of bush mango in agroforest areas. Agroforest and fallow areas are known to be important for obtaining wild foods and may contribute to increased dietary diversity in forest-edge areas (Powell et al., 2011). Alternatively, this could indirectly reflect widespread trade of bush mango seeds, known locally as “ogbono” and used in preparing Nigerian soups. Bush mango is mass-harvested in agroforests and in protected and unprotected forest areas in this region, with people setting up forest camps for the primary purpose of harvesting bush mango. The bush mango fruit is typically discarded, but sometimes consumed opportunistically when people are processing the fruit for the seed. Thus, increased consumption of wild vitamin-A rich fruits in marginal communities could reflect increased handling and opportunistic consumption of bush mango in areas with better access to markets, demonstrating how commercial trade might affect diets, even in small and unexpected ways. Overall, the pathways by which forest-edge households achieve improved food access (e.g., direct subsistence from forest, agricultural, and market goods, or purchasing power gained from commercialization of these goods) is variable across food categories and systems.
Despite interior forest communities having more households that hunted and more hunters per household, forest-edge households sold a higher proportion of the meat they hunted to people outside of their communities. This switch toward income-driven hunting did not appear to result in nutrition-income trade-offs, likely due to the availability of alternatives. Interior and forest-edge communities had diets with similar proportions but different compositions of animal-based foods. Meat in interior forest diets was more likely to come from the wild than in forest-edge communities. Specific indicators of interior forest diets were porcupines and monkeys, whereas indicators of forest-edge diets were dried crustaceans, and domestic pig and cow meat/skin, which were imported into communities by traders. These findings align with previous studies showing that bushmeat consumption declines along the rural to urban gradient, being replaced by domestic and processed meat and fish (Van Vliet et al., 2015). Unlike those studies, however, dietary differences we documented were not associated with nutritional inadequacies in forest-edge communities (Sarti et al., 2015; Van Vliet et al., 2015), potentially because these communities still retained access to forests and bushmeat. However, hidden nutrition-related consequences could accrue via putative differences in micro and macro nutrient composition of wild animals compared to domestic animals and fish, though these are not well-understood (Cawthorn and Hoffman, 2015).
Differences in bushmeat consumption in interior forest communities may reflect differences in availability (i.e., animal biomass) and/or access (e.g., affordability). However, evidence from Central Africa indicates that mammalian biomass can actually be higher in marginal rainforest zones, despite higher biodiversity in interior forest zones (Fa et al., 2015). Market vicinity also influences rates of trade in bushmeat, with increased proximity related to higher extraction rates and concentration on large bodied species in the Amazon (Espinosa et al., 2014). If supply is limited, market proximity may reduce access to bushmeat within local communities, when profit margins for selling bushmeat are high. Reduced consumption of bushmeat in forest-edge communities could therefore reflect differences in availability due to ecological degradation associated with deforestation, or reduced access to the meat when hunters and traders prefer to sell outside of the community at higher profit margins.
Markets not only influence trade in goods, but also the values and taste preferences attached to those goods, which may accelerate dietary transitions or preserve the use of traditional foods (Bowles, 1998). Our results showed that the cultural salience of animals was similar across communities but differed across domains. Bushmeat was preferred and had more economic value than domestic animals and fish in both deep and forest-edge communities. Communities shared four out of five of the same preferred species (porcupine, pangolin, monkey, and red river hog) and economically valuable species (red river hog, Ogilby's duiker, African buffalo, and African forest elephant). While the importance of bushmeat likely has much to do with availability, during several interviews key informants referred to domestic animals as “dirty” compared to bushmeat which is “natural” and “sweet” (meaning it has good taste) as reason for their preference. Overall, bushmeat consumption in our study communities is shaped, in part, by preference for bushmeat over domestic species. This preference preserves the use of wild animals, even when other components of the diet differ.
Differences in consumption of bushmeat in forest-edge communities were mirrored by slight differences in value orientation toward domestic animals. Although domestic animals were not highly salient in either domain, they were listed as preferred species in forest-edge communities alone. Similarly, more domestic animals were listed as economically salient in forest-edge communities (e.g., goat, cow, and pig). Goat was listed in both interior and forest-edge communities but was listed more frequently and assigned higher rank in forest-edge communities. These data suggest that preferences can shift toward integration of domestic species as transitions progress. Importantly, rural diets are heavily intertwined with livelihood choices. Although bushmeat is highly preferred, hunting within this region, and in many of the same communities, is considered a low-merit livelihood described as full of suffering and stress, unpredictable, and something people turn to for lack of better alternatives (Friant et al., 2015). Thus changes in livelihood opportunities may further modify consumption practices away from bushmeat consumption (Nasi et al., 2011; Van Vliet et al., 2015). However, high demand for bushmeat by urban populations (Fa et al., 2006; Macdonald et al., 2012) show that even when domestic animals are integrated into daily diets, preferences for bushmeat are maintained, and economic incentives from urban demand will motivate people to continue to hunt.
