Abstract
α-galactooligosaccharides (α-GOS) are a class of non-nutritional compounds present in pulses. These carbohydrates are associated with irritable bowel syndrome (IBS). This study explored the genotypic variability for α-GOS content in field pea and cowpea cultivars using high-performance liquid chromatography coupled with a refractive index detector (HPLC-RI). Verbacose, stachyose and raffinose were detected in field pea, whereas in cowpea, only stachyose and raffinose were detected, except the cultivar RC 101 in which verbascose was also found. The total α-GOS content varied from 65.89 ± 0.21 mg/g DW to 101.84 ± 0.33 mg/g DW and 78.78 ± 0.65 mg/g DW to 108.00 ± 0.49 mg/g DW in field pea and cowpea, respectively. The cultivars IPFD 16–13 and KBC 7 were identified as low α-GOS cultivars of field pea and cowpea, respectively. Further, three low-cost, sustainable processing methods, viz. soaking, cooking, and malting (12, 24, 48 and 72 h), were investigated for their effect on the α-GOS content of field pea and cowpea. The results from this study show that malting with 72 h of germination emerged as the most effective method in reducing the total α-GOS content. It reduced verbascose and stachyose content by 74.6 and 67.43% in field pea and by 44.82 and 69.64% in cowpea.
Introduction
Field pea (Pisum sativum L.) and Cowpea (Vigna unguiculata (L.) Walp.) are nutritionally significant pulses, especially for the people of Africa and South Asia. The high level of protein and various micronutrients in them underscores their potential to alleviate malnutrition prevalent in these regions (Hall et al., 2017; Schiermeier, 2019; Hughes et al., 2022). However, the abundance of alpha galactooligosaccharides (α-GOS), a category of FODMAPs (Fermentable oligo-, di-, and mono-saccharides and polyols) in these pulses, limits their consumption and nutritional value. Generally, the α-GOS content of pulses varies from less than 1% to more than 10% (Varney et al., 2017; Ispiryan et al., 2020; Elango et al., 2022). Structurally, α-GOS (including raffinose, stachyose, verbascose, etc.) are made up of different units of galactose molecules linked to sucrose through α-D-1,6-glycosidic bonds (Figure 1).
Figure 1
Humans lack the enzyme alpha-galactosidase, which hydrolyses α-GOS. As a consequence, these carbohydrates are poorly absorbed in the small intestine and pass unbroken to the colon, where they get rapidly fermented by bacterial microflora, producing hydrogen and methane. Besides this, the increased water retention caused by the osmotically active nature of α-GOS (Gibson et al., 2020; Gibson and Halmos, 2020) leads to colon extension. This results in unpleasant gastrointestinal symptoms like excessive flatus, abdominal pain, and changes in bowel frequency in individuals with gastrointestinal disorders, such as irritable bowel syndrome (IBS) (Gibson and Shepherd, 2010; Lenhart and Chey, 2017; Sasi et al., 2023). A low FODMAP diet is widely recognised as an effective therapy for managing the symptoms of IBS (Staudacher et al., 2014; Varney et al., 2017; Gibson and Halmos, 2020; Sperber et al., 2021). Generally, the amount of FODMAPs in the normal diet of an IBS patient varies from 15 to 30 g/day, whereas in a low FODMAP diet, it is restricted to nearly 5 to 18 g/day (Böhn et al., 2015).
Various strategies have been used to reduce the α-GOS content of legumes for nutritional enhancement (Samal et al., 2023). These include enzymatic and bioprocessing methods such as germination and fermentation. Downregulation of galactinol synthase, raffinose synthase, and stachyose synthase has been reported to reduce the α-GOS content of plants (Polowick et al., 2009; Kannan et al., 2018). However, these strategies could not be very successful given the important role of α-GOS in providing abiotic stress tolerance, seed germination, maintaining osmotic balance, and enhancing seed storability. Some commercial supplements (Beano® or Bean Relief™) containing α-GOS hydrolysing enzymes like alpha-galactosidase are available in the market to reduce flatulence and gastrointestinal discomfort; however, their high cost and suboptimal efficiency in the gastrointestinal tract are limitations. Moreover, the α-GOS are also regarded as dietary fibre and possess prebiotic properties that provide numerous health benefits to the human body (Brouns et al., 2019). Therefore, strategies such as the complete dietary exclusion of α-GOS, their enzymatic removal or transgenic approaches aiming for the elimination of α-GOS in seeds may result in low dietary fibre intake and have potentially negative health impacts (Hill et al., 2017). Moreover, in light of the growing demand for minimally processed and clean foods, the research on traditional low-cost, sustainable processing has gained momentum (Nyyssölä et al., 2020). Identifying cultivars with low α-GOS content and targeted reduction of α-GOS using low-cost, sustainable, clean food processing strategies can increase consumer acceptability of field pea and cowpea (Grausgruber et al., 2020).
A significant genotypic variation has been reported for α-GOS content of pulses such as soybean, lupin, and faba bean (Kasprowicz-Potocka et al., 2022). However, there are substantially fewer reports on the variations in α-GOS content of Indian field pea and cowpea cultivars. Hence, the present study was designed to investigate genotypic variation for the α-GOS profile and to investigate the effect of low-cost, sustainable processing methods, viz. soaking, cooking and malting for different time durations to reduce the α-GOS content of field pea and cowpea. The activation of endogenous galactosidases during malting can be harnessed to modulate the α-GOS content of field pea and cowpea. Unlike germination, the scientific literature malting of legumes is very scarce, and the reports on the effect of malting on the α-GOS content of legumes are even more scarce (El-Adawy, 2002; Martínez-Villaluenga et al., 2008; Pérez-Ramírez et al., 2023). Moreover, this is the first study which reports the effect of malting on the α-GOS content of field pea and cowpea, which is an underutilized pulse crop.
Materials and methods
Seed materials
Seeds of 15 field pea cultivars (IPF 99–25, IPF 5–19, IPFD 10–12, IPFD 1–10, IPFD 12–2, IPFD 2014–2, Arkel, Azad P-3, IPFD 16–13, P-1586, P-489, IPF 12–20, EC 328758, EC 341792, and B-22) and 12 cowpea cultivars (TPTC 29, TCS 160, GC 901, TC 901, KBC 7, KBC 9, PL2, PL3, DC 15, DC 16, GC 3, and RC 101) were obtained from All India Coordinated Research Project-MuLLaRP, Indian Institute of Pulses Research, Kanpur, India.
Cultivation practices
The field pea and cowpea cultivars were grown at ICAR-Indian Institute of Pulses Research, Kanpur, Uttar Pradesh, India (situated at 26°29′27.38” N latitude, 80°16′32.319″E longitude, and 152 m above mean sea level) in a randomised complete block design (RCBD) with three replications in respective seasons. This location’s mean annual temperature and rainfall were 26°C and 750 mm, respectively. The soil belongs to the order Inceptisols and has a sandy loam texture, with a mean pH value of 8.0, and an organic carbon content of 2.5 g/kg. The experimental field was prepared by two ploughing followed by harrowing and planking. Cultivars were planted in replication in four rows, each of 4 meters, with a standard row-to-row spacing of 30 cm and plant-to-plant spacing of 5 cm. The spacing of 60 cm was kept between the cultivars. In field pea, the application of nitrogen: phosphorus: potassium was in the ratio 30: 60: 30 Kg/ha, whereas in cowpea, this ratio was 20: 40: 20 Kg/ha. The crops before cowpea and field pea were mungbean and urdbean, respectively. Field pea was irrigated twice, at 45 days after sowing (DAS) and next at the pod filling stage. Cowpea is a rainfed crop grown in the kharif season. To ensure optimal crop health, recommended plant protection measures were also implemented to control insect pests and diseases. The seasonal weed infestation was managed by hand weeding at 20–25 DAS in all the cultivars. To avoid the border effect, out of the four rows, two inner rows were harvested separately, and the harvested seeds were stored in bags at 4°C until use.
Chemicals and reagents
Analytical standards used in the study, including raffinose, stachyose, verbascose, sucrose, maltose and glucose, were purchased from Sigma-Aldrich (India). Ethanol (ACS) was obtained from HiMedia (India). Hexane (HPLC) and water (HPLC) were purchased from Merck-Millipore (India). Hydrogen peroxide (AR) and sodium bicarbonate (ACS) were purchased from Sigma -Aldrich (India).
Processing of field pea and cowpea seed samples
The field pea (IPFD 10–12) and cowpea (RC 101) varieties were selected to assess the effect of different processing treatments because they are popular varieties in India and because of the sufficient availability of their seeds. The various processing treatments used in this study are presented in Table 1. All parameters studied were replicated three times for technical accuracy (n = 3). To prevent microbial contamination, field pea (IPFD 10–12) and cowpea (RC 101) seeds were treated with 10% (w/v) hydrogen peroxide (H2O2), according to Oliveira et al. (2012). Soaking was done by placing 50 g pulse seeds in double distilled water (1:5 ratio) for 12 h at 20°C temperature. Chemical soaking was done by soaking 50 g seeds of field pea and cowpea in 0.03% sodium bicarbonate (NaHCO3) solution in a 1:5 ratio for 12 h at 20°C. For pressure cooking, 100 g seeds were soaked overnight in double distilled water (1:5 ratio) and cooked in fresh water in a pressure cooker for 15 min. All the treated seeds were dried for 48 h at 35°C in an oven and then ground to powder form to be stored in an airtight bag at −20°C till their analysis. Malting of field pea and cowpea seeds was done as per the standard micro malting method (MEBAK 1.5.3) from Mitteleuropäische Brautechnische Analysenkommission MEBAK MEBAK (2011) with some modifications (Onwurafor et al., 2020). Steeping and germination were conducted in a growth chamber (Hi Point 700 FHC LED) with controlled temperature and humidity. Four lots of disinfected field pea and cowpea grains (50 g each) were kept in perforated stainless-steel boxes and were soaked as typically done for the malting of barley seeds, i.e., (soaking-air rest-soaking-air rest-soaking). Initially, seeds were soaked for 5 h at 25°C and then given an air-rest of 2 h. Another round of soaking was done for 16 h at 25°C, followed by air rest of 2 h. Seeds were again soaked for 2 h at 25°C, after which the water was drained, and field pea and cowpea grain lots were kept for germination for different time duration (12 h, 24 h, 48 h and 72 h) at 25°C. The malts were kilned for 17 h at 50°C, 1 h at 60°C, and 5 h at 65°C, after which a characteristic malt aroma was obtained. Subsequently, each lot was milled using a cyclone mill (UDY Corporation, Fort Collins, Colorado, USA) and sieved through a 200 μm sieve. The resultant flour was stored in airtight bags at 4°C until use.
