- 1Department of Chemical, Biological, Pharmaceutical and Environmental Sciences, University of Messina, Messina, Italy
- 2Department of Mathematical and Computer Sciences, Physical Sciences and Earth Sciences, University of Messina, Messina, Italy
Investigations carried out on the geological/ecological asset of Peloro Cape, northeastern Sicily (Italy), revealed the presence of hundreds of asbestos cement material (ACM) fragments, whose features indicated their origin from undulated roofing sheets left in demolition waste dumps, later exposed to marine coastal erosion. Some fragments showed clear evidence of biotic activity, both in the form of corrosion and encrustation. The first stage of colonization was indicated by organic weathering under subaerial conditions, evidenced by bare substrates left by lichens. A second stage of colonization was recognized through the calcareous remains of marine encrusting organisms found in shallow marine environments. In this latter phase, evidence of biotic overgrowth of spirorbids, large serpulids, oysters, bryozoans, and vermetid gastropods indicated a successional process likely developed over a timespan of several decades. Repeated phases of immersion and stranding due to storms, however, may also be considered. Subaerial and subaqueous colonization may be indirectly tied, since lichen may contribute to make inert Asbestos materials, facilitating the later settlement of marine encrusting biota. The combined action of continental and marine biota thus reduces the release of hazardous asbestos fibers into the environment. Such a process, nevertheless, cannot affect the smallest fragments, which are too unstable on the sea bottom to be colonized. In conclusion, multiple interferences from uncontrolled ACM waste have affected both emerged and submerged coastal environments. These impacts need to be evaluated, particularly with regard to their threat to human health. Areas affected by environmental and health risks due to asbestos exposure should be remediated to avoid damage to humans, living organisms, and ecosystems, whose relationships in marine environments have been almost neglected. This research addresses the knowledge gap regarding the sources and fate of hazardous ACM waste after being transferred and transported by alluvial and marine waters. It may support competent public authorities and technicians in planning real remediation activities once the full complexity of the coastal area contamination is understood.
Introduction
Asbestos minerals are characteristic fibrous minerals belonging to the groups of amphiboles (amosite, actinolite, anthophyllite, crocidolite, and tremolite) and serpentine (chrysotile). Since the early–mid-19th century, mines have extracted asbestos minerals, primarily used for producing fiber cement employed in industries and constructions for manufacturing corrugated roofing, water tanks, and tubes (Somma et al., 2024 and references therein). These cements degrade, losing asbestos fibers after being exposed to chemical and physical weathering, abrasion, and erosion. Consequently, asbestos fiber dispersion in the environmental matrices may be responsible for severe environmental and health risks due to the toxicity and potential high hazard of some of these mineral species. Asbestos cement materials (ACMs), after their use, must be treated/disposed of as dangerous waste [European Waste Catalogue (EWC)—code 17 06 01* 17 06 05*]. The toxicity of the asbestos fibers is mainly due to their fracture, length, and diameter. The primary asbestos-related diseases are asbestosis; tumors of the lung, larynx, ovaries, and serosal membranes; serous covering of the testis; and mesothelioma. The latter is mainly related to prior extensive inhalation of crocidolite, tremolite, or amosite fibers (Somma, 2024; Somma et al., 2024 and references therein). For all these reasons, the production and use of ACMs have been banned in Italy since 1992 under Law No. 257, and the regional governments were entrusted to implement environmental protection plans and activities for the decontamination and remediation of the environmental matrices in order to contrast the hazards and risks related to the human and environment interactions with asbestos. In 1994, a ministerial decree ruled on the activities for the removal of ACMs and environmental remediation. In 1997, the government established that ACMs had to be carried out in controlled landfills after thermal, chemical, or mechanical treatments. Due to the high taxes imposed on private entities for ACM disposal, illegal dumping in the countryside has greatly increased since the 1990s (Somma et al., 2024). Disposed aged man-made ACMs may be commonly found as anthropogenic hazardous wastes in urbanized and industrialized areas of many countries (Camplin, 2008; Jacoby, 2011; Lisco et al., 2023). ACMs may have formed historical solid waste disposals with other demolition materials (Vimercati et al., 2019; Paglietti et al., 2022), often localized along the alluvial and coastal areas (Nicholls et al., 2021), as frequently recorded in Italy. After the ban on ACMs, in most cases, asbestos wastes were illegally buried along the Italian coastal areas (Lisco et al., 2023; Somma et al., 2024) to avoid detection by control authorities. This activity was of no avail as the shallowly buried waste was, in most cases, brought to the surface by coastal erosion caused by storm waves, tides, currents, and increased global sea levels (D'Alessandro et al., 2008). Consequently, dispersal of such pollutants in shallow marine and beach environments also occurred, as observed in the Bay of Fundy in Canada (Goodman et al., 2020). Extreme events, such as tidal surges associated with tropical cyclones, like the 2011 tropical cyclone Yasi in Queensland, Australia (Ryan et al., 2014), or exceptional floods, such as the flood that occurred in 2009 at Giampilieri, Italy (Aronica et al., 2012; Di Gregorio et al., 2024), were identified as responsible for the commingling of ACMs with soil, vegetation, and other debris on marine beaches. Notwithstanding ordinary cleaning or faint reclamation activities, such as the physical waste removal from the beach surface or the movement of beach sediment using mechanical excavators, the pollutant is continuously increasing. Several cases of ACMs were reported during reclamation campaigns along marine beaches, where their reappearance was documented after some time (Somma et al., 2024). Consequently, the determination of the provenance of beached asbestos waste, whether from continental or marine environments, may be a crucial task for adopting effective strategies for proper environmental management and planning activities aimed at protecting human and ecosystem health. In recent years, after decades of reports on the contamination of Italian marine beaches by ACMs from esteemed environmental associations and newspapers (Somma et al., 2024), the first scientific research on this growing issue was carried out along the Puglia coasts (southern Italy) (Lisco et al., 2023). Similar research was carried out along the coasts of Sicily (southern Italy) (Somma, 2024; Somma et al., 2024), where several hundred ACM waste fragments were found on the beaches surrounding the Cape Peloro peninsula, facing the Strait of Messina. Fragments were found as beached waste or contained in the beach sands and gravels, as technofossils (Zalasiewicz et al., 2014). Several abandoned dumps of construction waste dating back to the 1970s were identified, most of which are located along the Ionian beaches, particularly in the fishing villages of Torre Faro and Ganzirri (well-known tourist destinations crowded with bathers during the summer), recently awarded the blue flag. The coastal erosion and marine currents affecting this area of the Strait of Messina were responsible for facilitating the dispersal of ACM wastes (including bricks, tiles, glass, metal, and plastic) on and in the beach and shallow marine deposits, mainly composed of silicate sandy mono- to polymineral grains (quartz, feldspars, biotite, muscovite, garnets, and staurolite) and pebbles derived from metamorphic and igneous silicate rocks, such as gneiss, augen gneiss, granitoids, and quartz (Somma, 2024; Somma et al., 2024).
Biotic interactions in ACM wastes
The significant mobility of waste in the continental and marine environments, due to natural and anthropogenic activities, may involve strict interactions of the waste with the biotic compartment (terrestrial vegetation and marine organisms). Thus, the organic encrustations on the ACM wastes’ surface may provide useful indications on the prevalent environment in which the materials settled and the possible sequence of depositional environments in which they were transported by floods or coastal erosion and currents.
ACMs, during the first stages of their use, mainly occur in the external parts of the buildings. In such anthropogenic subaerial environments, these materials may provide available substrata for primary organic colonization by cyanobacteria, algae, fungi, mosses, and lichens (Gilbert, 1990). Their settlement is favored by the rough texture and water-absorbing property of the weathered material. Epilithic lichens, as primary colonizers of bare substrates, regularly occur in slag dumps, and frequently settle on ACM waste. Their proliferation may be further enhanced by their ability to hyperaccumulate heavy metals, especially in crustose thalli (Rola et al., 2016). When growing on ACMs, these thalli play a remarkable role in biocovering and bioweathering, providing a physical barrier that helps prevent the detachment and environmental dispersion of asbestos fibers with significant bio-attenuation effects (Favero-Longo et al., 2009). Moreover, despite the alleged role of lichen acid secretion in accelerating the deterioration of asbestos materials, recent investigation proved that the released oxalic acids act in converting asbestos fibers into less hazardous amorphous and non-fibrous silica debris (Favero-Longo et al., 2005; Turci et al., 2007). Secondary colonizers, favored by such biological stabilization of the asbestos substrate, may consequently settle (Gilbert, 1971; Rajandu et al., 2021).
Even in modern times, the frequent findings of ACM wastes in coastal and marine environments (also known as recent dumping) further prove that not all of them are carried out in legal landfills after their use. The occurrence of ACM wastes is only incidentally mentioned in research concerning the effects of sunken waste on the marine environment (Rizzo et al., 2021), although different sources releasing ACM wastes are known (Lakhal et al., 2009; Liu, 2013; Rizzo et al., 2021; Dąbrowska et al., 2021; Afgatiani et al., 2023). Such a lack of data on the ACM contamination of marine environments strikingly contrasts with data provided by manipulative experiments using asbestos-cement panels as the bare substrate in benthic colonization processes in tropical (Jackson and Buss, 1975), temperate (Relini et al., 1994), and Arctic environments (Meyer-Kaiser et al., 2019).