Heavy regional involvement in the bushmeat trade is associated with wildlife declines and expected species extinctions that may decrease availability of this preferred and nutritionally rich resource (Fa et al., 2002, 2006; Ripple et al., 2016). Indeed, hunters report having to travel further distances and stay longer in the forest to obtain meat, and community members report reduced availability of wild fish due to the use of unsustainable fishing practices (e.g., use of poison and dynamite in streams). When faced with declining availability of meat, especially during lean seasons, interior forest communities have limited ability to supplement wild resources with domestic and imported alternatives. Forest-edge communities may therefore have a dietary advantage, in that they are able to switch between consumption of wild and domesticated meat. Thus, forest-edge communities may be better able to cope with declines in bushmeat by importing meat and using capital from traded goods. Meanwhile, when households lack funds, they may still fall back on forest resources for food in times of need. Indeed, results from Congolese agricultural communities indicate that wild foods play a small role in household consumption but a major role in household income with 90% of bushmeat and fish sold at the market and increased value of these resources during the lean season (de Merode et al., 2004).
Overall, increasing commercialization of forest resources, coupled with high rates of extraction and land conversion in this region is unsustainable (Fa et al., 2006; Schoneveld, 2014), and our data support the notion that ensuing ecological change may disproportionately affect different members of society (Myers et al., 2013). Our results imply that continued heavy extraction from communities for sale of bushmeat would more heavily impact the diets of interior forest communities that lack alternatives. Improved access to markets, when coupled with forest protection, could help enhance dietary diversity and preserve the use wild foods for rural communities. Inclusion of alternative animal-based foods, especially in interior forest communities, will be important for maintaining high quality diets in the face of increased deforestation, agricultural expansion, and improved conservation efforts. Although cultural preference for wild foods is often seen as a barrier to acceptance of new or alternative foods, our results indicate that food preferences may shift as alternatives are introduced and become more culturally salient. However, access to alternative meat sources in rural forested communities may have very little effect on hunting, given that in the presence of alternatives people tend to shift to income-driven hunting and supplement their diets with alternatives. We argue that forest protection and economic alternatives, alongside improved access to alternative animal-based, will be critical for protection of bio- and dietary diversity.
Our study offers an in-depth analysis of food systems in a region of Nigeria undergoing rampant and unregulated environmental change. However, our study has several limitations, including non-random sampling of a small number of communities (n = 6) and lack of associated ecological and landscape data, which together limit the generalizability of our results. Due to logistical constraints of accessing our remote study communities, and the potential that these communities would be uncooperative based on the complicated history of their relationship with the park, we strategically selected a small number of study sites that were representative of the area, but where we could feasibly carryout the study (i.e., connections with people who could favorably introduce us and our research and ability to stay in communities for up to a month). Despite the importance of Cross River as a unique biodiversity reserve in Africa, limited research effort in these areas has severely limited availability of data on deforestation and land-use changes, and thus prohibited a quantitative comparison of the ecological differences between our interior and forest-edge study communities. We have therefore evoked the complicated history of CRNP to aid in explaining site specific differences. Despite the myriad challenges faced during the formation of Cross River National Park, its existence has so far prevented interior forest areas from experiencing the large-scale land conversion that is typical of forest-edge communities. CRNP has also prevented the construction of access roads that link interior communities to major roads and markets. Despite these key differences, we cannot assume that food systems are homogenously impacted based on proximity to CRNP alone. For example, communities in the northern Okwangwo division of the park have been more heavily impacted by conservation policies due to the presence of the Cross-River Gorilla, whereas communities in the southern Oban division have been more heavily impacted by industrial agriculture. Thus, while our data describe the responses of food systems to differences in locations and landscapes, we cannot infer causal processes due to the multiple interacting pathways by which communities might be affected by and respond to tropical land-use change.