Table 1
| Sl. No | Samples | Processing treatments |
|---|---|---|
| 1. | Field pea (IPFD 10–12) |
|
| 2. | Cowpea (RC 101) |
|
Low cost processing treatments for α-GOS modulation of field pea and cowpea.
Extraction of α-GOS
The extraction of α-GOS from raw and processed pulse samples was done as per the method described by Xiaoli et al. (2008) with slight modifications. In brief, 200 mg of each sample (field pea and cowpea flour) was treated with hexane to remove the fats. The sugars were extracted from the defatted flour by adding 2 mL of 50% ethanol and placing the tubes in a water bath set at 50°C for 30 min. They were centrifuged at a relative centrifugal force of 2,500 x g (times gravity) for 20 min, and the supernatant was stored. Re-extraction of α-GOS was done by adding 2 mL of 50% ethanol to the pellet and incubating it at 50°C for 30 min. The supernatant was again separated using a relative centrifugal force of 2,500 x g (times gravity) for 20 min. The pooled supernatants were evaporated at room temperature, and finally, the sugar left was solubilised in 1.5 mL of HPLC-grade water. The solution was filtered with a 4 μm filter before the chromatographic analysis.
Quantification of α-GOS and other sugars
The Agilent 1,260 infinity series HPLC system coupled to a refractive index (RI) detector was used to quantify α-GOS and other sugars. The HiPlex Column (Agilent, USA) was used, and HPLC-grade water served as a mobile phase (flow rate: 0.6 mL/min). The column temperature was 85°C, the injection volume was 10 μL, and the total run time was 25 min. The retention time of standard raffinose, stachyose and verbascose were 8.91 min, 8.41 min, and 8.16 min, respectively (Figure 2).
Figure 2
The method was validated by assessing linearity, precision (both repeatability and reproducibility), accuracy, the limit of detection (LOD), and the limit of quantification (LOQ). Linearity was determined through linear regression analysis of calibration curves using various concentrations of each analyte, ranging from 25 ppm to 100 ppm. Calibration curves were created by plotting the peak area against the concentration of each standard in MS Excel. Precision was assessed in terms of repeatability and reproducibility and expressed as the percentage of relative standard deviation (RSD) from triplicate measurements. For repeatability, three different concentrations (25 ppm, 50 ppm, and 100 ppm) of the standard analytes were analysed on the same day. For reproducibility, the same concentrations were analysed over three consecutive days. The LOD and LOQ were determined using a statistical method that involves the standard deviation of the response and the slope method. The accuracy of the method was evaluated by adding standard α-GOS at varying concentrations (25 ppm, 50 ppm, 75 ppm, and 100 ppm) to the sample, and the mean percentage recovery values were calculated. Each oligosaccharide in the samples was quantified by comparing its peak area with the peak area of the respective standard analyte.
Total protein estimation
The total protein was determined by Kjeldahl’s method by following AOAC (2005). In this method, the sample is digested in strong acid, which releases nitrogen that is then determined by titration. Briefly, 02 g of powdered sample was transferred to a digestion tube and 3.99 g of catalyst mixture was added to it. Next, 10 mL of 96% concentrated H2SO4 was added to the tube and it was placed in the digestion block preheated to 420°C. The sample in the digestion tube was diluted with 5 mL distilled water after which distillation was carried out. 25 mL of 4% boric acid was transferred in a 250 mL conical flask and kept at the receiver end and 40 mL of 40% NaOH was added using the control panel. The distillate was then titrated against 0.1 N HCl and % Nitrogen and % Protein were calculated by using the following formula.
The total protein was determined by Kjeldahl’s method by following AOAC (2005). In this method, the sample is digested in strong acid, which releases nitrogen that is then determined by titration. Briefly, 02 g of powdered sample was transferred to a digestion tube and 3.99 g of catalyst mixture was added to it. Next, 10 mL of 96% concentrated H2SO4 was added to the tube and it was placed in the digestion block preheated to 420°C. The sample in the digestion tube was diluted with 5 mL distilled water after which distillation was carried out. 25 mL of 4% boric acid was transferred in a 250 mL conical flask and kept at the receiver end and 40 mL of 40% NaOH was added using the control panel. The distillate was then titrated against 0.1 N HCl and % Nitrogen and % Protein were calculated by using the following formula.
Total carbohydrate estimation
Total carbohydrate was determined through the spectrophotometric method using Anthrone’s reagent (Sadasivam, 1996). Carbohydrates were hydrolysed to simple sugars using dilute hydrochloric acid (HCl). Under hot acidic conditions, glucose is dehydrated to hydroxymethyl furfural, which reacts with anthrone reagent to form a green coloured adduct having absorption maxima at 630 nm. 0.1 gram sample was hydrolysed in a boiling water bath for 3 h with 5 mL 2.5 N HCl. After cooling, it was neutralised with sodium carbonate, and volume was made to 100 mL with double-distilled water. 0.5 mL aliquot of it was mixed with 4 mL of anthrone reagent and heated for 8 min in a boiling water bath. Absorbance was noted at 630 nm. A glucose standard varying in concentration from 10 to 100 μg was prepared simultaneously.
Total fat estimation
The gravimetric method was used to estimate the total fat content. Total lipids were extracted from the sample (1.0 gram) in chloroform: methanol: water in the ratio 2:2:1.8. The centrifuge tubes were capped and centrifuged at 2,300 rpm at room temperature for 10 min. Precisely, the chloroform layer was pipetted out and evaporated to dryness at 100°C. The crude fat content was then determined gravimetrically.
Crude fibre estimation
Crude fibre was estimated as described in AOAC method (1995). 2 gram defatted sample was weighed and to it was added 200 mL of 0.255 M H2SO4. It was boiled for 30 min, filtered through Whatman No. 54 filter paper and then washed with distilled water in a Buchner funnel. Next, 200 mL 0.313 M NaOH was added to the residue and it was again boiled for 30 min. The contents were then filtered through Whatman No. 54 filter paper and washed with distilled. The residue was dried in an oven set at 105°C for 3 h till constant weight was attained. It was cooled in a desiccator and weighed again. The crude fibre content was calculated using the following formula:
Where,
W1 = Weight of filter paper.
W2 = Weight of residue + filter paper.
W = Weight of sample.
Ash content estimation
The ash content was determined using AOAC (1995) method. Briefly, 5 gram sample was weighed in a crucible and incinerated in a muffle furnace at 550°C until it turned light grey. It was then cooled in a desiccator and weighed again. The ash content was calculated as follows:
Where,
W1 = Weight of crucible (before incineration).
W2 = Weight of crucible + Weight of sample (after incineration).
W = Weight of sample.
Statistical analysis
All experiments were conducted with three biological replicates. Each biological replicate consisted of three technical replicates, and the mean of the technical replicates was calculated and considered as one biological replicate. For statistical analysis, three biological replicates (n = 3) were used. Statistical analysis was carried out with JMP Pro 16 (JMP®, Version <16>. SAS Institute Inc., Cary, NC, 1989–2023). The results are shown as mean ± standard deviation. The significant difference between different cultivars and treatments was determined through one-way analysis of variance (ANOVA) and posthoc Tukey test. Means were considered statistically different at a p-value < 0.05. The Kolmogorov–Smirnov test was used to confirm that the biochemical parameters in both the field pea and cowpea cultivars are normally distributed. The test results indicated that all samples exhibited a normal distribution for these biochemical parameters in both field pea and cowpea. The box plots showing the distribution of α-GOS data were made using Origin. Pearson’s correlation analysis, hierarchical cluster analysis and principal component analysis were conducted using JMP Pro 16.
Results
HPLC method validation
For validation of the HPLC-RI method, the linearity, repeatability, reproducibility, limits of detection (LODs), and limit of quantification (LOQs) were estimated and the values are enlisted in Table 2. The calibration curve was linear for the three α-GOS standards (raffinose, stachyose and verbascose) and other sugars (sucrose + maltose and glucose). The correlation coefficient value was > 0.99 for each standard. The LOD values ranged from 0.70 to 4.19 ppm, while the LOQ values varied from 2.14 to 12.71 ppm, which shows the good resolution and sensitivity of the analytical method. The method was thoroughly checked for accuracy. Each standard α-GOS was added at different concentrations (25 ppm, 50 ppm, 75 ppm, and 100 ppm) to the field pea sample (IPF 99–25), and the mean percentage recovery values were calculated (97.65% for raffinose, 97.62% for stachyose, and 97.51% for verbascose) which demonstrates the good accuracy of the method. The percentage recovery at different spiking levels is shown in Table 3.
Table 2
| Analytes | Rt (min) | Regression equations | R2 | LODs (ppm) | LOQs (ppm) | Repeatability (% RSD) | Reproducibility (% RSD) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C1 (25 ppm) | C2 (50 ppm) | C3 (100 ppm) | C1 (25 ppm) | C2 (50 ppm) | C3 (100 ppm) | |||||||
| 1 | Raffinose | 8.911 | y = 129.22x – 67.85 | 0.995 | 3.140 | 9.518 | 1.286 | 1.019 | 0.630 | 1.838 | 1.525 | 1.679 |
| 2 | Stachyose | 8.424 | y = 126.54x + 1,408 | 0.997 | 0.704 | 2.136 | 1.643 | 1.264 | 0.948 | 1.823 | 1.061 | 0.935 |
| 3 | Verbascose | 8.163 | y = 126.65x + 874.64 | 1.000 | 1.502 | 4.553 | 0.985 | 0.877 | 0.696 | 1.118 | 1.078 | 0.932 |
| 4 | Sucrose + Maltose | 9.680 | y = 141.95x + 3.63 | 0.998 | 1.527 | 4.627 | 1.245 | 1.221 | 0.741 | 1.513 | 1.234 | 0.735 |
| 5 | Glucose | 11.363 | y = 152.43x – 187.48 | 0.997 | 4.194 | 12.709 | 0.998 | 1.032 | 0.587 | 1.391 | 0.577 | 0.423 |
Retention times (Rt), regression equations, linearity (R2), limits of detection (LODs) and limits of quantification (LOQs), precision (repeatability and reproducibility) of the standard FODMAPs (α-GOS) and other sugars.
