- 1Center for the Pan-Third Pole Environment, Lanzhou University, Lanzhou, China
- 2College of Earth and Environmental Sciences, Lanzhou University, Lanzhou, China
- 3Chayu Monsoon Corridor Observation and Research Station for Multi-Sphere Changes, Chayu, Xizang Autonomous Region, China
- 4Department of Earth Sciences, Graduate School of Science, Chiba University, Chiba, Japan
- 5State Key Laboratory of Cryospheric Sciences/Tien Shan Glaciological Station, Northwest Institute of Eco-Environment and Resources, Chinese Academy of Sciences, Lanzhou, China
Cryoconite granules are microbial aggregations formed by filamentous cyanobacteria with mineral particles on the bare ice surface of glaciers worldwide. Multiple species of filamentous cyanobacteria in cryoconite granules can usually be microscopically and phylogenetically identified. However, the roles of each species in the formation process of granules remain unclear. In this study, the compositions of filamentous cyanobacteria and minerals, as well as the microstructure of cryoconite granules of different sizes collected on a mountain glacier in central Asia, were analyzed. Three distinct morphological taxa of filamentous cyanobacteria (Types A, B, and C) were observed in cryoconite granules, with Types B and C being dominant across all size fractions. Scanning electron microscope observation revealed that Type B often had abundant small mineral particles on its filament surface, while Type C was mostly without or with only a few mineral particles. Abundant clay minerals were found in all size fractions of granules. Our results suggest that cyanobacterial taxa play a different role in binding mineral particles within cryoconite granules and that the presence of multiple taxa of filamentous cyanobacteria contributes to the effective growth of these granules on the glacier surface.
1 Introduction
Cryoconite is an association of biogenic aggregates, consisting of organic matter, mineral particles, and microbes, including phototrophs and heterotrophs. They form small spherical aggregates, widely referred to as cryoconite granules, which are entangled by filamentous cyanobacteria. The cryoconite granules often cover bare ice surfaces of many glaciers in Asia (e.g., Segawa et al., 2017; Takeuchi, 2002; Takeuchi et al., 2010; Rozwalak et al., 2022). Since the granules are usually dark in color, they induce a reduction of the albedo of the bare ice surface and accelerate the melt rate of the ice. For example, the cryoconite granules dispersed on the entire glacier surface on a Himalayan glacier accelerate the melt rate of the ice surface threefold faster than that without cryoconite (Kohshima et al., 1993). Thus, it is necessary to understand the processes of their formation and growth.
Filamentous cyanobacteria are the predominant primary producers in Asian glacier ecosystems (Segawa et al., 2017; Segawa et al., 2020; Murakami et al., 2022). Their filamentous cells of cyanobacteria are also able to ensnare both mineral and organic particles, with their extracellular polymeric substances (EPS) serving to fortify these granular structures (Stibal et al., 2012; Yallop et al., 2012; Musilova et al., 2016). Filamentous cyanobacteria mainly grow at the surface of the granules, whereas their dead body and heterotrophic bacteria are distributed in the inner part of the granules (Segawa et al., 2020). The size of the granules presents significant spatial variability (Cook et al., 2016), likely constrained by the binding capacity of filamentous autotrophs, the adhesive potential of other organic matter (OM), and the decomposition rates facilitated by heterotrophs (Takeuchi et al., 2010).
Microbial communities within cryoconite granules vary with their size, suggesting their different functions in the growth stages of the granules (Uetake et al., 2019; Segawa et al., 2020). However, the functions of microbial communities, especially multiple species of filamentous cyanobacteria, remain unknown. This is primarily due to the limitations of 16S rRNA analysis, which tests all gene sequences within the granules, including both living and non-living cells. Furthermore, cryoconite formation and growth may be influenced by a specific cyanobacterial taxon (Park and Takeuchi, 2021). Filamentous cyanobacteria identified as Oscillatoriales order on glacier surfaces of the Tien Shan Mountains in Asia (Segawa et al., 2017) are associated with the formation of in situ cryoconite granules (Takeuchi et al., 2010).
Urumqi Glacier No. 1, a typical mountain glacier located in the Tianshan Mountains of central Asia, is covered with abundant cryoconite granules (Takeuchi and Li, 2008). 16S rRNA gene analysis has revealed that filamentous cyanobacteria in the cryoconite consisted of multiple species belonging to the order Oscillatoriales (Segawa et al., 2017). The identification of the cyanobacterial species in microscopy is generally difficult due to similar cell morphology among there species. However, microscopic observations based on the presence of sheath and filament thickness allow for the classification of the filamentous cyanobacteria into three distinct morphological taxa, namely, Oscillatoriales A, B, and C (Segawa et al., 2017). Oscillatoriales A (Type A) has a bead-like cellular arrangement without a mucilaginous sheath and represents the smallest of the three taxa. Oscillatoriales B (Type B) possesses a mucilaginous sheath and green granules within the cells, making it the largest of the three taxa. On the other hand, Oscillatoriales C (Type C) is a smaller taxon, characterized by a mucilaginous sheath (Segawa et al., 2017).
The cyanobacteria present in the cryoconite granules exhibit distinct regional characteristics in terms of taxa, light harvesting patterns, and nitrogen utilization, significantly differing from those found in the polar regions (Segawa et al., 2017; Murakami et al., 2022). They may have different ecological characteristics, preferred conditions for growth, and roles in building the cryoconite granules. However, the difference in the ecological role of each cyanobacterial taxon in cryoconite granules and in glacier ecosystems is still unknown.
One of the possible distinctive functions among the cyanobacterial species may be association with mineral particles, which play a crucial role in the formation of cryoconite granules on the glacier surface. Certain minerals contain nutrients essential to microbial growth, and microbes actively extract nutrients from solid materials (Uroz et al., 2009). The abundance of mineral particles on the glacier surface affects the spatial distribution of cyanobacteria, thereby influencing the development of cryoconite granules (Uetake et al., 2016). Phosphorus (P), primarily in the form of PO43-, is mainly derived from minerals such as apatite, which serves as a significant source of P on P-limited glacier surfaces (Nagatsuka et al., 2010). Carbonate minerals (e.g., calcite) not only regulate the stabilization of pH in the extracellular environment of microbes but also provide inorganic carbon for phototrophs (Guida et al., 2017). Cyanobacteria may use such nutrients from mineral particles as well as dissolved inorganics in snow and ice. Each cyanobacterial species may have preferred minerals and sizes to build the cryoconite granules.
Glaciers in central Asia are subject to frequent dust storms originating from the surrounding deserts. These dust storms result in the deposition of mineral particles onto the glacier surface, thereby triggering the formation and growth of cryoconite granules. Several studies have been conducted to investigate the mineral composition of cryoconite on glaciers. Silicate minerals have been identified as the predominant components within cryoconite granules, and the mineral composition exhibits spatial uniformity across the glacier (Nagatsuka et al., 2010), which also bears similarities to that found in cryoconite from other Asian glaciers, despite having different sources (Nagatsuka et al., 2014a). Clay minerals are commonly present in both cryoconite granules and on the glacier surfaces (e.g., Nagatsuka et al., 2010; Nagatsuka et al., 2014a). Notably, clay minerals are surrounded by unfrozen water, even at very low temperatures (Price, 2007), which creates favorable conditions for overwintering microorganisms. However, these studies have primarily focused on the mineral composition of bulk cryoconite, and the potential variations in mineral particles across different granule sizes have not been thoroughly examined. The composition of mineral dust may change in different sizes of granules, since cyanobacteria may selectively bind certain sizes or kinds of mineral dust for their growth.
This study aims to describe the variations in morphological type compositions of filamentous cyanobacteria and mineral composition in cryoconite granules of various sizes on Urumqi Glacier No.1 in central Asia. We discussed the growth process of cryoconite granules incorporating microbes and mineral particles, focusing on the role of each taxon of filamentous cyanobacteria in cryoconite granules at different growth stages. We proposed the hypothesis that distinct cyanobacterial taxa contribute differently to the development of cryoconite granules.