Conclusions
Diets at the agricultural-forest frontier of southern Nigeria are characterized by fewer forest-based resources, specifically nutrient-rich foods such as bushmeat and dark green leafy vegetables. Bushmeat was consumed less but traded more often by in forest-edge communities, illustrating potential nutrition-income tradeoffs. However, forest-edge communities appear to compensate for the reduction of forest foods in diets by incorporating alternative animal-based foods (e.g., fish and domestic animals) and other nutritionally important foods, including small proteins, beans, dairy, eggs, and other fruits and vegetables. These data also highlight the heterogeneity in the effect of tropical land use change in diets overtime, suggesting that in the intermittent stages of tropical deforestation, communities experience the best of two worlds—the agricultural and forest frontier. In our study sites, these dietary differences led to improved nutrition and dietary diversity in forest-edge communities. We explain these differences through trade-offs between market access, agricultural expansion and deforestation, and conservation policies. Understanding “micro-transitions” at intermediate stages of land use change will be necessary to provide a clearer picture of the trajectory of livelihood responses to ecological transitions and their associated consequences for human and ecosystem health.
Statements
Data availability statement
The datasets generated for this study are available on request to the corresponding author.
Ethics statement
Nigeria Health Research Ethics Committee (# NHREC/01/01/2007-18/05/2017), City University of New York Integrated Institutional Review Board (#2016-0352), and The Pennsylvania State University Institutional Review Board (#00011190) approved all research activities prior to the initiation of data acquisition. Study activities were also approved by Nigeria National Parks Service and community leaders of each study community. Methods were carried out in accordance with the relevant guidelines and regulations of these institutions and with oral informed consent from all subjects.
Author contributions
SF designed the study, contributed to data collection, cleaned and analyzed data, and drafted the manuscript. WA, AA, NI, and OO contributed to data collection and analysis. JR, TG, JJ, CA, and DO contributed to study design.
Funding
This research was funded by the National Science Foundation (SBE #1604902), Primate Conservation Inc. (PCI# 1381), and The Professional Staff Congress-City University of New York (PSC-CUNY) Research Award Program.
Acknowledgments
We thank the Nigeria National Parks Service, University of Calabar, and our study communities for their support in the field. Specifically, we would like to thank Charles, Ntui Ewu-Ogar, Basil, Imoh Ejen, Caring, Nse Ndoma for their assistance as liaisons and translators within the communities. We also thank Paschal Oshen, Emelia Loomis, and Bill Rohde for their assistance with translations, logistics and training, and manuscript editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsufs.2019.00113/full#supplementary-material
References
1
AbereS. A.LateefF. L.LameedG. A. (2016). Assessment of hunters and other rate of illegal activities in Afi-Mbe-Okwango Division, Cross River State, Nigeria. Nat. Resour.7, 287–294. 10.4236/nr.2016.75025
2
BowlesS. (1998). Endogenous preferences: the cultural consequences of markets and other economic institutions. J. Econ. Lit. 37, 75–111. Available online at: https://www.jstor.org/stable/2564952
3
BroegaardR. B.RasmussenL. V.DawsonN.MertzO.VongvisoukT.GroganK. (2017). Wild food collection and nutrition under commercial agriculture expansion in agriculture-forest landscapes. Forest Policy Econ.84, 92–101. 10.1016/j.forpol.2016.12.012
4
CawthornD.-M.HoffmanL. C. (2015). The bushmeat and food security nexus: a global account of the contributions, conundrums and ethical collisions. Food Res. Int.76, 906–925. 10.1016/j.foodres.2015.03.025
5
ChrisomalisS. (2006). Comparing cultures and comparing processes: diachronic methods in cross-cultural anthropology. Cross Cult. Res.40, 377–404. 10.1177/1069397106287926
6
ClaryC.MatthewsS. A.KestensY. (2017). Between exposure, access and use: reconsidering foodscape influences on dietary behaviours. Health Place44, 1–7. 10.1016/j.healthplace.2016.12.005
7
CoatesJ.SwindaleA.BilinskyP. (2007). Household Food Insecurity Access Scale (HFIAS) for Measurement of Food Access: Indicator Guide. Washington, DC: Food and Nutrition Technical Assistance Project, Academy for Educational Development.
