- 1Xi’an University of Architecture and Technology, Xi’an, Shaanxi, China
- 2State Key Laboratory of Green Building, Xi’an, Shaanxi, China
- 3Engineering Research Institute, China Construction Eighth Engineering Division Corp., Ltd., Shanghai, China
Electroosmosis method is an environmentally friendly and low-carbon foundation treatment technique that can quickly improve soil shear strength. To investigate the effect of electric potential on water migration in unsaturated loess, this study employed a custom-designed one-dimensional laboratory experimental device to conduct electroosmosis experiments with six different electrode materials: copper, aluminum, stainless steel, nickel, graphite, and iron. Changes in water content within the unsaturated loess were monitored before and after the experiments. The internal current, electric potential distribution, interface resistance, and soil resistivity were observed during the experiments. The results indicated that: (1) the maximum and minimum water migration amounts were 8% and 3.4%, respectively, with the maximum being 2.35 times the minimum. The preferred ranking of electrode materials for soil water migration efficiency was stainless steel > nickel > graphite > aluminum > copper > iron. (2) The effective electric potential of soil samples across all six test soil samples continuously decreased with increasing electrification duration, while the current initially increased and then decreased. The interface resistance continuously increased, while the soil resistivity for graphite electrode rapidly increased over time. Based on the variations in various parameters and energy efficiency during the experiments, the optimal ranking of electrode materials was as follows: graphite > stainless steel > nickel > aluminum > copper > iron; (3) Due to their high energy utilization rates and strong corrosion resistance, stainless steel, nickel, and graphite are recommended as the preferred electrode materials for both laboratory and field tests. In practical engineering applications, moisture migration volume is prioritized; thus, the final electrode selection order is: stainless steel > nickel > graphite > aluminum > copper > iron.
1 Introduction
Deep foundation pits and subway construction in the northwest China frequently encounter challenges due to soft soil layers with high water content. These soft soil layers possess low strength and significant deformation and exhibit properties similar to those of soft clay. Their presence complicates subway construction and increases safety risks during construction. Conventional techniques such as replacement filling, dynamic compaction, preloading, and vacuum preloading have stringent site requirements, lengthy construction durations, considerable carbon emissions, and can easily disturb the soil, making them unsuitable for rapid drainage construction in weak soil layers.
Electroosmosis method is capable of quickly draining water from the soil to facilitate consolidation and settlement, thereby improving the load-bearing capacity of the foundation (Gong, 2014; Ying, 2011; Zhuang, 2016). It is primarily a foundation treatment technique for soft clay with high water content. Water molecules in the soil are positively charged. Under the influence of a direct current electric field, water gradually moves from the anode to the cathode and finally accumulate at the cathode until it is discharged. In 1879, Helmholtz (Mitchell, 1976) firstly established a theoretical model to study the electro-osmotic mechanism. Casagrande (Casagrande, 1948) introduced electroosmotic phenomena into soil mechanics for the first time in 1939. Esrig (1968) and Lewis and Humpheson (1973) proposed one-dimensional and two-dimensional electroosmotic consolidation theories, respectively. Wan and Mitchell (1976) examined consolidation characteristics of soil under combined effects of electro-osmosis and pile load. In 1991, Lo and Ho (1991) conducted laboratory experiments on sensitive clay using a small electroosmotic device and compared the changes in soil strength before and after electroosmotic reinforcement. In 1997, Laursen (1997) conducted laboratory electroosmotic experiments on weathered soil and natural clay.
Wang (1999) was the first researcher in China to investigate the electroosmotic method. In 1956, Zeng and Gao (1956) conducted reinforcement tests on soft clay using the electroosmotic method and determined its effectiveness in improving soil properties for practical engineering applications. Zou et al. (2002) and Hu (2005) conducted laboratory electroosmotic experiments utilizing electric geosynthetic materials. Pang et al. (2006) performed laboratory model tests combining vacuum preloading and electroosmotic reinforcement. Zhuang and Wang (2004); Zhuang and Wang (2005) derived the expression for interface resistance based on the experiments and established a charge accumulation model. In 2010, Li et al. (Li et al., 2009; Li et al., 2010; Jiao et al., 2011) conducted a series of laboratory electroosmotic experiments on saturated soft clay and established a coupled consolidation equation that accounts for the combined effects of pile load and electroosmosis. Additionally, Tao et al. (2013a), Zhou et al. (2023), Gan et al. (2022), Tao et al. (2014) conducted systematic studies on electro-osmosis experiments under different electrode materials for soft clay and theoretical analysis of interfacial resistance in soft clay. Numerous scholars have extensively investigated electro-osmosis experiments (Lin and Hu, 2024; Ren et al., 2021; Huang et al., 2025; Yang et al., 2020; Xiang et al., 2022; Zhao, 2022; Mahalleh et al., 2021; Sun et al., 2024; Cui et al., 2021) on various soils and performed in-depth theoretical analyses (Yang et al., 2021; Qin et al., 2022).The experimental findings indicate that the conductivity of electrode materials significantly influences the effectiveness of water migration within the soil. Electrode materials with high conductivity not only enhance water migration efficiency and reduce energy consumption but also prolong the service life of electrodes.
Existing research on unsaturated loess (Wang and Lin, 1990; Gao et al., 2015; Lei et al., 2012) indicates that the engineering properties of unsaturated loess are closely related to the soil water content. Reducing the water content of loess can significantly improve its shear strength and deformation modulus. Therefore, electroosmotic method may be employed to reinforce high-water-content unsaturated loess, enhancing its engineering properties. This method is recognized as a green, environmentally friendly, and sustainable approach for foundation reinforcement. It presents significant promotion prospects in loess regions. On the basis of existing electroosmotic tests on soft clay, relevant theoretical frameworks (Qiu et al., 2017; Shen et al., 2015; Xue et al., 2019; Liu et al., 2019; Xie et al., 2018), and prior investigations into water migration in unsaturated loess under electric conditions (Wang et al., 2018; Jiao et al., 2019), this study conducted a series of laboratory experiments on water migration in unsaturated loess under the influence of an electric field utilizing various electrode materials. High-performance electrode materials suitable for unsaturated loess were identified by comparing the water migration within the loess using different electrode materials. This research provides a reference for the optimal electrode materials in subsequent on-site electroosmotic tests on unsaturated loess.
