- 1China Railway 15th Bureau Group Corporation Limited, Shanghai, China
- 2China Railway Construction Corporation Limited, beijing, China
- 3Hohai University College of Water Conservancy and Hydropower Engineering, Nanjing, China
- 4Key Laboratory of Ministry of Education for Geomechanics and Embankment Engineering, Nanjing, China
Calcium oxide (CaO) was used to condition urban sludge, with a focus on the impact of conditioning time, and its potential application in sustainable urban development was explored. In this study, the volume reduction ratio (VRR) was employed as the primary evaluation metric, and response surface methodology (RSM) was applied to investigate the combined effects of CaO dosage and conditioning time on sludge dewaterability. The optimal process conditions were determined, and the dewatering mechanism was analyzed. Results showed that the interaction between CaO dosage and conditioning time significantly influenced sludge dewatering performance. Under optimal conditions—0.48 g/g dry solids (DS) of CaO and 14.35 h of conditioning,the volume reduction ratio of sludge reached 72.76% ± 0.56%. Mechanistic analysis revealed that CaO conditioning promotes the embedding of calcium-based compounds into sludge flocs, forming a rigid skeletal structure that facilitates internal water release. These effects enhance with increasing conditioning time up to a certain threshold, thereby significantly improving sludge dewaterability. The findings highlight the potential of CaO conditioning as an effective strategy for sludge management in temporary lagoons and its broader implications for sustainable urban development.
1 Introduction
Most urban sewage treatment plants produce dewatered sludge with a high water content of about 80% (Wei et al., 2020), which is bulky and difficult to transport and store. However, the current relevant Chinese standards clearly stipulated that for landfill or agricultural application the sludge moisture content must be less than 60%. The dewatered sludge that over the local sludge treatment capacity is often stored in the temporary sludge lagoon as a temporary measure, waiting for subsequent treatment. If the increasing sludge is not treated in time, it will cause secondary pollution to the environment (Feng et al., 2015). The reduction of volume through deep dewatering is undoubtedly the key and focus of the sludge subsequent treatment. It can not only reduce the sludge volume and facilitate transportation, but also contribute to subsequent resource utilization and greatly reduce the sludge treatment cost (Wang et al., 2010; Colin and Gazbar, 1995; Zhang et al., 2014).
At present, the sludge deep dewatering usually adopts a combination of chemical conditioning and mechanical dewatering (Liu et al., 2013; He et al., 2015; Zhang et al., 2017). Among them, the dewatering process of adding CaO to sludge is simple and widely used, with low energy consumption (Wei et al., 2022). When the sludge and CaO are uniformly mixed, CaO reacts with the water in the sludge as follows:
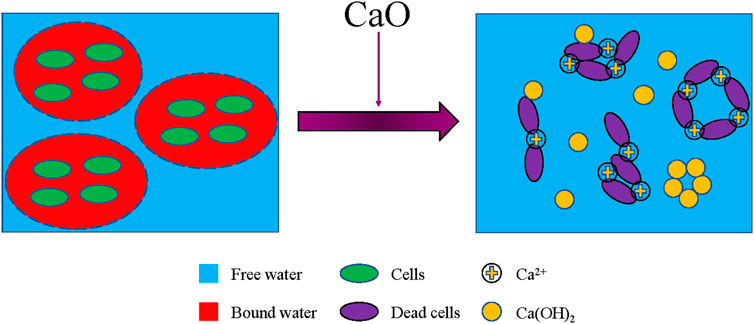
As presented in Equation 1, 0.32 kg of water is combined into Ca(OH)2 for every 1 kg of CaO added, and a large amount of heat is released at the same time, which can evaporate the water in the sludge. And Ca(OH)2 has three effects simultaneously in sludge conditioning: cell disruption, skeleton building, and flocculation. The cell disruption effect is that Ca(OH)2 kills microorganisms by increasing the sludge pH and destroying extracellular polymers, thereby releasing bound water (Garrec et al., 2003). The skeleton building effect is that a large amount of Ca(OH)2 precipitation will form a highly permeable rigid network structure, provide a stable drainage channel, and improve the sludge permeability (Deneux-Mustin et al., 2001; Liang et al., 2015). The flocculation effect is that Ca2+ can exert charge neutralization, bridging, and netting abilities, sweeping the fine organic colloidal particles in the aqueous phase of the sludge into the solid phase (Shanableh, 2017; Wang et al., 2019). Besides, Ca(OH)2 is considered to be the most common material to improve sludge stability. The Ca(OH)2-sludge mixture can maintain a high pH for a long time, which can significantly reduce the microbial content and the bioavailability of heavy metals in the sludge (Wong and Selvam, 2006; Akrivos et al., 2000).
Hwa and Jeyaseelan (1997) earlier studied the conditioning of oily digested sludge by lime and confirmed that it can enhance the dewaterability. Yu et al. (2015) studied the effect of CaO conditioning on the deep dewatering of dewatered sludge and believed that its dissolution exotherm, increase in pH, and skeleton building are all conducive to improving the sludge dewaterability. Liu et al. (2013) and Liang et al. (2015) studied the synergistic effect of lime and Fenton’s reagent in the deep dewatering of sludge, in which lime mainly acts as a skeleton builder. Qin et al. (2023) studied effects of pressure, dewatering time, and sludge cake thickness on the dewatering of dewatered sludge under the condition of adding CaO and FeSO4.