Results are the mean of three independent experiments.
Table 3
| Quantity spiked on sample (ppm) | Verbascose | Stachyose | Raffinose | Sucrose + Maltose | ||||
|---|---|---|---|---|---|---|---|---|
| Quantity recovered (ppm) | Recovery (%) | Quantity recovered (ppm) | Recovery (%) | Quantity recovered (ppm) | Recovery (%) | Quantity recovered (ppm) | Recovery (%) | |
| 25.00 | 24.15 ± 0.42 | 96.60 ± 1.69 | 24.11 ± 0.46 | 96.84 ± 1.85 | 24.07 ± 0.49 | 96.30 ± 1.95 | 23.85 ± 0.38 | 95.41 ± 1.52 |
| 50.00 | 48.56 ± 0.48 | 97.12 ± 0.96 | 49.08 ± 0.23 | 98.15 ± 0.46 | 48.10 ± 0.42 | 96.20 ± 0.83 | 48.99 ± 0.54 | 98.18 ± 1.16 |
| 75.00 | 74.24 ± 0.38 | 98.80 ± 0.28 | 74.05 ± 0.34 | 98.71 ± 0.46 | 74.24 ± 0.36 | 98.97 ± 0.48 | 73.86 ± 0.46 | 98.46 ± 0.60 |
| 100.00 | 98.30 ± 0.44 | 98.15 ± 0.44 | 98.50 ± 0.66 | 98.50 ± 0.66 | 98.34 ± 1.24 | 98.34 ± 1.24 | 98.93 ± 0.30 | 98.93 ± 0.30 |
| Mean | 97.67 | 98.05 | 97.45 | 97.75 | ||||
| SD | 0.99 | 0.84 | 1.41 | 1.59 | ||||
| % RSD | 1.02 | 0.86 | 1.45 | 1.62 | ||||
The accuracy of the HPLC method for verbascose, stachyose, raffinose, and sucrose + maltose analysis expressed as a percentage (%) recovery, determined using spiking experiments.
Genotypic variation in α-GOS profile of field pea and cowpea
The α-GOS (raffinose, stachyose and verbascose) were detected in field pea and cowpea cultivars using the HPLC-RI method. In field pea, the concentration of raffinose, stachyose and verbascose varied from 3.18 to 11.96 mg/g dry weight (DW), 19.28 to 48.25 mg/g DW and 10.28 to 50.55 mg/g DW, respectively (Table 4). The box plot distribution of mean α-GOS values in field pea cultivars is shown in Supplementary Figure S1A. The total α-GOS content of the field pea cultivars explored in this study varied from 65.89 to 101.84 mg/g DW (Table 4). The least total α-GOS content was observed in the green seeded variety IPFD 16–13 (65.89 ± 0.21 mg/g DW), whereas the α-GOS content was highest in a wrinkled variety Azad P-3 (101.84 ± 0.33 mg/g DW).
Table 4
| Pulse Cultivars | Seed coat characteristics (shape, color) | Raffinose (mg/g dry weight) | Stachyose (mg/g dry weight) | Verbascose (mg/g dry weight) | Total α-GOS (mg/g dry weight) | Total carbohydrates (%) | Total protein (%) | Total fat (%) | Crude fiber (%) | Ash (%) | Seed yield (kg/hact.) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Field pea | |||||||||||
| IPF 99–25 | Smooth, Yellow | 7.88 ± 0.53e | 29.62 ± 0.22gh | 37.78 ± 0.25ef | 75.28 ± 0.64g | 48.02 ± 0.40c | 19.63 ± 0.10i | 0.85 ± 0.05f | 3.07 ± 0.07a | 1.97 ± 0.12ef | 2,311 ± 15b |
| IPF 5–19 | Smooth, Yellow | 7.12 ± 0.45f | 31.98 ± 0.65f | 30.24 ± 0.17g | 69.34 ± 0.90l | 52.76 ± 0.31a | 19.44 ± 0.07i | 1.05 ± 0.06e | 2.88 ± 0.09ab | 2.15 ± 0.06de | 2,202 ± 20c |
| IPFD 10–12 | Smooth, Green | 5.39 ± 0.25f | 22.46 ± 0.32i | 46.77 ± 0.80ab | 74.62 ± 0.99i | 48.34 ± 0.21c | 20.38 ± 0.15h | 1.34 ± 0.07bcd | 2.43 ± 0.12cd | 2.05 ± 0.09ef | 2,176 ± 14c |
| IPFD 1–10 | Smooth, Yellow | 8.16 ± 0.09g | 33.90 ± 0.36e | 39.05 ± 0.39def | 81.11 ± 0.37f | 42.03 ± 0.11f | 21.24 ± 0.15f | 1.58 ± 0.03a | 1.38 ± 0.06g | 2.00 ± 0.04ef | 2,104 ± 14d |
| IPFD 12–2 | Smooth, Yellow | 5.89 ± 0.12g | 33.43 ± 0.42e | 28.31 ± 1.45g | 67.63 ± 2.39m | 49.98 ± 0.16b | 19.67 ± 0.12i | 1.50 ± 0.04ab | 2.31 ± 0.09d | 2.30 ± 0.05d | 2,434 ± 10a |
| IPFD 2014–2 | Smooth, Brown | 7.82 ± 0.34e | 30.27 ± 0.24g | 43.03 ± 0.34bcd | 81.12 ± 0.51e | 46.07 ± 0.16d | 20.61 ± 0.14gh | 1.24 ± 0.04cd | 2.96 ± 0.04a | 1.92 ± 0.05f | 2,270 ± 10c |
| Arkel | Wrinkled, Green | 8.36 ± 0.11de | 40.05 ± 0.48c | 50.55 ± 0.42a | 98.96 ± 0.51b | 40.58 ± 0.58f | 25.10 ± 0.29ab | 1.46 ± 0.05ab | 2.00 ± 0.07e | 3.07 ± 0.05a | 1845 ± 15e |
| Azad P-3 | Wrinkled, Yellow | 10.26 ± 0.10bc | 43.04 ± 0.01b | 48.54 ± 0.48a | 101.84 ± 0.33a | 41.90 ± 0.41f | 24.93 ± 0.04b | 1.39 ± 0.05bc | 1.54 ± 0.07fg | 2.87 ± 0.07ab | 2,210 ± 20c |
| IPFD 16–13 | Smooth, Green | 3.18 ± 0.03h | 19.28 ± 0.03j | 43.43 ± 0.43bc | 65.89 ± 0.21o | 43.26 ± 0.15e | 20.98 ± 0.43fg | 1.22 ± 0.04d | 2.34 ± 0.05d | 1.94 ± 0.04ef | 1752 ± 18f |
| P-1586 | Smooth, Black | 10.31 ± 0.01bc | 29.03 ± 0.31h | 35.47 ± 0.20f | 74.81 ± 0.14h | 49.35 ± 0.06b | 22.05 ± 0.19d | 1.40 ± 0.05bc | 1.63 ± 0.10f | 1.94 ± 0.05f | 1,224 ± 6jk |
| P-489 | Smooth, Yellow | 10.87 ± 0.26b | 38.51 ± 0.68d | 41.13 ± 0.12cde | 90.51 ± 0.73c | 48.28 ± 0.15c | 21.38 ± 0.13f | 0.82 ± 0.08f | 2.50 ± 0.09cd | 3.05 ± 0.06a | 1,196 ± 14k |
| IPF 12–20 | Smooth, Green | 10.56 ± 0.27b | 37.77 ± 0.66d | 26.14 ± 0.18g | 74.47 ± 0.73j | 52.19 ± 0.05a | 21.49 ± 0.09ef | 0.83 ± 0.04f | 2.67 ± 0.08bc | 2.74 ± 0.06b | 1,492 ± 8h |
| EC 328758 | Smooth, Reddish-brown | 8.77 ± 0.03d | 38.14 ± 0.09d | 36.12 ± 0.04f | 83.03 ± 0.04d | 43.54 ± 0.13e | 21.94 ± 0.14de | 1.50 ± 0.09ab | 2.53 ± 0.11cd | 2.53 ± 0.11c | 1,340 ± 15i |
| EC 341792 | Smooth, Brown | 11.96 ± 0.32a | 48.25 ± 0.27a | 10.28 ± 0.01i | 70.49 ± 0.40k | 39.82 ± 015g | 25.49 ± 0.14a | 1.42 ± 0.06ab | 1.47 ± 0.06fg | 2.93 ± 0.07ab | 1,258 ± 12j |
| B-22 | Smooth, Brown | 9.90 ± 0.03c | 39.87 ± 0.25c | 17.07 ± 0.21h | 66.84 ± 0.14n | 48.34 ± 0.11c | 23.49 ± 0.14c | 0.98 ± 0.03ef | 1.56 ± 0.11fg | 2.88 ± 0.07ab | 1,657 ± 13g |
| Cowpea | |||||||||||
| TPTC 29 | Smooth, Brown | 17.16 ± 0.26f | 78.38 ± 0.44a | ND | 95.54 ± 0.45bc | 43.67 ± 0.48d | 25.38 ± 0.24c | 1.33 ± 0.06e | 2.12 ± 0.07def | 2.97 ± 0.13a | 1,320 ± 10c |