2 Experimental design
2.1 Experimental materials
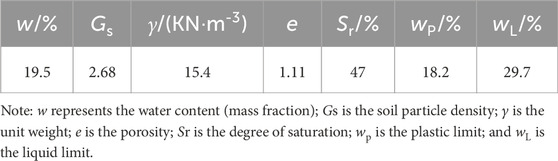
Laboratory experiments were conducted on water migration in unsaturated loess using different electrode materials. The results were then compared and analyzed to identify the optimal electrode material for water migration under the influence of an electric field in unsaturated loess. The soil used in this experiment was sourced from the loess region in Xi’an, and its physical properties in its natural state are presented in Table 1. The experiment utilized remolded soil samples. The preparation process involved mixing dried, crushed, and sieved soil powder with a suitable amount of water to achieve a uniform mixture, which was configured according to predetermined void ratios. The samples were sealed for 48 h to ensure uniform water content. The soil sample preparation process can be found in the “Specification for soil test” (SL237-1999).
Based on different electrical conductivity properties, the experiment utilized six metals as electrode materials: stainless steel, nickel, graphite, copper, aluminum, and iron. The potential within the soil was measured using probes made from fine iron wire with a diameter of 0.1 cm, while a geotextile was employed as a filter layer material between the electrodes and the soil.
2.2 Experimental device and methods
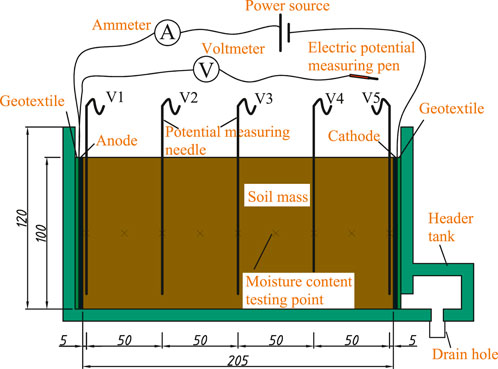
The experiments were conducted using a custom-made Miller soil box, which consists of a detachable main body and a water collection tank on the right side. The model chamber has internal net dimensions of 21.5 × 10 × 16 cm and is made of 0.8 cm thick glass. After the experiment, the glass sidewalls can be sequentially removed to extract the intact soil sample. A collection tank is positioned on the right side of the model chamber. A 1 cm high gap is provided at the connection point between the model chamber and the water collection tank to serve as a drainage channel for water. Additionally, a drainage hole is reserved at the bottom of the water collection tank to collect the water discharged during the experiment. The measurement apparatus for the experiment consists of a voltmeter, ammeter, potential measurement probes, and a graduated cylinder. The experiment utilized a constant voltage power supply with a maximum output voltage of 30V. The experimental device is presented in Figure 1.
Five potential measuring probes made from fine iron wire with a diameter of 0.1 cm were sequentially inserted into the potential testing holes. A voltmeter was used to measure the potential at each probe within the soil. The instantaneous current in the soil was measured using an ammeter. The water discharged from the soil was firstly channeled through the drainage hole at the bottom of the water collection tank into a flask and then transferred into a graduated cylinder for measurement. The electrode materials used in the experiments were six metal sheets: copper, aluminum, stainless steel, nickel, graphite, and iron. Each sheet measures 10 × 10 cm with a thickness of 0.1 cm. A total of six specimens were prepared for the experiments, with each test soil sample corresponding to a specific type of electrode material. The electrodes were embedded on both sides of the soil sample during preparation, while ensuring that they were closely attached to the sidewalls of the test box. Geotextile was placed between the electrodes and the model chamber to facilitate water drainage. The initial and electrical conditions for each soil specimen were identical, prior investigations into moisture migration dynamics in unsaturated loess under electric fields by our research group have established that reduced water content and degree of saturation enhance migration efficacy (Wang et al., 2018; Jiao et al., 2019). This study focuses on comparative evaluation of six electrode materials. To achieve measurable outcomes within a limited electrification period, remolded loess with controlled low moisture and saturation levels was utilized. Detailed physicochemical properties of the remolded loess are tabulated in Table 2.
The experiment is under an electric potential gradient of 1V/cm.After the electricity was applied in the experiment, measurements of the instantaneous current, potential at each probe, and drainage volume were performed every 3 h. The water contents at different locations in the soil were tested at the end of the experiment. The specific experimental procedures can be referenced from the “Specification for soil test” (SL237-1999). The measurement inaccuracies of current and voltage must be restricted to ±0.5%, whereas moisture content measurements should adhere to a tighter tolerance of ±(0.1–0.5)%,ensuring high-fidelity data acquisition for electro-osmotic treatment optimization.Due to the low initial water content of soil and the short electrification duration, no significant water discharge was observed during the experiment. The water only migrated and accumulated from the anode to the cathode.
3 Analysis of experimental results
3.1 Distribution of water content
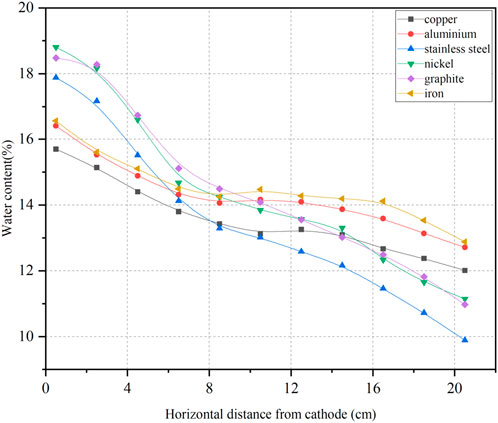
The changes in water content of soil during the experiment were primarily due to water migration caused by electric potential, while a smaller portion resulted from electrochemical reactions under the influence of the electric field. This section focused solely on water migration induced by electric potential. Eleven water content measurement points were established sequentially in each group of soil samples to assess the spatial distribution of water content from the cathode to the anode after the power supply was turned off. The spatial distribution of water content is presented in Figure 2. Figure 2 indicates that after the power supply was turned off, all six soil samples showed lower water content near the anode compared to their initial values, while the moisture content near the cathode was higher than the initial water content. The water content decreased more significantly as the distance to the anode decreased. The electrode materials that exhibited smaller reductions in water content at the anode and smaller increases at the cathode included aluminum, copper, and iron. In contrast, the electrode materials that showed greater reductions at the anode and larger increases at the cathode were stainless steel, nickel, and graphite. The reduction rates of water content at the anode are presented in Table 3. Table 3 illustrated that stainless steel, nickel, and graphite demonstrated effective water migration, with all reduction rates of water content in the soil at the anode exceeding 20%. Aluminum and copper followed with slightly lower reduction rates, while the iron electrode displayed the poorest water migration effect, with a reduction rate of only 8.7%.