8
CrittendenA. N.SchnorrS. L. (2017). Current views on hunter-gatherer nutrition and the evolution of the human diet. Am. J. Phys. Anthropol.162:e23148. 10.1002/ajpa.23148
9
de MerodeE.HomewoodK.CowlishawG. (2004). The value of bushmeat and other wild foods to rural households living in extreme poverty in Democratic Republic of Congo. Biol. Conserv.118, 573–581. 10.1016/j.biocon.2003.10.005
10
DufreneM.LegendreP. (1997). Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecol. Monogr.67, 345–366. 10.2307/2963459
11
EnuohO. O. O.OgogoA. U. (2018). Assessing tropical deforestation and biodiversity loss in the cross river rainforest of Nigeria. Open J. Forestry8, 393–408. 10.4236/ojf.2018.83025
12
EspinosaS.BranchL. C.CuevaR. (2014). Road development and the geography of hunting by an amazonian indigenous group: consequences for wildlife conservation. PLoS ONE.9:e114916. 10.1371/journal.pone.0114916
13
EwahJ. O. (2013). Survival strategies of support zone communities in Cross River National Park Okwangwo Division, 1990 – 2010. Int. J. Human. Soc. Sci. 3, 238–245. Available online at: www.ijhssnet.com
14
FaJ. E.CurrieD.MeeuwigJ. (2003). Bushmeat and food security in the Congo Basin: linkages between wildlife and people's future. Environ. Conserv.30, 71–78. 10.1017/S0376892903000067
15
FaJ. E.FarfánM. A.MarquezA. L.DuarteJ.NackoneyJ.HallA.et al. (2014). Mapping hotspots of threatened species traded in bushmeat markets in the Cross–Sanaga rivers region. Conserv. Biol.28, 224–233. 10.1111/cobi.12151
16
FaJ. E.OliveroJ.RealR.FarfánM. A.MárquezA. L.VargasJ. M.et al. (2015). Disentangling the relative effects of bushmeat availability on human nutrition in central Africa. Sci. Rep.5:8168. 10.1038/srep08168
17
FaJ. E.PeresC. A.MeeuwigJ. (2002). Bushmeat exploitation in tropical forests: an intercontinental comparison. Conserv. Biol.16, 232–237. 10.1046/j.1523-1739.2002.00275.x
18
FaJ. E.SeymourS.DupainJ.AminR.AlbrechtsenL.MacdonaldD. (2006). Getting to grips with the magnitude of exploitation: bushmeat in the Cross–Sanaga rivers region, Nigeria and Cameroon. Biol. Conserv.129, 497–510. 10.1016/j.biocon.2005.11.031
19
FAO (2010). Global Forest Resources Assessment 2010 - Main Report. Rome. Available online at: http://www.fao.org/3/i1757e/i1757e00.htm (accessed October 30, 2019).
20
FAO and FHI 360. (2016). Minimum Dietary Diversity for Women: A Guide for Measurement. Rome: FAO.
21
FriantS.PaigeS. B.GoldbergT. L. (2015). Drivers of bushmeat hunting and perceptions of zoonoses in Nigerian hunting communities. PLoS Negl. Trop. Dis.9:e0003792. 10.1371/journal.pntd.0003792
22
FrisanchoA. R. (2008). Anthropometric Standards: An Interactive Nutritional Reference of Body Size and Body Composition for Children and Adults. Ann Arbor, MI: University of Michigan Press.