| TCS 160 | Smooth, Pale yellow | 19.96 ± 0.19c | 74.52 ± 0.41cd | ND | 94.48 ± 0.35cd | 40.58 ± 0.47f | 25.79 ± 0.08abc | 2.01 ± 0.10abc | 2.26 ± 0.14de | 2.64 ± 0.05bc | 940 ± 20h |
| GC 901 | Smooth, Golden brown | 16.11 ± 0.01g | 72.46 ± 0.01e | ND | 88.57 ± 0.01h | 49.16 ± 0.33a | 23.42 ± 0.05d | 1.92 ± 0.05abcd | 2.55 ± 0.06bc | 2.87 ± 0.06ab | 1,020 ± 20fg |
| TC 901 | Smooth, Mosaic | 46.11 ± 0.28a | 36.19 ± 0.86h | ND | 82.30 ± 1.01i | 47.81 ± 0.22b | 21.21 ± 0.14f | 1.78 ± 0.05cd | 2.36 ± 0.08bcd | 2.38 ± 0.12cd | 980 ± 10gh |
| KBC 7 | Smooth, Dark brown | 16.99 ± 0.65f | 61.79 ± 0.03g | ND | 78.78 ± 0.65j | 42.72 ± 0.15e | 26.01 ± 0.38ab | 1.87 ± 0.06bcd | 1.97 ± 0.08f | 2.87 ± 0.13ab | 1,040 ± 30f |
| KBC 9 | Smooth, Dark brown | 24.21 ± 0.17b | 66.94 ± 0.13f | ND | 91.15 ± 0.18g | 38.96 ± 0.12g | 26.25 ± 0.25a | 2.13 ± 0.12a | 2.06 ± 0.05ef | 3.09 ± 0.11a | 1,170 ± 10e |
| PL2 | Smooth Reddish brown | 17.87 ± 0.06e | 78.51 ± 0.54a | ND | 96.38 ± 0.35b | 39.55 ± 0.23g | 22.91 ± 0.12d | 1.87 ± 0.06bcd | 3.03 ± 0.10a | 2.17 ± 0.10de | 1,437 ± 13b |
| PL3 | Smooth, Golden brown | 18.08 ± 0.20e | 75.32 ± 0.24bc | ND | 93.40 ± 0.26de | 45.06 ± 0.15c | 25.48 ± 0.10bc | 1.76 ± 0.05d | 2.99 ± 0.07a | 3.02 ± 0.11a | 1,510 ± 20a |
| DC 15 | Smooth, Dark brown | 46.67 ± 0.29a | 35.77 ± 0.02h | ND | 82.44 ± 0.29i | 44.11 ± 0.29d | 23.44 ± 0.10d | 2.00 ± 0.12abc | 2.34 ± 0.07cd | 2.31 ± 0.12de | 1,280 ± 20cd |
| DC 16 | Smooth, Dark brown | 18.80 ± 0.24d | 74.03 ± 0.37d | ND | 92.83 ± 0.37ef | 42.09 ± 0.29e | 25.97 ± 0.15ab | 1.88 ± 0.06bcd | 1.98 ± 0.11f | 2.03 ± 0.06ef | 1,235 ± 5d |
| GC 3 | Smooth, Pale yellow | 15.70 ± 0.05g | 75.94 ± 0.11b | ND | 91.64 ± 0.06fg | 47.96 ± 0.25b | 21.53 ± 0.18ef | 1.21 ± 0.06e | 2.94 ± 0.13a | 1.76 ± 0.09f | 1,150 ± 10e |
| RC 101 | Smooth, White | 13.46 ± 0.30h | 62.21 ± 0.78g | 32.33 ± 0.46a | 108.00 ± 0.49a | 38.87 ± 0.41g | 22.06 ± 0.07e | 2.02 ± 0.11ab | 2.62 ± 0.08b | 2.08 ± 0.06e | 840 ± 20i |
α-GOS, total carbohydrates, total protein and total fat, crude fiber, ash content and seed yield of raw field pea and cowpea cultivars.
Results are expressed as mean ± standard deviation of three independent replications of biological replicates (n = 3). Values are expressed as mean ± SD. Values followed by the different letters in the same column are significantly different (p < 0.05) by ANOVA and Tukey’s test.ND: not detected.
The median values of raffinose, stachyose, verbascose and total α-GOS along with other distribution parameters of field pea have been shown in Supplementary Figure S1A. Hierarchical cluster analysis grouped the studied field pea cultivars in three groups. The cultivars IPFD 1–10, IPFD 2014–2, EC328758, and P-489 were grouped in one cluster, Arkel and Azad P-3 were grouped in another cluster whereas the cultivars IPF99-25, IPFD 10–12, IPF 12–20, P-1586, IPF 5–19, EC-341792, IPFD 12–2, B-22, and IPF 16–13 were grouped in a separate cluster based on their total α-GOS content (Figure 3A). Correlation study showed that total α-GOS content is positively correlated to total protein content of field pea (r = 0.47). However, the total carbohydrate content of the field pea was negatively correlated with its total protein content (r = −0.68) and fat content (r = −0.59) (Figure 4A). PCA analysis further validated these findings and the two principal components (PC1 & PC 2) accounted for 74% of total variation (Figure 5A).
Figure 3
Figure 4
Figure 5
In contrast to the field pea, verbascose was detected in only one cowpea cultivar (RC 101). In other cowpea cultivars, only raffinose and stachyose were detected and their concentration varied from 13.46 to 46.67 mg/g DW and 35.77 to 78.51 mg/g DW, respectively (Table 4). The lowest total α-GOS content was noted in the cowpea cultivar KBC 7 (78.78 ± 0.65 mg/g DW), and the highest concentration was observed in the cultivar RC 101 (108.00 ± 0.49 mg/g DW). The median values of raffinose, stachyose, verbascose and total α-GOS along with other distribution parameters of cowpea have been shown in Supplementary Figure S1B. Hierarchical clustering showed that cultivars TC-901, DC-15 and KBC 7 were grouped in one cluster whereas the cultivars TPTC 29, PL2, TCS 160, PL3, DC 16, GC 901, KBC 9, GC 3 were grouped in another cluster and RC 101was grouped separately in next cluster based on their total α-GOS content (Figure 3B). Correlation study showed that total α-GOS content of cowpea exhibited weak positive correlation with its total protein content (r = 0.01). However, the total carbohydrate content of cowpea was negatively correlated with total protein content (r = −0.40) and fat content (r = −0.51) (Figure 4B). PCA analysis further validated these findings and the two principal components (PC1 & PC 2) accounted for 66.4% of the total variation (Figure 5B).
Effect of soaking on α-GOS content
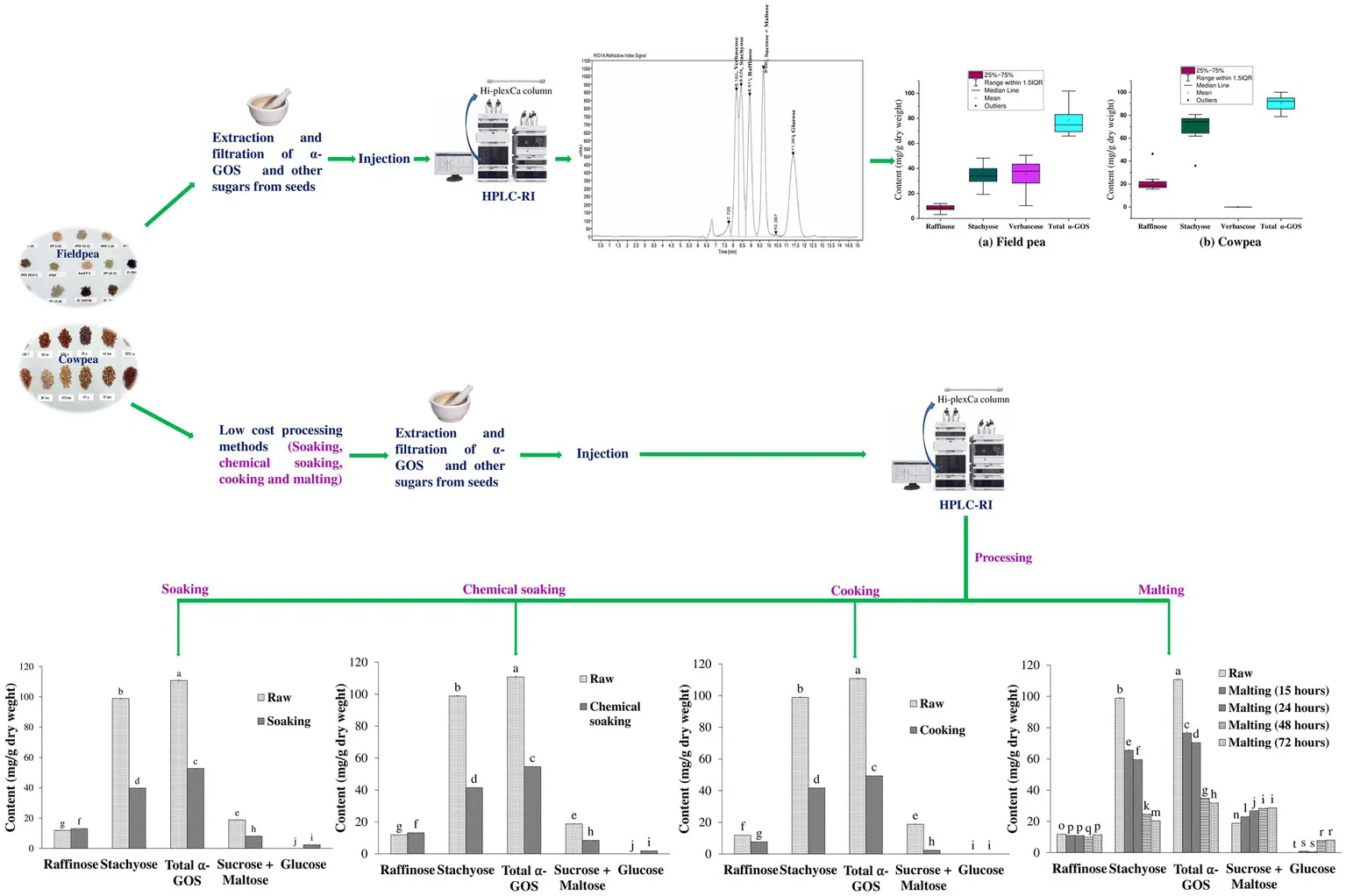
The results from this study exemplify the effectiveness of simple and sustainable processing methods in reducing the α-GOS content of pulses. It was observed that soaking field pea (IPFD 10–12) seeds in water for 12 h decreased their verbascose, stachyose, raffinose, total α-GOS, as well as sucrose + maltose content by 52.75, 15.47, 9.16, 38.27 and 2.56%, respectively (Table 5, Supplementary Figure S2). Chemical soaking in 0.3% sodium bicarbonate was found to be slightly more effective in reducing the verbascose, raffinose and sucrose + maltose content of field pea, and they were reduced by 54.12, 14.14 and 4.13%, respectively (Table 5, Supplementary Figure S2).
