- 1Institute for the Oceans and Fisheries, University of British Columbia, Vancouver, BC, Canada
- 2Department of Earth, Ocean and Atmospheric Sciences, University of British Columbia, Vancouver, BC, Canada
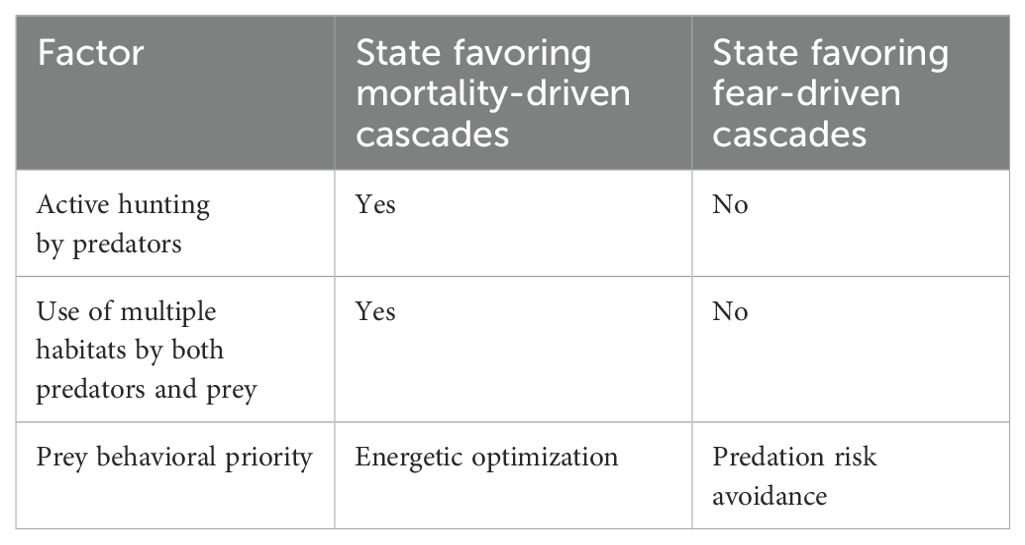
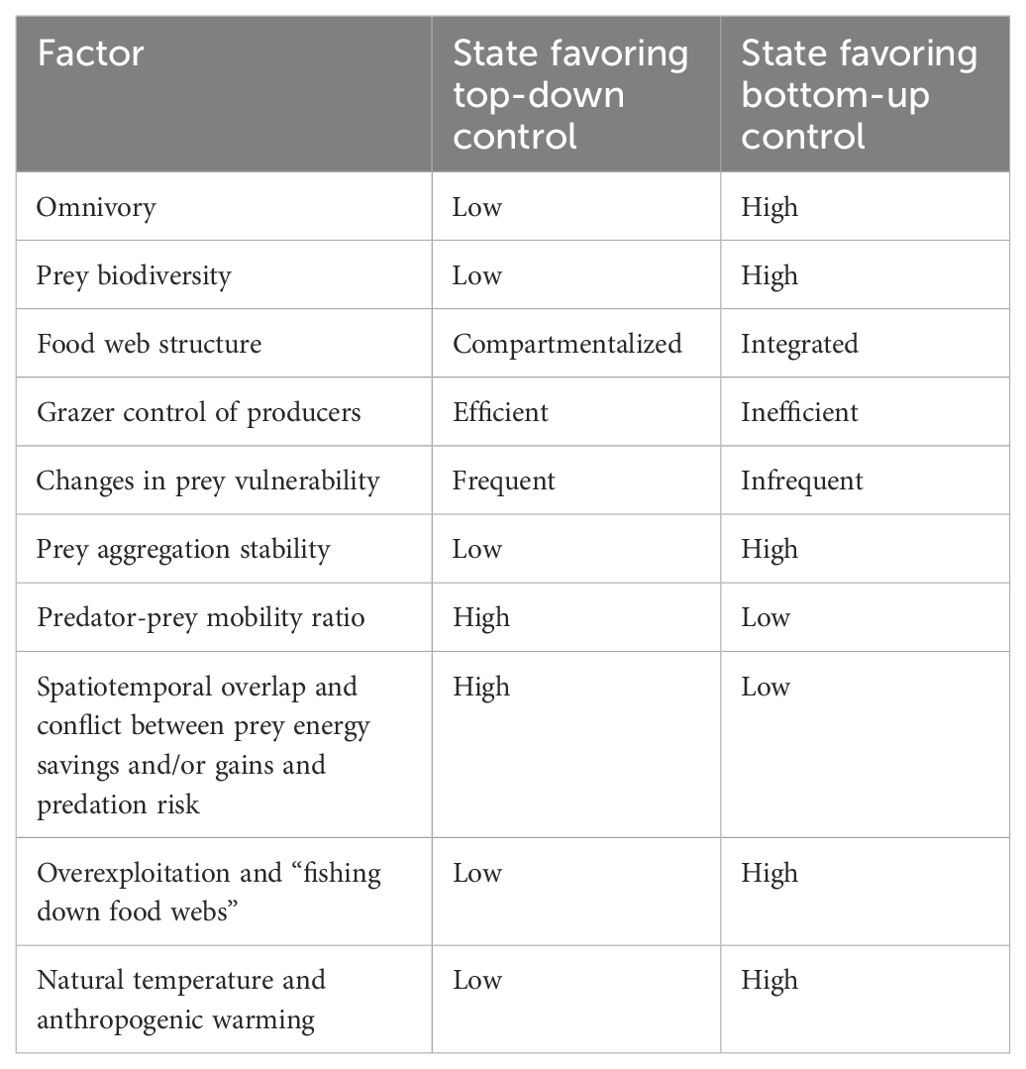
This review investigates the current state of knowledge on trophic control and cascades in marine ecosystems. It critically examines claims that top-down control and trophic cascades are rarer in saltwater ecosystems than in their freshwater counterparts, that these phenomena are scarcer in the marine water column than in intertidal or benthic habitats, and that various abiotic and/or biotic factors explain the incidence of top-down control and trophic cascades in neritic and pelagic ecosystems. This review suggests that top-down control is more widespread in neritic and pelagic ecosystems than species-level trophic cascades, which in turn are more frequent than community-level cascades. The latter occur more often in marine benthic ecosystems than in their lacustrine and neritic counterparts and are least frequently found in pelagic ecosystems. These distinctions among ecosystem types likely derive from differences in the spatial dimensionality and scale of physical processes through their effects on nutrient availability and community composition. The incidence of community-level trophic cascades among neritic and pelagic ecosystems is inversely related to biodiversity and omnivory, which are in turn associated with temperature. Regional variability in benthic and neritic trophodynamics also results from differences in producer and consumer traits and food web structure. Fear of predators, rather than predation mortality itself, drives many marine trophic cascades and massive vertical migrations. Paradoxical and synergistic trophic interactions, as well as positive feedback loops derived from biological nutrient cycling, complicate the conventional dichotomy between top-down and bottom-up control. Finally, this review presents a set of ecological factors whose alternative states favor top-down or bottom-up control in marine ecosystems.
At sea man sees daily how nature makes his conjectures vanish
Alejandro Malaspina (Kendrick, 1999, p. 146)
Introduction
The primary subjects of this paper are the importance and prevalence of trophic cascades and top-down trophic control in marine food webs, along with the nature and strength of the ecological and oceanographic mechanisms involved. These issues have stimulated perhaps the most contentious theoretical debate in marine ecology (Frank et al., 2015; Estes, 2018). Furthermore, they have strong and extensive implications for marine fisheries management and conservation, influencing prediction of fish production (Ware and Thomson, 2005; Chassot et al., 2010; Friedland et al., 2012; Ye and Carocci, 2018; Gregr et al., 2020; Marshak and Link, 2021) as well as design of protected areas and recovery plans for endangered species and depleted stocks (Salomon et al., 2008; Eddy et al., 2014; Gregr et al., 2020). Trophic control of marine ecosystem structure and dynamics is likewise relevant to broader environmental issues, including biodiversity conservation (Edwards and Konar, 2020; Eger et al., 2024), ecotourism (Gregr et al., 2020), and carbon sequestration (Pershing et al., 2010; Wilmers et al., 2012; Schmoker et al., 2013; Atwood et al., 2015, 2018; Gregr et al., 2020; Mariani et al., 2020). Long-term cascading impacts of marine predator-prey interactions also include maintenance of biodiversity and prevention of extinction (Donohue et al., 2017), as well as strong selective pressures shaping the morphology and behavior of organisms across multiple trophic levels (Verity and Smetacek, 1996).
This review examines the current state of knowledge on marine trophodynamics based on several decades of observational, experimental, and modeling studies. It harnesses the findings of this research to evaluate the empirical basis of claims that trophic cascades and top-down control are less prevalent in saltwater ecosystems than in their freshwater counterparts and that they occur less frequently in neritic and pelagic (i.e. deep, open ocean) environments than in benthic habitats. This review also assesses the support for arguments that pelagic ecosystems share abiotic and/or biotic traits that inhibit trophic cascades.
The development of trophic ecology
The debate on control of ecosystem structure and dynamics by top-down factors (i.e. predation and grazing) versus bottom-up drivers (i.e. food availability and ultimately primary productivity) originated with the study of energy flux from producers to top predators in lacustrine ecosystems (Lindeman, 1942). This bottom-up trophic paradigm (Figure 1a) dominated ecosystem ecology until the “green world” hypothesis attributed the prevalence of terrestrial vegetation to top-down control of herbivores by predators (Hairston et al., 1960). This publication was followed by two landmark studies from the Northeast Pacific demonstrating top-down control of ecosystem structure in the rocky intertidal (Paine, 1966) and a trophic cascade in kelp forests (Estes and Palmisano, 1974), although the term “trophic cascade” would only be coined six years later (Paine, 1980). Soon after, an investigation of Arctic tundra ecosystems revealed a positive relationship between top-down control and primary productivity (Oksanen et al., 1981). A further analysis demonstrated that cascades across four trophic levels (Figure 1b) could explain differences in plant communities among lakes with comparable nutrient availabilities (Carpenter et al., 1985). Conversely, later studies revealed that both top-down and bottom-up control weakened with each successive trophic level, and hence high nutrient supply would overpower the cascading impacts of predators on producers in both lacustrine (McQueen et al., 1986) and pelagic ecosystems (Micheli, 1999). This finding established the current understanding that the biomasses of all trophic levels are regulated by a pattern of alternating bottom-up and top-down control (Figure 1c) modulated by nutrient cycling and spatiotemporal variability (Leroux and Loreau, 2015).
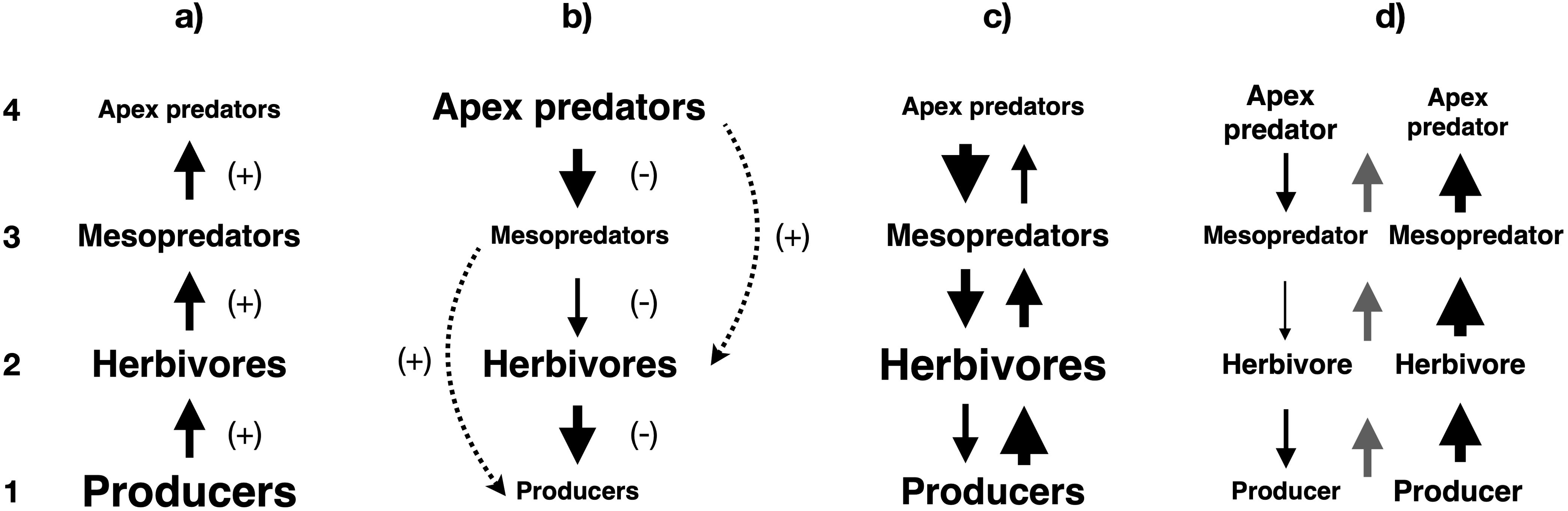
Figure 1. Trophic control scenarios in an idealized food chain with four trophic levels. (a) Biomass under community-level bottom-up control (this diagram also represents abundance or production under bottom-up or top-down control). (b) Biomass under community-level top-down control (trophic cascade). (c) Biomass under a combination of attenuating community-level top-down and bottom-up control. (d) Biomass under species-level top-down control (trophic cascade) and bottom-up control (gray arrows represent the direction of community-level trophic control). Solid lines indicate direct impacts, while dotted lines show indirect impacts. Arrow thickness and font size are approximately proportional to the strength of the impact and the biomass of the group in the ecosystem, respectively. Numbers on the left indicate trophic levels.
Community-level trophic cascades (Figure 1b), in which predators ultimately regulate the distribution of biomass across multiple trophic levels, can be distinguished from species-level cascades (Figure 1d), in which predators govern the biomasses of individual species but not entire trophic levels (Polis, 1999). However, even in a marine ecosystem regulated by a community-level cascade, distributions of abundance and production across trophic levels would likely follow the classic pyramid pattern (Figure 1a) due to trophic level increasing with individual mass (Potapov et al., 2019), and maximum growth rate decreasing with individual mass (Lynch et al., 2022). Trophic cascades can also be fruitfully classified (Shurin et al., 2002) as attenuating (wherein top-down interaction strength increases with trophic level), amplifying (in which this interaction strength decreases with trophic level), or neither (wherein top-down interaction strength is independent of trophic level).
Estuarine, intertidal, and benthic trophodynamics
Trophic cascades and top-down control in general, and in marine ecosystems in particular, were once considered to be restricted to rare, anomalous cases by food web complexity, omnivory, biodiversity, defensive adaptations, and spatial heterogeneity (Strong, 1992; Polis and Strong, 1996). However, meta-analyses (Pinnegar et al., 2000; Shurin et al., 2002; Borer et al., 2005) have demonstrated that, contrary to this dismissal, trophic cascades and top-down control (at both species and community levels) occur frequently in a wide range of marine benthic ecosystems. One of these analyses concluded that while cascades were typically attenuating in lacustrine benthic ecosystems, they were usually neither amplifying nor attenuating in their marine counterparts (Shurin et al., 2002). Furthermore, the latest of these meta-analyses found that marine benthic habitats hosted the strongest trophic cascades of all terrestrial, freshwater, and marine ecosystems studied (Borer et al., 2005). The fairly frequent occurrence of cascades and top-down control in salt marshes (Silliman and Bertness, 2002; Altieri et al., 2012), estuaries and seagrass beds (Myers et al., 2007; Lewis and Anderson, 2012; Baden et al., 2012; Hughes et al., 2019), rocky intertidal zones (Paine, 1966; Wootton, 1995; Schultz et al., 2016), shallow tropical banks (Parrish and Boland, 2004), coral reefs (Dulvy et al., 2004; Kroon et al., 2021; Mumby et al., 2012; McClanahan and Muthiga, 2016; Wolfe et al., 2025), kelp forests (Estes and Palmisano, 1974; Vicknair and Estes, 2012; Eisaguirre et al., 2020; Kumagai et al., 2024), coralline algal reefs (Rasher et al., 2020), soft-bottom coastal habitats (Kvitek et al., 1992; Kelaher et al., 2015), and continental shelves (Worm and Myers, 2003) is now well established.
However, trophic cascades and top-down control are not the sole organizing principles of marine benthic trophodynamics. While two studies (Silliman and Bertness, 2002; Altieri et al., 2012) found community-level trophic cascades regulating both herbivore and producer biomass in salt marshes of the eastern United States, a third analysis (Griffin et al., 2011) revealed that at another salt marsh site in this region, predatory crabs actually increased grazing by herbivorous snails by shifting their vertical distribution through non-consumptive (i.e. fear-mediated) impacts. In addition, studies of the Ythan estuary in Scotland (Hall and Raffaelli, 1991; Raffaelli and Hall, 1992) failed to demonstrate trophic cascades or top-down control at the ecosystem level, though a later analysis (Emmerson and Raffaelli, 2004) did find strong trophic interactions between species. Several studies on small to intermediate spatial scales have demonstrated top-down control (and, in the latter two cases, community-level trophic cascades) in rocky intertidal zones of the northeast Pacific Ocean (Paine, 1966; Wootton, 1995; Schultz et al., 2016). However, a global meta-analysis of studies on intermediate to large scales (Menge, 2000) and a novel modeling analysis (Robles and Desharnais, 2002) found that bottom-up as well as physical oceanographic influences interact with top-down impacts to shape rocky intertidal community structure and composition. Similarly, a study of seagrass bed condition in the Swedish Skagerrak found that a community-level trophic cascade caused by predator overfishing combined with eutrophication to yield an outbreak of epiphytic algae, reducing seagrass growth (Baden et al., 2012).
Finally, the role of the same species or functional group may differ among specific examples of an ecosystem type. While mesopredators hindered seagrass production in the Swedish Skagerrak by consuming grazers that would otherwise have limited epiphytic algal growth (Baden et al., 2012), mesopredators in a southern California estuary benefited seagrass beds by targeting epifauna that consumed or fouled seagrass blades (Lewis and Anderson, 2012). While predation on crabs by sea otters (Enhydra lutris) in a central California estuary supported seagrass through a community-level trophic cascade releasing grazers controlling epiphyte growth from crab predation pressure (Hughes et al., 2019), no significant impact on grazers of crab predation by sea otters was observed in southeast Alaska (Raymond et al., 2021). This unexpected result may stem from lower sea otter density and nutrient availability, as well as greater trophic complexity and spatial heterogeneity, in southeast Alaska compared to central California (Raymond et al., 2021). Furthermore, while sea otter presence is associated with reduced cancrid crab abundance and size in California estuaries, there is no evidence that sea otters negatively affect Dungeness crab (Cancer magister) landings in California (Grimes et al., 2020). Crab landings per trip increased across California over the past four decades regardless of sea otter presence (Boustany et al., 2021). In fact, the rate of increase in Dungeness crab landings per trip was positively associated with sea otter abundance near fishing ports, suggesting that otters may have indirect positive impacts on crab abundance at large spatial scales. Sea otters also exercise top-down control over the invasive green crab (Carcinus maenas) in central California (Jeppesen et al., 2025), potentially benefiting native crab species. Furthermore, otters serve as ecosystem engineers in eelgrass beds in central and southern British Columbia by promoting eelgrass sexual reproduction, and thus genetic diversity, through disturbance caused by digging for infaunal prey (Foster et al., 2021).
Trophic cascades are apparently less prevalent in coral reefs than in temperate benthic ecosystems, likely due to high biodiversity and food web complexity (Sandin et al., 2010; Roff et al., 2016a, Desbiens et al., 2021). Community-level cascades have been found in coral reefs off northwestern Australia (Ruppert et al., 2013) and the western Indian Ocean (McClanahan, 2000; McClanahan and Muthiga, 2016; Figure 2a). However, other studies in the Seychelles (Jennings et al., 1995), Fiji (Jennings and Polunin, 1997), the Caribbean Sea (Mumby et al., 2006), and the Great Barrier Reef (Rizzari et al., 2014a; Casey et al., 2017; Desbiens et al., 2021) failed to detect such trophic cascades. Furthermore, the northwestern Australian case, the only one involving sharks (Ruppert et al., 2013), is disputed (Roff et al., 2016a; Ruppert et al., 2016; Roff et al., 2016b). Several studies suggest that most reef sharks are in fact not apex predators but rather members of upper-level mesopredator guilds with high functional redundancy (Mourier et al., 2013; Heupel et al., 2014; Frisch et al., 2016; Roff et al., 2016a). This could explain the otherwise surprising paucity of community-level cascades involving these sharks.
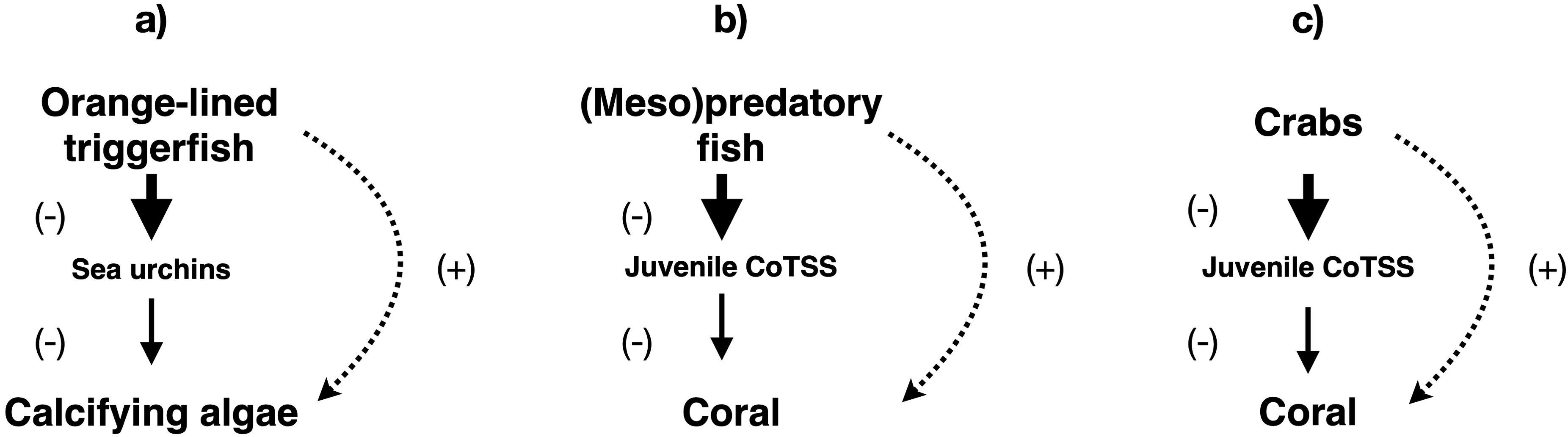
Figure 2. Community-level trophic cascades detected in coral reef ecosystems. (a) western Indian Ocean (McClanahan, 2000; McClanahan and Muthiga, 2016). (b) Fiji (Dulvy et al., 2004) and the Great Barrier Reef (Kroon et al., 2021). (c) Great Barrier Reef (Wolfe et al., 2025). CoTSS refers to crown-of-thorns sea star (Acanthaster spp.). Solid lines indicate direct impacts, while dotted lines show indirect impacts. Arrow thickness and font size are approximately proportional to the strength of the impact and the biomass of the group in the ecosystem, respectively.
It has been suggested that predatory fish exercise top-down control over crown-of-thorns sea stars (Acanthaster spp.), voracious corallivores capable of denuding reefs, by preying on juveniles in a community-level cascade sensitive to predator overfishing (Figure 2b). Such cascades have been recorded in Fiji (Dulvy et al., 2004) and on the Great Barrier Reef (Kroon et al., 2021). However, this trophic cascade may be mediated by mesopredatory fish switching from pelagic to benthic prey (including juvenile Acanthaster spp.) in the presence of apex predators (Meekan et al., 2025). A similar cascade, also capable of protecting coral reefs and resulting from predation by crabs on juvenile Acanthaster spp. (Figure 2c), has been found on the Great Barrier Reef (Wolfe et al., 2025).
Although unfished reefs in the Northwestern Hawaiian Islands (Friedlander and DeMartini, 2002) and Line Islands (Sandin et al., 2008) showed higher predator biomass and lower algal cover than fished reefs, herbivorous fish biomass was similar across fished and unfished sites in both island chains. However, herbivorous fish guild composition differed between fished and unfished atolls in the Line Islands (DeMartini et al., 2008). This suggests that cascading impacts of top predators on algae (via mesopredator biomass) on unfished reefs may affect the biodiversity or behavior of herbivores rather than their biomass (Sandin et al., 2008). Furthermore, high predator populations on unfished reefs are likely subsidized by exogenous pelagic production (McCauley et al., 2012a; Frisch et al., 2016; Mourier et al., 2016; Skinner et al., 2021). This is also apparently the case for two remote islets in the northern Galápagos Islands (Salinas-de-León et al., 2016), kelp forests in the neritic archipelago of Haida Gwaii in British Columbia, Canada (Trebilco et al., 2016), highly protected areas in the Mediterranean Sea (Guidetti et al., 2014), and other freshwater, marine, and terrestrial ecosystems where inverted trophic pyramids were reported (McCauley et al., 2017).
Trophic cascades in kelp forest ecosystems
The kelp forest is perhaps the ecosystem most readily associated with top-down control and trophic cascades. Similarly, the maintenance of Northeast Pacific kelp forests through intense predation on herbivorous sea urchins (Strongylocentrotus spp.) by sea otters (Estes and Palmisano, 1974; Figure 3a) is likely the most familiar trophic cascade, and the sea otter probably the most recognizable keystone species (Schiel and Foster, 2015). The otter - urchin - kelp community-level cascade was observed upon sea otter reintroduction or recovery in Alaska (Estes and Palmisano, 1974; Estes and Duggins, 1995), British Columbia (Breen et al., 1982; Burt et al., 2018; Langendorf et al., 2025), and California (Palumbi and Sotka, 2011; Nicholson et al., 2024; Langendorf et al., 2025) in the 20th century, following depletion and extirpation throughout the North Pacific Ocean by the maritime fur trade of the 18th and 19th centuries (Ogden, 1941; Jones, 2014; Gibson, 2024). This cascade was also widespread prior to European contact, despite localized sea otter depletion in the Aleutian Islands of Alaska (Simenstad et al., 1978), Haida Gwaii (Szpak et al., 2012, 2013), and the Channel Islands of California (Erlandson et al., 2005). However, there is surprisingly little evidence of this trophic cascade from the Northwest Pacific, despite the full recovery of Russian sea otter populations (Kornev and Korneva, 2006). In British Columbia (Burt et al., 2018; Langendorf et al., 2025) and central California (Selgrath et al., 2024), complementary predation on large sea urchins by sea otters and on smaller individuals by the sunflower sea star (Pycnopodia helianthoides) exerted cascading positive effects on kelp (Figure 3b).
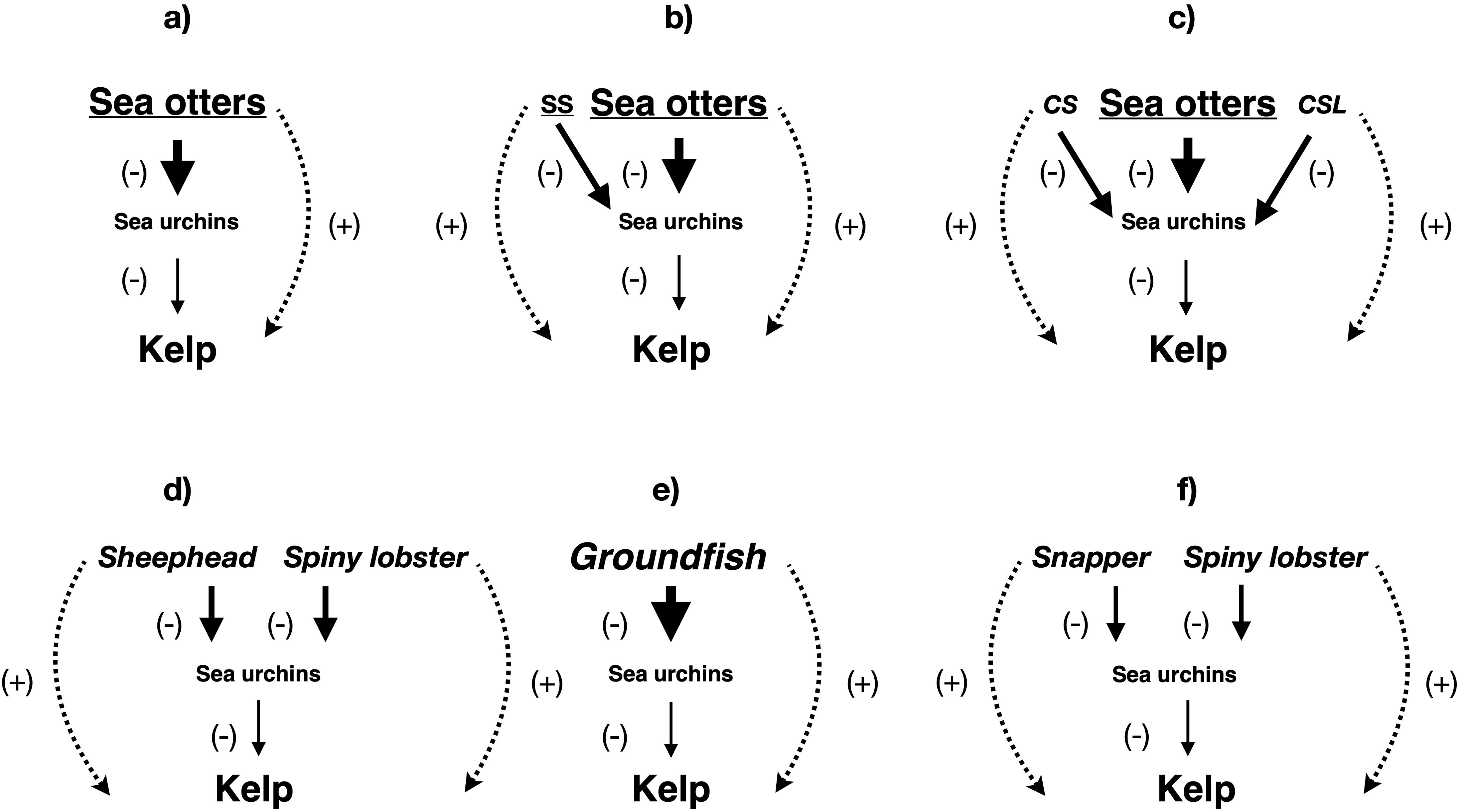
Figure 3. Community-level trophic cascades found in kelp forest ecosystems. (a) Aleutian Islands i) before sea otter depletion in the 19th century and ii) between sea otter recovery and collapse in the late 20th century. (b) south-central and southeast Alaska, central British Columbia, western Vancouver Island, and central California since sea otter population recovery; historically also northern British Columbia, Washington, Oregon, and northern California. SS refers to sunflower star (Pycnopodia helianthoides), functionally extinct since 2013. (c) San Nicolas Island, southern California; historically southern California (USA) and Baja California (Mexico). CS refers to California sheephead (Semicossyphus pulcher) and CSL to California spiny lobster (Panulirus interruptus). (d) southern California (USA) and Baja California (Mexico). (e) northwest Atlantic Ocean (Gulf of Maine to Labrador) before groundfish stock collapses in the late 20th century. (f) New Zealand. “Snapper” refers to Australasian snapper (Chrysophrys auratus) and “Spiny lobster” to southern rock lobster (Jasus edwardsii). Underlined names refer to species functionally extinct in part of their historical range. Italicized names refer to species fished outside marine protected areas. Solid lines indicate direct impacts, while dotted lines show indirect impacts. Arrow thickness and font size are approximately proportional to the strength of the impact and the biomass of the group in the ecosystem, respectively.
