- 1Department of Marine Chemistry and Biochemistry, Institute of Oceanology of the Polish Academy of Sciences, Sopot, Poland
- 2Department of Physical Oceanography, Institute of Oceanology of the Polish Academy of Sciences, Sopot, Poland
To assess the bioavailability in the soil-derived dissolved organic carbon (DOC), and to estimate potential remineralization kinetics for different bioavailable fractions of DOC, the long-lasting (180 days) incubation experiments of soil leachates were performed. The soil material was collected from the catchments of two contrasting rivers - Bayelva and Londonelva in Kongsfjorden (Arctic fjord in West Spitsbergen, Svalbard Archipelago). Both sampling sites were located close to the shore, where coastal erosion and tides directly affect the surface soil layer. The results indicate that the soil leachates contain a lot of DOC, which is highly bioavailable, even 68%–87% can be susceptible to biodegradation. The obtained decay curves allowed us to distinguish three DOC fractions: labile, semi-labile, and refractory. The contribution of the most labile DOC fraction is small and ranges from 13% in the Bayelva region to 25% in the Londonelva catchment but it remineralizes quickly once transported to the fjord, while the semi-labile DOC, whose half-life is measured in months, is much more abundant (74% and 43% of total DOC, respectively). These differences in the contribution of particular DOC fractions between stations can result from the different composition and provenance of organic matter. Nevertheless, this high lability of terrestrial DOC indicates that its supply to the fjords water column has the potential to play an essential role in sustaining the bacterial loop in the fjord and, through CO2 release, in amplifying ocean acidification in the coastal zone.
1 Introduction
Ongoing climate change significantly impacts the marine and terrestrial polar ecosystems, especially in the Arctic, where due to the Arctic Amplification, consequences of climate warming are much more visible (Rantanen et al., 2022). The increased melting and retreat of glaciers, as well as the thawing of permafrost, may increase the transport of dissolved organic matter (DOM) from land to fjords (Retelletti Brogi et al., 2018; Retelletti Brogi et al., 2019). It has been estimated that worldwide the permafrost surface layer contains as much as 1,035 ± 150 Pg of organic carbon (OC; Hugelius et al., 2013), so even a small release can significantly change the carbon loads to the fjords. Although there are quantitative estimations of the dissolved organic carbon (DOC) delivered from land (Nguyen et al., 2022; Retelletti Brogi et al., 2019), its fate in fjords remains highly unknown. It is still unclear to what extent this DOC pool is bioavailable and how fast can it be remineralized. This missing knowledge is extremely desired as it would allow for a better understanding of the processes shaping the carbon cycling in the Arctic fjords (and likely in other polar regions), but also nutrient availability as the DOM is an important carrier of nitrogen and phosphorus in the marine environment (Repeta, 2015). Current studies suggest that a relatively high proportion of DOM released from permafrost is bioavailable and can be relatively quickly mineralized by microbes or photo-oxidized during transport from land via rivers and surface runoff to the coastal Arctic Ocean. Vonk et al. (2015) estimated, based on literature synthesis and their own experiments, that the mean contribution of biodegradable DOC from soil leachates ranges between ∼9% in non-permafrost sites, ∼16% in discontinuous permafrost sites, and ∼20% in continuous permafrost sites. However, significantly higher shares of biodegradable OC have also been found. For instance, Elberling et al. (2013) based on long-term incubation, calculated that even up to 75% of total OC in Greenland permafrost soil can be bioavailable, and Hood et al. (2009) found 23%–66% of bioavailable DOC in heavily glaciated watersheds of the Gulf of Alaska. This shows, however, that despite the growing amount of data available, there is still a lot of variability in the assessment of biodegradable DOC contribution in permafrost regions. Furthermore, knowledge about the bioavailability of terrestrial organic matter and its reminaralization kinetics is essential to understanding the marine CO2 system variability, as DOC decay leads to seawater pCO2 increase and thus intensifies the seawater acidification (Polimene et al., 2022). The complexity of the marine CO2 system in the coastal Arctic waters may be exemplified by the study of Koziorowska-Makuch et al. (2023) who identified very high variability of pCO2 and pH (but also other CO2 system parameters) in the Spitsbergen fjords and especially close to the sources of land-delivered organic matter. Therefore, the following research objectives have been formulated: (1) to quantify the shares of labile (DOCL), semi-labile (DOCSL), and refractory (DOCR) fractions in the soil-derived DOC, and (2) to estimate the potential remineralization rate constants (k) and half-life times (t1/2) for different bioavailable fractions of DOC. This was done through long-lasting incubation experiments of the soil leachates containing terrestrial DOC. The study has been performed using soil material collected from two contrasting sites close to the shore in Kongsfjorden–the high Arctic fjord in West Spitsbergen, Svalbard Archipelago. The chosen study site is one of the fastest-warming polar regions, largely due to a process known as Atlanticification (Polyakov et al., 2017). This, combined with the projected 200% increase in freshwater discharge by 2,100 under the RCP4.5 scenario (Adakudlu et al., 2019), makes the region particularly important for the global carbon cycle. The increasing heat content in Kongsfjorden is affecting the stability of the ecosystem’s structure (Vihtakari et al., 2018), altering the hydrological cycle, and intensifying permafrost thaw. Measurements from 1998 to 2017 show a temperature increase of 0.18°C ± 0.07°C year-1 in the permafrost active layer at the Ny-Ålesund station (Boike et al., 2018). Furthermore, records from a longer period (1971–2017) indicate a significant increase in precipitation (Hanssen-Bauer et al., 2019), which may substantially enhance the supply of organic matter leached from land.
2 Methods
2.1 Study area
The catchments of two rivers in Kongsfjorden - Bayelva River and Londonelva River were selected for the study site, as they can be considered different in terms of the geology and the source of water supply (Figure 1). The Bayelva River catchment, which is almost entirely underlain by permafrost with a seasonal active layer (Killingtveit et al., 2004), may serve as a natural laboratory for understanding how other Arctic permafrost regions may respond to ongoing climate warming. In contrast, comparison with a station characterized by a deglacierized catchment area (Londonelva River) enables the assessment of differences in the quantity and reactivity of organic matter released to the coastal environments, depending on catchment type. Bayelva River is 4 km long, with a glacierised catchment area of 32 km2. The watershed is underlain by quartzite, and phyllite in the southern and eastern parts, and sedimentary rocks in the northern and western parts (Orvin, 1934; Hjelle, 1993; Nowak et al., 2021). Bayelva is fed by glacier melt, snow melt, rainfall, and ground ice melt. The average decadal discharge from the Bayelva River ranges from 25,696 to 33,683 × 103 m3 (measurements started in 1989; Nowak et al., 2021). Londonelva River is located on Blomstradøya (the largest island in Kongsfjorden) and is characterized by a small catchment area (0.7 km2), almost entirely underlain by carbonate rocks. Londonelva is fed by snow melt, rainfall, and ground ice melt, with the average decadal freshwater flux that increased from 271 × 103 m3 in 1991–2000 to 645 × 103 m3 in 2011–2019 (Nowak et al., 2021). Both rivers are restricted to the meltwater season only (usually May-October) (Nowak et al., 2021). Environmental conditions in both catchments are highly variable and subject to strong seasonal fluctuations. The 10-year mean monthly air temperature ranged from −10.6°C in March to 5.6°C in July, while monthly precipitation ranged from 6.4 mm in June to 79.9 mm in September (Supplementary Figure S1; climate data from the Ny-Ålesund station; Data source: Observations and weather statistics, Norwegian Centre for Climate Services; seklima.met.no; last access: 20.05.2025).