Table 5
| Field pea processing | Raffinose (mg/g dry weight) | Stachyose (mg/g dry weight) | Verbascose (mg/g dry weight) | Total α-GOS (mg/g dry weight) | Sucrose + Maltose (mg/g dry weight) | Glucose(mg/g dry weight) |
|---|---|---|---|---|---|---|
| Raw | 6.22 ± 0.09d | 20.42 ± 0.1ab | 44.66 ± 1.51a | 71.3 ± 2.38a | 31.16 ± 0.11e | ND |
| Soaking | 5.65 ± 0.03e | 17.26 ± 0.21c | 21.1 ± 0.13d | 44.01 ± 0.09e | 30.36 ± 0.06f | 2.26 ± 0.1a |
| Chemical Soaking | 5.34 ± 0.02e | 17.73 ± 0.01c | 20.49 ± 0.03d | 43.56 ± 0.02e | 29.87 ± 0.08g | 2.01 ± 0.15b |
| Cooking | 8.15 ± 0.02b | 16.30 ± 0.53c | 23.16 ± 0.03c | 47.61 ± 0.30d | 6.15 ± 0.12h | 0.76 ± 0.14e |
| Mating for 15 h | 6.43 ± 0.00d | 21.83 ± 2.01a | 26.16 ± 0.81b | 54.42 ± 4.69b | 39.63 ± 0.02d | 1.06 ± 0.09d |
| Mating for 24 h | 9.18 ± 0.07a | 19.52 ± 0.03b | 23 ± 0.07c | 51.7 ± 0.07c | 42.65 ± 0.25c | 1.03 ± 0.05d |
| Mating for 48 h | 6.57 ± 0.05d | 8.01 ± 0.16d | 12.74 ± 0.22e | 27.32 ± 0.12f | 51.1 ± 0.73a | 1.82 ± 0.2d |
| Mating for 72 h | 7.19 ± 0.73c | 6.65 ± 0.02d | 11.34 ± 0.13f | 25.18 ± 0.74g | 45.73 ± 0.67b | 1.72 ± 0.2a |
α-GOS content and mono−/disaccharides in raw and processed field pea seeds (IPFD 10–12).
Results are expressed as mean ± standard deviation of three independent replications of technical replicates (n = 3). Values are expressed as mean ± SD. Values followed by the different letters in the same column are significantly different (p < 0.05) by ANOVA followed by Tukey’s test.ND: not detected.
Soaking for 12 h also decreased the verbascose, stachyose, total α-GOS, and sucrose + maltose content of cowpea (RC 101) seeds by 36.21, 40.66, 34.1 and 57.12%, respectively (Table 6) but it increased the raffinose content by 8.31%. Chemical soaking of cowpea (RC 101) seeds in 0.3% sodium bicarbonate solution also decreased their verbascose, stachyose, total α-GOS, and sucrose + maltose content by 37.08, 38.14, 32.75 and 55%, respectively but increased their raffinose content by 9.65% (Table 6; Supplementary Figure S3).
Table 6
| Cowpea processing | Raffinose (mg/g dry weight) | Stachyose (mg/g dry weight) | Verbascose (mg/g dry weight) | Total α-GOS (mg/g dry weight) | Sucrose + Maltose (mg/g dry weight) | Glucose (mg/g dry weight) |
|---|---|---|---|---|---|---|
| Raw | 11.91 ± 0.20b | 67.01 ± 0.16a | 35.43 ± 0.07a | 114.35 ± 0.29a | 18.80 ± 0.3d | ND |
| Soaking | 12.99 ± 0.18a | 39.76 ± 0.16cd | 22.60 ± 0.19c | 75.35 ± 0.43c | 8.06 ± 0.06e | 2.37 ± 0.08c |
| Chemical soaking | 13.15 ± 0.10a | 41.45 ± 0.23b | 22.29 ± 0.64c | 76.89 ± 0.15b | 8.46 ± 0.03e | 1.85 ± 0.03d |
| Cooking | 7.66 ± 0.02f | 41.71 ± 0.11b | 14.18 ± 0.58f | 63.54 ± 0.51e | 2.40 ± 0.22f | ND |
| Mating for 15 h | 11.00 ± 0.27d | 39.34 ± 0.11d | 25.08 ± 0.16b | 75.43 ± 0.03c | 22.92 ± 0.07c | 0.96 ± 0.08e |
| Mating for 24 h | 10.86 ± 0.05d | 39.87 ± 0.14c | 19.54 ± 0.10d | 70.28 ± 0.16d | 26.83 ± 0.03b | 0.52 ± 0.00f |
| Mating for 48 h | 10.12 ± 0.08e | 24.54 ± 0.18e | 17.73 ± 0.06e | 52.39 ± 0.07f | 28.36 ± 0.01a | 7.68 ± 0.04b |
| Mating for 72 h | 11.49 ± 0.01c | 20.34 ± 0.06f | 19.55 ± 0.10d | 51.39 ± 0.14g | 28.66 ± 0.18a | 8.01 ± 0.17a |
α-GOS content and mono−/disaccharides in raw and processed cowpea seeds (RC 101).
Results are expressed as mean ± standard deviation of three independent replications of technical replicates (n = 3). Values are expressed as mean ± SD. Values followed by the different letters in the same column are significantly different (p < 0.05) by ANOVA followed by Tukey’s test.ND: not detected.
Effect of cooking on α-GOS content
Pressure cooking of field pea (IPFD 10–12) seeds (soaked overnight in water) for 15 min reduced their verbascose, stachyose and total α-GOS content by 48.14, 20.17, and 33.22%, respectively, but it increased the raffinose content of field pea by 23.68% (Table 5; Supplementary Figure S2). Pressure cooking also reduced the α-GOS content of cowpea (RC 101) seeds. The verbascose, stachyose, raffinose, α-GOS, and sucrose + maltose content of cowpea (soaked overnight in water) were reduced by 59.97, 37.75, 35.68, 44.43, and 87.20%, respectively, after pressure cooking for 15 min (Table 6; Supplementary Figure S3).
Effect of malting on α-GOS content
It was observed that malting of field pea (IPFD 10–12) and cowpea (RC 101) seeds drastically reduced their α-GOS concentration, particularly after 48 h of malting. Verbascose content of field pea was reduced by 71.47% (12.74 mg/g) and 74.6% (11.34 mg/g) upon malting for 48 h and 72 h, respectively, whereas their stachyose content decreased by 60.77% (8.01 mg/g) and 67.43% (6.65 mg/g) upon malting for 48 h and 72 h, respectively (Table 5; Supplementary Figure S2). In contrast to verbascose and stachyose, the raffinose content of field pea increased upon malting and a maximum enhancement of 13.49% was noted after germination for 72 h, suggesting the increased breakdown of higher oligosaccharides such as verbascose and stachyose into raffinose. Malting also increased the sucrose + maltose content of field pea, and their concentration peaked after 48 h (Table 5; Supplementary Figure S2).
Similar to field pea, the verbascose content of cowpea was reduced by 49.95 and 44.82% after malting for 48 h and 72 h, respectively. Likewise, the stachyose content of cowpea was also reduced by 63.37 and 69.64% after malting for 48 h and 72 h, respectively (Table 6; Supplementary Figure S3). Maximum reduction in raffinose content of cowpea (15.00%) was observed after malting for 48 h. However, Cimini et al. (2024) have reported even up to 80% reduction in the raffinose content of lentil malted for 72 h. Like in field pea, the sucrose + maltose concentration also increased with the progression of malting in cowpea (Table 6). Glucose was absent in raw cowpea seeds but was detected after malting. Generally, the raw seeds have a very low concentration of monosaccharides. However, during malting, the activity of the starch-hydrolysing enzymes, such as α- and β-amylases is increased thereby increasing the glucose content in the malts (Ispiryan et al., 2020).