The reduction of sea urchin grazing by sea otters also yielded many indirect impacts on kelp forest ecosystems (Estes, 2018). Firstly, high concentrations of suspended detritus particles derived from dead kelp (Ramshaw et al., 2017) stimulated the growth of filter feeders such as mussels and barnacles (Duggins et al., 1989) as well as heterotrophic bacteria (Clasen and Shurin, 2015). Filter feeder biomass was also supported, in an independent community-level trophic cascade, by sea otter consumption of carnivorous sea stars and the resulting release of mussels and barnacles from predation pressure (Vicknair and Estes, 2012). Secondly, high kelp cover augmented the biomass of inshore fish such as greenlings (Hexagrammidae) and rockfish (Sebastidae) through increased supply of kelp detritus (and thus small detritivores as prey) and/or improved nursery habitat in kelp forests (Reisewitz et al., 2006; Markel and Shurin, 2015). Kelp forests are globally important as fish nurseries (Pérez-Matus et al., 2025). The diets of Aleutian glaucous-winged gulls (Larus glaucescens) and bald eagles (Haliaeetus leucocephalus) shifted from fish to invertebrates (Irons et al., 1986) and seabirds (Anthony et al., 2008), respectively, likely due to reduced inshore fish biomass resulting from a decline in sea otters and the consequent loss of kelp forests. Thirdly, in an example of an apparently global pattern (Eger et al., 2024), Aleutian kelp forests supported by sea otters demonstrated significantly higher species richness and spatial heterogeneity than urchin barrens (Edwards and Konar, 2020). Finally, kelp forest recovery due to otter predation on urchins has increased carbon fixation and reduced ocean acidification in Alaska and British Columbia (Wilmers et al., 2012). A recent ecosystem modeling analysis concluded that the total economic benefits of sea otter recovery, including increases in ecotourism, finfish yields, and sequestration of carbon by kelp, outweigh the costs of drastically reduced invertebrate yields (Gregr et al., 2020).
While sea otters were originally abundant in southern California, they are now absent there except around San Nicolas Island. Here, the classic sea otter–sea urchin–kelp cascade was dampened by additional interactions in the kelp forest food web, particularly competition among herbivores and seaweeds, although interaction strengths in the cascade itself were not weakened (Langendorf et al., 2025; Figure 3c). However, numerous kelp forests remain elsewhere in southern California, raising the question of the mechanisms responsible (Schiel and Foster, 2015). Unlike waters to the north, southern California hosts two additional urchin predators (Figure 3d), the California spiny lobster (Panulirus interruptus) and California sheephead (Semicossyphus pulcher). Early findings of community-level trophic cascades driven by spiny lobsters (Tegner and Dayton, 1981; Tegner and Levin, 1983), sheephead (Cowen, 1983), and both species (Lafferty, 2004; Behrens and Lafferty, 2004; Halpern et al., 2006) have been disputed on methodological grounds (Schiel and Foster, 2015), while several later analyses (Foster and Schiel, 2010; Guenther et al., 2012; Malakhoff and Miller, 2021) failed to detect such cascades. Nevertheless, more convincing cases for the importance of predation on sea urchins by sheephead (Hamilton and Caselle, 2015) and sheephead and lobsters (Eisaguirre et al., 2020; Kumagai et al., 2024) in preserving southern California kelp forests have since been made. Furthermore, large fish such as sheephead support these forests through nutrient recycling (Shrestha et al., 2023, 2024; Peters et al., 2025). Another trophic cascade links inshore planktivorous fish to kelp forest maintenance in southern California through predation on mesograzers, which would otherwise reduce kelp condition and biomass (Davenport and Anderson, 2007). Nevertheless, kelp cover in this region declined between 1910–1912 and 2014–2016, likely due to the longstanding absence of sea otters (Nicholson et al., 2024). In central California, complementary predation on sea urchins by sea otters and sunflower stars prevented kelp cover from decreasing to extremely low levels from 1897–1899 until the sea star epizootic of 2013 (Selgrath et al., 2024), while sea otter recovery allowed it to increase between 1910–1912 and 2014–2016 (Nicholson et al., 2024). Sea otter control of sea urchins in the latter period was supported by the release of California mussels (Mytilus californianus), a sea otter prey item, from top-down control by the ochre sea star (Pisaster ochraceus), the keystone species of the Northeast Pacific rocky intertidal, devastated by the same epizootic as the sunflower star (Smith et al., 2025). Considering the decline in southern California kelp cover during this period (Nicholson et al., 2024), the low proportion (3.6%) of the total kelp forest area protected by marine reserves in southern California is a cause for concern (Arafeh-Dalmau et al., 2021), particularly compared to the increase in kelp cover (Nicholson et al., 2024) and higher (12.8%) proportion of kelp forest area protected in central California (Arafeh-Dalmau et al., 2021). Given projected increases in marine heatwave exposure (Cheung and Frölicher, 2020), stronger protection would likely be needed to safeguard kelp forests in southern California (Arafeh-Dalmau et al., 2025).
In northern California, where sea otters have long been absent, the sunflower star exercised top-down control over sea urchins, thus maintaining healthy kelp forests (Byrnes et al., 2006), until its population collapsed in the 2013 epizootic (Harvell et al., 2019; McPherson et al., 2021). However, unlike in southern and central California, where sunflower star abundance likewise plummeted (Harvell et al., 2019), in northern California no effective sea urchin predators remained due to the longstanding absence of sea otters and lower functional complementarity and redundancy in the predator guild. Thus, due to overgrazing and the 2014–16 Northeast Pacific marine heatwave, kelp forests were replaced by urchin barrens, devastating red abalone (Haliotis rufescens) recruitment and the associated fishery (Rogers-Bennett et al., 2024). Considering forecast increases in the future impacts of marine heatwaves in the Northeast Pacific (Cheung and Frölicher, 2020) sea otter and/or sunflower star population recovery would likely be required to restore kelp forests in northern California.
In Oregon, where sea otters have likewise long been absent and may never have been abundant (Ogden, 1941; p. 6), reduced kelp condition, likely due to the marine heatwave and epizootic, was associated with lower zooplankton abundance and gray whale (Eschrichtius robustus) feeding time, suggesting indirect benefits of kelp to gray whales through habitat provision to their zooplankton prey (Hildebrand et al., 2024). Off western Vancouver Island (Langendorf et al., 2025) and central British Columbia (Burt et al., 2018), sea otters and sunflower stars functioned in a complementary fashion, controlling large and medium-sized urchins, respectively, with an increase in the latter and a slight reduction in kelp density in central British Columbia after the epizootic and marine heatwave (Burt et al., 2018). These studies illuminate the importance of coastal biogeography and functional complementarity and redundancy among sea urchin predators to trophic cascade and kelp forest persistence across the Northeast Pacific. They also underscore the unique benefits of sea otters as keystone predators in these ecosystems. These exceptional benefits almost certainly result from the extremely high prey consumption rates of endothermic sea otters (Borer et al., 2005), which lack blubber and rely solely on their extremely dense fur for insulation in cold seas.
In the Northwest Atlantic, a community-level trophic cascade linking large demersal fish, mainly wolffish (Anarhichas spp.) and Atlantic cod (Gadus morhua), to kelp forests through predation on sea urchins (Figure 3e) operated prior to overfishing of these fish (Vadas and Steneck, 1995). Groundfish stock collapses caused by overfishing released sea urchins from predation pressure, precipitating the loss of kelp forests, which was then reversed by the reimposition of top-down control over sea urchins by the introduction of a new fishery (Jackson et al., 2001). This sequence of events provides a classic example of “fishing down marine food webs” (Pauly et al., 1998). In this process, fisheries serially target and deplete biomass at successively lower trophic levels (in this case predatory groundfish and herbivorous sea urchins). However, in the Northeast Atlantic evidence for trophic cascades perpetuating kelp forests is relatively sparse (Steneck et al., 2002), despite similar large groundfish guilds operating across the North Atlantic Ocean. While edible crabs (Cancer pagurus) may support Norwegian kelp forests by preying on sea urchins (Fagerli et al., 2014), the persistence of these forests is influenced by temperature as well as cascading impacts of crab abundance (Christie et al., 2018). Furthermore, in a surprising contrast to the Northwest Atlantic case, the Norwegian coastal cod stock negatively impacts kelp by consuming crabs, thereby releasing sea urchins from predation (Christie et al., 2018). Thus, Northeast Atlantic kelp forests include a mesopredator (the edible crab) absent from their Northwest Atlantic counterparts, resulting in opposite impacts of the same predator (Atlantic cod) on kelp in these two regions. These cases again highlight the influences of biogeography and predator community composition on the strength of trophic cascades in kelp forest ecosystems.
Trophic cascades are apparently less prevalent in Southern Hemisphere kelp forests than in their northern equivalents (Tegner and Dayton, 2000; Steneck et al., 2002; Schiel and Foster, 2015). In the absence of a keystone predator such as the sea otter, an evolutionary arms race between kelps and herbivores is apparently occurring in New Zealand, with kelps developing stronger chemical defenses, and herbivores acquiring greater tolerance for these compounds, than their northern kin (Steinberg et al., 1995). However, community-level kelp forest trophic cascades due to predation on sea urchins by spiny lobsters (Jasus spp.) occur in New Zealand (J. edwardsii; Babcock et al., 1999; Shears and Babcock, 2002; Eddy et al., 2014; Edgar et al., 2017; Figure 3f) and Western Cape Province, South Africa (J. lalandii; Barkai and McQuaid, 1988; Anderson et al., 1997; Blamey and Branch, 2010). The Australasian snapper (Chrysophrys auratus) likewise controls sea urchins and supports kelp forests in New Zealand (Cole and Keuskamp, 1998; Babcock et al., 1999; Shears and Babcock, 2002; Edgar et al., 2017; Figure 3f). There is also evidence of non-consumptive (fear-mediated) impacts of predators on kelp grazing by sea urchins in New Zealand (Spyksma et al., 2017; Curtis and Wing, 2024) and in northern California (Byrnes et al., 2006). Top-down, bottom-up, and physical oceanographic processes may all combine to determine kelp forest persistence in Tasmania (Ling et al., 2009) and New Zealand (Salomon et al., 2008). In southern Patagonia and the subantarctic archipelagoes, sea urchin larvae are largely dispersed by the powerful Antarctic Circumpolar Current, impairing juvenile urchin settlement and maintaining kelp forests in the apparent absence of top-down control (Tegner and Dayton, 2000).
While community-level trophic cascades are common in kelp forests, bottom-up and non-trophic factors (e.g. physical stress or infectious disease) often complicate and moderate the impacts of these cascades (Schiel and Foster, 2015). For example, direct physical effects of wave action often overpower both top-down and bottom-up impacts in California kelp forests (Reed et al., 2011). In the Gulf of Maine, ocean warming is driving kelp forest declines even without overgrazing by sea urchins (Suskiewicz et al., 2024), while allelopathic (i.e. adverse chemical) effects of competing turf algae hinder kelp recovery (Farrell et al., 2025), shifting the base of coastal food webs from benthic kelp to neritic phytoplankton (Yiu et al., 2025). Mesocosm experiments reveal that besides its direct negative impacts, warming indirectly harms Australian kelp by increasing consumption rates in sea urchins and reducing predation rates in spiny lobsters (Sagmariasus verreauxi; Provost et al., 2017). In central California, the epizootic among sunflower stars and the 2014–2016 marine heatwave produced a mosaic of urchin barrens and kelp forests (Smith et al., 2024). However, sea otter presence prevented a complete collapse of kelp forests in the region (Smith et al., 2021).
A catastrophic decline in Aleutian sea otter abundance in the late 20th century (Doroff et al., 2003) released sea urchins from top-down control, causing a transition from kelp forests to urchin barrens (Estes et al., 1998) and combining with ocean warming and acidification to imperil coralline algal reefs (Rasher et al., 2020). These events were attributed to killer whale (Orcinus orca) predation adding a trophic level to the otter – urchin – kelp trophic cascade (Estes et al., 1998). The sequential megafaunal collapse hypothesis proposed that whaling had removed prey biomass from the ecosystem, leading transient killer whales to successively overexploit pinniped and sea otter populations (Springer et al., 2003). Demographic and bioenergetic modeling studies conditionally supported this hypothesis (Springer et al., 2003; Williams et al., 2004; Estes et al., 2009). However, several critical analyses successfully challenged its core tenets, i.e. that large whales were important prey for killer whales, that whale, pinniped, and otter population declines in western Alaska in the late 20th century were sequential, and that pinniped declines were due to predation (DeMaster et al., 2006; Mizroch and Rice, 2006; Mehta et al., 2007; Trites et al., 2007; Wade et al., 2007, 2009). Furthermore, the original attribution of the sea otter decline to killer whale predation (Estes et al., 1998) has been reassessed as an overinterpretation of the limited existing data (Kuker and Barrett-Lennard, 2010). Only two indisputable records of sea otter consumption by killer whales exist; one from Prince William Sound, Alaska (Vos et al., 2006) and the other from the Commander Islands, Russia (Fomin et al., 2023).
Neritic and pelagic trophodynamics
Trophic cascades and top-down control are apparently weaker and less frequent in neritic and pelagic ecosystems than in their intertidal, benthic, and lacustrine counterparts (Shurin et al., 2002; Borer et al., 2005). Furthermore, while top-down control is quite common in pelagic ecosystems, community-level cascades are distinctly less frequent (Baum and Worm, 2009; Essington, 2010). While such cascades in the lacustrine water column were originally observed to be attenuating (McQueen et al., 1986) and later found to be neither amplifying nor attenuating (Shurin et al., 2002) or non-attenuating (Carpenter et al., 2010), their marine counterparts were observed to be generally attenuating (Shurin et al., 2002). Although community-level cascades in neritic ecosystems were found to occupy the neutral border space between amplification and attenuation (Rossberg et al., 2019), such cascades in pelagic ecosystems were observed to be attenuating (Micheli, 1999). It may be argued that this cross-ecosystem pattern is due to a chain of physical, chemical, and biological factors.
The spatial structure of the water column has been suggested to promote bottom-up control in pelagic ecosystems by spreading predation pressure across three dimensions, rather than two as in benthic and terrestrial habitats (McCann et al., 2005). Although lacustrine and neritic ecosystems, where trophic cascades are more prevalent (Essington, 2010) and less susceptible to attenuation (Carpenter et al., 2010; Rossberg et al., 2019), are likewise three-dimensional, the magnitude of the third dimension (i.e. depth) is one to two orders greater (103 vs. 101–102 m) in pelagic ecosystems. A related explanation for the decreasing frequency of community-level trophic cascades from lacustrine through neritic to pelagic ecosystems involves the increasing scale of physical processes, particularly advection (Essington, 2010; Pershing et al., 2015). Advective transport of nutrients and biota is negligible in lakes, driven by tides on modest scales (103–105 m) in coastal waters, and by currents on enormous scales (106–107 m) in the open ocean. While such transport, combined with stronger mixing and weaker stratification resulting from longer wind and wave fetch, and with upwelling in several key ecosystems, renders saltwater fisheries more productive than their freshwater counterparts (Nixon, 1988), it may also disrupt the strong trophic interactions required for community-level cascades. The spatial scale of advection and mixing is smaller in neritic ecosystems than in their pelagic counterparts, which is reflected in the higher frequency of known trophic cascades in neritic waters (Essington, 2010). However, exhaustively testing this hypothesis would require long-term ecological research involving intensive and extensive sampling of large pelagic ecosystems across multiple scales, which would be both logistically challenging and costly.
Nutrient availability has been suggested to favor bottom-up control (Gasol et al., 2003; Banse, 2013), allowing primary productivity to overpower grazing and attenuate trophic cascades in both lakes (McQueen et al., 1986) and oceans (Micheli, 1999). However, a more recent study of lacustrine ecosystems casts doubt on this hypothesis (Carpenter et al., 2010). In addition, as was originally observed in the Arctic tundra (Oksanen et al., 1981), nutrient availability may promote trophic cascades in estuaries (Stoecker et al., 2008) as well as neritic and pelagic ecosystems (Rossberg et al., 2019), particularly where zooplankton are small relative to their food (Fuchs and Franks, 2010), although this effect is not universal (Stibor et al., 2004a). Furthermore, despite the wide ranges of eutrophic, mesotrophic, and oligotrophic conditions occurring in lacustrine, neritic, and pelagic ecosystems, theoretical modeling suggests that decreasing nutrient availability with increasing spatial scale (i.e. from lakes and coastal waters to open oceans) could explain the pattern of cascade prevalence and attenuation across these three ecosystem types (Rossberg et al., 2019). While the productivity of saltwater fisheries is elevated relative to their freshwater counterparts by increased nutrient availability due to mixing and upwelling (Nixon, 1988), most productive marine fisheries occur in neritic rather than pelagic waters. This is most likely due to the higher input, lower export, and more efficient recycling of nutrients on continental shelves and slopes relative to the deep sea.
While global abundances of all zooplankton taxa are significantly correlated with macronutrient (nitrate, phosphate, and silicate) concentrations, correlations with depth-integrated chlorophyll a are far more ambiguous (Brandão et al., 2021). This suggests that control of trophic interactions between phytoplankton and zooplankton varies across the world ocean. In macronutrient-limited oligotrophic and micronutrient-limited high nutrient – low chlorophyll (HNLC) ecosystems, which predominate at low and high latitudes, respectively, phytoplankton is subject to top-down control by grazing (Banse, 2013). This allows the biomass of zooplankton to exceed that of phytoplankton (Gasol et al., 2003), as in the HNLC Southern Ocean (Yang et al., 2022). If macronutrients were assigned a trophic level of zero, a type of community-level trophic cascade could be postulated for HNLC waters in which herbivorous zooplankton exercised top-down control over phytoplankton, with indirect positive effects on macronutrient concentrations. Such a cascade, albeit with four trophic levels (plus nutrients) has indeed been observed in a Norwegian fjord (Sommer et al., 2004). In HNLC ecosystems, phytoplankton biomass could be caught in a “trophic vise” of top-down and bottom-up control by grazing and micronutrient limitation, respectively. This hypothesis could be tested by micronutrient fertilization experiments in HNLC waters. An increase in zooplankton biomass exceeding that detected in phytoplankton in response to fertilization would support the “trophic vise” hypothesis, while the opposite pattern of biomass increases would contradict it.
Plankton community composition and trophic ecology also influence the pattern of trophic cascade incidence and attenuation across lacustrine, neritic, and pelagic ecosystems (Sommer and Sommer, 2006; Sommer, 2008). A study using freshwater, brackish, and saltwater mesocosm experiments (Sommer and Sommer, 2006) revealed that grazing by copepods, a dominant group of saltwater zooplankton, mainly reduces microphytoplankton biomass (Figure 4a). By contrast, cladocerans, the foremost components of freshwater zooplankton, primarily deplete nano- and picophytoplankton biomass (Figure 4b). These small size classes are favored by the strong stratification and resulting nutrient limitation characteristic of many lacustrine ecosystems. Since small phytoplankton can increase in biomass more rapidly than their larger counterparts due to their higher turnover rates and more efficient nutrient uptake resulting from higher surface area to volume ratios, cladocerans, which mainly consume nano- and picophytoplankton, can regulate total phytoplankton biomass more strongly than copepods, which focus on microphytoplankton. Thus, predation on cladocerans, whose dominance in freshwater ecosystems likely results at least partly from their efficient grazing of small phytoplankton, is more likely to cause non-attenuating, community-level trophic cascades than comparably intense predation on copepods, which likely predominate in saltwater ecosystems due to their effective grazing of large phytoplankton (Sommer and Sommer, 2006). Furthermore, both laboratory bottle incubation studies (Nejstgaard et al., 2001; Leising et al., 2005a, b; Olson et al., 2006; Stoecker et al., 2008; Sherr et al., 2009) and mesocosm experiments (Stibor et al., 2004a; Vadstein et al., 2004; Sommer and Sommer, 2006) based on sampling of the North Atlantic, North Pacific, and Arctic Oceans indicate that marine copepod predation on microzooplankton releases nano- and picophytoplankton from top-down control through species-level trophic cascades (Figure 4a). This indirect positive impact further increases the resistance of total saltwater phytoplankton biomass to herbivory, contributing to attenuation of community-level cascades in the marine water column (Sommer and Sommer, 2006).
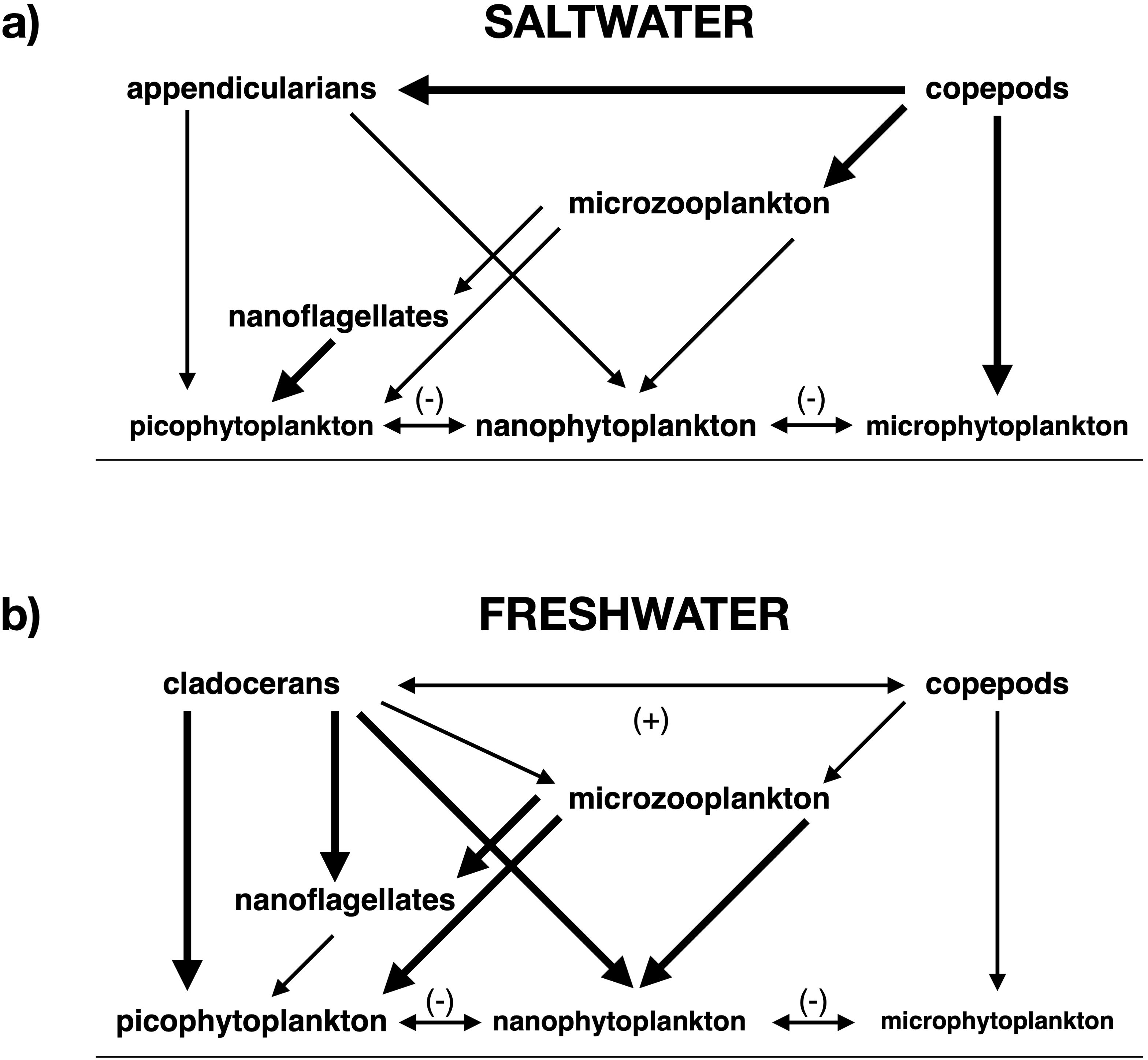
Figure 4. Trophic structure of plankton communities. (a) saltwater and copepod-dominated, after Sommer and Sommer (2006, Figure 5, left panel), (b) freshwater and cladoceran-dominated, after Sommer and Sommer (2006, Figure 5, right panel). Directional arrows indicate direct negative (top-down) impacts, while double-headed arrows indicate bidirectional interactions. The double-headed arrow accompanied by a plus sign indicates improved feeding opportunities for copepods in the presence of cladocerans and vice versa, while the double-headed arrows with minus signs represent competition for nutrients among phytoplankton groups. Arrow thickness and font size are approximately proportional to the strength of the impact and the importance of the plankton group in the ecosystem, respectively.
The trophic impacts of global warming also differ between freshwater and saltwater ecosystems (Murphy et al., 2020). In fresh water, warming reduces zooplankton biomass, causing a top-down release of phytoplankton from grazing pressure. In salt water, it reduces phytoplankton biomass, exerting a bottom-up adverse impact on zooplankton. In both cases, effect size is attenuating and only the direct impacts of warming are statistically significant, suggesting that both top-down and bottom-up control operate in each ecosystem type (Murphy et al., 2020). The mechanism driving the adverse impacts of warming on marine phytoplankton likely depends on nutrient availability (Lewandowska et al., 2014). In waters with strong mixing, weak stratification, and high nutrient supply, warming increases copepod grazing rates, creating a top-down impact. In seas with weak mixing, strong stratification, and low nutrient availability, it reduces the depth of the thermocline, further decreasing nutrient availability and generating a bottom-up impact. Given the declines in phytoplankton detected across much of the world ocean during the 20th century (Boyce et al., 2010, 2014; Boyce and Worm, 2015), these impacts of warming may have overpowered or masked many pelagic trophic cascades. Increased feeding rates would likely not prevent copepod biomass from decreasing but would cause phytoplankton biomass to decline, thus creating a positive correlation between copepod and phytoplankton biomasses and obscuring the lowest link in a trophic cascade.
Plankton community ecology likewise helps explain the differences in trophic cascade frequency and attenuation among marine water column ecosystems (Sommer et al., 2002; Sommer and Stibor, 2002; Sommer, 2008; Figure 5). In polar and subpolar waters (Figure 5a), frequent storms increase mixing and decrease stratification, improving nutrient availability and thus raising the importance of large phytoplankton (Sommer et al., 2017). In these ecosystems, large copepods forage on microphytoplankton and euphausiids feed on micro- and nanophytoplankton, while salps consume all size classes from micro- to picophytoplankton (Sommer and Stibor, 2002). Thus, the entire phytoplankton size spectrum is exposed to grazing in subpolar and polar waters, supporting top-heavy plankton biomass distributions (Gasol et al., 2003), top-down control (Strom et al., 2007; Banse, 2013; Yang et al., 2022), and unattenuated community-level trophic cascades (Sommer, 2008).