These studies were mechanical dewatering immediately after chemical conditioning, and did not consider the time required for mixing and various reactions during conditioning. Gehrke (1978) added a quantitative amount of CaO to distilled water and sludge filtrate. Under the same stirring condition, it was found that it took 12 min and 18 min to reach the maximum temperature of the solution respectively. This indicates that only the turbidity in the solution will significantly retard the reaction rate of CaO and water. Fries and Rueffer (1979) mixed CaO (88% purity) with dewatered sludge. The experimental results showed that the temperature of dewatered sludge continues to rise, and only begins to slow down or decrease after 80 min. This shows that CaO conditioning requires a certain amount of time during the mixing reaction stage of CaO and sludge. Also, the cell disruption effect of Ca(OH)2 is related to the hydrolysis reaction. It took about 1 h for Ca(OH)2 to hydrolyze sludge at a high temperature of 100°C (Neyens et al., 2003), 3 h at a temperature of 70°C (Collivignarelli et al., 2017), and more time at normal temperature.
The existing research on the application of CaO conditioning in mechanical dehydration was not sufficient, especially the influence of conditioning time was not considered. Therefore, this article aimed to study the combined effect of CaO and conditioning time on deep dewatering of urban dewatered sewage sludge. Through a comparative analysis of the changes in water content, volume reduction ratio, filtrate pH, and scanning electron microscopy (SEM), this paper can provide a reference for the application of CaO in deep dewatering. The response surface methodology (RSM) was used to optimize the combined conditioning of CaO and conditioning time, by taking the volume reduction ratio as the response index.
2 Materials and methods
2.1 Materials
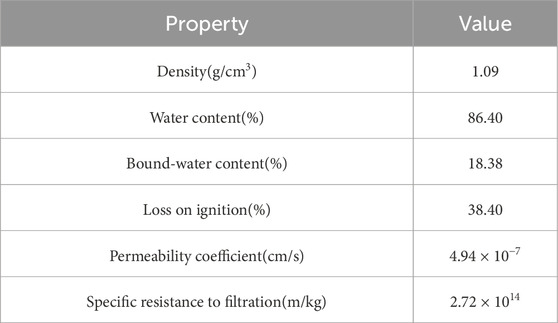
The sludge samples used in this experiment contained dewatered sludge from the Laogang temporary sludge lagoons, which was from the Shanghai Urban Sewage Treatment Plant. They were from the upper part of the temporary sludge lagoons. The dewatered sludge was mainly produced by the water treatment process after adding two kinds of agents, poly aluminum chloride and polyacrylamide, and was piled up in temporary sludge lagoons from 2010 to 2014. Figure 1 displays an actual site photograph, which reveals that after an extended period of storage, the sludge in the temporary sludge lagoon exhibits surface desiccation. However, the moisture content beneath the surface has not significantly diminished, indicating that the sludge retains a high water retention capacity. Sludge samples were taken by the bucketful to a shady area. Properties of the raw sludge (RS) are listed in Table 1. CaO reagent was in powder form, chemically pure.
2.2 Conditioning and dewatering experiments
An electric stirrer was used to condition the sludge. The sludge conditioning method was adding quantitative CaO to 200 mL of sludge, stirring the resulting mixture evenly with a glass rod, and stirring it with an electric stirrer at 300 r/min for 10 min.
The sludge was loaded with a ring knife into an improved consolidometer, and the dewatering experiment was carried out after the conditioning time. In this experiment, based on the previous research (O'Kelly 2005), the standard consolidometer was improved. To reduce the impacts of sludge extrusion and loading cap tilt, a plastic light loading cap was used. At the same time, the consolidation path increased from 2 cm to 4 cm, which reduces the measurement error caused by too much compression deformation of the sludge itself. The dewatering process was pressurizing step by step every 5 min to 0.8 MPa and then lasting for 2 h. The sludge water content, the amount of compression, and the filtrate pH were measured. Volume reduction ratio was calculated by the following equation:
where
2.3 Analytical methods
Prior to examination under a scanning electron microscope (Hitachi SU3500), the sludge samples were subjected to vacuum freeze-drying at −60°C for 48 h. Subsequently, an appropriate amount of the sample was secured onto the specimen stage using a conductive adhesive, followed by gold sputtering to enhance its electrical conductivity. The samples were then placed into the scanning electron microscope chamber for observation of the micromorphology of the sludge.
2.4 Experimental design and data analysis
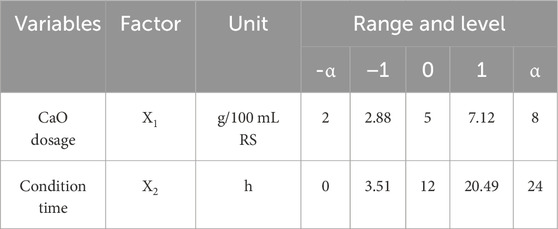
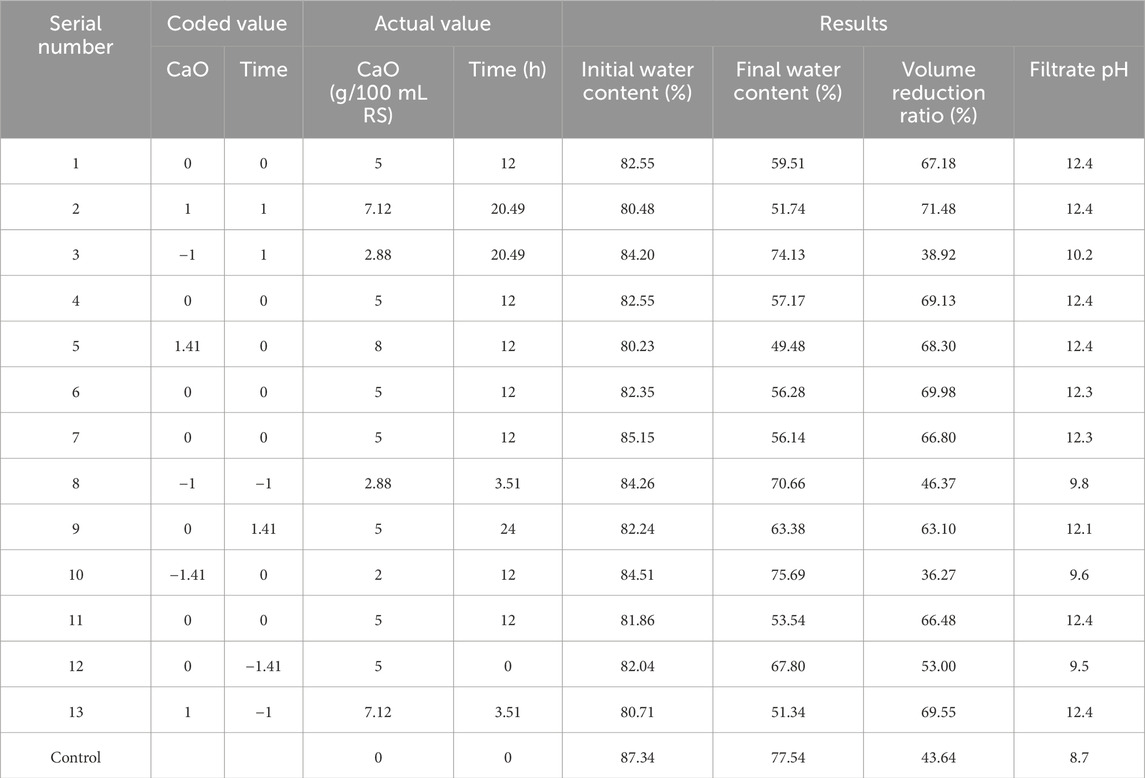
