- 1College of Animal Husbandry and Veterinary Medicine, Jinzhou Medical University, Jinzhou, China
- 2Liaoning Kaiwei Biotechnology Co., Ltd., Jinzhou, China
- 3College of Animal Science and Technology, Northeast Agricultural University, Harbin, China
Sodium dichloroisocyanurate (NaDCC) is commonly used for treating drinking water, industrial water, and wastewater. This study aimed to investigate the potential effects of NaDCC-treated waterline drinking water on the growth of AA+ broilers by reducing microbial levels in the waterline. A total of 480 healthy 1-day-old AA+ broilers (46.77 ± 0.50 g) were selected for the experiment and randomly divided into four groups with six replicates of 20 birds each. The control group received regular drinking water, while the test groups received drinking water with NaDCC concentrations of 10, 30, and 50 mg/L. The test groups consumed the treated water on specific days throughout the 42-day experimental period. Results showed that NaDCC treatment significantly reduced the levels of E. coli, Salmonella, S. aureus and Moulds in the drinking water at the waterline (p < 0.05). Drinking water with NaDCC also led to reduced broiler fecal emissions of NH3 and H2S, as well as reduced counts of E. coli, Salmonella, S. aureus and Moulds (p < 0.05), particularly at 30 mg/L and 50 mg/L concentrations. Broilers consuming NaDCC at 50 mg/L exhibited a significant increase in ADG from days 1–42 (p < 0.05). The levels of E. coli, Salmonella, S. aureus and Moulds in the drinking water at the waterline were significantly and positively correlated with the bacterial count in the feces (p < 0.05, R > 0.6). Additionally, bacterial levels in drinking water and broiler feces were negatively correlated with broiler production performance indicators, including ADG, ADFI, F/G and AWC. In conclusion, NaDCC can indirectly enhance broiler performance by reducing the levels of harmful bacteria in the waterline without affecting normal drinking water. The addition of 30 mg/L or 50 mg/L of NaDCC to the waterline in poultry production can effectively control harmful microorganisms and improve poultry health. Based on the experiment’s results, it is recommended to preferentially use 30 mg/L NaDCC in the waterline to reduce farming costs.
Introduction
Agriculture is an important part of the global economy and livestock production contributes significantly to gross domestic product (1). Poultry meat is becoming one of the most important sources of animal protein for humans in terms of health benefits, cost and production efficiency (2). And pathogenic microorganisms in the water are one of the main culprits of broiler diseases. The use of better water sources and upgraded water treatment processes to enhance drinking water quality has become a widespread practice worldwide, driven by advancements in water purification technology and regulations governing water quality (3). However, despite these efforts, chemical and microbiological contamination of drinking water remains a significant health concern. People strive for pathogen-free drinking water and employ disinfection measures to eliminate most microorganisms from the water. Nonetheless, treated water is not entirely sterile, and small amounts of microorganisms can still be present in drinking water systems (DWS) (4, 5). In the case of livestock, including broilers, the quality of drinking water and DWS play a crucial role in overall health and performance (6, 7).
Research indicates that over 95% of the biomass in water pipes adheres to the pipe walls as biofilm, while only 5% remains suspended in the bulk water (8, 9). Under favorable conditions such as suitable coop temperature, low water flow in the waterline, and sufficient nutrients, DWS can create an ideal environment for microbial growth (10). Common microorganisms found in broiler drinking water (11), including Campylobacter jejuni, E. coli, Pseudomonas and Salmonella, have been identified as major biofilm-forming organisms in DWS (12–14). Although biofilms themselves do not contain pathogens, they provide a favorable surface for microbial attachment (15, 16), allowing microorganisms in biofilms to evade clearance mechanisms (17). When pathogen-rich biofilms detach and contaminate the water, they can pose a potential risk to animal and human health if consumed (18).
In daily production practices, various strategies are employed to improve water quality, including acidification and magnetization of drinking water, which have proven effective in inhibiting pathogen growth (19, 20). Treatment with these methods has shown improvements in animal growth performance and reduced pathogen spread (19–21). Chlorinated drinking water is commonly used in the livestock industry due to its ease of application, cost-effectiveness, and broad antimicrobial properties (7). Sodium dichloroisocyanurate (NaDCC), a white powder with a molecular weight of 219.9 g/mol (22), is considered safe and effective for disinfection of drinking water and domestic environments in meat animal treatment (23–25).
NaDCC is widely employed as a bactericide in various setting, such as swimming pool disinfection, drinking water treatment, and industrial deodorization (26, 27). However, there is limited information on the effects of NaDCC waterline disinfection in broiler farming. This experiment hypothesized that the addition of NaDCC to broiler drinking water pipes would enhance broiler health by improving water cleanliness through the reduction of biofilm microbial levels in the pipes. Thus, the primary objective of this study was to evaluate whether the addition of NaDCC to broiler drinking water could enhance broiler health by reducing microbial levels in the waterline. This research will provide a theoretical basis for the future application of NaDCC in broiler production.
Materials and methods
Ethics statement
The Animal Conservation and Utilization Committee of the Jinzhou Medical University approved the animal use agreement (Grant No. JZMULL2022011).
Animals and experimental design
The test waterline had a length of 60 meters, with two test cages at the front, end, and 30 meters in the middle, resulting in a total of six test cages per waterline. Four waterlines comprising a total of 24 test cages, were selected for the experiment. A total of 480 healthy 1-day-old AA+ broilers weighting 46.77 ± 0.50 g were randomly divided into four groups with six replicates of 20 birds per group. The broilers were housed in test cages along each waterline. The experiment lasted for 42 days. In the control group, tap water was provided through the waterline, while in the test groups, the water lines were supplemented with NaDCC at concentrations of 10, 30, and 50 mg/L. The test group consumed tap water containing NaDCC on specific days during the experiment. For the rest of the time, the treatment was the same as the control group. It should be noted that long-term use of chlorinated water can damage the waterline and may have some impact on the broiler organism.
Material sources
The NaDCC used in this experiment was provided free of charge by Jinzhou Zhongke Genetic Testing Service Co. It was in powder form and had an effective chlorine content of 60.35%.