23
GalwayL. P.AcharyaY.JonesA. D. (2018). Deforestation and child diet diversity: a geospatial analysis of 15 Sub-Saharan African countries. Health Place51, 78–88. 10.1016/j.healthplace.2018.03.002
24
GeistH. J.LambinE. F. (2002). Proximate causes and underlying driving forces of tropical deforestation tropical forests are disappearing as the result of many pressures, both local and regional, acting in various combinations in different geographical locations. Bioscience52, 143–150. 10.1641/0006-3568(2002)052[0143:PCAUDF]2.0.CO;2
25
GodoyR.WilkieD. S.Reyes-GarcíaV.LeonardW. R.HuancaT.McdadeT.et al. (2006). Human Body-mass Index (Weight in kg/stature in m2) as a useful proxy to assess the relation between income and wildlife consumption in poor rural societies. Biodivers. Conserv.15, 4495–4506. 10.1007/s10531-005-5100-y
26
GoldenC. D.FernaldL. C.BrasharesJ. S.RasolofoniainaB. R.KremenC. (2011). Benefits of wildlife consumption to child nutrition in a biodiversity hotspot. Proc. Natl. Acad. Sci. U.S.A.108, 19653–19656. 10.1073/pnas.1112586108
27
HerforthA.AhmedS. (2015). The food environment, its effects on dietary consumption, and potential for measurement within agriculture-nutrition interventions. Food Sec.7, 505–520. 10.1007/s12571-015-0455-8
28
IckowitzA.PowellB.RowlandD.JonesA.SunderlandT. (2019). Agricultural intensification, dietary diversity, and markets in the global food security narrative. Global Food Security20, 9–16. 10.1016/j.gfs.2018.11.002
29
IckowitzA.PowellB.SalimM. A.SunderlandT. C. H. (2014). Dietary quality and tree cover in Africa. Glob. Environ. Change24, 287–294. 10.1016/j.gloenvcha.2013.12.001
30
IteU.AdamsW. (2000). Expectations, impacts and attitudes: conservation and development in Cross River National Park, Nigeria. J. Int. Dev.12, 325–342. 10.1002/(SICI)1099-1328(200004)12:3<325::AID-JID655>3.0.CO;2-X
31
IteU. E. (1998). New wine in an old skin: the reality of tropical moist forest conservation in Nigeria. Land Use Policy15, 135–147. 10.1016/S0264-8377(98)80010-0
32
JohnsonK. B.JacobA.BrownM. E. (2013). Forest cover associated with improved child health and nutrition: evidence from the Malawi Demographic and Health Survey and satellite data. Glob. Health Sci. Pract.1, 237–248. 10.9745/GHSP-D-13-00055
33
JonesA. D. (2017). Critical review of the emerging research evidence on agricultural biodiversity, diet diversity, and nutritional status in low- and middle-income countries. Nutr. Rev.75, 769–782. 10.1093/nutrit/nux040
34
Kamden-TohamA.D'AmicoJ.OlsonO. (2006). A Vision for Biodiversity Conservation in Central Africa: Biological Priorities for Conservation in the Guinean-Congolian Forest and Freshwater Region: Annex A. Libreville: World Wide Fund for Nature. Available online at: https://www.fws.gov/international/pdf/results-based-vision-for-conservation-in-Central-Africa.pdf
35
KennedyG.BallardT.DopM. C.European Union (2011). Guidelines for Measuring Household and Individual Dietary Diversity. Rome: Food and Agriculture Organization of the United Nations.
36
KingdonJ. (2005). The Kingdon Pocket Guide to African Mammals. Princeton: Princeton University Press.
37
KoppmairS.KassieM.QaimM. (2017). Farm production, market access and dietary diversity in Malawi. Public Health Nutr.20, 325–335. 10.1017/S1368980016002135
38
KuhnleinH. V.ErasmusB.SpigelskiD.FAO. (2009). Indigenous Peoples' Food Systems: The Many Dimensions of Culture, Diversity and Environment for Nutrition and Health. Rome: Food and Agriculture Organization of the United Nations.
39
KuhnleinH. V.ReceveurO. (1996). Dietary change and traditional food systems of indigenous peoples. Annu. Rev. Nutr.16, 417–442. 10.1146/annurev.nu.16.070196.002221
40
LameedG. A.OmifolajiJ. K.AbereA. S.IloriS. O. (2015). Hunting intensity on wildlife population in oban sector of cross river National Park. Nat. Resour.6, 325–330. 10.4236/nr.2015.64029
41
MacdonaldD. W.JohnsonP. J.AlbrechtsenL.SeymourS.DupainJ.HallA.et al. (2012). Bushmeat trade in the Cross–Sanaga rivers region: evidence for the importance of protected areas. Biol. Conserv.147, 107–114. 10.1016/j.biocon.2011.12.018
42
MahmoudM. I.SloanS.CampbellM. J.AlamgirM.ImongI.OdighaO.et al. (2017). Alternative routes for a proposed nigerian superhighway to limit damage to rare ecosystems and wildlife. Trop. Conserv. Sci.10:1940082917709274. 10.1177/1940082917709274
43
MallesonR.AsahaS.SunderlandT.BurnhamP.EgotM.Obeng-OkrahK.et al. (2008). A methodology for assessing rural livelihood strategies in West/Central Africa: lessons from the field. Ecol. Environ. Anthropol. 25. Available online at: http://digitalcommons.unl.edu/icwdmeea/25
44
MurphyS. P.AllenL. H. (2003). Nutritional importance of animal source foods. J. Nutr.133, 3932S−3935S. 10.1093/jn/133.11.3932S
45
MyersN.MittermeierR. A.MittermeierC. G.Da FonsecaG. A.KentJ. (2000). Biodiversity hotspots for conservation priorities. Nature403, 853–858. 10.1038/35002501
46
MyersS. S.GaffikinL.GoldenC. D.OstfeldR. S.RedfordK. H.RickettsT. H.et al. (2013). Human health impacts of ecosystem alteration. Proc. Natl. Acad. Sci.110, 18753–18760. 10.1073/pnas.1218656110
47
NasiR.TaberA.VlietN. V. (2011). Empty forests, empty stomachs? bushmeat and livelihoods in the Congo and Amazon Basins. Int. Forestry Rev.13, 355–368. 10.1505/146554811798293872
48
OatesJ. (1999). Myth and Reality in the Rain Forest. Berkeley, Los Angeles, CA: University of California Press.