With this in mind, the present research focused on the reconstruction of the spatio-temporal dynamics of such organically altered ACM beached waste. Encrusting organisms, depositional environments, residence time, superposition succession, and organic–inorganic interactions with ACM substrates were characterized.
Materials and methods
The study area was located in the Holocene Tyrrhenian and Ionian coasts of the Cape Peloro peninsula in northeastern Sicily. Since the coastline extends equally on both sides of the peninsula, it experiences two distinct storm surge exposures, being approximately N–NE and S–SE in the Tyrrhenian and Ionian seas, respectively (Figure 1). A stretch of approximately 4.5 km of beach was surveyed during the winters of 2023/2024 and 2024/2025, aiming to detect ACM waste in the beach deposits and the shallow marine environment. A total of 520 ACM fragments were documented in the field (Figure 1).
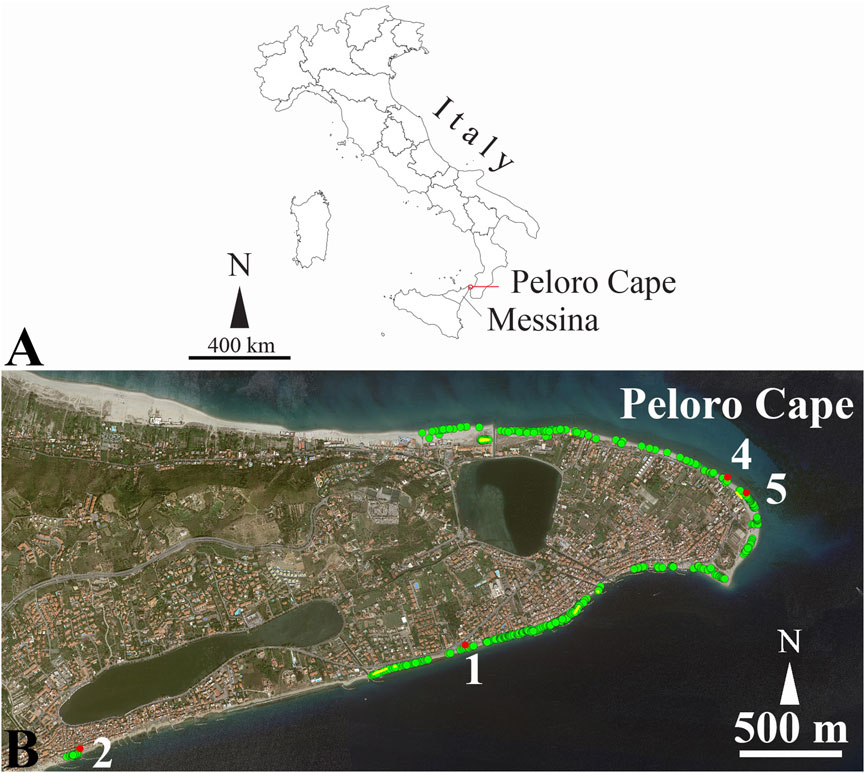
Figure 1. Fragments of ACM waste found along the beaches of the Cape Peloro peninsula in the Messina Straits. The study area extended from the “Beach of the Carabiniere” (latitude: 38°16′20.03″N; longitude: 15°37′53.07″E) on the Tyrrhenian coast to Canal Carmine (latitude: 38°15′39.94″N; longitude: 15°37′37.33″E) on the Messina Straits. (A) Localization of the study area. (B) Green dots indicate the fragments of ACM waste (n. 520); red dots indicate the four organic encrusted fragments shown in Figures 5, 6, 9, 13.
Asbestos cement is composed of Portland cement (CaCO3), with asbestos fibers appearing as white and bluish millimetric tufts. These were identified (Figure 2) as chrysotile (also known as white asbestos) and crocidolite (also known as blue asbestos) within the matrix of the ACM fragments, distinguishable to the naked eye after prior identification (Somma et al., 2024). Due to the hazardous nature of the asbestos-bearing cement, a photographic survey (Nikon Z30 camera) of the detected ACM waste was conducted directly in the field to preliminarily assess the morphology and eventual evidence of biocovering, bioerosion, and weathering processes. Based on the photographic survey, 400 frames were specifically analyzed to detect eventual traces of biotic encrusting, distinguishing the largest (≥10 cm) from the smallest (<10 cm) fragments. Moreover, a sampling of 50 randomly collected fragments was carried out safely for further observations. In the laboratory, since all fragments were flattened, both sides were photographed for preliminary assessing their morphology, along with the extent of biological alteration and weathering. The general shape, roundness, and consumption grade were reported, and the maximum size was measured using a Vernier caliper (±1 mm). Moreover, the same fragments were examined under a stereomicroscope (Zeiss, Stereo Discovery V20 model), coupled with a telecamera and workstation. Three Zeiss objectives (from ×3.8 to ×75 with optical zoom and resolution of 2.3 µ; from ×11.2 to ×225 with optical zoom and resolution of 0.8 µm; and from ×26 to ×530 with optical zoom and resolution of 0.4 µm) were used, keeping the two sides distinct. In such a phase, traces of biological activity, of both terrestrial and marine organisms, were detected and quantified. The main colonizing organisms were identified based on their calcareous remains, at least to broad categories (e.g., crustose algae, bryozoans, and serpulid polychaetes), and to the species level whenever possible. Scientific nomenclature was updated according to the WoRMS database (https://www.marinespecies.org/).
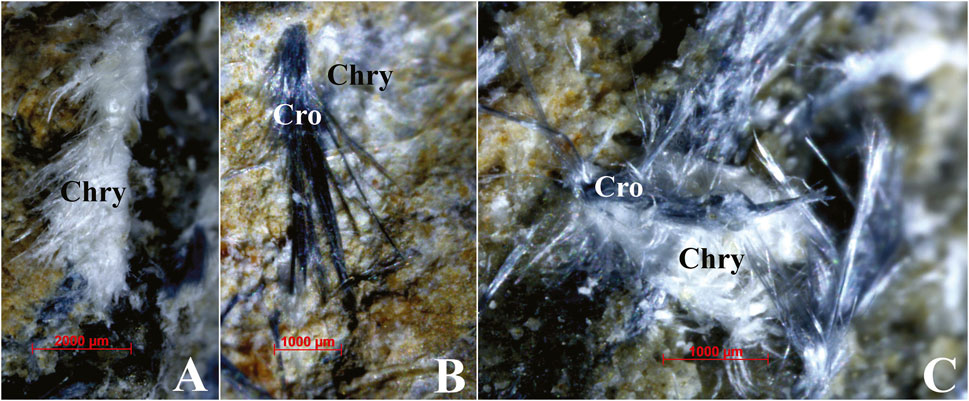
Figure 2. White and bluish millimetric tufts of asbestos fibers of the studied ACM surface wastes, observed under stereomicroscope. (A) Typical woolly appearance of chrysotile fibers. (B) Crocidolite fibers lying on chrysotile fibers. (C) Intertwining of chrysotile and crocidolite fibers. Acronyms: Chry, chrysotile; Cro, crocidolite. See text for detailed discussion.
Results
The ACM fragments were distributed in the study area unevenly, with the major amounts present on the Ionian coast of the Messina Straits (Figure 1). Such beached fragments, ranging from centimetric to decimetric size, all showed flattened shapes with a minimum thickness of less than 3 mm, coupled with well-rounded and elongated profiles. In the largest fragments, a concave and a convex side (due to the undulated shape of the original material), were recognized, occasionally maintaining the typical crossed textures.
Of the 400 frames examined in detail, 140 specimens were characterized by a length ≥10 cm. Among them, only 59 (42% of the sample) showed traces of colonization, ranging from easily recognizable encrusting organisms, such as serpulid polychaetes, to simple calcareous patinas. Among the 260 remaining fragments with a length ≤10 cm, only 68 (26%) showed calcareous remains. It should be noted that in addition to the ACM fragments, many other anthropogenic platy fragments rich in CaCO3 (bricks) showed bioencrustation.
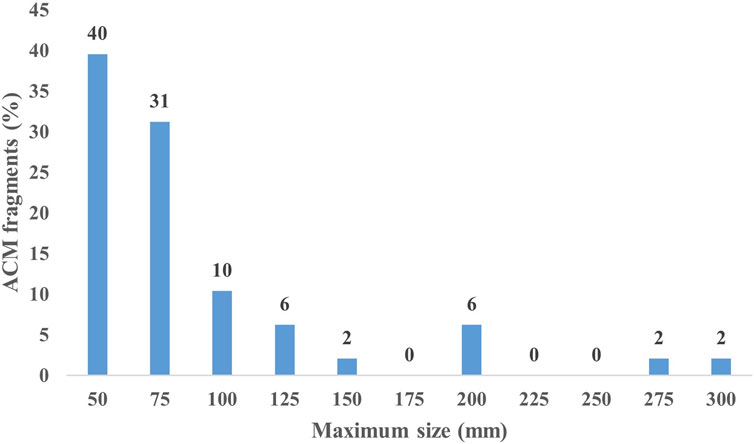
Most of the ACM waste fragments (79%) showed both surfaces totally abraded, with a prevalent abrasion extension on the convex sides. A single fragment, 75 mm long, locally maintained traces of the original texture with a honey-comb texture. Among the 50 collected fragments, only 48 were analyzed in the laboratory due to the accidental crumbling of two. The fragment sizes, ranging from 30 mm to 280 mm, showed a clear decreasing trend in the frequency of size classes (25 mm spaced) from 50 mm (40%) to 150 mm (2%), with occasional records of fragments larger than 150 mm (Figure 3).