Animals feeding management
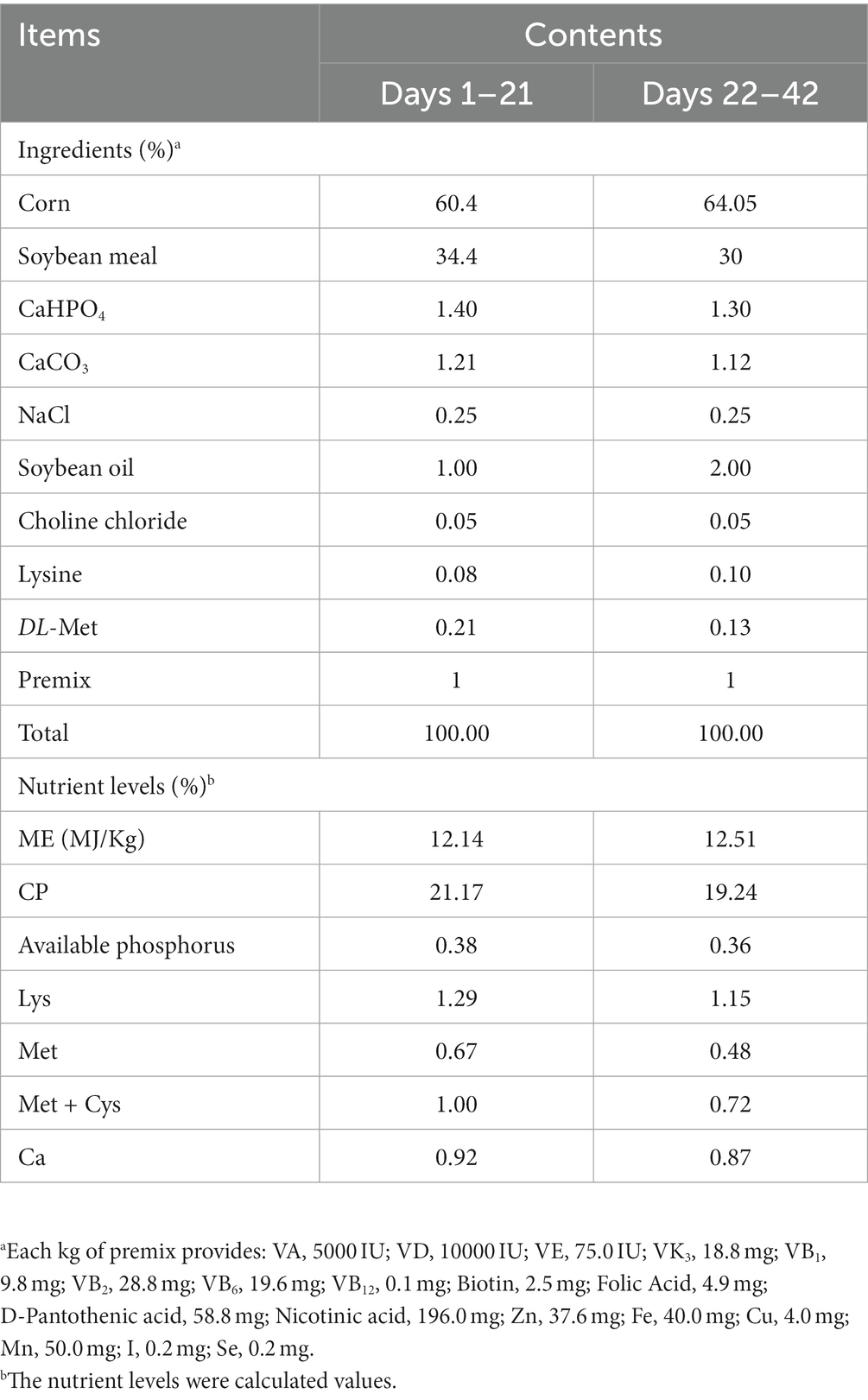
All birds were housed in experimental cages measuring 1.25 × 0.80 × 0.50 m3 per cage, located in a dedicated test broilers room. The test broilers room was fully enclosed and equipped with an automatic environmental control system to maintain optimal temperature and humidity conditions. The temperature was initially set at 33°C and gradually reduced by 3°C every week until reaching 22°C, while the relative humidity was maintained at 65%. The birds had unrestricted access to feed and water. The immunization program for the test broilers include vaccination against Newcastle disease and avian influenza at 7 days of age, followed by a Newcastle disease vaccination at 21 days of age administered through the water. The diets provided to the broiler were formulated to meet the nutritional requirements recommended by the NRC (1994) and were modified to suit the specific nutritional needs of Chinese broilers. The diets were provided in mash form and their composition is detailed in Table 1.
Measurement indicators
Growth performance
The broilers’ weights were measured at 1 day, 21 days, and 42 days of age. The feed intake was recorded for each replicate throughout the entire experiment, and from this data, average daily feed intake (ADG), average daily weight gain (ADFI), and meat to feed ratio (F/G) were calculated. Additionally, the water consumption of the broilers in each treatment group was carefully recorded, and the average daily water consumption (AWC) was calculated based on this data.
Blood indicators
On days 21 and 42 of the experiment, a total of 4 mL of blood was collected from the broiler’s lower wing vein. The blood samples were allowed to stand for 30 min and then centrifuged at 1200 r/min for 15 min to separate the supernatant. The potency of serum antibodies against Newcastle disease and avian influenza H9 was determined using a haemagglutination inhibition test. Furthermore, serum levels of albumin (ALB), total protein (TP), globulin (GLOB), alanine transaminase (ALT), alkaline phosphatase (ALP), and glucose (GLU) were measured using a fully automated biochemical analyzer.
Harmful gas emissions
On day 21 and 42 of the experiment, fresh broiler manure samples were collected from each replicate. The emission of ammonia and hydrogen sulphide from the manure were determined using the method described by Dang et al. (28). The collected manure samples were placed in a 2-liter plastic box with small holes attached to the side. The boxes were then left to ferment at room temperature (25°C) for specific durations of 12 h, 24 h, 48 h, and 72 h. Air samples were collected from above the small holes on either side of the box using a gas collection pump. The concentrations of NH3 and H2S were measured within the range of 0.00–100.00 mg/m3.
Waterline and fecal microbiota
On days 21 and 42 of the experiment, 10 mL of NaDCC-free drinking water was collected from the nipple drinker above each test cage. Additionally, 1 g broiler fecal samples was collected from each replicate and immediately transported to the laboratory on ice, following the method described by Dang et al. (28). The number of E. coli, Salmonella, S. aureus and Moulds in the water samples was determined using the plate count method. The number of microorganisms in the waterline was expressed as log10 colony forming units per mL of water. Similarly, the number of microorganisms in the fecal samples was expressed as log10 colony forming units per gram of feces.
Data analysis
The data was analyzed using a completely randomized grouping design. Multiple comparisons of significant differences in means were conducted using the one-way ANOVA LSD method in SPSS 25.0, and the results were visualized using Graphpad Prism 8. The results were presented as the mean and standard deviation, with a significance level of p < 0.05 indicating a significant difference. Correlation analysis was performed using R (V4.0.2), and the results were displayed using the R (V4.0.2) corrplot package. Pearson correlation analysis was carried out for the 21 and 42-day indicators, and correlations with a significance level of p < 0.05 and an absolute correlation coefficient greater than 0.6 or less than-0.6 were considered significant.