2 Study site and methods
2.1 Study site
Urumqi Glacier No.1, situated at coordinates 43°06′N and 86°49′E, is located in the Tien Shan Mountains within the Xinjiang Uyghur Autonomous Region of western China (Figure 1a). This glacier, facing northeast, extends from an elevation of 3,740 to 4,486 m above sea level (a.s.l). Due to the terminus retreat of Urumqi Glacier No.1, the confluence of the two branches at the glacier’s terminus ceased in 1993, resulting in the spatial segregation of the glacier into distinct east and west branches (Ye et al., 2005). The total area of this catchment is approximately 1.73 km2. The mass acquirement of this glacier primarily relies on summer precipitation, which presents the highest rates. According to observational data from the Daxigou Meteorological Station (3,593 m a.s.l.), located 2 km east of Urumqi Glacier No.1, the region experienced a mean annual air temperature of −5.0°C and a mean annual precipitation of 460 mm from 1959 to 2015 (Yue et al., 2017). The mean equilibrium line altitude measured from 1997 to 2003 was 4110 m a.s.l. (Ye et al., 2005).
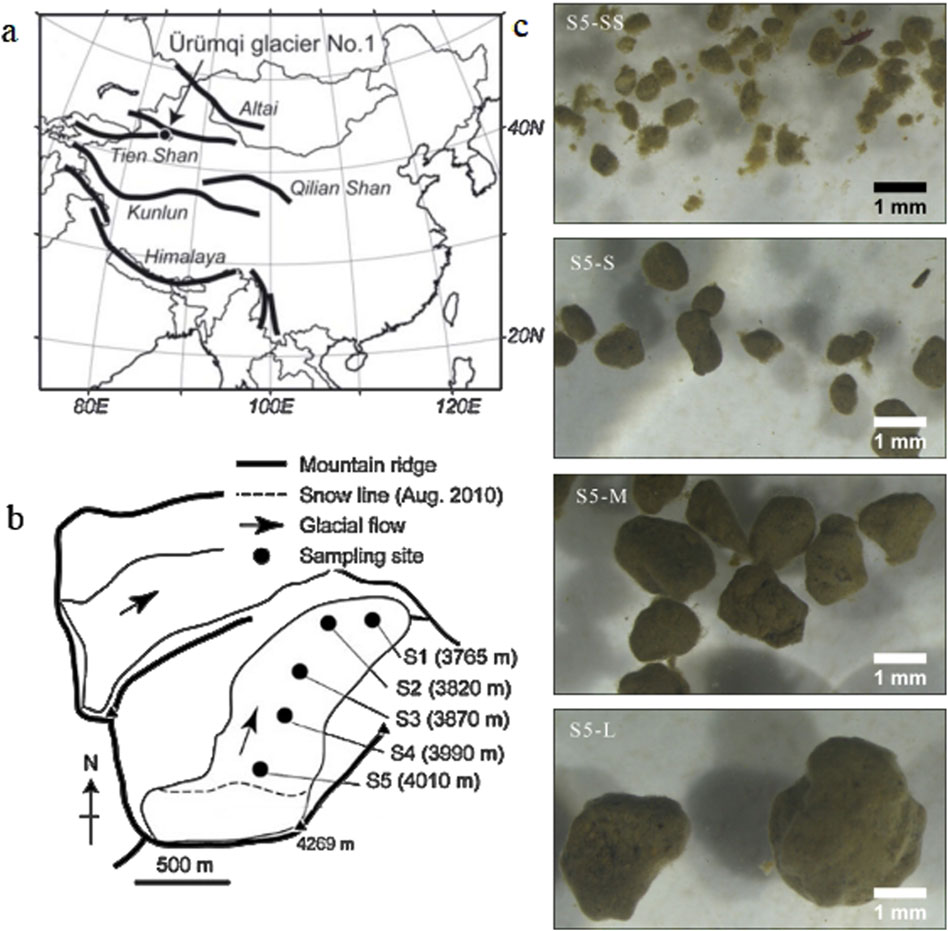
Figure 1. Location maps and photographs of Urumqi Glacier No.1. (a) Location of Urumqi Glacier No.1 in the Tien Shan Mountains. (b) Map of sampling sites (S1 - S5) in this study. (c) Selected photographs of cryoconite granules in difference sizes collected from S5 under stereomicroscope.
The ablation area of this glacier is characterized by the presence of substantial cryoconite granules on the surface, with a mean dry weight of 335 g m-2, and their organic content was 9.4% ± 1.6% (Takeuchi and Li, 2008). Cryoconite holes are not prominent on the glacier surface (Takeuchi and Li, 2008). Cryoconite granules’ sizes ranged from 0.3 to 3.5 mm in diameter with a mean of 1.1 ± 0.39 mm (Takeuchi et al., 2010). Cryoconite granules exhibit stratified layers with an annual thickness increment of 0.2 mm. These layers have been observed to endure for an average duration of approximately 3.5 years, which aligns with their mean longevity (Takeuchi et al., 2010). Microscopy has revealed that cryoconite granules contain mineralogical particles, filamentous and coccoid cyanobacteria (Takeuchi and Li, 2008).
2.2 Methods
2.2.1 Sampling and pretreatment
Surface snow and ice samples were collected from the East Branch of Urumqi Glacier No. 1 on 2 August 2013. Surface samples were collected at five different elevations of the glacier (S1-S5, Figure 1b). The surface ice, 1–2 cm thick, containing cryoconite granules, was collected from three surfaces selected randomly at each site, using a sterilized stainless-steel scoop. The samples were preserved in 50 mL sterile polyethylene bottles (I-boy, As One, Japan) and treated with 3% formalin to inhibit microbial activities after melting. Subsequently, all samples were transported to the laboratory at Chiba University, Japan, for further analysis. The samples were preserved at room temperature (25°C) until analysis.
To analyze the characteristics of cryoconite granules in different size fractions, the samples were wet-sieved through four type meshes with different pore size (Figure 1c). The cryoconite granules were separated into four size fractions (SS: 0.3–0.59; S: 0.6–0.99; M:1.0–1.59; and L: >1.6 mm) before analysis. The samples were subjected to repeated flushing with Milli-Q water to ensure complete dispersion of cryoconite granules during the filtration process.
2.2.2 Analysis of organic and inorganic matter content
The contents of organic and inorganic matter in cryoconite within each size fraction were measured by combustion. The samples were dried in pre-weighed crucibles at an electrical dryer (DO-450FA, As One, Japan) at 60°C for 24–48°h until complete evaporation of water from the samples. The cooled samples were weighed to obtain the dry weight (md). These dried samples were then combusted for 3°h at 500°C in an electric furnace and weighed again (mc). The difference in weight between the dried and combusted samples (md - mc) was used to calculate the amount of organic matter. This procedure was repeated for three different samples at each sampling site. The organic matter content, expressed as a percentage of dry weight, was calculated as the mean amount of organic matter to the total weight within each size fraction.
2.2.3 Quantification of filamentous cyanobacterial biomass
The abundance of filamentous cyanobacteria in the different size fractions of cryoconite granules was quantified through direct cell counting using an optical microscope. To understand the local distribution of cyanobacteria, cyanobacterial biomass within cryoconite granules of different size fractions was quantified. However, due to the limited sample quantity in the smallest size fraction (referred to as SS), only samples from the other three size fractions were analyzed. In order to loosen the solid sediment and facilitate individual cyanobacteria observation, the samples were supplemented with additional Milli-Q water in 6 mL tubes and subjected to ultrasonication for 10–20 min. Then, a water sample (10 μL) along with some extra water was filtered through a 0.45 μm PTFE membrane filter (JHWP01300, Merck Millipore, Germany). Photographs containing filamentous cyanobacteria around the center of the filter were randomly taken in the darkroom, using an optical and fluorescence microscope (BX51, Olympus, Japan) under ×200 magnification.
The obtained photographs were subsequently analyzed using an image processing software (ImageJ, National Institutes of Health, USA). Cyanobacterial cells with lengths greater than 10 μm, showing chlorophyll autofluorescence (indicating active cells), were manually measured. The biomass of filamentous cyanobacteria for each taxon was represented by its biovolume, which is the volume of geometric analogs of microbial cells. The biovolume of a single cyanobacterium was considered as that of a cylinder. The biomass of each cyanobacterial taxon within each size fraction at each sampling site was calculated as the average of three measurements taken from three different samples collected at that site. The morphological type composition (proportion) of the filamentous cyanobacteria across all size fractions was expressed as the mean proportion of each taxon to the total filamentous cyanobacterial biomass at each sampling site.