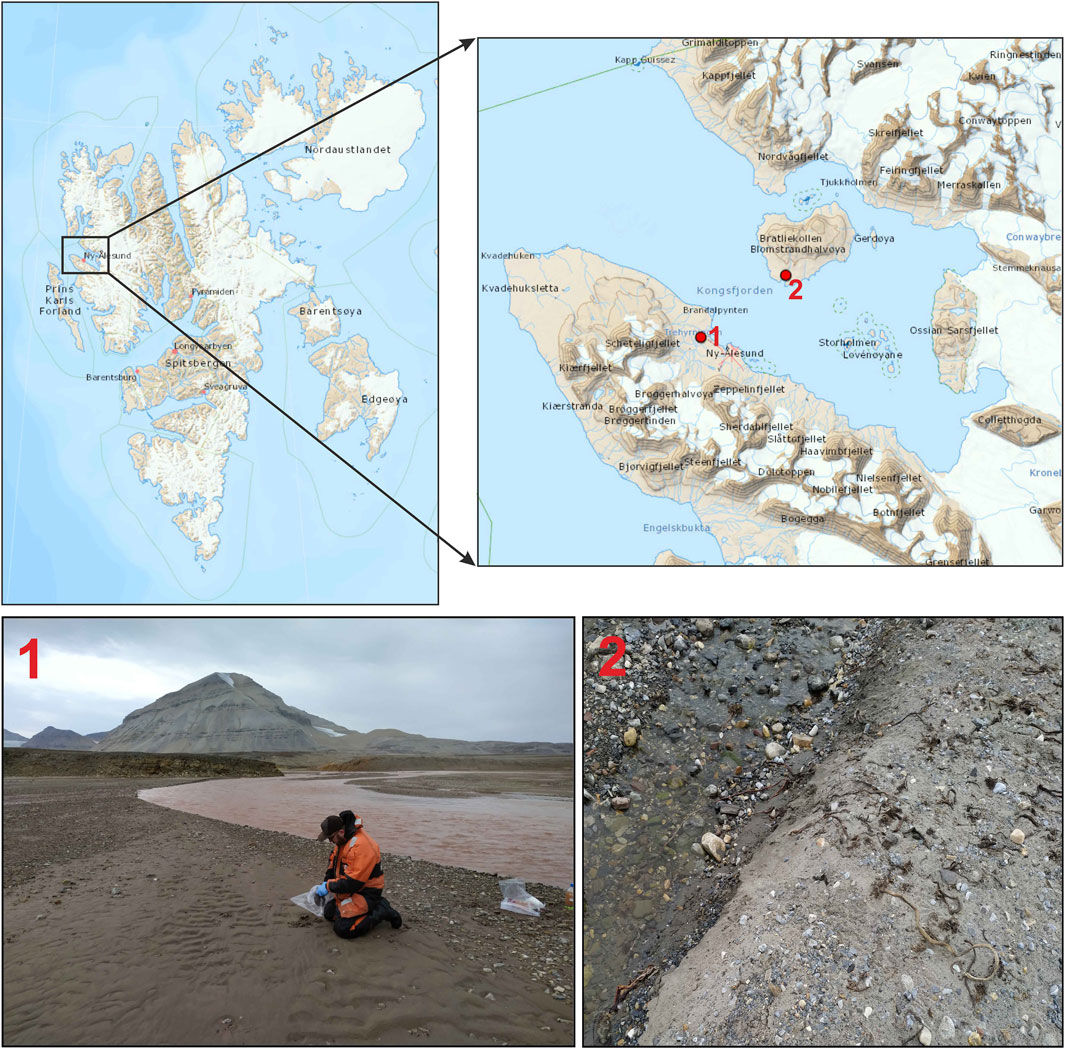
Figure 1. Map of the study area with photos from sampling stations: 1 - Bayelva River, 2 - Londonelva River. Map modified from https://toposvalbard.npolar.no.
2.2 Incubation experiment
The incubation experiment was designed based on the ‘Standardized DOC incubation protocol’ proposed by Vonk et al. (2015). Sampling stations were located close to the shore in the catchments of Bayelva (78 56.05′N and 11 51.28′E) and Londonelva Rivers (78 57.77′N and 12 02.88′E; hereinafter referred to as Bayelva station and Londonelva station). Soil samples were collected from the surface layer (0–10 cm) and frozen at −20°C in ziplock bags until further processing. In the laboratory, frozen samples were slowly thawed overnight at 4°C and gently homogenized. Then, subsamples (in triplicate) were collected to determine the density, moisture of the soil (sediment samples were weighed before and after drying at 60°C for 24 h), total nitrogen (TN), organic carbon concentrations (OC), the stable isotopic composition of TN and OC (δ15TN and δ13OC). Both sampling sites were located next to the fjord, where coastal erosion and tides directly affect the surface layer of soil, and where soil is constantly washed away by seawater, thus flushing out the terrestrial DOC it contains. Therefore, soil leachates were prepared by adding surface seawater from the adjacent fjord (78 59.47′N and 11 34.39′E) to the soil in a volume ratio of 1:4, letting this stand in glass flasks for 24 h at 4°C in the dark, and extracting liquids using a centrifuge. Obtained soil leachates were filtered through glass fibre filters (Whatman GF/F, pore size 0.7 µm), transported to 40 mL glass incubation vials, and stored in the dark. In parallel to the soil leachate from two stations, the filtered seawater was also incubated and used as a control. All laboratory glassware and filters used during the experiment were previously pre-combusted (450°C, 6 h) to reduce the possibility of sample contamination. To ensure aerobic conditions throughout the experiment, the vials were left open to the atmosphere and only covered with sterile gauze to prevent any contamination from the air. The dissolved oxygen concentration was monitored using a WTW multi 3400i Multi-Parameter Field Metre. Incubation was conducted for 180 days at 22°C ± 0.5°C. This high temperature was selected following the recommendations by Vonk et al. (2015) to accelerate the DOC remineralization and obtain the DOC loss high enough to quantify the contributions of different DOC fractions (DOCL, DOCSL, and DOCR) in the time frame of the experiment. During the experiment, the samples were carefully shaken daily to ensure homogeneous conditions. At the beginning (t = 0) and after 1, 2, 3, 5, 9, 19, 29, 65, 90, and 180 days of the incubation (three replicates for time steps t = 0, t = 19, and t = 180), the individual samples were filtered using pre-combusted filters (Whatman GF/F), acidified with 50 µL concentrated HCl and stored in a dark until analysis for DOC.