Results
Growth performance
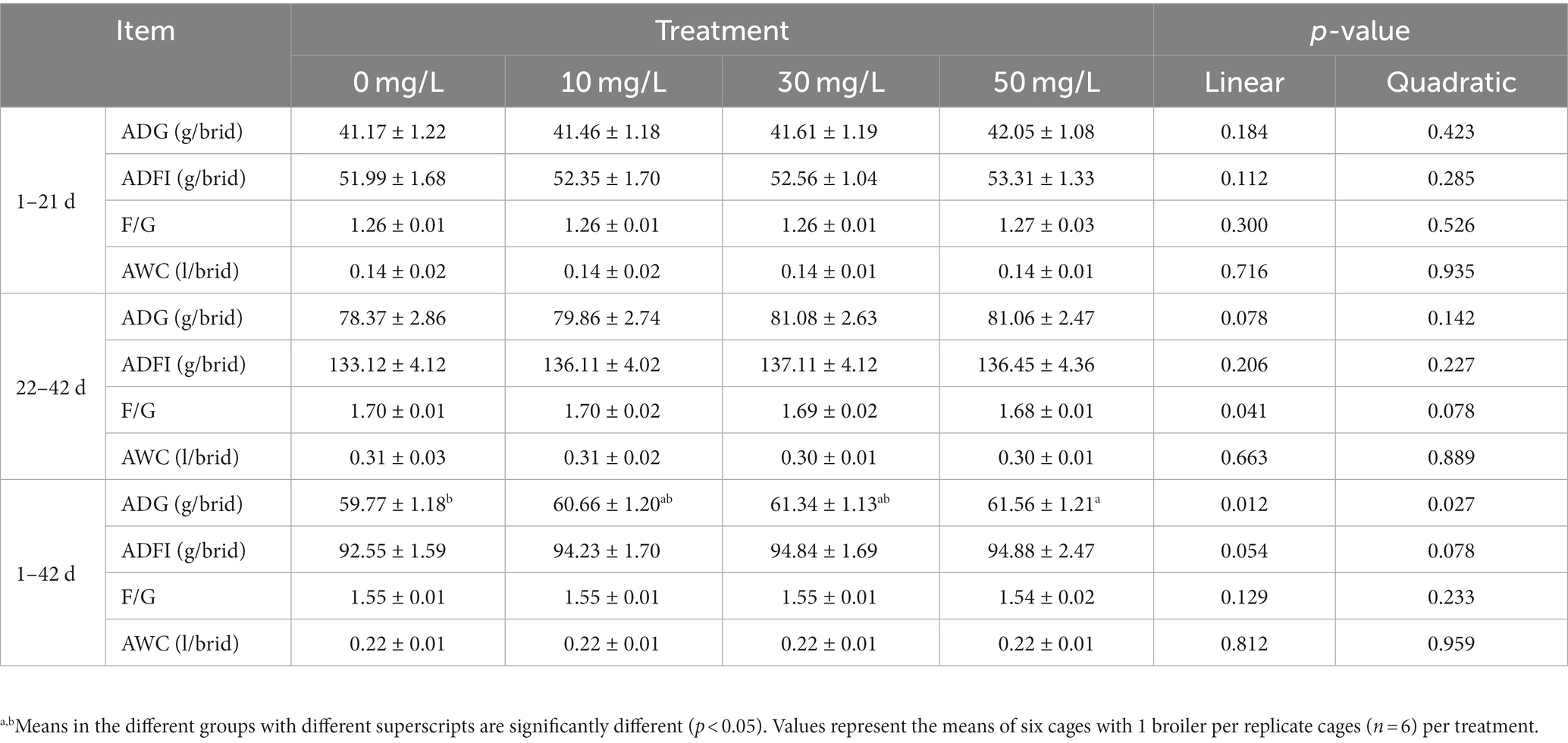
As shown in Table 2, the addition of 50 mg/L NaDCC to the waterline significantly increased ADG in broilers from 1 to 42 days (p < 0.05). The addition of NaDCC at 10 mg/L and 30 mg/L also increased ADG in broilers compared to the control, but not at a significant level (p > 0.05). The addition of NaDCC to the waterline had no significant effect on ADFI, F/G, and AWC in broilers (p > 0.05). NaDCC supplementation in drinking water had a negative linear effect on F/G in 22–42 day old broilers (p = 0.041) and a positive linear effect on ADG in 1–42 day old broilers (p = 0.012), with a quadratic curve effect (p = 0.027).
Blood indicators
As shown in Figures 1, 2, the addition of NaDCC to the waterline did not have a significant effect on antibody potency and various biochemical parameters in broiler serum (p > 0.05).
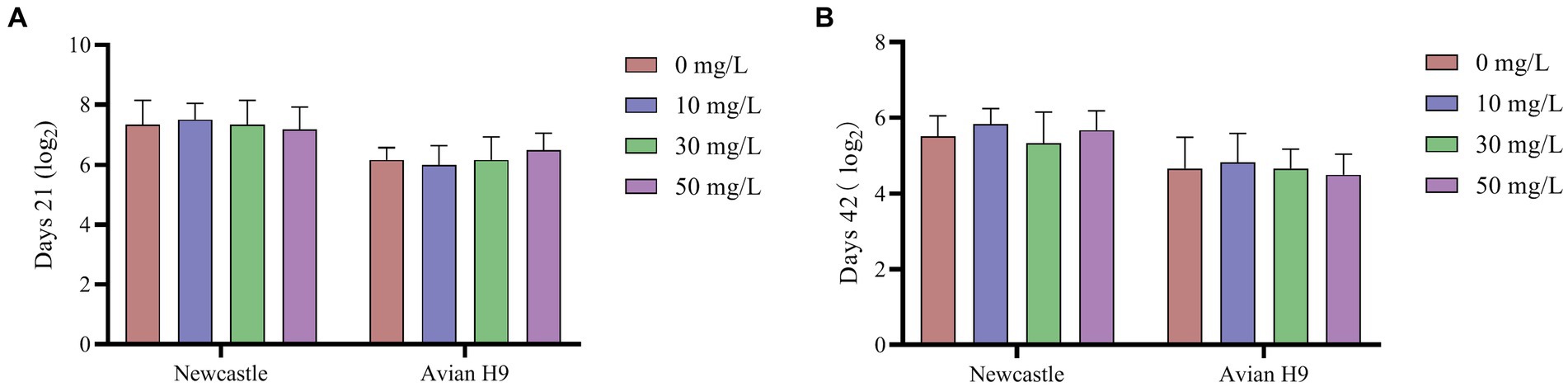
Figure 1. Effect of NaDCC on serum antibody potency of AA+ broiler. (A) Days 21 Serum antibody potency; (B) Days 42 Serum antibody potency. Values represent the means of six cages with 1 broiler per replicate cages (n = 6) per treatment.