Subaerial biological corrosion
Despite the highly abraded surfaces of the fragments, 42% of the entire sample showed extensive traces of biological corrosion—27% on both sides and 15% on the concave surface. Although clearly recognizable remains of subaerial organisms were not found, traces of their biological activity were frequently recorded. Due to their peculiar appearance, such traces of corrosion were attributed to the weathering activity of lichens (Figure 4), recognizable in the glaze of non-fibrous minerals as background and radial engraves left by mycelium, as documented both in asbestos cement (Favero-Longo et al., 2009) and other porous substrates (De La Rosa et al., 2013).

Figure 4. Surface of beached ACM waste fragment altered by subaerial organic corrosion, observed under stereomicroscope. See text for detailed discussion.
Marine benthic colonizers
Abundant remains of marine benthic colonizers were especially recognized in the 10 largest-sized fragments. The concave surfaces were, in general, more extensively colonized than the convex surfaces, ranging from 40% to 100% (average: 65% ± 30) and from 1% to 100% (average: 35% ± 45), respectively. The remains of different organisms showed a patchy distribution on the fragment surface, close to emerging tufts of both chrysotile and crocidolite fibers (Figure 2). Almost exclusively, carbonate skeletons were recorded, and the zoobenthic fraction was highly prevalent. Bryozoan colonies covered most of the surface, followed by serpulids and vermetid gastropods (Figures 5, 6A,A’). Rare encrusting rhodophytes mostly occurred on the concave fragment surface, occasionally maintaining traces of their original pigmentation (Figures 5A,A’).
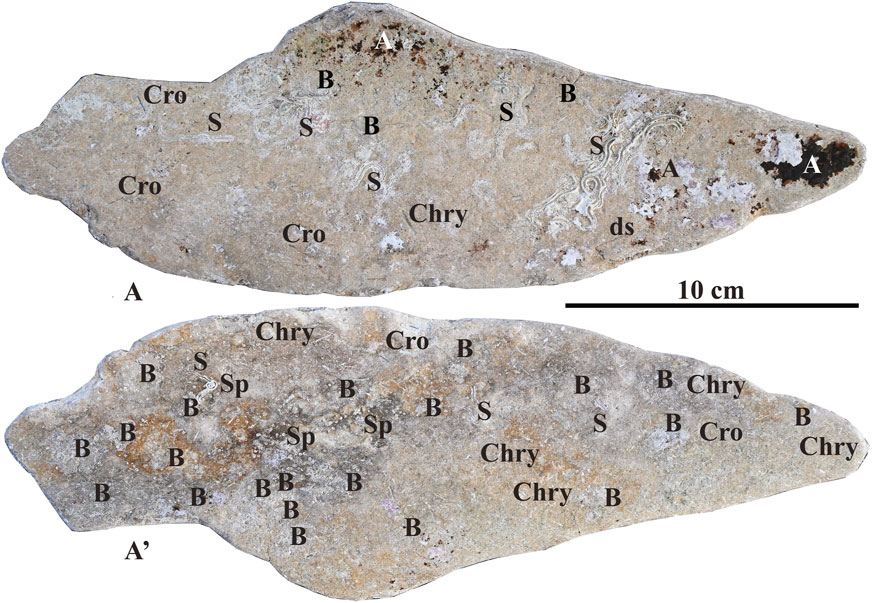
Figure 5. Fragment no. 4 of beached ACM waste encrusted by benthic organisms. (A) Concave side; (A’) convex side. Acronyms: A, encrusting red algae (see details in Figure 7); B, bryozoans; Chry, chrysotile; Cro, crocidolite; S, serpulids (see details in Figure 8A); Sp, spirorbids. For the localization of the fragment, see Figure 1. See text for detailed discussion.
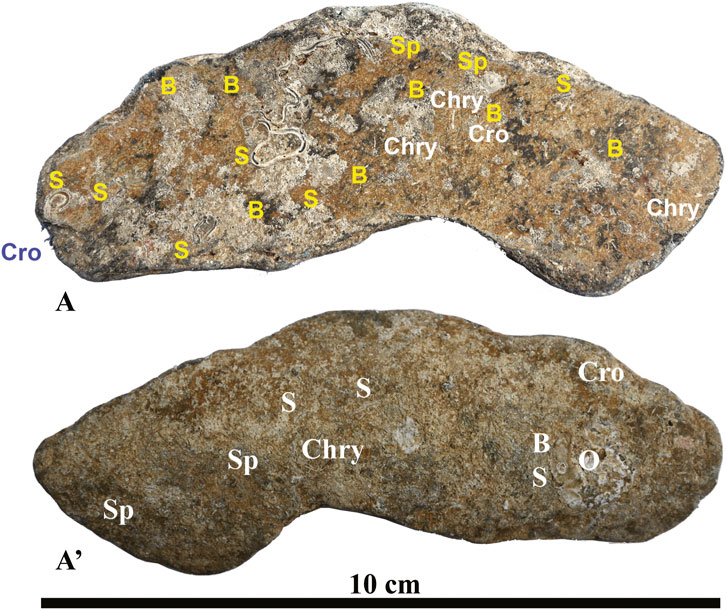
Figure 6. Fragment no. 5 of beached ACM waste encrusted by benthic organisms. (A) Concave side; (A’) convex side. Acronyms: B, bryozoans; Chry, chrysotile; Cro, crocidolite; O, Ostrea sp. (see details in Figure 10B); S, serpulids; Sp, spirorbids. For the localization of the fragment, see Figure 1. See text for detailed discussion.
Algal thalli
Brown-pinkish remains of algal thallus recognizable as laminar and encrusting rhodophytes were occasionally found on the ACM fragments, ranging from conspicuous mats to pigmented halos (Figures 5A,A’, 7). In general, the algal-covered surface was centimetric, with less coverage on the convex side than on the concave side. Due to their poor state of preservation and high susceptibility to abrasion, they could not be further identified, except for a single specimen, which was attributed to an unidentified calcareous red alga of the subclass Corallinophicydae (Figures 5A, 7).
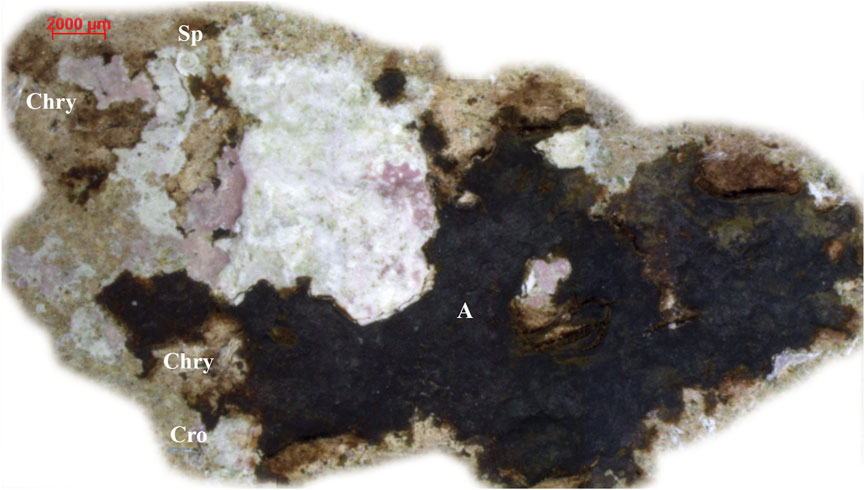
Figure 7. Remains of crustose red algae on the concave side of ACM fragment no. 4 (observed under stereomicroscope). Acronyms: A, algae (details from Figure 5A); Chry, chrysotile; Cro, crocidolite; Sp, spirorbid polychaete (details from Figure 5A). For a better image restitution, the algae image was cut out from the surrounding substrate using image treatment software. For the localization of the fragment, see Figure 1. See text for detailed discussion.
Serpulid polychaetes
Whitish calcareous tubes of serpulid polychaetes were found on both sides of the ACM fragments, locally in dense aggregations (up to 10% cover). Largest calcareous tubes, when not heavily eroded and damaged by abrasion, were recognizable as belonging to the genera Pomatoceros, Hydroides (Figure 8A), and Serpula (Figures 8A,B). Their conservation grade only occasionally allowed classification at the species level, namely, for Serpula vermicularis (Figure 8B). Although large serpulid species affected relatively wide surfaces, the total cover by the millimetric spirorbids was very limited, despite their abundance (up to 100 on a single fragment side). Due to the lack of some diagnostic characteristics, primarily the operculum, it was not possible to classify them with certainty at the species level. However, with some uncertainty due to the variable degree of conservation, Pileolaria cf. berkeleyana (Rioja, 1942) (Figures 8C,D) was the most frequently recorded species, accounting for almost 40% of spirorbid specimens in all samples. Other frequent species, reported in this study in dubitative terms, were Vinearia cf. koehleri (Caullery and Mesnil, 1897) (Figure 8E), Janua cf. heterostropha (Montagu, 1803) (Figure 8F), and Neodexiospira cf. pseudocorrugata (Bush, 1905) (Figure 8G). Several specimens of Josephella cfr. marenzelleri (Caullery and Mesnil, 1896) (Figure 8H) were also recognized despite their minute and fragile tubes. Moreover, highly altered tubes of non-determinable filograninae (Figure 8G) were rarely recorded.