2.3 Chemical analyses
DOC samples were analyzed on the automated total organic carbon analyzer TOC-L (Shimadzu Corp., Japan) equipped with an NDIR CO2 detector. Samples were measured using a high-temperature (680°C) oxidation method and a Pt catalyst. The quality of the measurements was examined by the regular analyses of certified reference material - North Atlantic water obtained from the Hansell Laboratory (recovery: 95%, precision: 4 μmol/L; n = 5).
The analyses of OC, TN, δ13OC, and δ15TN were performed in an Elemental Analyzer Flash EA 1112 Series combined with an Isotopic Ratio Mass Spectrometer (IRMS Delta V Advantage; Thermo Electron Corp., Germany). Samples were weighed into silver capsules, acidified with 2 M HCl to remove carbonates, and dried at 60°C for 24 h (the procedure was repeated until a constant weight was obtained). The OC and TN concentrations were calibrated against certified reference materials provided by HEKAtech GmbH (Germany). δ13OC and δ15TN were calculated using the laboratory working pure reference gases (CO2 and N2) calibrated against IAEA standards.
2.4 Data interpretation
To quantify the DOC remineralization during the experiment, the first-order kinetics (Equation 1) was applied, which assumes that the DOC concentration changes over time depend on the initial DOC concentration (time t = 0) and the remineralization rate constant (k). The use of this assumption was possible as the experiments were carried out in aerobic conditions (the oxygen conditions did not limit the growth of the microorganisms).
where:
The contributions of fractions having different bioavailability, as well as their remineralization rate constants, and half-life times were calculated using the procedures published by Kuliński et al. (2016) and briefly described further in the text.
3 Results
All the results obtained in the incubation experiments are presented in Supplementary Table S1. They consist of DOC concentrations measured at different time steps (with standard deviation for time steps t = 0, t = 19, and t = 180, representing three replicates) of the experiment, both in the control samples (seawater) and in the soil leachates (seawater enriched with soil extracts). For further elaboration in the text, however, the raw DOC results for the soil leachates have been corrected to remove from them the contribution of seawater DOC changes (Supplementary Figure S2) and to obtain in that way changes over time of soil-derived DOC only (Figures 2a,b). This has been done by proportional subtracting the control (seawater) DOC results from the raw DOC results obtained for the soil leachates at a given time. The calculations took into account: soil moisture, the initial volume of seawater added to the soils, the volume of water extracted from the soils, and the DOC concentrations in both runs (control and the soil leachates) at each time step.
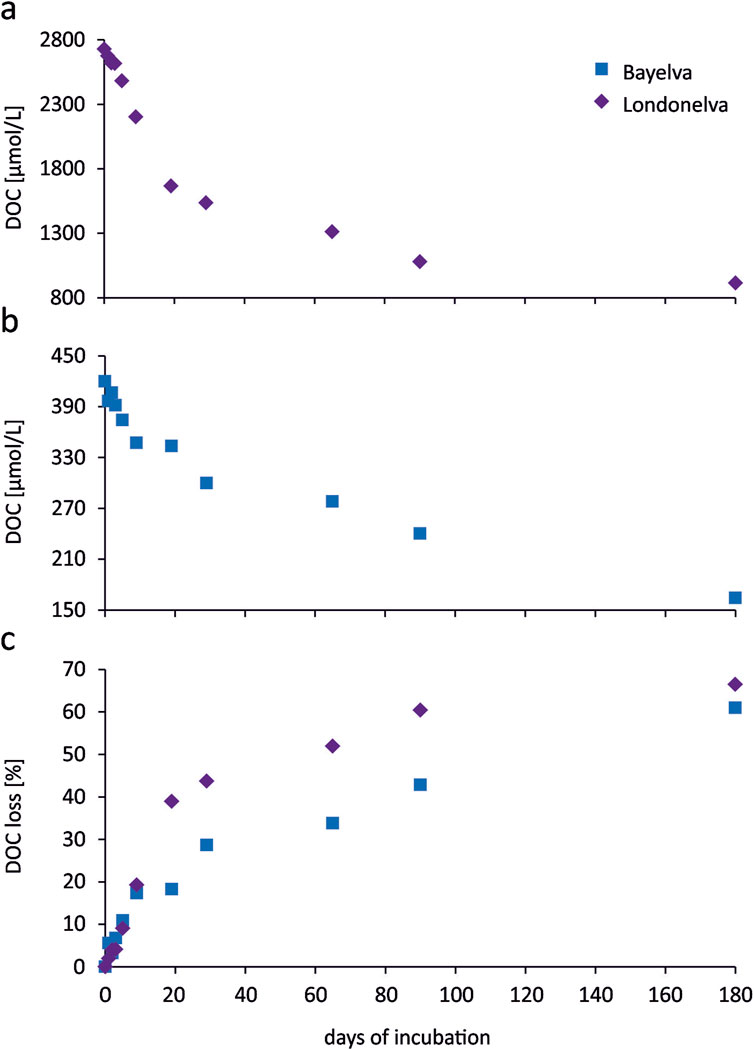
Figure 2. The decay of soil-derived DOC (corrected for the contribution of seawater DOC changes) during incubation of soil leachates from the Bayelva River catchment (a) and the Londonelva River catchment (b), cumulative losses of soil-derived DOC during the incubation presented as a percentage of initial concentrations (c).
In the control run the DOC concentration decreased after 20 days from 107 ± 2 to 69 ± 6 μmol/L and since that time remained almost unchanged until the end of the experiment. This suggests that the surface seawater in Kongsjorden contained about 35% bioavailable DOC at the time of sampling. On the other hand, the DOC level of 69 ± 6 μmol/L that was continuously observed at the later stage of the experiment can be considered as the refractory DOC in that region. This is more from what has been found for the open waters of the North Atlantic, for which the refractory DOC amounts to ±45 μmol/L (Hansell and Orellane, 2021). These differences are probably due to the influence of terrestrial OM in the fjords.