Figure 2. Effect of NaDCC on serum biochemical parameters of AA+ broiler. (A) Days 21 Albumin and Globulin. (B) Days 21 Alanine transaminase. (C) Days 21 Alkaline phosphatase. (D) Days 21 Glucose. (E) Days 42 Albumin and Globulin. (F) Days 42 Alanine transaminase. (G) Days 42 Alkaline phosphatase. (H) Days 42 Glucose. Values represent the means of six cages with 1 broiler per replicate cages (n = 6) per treatment.
Harmful gas
As shown in Figure 3, NH3 emissions from broiler feces continue to rise over time, while H2S emissions increase initially and then decline. Additionally, both NH3 and H2S emissions were consistently lower in the treatment group compared to the control group.

Figure 3. Effect of NaDCC on harmful gas emissions of AA+ broiler. (A) Days 21 NH3. (B) Days 21 H2S. (C) Days 42 NH3. (D) Days 42 H2S. Values represent the means of six cages with 1 broiler per replicate cages (n = 6) per treatment.
Waterline and fecal microbiota
According to Figure 4, the addition of NaDCC to the waterline significantly reduced the levels of E. coli, Salmonella, S. aureus, and Moulds in the waterline (p < 0.05).
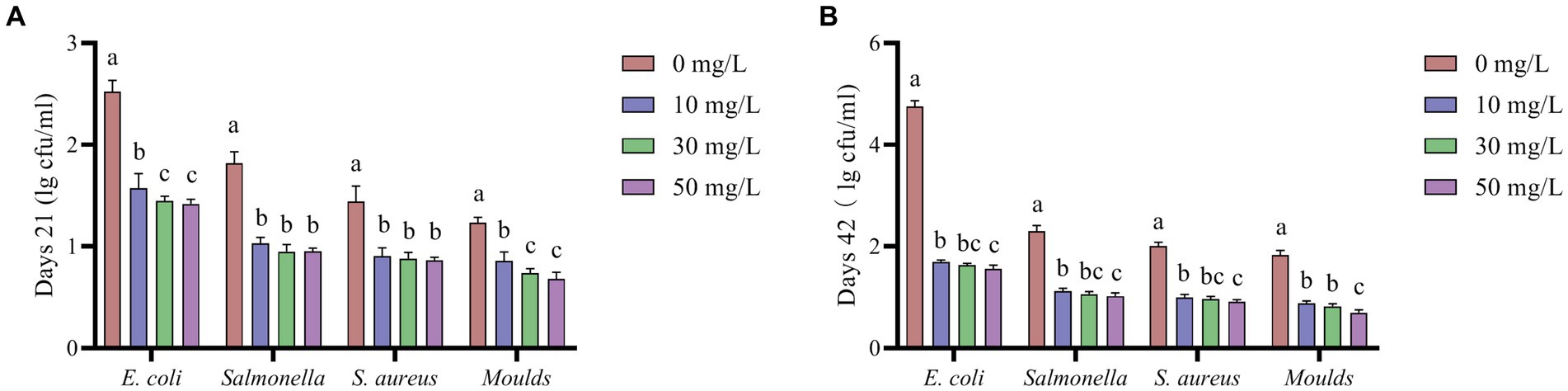
Figure 4. Effect of NaDCC on waterline microbiota of AA+ broiler. (A) Days 21 Microbial content. (B) Days 42 Microbial content. a,b,cMeans in the different groups with different superscripts are significantly different (p < 0.05). Values represent the means of six cages with 1 broiler per replicate cages (n = 6) per treatment.
As shown in Figure 5, the addition of NaDCC to the waterline resulted in significantly lower levels of E. coli, S. aureus and Moulds in broiler feces at 21 and 42 days (p < 0.05). The addition of NaDCC to the waterline reduced Salmonella levels in broiler feces at 21 days, but not to a significant level (p > 0.05), while it significantly reduced Salmonella levels in broiler feces at 42 days (p < 0.05).
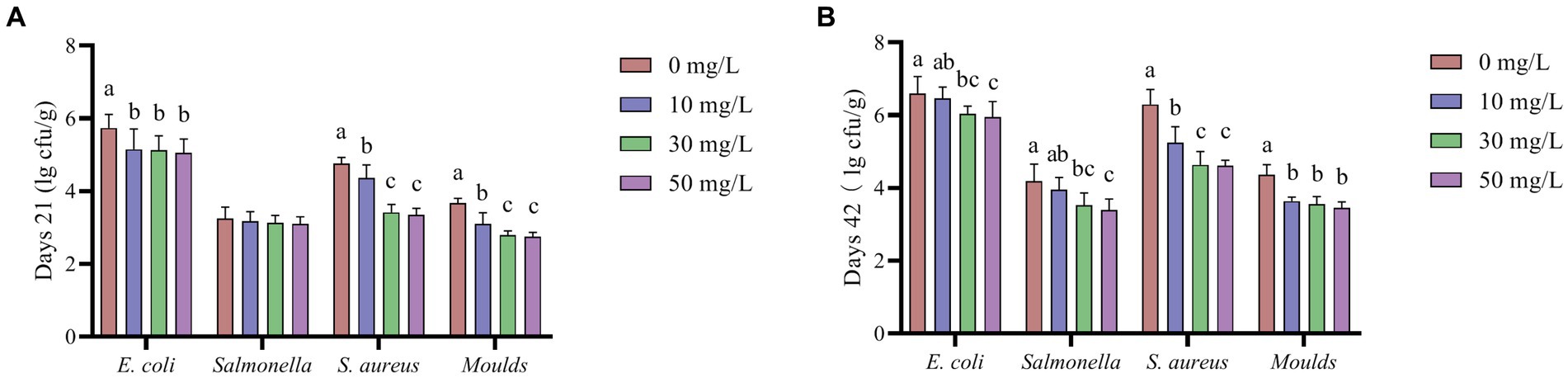
Figure 5. The effect of NaDCC on the microbial content of AA+ broiler feces. (A) Days 21 Microbial content. (B) Days 42 Microbial content. a,b,cMeans in the different groups with different superscripts are significantly different (p < 0.05). Values represent the means of six cages with 1 broiler per replicate cages (n = 6) per treatment.