49
OatesJ. F.BerglR. A.LinderJ. M. (2004). Africa's Gulf of Guinea forests: biodiversity patterns and conservation implications. Adv. Appl. Biodivers. Sci.6, 1–90. Available online at: www.biodiversityscience.org; http://s3.amazonaws.com/WCSResources/file_20110823_034557_Oates+Africa+Gulf+Guinea+Biodiversity+Patterns+Priorities_fqyVv.pdf
50
PingaliP. (2015). Agricultural policy and nutrition outcomes – getting beyond the preoccupation with staple grains. Food Sec.7, 583–591. 10.1007/s12571-015-0461-x
51
PiperataB. A.IvanovaS. A.Da-gloriaP.VeigaG.PolskyA.SpenceJ. E.et al. (2011). Nutrition in transition: dietary patterns of rural Amazonian women during a period of economic change. Am. J. Hum. Biol.23, 458–469. 10.1002/ajhb.21147
52
PopkinB. M. (2004). The nutrition transition: an overview of world patterns of change. Nutr. Rev.62, S140–143. 10.1111/j.1753-4887.2004.tb00084.x
53
PowellB.HallJ.JohnsT. (2011). Forest cover, use and dietary intake in the East Usambara Mountains, Tanzania. Int. Forestry Rev.13, 305–317. 10.1505/146554811798293944
54
RasolofosonR. A.HanauerM. M.PappinenA.FisherB.RickettsT. H. (2018). Impacts of forests on children's diet in rural areas across 27 developing countries. Sci. Adv.4:eaat2853. 10.1126/sciadv.aat2853
55
Reyes-GarcíaV.PowellB.Díaz-ReviriegoI.Fernández-LlamazaresÁ.GalloisS.GuezeM. (2019). Dietary transitions among three contemporary hunter-gatherers across the tropics. Food Sec.11, 109–122. 10.1007/s12571-018-0882-4
56
RibotJ. C.AgrawalA.LarsonA. M. (2006). Recentralizing while decentralizing: how national governments reappropriate forest resources. World Dev.34, 1864–1886. 10.1016/j.worlddev.2005.11.020
57
RippleW. J.AbernethyK.BettsM. G.ChapronG.DirzoR.GalettiM.et al. (2016). Bushmeat hunting and extinction risk to the world's mammals. R. Soc. Open Sci.3:160498. 10.1098/rsos.160498
58
RuelM. T.AldermanH.Maternal Child Nutrition Study Group (2013). Nutrition-sensitive interventions and programmes: how can they help to accelerate progress in improving maternal and child nutrition?Lancet382, 536–551. 10.1016/S0140-6736(13)60843-0
59
RuppS. (2003). Interethnic Relations in Southeastern Cameroon: Challenging the “Hunter- Gatherer” – “Farmer” Dichotomy. Available online at: http://repository.kulib.kyoto-u.ac.jp/dspace/handle/2433/68427
60
SandbrookC.NelsonF.AdamsW. M.AgrawalA. (2010). Carbon, forests and the REDD paradox. Oryx44, 330–334. 10.1017/S0030605310000475
61
SartiF. M.AdamsC.MorselloC.Van VlietN.SchorT.YagüeB.et al. (2015). Beyond protein intake: bushmeat as source of micronutrients in the Amazon. Ecol. Soc.20:22. 10.5751/ES-07934-200422
62
SchoneveldG. C. (2014). The politics of the forest frontier: negotiating between conservation, development, and indigenous rights in Cross River State, Nigeria. Land Use Policy38, 147–162. 10.1016/j.landusepol.2013.11.003
63
SibhatuK. T.KrishnaV. V.QaimM. (2015). Production diversity and dietary diversity in smallholder farm households. Proc. Natl. Acad. Sci. U.S.A.112, 10657–10662. 10.1073/pnas.1510982112
64
SirénA.MachoaJ. (2008). Fish, wildlife, and human nutrition in tropical forests: a fat gap? Interciencia 33:186. Available online at: https://www.redalyc.org/articulo.oa?id=33933306
65
TangA. M.DongK.DeitchlerM.ChungM.Maalouf-ManassehZ.TumilowiczA.et al. (2013). Use of Cutoffs for Mid-Upper Arm Circumference (MUAC) as an Indicator or Predictor of Nutritional and Health-Related Outcomes in Adolescents and Adults: A Systematic Review. Washington, DC: FHI 360/FANTA.