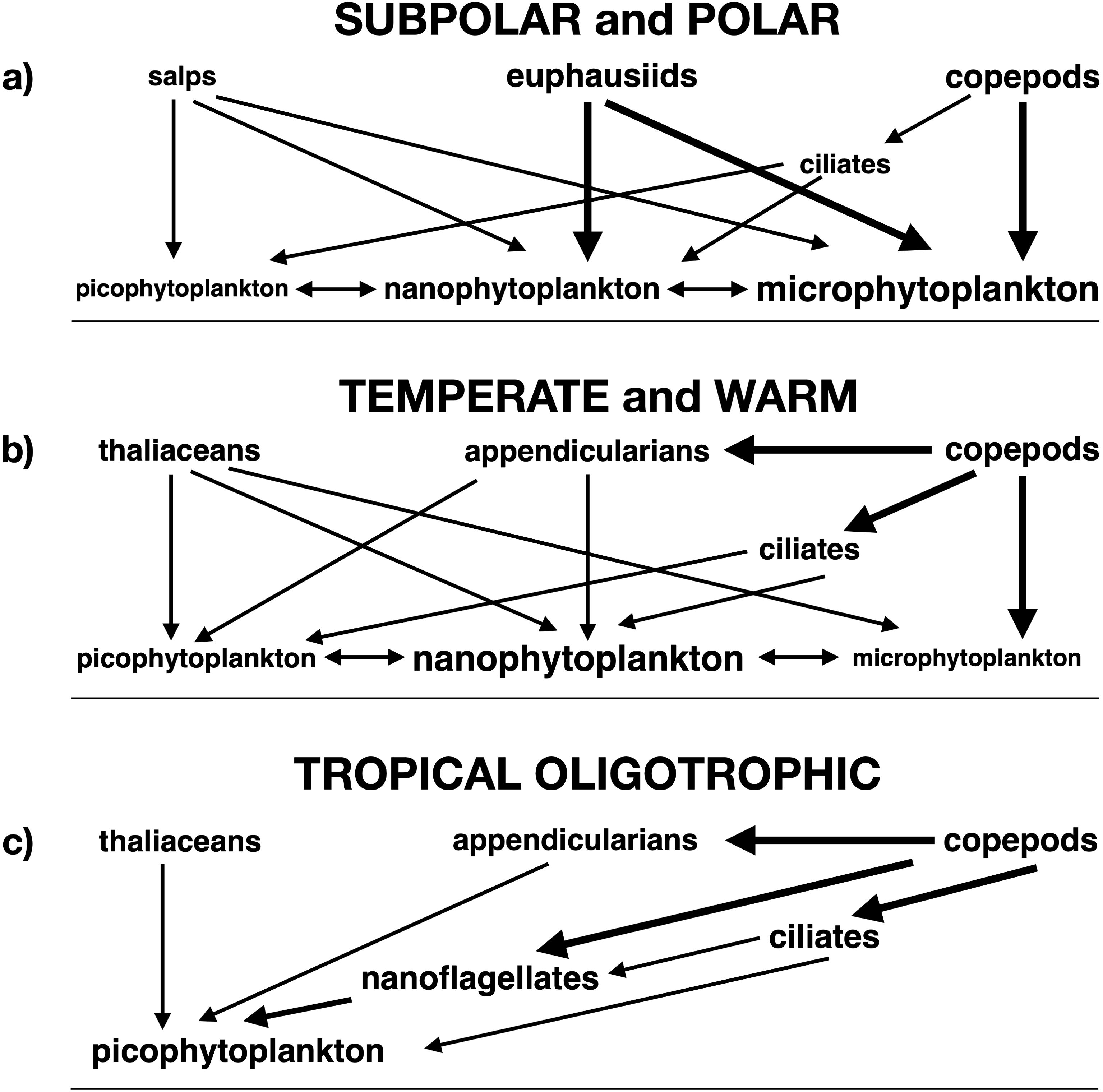
Figure 5. Trophic structure of plankton communities. (a) subpolar and polar, (b) temperate and warm, (c) tropical oligotrophic. Directional arrows indicate direct negative (top-down) impacts, while double-headed arrows represent competition for nutrients among phytoplankton groups. Arrow thickness and font size are approximately proportional to the strength of the impact and the importance of the plankton group in the ecosystem, respectively.
It is thus not surprising that the only universally accepted community-level cascade in a pelagic ecosystem was detected in decadal (1985-1994) time series from the central subarctic Pacific Ocean south of the Aleutian Islands (Shiomoto et al., 1997). These time series revealed a biennial cycle in pink salmon (Oncorhynchus gorbuscha) catch per unit effort in phase with chlorophyll a concentration, while large zooplankton biomass cycled out of phase with both of these groups (Figure 6). This pattern represents the classic signature of a community-level trophic cascade, a conclusion supported by somewhat longer and later (2000-2015) pink salmon, large copepod, and large diatom time series from the same area (Batten et al., 2018). Moreover, a further study found an inverse relationship between pink salmon abundance and seabird reproductive success in the subarctic Pacific, indicating that birds suffer from competition with pink salmon for shared prey, particularly large copepods (Springer and van Vliet, 2014). The causes of this cascade likely lie in the biomass fluctuations driven by the unique biennial life cycle of pink salmon and in a chain of physical, chemical, and ecological features of the subarctic Pacific. This chain links frequent storms, large waves, powerful mixing, low stratification, high nutrient availability, intense microphytoplankton blooms, and abundant grazing copepods and euphausiids (Sommer, 2008; Sommer et al., 2017).
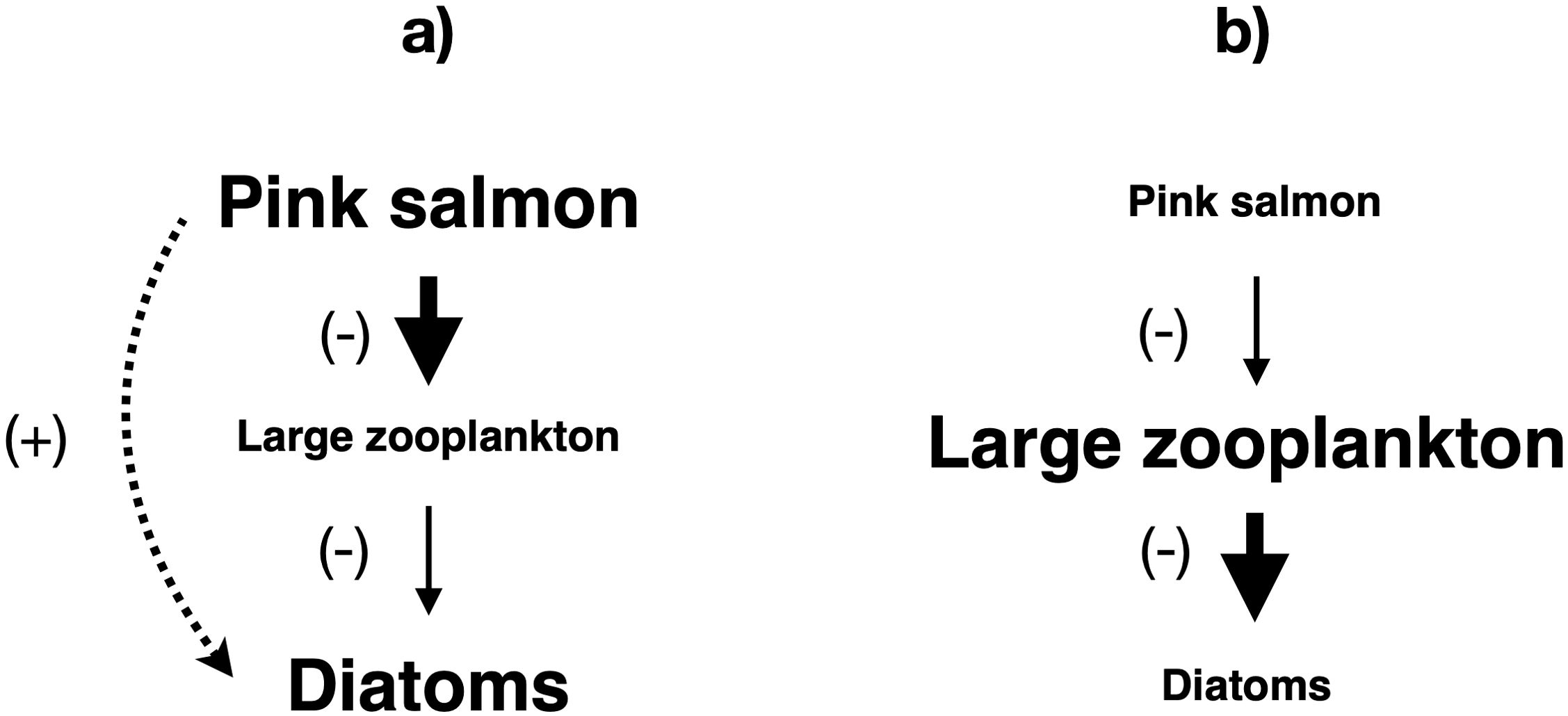
Figure 6. The only community-level trophic cascade known from a pelagic ecosystem, in this case driven by the biennial population cycle of pink salmon (Oncorhynchus gorbuscha) in the subarctic North Pacific (Shiomoto et al., 1997). (a) High pink salmon biomass (odd years). (b) Low pink salmon biomass (even years). Solid lines indicate direct impacts, while dotted lines show indirect impacts. Arrow thickness and font size are approximately proportional to the strength of the impact and the biomass of the group in the ecosystem, respectively.
Upwelling zones are found primarily in tropical to temperate eastern boundary currents flowing toward the Equator, although they also occur in the equatorial Atlantic and Pacific Oceans and the Southern Ocean. Despite their latitudes, eastern boundary upwelling zones share a similar plankton community composition (i.e. the importance of large phytoplankton and herbivorous zooplankton) with other cold waters (Sommer et al., 2002). However, in these unique ecosystems, interannual and decadal variability in upwelling strength, and thus in nutrient supply, appears to be sufficiently strong to overpower top-down control and cause attenuation of community-level trophic cascades.
In comparison to polar and subpolar waters, temperate and warm seas (Figure 5b) show less frequent storms, weaker mixing, and stronger stratification, reducing nutrient availability and thus the importance of large phytoplankton (Sommer et al., 2017). Copepods in these waters also consume microphytoplankton, while thaliaceans (salps, doliolids, and pyrosomes) feed on all phytoplankton size classes. However, euphausiids foraging on micro- and nanophytoplankton are less abundant in these ecosystems than in subpolar and polar seas, while appendicularians feeding on nano- and picophytoplankton appear (Sommer and Stibor, 2002). Appendicularian eggs and juveniles are in turn consumed by copepods, releasing nano- and picophytoplankton from grazing pressure (Stibor et al., 2004b). Furthermore, copepods also feed on microzooplankton, with similar indirect impacts on nano- and picophytoplankton (Stibor et al., 2004a; Vadstein et al., 2004; Sommer and Sommer, 2006). Thus, micro-, nano-, and picophytoplankton are at least partially protected from grazing in temperate and warm waters, leading to attenuation of community-level cascades (Sommer, 2008).
In tropical and subtropical seas (Figure 5c), storms are infrequent, mixing weak, and stratification strong, leading to oligotrophic conditions and picophytoplankton dominance, except in eastern boundary upwelling zones (Sommer et al., 2017). The dominant picophytoplankton is consumed by nanoflagellates and ciliates. The latter in turn forage on nanoflagellates and picophytoplankton, while copepods feed on ciliates and nanoflagellates (Sommer et al., 2002; Sommer and Stibor, 2002). This high level of omnivory in meso- and microzooplankton permits zooplankton biomass to exceed that of phytoplankton (Gasol et al., 2003) due to top-down control (Banse, 2013), but does not favor non-attenuating, community-level cascades with well-defined trophic levels.
Copepod functional diversity displays consistent patterns across the world ocean (Benedetti et al., 2025). In the oligotrophic gyres of tropical and subtropical oceans, copepods show the highest functional richness and lowest functional evenness (i.e. functional types are most numerous but the distribution of biomass among them is least even). These waters feature top-down control of phytoplankton by zooplankton (Gasol et al., 2003; Banse, 2013) but apparently no community-level trophic cascades. This may be partly due to the many weak links in the planktonic food web created by the high richness and low evenness of copepod functional types. In the subarctic Pacific and the Southern Ocean, copepods demonstrate the lowest functional richness and intermediate functional evenness. These waters host the only known non-attenuating, community-level pelagic trophic cascade (in the subarctic Pacific; Shiomoto et al., 1997), as well as top-down control of phytoplankton by zooplankton (in the Southern Ocean; Yang et al., 2022). This may partly result from the few strong links in the planktonic food web yielded by the low richness and intermediate evenness of copepod types. In temperate seas, copepods show intermediate functional richness and the highest functional evenness. These waters feature attenuating trophic cascades (Sommer, 2008). This may partly stem from the substantial number of moderately strong links in the planktonic food web generated by the intermediate richness and high evenness of copepod functional types.
In temperate and polar waters, reduced nutrient availability can cause zooplankton to switch from trophic cascades based on photosynthesis to ones founded on detritus (Stibor et al., 2019). Under eutrophic conditions, copepods in the Baltic Sea forage on microphytoplankton (Lewandowska et al., 2014) and rotifers, which in turn consume picophytoplankton. The latter interactions belong to a cascade linking mesopredators (Berthold et al., 2023), copepods, rotifers, and picophytoplankton (Heiskanen et al., 1996; Berthold et al., 2023). However, under oligotrophic conditions, copepods switch to foraging on ciliates, which consume flagellates, which in turn feed on bacterioplankton, forming a second trophic cascade (Lewandowska et al., 2014). In Kongsfjorden, an inlet of the Greenland Sea in western Spitzbergen, copepods consume ciliates. Under eutrophic conditions, the latter feed on autotrophic flagellates, but in oligotrophic conditions they switch to consuming heterotrophic flagellates, which in turn forage on bacterioplankton (Thingstad, 2020). The detritus-based trophic cascade observed under oligotrophic conditions in Kongsfjorden is nearly identical to one detected by mesocosm experiments in a Norwegian fjord further south (Zöllner et al., 2009).
In a different example of plankton-mediated switching between two trophic cascades, a mesocosm experiment conducted in a Norwegian fjord detected that jellyfish predation on calanoid copepods triggered positive effects on phytoplankton biomass in a cascade featuring three trophic levels and an algal guild initially dominated by large phytoplankton. However, these indirect impacts became negative in a cascade including four trophic levels and an algal community originally characterized by small phytoplankton. In each case, a species-level trophic cascade was found but a community-level cascade was not detected when both food chains were examined together (Stibor et al., 2004a).
Similarly, while species-level trophic cascades driven by jellyfish predation and involving meso- and microzooplankton were obtained by mesocosm experiments in Lake Illawarra (a saline coastal lagoon in New South Wales, Australia), neither a community-level cascade nor top-down impacts on phytoplankton occurred (Pitt et al., 2007; West et al., 2009). Furthermore, bottle incubation experiments based on large-scale sampling in the North Atlantic Ocean failed to find a community-level cascade connecting copepods, microzooplankton, and phytoplankton (Morison et al., 2020). However, a global marine biogeochemical model predicted community-level trophic cascades driven by jellyfish predation on crustacean macrozooplankton (Wright et al., 2021).
Ctenophores also appear capable of causing community-level cascades in marine ecosystems. In the most famous example, “fishing down” the Black Sea food web triggered a cascade involving the invasive ctenophore Mnemiopsis leidyi suppressing other zooplankton and thereby releasing phytoplankton from control by grazers. These impacts were later partly reversed by predation on M. leidyi by a later-invading ctenophore, Beroe ovata (Daskalov et al., 2007; Oguz and Gilbert, 2007; Oguz et al., 2012). However, top-down control of planktonic bivalve larvae by M. leidyi coupled the pelagic and benthic ecosystems through reduced larval bivalve settlement, causing a shift from bivalve to polychaete dominance in the benthos (Oguz et al., 2012). Invasions by M. leidyi also triggered community-level cascades involving mesozooplankton and phytoplankton in the Baltic Sea (Dinasquet et al., 2012) and a Norwegian fjord (Tiselius and Møller, 2017), while the native ctenophore Pleurobrachia pileus exerted cascading impacts on copepods, ciliates, and autotrophic flagellates in an inlet of the Skagerrak (Granéli and Turner, 2002). The high biomasses and consumption rates of cestid and lobate ctenophores in the open ocean suggest the potential for top-down impacts of these pelagic groups (Potter et al., 2023; Child et al., 2025; Irvine et al., 2025), including community-level trophic cascades similar to those observed in neritic waters.
In addition to the biotic and abiotic factors discussed above, several less objective circumstances may contribute to the apparent paucity of non-attenuating, community-level trophic cascades in pelagic ecosystems. Firstly, the bottom-up paradigm which continues to prevail in marine science despite increasing evidence of top-down control and trophic cascades (Verity and Smetacek, 1996; Estes, 2018) may discourage researchers from investigating potential instances of these processes. Secondly, the academic culture of excessive specialization (Essington, 2010; Sergio et al., 2014) may impair the development of an ecosystem perspective in marine science and the flow of ideas between oceanography (which is focused on the marine water column and remains dominated by the bottom-up paradigm) and marine ecology (which is mainly concerned with benthic ecosystems and more open to a top-down perspective). Thirdly, the impracticability of conducting large-scale experiments in pelagic ecosystems may hinder the detection of community-level trophic cascades. Mesocosm experiments, while valuable, cannot account for the massive three-dimensional scale and spatiotemporal variability of the open ocean. This obstacle necessitates a reliance on time series in investigations of pelagic ecosystem dynamics (e.g. Shiomoto et al., 1997; Batten et al., 2018), which in turn presents methodological difficulties for excluding alternate hypotheses based on bottom-up or non-trophic drivers (Essington, 2010). Fourthly, due to the enormous logistical challenges and costs of conducting intensive and extensive sampling and long-term ecological research programs in the open ocean, as well as the typically low perceived relevance of pelagic trophic ecology to fisheries management (except in Japan, Russia, and Norway), the availability of sufficiently fine-grained data on the pelagic biota across all trophic levels is typically poor (with the laudable exceptions of the intensively investigated western North Pacific and Norwegian Sea).
Furthermore, overexploitation may obscure the past trophic importance of currently depleted large predators and grazers (Jackson et al., 2001; Jackson, 2006; O'Dea et al., 2025) and favor bottom-up control in neritic and pelagic ecosystems (Essington, 2010; Boyce et al., 2015). Such masking of knowledge of past species abundances and ecosystem structures by the shortness of human memory and skepticism towards oral and written records of richer past ecosystem states is known as “the shifting baseline syndrome of fisheries” (Pauly, 1995). The widespread occurrence of the related phenomenon of “fishing down marine food webs” (Pauly et al., 1998), discussed above, which was first detected in modern industrial fisheries but dates back for millennia in the Americas (Bourque et al., 2008; Fitzpatrick et al., 2008; Kennett et al., 2008; Steneck and Pauly, 2019), implies that top-down control and mesopredator release, and potentially community-level trophic cascades, occurred in many neritic and pelagic ecosystems.
While high productivity and past overexploitation may now favor bottom-up control in marine ecosystems at large spatial scales (Mcowen et al., 2014), pristine neritic and pelagic ecosystems may have featured an hourglass-shaped trophic structure with biomasses alternately increasing and decreasing with successive trophic levels (Woodson et al., 2018, 2020). This structure suggests a somewhat attenuating community-level cascade regulating higher trophic levels (thus the inverted pyramid above) and overlapping with likewise attenuating bottom-up control governing lower trophic levels (hence the classic pyramid below). Such a structure would have been supported by high food web complexity, generalist predation, and rapid biomass turnover at low trophic levels (Woodson et al., 2018, 2020). As large cetaceans (Rocha et al., 2014) and predatory fishes (Myers and Worm, 2003; Pacoureau et al., 2021; Juan-Jordá et al., 2022) were depleted and “fishing down the food web” intensified (Pauly et al., 1998), the hourglass-shaped trophic structure would have been replaced by the classic trophic pyramid (Elton, 1927). Such a drastic shift would have notably reduced marine carbon sequestration by reducing the number of carcasses of whales (Smith, 2006; Pershing et al., 2010) and large pelagic fish (Mariani et al., 2020) sinking into the deep ocean.
However, hourglass-shaped trophic structures could also result from exogenous prey subsidies to predators (McCauley et al., 2012a; Skinner et al., 2021) and from trade-offs between attack and growth rates in mesopredators (Gibert and Yeakel, 2019). Furthermore, the apparent dominance of generalist predators in aquatic ecosystems has recently been challenged by a study emphasizing the importance of two additional predator types specializing in prey larger and smaller than expected based on predator size (García-Oliva and Wirtz, 2025). These types are exemplified by anglerfish (Lophiiformes) and baleen whales (Mysticeti), and together they predict approximately half of food web organization. The impacts of this tripartite structure of predator guilds on trophic control and cascades in aquatic ecosystems remain to be investigated, but may well be significant.
Trophic cascades and top-down control, revealed by the widespread overfishing of top predators, could still govern entire neritic food webs in the North Atlantic Ocean, especially northern seas characterized by low temperature and species richness (Frank et al., 2005, 2006, 2007, 2013, 2015). These conclusions, at least as originally stated for the Northwest Atlantic (Frank et al., 2005, 2006), were disputed by several analyses seeking to explain the data through climatic and oceanographic change in the Arctic (Greene and Pershing, 2007; Greene, 2013; Pershing et al., 2015). The authors of the original studies responded with rebuttals based on data from the entire North Atlantic which expanded and strengthened their argument (Frank et al., 2007, 2013, 2015; Petrie et al., 2009).
While one global study (Chassot et al., 2010) did not support inclusion of temperature or species richness as explanatory variables in a cross-ecosystem model of marine fisheries catches, another worldwide modeling analysis (Ye and Carocci, 2018) recovered temperature as an explanatory factor. Furthermore, a third global study (Boyce et al., 2015) demonstrated that temperature and biodiversity predict the direction of trophic control in neritic and pelagic ecosystems, providing ultimate and proximate explanations of the observed patterns, respectively. Thus, this analysis corroborated the conclusions originally drawn from the North Atlantic data (Frank et al., 2007, 2013, 2015; Petrie et al., 2009). Globally, marine biodiversity is positively associated with temperature, especially for ectotherms, perhaps due to increased metabolic and speciation rates or greater tolerance for higher temperatures in ectothermic species (Tittensor et al., 2010). It is thus not surprising that the number of links in marine fish food webs is likewise positively correlated with temperature, while connectance (i.e. the number of actual food web links divided by the number of possible links) and the mean number of predator species targeting a prey species are both negatively associated with temperature (Albouy et al., 2019). This suggests that temperature could indirectly inhibit trophic cascades in neritic and pelagic ecosystems by increasing food web complexity while decreasing functional complementarity and/or redundancy in predator guilds.
Several community-level trophic cascades associated with predator overexploitation are known from neritic ecosystems in coastal or semi-enclosed seas (Figure 7). Firstly, depletion of Atlantic cod (Gadus morhua) and other groundfish in the Northwest Atlantic triggered cascading effects on forage fish (i.e. small, schooling planktivores), zooplankton, and phytoplankton (Frank et al., 2005, 2006; Scheffer et al., 2005; Figure 7a). This cascade may have partly stemmed not from reduced cod abundance but from decreased adult size of these predators due to evolutionary effects of fishing (Shackell et al., 2010). Furthermore, the release of forage fish from cod predation may have inhibited cod recovery through predation by forage fish on cod larvae, in what is known as cultivation/depensation, reinforcing the trophic cascade (Walters and Kitchell, 2000). However, as discussed above, this putative cascade has been disputed in several studies (Greene and Pershing, 2007; Greene, 2013; Pershing et al., 2015). Secondly, in the Gulf of Saint Lawrence, top-down impacts of predation by marine mammals inhibited the recovery of cod and other groundfish, with cascading positive impacts on small demersal fish and benthos (Morissette et al., 2006). It has also been suggested that the wider Northeast Atlantic cascade (Frank et al., 2005; Scheffer et al., 2005) had reduced euphausiid availability to endangered blue whales (Balaenoptera musculus) in the Gulf of Saint Lawrence (Comtois, 2010). However, a later study has cast doubt on this hypothesis (Savenkoff et al., 2013). Thirdly, overfishing of cod in the Baltic Sea initiated a trophic cascade in Atlantic sprat (Sprattus sprattus), zooplankton, and phytoplankton (Möllmann and Köster, 2002; Essington and Hansson, 2004; Casini et al., 2009), while interruption of cod immigration from the central Baltic into the Gulf of Riga triggered a nearly identical cascade involving Atlantic herring (Clupea harengus; Casini et al., 2012; Figure 7b).
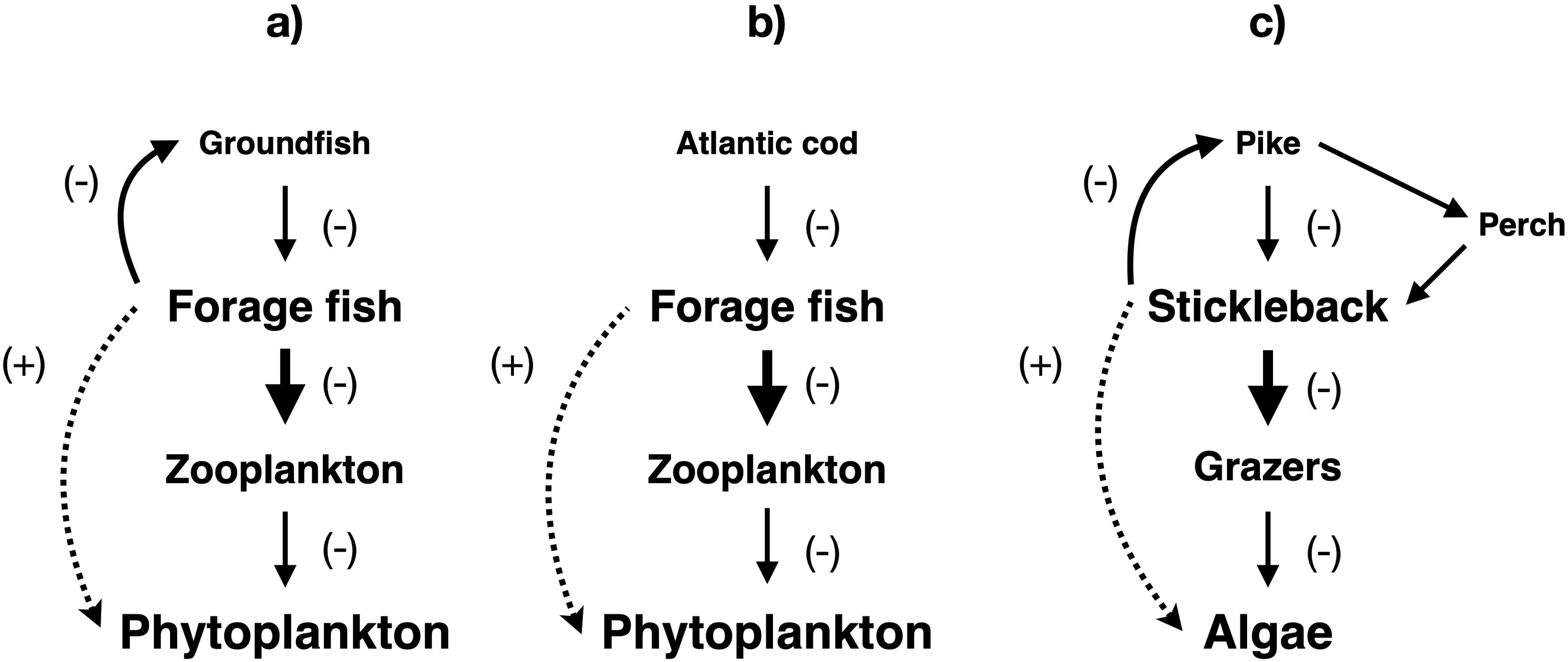
Figure 7. Community-level trophic cascades driven by groundfish overfishing in northern neritic ecosystems. (a) Northwest Atlantic. (b) Baltic Sea (deeper, saltier waters, including Gulf of Riga). (c) Baltic Sea (coastal brackish waters). Solid straight lines indicate top-down negative impacts, solid curved lines represent negative impacts of predation by planktivorous fish on eggs and larvae of predatory fish (cultivation/depensation), while dotted curved lines show indirect impacts. Arrow thickness and font size are approximately proportional to the strength of the impact and the biomass of the group in the ecosystem, respectively.
Fourthly, in the brackish coastal waters of the Baltic Sea, fishing, habitat loss, and predation by gray seals (Halichoerus grypus) and great cormorants (Phalacrocorax carbo), exacerbated by eutrophication and warming, reduced the biomasses of northern pike (Esox lucius) and Eurasian perch (Perca fluviatilis), both important piscivores (Olin et al., 2022). These predator declines, compounded by reduced herring biomass (Donadi et al., 2024), triggered mesopredator release of three-spined stickleback (Gasterosteus aculeatus), which has proliferated and now consumes pike eggs and larvae, reducing recruitment (Nilsson, 2006; Byström et al., 2015; Nilsson et al., 2019; Eklöf et al., 2020) through the cultivation/depensation mechanism (Walters and Kitchell, 2000). Furthermore, stickleback exerts negative impacts on grazers and cascading positive impacts on filamentous algae (Figure 7c), exacerbating the effects of the eutrophication that contributed to the original decline in predatory fish (Eriksson et al., 2009; Candolin et al., 2016; Donadi et al., 2017).
Fifthly, overexploitation of marine mammals in the Bering Sea may have released groundfish, mainly Alaska pollock (Gadus chalcogrammus), from predation, with cascading negative impacts on forage fish, especially capelin (Mallotus villosus), thus reducing the availability of energy-rich prey to marine mammals and exacerbating their decline (Merrick, 1997). Sixthly, predation by Antarctic minke whales (Balaenoptera bonaerensis) and Adélie penguins (Pygoscelis adeliae) on crystal krill (Euphausia crystallorophias), and by penguins and killer whales on Antarctic silverfish (Pleuragramma antarcticum), combine to exert cascading positive impacts on phytoplankton in the marginal ice zone of the Ross Sea (Ainley et al., 2006, 2015). However, this cascade, unlike the previous five, is not due to overfishing and may be spatially constrained by predator distributions (Ainley, 2007; Smith et al., 2007).
Pelagic ecosystems in the tropical Pacific Ocean demonstrate top-down control of mesopredators by upper-level predators, including large fish (Ward and Myers, 2005; Hunsicker et al., 2012), sperm whales (Physeter macrocephalus; Essington, 2006) and Hawaiian monk seals (Monachus schauinslandi; Parrish, 2009), but no trophic cascades, despite the massive depletion of pelagic sharks (Pacoureau et al., 2021) and sperm whales (Whitehead and Shin, 2022). Cascade attenuation is likely due to plankton community structure in these waters, as discussed above (Sommer et al., 2002; Sommer and Stibor, 2002; Sommer et al., 2017; Benedetti et al., 2025). Reviews of the trophic roles of sharks (Ferretti et al., 2010; Dedman et al., 2024) found that while overfishing of apex predatory species could cause mesopredator release and occasionally cascading suppression of lower-order consumers, such impacts occurred more frequently in coastal than pelagic waters.