Discussion
This study shows that stachyose and verbascose are the predominant α-GOS in field pea. Verbascose has a more linear molecular structure than stachyose and raffinose, and thus it is better utilised by probiotic bacteria and, therefore, has a higher flatus-causing potential (Mei et al., 2011). The presence of verbascose in field pea also explains the high flatulence associated with their consumption. Vidal-Valverde et al. (2003) investigated the α-GOS content of 18 field pea cultivars (12 commercial varieties, 2 local varieties and 4 improved lines) from Valladolid (Spain) and reported a raffinose range that varied from 4.1 to 10.3 mg/g DW, which is comparable to our findings. However, the stachyose (10.7 to 26.7 mg/g DW) and verbascose content (0 to 26.7 mg/g DW) reported by them are less as compared to our findings, which might be due to genotypic variations. They also identified superior pea lines having higher nutritional content and fewer non-nutritional compounds. The total α-GOS content was lowest in the green-seeded variety IPFD 16–13 (65.89 ± 0.21 mg/g DW), and it was highest in the wrinkled variety Azad P-3 (101.84 ± 0.33 mg/g DW). Gawłowska et al. (2017) investigated the total α-GOS content of 248 field pea accessions from the Polish Pisum Genebank and reported that it varied from 37.7 to 177.6 mg/g DW. A wider range of total α-GOS content was reported compared to this study, which may be attributed to the large number of accessions they screened, including the wild-type accessions. The wrinkled pea lacks starch branching enzyme 1 (SBE1), which alters not just their starch composition and seed structure but also leads to a higher accumulation of lipids, proteins and α-GOS as compared to the round varieties of pea (Górecki et al., 2000; Mei et al., 2011). Reduced starch accumulation leads to a high sucrose concentration in cotyledons and higher accumulation of α-GOS. This can be further explained by the fact that sucrose is the first acceptor of galactosyl residue during raffinose biosynthesis, and it is also a source of UDP-glucose, which epimerizes to UDP-galactose and is used for galactinol synthesis, which serves as the donor of galactosyl residues in the biosynthesis of α-GOS. The higher sucrose and α-GOS content in wrinkled varieties of pea was also confirmed by Borisjuk et al. (2003). Interestingly, the green-seeded variety IPFD 16–13 that had the least total α-GOS content (65.89 ± 0.43 mg/g DW) also showed the highest verbascose percentage (65.91% of total α-GOS) and thus possibly could have a higher flatus-causing effect despite its low total α-GOS content. This emphasises that even cultivars having low total α-GOS must be checked for their verbascose content and clinically evaluated for flatulence before recommending them for a low FODMAP diet. In contrast to the field pea, verbascose was detected in only one cowpea cultivar (RC 101). In other cowpea cultivars, only raffinose and stachyose were detected. Gonçalves et al. (2016) have also reported the presence of verbascose (0.6–1.3% w/w) in cowpea, albeit at very low concentrations. Apart from cowpea, a few studies in field pea and lentils have also reported that verbascose was undetectable. A study of field pea found verbascose values varying from undetectable concentration to 3.1% of dry matter (Jones et al., 1999). Likewise, a wide variation in the verbascose content of lentils, which ranged from undetectable concentration to 1% of the seed dry weight, was reported by Frias et al. (1994). Vidal-Valverde et al. (2003) investigated the variation in verbascose content of field pea and also observed that the brown colour of the seed coat was correlated with low verbascose content. Possibly, such a correlation between seed coat colour and verbascose content could be there in cowpea as well. Interestingly, all the cowpea varieties that we studied were of brown or reddish-brown colour, except RC 101, which had a white seed coat and in which verbascose was detected. Overall, the results show that cultivar type significantly affects the α-GOS content of field pea and cowpea, and breeding could be exploited as a potential tool to produce low α-GOS cultivars of these pulse crops. Breeders can develop low α-GOS content varieties of field pea and cowpea by selecting parent plants with contrasting α-GOS content and crossing them to combine desirable traits. Multiple generations of selection are necessary to stabilise the low α-GOS content and develop a variety having low α-GOS content (Kumar et al., 2020).
Soaking of pulses in water is done primarily to improve seed texture, reduce cooking time and also to reduce the concentration of non-nutritive compounds. Ibrahim et al. (2002) reported that soaking in alkaline water for 16 h reduced the stachyose content of cowpeas by more than 80%. α-GOS are water-soluble and are leached out during soaking. Besides, the leaching of α-GOS in water soaking could also cause enzymatic degradation of α-GOS (Samtiya et al., 2020). Coffigniez et al. (2018) studied the water uptake kinetics of the cowpea seeds during soaking and cooking. It was reported that soaking at 30°C resulted in low diffusion of α-GOS, whereas at higher soaking temperatures (60°C and 95°C) the rate of diffusion increased substantially. They even studied the cytohistological mechanisms involved in soaking-induced diffusion of α-GOS in cowpeas. Soaking disrupted the pectin and cellulose in the cell wall, and it was more prominent at 60°C and 95°C. Thus, cell wall disruption is responsible for the diffusion of α-GOS during soaking. The higher effectiveness of chemical soaking in reducing these α-GOS and other sugars could be attributed to the change in seed coat permeability in a high-pH solution, which facilitates the α-GOS diffusion (Vijayakumari et al., 2007). Kaur and Prasad (2021) stated that higher α-GOS reduction upon chemical soaking could be explained by the salt-induced dispersion, which increases the solubility of α-GOS in water. Intriguingly, the raffinose content of cowpea (RC 101) seeds increased by 8.31% after soaking (Table 6), which indicates that soaking can induce the metabolic breakdown of stachyose to raffinose. The activation of endogenous alpha-galactosidase in seeds induces stachyose formation from verbascose and raffinose formation of stachyose. Thus, it is evident that soaking effectively modulates the α-GOS content of pulses, and the extent of reduction achieved depends on pulse species and the oligosaccharide being investigated (Acquah et al., 2021).
Pressure cooking is the most common and time-efficient way of cooking pulses. The reduction in verbascose, stachyose and total α-GOS content after pressure cooking can be attributed to the leaching of oligosaccharides and their heat hydrolysis (Thirunathan and Manickavasagan, 2019). However, pressure cooking increased the raffinose content of field pea by 23.68%, which could be due to the pre-soaking-induced enzymatic breakdown of higher oligosaccharides such as verbascose and stachyose into raffinose. Njoumi et al. (2019) investigated the effect of soaking and cooking on α-GOS content of lentil, chickpea and faba bean. They found that soaking decreased the total α-GOS content of lentil and faba bean by 10% and of chickpea by 40%. Cooking of a dish prepared from these pulses further decreased its raffinose, stachyose and verbascose content by 32, 25 and 35%, respectively. This reduction was attributed to the increase in the activity of α-galactosidase, which is active even up to 65°C in lentil.
A comparison of the effect of soaking versus cooking pre-soaked field pea seeds showed that while the former reduced the total α-GOS by 38.27%, the latter reduced it by only 33.22%. This shows that the reduction of total α-GOS in field pea is more effective through soaking as compared to cooking pre-soaked seeds. This could be explained as some protein-bound α-GOS possibly gets released in field pea when these proteins are denatured by cooking (Han and Baik, 2006). Liu et al. (2020) reported that pressure cooking is more effective in reducing the α-GOS concentration than microwave cooking and boiling. In another study by Wang et al. (2008) it is reported that soaking followed by cooking decreased the raffinose, stachyose, and verbascose content of field peas decreased by 12–48%, 34–58%, and 16–42%, respectively, in different cultivars.
A comparison of the effect of soaking versus cooking pre-soaked cowpea seeds showed that while the former reduced the total α-GOS by 34.1%, the latter reduced it by 44.43%. Thus, cooking pre-soaked cowpea seeds is more effective in reducing total α-GOS as compared to soaking. This is in contrast with the observation in field pea, where soaking was more effective in reducing total α-GOS. This highlights the pulse species-dependent manifestation of the effect of various processing treatments in modulating the α-GOS content.
Malting is the controlled germination of seeds, and it is primarily practised in the brewing and distilling industries. However, malting is also used to improve the organoleptic and sensory properties, such as flavour, taste, colour, and tenderness of grains. Malting has three distinct steps: 1. steeping, 2. germination, and 3. kilning. During steeping, the raw grain is submerged in water, increasing the grain moisture to above 42%. This initiates many biochemical pathways involved in synthesising enzymes for the breakdown of non-starch polysaccharides, storage proteins, and starch-degrading enzymes. The next step, germination, is characterised by the mobilisation of these enzymes from the embryo and aleurone layer to the endosperm (Briggs and Hough, 1981). Various enzymes such as proteases, amylases, oxidases, and hemicellulases are active during this stage and result in the formation of green malt. The last step in the process of malting is kilning, during which the green malt is heated to initially bring down the moisture to 12% and then finally to 4–5%. Kilning also induces colour reactions (Maillard and Strecker Degradation compounds) and aroma production.
A recent proteomics study on barley seeds revealed that during malting, the enzyme expression is altered, and storage proteins are lost (Jin et al., 2013; Kerr et al., 2019; Osama et al., 2021). The loss of late embryogenesis proteins (LEA), such as dehydrins, is prominent in germinating seeds. The LEA proteins impart desiccation tolerance to the seed during storage but do not play a significant role after the onset of germination. Likewise, there is a loss of linoleate 9S-lipoxygenase 1 (LOX1), an enzyme involved in the biosynthesis of oxylipins that are known to inhibit germination. Further, enhancement in sugars, amino acids, fatty acids, and Maillard and Strecker compounds has also been reported (Bettenhausen et al., 2018). It was observed in C-hordein-reduced lines of barley that during malting, the enzymes involved in the tricarboxylic acid cycle and fatty acid peroxidation are upregulated to meet the higher energy requirements for seed germination (Bahmani et al., 2023). Beta-amylases and lipid transfer proteins, serpin Z4 and serpin Z7, are also known to be upregulated and mobilise metabolites from the endosperm to the developing embryo. These biochemical changes during malting can thus be harnessed to modulate the α-GOS content of pulses.
The findings from this study make it evident that the malting of field pea and cowpea seeds markedly reduces their α-GOS content and is an efficient and sustainable way of processing pulses. The observation that the duration of germination in malting significantly affects the α-GOS concentration is substantiated by Tian et al. (2010) and Zhang et al. (2021), as they too reported that the hydrolysis of starch and α-GOS peaks between 48 h and 72 h of germination when amylase and alpha-galactosidase activity is at its maximum. Ibrahim et al. (2002) reported complete elimination of α-GOS after germination. Further, Ispiryan et al. (2020) investigated the effect of malting on the FODMAP content of chickpea and lentil and reported that the α-GOS content of chickpea malts was reduced by 87% (0.49 g/100 g DM) and of lentil malts by 72% (0.84 g/100 g DM), respectively. The increase in sucrose and maltose concentration of malted legumes has been previously reported by Gangola et al. (2016) and Arunraj et al. (2020), and it sustains the energy needs of the seed during malting (Kalaivani et al., 2021). Gasiński et al. (2022) investigated the effect of malting on the α-GOS content of lentils. They observed that malting with 6-day germination radically reduced their raffinose and stachyose content. The average stachyose and raffinose content of black lentil malts was 20 times and 23 times lower than the stachyose and raffinose content of black lentils, respectively.