The popular central composite design (CCD) was used for the RSM in the experimental design. In 1951, Box and Wilson introduced the CCD method, which is very suitable for fitting multiple surfaces and is widely used in process optimization (Myers et al., 2004). The effects of two variables in the sludge dewatering process including CaO dosage and conditioning time was investigated. According to the 2-factor CCD, there were 13 experiments in total, consisting of 4 corner points (coded to the usual±1 notation), 4 axial points (±α, 0), (0, ±α), and 5 repeated center points (0, 0). The value of α for rotatability depended on the number of factors of the design, which is given in Equation 3:
where F is decided by the number of factors (F = 2k, k is the number of factors). Therefore, according to Equation 3, where α is equal to (22)1/4 = 1.414.
First, the preliminary single-factor pre-experiment was used to determine the approximate good range of CaO dosage and conditioning time. With the increase of CaO dosage, the volume reduction ratio first increased and then decreased. As the conditioning time increases, the volume reduction ratio increased rapidly and then fluctuated smoothly. Based on the results of the preliminary experiment, we had determined the scope of two factors, as shown in Table 2. In statistical calculations, the variable Xi (the true value of the independent variable) was coded as xi (the dimensionless value of the independent variable), according to the following equation:
where X0 is the value of Xi at the center point, and
Experimental data were analyzed by Design Expert 10. Polynomial model fitting is generally used for optimization in engineering practice. The applicability of the proposed model was diagnosed by analysis of variance (ANOVA). The accuracy of the fit polynomial model was represented by the coefficient of determination R2, which is a measure of the amount of variation around the mean explained by the model. These analyses were all done through Fisher’s ‘F’ test and P-value (probability). The model used a P-value of 95% as the confidence level. Finally, the optimal value of the key parameter was obtained by analyzing the response surface model and solving the fitting equation.
3 Results and discussion
3.1 Overall analysis
The experiment was carried out according to CCD, and the experimental design and results are shown in Table 3. The initial water content is the water content of the sludge after CaO conditioning. CaO conditioning can significantly reduce the water content in the conditioning stage. CaO will react with the water to form Ca(OH)2, which means that while the reaction reduces the water in the sludge, it also increases the amount of solids. So, a significant change can be seen after the reaction to the water content. When CaO dosage was 8 g/100 mL RS, the initial water content changed from 87.34% to 80.23%. This shows that due to a large amount of conditioning agent added, it will have a greater impact on the water content, and the commonly used water content index cannot accurately characterize the conditioning effect alone.
The final water content is the water content of the sludge after dewatering experiments. It can be seen from Table 3 that the most final water content is less than 60% (control group is 77.54%), which meets the basic requirements of deep dewatering. The real goal of deep dewatering is sludge reduction, not simply reducing sludge water content. The conditioner CaO is solid and its density is much higher than sludge. Adding CaO has little effect on the sludge volume. Therefore, this article used the sludge volume reduction ratio as the main indicator to accurately characterize the sludge conditioning effect on deep dewatering.
3.2 Volume reduction ratio analysis
Taking the volume reduction ratio as the response value, the data obtained from the experiment was fitted with the least square method in the form of coded factors. Equation 4 is a regression model of the volume reduction ratio affected by CaO dosage and conditioning time with the best fitting effect.
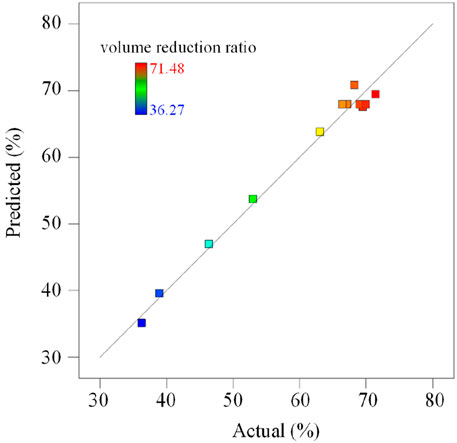
In Equation 5, a positive sign in front of the terms indicates synergistic effects, whereas a negative sign indicates antagonistic effects. The reliability requires the model to achieve reasonable accuracy when predicting the response value. Figure 2 compares the volume reduction ratio of the experiment with the predicted value obtained from the model. It shows that the experimental value and the predicted value are in good agreement.