Humpback whales (Megaptera novaeangliae) may exercise top-down control over Pacific herring (Clupea pallasii) off Alaska (Heintz et al., 2010; Boswell et al., 2015; Moran et al., 2018; Straley et al., 2018) and British Columbia (Surma and Pitcher, 2015; Doherty et al., 2024), although this hypothesis has been disputed (Fu et al., 2017; Tanasichuk, 2017; Ward et al., 2017; Sewall et al., 2018). Multispecies modeling studies show that northern minke whales (B. acutorostrata) exert top-down impacts on herring and juvenile cod in the Norwegian and Barents Seas (Bogstad et al., 1997; Schweder et al., 2000; Lindstrøm et al., 2009) and on capelin in the Barents Sea (Smout and Lindstrøm, 2007). Finally, abundant baleen whale populations apparently exercised top-down control over Antarctic krill (E. superba), a crucial forage species in the Southern Ocean, before their depletion by whaling in the 20th century, with ecosystem-wide impacts on other predators (Laws, 1977; Reid and Croxall, 2001; Murphy et al., 2007; Ainley et al., 2007, 2009; Trivelpiece et al., 2011; Trathan et al., 2012; Surma et al., 2014; Tulloch et al., 2018, 2019). A scenario nearly identical to this “krill surplus” hypothesis involves top-down control of copepods by bowhead whales (Balaena mysticetus), and of bivalves by walrus (Odobenus rosmarus), off Svalbard before the overhunting of these once common mammals in the early modern period (Hacquebord, 2001).
Some abundant species occupying intermediate trophic levels in neritic and pelagic ecosystems, particularly forage fish in eastern boundary upwelling zones, have been suggested to exhibit wasp-waist dynamics, i.e. simultaneously exercise top-down control over prey and bottom-up control over predators (Cury et al., 2000; Figure 8a). However, the high levels of omnivory in many marine predators, e.g. Pacific bluefin tuna (Thunnus orientalis) and yellowfin tuna (T. albacares) in the southern California Current (Madigan et al., 2012), and of functional redundancy at intermediate trophic levels in numerous ecosystems (Fréon et al., 2009; Bundy and Guénette, 2014; Cardona et al., 2015; Gaichas et al., 2015), have cast doubt on this hypothesis. The wasp-waist idea has been questioned in studies of the southern Caribbean Sea (Duarte and García, 2004), eastern boundary upwelling zones (Fréon et al., 2009), including the northern Humboldt Current (Ayón et al., 2008; Taylor et al., 2008) and southern California Current ecosystems (Madigan et al., 2012); the western Mediterranean Sea (Cardona et al., 2015), and the Gulf of Alaska and Bering Sea (Gaichas et al., 2015), as well as in a global analysis (Bundy and Guénette, 2014). A suggested refinement to the wasp-waist concept would only require local top-down impacts on prey of the mid-trophic species, allowing Antarctic krill to qualify (Atkinson et al., 2014). Nevertheless, wasp-waist ecosystem structures have been found in the Arctic Ocean (Thingstad, 2021), the tropical Pacific Ocean (Griffiths et al., 2013), the southwest Atlantic Ocean (Padovani et al., 2012; Laptikhovsky et al., 2013; Saporiti et al., 2015; Riccialdelli et al., 2020; Büring et al., 2024; Marina et al., 2024), the adjacent Drakes Passage (Scian et al., 2025), and the South Shetland Islands in the Southern Ocean (Rodriguez and Savaria, 2024). The species found to exercise wasp-waist control include copepods (Thingstad, 2021), amphipods (Padovani et al., 2012; Rodriguez and Savaria, 2024; Scian et al., 2025), the Patagonian squid Doryteuthis gahi (Laptikhovsky et al., 2013; Büring et al., 2024; Marina et al., 2024), the squat lobster Munida gregaria (Riccialdelli et al., 2020), and many small pelagic fish (Padovani et al., 2012; Griffiths et al., 2013; Laptikhovsky et al., 2013; Riccialdelli et al., 2020; Büring et al., 2024; Rodriguez and Savaria, 2024). For clupeid forage fish in the North Sea, bottom-up control of predators was only demonstrated for seabirds (Fauchald et al., 2011). Wasp-waist control should not be confused with the hourglass-shaped trophic structure associated with community-level trophic cascades in pristine marine ecosystems (Woodson et al., 2018, 2020). In fact, wasp-waist ecosystems would likely show the rare diamond-shaped distribution of biomass across trophic levels, with several wasp-waist species at intermediate trophic levels concentrating much of the total ecosystem biomass (Gibert and Yeakel, 2019).
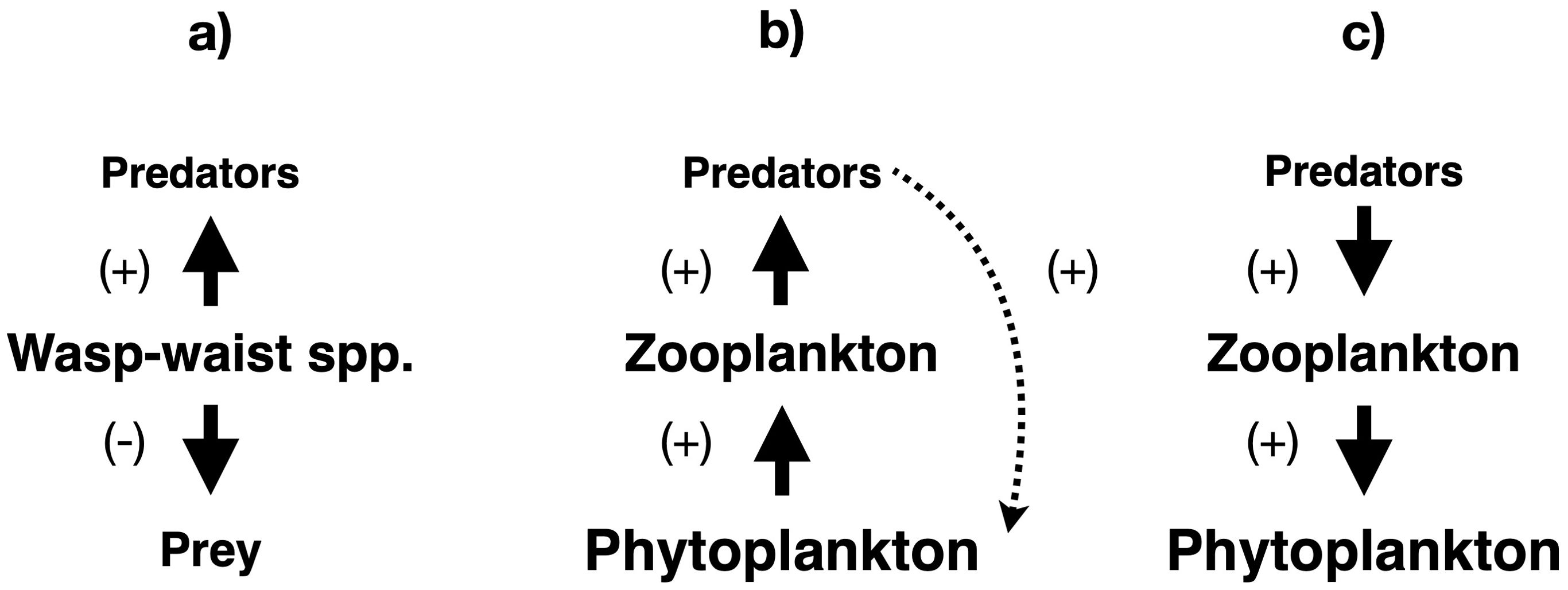
Figure 8. Unconventional patterns of trophic control in marine ecosystems. (a) wasp-waist control (Cury et al., 2000). (b) Trophic feedback loop (e.g. Smetacek and Nicol, 2005). (c) Synergistic trophic interactions (Johannessen, 2014). Solid lines indicate direct impacts, while dotted lines show indirect impacts. Arrow thickness and font size are approximately proportional to the strength of the impact and the biomass of the group in the ecosystem, respectively.
Forage fish exercise top-down control over zooplankton in some cases (Cury et al., 2000; Yebra et al., 2020) and are controlled by them in a bottom-up manner in others (Duarte and García, 2004; Ayón et al., 2008; Taylor et al., 2008; Engelhard et al., 2014). These fish also demonstrate bottom-up control over diverse predators (Smith et al., 2011; Engelhard et al., 2014; Pikitch et al., 2012, 2018), notably central place foragers and mobile predators forming feeding aggregations. These groups encompass most seabirds (Cury et al., 2011; Sydeman et al., 2017) and marine mammals (e.g. McClatchie et al., 2016), as well as some piscivorous fish (Hannesson, 2013; Kaplan et al., 2017; Koehn et al., 2017). Conversely, a theoretical modeling study predicted top-down control over forage fish by predators (Houle et al., 2013), which has been detected for demersal fish and seals in the North Sea (Engelhard et al., 2014).
Many statistical and modeling studies of marine ecosystem structure and fisheries dynamics in European neritic seas (Chassot et al., 2007), pelagic waters of the tropical and subtropical Atlantic (Finenko et al., 2003), Northeast Pacific continental shelf ecosystems (Ware and Thomson, 2005), large marine ecosystems of the United States (Marshak and Link, 2021), and globally (Chassot et al., 2010; Friedland et al., 2012; Schlenger et al., 2018; Ye and Carocci, 2018; Marshak and Link, 2021), support the primacy of bottom-up control at large spatial scales. However, while primary productivity constrains fish production across large marine ecosystems in the USA and worldwide (Marshak and Link, 2021), bottom-up, top-down, and wasp-waist control may all act at smaller scales (Hunt, 2006; Hunt and McKinnell, 2006). Other studies indicate that top-down and bottom-up control often occurs simultaneously or successively in pelagic and neritic ecosystems, including the Southern Ocean (Smetacek and Nicol, 2005; Ainley et al., 2007; Nicol et al., 2007), Barents Sea (Johannesen et al., 2012; Dalpadado et al., 2014), and North Sea (Lynam et al., 2017). Furthermore, both top-down processes (i.e. predation and fishing pressure) and bottom-up forcing (i.e. climate, physical oceanography, and primary productivity) are necessary to explain ecosystem and fisheries dynamics in the Gulf of Alaska (Gaichas et al., 2011), North Sea (Lynam et al., 2017), and worldwide (Mackinson et al., 2009; Chassot et al., 2010; Fu et al., 2012; Mcowen et al., 2014; Ye and Carocci, 2018).
Behaviorally mediated trophic cascades and marine ecosystem dynamics
Optimal foraging theory suggests that three-level trophic cascades can operate not only through predation mortality of organisms at the intermediate trophic level (as in the cases discussed above), but also through alteration of their foraging behavior by fear of predators, with these two pure states bounding a continuum of intermediate cases (Brown et al., 1999). This theoretical argument is supported by numerous fear-mediated cascades observed in the field (Schmitz et al., 2004). In some cases, these fear-mediated cascades may yield indirect effects that appear counterintuitive from the perspective of a classic trophic cascade (e.g. Griffin et al., 2011). Furthermore, predator hunting strategies, combined with proportions of all available habitats in the ecosystem used by predators and prey, could determine whether mortality or fear will drive cascades, with the latter predicted to dominate across most combinations of predator and prey behaviors (Schmitz et al., 2004). Specifically, classical mortality-driven trophic cascades should occur only when predators hunt actively and both predators and prey use multiple habitats. Although this hypothesis requires further testing, it is thus not surprising that fear-mediated cascades occur across terrestrial and freshwater ecosystems (Schmitz et al., 2004).
Marine fear-mediated trophic cascades, or “seascapes of fear” (Wirsing et al., 2008), are likewise widespread, occurring in salt marsh (Griffin et al., 2011), rocky intertidal (Trussell et al., 2004; Donohue et al., 2017), oyster bed (Grabowski and Kimbro, 2005), seagrass bed (Wirsing et al., 2007; Burkholder et al., 2013; Heithaus et al., 2012, 2014), kelp forest (Duggins, 1983; Byrnes et al., 2006; Spyksma et al., 2017; Haggerty et al., 2018; Curtis and Wing, 2024), coral reef (Madin et al., 2011, 2019; Rizzari et al., 2014b; Rasher et al., 2017; Atwood et al., 2018; Meekan et al., 2025), and neritic ecosystems (Frid et al., 2008, 2009; Matthews et al., 2020). In agreement with optimal foraging theory (Brown et al., 1999), these fear-mediated cascades typically span three trophic levels. However, a recent study failed to detect a “seascape of fear” on a coral reef (Tebbett et al., 2024). Potential cascades involving at least partly fear-mediated interactions between two top predators, the killer whale and white shark (Carcharodon carcharias), have been reported from neritic waters off central California (Jorgensen et al., 2019) and South Africa (Towner et al., 2022). However, further research is necessary to establish that intraguild impacts of killer whales on white sharks have cascading effects on lower trophic levels. While the existence of fear-mediated trophic cascades in pelagic ecosystems has likewise yet to be conclusively demonstrated, this may result from some of the same causes as the apparent dearth of mortality-driven pelagic cascades discussed above, particularly the logistical challenges to ecological research in the open ocean. Furthermore, there are strong indications that pelagic “seascapes of fear” not only exist but may in fact be a ubiquitous feature of ocean ecology, driving and modulating spatiotemporal activity patterns of plankton and nekton across multiple trophic levels and timescales (Urmy and Benoit-Bird, 2021).
It is also increasingly recognized that predator and prey bioenergetics influence trade-offs between foraging and predation risk avoidance (Gallagher et al., 2017), potentially modulating mortality- and fear-driven trophic cascades (Papastamatiou et al., 2023). Examples of such influences have been documented in terrestrial (Gallagher et al., 2017), fluvial (Gil et al., 2025), coral reef (Papastamatiou et al., 2021, 2023), and pelagic ecosystems (Beltran et al., 2023). Theory predicts that top-down impacts should increase with spatiotemporal overlap and conflict between prey energy savings (and/or gains) and predation risk (Gil et al., 2025). Thus, according to this argument, a species-level trophic cascade would occur if the interaction between the prey and the next trophic level involved a similarly irreconcilable conflict between energy optimization and the risk of being consumed. Community-level cascades would occur if strong interactors (e.g. foundation, keystone, or wasp-waist species) were involved. Trophic cascades would be primarily fear-mediated if prey more frequently prioritized risk avoidance over foraging optimization, and mainly mortality-driven in the opposite situation (Gil et al., 2025; Table 1). However, the multiple spatiotemporal scales of factors affecting energetics and predation risk for pelagic organisms, which move in three dimensions according to circadian and seasonal rhythms (Bandara et al., 2021; Urmy and Benoit-Bird, 2021; Beltran et al., 2023), along with the logistical challenges to research in the open ocean discussed above, render the frequency and drivers of pelagic cascades highly challenging to predict.
Foraging arena theory predicts the direction of control over a trophic interaction using the rate at which prey switch between states of vulnerability and invulnerability to a given predator (Walters and Juanes, 1993; Ahrens et al., 2012). This rate is affected by factors including prey aggregation, predator attack strategy, predator and prey mobility, and spatiotemporal refuge availability. Prey aggregation behavior (fish schooling and krill swarming) involves trade-offs between predation risk and other considerations, e.g. oxygen availability (Brierley and Cox, 2010). Foraging arena theory predicts that ephemeral aggregations should favor top-down control and non-attenuation of trophic cascades, while stable aggregations should promote bottom-up control and attenuation of cascades. In turn, prey aggregation stability is affected by predator attack strategy. Atlantic herring schools in the Norwegian Sea remained stable in response to attacks by individual Atlantic cod and haddock (Melanogrammus aeglefinus), but were disrupted by aggregations of predatory saithe (Pollachius virens; Pitcher et al., 1996). Similarly, large schools of overwintering Pacific herring (Clupea pallasii) in Lynn Canal, a Pacific fjord in southeast Alaska, were dissipated and forced to surface by lunge-feeding humpback whales, facilitating attacks by Steller sea lions (Eumetopias jubatus; Boswell et al., 2015). Thus, predators whose attack strategies allow them to disrupt prey aggregations are more likely to exercise top-down control over prey, promoting trophic cascades.
Predator mobility has been suggested to promote bottom-up control and weaken trophic cascades in marine ecosystems by spreading predation pressure in three-dimensional space (McCann et al., 2005). However, mobile predators have also been hypothesized to promote top-down control and trophic cascades (Borer et al., 2005), particularly in spatially confined habitats contiguous with open ecosystems (McCann et al., 2005). For example, sharks which often forage in the epipelagic zone surrounding Palmyra Atoll, a near-pristine coral reef in the South Pacific, exert cascading negative impacts on algal cover, mediated by herbivore diversity and/or behavior (Sandin et al., 2008; McCauley et al., 2012a). More generally, the direction of trophic control in a marine predator-prey interaction could depend on the balance between predator and prey mobility (Hunt, 2006). When predators are more mobile than prey (e.g. in the cases of sharks and resident fish on coral reefs, swimming predators and benthic grazers in kelp forests, and baleen whales and their prey), top-down impacts (whether mediated by mortality of fear) would be expected. Conversely, when prey are more mobile than predators (e.g. in the case of pelagic fish and central place foragers or epipelagic visual predators and vertically migrating prey), bottom-up control would be favored. Perhaps due to this multiplicity of possible predator-prey interaction scenarios in every ecosystem, predator mobility does not significantly affect the prevalence of trophic cascades across ecosystem types (Borer et al., 2005). This result may also stem from the fact that along the spatial scale from lacustrine through neritic to pelagic habitats, potential predator mobility increases but so does the influence of advection on prey, yielding a constantly shifting balance between predator and prey mobility. Furthermore, the effects of scale and advection in pelagic ecosystems on the trade-off between prey energy optimization and predation risk avoidance, and thus on mortality- versus fear-mediated trophic cascades (Gil et al., 2025), are highly variable and therefore difficult to predict.
Diel vertical migration is determined primarily by trophic factors (Pinti et al., 2019), with studies revealing predation risk and prey availability as the principal drivers for plankton and large nekton, respectively (Bandara et al., 2021). However, there is a great deal of plasticity in the recorded responses to these factors among and within zooplankton species (Bandara et al., 2021), and typical migration patterns may be modulated by fear-mediated avoidance responses to encounters with pelagic predators (Urmy and Benoit-Bird, 2021). Organisms avoiding visual predation typically migrate upward at dusk and downward at dawn, but zooplankton may also demonstrate the reverse pattern, which was first recorded in the 19th century (Brook and Calderwood, 1885). Reverse diel vertical migration occurs in both lacustrine (Farrell and Hodgson, 2012) and neritic ecosystems (Ohman et al., 1983; Frost and Bollens, 1992), and may be more frequent in deeper waters (Irigoien et al., 2004). Like the normal zooplankton pattern, it is driven by predation avoidance (Ohman et al., 1983; Frost and Bollens, 1992; Pinti et al., 2019). Normal and reverse diel vertical migrations may alternate across trophic levels, forming a cascade driven by avoidance of the highest predator, with potentially notable implications for vertical biogeochemical fluxes (Bollens et al., 2010). This phenomenon may be limited to lower trophic levels, with visual predation by nekton as the driver of migration cascades in plankton (Bandara et al., 2021). However, a recent study indicates that vertical movements of predators, including echolocating odontocetes and epipelagic schooling fish, modulate diel vertical migration in their prey, potentially affecting ocean biogeochemical cycles (Urmy and Benoit-Bird, 2021). From the perspective of foraging arena theory, such switching between vulnerability and invulnerability of prey to a given predator on diel and shorter timescales across multiple trophic levels may be expected to promote top-down control and trophic cascades.
It has been suggested that the vast movement of biomass involved in diel vertical migration may contribute to noticeably to mixing of the upper ocean (Huntley and Zhou, 2004; Dewar et al., 2006). This radical hypothesis has been supported by field studies (Katija and Dabiri, 2009) and modeling analyses (Dabiri, 2010), critiqued in a review (Kunze, 2019), and corroborated by laboratory experiments (Wilhelmus and Dabiri, 2014; Houghton et al., 2018; Houghton and Dabiri, 2019) and further field observations (Fernández Castro et al., 2022). If diel vertical migration indeed contributes significantly to ocean mixing, fear-mediated cascading impacts of predation may affect ocean biogeochemical cycling (Bollens et al., 2010; Urmy and Benoit-Bird, 2021), potentially creating trophic feedback loops supporting the top predators modulating the migrations themselves.
Complications and paradoxes in marine trophodynamics
A complication to the conventional dichotomy of top-down versus bottom-up control stems from trophic feedback loops generated by positive biogeochemical impacts of predators on producers (Figure 8b). A small-scale trophic feedback loop has been observed at Palmyra Atoll, where nutrients in seabird guano stimulate phytoplankton blooms, increasing zooplankton biomass, which in turn supports giant manta rays (Manta birostris; McCauley et al., 2012b). In another local feedback loop detected in southern California, ammonium ions recycled in the excretions of fish inhabiting kelp forests improve kelp productivity (Shrestha et al., 2024; Peters et al., 2025).
However, the classic example of this effect is the “whale pump” concept, first mooted in a review of polar marine ecology (Smetacek and Nicol, 2005). This hypothesis states that unexploited whale populations fertilized surface waters with feces rich in bioavailable nutrients (particularly iron), thus recycling them in the euphotic zone and raising primary productivity (Smetacek and Nicol, 2005; Smetacek, 2008; Nicol et al., 2010; Roman and McCarthy, 2010; Pershing et al., 2010; Lavery et al., 2010, 2014; Smith et al., 2013; Willis, 2014; Ratnarajah et al., 2014, 2016, 2018; Woodstock et al., 2023; Monreal et al., 2025). However, an ecosystem modeling analysis of historical and recent biological iron cycling in the Southern Ocean indicates that this recycling was, and is, carried out predominantly by zooplankton (Maldonado et al., 2016). Nevertheless, this study also found that the iron demands of the primary production required to sustain baleen whales could be met entirely by the iron recycled by these same whales if the iron content of krill was sufficiently high (Maldonado et al., 2016). Furthermore, this analysis did not include recycling by sperm whales, which could be particularly effective in recirculating iron from the bathypelagic to the euphotic zone (Lavery et al., 2010). In addition, iron excreted by whales is bound by organic ligands, rendering it highly bioavailable (Monreal et al., 2025). A recent study found that the roles of cetaceans in nutrient recycling vary globally, with greater amounts of nutrients recycled by whales associated with higher primary productivity and their elemental composition dependent on cetacean functional diversity (Gilbert et al., 2023).
In addition to the classic trophic cascade, in which biomasses at successive trophic levels show alternating negative and positive correlations with top predator biomass, “paradoxical top-down control” (Morozov et al., 2005), characterized by trophic effects with signs opposite to those in the classic cascade, is occasionally observed. This counterintuitive situation has been documented in the plankton of a shallow embayment of the northwest Atlantic Ocean (Hargrave et al., 1985), as well as in freshwater (Leibold et al., 1997; Alimov, 2000) and terrestrial ecosystems (Halaj and Wise, 2001). Such behavior is also predicted by a simple model of eutrophic pelagic ecosystems featuring high nutrient fluxes across the pycnocline (Morozov et al., 2005). Both empirical and model findings suggest that predation could prevent short-term overgrazing of phytoplankton by zooplankton, thus preventing consumer-resource cycles and effectively increasing and decreasing long-term zooplankton and phytoplankton biomass, respectively (Hargrave et al., 1985; Morozov et al., 2005). However, given how infrequently they have been observed, such dynamics are likely exceptional. Furthermore, empirical and modeling studies indicate that the increased zooplankton biomass generated in this way is prone to sudden collapses (Alimov, 2000; Morozov et al., 2005).
The hypothesis of synergistic trophic interactions (Johannessen, 2014) also departs radically from the traditional understanding of top-down versus bottom-up control (Figure 8c). It was advanced to explain observed patterns in data on phytoplankton, zooplankton, and planktivorous fish in the Skagerrak. This hypothesis states that high consumption by fish and zooplankton could reduce exploitation competition among zooplankton and phytoplankton. Thus, through multiple species-level trophic cascades, consumers could increase rather than decrease total biomasses at lower trophic levels. Such synergistic predator-prey interactions, associated with functional redundancy at lower trophic levels, would generate mutually supporting standing biomasses at adjacent trophic levels and constitute an evolutionarily stable strategy, i.e. a system of trophic interactions resistant to selective pressures favoring a classic evolutionary arms race. While this hypothesis is potentially revolutionary, it remains untested beyond the original study area off southern Norway.
Unconventional trophic interactions may also be involved in the famous “paradox of the plankton” (Hutchinson, 1961), one of the greatest unsolved problems in aquatic ecology. The paradox stems from the apparent conflict between phytoplankton species richness in well-mixed waters and the competitive exclusion principle (Gause, 1934). Suppression of strong competitors by zooplankton grazing was advanced as a potential resolution but initially considered incapable of explaining its paradoxical magnitude (Hutchinson, 1961). However, a more recent study suggests that disruption of competition by grazing could indeed support high phytoplankton species richness (Prowe et al., 2012) through the synergistic trophic interactions described above (Johannessen, 2014). While horizontal spatial heterogeneity generated by mesoscale vortices in the epipelagic zone could also interfere with interspecific competition (Bracco et al., 2000), this mechanism cannot explain the paradoxically high species richness of lacustrine phytoplankton. Chaotic phytoplankton guild dynamics have also been invoked to resolve this paradox. While chaotic dynamics could stem from multiple biotic and abiotic factors, including mesoscale vortices, trophic interactions are likely one of the mechanisms involved in generating such dynamics (Scheffer et al., 2003).
Patterns and processes in marine trophodynamics
Several biotic factors have been suggested to promote trophic cascades and top-down control in marine ecosystems. Firstly, palatability of dominant producers (e.g. Northern Hemisphere kelps and many phytoplankton) is associated with trophic cascades (Strong, 1992; Steinberg et al., 1995). Secondly, keystone species (Strong, 1992) and endothermic vertebrates with high metabolic rates (Borer et al., 2005) are frequently involved in trophic cascades and top-down control. The sea otter belongs to both of these categories, which explains the strength and variety of its top-down impacts on North Pacific coastal ecosystems. Thirdly, highly compartmentalized cold-water food webs, in which a few strong interactions among several species channel much of the total energy flow, e.g. the Antarctic Scotia Sea (Murphy et al., 2007), subarctic Atlantic (Frank et al., 2007), and North Pacific kelp forests (Estes and Palmisano, 1974) often demonstrate trophic cascades and top-down control. This is likely at least partly due to the fact that among marine fish, both connectance and the average number of predator species consuming a prey species are negatively correlated with temperature (Albouy et al., 2019).
Conversely, several producer traits have been proposed to favor bottom-up control in marine ecosystems. Firstly, strong chemical defenses in the kelps of temperate Oceania (Steinberg et al., 1995) weaken grazing pressure and thus should attenuate community-level trophic cascades, yet such cascades occur in New Zealand kelp forests (Babcock et al., 1999; Shears and Babcock, 2002; Edgar et al., 2017), albeit not as frequently as in their North Pacific counterparts. Secondly, small phytoplankton size and short lifespan are globally associated with bottom-up control (Sommer, 2008; Boyce et al., 2015). Phytoplankton size and lifespan decrease with increasing temperature, most likely due to increased stratification resulting in decreased nutrient availability (Sommer et al., 2017). This suggests that warmer waters should favor bottom-up control and attenuate trophic cascades (Sommer, 2008; Boyce et al., 2015; Murphy et al., 2020). While phytoplankton in tropical pelagic ecosystems is subject to top-down control (Gasol et al., 2003; Banse, 2013), community-level cascades are unlikely to occur due to the dominance of small phytoplankton and the resulting high levels of omnivory within the zooplankton guild (Sommer et al., 2002; Sommer and Stibor, 2002).
Omnivory is a consumer trait often suggested to favor bottom-up control in marine ecosystems (Strong, 1992; Bascompte et al., 2005; Bruno and O’Connor, 2005; Boyce et al., 2015) by distributing predation pressure across trophic levels and thus increasing food web complexity. It is significantly more widespread in saltwater ecosystems than in their freshwater or terrestrial counterparts (Thompson et al., 2007). The typical degrees of omnivory among two zooplankton taxa dominating marine and lacustrine ecosystems (i.e. copepods and cladocerans, respectively) are a case in point (Sommer and Sommer, 2006). In marine ecosystems, omnivory is positively associated with temperature, again suggesting that bottom-up control is favored in warmer waters (Boyce et al., 2015) and could increase with climate change (Murphy et al., 2020). While top-down control does occur in tropical pelagic ecosystems (Gasol et al., 2003; Banse, 2013; Ward and Myers, 2005; Essington, 2006; Parrish, 2009; Hunsicker et al., 2012), community-level trophic cascades are unlikely due to high omnivory among several zooplankton groups (Sommer et al., 2002; Sommer and Stibor, 2002). The converse of omnivory is subsidized predation, in which feeding on one resource strengthens the impact of a predator on another (Polis, 1999; Borer et al., 2005). The top-down impact in this case is analogous to that of a mobile predator on resident prey inhabiting a spatially constrained habitat (McCann et al., 2005), such as the sharks feeding on coral reef fish at Palmyra Atoll (Sandin et al., 2008; McCauley et al., 2012a).