Conclusion
Both field pea and cowpea are a cheap source of protein in many parts of the world, particularly in Asia and Africa. The high α-GOS content of these pulses restricts the harnessing of their nutritional worth and limits their consumer acceptability. The data from this study shows that verbascose and stachyose are the predominant α-GOS in field pea and cowpea, respectively, and the cultivar type significantly affects their α-GOS concentration. Further, it can be inferred from this study that low-cost processing methods, viz. soaking, cooking and malting, are effective enough in reducing the α-GOS concentration of field pea and cowpea. Among all the treatments investigated here, malting for 72 h was found to be the most effective method in reducing their α-GOS concentration. Future studies in this direction may investigate the effect of malting beyond 72 h of germination on the α-GOS content, which has not been explored here. The low α-GOS field pea and cowpea malts can be utilised for developing functional food products and also in the formulation of low FODMAP diets. The in vivo studies using animal models can further validate the effectiveness of these field pea and cowpea malts in alleviating the symptoms of IBS.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
KT: Conceptualization, Investigation, Methodology, Writing – original draft, Writing – review & editing. VK: Conceptualization, Investigation, Methodology, Writing – original draft, Writing – review & editing. AP: Methodology, Writing – review & editing. SJ: Methodology, Writing – original draft. KK: Methodology, Writing – review & editing. RiK: Formal analysis, Validation, Writing – review & editing. MD: Formal analysis, Validation, Writing – review & editing. SM: Writing – original draft. RaK: Formal analysis, Validation, Writing – review & editing. MS: Writing – review & editing. GD: Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. ICAR-Indian Institute of Pulses Research financially supported this work under the research project number CRSCIIPRSIL201701200151.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer GPN declared a shared parent affiliation with the authors to the handling editor at the time of review.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsufs.2025.1600726/full#supplementary-material
References
1
AcquahC.Ohemeng-BoahenG.PowerK. A.ToshS. M. (2021). The effect of processing on bioactive compounds and nutritional qualities of pulses in meeting the sustainable development goal. Front. Sustain. Food Syst.5:681662. doi: 10.3389/fsufs.2021.681662
2
AOAC (1995). Official methods of analysis. 16th Edn. Washington DC: Association of Official Analytical Chemists.
3
AOAC (2005). Official methods of analysis. 18th Edn. Washington DC: Association of Officiating Analytical Chemist.
4
ArunrajR.SkoriL.KumarA.HickersonN. M.ShomaN.SamuelM. A. (2020). Spatial regulation of alpha-galactosidase activity and its influence on raffinose family oligosaccharides during seed maturation and germination in Cicer arietinum. Plant Signal. Behav.15:1709707. doi: 10.1080/15592324.2019.1709707
5
BahmaniM.JuhászA.BoseU.Nye-WoodM. G.BlundellM.HowittC. A.et al. (2023). Proteome changes resulting from malting in hordein-reduced barley lines. J. Agric. Food Chem.71, 14079–14091. doi: 10.1021/acs.jafc.3c02292
6
BettenhausenH. M.BarrL.BroecklingC. D.ChaparroJ. M.HolbrookC.SedinD.et al. (2018). Influence of malt source on beer chemistry, flavour, and flavor stability. Food Res. Int.113, 487–504. doi: 10.1016/j.foodres.2018.07.024
7
BöhnL.StörsrudS.LiljeboT.CollinL.LindforsP.TörnblomH.et al. (2015). Diet low in FODMAPs reduces symptoms of irritable bowel syndrome as well as traditional dietary advice: a randomized controlled trial. Gastroenterology149, 1399–1407. doi: 10.1053/j.gastro.2015.07.054
8
BorisjukL.RolletschekH.WobusU.WeberH. (2003). Differentiation of legume cotyledons as related to metabolic gradients and assimilate transport into seeds. JXB54, 503–512. doi: 10.1093/jxb/erg051
9
BriggsD. E.HoughJ. S. (1981). “Malting and brewing science” in Malt and sweet wort, vol. 1. 2nd ed (London: Chapman and Hall). Available at: https://search.worldcat.org/en/title/768807527
10
BrounsF.van RooyG.ShewryP.RustgiS.JonkersD. (2019). Adverse reactions to wheat or wheat components. CRFSFS18, 1437–1452. doi: 10.1111/1541-4337.12475
11
CiminiA.PolizianiA.MorganteL.MoresiM. (2024). Antinutrient removal in yellow lentils by malting. J. Sci. Food Agric.104, 508–517. doi: 10.1002/jsfa.12950
12
CoffigniezF.BriffazA.MestresC.AlterP.DurandN.BohuonP. (2018). Multi-response modeling of reaction-diffusion to explain alpha-galactoside behavior during the soaking-cooking process in cowpea. Food Chem.242, 279–287. doi: 10.1016/j.foodchem.2017.09.057
13
El-AdawyT. A. (2002). Nutritional composition and antinutritional factors of chickpeas (Cicer arietinum L.) undergoing different cooking methods and germination. Plant Foods Hum. Nutr.57, 83–97. doi: 10.1023/A:1013189620528
14
ElangoD.WangW.ThudiM.SebastiarS.RamadossB. R.VarshneyR. K. (2022). Genome-wide association mapping of seed oligosaccharides in chickpea. Front. Plant Sci.13:1024543. doi: 10.3389/fpls.2022.1024543
15
FriasJ.Vidal-ValverdeC.BakhshA.ArthurA. E.HedleyC. (1994). An assessment of variation for nutritional and non-nutritional carbohydrates in lentil seeds (Lens culinaris). Plant Breed.113, 170–173. doi: 10.1111/j.1439-0523.1994.tb00719.x
16
GangolaM. P.JaiswalS.KannanU.GaurP. M.BågaM.ChibbarR. N. (2016). Galactinol synthase enzyme activity influences raffinose family oligosaccharides (RFO) accumulation in developing chickpea (Cicer arietinum L.) seeds. Phytochemistry125, 88–98. doi: 10.1016/j.phytochem.2016.02.009
17
GasińskiA.Kawa-RygielskaJ.MikulskiD.KłosowskiG. (2022). Changes in the raffinose family oligosaccharides content in the lentil and common bean seeds during malting and mashing processes. Sci. Rep.12:17911. doi: 10.1038/s41598-022-22943-1
18
GawłowskaM.ŚwięcickiW.LahutaL.KaczmarekZ. (2017). Raffinose family oligosaccharides in seeds of Pisum wild taxa, type lines for seed genes, domesticated and advanced breeding materials. Genet. Resour. Crop. Evol.64, 569–578. doi: 10.1007/s10722-016-0384-1
19
GibsonP. R.HalmosE. P. (2020). “FODMAPs and carbohydrate intolerance” in Neurogastroenterol Motil. eds. RaoS. S. C.LeeY. Y.GhoshalU. C.. (Netherlands: Academic Press), 371–386. doi: 10.1016/B978-0-12-813037-7.00026-1
20
GibsonP. R.HalmosE. P.MuirJ. G. (2020). FODMAPS, prebiotics and gut health-the FODMAP hypothesis revisited. Aliment. Pharmacol. Ther.52, 233–246. doi: 10.1111/apt.15818
21
GibsonP. R.ShepherdS. J. (2010). Evidence-based dietary management of functional gastrointestinal symptoms: the FODMAP approach. JGH25, 252–258. doi: 10.1111/j.1440-1746.2009.06149.x
22
GonçalvesA.GoufoP.BarrosA.Domínguez-PerlesR.TrindadeH.RosaE. A.et al. (2016). Cowpea (Vigna unguiculata L. Walp), a renewed multipurpose crop for a more sustainable Agri-food system: nutritional advantages and constraints. J. Sci. Food Agric.96, 2941–2951. doi: 10.1002/jsfa.7644
23
GóreckiR. J.LahutaL. B.JonesA. D.HedleyC. L. (2000) Soluble sugars in maturing pea seeds of different lines in relation to desiccation tolerance. In Seed biology: Advances and applications. Proceedings of the sixth international workshop on seeds, Merida, Mexico, 1999. (pp. 67–74). Wallingford UK: CABI Publishing.
24
GrausgruberH.LovegroveA.ShewryP.BékésF. (2020). “FODMAPs in wheat” in Wheat quality for improving processing and human health. eds. IgrejasG.IkedaT. M.GuzmánC.. (New York: Springer), 517–534. doi: 10.1007/978-3-030-34163-3
25
HallC.HillenC.Garden RobinsonJ. (2017). Composition, nutritional value, and health benefits of pulses. Cereal Chem.94, 11–31. doi: 10.1094/CCHEM-03-16-0069-FI
26
HanI. H.BaikB. K. (2006). Oligosaccharide content and composition of legumes and their reduction by soaking, cooking, ultrasound, and high hydrostatic pressure. Cereal Chem.83, 428–433. doi: 10.1094/CC-83-0428
27
HillP.MuirJ. G.GibsonP. R. (2017). Controversies and recent developments of the low-FODMAP diet. Gastroenterol. Hepatol.13:36:PMC5390324. Available at: https://pubmed.ncbi.nlm.nih.gov/28420945/
28
HughesJ.PearsonE.GrafenauerS. (2022). Legumes—a comprehensive exploration of global food-based dietary guidelines and consumption. Nutrients14:3080. doi: 10.3390/nu14153080
29
IbrahimS. S.HabibaR. A.ShattaA. A.EmbabyH. E. (2002). Effect of soaking, germination, cooking and fermentation on antinutritional factors in cowpeas. Food Nahrung46, 92–95. doi: 10.1002/1521-3803(20020301)46:2<92::AID-FOOD92>3.0.CO;2-P
30
IspiryanL.ZanniniE.ArendtE. K. (2020). Characterization of the FODMAP profile in cereal-product ingredients. J. Cereal Sci.92:102916. doi: 10.1016/j.jcs.2020.102916
31
JinZ.LiX. M.GaoF.SunJ. Y.MuY. W.LuJ. (2013). Proteomic analysis of differences in barley (Hordeum vulgare) malts with distinct filterability by DIGE. J. Proteome93, 93–106. doi: 10.1016/j.jprot.2013.05.038
32
JonesD. A.DuPontM. S.AmbroseM. J.FríasJ.HedleyC. L. (1999). The discovery of compositional variation for the raffinose family of oligosaccharides in pea seeds. Seed Sci. Res.9, 305–310. doi: 10.1017/S0960258599000318
33
KalaivaniV.NikarikaR.ShomaN.ArunrajR. (2021). Delayed hydrolysis of Raffinose family oligosaccharides (RFO) affects critical germination of chickpeas. 3 Biotech.11:298. doi: 10.1007/s13205-021-02764-1
34
KannanU.SharmaR.GangolaM. P.ChıbbarR. N. (2018). Improving grain quality in pulses: strategies to reduce raffinose family oligosaccharides in seeds. EKIN4, 70–88. Available at: https://dergipark.org.tr/en/download/article-file/445467
35
Kasprowicz-PotockaM.GulewiczP.Zaworska-ZakrzewskaA. (2022). The content of raffinose oligosaccharides in legumes and their importance for animals. JAFS31, 265–275. doi: 10.22358/jafs/149656/2022
36
KaurR.PrasadK. (2021). Technological, processing and nutritional aspects of chickpea (Cicer arietinum)-a review. TFST109, 448–463. doi: 10.1016/j.tifs.2021.01.044
37
KerrE. D.CabocheC. H.SchulzB. L. (2019). Posttranslational modifications drive protein stability to control the dynamic beer brewing proteome*[S]. MCP18, 1721–1731. doi: 10.1074/mcp.RA119.001526
38
KumarS.GuptaP.ChoukriH.SiddiqueK. H. M. (2020). “Efficient breeding of pulse crops” in Accelerated plant breeding. eds. GosalS. S.WaniS. H. (Cham: Springer).