The decay of the soil-derived DOC (corrected for the contribution of seawater DOC changes) induced by microbial remineralization was tracked by interpreting the concentration changes during the incubation experiments (Figures 2a,b). A pronounced DOC decrease over time was observed for both incubations, with the highest dynamics at the beginning, and slower in the middle and the end of the experiments (an exponential character). After 180 days of incubation, the cumulative DOC loss, expressed as a percentage of initial DOC concentration (t = 0), was 61% for the material from the Bayelva River catchment and 66% for the one from the Londonelva River catchment, which corresponds to the DOC decay from 2,728 ± 33 to 916 ± 28 μmol/L, and from 420 ± 12 to 164 ± 14 μmol/L, respectively (Figure 2c).
The exponential character of the decay curves (Figure 2) justified the use of first-order kinetics in interpreting the remineralization dynamics. The integration of Equation 1 gives:
Through plotting the natural logarithms of DOC concentrations versus time (Equation 2), it is possible to estimate the potential remineralization rate constant, which is the slope in the linear regression of the resulting relationship. This has been done for both datasets originating from incubation of soil-derived DOC obtained from the Bayelva and Londonelva River catchments (Supplementary Figure S3). This exercise revealed in both investigated DOC data series the inflection points separating two different linearities, each having a different slope, higher at the beginning and lower in the later phase of the experiment. This indicates the presence of two bioavailable DOC fractions, each having different lability: (i) the labile DOC, present only in the initial stage of the experiment, and (ii) the semi-labile DOC, undergoing remineralization during the entire incubation. In the case of soil-derived DOC from the Bayelva station, the DOCL has been remineralized by the ninth day of the incubation and the sampling conducted in the later time steps shows the DOCSL decrease only. For the DOC from the Londonelva station, the remineralization of DOCL continued until the 19th day, while later on the DOC drop can be attributed to the DOCSL decay. The strong linearity (R2) of the lnDOC(t) dependence (Supplementary Figure S3) indicates that the uneven distribution of sampling points (higher density of measurements at the beginning of the experiment, followed by a smaller number of measurements later), which was planned according to the available literature (e.g., Kuliński et al., 2016), does not affect the calculations and confirms the appropriateness of our sampling strategy. The R2 values obtained for the most rapidly remineralizing DOC fraction were 0.949 for the Bayelva station and 0.993 for the Londonelva station, while for the less reactive fraction, the R2 values were 0.987 and 0.921, respectively.
In addition to the labile and semi-labile DOC fractions that decrease over time at different rates, the background of refractory DOC, which is not subjected to remineralization, must be taken into account. Therefore, the bulk description of total DOC remineralization can be given by (Equation 3):
where:
The DOCR values for both experiments were determined by interpreting the DOC decay curves at the later stages of the incubations (starting from day 19 for Bayelva and day 29 for Londonelva), i.e., when there was no more DOCL available for remineralization and only DOCSL and DOCR were present in the samples. This was done by mathematical fitting, in which DOCR value was searched to obtain the highest possible determination coefficient for linear regression of ln (DOC-DOCR) vs time (Supplementary Figures S4, S5A). The intercepts in these linear regressions represent the ln DOCSL at t = 0, from which the initial concentrations of DOCSL were determined, while the slopes correspond to the remineralization rate constants for the DOCSL fractions, k(SL) expressed in a unit of day-1 (Supplementary Figure S5A). Then, knowing the DOCSL concentrations at t = 0 for both data sets and the corresponding k(SL) values, it was possible to calculate DOCSL at each time step of the incubations. Finally, the concentrations of DOCL at each time step were calculated by subtracting the concentrations of DOCR and DOCSL from the total DOC. Similarly, as for DOCSL, initial DOCL concentrations and k(L) values were determined from the intercept and the slope, respectively, of the linear regressions (Supplementary Figure, S5B).
Additionally, for the bioavailable DOC fractions, DOCL and DOCSL, their half-life times were established following the first-order kinetics (Equation 4):
4 Discussion
The results indicate that the soil leachates contained a lot of soil-derived DOC, namely, 420 ± 12 μmol/L from the Bayelva River catchment and 2,728 ± 33 μmol/L from the Londonelva catchment (initial concentrations at t = 0). These significant disproportions reflect the organic carbon content in soils at both investigated sites, which amounted to 0.16% ± 0.02% for the Bayelva station and 1.95% ± 0.1% for the Londonelva station (Supplementary Table S2). This suggests different characteristics of deposits accumulated in the vicinity of both rivers. The Bayelava River is mainly fed with meltwater from surrounding glaciers, thus the organic matter in the river delta is strongly diluted with mineral material (Sund, 2008). The dilution effect is also confirmed by DOC concentrations in the river water ranging from 50 to 125 μmol/L during the meltwater season (Zhu et al., 2016; Retelletti Brogi et al., 2019; Gödde et al., 2024). On the contrary, the glacier-free Blomstradøya Island which is drained by the Londonelva River is the source of contemporarily produced organic matter (Hop and Wiencke, 2019) with a DOC concentration of 380 μmol/L measured in summer (own result, unpublished data). Moreover, high variability, both spatial and temporal, of DOC concentrations in the soil leachates has been already reported for other Arctic regions. For instance, Vonk et al. (2015) found the DOC concentrations in soil leachates from Toolik Field Station (Alaska) ranging from 175 μmol/L in samples collected in May to 1930 μmol/L in samples collected in September, while MacDonald et al. (2021) measured DOC from about 800 to 4170 μmol/L in leachates from the permafrost active layer in the western Canadian Arctic.
The contributions of different DOC stability fractions are shown in Figure 3. Although this theoretical division into three classes of biological stability is undoubtedly only an approximation of the complex structure of DOM, it clearly shows that DOC released from soils is highly bioavailable. Only 54 μmol/L and 871 μmol/L, which corresponds to 13% and 32% of total DOC, can be considered as refractory DOC in soils from the Bayelva and Londonelva catchment, respectively. These values are often substantially lower than those reported in the literature so far. For instance, Bristol et al. (2024) examined the bioavailability of DOC leached from active layer and permafrost soils at Drew Point, Alaska. The authors followed the protocol published by Vonk et al. (2015) and, similarly to our approach, extracted leachates with seawater. DOC loss during incubation was 9.0% ± 3% over 26 days and 23% ± 5% over 90 days for active layer soils. For Holocene terrestrial permafrost, DOC loss was 9.1% ± 3% over 26 days and 14% ± 3% over 90 days Speetjens et al. (2022) measured 5%–17% DOC loss in 21-day porewater incubation experiments, with higher losses from the active layer than from permafrost (coastal catchment, western Canadian Arctic). Li et al. (2022) estimated DOM biodegradation after the 14-day incubation from 3.86% to 23.4%. Vonk et al. (2015 and references therein) obtained the maximum DOC loss of up to ∼50% during incubation experiments of soil leachates within the Arctic Ocean watershed. Higher DOC bioavailability values, but still in the lower range of results obtained in this study, were obtained by Spencer et al. (2015) for Siberian permafrost (∼40%–60% over 28 days incubation) and Abbott et al. (2014) for thawing and collapsing permafrost on the North Slope of Alaska (DOC loss exceeded 50% after 10 days at several sites and reached even 67% loss after 40 days). However, most of these previous estimations were based on the DOC loss observed during incubation experiments lasting up to 28–40 days (maximum 90 days by Bristol et al., 2024), with the most commonly used incubation temperature between 15°C and 25°C (Vonk et al., 2015). Therefore, they may potentially underestimate the bioavailability as some of the semi-labile DOC fraction was likely not remineralized during the incubations. Also in our experiments, though they were much longer, the constant DOC level has not been reached. This justifies the use of a mathematical model based on the first-order kinetics assumptions to approximate the DOCR and to avoid underestimating the contribution of the bioavailable DOC due to too short incubation time.