2.2.4 Scanning electron microscopy (SEM) observation
In order to observe an association between cyanobacteria and mineral particles, SEM observation of the cryoconite granules was performed. Cryoconite granules were dehydrated by freezing-drying prior to SEM observation. Samples of cryoconite granules in different size fractions (S, M and L for S1 and S2; all size fractions for S3) were washed with Milli-Q water, and soaked into ethanol series 50%, 70%, 80%, 90%, 95%, and 100% for 4 h each to dehydrate. After the samples were treated in 100% t-butyl alcohol for 24 h, they were frozen in the freezer compartment of the refrigerator (−18°C) and lyophilized by using a freezing drying device (JFD-320, JEOL, Japan). The freeze-dried samples were observed with a low vacuum mode of the SEM (JSM-6010PLUS/LA, JEOL, Japan) under the conditions of 10 kV acceleration voltage and 11 mm working distance. The size (width) and number of filamentous cyanobacteria on the SEM images were manually measured using an image processing application of ImageJ (National Institutes of Health, USA).
The filamentous cyanobacteria often attached fine mineral particles to their filaments' surfaces, but the abundance varied with filament. Based on the abundance of mineral particles attached on their surface, cyanobacterial filaments were divided into three levels of mineral abundance: Level H: Mineral particles were densely attached on the entire filament surface; Level M: Mineral particles were attached on the filament surface, but the cell surface was visible; Level L: No mineral particles on the filament surface (Figure 2). The proportions of these three mineral particle levels were determined for each cyanobacterial taxon in each cryoconite granules, calculated as the mean of 10 different single granules, with each taxon of cyanobacteria being counted for 20 filaments.
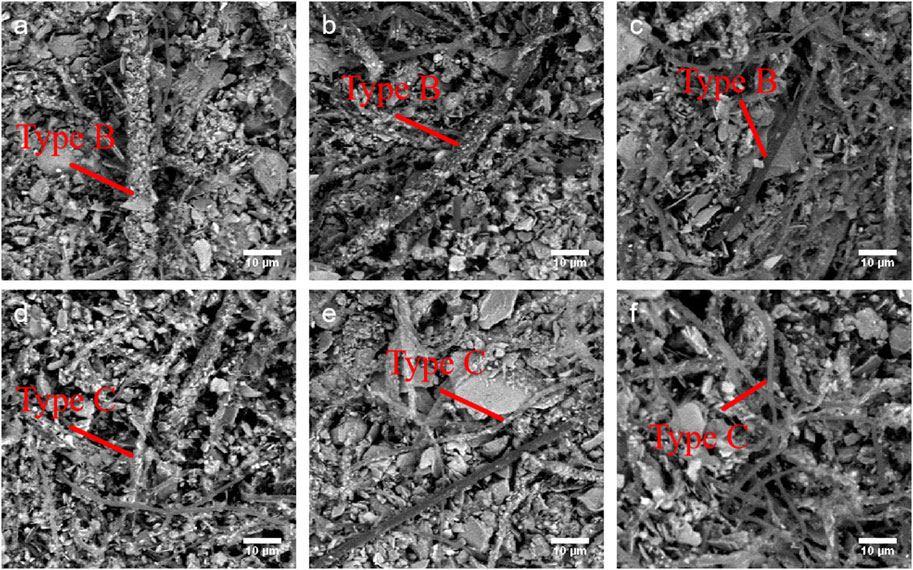
Figure 2. SEM images of two filamentous cyanobacterial taxa of Type B (top) and Type C (bottom) under different level of mineral loading. (a) Level H of Type B; (b) Level M of Type B; (c) Level L of Type B; (d) Level H of Type C; (e) Level M of Type C; (f) Level L of Type C. Scale bar = 10 μm.
2.2.5 X-ray diffraction (XRD) analysis and mineralogical composition
To determine the mineralogical composition in the different size fractions of cryoconite granules, powder XRD analysis was conducted using an X-ray diffractometer (Ultima Ⅳ RAD 11-B, Rigaku, Japan). Samples were dried at 60°C for 24–48 h in an electrical dryer (DO-450FA, As One, Japan), and then finely ground into powder in a mortar. The powdered samples were evenly spread on a reflection-free specimen holder. The X-ray target was Cu-Kα, the tube voltage was 40 kV, and the tube current was 30 mA. Scans were performed from 4° to 60° (2θ) at a rate of 2° (2θ) per minute. The mineralogical composition of the cryoconite was identified by an XRD analysis software (X’Pert Highscore Plus, Malvern Panalytical, UK), using the obtained diffraction spectra of minerals in the samples. Semi-quantification of each mineral was automatically estimated with the software, using the reference intensity ratio (RIR) method. The precision of the RIR method has been estimated to be 3%–8% (Chung, 1975).
2.2.6 Statistical analysis
One-way analysis of variance (ANOVA) was conducted using Origin software to assess differences in organic matter content across study sites and among all size fractions of cryoconite granules. When a significant effect was detected (p < 0.01), a post hoc Tukey’s honest significant difference test was performed to identify which specific groups differed significantly from each other. Differences in cyanobacterial morphological type composition and mineralogical composition across sites and among size fractions were tested using permutational multivariate analysis of variance (PERMANOVA) based on Bray-Curtis distance metrics with the “adonis” function in the R package “vegan” (Oksanen et al., 2010). Statistical significance was defined as p < 0.05.
3 Results
3.1 Contents of organic matter in cryoconite granules of different size fractions
There was a general decrease of organic content from S1 to S5, despite a slight increase observed at S4, ranged from 6.9% to 10.5%, with an mean of 9.1% (Figure 2). The difference among size fractions was statistically significant at sampling sites (one-way ANOVA, F = 6.5, p < 0.01). Post-hoc analysis using Tukey’s honest significant difference test revealed that significant differences existed between S5 and S2, S5 and S1, as well as between S3 and S2, and S3 and S1. Larger sizes (M and L) of cryoconite granules tend to have higher organic content across different sites, while smaller sizes (S and SS) showed generally lower contents, especially at sites with overall lower organic content (Figure 3). However, there was no significant difference among all size fractions (one-way ANOVA, F = 0.86, p = 0.47 > 0.05).
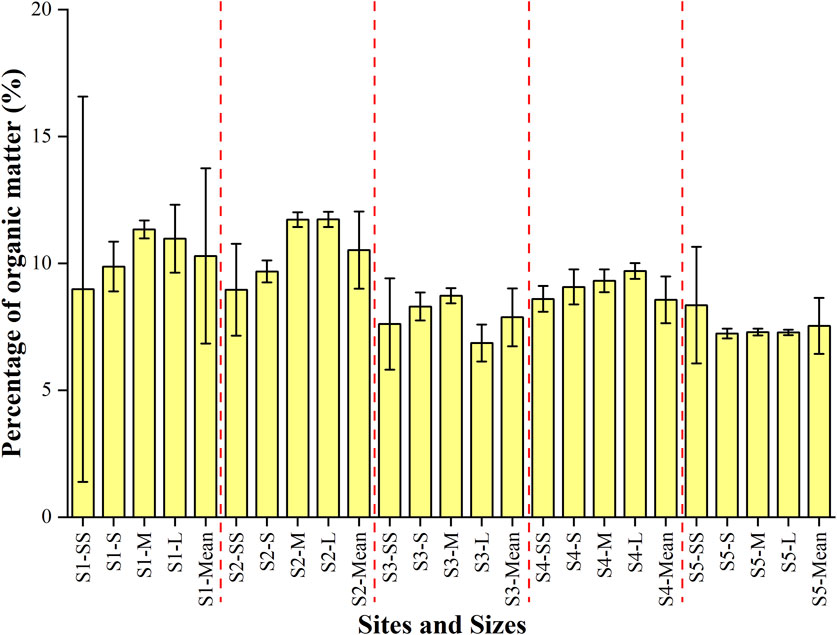
Figure 3. Contents of organic matter in cryoconite granules with different size fractions across Urumqi Glacier No.1.
Despite the lack of significant differences, the contents of OM tended to be associated with granule size. At the lower four sites (S1-S4), the OM contents of the smallest size fractions (SS) were generally smaller compared with those of the medium (M) or large (L) size fractions. For instance, at S1, the OM content was 9.0% in the SS size, while it was 11.3% and 11.0% in the M and L size fractions, respectively. Similar trends were observed at S2, S3, and S4, where the OM content was lowest for the SS size fraction, but greater for the L size fractions. In contrast, the highest site (S5) exhibited a distinct pattern. The OM content was lowest for the S size (7.2%) and generally low in all of the size fractions, including the SS size (8.4%).
3.2 Morphological characteristics of observed filamentous cyanobacteria
Three distinct morphological taxa of filamentous cyanobacteria (Types A, B, and C) were consistently observed across all size fractions using an optical microscope. The mean cell sizes (diameters) of Type A, Type B, and Type C were measured to be 1.4 ± 0.2 μm, 3.2 ± 0.6 μm, and 1.4 ± 0.3 μm, respectively (mean ± standard deviation; Supplementary Figure S1), across all samples. Notably, all Type A and most of Type C (>96.3%) exhibited cell sizes below 2 μm, while all Type B displayed a larger cell size exceeding 2 μm.