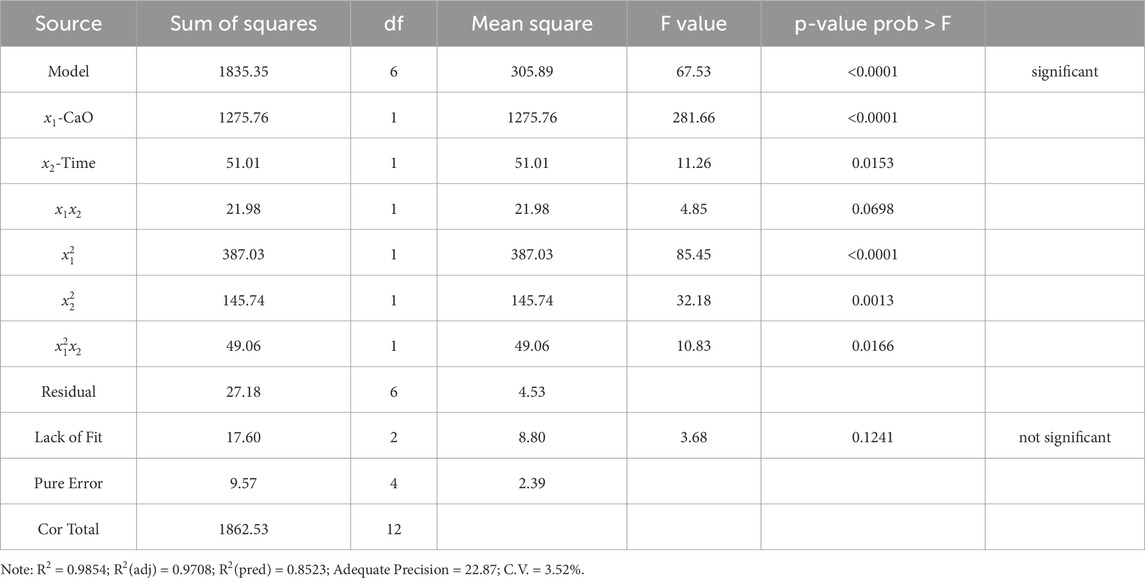
The analysis of variance for the volume reduction ratio model is shown in Table 4. The regression coefficient R2 is 0.9854, R2(adj) is 0.9708, R2(pred) is 0.8523, indicating that there is a strong correlation between the model prediction results and the experiment results. The F value of the model is 67.53, and the P-value is less than 0.0001. When the P-value is less than 0.05, it indicates that the model is statistically significant. The F value of the lack-of-fit term is 3.68 and the P-value is 0.1241, which means that the lack-of-fit term is less obvious than the pure error. This indicates that the model has a good predictive fit. Adequate Precision is a signal-to-noise ratio, >4 proves that the average prediction error of the model is small. The coefficient of variation (C.V.) is less than 10%, which proves that the model has high reliability and good repeatability.
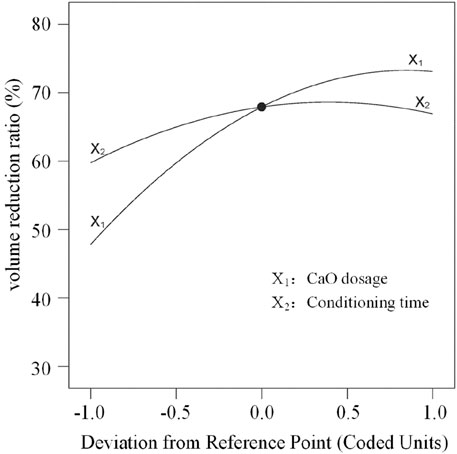
To compare the sensitivity of the volume reduction ratio to CaO dosage and conditioning time, a perturbation graph of the experimental conditions at the center point is shown in Figure 3. The coded value at the center point x1 = 0, x2 = 0 (actual value X1 = 5 g/100 mL RS, X2 = 12 h). It can be clearly seen from Figure 3 that the volume reduction ratio near the center point changes more drastically with CaO dosage than with conditioning time. That is, the volume reduction ratio is more sensitive to the CaO dosage than conditioning time.
Three-dimensional plots and contour plots of the interaction effect of CaO dosage and conditioning time on volume reduction ratio are shown in Figure 4. It more intuitively illustrates the combined effects of CaO dosage and conditioning time on the sludge volume reduction ratio and characterizes the shape of the response surface model. Within a certain range, the sludge volume reduction ratio shows an upward trend with the increase of the CaO dosage. If the CaO dosage continues to be increased, the sludge volume reduction ratio shows a downward trend, indicating that excessive CaO will curb the sludge volume reduction ratio. This is because the skeleton building effect of CaO will increase the permeability and reduce the compressibility (Wang et al., 2019; Liu et al., 2017). Excessive CaO makes the sludge more difficult to compress. The increase of conditioning time will also cause the volume reduction ratio to increase first and then decrease slightly. The rising part is because it takes a certain time for CaO and sludge to fully mix, and it also takes a certain time for Ca(OH)2 to participate in the hydrolysis reaction to produce the cell disruption effect. The descending section is because Ca(OH)2 is consumed in the process of the hydrolysis reaction, which leads to the weakening of the skeleton building effect, reduced sludge permeability, and slower dehydration speed. And the dewatering experiment limited the time, so the experiment results of the volume reduction ratio showed a small drop.