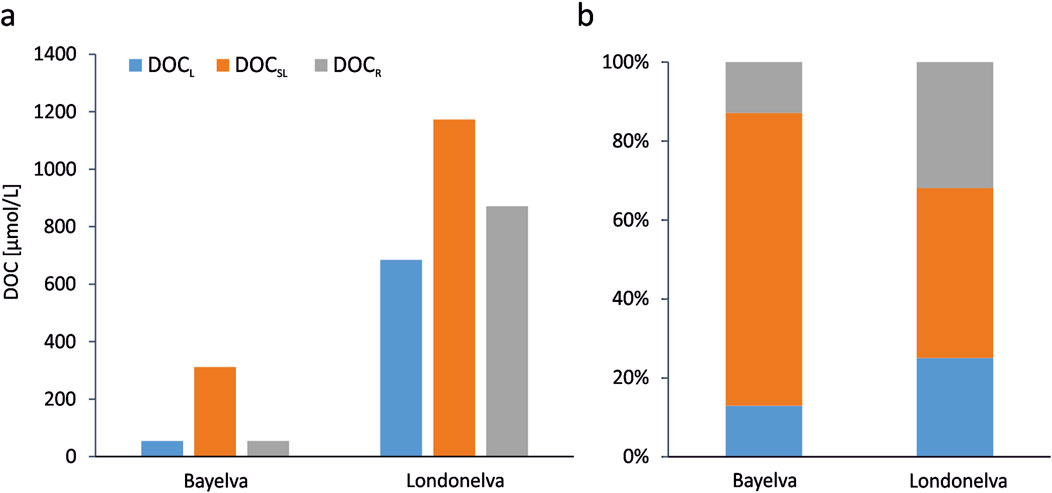
Figure 3. Concentrations (a) and relative contributions (b) of labile (DOCL), semi-labile (DOCSL) and refractory (DOCR) fractions of soil-derived DOC in the soil leachates from the Bayelva and Londonelva catchments.
Although the leachates from both investigated sites are characterized by a high contribution of bioavailable soil-derived DOC, there are clear differences between the shares of DOCL and DOCSL fractions. In the case of material from the Londonelva station, a higher contribution (25%, compared to 13% for the Bayelva station) of the most labile DOC fraction, DOCL, suggests a higher supply of relatively fresh DOC from the contemporary biological production, which can be rapidly remineralized. This corresponds well with the results published by Lods-Crozet et al. (2007), who found lower turbidity, higher temperatures, and greater primary production in the Londonelva than in the Bayelva River. Glacial basin rivers, like Bayelva, are usually characterized by much higher levels of mineral suspension (Dittmar and Kattner, 2003), which can strongly dilute the organic matter signal but also through increased turbidity hamper OM production in rivers. Furthermore, glacier-derived DOC is usually substantially older as indicated by 14C ages (Behnke et al., 2021; Holt et al., 2021). The high contribution of ancient OM in the deposits at the Bayelva station can likely explain the lower share of DOCL observed there as compared to the rather more recent OM at the Londoelva station. Still, it is worth noting the high share of DOCSL and in consequence, the overall very high contribution (87%) of bioavailable DOC fraction (DOCL + DOCSL) observed in the material extracted from the soil at the Bayelva station.
The high temperature at which the experiments have been conducted allowed us to obtain distinct and measurable DOC decay and thus quantify the potential shares of DOC fractions having different lability. However, the parameters describing the OM remineralization kinetics are temperature-dependent and thus they have been recalculated to the in situ temperatures. As a site-specific reference, the temperature of 2.2°C has been used, which is the 10-year mean temperature for the melt season in Ny-Ålesund (Kongsfjorden; Supplementary Figure S6; Data source: Observations and weather statistics, Norwegian Centre for Climate Services; seklima.met.no; last access: 20.05.2025). To recalculate the potential remineralization rate constants and corresponding half-life times, determined in the experiments (Table 1), the Q10 factor was used (Equation 5), which describes the multiplicity of the increase of a rate constant of any biochemical reaction due to temperature increase by 10°C. Based on literature data, the Q10 for remineralization of permafrost- and soil-derived OC is between 1.3 and 2.6. These values were further used for estimating the expected range of remineralization rate constants and half-life times at the local mean temperature of 2.2°C (Meyer et al., 2018; Qin et al., 2021; Quinlan, 1980; Quinlan, 1981). The calculated in this study OM remineralization kinetics should be considered as a first approximation for this region. This is due to the fact that the Q10 values, although well characterized for the polar regions, may still be site-specific, and also constants determined with first-order kinetics (k and t1/2) may be influenced by elevated concentrations of the limiting element (Payn et al., 2005; Mulholland et al., 2002).
where: k1 and k2 are the rates at which products of a reaction are produced (mmol/s) at two different corresponding temperatures: T1 and T2 in which the reaction occurred.
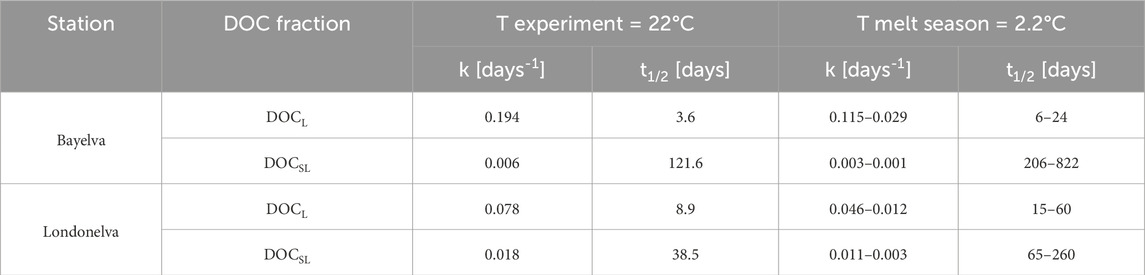
Table 1. Remineralization rate constants (k) and half-life times (t1/2) of semi-labile (DOCSL), and labile (DOCL) DOC for Bayelva and Londonelva sampling stations.