3.3 Morphological type composition of filamentous cyanobacteria in cryoconite granules with different sizes
The morphological type composition of filamentous cyanobacteria generally showed no significant difference among the size fractions of the granules (PERMANOVA, p > 0.05). The two cyanobacterial taxa, Types B and C, were dominant in cyanobacterial communities in all size fractions of cryoconite granules across the glacier, whereas Type A accounted for less than 2.1% of the total biomass (Figure 4). Notably, Type A taxon constituted the highest percentage in the SS size at S1, with a mean value of 2.1%. Conversely, it was completely absent in the M size at S4. The biomass of Type A did not show significant variations in the samples.
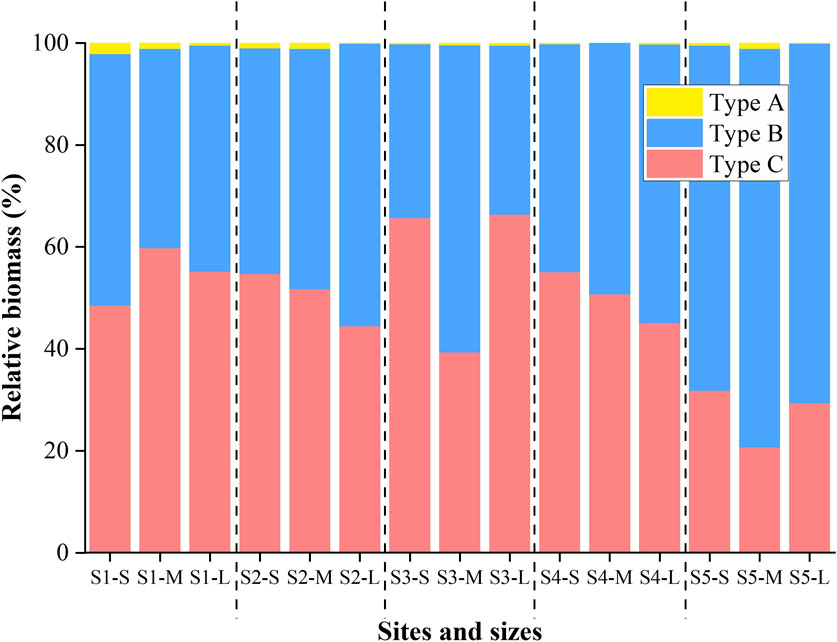
Figure 4. Cyanobacteria morphological composition in different sizes of cryoconite granules across Urumqi Glacier No.1.
The proportions of Type B and Type C did not differ significantly from S1 to S4. The proportions of Type B at S2 and S4 displayed a similar pattern, gradually increasing from small to large size fractions. In contrast, the proportions of Type C showed the opposite trend at the same sampling site. However, the proportion of Type B was consistently higher at the highest site (S5) compared to the lower sites, fluctuating between 67.8% and 78.2% across the S to L size fractions. PERMANOVA analysis revealed that the community composition at S5 differed significantly from that at S1–S4 (p < 0.05).
3.4 SEM observation
SEM observations revealed that filamentous cyanobacteria densely covered the surface of cryoconite granules of all size fractions (Figure 5). Mineral particles, which appeared to be brighter in the backscattered electrons, were also observed within the cryoconite granules.
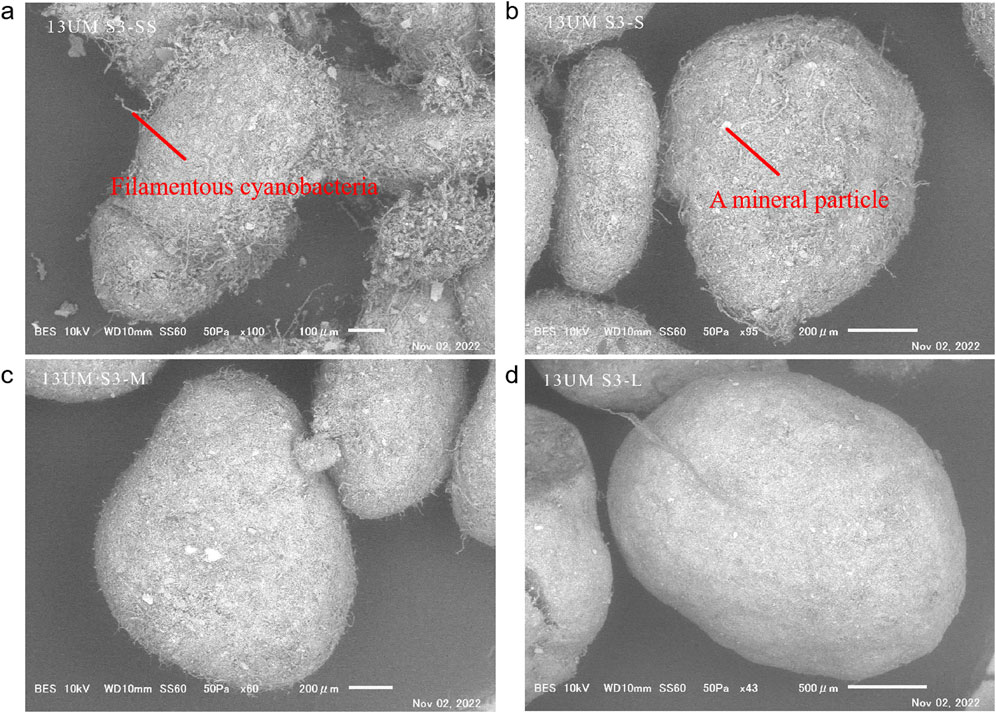
Figure 5. SEM images of cryoconite granules of different sizes at S3 under SEM observation. (a) SS size; (b) S size; (c) M size; (d) L size.
Based on cell size, two distinct taxa of filamentous cyanobacteria were identified in the SEM observation. The thicker filament is likely to correspond to Type B, while the thinner filament (<2 μm in diameter) probably corresponds to either Type A or Type C. As the presence of a sheath of cyanobacteria could not be clearly observed in the SEM images, it was not possible to distinguish between Type A and Type C, which have thinner filaments. As optical microscopy showed that the proportion of Type A was generally very low, less than 2.1%, the thinner filament observed in the SEM images was mostly considered Type C.
The two taxa of filamentous cyanobacteria appeared to have distinctive associations with minerals in the SEM images.: (1) Large-sized mineral particles (5–100 μm in diameter) were entangled by multiple cyanobacterial filaments and (2) numerous small mineral particles (<5 μm in diameter) were attached to the surface of a single filament (Figure 6).
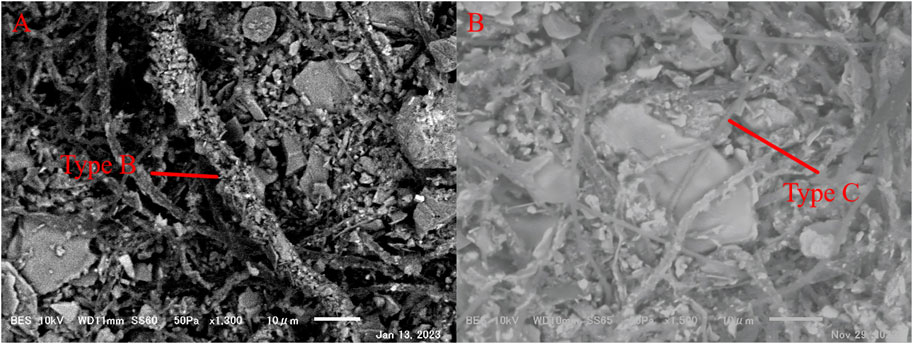
Figure 6. SEM images of the association status of filamentous cyanobacteria Type B and Type C with minerals in cryoconite granules under SEM observation. (a) Large sized mineral particles (5–100 μm in diameter) were entangled by multiple Type C, taken in S size at S3; (b) massive small mineral particles (<5 μm in diameter) were attached at the surface of a single Type B, taken in SS size at S3. Scale bar = 10 μm.