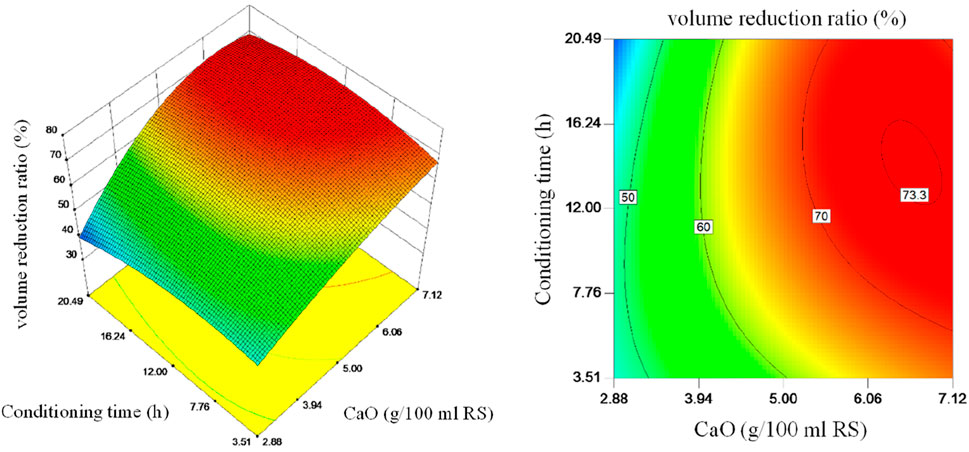
Figure 4. 3D response surface and contour plots: interaction effect of CaO dosage and conditioning time on volume reduction ratio.
The response surface model was used to determine the optimal conditions for variables operation in the conditioning process. The volume reduction ratio regression equation Equation 4 taken the maximum value when the coding variables x1 = 0.75 and x2 = 0.28, which is 73.54%. The corresponding CaO dosage was 6.59 g/100 mL RS, [i.e., a CaO dosage of 48% dry solids (DS)], and the conditioning time was 14.35 h. In order to investigate the accuracy and practicability of the response surface model equations, experiments were conducted under optimal conditions. The results showed that the volume reduction rate of sludge is 72.76% ± 0.56%, which is consistent with the predicted value of the model. Therefore, the optimal process parameters obtained based on the response surface method were accurate and reliable, which had certain guiding significance for related sludge dewatering and condition optimization.
3.3 Filtrate pH analysis
CaO reacts with water to form Ca(OH)2 which is slightly soluble in water. At 20°C, the pH of a Ca(OH)2 saturated solution is 12.65. The filtrate pH can directly reflect the Ca(OH)2 concentration in the liquid phase, and it can also reflect whether the CaO dosage is appropriate and whether the CaO and sludge are fully mixed.
Taking the filtrate pH as the response, the data obtained from the experiment was fitted with the least square method in the form of coded factors. Equation 6 is a regression model of the filtrate pH affected by CaO dosage and conditioning time.
The P-value of the model is 0.0014, the regression coefficient R2 is 0.9130, and R2(adj) is 0.8509, indicating that there is a strong correlation between the model prediction results and the experimental results.
Three-dimensional plots and contour plots of the interaction effect of CaO dosage and conditioning time on filtrate pH are shown in Figure 5. It more intuitively illustrates the combined effects of CaO dosage and conditioning time on filtrate pH and characterizes the shape of the response surface model. It can be seen from Figure 5 that the filtrate pH increases with the increase of CaO dosage within the research range, and the two are completely positively correlated. The filtrate pH first increases and then decreases with the increase of conditioning time. The rising section is because it takes a certain time for CaO to react with water, and it also takes a certain time for Ca(OH)2 obtained by the reaction and sludge to be fully mixed; the falling section is because the partial OH− in the liquid phase is consumed by hydrolysis.
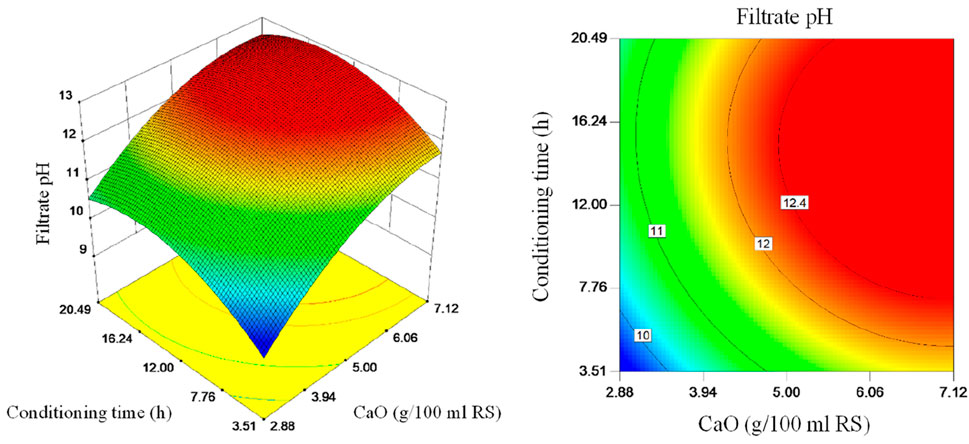
Figure 5. 3D response surface and contour plots: interaction effect of CaO dosage and conditioning time on filtrate pH.
Comparing Figures 4, 5, the filtrate pH change trend is similar to the volume reduction ratio before the pH value increases to the highest value. They all increase with the increase of CaO dosage and conditioning time. High pH means stronger hydrolysis capacity (Na et al., 2010). With the increase of CaO dosage and conditioning time, when filtrate pH is close to the highest value, the optimal conditioning conditions are approaching. Therefore, the filtrate pH can be used as an indicator of whether CaO dosage and conditioning time are suitable, and whether CaO and sludge are fully mixed.
3.4 SEM analysis
The RS, the sludge conditioned by CaO 5 g/100 mL RS, and the sludge conditioned by CaO 5 g/100 mL RS and 24 h were freeze-dried and observed with SEM to obtain Figure 6.