39
LenhartA.CheyW. D. (2017). A systematic review of the effects of polyols on gastrointestinal health and irritable bowel syndrome. Adv. Nutr.8, 587–596. doi: 10.3945/an.117.015560
40
LiuY.RagaeeS.MarconeM. F.Abdel-AaE. S. M. (2020). Effect of different cooking methods and heating solutions on nutritionally important starch fractions and flatus oligosaccharides in selected pulses. Cereal Chem.97, 1216–1226. doi: 10.1002/cche.10344
41
Martínez-VillaluengaC.FriasJ.Vidal-ValverdeC. (2008). Alpha-galactosides: antinutritional factors or functional ingredients?Crit. Rev. Food Sci. Nutr.48, 301–316. doi: 10.1080/10408390701326243
42
MEBAK (2011): Raw materials: collection of brewing analysis methods of the Mitteleuropäische Brautechnische Analysenkommission (MEBAK), Germany, Freising-Weihenstephan.
43
MeiG. Y.CareyC. M.ToshS.KostrzynskaM. (2011). Utilization of different types of dietary fibres by potential probiotics. Can. J. Microbiol.57, 857–865. doi: 10.1139/w11-077
44
NjoumiS.Josephe AmiotM.RochetteI.BellaghaS.Mouquet-RivierC. (2019). Soaking and cooking modify the alpha-galacto-oligosaccharide and dietary fibre content in five Mediterranean legumes. Int. J. Food Sci. Nutr.70, 551–561. doi: 10.1080/09637486.2018.1544229
45
NyyssöläA.ElliläS.NordlundE.PoutanenK. (2020). Reduction of FODMAP content by bioprocessing. TFST99, 257–272. doi: 10.1016/j.tifs.2020.03.004
46
OliveiraP. M.MauchA.JacobF.WatersD. M.ArendtE. K. (2012). Fundamental study on the influence of fusarium infection on quality and ultrastructure of barley malt. Int. J. Food Microbiol.156, 32–43. doi: 10.1016/j.ijfoodmicro.2012.02.019
47
OnwuraforE. U.UzodinmaE. O.UchegbuN. N.AniJ. C.UmunnakweI. L.ZieglerG. (2020). Effect of malting periods on the nutrient composition, antinutrient content and pasting properties of mungbean flour. Agro Sci.19, 18–24. doi: 10.4314/as.v19i1.3
48
OsamaS. K.KerrE. D.YousifA. M.PhungT. K.KellyA. M.FoxG. P.et al. (2021). Proteomics reveals commitment to germination in barley seeds is marked by loss of stress response proteins and mobilisation of nutrient reservoirs. J. Proteome242:104221. doi: 10.1016/j.jprot.2021.104221
49
Pérez-RamírezI. F.Escobedo-AlvarezD. E.Mendoza-SánchezM.Rocha-GuzmánN. E.Reynoso-CamachoR.Acosta-GallegosJ. A.et al. (2023). Phytochemical profile and composition of chickpea (Cicer arietinum L.): varietal differences and effect of germination under elicited conditions. Plan. Theory12:93. doi: 10.3390/plants12173093
50
PolowickP. L.BaliskiD. S.BockC.RayH.GeorgesF. (2009). Over-expression of α-galactosidase in pea seeds to reduce raffinose oligosaccharide content. Botany87, 526–532. doi: 10.1139/B09-020
51
SadasivamS. (1996). Biochemical methods. New Delhi: New Age International (P) Limited.
52
SamalI.BhoiT. K.RajM. N.MajhiP. K.MurmuS.PradhanA. K.et al. (2023). Underutilized legumes: nutrient status and advanced breeding approaches for qualitative and quantitative enhancement. Front. Nutr.10:1110750. doi: 10.3389/fnut.2023.1110750
53
SamtiyaM.AlukoR. E.DhewaT. (2020). Plant food anti-nutritional factors and their reduction strategies: an overview. FPPN2, 1–14. doi: 10.1186/s43014-020-0020-5
54
SasiM.KumarS.HasanM.Garcia-GutierrezE.KumariS.PrakashO.et al. (2023). Current trends in the development of soy-based foods containing probiotics and paving the path for soy-synbiotics. Crit. Rev. Food Sci. Nutr.63, 9995–10013. doi: 10.1080/10408398.2022.2078272
55
SchiermeierQ. (2019). Eat less meat: UN climate-change report calls for change to human diet. Nature572, 291–292. doi: 10.1038/d41586-019-02409-7
56
SperberA. D.BangdiwalaS. I.DrossmanD. A.GhoshalU. C.SimrenM.TackJ.et al. (2021). Worldwide prevalence and burden of functional gastrointestinal disorders, results of Rome foundation global study. Gastroenterology160, 99–114.e3. doi: 10.1053/j.gastro.2020.04.014
57
StaudacherH. M.IrvingP. M.LomerM. C.WhelanK. (2014). Mechanisms and efficacy of dietary FODMAP restriction in IBS. Nat. Rev. Gastroenterol. Hepatol.11, 256–266. doi: 10.1038/nrgastro.2013.259
58
ThirunathanP.ManickavasaganA. (2019). Processing methods for reducing alpha-galactosides in pulses. Crit. Rev. Food Sci. Nutr.59, 3334–3348. doi: 10.1080/10408398.2018.1490886
59
TianB.XieB.ShiJ.WuJ.CaiY.XuT.et al. (2010). Physicochemical changes of oat seeds during germination. Food Chem.119, 1195–1200. doi: 10.1016/j.foodchem.2009.08.035
60
VarneyJ.BarrettJ.ScarlataK.CatsosP.GibsonP. R.MuirJ. G. (2017). FODMAPs: food composition, defining cutoff values and international application. JGH32, 53–61. doi: 10.1111/jgh.13698
61
Vidal-ValverdeC.FriasJ.HernándezA.Martín-AlvarezP. J.SierraI.RodríguezC.et al. (2003). Assessment of nutritional compounds and antinutritional factors in pea (Pisum sativum) seeds. J. Sci. Food Agric.83, 298–306. doi: 10.1002/jsfa.1309
62
VijayakumariK.PugalenthiM.VadivelV. (2007). Effect of soaking and hydrothermal processing methods on the levels of antinutrients and in vitro protein digestibility of Bauhinia purpurea L. seeds. Food Chem.103, 968–975. doi: 10.1016/j.foodchem.2006.07.071
63
WangN.HatcherD.GawalkoE. (2008). Effect of variety and pro- cessing on nutrients and certain anti-nutrients in field peas (Pisum sativum). Food Chem.111, 132–138. doi: 10.1016/j.foodchem.2008.03.047
64
XiaoliX.LiyiY.ShuangH.WeiL.YiS.HaoM.et al. (2008). Determination of oligosaccharide contents in 19 cultivars of chickpea (Cicer arietinum L) seeds by high performance liquid chromatography. Food Chem.111, 215–219. doi: 10.1016/j.foodchem.2008.03.039
65
ZhangY.LiD.DirkL. M.DownieA. B.ZhaoT. (2021). ZmAGA1 hydrolyzes RFOs late during the lag phase of seed germination, shifting sugar metabolism toward seed germination over seed aging tolerance. JAFC69, 11606–11615. doi: 10.1021/acs.jafc.1c03677
Summary
Keywords
cowpea, field pea, α-GOS/RFO, legumes, malting, non-nutritional compounds, processing
Citation
Tewari K, Kumar V, Parihar AK, Jha SK, Kumar K, Kumar R, Deo MM, Meena SK, Kumar R, Senthil Kumar M and Dixit GP (2025) Characterisation of the α-GOS profile of field pea and cowpea cultivars and their modulation through sustainable processing methods. Front. Sustain. Food Syst. 9:1600726. doi: 10.3389/fsufs.2025.1600726
Received
26 March 2025
Accepted
02 May 2025
Published
22 May 2025
Volume
9 - 2025
Edited by
Raul Avila-Sosa, Benemérita Universidad Autónoma de Puebla, Mexico
Reviewed by
Guru P. N., Central Institute of Post-Harvest Engineering and Technology (ICAR), India
Alfredi Moyo, National Institute of Medical Research, Tanzania
Sayed Smuda, Cairo University, Egypt
Updates
Copyright
© 2025 Tewari, Kumar, Parihar, Jha, Kumar, Kumar, Deo, Meena, Kumar, Senthil Kumar and Dixit.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kalpana Tewari, tewarikalpana44@gmail.comM. Senthil Kumar, senthil_iari@yahoo.co.in
†These authors have contributed equally to this work and share first authorship
‡ORCID: Kalpana Tewari, https://orcid.org/0000-0001-5978-4653
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.