The mineral attachment levels of filaments varied significantly across cyanobacterial taxa (Figure 7), with Type C filaments predominantly lacking mineral particles, while Type B filaments exhibited a higher proportion of filaments with dense mineral particles. Filaments without mineral particles (Level L) were more abundant in Type C compared to Type B (mean: 37.3% vs 8.1%). Conversely, filaments with dense mineral particles (Level H) were more abundant in Type B than in Type C (mean: 28.8% vs 7.8%). There was no significant difference in the proportions of Level L, M, and H among the different granule sizes.
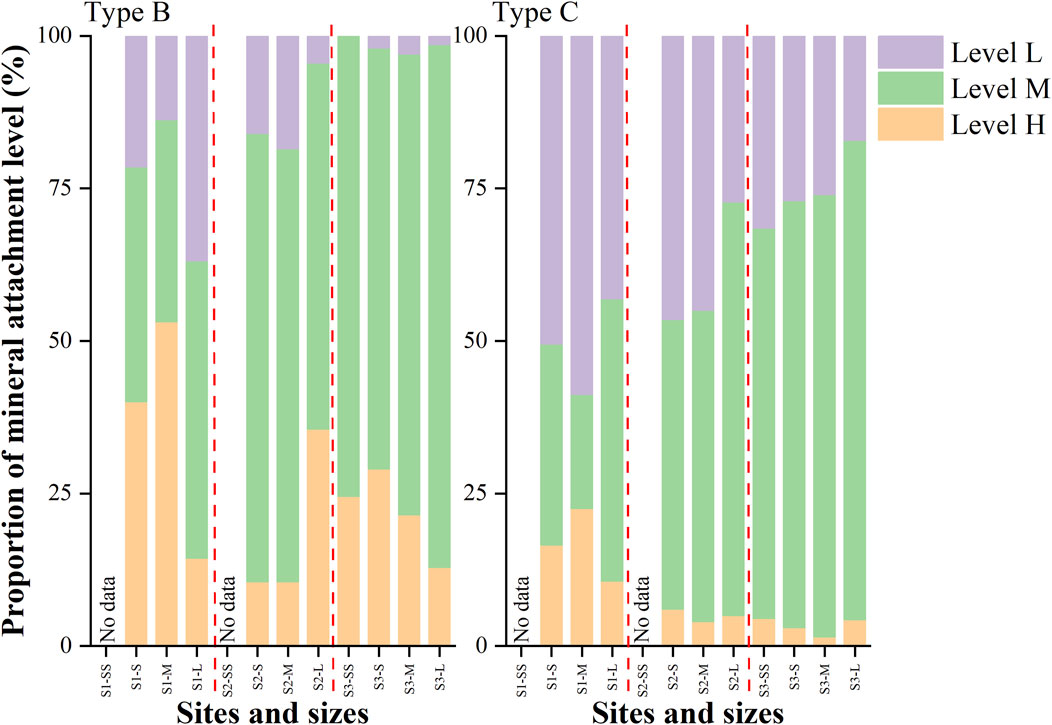
Figure 7. Proportions of filamentous cyanobacteria Type B and Type C under different mineral loading at each sampling site.
The level of attached mineral particles also differed among the granule size fractions. Filaments without mineral particles (Level L) of Type C were more abundant in smaller size fractions. Filaments with dense mineral particles (Level H) of Type B were more abundant in smaller size fractions at S1, while they were more abundant in larger size fractions at S2.
Significant differences in the level of minerals of each cyanobacterial taxon were also observed among the altitudes. Both Type B and Type C filaments, without mineral particles (Level L) and with dense mineral particles (Level H), were most abundant at S1 compared to the upper sites (S2 and S3). Specifically, filaments with dense mineral particles (Level H) of Type B were abundant at S1 (43.3%). At the upper two sites (S2 and S3), filaments with medium mineral particles (Level M) dominated (>50%) for both Type B and Type C.
3.5 XRD spectra and composition of minerals in cryoconite granules with different sizes
The XRD spectra of cryoconite granules in all size fractions across Urumqi Glacier. No.1 were generally similar, and showed peaks for several silicate minerals (Figure 8a). Specifically, the observed peaks were identified as quartz (26.7°), albite (23.5, 27.9°), orthoclase (26.9, 27.6°) and clay minerals including illite (8.9°), and chlorite (6.2, 12.5°). Hornblende (10.5°) was detected in all samples except S1-M, S1-L, S2-S, and S3-L. Calcite (CaCO3, 29.4°) was not identified in all samples. There were no significant differences in the positions and intensities of these peaks among size fractions and sites, indicating a consistent mineralogical composition of cryoconite granules across various size fractions and different altitudes on the glacier surface.
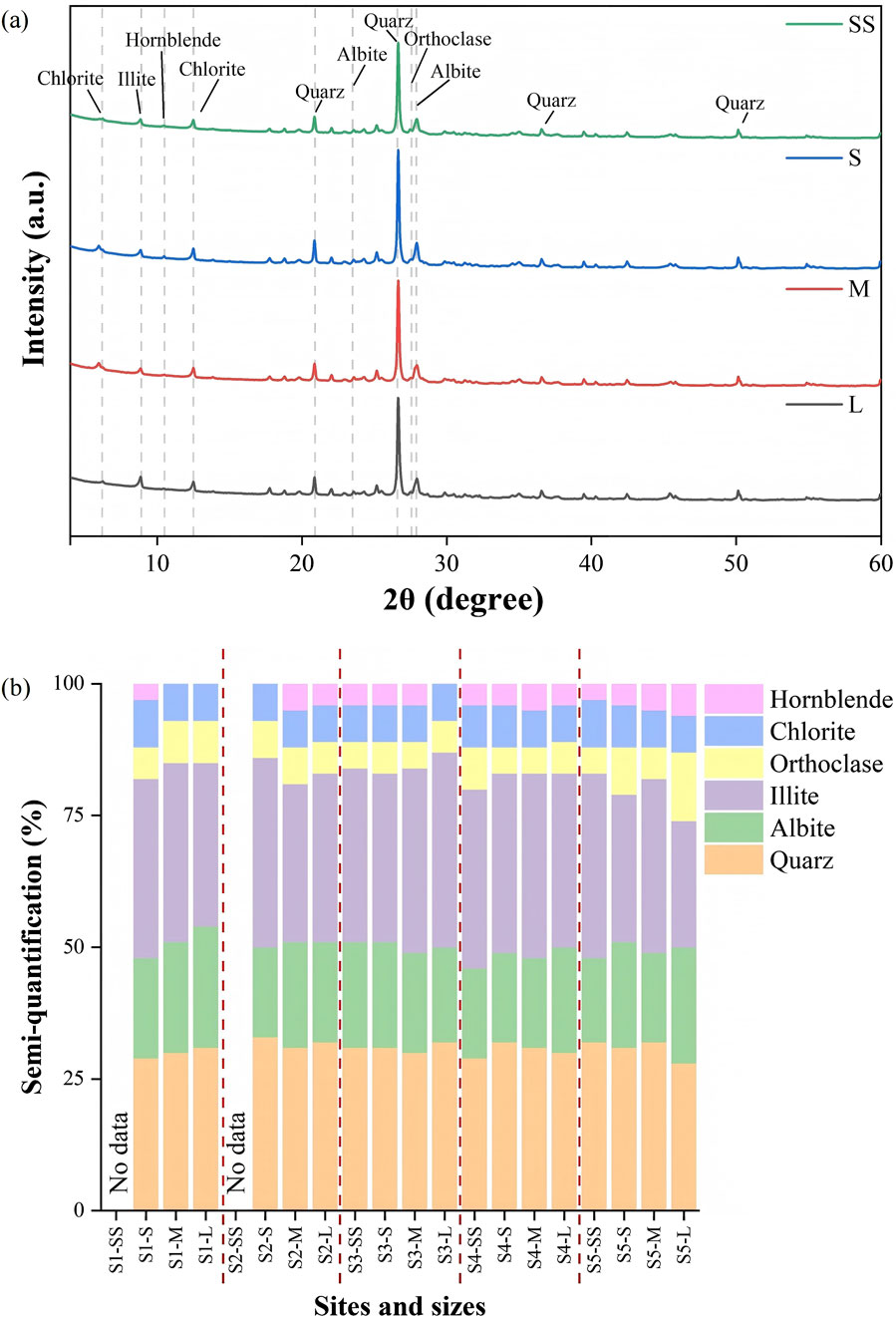
Figure 8. (a) XRD spectra of minerals contained in cryoconite granules with different sizes (SS, S, M and L) at S3; (b) Semi-quantitative mineralogical composition in cryoconite granules of all size fractions across different altitudes.