66
USAID FANTA III, and FHI 360. (2018). Global MUAC Cutoffs for Adults: A Technical Consultation. Washington, DC.
67
USDA (1996). The U.S. Contribution to World Food Security. The U.S. Position Paper Prepared for the World Food Summit. Washington, DC: United States Department of Agriculture.
68
van NoordwijkM.BizardV.WangpakapattanawongP.TataH. L.VillamorG. B.LeimonaB. (2014). Tree cover transitions and food security in Southeast Asia. Glob. Food Security3, 200–208. 10.1016/j.gfs.2014.10.005
69
Van VlietN.AdamsC.MorselloC.NasiR. (2015). From fish and bushmeat to chicken nuggets: the nutrition transition in a continuum from rural to urban settings in the Colombian Amazon region. Ethnobiol. Conserv. 4, 1–12. 10.15451/ec2015-7-4.6-1-12
70
van VlietN.MertzO.HeinimannA.LangankeT.PascualU.SchmookB.et al. (2012). Trends, drivers and impacts of changes in swidden cultivation in tropical forest-agriculture frontiers: a global assessment. Glob. Environ. Change22, 418–429. 10.1016/j.gloenvcha.2011.10.009
71
VincetiB.TermoteC.IckowitzA.PowellB.KehlenbeckK.HunterD. (2013). The contribution of forests and trees to sustainable diets. Sustainability5, 4797–4824. 10.3390/su5114797
72
ViraB.AgarwalB.JamnadassR.KleinschmitD.McMullinS.MansourianS.et al. (2015). Forests, trees and landscapes for food security and nutrition, in Forests and Food: Addressing Hunger and Nutrition Across Sustainable Landscapes, eds ViraB.WildburgerC.MansourianS. (Vienna: International Union of Forest Research Organizations (IUFRO)), 9–26. 10.11647/OBP.0085.01
73
WilkieD. S.WielandM.BouletH.Le BelS.van VlietN.CornelisD.et al. (2016). Eating and conserving bushmeat in Africa. Afr. J. Ecol.54, 402–414. 10.1111/aje.12392
74
WWF (2016). Western Africa: Coastal parts of Cameroon, Equator | Ecoregions | WWF. World Wildlife Fund. Available online at: http://www.worldwildlife.org/ecoregions/at0107
Summary
Keywords
agriculture, deforestation, bushmeat, conservation, diet, food security, West Africa
Citation
Friant S, Ayambem WA, Alobi AO, Ifebueme NM, Otukpa OM, Ogar DA, Alawa CBI, Goldberg TL, Jacka JK and Rothman JM (2019) Life on the Rainforest Edge: Food Security in the Agricultural-Forest Frontier of Cross River State, Nigeria. Front. Sustain. Food Syst. 3:113. doi: 10.3389/fsufs.2019.00113
Received
21 April 2019
Accepted
27 November 2019
Published
20 December 2019
Volume
3 - 2019
Edited by
Laura Vang Rasmussen, University of British Columbia, Canada
Reviewed by
Alisher Mirzabaev, Center for Development Research (ZEF), Germany; Sylvia Wood, Université du Québec en Outaouais, Canada; Christopher D. Golden, Harvard University, United States
Updates
Copyright
© 2019 Friant, Ayambem, Alobi, Ifebueme, Otukpa, Ogar, Alawa, Goldberg, Jacka and Rothman.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sagan Friant sagan.friant@psu.edu
This article was submitted to Land, Livelihoods and Food Security, a section of the journal Frontiers in Sustainable Food Systems
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.