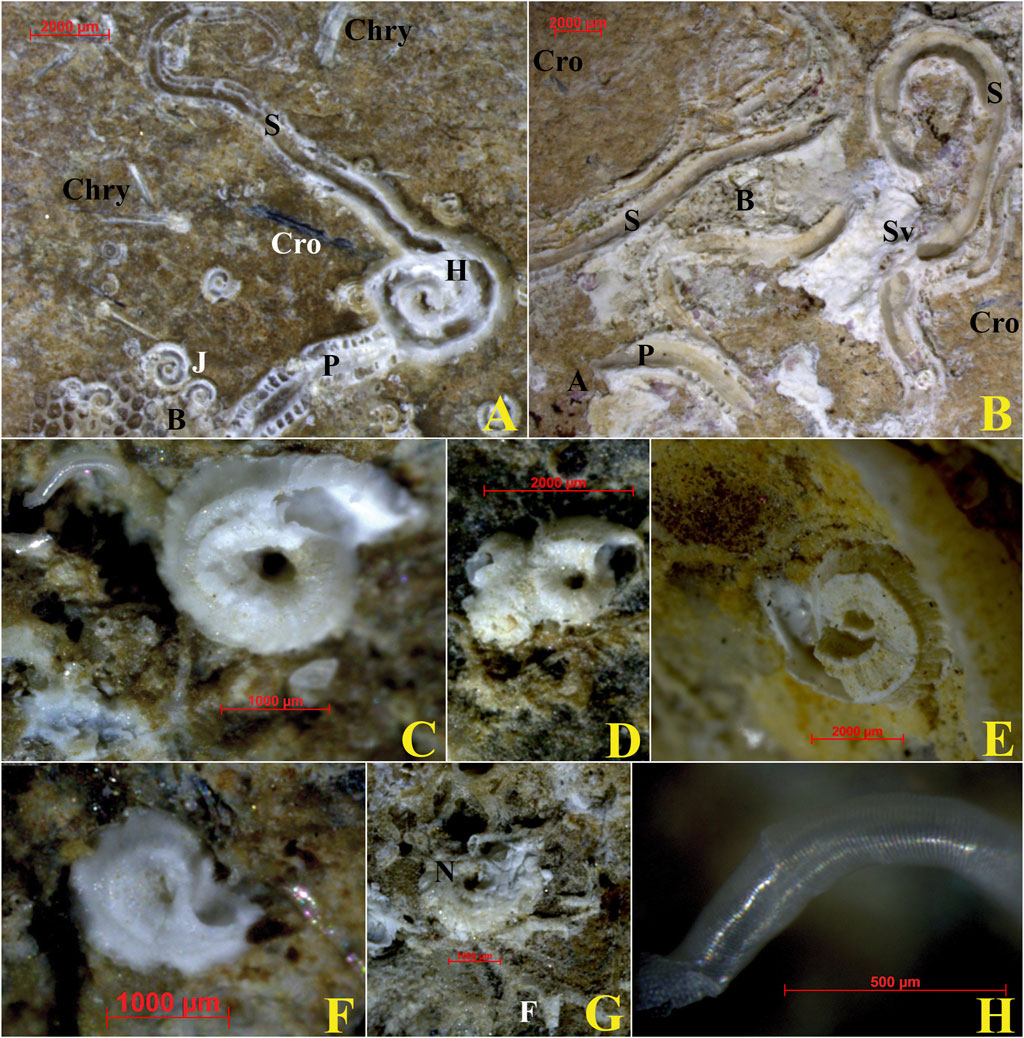
Figure 8. Encrusting serpula on the concave side of ACM fragment no. 4 with evidence of chrysotile and crocidolite fibers (observed under stereomicroscope). (A) Abraded remains of Pomatoceros sp. and Hydroides sp. tubes. Specimens of the spirorbids Janua (Dexiospira) pagenstecheri overgrown by a bryozoan colony are also recognizable. (B) Fragment of the Serpula vermicularis tube, along with altered remains of Pomatoceros sp. and Serpula sp. tubes, associated with bryozoan and algae residuals. (C) Pileolaria cf. berkeleyana. (D) Pileolaria cf. berkeleyana (Rioja, 1942). (E) Vinearia cfr. koehleri. (F) Janua cf. heterostropha. (G) Neodexiospira cf. pseudocorrugata and remains of Filograninae tubes. (H) Josephella cfr. marenzelleri. Acronyms: A, algae; B, bryozoan; Cro, crocidolite; F, filograninae; H, Hydroides sp.; J, Janua (Dexiospira) pagenstecheri; N, Neodexiospira cf. pseudocorrugata; P, Pomatoceros sp.; S, Serpula sp.; Sv, Serpula vermicularis. For the localization of the fragment, see Figure 1. See text for detailed discussion.
Mollusks
Shells of the encrusting gastropod Vermetus triquetrus were found only once, counting five specimens settled on the concave side of an ACM fragment, with a single and highly abraded specimen recognized on the convex side of the same fragment. The specimens, showing different grades of abrasion, were also overgrown by numerous spirorbids, belonging to the same colony, densely covering the ACM fragment surface (Figures 9A, A’). The best-preserved V. triquetrus specimen (Figure 9A) is shown in detail in Figure 10A. The size of the gastropod ranged from 8 mm to 10 mm. Bivalves occurred only once, represented by the cemented left valva of a heavily corroded Ostraea sp. (Figure 10B).

Figure 9. Fragment no. 2 of beached ACM waste encrusted by benthic organisms. (A) Concave side; (A’) convex side. Acronyms: S, spirorbids; V, vermetid (Vermetus triquetrus) (see details in Figure 10A). For the localization of the fragment, see Figure 1. See text for detailed discussion.
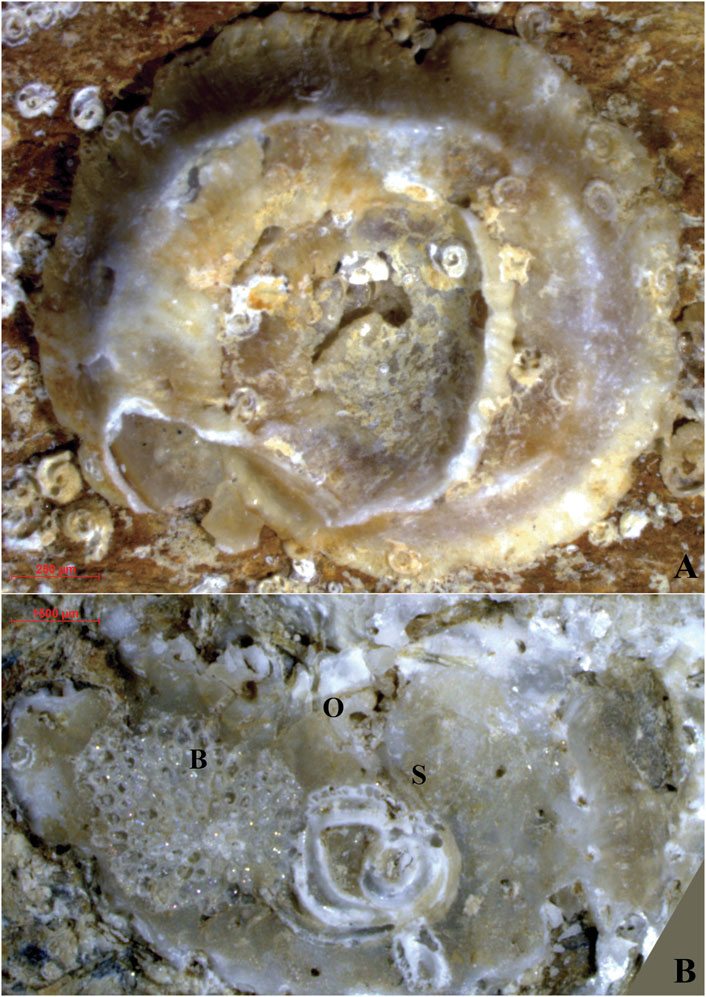
Figure 10. Calcareous remains of encrusting mollusks (observed under stereomicroscope). (A) Vermetus triquetrus from fragment no. 2 on the concave side (details from Figure 9A). (B) Cemented left valva of Ostraea sp. from fragment no. 5 convex side (Figure 6A’; the image was rotated of 90° with respect to Figure 6A’). Acronyms: B, bryozoan; S, Serpula sp.; O, Ostraea sp. For the localization of the fragment, see Figure 1. See text for detailed discussion.
Bryozoans
Skeletons of bryozoans (Figures 11A,B) were recognized on both sides of the ACM fragments, covering from 5% to 30% of the available surface. Most colonies appeared abraded to various grades (Figures 11C,D), making it difficult to associate the observed different morphologies precise taxa, except for few well-preserved colonies recognizable as Reptadeonella violacea (Johnston, 1847) (Figure 11B).