Semi-quantification results showed that clay minerals were the dominant components in all samples (Figure 8b), constituting a range of 31% and 44%. Quartz and albite were present in lower relative abundances across all samples, comprising 22%–28% for quartz and 16%–23% for albite. The proportion of hornblende was the least, accounting for ≤5%. There was no statistically significant difference in mineralogical composition among sizes and sites (PERMANOVA, p > 0.05; Supplementary Figure S2).
4 Discussion
4.1 Potential factors of organic matter content difference in cryoconite granules
The mean organic matter (OM) content varied significantly across different altitudes; however, no significant differences were observed among size fractions. This suggests that OM content may be influenced by site-specific environmental conditions, including photosynthesis by phototrophs (e.g., cyanobacteria), input of allochthonous organic matter, OM decomposition by heterotrophic microorganisms, and mineral availability (Xu et al., 2010; Cook et al., 2016).
The mineral content displayed a spatial pattern similar to that of OM (Takeuchi and Li, 2008), indicating that mineral supply does not markedly affect OM content in cryoconite. A positive correlation between mineral particles and cyanobacterial abundances has been observed on a glacier in northwest Greenland (Uetake et al., 2016); however, this relationship was not found on Urumqi Glacier No.1 (Spearman, p > 0.05). This suggests that the relationship appears to be specific to certain environmental conditions or local factors unique to the Greenland glaciers, and similar patterns may not necessarily be observed in different glacier ecosystems. On some glaciers in the Arctic, OM content increases with elevation. The main factors contributing to this increase include the generation of EPS, which leads to an increase in degradable carbohydrates, as well as changes in the quantity and activity of photosynthetic microorganisms (Langford et al., 2014; Stibal et al., 2010). Therefore, the altitudinal differences in organic content within cryoconite granules are mainly due to microbial activity on Urumqi Glacier No.1.
The mean OM content across all size fractions was generally higher at lower altitudes (S1 and S2) and progressively lower at higher altitudes (S3-S5), likely because OM production by primary producers within cryoconite granules outpaces consumption by heterotrophic microorganisms at these sites. At lower altitudes, with warmer temperatures and stronger sunlight, the microbial growth period is extended (Yoshimura et al., 1997), resulting in higher photosynthetic production. Moreover, this pattern may reflect the accumulation of microbially produced OM as granules are transported downstream. Cryoconite granules are likely to form upstream on glaciers (Hodson et al., 2010) and, as they develop, are transported downstream by surface meltwater, resulting in an accumulation of OM content in downstream areas.
The OM content in cryoconite granules generally increases with granule size, which may be attributed to the distinct layer structures that vary with size. In smaller cryoconite granules, filamentous cyanobacteria are distributed throughout the granule, while in larger granules, cyanobacteria are predominantly active in the surface layer, with heterotrophic microorganisms colonizing the inner layers (Segawa et al., 2020). Cyanobacteria contribute 75%–95% of the available organic carbon in the cryoconite hole food web, positioning them as primary producers in these habitats (Stibal and Tranter, 2007). This suggests different states of balance between organic matter production and decomposition. For example, in cryoconite granules from glaciers in Svalbard, a net autotrophic rate has been observed in smaller-sized granules (Telling et al., 2012). However, in certain instances, such as medium to large granules at sites S1 and S3, OM content was found to be lower in larger granule sizes. This could result from the rate of OM consumption exceeding the rate of production in larger granules, possibly due to the activity of internal heterotrophic microbes and re-assimilated cyanobacteria (Stuart et al., 2016; Segawa et al., 2020). Further analysis of the organic matter composition across different granule sizes is needed to elucidate the factors driving the observed variations in OM content.
4.2 Effects of minerals on cryoconite granules
SEM observation revealed that filamentous cyanobacteria colonize the surface of cryoconite granules and utilize “glue-like” extracellular polymeric substances (EPS) to bind mineral particles in all size fractions (e.g., Takeuchi et al., 2010; Hodson et al., 2010; Segawa et al., 2020). Although previous studies have suggested that minerals play a role in the early formation of cryoconite (Uetake et al., 2016), observations indicate that the interaction between cyanobacteria and minerals occurs across various granule sizes. Furthermore, two distinct associations between cyanobacteria and minerals suggest that minerals are capable of binding with cyanobacteria within a suitable size range. This suggests that minerals may play a role not only in the initial formation but also in the growth and development of cryoconite granules.
The similar composition of minerals across the study sites on this glacier suggests a supply of the minerals from similar sources. Clay minerals, which are typically concentrated in finer sediments during long-distance transport, were abundant in cryoconite granules, indicating a distant provenance, possibly from the Taklamakan Desert (Nagatsuka et al., 2014b). Based on the alignment of XRD spectra with surface dust (Nagatsuka et al., 2010), mineral particles may interact with cyanobacteria to form cryoconite granules after deposition on the glacier surface. Due to their similar size, some minerals may also derive from the glacial ice (Chen et al., 2022). The uniform mineralogical composition of cryoconite granules across different size fractions further indicates that minerals are not selectively utilized during granule growth.
4.3 Roles of filamentous cyanobacteria taxa in cryoconite granules
Microscopic observation identified three distinct morphological taxa of filamentous cyanobacteria in cryoconite granules, indicating that these taxa may be potential cryoconite-forming cyanobacteria. The three observed cyanobacteria correspond to five species (a total of 20 OTUs) at the molecular level using single-filament PCR analysis, and their metabolism-related functions have been previously reported (Segawa et al., 2017; Murakami et al., 2022). Type A, corresponding to OTU5 (Pseudanabaena), is primarily involved in photoadaptation under blue light conditions. Type B, corresponding to OTU4 (Microcoleus), integrates nitrogen metabolism and synthesizes both phycocyanin and phycoerythrin, facilitating adaptation to diverse light environments. Type C, corresponding to OTUs 0, 8 (Oscillatoriales), and 9 (Geitlerinema), participates in nitrogen cycling but exhibits differences in light utilization. OTU0 can utilize both blue and red light, whereas OTUs 8 and 9 are more specialized for adaptation to blue light conditions. Cyanobacteria adhering to minerals on the surface are likely essential for the formation and growth of cryoconite, whereas cyanobacteria without mineral attachment do not contribute significantly to the aggregate formation of cryoconite granules.
Although Type A was present in all sampling sites, its contribution to total cyanobacterial biomass was less than 2.1%, and its proportion remained relatively constant across granule size fractions, suggesting that it does not play a prominent role in cryoconite formation. During the formation of cryoconite granules, cyanobacterial EPS play a vital role in shaping the physical structure and acting as an adhesive that binds together inorganic particles and living microbes (Langford et al., 2010). The low abundance of Type A likely results in insufficient EPS production to bind enough minerals for cryoconite formation. Furthermore, Type A is a minor constituent but widely distributed in glaciers across the Northern Hemisphere (Segawa et al., 2017). Its low abundance, along with a lack of nitrogen utilization functions, suggests that it is not necessarily essential for granule formation, probably an opportunistic species that proliferates widely in cold environments.
Type B appears to preferentially bind with smaller minerals (<5 μm) through EPS. Type B is widely distributed and dominant on Asian glaciers but is relatively scarce in the Arctic (Segawa et al., 2017). This may be attributed to their preference for nutrient- and mineral-rich environments, which facilitate nitrogen uptake. In high-altitude and high-radiation alpine regions, prolonged exposure to short-wavelength radiation may provide cyanobacteria possessing phycoerythrin with additional energy (Murakami et al., 2022). Given their dominance within cryoconite granules, Type B is likely a key cyanobacterial taxon in cryoconite formation. This association may initiate from the early stages of cryoconite formation, as numerous smaller mineral particles were often observed adhering to Type B surfaces, and the proportion of Level L remained consistently low across all cryoconite size fractions. Analysis of the morphological composition of cyanobacteria revealed a significantly higher abundance of Type B at the highest site (S5), suggesting its crucial role in initiating cryoconite formation, especially given that cryoconite granules are thought to originate from the upstream regions of glaciers (Hodson et al., 2010). Hence, the abundant formation of cryoconite granules in Asian glaciers, including those in Urumqi, is likely attributable to the presence of this species.