Biodiversity has also been suggested to promote bottom-up control in marine ecosystems (Strong, 1992; Frank et al., 2006, 2007, 2015; Petrie et al., 2009; Pershing et al., 2015; Boyce et al., 2015) by increasing the number of possible trophic interactions (Albouy et al., 2019) and thus decreasing the influence of each interaction through compensatory responses (Fahimipour et al., 2017). While biodiversity of prey (Stachowicz et al., 2007) and copepods (Benedetti et al., 2025) is associated with bottom-up control in marine ecosystems, the trophic effects of predator biodiversity vary (Stachowicz et al., 2007). Although the species richness of sharks (Boyce et al., 2015) and other marine predators (Baum and Worm, 2009) is linked to bottom-up control and inimical to trophic cascades, predator species richness in Northeast Pacific kelp forests strengthens trophic cascades, and thus kelp cover, through functional complementarity (Byrnes et al., 2006; Burt et al., 2018; Selgrath et al., 2024; Langendorf et al., 2025) and redundancy (Eisaguirre et al., 2020; Kumagai et al., 2024). Since marine species richness increases with temperature (Tittensor et al., 2010; Albouy et al., 2019), it is not surprising that tropical seas feature many species and few trophic cascades (Boyce et al., 2015). It may be relevant that prey consumption by coastal mesopredators (Whalen et al., 2020) and large pelagic fish (Roesti et al., 2020) peaks in subtropical and temperate waters, respectively, i.e. at higher latitudes than marine species richness (Tittensor et al., 2010; Albouy et al., 2019), although community-level cascades only appear in North Atlantic neritic ecosystems at subarctic latitudes with low species richness (Frank et al., 2007, 2015; Petrie et al., 2009). However, species richness is not a significant factor in the relative prevalence of trophic cascades across ecosystem types (Borer et al., 2005).
Conclusions
The studies discussed above reveal three general patterns in the prevalence of top-down control and trophic cascades across marine ecosystems. Firstly, top-down control of individual trophic interactions is more common in neritic and pelagic ecosystems than are species-level cascades, which in turn are more frequent than community-level cascades. Secondly, although top-down control and species-level trophic cascades are relatively common in neritic and pelagic ecosystems, community-level cascades are indeed more prevalent in lacustrine and marine benthic ecosystems than in their neritic counterparts, and occur least frequently in pelagic ecosystems. Thirdly, the incidence and strength of community-level trophic cascades in neritic and pelagic ecosystems is inversely related to species richness and omnivory, both of which are positively associated with temperature at large spatial scales.
These studies also identify factors that provide plausible explanations of these patterns (Table 2). The distinctions between lacustrine, neritic, and pelagic trophodynamics apparently stem from the increasing scale of physical processes from lacustrine to pelagic ecosystems and from the effects of these processes on nutrient availability, plankton community composition, and the efficiency of phytoplankton control by grazing. This chain of physical, chemical, and biological influences also explains the differences in trophic structure and control among neritic and pelagic ecosystems in subpolar and polar, temperate and warm, and oligotrophic tropical waters. Top-down control and trophic cascades in marine ecosystems are seemingly hindered by high omnivory and prey species richness through their disruption of strong predator-prey interactions. The influence of temperature on these ecological variables is likely mediated by the effects of evolutionary and physiological factors on marine organisms. Ecological theory predicts that top-down control, and thus trophic cascades, should be favored by frequent changes in the vulnerability of prey to predators (including those caused by ephemeral prey aggregations and high predator-prey mobility ratios), as well as by overlap and conflict between prey energy gains and/or savings and predation risk avoidance. Overexploitation and anthropogenic warming weaken or obscure trophic cascades in many marine ecosystems. The logistical challenges of conducting ecological research in the open ocean hinder the detection of pelagic trophic cascades. Fear of predators drives trophic cascades and vertical migrations in numerous marine ecosystems. Paradoxical and synergistic trophic interactions, as well as feedback loops mediated by nutrient cycling, complicate the conventional dichotomy between top-down and bottom-up control. Finally, both of these types of trophic control often operate simultaneously, successively, or at different spatial scales in numerous marine ecosystems.
Best practices and new research directions for marine trophic ecology
While there may well be objective reasons for the paucity of observed trophic cascades in pelagic ecosystems, it is also highly likely that a dearth of research effort has contributed to this situation. Thus, formulation and implementation of best research practices for marine trophic ecology could help evaluate the true importance of trophic cascades and top-down control in pelagic ecosystems.
Firstly, biological sampling across multiple trophic levels could be emphasized more strongly in planning standard oceanographic and fisheries research cruises. For example, net tows producing biomass and diet composition indices for pelagic species (i.e. fish, squid, and zooplankton) and physical and chemical sampling of the water column could be integrated with visual and acoustic surveys of marine mammals, seabirds, and fish and mark-recapture estimation of abundance and mortality to yield an end-to-end view of the abiotic and biotic dynamics of pelagic ecosystems. Currently, such comprehensive surveys of pelagic ecosystems are exceptional, as most research cruises focus on gathering data on physical and NPZ (nutrient, phytoplankton, and zooplankton) dynamics and/or providing inputs for fisheries stock assessments.
Secondly, biological data from research cruises could be more effectively harnessed to investigate the prevalence of trophic cascades and top-down control in neritic and pelagic ecosystems. These data (with particular emphasis on biomass and diet composition indices) could be assembled into time series documenting seasonal, interannual, and decadal patterns in ecosystem dynamics. Such patterns could then be analyzed using a variety of statistical techniques and food web models to determine the likely type of trophic control governing each predator-prey interaction. Currently, such an approach is limited to the best-funded and staffed marine and fisheries research agencies and rarely applied to the open ocean, particularly the high seas.
Thirdly, research cruises could be planned to facilitate comparative investigations of ecosystem dynamics across gradients in selected variables known or suggested to influence trophic control. On the scale of one or more large marine ecosystems, relevant variables could include latitude, temperature (by depth), mixed layer depth, nutrient concentrations (by depth), and biodiversity. To minimize confounding effects, these studies could be designed as “natural experiments” by selecting stations and transects known to differ in as few variables as possible.
Finally, the current state of knowledge permits the formulation of several research questions to be addressed in the future using the best practices discussed above.
1. Do as yet undetected community-level trophic cascades operate in pelagic ecosystems?
2. Is the HNLC character of subpolar and polar pelagic waters due entirely to micronutrient limitation or do zooplankton – phytoplankton – macronutrient cascades also contribute?
3. How does the tripartite functional classification of marine predators (i.e. generalists, large prey specialists, and small prey specialists) impact trophic cascades?
4. Are community-level marine trophic cascades typically driven by mortality or by fear?
5. Do cascading diel vertical migrations, driven and modulated by trophic interactions, trigger community-level trophic cascades in pelagic ecosystems?
Author contributions
SS: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. EP: Conceptualization, Funding acquisition, Supervision, Writing – review & editing. TP: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. EP was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant RGPIN-2014-05107.
Acknowledgments
The lead author thanks Andrew Trites (Director, Marine Mammal Research Unit, Institute for the Oceans and Fisheries, University of British Columbia) for stimulating and cultivating his interest in marine ecosystem dynamics in general and the trophic ecology of marine mammals in particular.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahrens R. N. M., Walters C. J., and Christensen V. (2012). Foraging arena theory. Fish Fish. 13, 41–59. doi: 10.1111/j.1467-2979.2011.00432.x
Ainley D. G. (2007). Insights from study of the last intact neritic marine ecosystem. Trends Ecol. Evol. 22, 444–445. doi: 10.1016/j.tree.2007.06.007
Ainley D. G., Ballard G., Ackley S., Blight L. K., Eastman J. K., Emslie S. D., et al. (2007). Paradigm lost, or is top-down forcing no longer significant in the Antarctic marine ecosystem? Antarct. Sci. 19, 283–290. doi: 10.1017/S095410200700051X
Ainley D. G., Ballard G., Blight L. K., Ackley J. K., Emslie S. D., Lescroël A., et al. (2009). Impacts of cetaceans on the structure of Southern Ocean food webs. Mar. Mamm. Sci. 26, 1–17. doi: 10.1111/j.1748-7692.2009.00337.x
Ainley D. G., Ballard G., and Dugger K. M. (2006). Competition among penguins and cetaceans reveals trophic cascades in the western Ross Sea, Antarctica. Ecology 87, 2080–2093. doi: 10.1890/0012-9658(2006)87[2080:CAPACR]2.0.CO;2
Ainley D. G., Ballard G., Jones R. M., Jongsomjit D., Pierce S. D., Smith W. O. Jr., et al. (2015). Trophic cascades in the western Ross Sea, Antarctica: revisited. Mar. Ecol. Prog. Ser. 534, 1–16. doi: 10.3354/meps11394
Albouy C., Archambault P., Appeltans W., Araújo M. B., Beauchesne D., Cazelles K., et al. (2019). The marine fish food web is globally connected. Nat. Ecol. Evol. 3, 1153–1161. doi: 10.1038/s41559-019-0950-y
Alimov A. F. (2000). Elements of Theory of Functioning of Aquatic Systems (St. Petersburg, Russia: St. Petersburg, Nauka).
Altieri A. H., Bertness M. D., Coverdale T. C., Herrmann N. C., and Angelini C. (2012). A trophic cascade triggers collapse of a salt-marsh ecosystem with intensive recreational fishing. Ecology 93, 1402–1410. doi: 10.1890/11-1314.1
Anderson R. J., Carrick P., Levitt G. J., and Share A. (1997). Holdfasts of adult kelp Ecklonia maxima provide refuges from grazing for recruitment of juvenile kelps. Mar. Ecol. Prog. Ser. 159, 265–273. doi: 10.3354/meps159265
Anthony R. G., Estes J. A., Ricca M. A., Miles A. K., and Forsman E. D. (2008). Bald eagles and sea otters in the Aleutian Archipelago: Indirect effects of trophic cascades. Ecology 89, 2725–2735. doi: 10.1890/07-1818.1
Arafeh-Dalmau N., Kavanaugh K. C., Possingham H. P., Munguia-Vega A., Montaño-Moctezuma G., Bell T. W., et al. (2021). Southward decrease in the protection of persistent giant kelp forests in the northeast Pacific. Commun. Earth Environ. 2, 119. doi: 10.1038/s43247-021-00177-9
Arafeh-Dalmau N., Villaseñor-Derbez J. C., Schoeman D. S., Mora-Soto A., Bell T. W., Butler C. L., et al. (2025). Global floating kelp forests have limited protection despite intensifying marine heatwave threats. Nat. Commun. 16, 3173. doi: 10.1038/s41467-025-58054-4
Atkinson A., Hill S. L., Barange M., Pakhomov E. A., Raubenheimer D., Schmidt K., et al. (2014). Sardine cycles, krill declines, and locust plagues: revisiting ‘wasp-waist’ food webs. Trends Ecol. Evol. 29, 309–316. doi: 10.1016/j.tree.2014.03.011
Atwood T. B., Connolly R. M., Ritchie E. G., Lovelock C. E., and Heithaus M. R. (2015). Predators help protect carbon stocks in blue carbon ecosystems. Nat. Clim. Change 5, 1038–1045. doi: 10.1038/nclimate2763
Atwood T. B., Madin E. M. P., Harborne A. R., Hammill E., Luiz O. J., Ollivier Q. R., et al. (2018). Predators shape sedimentary organic carbon storage in a coral reef ecosystem. Front. Ecol. Evol. 6. doi: 10.3389/fevo.2018.00110
Ayón P., Swartzman G., Bertrand A., Gutiérrez M., and Bertrand S. (2008). Zooplankton and forage fish species off Peru: Large-scale bottom-up forcing and local-scale depletion. Prog. Oceanogr. 79, 208–214. doi: 10.1016/j.pocean.2008.10.023
Babcock R. C., Kelly S., Shears N. T., Walker J. W., and Willis T. J. (1999). Changes in community structure in temperate marine reserves. Mar. Ecol. Prog. Ser. 189, 125–134. doi: 10.3354/meps189125
Baden S., Emanuelsson A., Pihl L., Svensson C.-J., and Åberg P. (2012). Shift in seagrass food web structure over decades is linked to overfishing. Mar. Ecol. Prog. Ser. 451, 61–73. doi: 10.3354/meps09585
Bandara K., Varpe Ø., Wijewardene L., Tverberg V., and Eiane K. (2021). Two hundred years of zooplankton vertical migration research. Biol. Rev. 96, 1547–1589. doi: 10.1111/brv.12715
Banse K. (2013). Reflections about chance in my career, and on the top-down regulated world. Annu. Rev. Mar. Sci. 5, 1–19. doi: 10.1146/annurev-marine-121211-172359
Barkai A. and McQuaid C. (1988). Predator-prey role reversal in a marine benthic ecosystem. Science 242, 62–64. doi: 10.1126/science.242.4875.62
Bascompte J., Melián C. J., and Sala E. (2005). Interaction strength combinations and the overfishing of a marine food web. PNAS 102, 5443–5447. doi: 10.1073/pnas.0501562102
Batten S. D., Ruggerone G. T., and Ortiz I. (2018). Pink salmon induce a trophic cascade in plankton populations in the southern Bering Sea and around the Aleutian Islands. Fish. Oceanogr. 27, 548–559. doi: 10.1111/fog.12276
Baum J. K. and Worm B. (2009). Cascading top-down effects of changing oceanic predator abundances. J. Anim. Ecol. 78, 699–714. doi: 10.1111/j.1365-2656.2009.01531.x
Behrens M. D. and Lafferty K. D. (2004). Effects of marine reserves and urchin disease on southern Californian rocky reef communities. Mar. Ecol. Prog. Ser. 279, 129–139. doi: 10.3354/meps279129
Beltran R. S., Kendall-Bar J. M., Pirotta E., Adachi T., Naito Y., Takahashi A., et al. (2023). Lightscapes of fear: How mesopredators balance starvation and predation in the open ocean. Sci. Adv. 7, eabd9818. doi: 10.1126/sciadv.abd9818
Benedetti F., Wydler J., Clerc C., Knecht N., and Vogt M. (2025). Emergent relationships between the functional diversity of marine planktonic copepods and ecosystem functioning in the global ocean. Glob. Change Biol. 31, e70094. doi: 10.1111/gcb.70094
Berthold M., Schumann R., Reiff V., Wulff R., and Schubert H. (2023). Mesopredator-mediated trophic cascade can break persistent phytoplankton blooms in coastal waters. Oikos 2023, e09469. doi: 10.1111/oik.09469
Blamey L. K. and Branch G. M. (2010). Regime shift of a kelp-forest benthic community induced by an ‘invasion’ of the rock lobster Jasus lalandii. J. Exp. Mar. Biol. Ecol. 420–421, 33–47. doi: 10.1016/j.jembe.2012.03.022
Bogstad B., Hauge K. H., and Ulltang Ø. (1997). MULTSPEC – A multi-species model for fish and marine mammals in the barents sea. J. North. Atl. Fish. Sci. 22, 317–341. doi: 10.2960/J.v22.a23
Bollens S. M., Rollwagen-Bollens G., Quenette J. A., and Bochdansky A. B. (2010). Cascading migrations and implications for vertical fluxes in pelagic ecosystems. J. Plankt. Res. 33, 349–355. doi: 10.1093/plankt/fbq152
Borer E. T., Seabloom E. W., Shurin J. B., Anderson K. E., Blanchette C. A., Broitman B., et al. (2005). What determines the strength of a trophic cascade? Ecology 86, 528–537. doi: 10.1890/03-0816
Boswell K. M., Rieucau G., Vollenweider J. J., Moran J. R., Heintz R. A., Blackburn J. K., et al. (2015). Are spatial and temporal patterns in Lynn Canal overwintering Pacific herring related to top predator activity? Can. J. Fish. Aquat. Sci. 73, 1307–1318. doi: 10.1139/cjfas-2015-0192
Bourque B. J., Johnson B. J., and Steneck R. S. (2008). “Possible Prehistoric Fishing Effects on Coastal Marine Food Webs in the Gulf of Maine,” in Human impacts on ancient marine ecosystems: a global perspective. Eds. Rick T. C. and Erlandson J. M. (University of California Press, Berkeley, CA), 165–186. doi: 10.1525/9780520934290-010
Boustany A. M., Hernandez D. A., Miller E. A., Fujii J. A., Nicholson T. E., Tomoleoni J. A., et al. (2021). Examining the potential conflict between sea otter recovery and Dungeness crab fisheries in California. Biol. Conserv. 253, 108830. doi: 10.1016/j.biocon.2020.108830
Boyce D. G., Dowd M., Lewis M. R., and Worm B. (2014). Estimating global chlorophyll changes over the past century. Prog. Oceanogr. 122, 163–173. doi: 10.1016/j.pocean.2014.01.004
Boyce D. G., Frank K. T., Worm B., and Leggett W. C. (2015). Spatial patterns and predictors of trophic control in marine ecosystems. Ecol. Lett. 18, 1001–1011. doi: 10.1111/ele.12481
Boyce D. G., Lewis M. R., and Worm B. (2010). Global phytoplankton decline over the past century. Nature 466, 591–596. doi: 10.1038/nature09268
Boyce D. G. and Worm B. (2015). Patterns and ecological implications of historical marine phytoplankton change. Mar. Ecol. Prog. Ser. 534, 251–272. doi: 10.3354/meps11411
Bracco A., Provenzale A., and Scheuring I. (2000). Mesoscale vortices and the paradox of the plankton. Proc. R. Soc B 267, 1795–1800. doi: 10.1098/rspb.2000.1212
Brandão M. C., Benedetti F., Martini S., Soviadan Y., Irisson J.-O., Romagnan J.-B., et al. (2021). Macroscale patterns of oceanic zooplankton composition and size structure. Sci. Rep. 11, 15714. doi: 10.1038/s41598-021-94615-5
Breen P. A., Carson T. A., Foster J. B., and Stewart E. A. (1982). Changes in subtidal community structure associated with british columbia sea otter transplants. Mar. Ecol. Prog. Ser. 7, 13–20. doi: 10.3354/meps007013
Brierley A. S. and Cox M. J. (2010). Shapes of krill swarms and fish schools emerge as aggregation members avoid predators and access oxygen. Curr. Biol. 20, 1758–1762. doi: 10.1016/j.cub.2010.08.041
Brook G. and Calderwood W. (1885). Report on the food of the herring. Fourth Ann. Rep. Fish. Board Scot. 3, 102–128.
Brown J. S., Laundré J. W., and Gurung M. (1999). The ecology of fear: optimal foraging, game theory, and trophic interactions. J. Mammal. 80, 385–399. doi: 10.2307/1383287
Bruno J. F. and O'Connor M. I. (2005). Cascading effects of predator diversity and omnivory in a marine food web. Ecol. Lett. 8, 1048–1056. doi: 10.1111/j.1461-0248.2005.00808.x
Bundy A. and Guénette S. (2014). “Loosening the corset: how real are wasp-waist ecosystems?,” in Ecopath 30 Years Conference Proceedings: Extended Abstracts, vol. 22 . Eds. Steenbeek J., Piroddi C., Coll M., Heymans J. J., Villasante S., et al (Vancouver, BC: UBC Fish. Centre Res. Rep), 33. doi: 10.14288/1.0354312
Büring T., van der Grient J., Pierce G., Bustamante P., Scotti M., Jones J. B., et al. (2024). Unveiling the wasp-waist structure of the Falkland shelf ecosystem: the role of Doryteuthis gahi as a keystone species and its trophic influences. J. Mar. Biol. Assoc. U.K. 104, e2. doi: 10.1017/S0025315423000887
Burkholder D. A., Heithaus M. R., Fourqurean J. W., Wirsing A., and Dill L. M. (2013). Patterns of top-down control in a seagrass ecosystem: could a roving apex predator induce a behaviour-mediated trophic cascade? J. Anim. Ecol. 82, 1192–1202. doi: 10.1111/1365-2656.12097
Burt J. M., Tinker M. T., Okamoto D. K., Demes K. W., Holmes K., and Salomon A. K. (2018). Sudden collapse of a mesopredator reveals its complementary role in mediating rocky reef regime shifts. Proc. R. Soc B 285, 20180553. doi: 10.1098/rspb.2018.0553
Byrnes J., Stachowicz J. J., Hultgren K. M., Hughes A. R., Olyarnik S. V., and Thornber C. S. (2006). Predator diversity strengthens trophic cascades in kelp forests by modifying herbivore behaviour. Ecol. Lett. 9, 61–71. doi: 10.1111/j.1461-0248.2005.00842.x
Byström P., Bergström U., Hjälten A., Ståhl S., Jonsson D., and Olsson J. (2015). Declining coastal piscivore populations in the Baltic Sea: Where and when do sticklebacks matter? Ambio 44, 462–471. doi: 10.1007/s13280-015-0665-5
Candolin U., Johanson A., and Budria A. (2016). The influence of stickleback on the accumulation of primary production: a comparison of field and experimental data. Estuar. Coast. 39, 248–257. doi: 10.1007/s12237-015-9984-9
Cardona L., Martínez-Iñigo L., Mateo R., and González-Solís J. (2015). The role of sardine as prey for pelagic predators in the western Mediterranean Sea assessed using stable isotopes and fatty acids. Mar. Ecol. Prog. Ser. 531, 1–14. doi: 10.3354/meps11353
Carpenter S. R., Cole J. J., Kitchell J. F., and Pace M. L. (2010). “Trophic Cascades in Lakes: Lessons and Prospects,” in Trophic Cascades: Predators, Prey, and the Changing Dynamics of Nature. Eds. Terborgh J. and Estes J. A. (Island Press, Washington, DC), 55–70.
Carpenter S. R., Kitchell J. F., and Hodgson J. R. (1985). Cascading trophic interactions and lake productivity. Bioscience 35, 634–639. doi: 10.2307/1309989
Casey J. M., Baird A. H., Brandl S. J., Hoogenboom M. O., Rizzari J. R., Frisch A. J., et al. (2017). A test of trophic cascade theory: fish and benthic assemblages across a predator density gradient on coral reefs. Oecologia 183, 161–175. doi: 10.1007/s00442-016-3753-8
Casini M., Blenckner T., Möllmann C., Gårdmark A., Lindegren M., Llope M., et al. (2012). Predator transitory spillover induces trophic cascades in ecological sinks. PNAS 109, 8185–8189. doi: 10.1073/pnas.1113286109
Casini M., Hjelm J., Molinero C., Lövgren J., Cardinale M., Bartolino V., et al. (2009). Trophic cascades promote threshold–like shifts in pelagic marine ecosystems. PNAS 106, 197–202. doi: 10.1073/pnas.0806649105
Chassot E., Bonhommeau S., Dulvy N. K., Mélin F., Watson R., Gascuel D., et al. (2010). Global marine primary production constrains fisheries catches. Ecol. Lett. 13, 495–505. doi: 10.1111/j.1461-0248.2010.01443.x
Cheung W. W. L. and Frölicher T. L. (2020). Marine heatwaves exacerbate climate change impacts for fisheries in the northeast Pacific. Sci. Rep. 10, 6678. doi: 10.1038/s41598-020-63650-z
Child T., Costello J. H., Gemmell B. J., Sutherland K. R., and Colin S. P. (2025). High prey capture efficiencies of oceanic epipelagic lobate and cestid ctenophores. J. Plankton Res. 47, fbae044. doi: 10.1093/plankt/fbae044
Christie H., Gundersen H., Rinde E., Filbee–Dexter K., Norderhaug K. M., Pedersen T., et al. (2018). Can multitrophic interactions and ocean warming influence large-scale kelp recovery? Ecol. Evol. 9, 2847–2862. doi: 10.1002/ece3.4963
Clasen J. L. and Shurin J. B. (2015). Kelp forest size alters microbial community structure and function on Vancouver Island, Canada. Ecology 96, 862–872. doi: 10.1890/13-2147.1
Cole R. G. and Keuskamp D. (1998). Indirect effects of protection from exploitation: patterns from populations of Evechinus chloroticus (Echinoidea) in northeastern New Zealand. Mar. Ecol. Prog. Ser. 73, 215–226. doi: 10.3354/meps173215
Comtois S. (2010). Effets des changements de la structure trophique suivant l’effondrement des stocks de poissons de fond sur l’abondance et la distribution du rorqual bleu, de ses proies et compétiteurs dans le nord du golfe du Saint-Laurent. Rimouski, QC: Université du Québec à Rimouski.
Cowen R. K. (1983). The effect of sheephead (Semicossyphus pulcher) predation on red sea urchin populations: an experimental analysis. Oecologia 58, 249–255. doi: 10.1007/BF00399225
Curtis J. S. and Wing S. R. (2024). Size-specific reduction in kelp consumption by New Zealand urchins exposed to chemical cues from the red rock lobster. Ecosphere 15, e4932. doi: 10.1002/ecs2.4932
Cury P., Bakun A., Crawford R. J. M., Jarre A., Quiñones R. A., Shannon L. J., et al. (2000). Small pelagics in upwelling systems: patterns of interaction and structural changes in “wasp-waist” ecosystems. ICES J. Mar. Sci. 57, 603–618. doi: 10.1006/jmsc.2000.0712
Cury P. M., Boyd I. L., Bonhommeau S., Anker-Nilssen T., Crawford R. J., Furness R. W., et al. (2011). Global seabird response to forage fish depletion - one-third for the birds. Science 334, 1703–1706. doi: 10.1126/science.1212928
Dabiri J. O. (2010). Role of vertical migration in biogenic ocean mixing. Geophys. Res. Lett. 37, L11602. doi: 10.1029/2010GL043556
Dalpadado P., Arrigo K. R., Hjøllo S. S., Rey F., Ingvaldsen R. B., Sperfeld E., et al. (2014). Productivity in the barents sea - response to recent climate variability. PloS One 9, e95273. doi: 10.1371/journal.pone.0095273
Daskalov G. M., Grishin A. N., Rodionov S., and Mihneva V. (2007). Trophic cascades triggered by overfishing reveal possible mechanisms of ecosystem regime shifts. PNAS 104, 10518–10523. doi: 10.1073/pnas.0701100104
Davenport A. C. and Anderson T. W. (2007). Positive indirect effects of reef fishes on kelp performance: the importance of mesograzers. Ecology 88, 1548–1561. doi: 10.1890/06-0880
Dedman S., Moxley J. H., Papastamatiou Y. P., Braccini M., Caselle J., Chapman D. D., et al. (2024). Ecological roles and importance of sharks in the Anthropocene Ocean. Science 385, eadl2362. doi: 10.1126/science.adl2362
DeMartini E. E., Friedlander A. M., Sandin S. A., and Sala E. (2008). Differences in fish-assemblage structure between fished and unfished atolls in the northern Line Islands, central Pacific. Mar. Ecol. Prog. Ser. 365, 199–215. doi: 10.3354/meps07501
DeMaster D. P., Trites A. W., Clapham P., Mizroch S., Wade P., Small R. J., et al. (2006). The sequential megafaunal collapse hypothesis: testing with existing data. Prog. Oceanogr. 68, 329–342. doi: 10.1016/j.pocean.2006.02.007
Desbiens A. A., Roff G., Robbins W. D., Taylor B. M., Castro-Sanguino C., Dempsey A., et al. (2021). Revisiting the paradigm of shark-driven trophic cascades in coral reef ecosystems. Ecology 102, e03303. doi: 10.1002/ecy.3303
Dewar W. K., Bingham R. J., Iverson R. L., Nowacek D. P., St. Laurent L. C., and Wiebe P. H. (2006). Does the marine biosphere mix the ocean? J. Mar. Res. 64, 541–561. doi: 10.1357/002224006778715720
Dinasquet J., Titelman J., Møller L. F., Setälä O., Granhag L., Andersen T., et al. (2012). Cascading effects of the ctenophore Mnemiopsis leidyi on the planktonic food web in a nutrient-limited estuarine system. Mar. Ecol. Prog. Ser. 460, 49–61. doi: 10.3354/meps09770
Doherty B., Johnson S. D. N., Benson A. J., Cox S. P., Cleary J. S., and Lane J. (2024). Predation by marine mammals explains recent trends in natural mortality of Pacific Herring (Clupea pallasii) and changes expectations for future biomass. ICES J. Mar. Sci. 82, fsae183. doi: 10.1093/icesjms/fsae183
Donadi S., Austin Å.N., Bergström U., Eriksson B. K., Hansen J. P., Jacobson P., et al. (2017). A cross-scale trophic cascade from large predatory fish to algae in coastal ecosystems. Proc. R. Soc B 284, 20170045. doi: 10.1098/rspb.2017.0045
Donadi S., Olin A., Casini M., Eklöf J., Erlandsson M., Fredriksson R., et al. (2024). Reduced predation and competition from herring may have contributed to the increase of three-spined stickleback in the Baltic Sea. ICES J. Mar. Sci. 82, fsae168. doi: 10.1093/icesjms/fsae168
Donohue I., Petchey O. L., Kéfi S., Génin A., Jackson A. L., Yang Q., et al. (2017). Loss of predator species, not intermediate consumers, triggers rapid and dramatic extinction cascades. Glob. Change Biol. 23, 2962–2972. doi: 10.1111/gcb.13703
Doroff A. M., Estes J. A., Tinker M. T., Burn D. M., and Evans T. J. (2003). Sea otter population declines in the Aleutian Archipelago. J. Mammal. 84, 55–64. doi: 10.1644/1545-1542(2003)084<0055:SOPDIT>2.0.CO;2
Duarte L. O. and García C. B. (2004). Trophic role of small pelagic fishes in a tropical upwelling ecosystem. Ecol. Modell. 172, 323–338. doi: 10.1016/j.ecolmodel.2003.09.014
Duggins D. O. (1983). Starfish predation and the creation of mosaic patterns in a kelp-dominated community. Ecology 64, 1610–1619. doi: 10.2307/1937514
Duggins D. O., Simenstad C. A., and Estes J. A. (1989). Magnification of secondary production by kelp detritus in coastal marine ecosystems. Science 245, 170–173. doi: 10.1126/science.245.4914.170
Dulvy N. K., Freckleton R. P., and Polunin N. V. C. (2004). Coral reef cascades and the indirect effects of predator removal by exploitation. Ecol. Lett. 7, 410–416. doi: 10.1111/j.1461-0248.2004.00593.x
Eddy T. D., Pitcher T. J., MacDiarmid A. B., Byfield T. T., Tam J. C., Jones T. T., et al. (2014). Lobsters as keystone: only in unfished ecosystems? Ecol. Modell. 275, 48–72. doi: 10.1016/j.ecolmodel.2013.12.006
Edgar G. J., Stuart-Smith R. D., Thomson R. J., and Freeman D. J. (2017). Consistent multi-level trophic effects of marine reserve protection across northern New Zealand. PloS One 12, e0177216. doi: 10.1371/journal.pone.0177216
Edwards M. S. and Konar B. (2020). Trophic downgrading reduces spatial variability on rocky reefs. Sci. Rep. 10, 18079. doi: 10.1038/s41598-020-75117-2
Eger A. M., Blain C. O., Brown A. L., Chan S. S. W., Miller K. I., and Vergés A. (2024). Kelp forests versus urchin barrens: a comparison of ecosystem functions and services provided by two alternative stable marine habitats. Proc. R. Soc B 291, 20241539. doi: 10.1098/rspb.2024.1539
Eisaguirre J. H., Eisaguirre J. M., Davis K., Carlson P. M., Gaines S. D., and Caselle J. E. (2020). Trophic redundancy and predator size class structure drive differences in kelp forest ecosystem dynamics. Ecology 101, e02993. doi: 10.1002/ecy.2993
Eklöf J. S., Sundblad G., Erlandsson M., Donadi S., Hansen J. P., Eriksson B. K., et al. (2020). A spatial regime shift from predator to prey dominance in a large coastal ecosystem. Nat. Commun. Biol. 3, 459. doi: 10.1038/s42003-020-01180-0
Emmerson M. C. and Raffaelli D. (2004). Predator-prey size, interaction strength, and the stability of real food webs. J. Anim. Ecol. 73, 399–409. doi: 10.1111/j.0021-8790.2004.00818.x
Engelhard G. H., Peck M. A., Rindorf A., Smout S. C., van Deurs M., Raab K., et al. (2014). Forage fish, their fisheries, and their predators: who drives whom? ICES J. Mar. Sci. 71, 90–104. doi: 10.1093/icesjms/fst087
Eriksson B. K., Ljunggren L., Sandström A., Johansson G., Mattila J., Rubach A., et al. (2009). Declines in predatory fish promote bloom-forming macroalgae. Ecol. Appl. 19, 1975–1988. doi: 10.1890/08-0964.1
Erlandson J. M., Rick T. C., Estes J. A., Graham M. H., Braje T. J., and Vellanoweth R. L. (2005). “Sea otters, shellfish, and humans: 10,000 years of ecological interaction on San Miguel Island, California,” in Proceedings of the Sixth California Islands Symposium. Eds. Schwemm C. A. and Garcelon D. K. (Institute for Wildlife Studies, Arcata, CA), 9–21.