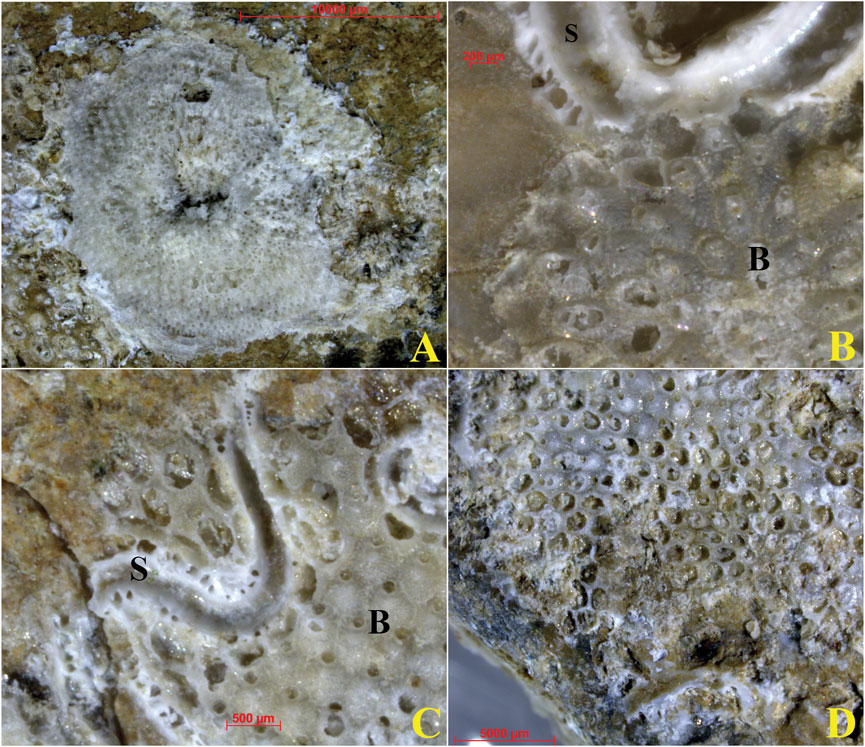
Figure 11. Calcareous remains of encrusting bryozoans (observed under stereomicroscope). (A) Discoporella sp. (B) Bryozoan Reptadeonella violacea is recognizable in the lower right corner of the image. (C) Bryozoan overgrowths on serpulid. (D) Strongly eroded bryozoan colony. Acronyms: B, bryozoan; S, Serpula sp. See text for detailed discussion.
Encruster interaction
Encrusting biota mostly occurred as heterogeneous aggregates, often stratified or at least overlapping, showing indications of a biological succession. Among the observed serpulids, some specimens of Janua (Dexiospira) cf. pagenstecheri spirorbids appeared incorporated in an overgrowing bryozoan colony (Figures 8A, 12A,B). Other specimens, belonging to the species Janua cf. heterostropha, appeared in close contact with older remains of serpulid Hydroides sp. (Figure 8A). The whole association thus showed indications of a clear successional pattern with different overlapping phases, including serpulids as primary colonizers, followed by bryozoan colonies as secondary colonizers. Bryozoan overgrowth on spirorbids of the genus Janua (Figures 8A, 12A,B) was an almost constant condition in the studied ACM waste fragments, whose accidental detachment locally determined asbestos fiber exposure (Figure 12B). The role of large serpulid polychaetes and bryozoans among the primary to secondary colonizers (Figures 12C–F) is confirmed by the successional pattern shown in Figures 12C–E which documents their overgrowth on the inner surface of a dead ostreidae bivalve, encrusting the concave side of fragment 4 (Figures 5A, 12C). The timing of algal settlement remains unclear; although it was usually recorded on bare substrates, but it was also observed on bryozoan remains and other bioconcretions (Figure 12D). Moreover, contrasting processes of ACM biocovering and bioerosion/abrasion were documented (Figure 12). A singular aggregation of serpulids, widely cementing beach-sand lithoclasts (biotite and quartz) and covering almost 60% of the concave surface of fragment no. 1, was also recognized (Figures 13A,A’, 14A–C).
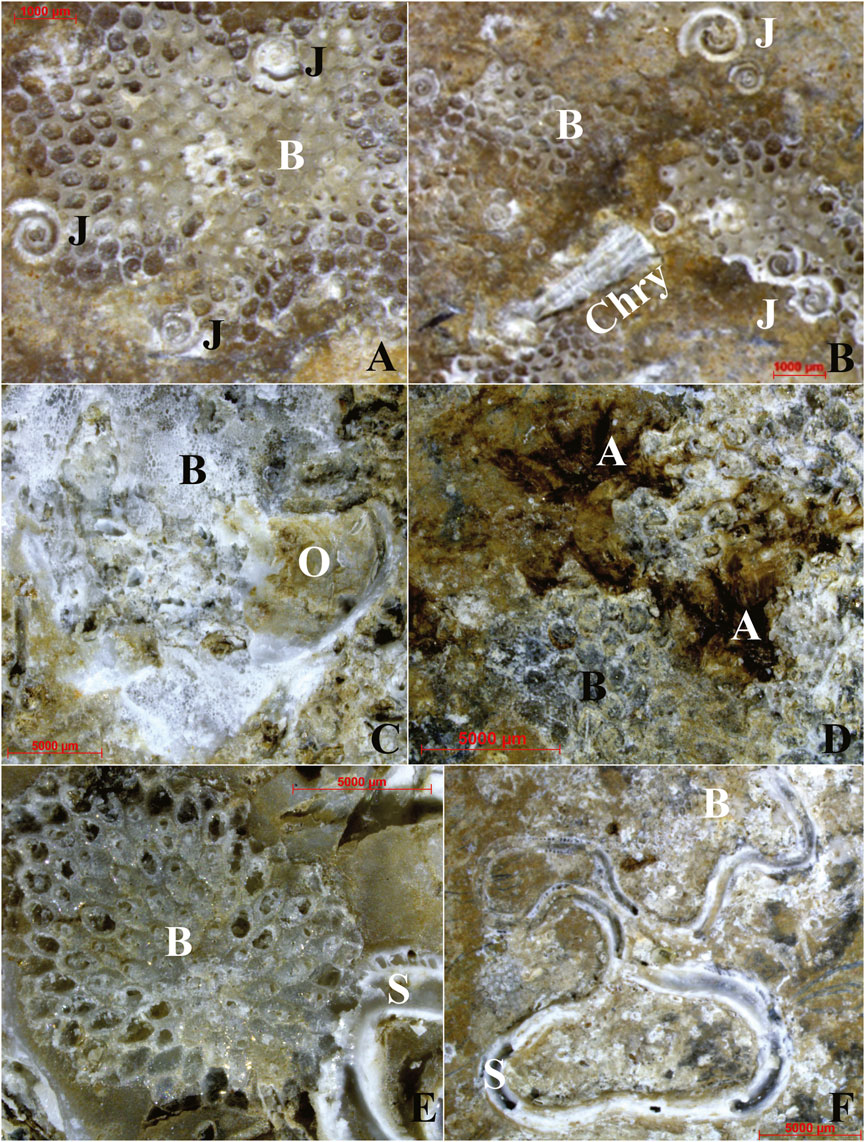
Figure 12. Successional patterns with different overlapping generations of calcareous remains of encrusting marine organisms, observed under stereomicroscope. (A,B) Remains of abraded Janua sp. tubes associated with residual bryozoan colonies. Surfacing asbestos fibers of chrysotile are also recognizable. (C) Bryozoan colony and serpulid tube growth on the inner surface of a dead ostreidae valva. (D) Evidence of biocovering and bioerosion of ACM waste fragment. Not determined algal thalli associated with bryozoan colonies. (E) Inner surface of a dead ostreidae valva biocovered by a serpulid tube. Both of them are biocovered by a bryozoan colony. (F) Serpulid growth on abraded bryozoan colony. Acronyms: A, algae; B, bryozoan; Chry, chrysotile; J, Janua (Dexiospira) pagenstecheri; S, Serpula sp.; O, Ostreidae. For the localization of the fragment, see Figure 1. See text for detailed discussion.

Figure 13. Fragment no. 1 of beached ACM waste encrusted by benthic organisms (see details in Figure 14). (A) Concave side; (A’) convex side. Acronyms: B, bryozoans; Chry, chrysotile; Cro, crocidolite; ds, degradation surface; S, serpulids; Qz, quartz (see details in Figure 14A). For the localization of the fragment, see Figure 1. See text for detailed discussion.
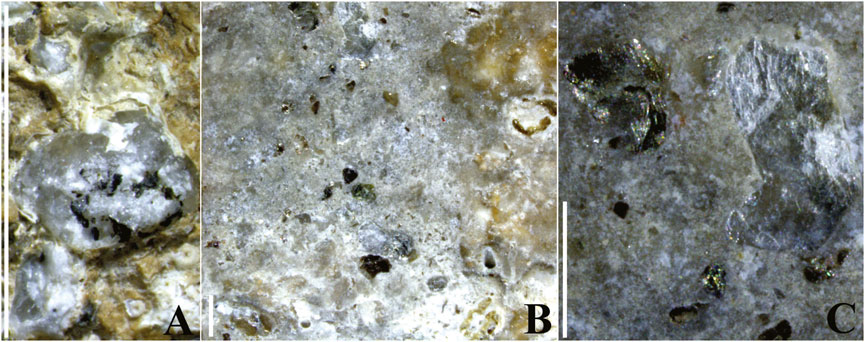
Figure 14. Beach sand lithoclasts included by the biocovering carbonates secreted by serpulids on the concave surface of fragment no. 1 (Figure 13A) (observed under stereomicroscope). (A) Polymineral lithoclasts composed of quartz with biotite. (B) Lithoclasts included by biocovering carbonates secreted by serpulids. (C) Monomineral lithoclasts composed of biotite (on the left) and white mica (on the right) included in the carbonates. Vertical scale bar: 2 mm. For the localization of the fragment, see Figure 1. See text for detailed discussion.