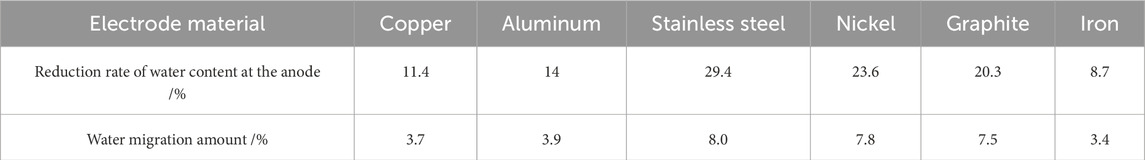
Table 3. Comparison of changes in water content at the anode and water migration amount after the experiment.
Under the influence of electric potential, water in the soil moved from the anode to the cathode and accumulates near the cathode. The soil near the cathode gradually transitioned from unsaturated to saturated state. The water content of the soil at the cathode continuously increased; however, it never reached the saturation level calculated based on the initial void ratio. Consequently, no water was discharged from the soil during the experiment. The total amount of water migration in the soil can be approximated as the total reduction in water content in the anode region. The longer the electrification duration, the greater the effect of electro-osmosis. As a result, the amount of water migration driven by the electric potential increased. The approximate formula for calculating the water migration amount can be calculated using Equation 1:
where Q represents the total amount of water migration, △wl is the reduction in water content of the soil at different locations, l is the distance from each location to the anode, and i is the distance between the anode and cathode.
Table 3 compares the water migration amounts in the soil across the six experimental samples after the experiments. The efficacy of electro-osmotic moisture migration is rigorously evaluated through two key parameters: the anode water content reduction rate and moisture migration percentage (Table 3). A direct correlation exists between these metrics and migration efficiency, where elevated values signify enhanced performance in reducing soil moisture content.The findings revealed that the electrode materials with the higher water migration amounts are stainless steel, nickel, and graphite, whereas aluminum, copper, and iron exhibited lower water migration amounts. Under identical initial conditions, the maximum and minimum water migration amounts were 8% and 3.4%, respectively, with the maximum being 2.35 times the minimum for different electrode materials.Considering both the reduction rate of water content at the anode and the amount of water migration within the soil, the optimal order of electrode materials for water migration experiments in unsaturated loess under an electric field was as follows: stainless steel > nickel > graphite > aluminum > copper > iron.
3.2 Distribution of electric potential
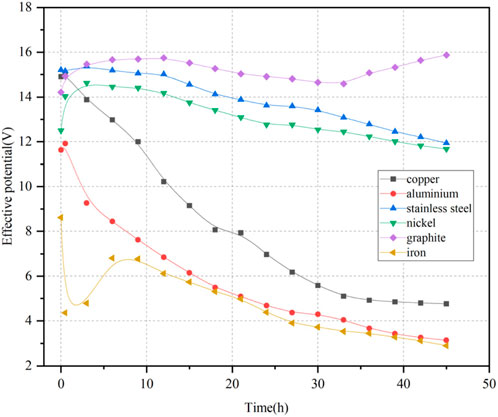
The presence of electrode corrosion and interface resistance at the contact surface between the electrode and the soil can increase voltage loss during the experiment. This voltage loss does not affect water migration within the soil. To minimize its impact on the distribution of electric potential in the soil, two electric potential measurement probes near the electrodes were maintained at a distance of 0.5 cm from both electrodes. Five electric potential measurement probes were sequentially placed within the soil from the anode to the cathode. The potential difference between measurement probes one and five near the electrodes represents the effective voltage driving water migration in the soil, which is referred to as the effective electric potential. The ratio of the effective electric potential to the distance between probes one and five is defined as the electric potential gradient, which serves as a crucial parameter affecting water migration in the electric field. The electric potential gradient used in all six experimental soil samples was set at 1 V/cm. Figure 3 illustrates the distribution of effective electric potential within the soil for different electrode materials. The effective electric potential decreased continuously with increasing electrification duration, where the iron electrode exhibited the most significant decline, while the graphite electrodes showed the least reduction. When considering only the effects of different electrode materials on the effective electric potential in the soil, the optimal selection order was as follows: graphite > stainless steel > nickel > copper > aluminum > iron.
Figure 4 displays the variations of electric potential for three representative electrode materials: iron, graphite, and stainless steel. The results suggested that the electric potential distribution across various locations is relatively uniform and resembles a linear distribution. The slopes of the electric potential distribution curves near the cathode and anode were greater than that of the curve in the middle of the soil. The voltage measured at all five probes continuously increased over time, while the voltage difference between probes one and 5, representing the effective electric potential in the soil, steadily decreased. At the moment of power application, the effective electric potential of the iron electrode was 43% of the power supply voltage and decreased to 15% by the end of the experiment. For the stainless steel electrode, the effective electric potential started at 76% of the power supply voltage and dropped to 60% by the conclusion of the experiment. Conversely, the effective electric potential of the graphite electrode began at 71% of the power supply voltage and slightly increased to 80% by the experiment’s end. This slight increase is attributed to the good chemical stability of the graphite electrode, which experienced minimal corrosion. The accumulation of water at the cathode increased the contact between the soil and the cathode plate, which in turn reduced the voltage near the cathode. The graphite and stainless steel electrodes are superior to the iron electrode regarding the effective electric potential loss.
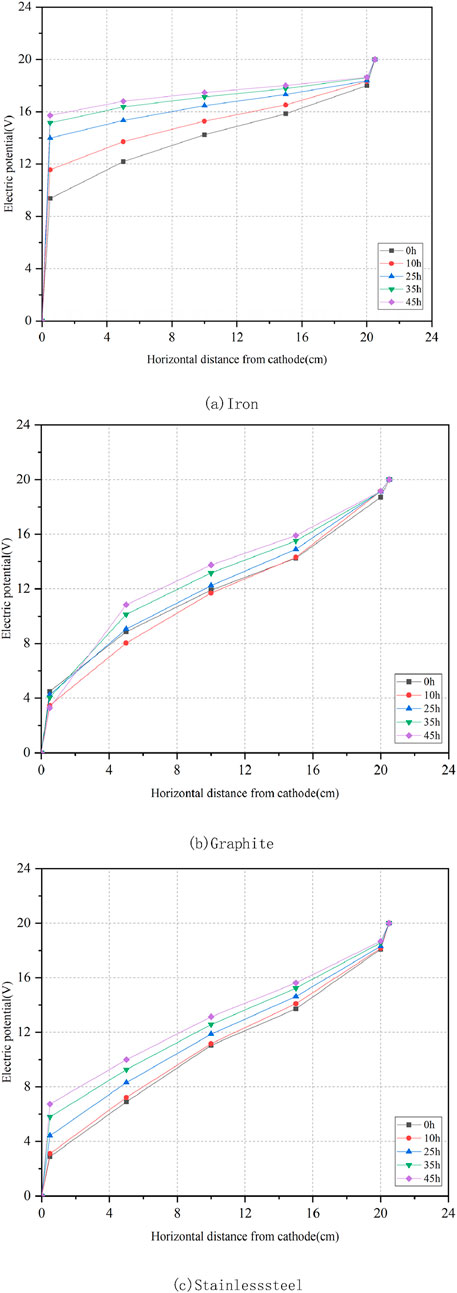
Figure 4. (a) Iron. (b) Graphite. (c) Stainless steel. Distribution of electric potential in the soil for different electrode materials.