Essington T. E. (2006). “Pelagic Ecosystem Response to a Century of Commercial Fishing and Whaling,” in Whales, Whaling, and Ocean Ecosystems. Eds. Estes J. A., DeMaster D. P., Doak D. F., Williams T. M., and Brownell R. L. (University of California Press, Berkeley, CA), 38–49. doi: 10.1525/9780520933200-009
Essington T. E. (2010). “Trophic Cascades in Open Ocean Ecosystems,” in Trophic Cascades: Predators, Prey, and the Changing Dynamics of Nature. Eds. Terborgh J. and Estes J. A. (Island Press, Washington, DC), 91–105.
Essington T. E. and Hansson S. (2004). Predator-dependent functional responses and interaction strengths in a natural food web. Can. J. Fish. Aquat. Sci. 61, 2215–2226. doi: 10.1139/f04-146
Estes J. A. (2018). The history and future of marine mammal ecology. Aquat. Mamm. 44, 580–594. doi: 10.1578/AM.44.5.2018.580
Estes J. A., Doak D. F., Springer A. M., and Williams T. M. (2009). Causes and consequences of marine mammal population declines in southwest Alaska: a food-web perspective. Phil. Trans. R. Soc B 364, 1647–1658. doi: 10.1098/rstb.2008.0231
Estes J. A. and Duggins D. O. (1995). Sea otters and kelp forests in Alaska: generality and variation in a community ecological paradigm. Ecol. Monogr. 65, 75–100. doi: 10.2307/2937159
Estes J. A. and Palmisano J. F. (1974). Sea otters: their role in structuring nearshore communities. Science 185, 1058–1060. doi: 10.1126/science.185.4156.1058
Estes J. A., Tinker M. T., Williams T. M., and Doak D. F. (1998). Killer whale predation on sea otters linking oceanic and nearshore ecosystems. Science 282, 473–476. doi: 10.1126/science.282.5388.47
Fagerli C. W., Norderhaug K. M., Christie H., Pedersen M. F., and Fredriksen S. (2014). Predators of the destructive sea urchin Strongylocentrotus droebachiensis on the Norwegian coast. Mar. Ecol. Prog. Ser. 502, 207–218. doi: 10.3354/meps10701
Fahimipour A. K., Anderson K. E., and Williams R. J. (2017). Compensation masks trophic cascades in complex food webs. Theor. Ecol. 10, 245–253. doi: 10.1007/s12080-016-0326-8
Farrell A. M. and Hodgson J. R. (2012). Zooplankton diel vertical migrations in lakes of contrasting food webs. Bios 83, 12–16. doi: 10.1893/0005-3155-83.1.12
Farrell S. P., Petras D., Stincone P., Yiu D. S., Burns J. A., Shah A. K. P., et al. (2025). Turf algae redefine the chemical landscape of temperate reefs, limiting kelp forest recovery. Science 388, 876–880. doi: 10.1126/science.adt6788
Fauchald P., Skov H., Skern-Mauritzen M., Johns D., and Tveraa T. (2011). Wasp-Waist interactions in the north sea ecosystem. PloS One 6, e22729. doi: 10.1371/journal.pone.0022729
Fernández Castro B., Peña M., Nogueira E., Gilcoto M., Broullón E., Comesaña A., et al. (2022). Intense upper ocean mixing due to large aggregations of spawning fish. Nat. Geosci. 15, 287–292. doi: 10.1038/s41561-022-00916-3
Ferretti F., Worm B., Britten G. L., Heithaus M. R., and Lotze H. K. (2010). Patterns and ecosystem consequences of shark declines in the ocean. Ecol. Lett. 13, 1055–1071. doi: 10.1111/j.1461-0248.2010.01489.x
Finenko Z. Z., Piontkovski S. A., Williams R., and Mishonov A. V. (2003). Variability of phytoplankton and mesozooplankton biomass in the subtropical and tropical Atlantic Ocean. Mar. Ecol. Prog. Ser. 250, 125–144. doi: 10.3354/meps250125
Fitzpatrick S. M., Keegan W. F., and Sealey K. S. (2008). “Human Impacts On Marine Environments In The West Indies During The Middle To Late Holocene,” in Human impacts on ancient marine ecosystems: a global perspective. Eds. Rick T. C. and Erlandson J. M. (University of California Press, Berkeley, CA), 147–164. doi: 10.1525/9780520934290-009
Fomin S. V., Fedutin I. D., Borisova E. A., Meschersky I. G., and Filatova O. A. (2023). Sea Otters (Enhydra lutris) Found in the Stomach of a Stranded Killer Whale (Orcinus orca) in the Commander Islands, Western North Pacific. Aquat. Mamm. 49, 462–467. doi: 10.1578/AM.49.5.2023.462
Foster M. S. and Schiel D. R. (2010). Loss of predators and the collapse of southern California kelp forests ()?: Alternatives, explanations and generalizations. J. Exp. Mar. Biol. Ecol. 393, 59–70. doi: 10.1016/j.jembe.2010.07.002
Foster E., Watson J., Lemay M. A., Tinker M. T., Estes J. A., Piercey R., et al. (2021). Physical disturbance by recovering sea otter populations increases eelgrass genetic diversity. Science 374, 333–336. doi: 10.1126/science.abf2343
Frank K. T., Fisher J. A. D., and Leggett W. C. (2015). “The spatio-temporal dynamics of trophic control in large marine ecosystems,” in Trophic Ecology: Bottom-up and Top-down Interactions across Aquatic and Terrestrial Systems. Eds. Hanley T. C. and LaPierre K. J. (Cambridge University Press, Cambridge), 31–54. doi: 10.1017/CBO9781139924856.003
Frank K. T., Petrie B., Choi J. S., and Leggett W. C. (2005). Trophic cascades in a formerly cod-Dominated ecosystem. Science 308, 1621–1623. doi: 10.1126/science.1113075
Frank K. T., Petrie B., Fisher J. A. D., and Leggett W. C. (2013). Setting the record straight on drivers of changing ecosystem states. Fish. Oceanogr. 22, 143–146. doi: 10.1111/fog.12007
Frank K. T., Petrie B., and Shackell N. L. (2007). The ups and downs of trophic control in continental shelf ecosystems. Trends Ecol. Evol. 22, 236–242. doi: 10.1016/j.tree.2007.03.002
Frank K. T., Petrie B., Shackell N. L., and Choi J. S. (2006). Reconciling differences in trophic control in mid-latitude marine ecosystems. Ecol. Lett. 9, 1096–1105. doi: 10.1111/j.1461-0248.2006.00961.x
Fréon P., Arístegui J., Bertrand A., Crawford R. J. M., Field J. C., and Gibbons M. J. (2009). Functional group biodiversity in Eastern Boundary Upwelling Ecosystems questions the wasp-waist trophic structure. Prog. Oceanogr. 83, 97–106. doi: 10.1016/j.pocean.2009.07.034
Frid A., Baker G. G., and Dill L. M. (2008). Do shark declines create fear-released systems? Oikos 117, 191–201. doi: 10.1111/j.2007.0030-1299.16134.x
Frid A., Burns J., Baker G. G., and Thorne R. E. (2009). Predicting synergistic effects of resources and predators on foraging decisions by juvenile Steller sea lions. Oecologia 158, 775–786. doi: 10.1007/s00442-008-1189-5
Friedland K. D., Stock C., Drinkwater K. F., Link J., Leaf R. T., Shank B. V., et al. (2012). Pathways between primary production and fisheries yields of large marine ecosystems. PloS One 7, e28945. doi: 10.1371/journal.pone.0028945
Friedlander A. M. and DeMartini E. E. (2002). Contrasts in density, size, and biomass of reef fishes between the northwestern and the main Hawaiian islands: the effects of fishing down apex predators. Mar. Ecol. Prog. Ser. 230, 253–264. doi: 10.3354/meps230253
Frisch A. J., Ireland M., Rizzari J. R., Lönnstedt O. M., Magnenat K. A., Mirbach C. E., et al. (2016). Reassessing the trophic role of reef sharks as apex predators on coral reefs. Coral Reefs 35, 459–472. doi: 10.1007/s00338-016-1415-2
Frost B. W. and Bollens S. M. (1992). Variability of diel vertical migration in the marine planktonic copepod pseudocalanus newmani in relation to its predators. Can. J. Fish. Aquat. Sci. 49, 1137–1141. doi: 10.1139/f92-126
Fu C., Gaichas S., Link J., Bundy A., Boldt J. L., Cook A. M., et al. (2012). Relative importance of fisheries, trophodynamic and environmental drivers in a series of marine ecosystems. Mar. Ecol. Prog. Ser. 459, 169–184. doi: 10.3354/meps09805
Fu C., Olsen N., Taylor N., Grüss A., Batten S., Liu H., et al. (2017). Spatial and temporal dynamics of predator-prey species interactions off western Canada. ICES J. Mar. Sci. 74, 2107–2119. doi: 10.1093/icesjms/fsx056
Fuchs H. L. and Franks P. J. S. (2010). Plankton community properties determined by nutrients and size-selective feeding. Mar. Ecol. Prog. Ser. 413, 1–15. doi: 10.3354/meps08716
Gaichas S. K., Aydin K. Y., and Francis R. C. (2011). What drives dynamics in the Gulf of Alaska? Integrating hypotheses of species, fishing, and climate relationships using ecosystem modeling. Can. J. Fish. Aquat. Sci. 68, 1553–1578. doi: 10.1139/f2011-080
Gaichas S., Aydin K., and Francis R. C. (2015). Wasp waist or beer belly? Modeling food web structure and energetic control in Alaskan marine ecosystems, with implications for fishing and environmental forcing. Prog. Oceanogr. 138, 1–17. doi: 10.1016/j.pocean.2015.09.010
Gallagher A. J., Creel S., Wilson R. P., and Cooke S. J. (2017). Energy landscapes and the landscape of fear. Trends Ecol. Evol. 32, 88–96. doi: 10.1016/j.tree.2016.10.010
García-Oliva O. and Wirtz K. (2025). The complex structure of aquatic food webs emerges from a few assembly rules. Nat. Ecol. Evol. 9, 576–588. doi: 10.1038/s41559-025-02647-1
Gasol J. M., del Giorgio P. A., and Duarte C. M. (2003). Biomass distribution in marine planktonic communities. Limnol. Oceanogr. 42, 1353–1363. doi: 10.4319/lo.1997.42.6.1353
Gibert J. P. and Yeakel J. D. (2019). Eco-evolutionary origins of diverse abundance, biomass, and trophic structures in food webs. Front. Ecol. Evol. 7. doi: 10.3389/fevo.2019.00015
Gibson J. R. (2024). Otter Skins, Boston Ships, and China Goods. Voices of the Maritime Fur Trade of the Northwest Coast 1785–1841 (Montreal: McGill-Queen’s University Press). doi: 10.1515/9780228007326
Gil M. A., Michel C. J., Olivetti S., Sridharan V., and Hein A. M. (2025). Integrating Landscapes of fear and energy reveals the behavioural strategies that shape predator–Prey interactions. Ecol. Lett. 28, e70068. doi: 10.1111/ele.70068
Gilbert L., Jeanniard-du-Dot T., Authier M., Chouvelon T., and Spitz J. (2023). Composition of cetacean communities worldwide shapes their contribution to ocean nutrient cycling. Nat. Commun. 14, 5823. doi: 10.1038/s41467-023-41532-y
Grabowski J. H. and Kimbro D. L. (2005). Predator-avoidance behavior extends trophic cascades to refuge habitats. Ecology 86, 1312–1319. doi: 10.1890/04-1216
Granéli E. and Turner J. T. (2002). Top-down regulation in ctenophore-copepod-ciliate-diatom-phytoflagellate communities in coastal waters: a mesocosm study. Mar. Ecol. Prog. Ser. 239, 57–68. doi: 10.3354/meps239057
Greene C. H. (2013). Towards a more balanced view of marine ecosystems. Fish. Oceanogr. 22, 140–142. doi: 10.1111/fog.12006
Greene C. H. and Pershing A. J. (2007). Climate drives sea change. Science 315, 1084–1085. doi: 10.1126/science.1136495
Gregr E. J., Christensen V., Nichol L., Martone R. G., Markel. R. W., Watson J. C., et al. (2020). Cascading social-ecological costs and benefits triggered by a recovering keystone predator. Science 368, 1243–1247. doi: 10.1126/science.aay5342
Griffin J. N., Butler J., Soomdat N. N., Brun K. E., Chejanovski Z. A., and Silliman B. R. (2011). Top predators suppress rather than facilitate plants in a trait-mediated tri-trophic cascade. Biol. Lett. 7, 710–713. doi: 10.1098/rsbl.2011.0166
Griffiths S. P., Olson R. J., and Watters G. M. (2013). Complex wasp-waist regulation of pelagic ecosystems in the Pacific Ocean. Rev. Fish Biol. Fisheries. 23, 459–475. doi: 10.1007/s11160-012-9301-7
Grimes T. M., Tinker M. T., Hughes B. B., Boyer K. E., Needles L., and Lewison R. L. (2020). Characterizing the impact of recovering sea otters on commercially important crabs in California estuaries. Mar. Ecol. Prog. Ser. 655, 123–137. doi: 10.3354/meps13530
Guenther C. M., Lenihan H. S., Grant L. E., Lopez-Carr D., and Reed D. C. (2012). Trophic cascades induced by lobster fishing are not ubiquitous in southern california kelp forests. PloS One 7, e49396. doi: 10.1371/journal.pone.0049396
Guidetti P., Baiata P., Ballesteros E., Di Franco A., Hereu B., Macpherson E., et al. (2014). Large-scale assessment of mediterranean marine protected areas effects on fish assemblages. PloS One 9, e91841. doi: 10.1371/journal.pone.0091841
Hacquebord L. (2001). Three centuries of whaling and walrus hunting in svalbard and its impact on the arctic ecosystem. Env. Hist. 7, 169–185. doi: 10.3197/096734001129342441
Haggerty M. B., Anderson T. W., and Long J. D. (2018). Fish predators reduce kelp frond loss via a trait-mediated trophic cascade. Ecology 99, 1574–1583. doi: 10.1002/ecy.2380
Hairston N. G., Smith F. E., and Slobodkin L. B. (1960). Community structure, population control, and competition. Am. Nat. 94, 421–425. doi: 10.1086/282146
Halaj J. and Wise D. H. (2001). Terrestrial trophic cascades: how much do they trickle? Am. Nat. 157, 262–281. doi: 10.1086/319190
Hall S. J. and Raffaelli D. (1991). Food-web patterns: lessons from a species-rich web. J. Anim. Ecol. 60, 823–842. doi: 10.2307/5416
Halpern B. S., Cottenie K., and Broitman B. R. (2006). Strong top-down control in southern california kelp forest ecosystems. Science 312, 1230–1232. doi: 10.1126/science.1128613
Hamilton S. L. and Caselle J. E. (2015). Exploitation and recovery of a sea urchin predator has implications for the resilience of southern California kelp forests. Proc. R. Soc B 282, 20141817. doi: 10.1098/rspb.2014.1817
Hannesson R. (2013). Strictly for the birds? On ecosystem services of forage fish. Mar. Pol. 38, 109–115. doi: 10.1016/j.marpol.2012.05.026
Hargrave B. T., Harding G. C., Drinkwater K. F., Lambert T. C., and Harrison W. G. (1985). Dynamics of the pelagic food web in St. Georges Bay, southern Gulf of St. Lawrence. Mar. Ecol. Prog. Ser. 20, 221–240. doi: 10.3354/meps020221
Harvell C. D., Montecino-Latorre D., Caldwell J. M., Burt J. M., Bosley K., Keller A., et al. (2019). Disease epidemic and a marine heat wave are associated with the continental-scale collapse of a pivotal predator (Pycnopodia helianthoides). Sci. Adv. 5, eaau7042. doi: 10.1126/sciadv.aau7042
Heintz R., Moran J., Vollenweider J., Straley J., Boswell K., and Rice J. (2010). Humpback whale predation and the case for top-down control of local herring populations in the gulf of Alaska. AFSC Quart. Rep. October–November-December 2010, 1–6.
Heiskanen A.-S., Tamminen T., and Gundersen K. (1996). Impact of planktonic food web structure on nutrient retention and loss from a late summer pelagic system in the coastal northern Baltic Sea. Mar. Ecol. Prog. Ser. 145, 195–208. doi: 10.3354/meps145195
Heithaus M. R., Alcoverro T., Arthur R., Burkholder D. A., Coates K. A., Christiansen M. J. A., et al. (2014). Seagrasses in the age of sea turtle conservation and shark overfishing. Front. Mar. Sci. 1. doi: 10.3389/fmars.2014.00028
Heithaus M. R., Wirsing A. J., and Dill L. M. (2012). The ecological importance of intact top-predator populations: a synthesis of 15 years of research in a seagrass ecosystem. Mar. Freshw. Res. 63, 1039–1050. doi: 10.1071/MF12024
Heupel M. R., Knip D. M., Simpfendorfer C. A., and Dulvy N. K. (2014). Sizing up the ecological role of sharks as predators. Mar. Ecol. Prog. Ser. 495, 291–298. doi: 10.3354/meps10597
Hildebrand L., Derville S., Hildebrand I., and Torres L. G. (2024). Exploring indirect effects of a classic trophic cascade between urchins and kelp on zooplankton and whales. Sci. Rep. 14, 9815. doi: 10.1038/s41598-024-59964-x
Houghton I. A. and Dabiri J. O. (2019). Alleviation of hypoxia by biologically generated mixing in a stratified water column. Limnol. Oceanogr. 64, 2161–2171. doi: 10.1002/lno.11176
Houghton I. A., Koseff J. R., Monismith S. G., and Dabiri J. O. (2018). Vertically migrating swimmers generate aggregation-scale eddies in a stratified column. Nat. volume 556, 497–500. doi: 10.1038/s41586-018-0044-z
Houle J. E., Andersen K. H., Farnsworth K. D., and Reid D. G. (2013). Emerging asymmetric interactions between forage and predator fisheries impose management trade-offs. J. Fish Biol. 83, 890–904. doi: 10.1111/jfb.12163
Hughes B. B., Wasson K., Tinker M. T., Williams S. L., Carswell L. P., Boyer K. E., et al. (2019). Species recovery and recolonization of past habitats: lessons for science and conservation from sea otters in estuaries. PeerJ 7, e8100. doi: 10.7717/peerj.8100
Hunsicker M. E., Olson R. J., Essington T. E., Maunder M. N., Duffy L. M., and Kitchell J. F. (2012). Potential for top-down control on tropical tunas based on size structure of predator-prey interactions. Mar. Ecol. Prog. Ser. 445, 263–277. doi: 10.3354/meps09494
Hunt G. L. Jr. (2006). “Evidence for Bottom-Up Control of Upper-Trophic-Level Marine Populations: Is it Scale-Dependent?,” in Whales, Whaling, and Ocean Ecosystems. Eds. Estes J. A., DeMaster D. P., Doak D. F., Williams T. M., and Brownell R. L. Jr (University of California Press, Berkeley, CA), 50–66. doi: 10.1525/9780520933200-010
Hunt G. L. and McKinnell S. (2006). Interplay between top-down, bottom-up, and wasp-waist control in marine ecosystems. Prog. Oceanogr. 68, 115–124. doi: 10.1016/j.pocean.2006.02.008
Huntley M. E. and Zhou M. (2004). Influence of animals on turbulence in the sea. Mar. Ecol. Prog. Ser. 273, 65–79. doi: 10.3354/meps273065
Irigoien X., Conway D. V. P., and Harris R. P. (2004). Flexible diel vertical migration behaviour of zooplankton in the Irish Sea. Mar. Ecol. Prog. Ser. 267, 85–97. doi: 10.3354/meps267085
Irons D. B., Anthony R. G., and Estes J. A. (1986). Foraging strategies of glaucous–winged gulls in a rocky intertidal community. Ecology 67, 1460–1474. doi: 10.2307/1939077
Irvine T., Costello J. H., Gemmell B. J., Sutherland K. R., Corrales-Ugalde M., Townsend J. P., et al. (2025). Ctenophores are a highly impactful predatory guild in open oceanic ecosystems. Curr. Biol. 35, 2467–2473. doi: 10.1016/j.cub.2025.04.029
Jackson J. B. C. (2006). “When Ecological Pyramids Were Upside Down,” in Whales, Whaling, and Ocean Ecosystems. Eds. Estes J. A., DeMaster D. P., Doak D. F., Williams T. M., and Brownell R. L. Jr (University of California Press, Berkeley, CA), 27–37. doi: 10.1525/9780520933200-008
Jackson J. B. C., Kirby M. X., Berger W. H., Bjorndal K. A., Botsford L. W., Bourque B. J., et al. (2001). Historical overfishing and the recent collapse of coastal ecosystems. Science 293, 629–638. doi: 10.1126/science.105919
Jennings S., Grandcourt E. M., and Polunin N. V. C. (1995). The effects of fishing on the diversity, biomass and trophic structure of Seychelles' reef fish communities. Coral Reefs 14, 225–223. doi: 10.1007/BF00334346
Jennings S. and Polunin N. V. C. (1997). Impacts of predator depletion by fishing on the biomass and diversity of non-target reef fish communities. Coral Reefs 16, 71—82. doi: 10.1007/s003380050061
Jeppesen R., de Rivera C. E., Grosholz E. D., Tinker M. T., Hughes B. B., Eby R., et al. (2025). Recovering population of the southern sea otter suppresses a global marine invader. Biol. Invasions 27, 33. doi: 10.1007/s10530-024-03467-3
Johannesen E., Ingvaldsen R. B., Bogstad B., Dalpadado P., Eriksen E., Gjøsæter H., et al. (2012). Changes in Barents Sea ecosystem state 1970–2009: climate fluctuations, human impact, and trophic interactions. ICES J. Mar. Sci. 69, 880–889. doi: 10.1093/icesjms/fss046
Johannessen T. (2014). From an antagonistic to a synergistic predator prey perspective (Amsterdam: Academic Press). doi: 10.1016/C2012-0-13683-8
Jones R. T. (2014). Empire of Extinction: RUSSIAns and the North Pacific's Strange Beasts of the Ses 1741–1867 (Oxford: Oxford University Press). doi: 10.1093/acprof:oso/9780199343416.001.0001
Jorgensen S. J., Anderson S., Ferretti F., Tietz J. R., Chapple T., Kanive P., et al. (2019). Killer whales redistribute white shark foraging pressure on seals. Sci. Rep. 9, 6153. doi: 10.1038/s41598-019-39356-2
Juan-Jordá M. J., Murua H., Arrizabalaga H., Merino G., Pacoureau N., and Dulvy N. K. (2022). Seventy years of tunas, billfishes, and sharks as sentinels of global ocean health. Science 378, eabj0211. doi: 10.1126/science.abj0211
Kaplan I. C., Koehn L. E., Hodgson E. E., Marshall K. N., and Essington T. E. (2017). Modeling food web effects of low sardine and anchovy abundance in the California Current. Ecol. Modell. 359, 1–24. doi: 10.1016/j.ecolmodel.2017.05.007
Katija K. and Dabiri J. O. (2009). A viscosity-enhanced mechanism for biogenic ocean mixing. Nature 460, 624–626. doi: 10.1038/nature08207
Kelaher B. P., Tan M., Figueira F., Gillanders B. M., Connell S. D., Goldsworthy S. D., et al. (2015). Fur seal activity moderates the effects of an Australian marine sanctuary on temperate reef fish. Biol. Conserv. 182, 205–214. doi: 10.1016/j.biocon.2014.12.011
Kendrick J. (1999). Alejandro Malaspina: Portrait of a Visionary (Montréal: McGill-Queen’s University Press).