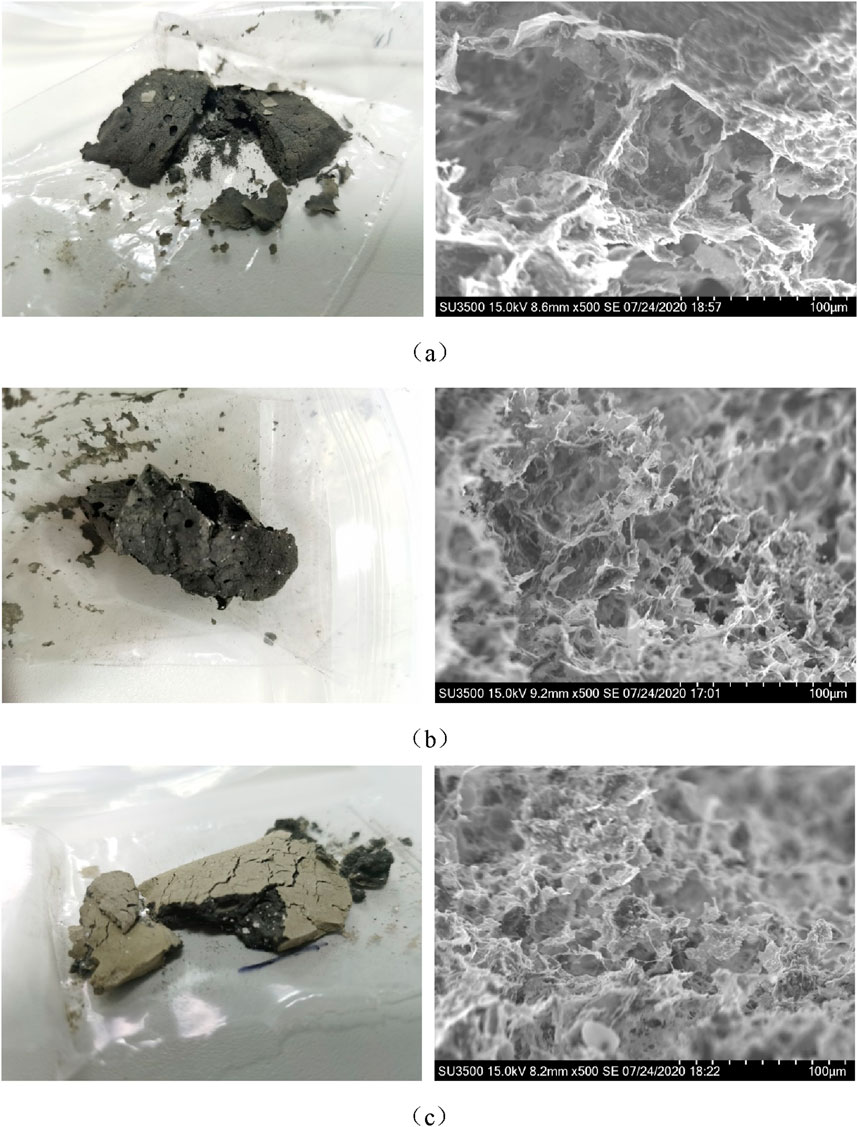
Figure 6. Appearance and SEM images of sludge samples: (a) the RS, (b) the sludge conditioned by CaO 5 g/100 mL RS, (c) the sludge conditioned by CaO 5 g/100 mL RS and 24 h.
For the appearance photos of the freeze-dried sludge, it can be seen from Figure 6a that the RS has a dense structure and fewer voids after freeze-drying, which makes it difficult to remove the internal water. The sludge conditioned by CaO has a loose structure and many voids, as shown in Figure 6b. In Figure 6c, after 24 h of CaO conditioning, the strength of the sludge sample increased significantly. The sludge in Figure 6a is all black, and a layer of off-white substance appears on the surface in Figures 6b,c. This is because facultative and anaerobic bacteria are black when the sludge is stored in closed storage. After long-term contact with air on the outer surface, aerobic bacteria are yellowish-brown, and this part is off-white after freeze-drying. Subsequent research will draw upon methodologies employed in the study of soil cracking characteristics to investigate the crack propagation mechanisms and the evolution of mechanical behavior during the sludge deep dewatering process (Yang et al., 2024a; Yang et al., 2024b).
For the SEM images of the freeze-dried sludge, it can be seen from Figure 6a that the RS is densely agglomerated, mainly composed of coarse flocs with large particle diameters, tightly packed, and strong adsorption. These make it difficult to remove the internal water. As shown in Figure 6b, after CaO conditioning, the sludge floc has cracks and holes from the previous dense mass, and the surface becomes honeycomb. At the same time, the calcium mixture is embedded in the sludge flocs to form a rigid skeleton structure, which provides a channel for internal water and significantly improves the dewatering performance. The corresponding mechanism of action is illustrated in Figure 7. In Figure 6c, after 24 h of CaO conditioning, the sludge structure does not change significantly on the microscopic picture. But combined with appearance performance, the effect of CaO conditioning is greatly enhanced.
3.5 Urban sustainable development
Currently, the resource utilization of the temporary sludge lagoon is low, and environmental risks are prominent. The use of calcium oxide (CaO) for the deep dewatering of municipal dewatered sludge offers the advantages of high efficiency, economy, and safety.
Firstly, deep dewatering significantly reduces the volume and weight of the sludge, thereby minimizing the potential environmental pollution during sludge treatment and transportation, protecting water bodies and soil from organic matter and heavy metal contamination. Secondly, dewatered sludge is more amenable to resource utilization, such as conversion into fertilizers or soil conditioners, which not only reduces the exploitation of natural resources but also promotes the development of a circular economy, enhancing the efficiency of resource use (Yuan et al., 2023; Yuan et al., 2024). Lastly, as environmental regulations become increasingly stringent, cities must comply with the relevant sludge treatment and disposal regulations. Deep dewatering is an effective means to meet these regulatory requirements, helping cities avoid legal risks and economic penalties associated with non-compliant sludge treatment.