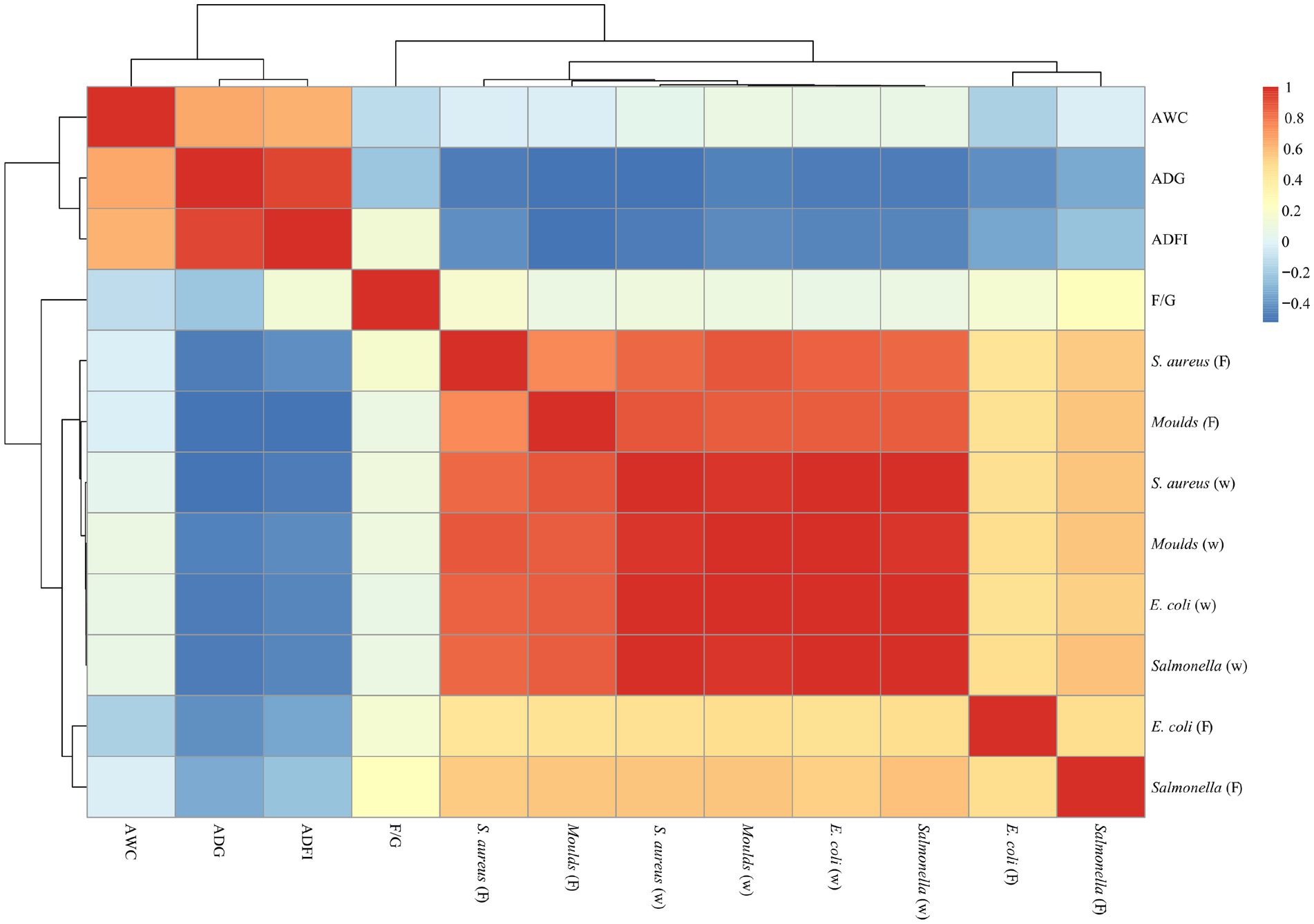
Correlation analysis of microorganisms and production performance
As shown in Figures 6, 7, a significant positive correlation (p < 0.05, R > 0.6) was observed between the microbial content (E. coli, Salmonella, S. aureus and Moulds) in the waterline and feces. However, the microbial content in the waterline and manure showed a negative correlation with production performance indicators (ADG, ADFI, F/G and AWC), although these correlations did not reach significant levels (p > 0.05).
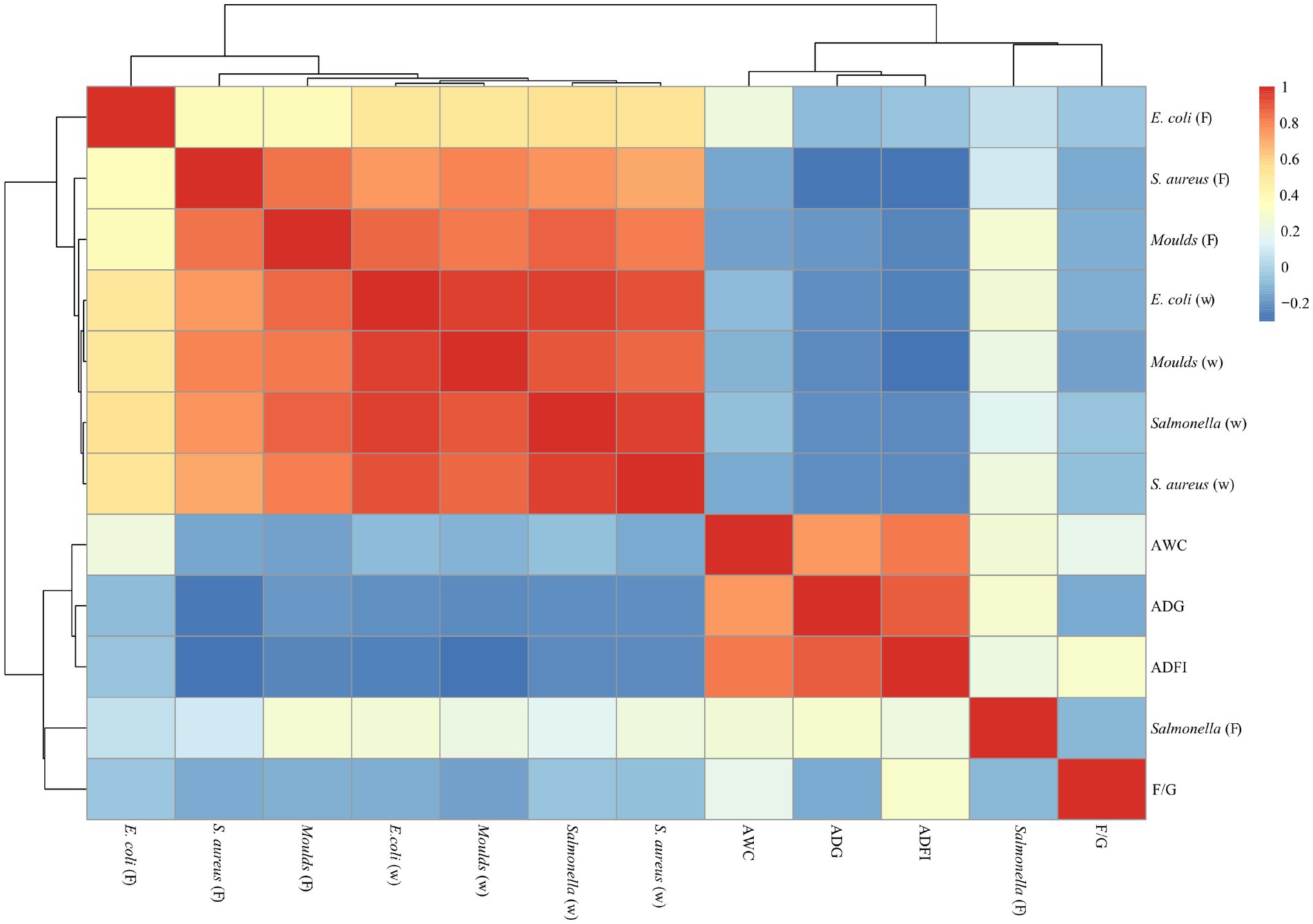
Figure 6. Correlation analysis of microorganisms and production performance on days 21. “W” means microbial content in the waterline; “F” means microbial content in the feces. The same as below.