The highest potential remineralization rate constant at 2.2°C was found for the labile fraction of the soil-derived DOC from the Bayelva catchment and ranged from 0.115 to 0.029 d-1 (t1/2 = 6–24 days; Table 1). This value is more than twice as high as the k(L) value for Londonelva catchment (k(L) = 0.046–0.012 d-1 and t1/2 = 15–60 days), and indicates that despite a lower contribution of the labile DOC in Bayelva region, it is more reactive than DOCL from Londonelva catchment. The opposite situation has been observed for the contribution and potential remineralization rate constants for DOCSL. At the Bayelva station, it is characterized by three times lower k(SL) and triply higher t1/2 values, while DOCSL share of the total DOC pool is much higher than at the Londonelva station. The substantial differences in k(L) and k(SL) between both sites influence likely the final shares of both labile and semi-labile fractions. The soil-derived DOC from Londonelva is richer in DOCL as the latter remineralizes slower than at the Bayelva site, while the fast remineralization of DOCL at the Bayelva catchment makes the DOC overall enriched in DOCSL. To unravel fully these dependencies it would be necessary to have information about inputs of different fractions (source term) and the time of their exposure before sampling. However, it is worth emphasizing that the k(L) and k (SL) results obtained for both stations are closer to the values observed in the coastal seas than in the freshwater environment. Lonborg and Alvarez-Salgado (2012) found that the decay constant for bioavailable DOC is 0.066 ± 0.065 d-1 (n = 127) in the marginal and shelf seas, while Hopkinson et al. (2002) reported for continental margins mean values of 0.219 d-1 and 0.01 d-1 for very labile and labile DOC fraction, respectively. While for the freshwater environment, Koehler et al. (2012) found the initial first-order decay coefficient for DOC ranged from 0.0009 to 0.0043 d-1 in the waters of six Swedish lakes.
All these results suggest that the composition and provenance of soil-derived DOC are different in both studied regions. This can be also confirmed by the isotopic composition of organic matter in soils. The material from the Bayelva catchment had higher δ13OC and δ15TN (−23.7‰ ± 0.2‰ and 3.7‰ ± 0.3‰, respectively) compared to the results from the Londonelva station (−27.5‰ ± 0.2‰ and 2.3‰ ± 0.3‰, respectively) (Supplementary Table S2). According to the commonly used interpretation (e.g., Ruttenberg and Goni, 1997; Kuliński et al., 2014), the isotopically heavier carbon (higher δ13C) characterizes organic matter of marine provenance, while higher δ15N values indicate a higher contribution of organic matter reworked by organisms from higher trophic levels. For terrestrial OM, Kim et al. (2011) distinguished a few main fractions and estimated that δ13OC of particulate sources differ: 27.1 to −25.2‰ for soil OM, −25.0‰ for coal, and −23.8 to −20.3‰ for ice-rafted debris (IRD). The higher δ13OC values in the Bayelva catchment could be related to the latter source–IRD, as large amounts of algae-derived OM were previously observed in this region (Chen et al., 2021). However, as was shown for instance by Kuliński et al. (2014), the high variety of isotopic composition of organic matter originating from multiple sources makes the interpretation of the isotopic signatures in the Arctic challenging. This makes concluding about the provenance of organic matter in the investigated regions difficult. Nevertheless, the variability observed in the isotopic results indicates that differences occur.
5 Conclusion
The obtained results indicate that the soils in catchments of rivers draining into Kongsfjorden contain a large quantity of bioavailable DOC, with as much as 68%–87% of the total leachable DOC being susceptible to degradation. The contribution of the most labile DOC fraction is small and ranges from 13% in the Bayelva region to 25% in the Londonelva catchment, but it remineralizes quickly once transported to the fjord - within days or weeks, while the semi-labile DOC, whose half-life is measured in months, is much more abundant (74% and 43% of total soil-derived DOC, respectively). Despite the high bioavailability of soil-derived DOC in both studied catchments, there are significant differences in the contribution of particular DOC fractions between stations, which can result from the different composition and provenance of OM as well as different times of their exposure before sampling. Higher DOC losses, compared to previous studies, indicate that the incubation experiment setup should consider the potentially significant contribution of semi-labile DOC. Since the remineralization of this fraction is slow (half-life time measured in months), too short experiments may underestimate the bioavailability of DOC. Thus, we recommend applying the reaction kinetics models, which together with the appropriately adjusted duration time of the incubation experiment may substantially improve the reliability of the results.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
KKo: Conceptualization, Formal Analysis, Investigation, Writing – original draft. PM: Investigation, Writing – review and editing. FA: Writing – review and editing. KKu: Conceptualization, Funding acquisition, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Funding for incubation experiments, data analysis and interpretation was provided by the National Science Centre, Poland (2019/34/E/ST10/00167). Fieldwork and environmental characteristics were financially supported by the Norwegian Financial Mechanism 2014–2021 (85%) National Science Centre (15%) within GRIEG Programme (2019/34/H/ST10/00504 and 2019/34/H/ST10/00645). Authors also acknowledge the support of the Institute of Oceanology of the Polish Academy of Sciences.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/feart.2025.1456404/full#supplementary-material
References
Abbott, B. W., Larouche, J. R., Jones, Jr., J. B., Bowden, W. B., and Balser, A. W. (2014). Elevated dissolved organic carbon biodegradability from thawing and collapsing permafrost. J. Geophys. Res. Biogeosci. 119, 2049–2063. doi:10.1002/2014JG002678
Adakudlu, M., Andersen, J., Bakke, J., Beldring, S., Benestad, R., Bilt, W. V., et al. (2019). Climate in Svalbard 2100–a knowledge base for climate adaptation. Available online at: www.miljodirektoratet.no/globalassets/publikasjoner/M1242/M1242.pdf.