Discussion
Biodeterioration, along with the opposite yet concurrent bioprotection processes affecting man-made structures, has been extensively investigated as a natural phenomenon in the marine environment. Biota such as algae, bivalves, and gastropods notoriously act in directly and indirectly damaging concrete substrata; at the same time, these same organisms may also play protective functions so that the balance of benefit or detriment deriving from biotic action on such materials is increasingly debated (Bone et al., 2022; Chen et al., 2024). Due to a preeminent interest in marine geology and engineering, successional processes on bare concrete substrates have been widely investigated, confirming that approximately 90 days are sufficient for the early production of bacterial biofilm to be followed by an appreciable colonization by sessile fauna and some macroalgae. Bryozoans, sponges, and serpulids (mainly spirorbids) are precocious colonizers, making it difficult to generalize their exact successional sequence (Georges et al., 2021). In contrast, since ACMs are not used in marine constructions, there is no literature reporting on their biodegradation processes, except for the aforementioned investigations on biofouling (Jackson and Buss, 1975; Relini et al., 1994; Meyer-Kaiser et al., 2019), which, in general, confirm the successional trends observed in concrete materials (Bone et al., 2022; Georges et al., 2021; Chen et al., 2024) and reported in this study.
Sources of contamination
Flat and undulated shapes of the beached ACM wastes and the honey-comb texture of fragment surfaces make them easily recognizable as building materials, namely, undulated slabs for roofing. These slabs were widely used during the 1960s and 1970s along the coastal plains and the hills surrounding Messina as roofing for small buildings, including rudimentary cottages, garages, and fishermen’s or farmers’ sheds and shacks. Although most of them were demolished, some residual roofs with extensive lichen colonization, among which the peculiar yellow-orange-colored Candelariella sp., can still be observed.
These findings, consistent with the recognized role of lichens in weathering asbestos cement materials and reducing fiber release (Ervik et al., 2021; Łuniewski et al., 2024), support the hypothesis that the traces found on stranded ACM waste represent an initial step of subaerial organic corrosion. This process likely left behind a bared but less-corrodible substrate after the decay of the lichen thallus.
The source areas of the beached ACM waste, searched in the surrounding areas, were easily identified as demolition waste dumps on the backshore near the accumulation zones. Subsequent coastal erosion may expose this waste along the scarps and facilitate its accumulation at the base, further supplied by irresponsible ACM waste disposal on the backshore.
Once ACM waste reached the shoreline, the ACM fragments could be easily transported underwater by sea waves, as proven by their detection in shallow waters and the observation of ACM waste encrusted by marine organisms. Such bioconcreted fragments, when transported landward by storms as beached ACM, indicate that shallow marine environments can serve as secondary sources of contamination.
Biotic interactions
Among the beached ACM fragments, a high percentage showed evidence of a submerged phase sufficiently long (at least 90 days) to allow the settlement of encrusting marine organisms, even determining competitive processes. The real extent of such a submerged phase, however, is certainly underestimated, considering the powerful abrasive action of the waves and the poor resistance of ACM. In this respect, the greater incidence of encrusting organisms found in large fragments may be explained by several concomitant causes, such as their preferential settlement and survival on more stable, platy substrates and the availability of larger surfaces, not excluding the possibility that small ACM fragments are the result of prolonged reworking of larger, originally colonized blocks.
As for the attractiveness of ACM for biotic colonization, it can be similar to that of any other porous platy substrates, especially those that are totally or partially calcareous; however, its fragility and the ease of fiber detachment can hinder the settlement and persistence of epibiota.
If the colonized surfaces of beached ACM waste reflect alternating phases of aerial and wave exposure, shallow-water residence, and subsequent beaching, then marine sessile encrusters with mineralized skeletons may provide additional and more detailed insights into the marine-submerged continental wastes, as suggested by overgrowth characteristics and other interactions (Taylor and Wilson, 2003). ACM fragments are particularly suitable for this purpose due to their small size, flat shapes, and fragile structures, recalling other, better investigated substrates, such as the shell surfaces (Rosso and Sanfilippo, 2005). A peculiarity of the ACM-stranded fragments is the temporal sequence of colonization, starting from the subaerial environment where lichens acted as primary colonizers capable of modifying the texture and mechanical properties of the ACM surface. Once asbestos fibers are converted into less hazardous amorphous and non-fibrous silica debris (Favero-Longo et al., 2005; Turci et al., 2007), ACM becomes more available for subsequent colonization. This partially inert material, once it enters the marine environment, will easily undergo a new primary succession. The stages of this process have been recognized in some beached ACM fragments, according to the observed biota overgrowth on skeletons of previous, already dead encrusting organisms. In general, opportunistic, short-lived spirorbids acted as pioneer species, as is typical in some impacted environments (Danovaro et al., 2018; Tamburini et al., 2022). The settlement of bryozoans might be immediately subsequent, which is different from some literature studies reporting an ensemble of spirorbids, bryozoans, and oysters as synchronous early colonizers (Relini et al., 1994; Stark, 2008). Bryozoans, along with serpulid polychaetes and vermetid gastropods, appeared as later colonizers than spirorbids, likely taking advantage of their larger size and longer lifespan. However, since the observations have been carried out on dead assemblages, it was difficult to determine whether a certain biota had overgrown on the skeleton of an already dead organism, rather than whether different species interacted after simultaneous settling. Notwithstanding, in a few cases, the differential preservation state of skeletobionts coupled with the position of the overgrowing biota unequivocally testified for a sequential settlement. An example of a three-step succession is shown in Figure 10B, 11B, where a well-preserved Bryozoan colony is growing on the margin of an abraded serpulid tube, which, in turn, colonized the inner valve of a dead oyster. According to some studies (Gappa, 1989; Rosso and Sanfilippo, 2005), early-settling spirorbids appeared particularly prone to overgrowth, especially by bryozoans. Nevertheless, the settlement of spirorbids on secondary substrata as an effect of repeated recruitment has also been recorded, for example, on Vermetus triquetrus shells (Figures 9A, 10A). Elsewhere, evidence of spatial competition between different encrusting organisms was recognized, especially involving large bryozoans, which appeared to be the most long-lived colonizers of the studied ACM waste substrates. These bryozoans often surrounded and then incorporated smaller spirorbids, which, however, were able to persist (Figures 12A,B), as also reported by Stebbing (1973). As far as the role of algae and their contribution to the colonization process are concerned, it may be largely underestimated. Only calcareous corallinaceans left recognizable traces of their settlement and growth. Finally, traces left by terrestrial organisms (notably lichens), along with remains of marine encrusting invertebrates and plants (algae, mollusks, polychaetes, and bryozoans), and signs of erosive processes recognized in the studied marine-beached ACM fragments, provided evidence of repeated displacements between continental (sub-aerial) and shallow marine environments and vice versa. Traces of the first subaerial colonization were rare (Figure 4), but the effects of oxalic acids released by lichens, in converting ACM into amorphous and non-fibrous matter could have facilitated the subsequent settlement by marine benthic colonizers. Although early underwater colonization of ACM waste is scarcely described in the literature, it may be assumed that early microbial biofilm production could play a fundamental role in attracting benthic larvae and propagules (Hadfield, 2011). It is known that asbestos cement materials may be particularly attractive for benthic diatoms (Nenadović et al., 2015), which may form a first autotrophic layer. Such behavior may explain the reason why larval asbestos cement-made collectors resulted in oyster spatfall more efficiently than other carbonate and wooden devices (Hartati and Suryono, 2000). In the Mediterranean Sea, where diversified communities have been described for experimental asbestos cement-made collectors, the colonization process appeared rapid, and a relative stabilization of the sessile communities after the first year of immersion was documented (Relini et al., 1994; Relini et al., 1998).
The studied beached asbestos fragments, once exposed to subaerial weathering, may have rapidly lost most of the associated biota since they are easily detachable or rapidly decomposed. Nevertheless, carbonate remains of encrusting organisms, due to their stronger adhesion to the substrate and greater resistance to abrasion for a relatively long time, persisted. Among them, encrusting bryozoan remains were frequently found, such as the identified R. violacea (Figure 11B). Such species, which have also been reported from marine biofouling on beached plastic debris (Subías-Baratau et al., 2022), may colonize the sciaphilous lower side of boulders (Canessa et al., 2020), thus explaining the observed prevalence of this biota on the ACM waste concave side. Bryozoans, as major biota of ACM biofouling, may help understand the spatio-temporal evolution of the colonized substrates. It is known that in the first stages of growth, encrusting bryozoans appear prevalent at shallow depths, but after 12–25 months, these biota are dramatically replaced by “tuft-like types,” namely, erect, ramified, and flexible zoaries (Pisano and Boyer, 1985). In this respect, the occurrence of exclusively encrusting bryozoans on beached ACM waste may suggest that the substrate materials remained under shallow marine conditions for less than 1–2 years. In contrast, the occurrence of beached ACM waste of more complex associations of algae, mollusks, bryozoans, and polychaetes may suggest evidence of a wider spatio-temporal evolution, probably over a timespan of several decades. Repeated phases of less than 1–2 years of staying of the substrate material in shallow marine environments could have alternated, as a consequence of storm events, with periods of subaerial staying along the marine shoreline. Wave action, in general, is certainly a primary force in determining the biological colonization success on ACM fragments with the related consequences, as proven by the lack of encrusters on fragments smaller than decimetric size. Biota propagules select substrates based on their chemical and physical characteristics rather than size. A small substrate, however, does not allow an effective settlement because it is unstable. It follows that the smallest fragments of ACM, once they reach the marine environment, cannot be rendered inert by biological action, being much more susceptible to wear and the release of hazardous fibers than the largest fragments.