Type C possibly serves a different role in cryoconite formation. It consists of three phylotypes (OTUs 0, 8, and 9). Among them, OTUs 8 and 9 are exclusively distributed on Asian glaciers, indicating that these cyanobacteria are specific to the Asian region but are likely minor species. On the other hand, OTU0 is distributed from Asia to the Arctic, with dominance particularly pronounced in Asia (Segawa et al., 2017), especially in the southern Himalayas. Given its functional similarity to Type B, it suggests that OTU0 is also capable of thriving and proliferating extensively in Asian glacier environments. This underscores the importance of OTU0 as a key species in cryoconite granule formation across Asian glaciers. The proportion of Type C without mineral particles (Level L) is higher in smaller granular sizes and decreases with size, indicating a smaller role in cryoconite formation. Multiple Type C can bundle larger mineral particles (>5 μm), a binding mode observed across all granule size fractions, suggesting a critical role in granule development.
Based on the morphological composition of filamentous cyanobacteria and their interactions with minerals, we hypothesize the roles of cyanobacteria in the formation and growth of cryoconite particles. Firstly, Type B, due to its largest size, likely produces more EPS, allowing it to initially capture fine minerals and form the small cryoconite granules. Type C, being smaller in size, captures minerals more slowly than Type B but can combine with larger minerals, contributing to the growth of cryoconite granules. Type A, due to its minimal proportion in the community, has a negligible role in particle formation and proliferates within the cryoconite granules as they grow.
XRD analysis identified clay minerals as the dominant component across all size fractions, highlighting their importance in cryoconite granule growth. These minerals, surrounded by unfrozen water even at low temperatures, support cyanobacterial growth during winter (Price, 2007). Anaerobic bacteria in the unfrozen water may also utilize nitrate ions for denitrification. Specific microbes in cryoconite granules contribute to nitrogen cycles on glacier surfaces (Segawa et al., 2017; Segawa et al., 2020), with the nitrogen cycle’s establishment closely linked to clay minerals. The presence of clay minerals may also contribute to variations in microbial communities across different granule sizes. Microbes can assimilate nutrients from minerals through bioerosion processes (Zawierucha et al., 2019) or via mineral grain edges using an electron shuttle mechanism (Price, 2007). As cryoconite granules grow, the increased presence of clay minerals provides more binding sites for filamentous cyanobacteria, leading to higher bacterial diversity and abundance with increasing granule size (e.g., Uetake et al., 2019; Segawa et al., 2020). Additionally, this process may be influenced by predation pressure from glacier-specific tardigrades (Zawierucha et al., 2018). Filamentous cyanobacteria lacking mineral attachment might be more susceptible to predation, potentially impacting their role in cryoconite formation and morphological composition.
While laboratory simulations of Arctic cryoconite formation suggest that various filamentous cyanobacteria possess the potential to form cryoconite granules (Wejnerowski et al., 2023), the spatial heterogeneity of cyanobacterial species highlights the need for further studies. Future research should focus on separately simulating the formation of cryoconite granules using these four cyanobacterial species identified with Type B and Type C to confirm their distinct roles. Additionally, further functional analyses, including the characterization of light-utilizing proteins and other metabolic pathways, are essential to elucidate the mechanisms underlying their contributions to cryoconite formation.
5 Conclusion
Filamentous cyanobacteria community within cryoconite granules of Urumqi Glacier No.1 consists of three morphological taxa (Type A, Type B, and Type C). Type B or Type C was dominant in all size fractions of cryoconite granules, while Type A was present in lower abundance. The structure of the cyanobacterial community did not significantly vary with the size of cryoconite granules but showed significant variation with altitude. This indicates that the variation in cyanobacterial morphological type composition depends on the local conditions on the glacier surface.
There were no significant variations in mineral composition and abundances among all size fractions, suggesting that local minerals are used to form cryoconite granules, and these minerals mainly originate from the same source. Scanning electron microscopy (SEM) observations revealed that Type B often had abundant small mineral particles attached to its filament surface, while Type C was mostly devoid of or had only a few mineral particles. This suggests that these two cyanobacterial taxa may play different roles in binding mineral particles within cryoconite granules. The findings from this study provide insights into the process of cryoconite formation and growth by examining the variations in filamentous cyanobacterial species and mineralogical composition in different sizes of cryoconite granules.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
YC: Methodology, Data curation, Validation, Conceptualization, Software, Writing – original draft, Writing – review and editing, Formal Analysis. NT: Validation, Writing – review and editing, Methodology, Data curation, Supervision, Conceptualization, Funding acquisition, Writing – original draft. ST: Writing – review and editing, Investigation. ZL: Writing – review and editing, Investigation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study were financially supported by the JSPS KAKENHI (19H01143, 20H00196, 21H04357, and 24H00260) and Young Scientists Fund of the National Natural Science Foundation of China (42306268).
Acknowledgments
We wish to thank the staff and students of the Northwest Institute of Eco-Environment and Resources of the Chinese Academy of Sciences in Lanzhou, China, for their invaluable support during the fieldwork. We extend our gratitude to Professor Noboru Furukawa from Chiba University for providing worthwhile assistance with the XRD analysis. We are also very grateful to Zoffy Yu for his assistance in refining the figures for our paper. We also thank two reviewers for their helpful suggestions that significantly enhanced the quality of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/feart.2025.1596472/full#supplementary-material
References
Chen, Y., Takeuchi, N., Wang, F., and Li, Z. (2022). Characteristics of chemical solutes and mineral dust in ice of the ablation area of a glacier in tien Shan mountains, central Asia. Front. Earth Sci. 10, 904261. doi:10.3389/feart.2022.904261
Chung, F. H. (1975). Quantitative interpretation of X-ray diffraction patterns of mixtures. III. Simultaneous determination of a set of reference intensities. Appl. Crystallogr. 8 (1), 17–19. doi:10.1107/s0021889875009454
Cook, J., Edwards, A., Takeuchi, N., and Irvine-Fynn, T. (2016). Cryoconite: the dark biological secret of the cryosphere. Prog. Phys. Geogr. 40 (1), 66–111. doi:10.1177/0309133315616574
Guida, B. S., Bose, M., and Garcia-Pichel, F. (2017). Carbon fixation from mineral carbonates. Nat. Commun. 8 (1), 1025. doi:10.1038/s41467-017-00703-4
Hodson, A., Cameron, K., Bøggild, C., Irvine-Fynn, T., Langford, H., Pearce, D., et al. (2010). The structure, biological activity and biogeochemistry of cryoconite aggregates upon an Arctic valley glacier: longyearbreen, Svalbard. J. Glaciol. 56 (196), 349–362. doi:10.3189/002214310791968403
Kohshima, S., Seko, K., and Yoshimura, Y. (1993). “Biotic acceleration of glacier melting in Yala glacier, Lantang region, Nepal Himalayas,” 218. Wallingford, UK: IAHS Publ, 309–316.
Langford, H., Hodson, A., Banwart, S., and Bøggild, C. (2010). The microstructure and biogeochemistry of Arctic cryoconite granules. Ann. Glaciol. 51 (56), 87–94. doi:10.3189/172756411795932083
Langford, H. J., Irvine-Fynn, T. D. L., Edwards, A., Banwart, S. A., and Hodson, A. J. (2014). A spatial investigation of the environmental controls over cryoconite aggregation on Longyearbreen glacier, Svalbard. Biogeosciences 11 (19), 5365–5380. doi:10.5194/bg-11-5365-2014
Murakami, T., Takeuchi, N., Mori, H., Hirose, Y., Edwards, A., Irvine-Fynn, T., et al. (2022). Metagenomics reveals global-scale contrasts in nitrogen cycling and cyanobacterial light-harvesting mechanisms in glacier cryoconite. Microbiome 10 (1), 50–14. doi:10.1186/s40168-022-01238-7
Musilova, M., Tranter, M., Bamber, J. L., Takeuchi, N., and Anesio, A. M. (2016). Experimental evidence that microbial activity lowers the albedo of glaciers. Geochem. Perspect. Lett. 2, 106–116. doi:10.7185/geochemlet.1611
Nagatsuka, N., Takeuchi, N., Nakano, T., Kokado, E., and Li, Z. (2010). Sr, Nd and Pb stable isotopes of surface dust on Ürümqi glacier No. 1 in western China. Ann. Glaciol. 51 (56), 95–105. doi:10.3189/172756411795931895
Nagatsuka, N., Takeuchi, N., Nakano, T., Shin, K., and Kokado, E. (2014b). Geographical variations in Sr and Nd isotopic ratios of cryoconite on Asian glaciers. Environ. Res. Lett. 9 (4), 045007. doi:10.1088/1748-9326/9/4/045007
Nagatsuka, N., Takeuchi, N., Uetake, J., and Shimada, R. (2014a). Mineralogical composition of cryoconite on glaciers in northwest Greenland. Bull. Glaciol. Res. 32, 107–114. doi:10.5331/bgr.32.107
Oksanen, J., Blanchet, F. G., Kindt, R., Legendre, P., O’Hara, R. B., Simpson, G. L., et al. (2010). Package ‘vegan’. Community ecology package.