Behnke, M. I., Stubbins, A., Fellman, J. B., Hood, E., Dittmar, T., and Spencer, R. G. M. (2021). Dissolved organic matter sources in glacierized watersheds delineated through compositional and carbon isotopic modeling. Limnol. Oceanogr. 66 (2), 438–451. doi:10.1002/lno.11615
Boike, J., Juszak, I., Lange, S., Chadburn, S., Burke, E., Overduin, P. P., et al. (2018). A 20-year record (1998–2017) of permafrost, active layer and meteorological conditions at a high Arctic permafrost research site (Bayelva, Spitsbergen). Earth Syst. Sci. Data 10, 355–390. doi:10.5194/essd-10-355-2018
Bristol, E. M., Behnke, M. I., Spencer, R. G., McKenna, A., Jones, B. M., Bull, D. L., et al. (2024). Eroding permafrost coastlines release biodegradable dissolved organic carbon to the Arctic Ocean. J. Geophys. Res. Biogeosciences 129 (7), e2024JG008233. doi:10.1029/2024JG008233
Chen, M., Kim, J.-H., Hong, S., Lee, Y. K., Kang, M. H., Jin, Y. K., et al. (2021). Spectral characterization of dissolved organic matter in seawater and sediment pore water from the arctic fjords (west svalbard) in summer. Water 13, 202. doi:10.3390/w13020202
Dittmar, T., and Kattner, G. (2003). The biogeochemistry of the river and shelf ecosystem of the Arctic Ocean: a review. a Rev. Mar. Chem. 83 (3–4), 103–120. doi:10.1016/S0304-4203(03)00105-1
Elberling, B., Michelsen, A., Schäde, C., Schuur, E. A. G., Christiansen, H. H., Berg, L., et al. (2013). Long-term CO2 production following permafrost thaw. Nat. Clim. Change 3, 890–894. doi:10.1038/nclimate1955
Gödde, A., Moe, B., and Mikkelse, Ø. (2024). Seasonal dynamics of chemistry in an Arctic glacier-fed river. Sci. Total Environ. 954, 176645. doi:10.1016/j.scitotenv.2024.176645
Hansell, D. A., and Orellana, M. V. (2021). Dissolved organic matter in the global ocean: a primer. Gels 7 (3), 128. doi:10.3390/gels7030128
Hanssen-Bauer, I., Førland, E., Hisdal, H., Mayer, S., Sandø, A., and Sorteberg, A. (2019). “Climate in Svalbard 2100 – a knowledge base for climate adaptation,”. NCCS report no. 1/2019.
Holt, A. D., Fellman, J., Hood, E., Kellerman, A. M., Raymond, P., Stubbins, A., et al. (2021). The evolution of stream dissolved organic matter composition following glacier retreat in coastal watersheds of Southeast Alaska. Biogeochemistry 164, 99–116. doi:10.1007/s10533-021-00815-6
Hood, E., Fellman, J., Spencer, R. G. M., Hernes, P. J., Edwards, R., D’Amore, D., et al. (2009). Glaciers as a source of ancient and labile organic matter to the marine environment. Nature 462 (7276), 1044–1047. doi:10.1038/nature08580
Hop, H., and Wiencke, C. (2019). The ecosystem of kongsfjorden, svalbard, springer cham. Ser. Title Adv. Polar Ecol. doi:10.1007/978-3-319-46425-1
Hopkinson, Jr., C. S., Vallino, J. J., and Nolin, A. (2002). Decomposition of dissolved organic matter from the continental margin. Deep-Sea Res. II 49, 4461–4478. doi:10.1016/S0967-0645(02)00125-X
Hugelius, G., Tarnocai, C., Broll, G., Canadell, J. G., Kuhry, P., and Swanson, D. K. (2013). The Northern Circumpolar Soil Carbon Database: spatially distributed datasets of soil coverage and soil carbon storage in the northern permafrost regions. Earth Syst. Sci. Data 5, 3–13. doi:10.5194/essd-5-3-2013
Killingtveit, A., Kane, D. L., and Yang, D. (2004). “Water balance studies in two catchments on Spitsbergen. Northern research basins water balance,” in Proceedings of a workshop held at Victoria, Canada, March 2004 (Wallingford: International Association of Hydrological Sciences Press), 120–128.
Kim, J. H., Peterse, F., Willmott, V., Kristensen, D. K., Baas, M., Schouten, S., et al. (2011). Large ancient organic matter contributions to Arctic marine sediments (Svalbard). Limnol. Oceanogr. 56, 1463–1474. doi:10.4319/lo.2011.56.4.1463
Koehler, B., von Wachenfeldt, E., Kothawala, D., and Tranvik, L. J. (2012). Reactivity continuum of dissolved organic carbon decomposition in lake water. J. Geophys. Res. Biogeosciences 117 (Issue G1). doi:10.1029/2011JG001793
Koziorowska-Makuch, K., Szymczycha, B., Thomas, H., and Kuliński, K. (2023). The marine carbonate system variability in high meltwater season (Spitsbergen fjords, Svalbard). Prog. Oceanogr. 211, 102977. doi:10.1016/j.pocean.2023.102977
Kuliński, K., Hammer, K., Schneider, B., and Schulz-Bull, D. (2016). Remineralization of terrestrial dissolved organic carbon in the Baltic Sea. Mar. Chem. 181, 10–17. doi:10.1016/j.marchem.2016.03.002
Kuliński, K., Kędra, M., Legeżyńska, J., Gluchowska, M., and Zaborska, A. (2014). Particulate organic matter sinks and sources in high Arctic fjord. J. Mar. Syst. 139, 27–37. doi:10.1016/j.jmarsys.2014.04.018
Li, Y., Chen, Z., Chen, J., Castellano, M. J., Ye, C., Zhang, N., et al. (2022). Oxygen availability regulates the quality of soil dissolved organic matter by mediating microbial metabolism and iron oxidation. Glob. Chang. Biol. 28, 7410–7427. doi:10.1111/gcb.16445
Lods-Crozet, B., Lencioni, V., Brittain, J. E., Marziali, L., and Rossaro, B. (2007). Contrasting chironomid assemblages in two high Arctic streams on Svalbard. Fundam. Appl. Limnol. 170 (3), 211–222. doi:10.1127/1863-9135/2007/0170-0211
Lonborg, C., and Alvarez-Salgado, A. X. (2012). Recycling versus export of bioavailable dissolved organic matter in the coastal ocean and efficiency of the continental shelf pump. Glob. Biogeochem. Cycles 26. doi:10.1029/2012GB004353
MacDonald, E. N., Tank, S. E., Kokelj, S. V., Froese, D. G., and Hutchins, R. H. S. (2021). Permafrost-derived dissolved organic matter composition varies across permafrost end-members in the western Canadian Arctic. Environ. Res. Lett. 16, 024036. doi:10.1088/1748-9326/abd971
Meyer, N., Welp, G., and Amelung, W. (2018). The temperature sensitivity (Q10) of soil respiration: controlling factors and spatial prediction at regional scale based on environmental soil classes. Glob. Biogeochem. Cycles 32, 306–323. doi:10.1002/2017GB005644
Mulholland, P. J., Tank, J. L., Webster, J. R., Bowden, W. B., Dodds, W. K., Gregory, S. V., et al. (2002). Can uptake length in streams be determined by nutrient addition experiments? Results from an interbiome comparison study. J. North Am. Benthol. Soc. 21 (4), 544–560. doi:10.2307/1468429
Nguyen, H. T., Lee, Y. M., Hong, J. K., Hong, S., Chen, M., and Hur, J. (2022). Climate warming-driven changes in the flux of dissolved organic matter and its effects on bacterial communities in the Arctic Ocean: a review. Front. Mar. Sci. 9, 968583. doi:10.3389/fmars.2022.968583
Nowak, A., Hodgkins, R., Nikulina, A., Osuch, M., Wawrzyniak, T., Kavan, J., et al. (2021). “From land to fjords: the review of Svalbard hydrology from 1970 to 2019 (SvalHydro),” in SESS report 2020 - the state of environmental science in svalbard - an annual report (176–201). Svalbard integrated arctic Earth observing system. doi:10.5281/zenodo.4294063
Orvin, A. K. (1934). “Geology of the kings bay region, Spitsbergen. Skrifter om Svalbard og Ishavet 57,” in Norway’s Svalbard and Arctic Ocean Survey. Oslo: Norwegian Polar Institute, I kommisjon hos J. Dybwad.