Therefore, deep dewatering of municipal dewatered sludge is a win-win strategy for environmental protection, resource recycling, and regulatory compliance, playing a significant role in promoting sustainable urban development.
4 Conclusion
This paper studied the time-dependent dewatering effects on the deep dewatering of urban dewatered sludge in the temporary sludge lagoon. All the results of water content change, volume reduction ratio, and filtrate pH indicated that it is necessary to consider conditioning time when using CaO as a conditioning agent. In addition, RSM was used to optimize the combined conditioning, and the volume reduction ratio was used as the response. After the RSM optimization, experiments were conducted to verify that the sludge volume reduction ratio is increased to 72.76% ± 0.56% when CaO dosage was 0.48 g/g DS and conditioning time was 14.35 h. In the dewatering process, the filtrate pH can be used as an indicator of whether CaO dosage and conditioning time are suitable, and whether CaO and sludge are fully mixed. After CaO conditioning, the sludge flocs appeared cracks and holes from the previous dense mass, and the surface became honeycomb. At the same time, the calcium mixture is embedded in the sludge flocs to form a rigid skeleton structure, which significantly improves the sludge dewaterability. These effects will increase over time. The results indicate that the treatment method proposed in this article contributes to the sustainable development of cities.
Future work will incorporate microscopic characterization (e.g., EPS component analysis, water form distribution) and macro-micro mechanical tests to further elucidate the time-dependent effects of CaO conditioning on sludge dewatering.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
YZ: Writing – review and editing, Writing – original draft, Conceptualization. CH: Data curation, Writing – review and editing. YW: Methodology, Supervision, Writing – original draft. XZ: Writing – original draft. HL: Writing – review and editing, Software. SZ: Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by National Key R&D Program of China (2023YFC3707804), Henan Province Science and Technology Development Project N. 232102320048. The authors declare that this study received funding from Major Science and Technology Projects of China Railway Construction Corporation Limited (2023A02). The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Conflict of interest
Authors YZ, HL and SZ were employed by China Railway 15th Bureau Group Corporation Limited.
Author CH was employed by China Railway Construction Corporation Limited.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Akrivos, J., Mamais, D., Katsara, K., and Andreadakis, A. (2000). Agricultural utilisation of lime treated sewage sludge. Water Sci. Technol. 42 (9), 203–210. doi:10.2166/wst.2000.0207
Colin, F., and Gazbar, S. (1995). Distribution of water in sludges in relation to their mechanical dewatering. Water Res. 29 (8), 2000–2005. doi:10.1016/0043-1354(94)00274-b
Collivignarelli, M. C., Sordi, M., Abba, A., Castagnola, F., and Bertanza, G. (2017). Treatment of waste activated sludge by means of alkaline hydrolysis under 'mild' conditions. Int. J. Of Glob. Warming 11 (3), 1–316. doi:10.1504/ijgw.2017.10001247
Deneux-Mustin, S., Lartiges, B. S., Villemin, G., Thomas, F., Yvon, J., Bersillon, J. L., et al. (2001). Ferric chloride and lime conditioning of activated sludges: an electron microscopic study on resin-embedded samples. Water Res. 35 (12), 3018–3024. doi:10.1016/s0043-1354(01)00003-3
Feng, L., Luo, J., and Chen, Y. (2015). Dilemma of sewage sludge treatment and disposal in China. Environ. Sci. Technol. 49 (8), 4781–4782. doi:10.1021/acs.est.5b01455
Fries, D. R. K.-H., and Rueffer, H. (1979). Einsatz von Kalk bei der weitergehenden Schlammentwaesserung. Korresp. Abwasser 26 (6), 291–295.
Garrec, N., Picard-Bonnaud, F., and Pourcher, A. M. (2003). Occurrence ofListeriasp. andL. monocytogenesin sewage sludge used for land application: effect of dewatering, liming and storage in tank on survival ofListeriaspecies. FEMS Immunol. Med. Microbiol. 35 (3), 275–283. doi:10.1016/s0928-8244(02)00443-1
Gehrke, R. (1978). Anwendung von Kalk in kommunalen Kläranlagen. Korresp. Abwasser 25 (11), 375–389.