Discussion
Effect of sodium dichloroisocyanurate on microorganisms in waterline and broiler feces
Drinking water and drinking water systems play an important role in the overall health and performance of broiler chickens (29). This pilot study found that the addition of NaDCC to broiler drinking water systems significantly reduced the levels of E. coli, Salmonella, S. aureus, and Moulds in both the waterline and broiler feces. Correlation analysis revealed a significant positive correlation between microbial levels in the waterline and feces. Generally, bacterial infections in broilers are caused by microorganisms commonly found in the poultry environment. In this context, colibacillosis caused by E. coli (30), paratyphoid infection caused by Salmonella (31), necrotizing enteritis caused by Clostridium perfringens (32), as well as S. aureus infection (33) and Pseudomonas aeruginosa infection (34) could be important contaminants leading to issues in 3–6 week old chickens.
NaDCC is a highly effective disinfectant containing two active chlorine atoms that disrupt cell membranes, nucleic acids, and proteins, thereby causing oxidative degradation of microorganisms (35). It has been reported to immediately kill E. coli, S. aureus, and fungi (36). Jang et al. (37) demonstrated that a 0.3% NaDCC solution effectively kills Salmonella even at −10°C after only 1 min of treatment, while a 0.1% glutaraldehyde solution requires 10 min of treatment at 25°C. Ferreira et al. (38) found that using a disinfectant containing 0.67 g/L sodium dichloroisocyanurate for 15 min effectively kills Pseudomonas aeruginosa, E. coli, Salmonella, and Clostridium perfringens in disinfection tests. Increasing the concentration to 0.93 g/L and treating for 20 min eliminated all Staphylococcus aureus. In this experiment, the addition of NaDCC to the drinking water of broilers demonstrated a reduction in the number of pathogenic bacteria entering the broiler by reducing the amount of these bacteria in the waterline, consequently leading to a decrease in pathogenic bacteria in the feces.
Effect of sodium dichloroisocyanurate on the health of broiler organisms
Drinking clean and hygienic water is essential for the welfare of broilers. This pilot study found that drinking water containing NaDCC did not affect the normal water intake of broilers. However, it significantly increased the ADG when consumed by broilers at a concentration of 50 mg/L. Nonetheless, at this stage, there is insufficient information to confirm that NaDCC can directly increase the body weight of broilers. Based on the results of this experiment, it is hypothesized that the potential cause of weight gain in broilers is the addition of NaDCC to their drinking water, which eliminates pathogenic microorganisms in the drinking water and waterline biofilm. When chickens consume healthy and clean water, it reduces their intake of pathogenic bacteria, leading to an increase in beneficial bacteria in their gut. By improving the micro-ecological environment of the gut, broilers tend to have increased feed intake and subsequently gain more body weight. However, the exact mechanism underlying this hypothesis requires further in-depth study.
It has been demonstrated that birds can tolerate free chlorine residues of 50 ppm in drinking water without any adverse effects (39, 40). Similar results were obtained in this experiment, as the broiler serum antibodies and biochemical parameters were not affected by the drinking water containing NaDCC. More-Bayona et al. (41) reported an increase in macrophages and a decrease in CD8+ lymphocytes in the peritoneal cavity of broilers that consumed well water containing a mixture of biological preparations for an extended period. They also observed varying degrees of inflammatory responses in the organism caused by fungi and bacteria present in the water. Therefore, occasional consumption of chlorinated drinking water is necessary for the health of broilers.
Under anaerobic conditions, microbial decomposition of feces produces certain gaseous compounds, mainly from carbohydrates and proteins (42). In this experiment, drinking water containing NaDCC significantly reduced NH3 and H2S emissions from feces. Previous reports have shown that when the number of E. coli and Salmonella in feces is reduced, fecal NH3 and H2S emissions are also reduced (43). Prolonged exposure of broilers to NH3 has been shown to affect the structure of their cecal flora and, consequently, their growth performance (44). Additionally, sudden releases of H2S from manure stored in chicken houses can increase the concentration of toxic substances in the room, leading to the death of many animals and humans (45). While birds are not as sensitive to the toxic effects of hydrogen sulfide as mammals, high concentrations of this gas in the air can still negatively impact production parameters in laying hens and broilers (46). Therefore, improving the farm environment is equally important for the healthy growth of broilers. Based on the results of this experiment, NaDCC is highly effective in waterline treatment. Further research can focus on optimizing the dosage to reduce resource consumption while maintaining efficacy.
Conclusion
The addition of sodium dichloroisocyanurate to broiler drinking water systems does not impact broiler feed intake, immune function, or the normal physiological functions of the organism. By reducing the levels of pathogenic microorganisms in the waterline, it is possible to also reduce their presence in broiler feces, ultimately improving the overall growth of the broilers. Taking all factors into consideration, it is recommended to use a concentration of 30 mg/L of sodium dichloroisocyanurate in poultry production to enhance the health of broilers.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was reviewed and approved by Animal Ethics Committee of Jinzhou Medical University.
Author contributions
QZ: data curation and writing-original draft. WM: investigation. CW: writing-review and editing. TW: resources. XL: project administration, supervision, and validation. DL: resources, supervision, validation, writing, and writing-review and editing. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by the 2022 Proposed Student Innovation and Entrepreneurship Project (Grant no. S202210160027).
Acknowledgments
The authors thank the College of Animal Husbandry and Veterinary Medicine of Jinzhou Medical University for its support of the experiment. The authors thank Liaoning Kaiwei Biological Co Ltd. for providing the breeding base for this experiment. The authors thank Jinzhou Zhongke Genetic Testing Service Co., Ltd. for providing the test materials and assisting with the microbiological testing.