3.3 Current variation and electric energy consumption
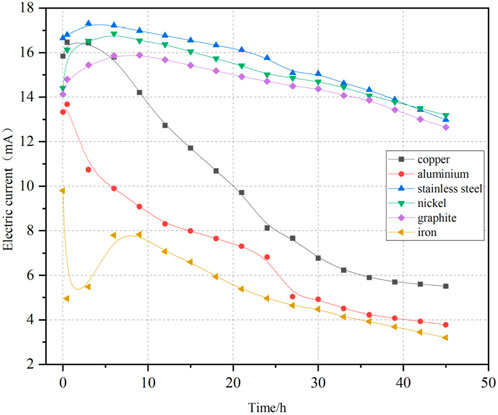
During the experiment, the current in the six soil samples varied continuously over time. Figure 5 depicts the variation of current in the soil over time. It was observed that the currents associated with the graphite, stainless steel, nickel, copper, and aluminum electrodes rise initially and subsequently decline over time. In contrast, the current linked to the iron electrode initially declined rapidly, then increased, and ultimately exhibited a continuous decrease. The currents within the soil associated with the graphite, stainless steel, and nickel electrodes were generally higher than linked to the copper, aluminum, and iron electrodes.
The current magnitude within a unit of time reflects the electric energy consumption of the experiment to some extent. The electric energy consumption over a fixed time interval can be estimated using the following formula:
where W represents the cumulative electric energy consumption during the experiment, T is the electrification duration, and I is the current for different electrification durations.
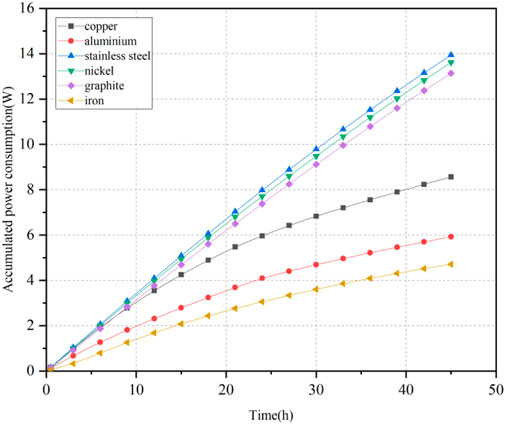
The variations of electric energy consumption over time for the six experimental soil samples can be calculated using Equation 2, as illustrated in Figure 6. The order of cumulative electric energy consumption for electrode materials from highest to lowest was as follows: stainless steel > nickel > graphite > copper > aluminum > iron.
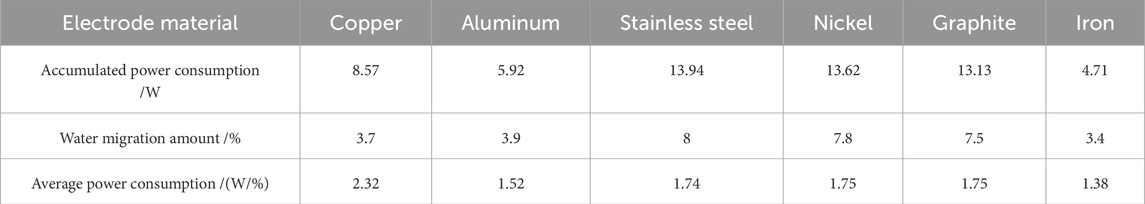
Table 4 quantifies the average energy consumption per unit moisture migration percentage. Economically, copper demonstrates the highest energy demand and least cost-effectiveness, whereas aluminum and iron offer superior economic performance due to lower energy requirements. Stainless steel, nickel, and graphite represent intermediate solutions with balanced energy consumption and financial efficiency.
3.4 Variations in interface resistance and soil resistivity
Interface resistance arises at the contact surface between the soil and metal electrodes due to differences in conductive area and electrode corrosion during power application. This resistance consumes a portion of the power supply voltage, which reduces its utilization efficiency. The energy consumed by the interface resistance does not contribute to water migration. Additionally, the magnitude of the interface resistance reflects the conductivity properties of the electrode materials to some extent.
The magnitude of interface resistance can be calculated using Equation 3:
where Rinterface is the interface resistance of the soil in Ω; Utotal is the power supply voltage in V; Ueffective is the effective electric potential of the soil in V; I is the current in the soil in A.
The interfacial resistance was inferred computationally via Equation 3, as direct measurement was omitted in this study. A stabilized 20 V power supply ensured consistent experimental conditions. The effective potential was quantified as the potential difference between Probe one and Probe 5, while the instantaneous soil current was recorded synchronously. This methodology allowed precise determination of time-resolved interfacial resistance.
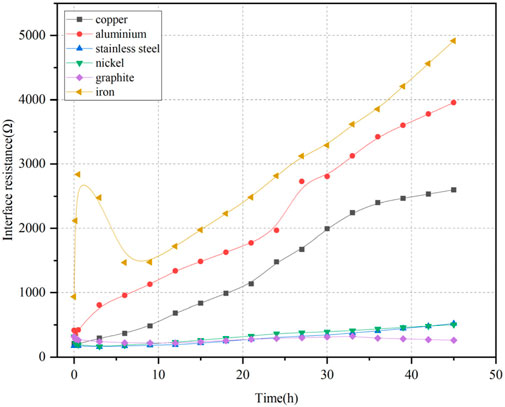
Figure 7 illustrates the variation of interface resistance at the anode over time. The interface resistance of the iron electrode first increased, then rapidly decreased, and finally increased slowly. For the other five electrode materials, interface resistance steadily increased over time. The ranking of interface resistance at the anode from highest to lowest was iron > aluminum > copper. These were significantly higher than those of stainless steel, nickel, and graphite, which exhibited smaller and more stable resistance values over time.