Kennett D. J., Voorhies B., Wake T. A., and Martínez N. (2008). “Long-Term Effects of Human Predation on Marine Ecosystems in Guerrero, Mexico,” in Human impacts on ancient marine ecosystems: a global perspective. Eds. Rick T. C. and Erlandson J. M. (University of California Press, Berkeley, CA), 103–124. doi: 10.1525/9780520934290-007
Koehn L. E., Essington T. E., Marshall K. N., Sydeman W. J., Szoboszlai A. I., and Thayer J. A. (2017). Trade-offs between forage fish fisheries and their predators in the California Current. ICES J. Mar. Sci. 74, 2448–2458. doi: 10.1093/icesjms/fsx072
Kornev S. I. and Korneva S. M. (2006). Some criteria for assessing the state and dynamics of sea otter (Enhydra lutris) populations in the Russian part of the species range. Russ. J. Ecol. 37, 172–179. doi: 10.1134/S1067413606030052
Kroon F. J., Barneche D. R., and Emslie M. J. (2021). Fish predators control outbreaks of Crown-of-Thorns Starfish. Nat. Commun. 12, 6986. doi: 10.1038/s41467-021-26786-8
Kuker K. and Barrett-Lennard L. (2010). A re-evaluation of the role of killer whales Orcinus orca in a population decline of sea otters Enhydra lutris in the Aleutian Islands and a review of alternative hypotheses. Mamm. Rev. 40, 103–124. doi: 10.1111/j.1365-2907.2009.00156.x
Kumagai J. A., Goodman M. C., Villaseñor-Derbez J. C., Schoeman D. S., Cavanaugh K. C., Bell T. W., et al. (2024). Marine protected areas that preserve trophic cascades promote resilience of kelp forests to marine heatwaves. Glob. Change Biol. 30, e17620. doi: 10.1111/gcb.17620
Kunze E. (2019). Biologically generated mixing in the ocean. Annu. Rev. Mar. Sci. 11, 215–226. doi: 10.1146/annurev-marine-010318-095047
Kvitek A. R. G., Oliver J. S., Degange A. R., and Anderson B. S. (1992). Changes in Alaskan soft-bottom prey communities along a gradient in sea otter predation. Ecology 73, 413–428. doi: 10.2307/1940749
Lafferty K. D. (2004). Fishing for lobsters indirectly increases epidemics in sea urchins. Ecol. Appl. 14, 1566–1573. doi: 10.1890/03-5088
Langendorf R. E., Estes J. A., Watson J. C., Kenner M. C., Hatfield B. B., Tinker M. T., et al. (2025). Dynamic and context-dependent keystone species effects in kelp forests. PNAS 122, e2413360122. doi: 10.1073/pnas.2413360122
Laptikhovsky V., Arkhipkin A., and Brickle P. (2013). From small bycatch to main commercial species: Explosion of stocks of rock cod Patagonotothen ramsayi (Regan) in the Southwest Atlantic. Fish. Res. 147, 399–403. doi: 10.1016/j.fishres.2013.05.006
Lavery T. J., Roudnew B., Gill P., Seymour J., Seuront L., Johnson G., et al. (2010). Iron defecation by sperm whales stimulates carbon export in the Southern Ocean. Proc. R. Soc B 277, 3527–3531. doi: 10.1098/rspb.2010.0863
Lavery T. J., Roudnew B., Seymour J., Mitchell J. G., Smetacek V., and Nicol S. (2014). Whales sustain fisheries: Blue whales stimulate primary production in the Southern Ocean. Mar. Mamm. Sci. 30, 888–904. doi: 10.1111/mms.12108
Laws R. M. (1977). Seals and whales of the southern ocean. Philos. Trans. R. Soc B 279, 81–96. doi: 10.1098/rstb.1977.0073
Leibold M. A., Chase J. M., Shurin J. B., and Downing A. L. (1997). Species turnover and the regulation of trophic structure. Annu. Rev. Ecol. Syst. 28, 467–494. doi: 10.1146/annurev.ecolsys.28.1.467
Leising A. W., Horner R., Pierson J. J., Postel J., and Halsband-Lenk C. (2005a). The balance between microzooplankton grazing and phytoplankton growth in a highly productive estuarine fjord. Prog. Oceanogr. 67, 366–383. doi: 10.1016/j.pocean.2005.09.007
Leising A. W., Pierson J. J., Halsband-Lenk C., Horner R., and Postel J. (2005b). Copepod grazing during spring blooms: Can Pseudocalanus newmani induce trophic cascades? Prog. Oceanogr. 67, 406–421. doi: 10.1016/j.pocean.2005.09.009
Leroux S. J. and Loreau M. (2015). “Theoretical perspectives on bottom-up and top-down interactions across ecosystems,” in Trophic Ecology: Bottom-up and Top-down Interactions across Aquatic and Terrestrial Systems. Eds. Hanley T. C. and LaPierre K. J. (Cambridge University Press, Cambridge), 3–30. doi: 10.1017/CBO9781139924856.002
Lewandowska A. M., Boyce D. G., Hofmann M., Matthiessen B., Sommer U., and Worm B. (2014). Effects of sea surface warming on marine plankton. Ecol. Lett. 17, 614–623. doi: 10.1111/ele.12265
Lewis L. S. and Anderson T. W. (2012). Top-down control of epifauna by fishes enhances seagrass production. Ecology 93, 2746–2757. doi: 10.1890/12-0038.1
Lindeman R. L. (1942). The trophic-dynamic aspect of ecology. Ecology 23, 399–417. doi: 10.2307/1930126
Lindstrøm U., Smout S., Howell D., and Bogstad B. (2009). Modelling multi-species interactions in the Barents Sea ecosystem with special emphasis on minke whales and their interactions with cod, herring and capelin. Deep-Sea Res. II 56, 2068–2079. doi: 10.1016/j.dsr2.2008.11.017
Ling S. D., Johnson C. R., Frusher S. D., and Ridgway K. R. (2009). Overfishing reduces resilience of kelp beds to climate-driven catastrophic phase shift. PNAS 106, 22341–22345. doi: 10.1073/pnas.0907529106
Lynam C. P., Llope M., Möllmann C., Helaouët P., Bayliss-Brown G. A., and Stenseth N. C. (2017). Interaction between top-down and bottom-up control in marine food webs. PNAS 114, 1952–1957. doi: 10.1073/pnas.1621037114
Lynch M., Trickovic B., and Kempes C. P. (2022). Evolutionary scaling of maximum growth rate with organism size. Sci. Rep. 12, 22586. doi: 10.1038/s41598-022-23626-7
Mackinson S., Daskalov G., Heymans J. J., Neira S., Arancibia H., Zetina-Rejón M., et al. (2009). Which forcing factors fit? Using ecosystem models to investigate the relative influence of fishing and changes in primary productivity on the dynamics of marine ecosystems. Ecol. Modell. 220, 2972–2987. doi: 10.1016/j.ecolmodel.2008.10.021
Madigan D. J., Carlisle A. B., Dewar H., Snodgrass O. E., Litvin S. Y., Micheli F., et al. (2012). Stable isotope analysis challenges wasp-waist food web assumptions in an upwelling pelagic ecosystem. Sci. Rep. 2, 654. doi: 10.1038/srep00654
Madin E. M. P., Madin J. S., and Booth D. J. (2011). Landscape of fear visible from space. Sci. Rep. 1, 14. doi: 10.1038/srep00014
Madin E. M. P., Precoda K., Harborne A. R., Atwood T. B., Roelfsema C. M., and Luiz O. J. (2019). Multi-Trophic species interactions shape seascape-Scale coral reef vegetation patterns. Front. Ecol. Evol. 7. doi: 10.3389/fevo.2019.00102
Malakhoff K. D. and Miller R. J. (2021). After 15 years, no evidence for trophic cascades in marine protected areas. Proc. R. Soc B 288, 20203061. doi: 10.1098/rspb.2020.3061
Maldonado M. T., Surma S., and Pakhomov E. A. (2016). Southern Ocean biological iron cycling in the pre-whaling and present ecosystems. Phil. Trans. R. Soc A 374, 20150292. doi: 10.1098/rsta.2015.0292
Mariani G., Cheung W. W. L., Lyet A., Sala E., Mayorga J., Velez L., et al. (2020). Let more big fish sink: Fisheries prevent blue carbon sequestration—half in unprofitable areas. Sci. Adv. 6, eabb4848. doi: 10.1126/sciadv.abb4848
Marina T. I., Schloss I. R., Lovrich G. A., Boy C. C., Bruno D. O., Capitanio F. L., et al. (2024). Complex network of trophic interactions in Burdwood Bank, a sub-Antarctic oceanic marine protected area. Mar. Ecol. Prog. Ser. 736, 1–18. doi: 10.3354/meps14600
Markel R. M. and Shurin J. B. (2015). Indirect effects of sea otters on rockfish (Sebastes spp.) in giant kelp forests. Ecology 96, 2877–2890. doi: 10.1890/14-0492.1
Marshak A. R. and Link J. S. (2021). Primary production ultimately limits fisheries economic performance. Sci. Rep. 11, 12154. doi: 10.1038/s41598-021-91599-0
Matthews C. J. D., Breed G. A., LeBlanc B., and Ferguson S. H. (2020). Killer whale presence drives bowhead whale selection for sea ice in Arctic seascapes of fear. PNAS 117, 6590–6598. doi: 10.1073/pnas.1911761117
McCann K. S., Rasmussen J. B., and Umbanhowar J. (2005). The dynamics of spatially coupled food webs. Ecol. Lett. 8, 513–523. doi: 10.1111/j.1461-0248.2005.00742.x
McCauley D. J., DeSalles P. A., Young H. S., Dunbar R. B., Dirzo R., Mills M. M., et al. (2012b). From wing to wing: the persistence of long ecological interaction chains in less-disturbed ecosystems. Sci. Rep. 2, 409. doi: 10.1038/srep00409
McCauley D. J., Gellner G., Martinez N. D., Williams R. J., Sandin S. A., Micheli F., et al. (2017). On the prevalence and dynamics of inverted trophic pyramids and otherwise top-heavy communities. Ecol. Lett. 21, 439–454. doi: 10.1111/ele.12900
McCauley D. J., Young H. S., Dunbar R. B., Estes J. A., Semmens B. X., and Micheli F. (2012a). Assessing the effects of large mobile predators on ecosystem connectivity. Ecol. Appl. 22, 1711–1717. doi: 10.1890/11-1653.1
McClanahan T. R. (2000). Recovery of a coral reef keystone predator, Balistapus undulatus, in East African marine parks. Biol. Conserv. 94, 191–198. doi: 10.1016/S0006-3207(99)00176-7
McClanahan T. R. and Muthiga N. A. (2016). Geographic extent and variation of a coral reef trophic cascade. Ecology 97, 1862–1872. doi: 10.1890/15-1492.1
McClatchie S., Field J., Thompson A. R., Gerrodette T., Lowry M., Fiedler P. C., et al. (2016). Food limitation of sea lion pups and the decline of forage off central and southern California. R. Soc Open Sci. 3, 150628. doi: 10.1098/rsos.150628
Mcowen C. J., Cheung W. W. L., Rykaczewski R. R., Watson R. A., and Wood L. J. (2014). Is fisheries production within Large Marine Ecosystems determined by bottom-up or top-down forcing? Fish Fish. 16, 623–632. doi: 10.1111/faf.12082
McPherson M. L., Finger D. J. I., Houskeeper H. F., Bell T. W., Carr M. H., Rogers-Bennett L., et al. (2021). Large-scale shift in the structure of a kelp forest ecosystem co-occurs with an epizootic and marine heatwave. Commun. Biol. 4, 298. doi: 10.1038/s42003-021-01827-6
McQueen D. J., Post J. R., and Mills E. L. (1986). Trophic relationships in fresh-water pelagic ecosystems. Can. J. Fish. Aquat. Sci. 43, 1571–1581. doi: 10.1139/f86-195
Meekan M. G., Lester E. K., Kroon F. J., and Barneche D. R. (2025). Predator removals, trophic cascades and outbreaks of crown-of-thorns starfish on coral reefs. Commun. Biol. 8, 305. doi: 10.1038/s42003-025-07716-6
Mehta A. V., Allen J. M., Constantine R., Garrigue C., Jann B., Jenner C., et al. (2007). Baleen whales are not important as prey for killer whales Orcinus orca in high-latitude regions. Mar. Ecol. Prog. Ser. 348, 297–307. doi: 10.3354/meps07015
Menge B. A. (2000). Top-down and bottom-up community regulation in marine rocky intertidal habitats. J. Exp. Mar. Biol. Ecol. 250, 257–289. doi: 10.1016/S0022-0981(00)00200-8
Merrick R. L. (1997). Current and historical roles of apex predators in the bering sea ecosystem. J. Northw. Atl. Fish. Sci. 22, 343–355. doi: 10.2960/J.v22.a24
Micheli F. (1999). Eutrophication, fisheries, and consumer-resource dynamics in marine pelagic ecosystems. Science 285, 1396–1398. doi: 10.1126/science.285.5432.1396
Mizroch S. A. and Rice D. W. (2006). Have North Pacific killer whales switched prey species in response to depletion of the great whale populations? Mar. Ecol. Prog. Ser. 310, 235–246. doi: 10.1126/science.285.5432.139
Möllmann C. and Köster F. W. (2002). Population dynamics of calanoid copepods and the implications of their predation by clupeid fish in the Central Baltic Sea. J. Plankt. Res. 24, 959–977. doi: 10.1093/plankt/24.10.959
Monreal P. J., Savoca M. S., Babcock-Adams L., Moore L. E., Ruacho A., Hull D., et al. (2025). Organic ligands in whale excrement support iron availability and reduce copper toxicity to the surface ocean. Commun. Earth Environ. 6, 20. doi: 10.1038/s43247-024-01965-9
Moran J. R., Heintz R. A., Straley J. R., and Vollenweider J. J. (2018). Regional variation in the intensity of humpback whale predation on Pacific herring in the Gulf of Alaska. Deep-Sea Res. II 147, 187–195. doi: 10.1016/j.dsr2.2017.07.010
Morison F., Pierson J. J., Oikonomou A., and Menden-Deuer S. (2020). Mesozooplankton grazing minimally impacts phytoplankton abundance during spring in the western North Atlantic. PeerJ 8, e943. doi: 10.7717/peerj.9430
Morissette L., Hammill M. O., and Savenkoff C. (2006). The trophic role of marine mammals in the northern Gulf of St. Lawrence. Mar. Mamm. Sci. 22, 74–103. doi: 10.1111/j.1748-7692.2006.00007.x
Morozov A. Y., Nezlin N. P., and Petrovskii S. V. (2005). Invasion of a top predator into an epipelagic ecosystem can bring a paradoxical top-down trophic control. Biol. Invasions 7, 845–861. doi: 10.1007/s10530-005-5213-y
Mourier J., Maynard J., Parravicini V., Ballesta L., Clua E., Domeier M. L., et al. (2016). Extreme inverted trophic pyramid of reef sharks supported by spawning groupers. Curr. Biol. 26, 2011–2016. doi: 10.1016/j.cub.2016.05.058
Mourier J., Planes S., and Buray N. (2013). Trophic interactions at the top of the coral reef food chain. Coral Reefs 32, 285. doi: 10.1007/s00338-012-0976-y
Mumby P. J., Dahlgren C. P., Harborne A. R., Kappel C. V., Micheli F., Brumbaugh D. R., et al. (2006). Fishing, trophic cascades, and the process of grazing on coral reefs. Science 311, 98–101. doi: 10.1126/science.1121129
Mumby P. J., Steneck R. S., Edwards A. J., Ferrari R., Coleman R., Harborne A. R., et al. (2012). Fishing down a Caribbean food web relaxes trophic cascades. Mar. Ecol. Prog. Ser. 445, 13–24. doi: 10.3354/meps09450
Murphy G. E. P., Romanuk T. N., and Worm B. (2020). Cascading effects of climate change on plankton community structure. Ecol. Evol. 10, 2170–2181. doi: 10.1002/ece3.6055
Murphy E. J., Watkins J. L., Trathan P. N., Reid K., Meredith M. P., Thorpe S. E., et al. (2007). Spatial and temporal operation of the Scotia Sea ecosystem: a review of large-scale links in a krill centred food web. Philos. Trans. R. Soc B. 363, 113–148. doi: 10.1098/rstb.2006.1957
Myers R. A., Baum J. K., Shepherd T. D., Powers S. P., and Peterson C. H. (2007). Cascading effects of the loss of apex predatory sharks from a coastal ocean. Science 315, 1846–1850. doi: 10.1126/science.1138657
Myers R. A. and Worm B. (2003). Rapid worldwide depletion of predatory fish communities. Nature 423, 280–283. doi: 10.1038/nature01610
Nejstgaard J. C., Naustvoll L.-J., and Sazhin A. (2001). Correcting for underestimation of microzooplankton grazing in bottle incubation experiments with mesozooplankton. Mar. Ecol. Prog. Ser. 221, 59–75. doi: 10.3354/meps221059
Nicholson T. E., McClenachan L., Tanaka K. R., and Van Houtan K. S. (2024). Sea otter recovery buffers century-scale declines in California kelp forests. PLOS Clim 3 (1), e0000290. doi: 10.1371/journal.pclm.0000290
Nicol S., Bowie A., Jarman S., Lannuzel D., Meiners K. M., and van der Merwe P. (2010). Southern Ocean iron fertilization by baleen whales and Antarctic krill. Fish Fish. 11, 203–209. doi: 10.1111/j.1467-2979.2010.00356.x
Nicol S., Croxall J., Trathan P., Gales N., and Murphy E. (2007). Paradigm misplaced? Antarctic marine ecosystems are affected by climate change as well as biological processes and harvesting. Antarct. Sci. 19, 291–295. doi: 10.1017/S0954102007000491
Nilsson J. (2006). Predation of northern pike (Esox lucius L.) eggs: a possible cause of regionally poor recruitment in the Baltic Sea. Hydrobiologia 553, 161–169. doi: 10.1007/s10750-005-1949-8
Nilsson J., Flink H., and Tibblin P. (2019). Predator–prey role reversal may impair the recovery of declining pike populations. J. Anim. Ecol. 88, 927–939. doi: 10.1111/1365-2656.12981
Nixon S. W. (1988). Physical energy inputs and the comparative ecology of lake and marine ecosystems. Limnol. Oceanogr. 33, 1005–1025. doi: 10.4319/lo.1988.33.4part2.1005
O'Dea A., Dillon E. M., Brandl S. J., Cramer K. L., Cybulski J. D., de Gracia B., et al. (2025). Prehistoric archives reveal evidence of predator loss and prey release in Caribbean reef fish communities. PNAS 122, e2503986122. doi: 10.1073/pnas.2503986122
Ogden A. (1941). The California Sea Otter Trade 1784–1848 (Berkeley: University of California Press). doi: 10.1525/9780520316683
Oguz T. and Gilbert D. (2007). Abrupt transitions of the top-down controlled Black Sea pelagic ecosystem during 1960–2000: Evidence for regime-shifts under strong fishery exploitation and nutrient enrichment modulated by climate-induced variations. Deep-Sea Res. I 54, 220–242. doi: 10.1016/j.dsr.2006.09.010
Oguz T., Salihoglu B., Moncheva S., and Abaza V. (2012). Regional peculiarities of community-wide trophic cascades in strongly degraded Black Sea food web. J. Plankt. Res. 34, 338–343. doi: 10.1093/plankt/fbs002
Ohman M. D., Frost B. W., and Cohen E. B. (1983). Reverse diel vertical migration: an escape from invertebrate predators. Science 220, 1404–1407. doi: 10.1126/science.220.4604.1404
Oksanen L., Fretwell S. D., Arruda J., and Niemela P. (1981). Exploitation ecosystems in gradients of primary productivity. Am. Nat. 118, 240–261. doi: 10.1086/283817
Olin A. B., Olsson J., Eklöf J. S., Eriksson B. K., Kaljuste O., Briekmane L., et al. (2022). Increases of opportunistic species in response to ecosystem change: the case of the Baltic Sea three-spined stickleback. ICES J. Mar. Sci. 79, 1419–1434. doi: 10.1093/icesjms/fsac073
Olson M. B., Lessard E. J., Wong C. H. J., and Bernhardt M. J. (2006). Copepod feeding selectivity on microplankton, including the toxigenic diatoms Pseudo-nitzschia spp., in the coastal Pacific Northwest. Mar. Ecol. Prog. Ser. 326, 207–220. doi: 10.3354/meps326207
Pacoureau N., Rigby C. L., Kyne P. M., Sherley R. B., Winker H., Carlson J. K., et al. (2021). Half a century of global decline in oceanic sharks and rays. Nature 589, 567–571. doi: 10.1038/s41586-020-03173-9
Padovani L. N., Viñas M. D., Sánchez F., and Mianzan H. (2012). Amphipod-supported food web: Themisto gaudichaudii, a key food resource for fishes in the southern Patagonian Shelf. J. Sea Res. 67, 85–90. doi: 10.1016/j.seares.2011.10.007
Paine R. T. (1966). Food web complexity and species diversity. Am. Nat. 100, 65–75. doi: 10.1086/282400
Paine R. T. (1980). Food webs: linkage, interaction strength, and community infrastructure. J. Anim. Ecol. 49, 667–685. doi: 10.2307/4220
Palumbi S. R. and Sotka C. (2011). The Death and Life of Monterey Bay: A Story of Revival (Washington, DC: Island Press).
Papastamatiou Y. P., Binder B. M., Boswell K. M., Malone M. A., Heithaus M. R., Huveneers C., et al. (2023). Dynamic energy landscapes of predators and the implications for modifying prey risk. Funct. Ecol. 38, 284–293. doi: 10.1111/1365-2435.14478
Papastamatiou Y. P., Iosilevskii G., Di Santo V., Huveneers C., Hattab T., Planes S., et al. (2021). Sharks surf the slope: Current updrafts reduce energy expenditure for aggregating marine predators. J. Anim. Ecol. 90, 2302–2314. doi: 10.1111/1365-2656.13536
Parrish F. A. (2009). Do monk seals exert top-down pressure in subphotic ecosystems? Mar. Mamm. Sci. 25, 91–106. doi: 10.1111/j.1748-7692.2008.00245.x
Parrish F. A. and Boland R. C. (2004). Habitat and reef-fish assemblages of banks in the Northwestern Hawaiian Islands. Mar. Biol. 144, 1065–1073. doi: 10.1007/s00227-003-1288-0
Pauly D. (1995). Anecdotes and the shifting baseline syndrome of fisheries. Trends Ecol. Evol. 10, 430. doi: 10.1016/S0169-5347(00)89171-5
Pauly D., Christensen V., Dalsgaard J., Froese R., and Torres F. Jr. (1998). Fishing down marine food webs. Science 279, 860–863. doi: 10.1126/science.279.5352.860
Pérez-Matus A., Micheli F., Konar B., Shears N., Low N. H. N., Okamoto D. K., et al. (2025). Kelp forests as nursery and foundational habitat for reef fishes. Ecology 106, e70007. doi: 10.1002/ecy.70007
Pershing A. J., Christensen L. B., Record N. R., Sherwood G. D., and Stetson P. B. (2010). The impact of whaling on the ocean carbon cycle: why bigger was better. PloS One 5, e12444. doi: 10.1371/journal.pone.0012444
Pershing A. J., Mills K. E., Record N. R., Stamieszkin K., Wurtzell K. V., Byron C. J., et al. (2015). Evaluating trophic cascades as drivers of regime shifts in different ocean ecosystems. Phil. Trans. R. Soc B 370, 20130265. doi: 10.1098/rstb.2013.0265
Peters J. R., Reed D. C., Shrestha J., Hamilton S. L., and Burkepile D. E. (2025). Frequent disturbance to a foundation species disrupts consumer-mediated nutrient cycling in giant kelp forests. Ecology 106, e70019. doi: 10.1002/ecy.70019
Petrie B., Frank K. T., Shackell N. L., and Leggett W. C. (2009). Structure and stability in exploited marine fish communities: quantifying critical transitions. Fish. Oceanogr. 18, 83–101. doi: 10.1111/j.1365-2419.2009.00500.x
Pikitch E. K., Boersma P. D., Boyd I. L., Conover D. O., Cury P., Essignton T., et al. (2018). The strong connection between forage fish and their predators: a response to Hilborn et al., (2017). Fish. Res. 198, 220–223. doi: 10.1016/j.fishres.2017.07.022
Pikitch E. K., Rountos K. J., Essington T. E., Santora C., Pauly D., Watson R., et al. (2012). The global contribution of forage fish to marine fisheries and ecosystems. Fish Fish. 15, 43–64. doi: 10.1111/faf.12004
Pinnegar J. K., Polunin N. V. C., Francour P., Badalamenti F., Chemello R., Harmelin-Vivien M.-L., et al. (2000). Trophic cascades in benthic marine ecosystems: lessons for fisheries and protected-area management. Env. Conserv. 27, 179–200. doi: 10.1017/S0376892900000205
Pinti J., Kiørboe T., Thygesen U. H., and Visser A. W. (2019). Trophic interactions drive the emergence of diel vertical migration patterns: a game-theoretic model of copepod communities. Proc. R. Soc B 286, 20191645. doi: 10.1098/rspb.2019.1645
Pitcher T. J., Misund O. A., Fernö A., Totland B., and Melle V. (1996). Adaptive behaviour of herring schools in the Norwegian Sea as revealed by high-resolution sonar. ICES J. Mar. Sci. 53, 449–452. doi: 10.1006/jmsc.1996.0063
Pitt K. A., Kingsford M. J., Rissik D., and Koop K. (2007). Jellyfish modify the response of planktonic assemblages to nutrient pulses. Mar. Ecol. Prog. Ser. 351, 1–13. doi: 10.3354/meps07298
Polis G. A. (1999). Why are parts of the world green? Multiple factors control productivity and the distribution of biomass. Oikos 86, 3–15. doi: 10.2307/3546565
Polis G. A. and Strong D. R. (1996). Food web complexity and community dynamics. Am. Nat. 147, 813–846. doi: 10.1086/285880
Potapov A. M., Brose U., Scheu S., and Tiunov A. V. (2019). Trophic position of consumers and size structure of food webs across aquatic and terrestrial ecosystems. Am. Nat. 194, 823–839. doi: 10.1086/705811
Potter B., Corrales-Ugalde M., Townsend J. P., Colin S. P., Sutherland K. R., Costello J. H., et al. (2023). Quantifying the feeding behaviorand trophic impact of a widespread oceanic ctenophore. Sci. Rep. 13, 2292. doi: 10.1038/s41598-023-27955-z
Provost E. J., Kelaher B. P., Dworjanyn S. A., Russell B. D., Connell S. D., Ghedini G., et al. (2017). Climate-driven disparities among ecological interactions threaten kelp forest persistence. Glob. Change Biol. 23, 353–361. doi: 10.1111/gcb.13414
Prowe A. E. F., Pahlow M., Dutkiewicz S., Follows M., and Oschlies A. (2012). Top-down control of marine phytoplankton diversity in a global ecosystem model. Prog. Oceanogr. 101, 1–13. doi: 10.1016/j.pocean.2011.11.016
Raffaelli D. and Hall S. J. (1992). Compartments and predation in an estuarine food web. J. Anim. Ecol. 61, 551–560. doi: 10.2307/5610
Ramshaw B. C., Pakhomov E. A., Markel R. W., and Kaehler S. (2017). Quantifying spatial and temporal variations in phytoplankton and kelp isotopic signatures to estimate the distribution of kelp-derived detritus off the west coast of Vancouver Island, Canada. Limnol. Oceanogr. 62, 2133–2153. doi: 10.1002/lno.10555
Rasher D. B., Hoey A. S., and Hay M. E. (2017). Cascading predator effects in a Fijian coral reef ecosystem. Sci. Rep. 7, 15684. doi: 10.1038/s41598-017-15679-w
Rasher D. B., Steneck R. S., Halfar J., Kroeker K. J., Ries J. B., Tinker M. T., et al. (2020). Keystone predators govern the pathway and pace of climate impacts in a subarctic marine ecosystem. Science 369, 1351–1354. doi: 10.1126/science.aav7515
Ratnarajah L., Bowie A. R., Lannuzel D., Meiners K. M., and Nicol S. (2014). The biogeochemical role of baleen whales and krill in southern ocean nutrient cycling. PloS One 9, e114067. doi: 10.1371/journal.pone.0114067
Ratnarajah L., Melbourne-Thomas J., Marzloff M. P., Lannuzel D., Meiners K. M., Chever F., et al. (2016). A preliminary model of iron fertilisation by baleen whales and Antarctic krill in the Southern Ocean: Sensitivity of primary productivity estimates to parameter uncertainty. Ecol. Modell. 320, 203–212. doi: 10.1016/j.ecolmodel.2015.10.007
Ratnarajah L., Nicol S., and Bowie A. R. (2018). Pelagic iron recycling in the southern ocean: exploring the contribution of marine animals. Front. Mar. Sci. 5. doi: 10.3389/fmars.2018.00109
Raymond W. W., Hughes B. B., Stephens T. A., Mattson C. R., Bolwerk A. T., and Eckert G. L. (2021). Testing the generality of sea otter-mediated trophic cascades in seagrass meadows. Oikos 130, 1–14. doi: 10.1111/oik.07681
Reed D. C., Rassweiler A., Carr M. H., Cavanaugh K. C., Malone D. P., and Siegel D. A. (2011). Wave disturbance overwhelms top-down and bottom-up control of primary production in California kelp forests. Ecology 92, 2108–2116. doi: 10.1890/11-0377.1
Reid K. and Croxall J. P. (2001). Environmental response of upper trophic-level predators reveals a system change in an Antarctic marine ecosystem. Philos. Trans. R. Soc B. 268, 377–384. doi: 10.1098/rspb.2000.1371
Reisewitz S. E., Estes J. A., and Simenstad C. A. (2006). Indirect food web interactions: Sea otters and kelp forest fishes in the Aleutian Archipelago. Oecologia 146, 623–631. doi: 10.1007/s00442-005-0230-1
Riccialdelli L., Becker Y. A., Fioramonti N. E., Torres M., Bruno D. O., Rey A. R., et al. (2020). Trophic structure of southern marine ecosystems: a comparative isotopic analysis from the Beagle Channel to the oceanic Burdwood Bank area under a wasp-waist assumption. Mar. Ecol. Prog. Ser. 655, 1–27. doi: 10.3354/meps13524
Rizzari J. R., Bergseth B. J., and Frisch A. J. (2014a). Impact of conservation areas on trophic interactions between apex predators and herbivores on coral reefs. Conserv. Biol. 29, 418–429. doi: 10.1111/cobi.12385
Rizzari J. R., Frisch A. J., Hoey A. S., and McCormick M. I. (2014b). Not worth the risk: apex predators suppress herbivory on coral reefs. Oikos 123, 829–836. doi: 10.1111/oik.01318
Robles C. and Desharnais R. (2002). History and current development of a paradigm of predation in rocky intertidal communities. Ecology 83, 1521–1536. doi: 10.1890/0012-9658(2002)083[1521:HACDOA]2.0.CO;2
Rocha R. C. Jr., Clapham P. J., and Ivashchenko Y. V. (2014). Emptying the oceans: A summary of industrial whaling catches in the 20th century. Mar. Fish. Rev. 76, 37–48. doi: 10.7755/MFR.76.4.3
Rodriguez I. D. and Savaria L. A. (2024). Potter cove's heavyweights: estimation of species' Interaction strength of an antarctic food web. Ecol. Evol. 14, e70389. doi: 10.1002/ece3.70389
Roesti M., Anstett D. N., Freeman B. G., Lee-Yaw J. A., Schluter D., Chavarie L., et al. (2020). Pelagic fish predation is stronger at temperate latitudes than near the equator. Nat. Commun. 11, 1527. doi: 10.1038/s41467-020-15335-4
Roff G., Doropoulos C., Rogers A., Bozec Y.-M., Krueck N. C., Aurellado E., et al. (2016a). The ecological role of sharks on coral reefs. Trends Ecol. Evol. 31, 395–407. doi: 10.1016/j.tree.2016.02.014
Roff G., Doropoulos C., Rogers A., Bozec Y.-M., Krueck N. C., Aurellado E., et al. (2016b). Reassessing Shark-Driven Trophic Cascades on Coral Reefs: A Reply to Ruppert et al. Trends Ecol Evol. 31, 587–589. doi: 10.1016/j.tree.2016.05.005
Rogers-Bennett L., Kawana S. K., Catton C. A., Klamt R., Dondanville R., Maguire A., et al. (2024). Abalone recruitment patterns before and after sea urchin barrens formation in northern California: incorporating climate change. N. Z. J. Mar. Freshw. Res. 59, 283–299. doi: 10.1080/00288330.2024.2403596
Roman J. and McCarthy J. J. (2010). The whale pump: marine mammals enhance primary productivity in a coastal basin. PloS One 5, e13255. doi: 10.1371/journal.pone.0013255
Rossberg A. G., Gaedke U., and Kratina P. (2019). Dome patterns in pelagic size spectra reveal strong trophic cascades. Nat. Commun. 10, 4396. doi: 10.1038/s41467-019-12289-0
Ruppert J. L. W., Fortin M.-J., and Meekan M. G. (2016). The ecological role of sharks on coral reefs. Trends Ecol. Evol. 31, 586–587. doi: 10.1016/j.tree.2016.05.003
Ruppert J. L. W., Travers M. J., Smith L. L., Fortin M.-J., and Meekan M. G. (2013). Caught in the middle: combined impacts of shark removal and coral loss on the fish communities of coral reefs. PloS One 8, e74648. doi: 10.1371/journal.pone.0074648
Salinas-de-León P., Acuña-Marrero D., Rastoin E., Friedlander A. M., Donovan M. K., and Sala E. (2016). Largest global shark biomass found in the northern Galápagos Islands of Darwin and Wolf. PeerJ 4, e1911. doi: 10.7717/peerj.1911
Salomon A. K., Shears N. T., Langlois T. J., and Babcock R. C. (2008). Cascading effects of fishing can alter carbon flow through a temperate coastal ecosystem. Ecol. Appl. 18, 1874–1887. doi: 10.1890/07-1777.1
Sandin S. A., Smith J. E., DeMartini E. E., Dinsdale E. A., Donner. S. D., Friedlaender A. M., et al. (2008). Baselines and degradation of coral reefs in the northern line islands. PloS One 3, e1548. doi: 10.1371/journal.pone.0001548
Sandin S. A., Walsh S. M., and Jackson J. B. C. (2010). “Prey Release, Trophic Cascades, and Phase Shifts in Tropical Nearshore Ecosystems,” in Trophic Cascades: Predators, Prey, and the Changing Dynamics of Nature. Eds. Terborgh J. and Estes J. A. (Island Press, Washington, DC), 71–90.