Conflict of interest
TW was employed by Liaoning Kaiwei Biotechnology Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Feshanghchi, M, Baghban-Kanani, P, Kashefi-Motlagh, B, Adib, F, Azimi-Youvalari, S, Hosseintabar-Ghasemabad, B, et al. Milk thistle (Silybum marianum), marine algae (Spirulina platensis) and toxin binder powders in the diets of broiler chickens exposed to aflatoxin-B1: growth performance, humoral immune response and Cecal microbiota. Agriculture. (2022) 12:805. doi: 10.3390/agriculture12060805
2. Choi, J, Kong, B, Bowker, BC, Zhuang, H, and Kim, WK. Nutritional strategies to improve meat quality and composition in the challenging conditions of broiler production: a review. Animals. (2023) 13:1386. doi: 10.3390/ani13081386
3. Liu, G, Zhang, Y, Knibbe, WJ, Feng, C, Liu, W, Medema, G, et al. Potential impacts of changing supply-water quality on drinking water distribution: a review. Water Res. (2017) 116:135–48. doi: 10.1016/j.watres.2017.03.031
4. Luis, LJ, Rafael, LE, and De La, P. A systematic review of microorganisms as indicators of recreational water quality in natural and drinking water systems. J Water Health. (2021) 19:20–8. doi: 10.2166/WH.2020.179
5. Novak Babič, M, Gostinčar, C, and Gunde-Cimerman, N. Microorganisms populating the water-related indoor biome. Appl Microbiol Biotechnol. (2020) 104:6443–62. doi: 10.1007/s00253-020-10719-4
6. Herman, L, Heyndrickx, M, Grijspeerdt, K, Vandekerchove, D, Rollier, I, and De Zutter, L. Routes for campylobacter contamination of poultry meat: epidemiological study from hatchery to slaughterhouse. Epidemiol Infect. (2003) 131:1169–80. doi: 10.1017/s0950268803001183
7. Maes, S, Vackier, T, Nguyen Huu, S, Heyndrickx, M, Steenackers, H, Sampers, I, et al. Occurrence and characterisation of biofilms in drinking water systems of broiler houses. BMC Microbiol. (2019) 19:77. doi: 10.1186/s12866-019-1451-5
8. Chan, S, Pullerits, K, Keucken, A, Persson, KM, Paul, CJ, and Rådström, P. Bacterial release from pipe biofilm in a full-scale drinking water distribution system. NPJ Biofilms Microbiomes. (2019) 5:9. doi: 10.1038/s41522-019-0082-9
9. Liu, S, Gunawan, C, Barraud, N, Rice, SA, Harry, EJ, and Amal, R. Understanding, monitoring, and controlling biofilm growth in drinking water distribution systems. Environ Sci Technol. (2016) 50:8954–76. doi: 10.1021/acs.est.6b00835
10. Sparks, N. The role of the water supply system in the infection and control of campylobacter in chicken. Worlds Poult Sci J. (2009) 65:459–74. doi: 10.1017/S0043933909000324
11. Zimmer, M, Barnhart, H, Idris, U, and Lee, MD. Detection of Campylobacter jejuni strains in the water lines of a commercial broiler house and their relationship to the strains that colonized the chickens. Avian Dis. (2003) 47:101–7. doi: 10.1637/0005-2086(2003)047[0101:DOCJSI]2.0.CO;2
12. Liu, G, Bakker, GL, Li, S, Vreeburg, JH, Verberk, JQ, Medema, GJ, et al. Pyrosequencing reveals bacterial communities in unchlorinated drinking water distribution system: an integral study of bulk water, suspended solids, loose deposits, and pipe wall biofilm. Environ Sci Technol. (2014) 48:5467–76. doi: 10.1021/es5009467
13. Reeser, RJ, Medler, RT, Billington, SJ, Jost, BH, and Joens, LA. Characterization of Campylobacter jejuni biofilms under defined growth conditions. Appl Environ Microbiol. (2007) 73:1908–13. doi: 10.1128/AEM.00740-06
14. Van Eenige, MJ, Counotte, GH, and Noordhuizen, JP. Drinking water for dairy cattle: always a benefit or a microbiological risk? Tijdschr Diergeneeskd. (2013) 138:86–99.
15. Trachoo, N, Frank, JF, and Stern, NJ. Survival of Campylobacter jejuni in biofilms isolated from chicken houses. J Food Prot. (2002) 65:1110–6. doi: 10.4315/0362-028x-65.7.1110
16. Zhang, J, Li, W, Chen, J, Qi, W, Wang, F, and Zhou, Y. Impact of biofilm formation and detachment on the transmission of bacterial antibiotic resistance in drinking water distribution systems. Chemosphere. (2018) 203:368–80. doi: 10.1016/j.chemosphere.2018.03.143
17. Yuan, L, Sadiq, FA, Wang, N, Yang, Z, and He, G. Recent advances in understanding the control of disinfectant-resistant biofilms by hurdle technology in the food industry. Crit Rev Food Sci Nutr. (2021) 61:3876–91. doi: 10.1080/10408398.2020.1809345
18. Hemdan, BA, El-Taweel, GE, Goswami, P, Pant, D, and Sevda, S. The role of biofilm in the development and dissemination of ubiquitous pathogens in drinking water distribution systems: an overview of surveillance, outbreaks, and prevention. World J Microbiol Biotechnol. (2021) 37:36. doi: 10.1007/s11274-021-03008-3
19. El-Katcha, M, Soltan, MA, El-Shobokshy, SA, and Kasser, M. Impact of water acidification or magnetic treatment on growth performance, health and oxidative status of broiler chicks challenged by Salmonella Enteritidis. Alex J Vet Sc. (2018) 59:154–68. doi: 10.5455/AJVS.293280
20. Guo, YJ, Wang, ZY, Wang, YS, Chen, B, Huang, YQ, Li, P, et al. Impact of drinking water supplemented 2-hydroxy-4-methylthiobutyric acid in combination with acidifier on performance, intestinal development, and microflora in broilers. Poult Sci. (2022) 101:101661. doi: 10.1016/j.psj.2021.101661
21. Zhang, H, Guo, Y, Wang, Z, Wang, Y, Chen, B, Du, P, et al. Acidification of drinking water improved tibia mass of broilers through the alterations of intestinal barrier and microbiota. Anim Biosci. (2022) 35:902–15. doi: 10.5713/ab.21.0455
22. Sigma-Aldrich. (2021). Safety DATA sheet for sodium dichloroisocyanurate. Available at: https://www.sigmaaldrich.com/KR/en/sds/aldrich/218928/ (accessed 10 May, 2021).