Soil resistivity is another fundamental parameter that reflects soil conductivity. It is defined as the resistance of a cubic soil body with a side length of 1 m when current flows perpendicularly through it. Factors influencing soil resistivity include soil grain composition, mineral content, bonding state, void ratio, degree of saturation, and resistivity of the pore liquid. Soil resistivity can be calculated using Equation 4:
where ρ represents the soil resistivity in Ω·m, E is the effective electric potential of the soil in V (i.e., the electric potential difference between probes one and five), A is the cross-sectional area of the soil (m2), L is the effective length of the soil sample in the electro-osmosis experiment in m, and I is the magnitude of the current passing through the soil in A.
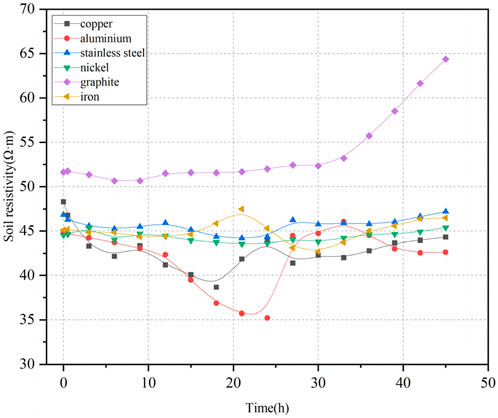
Figure 8 shows the variation of soil resistivity over time. For the graphite electrode, soil resistivity remained relatively stable over time and maintained a relatively high value. After 33 h, the resistivity began to increase gradually over time. For the other five electrode materials, soil resistivity showed minimal variations over time. For the iron, copper, and aluminum electrodes, soil resistivity fluctuated significantly around the 20-h mark during the experiment, while changes in soil resistivity at other times were relatively stable.
3.5 Electrode corrosion
After the experiment, the corrosion conditions of the six electrode materials are illustrated in Figure 9. The omission of pre- and post-test mass measurements was justified by two factors: (1) negligible mass loss due to short electrification time, and (2) simultaneous electrochemical oxidation of electrode surfaces that offset potential mass changes. As the pre- and post-test mass difference became unreliable, the gravimetric analysis of electrode mass variation was omitted. Building upon prior research on electrode materials (Tao et al., 2013a; Zhang et al., 2019; Tao et al., 2013b), as well as examining the corrosion photos. The anodic corrosion was significantly greater than the cathodic corrosion across all six electrode materials. Stainless steel, nickel, and graphite electrodes displayed relatively low corrosion, while iron, aluminum, and copper electrodes exhibited greater corrosion. From the perspective of electrode material corrosion, it is recommended to prioritize stainless steel, nickel, and graphite as electrode materials in future electro-osmosis experiments on loess.
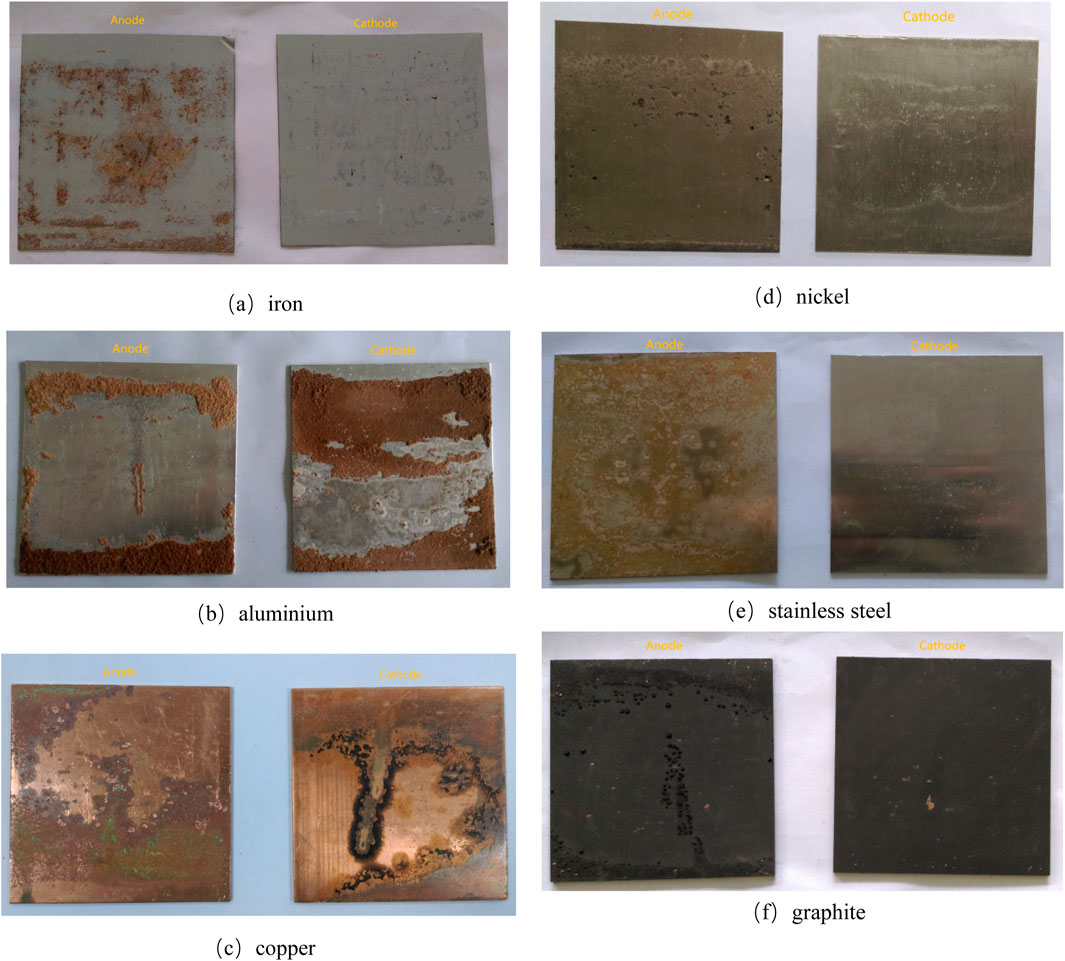
Figure 9. (a) iron. (b) aluminium. (c) copper. (d) nickel. (e) stainless steel. (f) graphite. Corrosion of electrode materials after the experiment.
After the experiment, cracks appeared on the surface of the soil. The maximum width of the cracks reached 0.3 cm. Cracks at the anode were wider than those at the cathode. All electric potential measurement probes showed some degree of corrosion and blackening, and obvious moisture migration phenomena were observed on the soil surface after the experiment,as depicted in Figure 10. White substances precipitated on the cathode surface. The soil near the anode exhibited acidity, while the soil near the cathode showed alkalinity. This indicates that certain chemical reactions occurred during the application of the electric field. The chemical reaction equations at the anode and cathode are expressed as Equations 5–8:
The white precipitate primarily consists of carbonate deposits of Ca2+ and Mg2+.