Saporiti F., Bearhop S., Vales D. G., Silva L., Zenteno L., Tavares M., et al. (2015). Latitudinal changes in the structure of marine food webs in the Southwestern Atlantic Ocean. Mar. Ecol. Prog. Ser. 538, 23–34. doi: 10.3354/meps11464
Savenkoff C., Comtois S., and Chabot D. (2013). Trophic interactions in the St. Lawrence Estuary (Canada): Must the blue whale compete for krill? Estuar. Coast. Shelf Sci. 129, 136e151. doi: 10.1016/j.ecss.2013.05.033
Scheffer M., Carpenter S., and de Young B. (2005). Cascading effects of overfishing marine systems. Trends Ecol. Evol. 20, 579–581. doi: 10.1016/j.tree.2005.08.018
Scheffer M., Rinaldi S., Huisman J., and Weissing F. J. (2003). Why plankton communities have no equilibrium: solutions to the paradox. Hydrobiologia 491, 9–18. doi: 10.1023/A:1024404804748
Schiel D. R. and Foster M. S. (2015). The Biology and Ecology of Giant Kelp Forests (Berkeley, CA: University of California Press). doi: 10.1525/9780520961098
Schlenger A. J., Libralato S., and Ballance L. (2018). Temporal variability of primary production explains marine ecosystem structure and function. Ecosystems 22, 331–345. doi: 10.1007/s10021-018-0272-y
Schmitz O. J., Krivan V., and Ovadia O. (2004). Trophic cascades: the primacy of trait-mediated indirect interactions. Ecol. Lett. 7, 153–163. doi: 10.1111/j.1461-0248.2003.00560.x
Schmoker C., Hernández-León S., and Calbet A. (2013). Microzooplankton grazing in the oceans: impacts, data variability, knowledge gaps and future directions. J. Plankton Res. 35, 691–706. doi: 10.1093/plankt/fbt023
Schultz J. A., Cloutier R. N., and Côté I. M. (2016). Evidence for a trophic cascade on rocky reefs following sea star mass mortality in British Columbia. PeerJ 4, e1980. doi: 10.7717/peerj.1980
Schweder T., Hagen G. S., and Hatlebakk E. (2000). Direct and indirect effects of minke whale abundance on cod and herring fisheries: A scenario experiment for the Greater Barents Sea. NAMMCO Sci. Publ. 2, 120–133. doi: 10.7557/3.2976
Scian M., Riccialdelli L., and Marina T. I. (2025). Food web structure and species’ role in the sub-Antarctic Marine Protected Area Yaganes. Polar Biol. 48, 49. doi: 10.1007/s00300-025-03368-8
Selgrath J. C., Carlton J. T., Pearse J., Thomas T., and Micheli F. (2024). Setting deeper baselines: kelp forest dynamics in California over multiple centuries. Reg. Environ. Change 24, 104. doi: 10.1007/s10113-024-02260-1
Sergio F., Schmitz O. J., Krebs C. J., Holt R. D., Heithaus M. R., Wirsing A. J., et al. (2014). Towards a cohesive, holistic view of top predation: a definition, synthesis and perspective. Oikos 123, 1234–1243. doi: 10.1111/oik.01468
Sewall F., Norcross B., Mueter F., and Heintz R. (2018). Empirically based models of oceanographic and biological influences on Pacific Herring recruitment in Prince William Sound. Deep-Sea Res. II 147, 127–137. doi: 10.1016/j.dsr2.2017.07.004
Shackell N. L., Frank K. T., Fisher J. A. D., and Leggett W. C. (2010). Decline in top predator body size and changing climate alter trophic structure in an oceanic ecosystem. Proc. R. Soc B 277, 1353–1360. doi: 10.1098/rspb.2009.1020
Shears N. T. and Babcock R. C. (2002). Marine reserves demonstrate top-down control of community structure on temperate reefs. Oecologia 132, 131–142. doi: 10.1007/s00442-002-0920-x
Sherr E. B., Sherr B. F., and Hartz A. J. (2009). Microzooplankton grazing impact in the Western Arctic Ocean. Deep-Sea Res. II 56, 1264–1273. doi: 10.1016/j.dsr2.2008.10.036
Shiomoto A., Tadokoro K., Nagasawa K., and Ishida Y. (1997). Trophic relations in the subarctic North Pacific ecosystem: possible feeding effect from pink salmon. Mar. Ecol. Prog. Ser. 150, 75–85. doi: 10.3354/meps150075
Shrestha J., Coale K. H., and Hamilton S. L. (2023). Empirical measurements of ammonium excretion in kelp forest fishes: Effects of body size, taxonomy and trophic guild. J. Exp. Mar. Biol. Ecol. 569, 151956. doi: 10.1016/j.jembe.2023.151956
Shrestha J., Peters J. R., Caselle J. E., and Hamilton S. L. (2024). Marine protection and environmental forcing influence fish-derived nutrient cycling in kelp forests. Funct. Ecol. 39, 403–417. doi: 10.1111/1365-2435.14708
Shurin J. B., Borer E. T., Seabloom E. W., Anderson K., Blanchette C. A., Broitman B., et al. (2002). A cross-ecosystem comparison of the strength of trophic cascades. Ecol. Lett. 5, 785–791. doi: 10.1046/j.1461-0248.2002.00381.x
Silliman B. R. and Bertness M. D. (2002). A trophic cascade regulates salt marsh primary production. PNAS 99, 10500–10505. doi: 10.1073/pnas.162366599
Simenstad C. A., Estes J. A., and Kenyon K. W. (1978). Aleuts, sea otters, and alternate stable-State communities. Science 200, 403–411. doi: 10.1126/science.200.4340.403
Skinner C., Mill A. C., Fox M. D., Newman S. P., Zhu Y., and Polunin N. V. C. (2021). Offshore pelagic subsidies dominate carbon inputs to coral reef predators. Sci. Adv. 7, eabf3792. doi: 10.1126/sciadv.abf379
Smetacek V. (2008). “Are Declining Antarctic Krill Stocks a Result of Global Warming or of the Decimation of the Whales?,” in Impacts of Global Warming on Polar Ecosystems. Ed. Duarte C. M. (Fundación BBVA, Bilbao), 46–83.
Smetacek V. and Nicol S. (2005). Polar ocean ecosystems in a changing world. Nature 437, 362–368. doi: 10.1038/nature04161
Smith C. R. (2006). “Bigger Is Better: The Role of Whales as Detritus in Marine Ecosystems,” in Whales, Whaling, and Ocean Ecosystem. Eds. Estes J. A., DeMaster D. P., Doak D. F., Williams T. M., and Brownell R. L. Jr (University of California Press, Berkeley, CA), 286–302. doi: 10.1525/9780520933200-026
Smith W. O. Jr., Ainley D. G., and Cattaneo-Vietti R. (2007). Trophic interactions within the Ross Sea continental shelf ecosystem. Phil. Trans. R. Soc B 362, 95–111. doi: 10.1098/rstb.2006.1956
Smith A. D., Brown C. J., Bulman C. M., Fulton E. A., Johnson P., Kaplan I. C., et al. (2011). Impacts of fishing low–trophic level species on marine ecosystems. Science 333, 1147–1150. doi: 10.1126/science.1209395
Smith J. G., Fujii J. A., Gaddam R., Konrad L., Lyon S., Nicholson T. E., et al. (2025). Keystone interdependence: Sea otter responses to a prey surplus following the collapse of a rocky intertidal predator. Sci. Adv. 11, eadu1028. doi: 10.1126/sciadv.adu1028
Smith J. G., Malone D., and Carr M. H. (2024). Consequences of kelp forest ecosystem shifts and predictors of persistence through multiple stressors. Proc. R. Soc B 291, 20232749. doi: 10.1098/rspb.2023.2749
Smith L. V., McMinn A., Martin A., Nicol S., Bowie A. R., Lannuzel D., et al. (2013). Preliminary investigation into the stimulation of phytoplankton photophysiology and growth by whale faeces. J. Exp. Mar. Biol. Ecol. 446, 1–9. doi: 10.1016/j.jembe.2013.04.010
Smith J. G., Tomoleoni J., Staedler M., Lyon S., Fujii J., and Tinker M. T. (2021). Behavioral responses across a mosaic of ecosystem states restructure a sea otter–urchin trophic cascade. PNAS 118, e2012493118. doi: 10.1073/pnas.2012493118
Smout S. and Lindstrøm U. (2007). Multispecies functional response of the minke whale Balaenoptera acutorostrata based on small-scale foraging studies. Mar. Ecol. Prog. Ser. 341, 277–291. doi: 10.3354/meps341277
Sommer U. (2008). Trophic cascades in marine and freshwater plankton. Internat. Rev. Hydrobiol. 93, 506–516. doi: 10.1002/iroh.200711039
Sommer U., Peter K. H., Genitsaris S., and Moustaka-Gouni M. (2017). Do marine phytoplankton follow Bergmann’s rule sensu lato? Biol. Rev. 92, 1011–1026. doi: 10.1111/brv.12266
Sommer U. and Sommer F. (2006). Cladocerans versus copepods: the cause of contrasting top-down controls on freshwater and marine phytoplankton. Oecologia 147, 183–194. doi: 10.1007/s00442-005-0320-0
Sommer U., Sommer F., Feuchtmayr H., and Hansen T. (2004). The influence of mesozooplankton on phytoplankton nutrient limitation: A mesocosm study with northeast atlantic plankton. Protist 155, 295–304. doi: 10.1078/1434461041844268
Sommer U. and Stibor H. (2002). Copepoda – Cladocera – Tunicata: The role of three major mesozooplankton groups in pelagic food webs. Ecol. Res. 17, 161–174. doi: 10.1046/j.1440-1703.2002.00476.x
Sommer U., Stibor H., Katechakis A., Sommer F., and Hansen T. (2002). Pelagic food web configurations at different levels of nutrient richness and their implications for the ratio fish production:primary production. Hydrobiologia 484, 11–20. doi: 10.1023/A:1021340601986
Springer A. M., Estes J. A., van Vliet G. B., Williams T. M., Doak D. F., Danner E. M., et al. (2003). Sequential megafaunal collapse in the North Pacific Ocean: An ongoing legacy of industrial whaling? PNAS 100, 12223–12228. doi: 10.1073/pnas.1635156100
Springer A. M. and van Vliet G. B. (2014). Climate change, pink salmon, and the nexus between bottom-up and top-down forcing in the subarctic Pacific Ocean and Bering Sea. PNAS 111, E1880–E1888. doi: 10.1073/pnas.1319089111
Spyksma A. J. P., Taylor R. B., and Shears N. T. (2017). Predation cues rather than resource availability promote cryptic behaviour in a habitat-forming sea urchin. Oecologia 83, 821–829. doi: 10.1007/s00442-017-3809-4
Stachowicz J. J., Bruno J. F., and Duffy J. (2007). Understanding the effects of marine biodiversity on communities and ecosystems. Annu. Rev. Ecol. Evol. Syst. 38, 739–766. doi: 10.1146/annurev.ecolsys.38.091206.095659
Steinberg P. D., Estes J. A., and Winter F. C. (1995). Evolutionary consequences of food chain length in kelp forest communities. PNAS 92, 8145–8148. doi: 10.1073/pnas.92.18.8145
Steneck R. S., Graham M. H., Bourque B. J., Corbett D., Erlandson J. M., Estes J. A., et al. (2002). Kelp forest ecosystems: biodiversity, stability, resilience and future. Env. Conserv. 29, 436–459. doi: 10.1017/S0376892902000322
Steneck R. S. and Pauly D. (2019). Fishing through the anthropocene. Curr. Biol. 29, R942–R995. doi: 10.1016/j.cub.2019.07.081
Stibor H., Stockenreiter M., Nejstgaard J. C., Ptacnik R., and Sommer U. (2019). Trophic switches in pelagic systems. Curr. Opin. Syst. Biol. 13, 108–114. doi: 10.1016/j.coisb.2018.11.006
Stibor H., Vadstein O., Diehl S., Gelzleichter A., Hansen T., Hantzsche F., et al. (2004a). Copepods act as a switch between alternative trophic cascades in marine pelagic food webs. Ecol. Lett. 7, 321–328. doi: 10.1111/j.1461-0248.2004.00580.x
Stibor H., Vadstein O., Lippert B., Roederer W., and Olsen Y. (2004b). Calanoid copepods and nutrient enrichment determine population dynamics of the appendicularian Oikopleura dioica: a mesocosm experiment. Mar. Ecol. Prog. Ser. 270, 209–215. doi: 10.3354/meps270209
Stoecker D. K., Thessen A. E., and Gustafson D. E. (2008). Windows of opportunity” for dinoflagellate blooms: Reduced microzooplankton net growth coupled to eutrophication. Harmful Algae 8, 158–166. doi: 10.1016/j.hal.2008.08.021
Straley J. M., Moran J. R., Boswell K. M., Vollenweider J. J., Heintz R. A., Quinn T. J. II, et al. (2018). Seasonal presence and potential influence of humpback whales on wintering Pacific herring populations in the Gulf of Alaska. Deep-Sea Res. II 147, 173–186. doi: 10.1016/j.dsr2.2017.08.008
Strom S. L., Macri E. L., and Olson M. B. (2007). Microzooplankton grazing in the coastal Gulf of Alaska: Variations in top-down control of phytoplankton. Limnol. Oceanogr. 52, 1480–1494. doi: 10.4319/lo.2007.52.4.1480
Strong D. R. (1992). Are trophic cascades all wet? Differentiation and donor control in speciose ecosystems. Ecology 73, 747–754. doi: 10.2307/1940154
Surma S., Pakhomov E. A., and Pitcher T. J. (2014). Effects of whaling on the structure of the southern ocean food web: insights on the ‘‘Krill surplus’’ from ecosystem modelling. PloS One 9, e114978. doi: 10.1371/journal.pone.0114978
Surma S. and Pitcher T. J. (2015). Predicting the effects of whale population recovery on Northeast Pacific food webs and fisheries: an ecosystem modelling approach. Fish. Oceanogr. 24, 291–305. doi: 10.1111/fog.12109
Suskiewicz T. S., Byrnes J. E. K., Steneck R. S., Russell R., Wilson C. J., and Rasher D. B. (2024). Ocean warming undermines the recovery resilience of New England kelp forests following a fishery-induced trophic cascade. Ecology 105, e4334. doi: 10.1002/ecy.4334
Sydeman W. J., Thompson S. A., Anker-Nilssen T., Arimitsu M., Bennison A., Bertrand S., et al. (2017). Best practices for assessing forage fish fisheries-seabird resource competition. Fish. Res. 194, 209–221. doi: 10.1016/j.fishres.2017.05.018
Szpak P., Orchard T. J., McKechnie I., and Gröcke D. R. (2012). Historical ecology of late Holocene sea otters (Enhydra lutris) from northern British Columbia: isotopic and zooarchaeological perspectives. J. Archaeol. Sci. 39, 1553–1571. doi: 10.1016/j.jas.2011.12.006
Szpak P., Orchard T. J., Salomon A., and Gröcke D. R. (2013). Regional ecological variability and impact of the maritime fur trade on nearshore ecosystems in southern Haida Gwaii (British Columbia, Canada): evidence from stable isotope analysis of rockfish (Sebastes spp.) bone collagen. Archaeol. Anthropol. Sci. 5, 159–182. doi: 10.1007/s12520-013-0122-y
Tanasichuk R. W. (2017). An investigation of the biological basis of recruitment, growth and adult survival rate variability of Pacific herring (Clupea pallasi) from British Columbia: a synthesis. Fish. Oceanogr. 26, 413–438. doi: 10.1111/fog.12206
Taylor M. H., Tam J., Blaskovic V., Espinoza P., Ballón R. M., Wosnitza-Mendo C., et al. (2008). Trophic modeling of the Northern Humboldt Current Ecosystem, Part II: Elucidating ecosystem dynamics from 1995 to 2004 with a focus on the impact of ENSO. Prog. Oceanogr. 79, 366–378. doi: 10.1016/j.pocean.2008.10.008
Tebbett S. B., Faul S. I., and Bellwood D. R. (2024). Quantum of fear: Herbivore grazing rates not affected by reef shark presence. Mar. Environ. Res. 196, 106442. doi: 10.1016/j.marenvres.2024.106442
Tegner M. J. and Dayton P. K. (1981). Population Structure, Recruitment and Mortality of Two Sea Urchins (Strongylocentrotus franciscanus and S. purpuratus) in a Kelp Forest. Mar. Ecol. Prog. Ser. 5, 255–268. doi: 10.3354/meps005255
Tegner M. J. and Dayton P. K. (2000). Ecosystem effects of fishing in kelp forest communities. ICES J. Mar. Sci. 57, 579–589. doi: 10.1006/jmsc.2000.0715
Tegner M. J. and Levin L. A. (1983). Spiny lobsters and sea urchins: analysis of a predator-prey interaction. J. Exp. Mar. Biol. Ecol. 73, 125–150. doi: 10.1016/0022-0981(83)90079-5
Thingstad T. F. (2020). How trophic cascades and photic zone nutrient content interact to generate basin-scale differences in the microbial food web. ICES J. Mar. Sci. 77, 1639–1647. doi: 10.1093/icesjms/fsaa028
Thingstad T. F. (2021). Reproducing the virus-to-copepod link in Arctic mesocosms using host fitness optimization. Limnol. Oceanogr. 66, S303–S313. doi: 10.1002/lno.11549
Thompson R. M., Hemberg M., Starzomski B. M., and Shurin J. B. (2007). Trophic levels and trophic tangles: the prevalence of omnivory in real food webs. Ecology 88, 612–617. doi: 10.1890/05-1454
Tiselius P. and Møller L. F. (2017). Community cascades in a marine pelagic food web controlled by the non-visual apex predator Mnemiopsis leidyi. J. Plankton Res. 39, 271–279. doi: 10.1093/plankt/fbw096
Tittensor D. P., Mora C., Jetz W., Lotze H. K., Ricard D., Vanden Berghe E., et al. (2010). Global patterns and predictors of marine biodiversity across taxa. Nature 466, 1098–1101. doi: 10.1038/nature09329
Towner A. V., Watson R. G. A., Kock A. A., Papastamatiou Y., Sturup M., Gennari E., et al. (2022). Fear at the top: killer whale predation drives white shark absence at South Africa’s largest aggregation site. Afr. J. Mar. Sci. 44, 1–14. doi: 10.2989/1814232X.2022.2066723
Trathan P. N., Ratcliffe N., and Masden E. A. (2012). Ecological drivers of change at South Georgia: the krill surplus, or climate variability. Ecography 35, 983–993. doi: 10.1111/j.1600-0587.2012.07330.x
Trebilco R., Dulvy N. K., Anderson S. C., and Salomon A. K. (2016). The paradox of inverted biomass pyramids in kelp forest fish communities. Proc. R. Soc B 283, 20160816. doi: 10.1098/rspb.2016.0816
Trites A. W., Deecke V. B., Gregr E. J., Ford J. K. B., and Olesiuk P. F. (2007). Killer whales, whaling, and sequential megafaunal collapse in the North Pacific: a comparative analysis of the dynamics of marine mammals in Alaska and British Columbia following commercial whaling. Mar. Mamm. Sci. 23, 751–765. doi: 10.1111/j.1748-7692.2006.00076.x
Trivelpiece W. Z., Hinke J. T., Miller A. K., Reiss C. S., Trivelpiece S. G., and Watters G. M. (2011). Variability in krill biomass links harvesting and climate warming to penguin population changes in Antarctica. PNAS 108, 7625–7628. doi: 10.1073/pnas.1016560108
Trussell G. C., Ewanchuk P. J., Bertness M. D., and Silliman B. R. (2004). Trophic cascades in rocky shore tide pools: distinguishing lethal and nonlethal effects. Oecologia 139, 427–432. doi: 10.1007/s00442-004-1512-8
Tulloch V. J. D., Plagányi É. E., Brown C., Richardson A. J., and Matear R. (2019). Future recovery of baleen whales is imperiled by climate change. Glob. Change Biol. 25, 1263–1281. doi: 10.1111/gcb.14573
Tulloch V. J. D., Plagányi É. E., Matear R., Brown C. J., and Richardson A. J. (2018). Ecosystem modelling to quantify the impact of historical whaling on Southern Hemisphere baleen whales. Fish Fish. 19 (1), 117–137. doi: 10.1111/faf.12241
Urmy S. S. and Benoit-Bird K. J. (2021). Fear dynamically structures the ocean’s pelagic zone. Curr. Biol. 31, 5086–5092. doi: 10.1016/j.cub.2021.09.003
Vadas R. L. and Steneck R. S. (1995). “Overfishing and inferences in kelp-sea urchin interactions,” in Ecology of Fjords and Coastal Waters. Eds. kjoldal H. R., Hopkins C., Erikstad K. E., and Leinaas H. P. (Elsevier Science B.V., Amsterdam), 509–524.
Vadstein O., Stibor H., Lippert, Løseth K., Roederer W., Sundt-Hansen L., et al. (2004). Moderate increase in the biomass of omnivorous copepods may ease grazing control of planktonic algae. Mar. Ecol. Prog. Ser. 270, 199–207. doi: 10.3354/meps270199
Verity P. G. and Smetacek V. (1996). Organism life cycles, predation, and the structure of marine pelagic ecosystems. Mar. Ecol. Prog. Ser. 130, 277–293. doi: 10.3354/meps130277
Vicknair K. and Estes J. A. (2012). Interactions among sea otters, sea stars, and suspension-feeding invertebrates in the western Aleutian archipelago. Mar. Biol. 159, 2641–2649. doi: 10.1007/s00227-012-2021-7
Vos D. J., Quakenbush L. T., and Mahoney B. A. (2006). Documentation of sea otters and birds as prey for killer whales. Mar. Mamm. Sci. 22, 201–205. doi: 10.1111/j.1748-7692.2006.00014.x
Wade P. R., Burkanov V. N., Dahlheim M. E., Friday N. A., Fritz L. W., Loughlin T. R., et al. (2007). Killer whales and marine mammal trends in the North Pacific - a re-examination of evidence for sequential megafaunal collapse and the prey-switching hypothesis. Mar. Mamm. Sci. 23, 766–802. doi: 10.1111/j.1748-7692.2006.00093.x
Wade P. R., Ver Hoef J. M., and Demaster D. P. (2009). Mammal-eating killer whales and their prey—trend data for pinnipeds and sea otters in the North Pacific Ocean do not support the sequential megafaunal collapse hypothesis. Mar. Mamm. Sci. 25, 737–747. doi: 10.1111/j.1748-7692.2009.00282.x
Walters C. J. and Juanes F. (1993). Recruitment limitation as a consequence of natural selection for use of restricted feeding habitats and predation risk taking by juvenile fishes. Can. J. Fish. Aquat. Sci. 50, 2058–2070. doi: 10.1139/f93-229
Walters C. and Kitchell J. F. (2000). Cultivation/depensation effects on juvenile survival and recruitment: implications for the theory of fishing. Can. J. Fish. Aquat. Sci. 58, 39–50. doi: 10.1139/f00-160
Ward E. J., Adkison M., Couture J., Dressel S. C., Litzow M. A., Moffitt S., et al. (2017). Evaluating signals of oil spill impacts, climate, and species interactions in Pacific herring and Pacific salmon populations in Prince William Sound and Copper River, Alaska. PloS One 12, e0172898. doi: 10.1371/journal.pone.0172898
Ward P. and Myers R. A. (2005). Shifts in open-ocean communities coinciding with the commencement of commercial fishing. Ecology 86, 835–847. doi: 10.1890/03-0746
Ware D. M. and Thomson R. E. (2005). Bottom-up ecosystem trophic dynamics determine fish production in the northeast pacific. Science 308, 1280–1284. doi: 10.1126/science.1109049
West E. J., Pitt K. A., Welsh D. T., Koop K., and Rissik D. (2009). Top-down and bottom-up influences of jellyfish on primary productivity and planktonic assemblages. Limnol. Oceanogr. 54, 2058–2071. doi: 10.4319/lo.2009.54.6.2058
Whalen M. A., Whippo R. D. B., Stachowicz J. J., York P. H., Aiello E., Alcoverro T., et al. (2020). Climate drives the geography of marine consumption by changing predator communities. PNAS 117, 28160–28166. doi: 10.1073/pnas.2005255117
Whitehead H. and Shin M. (2022). Current global population size, post-whaling trend and historical trajectory of sperm whales. Sci. Rep. 12, 19468. doi: 10.1038/s41598-022-24107-7
Wilhelmus M. M. and Dabiri J. O. (2014). Observations of large-scale fluid transport by laser-guided plankton aggregations. Phys. Fluids 26, 101302. doi: 10.1063/1.4895655
Williams T. M., Estes J. A., Doak D. F., and Springer A. M. (2004). Killer appetites: assessing the role of predators in ecological communities. Ecology 85, 3373–3384. doi: 10.1890/03-0696
Willis J. (2014). Whales maintained a high abundance of krill; both are ecosystem engineers in the Southern Ocean. Mar. Ecol. Prog. Ser. 513, 51–69. doi: 10.3354/meps10922
Wilmers C. C., Estes J. A., Edwards M., Laidre K. L., and Konar B. (2012). Do trophic cascades affect the storage and flux of atmospheric carbon? An analysis of sea otters and kelp forests. Front. Ecol. Environ. 10, 409–415. doi: 10.1890/110176
Wirsing A. J., Heithaus M. R., and Dill L. M. (2007). Living on the edge: dugongs prefer to forage in microhabitats that allow escape from rather than avoidance of predators. Anim. Behav. 74, 93–101. doi: 10.1016/j.anbehav.2006.11.016
Wirsing A. J., Heithaus M. R., Frid A., and Dill L. M. (2008). Seascapes of fear: evaluating sublethal predator effects experienced and generated by marine mammals. Mar. Mamm. Sci. 21, 1–15. doi: 10.1111/j.1748-7692.2007.00167.x
Wolfe K., Desbiens A. A., Patel F., Kwong S., Fisher E., Mumby P. J., et al. (2025). eDNA confirms lower trophic interactions help to modulate population outbreaks of the notorious crown-of-thorns sea star. PNAS 122, e2424560122. doi: 10.1073/pnas.2424560122
Woodson C. B., Schramski J. R., and Joye S. B. (2018). A unifying theory for top-heavy ecosystem structure in the ocean. Nat. Commun. 9, 23. doi: 10.1038/s41467-017-02450-y
Woodson C. B., Schramski J. R., and Joye S. B. (2020). Food web complexity weakens size-based constraints on the pyramids of life. Proc. R. Soc B 287, 20201500. doi: 10.1098/rspb.2020.1500
Woodstock M. S., Kiszka J. J., Ramírez-León M. R., Sutton T. T., Fennel K., Wang B., et al. (2023). Cetacean-mediated vertical nitrogen transport in the oceanic realm. Limnol. Oceanogr. 68, 2445–2460. doi: 10.1002/lno.12433
Wootton J. T. (1995). Effects of birds on sea urchins and algae: A lower-intertidal trophic cascade. Ecoscience 2, 321–328. doi: 10.1080/11956860.1995.11682299
Worm B. and Myers R. A. (2003). Meta-analysis of cod-shrimp interactions reveals top-down control in oceanic food webs. Ecology 84, 162–173. doi: 10.1890/0012-9658(2003)084[0162:MAOCSI]2.0.CO;2
Wright R. M., Le Quéré C., Buitenhuis E., Pitois S., and Gibbons M. J. (2021). Role of jellyfish in the plankton ecosystem revealed using a global ocean biogeochemical model. Biogeosciences 18, 1291–1320. doi: 10.5194/bg-18-1291-2021
Yang G., Atkinson A., Pakhomov E. A., Hill S. L., and Racault M.-F. (2022). Massive circumpolar biomass of Southern Ocean zooplankton: Implications for food web structure, carbon export, and marine spatial planning. Limnol. Oceanogr. 67, 2516–2530. doi: 10.1002/lno.12219
Ye Y. and Carocci F. (2018). Control mechanisms and ecosystem-based management of fishery production. Fish Fish. 20, 15–24. doi: 10.1111/faf.12321
Yebra L., Espejo E., Putzeys S., Giráldez A., Gómez-Jakobsen F., León P., et al. (2020). Zooplankton biomass depletion event reveals the importance of small pelagic fish top-down control in the western mediterranean coastal waters. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.608690
Yiu D. S., Elliott Smith E. A., Farrell S. P., and Rasher D. B. (2025). Kelp forest loss and emergence of turf algae reshapes energy flow to predators in a rapidly warming ecosystem. Sci. Adv. 11, eadw7396. doi: 10.1126/sciadv.adw7396
Keywords: trophodynamics, trophic cascades, top-down-control, marine ecology, marine ecosystems, benthic ecosystems, neritic ecosystems, pelagic ecosystems
Citation: Surma S, Pakhomov EA and Pitcher TJ (2025) Trophic cascades and top-down control: found at sea. Front. Ecol. Evol. 13:1587171. doi: 10.3389/fevo.2025.1587171
Received: 04 March 2025; Accepted: 23 June 2025;
Published: 16 July 2025.
Edited by:
Christian Henri Nozais, Université du Québec à Rimouski, CanadaReviewed by:
Jason Link, National Marine Fisheries Service (NOAA), United StatesJory Cabrol, Fisheries and Oceans Canada (DFO), Canada
Copyright © 2025 Surma, Pakhomov and Pitcher. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Szymon Surma, cy5zdXJtYUBvY2VhbnMudWJjLmNh
 Szymon Surma
Szymon Surma Evgeny A. Pakhomov
Evgeny A. Pakhomov Tony J. Pitcher
Tony J. Pitcher