Payn, R. A., Webster, J. R., Mulholland, P. J., Valett, H. M., and Dodds, W. K. (2005). Estimation of stream nutrient uptake from nutrient addition experiments. Limnol. Oceanogr. Methods 3, 174–182. doi:10.4319/lom.2005.3.174
Polimene, L., Torres, R., Powley, H. R., Bedington, M., Juhls, B., Palmtag, J., et al. (2022). Biological lability of terrestrial DOM increases CO2 outgassing across Arctic shelves. Biogeochemistry 160, 289–300. doi:10.1007/s10533-022-00961-5
Polyakov, I. V., Pnyushkov, A. V., Alkire, I. M., Baumann, T. M., Carmack, E. C., Goszczko, I., et al. (2017). Greater role for Atlantic inflows on sea-ice loss in the Eurasian basin of the Arctic Ocean. Science 356, 285–291. doi:10.1126/science.aai8204
Qin, S., Kou, D., Mao, C., Chen, Y., Chen, L., and Yang, Y. (2021). Temperature sensitivity of permafrost carbon release mediated by mineral and microbial properties. Sci. Adv. 7, eabe3596. doi:10.1126/sciadv.abe3596
Quinlan, A. V. (1980). The thermal sensitivity of Michaelis-Menten kinetics as a function of substrate concentration. J. Frankl. Inst. 310, 325–342. doi:10.1016/0016-0032(80)90011-3
Quinlan, A. V. (1981). The thermal sensitivity of generic Michaelis-Menten processes without catalyst denaturation or inhibition. J. Therm. Biol. 6, 103–114. doi:10.1016/0306-4565(81)90061-9
Rantanen, M., Karpechko, A. Y., Lipponen, A., Nordling, K., Hyvärinen, O., Ruosteenoja, K., et al. (2022). The Arctic has warmed nearly four times faster than the globe since 1979. Commun. Earth Environ. 3, 168. doi:10.1038/s43247-022-00498-3
Repeta, D. J. (2015). “Chemical characterization and cycling of dissolved organic matter,” in Biogeochemistry of marine dissolved organic matter (Elsevier), 21–63.
Retelletti Brogi, S., Ha, S. Y., Kim, K., Derrien, M., Lee, Y. K., and Hur, J. (2018). Optical and molecular characterization of dissolved organic matter (DOM) in the Arctic ice core and the underlying seawater (Cambridge bay, Canada): implication for increased autochthonous DOM during ice melting. Sci. Total Environ. 627, 802–811. doi:10.1016/j.scitotenv.2018.01.251
Retelletti Brogi, S., Jung, J. Y., Ha, S. Y., and Hur, J. (2019). Seasonal differences in dissolved organic matter properties and sources in an Arctic fjord: implications for future conditions. Sci. Total Environ. 694, 133740. doi:10.1016/j.scitotenv.2019.133740
Ruttenberg, K. C., and Goni, M. A. (1997). Phosphorus distribution, C:N:P ratios, and δ13Coc in arctic, temperate, and tropical coastal sediments: tools for characterizing bulk sedimentary organic matter. Mar. Geol. 139 (1–4), 123–145. doi:10.1016/S0025-3227(96)00107-7
Speetjens, N. J., Tanski, G., Martin, V., Wagner, J., Richter, A., Hugelius, G., et al. (2022). Dissolved organic matter characterization in soils and streams in a small coastal low-Arctic catchment. Biogeosciences 19, 3073–3097. doi:10.5194/bg-19-3073-2022
Spencer, R. G. M., Mann, P. J., Dittmar, T., Eglinton, T. I., McIntyre, C., Holmes, R. M., et al. (2015). Detecting the signature of permafrost thaw in Arctic rivers. Geophys. Res. Lett. 42 (8), 2830–2835. doi:10.1002/2015GL063498
Sund, M. (2008). Report nr 2 -2008, polar hydrology, published by: Norwegian water resources and energy directorate’s work in svalbard .
Vihtakari, M., Welcker, J., Moe, B., Chastel, O., Tartu, S., Hop, H., et al. (2018). Black-legged kittiwakes as messengers of atlantification in the arctic. Sci. Rep. 8, 1178. doi:10.1038/s41598-017-19118-8
Vonk, J. E., Tank, S. E., Mann, P. J., Spencer, R. G. M., Treat, C. C., Striegl, R. G., et al. (2015). Biodegradability of dissolved organic carbon in permafrost soils and aquatic systems: a meta-analysis. Biogeosciences 12, 6915–6930. doi:10.5194/bg-12-6915-2015
Keywords: DOC (dissolved organic carbon), remineralization, lability, biodegradation, Spitsbergen (Svalbard)
Citation: Koziorowska K, Makuch P, Aguado Gonzalo F and Kuliński K (2025) High bioavailability of soil-derived dissolved organic carbon in the high Arctic fjord (Kongsfjorden, Svalbard Archipelago). Front. Earth Sci. 13:1456404. doi: 10.3389/feart.2025.1456404
Received: 28 June 2024; Accepted: 05 June 2025;
Published: 20 June 2025.
Edited by:
Patrick G. Hatcher, Old Dominion University, United StatesReviewed by:
Xiangjin Shen, Chinese Academy of Sciences (CAS), ChinaLouis Kaplan, Stroud Water Research Center, United States
Copyright © 2025 Koziorowska, Makuch, Aguado Gonzalo and Kuliński. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Katarzyna Koziorowska, a2tvemlvQGlvcGFuLnBs
 Katarzyna Koziorowska
Katarzyna Koziorowska Przemysław Makuch
Przemysław Makuch Fernando Aguado Gonzalo
Fernando Aguado Gonzalo Karol Kuliński
Karol Kuliński