3.6 Causes of water migration in unsaturated loess under an electric field
Water molecules in soil are polar. Under the influence of an external electric field, these molecules exhibit specific alignment characteristics and readily combine with dissolved cations in water to form hydrated cations. The surface of soil particles carries a negative charge. Due to this surface charge, polar water molecules and hydrated cations near the particle surfaces experience strong electrostatic attraction, forming a tightly bound fixed layer (strongly bound water layer). Strongly bound water is difficult to remove due to intense adsorption and remains close to the solid phase.Outside the fixed layer, polar water molecules and hydrated cations experience weaker electrostatic forces. Molecular diffusion dominates here, forming the diffuse layer (weakly bound water layer). In this layer, water molecules and hydrated cations are less influenced by electrostatic forces. Electro-osmosis disrupts the original electrostatic equilibrium by applying a voltage across the soil, enabling the migration of weakly bound water. Beyond the diffuse layer, water unaffected by the surface charge is termed free water. These water molecules, either polarized or hydrated with cations, can rapidly migrate under an applied electric field.
Due to the charged nature of clay particles and the formation of a diffuse double layer, when an external electric field is applied to the soil-water system, exchangeable cations in the diffuse layer and free pore water migrate from the anode to the cathode. Concurrently, these cations drag polar water molecules toward the cathode, resulting in water migration from the anode side to the cathode side within the soil. This microscale mechanism underpins the electro-osmotic phenomenon.
The water in saturated loess migrates and accumulates at the cathode before being gradually discharged. As water is continuously discharged from the soil, saturated loess gradually transitions to unsaturated loess. The water in unsaturated loess migrates from the anode to the cathode. It accumulates only at the cathode and does not exit the soil. Key factors influencing water migration in unsaturated loess include soil structure, current propagation paths within the soil, and the driving forces for water migration.
The microstructure of loess (Hou et al., 2007) reveals that unsaturated loess is composed of sand grains, coarse silt, binding agents, pores, and water in those pores. Soil particles are negatively charged, while water molecules are positively charged. Under the influence of electric potential, water molecules move through the pores of soil particles from the anode to the cathode. The water migration in the soil due to electric potential mainly involves free water in the soil and weakly bound water on the soil particle surfaces. However, strongly bound water cannot migrate within the soil due to the strong adsorption effects of the particles.
There are three current propagation paths in soil (Corwin and Lesh, 2005), as depicted in Figure 11. Given the presence of water films on the soil particle surfaces in unsaturated soil, there is inadequate direct contact between particles, which complicates the current propagation through path 3. Additionally, the abundant large pores in unsaturated loess lead to water preferentially filling these pores, which makes it difficult to form interconnected conductive paths. Consequently, the current propagation through path two is likewise challenging. Therefore, current in unsaturated loess primarily propagates through path 1, which is formed by the series connection of soil and water.
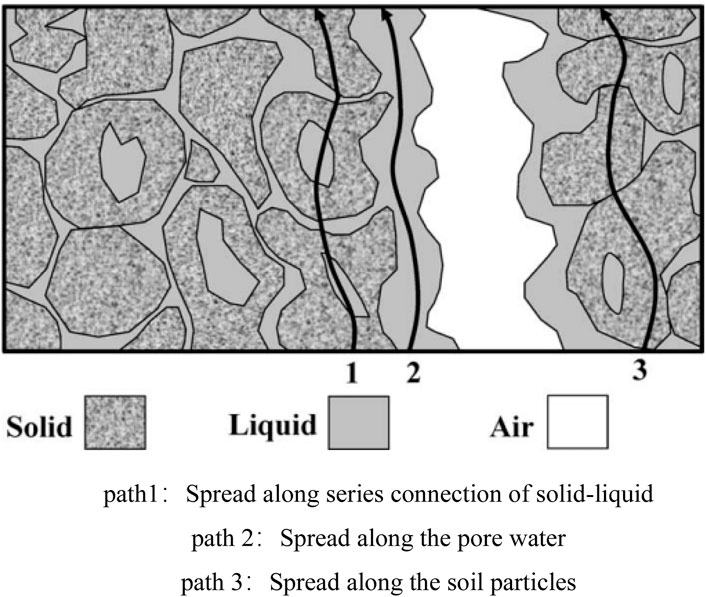
Figure 11. path1:Spread along series connection of solid-liquid. path 2:Spread along the pore water. path 3:Spread along the soil particles. Schematic diagram of three current propagation paths in soil.
Water in unsaturated loess migrates from a high-energy state to a low-energy state. In the electric field generated by the water-soil series circuit, positively charged free water molecules and some weakly bound water molecules moves from high-potential energy regions to low-potential energy regions in unsaturated loess. The energy of water in the soil consists of both kinetic and potential energy. Given the slow movement of water in the soil, its kinetic energy can be considered negligible. Therefore, the driving force for water migration in unsaturated soil is predominantly derived from potential energy.
The total potential energy of water in the soil is referred to as water potential, which is expressed as Equation 9:
where
Compared to clay, the pore structure of loess significantly impacts moisture migration within the soil: (1) Initial Efficiency: Loess exhibits higher moisture migration efficiency initially due to its macropores enabling rapid water transport. (2) Later Decline: Efficiency drops sharply as macropores become air-filled after water content reduction, increasing electrical resistance and weakening the electric field effect. (3) Clay Behavior: In contrast, clay’s poor pore connectivity causes minimal disruption during dewatering. Slow pore compression gradually increases resistance, leading to a slow decline in effective potential and prolonged moisture migration.
4 Conclusion
This study employed six different electrode materials with varying conductivity, including copper, aluminum, stainless steel, nickel, graphite, and iron, to conduct water migration experiments on unsaturated loess sourced from northwest China under an electric potential gradient of 1V/cm. The findings demonstrated that the electric field significantly influences water migration in unsaturated loess. During the experiments, water moved from the anode to the cathode and accumulated at the cathode. The loess at the cathode gradually transitioned from an unsaturated to a saturated state. A small amount of liquid water was discharged from the unsaturated loess with a high initial water content at the cathode. The following conclusions are drawn:
(1) After the experiment, the stainless steel electrode had the highest water migration amount of 8%, while the iron electrode yielded the lowest water migration amount of 3.4%. The maximum water migration amount was 2.35 times the minimum. From the perspective of water migration efficiency, the electrode materials can be ranked in the following order: stainless steel > nickel > graphite > aluminum > copper > iron.