Environmental implications
The renowned Mediterranean coasts, whose high recreational and touristic values are globally recognized, should be healthy areas, properly managed and monitored by competent authorities. Unfortunately, from northern to southern Italy, even renovated and touristic beaches are also affected by beached ACM waste, making coastal contamination an increasingly critical issue along much of the Italian coastline (Lisco et al., 2023; Somma et al., 2024). It follows that the sites affected by environmental and health risks from asbestos exposure should be reclaimed everywhere to avoid damage to humans and ecosystems.
Based on this, it is imperative that the safety and health of these environments must be guaranteed, especially for particularly sensitive beach stakeholders, such as children and elderly people. Reclamation activities cannot consist of mechanical removal of the ACM fragments on the beach as fragments have been found in the sediments at different depths and the shallow marine sediments. On the other hand, the mechanical removal of fragments on the beaches, beyond being ineffective, is also risky because this activity can cause the fiber release.
In any conceptual model aimed to a correct planning of remediation activities, knowledge of the texture and the related organic encrustations of the beached ACM waste can play a basic role. Such a possible model should consider the following information:
i. Sedimentological characterization of the beach deposits.
ii. Thickness of the beach deposits determined using geophysical surveys or drillings.
iii. Identification of the main coastal physiographic units.
iv. Characterization of the coastal dynamics, including the closure depth (i.e., seaward boundary of the active coastal zone).
v. Annual monitoring of marine coastal hydrodynamics.
vi. GIS mapping of the beached organic encrusted and non-encrusted ACM waste on the beaches.
vii. GIS mapping and depth, whenever possible, of the ACM waste contained in the beach deposits along natural or artificial trenches.
viii. GIS monitoring of the beached ACM waste on the beaches after storms, also using GPS microchip technology applied to ACM.
ix. GIS mapping of the primary sources of ACM contamination onshore.
x. GIS mapping of the secondary sources of ACM contamination offshore through scuba diving surveys.
xi. Search for possible contiguity of coastal areas with the contamination sources.
Concluding remarks
Among the asbestos cement materials stranded along the Cape Peloro coasts, fragments colonized by encrusting marine biota testified to their transitional residence in the shallow water environment. On the same fragments, residual traces of lichen thalli suggested a continuity in the ACM alteration by subaerial and underwater biota. The submerged phase, whose extent may be evaluated over a timespan of several decades on the basis of the observed biotic successional processes, was preluding to subsequent washing up on the subaerial beach environment, as suggested by the abrasion grade of the biota calcareous remains, in accordance with local high-energy marine conditions. Some evidence suggests that lichen colonization, in addition to contributing to inertization of asbestos materials in the continental environment, may facilitate the settlement of marine encrusting biota once the waste reaches the marine environment. The calcareous skeletons of benthic colonizers, in turn, can significantly reduce the breakdown of fragments reaching shallow water marine environments. Marine biota, however, is generally unable to colonize the smallest fragments, which retain their hazardous nature due to the release of harmful asbestos fibers.
The time sequence of biological colonization processes was rather difficult to define and probably not generalizable. Very few traces of a possible algal colonization, except for some Rhodophyceae, were detected. Early colonizers, such as bryozoans and serpulid polychaetes, could also settle simultaneously, although overgrowth phenomena by colonies of encrusting bryozoans seemed to demonstrate their competitive advantage. Large encrustings, in turn, were secondarily colonized by several species of small spirorbids, which recruited repeatedly and successfully.
In conclusion, multiple interferences from uncontrolled ACM waste have affected both emerged and submerged coastal environments; these impacts need to be evaluated, particularly with regard to their threat to human health.
The results of the present investigation, which focused on the origin and fate of ACMs reaching the coastal environment, address the knowledge gap concerning a hazardous waste type that remains adequately studied. Consequently, effective remediation activities in coastal areas should consider the findings presented in this study, within the broader context of the complex processes that characterize coastal contamination.
Data availability statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Author contributions
SG: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review and editing. RS: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors are grateful to the reviewers and the academic editors for strongly improving the present paper.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Afgatiani, P. M., Herdanis, C., Wijaya, A. D., Kusuma, F. B., Dewi, E. K., Ulfa, A., et al. (2023). Spectral characteristics of marine debris in the coast of Padang city. AIP Conf. Proc. 2941 (1), 030003. doi:10.1063/5.0181394
Aronica, G. T., Brigandí, G., and Morey, N. (2012). Flash floods and debris flow in the city area of Messina, north-east part of Sicily, Italy in October 2009: the case of the Giampilieri catchment. Nat. Hazards Earth Syst. Sci. 12 (5), 1295–1309. doi:10.5194/nhess-12-1295-2012
Bone, J. R., Stafford, R., Hall, A. E., and Herbert, R. J. (2022). Biodeterioration and bioprotection of concrete assets in the coastal environment. Int. Biodeterior. and Biodegrad. 175, 105507. doi:10.1016/j.ibiod.2022.105507
Camplin, J. C. (2008). “Managing the new asbestos risks: amphiboles minerals and soils,” in ASSE Professional Development Conference and Exposition, Las Vegas, Nevada, ASSE-08-727.
Canessa, M., Bavestrello, G., Trainito, E., Navone, A., and Cattaneo-Vietti, R. (2020). Lithology could affect benthic communities living below boulders. J. Mar. Biol. Assoc. U. K. 100 (6), 879–888. doi:10.1017/S0025315420000818
Chen, X., Yu, C., Wang, L., and Yu, B. (2024). A comprehensive review of the bio-corrosion mechanisms, hydrodynamics and antifouling measures on marine concrete. Ocean. Eng. 310, 118696. doi:10.1016/j.oceaneng.2024.118696
Dąbrowska, J., Sobota, M., Świąder, M., Borowski, P., Moryl, A., Stodolak, R., et al. (2021). Marine waste-Sources, fate, risks, challenges and research needs. Int. J. Environ. Res. public health 18 (2), 433–33. doi:10.3390/ijerph18020433
D'Alessandro, F., Tomasicchio, G., Gencarelli, R., and Frega, F. (2008). “Design and verification of a contaminated material capping structure along the Adriatic coast, in the south of Italy,” in Proceedings International Conference on the Application of Physical Modelling to Port and Coastal Protection - Coastlab 08, Bari, 339–342.
Danovaro, R., Nepote, E., Martire, M. L., Ciotti, C., De Grandis, G., Corinaldesi, C., et al. (2018). Limited impact of beach nourishment on macrofaunal recruitment/settlement in a site of community interest in coastal area of the Adriatic Sea (Mediterranean Sea). Mar. Pollut. Bull. 128, 259–266. doi:10.1016/j.marpolbul.2018.01.033
De La Rosa, J. P. M., Warke, P. A., and Smith, B. J. (2013). Lichen-induced biomodification of calcareous surfaces: bioprotection versus biodeterioration. Prog. Phys. Geogr. 37 (3), 325–351. doi:10.1177/0309133312467660
Di Gregorio, S., Lupiano, V., Forno, F., Calidonna, C. R., Catelan, P., and Chidichimo, F. (2024). A transdisciplinary Analysis of the 2009 catastrophe in Giampilieri superiore by land use evolution over time. Earth Syst. Environ. 9, 505–517. doi:10.1007/s41748-024-00454-5
Ervik, T., Eriksen Hammer, S., and Graff, P. (2021). Mobilization of asbestos fibers by weathering of a corrugated asbestos cement roof. J. Occup. Environ. Hyg. 18 (3), 110–117. doi:10.1080/15459624.2020.1867730
Favero-Longo, S. E., Castelli, D., Fubini, B., and Piervittori, R. (2009). Lichens on asbestos-cement roofs: bioweathering and biocovering effects. J. Hazard. Mater. 162 (2-3), 1300–1308. doi:10.1016/j.jhazmat.2008.06.060
Favero-Longo, S. E., Turci, F., Tomatis, M., Castelli, D., Bonfante, P., Hochella, M. F., et al. (2005). Chrysotile asbestos is progressively converted into a non-fibrous amorphous material by the chelating action of lichen metabolites. J. Environ. Monit. 7 (8), 764–766. doi:10.1039/b507569f
Gappa, L. (1989). Overgrowth competition in an assemblage of encrusting bryozoans settled on artificial substrata. Mar. Ecol. Prog. Ser. Oldend. 51 (1), 121–130. doi:10.3354/meps051121
Georges, M., Bourguiba, A., Chateigner, D., Sebaibi, N., and Boutouil, M. (2021). The study of long-term durability and bio-colonization of concrete in marine environment. Environ. Sustain. Indic. 10, 100120. doi:10.1016/j.indic.2021.100120
Gilbert, O. L. (1971). Urban bryophyte communities in north-east England. Trans. Br. Bryological Soc. 6 (2), 306–316. doi:10.1179/006813871804146373
Gilbert, O. L. (1990). The lichen flora of urban wasteland. Lichenologist 22 (1), 87–101. doi:10.1017/S0024282990000056
Goodman, A., Walker, T. R., Brown, C., Wilson, B., Gazzola, V., and Sameoto, J. (2020). Benthic marine debris in the bay of Fundy, eastern Canada: spatial distribution and categorization using seafloor video footage. Mar. Pollut. Bull. 150, 110722. doi:10.1016/j.marpolbul.2019.110722
Hadfield, M. G. (2011). Biofilms and marine invertebrate larvae: what bacteria produce that larvae use to choose settlement sites. Annu. Rev. Mar. Sci. 3, 453–470. doi:10.1146/annurev-marine-120709-142753
Hartati, R., and Suryono, C. A. (2000). Oyster spatfall in Mlonggo Waters, Jepara, Indonesia. Phuket Mar. Biol. Cent. Spec. Publ. 21 (1), 183–186.