He, D. Q., Wang, L. F., Jiang, H., and Yu, H. Q. (2015). A Fenton-like process for the enhanced activated sludge dewatering. Chem. Eng. J. 272, 128–134. doi:10.1016/j.cej.2015.03.034
Hwa, T. J., and Jeyaseelan, S. (1997). Comparison of lime and alum as oily sludge conditioners. Water Sci. Technol. 36 (12), 117–124. doi:10.2166/wst.1997.0438
Liang, J., Huang, S., Dai, Y., Li, L., and Sun, S. (2015). Dewaterability of five sewage sludges in Guangzhou conditioned with Fenton's reagent/lime and pilot-scale experiments using ultrahigh pressure filtration system. Water Res. 84, 243–254. doi:10.1016/j.watres.2015.07.041
Liu, H., Yang, J. K., Zhu, N. R., Zhang, H., Li, Y., He, S., et al. (2013). A comprehensive insight into the combined effects of Fenton's reagent and skeleton builders on sludge deep dewatering performance. J. Hazard. Mater. 258, 144–150. doi:10.1016/j.jhazmat.2013.04.036
Liu, H. B., Xiao, H., Fu, B., and Liu, H. (2017). Feasibility of sludge deep-dewatering with sawdust conditioning for incineration disposal without energy input. Chem. Eng. J. 313, 655–662. doi:10.1016/j.cej.2016.09.107
Myers, R. H., Montgomery, D. C., Vining, G. G., Borror, C. M., and Kowalski, S. M. (2004). Response surface methodology: a retrospective and literature survey. J. Qual. Technol. 36 (1), 53–77. doi:10.1080/00224065.2004.11980252
Na, S. H., Shon, H. K., Kim, J. B., Park, H. J., Cho, D. L., El Saliby, I., et al. (2010). Recycling of excess sludge using titanium tetrachloride (TiCl4) as a flocculant aid with alkaline-thermal hydrolysis. J. Of Industrial And Eng. Chem. 16 (1), 96–100. doi:10.1016/j.jiec.2010.01.013
Neyens, E., Baeyens, J., and Creemers, C. (2003). Alkaline thermal sludge hydrolysis. J. Hazard. Mater. 97 (1-3), 295–314. doi:10.1016/s0304-3894(02)00286-8
Qin, W. H., Zhu, X. L., Liu, C., and Lin, F. (2023). Factors affecting the mechanical deep dewatering of sludge from wastewater treatment. Bioresources 18 (3), 5269–5282. doi:10.15376/biores.18.3.5269-5282
Shanableh, A. (2017). Modelling impact of precipitation of magnesium using lime on removal of select wastewater contaminants. Desalin. Water. Treat. 100, 287–294. doi:10.5004/dwt.2017.21202
Wang, W., Luo, Y., and Qiao, W. (2010). Possible solutions for sludge dewatering in China. Front. Of Environ. Sci. and Eng. China 4 (1), 102–107. doi:10.1007/s11783-010-0001-z
Wang, Y., Zhou, Y., Feng, D., Wu, C., Wang, X., and Min, F. (2019). Effects of chemical conditioners on deep dewatering of urban dewatered sewage sludge in the temporary sludge lagoon. J. Of Environ. Eng. 145 (10). doi:10.1061/(asce)ee.1943-7870.0001555
Wei, L. L., Zhu, F. Y., Li, Q. Y., Xue, C. H., Xia, X. H., Yu, H., et al. (2020). Development, current state and future trends of sludge management in China: based on exploratory data and CO2-equivaient emissions analysis. Environ. Int. 144, 106093. doi:10.1016/j.envint.2020.106093
Wei, R., Zhang, R. N., Song, L. J., Zhou, X., Lin, S. H., Zhao, Y. C., et al. (2022). Incineration disposal of organic waste bio-residue via a deep dewatering process using refuse incineration bottom ash: moisture transfer and low calorific value improvement. Environ. Sci. Pollut. Res. 29 (51), 78107–78119. doi:10.1007/s11356-022-22645-1
Wong, J. W. C., and Selvam, A. (2006). Speciation of heavy metals during co-composting of sewage sludge with lime. Chemosphere 63 (6), 980–986. doi:10.1016/j.chemosphere.2005.08.045
Yang, B. B., Chen, Y., and Yuan, S. C. (2024a). Investigation on structure, evaporation, and desiccation cracking of soil with straw biochar. Land Degrad. Dev. 35 (9), 3126–3135. doi:10.1002/ldr.5122
Yang, B. B., Chen, Y., Zhao, C., and Li, Z. L. (2024b). Effect of geotextiles with different masses per unit area on water loss and cracking under bottom water loss soil conditions. Geotext. Geomembranes 52 (2), 233–240. doi:10.1016/j.geotexmem.2023.10.006
Yu, L., Yu, Y., Jiang, W. T., Wei, H. Z., and Sun, C. L. (2015). Integrated treatment of municipal sewage sludge by deep dewatering and anaerobic fermentation for biohydrogen production. Environ. Sci. And Pollut. Res. 22 (4), 2599–2609. doi:10.1007/s11356-014-3514-3
Yuan, B. X., Liang, J. K., Huang, X. L., Huang, Q. Y., Zhang, B. F., Yang, G. H., et al. (2024). Eco-efficient recycling of engineering muck for manufacturing low-carbon geopolymers assessed through LCA: exploring the impact of synthesis conditions on performance. Acta Geotech. doi:10.1007/s11440-024-02395-9
Yuan, B. X., Chen, W. J., Li, Z. H., Zhao, J., Luo, Q. Z., Chen, W. W., et al. (2023). Sustainability of the polymer SH reinforced recycled granite residual soil: properties, physicochemical mechanism, and applications. J. Soils Sediments 23 (1), 246–262. doi:10.1007/s11368-022-03294-w
Zhang, H., Yang, J. K., Yu, W. B., Luo, S., Peng, L., Shen, X. X., et al. (2014). Mechanism of red mud combined with Fenton's reagent in sewage sludge conditioning. Water Res. 59, 239–247. doi:10.1016/j.watres.2014.04.026
Keywords: CaO, conditioning time, deep dewatering, temporary sludge lagoons, response surface methodology, volume reduction ratio, filtrate pH, sustainable development
Citation: Zhou Y, Huang C, Wang Y, Zhao X, Luan H and Zhang S (2025) Time-dependent effects of calcium oxide on urban sludge dewatering in temporary lagoons and its application in sustainable urban development. Front. Earth Sci. 13:1605262. doi: 10.3389/feart.2025.1605262
Received: 03 April 2025; Accepted: 20 June 2025;
Published: 09 July 2025.
Edited by:
Binbin Yang, Xuchang University, ChinaReviewed by:
Hao Wang, University of Science and Technology Beijing, ChinaXiangchun Xu, Henan University, China
Copyright © 2025 Zhou, Huang, Wang, Zhao, Luan and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Changfu Huang, SHVhbmdjaGYwMTJAMTYzLmNvbQ==
 YunTian Zhou1
YunTian Zhou1 Changfu Huang
Changfu Huang Shuailong Zhang
Shuailong Zhang