(2) During the experiment, the effective electric potential of the soil samples with six different electrode materials decreased continuously with increasing electrification duration. The current exhibited an overall pattern of first increasing and then decreasing. The interface resistance generally increased over time. The soil resistivity with the graphite electrode increased rapidly, while resistivity with the other five materials remained relatively stable. Based on the changes in effective electric potential, current, interface resistance, and resistivity, the electrode materials can be prioritized in the following order: graphite > stainless steel > nickel > aluminum > copper > iron.
(3) From the perspectives of energy consumption and electrode corrosion, the stainless steel, nickel, and graphite electrode materials demonstrated higher energy utilization rates and strong corrosion resistance. Considering the comprehensive effects of water migration and parameter variations during the experiments, the stainless steel, nickel, and graphite are optimal choices for electrode materials in laboratory and field tests on unsaturated loess.In field applications, moisture migration efficiency is the primary criterion for electrode selection. Accordingly, the optimized electrode sequence is: stainless steel > nickel > graphite > aluminum > copper > iron, balancing energy consumption and long-term stability.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
DJ: Supervision, Writing – original draft, Formal Analysis. ZH: Data curation, Writing – review and editing, Resources. TW: Investigation, Writing – review and editing. YZ: Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The project team at Xi’an University of Architecture and Technology appreciates financial support from Major Program of National Natural Science Foundation of China under Contract No.52394221. This study’s experimental costs were funded by the program.
Conflict of interest
Author YZ was employed by China Construction Eighth Engineering Division Corp., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Casagrande, L. (1948). Electroosmosis in soils. Geotechnique 1 (1), 159–177. doi:10.1680/geot.1949.1.3.159
Corwin, D. L., and Lesh, S. M. (2005). Apparent soil electrical conductivity measurements in agriculture. Comput. Electron. Agric. 46 (1–3), 11–43. doi:10.1016/j.compag.2004.10.005
Cui, K., Cheng, F., and Chen, W. (2021). Experimental study on electro-osmosis grouting reinforcement of scaling off earthen sites. Chin. J. Rock Mech. Eng. 40 (2), 390–398. doi:10.13722/j.cnki.jrme.2020.0294
Esrig, M. I. (1968). Pore pressures, consolidation, and electrokinetics. J. SMFD, ASCE 94 (SM4), 899–921. doi:10.1061/jsfeaq.0001178
Gan, Q., Zhou, J., Tao, Y., and Jiang, Y. (2022). Interfacial resistance model for electro-osmotic system. Geotechnique 74 (3), 221–237. doi:10.1680/jgeot.21.00211
Gao, S., Luo, Y., and Hu, H. (2015). Triaxial tests on water immersion of unsaturated and undisturbed loess. Chin. J. Geotechnical Eng. (7), 1313–1318. doi:10.11779/CJGE201507019
Gong, X. (2014). Foundation treatment technology and development prospects. Beijing: China Architecture and Building Press.
Hou, Z., Liu, Z., and Wu, C. (2007). Special soil foundation. Beijing: China Building Materials Industry Press, 50–62.
Hu, Y. (2005). Experimental study on electro-osmotic consolidation of soft clay using EKG electrodes. Chin. J. Geotechnical Eng. 27 (5), 582–586. doi:10.3321/j.issn:1000-4548.2005.05.020
Huang, W.-C., Li, G., Luo, S., Wei, W., Nan, C.-Z., and Zhang, W.-F. (2025). Impact of potential gradient on soft soil electro-osmotic consolidation effect and energy consumption. J. Changjiang River Sci. Res. Inst. 42 (4), 134–141. doi:10.11988/ckyyb.20240023
Jiao, D., Gong, X., and Li, Y. (2011). Experimental study of consolidation of soft clay using electro-osmosis method. Chin. J. Rock Mech. Eng. (S1), 3208–3216.
Jiao, D., Ren, R., and Wang, T. (2019). Experimental research on effect of soil salinity in loess moisture migration under action of electric potential. J. Central South Univ. 50 (12), 3075–3083. doi:10.11817/j.issn.1672-7207.2019.12.017
Laursen, S. (1997). Laboratory investigation of electroosmosis in bentonites and natural clays. Can. Geotechical J. 34 (5), 664–671. doi:10.1139/t97-043
Lei, S., Li, Z., and Wang, J. (2012). Effect of water content on strength of unsaturated loess. J. Traffic Transp. Eng. (1), 1–5. doi:10.19818/j.cnki.1671-1637.2012.01.001
Lewis, W. R., and Humpheson, C. (1973). Numerical analysis of electroosmotic flow in soils. J. SMFD, ASCE 95 (SM4), 603–616. doi:10.1061/jsfeaq.0001912
Li, Y., Gong, X., Jiao, D., and Liu, Z. (2009). Experimental study on two-dimensional electro-osmotic consolidation of soft clay. Chin. J. Rock Mech. Eng. (9), 4034–4039. doi:10.3321/j.issn:1000-6915.2009.z2.108
Li, Y., Gong, X., and Lu, M. (2010). Coupling consolidation theory under combined action of load and electro-osmosis. Chin. J. Geotechnical Eng. (1), 77–81.
Li, Y. (2011). Experimental and theoretic study on electro-osmotic consolidation of soft clay foundation. Hangzhou: Zhejiang University.