Jackson, J., and Buss, L. W. (1975). Alleopathy and spatial competition among coral reef invertebrates. Proc. Natl. Acad. Sci. 72, 5160–5163. doi:10.1073/pnas.72.12.5160
Jacoby, G. S. (2011). Asbestos exposure at the long beach naval shipyard; reproducibility and validity of self reported exposure. Irvine: University of California, 66.
Lakhal, S. Y., Khan, M. I., and Islam, M. R. (2009). An “Olympic” framework for a green de-commissioning of an offshore oil platform. Ocean and Coast. Manag. 52 (2), 113–123. doi:10.1016/j.ocecoaman.2008.10.007
Lisco, S., Lapietra, I., Laviano, R., Mastronuzzi, G., Fracchiolla, T., and Moretti, M. (2023). Sedimentological features of asbestos cement fragments in coastal environments (Taranto, southern Italy). Mar. Pollut. Bull. 187, 114469. doi:10.1016/j.marpolbul.2022.114469
Liu, N. (2013). Marine pollution: A. Protection of the marine environment from land-based activities. Yearb. Int. Environ. Law 24 (1), 243–247. doi:10.1093/yiel/yvu010
Łuniewski, S., Rogowska, W., Łozowicka, B., and Iwaniuk, P. (2024). Plants, microorganisms and their metabolites in supporting asbestos detoxification-A biological perspective in asbestos treatment. Materials 17 (7), 1644. doi:10.3390/ma17071644
Meyer-Kaiser, K., Bergmann, M., Soltwedel, T., and Klages, M. (2019). Recruitment of arctic deep-sea invertebrates: results from a long-term hard-substrate colonization experiment at the long-term ecological research observatory hausgarten. Limnol. Oceanogr. 64 (5), 1924–1938. doi:10.1002/lno.11160
Nenadović, T., Šarčević, T., Čižmek, H., Godrijan, J., Marić Pfannkuchen, D., Pfannkuchen, M., et al. (2015). Development of periphytic diatoms on different artificial sub-strates in the Eastern Adriatic Sea. Acta Bot. Croat. 74 (2), 377–392. doi:10.1515/botcro-2015-0026
Nicholls, R. J., Beaven, R. P., Stringfellow, A., Monfort, D., Le Cozannet, G., Wahl, T., et al. (2021). Coastal landfills and rising sea levels: a challenge for the 21st century. Front. Mar. Sci. 8, 710342. doi:10.3389/fmars.2021.710342
Paglietti, F., Malinconico, S., Bonifazi, G., Serranti, S., and Massaro, C. (2022). Classifying and managing of asbestos containing waste: Italian experience. Proc. World Conf. Waste Manag. 3 (1), 1–20. doi:10.17501/26510251.2022.3101
Pisano, E., and Boyer, M. (1985). Development pattern of an infralittoral bryozoan community in the western Mediterranean Sea. Mar. Ecol. Prog. Ser. 27 (1-2), 195–202. doi:10.3354/MEPS027195
Rajandu, E., Elvisto, T., Kappel, H. L., and Kaasik, M. (2021). Bryophyte species and commu-nities on various roofing materials, Estonia. Folia Cryptogam. Est. 58, 213–227. doi:10.12697/fce.2021.58.21
Relini, G., Tixi, F., Relini, M., and Torchia, G. (1998). The macrofouling on offshore platforms at Ravenna. Int. Biodeterior. and Biodegrad. 41 (1), 41–55. doi:10.1016/50964-8305(98)80007-3
Relini, G., Zamboni, N., Tixi, F., and Torchia, G. (1994). Patterns of sessile macrobenthos community development on an artificial reef in the Gulf of Genoa (northwestern Medi-terranean). Bull. Mar. Sci. 55 (2-3), 745–771. doi:10.1007/s10750-005-1133-1
Rizzo, L., Musco, L., and Crocetta, F. (2021). Cohabiting with litter: fish and benthic assemblages in coastal habitats of a heavily urbanized area. Mar. Pollut. Bull. 164, 112077. doi:10.1016/j.marpolbul.2021.112077
Rola, K., Osyczka, P., and Kafel, A. (2016). Different heavy metal accumulation strategies of epilithic lichens colonising artificial post-smelting wastes. Archives Environ. Contam. Toxicol. 70, 418–428. doi:10.1007/s00244-015-0180-5
Rosso, A., and Sanfilippo, R. (2005). Bryozoans and serpuloideans in skeletobiont communities from the Pleistocene of Sicily: spatial utilisation and competitive interactions. Sezione Museol. Sci. Nat., 115–124. doi:10.15160/1824-2707/361
Ryan, B., Kuhl, I., and Ware, R. (2014). Framework for handling asbestos after a tidal surge. J. Environ. Health 76 (6), 170–176.
Somma, R. (2024). Petrographic and textural characterization of beach sands contaminated by asbestos cement materials (Cape Peloro, Messina, Italy): hazardous human-environmental relationships. Geosciences 14 (6), 167. doi:10.3390/geosciences14060167
Somma, R., Giacobbe, S., La Monica, F. P., Molino, M. L., Morabito, M., Spoto, S. E., et al. (2024). Defense and protection of the marine coastal areas and human health: a case study of asbestos cement contamination (Italy). Geosciences 14 (4), 98. doi:10.3390/geosciences14040098
Stark, J. S. (2008). Patterns of higher taxon colonisation and development in sessile marine benthic assemblages at Casey Station, Antarctica, and their use in environmental monitoring. Mar. Ecol. Prog. Ser. 365, 77–89. doi:10.3354/meps07559
Stebbing, A. D. R. (1973). Observations on colony overgrowth and spatial competition. Living fossil Bryozoa, 173–183.
Subías-Baratau, A., Sanchez-Vidal, A., Di Martino, E., and Figuerola, B. (2022). Marine bio-fouling organisms on beached, buoyant and benthic plastic debris in the Catalan Sea. Mar. Pollut. Bull. 175, 113405. doi:10.1016/j.marpolbul.2022.113405
Tamburini, M., Occhipinti-Ambrogi, A., Vullo, M. L., and Ferrario, J. (2022). Biotic resistance of native fouling communities to bioinvasions could not be demonstrated by transplant experiments in Northern Italy. Mar. Pollut. Bull. 182, 113961. doi:10.1016/j.marpolbul.2022.113961
Taylor, P. D., and Wilson, M. A. (2003). Palaeoecology and evolution of marine hard substrate communities. Earth-Science Rev. 62 (1-2), 1–103. doi:10.1016/S0012-8252(02)00131-9
Turci, F., Favero-Longo, S. E., Tomatis, M., Martra, G., Castelli, D., Piervittori, R., et al. (2007). A biomimetic approach to the chemical inactivation of chrysotile fibres by lichen metabolites. Chemistry-A Eur. J. 13 (14), 4081–4093. doi:10.1002/chem.200600991
Vimercati, L., Cavone, D., Mansi, F., Cannone, E. S. S., De Maria, L., Caputi, A., et al. (2019). Health impact of exposure to asbestos in polluted area of Southern Italy. J. Prev. Med. Hyg. 60 (4), E407–E418. doi:10.15167/2421-4248/jpmh2019.60.4.1330
Keywords: asbestos cement materials, marine organisms, continental organisms, beach sands, environmental pollution
Citation: Giacobbe S and Somma R (2025) Spatio-temporal dynamics of beached asbestos cement wastes colonized by terrestrial and shallow marine organisms: new insights and environmental implications. Front. Earth Sci. 13:1570025. doi: 10.3389/feart.2025.1570025
Received: 02 February 2025; Accepted: 02 May 2025;
Published: 30 May 2025.
Edited by:
Jasmine Rita Petriglieri, University of Turin, ItalyReviewed by:
Alessandro Cavallo, University of Milano-Bicocca, ItalyDaniela Basso, University of Milano-Bicocca, Italy
Copyright © 2025 Giacobbe and Somma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Roberta Somma, cnNvbW1hQHVuaW1lLml0
 Salvatore Giacobbe1
Salvatore Giacobbe1 Roberta Somma
Roberta Somma