Park, C., and Takeuchi, N. (2021). Unmasking photogranulation in decreasing glacial albedo and net autotrophic wastewater treatment. Environ. Microbiol. 23 (11), 6391–6404. doi:10.1111/1462-2920.15780
Price, P. B. (2007). Microbial life in glacial ice and implications for a cold origin of life. FEMS Microbiol. Ecol. 59 (2), 217–231. doi:10.1111/j.1574-6941.2006.00234.x
Rozwalak, P., Podkowa, P., Buda, J., Niedzielski, P., Kawecki, S., Ambrosini, R., et al. (2022). Cryoconite–From minerals and organic matter to bioengineered sediments on glacier's surfaces. Sci. Total Environ. 807, 150874. doi:10.1016/j.scitotenv.2021.150874
Segawa, T., Takeuchi, N., Mori, H., Rathnayake, R. M., Li, Z., Akiyoshi, A., et al. (2020). Redox stratification within cryoconite granules influences the nitrogen cycle on glaciers. FEMS Microbiol. Ecol. 96 (11), fiaa199. doi:10.1093/femsec/fiaa199
Segawa, T., Yonezawa, T., Edwards, A., Akiyoshi, A., Tanaka, S., Uetake, J., et al. (2017). Biogeography of cryoconite forming cyanobacteria on polar and Asian glaciers. J. Biogeogr. 44 (12), 2849–2861. doi:10.1111/jbi.13089
Stibal, M., Lawson, E. C., Lis, G. P., Mak, K. M., Wadham, J. L., and Anesio, A. M. (2010). Organic matter content and quality in supraglacial debris across the ablation zone of the Greenland ice sheet. Ann. Glaciol. 51 (56), 1–8. doi:10.3189/172756411795931958
Stibal, M., Telling, J., Cook, J., Mak, K. M., Hodson, A., and Anesio, A. M. (2012). Environmental controls on microbial abundance and activity on the Greenland ice sheet: a multivariate analysis approach. Microb. Ecol. 63 (1), 74–84. doi:10.1007/s00248-011-9935-3
Stibal, M., and Tranter, M. (2007). Laboratory investigation of inorganic carbon uptake by cryoconite debris from Werenskioldbreen, Svalbard. J. Geophys. Res. Biogeosciences 112 (G4). doi:10.1029/2007jg000429
Stuart, R. K., Mayali, X., Lee, J. Z., Craig Everroad, R., Hwang, M., Bebout, B. M., et al. (2016). Cyanobacterial reuse of extracellular organic carbon in microbial mats. ISME J. 10 (5), 1240–1251. doi:10.1038/ismej.2015.180
Takeuchi, N. (2002). Optical characteristics of cryoconite (surface dust) on glaciers: the relationship between light absorbency and the property of organic matter contained in the cryoconite. Ann. Glaciol. 34, 409–414. doi:10.3189/172756402781817743
Takeuchi, N., and Li, Z. (2008). Characteristics of surface dust on ürümqi glacier No. 1 in the tien Shan mountains, China. Arct. Antarct. Alp. Res. 40 (4), 744–750. doi:10.1657/1523-0430(07-094)[takeuchi]2.0.co;2
Takeuchi, N., Nishiyama, H., and Li, Z. (2010). Structure and formation process of cryoconite granules on Ürümqi glacier No. 1, Tien Shan, China. Ann. Glaciol. 51 (56), 9–14. doi:10.3189/172756411795932010
Telling, J., Stibal, M., Anesio, A. M., Tranter, M., Nias, I., Cook, J., et al. (2012). Microbial nitrogen cycling on the Greenland ice sheet. Biogeosciences 9 (7), 2431–2442. doi:10.5194/bg-9-2431-2012
Uetake, J., Nagatsuka, N., Onuma, Y., Takeuchi, N., Motoyama, H., and Aoki, T. (2019). Bacterial community changes with granule size in cryoconite and their susceptibility to exogenous nutrients on NW Greenland glaciers. FEMS Microbiol. Ecol. 95 (7), fiz075. doi:10.1093/femsec/fiz075
Uetake, J., Tanaka, S., Segawa, T., Takeuchi, N., Nagatsuka, N., Motoyama, H., et al. (2016). Microbial community variation in cryoconite granules on Qaanaaq Glacier, NW Greenland. FEMS Microbiol. Ecol. 92 (9), fiw127. doi:10.1093/femsec/fiw127
Uroz, S., Calvaruso, C., Turpault, M. P., and Frey-Klett, P. (2009). Mineral weathering by bacteria: ecology, actors and mechanisms. Trends Microbiol. 17 (8), 378–387. doi:10.1016/j.tim.2009.05.004
Wejnerowski, Ł., Poniecka, E., Buda, J., Klimaszyk, P., Piasecka, A., Dziuba, M. K., et al. (2023). Empirical testing of cryoconite granulation: role of cyanobacteria in the formation of key biogenic structure darkening glaciers in polar regions. J. Phycol. 59 (5), 939–949. doi:10.1111/jpy.13372
Xu, Y., Simpson, A. J., Eyles, N., and Simpson, M. J. (2010). Sources and molecular composition of cryoconite organic matter from the Athabasca Glacier, Canadian Rocky Mountains. Org. Geochem. 41 (2), 177–186. doi:10.1016/j.orggeochem.2009.10.010
Yallop, M. L., Anesio, A. M., Perkins, R. G., Cook, J., Telling, J., Fagan, D., et al. (2012). Photophysiology and albedo-changing potential of the ice algal community on the surface of the Greenland ice sheet. ISME J. 6 (12), 2302–2313. doi:10.1038/ismej.2012.107
Ye, B., Yang, D., Jiao, K., Han, T., Jin, Z., Yang, H., et al. (2005). The Urumqi River source glacier No. 1, Tianshan, China: changes over the past 45 years. Geophys. Res. Lett. 32 (21). doi:10.1029/2005gl024178
Yoshimura, Y., Kohshima, S., and Ohtani, S. (1997). A community of snow algae on a Himalayan glacier: change of algal biomass and community structure with altitude. Arct. Alp. Res. 29 (1), 126–137. doi:10.2307/1551843
Yue, X., Zhao, J. U. N., Li, Z., Zhang, M., Fan, J. I. N., Wang, L., et al. (2017). Spatial and temporal variations of the surface albedo and other factors influencing Urumqi Glacier No. 1 in Tien Shan, China. J. Glaciol. 63 (241), 899–911. doi:10.1017/jog.2017.57
Zawierucha, K., Baccolo, G., Di Mauro, B., Nawrot, A., Szczuciński, W., and Kalińska, E. (2019). Micromorphological features of mineral matter from cryoconite holes on Arctic (Svalbard) and alpine (the Alps, the Caucasus) glaciers. Polar Sci. 22, 100482. doi:10.1016/j.polar.2019.100482
Zawierucha, K., Stec, D., Lachowska-Cierlik, D., Takeuchi, N., Li, Z., and Michalczyk, Ł. (2018). High mitochondrial diversity in a new water bear species (Tardigrada: eutardigrada) from mountain glaciers in central Asia, with the erection of a new genus Cryoconicus. Ann. Zool. 68 (1), 179–201. doi:10.3161/00034541anz2018.68.1.007
Keywords: cryoconite granules, cyanobacterial taxa, mineral categories, SEM observation, cyanobacteria-mineral interaction, glacier, central Asia
Citation: Chen Y, Takeuchi N, Tanaka S and Li Z (2025) Aggregation of mineral particles in cryoconite granules by multiple species of cyanobacteria on Urumqi Glacier No.1, China. Front. Earth Sci. 13:1596472. doi: 10.3389/feart.2025.1596472
Received: 19 March 2025; Accepted: 13 June 2025;
Published: 27 June 2025.
Edited by:
Eric Josef Ribeiro Parteli, University of Duisburg-Essen, GermanyReviewed by:
Kamil Wojciechowski, Polish Academy of Sciences, PolandNikita Mergelov, Institute of Geography (RAS), Russia
Copyright © 2025 Chen, Takeuchi, Tanaka and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nozomu Takeuchi, bnRha2V1Y2hAZmFjdWx0eS5jaGliYS11Lmpw
 Yunjie Chen
Yunjie Chen Nozomu Takeuchi
Nozomu Takeuchi Sota Tanaka4
Sota Tanaka4