Lin, Z., and Hu, L. (2024). Experimental study of effect of geochemical conditions on electro-osmotic consolidation of soft soils. J. Hydroelectr. Eng. 43 (6), 33–42. doi:10.11660/slfdxb.20240604
Liu, Z., Zhuang, Y., and Huang, Y. (2019). Field test on electroosmosis using EKG electrode. J. Water Resour. Archit. Eng. 17 (2), 46–51. doi:10.3969/j.issn.1672-1144.2019.02.008
Lo, K. Y., Inculet, I. I., and Ho, K. S. (1991). Electroosmotic strength of soft sensitive clays. Can. Geotechical J. 28 (1), 62–73. doi:10.1139/t91-007
Mahalleh, H. A. M., Siavoshnia, M., and Yazdi, M. (2021). Effects of electro-osmosis on the properties of high plasticity clay soil: chemical and geotechnical investigations. J. Electroanal. Chem. 880, 114890. doi:10.1016/j.jelechem.2020.114890
Pang, Y., Xu, M., and Zhu, Z. (2006). Experimental investigation into draining consolidation behavior of soda residue soil under vacuum preloading-electro-osmosis. J. South China Univ. Technology:Natural Sci. Ed. 34 (11), 70–75. doi:10.3321/j.issn:1000-565X.2006.11.015
Qin, A., Meng, H., and Jiang, L. (2022). Analysis of axisymmetric consolidation characteristics of unsaturated soils under surcharge loading and electro-osmosis. Rock Soil Mech. 43 (6), 97–106. doi:10.16285/j.rsm.2021.2137
Qiu, C.-C., Shen, Y., Li, Y.-D., You, Y.-F., and Rui, X.-X. (2017). Laboratory tests on soft clay using electro-osmosis in combination with vacuum preloading. Chin. J. Geotechnical Eng. 39 (S1), 251–255. doi:10.11779/CJGE2017S1050
Ren, L., Cao, H., and Kong, G. (2021). Experimental on treatment effect of chemical electroosmosis on soft clay influenced by reagent injection position. Rock Soil Mech. 42 (10), 1–9. doi:10.16285/j.rsm.2021.0116
Shen, Y., Xu, H., and You, Y. (2015). Cyclic electrode conversion law in treatment of soft soil foundation. Chin. J. Geotechnical Eng. 37 (S1), 65–71. doi:10.11779/CJGE2015S1014
Sun, H., Li, C.-A., and Qiu, J.-W. (2024). Experimental study on remediation of heavy metal contaminated kaolin by electrokinetic geosynthetics drainage board. J. Changjiang River Sci. Res. Inst. 41 (2), 123–127. doi:10.11988/ckyyb.20230173
Tao, Y., Zhou, J., and Gong, X. (2013a). Comparative experiment on influence of ferrum and cuprum electrodes on electroosmotic effects. Chin. J. Geotechnical Eng. 35 (2), 388–394.
Tao, Y., Zhou, J., and Gong, X. (2013b). Comparative experiment of electroosmosis of ferrum, graphite, copper and aluminum electrode. Chin. J. Rock Mech. Eng. 32 (A1), 3355–3362.
Tao, Y., Zhou, J., and Gong, X. (2014). Experimental Study on function mechanism of electrode materials upon electro-osmotic process. J. Zhejiang Univ. Sci. 48 (9), 1618–1623. doi:10.3785/j.issn.1008-973X.2014.09.011
Wan, T. Y., and Mitchell, J. K. (1976). Electro-osmotic consolidation of soils. J. Geotechnical Eng. Div. 102 (5), 473–491. doi:10.1061/ajgeb6.0000270
Wang, T., Cao, H., and Jiao, D. (2018). Experimental research on unsaturated loess moisture migration under the Action of electric potential. Chin. J. Undergr. Space Eng. (04), 994–998.
Wang, W. (1999). Selected works on geotechnical engineering of academician Wang Wenshao. Beijing: China Architecture & Building Press.
Wang, Y., and Lin, Z. (1990). Structural characteristics and physical and mechanical properties of Chinese loess. Beijing: Science Press, 181–194.
Xiang, P., Cui, Y., and Wei, G. (2022). Study on the effect of low-temperature anode filled with FeCl3 solution on electro-osmotic reinforcement of soft clay. Appl. Sci. 12 (5), 2517. doi:10.3390/app12052517
Xie, X., Li, Z., Zheng, L., Li, J., and Liu, Y. (2018). Experimental study on influencing factors of contact resistance on electroosmotic consolidation. J. Central South Univ. 49 (3), 655–662. doi:10.11817/j.issn.1672-7207.2018.03.019
Xue, Z., Tang, X., Yang, Q., Tian, Z., and Zhang, Y. (2019). Influence of salt content on clay electro-dewatering with copper and stainless steel anodes. Dry. Technol. 37 (15), 2005–2019. doi:10.1080/07373937.2018.1555709
Yang, K., Yuan, G., and Fu, H. (2020). Experimental study on influence of electrification method on soft soil enhanced by electroosmosis. Water Resour. Hydropower Eng. 51 (2), 170–176.
Yang, X., Dong, J., and Liu, G. (2021). Study on two-dimensional electroosmotic consolidation of punctiform electrode units with symmetric and asymmetric forms. Chin. J. Rock Mech. Eng. 40 (7), 1491–1502. doi:10.13722/j.cnki.jrme.2020.0983
Zeng, G., and Gao, Y. (1956). Electrochemical stabilization of soft clay. J. Zhejiang Univ. (02), 12–35.
Zhang, L., Wang, N., and Jing, L. (2019). Comparative experiment of different electrode materials on electro-osmotic consolidation. Rock Soil Mech. 40 (9), 3493–3501. doi:10.16285/j.rsm.2018.1109
Zhao, H. (2022). Mechanism of using electroosmosis and different chemical solutions to reinforce mucky clay. J. Yangtze River Sci. Res. Inst. 39 (5), 99–105. doi:10.11988/ckyyb.20210567
Zhou, J., Jiang, Y., Zhu, Z., and Gan, Q. (2023). Theoretical and experimental studies on interfacial resistance of electro-osmotic consolidation for soft ground improvement. Chin. J. Geotechnical Eng. 45 (10), 1995–2003. doi:10.11779/CJGE20220838
Zhuang, Y. (2016). Theory and design method for electro-osmotic consolidation. Chin. J. Geotechnical Eng. (A1), 152–155. doi:10.11779/CJGE2016S1028
Zhuang, Y., and Wang, Z. (2004). Study on interface electric resistance of electro-osmotic consolidation. Rock Soil Mech. 25 (1), 117–120. doi:10.3969/j.issn.1000-7598.2004.01.025
Zhuang, Y., and Wang, Z. (2005). Electric charge accumulation theory for electro-osmotic consolidation. Rock Soil Mech. 26 (4), 629–632. doi:10.3969/j.issn.1000-7598.2005.04.026
Keywords: unsaturated loess, electric potential effect, water migration, optimal ranking of electrode materials, experimental study
Citation: Jiao D, He Z, Wang T and Zhang Y (2025) Experimental study on water migration in unsaturated loess under an electric field: optimization of electrode material. Front. Earth Sci. 13:1606868. doi: 10.3389/feart.2025.1606868
Received: 06 April 2025; Accepted: 02 June 2025;
Published: 30 June 2025.
Edited by:
Binbin Yang, Xuchang University, ChinaReviewed by:
Junbiao Yan, Chinese Academy of Sciences (CAS), ChinaAbbas E. Rahma, King Faisal University, Saudi Arabia
Copyright © 2025 Jiao, He, Wang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhiheng He, MTc4MjkzODA1MTJAMTYzLmNvbQ==
 Dan Jiao
Dan Jiao Zhiheng He
Zhiheng He Tiehang Wang1
Tiehang Wang1 Yonggang Zhang
Yonggang Zhang