23. Keïta, A, Huneau-Salaün, A, Guillot, A, Galliot, P, Tavares, M, and Puterflam, J. A multi-pronged approach to the search for an alternative to formaldehyde as an egg disinfectant without affecting worker health, hatching, or broiler production parameters. Poult Sci. (2016) 95:1609–16. doi: 10.3382/ps/pew058
24. Mohammed, AN. Field study on evaluation of the efficacy and usability of two disinfectants for drinking water treatment at small cattle breeders and dairy cattle farms. Environ Monit Assess. (2016) 188:151. doi: 10.1007/s10661-016-5147-0
25. Wahman, DG. Chlorinated cyanurates: review of water chemistry and associated drinking water implications. J Am Water Works Ass. (2018) 110:E1–E15. doi: 10.1002/awwa.1086
26. Choi, HY, Lee, YH, Lim, CH, Kim, YS, Lee, IS, Jo, JM, et al. Assessment of respiratory and systemic toxicity of benzalkonium chloride following a 14-day inhalation study in rats. Part Fibre Toxicol. (2020) 17:5. doi: 10.1186/s12989-020-0339-8
27. Khazaei, A, Sarmasti, N, Seyf, JY, and Merati, Z. Anchoring N-halo (sodium dichloroisocyanurate) on the nano-Fe3O4 surface as “chlorine reservoir”: antibacterial properties and wastewater treatment. Arab J Chem. (2020) 13:2219–32. doi: 10.1016/j.arabjc.2018.04.007
28. Dang, DX, Yong, MK, and Kim, IH. Effects of a root extract from Achyranthes Japonica Nakai on the growth performance, blood profile, fecal microbial community, fecal gas emission, and meat quality of finishing pigs. Livest Sci. (2020) 239:1–5. doi: 10.1016/j.livsci.2020.104160
29. Maharjan, P, Clark, T, Kuenzel, C, Foy, MK, and Watkins, S. On farm monitoring of the impact of water system sanitation on microbial levels in broiler house water supplies. J Appl Poult Sci. (2016) 25:266–71. doi: 10.3382/japr/pfw010
30. Monroy, MA, Knöbl, T, Bottino, JA, Ferreira, CS, and Ferreira, AJ. Virulence characteristics of Escherichia coli isolates obtained from broiler breeders with salpingitis. Comp Immunol Microbiol Infect Dis. (2005) 28:1–15. doi: 10.1016/j.cimid.2004.03.001
31. Barrow, PA. The paratyphoid salmonellae. Revue scientifique et technique (International Office of Epizootics). (2000) 19:351–75. doi: 10.20506/rst.19.2.1225
32. Timbermont, L, Lanckriet, A, Gholamiandehkordi, AR, Pasmans, F, Martel, A, Haesebrouck, F, et al. Origin of Clostridium perfringens isolates determines the ability to induce necrotic enteritis in broilers. Comp Immunol Microbiol Infect Dis. (2009) 32:503–12. doi: 10.1016/j.cimid.2008.07.001
33. Huff, GR, Huff, WE, Rath, NC, and Balog, JM. Turkey osteomyelitis complex. Poult Sci. (2000) 79:1050–6. doi: 10.1093/ps/79.7.1050
34. Walker, SE, Sander, JE, Cline, JL, and Helton, JS. Characterization of Pseudomonas aeruginosa isolates associated with mortality in broiler chicks. Avian Dis. (2002) 46:1045–50. doi: 10.1637/0005-2086(2002)046[1045:COPAIA]2.0.CO;2
35. Lechevallier, MW, Evans, TM, and Seidler, RJ. Effect of turbidity on chlorination efficiency and bacterial persistence in drinking water. Appl Environ Microbiol. (1981) 42:159–67. doi: 10.1128/aem.42.1.159-167.1981
36. Proto, A, Zarrella, I, Cucciniello, R, Pironti, C, De Caro, F, and Motta, O. Bactericidal and fungicidal activity in the gas phase of sodium Dichloroisocyanurate (NaDCC). Curr Microbiol. (2016) 73:287–91. doi: 10.1007/s00284-016-1040-x
37. Jang, Y, Lee, K, Yun, S, Lee, M, Song, J, Chang, B, et al. Efficacy evaluation of commercial disinfectants by using Salmonella enterica serovar typhimurium as a test organism. J Vet Sci. (2017) 18:209–16. doi: 10.4142/jvs.2017.18.2.209
38. Ferreira, A, Ferreira, CA, Chacon, JL, Revolledo, L, Gross, R, and Cotrim, E. In vitro virucidal and bactericidal activities of aviclor (a formulation of sodium dichloroisocyanurate) against pathogens of poultry origin. J Appl Poult Res. (2010) 19:93–100. doi: 10.3382/japr.2009-00030
39. Hamdullah Khan, MZ, Khan, A, and Javed, I. Toxico-pathological effects of sodium hypochlorite administration through drinking water in female Japanese quail (Coturnix japonica). Hum Exp Toxicol. (2010) 29:779–88. doi: 10.1177/0960327110361755
40. Hulan, HW, and Proudfoot, FG. Effect of sodium hypochlorite (Javex) on the performance of broiler chickens. Am J Vet Res. (1982) 43:1804–6.
41. More-Bayona, JA, Torrealba, D, Thomson, C, Wakaruk, J, and Barreda, DR. Differential effects of drinking water quality on phagocyte responses of broiler chickens against fungal and bacterial challenges. Front Immunol. (2020) 11:584. doi: 10.3389/fimmu.2020.00584
42. Gutarowska, B, Matusiak, K, Borowski, S, Rajkowska, A, and Brycki, B. Removal of odorous compounds from poultry manure by microorganisms on perlite--bentonite carrier. J Environ Manag. (2014) 141:70–6. doi: 10.1016/j.jenvman.2014.03.017
43. Zou, Q, Fan, X, Xu, Y, Wang, T, and Li, D. Effects of dietary supplementation probiotic complex on growth performance, blood parameters, fecal harmful gas, and fecal microbiota in AA+ male broilers. Front Microbiol. (2022) 13:1088179. doi: 10.3389/fmicb.2022.1088179
44. Han, H, Zhou, Y, Liu, Q, Wang, G, Feng, J, and Zhang, M. Effects of ammonia on gut microbiota and growth performance of broiler chickens. Animals. (2021) 11:1716. doi: 10.3390/ani11061716
45. Oesterhelweg, L, and Püschel, K. “death may come on like a stroke of lightening”: phenomenological and morphological aspects of fatalities caused by manure gas. Int J Legal Med. (2008) 122:101–7. doi: 10.1007/s00414-007-0172-8
Keywords: sodium dichloroisocyanurate, broiler, microorganism, waterline, growth performance
Citation: Zou Q, Meng W, Wang C, Wang T, Liu X and Li D (2023) Sodium dichloroisocyanurate: improving broiler health by reducing harmful microbial levels in the waterline. Front. Vet. Sci. 10:1234949. doi: 10.3389/fvets.2023.1234949
Edited by:
Yangchun Cao, Northwest A&F University, ChinaReviewed by:
Ahsan Mustafa, Sichuan Agricultural University, ChinaJian Ma, Guangdong Ocean University, China
Copyright © 2023 Zou, Meng, Wang, Wang, Liu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Desheng Li, bGlkZXNoZW5nMDcyNjUyMUAxMjYuY29t
 Qiangqiang Zou
Qiangqiang Zou Weishuang Meng1
Weishuang Meng1 Desheng Li
Desheng Li